Abstract
Carbon-silicon-switch strategy, replacing one specific carbon atom in organic molecules with a silicon, has garnered significant interest for developing new functional molecules. However, the influence of a reaction regarding its selectivity and reactivity by carbon-silicon-switch strategy has far less been investigated. Here we discover an unusual carbon-silicon-switch effect in the enantioselective construction of silicon-stereogenic center. It is found that there has been a significant change in the desymmetrization reaction of silacyclohexadienones using asymmetric conjugate addition or oxidative Heck reaction with aryl/alkyl nucleophiles when compared with their carbon analogues cyclohexadienones. Specifically, the carbon-silicon-switch leads to a reversal in enantioselectivity with arylzinc as the nucleophile by the same chiral catalyst, and results in totally different reactivity with arylboronic acid as the nucleophile. Control experiments and density functional theory (DFT) calculations have shown that the unusual carbon-silicon-switch effect comes from the unique stereoelectronic feature of silicon.
Similar content being viewed by others
Introduction
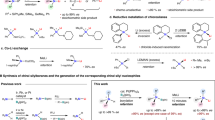
Silicon, positioned in the XIV group of the periodic table below carbon, has many similarities with the latter due to their close proximity. Carbon-silicon-switch strategy, replacing one specific carbon atom in organic molecules with a silicon, has garnered significant interest for developing new functional molecules (Fig. 1a)1,2,3,4,5,6,7. For examples, chiral molecules with silicon-stereogenic centers8,9,10,11,12,13,14,15,16,17,18,19,20,21 have been used in the development of advanced materials22, medical applications23,24,25,26,27,28, probes for mechanistic studies29, and ligands for asymmetric transformations30,31,32,33,34,35,36. However, the influence of a reaction regarding its selectivity and reactivity by carbon-silicon-switch has far less been investigated. Chemists generally believe that the reactions of silicon substrates exhibit the same or similar selectivity and reactivity when compared to their carbon analogues37,38.
Enantioselective construction of chirality at silicon is one of the most intriguing and challenging tasks in asymmetric synthesis and silicon chemistry37,38,39,40,41. Especially, synthesis of enantioenriched silicon-stereogenic silacycles is highly attractive and has drawn considerable attention42,43,44,45,46,47,48,49,50,51,52, given the increasing demand for the synthesis of functional silicon-bridged compounds (Fig. 1b)53,54,55,56. Therefore, we believe that the reaction for the construction of silicon-stereogenic center would be an ideal platform to investigate the carbon-silicon-switch effect. We then choose silacyclohexadienones as a type of model substrates and use well-estabilished asymmetric conjugate addition (the Hayashi-Miyaura reaction)57,58,59 and oxidative Heck reaction60,61,62,63 as the model reactions, which is based on the consideration of the following three aspects. First, the carbon analogues, cyclohexadienones, has been widely used in the asymmetric desymmetrization reaction for the construction of enantioenriched cyclohexanone-containing molecules64,65,66,67,68, while this strategy has not been used in construction of a silicon-stereogenic center, providing a good contrast when exploring the carbon-silicon-switch effect. Second, as a type of cyclic molecules, the structure distortion of silacyclohexadienones caused by the longer C–Si bond would be bigger than those of acyclic ones, allowing the possibility on the observation of different stereocontrol (Fig. 1c)69. Finally, the two adjacent alkenyl moieties to the Si center in silacyclohexadienones, featured with π-d conjugation between the olefin and the Si empty 3 d orbital, is a good example to study the reactivity changes derived from the silicon-carbon-switch.
In this work, an unusual carbon-silicon-switch effect is discovered in the desymmetrization reaction of silacyclohexadienones using the Hayashi-Miyaura reaction or the oxidative Heck reaction with aryl/alkyl nucleophiles, which challenges the traditional understanding of silicon chemistry (Fig. 1d). The reaction of silacyclohexadienone 1 using ArZnCl 2 as the nucleophile in the presence of ClSiMe3 catalyzed by Rh/(R)-DTBM-segphos (condition A) gave silacyclohexenone 4, with a silicon-stereogenic center as R-configuration (Fig. 1d, path 1). On the contrary, S-configuration is observed in the reaction of their carbon analogue cyclohexadienone under the same conditions. It overturned the traditional understanding of silicon chemistry that the reactions of silicon molecules follow the same or similar stereochemical pathways of the reactions of their carbon analogues. When using RB(OH)2 2’ (R = aryl/alkyl) as the nucleophile in the presence of a chiral (R)-DTBM-binap–rhodium catalyst (condition B), Heck-type product silacyclohexenone 5 was obtained exclusively via a sequence of nucleophilic addition and intramolecular hydrogen transfer process (Fig. 1d, path 2). Note that the addition of arylboronic acids to cyclohexadienones under the same conditions (condition B) did not take place, whereas arylboronic acids underwent hydrolysis instead. Therefore, the carbon-silicon-switch also leads to a reversal in reactivity by significantly increasing the reactivity of alkenyl moiety, which makes nucleophilic addition much more favorable than hydrolysis in the case of silacyclohexenone. Density functional theory (DFT) calculations were conducted and showed that the unusual carbon-silicon-switch effect is caused by the unique stereoelectronic feature of silicon, including longer C–Si bond than C–C bond, structure distortion and π-d conjugation from the Si empty 3 d orbital.
Results and discussion
Optimization of reaction conditions (condition A)
Silacyclohexadienones were prepared by DDQ oxidation of silacyclohexanones, which were prepared by a five-step approach from divinylsilanes via a base-induced reaction of borinic acid esters with α,α-dichloromethyl methyl ether70,71. Silacyclohexadienone 1a, in which silicon atom is bonded to a phenyl group and a methyl group, was selected as a model substrate to optimize the reaction conditions (Table 1). Notably, a challenge of the reaction is that the addition to 1a may lead to a mixture of mono- and bis-addition products72,73. The dienone 1a was allowed to react with PhZnCl (2a) and ClSiMe3 in the presence of a rhodium catalyst in situ generated from [RhCl(coe)2]2 and (R)-DTBM-segphos74 in THF at –10 °C for 12 h (condition A, entry 1). Hydrolysis of the silyl enol ether 3aa formed a 94% yield of the mono-1,4-addition product 4aa with > 50:1 trans:cis and 99% ee. The Heck-type product 5aa or the bis-addition product was not detected in 1H NMR and LC-Mass analyses of the crude reaction mixture. Other less bulky bisphosphine ligands, (R)-segphos and (R)-difluorphos, gave 4aa in lower yields with excellent chemo- and diastereoselectivity, but the enantioselectivity was much lower (entries 2 and 3). This indicates that the large sterically hindered bisphosphine ligand plays a key role in the control of enantioselectivity. To further support this conclusion, (R)-binap, (R)-DM-binap, and (R)-DTBM-binap75 were used as ligands in the present reaction under condition A (entries 4–6). The most sterically hindered (R)-DTBM-binap exhibited not only the best enantioselectivity, but also the highest selectivity in giving 5aa (4aa:5aa = 59:41) (entry 6). Thus, a large sterically hindered bisphosphine ligand with a large dihedral angle favors the formation of the oxidative Heck addition/intramolecular hydrogen transfer products. Chiral diene ligand (S,S)-Fc-tfb76,77, whose enantioselectivity is very high in most of the Hayashi-Miyaura reactions57,58,59, exhibited no reactivity (entry 7). A reaction in the absence of ClSiMe3 under otherwise identical conditions as in entry 1 did not give any addition product (entry 8). At 30 °C, the reaction proceeded smoothly to afford 4aa in a high yield with slightly lower enantioselectivity (entry 9).
Substrate scope under condition A
The desymmetrization of silacyclohexadienones via Rh-catalyzed asymmetric conjugate addition was also successful with several other silacyclohexadienones bearing various functional groups on the silicon atoms under condition A, and excellent chemoselectivity (4:5 = > 50:1) in giving the conjugate addition products was observed in the phenylation of these silacyclohexadienones, whose results are summarized in Fig. 2a. The desymmetrization of silacyclohexadienones 1b–1e, in which the silicon atoms are bonded to a methyl group and a substituted aryl group, gave high yields of the corresponding products 4ba–4ea with excellent chemo-, diastereo-, and enantioselectivity (4:5 = > 50:1, 93:7– > 50:1 dr, 99%– > 99.5% ee). The reaction of 1 f, bearing a methyl group and a thiophen-2-yl group, gave a high yield of the corresponding product 4fa with excellent chemo-, diastereo-, and enantioselectivity. The desymmetrization of 1g–1i, in which the methyl moiety of 1a was replaced by a larger alkyl group, proceeded smoothly to give high yields of the products 4ga–4ia with excellent chemo, diastereo-, and enantioselectivity. Excellent chemoselectivity, high yields, and high enantioselectivity were also achieved in the reactions of bulky silacyclohexadienones having a phenyl group and an ortho-substituted aryl group, giving exclusively the conjugate addition products 4ja–4la. The desymmetrization of 1 m, having two different alkyl groups (methyl and cyclohexyl), afforded the corresponding product 4ma in a high yield with excellent chemo-, diastereo-, and enantioselectivity. However, substrate 1n, featuring less pronounced differences (n-butyl versus methyl), gave the conjugate addition product 4na with high chemo- and enantioselectivity, but the diastereoselectivty sharply decreased. The condition A optimized for the reaction shown in Table 1 was also applied to reactions of silacyclohexadienone 1a with various arylzinc reagents 2, and the results are summarized in Fig. 2b. Under condition A, reactions of 1a with ArZnCl, in which aromatic groups are phenyls substituted with methoxy, methyl, trimethylsilyl, phenyl, and halo at para or meta position and 2-naphthyl, all gave the corresponding products 4ab–4ak in high yields with excellent chemoselectivity (4:5 = > 50:1), high diastereoselectivity, and high enantioselectivity, irrespective of the electronic properties of the substituents. It is also remarkable that the Heck-type products were not formed in the reactions shown under condition A in Fig. 2.
DFT calculations for opposite enantioselectivity
Here, we found the enantioselectivity in the reaction of silacyclohexadienone 1a catalyzed by the Rh/(R)-DTBM-segphos catalyst is opposite to that in the reaction of cyclohexadienone 6 under the same conditions (Fig. 3a). It overturned the traditional understanding of silicon chemistry that the reactions of silicon molecules follow the same/similar stereochemical pathways of the reactions of their carbon analogues78,79. To understand the opposite enantioselectivity caused by carbon-silicon-switch, DFT calculations have been performed using Gaussian 09 package80 (see DFT calculations section of supplementary Information for more details). The transition states (TSs) associated with the formation of C–C bonds for 1a and 6 mediated by L-Rh(I)-complex are located, and their corresponding optimized geometries are shown in Fig. 3b–e. For 1a, the relative Gibbs free energy (∆G) of TS-RSiSC is the lowest among the four TSs. That is, the pathway via TS-RSiSC is kinetically the most favorable. For TS-RSiSC, the C1…C2 distance is 2.04 Å. The Ph group of the silacyclohexadienone is positioned away from the bulky tBu group of the ligand, with a Ph…tBu distance of 3.80 Å, thus avoiding unfavorable steric effect. To minimize the repulsion between the two substrates and the bulky substituent in the ligand, the planar quadrilateral structure in the L-Rh(I)-complex slightly distorts, resulting in a C1-Rh-P1 bond angle of 147°. The Independent gradient model on Hirshfeld partition(IGMH) analysis81 also indicates that there are fewer steric repulsions among the Ph group of the silacyclohexadienone, the tBu groups of the ligand and the Rh(I)-Ph moiety in the TS-RSiSC (Fig. 3c). Accordingly, TS-RSiSC could predominantly form, yielding a major product with RSi,SC-configuration, as observed in the experiment. Different from 1a, the ∆G of TS-SR was the lowest among four TSs, leading to SR-configuration product in the reaction of carbon analog 6. Unlike the Si–C2 bonds in TSs, the C3–C2 bond lengths at the sp3 hybrid C3 atoms in TS-RR ~ TS-SS are shorter than the corresponding Si–C bonds by 0.34 ~ 0.35 Å. Consequently, the steric effect from the tBu groups of the ligand and the Ph group becomes more significant, especially for TS-SS and TS-RS (Fig. 3e). Due to relative weak repulsion between the small size Me at the C3 atom of 6 and the Ph group, TS-SR is more stable than TS-RR by 6.2 kcal mol-1.
a Opposite enantioselectivity due to carbon-silicon-switch. b Optimized geometries of four transition states in the Rh-catalyzed desymmetrization reaction of silacyclohexadienone 1a. Relative Gibbs free energy (in kcal mol-1) of TS-RSiSC was set to zero. c The IGMH analysis of key transition states, visualized by Multiwfn 3.8 (dev) and VMD (1.9.3 version) softwares (isovalue = 0.002 a.u.). d Optimized geometries of four transition states in the Rh-catalyzed desymmetrization reaction of cyclohexadienone 6. Relative Gibbs free energy (in kcal mol-1) of TS-SR was set to zero. e The IGMH analysis of key transition states, visualized by Multiwfn 3.8 (dev) and VMD (1.9.3 version) softwares (isovalue = 0.002 a.u.). a.u. = atomic units.
Optimization of reaction conditions (condition B)
The perfect chemoselectivity towards 5aa (4aa:5aa ≤ 1:50) was observed in the reaction of 1a with PhB(OH)2 (2a’) in the presence of KOH and a rhodium catalyst in situ generated from [RhCl(coe)2]2 and (R)-DTBM-binap in dioxane at 60 °C (condition B, Table 2, entry 1). Thus, 5aa was obtained in a high yield with high enantioselectivity, whereas 4aa or the bis-addition product was not detected. The binap derivates with the decrease of the bulkiness of the aryl substituents on phosphorus also gave mainly 5aa with lower chemo- and enantioselectivity (entries 2 and 3). The results obtained in the reactions using (R)-binap, (R)-MeO-biphep, (R)-synphos, and (R)-segphos, indicate that the ligand with the largest dihedral angle82 gave the highest chemoselectivity in giving 5aa (entries 3–6). Another bulky ligand, (R)-DTBM-segphos, exhibited perfect selectivity towards 5aa but with lower enantioselectivity (entry 7). (S,S)-Fc-tfb gave a mixture of 4aa and 5aa in a 56:44 ratio with low enantioselectivity, and the total yield was low due to the moderate conversion of 1a and the formation of the bis-addition products (entry 8). As excess water was added, the reaction gave a mixture of 4aa and 5aa with lower chemo- and enantioselectivity, and the second addition of PhB(OH)2 to 4aa did not take place (entry 9). In the presence of 0.02 equiv of KOH, the addition to 1a gave a moderate yield of 5aa with high chemoselectivity (entry 10). The reaction in the presence of a hydroxorhodium catalyst in situ generated from [RhOH(coe)2]2 and (R)-DTBM-binap gave a high yield of 5aa with excellent chemoselectivity and high enantioselectivity (entry 11). It demonstrates that the hydroxorhodium species plays an important role in the catalytic cycle83. Note that the addition of phenylboronic acid to cyclohexadienone 6 under the same conditions (condition B) did not take place, whereas phenylboronic acid underwent hydrolysis instead.

Substrate scope under condition B
The desymmetrization of silacyclohexadienones via Rh-catalyzed oxidative Heck reaction with intramolecular hydrogen transfer was also successful with several other silacyclohexadienones bearing various functional groups on the silicon atoms under condition B, and high chemoselectivity in giving the addition/intramolecular hydrogen transfer products was observed in the phenylation of these silacyclohexadienones, whose results are summarized in Fig. 4a. The desymmetrization of silacyclohexadienones 1b–1e, in which the silicon atoms are bonded to a methyl group and a substituted aryl group, with PhB(OH)2 proceeded well to give the addition/intramolecular hydrogen transfer products 5ba–5ea in high yields with high chemo- and enantioselectivity. In the reaction of 1 f bearing a methyl group and a thiophen-2-yl group, excellent chemoselectivity and high enantioselectivity were achieved, giving 5fa. The desymmetrization of 1g–1i with PhB(OH)2 proceeded smoothly to give the addition/intramolecular hydrogen transfer products 5ga–5ia with high chemo- and enantioselectivity. Excellent chemoselectivity, high yields, and high enantioselectivity were also achieved in the reactions of bulky silacyclohexadienones having a phenyl group and an ortho-substituted aryl group, giving exclusively the addition/intramolecular hydrogen transfer products 5ja–5la. The desymmetrization of 1 m and 1n, having two different alkyl groups, gave high yields of 5ma and 5na with high chemo- and enantioselectivity.
The condition B optimized for the reaction shown in Table 2 was also applied to reactions of silacyclohexadienone 1a with various organoboron reagents, and the results are summarized in Fig. 4b. Comparing to the results shown in Fig. 2b under condition A, the chemoselectivity was reversed in the reactions of 1a with arylboronic acids, in which aromatic groups are phenyls substituted with methoxy, methyl, trimethylsilyl, phenyl, and halo at para or meta position and 2-naphthyl, to give mainly the addition/intramolecular hydrogen transfer products 5ab–5ak under condition B. The substituents did not have a substantial effect on the enantioselectivity, with ee values of 85–91% for 5ab–5ak. Reaction of 1a with MeB(OH)2 under condition B also gave the methylation/intramolecular hydrogen transfer product 5a in a high yield with excellent chemoselectivity and high enantioselectivity. Reactions of MeB(OH)2 with some other representative silacyclohexadienones proceeded smoothly to give 5b–5 d with excellent chemoselectivity (4:5 = < 1:50) and high enantioselectivity.
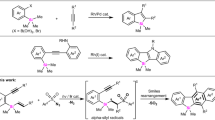
Substrate scope under condition C
In the oxidative Heck reaction giving 5, the remaining unreacted C = C double bond of silacyclohexadienones works as an oxidant. Next, we optimized the reaction conditions to achieve intermolecular redox version of this oxidative Heck reaction (see supplementary Table 1). The reaction of 1a with PhB(OH)2 (2a’) in the presence of the Rh/(R)-DTBM-binap catalyst and MVK in toluene at 120 °C gave silacyclohexadienone 9a as the main product (9a:5aa = 93:7) in 67% yield with high enantioselectivity, and 4aa was not detected (Fig. 5, entry 1). The reaction of cyclohexadienone 6 under condition C at 120 °C also did not take place to give any addition product. The condition C optimized for the reaction was also applied to reactions of silacyclohexadienones with various organoboron reagents, and the results are summarized in Fig. 5. Reactions of PhB(OH)2 with some other representative silacyclohexadienones, in which the silicon atoms are bonded to phenyl/n-butyl, phenyl/isopropyl, 1-naphthyl/phenyl, and 2-methylphenyl/phenyl, proceeded smoothly to give 9b–9e with high chemo- and enantioselectivity (entries 2–5). Oxidative Heck addition of some other arylboronic acids and MeB(OH)2 to 1a afforded the corresponding products 9f–9 h in moderate yields with high enantioselectivity (entries 6–8).
Proposed mechanism and mechanistic studies
Plausible reaction pathways for the formation of 3aa, 5aa, and 9a are proposed in Fig. 6a based on our experimental results and the catalytic cycles reported83. The addition of a phenylrhodium species to one of the two enantiotopic C = C double bonds in 1a generates an oxa-π-allyl-Rh intermediate A, which further gives 3aa, 5aa, and 9a via divergent reaction pathways (path 1, path 2, and path 3). In path 1, it shows a classical mechanism for the reaction of 1a under condition A giving 3aa as the exclusive product. Transmetalation of A with ClSiMe3 gives the product 2-silyoxy-1,3-diene 3aa, which undergoes hydrolysis to give 4aa. Under condition A, the transmetalation rate of A to 3aa is much higher than its isomerization rate to intermediate E, which undergoes transmetalation to give another 2-silyoxy-1,3-diene 3aa’. However, in path 2 under condition B, the protonolysis rate of A to 4aa is much lower than its isomerization rate. Thus, A first isomerizes to an alkyl–Rh species B, followed by syn-β-hydrogen elimination to form a H–Rh complex coordinated to the 2-arylethenyl group (C). Intramolecular H–Rh shift to the neighboring less-hindered double bond gives another olefin complex D, followed by hydrorhodation to form another oxa-π-allyl-Rh intermediate E. The protonolysis of E produces 5aa and regenerates [Rh]–OH species. The rate of protonolysis or transmetalation leading to conjugate addition products is finely tuned by varying the reaction conditions in our study. The ligand plays a crucial role in directing the reaction pathway. In particular, the unique characteristics of (R)-DTBM-binap, the large bite angle of P-Rh-P and great steric hindrance, slow down the transmetalation or protonolysis leading to the production of 3aa or 4aa and eventually to the production of 5aa or 9a. On the other side, a high temperature leads to the dissociation of the H–Rh species from C followed by its addition to MVK (path 3). It also gives the oxidized product 9a and another oxa-π-allyl-Rh intermediate F, which undergoes protonolysis to give the reduced product methylethylketone and regenerate [Rh]–OH. It is a type of oxidative Heck reaction, in which the catalysis remains rhodium-centered with the oxidation state of rhodium remaining unchanged.
Some experiments were carried out to gain further insight into the reaction mechanism (FigS. 6b–e). The reaction of 1a with PhB(OH)2 (1 equiv) in the presence of the Rh/(R)-DTBM-segphos catalyst under condition B gave a high yield of 5aa and <5% GC yield of benzene, whereas the reaction of its carbon carbon analog 6 did not gave any addition product (Fig. 6b). It may indicate that the addition of phenylrhodium species to silacyclohexadienones is much faster than its simple protonation under protic condition B, whereas the addition of phenylrhodium species to cyclohexadienones is prevented by its rapid protonolysis. The reaction of deuterium-labeled substrate 1a-d, where the two β-carbons are deuterated in 50%, with MeB(OH)2 under condition B gave the Heck-type methylation product 5a-d, where both of the two diastereotopic hydrogens on the β-position of -CH2CH2- moiety are incorporated with deuterium in 50%, demonstrating that the intramolecular Rh–D(H) shift is involved in the catalytic cycle (Fig. 6c). The reaction of (SSi)-9h with 4-MeOC6H4B(OH)2 under condition B gave the hydroarylation product 10 and the Heck-type product cis-11 in a 3:1 ratio (Fig. 6d). The selective formation of cis-isomer of 11 confirms that the Rh–H slipped from one double bond to the other on the same face of the silacyclohexadiene ring (from C to D in Fig. 6a, path 2). It is worthy of discussing the reaction of 1a with PhZnCl and ClSiMe3 in the presence of Rh/(R)-DTBM-binap catalyst, which gave a mixture of 4aa and 5aa in a 59:41 ratio with 99% ee and 85% ee, respectively (Table 1, entry 6). The difference in the % ee’s between 4aa and 5aa indicates that a kinetic resolution took place in the catalytic cycle (Fig. 6e). The addition of a phenylrhodium species coordinated with (R)-DTBM-binap to the pro-R C = C double bond of 1a generates (RSi)-A, which is the major diastereomeric intermediate. Transmetalation of (RSi)-A with ClSiMe3 produces silyl enol ether 3aa with RSi,SC configuration, whereas the product 3aa’ with SSi configuration is formed by the β-H elimination/Rh–H migration/hydrorhodation sequence. On the other hand, the minor diastereomer (SSi)-A does not undergo transmetalation which would generate (SSi)-3aa, but undergoes the sequence of reactions starting with β-H elimination leading to (RSi)-3aa’. As a result, the % ee of (RSi,SC)-4aa is very high, and the % ee of 5aa is lower than the selectivity at the phenylrhodation generating the intermediate A.
DFT calculations for different reactivity
To further elucidate the totally different reactivity of arylboronic acid as a nucleophile under condition B, DFT calculations were conducted to study the addition of phenylrhodium species to silacyclohexadienone 1a and its carbon analogue 6 (Fig. 7), as well as the hydrolysis of phenylrhodium species, based on the results of Fig. 6b. Compared to IM1-SR, the coordinated C = C bond in IM1-RSiSC was longer by 0.02 Å (Fig. 7d). Meanwhile, the corresponding Wiberg bons index was smaller than that in IM1-SR (1.365 vs 1.428). These results indicated that the C = C bond of 1a moiety in IM1-RSiSC was significantly weakened. Consequently, the activation energy via TS-RSiSC was 3.7 kcal mol⁻¹ lower than that via TS-SR (12.6 vs 16.3 kcal mol⁻¹). We speculate that π-d conjugation between the olefin and the Si empty 3 d orbital may be the main reason for their longer bond lengths. That is, 1a exhibited higher reactivity than 6 in the reaction. Notably, the ∆G≠ associated with the hydrolysis of Rh/(R)-DTBM-segphos catalyst was 13.9 kcal mol⁻¹, which was lower than that of C–C bond formation for 7. These results suggested that the Rh/(R)-DTBM-segphos catalyst would undergo hydrolysis in the presence of H₂O.
a Energy profiles for the C–C bond formation of 1a catalyzed by Rh/(R)-DTBM-segphos catalyst. b Energy profiles for the hydrolysis of Rh/(R)-DTBM-segphos catalyst. c Energy profiles for the C–C bond formation of 7 catalyzed by Rh/(R)-DTBM-segphos catalyst. d Comparison of optimized geometries IM1-RSiSC and IM1-SR.
Synthetic applications
The study not only demonstrates an unusual carbon-silicon-switch effect but also presents a efficient method for the asymmetric synthesis of enantioenriched silicon-stereogenic silacycles. Gram-scale reactions giving 4aa, 5aa, and 9a proceeds with equal efficiency without loss of enantioselectivity (Fig. 8a). Another great advantage of our methodology is the step-by-step installation of two different aryl groups on the two carbon–carbon double bonds of silacyclohexadienone to give pseudo C2-symmetric and pseudo-meso bis-addition products in a stereoselective manner (Fig. 8b). Thus, pseudo-meso trans,trans-12 was obtained in a high yield with high diastereo- and enantioselectivity by the second addition of 4-methoxyphenylboronic acid to 4aa in the presence of a Rh/(S)-binap catalyst, while its diastereoisomer trans,cis-12 was obtained by the use of (R)-binap as a ligand. The Rh-catalyzed oxidative Heck addition of 4-methoxyphenylboronic acid to 6 h in the presence of [RhCl(cod)]2 and MVK gave the diarylated diolefin 13 in a moderate yield (Fig. 8c). Enantioenriched silicon center functionalized compounds have shown a series of unique optical properties84,85,86,87,88,89. At present, molecules containing D-A (Donor-Acceptor) type have been widely used in optoelectronic devices and have shown good performance90,91, but the introduction of silicon into D-A structure molecules has not been reported yet. At 80 °C, the gram-scale reaction of 5-benzothiopheneboronic acid with silacyclohexadienone 1a proceeded smoothly to give the Heck-type product 14 with 88% yield and 91% ee (Fig. 8d). Subsequently, D-A type compound (SSi)-15 was obtained by a simple one-pot two-step method combining a DDQ oxidation reaction and a cyanidation reaction, with > 99.5% ee after recrystallization. 15 exhibited absorption maximum λabs at 410 nm with a molar absorption coefficient of 1.65 × 104 M–1 cm–1. 15 displayed bright blue and yellow fluorescence in CH2Cl2 solution and in the solid state under UV (Ultraviolet) light irradiation at 365 nm. Upon excitation at 410 nm, 15 in CH2Cl2 solution displayed broad emission peaked at around 532 nm, which was bathochromic shifted in the solid state. The absolute fluorescence quantum yield of 15 in CH2Cl2 solution was measured to be 8.2 %, while the solid-state was 21.6% (Figs. 8e, f). The electrochemical reduction of 15 in CH2Cl2 solution was studied with cyclic voltammetry (CV), the cyclic voltammogram with a scan rate of 100 mVs−1 exhibited two reversible reduction waves with half-wave potentials of –1.32 and –1.97 V versus ferrocenium/ferrocene (Fc+/Fc) as shown in Fig. 8g. Because of the electron accepting ability of 15, it is promising for applications in organic cells and n-type semiconductors. Subsequently, its enantioisomer (RSi)-15 was prepared using (S)-DTBM-binap as a ligand, and the CD spectra of two enantioisomers recorded in CH2Cl2 were clearly showed mirror images with broadband chiroptical activities in a range up to 470 nm, and the maximum Cotton effect were found at 262 nm with |Δε| of 30 M–1 cm–1 (Fig. 8h). The dissymmetry factors of |gabs| was recorded to be 2.0 × 10–3 (268 nm) (Fig. 8i). These results clearly indicate its potential for synthesizing chiral optical materials.
a–d Synthetic applications. e Pictures of the solution of 15 in UV cuvette under room light, under UV (365 nm) light, and solid under UV (365 nm) light. f UV-vis spectrum of 15 in CH2Cl2 (solid line, 50 µM), fluorescence spectra of 15 in CH2Cl2 (5.0 µM), and solid-state (λex = 410 nm). g Cyclic voltammograms of 15 (1 mM in CH2Cl2, 0.1 M n-Bu4NPF6, 100 mV/s). h CD (circular dichroism) spectra of 15 in CH2Cl2 (3.0 µM). i gabs spectra of 15. cod = 1,5-cyclooctadiene. Py = pyridine.
In summary, we discovered an unusual carbon-silicon-switch effect in the construction of silicon-stereogenic center. It was found that there was a significant change in the desymmetrization reaction of silacyclohexadienones using asymmetric conjugate addition or oxidative Heck reaction with aryl/alkyl nucleophiles when compared with their carbon analogues cyclohexadienones. Specifically, the carbon-silicon-switch leads to a reversal in enantioselectivity with arylzinc as the nucleophile by the same chiral catalyst, and results in totally different reactivity with arylboronic acid as the nucleophile. Control experiments and density functional theory (DFT) calculations showed that the unusual carbon-silicon-switch effect comes from the unique stereoelectronic feature of silicon. The longer C–Si bond than C–C bond, which distorts silacyclohexadienones as well as makes the reaction site more open, results in reversal in enantioselective control. In addition, the distorted structure and the π-d conjugation between the olefin and the Si 3 d orbital significantly activate the olefin moiety, which promotes the addition of aryl/alkylboronic acids to silacyclohexadienones rather than simple protonation in the cases with cyclohexadienones. Our findings overturn the traditional understanding of silicon chemistry, prompting scientists to focus more on the effects of reaction reactivity and stereochemistry caused by carbon-silicon-switch in future studies.
Methods
A typical procedure for Rh-catalyzed asymmetric conjugate addition (Table 1, entry 1, condition A)
An oven-dried sealed tube was charged with silacyclohexadienone 1a (40.0 mg, 0.20 mmol), [RhCl(coe)2]2 (3.6 mg, 5.0 μmol, 5.0 mol% of Rh), (R)-DTBM-segphos (14.2 mg, 12 μmol, 6.0 mol%), and THF (0.5 mL) under argon. The mixture was stirred at room temperature for 10 min and cooled to −10 °C. A solution of PhZnCl (2a, 1.5 mL, 0.40 M in THF, 0.60 mmol) and a solution of ClSiMe3 (0.6 mL, 1.0 M in THF, 0.60 mmol) were added dropwise simultaneously to the sealed tube over 15 min, and the mixture was stirred at −10 °C for additional 12 h. H2O (0.5 mL) was added, and the mixture was stirred at room temperature for 1 h. The mixture was passed through a short pad of silica gel with ethyl acetate as the eluent. The residue was subjected to NMR analysis. Purification by preparative TLC on silica gel (eluent: hexane/ethyl acetate (20/1)) gave compound (RSi,SC)-4aa (52.3 mg, 94% yield, 0.19 mmol) as a white solid.
A typical procedure for Rh-catalyzed asymmetric oxidative Heck reaction with intramolecular hydrogen transfer (Table 2, entry 1, condition B)
An oven-dried sealed tube was charged with silacyclohexadienone 1a (40.0 mg, 0.20 mmol), [RhCl(coe)2]2 (3.6 mg, 5.0 μmol, 5.0 mol% of Rh), (R)-DTBM-binap (14.3 mg, 12 μmol, 6.0 mol%), and dioxane (1.0 mL) under argon. The mixture was stirred at room temperature for 15 min. PhB(OH)2 (2a’, 48.4 mg, 0.40 mmol), and KOH (1.1 mg, 0.02 mmol) were added successively. The tube was placed in a preheated oil bath at 60 °C and stirred for 12 h. The mixture was passed through a short pad of silica gel with ethyl acetate as the eluent. The solvent was removed on a rotary evaporator. The residue was subjected to NMR analysis. Purification by preparative TLC on silica gel (eluent: hexane/ethyl acetate (20/1)) gave compound (SSi)-5aa (53.4 mg, 96% yield, 0.19 mmol) as a white solid.
A typical procedure for Rh-catalyzed asymmetric oxidative Heck reaction with intermolecular hydrogen transfer (Fig. 5, entry 1, condition C)
An oven-dried sealed tube was charged with silacyclohexadienone 1a (40.0 mg, 0.20 mmol), [RhCl(coe)2]2 (3.6 mg, 5.0 μmol, 5.0 mol% of Rh), (R)-DTBM-binap (14.3 mg, 12 μmol, 6.0 mol%), and toluene (1.0 mL) under argon. The mixture was stirred at room temperature for 15 min. PhB(OH)2 (2a’, 48.4 mg, 0.40 mmol), methyl vinyl ketone (MVK, 70.1 mg, 1.0 mmol), and KOH (5.5 mg, 0.10 mmol) were added successively. The tube was placed in a preheated oil bath at 120 °C and stirred for 12 h. The mixture was passed through a short pad of silica gel with ethyl acetate as the eluent. The solvent was removed on a rotary evaporator. The residue was subjected to NMR analysis. Purification by preparative TLC on silica gel (eluent: hexane/ethyl acetate (20/1)) gave compound (SSi)-9a (37.0 mg, 67% yield, 0.13 mmol) as a white solid.
Data availability
Detailed experimental procedures, characterization data, NMR spectra of new compounds, HPLC spectra for chiral compounds, detailed computational results, X-ray structural analysis, and calculated structures are available within Supplementary Information. Cartesian coordinates of the calculated structures are available from Supplementary Data 1. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2344298 (4aa) and 2344299 (4ba). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/ data_request/cif. Data supporting the findings of this manuscript are also available from the corresponding authors upon request.
References
Pooni, P. K. & Showell, G. A. Silicon switches of marketed drugs. Mini-Rev. Med. Chem. 6, 1169–1177 (2006).
Tacke, R. & Metz, S. Odorant design based on the carbon/silicon switch strategy. Chem. Biodivers. 5, 920–941 (2008).
Lazareva, N. F. & Lazarev, I. M. Drug design based on the carbon/silicon switch strategy. Russ. Chem. Bull. 64, 1221–1232 (2015).
Fujii, S. & Hashimoto, Y. Progress in the medicinal chemistry of silicon: C/Si exchange and beyond. Future Med. Chem. 9, 485–505 (2017).
Ramesh, R. & Reddy, D. S. Quest for novel chemical entities through incorporation of silicon in drug scaffolds. J. Med. Chem. 61, 3779–3798 (2018).
Kleemiss, F. et al. Sila-Ibuprofen. J. Med. Chem. 63, 12614–12622 (2020).
Panayides, J.-L., Riley, D. L., Hasenmaile, F. & van Otterlo, W. A. L. The role of silicon in drug discovery: a review. RSC Med. Chem. https://doi.org/10.1039/D4MD00169A (2024).
Chan, T. H. & Wang, D. Chiral organosilicon compounds in asymmetric synthesis. Chem. Rev. 92, 995–1006 (1992).
Oestreich, M. Silicon-stereogenic silanes in asymmetric catalysis. Synlett 1629–1643 (2007).
Xu, L.-W., Li, L., Lai, G.-Q. & Jiang, J.-X. The recent synthesis and application of silicon-stereogenic silanes: a renewed and significant challenge in asymmetric synthesis. Chem. Soc. Rev. 40, 1777–1790 (2011).
Min, G. K., Hernández, D. & Skrydstrup, T. Efficient routes to carbon-silicon bond formation for the synthesis of silicon-containing peptides and Azasilaheterocycles. Acc. Chem. Res. 46, 457–470 (2013).
Bauer, J. O. & Strohmann, C. Recent progress in asymmetric synthesis and application of difunctionalized silicon-stereogenic silanes. Eur. J. Inorg. Chem. 2016, 2868–2881 (2016).
Shintani, R. Recent progress in catalytic enantioselective desymmetrization of prochiral organosilanes for the synthesis of silicon-stereogenic compounds. Synlett 29, 388–396 (2018).
Ye, F., Xu, Z. & Xu, L.-W. The discovery of multifunctional chiral P ligands for the catalytic construction of quaternary carbon/silicon and multiple stereogenic centers. Acc. Chem. Res. 54, 452–470 (2021).
Zheng, L., Nie, X.-X., Wu, Y. & Wang, P. Construction of Si-stereogenic silanes through C−H activation approach. Eur. J. Org. Chem. 2021, 6006–6014 (2021).
Ye, F. & Xu, L.-W. A glimpse and perspective of current organosilicon chemistry from the view of hydrosilylation and synthesis of silicon-stereogenic silanes. Synlett 32, 1281–1288 (2021).
Huang, W.-S., Wang, Q., Yang, H. & Xu, L.-W. State-of-the-art advances in enantioselective transition-metal-mediated reactions of Silacyclobutanes. Synthesis 54, 5400–5408 (2022).
Wu, Y. & Wang, P. Silicon-stereogenic Monohydrosilane: synthesis and applications. Angew. Chem. Int. Ed. 61, e202205382 (2022).
Ge, Y., Huang, X., Ke, J. & He, C. Transition-metal-catalyzed enantioselective C−H silylation. Chem. Catal. 2, 2898–2928 (2022).
Gao, J. & He, C. Chiral silanols: strategies and tactics for their synthesis. Chem. Eur. J. 29, e202203475 (2023).
Wu, Y., Zheng, L., Wang, Y. & Wang, P. Catalytic asymmetric synthesis of silicon-stereogenic organosilanes. Chem. Catal. 9, 3461–3514 (2023).
Park, S. B. (C6F5)3-catalyzed sp3 C-Si bond forming consecutive reactions. Chin. J. Chem. 37, 1057–1071 (2019).
Showell, G. A. & Mills, J. S. Chemistry challenges in lead optimization: silicon isosteres in drug discovery. Drug. Discov. Today 8, 551–556 (2003).
Bains, W. & Tacke, R. Silicon chemistry as a novel source of chemical diversity in drug design. Curr. Opin. Drug Discov. Dev. 6, 526–543 (2003).
Gately, S. & West, R. Novel therapeutics with enhanced biological activity generated by the strategic introduction of silicon isosteres into known drug scaffolds. Drug Dev. Res. 68, 156–163 (2007).
Wang, J. et al. Exploring organosilane amines as potent inhibitors and structural probes of influenza a virus m2 proton channel. J. Am. Chem. Soc. 133, 13844–13847 (2011).
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Rémond, E., Martin, C., Martinez, J. & Cavelier, F. Silicon-containing amino acids: synthetic aspects, conformational studies, and applications to bioactive peptides. Chem. Rev. 116, 11654–11684 (2016).
Sommer, L. H. & Frye, C. L. Optically active organosilicon compounds having reactive groups bonded to asymmetric silicon. displacement reactions at silicon with pure retention and pure inversion of configuration. J. Am. Chem. Soc. 81, 1013 (1959).
Bai, X.-F. et al. Lewis-base-mediated diastereoselective Silylations of alcohols: synthesis of silicon-stereogenic Dialkoxysilanes controlled by chiral aryl BINMOLs. Chem. Asian J. 12, 1730–1735 (2017).
Chang, X., Ma, P.-L., Chen, H.-C., Li, C.-Y. & Wang, P. Asymmetric synthesis and application of chiral spirosilabiindanes. Angew. Chem. Int. Ed. 59, 8937–8940 (2020).
Zhang, H. & Zhao, D. Synthesis of silicon-stereogenic silanols involving iridium-catalyzed enantioselective c–h silylation leading to a new ligand scaffold. ACS Catal. 11, 10748–10753 (2021).
Yang, L. et al. Concise synthesis and applications of enantiopure Spirobiphenoxasilin-diol and its related chiral ligands. Chem. Commun. 57, 13365–13368 (2021).
Yang, B., Gao, J., Tan, X., Ge, Y. & He, C. Chiral PSiSi-ligand enabled iridium-catalyzed Atroposelective intermolecular C−H silylation. Angew. Chem. Int. Ed. 62, e202307812 (2023).
Liu, T. et al. Enantioselective nickel-catalyzed hydrosilylation of 1,1-disubstituted allenes. Angew. Chem. Int. Ed. 62, e202216878 (2023).
Li, H. et al. SPSiPs, a class of diphosphine ligands based on SPSiOL with a large dihedral angle. Org. Lett. 25, 3859–3863 (2023).
Igawa, K., Yoshihiro, D., Abe, Y. & Tomooka, K. Enantioselective synthesis of Silacyclopentanes. Angew. Chem. Int. Ed. 55, 5814–5818 (2016).
Wu, L. et al. Catalytic asymmetric construction of C‐ and Si‐stereogenic Silacyclopentanes via hydrosilylation of Arylmethylenecyclopropanes. Angew. Chem. Int. Ed. 63, e202413753 (2024).
An, K. et al. Rhodium hydride enabled enantioselective intermolecular C–H silylation to access acyclic stereogenic Si–H. Nat. Commun. 13, 847 (2022).
Wang, X. et al. Stereospecific synthesis of silicon-stereogenic optically active Silylboranes and general synthesis of chiral silyl Anions. Nat. Commun. 14, 5561 (2023).
Hu, T. et al. Enantioconvergent construction of stereogenic silicon via Lewis base-catalyzed dynamic kinetic Silyletherification of racemic Chlorosilanes. Nat. Commun. 14, 4900 (2023).
Rendler, S., Oestreich, M., Butts, C. P. & Lloyd-Jones, G. C. Intermolecular chirality transfer from silicon to carbon: interrogation of the two-silicon cycle for Pd-catalyzed hydrosilylation by Stereoisotopochemical crossover. J. Am. Chem. Soc. 129, 502–503 (2007).
Shintani, R., Moriya, K. & Hayashi, T. Palladium-catalyzed enantioselective desymmetrization of silacyclobutanes: construction of silacycles possessing a tetraorganosilicon stereocenter. J. Am. Chem. Soc. 133, 16440–16443 (2011).
Shintani, R., Otomo, H., Ota, K. & Hayashi, T. Palladium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzosiloles via enantioselective C–H bond functionalization. J. Am. Chem. Soc. 134, 7305–7308 (2012).
Shintani, R., Maciver, E. E., Tamakuni, F. & Hayashi, T. Rhodium-catalyzed asymmetric synthesis of silicon-stereogenic Dibenzooxasilines via enantioselective transmetalation. J. Am. Chem. Soc. 134, 16955–16958 (2012).
Shintani, R., Takagi, C., Ito, T., Naito, M. & Nozaki, K. Rhodium-catalyzed asymmetric synthesis of silicon-stereogenic Dibenzosiloles by enantioselective [2+2+2] cycloaddition. Angew. Chem. Int. Ed. 54, 1616–1620 (2015).
Kumar, R., Hoshimoto, Y., Yabuki, H., Ohashi, M. & Ogoshi, S. Nickel(0)-catalyzed enantio- and diastereoselective synthesis of Benzoxasiloles: ligand-controlled switching from inter- to intramolecular aryl-transfer process. J. Am. Chem. Soc. 137, 11838–11845 (2015).
Zhan, G. et al. Enantioselective construction of silicon-stereogenic silanes by scandium-catalyzed. Angew. Chem. Int. Ed. 57, 12342–12346 (2018).
Zeng, Y. et al. Rhodium-catalyzed dynamic kinetic asymmetric hydrosilylation to access silicon-stereogenic center. Angew. Chem. Int. Ed. 61, e202214147 (2022).
Wang, X.-C., Li, B., Ju, C.-W. & Zhao, D. Nickel(0)-catalyzed divergent reactions of silacyclobutanes with internal alkynes. Nat. Commun. 13, 3392 (2022).
Zhang, X.-X., Gao, Y., Zhang, Y.-X., Zhou, J. & Yu, J.-S. Highly enantioselective construction of multifunctional silicon-stereogenic silacycles by asymmetric enamine catalysis. Angew. Chem. Int. Ed. 62, e202217724 (2023).
Gan, W.-E. et al. Copper-catalyzed asymmetric synthesis of silicon-stereogenic Benzoxasiloles. Angew. Chem. Int. Ed. 63, e202317973 (2024).
Oestreich, M. & Rendler, S. True” chirality transfer from silicon to carbon: asymmetric amplification in a reagent-controlled palladium-catalyzed hydrosilylation. Angew. Chem. Int. Ed. 44, 1661–1664 (2005).
Daiss, J. O. et al. Sila-venlafaxine, a sila-analogue of the serotonin/noradrenaline reuptake inhibitor venlafaxine: synthesis, crystal structure analysis, and pharmacological characterization. Organometallics 25, 1188–1198 (2006).
Franz, A. K. & Wilson, S. O. Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388–405 (2013).
Luo, G. et al. Asymmetric total synthesis and antidepressant activity of (−)-sila-mesembranol bearing a silicon stereocenter. Org. Chem. Front. 8, 5941–5947 (2021).
Hayashi, T. & Yamasaki, K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 103, 2829–2844 (2003).
Tian, P., Dong, H.-Q. & Lin, G.-Q. Rhodium-catalyzed asymmetric arylation. ACS Catal. 2, 95–119 (2012).
Burns, A. R. & Lam, H. W. Enantioselective, rhodium-catalyzed 1,4-addition of organoboron reagents to electron-deficient alkenes. Org. React. 93, 1–686 (2017).
Ruan, J., Li, X., Saidi, O. & Xiao, J. Oxygen and base-free oxidative heck reactions of Arylboronic acids with olefins. J. Am. Chem. Soc. 130, 2424–2425 (2008).
Lee, A.-L. Enantioselective oxidative boron Heck reactions. Org. Biomol. Chem. 14, 5357–5366 (2016).
Lamb, C. J. C., Vilela, F. & Lee, A.-L. Pd(II)-catalyzed enantioselective desymmetrization of polycyclic cyclohexenediones: conjugate addition versus oxidative heck. Org. Lett. 21, 8689–8694 (2019).
Lv, H. et al. Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation. Nat. Commun. 10, 5025 (2019).
Yang, X., Wang, J. & Li, P. Recent progress on asymmetric organocatalytic construction of chiral cyclohexenone skeletons. Org. Biomol. Chem. 12, 2499–2513 (2014).
Chen, B., He, C.-Y., Chu, W.-D. & Liu, Q.-Z. Recent advances in the asymmetric transformations of achiral cyclohexadienones. Org. Chem. Front. 8, 825–843 (2021).
Qiao, Y. et al. Rhodium-catalyzed Desymmetric arylation of γ,γ-disubsituted cyclohexadienones: asymmetric synthesis of chiral all-carbon quaternary centers. Org. Lett. 24, 1556–1560 (2022).
Xu, P., Zhou, F., Zhu, L. & Zhou, J. Catalytic desymmetrization reactions to synthesize all-carbon quaternary stereocentres. Nat. Synth. 2, 1020–1036 (2023).
Zeng, Q.-Q. et al. Biocatalytic desymmetrization for Synthesis of chiral enones using flavoenzymes. Nat. Synth. https://doi.org/10.1038/s44160-024-00596-4 (2024).
Wang, Q. et al. Copper-catalyzed enantioselective desymmetrization of prochiral tetrasubstituted Siladiols: access toward optically active silicon-stereogenic Silylmethanols. Catal. Commun. 138, 105950 (2020).
Soderquist, J. A., Shiau, F.-Y. & Lemesh, R. A. 1,1-Dimethyl-1-silacyclohexan-4-one and Its Germanium analogue via boracyclic intermediates. J. Org. Chem. 49, 2565–2569 (1984).
Guo, J. et al. Synthesis of Silacyclohexanones from Divinylsilanes and Allylamines by a Rh-catalyzed cyclization. Org. Lett. 24, 726–730 (2022).
Yin, L. et al. Enantioselective synthesis of 3,3′-Diaryl-SPINOLs: rhodium-catalyzed asymmetric Arylation/BF3-promoted Spirocyclization sequence. Angew. Chem. Int. Ed. 58, 2474–2478 (2019).
Liu, N. et al. Catalyst-controlled Chemodivergent synthesis of Spirochromans from Diarylideneacetones and organoboronic acids. ACS Catal. 10, 2596–2602 (2020).
Saito, T. et al. New chiral diphosphine ligands designed to have a narrow dihedral angle in the biaryl backbone. Adv. Synth. Catal. 343, 264–267 (2001).
Goto, M. et al. Process research on the asymmetric hydrogenation of a benzophenone for developing the manufacturing process of the squalene synthase inhibitor TAK-475. Org. Process Res. Dev. 15, 1178–1184 (2011).
Dong, H.-Q., Xu, M.-H., Feng, C.-G., Sun, X.-W. & Lin, G.-Q. Recent applications of chiral N-tert-butanesulfinyl imines, chiral diene ligands and chiral sulfur–olefin ligands in asymmetric synthesis. Org. Chem. Front. 2, 73–89 (2015).
Huang, Y. & Hayashi, T. Chiral diene ligands in asymmetric catalysis. Chem. Rev. 122, 14346–14404 (2022).
Shintani, R., Okamoto, K. & Hayashi, T. Carbon‒Carbon bond-forming enantioselective synthesis of chiral organosilicon compounds by rhodium/chiral diene-catalyzed asymmetric 1, 4-addition reaction. Org. Lett. 7, 4757–4759 (2005).
Shintani, R., Ichikawa, Y., Takatsu, K., Chen, F.-X. & Hayashi, T. Tuning the chiral environment of C2-symmetric diene ligands: development of 3,7-disubstituted bicyclo [3.3.1]nona-2,6-dienes. J. Org. Chem. 74, 869–873 (2009).
Frisch, M. et al. Gaussian 09 (Revision D01) (Gaussian, Inc., 2013).
Lu, T. & Chen, Q. Independent gradient model based on Hirshfeld partition: a new method for visual study of interactions in chemical systems. J. Comput Chem. 43, 539–555 (2022).
Berthod, M., Mignani, G., Woodward, G. & Lemaire, M. Modified BINAP: the how and the why. Chem. Rev. 105, 1801–1836 (2005).
Hayashi, T., Takahashi, M., Takaya, Y. & Ogasawara, M. Catalytic cycle of rhodium-catalyzed asymmetric 1,4-addition of organoboronic acids. arylrhodium, oxa-π-allylrhodium, and Hydroxorhodium intermediates. J. Am. Chem. Soc. 124, 5052–5058 (2002).
Guo, Y., Liu, M.-M., Zhu, X., Zhu, L. & He, C. Catalytic asymmetric synthesis of silicon-stereogenic Dihydrodibenzosilines: silicon central-to-axial chirality relay. Angew. Chem. Int. Ed. 60, 13887–13891 (2021).
Zhu, J., Chen, S. & He, C. Catalytic enantioselective Dehydrogenative Si–O coupling to access chiroptical Silicon-stereogenic siloxanes and alkoxysilanes. J. Am. Chem. Soc. 143, 5301–5307 (2021).
Chen, S. et al. Enantioselective construction of six- and seven-membered Triorgano-substituted silicon-stereogenic heterocycles. Nat. Commun. 12, 1249 (2021).
Zhang, J., Yan, N., Ju, C.-W. & Zhao, D. Nickel(0)-catalyzed asymmetric ring expansion toward enantioenriched silicon-stereogenic Benzosiloles. Angew. Chem. Int. Ed. 60, 25723–25728 (2021).
Chen, H. et al. Enantioselective synthesis of Spirosilabicyclohexenes by asymmetric dual ring expansion of Spirosilabicyclobutane with alkynes. Angew. Chem. Int. Ed. 61, e202212889 (2022).
Chen, S., Zhu, J., Ke, J., Li, Y. & He, C. Enantioselective intermolecular C−H silylation of heteroarenes for the synthesis of acyclic Si-stereogenic silanes. Angew. Chem. Int. Ed. 61, e202117820 (2022).
Zhao, J., Yao, C., Ali, M. U., Miao, J. & Meng, H. Recent advances in high-performance organic solar cells enabled by acceptordonor-acceptor-donor-acceptor (A-DA′D-A) type acceptors. Mater. Chem. Front. 4, 3487–3504 (2020).
Yang, T. & Zhan, C. Polymerized A-DA′D-A type small-molecule acceptors for high performance all-polymer solar cells: progress and perspective. Sci. China Chem. 66, 2513–2531 (2023).
Acknowledgements
The authors are thankful for financial support from the National Natural Science Foundation of China (22261039 and 21973066). T.H. thanks the Taiwan Ministry of Education for support through the Yushan Fellow Program. We are also very grateful to Xiaoying Zhang and He Meng (both at IMU) for optical experiments and NMR, respectively.
Author information
Authors and Affiliations
Contributions
J.M. conceived the idea and guided the project. Y.Y. and N.H. performed the experiments and analyzed the data. Z.S. directed the part of computational study. Q.W., Y.Y., and Z.S. carried out the computational study. J.M., T.H., and Z.S. wrote the manuscript. All authors approved the submission of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chuan He and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, Y., Wei, Q., Su, Z. et al. Carbon-silicon-switch effect in enantioselective construction of silicon-stereogenic center from silacyclohexadienones. Nat Commun 15, 9915 (2024). https://doi.org/10.1038/s41467-024-54241-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54241-x
This article is cited by
-
Desymmetric sulfonylation of prochiral siladiols and related tandem sequences to multifunctional Si-chiral platform molecules
Science China Chemistry (2026)
-
Permethylated Silicon: A Structural Motif with a Critical Role in Shaping the Properties of Organic–Inorganic Compounds
Journal of Inorganic and Organometallic Polymers and Materials (2025)