Abstract
Currently there are no effective treatments for an array of neurodegenerative disorders to a large part because cell-based models fail to recapitulate disease. Here we develop a reproducible human iPSC-based model where laser axotomy causes retrograde axon degeneration leading to neuronal cell death. Time-lapse confocal imaging revealed that damage triggers an apoptotic wave of mitochondrial fission proceeding from the site of injury to the soma. We demonstrate that this apoptotic wave is locally initiated in the axon by dual leucine zipper kinase (DLK). We find that mitochondrial fission and resultant cell death are entirely dependent on phosphorylation of dynamin related protein 1 (DRP1) downstream of DLK, revealing a mechanism by which DLK can drive apoptosis. Importantly, we show that CRISPR mediated Drp1 depletion protects mouse retinal ganglion neurons from degeneration after optic nerve crush. Our results provide a platform for studying degeneration of human neurons, pinpoint key early events in damage related neural death and provide potential focus for therapeutic intervention.
Similar content being viewed by others
Introduction
Neuronal death and axon loss are universal hallmarks of neurodegeneration, relevant to Alzheimer’s disease, ALS and other neurodegenerative diseases. However, our understanding of the mechanisms controlling axon degeneration and neuron death is incomplete. Advances in this area have enormous potential to benefit all types of neurodegenerative conditions including those arising from nerve injury and traumatic brain injury.
Separate mechanisms regulate axon degeneration and neuronal apoptosis1. For example, deletion of BAX (BCL-2-associated X protein), a central regulator of apoptosis, does not protect axons from Wallerian degeneration. On the other hand, BAX deletion protects neurons following trophic factor withdrawal, a developmental model of axon degeneration and death2,3,4. SARM1 is the central executioner of Wallerian axon degeneration5,6. However, it is unclear how universal this pathway is for neuron degeneration in the context of disease since neuron death is rescued by loss of SARM1 in some models7,8 but not others9,10. Furthermore, while most neurodegenerative diseases involve loss of axons and cell bodies, Wallerian axon degeneration occurs independently from neuronal cell death.
Mitochondria are a central hub of axon health regulation, at the nexus of axonal energy production, oxidative stress, and calcium buffering11,12. They also play critical roles in degeneration and regeneration13,14. Healthy maintenance of the mitochondrial network is mediated by a balance of mitochondrial fission and fusion regulated by specialized machinery15. Interestingly, mutations in some of these genes cause neurodegenerative conditions such as motor and sensory neuropathy e.g. MFN1 in Charcot-Marie-Tooth16. A key player in the mitochondrial fission process is dynamin related protein 1 or DRP1, a GTPase that oligomerizes around the outer mitochondrial membrane to cause mitochondrial fission17. DRP1 also regulates apoptosis15,18,19,20. Under conditions that drive apoptosis, DRP1 promotes a type of apoptotic mitochondrial fission in which BAX permeabilizes the outer mitochondrial membrane, allowing the release of pro-apoptotic factors such as cytochrome c21,22.
Much of what we know about mechanisms regulating neuron degeneration and death comes from a wealth of studies of apoptosis in cell lines (but not neurons), from primary mouse neurons or from animal models (neurons, but not human). How translatable this work is and how applicable to human neurons remains to be determined. Recent advances in iPSC-derived neuron techniques now enable the generation of a diverse range of human neuron subtypes. These efforts have already generated exciting findings in the neurodegeneration field23,24,25. We therefore set out to study axon injury using a widely adopted glutamatergic neuron model produced by directed differentiation of human iPSCs (i3Neurons26,27).
We and others have shown that the dual leucine zipper kinase (DLK, or MAP3K12), is a key regulator of axon degeneration and a driver of neuron cell death28,29,30,31,32. The canonical DLK pathway involves a phosphorylation cascade downstream of DLK homodimerization and a transcriptional stress response, including phosphorylation of the transcription factor cJun33. Despite its consideration as a drug target for several neurological conditions33,34,35, relatively little is known about DLK function in human neurons.
Here, we investigate the behavior of i3Neurons following laser axotomy and the role of DLK in the injury response of human neurons. Following axotomy, we observe progressive retrograde axon degeneration from the injury site back towards the cell body, causing subsequent neuronal death, and establishing a unique human model of neurodegeneration. This resembles the pattern of neurodegeneration observed in optic nerve injuries in living mice36.
We find that in axons proximal to the injury, mitochondria shrink and undergo DRP1-dependent fission in a retrograde wave from the damage site, leading to apoptosis of the cell body. The DLK-JNK pathway regulates this mitochondrial fission via phosphorylation of DRP1 at serine 616. This DRP1 phosphorylation is necessary for localization of BAX to axonal mitochondria, which causes axon degeneration. Blocking DLK or DRP1 protects axons from degeneration and neurons from cell death both in human i3Neurons in vitro and in vivo in the mouse. This study identifies a signaling mechanism for DLK that drives local axon degeneration without the need for transcription. Our findings reveal an apoptotic cascade that is initiated in the axon that underlies a form of non-Wallerian axon degeneration and drives neuronal cell death.
Results
Axotomy in human neurons causes both proximal and distal axon degeneration
Axotomy is one established method to study axon degeneration. After axotomy, the distal portion of the axon undergoes Wallerian degeneration, a phenomenon controlled by the NADase SARM1, the key executioner of this type of axon degeneration5,6. To study the response of human neurons to axotomy, we performed laser axotomies in i3Neurons that were sparsely transduced with a fluorescent protein to visualize individual neurons (Fig. 1a). The distal portion of these axons underwent Wallerian degeneration as expected (Fig. 1b, c). To our surprise, we observed that the portion of the axon proximal to the injury and still connected to the cell body underwent progressive retrograde axon degeneration (Fig. 1b, c). Because SARM1-dependent degeneration relies on the loss of the axon survival factor NMNAT2, which occurs when the axon is severed from the cell body, we hypothesized that the proximal axon degeneration we observed might not rely on SARM1 and could provide an opportunity to elucidate pathways of axon degeneration.
a Schematic representation of laser axotomy. Created in BioRender. Le Pichon, C. E. (2022) BioRender.com/z21b395. b Representative images of WT and SARM1 KO axons expressing cytoplasmic mApple (red) pre- and 4 h post-axotomy (PA). Scalebar 100 µm. c Axon degeneration index (ADI) in WT and SARM1 axons 4 h post-axotomy. n = 38. Two-way ANOVA (p = 0.0086 **, not significant, ns). d Representative images of mitoGFP (green) in axons pre- and 5 min post-axotomy (PA). Scalebar 20 µm. e Representative time-lapse images of mitochondria undergoing fission after axon injury. Scalebar 2 µm (valid for all 5 examples). f Schematic. g Quantification of mitochondrial length pre- (green) and 5 min post-axotomy (gray). n = 29. Two-way ANOVA (10 min post-axotomy, 0–100 µm vs 200–300 µm p = 0.0021; vs 400–500 µm p < 0.0001. 20 min post-axotomy, 0–100 vs 200–300 µm p = 0.0019). h Axonal mitochondrial particles in control and DRP1 knockdown (KD) neurons. n = 26 WT, n = 25 DRP1 KD. Two-way ANOVA (p ≤ 0.05 *). i Axonal mitochondrial particles 3 min post-axotomy in DMSO and P110 treated neurons. n = 27 DMSO n = 26 P110. Two-way ANOVA (p = 0.0467 *). j Mitochondrial particles 0–100, 200–300 and 400–500 µm from axotomy. n = 23. One-way ANOVA (*p ≤ 0.05, 0–100 µm vs 200–300 µm, # p ≤ 0.05, 0–100 µm vs 400–500 µm). k Representative images of axotomy-induced mitochondrial fission wave. mitoGFP (green). Scalebar 25 µm. l Axon degeneration index (ADI) 0–100, 200–300 and 400–500 µm from axotomy. n = 14. One-way ANOVA (*p ≤ 0.05, 0–100 vs 200–300 µm, ## p ≤ 0.01, ### p ≤ 0.005, 0–100 vs 400–500 µm). m Representative images of retrograde axon degeneration 0, 10-, 30- and 120-min post-axotomy. Cyto-mApple (red). Scalebar 25 µm. n Representative images of axotomy-induced neuron death, pre- and 12-h post axotomy. Arrowhead indicates axotomized neuron; the other neuron is uninjured. Scalebar 40 µm. o Quantification of axotomy-induced neuron death. Percentage of dead neurons 12 h post-axotomy. n = 30. Unpaired, two-tailed, t test (p = 0.004 **). Graphical representations show mean ± SEM. Dashed box in (a, f) indicates field of view. Asterisks * in (a, b, d) mark site of axotomy. All experiments include axotomies from N = 3 independent differentiations. Bonferroni correction applied to all ANOVAs. Schematics in a and f made using Biorender.
We tested the requirement for SARM1 in the degeneration of axons both proximal and distal to the injury site. Using CRISPR/Cas9, we knocked out SARM1 in i3Neurons37. We then axotomized these neurons and control neurons and imaged the axon on both sides of the injury. As expected, SARM1 was necessary for distal, but not proximal, axon degeneration (Fig. 1b, c). We therefore investigated the mechanisms underlying proximal axon degeneration in these neurons. For the remainder of the study, we focus specifically on the axon segment proximal to the injury site.
Axotomy in human neurons triggers a wave of DRP1-dependent mitochondrial fission
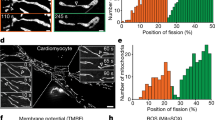
Mitochondria play pivotal roles in axon degeneration, due to their importance in energy production, calcium homeostasis, production of reactive oxygen species and signaling38,39. We transduced i3Neurons with a mitochondrial-targeted GFP and plated them in the center of wells to allow axons to project outwards and visualize individual axons. After axotomy, mitochondria underwent rapid shrinkage (Fig. 1d–g; Video S1) and increased in number (Fig. 1h). Using live imaging, we determined that the increase in mitochondrial number was due to fission events rather than mitochondrial transport and localization to the site of injury. Mitochondrial fission was most evident from close analysis of live imaging (Video S1 and Fig. 1d, e). This phenomenon was observed within 5 s after axon injury and was not affected by distance from the damage site from the cell body (Fig. S1a).
Mitochondrial fission is a dynamic process that is regulated by proteins localized in the inner and outer mitochondrial membranes17. A critical regulator of fission is dynamin related protein 1 (DRP1), a cytoplasmic GTPase. DRP1 is recruited to the outer mitochondrial membrane and helically oligomerizes around the mitochondrion to constrict it and bring about scission of the membrane. To test whether the fission we observed was dependent on DRP1, we knocked down DRP1 using CRISPR interference by expressing a previously validated guide RNA to DRP1 in i3Neurons expressing a catalytically dead Cas9 (dCAS9)24,37. DRP1 knockdown prevented the increase in mitochondrial particle number (Fig. 1h). We confirmed this result by inhibiting DRP1 using P110, a peptide that blocks the interaction between DRP1 and FIS1 (mitochondrial fission protein 1), another protein that is essential for fission (Fig. 1i). In line with previous reports40,41, these events were dependent on calcium influx into the injured axon which, when buffered using BAPTA-AM, also prevented mitochondrial fission (Fig. S1a–d; Videos S2–5).
Mitochondrial fission precedes proximal axon degeneration
To characterize the spatial and temporal dynamics of the axonal response to injury, we analyzed mitochondrial fission and axon degeneration in 100 µm segments of the axon relative to the injury site every 10 min following axotomy. This analysis revealed that mitochondrial fission began seconds after injury and progressed in a retrograde manner back towards the cell body. One hour after axotomy, mitochondria 500 µm away from the damage site had undergone fission (Fig. 1j, k, Video S6). This fission preceded a slower retrograde wave of axon degeneration back towards the cell body (Fig. 1l, m, Video S7).
Time lapse imaging of the cell bodies of axotomized neurons revealed that the retrograde wave of axon degeneration was followed by fragmentation of mitochondria in the soma and cell death (Fig. 1n, o; Videos S8–9). Axotomy led to death of 84% of cells analyzed. We have thus uncovered a human neuron model in which axon injury initiates progressive retrograde axon degeneration that ultimately results in neuronal cell death.
DLK regulates axotomy-induced mitochondrial fission
To understand mechanisms governing neurodegeneration in this model, we started by examining a pathway involved in the cellular response to axon injury. The dual leucine zipper kinase (DLK; MAP3K12) is a key regulator of the axon damage response and neuronal cell death31,42. Because mitochondria are critical regulators of axon degeneration, we were curious whether DLK protein might localize to axonal mitochondria. In i3Neurons, we observed association of endogenous DLK with 77% of axonal mitochondria (Fig S2a–c). We further corroborated this association by immuno-EM (Fig. S2d), and through observation of DLK co-trafficking with mitochondria (Fig. S2e).
To investigate how DLK responds to axon damage in human neurons, we performed laser axotomies and live imaging of GFP-tagged DLK and mitochondria. We observed an intriguing presence of DLK puncta at 45% of sites of mitochondrial fission (Fig. 2a, b, Video S10). To determine whether DLK influences mitochondrial fission, we generated DLK KO neurons using CRISPR/Cas9 (Fig. S2f). The knockout neurons were validated for absence of DLK protein by immunofluorescent staining and western blotting (Fig. S2g, h). In the undamaged condition, we observed no mitochondrial phenotypes in these cells including number, size, movement, and oxygen consumption rate (Fig. S2i–p). Strikingly, DLK KO neurons did not exhibit the axonal mitochondrial fission we had observed in response to axotomy in wild type neurons (Fig. 2c, d; Videos S11–12). Corroborating this result, pharmacological inhibition of DLK using GNE3511 prevented mitochondrial fission after injury (Fig. 2e, Videos S13–14).
a Representative images of DLK colocalization at the site of mitochondrial fission after axotomy. DLK-GFP (green) and mitochondria (mitoRFP, magenta). Scalebar = 10 µm. b Percentage mitochondrial fission events where DLK is localized at the site of fission. N = 3 independent differentiations, 74 fission events from 33 axotomized neurons. c Representative images of WT and DLK KO neurons transduced with mitoGFP (green) pre (Pre) and 5 min post axotomy (PA). * Marks the site of axotomy. Scalebar = 25 µm. d Normalized number of mitochondrial particles post axotomy in WT (black) and DLK KO (green) neurons. N = 3 independent differentiations, N = 29 WT and N = 30 DLK KO axotomized neurons (Two-way ANOVA, Bonferroni correction (p < 0.0001 ****). e Normalized number of mitochondrial particles post axotomy in DMSO (black) and GNE3511 (green)-treated neurons. N = 3 independent differentiations, N = 23 DMSO and N = 25 GNE3511 axotomized neurons. (Two-way ANOVA, Bonferroni correction, p < 0.0001 ****). f Normalized number of mitochondrial particles post axotomy in DLK KO neurons transduced with WT DLK-GFP (green), DLK-C127S-GFP (black) or DLK-S302A-GFP (purple). N = 3 independent differentiations, N = 31 DLK WT and N = 27 DLK-C127S and N = 23 DLK-S302A axotomized neurons. Two-way ANOVA, Bonferroni correction (p < 0.0001 ****, WT DLK vs DLK C127S, p < 0.0001 ####, WT DLK vs S302A). g Representative images of neurons transduced with mitoGFP (green) treated with DMSO and JNKi pre (Pre) and 5 min post axotomy (PA). * Marks the site of axotomy. Scalebar = 25 µm. h Normalized number of mitochondrial particles post axotomy in DMSO (black) and JNKi (green)-treated neurons. N = 3 independent differentiations, N = 32 DMSO and N = 31 JNKi axotomized neurons. (Two-way ANOVA, Bonferroni correction, p < 0.0001 ****). Graphical representations show mean ± SEM.
To better understand the mechanism by which DLK regulates mitochondrial fission, DLK KO neurons were transduced with wild type DLK or 2 mutant versions of DLK. DLK C127S cannot be palmitoylated, hindering its ability to properly localize and signal43. Kinase dead DLK (S302A) cannot be trans-phosphorylated and is catalytically inactive29,44. Only the expression of wild type DLK rescued the fission phenotype in DLK KO neurons (Fig. 2f; Videos S15–17), indicating DLK kinase activity is critical for this process.
We next tested whether inhibiting c-Jun N-terminal kinases (JNK), known downstream players of the DLK pathway, reduced mitochondrial fission after axotomy. Neurons exposed to JNK inhibitor (JNKi) showed a significant reduction in mitochondrial fission following axon injury (Fig. 2g, h). We thus uncover a role for DLK in initiating a wave of mitochondrial fission after injury.
DLK kinase is necessary for DRP1 to induce mitochondrial fission
DRP1 phosphorylation state regulates its function, with phosphorylation at serine 616 promoting mitochondrial fission45. We speculated that DLK might be required for phosphorylation of DRP1 at this residue. In order to more easily study this signaling mechanism, we turned to a heterologous system, HEK-293 cells, that do not express DLK. Overexpression of DLK in these cells can be used to study DLK signaling since its homodimerization is sufficient to drive pathway activity44,46.
Expression of wild type DLK, but not DLK C127S (palmitoyl-site mutant) or DLK S302A (kinase dead), resulted in activation of DLK signaling as reflected by the increased phosphorylation of its downstream target c-Jun (Fig. 3a, b). Interestingly, when we co-expressed DLK and DRP1, phosphorylation at S616 was increased in this model of DLK activation (Fig. 3a, c), but not with mutant DLK, showing that DLK activity is sufficient to cause DRP1 S616 phosphorylation. Levels of total DRP1 remained unchanged, as did levels of S637 pDRP1, a phosphorylation event that impairs DRP1 GTPase activity47,48 (Fig. 3a, d, e).
a Representative western blots of HEK-293A cells transfected for 24 h with WT, C127S and S302A DLK-GFP. Immunoblot for pS616-DRP1, pS637-DRP1, total DRP1, DLK, pS63-cJun and loading control ß actin. b Quantification of pS63-cJun/ ß actin levels after expression of WT, C127S and S302A DLK-GFP in HEK-293A cells. N = 3 independent transfections. One-way ANOVA, Bonferroni correction (DLK WT vs DLK C127S p = 0.0335 *, DLK WT vs DLK C127S p = 0.0373 *). c Quantification of pS616-DRP1/total DRP1 levels after expression of WT, C127S and S302A DLK-GFP in HEK-293A cells. N = 3 independent transfections. One-way ANOVA, Bonferroni correction (DLK WT vs DLK C127S p = 0.0103 *, DLK WT vs DLK C127S p = 0.0105 *). d Quantification of total DRP1/ ß actin levels after expression of WT, C127S and S302A DLK-GFP in HEK-293A cells. N = 3 independent transfections. One-way ANOVA, Bonferroni correction (not significant, ns). e Quantification of pS637 DRP1/ ß actin levels after expression of WT, C127S and S302A DLK-GFP in HEK-293A cells. N = 3 independent transfections. One-way ANOVA, Bonferroni correction (not significant, ns). f Phospho-mass spectrometry. pS616-DRP1 peptide / total DRP1 peptide levels from purified GST-DRP1 in HEK-293A cells expressing GST-DRP1, GST-DRP1 and DLK-GFP or GST-DRP1 and DLK-S302A-GFP. N = 4 independent transfections. One-way ANOVA, Bonferroni correction (p = 0.0046 **). g Representative images of HEK-293A cells expressing WT, C127S and S302A DLK-GFP (green) and mitoTracker (orange) for 24 h. Scalebar = 10 µm. h Quantification of mitochondrial morphology in HEK-293A cells transfected with WT, C127S and S302A DLK-GFP. N = 3 individual transfections. Two-way ANOVA, Bonferroni correction (WT vs C127S p ≤ 0.0001 ****, WT vs S302A p ≤ 0.0001 ****, C127S vs S302A not significant, ns). i Representative western blots HEK-293A cells expressing dCAS9 transduced with control and DRP1 gRNAs. Immunoblot for total DRP1 and loading control ß actin. Replicated twice with similar results. j Representative images of Control and DRP1 KD HEK-293A cells expressing DLK-HALO for 24 h. NLS-GFP (green), DLK-HALO (white), mitoTracker (orange). Scalebar = 20 µm. k Quantification of Control and DRP1 KD HEK-293A cells mitochondrial morphology. Cells transfected with DLK-HALO. N = 3 individual transfections. (Two-way ANOVA, Bonferroni correction (p = 0.0024 **) All graphical representations show mean ± SEM.
To further validate that DLK signaling causes DRP1 phosphorylation, we purified GST-DRP1 from HEK-293 cells expressing wild type DLK or DLK S302A, and performed targeted mass spectrometry to quantify DRP1 phosphorylation. The relative abundance of phospho-DRP1 S616 peptides was significantly increased only in the HEK-293 cells expressing wild type but not kinase dead DLK (Fig. 3f). These results demonstrate that DLK kinase activity can promote DRP1 phosphorylation at S616.
To further examine this phosphorylation event, we performed an in vitro kinase assay using proteins separately purified from HEK-293 cells. Incubation of DRP1 with wild type DLK increased pDRP1 S616, whereas incubation with kinase dead DLK did not (Fig. S3a–c). We wondered whether this DLK kinase activity on DRP1 was occurring via the canonical DLK kinase cascade, since purification of DLK from HEK cells could result in isolation of the entire DLK signaling complex including downstream kinases MAP2K and JNK. We detected JNK to be associated with the DLK we pulled down, suggesting this was via canonical MAP3K signaling (Fig S3d). We also found that DLK inhibitor and JNK inhibitor (but not p38 inhibitor) could block the phosphorylation of DRP observed in the heterologous expression of DLK in HEK cells (Fig S3d–f). Together, these data demonstrate that DRP1 is a substrate of DLK signaling that occurs via the canonical DLK kinase cascade. To our knowledge, DRP1 is only the second substrate of DLK/JNK signaling to be defined after cJun.
We analyzed mitochondrial morphology in the HEK-293 cells to examine whether DRP1 phosphorylation caused by heterologous expression of DLK in HEK cells led to fission. Similar to what we had observed in injured axons, DLK signaling resulted in an increase in mitochondrial fragmentation (Fig. 3g, h). DLK-induced mitochondrial fragmentation was prevented by knocking down DRP1 (Fig. 3i–k). These data identify DLK as a kinase upstream of DRP1 that drives mitochondrial fission via phosphorylation of DRP1 at serine 616.
Axotomy causes DLK-dependent phosphorylation of DRP1
To validate these results in the i3Neurons, DLK KO or WT neurons were center-plated such that axon fractions could be harvested to examine axonal DRP1 phosphorylation after injury (Fig. 4a). Two hours after axotomy, phospho-DRP1 S616 (pDRP1 S616) was increased in axons but not cell bodies of wild type neurons. However, as predicted, the increase in p-DRP1 did not occur in DLK KO axons (Fig. 4b–e). The increase in pDRP1 S616 in wild type but not DLK KO axons was further validated by immunostaining (Fig. 4f, g).
a Schematic representation of i3Neuron center plating and axotomy for protein harvesting after injury. b Representative western blots of WT and DLK KO neuron cell bodies untreated (UT), 2 and 4 h post axotomy. Immunoblot for p-S616 DRP1, total DRP1 and loading control ß actin. c Quantification of pDRP1 (S616)/ total DRP1 levels in WT and DLK KO neuron cell bodies untreated (UT), 2 and 4 h post axotomy. Results normalized to UT. N = 3 independent differentiations. One-way ANOVA. No significant changes observed. d Representative Western blots of WT and DLK KO neuron axons untreated (UT), 2 and 4 h post axotomy. Immunoblot for p-S616 DRP1, total DRP1 and loading control ß actin. e Quantification of pDRP1 (S616)/ total DRP1 levels in WT and DLK KO neuron axons 0, 2 and 4 h post axotomy. Results normalized to UT. N = 5 independent differentiations. Two-way ANOVA. Bonferroni correction (p = 0.0058 **). f Representative images of WT and DLK KO axons stained for ßIII tubulin (red) and pDRP1 S616 (white) untreated (UT), 30 min and 1 h post axotomy. Scalebar 25 µm. g Quantification of pDRP1 S616 fluorescence in WT and DLK KO neuron axons after axotomy. Results normalized to untreated axons. N = 3 independent differentiations. Two-way ANOVA, Bonferroni correction (UT vs 30 min p = 0.0156*, UT vs 1 h p = 0.0185*). h Schematic representation of optic nerve crush injury model. Bracket and * indicate the proximal portion of the injured nerve harvested for Western blotting. i Representative Western blots of proximal portion of ipsilateral (I) injured and contralateral (C) optic nerves 24 h after optic nerve crush. Immunoblot for p-S616 DRP1, total DRP1 and loading control ß actin. j Quantification of pDRP1 (S616)/ total DRP1 levels after optic nerve crush. Results normalized to contralateral side (C). N = 4 samples. Unpaired, two-tailed, t test (p = 0.0346 *). k Quantification of total DRP1/ ß actin levels after optic nerve crush. Results normalized to contralateral side (C). N = 4 samples. Unpaired, two-tailed t test. not significant, ns. All graphical representations show mean ± SEM.
To examine whether DRP1 phosphorylation at S616 also occurs in vivo, we performed optic nerve crush in mice. Increased pDRP1 S616 relative to total DRP1 was detected in optic nerve proximal to the crush site 24 h after injury (Fig. 4h–j). Levels of total DRP1 in injured nerves remained unchanged (Fig. 4i, k).
Inhibiting DRP1 delays axon degeneration and neuron death
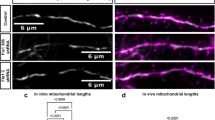
Since axotomy leads to progressive degeneration of the axon proximal to the injury and eventually to cell death, we asked to what extent DLK and DRP1 control the degeneration of each compartment. DLK has been shown to contribute to axon degeneration and to neuron death in many contexts28,29,30,31,42. We hypothesized that the DLK pathway would promote axon degeneration in axotomized human neurons. Whereas wild type axons had degenerated as early as 4 h after injury, DLK KO axons were still present after 24 h (Fig. 5a–c). Axons of neurons treated with JNK inhibitor were similarly protected (Fig S4c, d). Interestingly, in neurons with CRISPRi knockdown of DRP1 axons were also protected from degeneration (Fig. 5d–f).
a Schematic representation of i3Neuron laser axotomy. Dashed box indicates the field of view. b Representative images of WT and DLK KO neuron axons transduced with cyto mApple (red) proximal to the site of injury 0,4, 8 and 24 h post axotomy. Scalebar 25 µm. c Quantification of axon degeneration index (ADI) in WT and DLK KO neurons 0, 4, 8 and 24 h post axotomy. N = 3 independent differentiations, N = 33 WT and N = 35 DLK KO axotomized axons. Two-way ANOVA, Bonferroni correction (p ≤ 0.005 ***, p ≤ 0.001 ****). d Schematic representation of i3Neuron laser axotomy. Dashed box indicates field of view. e Representative images of Control and DRP1 KD neuron axons transduced with cyto mApple (red) proximal to the site of injury 0, 4, 8 and 24 h post axotomy. Scalebar 25 µm. f Quantification of axon degeneration index (ADI) in WT and DRP1 KD neurons 0, 4, 8 and 24 h post axotomy. N = 3 independent differentiations, N = 35 control gRNA and N = 34 DRP1 gRNA axotomized axons. Two-way ANOVA, Bonferroni correction (p ≤ 0.005 ***, p ≤ 0.001 ****). g Schematic representation of i3Neuron laser axotomy. Dashed box indicates field of view. h Representative images of WT and DLK KO neuron cell bodies transduced with cyto mApple (red) pre and 18 h post axotomy (PA). Scalebar 40 µm. i Percentage degenerated WT and DLK KO 18 post axotomy. N = 3 independent differentiations. Unpaired, two-tailed, t test (p = 0.0129 *). j Schematic representation of i3Neuron laser axotomy. Dashed box indicates field of view. k Representative images of Control and DRP1 KD neuron cell bodies transduced with cyto mApple (red) pre and 18 h post axotomy (PA). Scalebar 40 µm. l Percentage degenerated Control and DRP1 KD 18 post axotomy. Results are represented as mean ± SEM. N = 3 independent differentiations. Unpaired, two-tailed, t test (p = 0.0174 *). All graphical representations show mean ± SEM.
To determine whether loss of DLK or DRP1 also protected neurons from death, we followed the fate of cell bodies of axotomized neurons. In control neurons we observed cell death in about 70% of cells after axotomy whereas only 18% of DLK KO cells and 33% of DRP1 knockdown cells died (Fig. 5g–l). These results demonstrate that both DRP1 and DLK are key regulators of neuronal cell death after axotomy. Interestingly, inhibiting transcription using actinomycin D (ActD) protected neurons from cell death but did not prevent the retrograde axon degeneration (Fig S4a, b), highlighting mechanistic differences for DLK-driven degeneration of axon versus cell body.
Loss of DRP1 reduces neuron death after axotomy in vivo
We then asked how universal this pathway is, or whether it was unique to the i3Neuron model. It has been established that silencing DLK blocks retinal ganglion cell (RGC) death after optic nerve crush29,30,35,36. However, DRP1 has not previously been implicated downstream of this DLK signaling. To knock down DRP1, we used an AAV delivery strategy to express Cas9 (sa-Cas9) together with a guide RNA to DRP149 (Fig. 6a). AAVs were injected into the intravitreal space to transduce RGCs. Three weeks after the injection, DRP1 protein levels were significantly reduced in the optic nerve of mice that had received gRNA DRP1 but not control scrambled gRNA, validating this approach (Fig. 6b, c).
a Schematic representation of RGC survival following optic nerve crush (ONC). b Western blots of Control and DRP1 gRNA transduced optic nerved. Immunoblot for DRP1 and loading control ß actin. c Quantification of DRP1 knockdown by Western blot. N = 4 samples. d Representative images of cleaved caspase 3 positive cells in saCas9-Control and saCas9-DRP1 gRNA transduced RGCs 3 days after ONC. Scalebar 25 µm. e Average cleaved caspase 3 positive cells per section in saCas9-Control, saCas9-DRP1 gRNA and EGFP transduced RGCs after nerve crush. N = 6 Control gRNA, n = 4 DRP1 gRNA and n = 6 EGFP animals per condition. One-way ANOVA, Bonferroni correction. (Control gRNA vs EGFP, not significant, ns. Control gRNA vs DRP1 gRNA p = 0.025 *. DRP1 gRNA vs EGFP p = 0.0093 **). f Representative images of retinas 7 days post ONC and immunostained for RGC marker RBPMS (magenta). Images acquired at similar areas and the same distance from the optic nerve head (ONH). Scalebar 100 µm. g Quantification of percentage RBPMS-positive RGCs in the retinas of control and DRP1 gRNA mice at 7DPI (normalized to contralateral condition). (N = 8 mice per condition. Unpaired, two-tailed, t test. p < 0.0001 ****). h Representative isodensity maps display the topographical survival of RBPMS+RGCs at 7 days post-injury. DRP1 gRNA treatment delays ONC-induced RGC degeneration across the retina. Color scale for isodensity maps ranges from 0 (purple) to 3600 (red) RGCs/mm². All graphical representations show mean ± SEM.
Optic nerve crush was performed two weeks after AAV injection, and retinas were harvested 3 days post injury, a time point at which injured RGCs express cleaved caspase 3 (Fig. 6a). The number of cleaved caspase 3-positive RGCs with saCas9 and a control gRNA was similar to a control virus expressing just EGFP (55 ± 7.83 and 61 ± 6.24, respectively; average caspase-positive cell count per ROI± SEM). However, knocking down DRP1 significantly reduced the caspase positive cell count to 20 ± 8.93 (Fig. 6d, e), showing that apoptosis of RGCs partially relies on DRP1.
We performed a parallel experiment with the same viral strategy for DRP1 knockdown to assess RGC survival 7 days after the injury. Using the RGC marker RBPMS, we found that whilst silencing DRP1 does not affect RGC survival in uninjured retinas (Fig. S4g), DRP1 knockdown protected RGCs from axotomy-induced death (Fig. 6f–h), increasing the percent RGC number per retina from 33.46% ± 0.6% to 42.78% ± 1.5% (average ± SEM). Together, these experiments demonstrate that DRP1 contributes to neuronal cell death after axon injury in vivo.
BAX mediates axon degeneration and cell death after axotomy
To better understand the axon degeneration and cell death we observed, we asked whether they occurred via canonical pathways of apoptosis, and in particular whether they involved BAX. In models of apoptosis, DRP1 contributes to BAX recruitment to mitochondria50, and DRP1 and BAX interact to drive mitochondrial depolarization and fragmentation21,51. Jenner et al. showed a close interaction between DRP1 and BAX in HEK cells undergoing apoptosis using a fluorescence complementation biosensor (ddRFP52). This system produces enhanced red fluorescence upon dimerization of A1 and B1 proteins. Using A1-DRP1 and B1-BAX fusion constructs, we tested whether DRP1 and BAX closely interact in the axon after injury. One hour after axotomy, we observed a significant increase in red fluorescence. The interaction between DRP1 and BAX occurred at mitochondria as shown by co-localization with mito-GFP (Fig. 7a). However, DLK inhibitor GNE3511 reduced their interaction (Fig. 7a, b). To test whether the interaction between DRP1 and BAX was dependent on DRP1 phosphorylation at S616, we mutated the B1-DRP construct to carry a S616A point mutation, preventing its phosphorylation at this site. A1-BAX and B1-DRP1 S616A did not show the increase in red fluorescence, demonstrating that the BAX-DRP1 interaction is more likely to occur if DRP1 is phosphorylated at S616 (Fig. 7c), which requires DLK (Fig. 3).
a Representative images of WT neurons treated with DMSO or GNE3511 pre- and 1 h post-axotomy (PA) expressing mitoGFP (green) and A1-BAX/B1-DRP1 complexes (red). Scale bar = 5 µm. b Quantification of A1-BAX/B1-DRP1 fluorescence fold change in the mitochondria of DMSO or GNE3511 treated neurons 1-h post-axotomy. N = 3 independent differentiations, N = 35 DMSO and N = 34 GNE3511 axotomized neurons. Two-way ANOVA, Bonferroni correction. (p = 0.0168 *). c Quantification of A1-BAX/B1- WT DRP1 and A1-BAX/B1- DRP1 S616A fluorescence fold change in the mitochondria 1-h post-axotomy. N = 3 independent differentiations, N = 37 DRP1 WT and N = 38 DRP1 S616 axotomized neurons. Two-way ANOVA, Bonferroni correction. ( = 0.0192 *). d Representative images of DMSO and BAXi treated neuron cell bodies transduced with cyto mApple (red) pre and 18 h post axotomy (PA). Scalebar 40 µm. e Percent degenerated neurons 18 h post axotomy treated with either DMSO or BAXi. N = 3 independent differentiations. Unpaired, two-tailed, t test. (p = 0.0118 *). f Quantification of axon degeneration index (ADI) of DMSO and BAX-A-treated neurons 24 h after axotomy. N = 3 independent differentiations, total of 46 axons. One-way ANOVA, Bonferroni correction (p ≤ 0.0001 ****, not significant, ns). g Illustration of microfluidic devices used to separate axons from somas allowing for the local treatment of i3Neuron axons. h Representative images of WT axons separated using microfluidic chambers treated with DMSO, BAX-A or BAX-A + GNE3511 for 24 h. Scalebar 25 µm. i Quantification of axon degeneration index (ADI) of isolated axons treated with DMSO BAX-A and BAX-A + GNE3511 for 24 h. N = 3 independent differentiations, 10 images per differentiation. One-way ANOVA, Bonferroni correction (p ≤ 0.0001 ****, not significant, ns). All graphical representations show mean ± SEM.
We next tested whether inhibiting BAX would prevent cell death and axon degeneration in i3Neurons using BAI, a highly selective small molecule inhibitor of BAX53. BAX inhibition significantly preserved cell bodies of axotomized neurons (Fig. 7d, e). BAX inhibition also significantly reduced axon degeneration measured 24 h after injury (Fig. 7f), indicating that BAX is a key mediator of axon degeneration and cell death after axotomy.
Finally, we asked whether local activation of BAX in the axon was sufficient to cause axons to degenerate. Other studies have shown stimulation of BAX with a Bcl-x antagonist causes axon degeneration3,54. We grew i3Neurons in microfluidic chambers to physically isolate axons from cell bodies and dendrites. BAX activator (BAX-A), which binds to the N-terminus of BAX to promote its oligomerization55, was added to the axon compartment, and we measured axon integrity 24 h later. We also performed this experiment in the presence of DLK inhibitor GNE3511 (Fig. 7h). Local activation of BAX caused axons to degenerate, independently of DLK inhibition (Fig. 7h, i), suggesting BAX signaling is downstream of DLK.
Together, our data reveal a local DLK signaling cascade within the axon that drives retrograde axon degeneration after damage and does not require transcription. Starting at the site of damage, the DLK/JNK complex locally phosphorylates DRP1, recruiting BAX to mitochondria and producing an apoptotic cascade that spreads from the injury site back to the cell body where it eventually results in cell death.
Discussion
In this work, we establish a reproducible model of progressive axon degeneration and neuronal death in human neurons and unravel a cascade of events governing neurodegeneration after axon injury. We show that DLK kinase is required for DRP1 to drive mitochondrial fission and BAX-dependent apoptosis that originates in the axon and leads to degeneration of the axon and then the cell body. We demonstrate the relevance of this pathway in vivo, extending from cultured human neurons to retinal ganglion cells of mice with optic nerve crush injuries. We hypothesize this pathway is broadly implicated in neuronal degeneration caused by axon damage, such as spinal cord injury and traumatic brain injury.
While BAX and DLK have separately been implicated in neuronal apoptosis of retinal ganglion cells after optic nerve crush35,36,56,57, an interaction between the two has not previously been reported, nor with DRP1. One study reported that DRP1-dependent mitochondrial fission occurs in axons after damage58. Here, we show how DLK, DRP1, and BAX cooperate to cause neuronal apoptosis after axon damage, and in particular, how this apoptotic mechanism also causes axon degeneration.
Until now, it was also unclear whether pathways distinct from SARM1 can mediate axon degeneration. Many clinical descriptions of axon degeneration refer to “dying back” as a pattern in which distal axons are lost before cell bodies59,60. It has been suggested that dying back axon degeneration overlaps with Wallerian degeneration61, but this remains unclear62. Although neuron degeneration is rescued by SARM1 deletion in some models (e.g. chemotherapy-induced peripheral neuropathy7; TDP43-linked ALS8, it is not in others (SOD1 mouse model of ALS9; optic nerve crush10). We have shown that a type of non-Wallerian axon degeneration that is more akin to apoptosis can occur after axon damage, and that it spreads retrogradely, similar to a dying back. It is possible that additional axon degeneration pathways also exist.
Therapeutically targeting this pathway offers multiple options. There is precedence for testing some of these strategies, such as blocking BAX56,57, or targeting mitochondrial dynamics63, including promoting mitochondrial fusion64 and blocking mitochondrial fission65,66,67,68. Blocking DLK signaling using small molecules has been considered31,34,69, although a Phase I clinical trial in ALS patients was recently halted due to safety concerns70. However, specifically targeting the interaction between the DLK signaling complex and DRP1 has not been attempted.
In addition to protecting axons as we have shown, targeting DRP1 has the potential to protect dendrites71. However, it may cause toxicity as mitochondrial fission is critical for regeneration and motor neuron survival after sciatic nerve injury72. A more complete understanding of DRP1 regulation in homeostatic and injury conditions will be helpful. For example, future studies may identify other targetable DRP1 phosphorylation sites in addition to serine 616 as well as additional kinases. To date, kinases of DRP1 that have been identified include CDK573,74, TBK175, ERK1/276,77, CAMKIa78 and CAMK279; here, we highlight DLK/JNK as a regulatory kinase upstream of DRP180.
As a cytoplasmic facing kinase complex, DLK/JNK has the potential to regulate many substrates among which we now identify DRP1. Additional substrates of the pathway will be of great interest to uncover, as DLK is reported to associate with the plasma membrane, Rab3, Rab6, Rab7, Rab11, Golgi-derived, and LAMP1-positive vesicles43,81. Localization of DLK to mitochondria has also recently been described in cultured mouse embryonic DRG neurons81, corroborating our observation of DLK-mitochondrial association.
Cumulative evidence thus highlights a critical and complex role for DLK in axon degeneration and neuron death as it can act via multiple mechanisms. As shown in this study, DLK regulates retrograde axon degeneration and transcription-independent neuron death (DLK/JNK/DRP1/BAX). The DLK/JNK retrograde signal can also drive transcription-dependent cell death and axon degeneration (DLK/JNK/JUN/PUMA)3. Prior work had also shown that the DLK-JNK pathway signals locally in the distal axon to promote axon degeneration by regulating NMNAT2 turnover, thereby activating SARM182.
Our study highlights a signaling pathway downstream of DLK. It has generally been thought that DLK-dependent transcriptional events govern neuronal degeneration36, but here we find that transcription is dispensable for retrograde axon degeneration driven by DLK via DRP1. It will be of interest to examine how the retrograde axon degeneration mechanism regulated by DLK-DRP1 is coordinated with transcriptional regulation of cell death factors by DLK, for example BID and PUMA3.
DRP1-dependent apoptosis has been described in many cell types19 however, its regulation by DLK was not previously recognized. That axonal DLK can locally trigger this cellular apoptotic pathway from a site of axonal injury that then spreads retrogradely to the cell body is distinct from the previously recognized transcriptional regulation of apoptosis by DLK. Our results call to mind new questions, such as how is DLK localized to the site of mitochondrial fission so it can regulate DRP1 phosphorylation? Does it associate with factors like MFF, an essential factor in recruiting DRP1 to mitochondria83? And what is the mechanism of activation of DLK that would link it to mitochondrial fission? In the future, it will be of great interest to explore under which conditions this pathway is activated, as well as the specificity of this pathway to different neuronal subtypes.
Methods
Ethical compliance
Animal care and experimental procedures were performed in accordance with NICHD protocols 20-003 and 23-003 (Le Pichon lab) approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development ACUC, animal protocol number NEI-606 (Li lab) approved by the National Eye Institute ACUC and animal protocol AN-7208 (Watkins Lab) approved by the Baylor College of Medicine ACUC.
i3Neuron differentiation
The iPSC line (WTC11) used in this study was obtained from a central repository (Coriell), and this iPSC line was registered at the NIH IRP per established guidelines. i3Neurons were differentiated as previously described. Briefly, i3 iPSCs were dissociated using Accutase (Life Technologies, cat. no. A1110501). Cells were plated in Matrigel-coated (1:100 Corning) plates in Neuronal induction media on day 0 (Knockout Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium; Life Technologies Corporation, cat. no. 12660012), 1X N2 supplement (Life Technologies, cat. no. 17502048), 1× GlutaMAX (Thermofisher Scientific, cat. no. 35050061), 1× MEM nonessential amino acids (NEAA) (Thermofisher Scientific, cat. no. 11140050), 10 μM ROCK inhibitor (Y-27632; Tocris, cat. no. 1254), and 2 μg/ml doxycycline (Clontech, cat. no. 631311). Neuronal induction media was changed once a day for 2 more days. On day 3 of induction, cells were dissociated using Accutase and plated in dishes coated with poly-L-ornithine (PLO; 0.1 mg/ml; Sigma, cat. no. P3655-10MG). Cells were plated in neuronal maturation media (BrainPhys medium (STEMCELL Technologies, cat. no. 05790), 1× B27 Plus Supplement (ThermoFisher Scientific, cat. no. A3582801), 10 ng/ml BDNF (PeproTech, cat. no. 450-02), 10 ng/ml NT-3 (PeproTech, cat. no. 450-03), 1 mg/ml mouse laminin (Invitrogen, cat. no. 23017015), and 2 μg/ml doxycycline). Half of the neuronal maturation media was removed and replenished with fresh media every 2–3 days.
Lentivirus generation
Lentivirus were generated as previously described37, 7 million Lenti-X HEK-293T cells (Takara Bio, cat. no. 632180) were seeded in 9 mL DMEM. The next day, a transfection mix was prepared containing 1 µg of Lenti plasmid, 3 µg of third generation packaging mix (1:1:1 mix of three plasmids), 12 µL Lipofectamine 3000 Reagent (ThermoFisher, cat. no. L3000008), and 250 µL Opti-MEM I Reduced Serum Medium (GIBCO, cat. no. 31985070). The mix was vortexed, spun down briefly, incubated at room temperature for 40 min, then added dropwise to the HEK cells and gently swirled to mix. The next day, the media was replaced with 18 ml fresh 10% FBS DMEM supplemented with 1:500 ViralBoost (Alstem, Cat. No. VB100). Two days later, the media was collected into a 50 ml Falcon tube, supplemented with 6 ml Lenti-X Concentrator (Takara Bio; Cat. No. 631231), mixed thoroughly, and stored at 4 °C for 48 h. The supernatant was then spun down at 4 °C for 45 min at 1500 × g. The supernatant was aspirated, and the pellet was resuspended in 500 μL of PBS.
Plasmids and cloning
All DLK (Ensembl CCDS: 55831) constructs and mutants were generated by Epoch Live Science (Texas, USA) and cloned into a lentivirus-expressing backbone under a doxycycline-dependent promoter. DLK was C-terminally tagged by inserting a glycine rich linker (3x GGGGS) followed by EGFP, just before the stop codon. EYFP-DRP1 was obtained from Addgene (cat. no. 45160). For GST-purification, DLK and DRP1 were N-terminally tagged into a GST-containing backbone using In-fusion cloning (Takara Bio. cat. no. 638945). Cyto-mAPPLE plasmid was a gift from Michael E. Ward (NINDS). A1-BAX and B1-DRP1 were gifted by Dr. Ana J. Garcia-Saez (Max Plank Institute of Biophysics). Mito-EGFP and Mito-RFP plasmids were gifts from Dr. Zu-Hang Sheng (NINDS).
DRP1 knockdown using dead Cas9
For DRP1 knockdown, we used CRISPRi-i3 iPSCs containing a CAG promoter-driven dCas9-BFP-KRAB cassette inserted into the CLYBL safe harbor locus (Addgene #127968). iPSCs were transduced with control gRNA or a previously validated gRNA targeting DRP137 and differentiated as described above.
Laser Axotomies
Laser axotomies were performed on 10–14 day old neurons using a Zeiss LSM 880 Upright 2-Photon microscope equipped with an ablate laser. Temperature and CO2 levels were maintained using a Pecon microscope incubator. Ablation laser was set to 800 nm, 100% power and 100 iterations. Neurons were cultured in µ-Dish 35 mm dishes (Ibidi, Cat. No. 81156 or 81166 depending on the application) in Hybernate E (Gibco, Cat. No. A1247601), 1× B27 Plus Supplement (ThermoFisher Scientific, cat. no. A3582801), 10 ng/ml BDNF (PeproTech, cat. no. 450-02), 10 ng/ml NT-3 (PeproTech, cat. no. 450-03), 1 mg/ml mouse laminin (Invitrogen, cat. no. 23017015), and 2 μg/ml doxycycline.
Mitochondrial fission after axotomy
iPSCs were differentiated as described above. On day 1 of differentiation, cells were transduced with the appropriate lentivirus. Neuronal induction media was changed 24 h later. On day 3 of differentiation, 50,000 cells were plated in a 5 µL drop of neuronal maturation media in the middle of a 35 mm dish (ibidi, Cat. No. 81156) for the axons to grow out from the center. Dishes were incubated for 20 min at 37 °C to allow cells to attach, and 2 mL of neuronal maturation media was then added to each well. Cells were fed with half-media changes every two days until the day of the experiment. Laser axotomies were performed as described above. Single axons were randomly selected. Five images were taken before axotomy every 5 s and axons were imaged for 5 min after axotomy every 5 s. The number of mitochondrial particles was quantified using ImageJ imaging software and normalized to the number of mitochondria pre-axotomy. When treating neurons with different chemical compounds, neurons were pretreated for one hour prior to performing axotomies. The following compounds were used GNE3511 (500 nM, Sigma, Cat. No. 5331680001), JNKi (SP600125 1 µM, Selleckchem, Cat. No. S1460), P110 (1 µM, Tocris, Cat. No. 6897), BAPTA-AM (10 mM Tocris, Cat. No. 2787), BSTA1 (BAX activator, 10 µM, Sigma, Cat. No. SML2243) and BAI1 (BAX inhibitor, 10 µM, Medchemexpress, Cat. No. HY-103269).
Axon separation and axotomies
For axon separation experiments after axotomy, 1.5 million cells were plated in 150 µl of maturation media in a strip in the center of a 6 cm dish. Cells were allowed to attach for 15 min at 37 °C, after which 8 ml of media was added to the dish. Neurons were cultured for 1 month to allow for the axons to project outwards. Axotomy was performed using a sharp blade. Axons and cell bodies were separated and processed for western blotting.
Immunostaining of cells
Cells were fixed in cold 4% PFA for 5–10 min, permeabilized using 0.1% TritonX in PBS for 5 min and blocked in 5% normal donkey serum (NDS) in PBS at room temperature for 1 h. Cells were immunostained with the selected primary antibody in 2.5% NDS overnight at 4 °C. Cells were washed three times with PBS and stained with the appropriate secondary antibody at a concentration of 1:500 in 2.5% NDS for 1 h at room temperature. Cells were washed three times with PBS and DAPI was used as a nuclear counterstain. Antibodies used against: βIII tubulin (Thermo Fischer, cat. no. 2G10, 1:500), pDRP1 S616 (CST, cat. no. 3455S, 1:1000), DLK (DLK antibody developed and kindly provided by Cell Signaling Technology, clone cat. no. GF-23-1, 1:500), TOM20 (Santa Cruz, cat. no. SC-17764, 1:500).
Calcium imaging
Neurons were plated as described above in the center of a 35 mm dish, and their axons were allowed to grow outwards. 10–14 days post induction, neurons were treated with Fluo-4 AM cell permeable calcium dye (ThermoFischer, Cat. No. F14201) following the manufacturers indications. Axotomies were performed on single axons as described above and imaged every 2 s for 2 min after axotomy. Fluorescence intensities were calculated using ImageJ software and normalized to pre-axotomy levels.
HEK cell culture and transfection
HEK-293A cells were cultured in DMEM (Gibco, cat. no 11995065) supplemented with 10% FBS (Gibco; cat. no. 10437028). For transfection, cells were dissociated using Trypsin-EDTA (0.25%) (ThermoFischer, Cat. No. 25200056) and plated in DMEM, 10%FBS. The day after plating cells were transfected with 1 µg DNA plasmid, 8 µl lipofectamine 2000 (Invitrogen, Cat. No. 11668030) for 24 h. For western blotting, cells were harvested in ice cold RIPA buffer (Invitrogen, cat. no. 89901) supplemented with protease inhibitors (Sigma, 11836153001) and PhosStop (Sigma cat. no. 4906845001). For immunostaining, cells were fixed in 4% PFA for 10 min.
Western blotting
Protein concentrations were calculated using a BCA assay (ThermoFisher, cat. no. 23225). 10–20 µg of total protein lysate was loaded into a 4–20% Mini-PROTEAN® TGX™ Precast Protein Gels (Biorad, cat. no. 4561095). Gels were transferred into PVDF membranes and blocked for 1 h at room temperature in 5% BSA. Membranes were incubated with the appropriate primary antibody in 2.5% BSA solution in a cold room, rocking, overnight. Membranes were washed 3 times with PBS 0.1% Tween solution. Secondary antibody was incubated for 1 h at room temperature at a concentration of 1:8000 in 2.5% BSA solution. Membranes were washed 3 times with PBS 0.1% Tween solution prior to developing. Western blots were developed using Clarity Western ECL Substrate (Biorad, cat. no. 1705061) and imaged using a ChemiDoc MP Imaging system (Biorad). Band intensity was quantified using FIJI imaging analysis software. The following antibodies were used at the concentrations stated: anti-DRP1 (Santa Cruz Biotechnology, cat. no. sc-101270, 1:1000), anti-pDRP1 S616 (CST, cat. no. 3455S, 1:1000), anti-ß Actin (Sigma, cat. no. A1978, 1:5000), anti-p-cJun S63 (CST, cat. no. 9261, 1:1000), anti-pDRP1 S637 (CST, cat. no. 4867S, 1:1000).
Axon degeneration index and neuron survival
Induction of iPSCs into neurons was performed as described above. On day 1 of differentiation, neurons were transduced with a cytoplasmic mApple (cyto mApple)-expressing lentivirus, and the media was changed 24 h later. On day 3 of differentiation, transduced neurons were mixed with mixed with untransduced neurons at a ratio of 1:8000 to achieve sparse labeling of neurons, and re-plated on a 35 mm gridded dish (Ibidi Cat. No. 81166). On day 14 of differentiation, the positions of selected axons or neuron somas was recorded and laser axotomies were performed as described above. Dished were stored in a temperature and CO2 controlled incubator until imaged next. Injured axons and somas were re-located at different time points after axotomy using the gridded dish and imaged. Axons were injured <1 mm away from the soma. The axon degeneration index was calculated as previously described37. The following compounds were used: JNKi (SP600125 1 µM, Selleckchem, Cat. No. S1460), Actinomycin D (ActD) (Selleckchem, cat. no. S8964; 3 µM).
Airyscan imaging
Neurons were plated in 1 mm German Glass Coverslips thickness #1.5 (Electron Microscopy Sciences, Cat. No. 72290-04). 10-day old neurons were fixed with 4% PFA for 10 min at room temperature. Cells were permeabilized with 0.1% Triton-X for 5 min and blocked in 5% normal donkey serum (NDS) at room temperature for an hour. Primary antibody incubation was carried out in 2.5% NDS overnight at 4 oC. Cells were washed three times with PBS and stained with the appropriate secondary antibody at a concentration of 1:500 in 2.5% NDS for 1 h at room temperature. Cells were washed three times with PBS and DAPI was used as a nuclear counterstain. Coverslips were mounted using ProLong Glass antifade (Invitrogen, Cat. No. P36980).
Seahorse analysis
For Seahorse analysis, 50,000 cells per well were plated after 3 days of induction in a specialized Seahorse analyzer 96-well plate (Agilent, cat. no. 103774-100). Cells were washed in PBS once and incubated in a hypoxic chamber following the manufacturer’s procedure. Seahorse analysis was performed using a Seahorse XFe 96 Analyzer (Agilent). The average value for three wells was used for analysis.
Protein purification and in vitro kinase assay
DLK and DRP1 were cloned into a pcDNA3-C-terminal GST vector. 12 million HEK-293T cells were plated in 10 cm dished coated with Matrigel. The day after, cells were transfected with 10 µg plasmids and 40 µl lipofectamine. 24 h post transfection, cells were washed twice with PBS and harvested in 880 µl ice cold RIPA buffer (Invitrogen, cat. no. 89901) supplemented with protease inhibitors (Sigma, 11836153001) and PhosStop (Sigma cat. no. 4906845001). Lysates were sonicated and spun at 18000 g 10 min at 4 oC and the pellet was discarded. 500 µl Glutathione-Sepharose beads (Sigma Cat. No. GE17-0756-01) were washed twice in 1 ml in lysis buffer. Washed beads to 1 ml lysate and incubate end-over-end shaking in cold room overnight. The next morning. Beads were washed 4 times with lysis buffer + 0.4 M NaCl. The protein-containing beads were pelleted by spinning 1 min 8000 rpm at 4 oC, the supernatant was discarded, and the beads were resuspended in fresh 200 mM glutathione 1X PBS solution for elution of the desired protein. The beds were incubated for 20 min at room temperature shaking. The eluted protein and the beads were separated by briefly spinning in a SpinX column tubes (Sigma Cat. No. CLS8162-24EA). The purity of purified protein was by running 5 µg of protein on an SDS PAGE gel and performing Comassie stain. The purified protein was stored at −80 oC. For in vitro kinase assay, purified GST-DLK or GST-DLK-S302A was mixed with purified GST-DRP1 at a 1:1 ratio in 50 µl kinase buffer (50 mM TrisHCL pH 7.5, 150 mM NaCl, 10 mM MgCl, 10 mM MnCl2, 1.8 mM ATP) incubated for 60 mins at 37 oC. In vitro kinase assay was also performed in the presence of DLKi inhibitor GNE3511 (500 nM, Sigma, Cat. No. 5331680001) and JNKi (SP600125 1 µM, Selleckchem, Cat. No. S1460). The reaction was terminated by adding SDS gel loading buffer and the sample was subjected to western blotting.
For in vitro kinase assays using protein purified in Sf9 insect cells, all proteins and kinase buffers were purchased from Signal chem biotech unless otherwise stated. Active JNK1 (Cat. No. M33-10G), DLK (Cat. No. M20-11G), DRP1 (Novus biochemical, Cat. No. H00010059), ATF2 (Cat. No. A10-55G) were used and the in vitro kinase assays were performed according to the manufacturer’s protocols. Reactions were performed by incubating at 30 oC for 30 min, followed by 37 oC for 30 min. The reaction was terminated by adding SDS gel loading buffer and the sample was subjected to western blotting.
Phospho-mass spectrometry
DRP1 bands, with ~0.5 µg protein on each band, were cut from SDS-PAGE. In-gel samples were reduced with 10 mM Tris(2-carboxyethyl)phosphine hydrochloride, alkylated with N-Ethylmaleimide, and digested with trypsin at 37 °C for 18 h. Peptides were extracted, desalted and injected into a nano-LC-MS/MS system where an Ultimate 3000 HPLC was coupled to an Orbitrap Lumos mass spectrometer (Thermo Scientific) via an Easy-Spray ion source (Thermo Scientific). Peptides were separated on an ES902 Easy-Spray column (75-μm inner diameter, 25 cm length, 3 μm C18 beads; Thermo Scientific) with mobile phase B (0.1% formic acid in LC-MS grade acetonitrile) increased from 3 to 24% in 60 min. The flow rate was maintained at 300 nl/min. MS and MS/MS data were acquired on Thermo Scientific Orbitrap Lumos mass spectrometer. MS1 scans were acquired in orbitrap at of a resolution 120k with a mass range of m/z 400–1500. MS2 scans were acquired in ion trap with ETciD method at normal scan rate. The isolation width was 1.6 m/z. Ions were excluded after 1 acquisition and the exclusion duration is 9 s. MS1 scans were performed every 3 s. As many MS2 scans were acquired within each MS1 scan cycle. Proteome Discoverer software version 2.4 was used for protein identification and quantification. Database search was performed against Sprot Human database using Mascot search engine. Mass tolerances for MS1 and MS2 scans were set to 5 ppm and 0.4 Da, respectively. Up to 2 missed cleavage was allowed for trypsin digestion. NEM on cysteines was set as fixed modification. Oxidation (M) and Phosphorylation (STY) were searched as variable modifications. Spectra of phosphopeptides matched by database search were manually checked. The search results were filtered by a false discovery rate of 1% at the protein level. Proteins detected with 1–2 peptides were further filtered out. Protein abundance values were calculated by summing the abundance of unique and razor peptides matched to that protein. Each sample group contains 4 replicates. The ratios were calculated using protein abundance without normalization. Mass spectrometry raw data have been deposited at massive.ucsd.edu, accession code # MSV000096146.
Electron microscopy
Neurons were plated on 1 mm German Glass Coverslips thickness #1.5 (Electron Microscopy Sciences, Cat. No. 72290-04). 10 Day old neurons were fixed with in 4% PFA, 1% Glutaraldehyde for 40 min. Fixed cells were placed in phosphate buffer containing 0.1% sodium borohydride to inactivate residual aldehyde groups. Cells were then washed with phosphate buffer several times until the solution was clear of bubbles. To improve reagent penetration, the cells were then treated with phosphate buffer containing 0.05% Triton X-100. To prevent nonspecific binding of the immunoreagents, cells were incubated in Aurion blocking solution for Goat gold conjugates (Electron Microscopy Sciences, Hatfield, PA., containing phosphate buffer, pH 7.4, normal goat serum (NGS), bovine serum albumin (BSA), and cold water fish skin gelatin (CWFG)). After blocking, cells were incubated in the primary antibody, DLK (custom antibody from Cell Signaling Technology, clone GF-32-1, 1:100) diluted with incubation buffer (phosphate buffer containing 0.2% Aurion acetylated bovine serum albumin (BSA-c), Electron Microscopy Sciences, Hatfield, PA., 5% CWFG and 5% NGS, pH 7.4) for two hours at room temperature. After washes with incubation buffer, cells were incubated in the secondary antibody Aurion ultra-small gold-conjugated F(ab′)2 fragments of Goat anti-Rabbit IgG, (Electron Microscopy Sciences, Hatfield, PA.) diluted 1:100 with incubation buffer overnight at 4 oC. To remove unbound secondary antibody, cells were washed thoroughly with incubation buffer and then with PBS. After washes, cells were prepared for silver enhancement.
Silver enhancement and cell processing for electron microscopy
Cells were washed with MilliQ water and then transferred to Aurion R-Gent SE-EM silver enhancement solution, Electron Microscopy Sciences, Hatfield, PA. and incubated at room temperature for 35 min. The enhancement was stopped by washing cells several times in MilliQ water. The following processing steps were carried out using the variable wattage Pelco BioWave Pro microwave oven (Ted Pella, Inc., Redding, CA.): cells were rinsed in MilliQ water, post-fixed in 1% osmium tetroxide made in MilliQ water, ethanol dehydration series up to 100% ethanol, followed by a Embed-812 resin (Electron Microscopy Sciences, Hatfield, PA.) infiltration series up to 100% resin. The epoxy resin was polymerized for 20 h in an oven set at 60 °C. Ultra-thin sections (90 nm) were prepared on a Leica EM UC7 ultramicrotome. Ultra-thin sections were picked up and placed on 200-mesh cooper grids (Electron Microscopy Sciences, Hatfield, PA) and post-stained with uranyl acetate and lead citrate. Imaging was performed on a JEOL-1400 Transmission Electron Microscope operating at 80 kV and images were acquired on an AMT BioSprint 29 camera.
Generation of DLK KO iPSCs
WT iPSCs were transfected with Cas9-GFP, and two gRNA containing plasmids targeting exons 3 and 5. gRNA expressing plasmids were purchased from Sigma Aldrich (U6-gRNA:hPGK-puro-2A-tBFP, gRNA1: CCCAGGCTCCCTGCTACTGCAT, gRNA 2: TCCTTTGGCGTGGTGCTATGGG). 1 day after transfection, expression of Cas9 and gRNAs was validated by the expression of GFP and BFP respectively and iPSCs were treated for 3 days with 10 µM Puromycin (Invitrogen Cat. No. A11113803) and allowed to recover for 3 days. Cells were then dissociated with accutase for 10 min and dissociated my pipetting forcefully 10 times. A 1:2 serial dilution was then performed in two 6 well plates. iPSCs were fed every 3 days until individual colonies were figure visible (usually 10–14 days). Individually colonies were picked using a P1000 pipette and transferred into individual wells in a 24-well plate. Individual clones were allowed to expand, passaged and their DNA was extracted for DLK KO validation using PCR (Forward primer: TCAGGTGAATGCTGAGCCAGCT, reverse primer: TGGAGACTGTTGCTTCCCACAC). A shorter band from ~1200 bp to ~200 bp was expected if the KO was successful. Potential clones were sequenced. Further validation was performed by differentiating potential clones into neurons and knock out of DLK was validated by western blotting and immunofluorescence.
Animals
C57Bl/6 J (Jax#000664) mice of both sexes, aged between 6 and 10 weeks old, were used in this study. Mice were kept in their parent cages until 3 weeks of age, after which they were weaned into cages of up to five mice of the same sex. Water was available ad libidum. A 12-h light/dark cycle was maintained, with lights on from 6:00 a.m. to 5:59 p.m. Equivalent numbers of male and female mice were used in all experiments and were randomly allocated into experimental groups. We did not observe any sex-specific differences.
Mouse tissue preparation
The proximal segment of the injured nerve was harvested, and 4 nerves were pooled to obtain enough protein. Nerves were mechanically lysed in 40 µl ice cold RIPA buffer (Invitrogen, cat. no. 89901) supplemented with protease inhibitors (Sigma, 11836153001) and PhosStop (Sigma cat. no. 4906845001) for 2 min using a micro pestle. Nerves were then sonicated for 15 min at 4 °C. Protein lysates were centrifuged at 18,000 × g, 4 oC for 10 min and the pellet was discarded. The samples were subjected to the western blotting procedure described above. Abundance of phopho-DRP1/ total DRP1 was obtained running two western blots. Phosphorylated and unphosphorylated protein was divided by the total protein and then divided to each other to obtain the relative levels of phosphorylated protein.
Adeno-associated virus (AAV)
Adeno-associated viral vectors carrying human synapsin-1 (hSyn1) promoter driving expression of saCas9-U6-control, saCas9-U6-DRP1-gRNA, or eGFP were generated by Epoch Life Sciences Inc. The plasmids were then packaged into AAV2 capsids at the Optogenetics and Viral Vector Core at Duncan Neurological Research Institute, Houston.
Intravitreal injections
Mice to be injected were anesthetized with 3% isoflurane. The eyes were sterilized by 3 repeated applications of 5% ophthalmic betadine (Henry Schein #6900250), Opti-clear ophthalmic eye wash (Akorn #NDC 17478-620-04) and a dry wipe. A topical anesthetic 0.5% proparacaine HCl ophthalmic solution (Henry Schein #1365345) was applied. The eyeball was punctured using a 5-µl Hamilton syringe loaded with a custom 33-gauge needle (Hamilton #7803-5) and some of the intraocular pressure was relieved. The same puncture site was used to reinsert the Hamilton needle and 2 µl of AAV in titers ranging from 1012 −1013 vg/ml was delivered per eye.
Intra-orbital optic nerve crush (ONC)
Two weeks after intravitreal injections, animals underwent optic nerve crush surgeries. Mice undergoing surgery were dosed with 1 mg/kg buprenorphine sustained release formulation 1 h before surgery. Right before surgery, mice were anesthetized with 3% isoflurane. The non-surgical eye received artificial tears ointment (Covetrus #11695-6832-1. The surgical eye was sterilized as described in the intravitreal injections section above. Topical anesthetic 0.5% proparacaine HCl ophthalmic solution (Henry Schein #1365345) was applied on the surgical eye (left). A pair of Vannas scissors (World PrecisionInstruments #501777) were used to make incisions in the conjunctival layers. The optic nerve section in the intra-orbital space was exposed by using two pairs of suture-tying forceps (Fine Science Tools #1106307) to gently clear the soft tissue behind the eye until the optic nerve was visible. The optic nerve was manually crushed for 5 s by using a pair of Dumont forceps (Fine Science Tools #1125325). The eyeball was gently placed back into the orbit. Animals were post-surgically monitored in their home cages until sternal recumbency was observed.
Immunolabeling of retinae for cleaved caspase 3
At the experimental endpoint (3 days after optic nerve crush) mice were euthanized by an overdose of isoflurane and followed by decapitation. The eyes were enucleated and drop-fixed in 4% paraformaldehyde for 1 h. The retinae were dissected out in 1X phosphate-buffered saline (PBS) and then blocked with blocking buffer (5% goat serum, 0.5% Triton X-100 and 0.025% sodium azide in 1X PBS) for 30 min. The retinae were then immersed in primary antibody solution (anti-cleaved caspase 3, CST cat. no. 9661) prepared by dilution in blocking buffer solution and incubated for 5 days in 4 °C. They were then washed three times in 1X PBS with 0.5% TritonX-100 for 30 min each, and then moved to appropriate secondary antibody solution prepared in blocking buffer and incubated overnight in 4 °C. After secondary antibody incubation, they were washed again three times in 1X PBS with 0.5% TritonX-100 for 30 min each. Retinae were then mounted in Drop-n-Stain EverBriteTM Mounting Medium (Biotium #23008) onto slides and imaged using Zeiss Axio Imager Z1 fluorescence microscope. 5–8 images were taken per animal in a blinded manner for quantification.
Quantification of cleaved caspase 3-positive RGCs
The number of cleaved caspase 3 positive cells were quantified in 5–8 images per retina per animal in a blinded manner. The number of caspase 3 positive cells per section was averaged over all the images quantified to obtain the average number of cleaved caspase 3 cells per section.
Retinal dissection for RBPMS analysis
For RGC survival 7 days post injury, prior to fixation in 4% paraformaldehyde for 1 h, a burn signal was applied to the dorsal pole of enucleated eyeballs to maintain orientation. Retinas were then carefully dissected as flat whole-mounts employing four radial cuts, with the deepest incision previously marked at the dorsal pole. The flattened whole-mounts underwent an additional hour of post-fixation in 4% PFA, followed by a gentle removal of vitreous using brushes. Subsequently, they were kept in phosphate-buffered saline (PBS) for further processing.
Immunofluorescence for RBPMS
Retinal ganglion cells (RGCs) in whole-mount retinas were identified through immunodetection of the RBPMS (RNA-binding protein with multiple splicing) protein. Initially, all retinas were permeated (4 × 10 min) in PBS containing 0.5% Triton X-100 (Tx). The primary antibody (rabbit anti-RBPMS, GeneTex #GTX118619) was diluted at 1:500 and incubated overnight with shaking at room temperature in a blocking buffer (2% Normal Donkey Serum, 0.5% Tx in PBS). Next, the retinas were washed and incubated overnight with the appropriate secondary antibody (donkey anti-rabbit Alexa 649, Jackson ImmunoResearch, #706-495-148) diluted 1:500. Finally, retinas were washed in PBS and cover-slipped vitreal side up with antifading mounting medium.
Image acquisition
RBPMS+RGCs were imaged from flattened retinal whole-mounts using a 20x objective on an LSM 780 Zeiss confocal microscope equipped with a computer-driven motorized stage controlled by Zen Lite software (Black edition, Zeiss). Photomontage frames were captured contiguously side-by-side with an 8% overlap between them, and images within the same frame were acquired at intervals of 3.5 microns in the Z-dimension. The maximum-projection images were employed for subsequent analysis.
Quantification of RBPMS RGCs
Quantification of RBPMS+RGCs was conducted on entire retinas using an automated algorithm in ImageJ. Briefly, to minimize interference with background labeling, a rolling ball radius of 50 pixels was subtracted. The application of a median radius of 1 smoothed the edges, followed by a maximum radius of 0.0005 to fill and dilate the objects. Subsequently, all images underwent an 8-bit grayscale transformation to eliminate color information, and a predetermined lower threshold generate a binary mask-like images. The “watershed” segmentation automatically separated particles that were in contact, while the “despeckle” median filter efficiently removed noise. Positive objects were counted within defined parameters regarding shape and size, to exclude those considered either too small or too large to be classified as RGCs. The automatic routine extracted cell counts and their corresponding coordinates (x, y), which were exported to a spreadsheet (Microsoft Office Excel; Microsoft Corp., Redmond, WA, USA) for further analysis (see the next section).
Spatial analysis
Topographical distributions of RBPMS+RGC densities were assessed using color-filled contour maps generated with Sigma Plot 13.0 for Windows (Systat Software, Inc., Richmond, CA, USA). These heat maps assigned a color code to each area of interest (83.3 × 83.3 μm) based on its density values, ranging from 0 (purple) to 3600 RGCs/mm² (red) in a 10-step color scale.
Statistics and reproducibility
All quantifications in this study (except for Western blots), were performed blind to experimental groups and conditions. All statistical analysis was performed in GraphPad Prism 9 unless otherwise stated. All experiments in vitro or in cells were repeated independently 2–4 times (see Source Data file for information on replicates).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data are provided as a Source Data file. The mass spectrometry data generated in this study were deposited at the MassIVE database under accession code MSV000096146. Additional data files (including uncropped Western blots, raw image, video and other data files) are available as Supplementary information or have been deposited at synapse.org https://doi.org/10.7303/syn63835477. Source data are provided with this paper.
References
Whitmore, A. V., Lindsten, T., Raff, M. C. & Thompson, C. B. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 10, 260–261 (2003).
Deckwerth, T. L. et al. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17, 401–411 (1996).
Simon, D. J. et al. Axon degeneration gated by retrograde activation of somatic pro-apoptotic signaling. Cell 164, 1031–1045 (2016).
Simon, D. J. et al. A caspase cascade regulating developmental axon degeneration. J. Neurosci. 32, 17540–17553 (2012).
Osterloh, J. M. et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 337, 481–484 (2012).
Gerdts, J., Brace, E. J., Sasaki, Y., DiAntonio, A. & Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348, 453–457 (2015).
Geisler, S. et al. Vincristine and bortezomib use distinct upstream mechanisms to activate a common SARM1-dependent axon degeneration program. JCI Insight 4, e129920 (2019).
White, M. A. et al. Sarm1 deletion suppresses TDP-43-linked motor neuron degeneration and cortical spine loss. Acta Neuropathol. Commun. 7, 166 (2019).
Peters, O. M. et al. Loss of Sarm1 does not suppress motor neuron degeneration in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Hum. Mol. Genet 27, 3761–3771 (2018).
Fernandes, K. A. et al. Role of SARM1 and DR6 in retinal ganglion cell axonal and somal degeneration following axonal injury. Exp. Eye Res. 171, 54–61 (2018).
Mandal, A. & Drerup, C. M. Axonal transport and mitochondrial function in neurons. Front Cell Neurosci. 13, 373 (2019).
Smith, G. M. & Gallo, G. The role of mitochondria in axon development and regeneration. Dev. Neurobiol. 78, 221–237 (2018).
Han, S. M., Baig, H. S. & Hammarlund, M. Mitochondria localize to injured axons to support regeneration. Neuron 92, 1308–1323 (2016).
Lin, M.-Y. et al. Releasing syntaphilin removes stressed mitochondria from axons independent of mitophagy under pathophysiological conditions. Neuron 94, 595–610.e6 (2017).
Karbowski, M. & Youle, R. J. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 10, 870–880 (2003).
Palau, F., Estela, A., Pla-Martín, D. & Sánchez-Piris, M. Inherited neuromuscular diseases, translation from pathomechanisms to therapies. Adv. Exp. Med. Biol. 652, 129–137 (2009).
Tilokani, L., Nagashima, S., Paupe, V. & Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem 62, 341–360 (2018).
Youle, R. J. et al. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 (2012).
Lee, Y., Jeong, S.-Y., Karbowski, M., Smith, C. L. & Youle, R. J. Roles of the Mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 15, 5001–5011 (2004).
Frank, S. et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 (2001).
Jenner, A. et al. DRP1 interacts directly with BAX to induce its activation and apoptosis. EMBO J. 41, e108587 (2022).
Milani, M. et al. DRP-1 functions independently of mitochondrial structural perturbations to facilitate BH3 mimetic-mediated apoptosis. Cell Death Discov. 5, 117 (2019).
Goldsmith, J., Ordureau, A., Harper, J. W. & Holzbaur, E. L. F. Brain-derived autophagosome profiling reveals the engulfment of nucleoid-enriched mitochondrial fragments by basal autophagy in neurons. Neuron 110, 967–976.e8 (2022).
Tian, R. et al. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. Neuron 104, 239–255.e12 (2019).
Liao, Y.-C. et al. RNA granules Hitchhike on lysosomes for long-distance transport, using annexin A11 as a molecular tether. Cell 179, 147–164.e20 (2019).
Fernandopulle, M. S. et al. Transcription factor–mediated differentiation of human iPSCs into neurons. Curr. Protoc. Cell Biol. 79, e51 (2018).
Zhang, Y. et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013).
Miller, B. R. et al. A dual leucine kinase–dependent axon self-destruction program promotes Wallerian degeneration. Nat. Neurosci. 12, 387–389 (2009).
Huntwork-Rodriguez, S. et al. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J. Cell Biol. 202, 747–763 (2013).
Larhammar, M. et al. Dual leucine zipper kinase-dependent PERK activation contributes to neuronal degeneration following insult. Elife 6, e20725 (2017).
Pichon, C. E. L. et al. Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Sci. Transl. Med 9, eaag0394 (2017).
Adib, E. A., Smithson, L. J. & Collins, C. A. An axonal stress response pathway: Degenerative and regenerative signaling by DLK. Curr. Opin. Neurobiol. 53, 110–119 (2018).
Ferraris, D., Yang, Z. & Welsbie, D. Dual leucine zipper kinase as a therapeutic target for neurodegenerative conditions. Future Med Chem. 5, 1923–1934 (2013).
Siu, M., Ghosh, A. S. & Lewcock, J. W. Dual leucine zipper kinase inhibitors for the treatment of neurodegeneration. J. Med. Chem. 61, 8078–8087 (2018).
Welsbie, D. S. et al. Enhanced functional genomic screening identifies novel mediators of dual leucine zipper kinase-dependent injury signaling in neurons. Neuron 94, 1142–1154.e6 (2017).
Watkins, T. A. et al. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl Acad. Sci. 110, 4039–4044 (2013).
Gomez-Deza, J., Slavutsky, A. L., Nebiyou, M. & Pichon, C. E. L. Local production of reactive oxygen species drives vincristine-induced axon degeneration. Cell Death Dis. 14, 807 (2023).
Court, F. A. & Coleman, M. P. Mitochondria as a central sensor for axonal degenerative stimuli. Trends Neurosci. 35, 364–372 (2012).
Wang, B. et al. Mitochondrial behavior in axon degeneration and regeneration. Front Aging Neurosci. 13, 650038 (2021).
George, E., Glass, J. & Griffin, J. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 15, 6445–6452 (1995).
Villegas, R. et al. Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J. Neurosci. 34, 7179–7189 (2014).
Farley, M. M. & Watkins, T. A. Intrinsic neuronal stress response pathways in injury and disease. Annu Rev. Pathol. Mech. Dis. 13, 93–116 (2018).
Holland, S. M. et al. Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc. Natl Acad. Sci. 113, 763–768 (2016).
Nihalani, D., Merritt, S. & Holzman, L. B. Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation*. J. Biol. Chem. 275, 7273–7279 (2000).
Longo, D. L. & Archer, S. L. Mitochondrial dynamics — mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 369, 2236–2251 (2013).
Ghosh, A. S. et al. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J. Cell Biol. 194, 751–764 (2011).
Yu, R. et al. The phosphorylation status of Ser-637 in dynamin-related protein 1 (Drp1) does not determine Drp1 recruitment to mitochondria. J. Biol. Chem. 294, 17262–17277 (2019).
Chang, C.-R. & Blackstone, C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology*. J. Biol. Chem. 282, 21583–21587 (2007).
Hunker, A. C. et al. Conditional single vector CRISPR/SaCas9 viruses for efficient mutagenesis in the adult mouse nervous system. Cell Rep. 30, 4303–4316.e6 (2020).
Robitaille, K., Daviau, A., Lachance, G., Couture, J.-P. & Blouin, R. Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ. 15, 1522–1531 (2008).
Karbowski, M. et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938 (2002).
Alford, S. C., Abdelfattah, A. S., Ding, Y. & Campbell, R. E. A fluorogenic red fluorescent protein heterodimer. Chem. Biol. 19, 353–360 (2012).
Garner, T. P. et al. Small molecule allosteric inhibitors of BAX. Nat. Chem. Biol. 15, 322–330 (2019).
Cosker, K. E., Pazyra-Murphy, M. F., Fenstermacher, S. J. & Segal, R. A. Target-derived neurotrophins coordinate transcription and transport of Bclw to prevent axonal degeneration. J. Neurosci. 33, 5195–5207 (2013).
Reyna, D. E. et al. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 32, 490–505.e10 (2017).
Donahue, R. J., Maes, M. E., Grosser, J. A. & Nickells, R. W. BAX-depleted retinal ganglion cells survive and become quiescent following optic nerve damage. Mol. Neurobiol. 57, 1070–1084 (2020).
Goldenberg-Cohen, N., Dratviman-Storobinsky, O., El, S. D. B., Cheporko, Y. & Hochhauser, E. Protective effect of bax ablation against cell loss in the retinal ganglion layer induced by optic nerve crush in transgenic mice. J. Neuro-ophthalmol. 31, 331–338 (2011).
Kedra, J., Lin, S., Pacheco, A., Gallo, G. & Smith, G. M. Axotomy induces Drp1-dependent fragmentation of axonal mitochondria. Front Mol. Neurosci. 14, 668670 (2021).
Cavanagh, J. B. The significance of the “dying back” process in experimental and human neurological disease. Int Rev. Exp. Pathol. 3, 219–267 (1964).
Yaron, A. & Schuldiner, O. Common and divergent mechanisms in developmental neuronal remodeling and dying back neurodegeneration. Curr. Biol. 26, R628–R639 (2016).
Coleman, M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898 (2005).
Coleman, M. P. Axon biology in ALS: Mechanisms of axon degeneration and prospects for therapy. Neurotherapeutics 19, 1133–1144 (2022).
Yang, J. et al. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell 160, 161–176 (2015).
Franco, A., Dang, X., Zhang, L., Molinoff, P. B. & Dorn, G. W. Mitochondrial dysfunction and pharmacodynamics of mitofusin activation in murine Charcot-Marie-Tooth disease type 2A. J. Pharm. Exp. Ther. 383, 137–148 (2022). JPET-AR-2022-001332.
Guo, X. et al. Inhibition of mitochondrial fragmentation diminishes Huntington’s disease–associated neurodegeneration. J. Clin. Invest 123, 5371–5388 (2013).
Joshi, A. U. et al. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO Mol. Med. 10, e8166 (2018).
Chen, Z. et al. Inhibiting mitochondrial fission rescues degeneration in hereditary spastic paraplegia neurons. Brain 145, 4016–4031 (2022).
Flippo, K. H. et al. Deletion of a neuronal Drp1 activator protects against cerebral ischemia. J. Neurosci. 40, 3119–3129 (2020).
Patel, S. et al. Discovery of dual leucine zipper kinase (DLK, MAP3K12) inhibitors with activity in neurodegeneration models. J. Med. Chem. 58, 401–418 (2015).
Katz, J. S. et al. A Phase 1 study of GDC‐0134, a dual leucine zipper kinase inhibitor, in ALS. Ann. Clin. Transl. Neur 9, 50–66 (2022).
Chen, L. et al. Mitochondria and caspases tune nmnat-mediated stabilization to promote axon regeneration. Plos Genet 12, e1006503 (2016).
Kiryu-Seo, S. & Kiyama, H. Mitochondrial behavior during axon regeneration/degeneration in vivo. Neurosci. Res. 139, 42–47 (2019).
Jahani-Asl, A. et al. CDK5 phosphorylates DRP1 and drives mitochondrial defects in NMDA-induced neuronal death. Hum. Mol. Genet 24, 4573–4583 (2015).
Rong, R. et al. Cdk5-mediated Drp1 phosphorylation drives mitochondrial defects and neuronal apoptosis in radiation-induced optic neuropathy. Cell Death Dis. 11, 720 (2020).
Chen, S. et al. TBK1-mediated DRP1 targeting confers nucleic acid sensing to reprogram mitochondrial dynamics and physiology. Mol. Cell 80, 810–827.e7 (2020).
Gan, X. et al. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Biochimica Et. Biophysica Acta Bba - Mol. Basis Dis. 1842, 220–231 (2014).
Yu, T., Jhun, B. S. & Yoon, Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid. Redox Sign 14, 425–437 (2011).
Han, X.-J. et al. CaM kinase Iα–induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol. 182, 573–585 (2008).
Yang, D. et al. CaMK II -induced Drp1 phosphorylation contributes to blue light-induced AIF-mediated necroptosis in retinal R28 cells. Biochem Biophys Res Co. 559, 113–120 (2021).
Simula, L. et al. JNK1 and ERK1/2 modulate lymphocyte homeostasis via BIM and DRP1 upon AICD induction. Cell Death Differ. 27, 2749–2767 (2020).
Tortosa, E. et al. Stress‐induced vesicular assemblies of dual leucine zipper kinase are signaling hubs involved in kinase activation and neurodegeneration. EMBO J. 41, e110155 (2022).
Summers, D. W., Frey, E., Walker, L. J., Milbrandt, J. & DiAntonio, A. DLK activation synergizes with mitochondrial dysfunction to downregulate axon survival factors and promote SARM1-dependent axon degeneration. Mol. Neurobiol. 57, 1146–1158 (2019).
Otera, H., Ishihara, N. & Mihara, K. New insights into the function and regulation of mitochondrial fission. Biochimica Et. Biophys. Acta Bba - Mol. Cell Res. 1833, 1256–1268 (2013).
Acknowledgements
This work was supported by NIH intramural funding NICHD ZIA-HD008966 (C.E.L.P.), by a Milton-Safenowitz postdoctoral fellowship from the ALS Association (J.G.-.D.), by NIH grant R01NS112691 (T.A.W.) and grant #021-107 from Mission Connect, a project of the TIRR Foundation (TAW), and NIH intramural funding NINDS 1ZIANS003155-03 (MEW) and NEI ZIAEY000488 (W.L.). We would like to thank Drs. Vincent Schram and Chip Dye and the NICHD Microscopy core, Dr. Yan Li and the NINDS proteomics core, the NHLBI FACS core facility staff, Dr. Gareth Thomas (Temple University), Dr. Ana J. Garcia-Saez (Max Plank Institute of Biophysics) and Dr. Richard Cho (Cell Signaling Technologies) for reagents. We would also like to thank members of Dr. Michael Ward’s lab for technical and experimental advice, and Dr. Nicholas Ryba, Dr. Richard Youle, Dr. David Simon, Dr. Alexander Chesler, and Dr. Joseph Lewcock for helpful discussion and comments on the manuscript.
Funding
Open access funding provided by the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.G.-D. and C.E.L.P. Methodology and Investigation: all authors. Experiments using i3Neurons: M.N., A.L.S., J.G.-D. Optic nerve crush experiments: M.R.A., P.S. and F.N.N. Formal Analysis: M.N., F.N.N. and J.G.-D. Visualization: J.G.-D. and C.E.L.P. Writing: J.G.-D. and C.E.L.P. Supervision: C.E.L.P., T.A.W., W.L., M.E.W. Funding acquisition: C.E.L.P., T.A.W., W.L., M.E.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gómez-Deza, J., Nebiyou, M., Alkaslasi, M.R. et al. DLK-dependent axonal mitochondrial fission drives degeneration after axotomy. Nat Commun 15, 10806 (2024). https://doi.org/10.1038/s41467-024-54982-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54982-9
This article is cited by
-
Synaptic control of retinal ganglion cell survival and axon regeneration
Molecular Neurodegeneration (2026)