Abstract
Recent advances in machine perfusion have revolutionised the field of transplantation by prolonging preservation, permitting evaluation of viability prior to implant and rescue of discarded organs. Long-term perfusion for days-to-weeks provides time to modify these organs prior to transplantation. By using long-term normothermic machine perfusion to facilitate liver splitting and subsequent perfusion of both partial organs, possibilities even outside the clinical arena become possible. This model remains in its infancy but in the future, could allow for detailed study of liver injury and regeneration, and ex-situ treatment strategies such as defatting, genetic modulation and stem-cell therapies. Here we provide insight into this new model for research and highlight its great potential and current limitations.
Similar content being viewed by others
Introduction
Liver transplantation is the only treatment that provides a potential cure for patients with end-stage liver disease1. Despite an increasing need for donor livers due to an increasing number of patients with liver disease, there continues to be a shortage of available and suitable organs1,2. Although the number of organ donors has increased, the quality of available organs continues to decrease2,3,4. Consequently, strategies to increase the number of available organs have been required, such as using extended criteria or “marginal” donors, and split-liver transplantation to transplant two recipients using a single liver1. The use of these organs carries a theoretically increased risk of graft failure, and novel ways to assess this risk or improve these organs prior to implant are required.
Machine perfusion uses a pump, oxygenator and thermoregulator to keep an organ alive outside the body prior to transplantation. Preserving a liver in this way has a number of theoretical benefits including: improved preservation time, assessment of viability prior to transplant, rescue of organs that are otherwise not suitable for transplant and would otherwise be discarded and improved logistics for patients and treating teams. For the testing of viability prior to transplant, research to date has focused on short-term perfusion only for a few hours. However, long-term perfusion for days-to-weeks could facilitate more sophisticated viability assessment, the time and opportunity for modification of organs prior to transplant and even the possibility of liver regeneration5.
Preservation of livers for days to weeks using machine perfusion has enormous potential even outside the clinical arena. This “living” human tissue is a unique model that could be used for the study of liver injury and repair. Indeed, using techniques of liver splitting, two partial organs can be liberated, and both perfused in parallel. This has great potential for the testing of therapeutics and investigation into the possibility of ex-situ liver regeneration. This could pave the way for collaboration in the fields of transplantation and basic sciences. Herein, we will discuss the tremendous promise of long-term machine perfusion, the potential challenges to its use and the possibilities it provides in the fields of translational research and beyond.
Normothermic machine perfusion
Machine perfusion systems typically require a chamber to hold the organ; a pump to circulate the perfusate; an oxygenator to oxygenate the perfusate; a perfusion fluid (with or without an oxygen carrier); and a thermoregulator to maintain normothermic or hypothermic conditions6. The two main categories of systems are those that use hypothermic (4–12 °C) or normothermic (35–38 °C) conditions7. The current gold standard for preservation prior to transplant is using cold static storage, where an organ is kept cool using ice at 4 °C after perfusion with a preservation solution. Hypothermic perfusion systems extend on this principle by continuously circulating the preservation solution, but they have a limited capacity for viability testing (except perhaps the use of flavin mononucleotide)8. However, an oxygen carrier is not required due to the increased oxygen saturation and low metabolic activity at this temperature and supplemental nutrition is also not required. By contrast, normothermic perfusion systems simulate physiological conditions and do require an oxygen carrier and supplemental nutrition. However, they permit simulation of liver function in a near-physiological environment and assessment of liver function by traditional measures including bile production, biochemistry and histopathology5.
Short-term perfusion
A promising advantage of machine perfusion prior transplant is its use in the determination of graft viability prior to transplant and assessment of “marginal” organs. In this way, organs that would otherwise have been discarded may be able to be used safely for transplantation. The assessment of suitability for transplantation, or “viability” during machine perfusion is a field of active research. In the VITTAL clinical trial, livers that were rejected for transplantation were perfused under normothermic conditions. Livers that met a composite biochemical and haemodynamic viability (lactate ≤2.5 mmol/L, and two or more of: bile production, pH ≥ 7.30, glucose metabolism, hepatic arterial flow ≥150 ml/minute and portal vein flow ≥500 ml/minute, or homogeneous perfusion), were transplanted with 100% 90-day patient and graft survival9. The main criticism of the VITTAL trial criteria is the lack of assessment of bile. Other viability criteria, have therefore aimed to incorporate bile analysis with a view to predict those grafts that are at high risk of post-transplant biliary complications10. In the DHOPE-COR-NMP trial, bile pH >7.45 was a key viability criterion that was used in addition to perfusate lactate and pH to evaluate grafts for transplant11. Grafts that met viability criteria were transplanted with 100% 3- and 6-month patient and graft survival11.
Current viability criteria, however, remain imperfect and novel methods are emerging for assessment of graft viability prior to transplant. Flavin mononucleotide which is released during mitochondrial injury can be measured in real-time using fluorescent spectroscopy and can be assessed using hypothermic machine perfusion8,12,13 This marker has shown promise for predicting graft function but its role in clinical practice remains to be defined. The liver metabolic function has also been investigated using metabolomic profiling during machine perfusion. Although currently time consuming and at present only investigational, assessment of metabolites during machine perfusion may have a role in understanding metabolic liver failure during short-term and also long-term machine perfusion14.
Long-term perfusion
Compared to short-term perfusion (<24 hours), long-term perfusion (>7 days) is technically challenging and resource-intensive. However, perfusion in the range of days-to-weeks could facilitate more detailed and sophisticated assessment of organs and even provide time to regenerate or modify these organs prior to transplant5. In addition to the pump, oxygenator, thermoregulator and perfusate required for short-term perfusion, long-term perfusion requires supportive nutrition, precise control of acid-base balance and a dialysis filter5,15. Long-term perfusion, particularly under normothermic conditions, also carries additional challenges such as the prevention of microbial contamination. Contamination of the perfusate during perfusion of human kidneys16 and porcine livers17 has demonstrated both gram-positive and gram-negative organisms as well as fungi. Long-term perfusion of human livers by our group also demonstrated mixed growth of microbial contaminants in half of all perfused organs18.
Using subnormothermic conditions (34 °C), and a custom-built integrated system, long-term perfusion of livers for 7 days was reported by a group in Switzerland19,20. Perfusion for this duration allows for extended viability assessment and a prolonged period of perfusion time to facilitate graft resuscitation prior to transplant. The same group also recently reported the 1-year follow-up of a liver that was perfused using ex-situ normothermic preservation for 3 days (to allow time for histopathological clarification of a liver lesion in the donor) and then transplanted21.
Recently, the study of long-term normothermic perfusion of human livers for more than 7 days has increased in interest. Our group has recently reported our experience in long-term perfusion of human livers, with perfusion as long as 13 days22,23. By contrast to the Swiss group, a custom-built integrated system was not used and long-term perfusion was achieved by modifying a commercially available system (Liver assist, Xvivo, Gronigen, Netherlands). This has the advantage of being more easily reproducible and accessible. These modifications included exchange of short-term oxygenators for long-term oxygenators, addition of a parallel circuit including an adjustable dialysis filter and addition of a gas blender for fine control of oxygenation23,24. A group from Italy, also recently reported perfusion of a human liver for 17 days by integrating an extracorporeal blood purification system25. Although challenging and labour-intensive, this work demonstrates the potential role of long-term normothermic machine perfusion in the detailed assessment of organs and possibly even modification prior to transplant.
Liver splitting
Liver splitting is a technique used to permit the treatment of two recipients (typically an adult and a child) using a single donor liver. As described in the 1980s by Pichelmayr26 and Bismuth27 the liver is divided along the line of the falciform ligament into a left lateral segment graft (LLSG, segments 2 and 3) and an extended right graft (ERG, segment 1, 4–8)28. The smaller LLSG is then used for a paediatric recipient and the ERG for an adult. Full-left, full-right splitting where the liver is divided along Cantlie’s line and each partial graft is intended for an adult recipient remains controversial due to most groups reporting poor outcomes29.
There are currently two described methods for liver splitting for transplantation. The ex-situ method is performed in an ice bath on the back table after the whole liver has been retrieved from the donor29,30. Using the in-situ method, the liver splitting is performed during the warm phase of the donor operation prior to cold perfusion and the two partial grafts are then transported separately to the recipient transplant centres29,31. The ex-situ method requires a shorter donor surgery time, and specialist liver surgeons are not required to travel because the liver is retrieved whole, and the split occurs in the cold phase. However, this necessitates a longer cold ischaemic time which has associations with an increased risk of graft complications30,32,33. By contrast, the in-situ technique transfers the additional time required to the warm phase, which reduces the cold ischaemic time but requires a longer donor surgery, and for liver surgeons to travel to the donor hospital34,35.
Novel techniques for liver splitting using machine perfusion have recently been described36,37,38,39. This potentially combines the best of both the ex-situ and in-situ techniques. One technique utilises hypothermic oxygenated machine perfusion which maintains the organ under hypothermic conditions and maintains oxygen within the perfusate to essentially extend the preservation time. Another uses normothermic conditions and continuous perfusion during the splitting procedure, such that the liver and then both partial livers can be continuously assessed and monitored during perfusion39. In either case, liver splitting using machine perfusion certainly allows for the liberation of two partial organs from the same donor, which can be individually assessed for suitability for transplant.
Long-term perfusion of split livers
A major advantage of long-term normothermic machine perfusion is the ability to study living human tissue ex-situ, and the potential to use this as a model of liver injury and repair. A model of long-term perfusion that also utilises split livers could also unlock areas of research otherwise not thought possible. For instance, the detailed assessment of a liver after iatrogenic injury (splitting) and the process by which it undergoes regeneration. Indeed, a unique model with partial organs from the same liver could also act as an ideal model for the testing of therapeutics with a matched control.
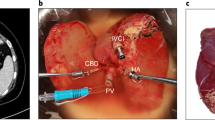
This idea was first explored by a group at Harvard, Boston, USA, that perfused partial liver after splitting for 3 hours under subnormothermic conditions40. Our group took this further and developed a long-term model for the ex-situ perfusion of split livers (Fig. 1). Using our modified commercial perfusion system, 10 whole livers were split into 20 partial livers without interruption to perfusion, and then both partial livers were perfused in parallel for more than 7 days. The median survival of the partial organs was 165 hours, and the longest surviving partial graft lasted for 327.5 hours41. Not only does this provide a model of living human tissue that can be analysed in detail without fear of harming a human participant; a built-in internal control compensates partially for the variability associated with each individual organ and the small numbers associated with research using donated organs.
After back table dissection, whole livers are resuscitated using normothermic machine perfusion for 12–24 hours. The liver is then surgically split without interruption to perfusion, and the two partial grafts are transferred to separate perfusion machines. The partial grafts are then perfused in the long-term, which allows for a number of experimental models, including testing therapeutics and the study of liver injury and liver regeneration. Surgery icon created by Yoyon Pujiono, https://thenounproject.com/icon/surgery-3681231/. Licensed under: https://creativecommons.org/licenses/by/3.0/”.
The other unique characteristic of this model is the ability to study the response of a partial liver when it has been injured in a predictable way (liver resection). Indeed, after liver resection, the pathways that lead to remnant hypertrophy in vivo are incompletely understood. A long-term perfusion model of partial livers provides an opportunity to study the changes that occur during injury, and potentially reveal the mechanisms required for regeneration.
The challenges in the long-term perfusion of split livers, however, should not be underestimated. This model required constant human supervision for manual titration of infusions and dialysis settings24. Automation by means of real-time sensors and artificial intelligence may be able to simplify this process, but each liver requires an individualized treatment paradigm (much as an individual patient would). This meant that medical expertise was almost always required, and our staff needed to draw on clinical knowledge to safely support the organs. Secondly, long-term perfusion using open reservoir systems is particularly susceptible to environmental contamination and infection of the organ. Perfusate cultures demonstrated the growth of gram-negative and gram-positive bacteria as well as fungi18. Not only could these microorganisms interfere with a scientific model, but infection of the organ through environmental contamination may act to damage the organ and shorten its lifespan.
Thirdly, perfusion of a partial organ is more difficult and less reliable than perfusion of a whole organ. Smaller arterial and venous cannulas were required, and due to the size of the organ relative to the organ chamber of the perfusion machine, the vessels were prone to kinking and inconsistent flow. Our perfusion system had a minimum perfusate volume of 2 L and utilized pressure control to regulate flow. Therefore, when smaller cannulas were used for the partial organs to account for smaller vessels, the flow provided exceeded normal physiological values. The effects of this on liver physiology in a partial organ remain to be quantified but may have induced a pseudo-small for size syndrome in these partial organs. Indeed, in the context of long-term perfusion, splitting the livers may have been a disadvantage. Differences in perfusion of the partial livers resulted in differences in the anatomy and degree of injury to each liver and this almost certainly compromised the potential lifespan of these organs. Until the technique and model is mature, this needs to be recognised as an important limitation.
Finally, despite our best efforts, all organs eventually failed without a specific explanation. This highlights that there must be either a missing metabolic signal required for the maintenance of energy homoeostasis or the buildup of a toxic metabolite that impacts graft function and leads to organ failure. It is possible that these organs were not thriving in the ex-situ environment and that conditions had not been met for them to even achieve growth or regeneration.
Machine perfusion as a platform for liver repair and regeneration
A potential application for long-term ex-situ perfusion is the repair of suboptimal grafts. Ischaemia-reperfusion, a process that is unavoidable during organ retrieval and preservation, plays a pivotal role in early organ failure, particularly in organs of lower quality. The release of cellular injury markers is indicative of this damage; these markers typically peak within the first 48 hours and then show signs of improvement, suggesting an ongoing repair process41. As a result, an enhancement in liver function is often observed, indicative of an active repair mechanism. Consequently, ex-situ liver repair and therapeutic intervention may require a perfusion duration extending beyond 48 hours. Long-term normothermic machine perfusion offers the unique advantage of a controllable environment, allowing for the introduction of therapeutic agents without any interference from the host immune system.
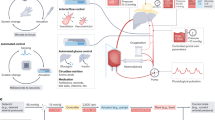
Therapeutics that are currently under investigation aim to repair and even regenerate injured livers during long-term ex-situ perfusion (Fig. 2). These include: defatting cocktails for hepatic steatosis, and RNA interference, and stem cell therapy to induce regeneration5. Overall, machine perfusion presents a novel strategy for treating and modifying an organ prior to transplantation. It effectively eliminates the challenges that typically accompany in vivo treatments, such as off-target effects and issues with over or under-dosage, and permits testing of these in the lab setting with living human tissue prior to translation into the clinical arena.
The machine perfusion system offers a uniquely controllable environment that potentially allows for the introduction of therapeutic agents without interference from the host immune system. The peak of cellular injury occurs 24–48 hours after reperfusion. The process of repair and regeneration occurs in the following days to weeks. Long-term perfusion provides an adequate time frame for these agents to be effective. Defatting cocktails, genetic modulation, stem cell therapy and agents for liver regeneration are under investigation.
Ex-situ treatment strategies
Defatting
Severely steatotic livers are typically excluded from transplantation due to the heightened risk of ischemia-reperfusion injury (IRI) and primary non-function4. Defatting cocktails, (including substances such as forskolin, scoparone, nuclear receptor ligands (GW7647, GW501516), hypericin, and visfatin) have been shown to reduce steatosis by 40% in studies using discarded human livers42. The safety of these cocktails for clinical use is currently under evaluation in a randomised controlled trial (RCT) (ISRCTN 14957538).
Sousa Da Silva et al.43 recently reported successful defatting of steatotic liver grafts using extended ex-situ normothermic perfusion. The authors credited the effective defatting to a combination of factors: prolonged perfusion, glucose management, circadian nutrition, and the addition of defatting agents such as L-Carnitine and fenofibrate. They suggested that secreted ketone bodies could be eliminated from the perfusate by dialysis, and fat could be excreted into the perfusate as very low-density lipoproteins, which are then removed by perfusate lipopheresis. Additionally, Kupffer cells might play a role in clearing the fat released into the sinusoids19.
Our group has recently demonstrated in 20 split human liver grafts that long-term perfusion result in significant reductions in both macrovesicular and microvesicular steatosis in steatotic liver, without the need for additional defatting agents41. This indicates the potential for long-term perfusion itself to be an effective method for defatting steatotic grafts.
Genetic modulation
Transient silencing of genes related to IRI or graft rejection may be able to minimise liver damage and post-transplantation complications44. Molecules such as small interfering RNA (siRNA), microRNA, and short-hairpin RNA, have been explored for this purpose44. The RNA-induced silencing complex has been used to target genes involved in IRI, including caspase-8 and caspase-3, that are involved in apoptosis, and nuclear factor-κB. In rodent models of liver transplantation, silencing these genes resulted in reduced apoptosis and better preservation of liver architecture, thereby demonstrating reduced IRI44,45,46.
While immunosuppressive therapy reduces rejection risk, it is associated with an increased risk of infection, malignancy and drug-related side-effects. Modification of the graft perioperatively may reduce immune system activation, and therefore the degree of immunosuppressive therapy required47. Nanoparticle therapy using compounds that release siRNA have been used during normothermic machine perfusion to ablate endothelial major histocompatibility complex class II molecules, and therefore reducing protein expression in the allograft and protect against rejection48. Furthermore, the CRISPR technique has been used to eliminate molecular antigens and to insert CD47 proteins49. This aims to, therefore, create hypo-immunogenic human-induced pluripotent stem cells and reduce the need for immunosuppression. Dendritic and regulatory T cells are also a potential target, whereby CRISPR/Cas9 gene editing can be used to modulate their activity and induce immunotolerance50, a strategy that could pave the way for organ bioengineering without the need for immunosuppressive drugs51. Machine perfusion has also recently been applied to remove blood group antigens. Notably, using a model of normothermic machine perfusion of human lungs, FpGalNAc deacetylase and FpGalactosaminidase were able to eliminate 97% of blood group A antigens from type A donor lungs52.
Stem cell and progenitor cell therapy
Hepatocytes constitute 80% of the liver’s mass. In the past two decades, hepatocyte transplantation has been explored for various diseases, showing short-term efficacy and safety53. However, its clinical application is limited by challenges such as the difficulty in isolating high-quality cells from “marginal” donor livers, low cell engraftment rates, inflammatory responses, and transient effects53. To address these issues, research is now focusing on alternative cell sources.
Mesenchymal stem cells (MSCs) are more readily isolated and cultured. They can be derived from different tissues and differentiated into hepatocyte-like cells, which may have anti-inflammatory and immunomodulatory effects54. Animal studies have demonstrated promising results when MSCs are combined with certain technologies, improving liver function, reducing mitochondrial and biliary epithelial cell damage, and extending survival times55. These encouraging findings have also been observed in donated human livers that were not suitable for transplant. Using a model of normothermic machine perfusion, multi-potent adult progenitor cells were directly infused into the right lobe via the right hepatic artery or the portal vein. This direct introduction of cells into the target organ yielded anti-inflammatory and immunomodulatory benefits without adversely affecting the perfusion process56.
Additionally, human-induced pluripotent stem cells can differentiate into multiple hepatic cell lineages, from embryoid bodies to mature hepatocytes, showing promise for the creation of human organoids57. Autologous hepatocytes can be cultured to form three-dimensional liver organoids, and potentially used for organ function restoration. Long-term normothermic machine perfusion offers an ideal setting for injecting organoids into injured donor livers. Sampaziotis et al. successfully delivered cholangiocyte organoids to donor human livers under normothermic machine perfusion, demonstrating the repair of intrahepatic ducts58. The team also injected protein-expressing gallbladder organoids into the hepatic duct of the liver and were able to demonstrate successful cholangiocyte engraftment and differentiation after 100 hours of perfusion58.
Liver regeneration
The liver is renowned for its remarkable regenerative capacity. After a reduction in volume, such as following liver resection or splitting, it can regenerate almost all of its original mass; although this can take up to several weeks59. The regenerative process involves compensatory hyperplasia of the remaining hepatocytes, which stops once the liver reaches an appropriate volume59. Machine perfusion presents a unique platform for regenerative therapy, providing a controlled environment for adding pro-regenerative compounds and offering the possibility to cultivate livers from partial grafts for transplantation.
In the context of partial hepatectomies, there is evidence of a transient accumulation of steatosis and an initial metabolic shift from glucose to lipid for energy production, fuelling the regenerative process59. This initial fat accumulation appears to be a regulated response, with upregulation of various gene expression markers60. Increased circulating cytokines stimulate the release of fatty acids from systemic adipose tissue, which are then stored as triacylglycerols in intracellular fat droplets within the liver, either for β-oxidation or cell membrane reconstruction60. These changes occur over a period of at least 7 days in mice, allowing the reduced liver to regenerate almost completely. It remains to be shown how long this takes, or whether it is possible in humans. Using models of long-term perfusion of human livers, the ability to study this has become reality41. In fact, this model offers a starting point for research into the induction of growth in undersized partial grafts for direct transplantation, potentially through genetic editing and therapeutics (Fig. 3).
Liver splitting and long-term perfusion provide the opportunity to induce liver regeneration and potentially improve partial organs prior to transplant. Multiple pathways have shown potential as mechanisms for liver regeneration, including phosphatase and tensin homologue (PTEN) inhibition, Hippo/Yap and Wnt-pathway activation, stem cell treatments, and modulation of cellular membrane potentials. An ex situ model of long-term perfusion of split livers allows a detailed study of liver regeneration and testing of these potential targets.
Current research in liver regeneration during normothermic machine perfusion focuses on the potential of stem cell-based therapies and organoids to foster repair and regeneration15. For instance, using machine perfusion, Xuan et al.61 demonstrated the repair and regeneration of bile duct injury in rat livers using haem oxygenase-1 modified bone marrow MSCs. Jassem et al.62 also showed upregulation of liver regeneration-promoting genes in human livers perfused for 3.5–18.5 hours, while Liu et al.63 observed biliary epithelial regeneration after 24 hours of normothermic perfusion in porcine livers.
Identifying key molecules that can induce liver growth during machine perfusion remains a challenge (Fig. 3). An important potential targets is phosphatase and tensin homologue (PTEN), which is a tumour suppressor that inhibits the PI3K/AKT pathway60. CRISPR-Cas9 targeting of PTEN in a murine model has demonstrated liver enlargement in four months after treatment64. PTEN inhibition, through drugs or RNAi, is another strategy, which has the additional benefit of potentially ameliorating IRI-related inflammation60. Other potential targets include the Hippo/Yap signalling pathway65, Wnt-pathway activators66, and the constitutive-androstane-receptor (CAR) agonists67. Additionally, the role of chemical gradients, endogenous ion flow, and cellular membrane potential in regulating cell behaviour during regeneration is worth exploring68. Several studies have implicated K+ currents in regulating cell division, suggesting that alterations in cellular membrane potential and hyperkalemia could significantly impact liver regeneration68,69. The importance of cellular membrane potential and hyperkalaemia on liver regeneration could easily be tested during long-term normothermic machine perfusion.
Biliary regeneration
Biliary strictures continue to be an important limiting factor in the utilisation of donated livers because they are an important contributor to post-transplant morbidity and the need for retransplantation70. Strictures are thought to originate from an ischaemic biliary injury that occurs at the time of organ donation71. Assessment of livers using normothermic machine perfusion has encouraged interest in assessing biliary injury prior to implant and the addition of biliary viability criteria has allowed for successful transplantation of livers with minimal rates of post-transplant biliary strictures72. The mechanism for the development of these biliary strictures, however, remains poorly understood.
A lack of biliary regeneration is thought to have a key role in the development of biliary strictures73. Injury to the peribiliary vascular plexus and peribiliary glands correlates with unfavourable biliary biochemistry and these structures are thought to be key to recovery and regeneration of the biliary epithelium after injury during preservation74,75. Indeed, the peribiliary glands are thought to store the biliary stem cells required for initiation of the regenerative response76,77. Evaluation of biliary regeneration, therefore may be key to predicting biliary strictures, and modulation of this phenomenon may be key to its prevention.
Importantly, biliary regeneration may require as long as 72 hours to commence, and therefore will be missed by traditional short-term normothermic machine perfusion74. Using a model of long-term perfusion permits a comprehensive evaluation of biliary regeneration and could provide a window for intervention. This may allow for identification of biomarkers of biliary regeneration during long-term perfusion, which could also be used for monitoring biliary regeneration, and even as targets for modulation of the process. Our group has also recently demonstrated evidence of biliary regeneration in human livers using our model of long-term normothermic machine perfusion78. Machine perfusion may also permit intervention in the biliary tree with therapeutics. In fact, using a model of normothermic machine perfusion of human livers, Sampatziotis et al.58 delivered gallbladder organoids into the intrahepatic ducts and demonstrated successful engraftment of the biliary tree and regeneration of cholangiocytes.
Beyond liver transplantation
Long-term normothermic machine perfusion provides a unique model for translational research enabling the introduction of therapeutic agents that would be ineffective in protocols of short duration15. The “living” organs maintained in this way can potentially provide valuable opportunities for translational research, allowing many types of research projects, including pharmacotherapy, gene therapy, stem-cell therapy, and organ/tissue engineering19. One of the most important advantages is that it allows researchers to overcome the known limitations of animal models79. The usefulness of animal models in Immunology, Transplantation, and Oncology has been questioned because of irreproducibility and poor simulation of human conditions79. Because human organs are used, the results obtained using human organs during machine perfusion are potentially highly translatable to clinical practice.
Preclinical drug testing has traditionally relied on animal models80. Despite their usefulness, these models often fail to predict human responses accurately due to interspecies differences in metabolism and drug processing. This limitation leads to high attrition rates in clinical trials80. Long-term normothermic machine perfusion emerges as a solution, offering a more human-relevant model by utilising human livers that are declined for transplantation. Partial livers obtained after liver resections that contain liver tumours may provide another unique model20. Models such as these can potentially be used to test new therapeutics and study the metabolism and growth patterns of tumours in a controlled, physiologically relevant environment, enhancing our understanding of cancer biology.
Gene therapy, particularly for liver-related diseases, stands to benefit significantly from long-term normothermic machine perfusion44,81. This technology can be used to test gene-editing tools like CRISPR/Cas9, RNAi and the effect of new vectors on human livers, providing insights into the efficacy and safety of these interventions44,45,46,47,48. Long-term normothermic machine perfusion may enhance these experiments by allowing the delivery of such therapeutics into the liver in isolation. This can minimise systemic toxicity and reduce associated costs by minimising the dose of the therapeutic agent required. In fact, our group’s model of long-term perfusion was recently used to evaluate adeno-associated viral vectors, which demonstrated the potential of using this model for testing liver-specific gene therapy82.
A potential limitation of testing therapeutics using human livers in this way is the heterogeneity of the livers available for research1,2,3,4. As such, the sample size required to achieve adequate power for meaningful results is often logistically challenging. Our group has developed a technique of ex-situ liver splitting whereby a whole liver can be anatomically split into left and right lobes during normothermic machine perfusion, and the two lobes can be perfused in parallel on separate perfusion machines. In this way, each experiment has an internal control, which can minimise the effect of heterogeneous grafts and increases the sample size of the organs available for research41.
Long-term normothermic machine perfusion can also serve as an incubator for growing tissues or engineering organs. By providing a controlled environment, researchers can explore tissue growth, stem cell differentiation, and organ development, potentially leading to breakthroughs in creating lab-grown organs15. Similarly, long-term normothermic machine perfusion can also provide an ideal environment to nurture the development of organoids or bioprinted tissues, benefiting from the dynamic flow of nutrients and oxygen15,83.
Moreover, long-term normothermic machine perfusion can be used to study cell-cell interactions in a dynamic environment, critical for understanding tissue homoeostasis and disease development84. Studying disease processes in real-time on human organs can yield invaluable insights. Diseases like hepatitis, cancer, and cirrhosis can be studied under more realistic conditions compared to traditional cell cultures or animal models, potentially leading to better understanding and treatments15,85. Indeed, long-term normothermic machine perfusion can also be valuable as an educational tool, providing medical professionals with a realistic platform to study human organ physiology and practice surgical techniques39.
Limitations
Long-term normothermic machine perfusion of the liver represents a groundbreaking advancement in organ transplantation and regenerative medicine. Despite its potential, this technology is not without challenges. Understanding these constraints is essential for guiding future research and clinical application. These include: technical challenges, ethical considerations regarding the use of human tissues, the need for advanced gene-editing tools, and the complexity of liver biology5,15,86.
Long-term normothermic machine perfusion systems are technically sophisticated and expensive, which limits their widespread adoption5. Our group developed a method to do this by modifying commercially available equipment, which makes this more accessible, but this method is labour intensive. The requirement for specialised trained personnel adds to the operational costs87. Future developments in this technology should aim to reduce its complexity and cost though the use of fully automatic systems that minimise the need for trained personnel5.
Prolonged perfusion can lead to cumulative stress on the liver, potentially causing damage. Balancing the perfusion duration to maximise benefits while minimising harm is a delicate and ongoing challenge5. The ideal parameters for organ survival and the required conditions for liver regeneration and repair remain to be defined. There is also a need to identify new viability criteria and understand the causes that lead to an organ’s death during long-term perfusion12,86. The challenge will be, particularly as we move to the clinical arena with this technology, to find consensus regarding definitions and define clear clinical endpoints such that inter-group variability can be minimised.
As a scientific model for study of human physiology, injury and for the study of therapeutics, this model suffers from inter-organ variability. Just as human participants vary from one to the other, each liver comes from a person with different co-morbidities and different genetic and environmental factors. The inter-organ variability may be partially compensated for by our model of split-liver perfusion by providing an in-built internal control, However, this model is labour intensive, requires a unique combination of scientists, surgeons and clinicians and a dedicated hybrid lab. Future work should aim to simplify the process, and perhaps develop a dedicated purpose-built perfusion machine with adaptations intended to allow easy replication of this model.
In all, despite great potential, this work to date largely remains theoretical, and preclinical. The challenge for the future will be to translate these concepts into practice, and to demonstrate their safety and efficacy in the clinical arena. Global collaboration is required, with an understanding of the potential of this technology but also acceptance of its limitations.
Future directions
Machine perfusion has revolutionised liver transplantation and opened numerous avenues for novel applications. Beyond viability testing, this technique may allow organs to be modified and improved prior to transplant. Liver splitting increases the utility of donated livers by allowing two recipients to be transplanted using the same donor liver. Machine perfusion can potentially be used to improve liver splitting and potentially provide the ability to assess individual partial livers prior to transplant. Long-term perfusion of split livers takes this to the next level by permitting perfusion in the range of days-to-weeks and with this, the potential to enhance, modify, and even regenerate these organs. Indeed, an accessible, robust model of long-term perfusion of split livers could bridge the gap between clinical sciences and translational research, paving the way for collaboration in the fields of transplantation, basic sciences and beyond. This field, however, remains in its infancy, with a number of known and unknown challenges to face. Future work should aim to understand in detail the requirements for the long-term ex-situ survival of organs and to understand the role of this model in the study of therapeutics, liver injury, genetic modulation, and liver regeneration.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
No data were generated in this study.
References
Pandya, K. et al. Differential impact of extended criteria donors after brain death or circulatory death in adult liver transplantation. Liver Transpl. 26, 1603–1617 (2020).
Tullius, S. G. & Rabb, H. Improving the supply and quality of deceased-donor organs for transplantation. N. Engl. J. Med. 378, 1920–1929 (2018).
Orman, E. S. et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 21, 1040–1050 (2015).
de Graaf, E. L. et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the donor risk index. J. Gastroenterol. Hepatol. 27, 540–546 (2012).
Lascaris, B. et al. Long-term normothermic machine preservation of human livers: what is needed to succeed? Am. J. Physiol. Gastrointest. Liver Physiol. 322, G183–G200 (2022).
Sousa Da Silva, R. X., Weber, A., Dutkowski, P. & Clavien, P.-A. Machine perfusion in liver transplantation. Hepatology 76, 1531–1549 (2022).
Linares, I., Selzner, N. & Selzner, M. Machine preservation of the liver: what is the future holding? Curr. Transplant. Rep. 5, 82–92 (2018).
Muller, X. et al. Novel real-time prediction of liver graft function during hypothermic oxygenated machine perfusion before liver transplantation. Ann. Surg. 270, 783–790 (2019).
Mergental, H. et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 11, 2939 (2020).
Brüggenwirth, I. M. A., de Meijer, V. E., Porte, R. J. & Martins, P. N. Viability criteria assessment during liver machine perfusion. Nat. Biotechnol. 38, 1260–1262 (2020).
van Leeuwen, O. B. et al. Transplantation of high-risk donor livers after ex situ resuscitation and assessment using combined hypo- and normothermic machine perfusion: a prospective clinical trial. Ann. Surg. 270, 906–914 (2019).
Brüggenwirth, I. M. A., van Leeuwen, O. B., Porte, R. J. & Martins, P. N. The emerging role of viability testing during liver machine perfusion. Liver Transpl. 28, 876–886 (2022).
Wang, L. et al. Flavin mononucleotide as a biomarker of organ quality-a pilot study. Transplant. Direct 6, e600 (2020).
Bruinsma, B. G. et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep. 6, 22415 (2016).
Lascaris, B., de Meijer, V. E. & Porte, R. J. Normothermic liver machine perfusion as a dynamic platform for regenerative purposes. What does the future have in store for us? J. Hepatol. 77, 825–836 (2022).
Phillips, B. L. et al. Microbial contamination during kidney ex vivo normothermic perfusion. Transplantation 102, e186-e188, (2018).
Eshmuminov, D. et al. Sources and prevention of graft infection during long-term ex situ liver perfusion. Transplant. Infect. Dis. 23, e13623 (2021).
Lau, N. S. et al. Microbial contamination during long-term ex vivo normothermic machine perfusion of human livers. Transplantation 108, 198–203 (2023).
Eshmuminov, D. et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 38, 189–198 (2020).
Mueller, M. et al. Long-term normothermic machine preservation of partial livers: first experience with 21 human hemi-livers. Ann. Surg. 274, 836–842 (2021).
Clavien, P.-A. et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat. Biotechnol. 40, 1610–1616 (2022).
Lau, N.-S. et al. Prolonged ex vivo normothermic perfusion of a split liver. Transplant. Direct 7, e763 (2021).
Lau, N. S. et al. Long‐term normothermic perfusion of human livers for longer than 12 days. Artif. Organs 46, 2504–2510 (2022).
Huang, J. et al. Incorporating a hemodialysis filter into a commercial normothermic perfusion system to facilitate long-term preservation of human split-livers. Artif. Organs, 48, 1008–1017 (2024).
Cillo, U., Nalesso, F., Bertacco, A., Indraccolo, S. & Gringeri, E. Normothermic perfusion of a human tumoral liver for 17 days with concomitant extracorporeal blood purification therapy: case description. J. Hepatol. 81, e96–e98 (2024).
Pichlmayr, R., Ringe, B., Gubernatis, G., Hauss, J. & Bunzendahl, H. Transplantation of a donor liver to 2 recipients (splitting transplantation)-a new method in the further development of segmental liver transplantation. J. Langenbeck Arch. Chir. 373, 127–130 (1988).
Bismuth, H. et al. Emergency orthotopic liver transplantation in two patients using one donor liver. Br. J. Surg. 76, 722–724 (1989).
Hackl, C. et al. Split liver transplantation: current developments. World J. Gastroenterol. 24, 5312–5321 (2018).
Lau, N. S. et al. Addressing the challenges of split liver transplantation through technical advances. A systematic review. Transplant. Rev. 35, 100627 (2021).
Battula, N. R. et al. Intention to split policy: a successful strategy in a combined pediatric and adult liver transplant center. Ann. Surg. 265, 1009–1015 (2017).
Lau, N. S. et al. Is it safe to expand the indications for split liver transplantation in adults? A single‐center analysis of 155 in‐situ splits. Clin. Transplant. 36, e14673 (2022).
Andrassy, J. et al. Higher retransplantation rate following extended right split-liver transplantation: an analysis from the eurotransplant liver follow-up registry. Liver Transpl. 24, 26–34 (2018).
Herden, U. et al. Outcome following right-extended split liver transplantation in the recent transplant era: single-center analysis of a German transplant center. Clin. Transplant. 32, e13288 (2018).
Yoon, K. et al. Survival outcomes in split compared with whole liver transplantation. Liver Transpl. 24, 1411–1424 (2018).
Cardillo, M. et al. Split and whole liver transplantation outcomes: a comparative cohort study. Liver Transpl. 12, 402–410 (2006).
Spada, M. et al. The new horizon of split-liver transplantation: ex situ liver splitting during hypothermic oxygenated machine perfusion. Liver Transpl. 26, 1363–1367 (2020).
Mabrut, J. Y. et al. Ex vivo liver splitting and hypothermic oxygenated machine perfusion: technical refinements of a promising preservation strategy in split liver transplantation. Transplantation 105, e89–e90 (2021).
Thorne, A. M. et al. Ex situ dual hypothermic oxygenated machine perfusion for human split liver transplantation. Transplant. Direct 7, e666 (2021).
Lau, N.-S. et al. Liver splitting during normothermic machine perfusion: a novel method to combine the advantages of both in-situ and ex-vivo techniques. HPB 25, 543–555 (2023).
Huang, V. et al. Split-liver ex situ machine perfusion: a novel technique for studying organ preservation and therapeutic interventions. J. Clin. Med. 9, 269 (2020).
Lau, N.-S. et al. Long-term ex situ normothermic perfusion of human split livers for more than 1 week. Nat. Commun. 14, 4755 (2023).
Boteon, Y. L. et al. Manipulation of lipid metabolism during normothermic machine perfusion: effect of defatting therapies on donor liver functional recovery. Liver Transpl. 25, 1007–1022 (2019).
Sousa Da Silva, R. X. et al. Defatting of human livers during long-term ex situ normothermic perfusion: novel strategy to rescue discarded organs for transplantation. Ann. Surg. 278, 669–675 (2023).
Brüggenwirth, I. M. A. & Martins, P. N. RNA interference therapeutics in organ transplantation: the dawn of a new era. Am. J. Transplant. 20, 931–941 (2020).
Li, X. et al. Alleviation of ischemia-reperfusion injury in rat liver transplantation by induction of small interference RNA targeting Fas. Langenbecks Arch. Surg. 392, 345–351 (2007).
Contreras, J. L. et al. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery 136, 390–400 (2004).
Martins, P. N., Chandraker, A. & Tullius, S. G. Modifying graft immunogenicity and immune response prior to transplantation: potential clinical applications of donor and graft treatment. Transpl. Int. 19, 351–359 (2006).
Cui, J. et al. Ex vivo pretreatment of human vessels with siRNA nanoparticles provides protein silencing in endothelial cells. Nat. Commun. 8, 191 (2017).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Zhang, Y. et al. In situ repurposing of dendritic cells with CRISPR/Cas9-based nanomedicine to induce transplant tolerance. Biomaterials 217, 119302 (2019).
Kuscu, C. et al. Applications of CRISPR technologies in transplantation. Am. J. Transplant. 20, 3285–3293 (2020).
Wang, A. et al. Ex vivo enzymatic treatment converts blood type A donor lungs into universal blood type lungs. Sci. Transl. Med. 14, eabm7190 (2022).
Iansante, V., Mitry, R. R., Filippi, C., Fitzpatrick, E. & Dhawan, A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr. Res. 83, 232–240 (2018).
Bogensperger, C. et al. Ex vivo mesenchymal stem cell therapy to regenerate machine perfused organs. Int. J. Mol. Sci. 22, (2021).
Yang, L. et al. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res. 381, 239–254 (2020).
Laing, R. W. et al. The delivery of multipotent adult progenitor cells to extended criteria human donor livers using normothermic machine perfusion. Front. Immunol. 11, 1226 (2020).
Septiana, W. L., Noviantari, A. & Antarianto, R. D. Induced pluripotent stem cells (Ipscs) based liver organoid: the benefits and challenges. Cell. Physiol. Biochem. 57, 345–359 (2023).
Sampaziotis, F. et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science 371, 839–846 (2021).
Taub, R. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 5, 836–847, (2004).
Kachaylo, E. et al. PTEN down-regulation promotes β-oxidation to fuel hypertrophic liver growth after hepatectomy in mice. Hepatology 66, 908–921 (2017).
Tian, X. et al. Heme oxygenase-1-modified bone marrow mesenchymal stem cells combined with normothermic machine perfusion repairs bile duct injury in a rat model of DCD liver transplantation via activation of peribiliary glands through the Wnt pathway. Stem Cells Int. 2021, 9935370 (2021).
Jassem, W. et al. Normothermic machine perfusion (NMP) inhibits proinflammatory responses in the liver and promotes regeneration. Hepatology 70, 682–695 (2019).
Liu, Q. et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 20, 987–999 (2014).
Wang, D. et al. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of cas9-specific immune responses. Hum. Gene Ther. 26, 432–442 (2015).
Tschuor, C. et al. Yes-associated protein promotes early hepatocyte cell cycle progression in regenerating liver after tissue loss. FASEB Bioadv. 1, 51–61 (2019).
Janda, C. Y. et al. Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signalling. Nature 545, 234–237 (2017).
Tschuor, C. et al. Constitutive androstane receptor (Car)-driven regeneration protects liver from failure following tissue loss. J. Hepatol. 65, 66–74 (2016).
McLaughlin, K. A. & Levin, M. Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev. Biol. 433, 177–189 (2018).
Sundelacruz, S., Moody, A. T., Levin, M. & Kaplan, D. L. Membrane potential depolarization alters calcium flux and phosphate signaling during osteogenic differentiation of human mesenchymal stem cells. Bioelectricity 1, 56–66 (2019).
Abt, P. et al. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation 75, 1659–1663 (2003).
de Jong, I. E. M. et al. Persistent biliary hypoxia and lack of regeneration are key mechanisms in the pathogenesis of posttransplant nonanastomotic strictures. Hepatology 75, 814–830 (2022).
van Leeuwen, O. B. et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am. J. Transplant. 22, 1658–1670 (2022).
Karimian, N., Op den Dries, S. & Porte, R. J. The origin of biliary strictures after liver transplantation: is it the amount of epithelial injury or insufficient regeneration that counts? J. Hepatol. 58, 1065–1067 (2013).
de Jong, I. E. M. et al. Peribiliary glands are key in regeneration of the human biliary epithelium after severe bile duct injury. Hepatology 69, 1719–1734 (2019).
de Jong, I. E. M. et al. Restoration of bile duct injury of donor livers during ex situ normothermic machine perfusion. Transplantation 107, e161–e172 (2023).
Sutton, M. E. et al. Regeneration of human extrahepatic biliary epithelium: the peribiliary glands as progenitor cell compartment. Liver Int. 32, 554–559 (2012).
op den Dries, S. et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J. Hepatol. 60, 1172–1179 (2014).
Ly, M. et al. Long term ex-situ normothermic machine perfusion allows regeneration of human livers with severe bile duct injury. Am. J. Transplant. S1600-6135, 00442-8 (2024).
Doncheva, N. T. et al. Human pathways in animal models: possibilities and limitations. Nucleic Acids Res. 49, 1859–1871 (2021).
Loewa, A., Feng, J. J. & Hedtrich, S. Human disease models in drug development. Nat. Rev. Bioeng. 1–15 (2023).
Baruteau, J., Waddington, S. N., Alexander, I. E. & Gissen, P. Gene therapy for monogenic liver diseases: clinical successes, current challenges and future prospects. J. Inherit. Metab. Dis. 40, 497–517 (2017).
Cabanes-Creus, M. et al. Harnessing whole human liver ex situ normothermic perfusion for preclinical AAV vector evaluation. Nat. Commun. 15, 1876 (2024).
Kuse, Y. & Taniguchi, H. Present and future perspectives of using human-induced pluripotent stem cells and organoid against liver failure. Cell Transplant. 28, 160s–165s (2019).
Hautz, T. et al. Immune cell dynamics deconvoluted by single-cell RNA sequencing in normothermic machine perfusion of the liver. Nat. Commun. 14, 2285 (2023).
Goldaracena, N. et al. Inducing hepatitis C virus resistance after pig liver transplantation-a proof of concept of liver graft modification using warm ex vivo perfusion. Am. J. Transplant. 17, 970–978 (2017).
Schlegel, A. et al. Machine perfusion of the liver and bioengineering. J. Hepatol. 78, 1181–1198 (2023).
Webb, A. N., Lester, E. L. W., Shapiro, A. M. J., Eurich, D. T. & Bigam, D. L. Cost-utility analysis of normothermic machine perfusion compared to static cold storage in liver transplantation in the Canadian setting. Am. J. Transplant. 22, 541–551 (2022).
Author information
Authors and Affiliations
Contributions
N.L. and C.P. drafted the manuscript. G.M., M.L., K.L. and M.C. critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Stefan Schneeberger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lau, NS., McCaughan, G., Ly, M. et al. Long-term machine perfusion of human split livers: a new model for regenerative and translational research. Nat Commun 15, 9809 (2024). https://doi.org/10.1038/s41467-024-54024-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54024-4