Abstract
Photoacoustic imaging is a promising non-invasive functional imaging modality for fundamental research and clinical diagnosis. However, achieving capillary-level resolution, wide field-of-view, and high frame rates remains challenging. To address this, we propose a transparent ultrasonic transducer design using our developed transparent Pb(In1/2Nb1/2)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3 crystals. Our fabrication technique incorporates quartz-glass-and-epoxy matching layers with low-resistance indium-tin-oxide electrodes through a brass-ring based structure, enabling a high frequency (28.5 MHz), wide bandwidth (78%), and enhanced pulse-echo sensitivity (2.5 V under 2-μJ pulse excitation). Our Pb(In1/2Nb1/2)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3-based transparent ultrasonic transducer demonstrates a four-fold enhancement in photoacoustic detection sensitivity when compared to the LiNbO3-based counterpart, leading to a 13 dB improvement of signal-to-noise ratio in microvascular photoacoustic imaging. This enables dynamic monitoring of mouse cerebral cortex microvasculature during seizures at 0.8 Hz frame rates over a 1.5 × 1.5 mm2 field-of-view. Our work paves the way for high-performance and compact photoacoustic imaging systems using advanced piezoelectric materials.
Similar content being viewed by others
Introduction
Photoacoustic imaging (PAI) combines the advantages of rich contrast from optical imaging and deep penetration capability from ultrasonic imaging1,2,3,4. In PAI, nanosecond laser pulses excite ultrasonic waves (known as photoacoustic signals) from light-absorbing chromophores in biological tissues, providing valuable anatomical and functional information for various research fields such as vasculature5,6,7,8, dermatology9,10, oncology11,12,13 and brain science14,15,16,17. Traditional photoacoustic microscopy systems can provide capillary-level resolution and exceptional signal-to-noise (SNR) ratio by using highly focused laser and high-frequency focused ultrasonic transducers5,6,16,17. However, the effective imaging field-of-view (FOV) is limited to the acoustically focal region, typically just several tens of microns for a 30 MHz transducer18. Consequently, the imaging system has to rely on a mechanical scanner, which is generally bulky, to move either the transducer or the imaging objects to cover a millimeter-range area. Thus, the imaging speed by this manner is always constrained by the mechanical scanning speed (~ 10 mm s−1 for state-of-the-art linear motor), which inherently hampers the improvement of imaging frame rate for dynamic in-vivo research.
Transparent ultrasonic transducers (TUTs), first proposed by S.-R. Kothapalli presents new opportunities to address the aforementioned challenges since they allow the passage of optical waves without compromising ultrasonic functionality19. Notably, the seamless integration of optical illumination and ultrasonic detection can be readily achieved by using TUTs, eliminating the need for complicated and bulky acoustic-optic coupling combiners and leading to a more compact imaging system suitable for wearable applications20,21,22,23. Even more appealingly, the TUT provides an expansive FOV that allows two-dimensional laser scanning from the top of the sensing element with on-axis alignment, overcoming any geometrical limitation imposed by the opacity of traditional transducers24,25. The elimination of the need for mechanical scanning greatly improves imaging speed, limited primarily by the laser repetition rate24. In addition, the TUT also facilitates multimodal fusion imaging due to its excellent compatibility with various optical microscopic imaging modalities, such as confocal, two-photon, and optical coherence tomography imaging23,26,27.
Although the functionalities of TUT have been demonstrated by many researchers, the relatively low performances of existing TUTs greatly impede their practical applications. For example, current TUTs exhibit a -6 dB fractional bandwidth only in the range of 10–60%, in stark contrast to traditional ultrasonic transducers, which offer more than 80% bandwidth26,27,28,29,30,31,32,33,34,35,36,37,38,39,40. This leads to extended pulse duration of the echo signal, resulting in a degraded axial resolution41,42. Moreover, due to the low sensitivity of existing TUTs, the imaging SNR is far from the expectation for high-contrast photoacoustic imaging24. The trade-off between imaging speed and SNR is widely recognized, necessitating a significant breakthrough in transducer performance, particularly in terms of transducer sensitivity (Supplementary Note 1). The underwhelming performances of existing TUTs can be attributed to the following two factors. First, the dielectric and electromechanical properties of state-of-the-art transparent piezoelectric materials, such as polyvinylidine fluoride (PVDF) polymers and LiNbO3 (LN) crystals, are significantly inferior to those of traditional transducer materials like Pb(Zr,Ti)O3 (PZT) piezoelectric ceramics, imposing severe limitations on the design of TUTs32. Second, the conventional fabrication strategy for high-frequency transducers is not compatible with the requirement of transparency, and the generally accepted acoustic impedance matching strategy for high-frequency transducers is not compatible with the requirement of transparency. For example, the silver-loaded epoxy is commonly used as an inner matching layer for high-frequency piezoelectric-crystal/ceramics-based transducers due to its moderate acoustic impedance (5-7 MRayl) and commendable electrical conductivity (< 1 Ω mm−1). However, its opacity prevents its application in the fabrication of TUTs43,44,45. Although extensive efforts have been made to develop various transparent matching layers for TUTs, there’s still a gap between TUTs and conventional high-frequency transducers. For example, Chen, H. et al. utilized quarter-wavelength glass and Parylene coating as matching layers for the TUT, resulting in a dual-frequency feature in the Fourier spectrum that exhibited a narrow bandwidth at its original resonant frequency35, while Zhang, J. et al. employed polymethyl methacrylate (PMMA) as the matching layer material, improving the bandwidth of the TUT to above 50%38. Recent studies have also employed silicon dioxide-epoxy composites to improve the bandwidth of TUTs. The acoustic impedance and transparency of matching and backing materials can be adjusted by doping silicon dioxide particles with different sizes and fraction ratios into epoxy. However, the introduction of silicon dioxide particles inevitably causes light scattering due to the difference in refractive index between silicon dioxide and epoxy, which reduces the lateral resolution of the photoacoustic microscopy system39,40.
Recently, Qiu et al. reported that a high piezoelectric coefficient (d33) of 2200 pC N−1, large dielectric constant ε33/ε0 of 8000, and high optical transparency can be simultaneously achieved in Pb(Mg1/3Nb2/3)O3-PbTiO3 (PMN-PT) relaxor ferroelectric crystals via ferroelectric domain engineering46. In this work, we realized high optical transparency in the ternary Pb(In1/2Nb1/2)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3 (PIN-PMN-PT) relaxor ferroelectric crystals by eliminating light-scattering ferroelectric domain walls. Compared with PMN-PT crystals, the PIN-PMN-PT crystals exhibit not only comparable dielectric and piezoelectric properties but also higher rhombohedral-tetragonal phase transition temperature (120 oC vs. 90 oC), being more suitable for transducer fabrication and practical applications47,48,49,50. By using the transparent PIN-PMN-PT crystals, we developed a high-performance TUT with a smaller aperture size (2 mm × 2 mm) to enhance the PA detection sensitivity. We employed a quartz glass and epoxy matching design, low-resistance (< 10 Ω square−1) ITO electrodes, and a brass-ring-based structure to simultaneously achieve high operating frequency (~ 30 MHz), wide bandwidth (~ 80%), and excellent pulse-echo sensitivity (> 1.2 V under 2 μJ electric pulse excitation) in our TUTs. Compared to the LN-based TUT, the PIN-PMN-PT-based TUT demonstrates a significantly improved SNR (13 dB) in microvascular photoacoustic imaging. This enhancement allows for the acquisition of capillary-level photoacoustic images with a single high-speed laser scan. The potential of this technology for hemodynamics studies was further demonstrated by monitoring dynamic changes in the microvasculature of the cerebral cortex in a mouse during a drug-induced epileptic seizure.
Results
The advantages of using PIN-PMN-PT for TUTs
Figure 1a shows the basic structure and working principle of a TUT used for PAI. The TUT mainly includes a transparent piezoelectric material, electrodes, matching layers, and backing layers, in which the piezoelectric material is essential for achieving high detection sensitivity43,51. Generally, the acoustic impedance of piezoelectric materials (e.g., piezoelectric ceramics, ~ 35 MRayl) mismatches that of biological tissues (~ 1.5 MRayl). Thus, the matching layers are required in the design of transducers to achieve high acoustic energy transmission efficiency. The backing layer is applied at the back of the piezoelectric material to damp out the ringing effect and provide reliable mechanical support43. For this design, the laser beam can be illuminated on the biological tissues directly through the transparent sensing elements. As shown in Fig. 1a, b, the tissue will generate spherical acoustic waves (i.e., the photoacoustic signal) under the laser excitation, and the piezoelectric material converts the acoustic signals into electrical signals, capturing essential anatomical information and functional information for imaging purpose3.
a Schematic diagram of the TUT and the working principle of the TUT-based PAI system. The TUT acts as a detector to capture the PA signals from the biological tissues excited by the pulsed laser. b Finite element simulation of the reception of a spherical wave by a planar transducer. As the wave font arrives at the detector surface, the electric signal is generated by the direct piezoelectric effect from the piezo-crystal. c Simulated peak-to-peak amplitude of the received voltage signal as a function of the aperture size (l) of the detector and the l/d ratio (the distance between the acoustic source and the detector), which was calculated with a constant d of 1.0 mm. d, e Received signals from the transducers with a diameter of 0.4 mm and 2.0 mm, respectively, by finite element simulations. The insets in (e) show the locally enlarged waveforms indicated by the red circles. The received signal was captured from the open-circuit voltage on the surfaces of the piezoelectric material.
We performed finite element analysis (FEA) to study the photoacoustic (PA) detection sensitivity for the planar piezoelectric ultrasonic transducer, as schematically shown in Fig. 1b. The PA signal was simulated by a spherical wave emitting from a point acoustic source at 1 mm away from the surface of the transducer. The point source is excited by a Gaussian pulse with a -6 dB bandwidth of 80% and center frequencies at 15 MHz, 30 MHz, and 45 MHz, respectively. The received signal was obtained from the open-circuit voltage on the piezoelectric material. More details about the simulation are given in the Method section. Figure 1c shows the received voltage with respect to the size of the piezoelectric material, as well as the ratio of aperture size to detection distance (l/d). A larger received voltage indicates a higher PA detection sensitivity from the corresponding aperture size. Due to the phase cancellation effect, the PA detection sensitivity suffers a significant degradation as the l/d ratio increases. With the increase in frequency, the cancellation effect becomes more pronounced and thus the PA detection sensitivity decreases. To clearly show this effect, Fig. 1d, e gives the received voltage signals from a l/d of 0.4 (aperture: 0.4 mm) and 2.0 (aperture: 2.0 mm), respectively. At the aperture of 0.4 mm, only one received voltage signal can be observed after the point acoustic source is excited by a 30 MHz Gaussian pulse (Fig. 1d and Supplementary Fig. 1). At the aperture of 2 mm, two received voltage signals can be distinguished at the time t1 and t2, as indicated by the red dashed circles (Fig. 1e). The received voltage signals between t1 and t2 cancel each other as a result of the phase mismatching, leading to a significantly weakened received voltage at t1 (Supplementary Fig. 2 and Supplementary Fig. 3). Therefore, a smaller aperture size of the planar detector can greatly benefit the detection sensitivity of PA signals.
However, for a specific piezoelectric material, there is minimal flexibility for adjusting its dimension in a transducer design, as explained below. The thickness of the piezoelectric material is determined by the operating frequency of the transducer. Once the thickness is fixed, the aperture is set to achieve electrical impedance matching (typically 50 Ω at the terminal). For example, to design a TUT made of LiNbO3 with an operating frequency of 30 MHz, the thickness of the LiNbO3 crystal should be around 130 μm, according to the equation vl = 2fa × t (vl is the longitude acoustic velocity of the piezoelectric material, t is the thickness, and fa is the corresponding anti-resonance frequency). Then, the aperture, which is determined by the Krimholtz, Leedom, and Matthae (KLM) equivalent circuit model with the consideration of electrical matching (50 Ω), is estimated to be ~ 6 mm in diameter for LiNbO352,53. Obviously, the ratio l/d (~ 6 at a detection distance of 1 mm) is too large to achieve the desired PA sensitivity in LiNbO3-based TUT. The larger diameter is due to the lower dielectric constant of the LiNbO3 crystal (ε33/ε0: ~ 65).
To address this challenge, we opted for the PIN-PMN-PT relaxor ferroelectric crystal as the piezoelectric material, given its notably higher dielectric constant (~ 7400) compared to LiNbO3. However, the [001]-oriented PIN-PMN-PT crystal typically exhibits opacity due to the dense distribution of light-scattering ferroelectric domain walls. Rhombohedral PIN-PMN-PT crystals possess two types of domain walls: 71o domain walls and 109o domain walls. Among them, the 71° domain walls are mainly responsible for the scattering when the light is incident from (001) surfaces, which can be attributed to the following reasons. First, the 71° domain walls contain four orientations: (\(011\)), (\(0\bar{1}1\)), (\(01\bar{1}\)), and (\(0\bar{1}\bar{1}\)), all positioned at 45° to (001) surfaces. Second, these domain walls are comparable in size to the wavelength of visible light and are randomly distributed inside the crystal (Fig. 2a). To eliminate these light-scattering domain walls, we employed a previously developed poling methodology, involving the application of an a.c. electric field in contrast to conventional d.c. electric field poling method46,47,48. During a.c. poling, the reversal of the electric field polarity can efficiently induce rotation of 71o domain walls, creating opportunities for the domains between 71° domain walls to coalesce. After 5~20 cycles of a.c. poling, almost all the 71° domain walls in PIN-PMN-PT crystals are eliminated, leading to a laminar domain structure as depicted in Fig. 2a. Therefore, significantly improved transparency can be achieved in [001]-oriented rhombohedral PIN-PMN-PT crystals. As shown in Fig. 2b, the PIN-PMN-PT crystal becomes transparent after a.c. poling. The patterns on the background are clearly viewed without any distortion through both a.c.-poled PIN-PMN-PT and LN crystals.
a Illustration of the transparency enhancement process via a.c. poling. The domain structures were characterized by scanning electron microscopy (SEM, upper figure) from the (100) surface and birefringence imaging microscopy (BIM, below the SEM figure) from the (001) surface. The 71o domain wall (DW) and 109o DW are indicated by the orange and red arrows in SEM figures, respectively. The colors in BIM images reveal the orientation angles (φ) of the projection of the principal optical axis of the ferroelectric domains on the (001) plane. Specifically, the blue and red colors represent the projection of the principal optical axis along the diagonal faces on the (001) plane, that is, φ of 45° and 135°, respectively. b Photo of a.c.-poled PIN-PMN-PT, LN, and traditional d.c.-poled PIN-PMN-PT crystals. c Piezoelectric coefficients (d33) of PIN-PMN-PT crystals poled by various amplitudes of a.c. electric field. d d33 with respect to cycle number of a.c. electric field. e d33 with respect to frequency of a.c. electric field. f Comparison of piezoelectric and dielectric properties of a.c.-poled PIN-PMN-PT and LN crystals. g Simulated electric impedance (at 34 MHz) of the PIN-PMN-PT- and LN-based transducers as a function of the aperture size. h Simulated peak-to-peak amplitude of pulse-echo response for the PIN-PMN-PT- and LN-based transducers with different aperture sizes. The pulse-echo amplitudes were normalized by the maximum amplitude of the PIN-PMN-PT-based transducer.
To determine the optimal a.c. poling conditions, we investigated the effects of cycle number, peak-to-peak amplitude, and frequency of the a.c. electric field on the piezoelectric coefficient (d33) of PIN-PMN-PT samples. The results show that the d33 value reaches its maximum, with minimal fluctuation across the test samples, at an electric field amplitude of 8 kV cm−1 (Fig. 2c). When the amplitude is further increased, the d33 value decreased, with greater fluctuations, which may be attributed to a field-induced phase transition. Regarding the cycle number, the d33 value significantly increases with considerable fluctuations during the first 5 cycles, after which it becomes relatively stable (Fig. 2d). For the frequency, a range of 5–10 Hz appears to yield higher d33, however, the fluctuations are also substantial (Fig. 2e). Typically, a higher d33 indicates fewer light-scattering 71o domain walls54, but performance fluctuations between samples should also be considered for robustness. Consequently, we selected 8 kV cm−1, 1 Hz, and 10 cycles as the optimal poling conditions to achieve higher d33 with minimal fluctuations.
Compared to LN, the ε33/ε0 and d33 of a.c.-poled PIN-PMN-PT are almost two orders higher (Fig. 2f), which will greatly benefit the miniaturization and PA detection sensitivity of TUTs. The variation of d33 over time for a.c.-poled PIN-PMN-PT crystals follow a logarithmic aging trend, which is typically for ferroelectrics55 (Supplementary Fig. 4). The detailed material properties are summarized in Supplementary Table 1. The crystal is oriented along the [001] direction in thickness, as evidenced by the XRD pattern (Supplementary Fig. 5). The rhombohedral-tetragonal phase transition temperature (TRT) is determined to be 120 °C according to the temperature-dependent dielectric constant measurements (Supplementary Fig. 6).
To assess the stability of the transparent PIN-PMN-PT crystals, we examined their domain structure and transparency using polarized light microscopy (PLM) and optical imaging at different temperatures during heating and cooling below the phase transition temperature (Supplementary Fig. 7). Neither domain configuration nor transparency shows discernible changes up to 100 °C, indicating the robustness of these crystals for transducer fabrication.
The design and fabrication of high-performance TUTs
We performed a comprehensive study to determine the structural parameters for the PIN-PMN-PT-based TUT. The center frequency (fc) of TUT was designed at 30 MHz to match the bandwidth of PA signals56. To determine the aperture size of TUTs, we initially calculated the electrical impedance at the designed frequency with different aperture sizes based on the KLM model. As shown in Fig. 2g, the electrical impedance increases dramatically as the aperture size decreases. For the LN-based TUT, the electrical impedance reaches 50 Ω at a diameter of 6 mm and progressively increases to ~ 480 Ω as the diameter decreases to 1 mm. In contrast, for the PIN-PMN-PT-based TUT, the electrical impedance approaches 50 Ω at a diameter of 1 mm, primarily due to the high dielectric constant of the PIN-PMN-PT crystal. Figure 2h shows the peak-to-peak amplitude of the pulse-echo response as a function of aperture size. The amplitude of the pulse-echo response reaches its maximum when the electrical impedance matches 50 Ω. It should be noted that the higher peak value of the PIN-PMN-PT-based TUT is attributed to the superior electromechanical coupling factor (kt) of the PIN-PMN-PT crystal (LN: kt = 47%, PIN-PMN-PT: kt = 60%). Considering that a small aperture size may limit the FOV of TUT during optical scanning, the aperture size of the PIN-PMN-PT-based TUT was designed to be 2 mm × 2 mm, striking a balance between the sensitivity and FOV. For the LN-based TUT, on the other hand, an aperture size with a diameter of 6 mm was chosen to achieve optimum electrical matching. The square shape of the PIN-PMN-PT crystal is for ease of cutting and has negligible effects on the beam pattern (Supplementary Fig. 8).
To optimize the bandwidth and sensitivity of TUT, we employed a transparent-double-matching-layer strategy. Supplementary Table 2 summarizes transparent materials that can be used as matching and backing layers for TUTs. After careful consideration of the acoustic impedance and processing compatibility for high-frequency design, we selected quartz glass with an acoustic impedance of ~ 12 MRayl as the first matching layer and transparent epoxy with an acoustic impedance of ~ 3 MRayl as the second matching layer. The selection of epoxy is mainly attributed to its physical properties, allowing it to be poured directly onto the transducer in its liquid state at room temperature. This fabrication process provides three key advantages: (1) precise control over the epoxy thickness within an error margin of 0.5 μm through lapping and polishing; (2) elimination of the need for any additional bonding layer; and (3) mitigation of the risk of crystal depolarization.
The pulse-echo response and the corresponding frequency spectrum of TUT with different schemes of acoustic matching layers were simulated and optimized based on the KLM model (Supplementary Fig. 9 and Supplementary Note 2). The transparent-double-matching-layer design results in a remarkable improvement in the -6 dB bandwidth, expanding it from 18% to 77%. The larger bandwidth can lead to a higher pulse-echo amplitude, consequently improving the transducer sensitivity. The optimized structural parameters for TUTs are given in Supplementary Table 3.
However, implementing a transparent double-matching layer in a high-frequency TUT presents significant challenges. Wiring the small-sized piezoelectric crystal while tightly attaching the rigid matching layer to the crystal proves to be a tough task. This is unlike conventional opaque ultrasonic transducers, which use an electrically conductive matching layer to allow for wiring from the outside. Therefore, to address this issue, we developed an innovative transducer fabrication process, as schematically shown in Fig. 3a. The fabrication process starts with securing the crystal plate into a brass ring using transparent epoxy, followed by precise optical lapping and polishing to achieve the designed thickness. After sputtering the ITO electrode on the crystal surface, we attached the matching layers (i.e., the ITO-coated quartz glass and the epoxy) onto the piezoelectric crystal and the brass ring. Finally, we inverted the transducer stack, connected the coaxial cable, and encapsulated it inside a brass tube. Further details on transducer fabrication are given in the Method section. Figure 3b shows the exploded view of the multilayer structure of the as-developed TUT. This innovative approach allows the negative electrode of TUT to be drawn from the piezoelectric material to the brass ring via the low-resistance ITO (sheet resistance: ~ 10 Ω square−1) on the quartz glass, which also serves as the first matching layer. By doing so, we addressed the technical difficulty in achieving electrical connection due to the non-conductive and rigid nature of the matching layer. The low-resistance ITO further improved the sensitivity of TUT (Supplementary Note 3). In addition, the brass-ring-based structure enables a larger effective optical aperture for focused laser without changing the size of the piezoelectric crystal, distinguishing it from the conventional TUTs (Supplementary Fig. 10).
a Schematic diagram of the key steps for assembling a TUT. (MLs: Matching layers). All the procedures were executed with the stack structure fixed onto a flat glass substrate to avoid cracking and ensure the precision of machining and assembling. b Explosion view of the TUT. c Cross section of a PIN-PMN-PT-based transducer stack. d Photograph of a PIN-PMN-PT-based TUT before encapsulating with the epoxy. The signal wire of the coaxial cable is connected to the upper ITO electrode of the PIN-PMN-PT crystal. e Photograph of the PIN-PMN-PT- and LN-based TUTs. f Frequency-dependent electrical impedance of the PIN-PMN-PT-based TUT. g Ultrasonic impulse and the corresponding frequency spectrum of the PIN-PMN-PT-based TUT. h Comparison of − 6 dB fractional bandwidth relative to center frequency for current TUTs. i, j PA impulse and the corresponding frequency spectrum of the PIN-PMN-PT- and LN-based TUTs, respectively. k PA sensitivity of representative TUTs measured in similar photoacoustic microscopic imaging systems35. The PA sensitivity is determined by the received voltage of the PA signal under the same condition, including the pulsed energy of the excitation laser and the gain of the ultrasonic amplifier (see Supplementary Note 4 for details on the calculation method).
Figure 3c shows the cross-section of the transducer stack, which is composed of two matching layers and a piezoelectric layer. Figure 3d shows a pre-encapsulation view of the PIN-PMN-PT-based TUT. The 65 μm-thick PIN-PMN-PT crystal plate was securely affixed in the middle of the brass ring, and connected to the coaxial cable by the conductive adhesive. The developed PIN-PMN-PT- and LN-based TUTs are compared in Fig. 3e, where the grid lines can be clearly seen through both TUTs, indicating the TUTs possess good light transmittance. The optical transmittance of both PIN-PMN-PT- and LN-based TUTs at 532 nm is ~ 70% (Supplementary Fig. 11). It should be noted that the nearly 30% transmittance loss is mainly attributed to the Fresnel reflection at the interfaces between the materials with different refractive indices (e.g., ~ 1.00 for air vs ~ 1.52 for epoxy).
The performance of TUTs
Figure 3f shows the measured electrical impedance spectrum of the PIN-PMN-PT-based TUT. Because of the double matching layers, the electrical impedance spectrum exhibits three characteristic peaks that cover a wide range of frequencies from 20 MHz to 45 MHz. The electrical impedance is 33 Ω at 30 MHz which is lower than 50 Ω. It should be noted that the optimal electrical impedance of the PIN-PMN-PT-based TUT is sacrificed for achieving a relatively large FOV. The ultrasonic impulse and the corresponding frequency spectrum of the PIN-PMN-PT-based TUT are displayed in Fig. 3g. The peak-to-peak amplitude of the unamplified echo signal is 1.2 V under 2-μJ pulse excitation. The center frequency and -6 dB relative bandwidth of the PIN-PMN-PT-based TUT are 31 MHz and 76%, respectively. The measured results are largely consistent with the simulations (Supplementary Fig. 12). The electrical impedance spectrum and the ultrasonic impulse of LN-based TUT are given in Supplementary Fig. 13. As observed, the echo amplitude of the LN-based TUT is 2.5 V under 2-μJ pulse excitation, which is at least 3 times higher than the-state-of-the-art LN-based TUT (Supplementary Fig. 14)40. In addition, both PIN-PMN-PT- and LN-based TUTs exhibit a substantial -6 dB bandwidth of nearly 80%. Figure 3h compares the -6 dB fractional bandwidth with respect to the center frequency for existing TUTs. Most previously reported TUTs have center frequencies ranging from 5 to 20 MHz, significantly lower than that of our newly developed TUTs, which reach up to 30 MHz. The -6 dB bandwidth of our newly developed TUTs is even 24% higher than that of the cutting-edge TUT using novel transparent matching and backing materials40. These superior performances indicate the advancement of our design and fabrication strategy for TUTs.
We characterized the receiving voltage sensitivity of our TUTs using a calibrated hydrophone (Supplementary Table 4)57. The receiving voltage sensitivity of PIN-PMN-PT- and LN-based TUT measured at 30 MHz was 2643 mV MPa−1 and 4919 mV MPa−1, respectively, which is more than 10 times higher than that of the hydrophone. Based on the receiving voltage sensitivity and the noise level of the entire system (~ 1.4 mV for both LN- and PIN-PMN-PT-based TUTs), the equivalent noise pressure (NEP) was calculated to be 530 Pa for LN-based TUT and 285 Pa for PIN-PMN-PT-based TUT. We also evaluated the insertion loss of TUTs as a function of frequency (Supplementary Fig. 15). At 30 MHz, the insertion loss is − 17.6 dB for LN-based TUT, and − 23.3 dB for PIN-PMN-PT-based TUT. These results align with the pulse-echo measurements, where the LN-based TUT outperforms the PIN-PMN-PT-based TUT, primarily due to its electrical impedance being much closer to 50 Ω.
To evaluate the PA detection sensitivity of TUTs, we measured the PA signal generated from a totally light-absorbing black target under 70-nJ pulsed laser excitation, as schematically shown in Supplementary Fig. 16. Figure 3i, j show the received PA signals from the PIN-PMN-PT-based TUT and the corresponding frequency spectra. The peak-to-peak amplitude is 185 mV after being amplified by 39 dB, which is more than fourfold higher than that of the LN-based counterpart. The photoacoustic bandwidths of TUTs align well with their pulse-echo bandwidths. Figure 3k compares the PA sensitivity of several representative TUTs, which is determined by the received voltage amplitude under focused laser excitation with the same pulse energy. The newly developed PIN-PMN-PT-based TUT shows significantly improved PA sensitivity when compared to the other TUTs, indicating its great potential for photoacoustic microscopy applications.
To further demonstrate the advantage of PIN-PMN-PT, we fabricated TUTs based on both PIN-PMN-PT and LN, varying their sizes, and tested their ultrasonic and photoacoustic performances without any matching layer (Supplementary Fig. 18 and Supplementary Fig. 19). As the aperture size decreases from 4.5 mm to 2.0 mm, the LN-based TUT shows decreased pulse-echo sensitivity, whereas the PIN-PMN-PT-based TUT exhibits increased sensitivity (Fig. 4a), consistent with the simulation results (Fig. 2h). When the aperture size exceeds 3 mm, the LN-based transducer exhibits higher pulse-echo sensitivity, because its electrical impedance comes closer to 50 Ω, while that of PIN-PMN-PT falls significantly below 50 Ω. However, the PA sensitivity of both LN- and PIN-PMN-PT-based TUTs increases as the aperture size decreases, which is attributed to the mitigation of the phase-canceling effect. Notably, the increase is more pronounced for the PIN-PMN-PT-based TUT in contrast to the LN-based counterpart, as their pulse-echo sensitivity increases with decreasing aperture size as well. Furthermore, the PIN-PMN-PT-based TUT consistently demonstrates higher PA sensitivity than the LN-based TUT, even as the aperture size increases, which highlights the clear advantage of PIN-PMN-PT crystals for photoacoustic applications. In terms of bandwidth, the PIN-PMN-PT-based TUT consistently outperforms the LN-based TUT in both pulse-echo (Fig. 4c) and photoacoustic modes (Fig. 4d), which can be attributed to the higher kt of PIN-PMN-PT crystals.
Based on our observations and finite-element simulations (Supplementary Fig. 17), we can further elucidate the higher PA detection sensitivity of the PIN-PMN-PT-based TUT as follows. Our use of a planar transducer to detect the photoacoustic signal, which propagates as a spherical wave, means that the detection sensitivity is primarily governed by the aperture size of the transducer. As the transducer aperture increases, the PA detection sensitivity sharply decreases due to the phase cancellation effect. Therefore, in this situation, a smaller transducer aperture is preferable for achieving higher PA detection sensitivity. The PIN-PMN-PT crystals exhibit significant advantages over LN crystals for small-aperture transducers because their high dielectric properties can greatly benefit the electrical impedance matching.
In vivo photoacoustic imaging
We characterized the imaging resolutions of the PIN-PMN-PT-based TUT. In ultrasound mode, the axial resolution was measured to be 37 μm, which is in good agreement with the theoretical value of 32 μm. Due to the unfocused nature of the TUT, the lateral resolution increased from 0.76 mm to 0.90 mm as the depth increased from 24.4 mm to 30.4 mm (Supplementary Fig. 20). In photoacoustic mode, the axial resolution was measured at 34 μm, comparable to that in ultrasound mode. Owing to the use of a focused laser, the lateral resolution reached 7 μm, which is sufficient to distinguish fine capillaries (Supplementary Fig. 21).
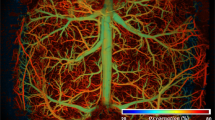
To demonstrate the advantages of the PIN-PMN-PT-based TUT for PAM imaging, we mapped the vasculature of a mouse ear in vivo, utilizing both PIN-PMN-PT and LN-based TUTs for comparison. A 532 nm pulsed laser was focused on the mouse ear through TUTs to excite the PA signals from the hemoglobin in red blood cells, thus enabling tracking and mapping of the vascular anatomy. Figure 5a shows the photoacoustic image of a 4 mm × 4 mm area captured by the PIN-PMN-PT-based TUT. The image was normalized logarithmically and displayed in a dynamic range of 40 dB. A densely packed capillary vessel network can be clearly imaged. The entangled concomitant venules and arterioles are observed and indicated by the white arrows58. The imaging depth is ~ 0.5 mm from the surface of the tissues. The maximum imaging depth is limited to ∼ 1 mm due to optical scattering in tissues42. Figure 5b shows the locally enlarged image of a bifurcation of arterial and venous trees in the area marked by a box in Fig. 5a. Figure 5c shows the PA intensity profiles of individual vessels in representative B-scans positioned by the white dash line in Fig. 5b. The diameter of the smallest vessel that can be distinguished from Fig. 5c is 13 μm, indicating a good lateral resolution of the PIN-PMN-PT-based TUT. To make a side-by-side comparison, the same area was imaged by the LN-based TUT, and the results are shown in Fig. 5d–f. Most of the PA signals from the complicated and detailed capillaries are overwhelmed by the background noise due to the poor sensitivity of the LN-based TUT (Fig. 5d). Clearly, only the major vessels with a diameter larger than 20 μm can be distinguished, as indicated by the red arrows in Fig. 5e, f. The small vessels marked by the blue arrows in Fig. 5b, c cannot be resolved by the LN-based TUT. This is also evidenced by the analysis of the vessel distribution. The total vessel density captured by the PIN-PMN-PT-based TUT is more than three times higher than that captured by the LN-based TUT (Supplementary Fig. 22). Meanwhile, the proportion of detected vessels with diameter below 20 μm is increased by 30%. Of particular significance is that the SNR of the PIN-PMN-PT-based TUT is calculated to be 18 dB from the in vivo imaging result, more than 13 dB higher than that of the LN-based TUT (Supplementary Fig. 23). We also noticed that the background noise levels from the photoacoustic images captured by LN and PIN-PMN-PT transducers are similar, suggesting that the improved SNR in PIN-PMN-PT-based TUT is mainly attributed to its higher PA sensitivity in contrast to LN-based TUT.
a Photoacoustic image of subcutaneous microvasculature networks in a mouse ear captured by the PIN-PMN-PT-based TUT. The white arrows indicate the bifurcations of venules and arterioles. b Locally enlarged image of the area positioned by the dashed line box in (a). c Normalized PA intensity with respect to lateral distance at the position marked by the white dash line in (b). The colored arrows in (b and c) indicate the vessels and corresponding PA intensity profiles, respectively. The vessel diameter was calculated by the full width at half maximum (FWHM) of the PA intensity profile. d Photoacoustic images captured by the LN-based TUT. e Locally enlarged image of the area positioned by the dashed line box in (d). f PA intensity at the position marked by the white dash line in (e). The red arrows highlight the vessels that are resolved by both PIN-PMN-PT- and LN-based TUTs, while the blue arrows highlight the vessels that cannot be clearly resolved by the LN-based TUT.
To demonstrate the potential of TUT for the fundamental research of brain science, we employed our newly developed PIN-PMN-PT-based TUT to visualize the dynamic change of the cerebral cortex microvasculature of a mouse with partial craniectomy, as schematically shown in Fig. 6a. Symptoms of epilepsy are produced by the intraperitoneal injection of Pentylenetetrazol (PTZ) solution59. Two-dimensional laser scanning was performed through the TUT. A laser repetition rate of 50 kHz provides a B-scan rate of 200 Hz with 250 A-lines, resulting in an acquisition time of 1.25 s for a 250 × 250-pixel image. Figure 6b and c show eight representative images illustrating the cortex vasculature at different time points. Prior to the injection, the vasculature was in a normal state and basically remained unchanged. Following the injection, the vessels exhibited dynamic contractions and expansions due to the drug-induced epilepsy. To quantitatively analyze the dynamic changes of the vessels, we calculated the vessel diameter at each frame, as shown in Fig. 6d. Within the initial 20 s, the vessel diameter was kept at ~ 30 μm. However, following the injection, the vessel diameter experienced abrupt reduction at intervals ranging from 5 to 15 s. In each spasm cycle, the vessel first contracted from 30 μm to 15 μm, then expanded to 50 μm before returning to its original size of 30 μm, where each cycle lasted about 3–5 s. These findings indicate that the PIN-PMN-PT-based TUT holds great potential as a powerful tool for hemodynamics studies.
a Schematic diagram of a mouse with a cranial window. The Pentylenetetrazol (PTZ) solution was injected into the mouse to induce an epileptic seizure. The laser was focused on the cerebral cortex through the TUT and performed a fast 2D optical scan. b, c Changes of the cortex vasculature over time at normal and epileptic states, respectively. d Vessel diameters with respect to the time before and after the injection. The vessel diameter was calculated at the position marked by the green line in (b). e, f Detailed views of the regions marked by the blue and orange boxes in (d), where the dots highlighted the time points corresponding to the images in (b).
Supplementary Table 5 provides a comprehensive comparison of the detailed parameters for the actively employed TUT-based PAI techniques. The PIN-PMN-PT-based TUT presented in this work has prominent advantages in terms of imaging speed. In comparison to the existing planar TUTs, the PIN-PMN-PT-based TUT offers the advantage of not requiring multiple data collection to achieve satisfactory SNR. This is due to the improvement in PA detection sensitivity, resulting in a substantial reduction in image acquisition time. When compared to the focused TUTs, on the other hand, the PIN-PMN-PT-based TUT provides a relatively larger FOV for 2D optical scanning, where its imaging speed is primarily limited by the laser repetition frequency. In this study, the image acquisition time for a scan range of 1.5 × 1.5 mm2 with 250 × 250 pixels has witnessed a drastic reduction to 1.25 s, representing a remarkable improvement of over 30 times in speed compared to traditional mechanical-scanning-based techniques with limited scanning speed of 10 mm/s, while also demonstrating higher stability60,61. Therefore, our newly designed TUT provides a critical platform to achieve ultrafast high-resolution PAI for fundamental brain research.
To demonstrate the robustness of the PIN-PMN-PT-based TUT for PA applications, we compared its optical transmittance before and after the PA experiments, revealing no noticeable changes (Supplementary Fig. 24a). Additionally, the a.c.-poled PIN-PMN-PT crystals remained consistent over time and exhibited homogeneity across the transducer surface (Supplementary Fig. 24b). We also checked the capacitance of the PIN-PMN-PT-based TUT, observing only a 2% degradation (before: ~ 1.20 ± 0.20 nF; after: ~ 1.18 ± 0.30 nF). These results indicate that the pulsed laser irradiation does not induce significant degradation in transparency or dielectric properties in these a.c.-poled PIN-PMN-PT crystals.
Discussion
The TUT has shown great potential to get rid of mechanical scanning by using optical scanning directly through the sensing element. However, the inferior sensitivity and bandwidth of existing TUTs have greatly limited their further applications. Our finite element simulations and experiments revealed that the PA sensitivity of a planar TUT is primarily influenced by phase cancellation, which depends on the ratio of the aperture size to detection distance. At a fixed distance, a planar transducer with a smaller aperture size achieves higher sensitivity to spherical PA signals, as confirmed by our experiments (Fig. 3b). This contrasts with conventional photoacoustic microscopy, where a larger aperture in focused transducers provides enhanced focusing and, consequently, higher PA sensitivity42,51. Furthermore, the electrical impedance of the TUT impacts sensitivity, with proper impedance matching improving both pulse-echo and PA sensitivity.
To develop a small-aperture TUT with improved sensitivity, we proposed using our newly developed transparent PIN-PMN-PT crystals, which exhibit significantly higher dielectric properties compared to commonly used LN crystals and offer better temperature stability compared to previously reported transparent PMN-PT crystals. This allows for a decreased aperture size of the TUT while maintaining satisfactory electrical impedance for optimal PA detection sensitivity. We have successfully demonstrated a 2 mm × 2 mm PIN-PMN-PT-based TUT, showcasing a 13-dB improvement in SNR and more than three times the number of resolved capillaries in PAM imaging when compared to a 6 mm diameter LN-based TUT with similar bandwidth and center frequency. In addition, our fabrication strategy, which employs an ITO-coated matching layer and the brass-ring-based structure, effectively addresses the challenge associated with wiring from small-sized piezoelectric materials while maximizing the aperture for light transmission. This strategy also enables optimal pulse-echo sensitivity, broad bandwidth (~ 80%), and high operating frequency (~ 30 MHz) in both LN- and PIN-PMN-PT-based TUTs, which further enhance the detection efficiency for high-frequency wideband PA signals. Compared with other reported TUTs, the pulse-echo sensitivity of our TUTs can reach up to 2.5 V under 2 μJ pulse excitation, which is at least 3 folds higher than that of the current cutting-edge TUTs (~ 1.1 V under 3 μJ electric pulse excitation)40. This advancement can be attributed to the following reasons: (1) The matching and backing materials we selected have minimal ultrasound attenuation compared with the silica-powder-doped epoxy. (2) The thickness of bonding layers was well controlled with a thickness of less than 1 μm. (3) The ITO electrodes we employed have a low sheet resistance of ~ 10 Ω square−1.
Building on these advantages, we have eliminated the need for time averaging during PAM imaging, significantly reducing the image acquisition time. We have demonstrated the ability of our PIN-PMN-PT-based TUT to capture the dynamic changes in the mouse cortex microvascular structure during an epileptic seizure with a frame rate of 0.8 Hz. Our insights are expected to guide the future design of advanced ultrasonic transducers for photoacoustic imaging, offering benefits across diverse biomedical applications, such as portable and wearable photoacoustic devices, ultrafast PAM imaging system and mutifunctional integrated biosensors.
This study primarily focused on developing high-performance TUTs based on our newly developed piezoelectric materials and demonstrating their potential in high-speed photoacoustic microscopy (PAM) imaging. With future development, the performance of the imaging system could be further enhanced.
First, the current TUT-based imaging system has limited time resolution. Although we have reduced the image acquisition time by eliminating the need for mechanical scanning and temporal averaging, the frame rate (0.8 Hz) is still insufficient for achieving real-time microvascular mapping, primarily due to the limited B-scan rate (200 Hz). Obtaining images with higher time resolution can provide more detailed insights into transient cerebrovascular behaviors. Thus, to improve the time resolution, the excitation laser with a higher pulse repetition frequency (RPF) may be needed to improve the B-scan rate. For example, utilizing the laser with an RPF of 1.5 MHz (vs. 50 kHz currently employed) can theoretically achieve a 30 Hz video-rate imaging in our case42,62. Moreover, employing the single-pixel non-scanning imaging technique may also be another choice to bypass this issue63.
Second, the PIN-PMN-PT-based TUT offers a limited FOV for optical scanning. While it provides a larger FOV compared to traditional focused transducers, it still cannot cover the entire mouse brain. Considering the trade-off between aperture size and PA sensitivity, developing TUT arrays along with corresponding synthetic aperture algorithms could be a promising solution to expand the FOV without sacrificing the PA sensitivity. In addition, the imaging SNR could also be enhanced through array beamforming.
Third, the current imaging setup followed the conventional guideline of using a water tank with a PE membrane for acoustic coupling. These coupling media may degrade imaging quality by introducing additional acoustic attenuation. Thus, further improvements in imaging quality could be achieved by opting for a wearable setup with minimal acoustic coupling media. For instance, the planar TUT can be directly attached to the target with only a thin layer of ultrasound gel27.
From a materials perspective, the cost and processing difficulties associated with relaxor-PT crystals may hinder their broader application. Although we have demonstrated minimal time-dependent performance degradation in transparent PIN-PMN-PT crystals and the robustness of these crystals for photoacoustic applications, achieving and maintaining transparency in these crystals may pose challenges for engineers and researchers without prior experience and expertize, due to the complexity of crystal composition and uncertainty regarding crystal quality. Rare-earth-doped transparent ferroelectric ceramics (e.g., Eu-doped PMN-PT64) could be a viable alternative in terms of cost-effectiveness and uniformity. From a transducer perspective, the electromechanical coupling factor of piezoelectric materials is the most critical intrinsic factor determining the sensitivity of TUTs. The dielectric constant is another key consideration when designing TUTs with varying aperture sizes, as electrical impedance matching directly affects the voltage received at the end of the cable. Moreover, the thermal stability of piezoelectric materials is crucial for ensuring the stability and reliability of TUTs during prolonged use. Therefore, improving the electromechanical and dielectric properties of these materials without compromising thermal stability would further benefit the development of high-performance TUTs, particularly for small-aperture designs and transducer arrays. We believe our work could serve as a guideline for designing high-performance TUTs, ultimately advancing the development of novel piezoelectric materials.
Methods
Material preparation
The PIN-PMN-PT single crystal was grown by a modified Bridgman method at Xi’an Jiaotong University. The starting composition we used for crystal growth is 0.26PIN-0.44PMN-0.30PT. The XRD data for the 0.26PIN-0.44PMN-0.30PT powder is illustrated in Supplementary Fig. 25. At room temperature, 0.26PIN-0.44PMN-0.30PT is in the rhombohedral state. The crystal boule was oriented by an X-ray diffraction (XRD) method and cut into [001]-oriented wafers with a thickness of 0.5 mm by a diamond wire saw (STX-600, MTI Corporation, China). Both sides of the wafer were lapped and polished to optical quality using colloidal silica polishing media with a grit size of 120 nm. Transparent Indium-tin-oxide films (ITO) with a thickness of 100 nm were deposited on the polished surfaces by electron beam evaporation (Peva600L, Advanced System Technology) to serve as electrodes. The sample was elevated at 300 oC, the deposition rate was controlled at 0.1 nm s−1, and the oxygen gas was supplied at 2.5 sccm. The sheet resistance of ITO electrodes was measured by a four-probe resistivity tester (EQ-JX2008-LD, MTI Corporation, China). The crystals were then poled by an a.c. electric field with a frequency of 1 Hz, a cycle number of 10, and a peak amplitude of 8 kV cm−1 to obtain high piezoelectricity together with good transparency. A customized brass fixture was designed to minimize the influence of ITO’s resistance during a.c. poling (Supplementary Fig. 27). The fixture can achieve full contact with the ITO electrodes on the sample surfaces and maintain a consistent electrical potential across the entire surface, thereby preventing any in-plane voltage drops.
The 36o rotated Y-cut lithium niobate (LN) wafer was purchased from Jiangxi Unicrystal Technology, China. Both sides of the wafer were also optically polished and coated by ITO electrodes as mentioned above.
Material characterizations
The domain morphology was characterized by field-emission SEM (Quanta F250, FEI, USA) and BIM (Metripol, Oxford Cryosystems, UK). For SEM, the scanning was performed on the (001) fracture surface of the PIN-PMN-PT samples. For BIM, the samples were lapped and optically polished to a thickness of 200 μm. The false-color images were reconstructed according to the optical anisotropy of the specimen and thus, enabled to distinguish the domain orientations46,65.
The d33 values were measured by a quasi-static d33 meter (ZJ-3A, Institute of Acoustics, Chinese Academy of Sciences, China), until its value stabilizes after 48 h of the poling. The dielectric permittivity was measured using an LCR meter (E4980A, KEYSIGHT technologies, USA). The longitudinal velocity and the thickness-mode electromechanical coupling factor (kt) were calculated using the resonance method according to the IEEE Standards on Piezoelectricity66.
Optical transmittance measurements
The transmission spectrum was measured by an ultraviolet-visible-infrared spectrophotometer (UV3600, Shimadzu, Japan) at wavelengths ranging from 300 to 2500 nm. The incident light transmits through the test sample perpendicular to the surface. For optical transmittance of TUTs, the measurements were conducted by placing the TUT inside the spectrophotometer. A black mold with a small hole was designed to hold the TUT and ensure that the light is transmitted only through the area where the crystal is located.
Finite element analysis
The simulation was performed using finite element software (COMSOL Multiphysics) with Acoustics and Piezoelectricity Modules. The model, including the transducer and water domain, was set in a two-dimensional axisymmetric configuration. The side of the backing layer is the only surface with a zero-displacement constraint to minimize unwanted reflections. Perfectly matched layers were applied to the boundary of the water domain to absorb the unwanted reflections. The [001]-poled PIN-PMN-PT crystal was adopted as the piezoelectric layer (thickness: 60 μm), the parameters of which were obtained from the ref. 67. The second matching layer (thickness: 20 μm) and the backing layer (thickness: 600 μm) were set to epoxy. And the first matching layer (thickness: 40 μm) was set to glass. The point acoustic source was excited by a Gaussian pulse generated from MATLAB (MathWorks Inc., USA), and the initial acoustic pressure was 1 kPa.
Transducer fabrication
The LN crystal wafers were machined into a 6 mm diameter circular shape by using a diamond wire saw (STX-100QX, MTI Corporation, China). The PIN-PMN-PT crystal wafers were cut into a 2 mm × 2 mm square shape by using a dicing saw (ADT 7022, Advanced Dicing Technologies, Israel). The crystals were then fixed into the center holes of brass rings by a non-conductive epoxy (Epo-Tek 301, Epoxy Technologies, USA) with the sides of negative polarization downwards. After being cured for 24 h at room temperature, the crystal, together with the brass ring, was closely fixed on a glass substrate using paraffin wax with a melting point of 90 oC and then lapped and polished to the required thickness. After that, ITO was sputtered on the polished crystal surface by d.c. magnetron sputtering at room temperature (NSC-3500, NANO-MASTER, Inc., USA). A 43 μm quartz glass plate with low-resistance ITO coating was carefully adhered on the top of the brass ring and the crystal using epoxy (Epo-Tek 301). The thickness of bond layers was controlled at less than 1 μm by pressing. A thin layer of epoxy (Epo-Tek 301) was then cured above the first matching layer and polished to 21 μm. Finally, the TUT was removed from the glass substrate and put into a 1.5 mm-height brass tube. The signal wire of a micro-coaxial cable (42 AWG, length: ~ 200 mm) was connected to the ITO electrode on the positive side of the crystal, and the shield wire was connected to the brass ring by conductive epoxy (E-solder 3022, Von Roll Inc., USA). The other end of the micro-coaxial cable was connected to a Subminiature Version A (SMA) connector. Finally, the degassed Epotek-301 was poured into the brass tube for encapsulation. The epoxy surfaces were polished using the polishing paste (PN05928, Finesse-ItTM Machine Polish, 3 M, USA). The time interval between a.c. poling and the implantation of samples into transducers was more than one week. The fabrication of transparent transducers took about two weeks. After the fabrication, the performance was characterized within one month.
Transducer characterization
The electrical impedance was measured by a precision impedance analyzer (E4991B, Keysight Technologies Inc., Santa Clara, CA, USA). The pulse-echo response of the transducer was tested in a deionized water bath at room temperature with a polished quartz plate as the reflected target. A pulser/receiver (5073PR, Olympus NDT Inc., USA), which was calibrated by the South China National Center of Metrology, Guangdong Institute of Metrology (Supplementary Fig. 14a), was used to activate the transducer and receive the echo. The damping factor of the pulser/receiver was set to 50 Ω. The output signal was recorded by a digital oscilloscope (DPO4104B, Tektronix, Inc., USA) with 50 Ω input impedance. The frequency spectrum of the signal was computed via a fast Fourier transform by the oscilloscope. The center frequency (fc) and fractional bandwidth (BW) are determined by the following equations:
where fl and fu are defined as the lower and upper -6 dB frequencies.
To measure the insertion loss (IL), the transducer was excited by a 5-cycle sinusoidal burst pulse with a peak-to-peak amplitude of 5 V. The oscilloscope was used to measure the input and output voltages at the end of the transducer coaxial cable. The IL was calculated from the following equation68:
where Vi and Vo are input and output voltage amplitudes, respectively, d is the distance from the transducer to the target (i.e., a quartz plate) in millimeters, and α is the attenuation coefficient of ultrasound in water, with a value of 2.2 × 10−4 dB mm−1·MHz2. The reflection loss from the quartz plate was compensated by a 1.9 dB gain.
The receiving voltage sensitivity was characterized in the frequency range from 10 to 40 MHz using a calibrated hydrophone (NH0500, Precision Acoustics) and a commercial probe (DOPPLER I2-25P3-H 25 MHz, diameter: 3 mm). The commercial probe was excited by a 10-cycle sinusoidal burst pulse with a peak-to-peak amplitude of 5 V, serving as an acoustic source. The TUTs and the calibrated hydrophone were placed 5 mm away from the source for receiving. The equivalent noise pressure (NEP) was calculated as the ratio of the receiving sensitivity to the noise level57. The noise level was 1.4 mV for both LN- and PIN-PMN-PT-based TUTs, which was calculated from the root-mean-square (RMS) noise recorded by the oscilloscope when the transducer was immersed in water without any acoustic input.
The ultrasound imaging resolution of the TUT was evaluated using a wire phantom composed of seven tungsten wires, each with a diameter of 10 µm (Supplementary Fig. 20a). The axial and lateral resolutions were determined from the full width at half maximum (FWHM) of the B-scan profile.
Animal preparation
For ear vasculature imaging, an athymic nude mouse (Zhuhai BesTest Bio-Tech Co., LTD., China; age: 6–8 weeks old) was anesthetized with 1.5% vaporized isoflurane, and its body temperature was maintained at 37 °C using a homemade heating pad with a temperature controller. For cortex vasculature imaging, a mouse (C57) was also anesthetized with 1.5% vaporized isoflurane and fixed onto a homemade holder. A partial skull of the mouse with an area of 5 mm × 5 mm was thinned carefully and removed surgically. 250 microliters of Pentylenetetrazol (PTZ) solution (concentration: 100 mg L−1) was injected intraperitoneally into the mouse to induce an epileptic seizure. All animal experimental procedures were performed in conformity with the protocol approved by the Animal Study Committee of Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences.
Photoacoustic imaging
A custom-made water tank filled with deionized water was placed on top of the imaging objects. The bottom of the water tank was made of transparent polyethylene membrane. A small amount of ultrasound gel was applied between the imaging target and the polyethylene membrane for efficient acoustic coupling. The mouse, as well as the water tank, was mounted on a two-axis linear motorized stage (PLS-85, PI miCos, GmbH, Germany) for mechanical raster scanning. A 532 nm Nd: YAG pulsed laser (GKNQL-532, Beijing Guoke Laser Co., LTD., China; pulse width: 2 ns, repetition rate: 50 kHz) was used as the illumination source. Two galvanometers (TSH8203, Sunny Technology, Beijing, China) were used to deflect the laser beam for optical scanning. The laser beam was focused by an achromatic doublet lens (f = 45 mm, Hengyang, China) and illuminated on the imaging target through the TUT. The laser pulse energy irradiated on the target was measured to be 500 nJ. The lower surface of the TUT was immersed in the water to detect the PA signals. The output signals of the TUT were amplified by an ultrasonic amplifier (5073PR, Olympus NDT Inc., Massachusetts, USA) with a gain of 39 dB and digitized by a high-speed data acquisition card (ATS9371, AlazarTech, Canada) with a sampling rate of 500 MS s-1. The axial resolution of the system was calculated from the full width at half maximum (FWHM) of the depth profile of a 10 μm-diameter tungsten wire. The lateral resolution was quantified by scanning across the sharp edge of a blade. The edge spread function (ESF) was obtained through the Boltzmann fitting of the data, and the corresponding line spread function (LSF) was obtained by taking the derivative of the ESF. The lateral resolution was then calculated from the FWHM of the LSF. For the in-vivo experiments, the images were reconstructed by the maximum amplitude projection of the PA signals and processed by non-local mean filtering to suppress the system noise. The imaging area of optical scanning is 1.5 mm × 1.5 mm, and the distance between each image pixel is 5 μm. For ear vasculature imaging, the step size of mechanical scanning is 1 mm. Sixteen images obtained by optical scanning were stitched together along the mechanical scanning (x-y) directions to form a relatively large area of 4 mm × 4 mm. The details of image stitching are given in Supplementary Fig. 28. For cortex vasculature imaging, only optical scanning was performed to study the dynamic change of vessels. After the injection of PTZ solution, a total of 1200 frames were acquired continuously over a period of approximately 20 min. And 20 frames were acquired before the injection for comparison. The experimental setup of the in-vivo PAI is shown in Supplementary Fig. 29. The distribution map of vessel diameter was obtained via the segmentation-based single-vessel analysis69. The SNR was calculated by using the following equation:
where meant and meann denote the mean values of the signal and the background noise, respectively.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data that support the findings of this study are included in the manuscript and Supplementary Information. Any other relevant data are also available upon request from corresponding authors.
References
Wang, L. V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photon. 3, 503–509 (2009).
Wang, L. V. & Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 13, 627–638 (2016).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Das, D., Sharma, A., Rajendran, P. & Pramanik, M. Another decade of photoacoustic imaging. Phys. Med. Biol. 66, 05TR01 (2021).
Kim, J. et al. Super-resolution localization photoacoustic microscopy using intrinsic red blood cells as contrast absorbers. Light Sci. Appl. 8, 103 (2019).
Zhu, X. et al. Real-time whole-brain imaging of hemodynamics and oxygenation at micro-vessel resolution with ultrafast wide-field photoacoustic microscopy. Light Sci. Appl. 11, 138 (2022).
Yang, J. M. et al. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat. Med. 18, 1297–1302 (2012).
Li, L. et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 1, 0071 (2017).
Deán‐Ben, X. L. & Razansky, D. Optoacoustic imaging of the skin. Exp. Dermatol. 30, 1598–1609 (2021).
Aguirre, J. et al. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 1, 0068 (2017).
Lin, L. et al. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 9, 2352 (2018).
Lin, L. et al. High-speed three-dimensional photoacoustic computed tomography for preclinical research and clinical translation. Nat. Commun. 12, 882 (2021).
Jang, J., Kim, J., Lee, H. J. & Chang, J. H. Transrectal ultrasound and photoacoustic imaging probe for diagnosis of prostate cancer. Sensors 21, 1217 (2021).
Wang, X. et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biomed. Eng. 21, 803–806 (2003).
Na, S. et al. Massively parallel functional photoacoustic computed tomography of the human brain. Nat. Biomed. Eng. 6, 584–592 (2022).
Yao, J. et al. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 12, 407–410 (2015).
Wong, T. T. et al. Label-free automated three-dimensional imaging of whole organs by microtomy-assisted photoacoustic microscopy. Nat. Commun. 8, 1386 (2017).
Zhang, D. et al. An ellipsoidal focused ultrasound transducer for extend-focus photoacoustic microscopy. IEEE Trans. Biomed. Eng. 68, 3748–3752 (2021).
Dangi, A., Agrawal, S. & Kothapalli, S.-R. Lithium niobate-based transparent ultrasound transducers for photoacoustic imaging. Opt. Lett. 44, 5326 (2019).
Hu, H. et al. A wearable cardiac ultrasound imager. Nature 613, 667–675 (2023).
Gao, X. et al. A photoacoustic patch for three-dimensional imaging of hemoglobin and core temperature. Nat. Commun. 13, 7757 (2022).
Park, J. et al. Opto-ultrasound biosensor for wearable and mobile devices: realization with a transparent ultrasound transducer. Biomed. Opt. Express 13, 4684 (2022).
Mirg, S. et al. Awake mouse brain photoacoustic and optical imaging through a transparent ultrasound cranial window. Opt. Lett. 47, 1121 (2022).
Liao, T. et al. Centimeter-scale wide-field-of-view laser-scanning photoacoustic microscopy for subcutaneous microvasculature in vivo. Biomed. Opt. Express 12, 2996 (2021).
Zhang, H. F., Xie, Z., Jiao, S. & Puliafito, C. A. Laser-scanning optical-resolution photoacoustic microscopy. Opt. Lett. 34, 1771–1773 (2009).
Park, J. et al. Quadruple ultrasound, photoacoustic, optical coherence, and fluorescence fusion imaging with a transparent ultrasound transducer. Proc. Natl. Acad. Sci. USA 118, e1920879118 (2021).
Park, J. et al. Bi-modal near-infrared fluorescence and ultrasound imaging via a transparent ultrasound transducer for sentinel lymph node localization. Opt. Lett. 47, 393 (2022).
Chen, H. et al. Optical-resolution photoacoustic microscopy using transparent ultrasound transducer. Sensors 19, 5470 (2019).
Chen, R. et al. Transparent high-frequency ultrasonic transducer for photoacoustic microscopy application. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 67, 1848–1853 (2020).
Park, S., Kang, S. & Chang, J. H. Optically transparent focused transducers for combined photoacoustic and ultrasound microscopy. J. Med. Biol. Eng. 40, 707–718 (2020).
Chen, H. et al. Transparent low intensity pulsed ultrasound (LIPUS) chip for high-throughput cell stimulation. Lab Chip 21, 4734–4742 (2021).
Ren, D., Sun, Y., Shi, J. & Chen, R. A review of transparent sensors for photoacoustic imaging applications. Photonics 8, 324 (2021).
Chen, M. et al. High-speed wide-field photoacoustic microscopy using a cylindrically focused transparent high-frequency ultrasound transducer. Photoacoustics 28, 100417 (2022).
Chen, H. et al. Transparent ultrasound array for real-time optical, ultrasound, and photoacoustic imaging. BME Front. https://doi.org/10.34133/2022/9871098 (2022).
Chen, H. et al. A high sensitivity transparent ultrasound transducer based on PMN-PT for ultrasound and photoacoustic imaging. IEEE Sens. Lett. 5, 1–4 (2021).
Fang, C., Hu, H. & Zou, J. A focused optically transparent PVDF transducer for photoacoustic microscopy. IEEE Sens. J. 20, 2313–2319 (2020).
Ilkhechi, A. K., Ceroici, C., Dew, E. & Zemp, R. Transparent capacitive micromachined ultrasound transducer linear arrays for combined realtime optical and ultrasonic imaging. Opt. Lett. 46, 1542 (2021).
Zhang, J. et al. Broadband transparent ultrasound transducer with polymethyl methacrylate as matching layer for in vivo photoacoustic microscopy. Photoacoustics 33, 100548 (2023).
Osman, M. S. et al. A novel matching layer design for improving the performance of transparent ultrasound transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 69, 2672–2680 (2022).
Cho, S. et al. An ultrasensitive and broadband transparent ultrasound transducer for ultrasound and photoacoustic imaging in-vivo. Nat. Commun. 15, 1444 (2024).
Shung, K. K. Diagnostic Ultrasound: Imaging and Blood Flow Measurements. (CRC Press, 2005).
Yao, J. & Wang, L. V. Photoacoustic microscopy. Laser Photonics Rev. 7, 758–778 (2013).
Zhou, Q., Lam, K. H., Zheng, H., Qiu, W. & Shung, K. K. Piezoelectric single crystal ultrasonic transducers for biomedical applications. Prog. Mater. Sci. 66, 87–11 (2014).
Cannata, J. M., Ritter, T. A., Chen, W.-H., Silverman, R. H. & Shung, K. K. Design of Efficient, Broadband Single-Element (20-80 MHz) Ultrasonic Transducers for Medical Imaging Applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 50, 1548–1557 (2003).
Rathod, V. T. A review of acoustic impedance matching techniques for piezoelectric sensors and transducers. Sensors 20, 4051 (2020).
Qiu, C. et al. Transparent ferroelectric crystals with ultrahigh piezoelectricity. Nature 577, 350–354 (2020).
Liu, X. et al. Ferroelectric crystals with giant electro-optic property enabling ultracompact Q-switches. Science 376, 371–377 (2022).
Sun, Y., Karaki, T. & Yamashita, Y. Recent progress on AC poling of relaxor-PbTiO3 ferroelectric single crystals: a review. Jpn. J. Appl. Phys. 61, SB0802 (2022).
Zhang, S. et al. Advantages and challenges of relaxor-PbTiO3 ferroelectric crystals for electroacoustic transducers–A review. Prog. Mater. Sci. 68, 1–66 (2015).
Jiang, X., Kim, J. & Kim, K. Relaxor-PT single crystal piezoelectric sensors. Crystals 4, 351–376 (2014).
Yao, J. & Wang, L. V. Sensitivity of photoacoustic microscopy. Photoacoustics 2, 87–101 (2014).
Krimholtz, R., Leedom, D. A. & Matthaei, G. L. New equivalent circuits for elementary piezoelectric transducers. Electron. Lett. 6, 398–399 (1970).
Desilets, C. S., Fraser, J. D. & Kino, G. S. The design of efficient broad-band piezoelectric transducers. IEEE Trans. Sonics Ultrason. 25, 115–125 (1978).
Qiu, C. et al. In-situ domain structure characterization of Pb(Mg1/3Nb2/3)O3-PbTiO3 crystals under alternating current electric field poling. Acta Mater. 210, 116853 (2021).
Chang, W. Y. et al. Dielectric and piezoelectric properties of 0.7Pb(Mg1/3Nb2/3)O3-0.3PbTiO3 single crystal poled using alternating current. Mater. Res. Lett. 6, 537–544 (2018).
Winkler, A. M., Maslov, K. I. & Wang, L. V. Noise-equivalent sensitivity of photoacoustics. J. Biomed. Opt. 18, 097003 (2013).
Liu, Y., Kurnikov, A., Li, W., Subochev, P. & Razansky, D. Highly sensitive miniature needle PVDF-TrFE ultrasound sensor for optoacoustic microscopy. Adv. Photon. Nexus 2, 056006 (2023).
Ning, B. et al. Simultaneous photoacoustic microscopy of microvascular anatomy, oxygen saturation, and blood flow. Opt. Lett. 40, 910–913 (2015).
Turker, S., Ilbay, G., Severcan, M. & Severcan, F. The investigation of compositional, structural and dynamical Changes of PTZ-induced seizures on a rat brain by FTIR spectroscopy. Anal. Chem. 86, 1395–1403 (2014).
Hu, S., Maslov, K. & Wang, L. V. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed. Opt. Lett. 36, 1134–1136 (2011).
Wang, K., Li, C., Chen, R. & Shi, J. Recent advances in high-speed photoacoustic microscopy. Photoacoustics 24, 100294 (2021).
Wang, L., Maslov, K., Xing, W., Garcia-Uribe, A. & Wang, L. V. Video-rate functional photoacoustic microscopy at depths. J. Biomed. Opt. 17, 106007 (2012).
Chen, N. et al. Video-rate high-resolution single-pixel nonscanning photoacoustic microscopy. Biomed. Opt. Express 13, 3823–3835 (2022).
Guo, P. et al. Advancement in PMN-PT transparent piezoelectric ceramic for photoacoustic/ultrasound dual-mode imaging. J. Mater. https://doi.org/10.1016/j.jmat.2024.100932 (2024).
Glazer, A. M., Lewis, J. G. & Kaminsky, W. An automatic optical imaging system for birefringent media. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 452, 2751–2765 (1996).
IEEE Standard on Piezoelectricity, ANSI/IEEE Standard 176-1987 (1987).
Qiao, L. et al. Temperature dependence of elastic, piezoelectric, and dielectric matrixes of [001]-poled rhombohedral PIN-PMN-PT single crystals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 66, 1786–1792 (2019).
Fei, C. et al. PMN-PT single crystal ultrasonic transducer with half-concave geometric design for IVUS imaging. IEEE Trans. Biomed. Eng. 65, 2087–2092 (2018).
Cao, R., Li, J., Zhang, C., Zuo, Z. & Hu, S. Photoacoustic microscopy of obesity-induced cerebrovascular alterations. Neuroimage 188, 369–379 (2019).
Acknowledgements
F.L. and C.Q. acknowledge the support of the National Natural Science Foundation of China (Grant Nos. 52325205 and U2441267) and the Fundamental Research Funds for the Central Universities (xzy022021034). C.L. and Z.Q.X. acknowledge the support of the National Key Research and Development Program of China (2021YFE0202200), the National Natural Science Foundation of China (Grant No. 82122034, 62205365), and the Youth Innovation Promotion Association (2019352). H.Z., W.Q., and Z.Z. acknowledge the support of the National Key R&D Program of China (2021ZD0200401, 2023YFF0715300, and 2023YFC2416400), National Science Foundation of China (Grant No. 82327805, 62022086, 12274430, 12204504), the Shenzhen Foundation (Grant No. JSGGZD20220822095602005, KCXFZ20230731093959009, GJHZ20220913142810021, JCYJ20200109114825064, ZDSYS201802061806314), the Shenzhen Medical Research Funds (B2302053), and the Youth Innovation Promotion Association (CAS 2018391). L.-Q.C. acknowledges the general support from the Hamer Foundation through the Hamer Professorship at Penn State.
Author information
Authors and Affiliations
Contributions
This work was conceived and designed by F.L., H.Z., C.L., and W.Q.; C.Q. prepared the piezoelectric crystals and fabricated the transducers. Z.Q.X. performed the photoacoustic imaging and data processing. Z.Z., H.Z., and W.Q. supervised the fabrication of the transducer; L.Q. assisted in the preparation of crystal samples; L.N., M.S., and W.W. assisted in the fabrication of the transducers; K.S., F.L., and Z.X. grew the crystals; C.L. supervised the photoacoustic imaging experiments; F.L. supervised the preparation of crystal samples; C.Q. and F.L. drafted the manuscript; S.Z. and L.-Q.C. revised the manuscript; and all authors discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qiu, C., Zhang, Z., Xu, Z. et al. Transparent ultrasonic transducers based on relaxor ferroelectric crystals for advanced photoacoustic imaging. Nat Commun 15, 10580 (2024). https://doi.org/10.1038/s41467-024-55032-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55032-0