Abstract
Late spring frosts, occurring after spring phenological events, pose a dire threat to tree growth and forest productivity. With climate warming, earlier spring phenological events have become increasingly common and led to plants experiencing more frequent and severe frost damage. However, the effect of late spring frosts after leaf-out on subsequent flowering phenology in woody species remains unknown. Utilizing 572,734 phenological records of 640 species at 5024 sites from four long-term and large-scale in situ phenological networks across the Northern Hemisphere, we show that late spring frosts following leaf-out significantly delay the onset of the subsequent flowering by approximately 6.0 days. Late-leafing species exhibit greater sensitivity to the frosts than early-leafing species, resulting in a longer delay of 2.5 days in flowering. Trees in warm regions and periods exhibit a more pronounced frost-induced flowering delay compared to those in cold regions and periods. A significant increase in the frequency of late spring frost occurrence is observed in recent decades. Our findings elucidate the intricate relationships among leaf-out, frost, and flowering but also emphasize that the sequential progression of phenological events, rather than individual phenological stages, should be considered when assessing the phenological responses to climate change.
Similar content being viewed by others
Introduction
Tree phenology, the periodic events of tree growth and development during the year1, influences species distribution and carbon, water, and nutrient cycles2,3,4. Under climate warming, advanced spring phenological events, such as budburst and leaf-out have been widely reported in recent decades5,6,7. Late spring frost (LSF), defined as frost events occurring after spring phenological events8,9, affects tree growth and causes disequilibrium in the terrestrial ecosystems10,11. Due to the warming-induced earlier spring phenology, newly developed organs in trees, such as buds and leaves, may be exposed to an increasing risk of LSF in temperate and boreal forests12. Therefore, it is essential to investigate the interaction between LSF events and tree spring phenology to accurately predict frost damage to tree growth and forest carbon cycling.
While trees are highly resistant to below-freezing temperatures in winter and can survive cold frosts through the development of cold hardiness during the endodormancy, newly emerging leaves and flowers are susceptible and vulnerable to cold temperatures as trees deharden and come into an active metabolism stage where freezing resistance drops irreversibly after warm spells in spring13,14,15. Therefore, LSF can cause more severe damage than frosts in winter and early spring. Freezing temperatures often result in severe tissue dehydration due to extracellular ice formation16 and irreversible cellular damage as the ice crystals inside the cells disrupt cell walls and membranes mechanically, and then lead to cellular rupture and tissue necrosis, impeding tree growth and life cycle17,18,19. In addition to structural damage, LSF events also impair critical physiological processes. For example, cold temperature conditions can induce the degradation of chlorophyll and consequently lead to a reduction in photosynthetic rate and carbon assimilation20.
LSF damage does not always diminish even though frost events generally occur less often21, as damage occurrence is co-determined by the timing of spring frost and freezing resistance of trees22. Trees need to extend the growing season and optimize photosynthetic carbon uptake by initiating leaf development as early as possible23, while they have to, on the other hand, minimize severe spring frost damage as a result of earlier spring budburst and leaf-out24,25. Therefore, trees need to balance maximizing photosynthetic productivity with mitigating the risk of LSF14,26. Overall, warming-induced advanced spring phenology increases the risk of LSF exposure and aggravates the subsequent potential damage27,28,29,30. However, responses of tree growth to LSF are often regionally different and species-specific12,24. Individuals and species in colder regions face more severe and frequent cold extremes, and their frost hardiness is often higher than those in warmer regions31,32. Besides, the widely observed significant correlation between freezing resistance and leaf-out date manifests the possibility that early-leafing species might have evolved stronger frost resistance than late-leafing species in order to minimize the risk of frost damage during leaf emergence33,34. These varied frost responses emphasize the importance of utilizing long-term and large-scale datasets across taxa to provide valid and reliable insights into the effects of LSF on tree phenology.
Flowering phenology, as the beginning of the reproductive process, impacts plants’ mating pattern, gene flow among populations, and interactions with pollinators, determines fruit and seed production, and then influences species fitness, community dynamics, and ecosystem structures35,36. Previous studies emphasize the effects of warming temperature and photoperiod on flowering phenology. However, extreme cold freezing events, especially occurring after spring leaf-out, can also impact the reproductive phases37,38. Most studies focus on one single phenological phase and its shift, such as budburst or flowering. However, the growth and development of trees is an ongoing process and the phenological cycle is an integrated system39,40. One phenological phase is jointly influenced by environmental conditions and the prior phenological phases, as evidenced by the observed correlations between leaf-out and leaf senescence41,42, and between leaf-out and flowering26. For leafing-first trees, which spread leaves first and then bloom flowers, LSF occurring in low-freezing resistance phases causes more than severe foliage setback and canopy destruction, which adversely impacts the photosynthetic process and carbon uptake43,44,45. This damage to leaves may carry over to flowering and fruiting because the reproductive processes are closely linked to the prior growth of leaves46. Leaf-out timing and leaf development are critical for flowering phenology as newly synthesized carbohydrates by vegetative tissues provide energy to initiate and maintain flowering47. As such, LSF events after leaf-out might have an extended effect on subsequent flowering events and even a lagged effect on trees growth in subsequent years by altering resource allocation and nutrient cycling11,48. However, the extent to which flowering is affected by LSF damage on leaves at a large spatial scale, and the interrelationships among leaf-out, flowering, and frost events remain uninvestigated.
Using 572,734 records of 640 leafing-first species at 5024 sites from four long-term and large-scale phenological observation datasets in the Northern Hemisphere, we investigate the subsequent effect of LSF-induced leaf damage on flowering phenology. Specifically, we compare the differences in flowering timing between scenarios with and without LSF occurrence. Furthermore, we compare the impact of LSF on flowering across different species, regions, and periods. We hypothesize that LSF occurrence would delay flowering because LSF could impair photosynthetic carbon assimilation in foliage, consequently leading to a carbon shortage for subsequent reproductive processes.
Results
Effect of LSF on the timing of flowering
We collected 572,734 phenological records of 640 woody species and from 5024 sites from four large-scale ground-observed phenological networks in Europe, the USA, China, and Russia across different climate regions in the Northern Hemisphere (see Supplementary Data 1-3). The onset of leaf unfolding and first flowering were appointed to represent leaf-out and flowering stages for each phenological network. The timings of these phenophases were recorded as or converted to the day of year (DOY). We defined late spring frost (LSF) occurrence as the daily minimum temperature fell below −2.2 °C during the period between leaf-out and flowering using climate data from the E-OBS and CRU JRA v2.4 datasets. To examine the effect of LSF-induced leaf damage on the subsequent flowering, we calculated differences in leaf-out timing (ΔDOYleaf-out) and in flowering (ΔDOYflowering) for each consecutive two-year per species at each site and compared the difference in flowering timing after excluding the effect of leaf-out (ΔDOYflowering,LSF) when LSF occurred and did not. Two additional baselines of long-term mean leaf-out and flowering dates for all years and for years without LSF presence were also utilized to calculate ΔDOYleaf-out, ΔDOYflowering, and ΔDOYflowering,LSF. Positive and negative values of ΔDOYflowering,LSF indicated delayed and advanced flowering timing, respectively. Linear mixed models were utilized to examine the differences in impacts of LSF on flowering across species, regions and periods (see Methods and Supplementary Data 4).
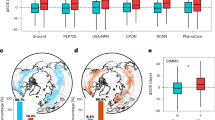
Using four ground-based phenological networks (i.e., the PEP725 network, USA-NPN, CPON, and RCNN), we observed a significant difference in the flowering timing between records with or without LSF occurrence. ΔDOYflowering,LSF remained centered at zero when LSF was absent, while it exhibited positive (6.14 ± 0.05 days) when LSF was present (Fig. 1b). This implied that LSF-induced damage on the leaf postponed flowering date significantly. Among all the four ground phenological observation datasets, the PEP725 network exhibited the most delayed flowering days, 6.30 ± 0.05 days, whereas RCNN showed the least delayed days, 3.49 ± 0.28 days (Fig. 1b and Supplementary Data 5a). The flowering timing delayed 4.77 ± 0.46 days and 3.56 ± 0.40 days in USA-NPN and CPON, respectively (Fig. 1b and Supplementary Data 5a). Using the long-term baselines, we also observed that flowering was delayed by LSF after leaf-out in the Northern Hemisphere (Supplementary Fig. 1ac). Additionally, flowering timing in Europe showed consistent delay responses when LSF occurred using CRU JRA v2.4 climate datasets (Supplementary Fig. 2).
Difference in ∆DOY between records in the presence and absence of LSF from all networks, the PEP725 network, USA-NPN, CPON, and RCNN (b) and for seven tree species in the PEP725 network (c). In a, Yellow dots represent the 3,969 sites selected from the PEP725 network. Green, blue, and red dots represent 918, 93, and 44 sites selected from USA-NPN, RCNN, and CNOP, respectively. In b, there are 57,001, 4,991, 734, and 1,239 qualified matched records in the PEP725 network, USA-NPN, CPON, and RCNN, respectively, in the presence of LSF. In the absence of LSF, these numbers are 203,950, 2,970, 10,017, and 5,465, respectively. In b and c, ∆DOY indicates ΔDOYflowering,LSF. Y and N indicate the presence and absence of LSF for each species in each year at each site, respectively. The black dashed line denotes when ∆DOY equals to zero. The boxes span from the first to the third quartile, with median values marked as the black lines in the middle of the boxes. The whiskers extend from the 5th to the 95th percentile. Values beyond the whiskers are hidden. The asterisk indicates a significant difference (two-side t-test, p < 0.05) in ∆DOY between LSF presence and absence using linear mixed models (Supplementary Data 5). Source data are provided in Source Data Fig. 1a–c.
To ensure the robustness of the results, we further employed linear mixed models to exclude the effects of climate variations between leaf-out and flowering on the date of flowering for each phenological dataset (see Methods). The linear mixed model outcomes highlighted that LSF significantly delayed flowering by 3.56 ± 0.04 days after excluding the influences of precipitation, radiation, and drought between leaf-out and flowering as well as leaf-out date (Supplementary Data 6). Moreover, we extended the investigation by utilizing different temperature thresholds (i.e., −2 °C, −5 °C, and −8 °C) to define frost occurrence. The flowering was observed delay in all phenological observation networks under each frost temperature threshold (Supplementary Data 7). Besides, the results underscored a significant difference among temperature thresholds in the PEP725 network and USA-NPN and an escalation in frost-induced delayed flowering as lower temperature threshold indicated exacerbated frost severity (Supplementary Fig. 3 and Supplementary Data 8).
Species-specific responses
For the PEP725 network, the largest ground phenological network used in our study, all of the selected species postponed flowering timing when LSF occurred (Fig. 1c and Supplementary Fig. 1bd). Among the seven species, Tilia platyphyllos and Aesculus hippocastanum delayed flowering over seven days, followed by Tilia cordata and Sorbus aucuparia, while Ribes uva-crispa, Fagus sylvatica, and Betula pendula delayed less than five days (Fig. 1c and Supplementary Data 5b). We categorized tree species into early-leafing and late-leafing groups based on the mean leaf-out dates across all the species. We consistently observed a delay in flowering for both early- and late-leafing groups using ground phenological datasets during LSF occurrence (Fig. 2ab). When encountering LSF, early- and late-leafing species delayed flowering by 5.05 ± 0.07 days and 7.50 ± 0.10 days, respectively, and demonstrated a significant difference in ΔDOYflowering,LSF (Fig. 2 and Supplementary Data 9a). However, in the absence of LSF, flowering timing change remained stably centered at zero and showed no evident difference between the two groups (Fig. 2c). We also observed a more pronounced delay in late-leafing species compared to early-leafing species using varying LSF temperature thresholds (Supplementary Fig. 4 and Supplementary Data 9b). When exposed to more severe LSF, indicated by lower frost temperature thresholds, both early- and late-leafing species exhibited escalated delays in flowering (Supplementary Data 10). The results of linear mixed models also supported that late-leafing species experienced more delayed flowering, as indicated by the positive interaction between LSF and leaf-out date (Supplementary Data 11 and Supplementary Data 12).
∆DOY indicates ΔDOYflowering,LSF. a–b The distributions of ∆DOY in the presence and absence of LSF and the estimates of ∆DOY and se (in parentheses) for early-leafing (a) and late-leafing species (b) using linear mixed models, respectively. c Differences in ∆DOY between early- and late-leafing species in the presence and absence of LSF. In a-c, Y and N indicate the presence and absence of LSF for each species in each year at each site, respectively. The asterisks indicate a significant difference (two-side t-test, p < 0.05) in ∆DOY using linear mixed models (Supplementary Data 9). In c, the black dashed line denotes when ∆DOY equals to zero. The boxes span from the first to the third quartile, with intermediate values marked as the black lines in the middle of the boxes. The whiskers extend from the 5th to the 95th percentile. Values beyond the whiskers are hidden. In the presence of LSF, there are 39,350 matched observations in the early-leafing group and 24,615 in the late-leafing group. In the absence of LSF, these numbers are 91,290 and 131,112, respectively. Source data are provided in Source Data Fig. 2.
Spatial and temporal variations
Using linear mixed models, we further examined the difference in the effect of LSF on flowering across different climate regions and periods (see Methods and Supplementary Data 4). We observed that when LSF occurred, the extent of the delay in flowering increased from the boreal region to the subtropical region (Fig. 3). The flowering timing of trees in the subtropical, temperate, and boreal regions was delayed by 5.36, 3.53, and 2.13 days, respectively (Supplementary Data 11). The positive interaction between LSF occurrence and mean temperature per site also suggested that this delaying effect was significantly more pronounced in warm regions than in cold ones (Supplementary Data 12).
In these models, all leafing-flowering matched observations (286,367 in total) were included. Species, region, and period were categorized into early-(130,640) and late-leafing (155,727) species, boreal (1994), temperate (6982), and subtropical (276,989) region, and period of 1950–1980 (118,591) and 1981–2021 (167,776), respectively. Numbers in the parentheses are the exact leafing-flowering matched sample size for each group. Flowering timing was the response variable, while species or region or period, LSF, and its interaction with each categorical variable were the predictors, with species and sites as random intercepts. The red and blue points indicate the negative and positive estimates of predictor effect size, respectively. Error bars represent 95% confidence intervals (CIs). The black vertical dashed line denotes when estimate of effect size equals to zero. The asterisks indicate a significant difference (two-side t-test, p < 0.05) from zero according to the mixed models results. Variables in these mixed models are standardized in order to compare the effect size within and between models. The detailed estimations of the mixed models are provided in Supplementary Data 11. Source data are provided in Source Data Fig. 3.
We also compared the difference in LSF-induced flowering delays between the periods of 1950–1980 and 1981–2021 using linear mixed models. We observed a significant increase in the delaying effect of LSF on flowering timing, from 3.36 days to 3.68 days (Fig. 3 and Supplementary Data 11). Furthermore, the positive interaction between LSF occurrence and the calendar year in the linear mixed model also suggested that the delay response of flowering timing to LSF intensified as climate warming progressed (Supplementary Data 12).
LSF frequency, FSI, and GDD
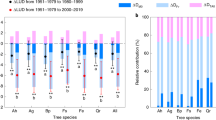
To assess LSF severity and prevalence, we calculated the LSF frequency occurring between leaf-out and flowering. We also calculated the FSI and accumulated GDD to assess tree frost susceptibility and vulnerability. The FSI was calculated as the number of days from leaf-out to the last LSF, and GDD was calculated as the sum of daily mean temperature above 5 °C from leaf-out date to the last LSF. Linear mixed models were used to examine the differences in LSF frequency, FSI, and GDD across species, climate regions, and periods. We found that early-leafing species experienced more frequent LSF events than late-leafing species. In addition, the LSF frequency between leaf-out and flowering was higher in the boreal region, followed by the temperate region, and lowest in the subtropical region. The LSF frequency during 1981-2021 was significantly higher than during 1950–1980 (Fig. 4 and Supplementary Data 13). Both FSI and GDD were significantly higher for early-leafing species than late-leafing species. Additionally, FSI and GDD exhibited significant increases from boreal to subtropical regions. The FSI and GDD were significantly lower in the period of 1950–1980 than in 1981−2021 (Fig. 4 and Supplementary Data 13). To test the robustness of our results, we applied linear mixed models incorporating continuous variables of mean site-species leaf-out date, mean temperature per site, and calendar year to examine the patterns of frost prevalence and tree frost vulnerability (Supplementary Data 14). We obtained similar results based on linear mixed models incorporating continuous and categorical variables.
In the mixed models, LSF frequency, FSI (the False Spring Index), and GDD (the accumulation of growing degree days) were response variables, while species, region, and period were the predictors, with species and sites as random intercepts. All records (81,093 in total) used in these models were in the presence of LSF, including 33,413 records during 1950–1980 and 47,680 records during 1981–2021. LSF frequency, FSI, and GDD were calculated as the proportion of days with LSF within the period between leaf-out and the last LSF, the days from leaf-out to the last LSF, and the sum of daily mean temperature above 5°C from leaf-out date to the last LSF, respectively. Species, region, and period were categorized into early- and late-leafing species, boreal, temperate, and subtropical region, and period of 1950-1980 and 1981-2021, respectively. The red and blue points indicate negative and positive estimates of predictor effect size, respectively. Error bars represent 95% confidence intervals (CIs). The black vertical dashed line denotes when estimate of effect size equals to zero. The asterisks indicate a significant difference (two-side t-test, p < 0.05) from zero according to the mixed models results. Variables in these mixed models are standardized in order to compare the effect size within and between models. The detailed estimations of the mixed models are provided in Supplementary Data 13. Source data are provided in Source Data Fig. 4.
Discussion
Delayed flowering timing induced by LSF
Utilizing four long-term and large-scale ground-based phenological datasets, our study revealed that LSF, taking place after leaf-out but before flowering, led to a delay in subsequent flowering in the Northern Hemisphere (Fig. 5). This observed LSF-induced delay in flowering could be due to reduced carbohydrates from frost damage to leaves. Leaf photosynthetic capacity is highly sensitive to temperature variation49. Chlorophyll, the photosynthetic pigment responsible for capturing light energy, degrades during cold temperatures, which leads to a decrease in light absorption rate20. In addition, energy from light cannot be employed for CO2 assimilation efficiently in below-freezing conditions, resulting in a reduction in carbon fixation and photosynthate production50,51. Adequate carbon assimilation and storage are essential for early spring growth processes such as leaf-out and flowering52. Nonstructural carbohydrates, comprising soluble sugars and starch, reach their peak in autumn prior to dormancy and subsequently diminish after the completion of spring growth53,54. LSF events taking place after leaf-out can reduce the photosynthetic area, thereby causing a shortfall in the supply of newly synthesized photosynthate55. After LSF events, tree leaves may require 16 to 34 days to regrow45,56. This prolonged regrowth period significantly hinders photosynthetic carbon assimilation57. When confronted with extreme climate events, trees typically prioritize survival by reallocating more carbohydrates to sustain basic metabolic activities and reduce investments to reproduce58,59. In the context of LSF, trees are compelled to invest more resources and stored carbon reserves in mitigating frost damage and facilitating the recovery of leaves and canopy55,60 to ensure survival, however, at the cost of growth and reproduction. Therefore, LSF events occurring after leaf-out can lead to a carbon shortage in flowering, adversely affecting the subsequent reproductive processes and resulting in a delay in flowering timing10,61.
Freezing resistance often varies among tree species and geographic regions. For instance, a temperature of −8 °C is still safe for conifers to grow young leaves in cold regions32,62, whereas −2 °C may cause serious damage to less cold-hardy species in subtropical region63. To evaluate the robustness of our conclusions, we further applied various frost temperature thresholds (−2 °C, −5 °C, and −8 °C) to define LSF presence. Across these thresholds, we consistently observed a significant delay in flowering, underscoring the influence of LSF irrespective of the specific temperature criteria. This confirmed that the delay effect of LSF on flowering was widespread and held true for most species. Additionally, the results from the linear mixed models demonstrated that while temperature is considered the primary driver of spring phenological events, the significance of extreme freezing events should not be disregarded. Substantial shifts might occur, particularly with the presence of LSF. The LSF-induced delay in flowering may have crucial ecological implications for ecosystem structures and functions. A mismatch between the emergence of pollinators and resource availability brought by the delayed flowering timing alters the dynamics of plant-pollinator interactions and results in decreased reproductive success and recruitment, which potentially declines both the abundance of plant and pollinator populations and genetic diversity64,65. Furthermore, the distinct extends of LSF-induced flowering delay can affect interspecific competition66, trophic cascades67, community composition68, and ecosystem functioning including nutrient cycling and energy flow26,69,70. And the observed delay in flowering is detrimental to ecosystem services provided by trees including carbon sequestration and biodiversity support71,72. Therefore, it is essential to consider the LSF-induced delay in flowering when assessing the impacts of climate change on tree phenology, allowing for a more comprehensive understanding of the potential cascading effects of climate extremes on the functions and services of forest ecosystems.
Early- and late-leafing species
Our research indicated that, when LSF was present, late-leafing species exhibited more pronounced delays in flowering timing compared to early-leafing species. Early-leafing species generally experience more frequent LSF events and accumulate more thermal units from leaf-out to the last LSF. This suggests that early-leafing species often face a higher risk of LSF than late-leafing species. However, in the presence of LSF, early-leafing species exhibit less delay in flowering compared to late-leafing species. This observed variation between early- and late-leafing species can be largely attributed to the distinct levels of freezing resistance found in these two groups of species. Early-leafing species are often considered as ‘opportunists’ becaues they act quickly to warming air temperatures and leaf-out with a low thermal accumulation, resulting in rapidly responding to early spring warm spells and optimizing photosynthesis through earlier leaf-out12,73. Due to their frequent exposure to severe frost, early-leafing species are likely to have undergone evolutionary adaptations, which equip them with enhanced resilience against the severe impacts of frost events74,75. In contrast, late-leafing species such as F. sylvatica rely on strict photoperiod cues and higher chilling requirements to break bud dormancy, a strategy potentially offering protection against LSF damage76,77. This aligns with research on frost resistance, which shows a high correlation between freezing tolerances (measured as lethal temperature for 50% mortality, LT50) and leaf-out dates, i.e., the earlier the leaf-out, the greater the frost resistance14,78. Therefore, early-leafing species, characterized by a higher degree of freezing hardiness, are expected to possess stronger frost resistance than late-leafing species. Consequently, the species-specific variation in frost resistance may account for the observed trend, wherein the flowering timing of early-leafing species exhibits a relatively conservative response to LSF events. The freezing resistance variations among species can provide cues to mitigate the detrimental impacts of LSF events on trees, such as breeding and selecting frost-resistant varieties capable of withstanding extreme cold events79,80.
Spatial and temporal variations
Our observations reveal varying effects of LSF on the timing of flowering across subtropical, temperate, and boreal regions. Trees in subtropical areas exhibited the most significant response to LSF, resulting in the greatest delay in flowering timing, whereas trees in boreal regions demonstrated the least delay. The observed spatial escalating response of LSF-induced flowering delay could be due to the distinct freezing resistances and thermal conditions of trees in each climatic region. In colder regions, trees experience lower temperatures and more fluctuating thermal conditions on average, resulting in stronger resistance to frosts through adaptive evolution32,81. However, in warmer areas, trees typically have weaker freezing resistance due to favorable thermal conditions with infrequent frost events. In addition, trees in warm areas often accumulate more thermal energy before the last frost event, making them more susceptible to frost damage. Although frost events are less frequent and the climates are milder in warm regions compared to cold regions, the LSF may cause devastating damage when it occurs in these warmer areas. Therefore, the delay in flowering induced by LSF is more pronounced in warmer regions than in colder regions.
We observed an increasing trend in the delayed responses of flowering to LSF from 1950–1980 to 1981–2021. This could be partly attributed to increased LSF risks denoted by higher LSF frequency and elevated prevalence. Besides, as indicated by the higher FSI and more GDD, the potential frost damage during 1981–2021 is exacerbated as the longer interval from leaf-out to the last frost and sufficient energy supply and thermal accumulation, resulting in full leaf development and vulnerable states56,61, which incurs more severe damage and more flowering delay. These findings suggest that spatial variations and temporal changes in thermal accumulation are key factors in the flowering response to LSF, explaining the distinct delays in flowering between warm and cold regions or periods. The LSF frequency and tree frost susceptibility are expected to become more intensified and aggravated with global climate change56. Therefore, the observed effect of LSF-induced damage on leaf-out on subsequent flowering should be integrated into current process-based phenological models to accurately predict changes in flowering phenology in the future.
Using in situ sequential phenological records of leaf-out and flowering in the Northern Hemisphere, our study revealed that LSF occurring after leaf-out significantly delayed the onset of the subsequent flowering phase. Late-leafing species exhibited a more pronounced flowering delay response compared to early-leafing species. Trees in warm regions and periods exhibited more pronounced delays in response to frost-induced damage compared to those in cold regions and periods, likely due to diminished freezing resistance from adaptive evolution. Moreover, favorable thermal conditions and rapid loss of freezing resistance also provide potential explanations for the pronounced LSF response in warmer regions and periods. Since 1980, an elevated prevalence of LSF has been observed, indicating a higher risk of LSF affecting flowering. Our findings emphasize the importance of considering the sequential progression of phenological stages when assessing responses to climate change and the necessity of incorporating the observed effects of extremes into current phenology models to enhance their accuracy, particularly under global warming scenarios.
Methods
In situ phenological datasets
Phenological datasets used in this study were obtained from the Pan European Phenology Network (the PEP725 network, www.pep725.eu), USA National Phenology Network (USA-NPN, https://www.usanpn.org/results/data), China Phenological Observation Network (CPON, http://www.cpon.ac.cn), and Russian ‘Chronicles of Nature’ Network (RCNN, https://doi.org/10.1038/s41597-020-0376-z). These datasets provide access to in situ phenological observation records for multiple phenophases of plant species across the Northern Hemisphere (Supplementary Data 1). The PEP725 network, the largest one of the four datasets, is comprised of 13 million phenological records of 201 species across central Europe at over 26,000 sites from 1868 to the present81. The CPON, RCNN, and USA-NPN provide records at 44 sites in China, at 471 sites across the Russian Federation, Ukraine, Uzbekistan, Belarus, and Kyrgyzstan, and at 3000 sites in the USA, respectively82,83,84. The onset of leaf unfolding and first flowering were appointed to represent leaf-out and flowering stages. The definitions for leaf-out and flowering were generally similar despite of subtle differences in the descriptions among phenological observation networks (Supplementary Data 2). The timings of leaf-out and flowering were recorded as or converted to the day of year (DOY).
Because we aimed to examine the effect of LSF-induced damage to leaves on subsequent flowering phenophase, we only selected leafing-first tree species observations, which spread leaves first and then bloomed flowers, and removed records with flowering earlier than leaf-out, and then matched leaf-out and flowering dates according to species, site, and year, retaining time series that recorded both timings of leaf-out and flowering. Records with leaf-out or flowering dates exceeding 2.5 times the median absolute deviation (MAD) for each species-site combination were removed to exclude potential biases from outliers. We then selected species-site combinations with at least 5-year records for USA-NPN, CPON, and RCNN during 1962–2021, 1964–2014, and 1950–2017, respectively (Supplementary Data 3a). To examine temporal changes in the relationship between phenology and LSF, we selected species with at least 100 sites for more than 15 years of observations during 1952-2021 for long-term records of seven widely distributed temperate tree species from the PEP725 network (Supplementary Data 3c). In total, we selected 572,734 qualified observations from 640 species (7, 98, 433, and 136 species from the PEP725 network, USA-NPN, CPON, and RCNN, respectively) at 5024 sites (3969 sites from the PEP725 network, 918 sites from USA-NPN, 44 sites from CPON, and 93 sites from RCNN) from 1950 to 2021 (Fig. 1a and Supplementary Data 3b).
Climate dataset and global climate zones
We obtained daily temperature data from a gridded climate dataset E-OBS, which is based on the EU-FP6 project ENSEMBLES and the ECA&D project (https://www.ecad.eu). E-OBS Version 28.0e data files contained daily minimum temperature between 1950 and 2021 for the European Continent, with a spatial resolution of 0.1°, and were used to match the PEP725 network phenological records considering the high-density distribution of PEP725 sites. We matched the other three phenological datasets with daily minimum temperature during 1950-2021 from the CRU JRA v2.4 datasets at 0.5° spatial resolution85. To test the robustness of the results and exclude the effect of spatial resolution, we further re-examined the effect of LSF on flowering in Europe using CRU JRA v2.4 datasets (Supplementary Fig. 2).
To exclude the potential effects of climate variables on the spring phenological events, we obtained climatic data, including daily mean temperature (TMP), daily total precipitation (PRE), and daily downward solar radiation flux (DSWRF) data from the global climate dataset CRU JRA v2.4 at a spatial resolution of 0.5° during 1950–2021. We also acquired monthly potential evapotranspiration and precipitation from CRU TS4.06 at 0.5° spatial resolution during 1950-2021 to estimate the standardized precipitation evapotranspiration index (SPEI)86. The bilinear interpolation method and the terra package87 were used to extract climate data for each observation site in R88. Then, the Global Ecological Zone (GEZ) of the Forest Resources Assessment was used to define the climate zone for each site with a spatial resolution of 0.05°89. We excluded tropical sites due to their aseasonal phenological cycles and the insufficient number of observations, and all sites were classified into subtropical, temperate, and boreal regions based on the GEZ 2010 map.
Statistical analyses
Effect of LSF on the timing of flowering
We defined late spring frosts (LSF) as frost events occurring after leaf-out and before flowering, with the daily minimum temperature below −2.2 °C25,90,91,92. This temperature criterion was used to qualify a hard freeze as potential damage might occur under this thermal condition91,93,94. We also employed frost temperature thresholds of −2 °C, −5 °C, and −8 °C to test the robustness of the results and investigate the phenological responses across different intensities of LSF through linear mixed models following ANOVA and Tukey HSD tests (Supplementary Data 4). All linear mixed models were performed using lmerTest package95.
To analyze the effect of LSF after leaf-out on flowering timing, we first calculated the difference in leaf-out date (ΔDOYleaf-out) and difference in flowering date (ΔDOYflowering) using Eqs. 1 and 2, respectively, for each consecutive two-year period. Considering the correlation between leafing and flowering, we excluded the effect of leaf-out date on flowering date and calculated ΔDOYflowering,LSF (Eq. 3).
Where DOYleaf-out,t and DOYflowering,t are leaf-out and flowering dates in year t of specific site-species combination, DOYleaf-out,t-1 and DOYflowering,t-1 are leaf-out and flowering dates in year t-1. ΔDOYleaf-out, ΔDOYflowering, and ΔDOYflowering,LSF represent change of leaf-out date, change of flowering date, and flowering timing change after excluding the effect of leaf-out, respectively. Positive and negative values of ΔDOYflowering,LSF indicated that flowering timing delayed and advanced, respectively. For year without LSF, controlling for temperature, precipitation, radiation, and dryness (SPEI), ΔDOYflowering,LSF was expected to be zero. Linear mixed models were carried out to test the statistical difference of ΔDOYflowering,LSF between LSF presence and absence and assess the magnitude of shifts in flowering dates due to LSF for each dataset (Supplementary Data 4). In the models, the response variable was the ΔDOYflowering,LSF, and the fixed effect was the LSF (presence or absence), with random intercepts among species and sites.
In addition, we examined the effect of LSF on flowering date using the long-term mean leaf-out and flowering dates across all years, and the mean leaf-out and flowering dates of years in the absence of LSF for each site-species combination as two additional baselines to calculate ΔDOYleaf-out, ΔDOYflowering, and ΔDOYflowering,LSF. Specifically, we calculated the ΔDOYflowering,LSF based on following Eqs. 4 and 5.
Where DOYflowering,t and DOYleaf-out,t are the flowering and leaf-out dates for species i at site j in year t, respectively. DOYflowering,all and DOYleaf-out,all are the mean flowering and leaf-out dates of all years for species i-site j combination, and ΔDOYflowering,LSF is the change of flowering date in year t to the mean flowering timing for species i at site j after excluding change of leaf-out date.
Similar to Eq. 4, however, DOYflowering,absence and DOYleaf-out,absence are the mean flowering and leaf-out dates of species i-site j combination using years without LSF occurrence, which characterize more normal leaf-out and flowering timings.
To ensure the robustness of results, we also examined the effect of LSF on flowering by pooling all the phenological records across all the selected species and sites using linear mixed models (Supplementary Data 4). In the mixed models, the response variable was flowering date, and the predictors were the LSF (presence or absence), leaf-out date, and the temperature, precipitation, radiation, and drought index calculated as the mean TMP (°C), PRE (mm), DSWRF (kJ/m2), and SPEI between leaf-out and flowering for each year, respectively, with random intercepts among species and sites.
Species-specific responses of flowering to LSF
To investigate species-specific responses of flowering to LSF, we compared the ΔDOYflowering,LSF between the presence and absence of LSF for each species in the PEP725 network. We categorized each species into early-leafing and late-leafing groups based on the mean leaf-out date across all the species of the four phenological observation networks and then tested the differences in ΔDOYflowering,LSF differences between early-leafing and late-leafing species when LSF was present and absent using linear mixed models (Supplementary Data 4). In the models, the response variable was the ΔDOYflowering,LSF, and the fixed effects were the LSF (presence and absence), species type (early- and late-leafing species), and their interaction, with random intercepts among species and sites. Moreover, we compared ΔDOYflowering,LSF between early- and late-leafing species when LSF occurred employing −2 °C, −5 °C, and −8 °C as frost temperature thresholds. Tukey HSD tests following the linear mixed models were further applied to compare the differences in ΔDOYflowering,LSF across different temperature thresholds for each species group.
To test the robustness of species-divergent responses, we further conducted linear mixed models, in which the response variable was flowering timing, and the categorical variable of species group and continuous variable of leaf-out date, and their interactions with LSF were incorporated as predictors, with random intercepts among species and sites. In this and following mixed models with interaction terms, all explanatory variables are standardized96 (Supplementary Data 4).
Spatial-temporal variation in the responses of flowering to LSF
To investigate the spatial difference in the response of flowering to LSF, a linear mixed model was used to compare the difference in LSF responses among different climate regions when LSF occurred and when it was absent. In this mixed model, we included the categorical variable of climate region and its interaction with LSF into the base model with climate variables (Supplementary Data 4). We further explored the temporal change in the LSF-induced effect on flowering with climate warming. Specifically, we divided the time series into two periods: 1950-1980 and 1981-2021, as the period since 1980 experienced unprecedented climate warming97,98. Then, we applied the mixed model by including the category of period and its interaction with LSF into the base model with climate variables to test the temporal difference of flowering response to LSF between 1950–1980 and 1981–2021 (Supplementary Data 4). To ensure the robustness of our results, we updated the linear mixed models of categorical variables to include continuous predictors, including mean temperature per site and the calendar year, and their interactions with LSF, and examined the phenological responses across continuous time and space.
LSF frequency, FSI, and GDD across species, regions, and periods
To evaluate the frost severity and prevalence across species, region, and period, we calculated the LSF frequency as the proportion of days with LSF within the period between leaf-out and flowering. The time gap between the date of leaf-out and the last LSF was referred to as the safety margin against frost or False Spring Index (FSI), which is used to assess frost damage90,92,93. A larger FSI indicates an increased potential frost damage as trees further develop into susceptible states with larger leaf area which may incur more severe damage38,92. We calculated FSI for each observation with the occurrence of LSF to examine the LSF potential damage. To assess tree frost vulnerability, the accumulation of growing degree days (GDD) was calculated as the sum of daily mean temperature above 5 °C from the leaf-out date to the last LSF for each LSF-present record99. More GDD accumulated before the last late spring frost (LSF) indicates higher frost vulnerability since trees that experience more thermal availability are more susceptible to frost damage12.
We applied linear mixed models to compare the difference in frost severity and frost damage factors across species groups, regions, and periods. In these models, all records were with the presence of LSF. The response variables were standardized LSF frequency, FSI, and GDD, and the predictors were categories of species groups, regions, and periods, each participating in separate regression analyses, with random intercepts among sites and species (Supplementary Data 4 and 13). We updated the linear mixed models by replacing categorical variables with standardized continuous predictors, including the mean leaf-out date for each species, the mean temperature per site, and the calendar year, and examined the differences in LSF frequency, FSI, and GDD across species, regions, and periods (Supplementary Data 4 and 14).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data used for this work are publicly available. The PEP725 phenological data was obtained from http://www.pep725.eu. USA-NPN data was accessed from https://www.usanpn.org/results/data. CPON data was accessed from http://www.cpon.ac.cn. RCNN data was downloaded from https://doi.org/10.1038/s41597-020-0376-z. Climate data were downloaded from E-OBS Version 28.0e dataset (https://surfobs.climate.copernicus.eu/dataaccess/access_eobs.php), CRU TS4.06 dataset (https://catalogue.ceda.ac.uk/uuid/e0b4e1e56c1c4460b796073a31366980), and CRU JRA v2.2 dataset (https://catalogue.ceda.ac.uk/uuid/4bdf41fc10af4caaa489b14745c665a6). Source data are provided as Source Data files. Source data are provided with this paper.
Code availability
The primary codes used in this study are available at https://doi.org/10.6084/m9.figshare.25678371.
References
Phenology and Seasonality Modeling. 8 (Springer Berlin Heidelberg, Berlin, Heidelberg, 1974).
Chuine, I. Why does phenology drive species distribution? Philos. Trans. R. Soc. B Biol. Sci. 365, 3149–3160 (2010).
Peñuelas, J., Rutishauser, T. & Filella, I. Phenology Feedbacks on Climate Change. Science 324, 887–888 (2009).
Richardson, A. D. et al. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. Meteorol. 169, 156–173 (2013).
Fu, Y. H. et al. Recent spring phenology shifts in western Central Europe based on multiscale observations. Glob. Ecol. Biogeogr. 23, 1255–1263 (2014).
Guo, J. et al. Meta-analytic and experimental evidence that warmer climate leads to shift from advanced to delayed spring phenology. Agric. Meteorol. 342, 109721 (2023).
Menzel, A. et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976 (2006).
Kollas, C., Körner, C. & Randin, C. F. Spring frost and growing season length co-control the cold range limits of broad-leaved trees. J. Biogeogr. 41, 773–783 (2014).
Körner, C. et al. Where, why and how? Explaining the low-temperature range limits of temperate tree species. J. Ecol. 104, 1076–1088 (2016).
Inouye, D. W. The ecological and evolutionary significance of frost in the context of climate change. Ecol. Lett. 3, 457–463 (2000).
Vitasse, Y., Lenz, A. & Körner, C. The interaction between freezing tolerance and phenology in temperate deciduous trees. Front. Plant Sci. 5, (2014).
Zohner, C. M. et al. Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proc. Natl Acad. Sci. 117, 12192–12200 (2020).
Kovaleski, A. P. Woody species do not differ in dormancy progression: Differences in time to budbreak due to forcing and cold hardiness. Proc. Natl Acad. Sci. 119, e2112250119 (2022).
Lenz, A., Hoch, G., Vitasse, Y. & Körner, C. European deciduous trees exhibit similar safety margins against damage by spring freeze events along elevational gradients. N. Phytol. 200, 1166–1175 (2013).
Vitasse, Y. & Rebetez, M. Unprecedented risk of spring frost damage in Switzerland and Germany in 2017. Clim. Change 149, 233–246 (2018).
Steponkus, P. L., Lynch, D. V. & Uemura, M. The influence of cold acclimation on the lipid composition and cryobehaviour of the plasma membrane of isolated rye protoplasts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 326, 571–583 (1990).
Meryman, H. T. Freezing Injury and its Prevention in Living Cells. Annu. Rev. Biophys. 3, 341–363 (1974).
Olien, C. R. Freezing Stresses and Survival. Annu. Rev. Plant Biol. 18, 387–408 (1967).
Panter, P. E., Panter, J. R. & Knight, H. Impact of Cell‐wall Structure and Composition on Plant Freezing Tolerance. In Annual Plant Reviews online (ed. Roberts, J. A.) 607–642 (Wiley, 2020). https://doi.org/10.1002/9781119312994.apr0746.
Chen, C. et al. Effect of Temperature on Photosynthetic Pigment Degradation during Freeze–Thaw Process of Postharvest of Celery Leaves. Horticulturae 10, 267 (2024).
Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change. (Cambridge University Press, 2012). https://doi.org/10.1017/CBO9781139177245.
Rigby, J. R. & Porporato, A. Spring frost risk in a changing climate. Geophys. Res. Lett. 35, L12703 (2008).
Cannell, M. G. R. Spring phenology of trees and frost avoidance*. Weather 52, 46–52 (1997).
Augspurger, C. K. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology 94, 41–50 (2013).
Ma, Q., Huang, J.-G., Hänninen, H. & Berninger, F. Divergent trends in the risk of spring frost damage to trees in Europe with recent warming. Glob. Change Biol. 25, 351–360 (2019).
Guo, L. et al. Climatic drivers and ecological implications of variation in the time interval between leaf-out and flowering. Curr. Biol. 0, (2023).
Allstadt, A. J. et al. Spring plant phenology and false springs in the conterminous US during the 21st century. Environ. Res. Lett. 10, 104008 (2015).
Lamichhane, J. R. Rising risks of late-spring frosts in a changing climate. Nat. Clim. Change 11, 554–555 (2021).
Liu, Q. et al. Extension of the growing season increases vegetation exposure to frost. Nat. Commun. 9, 426 (2018).
Richardson, A. D. et al. Ecosystem warming extends vegetation activity but heightens vulnerability to cold temperatures. Nature 560, 368–371 (2018).
Hofmann, M., Durka, W., Liesebach, M. & Bruelheide, H. Intraspecific variability in frost hardiness of Fagus sylvatica L. Eur. J. Res. 134, 433–441 (2015).
Kreyling, J., Schmid, S. & Aas, G. Cold tolerance of tree species is related to the climate of their native ranges. J. Biogeogr. 42, 156–166 (2015).
Vitasse, Y., Lenz, A., Hoch, G. & Körner, C. Earlier leaf-out rather than difference in freezing resistance puts juvenile trees at greater risk of damage than adult trees. J. Ecol. 102, 981–988 (2014).
Vitra, A., Lenz, A. & Vitasse, Y. Frost hardening and dehardening potential in temperate trees from winter to budburst. N. Phytol. 216, 113–123 (2017).
Elzinga, J. A. et al. Time after time: flowering phenology and biotic interactions. Trends Ecol. Evol. 22, 432–439 (2007).
Franks, S. J. The unique and multifaceted importance of the timing of flowering. Am. J. Bot. 102, 1401–1402 (2015).
Inouye, D. W. Effects of Climate Change on Phenology, Frost Damage, and Floral Abundance of Montane Wildflowers. Ecology 89, 353–362 (2008).
Schwartz, M. D. Assessing the onset of spring: a climatological perspective. Phys. Geogr. 14, 536–550 (1993).
Morellato, L. P. C., Alberti, L. F. & Hudson, I. L. Applications of Circular Statistics in Plant Phenology: a Case Studies Approach. in Phenological Research (eds. Hudson, I. L. & Keatley, M. R.) 339–359 (Springer Netherlands, Dordrecht, 2010). https://doi.org/10.1007/978-90-481-3335-2_16.
Buonaiuto, D. M. & Wolkovich, E. M. Differences between flower and leaf phenological responses to environmental variation drive shifts in spring phenological sequences of temperate woody plants. J. Ecol. 109, 2922–2933 (2021).
Fu, Y. H. et al. Nutrient availability alters the correlation between spring leaf-out and autumn leaf senescence dates. Tree Physiol. 39, 1277–1284 (2019).
Fu, Y. S. H. et al. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl Acad. Sci. 111, 7355–7360 (2014).
Hufkens, K. et al. Ecological impacts of a widespread frost event following early spring leaf-out. Glob. Change Biol. 18, 2365–2377 (2012).
Kreyling, J., Stahlmann, R. & Beierkuhnlein, C. Spatial variation in leaf damage of forest trees and the regeneration after the extreme spring frost event in May 2011. Allg. Forst- Jagdztg. 183, 15–22 (2012).
Menzel, A., Helm, R. & Zang, C. Patterns of late spring frost leaf damage and recovery in a European beech (Fagus sylvatica L.) stand in south-eastern Germany based on repeated digital photographs. Front. Plant Sci. 6, (2015).
Hänninen, H. & Tanino, K. Tree seasonality in a warming climate. Trends Plant Sci. 16, 412–416 (2011).
Gough, C. M., Flower, C. E., Vogel, C. S. & Curtis, P. S. Phenological and Temperature Controls on the Temporal Non-Structural Carbohydrate Dynamics of Populus grandidentata and Quercus rubra. Forests 1, 65–81 (2010).
Liu, W. et al. Importance of the memory effect for assessing interannual variation in net ecosystem exchange. Agric. Meteorol. 341, 109691 (2023).
Dreyer, E., Le Roux, X., Montpied, P., Daudet, F. A. & Masson, F. Temperature response of leaf photosynthetic capacity in seedlings from seven temperate tree species. Tree Physiol. 21, 223–232 (2001).
Corcuera, L., Morales, F., Abadia, A. & Gil-Pelegrin, E. The effect of low temperatures on the photosynthetic apparatus of Quercus ilex subsp. ballota at its lower and upper altitudinal limits in the Iberian peninsula and during a single freezing-thawing cycle. Trees 19, 99–108 (2005).
Tanaka, A. Photosynthetic activity in winter needles of the evergreen tree Taxus cuspidata at low temperatures. Tree Physiol. 27, 641–648 (2007).
Tissue, D. T. & Nobel, P. S. Carbon Relations of Flowering in a Semelparous Clonal Desert Perennial. Ecology 71, 273–281 (1990).
Davidson, A. M., Le, S. T., Cooper, K. B., Lange, E. & Zwieniecki, M. A. No time to rest: seasonal dynamics of non-structural carbohydrates in twigs of three Mediterranean tree species suggest year-round activity. Sci. Rep. 11, 5181 (2021).
Liu, W., Su, J., Li, S., Lang, X. & Huang, X. Non-structural carbohydrates regulated by season and species in the subtropical monsoon broad-leaved evergreen forest of Yunnan Province. China Sci. Rep. 8, 1083 (2018).
D’Andrea, E. et al. Winter’s bite: beech trees survive complete defoliation due to spring late-frost damage by mobilizing old C reserves. N. Phytol. 224, 625–631 (2019).
Augspurger, C. K. Spring 2007 warmth and frost: phenology, damage and refoliation in a temperate deciduous forest. Funct. Ecol. 23, 1031–1039 (2009).
Zohner, C. M., Rockinger, A. & Renner, S. S. Increased autumn productivity permits temperate trees to compensate for spring frost damage. N. Phytol. 221, 789–795 (2018).
Adams, H. D. et al. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl Acad. Sci. 106, 7063–7066 (2009).
O’Brien, M. J., Leuzinger, S., Philipson, C. D., Tay, J. & Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 4, 710–714 (2014).
D’Andrea, E. et al. Unravelling resilience mechanisms in forests: role of non-structural carbohydrates in responding to extreme weather events. Tree Physiol. 41, 1808–1818 (2021).
Sakai, A. & Larcher, W. Frost Survival of Plants: Responses and Adaptation to Freezing Stress. 62 (Springer Berlin Heidelberg, Berlin, Heidelberg, 1987).
Strimbeck, G. R., Schaberg, P. G., Fossdal, C. G., Schröder, W. P. & Kjellsen, T. D. Extreme low temperature tolerance in woody plants. Front. Plant Sci. 6, (2015).
Ren, H. et al. Molecular mechanisms of low-temperature sensitivity in tropical/subtropical plants: a case study of Casuarina equisetifolia. Res. 3, 0–0 (2023).
Kudo, G. & Ida, T. Y. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 94, 2311–2320 (2013).
Wei, N. et al. Pollinators contribute to the maintenance of flowering plant diversity. Nature 597, 688–692 (2021).
Li, X. et al. Warming-induced phenological mismatch between trees and shrubs explains high-elevation forest expansion. Natl Sci. Rev. 10, nwad182 (2023).
Renner, S. S. & Zohner, C. M. Climate Change and Phenological Mismatch in Trophic Interactions Among Plants, Insects, and Vertebrates. Annu. Rev. Ecol. Evol. Syst. 49, 165–182 (2018).
Greco, S. et al. Late Spring Frost in Mediterranean Beech Forests: Extended Crown Dieback and Short-Term Effects on Moth Communities. Forests 9, 388 (2018).
Škvareninová, J. et al. The effect of climate change on spring frosts and flowering of Crataegus laevigata – The indicator of the validity of the weather lore about “The Ice Saints”. Ecol. Indic. 145, 109688 (2022).
Satake, A., Chen, Y., Fletcher, C. & Kosugi, Y. Drought and cool temperature cue general flowering synergistically in the aseasonal tropical forests of Southeast Asia. Ecol. Res. 34, 40–49 (2019).
Devi, N. L., Singha, D. & Tripathi, S. K. Phenology, population structure and carbon sequestration potential of Parkia timoriana: a heirloom tree in traditional Meitei homegarden of northeast India. Vegetos 33, 222–228 (2020).
Nagai, S., Saitoh, T. M. & Yoshitake, S. Cultural ecosystem services provided by flowering of cherry trees under climate change: a case study of the relationship between the periods of flowering and festivals. Int. J. Biometeorol. 63, 1051–1058 (2019).
Körner, C. & Basler, D. Phenology Under Global Warming. Science 327, 1461–1462 (2010).
CaraDonna, P. J. & Bain, J. A. Frost sensitivity of leaves and flowers of subalpine plants is related to tissue type and phenology. J. Ecol. 104, 55–64 (2016).
Panchen, Z. A. et al. Leaf out times of temperate woody plants are related to phylogeny, deciduousness, growth habit and wood anatomy. N. Phytol. 203, 1208–1219 (2014).
Vitasse, Y. & Basler, D. What role for photoperiod in the bud burst phenology of European beech. Eur. J. Res. 132, 1–8 (2013).
Zohner, C. M., Benito, B. M., Svenning, J.-C. & Renner, S. S. Day length unlikely to constrain climate-driven shifts in leaf-out times of northern woody plants. Nat. Clim. Change 6, 1120–1123 (2016).
Lenz, A., Hoch, G., Körner, C. & Vitasse, Y. Convergence of leaf-out towards minimum risk of freezing damage in temperate trees. Funct. Ecol. 30, 1480–1490 (2016).
Khadivi, A., Montazeran, A. & Yadegari, P. Superior spring frost resistant walnut (Juglans regia L.) genotypes identified among mature seedling origin trees. Sci. Hortic. 253, 147–153 (2019).
Dumanoglu, H. et al. Spring late frost resistance of selected wild apricot genotypes (Prunus armeniaca L.) from Cappadocia region, Turkey. Sci. Hortic. 246, 347–353 (2019).
Templ, B. et al. Pan European Phenological database (PEP725): a single point of access for European data. Int. J. Biometeorol. 62, 1109–1113 (2018).
Ge, Q., Wang, H., Rutishauser, T. & Dai, J. Phenological response to climate change in China: a meta-analysis. Glob. Change Biol. 21, 265–274 (2015).
Ovaskainen, O. et al. Chronicles of nature calendar, a long-term and large-scale multitaxon database on phenology. Sci. Data 7, 47 (2020).
Schwartz, M. D., Betancourt, J. L. & Weltzin, J. F. From Caprio’s lilacs to the USA National Phenology Network. Front. Ecol. Environ. 10, 324–327 (2012).
Kobayashi, S. et al. The JRA-55 Reanalysis: General Specifications and Basic Characteristics. J. Meteorol. Soc. Jpn. Ser. II 93, 5–48 (2015).
Beguería, S. & Vicente-Serrano, S. M. SPEI: Calculation of the Standardized Precipitation-Evapotranspiration Index. 1.8.1 (2011) https://doi.org/10.32614/CRAN.package.SPEI.
Hijmans, R. J., Bivand, R., Pebesma, E. & Sumner, M. D. terra: Spatial Data Analysis. (2023).
R. Team. R: A language and environment for statistical computing. MSOR Connect. (2014).
Forest resources Assessment. Global ecological Zones for FAO forest reporting: 2010 update. For. Resour. Assess. Work. Pap. (2015).
Marino, G. P., Kaiser, D. P., Gu, L. & Ricciuto, D. M. Reconstruction of false spring occurrences over the southeastern United States, 1901–2007: an increasing risk of spring freeze damage? Environ. Res. Lett. 6, 024015 (2011).
Peterson, A. G. & Abatzoglou, J. T. Observed changes in false springs over the contiguous United States. Geophys. Res. Lett. 41, 2156–2162 (2014).
Schwartz, M. D., Ahas, R. & Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Change Biol. 12, 343–351 (2006).
Chamberlain, C. J., Cook, B. I., García De Cortázar-Atauri, I. & Wolkovich, E. M. Rethinking false spring risk. Glob. Change Biol. 25, 2209–2220 (2019).
Zhang, R., Hänninen, H., Wu, J. & Bhalerao, R. P. Bud endodormancy – a familiar but still unknown key adaptive trait in extratropical woody plants. Front. Plant Physiol. 1, (2023).
Kuznetsova, A., Brockhoff, P. B., Christensen, R. H. B. & Jensen, S. P. lmerTest: Tests in Linear Mixed Effects Models. (2020).
Schielzeth, H. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113 (2010).
Hansen, J., Ruedy, R., Sato, M. & Lo, K. Global Surface Temperature Change. Rev. Geophys. 48, (2010).
IPCC. Global Warming of 1.5 °C: IPCC Special Report on Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. (Cambridge University Press, 2022). https://doi.org/10.1017/9781009157940.
Wang, X. et al. Chilling and forcing proceed in parallel to regulate spring leaf unfolding in temperate trees. Glob. Ecol. Biogeogr. 32, 1914–1927 (2023).
Acknowledgements
We acknowledge all members of the PEP725 network, USA-NPN, CPON, and RCNN for collecting and providing the phenological data. This research was funded and supported by the National Natural Science Foundation of China (32271833 L.C., 32471912 R.Z., 32171832 R.Z.) and the National Key R&D Program of China (2023YFF0806600 L.C.).
Author information
Authors and Affiliations
Contributions
L.C. conceived and designed the study. H.Q., J.W., J.L., L.Z., and G.B collected the data. H.Q. and Q.Y. performed the data analyses and produced the figures with inputs from L.P., X.H., and Y.Y. H.Q., L.C., and Q.Y. wrote the first draft with inputs from Y.H.F., R.Z., C.W., and J.P. All coauthors interpreted the results and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiu, H., Yan, Q., Yang, Y. et al. Flowering in the Northern Hemisphere is delayed by frost after leaf-out. Nat Commun 15, 9123 (2024). https://doi.org/10.1038/s41467-024-53382-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-53382-3
This article is cited by
-
Coinciding spring and autumn frosts have a limited impact on carbon fluxes in a grassland ecosystem
Nature Communications (2025)
-
Applicability of Reanalysis Data in Analyzing the Occurrence time of Extreme Temperature Events in China
Asia-Pacific Journal of Atmospheric Sciences (2025)
-
Risk of extreme climate impacts on European Norway spruce forest: drought and frost in the climate emergency
Climatic Change (2025)
-
Nonlinear mixed effects crown width model for planted L. kaempferi at high altitudes in southern China
Trees (2025)