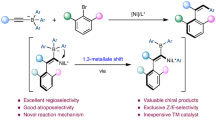
Abstract
The catalytic asymmetric synthesis of axially chiral alkenes remains a daunting challenge due to the lower rotational barrier, especially for longer stereogenic axis (e.g. C-B axis). The asymmetric radical difunctionalization of alkynes represents an efficient strategy for these targets. Key to the success of such transformations lies in aryl-stabilized highly reactive alkenyl radical intermediates, however, it remains an elusive whether a boryl group could play a similar role. Here we report a nickel-catalyzed atroposelective radical relayed reductive coupling reaction of our designed ethynyl-azaborines with simple alkyl and aryl halides through a boron-stabilized vinyl radical intermediate. This transformation enables a straightforward access to the challenging axially chiral alkenylborons bearing a C-B axis in generally high enantioselectivity and excellent stereoselectivity.
Similar content being viewed by others
Introduction
Axially chiral scaffolds are widely found in natural products, chiral catalysts, ligands, and materials1,2,3,4,5. Over the past decades, great efforts have been made for the synthesis of atropisomers featuring abundant skeletons, and biaryl derivatives are undoubtedly the most widely studied6,7,8,9,10,11,12,13. Recently, several challenging skeletons of axially chiral compounds have attracted considerable interest. For example, axially chiral styrenes are one challenging family of chiral compounds due to their relatively lower rotational barrier between different atropisomers compared to their diaryl counterparts14,15,16 (Fig. 1a). Despite significant achievements in the field, the investigations of axially chiral alkenes have focused on the C − C and C-N stereogenic axes. In this context and as part of our continued research interest in the C-B axial chirality17,18,19,20, we aimed to develop an alkenylboron scaffold based on the 1,2-azaborine units for expending the structural diversity of axial chirality (Fig. 1a). The interesting structure, derived from the replacement of the C = C bond of all-carbon aromatic rings with a B-N bond, can maintain the aromatic character and show great potential in functional materials, ligands and medicinal chemistry21,22,23,24,25,26,27 (Fig. 1b). For the designed alkenylboron atropisomers, their synthesis may be more challenging than the counterpart styrene atropisomers owing to the lower rotational barrier resulting from the fact that the Csp2-B bond is longer than the Csp2-Csp2 bond28,29,30,31.
a The design of C-B axially chiral alkenylborons. b Isoelectronic relationship between C = C and B-N bonds. c Diverse transformations via α-boryl carbon radical intermediates. d This work: nickel-catalyzed atroposelective relayed reductive coupling via α-borylvinyl radical. DPBNA = diphenyl-substituted 1,5-diaza-2,6- diboraanthracene.
Recently, tremendous progress has been made in the transition metal-catalyzed difunctionalization of alkynes via an aryl-stabilized vinyl radical intermediate, which serves as a powerful strategy to access multi-substituted alkenes32,33,34,35,36,37,38,39,40,41,42. Noteworthily, such a strategy has been successfully applied for the construction of axially chiral styrenes by Zhang43 and Liu44 groups respectively. Considering the feasibility of the process, we envisioned that an atroposelective difunctionalization of ethynyl-azaborines through an azaborine-stabilized vinyl radical intermediate could be an efficient approach to construct the designed C-B axially chiral alkenylborons. However, compared to the extensive studies on the α-borylalkyl radicals45,46,47,48,49,50 (Fig. 1c, left), the research on α-borylvinyl radicals is very rare and undeveloped51, which seems to totally be ignored by chemical community (Fig. 1c, right). Although α-borylvinyl radicals can theoretically serve as a fascinating intermediate to construct multi-substituted alkenylborons, only one example from iron-catalyzed radical addition of ethynylboronic acid pinacol ester involves such radical intermediate so far51. Herein, we report an atroposelective radical alkylarylation of ethynyl-azaborines to enable the axially chiral alkenylborons (Fig. 1d). Mechanistically, the catalytic protocol involves alkyl radical addition to the ethynyl moiety and cross-coupling of the resulting α-borylvinyl radical with nickel complex. This chemistry enables a straightforward access to the challenging axially chiral alkenylborons under mild conditions in generally high enantioselectivity and excellent stereoselectivity.
Results
Reaction conditions optimization
To overcome the above-mentioned challenges, we firstly designed and synthesized the sterically hindered ethynyl-azaborine 1a52,53,54. Then, ethynyl-azaborine 1a, 3-iodoanisole (2a) and tert-butyl iodide (3a) were employed to investigate the envisaged atroposelective radical relayed reductive coupling with NiBr2·DME as catalyst and tetrakis(dimethylamino)ethylene (TDAE) as reductant. To our delight, when pybox L1 was used as chiral ligand, the reaction could proceed smoothly (Table 1, entry 1), delivering the desired axially chiral alkenylboron 4a in 39% NMR yield and −30% enantiomeric excess (ee). The preliminary result encouraged us to find a more efficient ligand for this transformation. Other pybox ligands L2-L5 could efficiently promote this transformation in moderate enantioselectivities (Table 1, entries 2-5), and chlorine-substituted pybox ligand L4 was determined to be the best one (Table 1, entry 5, 48% yield and 86% ee). Chiral quinolin-2-yl pybox L6, bisoxazoline ligand L7, and diamine ligand L8 proved largely ineffective in this reaction (Table 1, entries 6-8). The mixed solvent (2-MeTHF/DCE) could slightly improve the yield and enantioselectivity (Table 1, entry 9), and the better enantioselectivity (89% ee) was obtained through decreased reaction temperature (Table 1, entry 10). The optimal reaction condition was furnished when MgCl2 was used as an additive (Table 1, entry 11, 72% yield and 92% ee). In addition, other Ni catalysts, such as NiCl2·DME and NiBr2, afforded inferior results (Table 1, entries 12 and 13).
Substrate scopes
With the optimized asymmetric radical relayed reductive coupling conditions in hand, we first evaluated the substrate scope of aryl iodides (Fig. 2). Generally, aryl iodides bearing electron-donating, -neutral and -withdrawing substituents proceeded smoothly under the standard conditions, furnishing the corresponding axially chiral alkenylborons 4a-4v with good to excellent enantioselectivities (83–93% ee). The lower to moderate yields for some axially chiral alkenylboron products were mainly due to the hydroalkylation process of ethynyl-azaborines51,55. The reaction showed good halogen compatibility, not only ethynyl-azaborine 1a but also aryl iodines (2c, 2i-2l and 2o), which offers valuable handles for further late-stage functionalization. Importantly, sensitive functional groups on the benzene ring, such as cyano (4 l and 4 m), aldehyde (4n), ester (4o-4s), alkene (4r), ketone (4t), trifluoromethyl (4 u), and trifluoromethoxy (4 v), also worked well. The absolute configuration of 4 m was determined by X-ray crystallographic analysis. Notably, aryl iodides derived from L(-)-borneol, geraniol and gemfibrozil were also well compatible, and furnished the desired three-component coupling products 4q–4 s in moderate yields with good to excellent diasteroselective (4q, >20:1 dr) and enantioselectivities (89% ee and 92% ee). 2-Naphthalene iodine is also a good substrate for this atroposelective radical relayed reductive coupling reaction (4w). Heterocyclic derivatives, such as thiophene and pyridine, were also suitable candidates for this reductive coupling to give axially chiral alkenylborons 4x and 4 y with good to excellent enantioselectivities (82% and 91% ee).
Next, the scope of ethynyl-azaborines in this protocol was also examined (Fig. 3). 1,2-benzazaborine ring of ethynyl-azaborines bearing alkyls, halides, and phenyl could all undergo this atroposelective coupling reaction to deliver the corresponding axially chiral alkenylborons 4z-4ae in 55%–74% yields with 89%–94% ee. Notably, when the bromo group on ethynyl-azaborine was replaced by phenyl group, the reaction also performed smoothly under the standard conditions, providing the corresponding product 4af in moderate yield and good enantioselectivity (41% yield, 83% ee). The installations of isopropyl or phenyl to the nitrogen atom had little effect on the enantioselectivities (4ag and 4ai, 89% and 92% ee) for this protocol. Of note, further reduction of the steric hindrance of ethynyl-azaborine would decrease the enantioselectivity of the target product (4ah, 88% yield, 81% ee). Finally, the reaction of other unactivated tertiary alkyl iodides also worked well to deliver the desired axially chiral alkenylboron products 4aj-4al in 42%–61% yields with 84%–87% ee.
Downstream applications and transformations
The potential value of this reaction was further illustrated by synthetic transformations (Fig. 4). The resulting axially chiral alkenylboron products have been pre-installed with a bromo group, providing a handle for later functionalizations, which could undergo Negishi and Sonogashira coupling to achieve alkylation, arylation and alkynylation (5, 6 and 7). In addition, the new chiral (P, olefin) ligand 8 was successfully synthesized via in situ axially chiral lithium intermediate with high retention of the enantiopurity.
Mechanism investigations
To elucidate the radical process of this reductive coupling, control experiments were carried out (Fig. 5a). Firstly, TEMPO as a radical scavenger was added to the three-component reaction, and the formation of coupling product 11 was completely inhibited. Meanwhile, the addition of 1,1-diphenylethylene almost interrupted this reductive coupling reaction, whereas affording compound 13, which may be derived from the addition of tert-butyl radical to 1,1-diphenylethylene. Fortunately, when the reaction of ethynyl-azaborine 10 was carried out in the presence of BrCCl3, the corresponding vinyl-CCl3 adduct (14) was detected by HRMS. Notably, the reaction of ethynyl-azaborine 10 with a smaller steric hindrance (ethyl group on N atom) in the absence of aryl iodide gave double addition products 15a and 15b as well as self-coupling product 15c. Moreover, hydrogenation of α-borylvinyl radical intermediates was detected by GC-MS in the process of substrate investigation. These experiments suggest that an α-borylvinyl radical is involved in this coupling reaction. Then, several control experiments with nickel species were also carried out (Fig. 5b). Firstly, the stoichiometric reaction of aryl iodide, Ni(COD)2 and 4,4’-dtbbpy furnished Ar-Ni(II)-I complex 16. Only trace amount of three-component coupling product 17 was obtained by mixing complex 16, ethynyl-azaborine 9 and tert-butyl iodide, while more obvious product 17 could be observed when reductant TDAE were added. Moreover, when the catalytic amount of the complex 16 was employed in the cross-over reaction with 3-iodoanisol, compounds 11 and 17 were obtained in 75% yield and 6% yield, respectively. Overall, these results suggest that the reduction of complex 16 is necessary for this catalytic cycle.
Based on the results of the above experiments and previous literatures43,56,57,58, we proposed a Ni(I)/Ni(II)/Ni(III) cycle for the asymmetric radical relayed reductive coupling (Fig. 6, left): the chiral Ni(I) species A generated in situ undergoes oxidative addition into the aryl iodine 2a to afford the Ar-Ni(III)L* intermediate B, which is reduced by TDAE to produce the Ar-Ni(I)L* complex C. The activation of alkyl iodide 3a by the Ar-Ni(I)L* complex C generates an alkyl radical D and a Ar-Ni(II)L* species E. Alkyl radical D undergoes regioselective addition to ethynyl-azaborine 1a to afford the α-borylvinyl radical F, which can combine with Ar-Ni(II)L* species E to deliver the Ni(III) intermediate G. The excellent E-stereoselectivity of alkene may stem from steric hindrance32,33,34,35,36,37,38,39,40. The final reductive elimination of the Ni(III) intermediate G yields axially chiral alkenylboron 4a and regenerates the chiral Ni(I) catalyst. However, Ni(0)/Ni(I)/Ni(II)/Ni(III) catalytic cycle can’t completely rule out (Fig. 6, right), which involves the oxidative addition of Ni(0) species to aryl iodides.
In conclusion, we have developed a nickel-catalyzed atroposelective radical relayed reductive coupling reaction, leading to the formation of the challenging C-B axially chiral alkenylborons. The method features mild conditions, high enantioselectivity and excellent stereoselectivity. The key mechanistic feature of the process is the generation and transformation of a boron-stabilized vinyl radical. It is expected that the concept obtained here will encourage the development of more asymmetric transformations around the multifunctional α-borylvinyl radical intermediates.
Methods
General procedure for the synthesis of C-B axially chiral alkenylborons
In a glove box, a 10 mL Schlenk tube was charged with NiBr2·DME (0.01 mmol, 10 mol%), ligand (0.01 mmol, 10 mol%), the bromination of B-ethynyl-2,1-borazaronaphthalene (0.1 mmol, 1.0 equiv), aryl iodide (0.13 mmol, 1.3 equiv), alkyl iodide (0.3 mmol, 3 equiv), MgCl2 (0.1 mmol, 1.0 equiv), 2-Me-THF (1 mL) and DCE (0.1 mL). The resulting mixture was stirred at room temperature for 1 min. Then TDAE (0.21 mmol, 2.1 equiv) was added. The reaction tube was taken out of the glove box and reacted at −5 oC for 24 h. Upon completion, proper amount of silica gel was added to the reaction mixture. After removal of the solvent, the crude reaction mixture was purified on silica gel to afford 4.
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information files. All other data are available from the corresponding author upon request. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2346898 (4 m). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
References
Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Perreault, S., Chandrasekhar, J. & Patel, L. Atropisomerism in Drug Discovery: A Medicinal Chemistry Perspective Inspired by Atropisomeric Class I PI3K Inhibitors. Acc. Chem. Res. 55, 2581–2593 (2022).
LaPlante, S. R. et al. Assessing Atropisomer Axial Chirality in Drug Discovery and Development. J. Med. Chem. 54, 7005–7022 (2011).
Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 111, 563–639 (2011).
Bisoyi, H. K. & Li, Q. Light-Driven Liquid Crystalline Materials: From Photo-Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 116, 15089–15166 (2016).
Li, T.-Z., Liu, S.-J., Tan, W. & Shi, F. Catalytic Asymmetric Construction of Axially Chiral Indole-Based Frameworks: An Emerging Area. Chem. Eur. J. 26, 15779–15792 (2020).
Bai, X.-F., Cui, Y.-M., Cao, J. & Xu, L.-W. Atropisomers with Axial and Point Chirality: Synthesis and Applications. Acc. Chem. Res. 55, 2545–2561 (2022).
Zhang, H.-H. & Shi, F. Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 55, 2562–2580 (2022).
Qin, W., Liu, Y. & Yan, H. Enantioselective Synthesis of Atropisomers via Vinylidene ortho-Quinone Methides (VQMs). Acc. Chem. Res. 55, 2780–2795 (2022).
Cheng, J. K., Xiang, S.-H. & Tan, B. Organocatalytic Enantioselective Synthesis of Axially Chiral Molecules: Development of Strategies and Skeletons. Acc. Chem. Res. 55, 2920–2937 (2022).
Feng, J., Lu, C.-J. & Liu, R.-R. Catalytic Asymmetric Synthesis of Atropisomers Featuring an Aza Axis. Acc. Chem. Res. 56, 2537–2554 (2023).
Zhang, H.-H., Li, T.-Z., Liu, S.-J. & Shi, F. Catalytic Asymmetric Synthesis of Atropisomers Bearing Multiple Chiral Elements: An Emerging Field. Angew. Chem. Int. Ed. 63, e202311053 (2024).
Xiang, S.-H., Ding, W.-Y., Wang, Y.-B. & Tan, B. Catalytic atroposelective synthesis. Nat. Catal. 7, 483–498 (2024).
Wu, S., Xiang, S.-H., Cheng, J. K. & Tan, B. Axially chiral alkenes: Atroposelective synthesis and applications. Tetrahedron Chem. 1, 100009 (2022).
Li, Z.-H., Li, Q.-Z., Bai, H.-Y. & Zhang, S.-Y. Synthetic strategies and mechanistic studies of axially chiral styrenes. Chem. Catal. 3, 100594 (2023).
Qian, P.-F., Zhou, T. & Shi, B.-F. Transition-metal-catalyzed atroposelective synthesis of axially chiral styrenes. Chem. Commun. 59, 12669–12684 (2023).
Yang, K. et al. Construction of Axially Chiral Arylborons via Atroposelective Miyaura Borylation. J. Am. Chem. Soc. 143, 10048–10053 (2021).
Xu, J. et al. Palladium-Catalyzed Atroposelective Kinetic C−H Olefination and Allylation for the Synthesis of C−B Axial Chirality. Angew. Chem. Int. Ed. 62, e202313388 (2023).
Yang, K. et al. Construction of C-B axial chirality via dynamic kinetic asymmetric cross-coupling mediated by tetracoordinate boron. Nat. Commun. 14, 4438 (2023).
Wang, H. et al. Enantio- and regioselective [2 + 2 + 2] cycloaddition of BN-diynes for construction of C-B axial chirality. Chem. 10, 317–329 (2023).
Wang, X., Wang, J. & Pei, J. BN Heterosuperbenzenes: Synthesis and Properties. Chem. Eur. J. 21, 3528–3539 (2015).
Giustra, Z. X. & Liu, S.-Y. The State of the Art in Azaborine Chemistry: New Synthetic Methods and Applications. J. Am. Chem. Soc. 140, 1184–1194 (2018).
McConnell, C. R. & Liu, S.-Y. Late-stage functionalization of BN-heterocycles. Chem. Soc. Rev. 48, 3436–3453 (2019).
Bhattacharjee, A., Davies, G. H. M., Saeednia, B., Wisniewski, S. R. & Molander, G. A. Selectivity in the Elaboration of Bicyclic Borazarenes. Adv. Synth. Catal. 363, 2256–2273 (2021).
Xu, X., Liu, M., Li, C. & Liu, X. Recent Advance of 1,2-BN Heteroaromatics in China. Chin. J. Org. Chem. 43, 1611 (2023).
Lyu, H. et al. Modular synthesis of 1,2-azaborines via ring-opening BN-isostere benzannulation. Nat. Chem. 16, 269–276 (2024).
Su, W. et al. Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres. Nat. Chem. 16, 1312–1319 (2024).
Mancinelli, M., Bencivenni, G., Pecorari, D. & Mazzanti, A. Stereochemistry and Recent Applications of Axially Chiral Organic Molecules. Eur. J. Org. Chem. 2020, 4070–4086 (2020).
Yang, J. et al. Chiral Phosphoric Acid-Catalyzed Remote Control of Axial Chirality at Boron–Carbon Bond. J. Am. Chem. Soc. 143, 12924–12929 (2021).
Zhang, X.-L. et al. Stepwise Asymmetric Allylic Substitution-Isomerization Enabled Mimetic Synthesis of Axially Chiral B,N-Heterocycles. Angew. Chem. Int. Ed. 61, e202210456 (2022).
Ping, Y., Shi, X., Lei, M. & Wang, J. Atroposelective Construction of Carbon–Boron Axial Chirality through Rh-Catalyzed [2 + 2 + 2] Cycloaddition. ACS Catal. 14, 5064–5076 (2024).
Li, Z., García-Domínguez, A. & Nevado, C. Pd-Catalyzed Stereoselective Carboperfluoroalkylation of Alkynes. J. Am. Chem. Soc. 137, 11610–11613 (2015).
Li, Z., García-Domínguez, A. & Nevado, C. Nickel-Catalyzed Stereoselective Dicarbofunctionalization of Alkynes. Angew. Chem. Int. Ed. 55, 6938–6941 (2016).
Guo, L., Song, F., Zhu, S., Li, H. & Chu, L. syn-Selective alkylarylation of terminal alkynes via the combination of photoredox and nickel catalysis. Nat. Commun. 9, 4543 (2018).
Zhu, C. et al. A multicomponent synthesis of stereodefined olefins via nickel catalysis and single electron/triplet energy transfer. Nat. Catal. 2, 678–687 (2019).
Israr, M., Xiong, H., Li, Y. & Bao, H. Copper(I)-Catalyzed Cyanoperfluoroalkylation of Alkynes. Org. Lett. 21, 7078–7083 (2019).
Zhu, S., Zhao, X., Li, H. & Chu, L. Catalytic three-component dicarbofunctionalization reactions involving radical capture by nickel. Chem. Soc. Rev. 50, 10836 (2021).
Tao, Z.-K. et al. Copper-Catalyzed Vicinal Cyano-, Thiocyano-, and Chlorophosphorylation of Alkynes: A Phosphinoyl Radical-Initiated Approach for Difunctionalized Alkenes. Org. Lett. 23, 4342–4347 (2021).
Chalotra, N., Kumar, J., Naqvi, T. & Shah, B. A. Photocatalytic functionalizations of alkynes. Chem. Commun. 57, 11285–11300 (2021).
Li, H., Wang, F., Zhu, S. & Chu, L. Selective Fluoromethyl Couplings of Alkynes via Nickel Catalysis. Angew. Chem. Int. Ed. 61, e202116725 (2022).
Tang, J.-B. et al. Copper-Catalyzed anti-Selective Radical 1,2-Alkylarylation of Terminal Alkynes. Org. Lett. 24, 2536–2540 (2022).
Long, T. et al. Ligand-controlled stereodivergent alkenylation of alkynes to access functionalized trans- and cis-1,3-dienes. Nat. Commun. 14, 55 (2023).
Li, Q.-Z., Li, Z.-H., Kang, J.-C., Ding, T.-M. & Zhang, S.-Y. Ni-catalyzed, enantioselective three-component radical relayed reductive coupling of alkynes: Synthesis of axially chiral styrenes. Chem. Catal. 2, 3185–3195 (2022).
Fu, L., Chen, X., Fan, W., Chen, P. & Liu, G. Copper-Catalyzed Asymmetric Functionalization of Vinyl Radicals for the Access to Vinylarene Atropisomers. J. Am. Chem. Soc. 145, 13476–13483 (2023).
Gao, P., Niu, Y.-J., Yang, F., Guo, L.-N. & Duan, X.-H. Three-component 1,2-dicarbofunctionalization of alkenes involving alkyl radicals. Chem. Commun. 58, 730–746 (2022).
Leonori, D. & Aggarwal, V. K. Lithiation–Borylation Methodology and Its Application in Synthesis. Acc. Chem. Res. 47, 3174–3183 (2014).
Kumar, N., Reddy, R. R., Eghbarieh, N. & Masarwa, A. α-Borylalkyl radicals: their distinctive reactivity in modern organic synthesis. Chem. Commun. 56, 13–25 (2019).
Kischkewitz, M., Friese, F. W. & Studer, A. Radical-Induced 1,2-Migrations of Boron Ate Complexes. Adv. Syn. Catal. 362, 2077–2087 (2020).
Lovinger, G. J. & Morken, J. P. Recent Advances in Radical Addition to Alkenylboron Compounds. Eur. J. Org. Chem. 2020, 2362–2368 (2020).
Wang, D. & Xu, T. Recent Advances in the Preparation and Asymmetric Transformation of α-Haloboron Compounds. Synlett 34, 2085–2096 (2023).
Barzanò, G., Cheseaux, A. & Hu, X. Z-Selective Synthesis of Vinyl Boronates through Fe-Catalyzed Alkyl Radical Addition. Org. Lett. 21, 490–493 (2019).
Jiao, J. & Nishihara, Y. Alkynylboron compounds in organic synthesis. J. Org. Chem. 721–722, 3–16 (2012).
Nandy, S. et al. Synthesis and reactivity of alkynyl boron compounds. Org. Biomol. Chem. 19, 7276–7297 (2021).
He, Y., Wang, H., Zhou, Y., Yang, K. & Song, Q. Divergent synthesis of 5- and 4-(2,1-azaborine) substituted isoxazoles via regioselective [3 + 2] cycloadditions of nitrile oxides and B-ethynyl-1,2-azaborines. Org. Chem. Front. 10, 127–132 (2022).
Li, X.-R. et al. Stereoselective synthesis of fluoroalkylated (Z)-alkene via nickel-catalyzed and iron-mediated hydrofluoroalkylation of alkynes. Org. Chem. Front. 8, 6377–6383 (2021).
García-Domínguez, A., Li, Z. & Nevado, C. Nickel-Catalyzed Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 139, 6835–6838 (2017).
Shu, W. et al. Ni-Catalyzed Reductive Dicarbofunctionalization of Nonactivated Alkenes: Scope and Mechanistic Insights. J. Am. Chem. Soc. 141, 13812–13821 (2019).
Du, X., Cheng-Sánchez, I. & Nevado, C. Dual Nickel/Photoredox-Catalyzed Asymmetric Carbosulfonylation of Alkenes. J. Am. Chem. Soc. 145, 12532–12540 (2023).
Acknowledgements
Financial support from the National Key R&D Program of China (2023YFF0723900 to Q.S.), National Natural Science Foundation of China (22271048 to K.Y.; 21931013 and 22271105 to Q.S.), Natural Science Foundation of Fujian Province (2022J02009 to Q.S.; 2022J05016 to K.Y.) and Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Q.S. and K.Y. conceived and directed the project. Q.W., T.R., H.Y., and Z.Y. performed experiments. Q.W. prepared the Supplementary Information. Q.S. and K.Y. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jianbo Wang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiu, W., Tao, R., He, Y. et al. Enantioselective construction of C-B axially chiral alkenylborons by nickel-catalyzed radical relayed reductive coupling. Nat Commun 15, 10432 (2024). https://doi.org/10.1038/s41467-024-54597-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54597-0
This article is cited by
-
Rhodium-catalyzed construction of boron-based point and axial chirality via asymmetric annulation of alkynylborons
Nature Communications (2025)