Abstract
Microthrombus formation is associated with COVID-19 severity; however, the detailed mechanism remains unclear. In this study, we investigated mouse models with severe pneumonia caused by SARS-CoV-2 infection by using our in vivo two-photon imaging system. In the lungs of SARS-CoV-2-infected mice, increased expression of adhesion molecules in intravascular neutrophils prolonged adhesion time to the vessel wall, resulting in platelet aggregation and impaired lung perfusion. Re-analysis of scRNA-seq data from peripheral blood mononuclear cells from COVID-19 cases revealed increased expression levels of CD44 and SELL in neutrophils in severe COVID-19 cases compared to a healthy group, consistent with our observations in the mouse model. These findings suggest that pulmonary perfusion defects caused by neutrophil adhesion to pulmonary vessels contribute to COVID-19 severity.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative virus of COVID-19 (coronavirus disease 2019) and is transmitted via droplets/aerosols containing the virus. SARS-CoV-2 invasion of the lung is initiated by the binding of the virus to the alveolar epithelium using angiotensin-converting enzyme-2 (ACE-2) as a receptor1,2. Most cases of COVID-19 are mild with respiratory symptoms such as fever, cough, nasal discharge, and pharyngitis; however, the elderly and those with underlying medical conditions such as obesity, diabetes, and hypertension develop severe viral pneumonia, which often leads to serious complications and even death3,4. Patients presenting with severe viral pneumonia are treated according to their respiratory and systemic symptoms, but the mechanism of COVID-19 severity is not fully understood and an optimal treatment strategy has not been established.
Immunopathology, including neutrophilia and lymphopenia, decreased or delayed type I interferon response, and the cytokine storm caused by dysregulation of monocytes and granulocytes may play a role in the pathogenesis of COVID-195. In addition to these immunopathological events, thrombosis has frequently been reported in patients with severe COVID-19, which may also contribute to the severity of the disease6,7,8. Platelets bind to neutrophils through neutrophil extracellular traps (NETs) released by the neutrophils to form large thrombus-like aggregates; they also form thrombi independently of neutrophils in response to tissue factors exposed to damaged endothelial cells. Single-cell RNA-seq and proteomic approaches using clinical samples and animal models of SARS-CoV-2 infection have revealed that factors associated with inflammation, coagulation, and NETs release are activated during SARS-CoV-2 infection. The factors involved in thrombus formation are spatiotemporally regulated and the thrombogenic process is thought to be a multistep process; however, these dynamic events are difficult to observe using conventional histological approaches with COVID-19 autopsy samples or SARS-CoV-2-infected animals. Accordingly, it remains unclear how the process of thrombus formation proceeds in patients with severe COVID-19.
To establish an effective treatment for patients with severe COVID-19, we must elucidate the mechanisms involved in the severity of the disease. To understand how SARS-CoV-2 affects the responses of platelets and neutrophils in the pulmonary vasculature and the thrombogenic response, we performed two-photon in vivo imaging analysis of cellular responses after SARS-CoV-2 infection by using SARS-CoV-2 strains and lethal mouse models. Our in vivo imaging analysis revealed increased neutrophil numbers in the pulmonary vasculature, prolonged neutrophil adhesion to pulmonary vessels, and enhanced platelet aggregation in SARS-CoV-2-infected mice. The increased expression of adhesion factors on the neutrophils in pulmonary blood vessels promoted thrombus formation and impaired pulmonary blood flow in the lungs of the SARS-CoV-2-infected mice. In addition, re-analysis of data from human clinical samples9 demonstrated the relevance of the findings in mice.
Results
In vivo imaging of SARS-CoV-2-infected lung
To elucidate the pathological changes of SARS-CoV-2-infected lungs in living animals, we examined K18-human angiotensin-converting enzyme-2 transgenic (K18-hACE2 Tg) mice by using an in vivo imaging system we previously established in a biosafety level (BSL) 3 facility10,11. hACE2 is expressed on the epithelial cells of the lungs, kidneys, liver, intestine, and brain of K18-hACE2 mice, and contributes to the establishment of SARS-CoV-2 infection as a receptor for the S protein1,2. To visualize SARS-CoV-2-infected cells, K18-hACE2 Tg mice were intranasally inoculated with SARS-CoV-2-Venus, which expresses the Venus fluorescent reporter gene in infected cells. SARS-CoV-2-Venus, generated by reverse genetics in the backbone of pBAC SARS2 WK521 (derived from SARS-CoV-2/JPN/TY/WK521), exhibited lethal infection with an MLD50 (mouse lethal dose 50; the dose required to kill 50% of infected mice) value of 103.83 plaque-forming units (PFU) in K18-hACE2 Tg mice (Fig. 1a). To analyze the pathobiological effects of SARS-CoV-2, we infected K18-hACE2 Tg mice with 105 PFU of SARS-CoV-2-Venus. Fluorescent dextran, as well as fluorochrome-conjugated anti-mouse Ly-6G and CD41 antibodies, were also intravenously (i.v.) administered to the mice to visualize lung vascular structures, neutrophils, and platelets, respectively. Virus-infected Venus-positive epithelial cells began to be observed in the lungs of SARS-CoV-2-infected mice on day 4 post-infection (p.i.), and their number had visibly increased at day 6 p.i. (Fig. 1b and Supplementary Movie 1). The Venus-positive infected cells appeared to be morphologically flat type I alveolar epithelial cells surrounding the alveoli, and spherical large type II alveolar epithelial cells were infected with SARS-CoV-2-Venus (Fig. 1c), consistent with previous studies12,13. Platelets and neutrophils flowed smoothly in the pulmonary capillaries of uninfected mice, whereas in the mice infected with SARS-CoV-2-Venus, their movements were slower in the capillaries (Supplementary Movie 1). On day 6 p.i., an increase in the number of neutrophils was apparent in the pulmonary capillaries of the infected-K18-hACE2 Tg mice (Fig. 1d).
a Survival and body weight changes of SARS-CoV-2-Venus-infected mice. K18-hACE2 C57BL/6J mice were infected with 101–105 PFU of SARS-CoV-2-Venus, and survival and body weight were monitored daily for 10 days. n = 6 biologically independent animals. The results are expressed as the mean ± SD. b Pathophysiological changes in SARS-CoV-2-infected lungs. K18-hACE2 C57BL/6J mice were intranasally infected with SARS-CoV-2-Venus and observed at the indicated timepoints. White arrows indicate infected cells. c In vivo imaging of SARS-CoV-2-infected cells on 4-day post-infection (dpi). (i) and (ii) are enlarged images of the leftmost image. d The number of intravascular neutrophils in SARS-CoV-2-infected lungs. On 6 dpi, SARS-CoV-2-Venus-infected K18-hACE2 C57BL/6J mice were observed, and neutrophils in the pulmonary capillaries in the microscopic field of view were counted. n = 6 biologically independent animals. Each dot represents data from an individual animal and the horizontal bars represent means ± SD. b–d Green indicates virus-infected cells. At the indicated timepoints, fluorescent dextran (white), fluorochrome-conjugated anti-mouse Ly-6G antibody (red), and anti-mouse CD41 (cyan) were i.v. administered to visualize lung architecture, neutrophils, and platelets, respectively. Source data are provided as a Source Data file.
SARS-CoV-2 alters the motility of intravascular neutrophils and impairs blood flow in infected lung
To analyze the functional changes of neutrophils in SARS-CoV-2-infected lungs, we quantitatively assessed the neutrophil movement. In our previous studies, we reported that pulmonary neutrophils in influenza virus-infected mice show two types of motility10: “slow movement”, which is considered tethering or rolling, in which neutrophils move along the vessel wall at a speed of 50 µm/s or less; and “rapid motion”, which is moving in the blood flow at a speed of more than 50 µm/s. We analyzed the amount of time individual neutrophils engaged in each motion in the lungs of K18-hACE2 Tg mice infected with SARS-CoV-2-Venus. Pulmonary neutrophils observed in SARS-CoV-2-infected lungs were tracked (Fig. 2a left panels) and ordered according to the amount of time they spent in slow motion, and the total time they spent in slow (Fig. 2a right panels, blue bars) and rapid (Fig. 2a right panels, red bars) motion was plotted for each neutrophil (Supplementary Movie 2). In SARS-CoV-2-Venus-infected lung, the amount of time the neutrophils spent in slow motion was significantly increased compared with uninfected lung (Fig. 2b). We also found that the percentage of neutrophils that engaged in rapid motion decreased (Fig. 2b). These results indicate that vascular neutrophils in SARS-CoV-2-infected lungs have increased contact time with the pulmonary vessel wall.
a Motility of pulmonary intravascular neutrophils in response to SARS-CoV-2 infection. Tracking analyses were performed to obtain the trajectories of individual neutrophils (left panel, vascular structures are shown in white, and individual trajectories are coded in different colors). For each neutrophil, speeds were measured for individual steps in its trajectory and subsequently defined as slow (≤50 µm/s) or rapid (>50 µm/s). All neutrophils observed under the same conditions were arranged along the y-axis according to the duration of their slow motion. The amount of time each neutrophil was engaged in slow (blue) or rapid (red) motion was plotted along the x-axis (right panel). n = 4 biologically independent animals. b The average time for which neutrophils engaged in slow movement (left panel) and the average percentage of neutrophils with rapid movement (right panel) in SARS-CoV-2-Venus-infected lung. c Histograms were plotted based on the size of the detected platelet signals at the indicated timepoints. n = 4 biologically independent animals. d K18-hACE2 C57BL/6J mice were intranasally infected with SARS-CoV-2-Venus and observed at 6 dpi. At this timepoint, fluorescent dextran (white), fluorochrome-conjugated anti-mouse Ly-6G antibody (red), and anti-mouse CD41 (cyan) were i.v. administered to visualize the lung architecture, neutrophils, and platelets, respectively (left panel). Arrows indicate platelet aggregates. The number of neutrophils complexed with platelets in the pulmonary capillaries in the microscopic field of view was counted, and the ratio to the total number of neutrophils was plotted (right panel). n = 4 biologically independent animals. e Pulmonary blood perfusion in SARS-CoV-2-Venus-infected mice. To visualize blood vessels through which erythrocytes passed, SARS-CoV-2-Venus-infected K18-hACE2 C57BL/6J mice were i.v. inoculated with fluorescently labeled erythrocytes (red) at the indicated timepoint. The mice were also i.v. inoculated with fluorescent dextran (blue) to visualize the lung architecture. White circles indicate vessels in which the transferred erythrocytes did not pass during the observation period in the lungs of the SARS-CoV-2-Venus-infected K18-hACE2 C57BL/6J mice. Each dot represents data from an individual animal and the horizontal bars represent means ± SD. Source data are provided as a Source Data file.
Thrombus formation in pulmonary vessels has emerged as a typical clinical feature of COVID-19 pneumonia14. To assess whether intravascular neutrophils contribute to thrombus formation during SARS-CoV-2 pathogenesis, we performed an in vivo imaging analysis by co-staining platelets and neutrophils. To quantify the size of the platelet aggregates in the infected lungs, we assessed the fluorescent signals of CD41 as a surface marker of platelets15 from the original image, measured the area of each platelet, and performed a frequency analysis (Fig. 2c). CD41 signals occupying a small area (<50 pixels, which is approximately 8.57 µm2 in our imaging setting) and flowing in the blood vessels were considered to be single platelets. In SARS-CoV-2-infected lungs, an increased percentage of aggregated platelets with a large area of CD41 signal (≥50 pixels and <200 pixels, which is approximately 34.28 µm2 in our imaging setting) and thrombi (>200 pixels) were also observed to block the vessels as emboli. The percentage of aggregated platelets and thrombi was significantly increased in SARS-CoV-2-infected lungs (Fig. 2c). Interestingly, aggregated platelets frequently interacted with intravascular neutrophils to form microthrombus-like structures (neutrophil-platelet complexes) in the lungs infected with SARS-CoV-2-Venus (Fig. 2d and Supplementary Fig. 1). The ratio of intravascular neutrophils in contact with platelets was significantly increased in SARS-CoV-2-infected lungs compared to that of uninfected mice (Fig. 2d). These observations suggest that neutrophils adhering to pulmonary vessels in SARS-CoV-2-infected lungs participate in the formation of platelet aggregates.
The stagnation of blood neutrophils, aggregation of platelets, and formation of platelet-neutrophil complexes observed in SARS-CoV-2-infected lungs may have a significant effect on pulmonary hemodynamics and oxygen exchange mediated by erythrocytes. To test this concept, we transferred fluorescently labeled erythrocytes into SARS-CoV-2-infected K18-hACE2 Tg mice and observed their trajectories for 10 min. The time-lapse images were overlapped every 50 frames to visualize the areas where labeled erythrocytes flowed (Fig. 2e and Supplementary Movie 3). In naive mice, the blood vessels visualized by use of fluorescent dextran and the trajectories of the labeled erythrocytes almost overlapped, indicating that most pulmonary vessels were functional for oxygen exchange. In contrast, in SARS-CoV-2-infected lungs, there were areas where the vascular structures and the trajectories of the labeled erythrocytes did not overlap, indicating that the availability of functional capillaries for oxygen exchange was reduced. Blood neutrophil stagnation, platelet aggregation, and platelet-neutrophil complexes may disturb the pulmonary microcirculation in the SARS-CoV-2-infected lungs. Our in vivo imaging data revealed that the functional failure of lung capillaries is likely due to microthrombi formation in mice infected with SARS-CoV-2.
Development of a mouse model of severe SARS-CoV-2 pneumonia
Although K18-hACE2 Tg mice have been widely used to study SARS-CoV-2 pathogenesis16,17, intranasal inoculation of these mice with SARS-CoV-2 can cause severe neurological disease, including encephalitis, with features that differ from severe COVID-19 cases18,19. We previously established a mouse-adapted (MA) SARS-CoV-2 and its derivative expressing the Venus protein (named MA-SARS-CoV-2-Venus), which causes severe lung inflammation in C57BL/6J mice similar to severe COVID-19 cases20.
To establish an animal model that can mimic the symptoms of severe COVID-19 patients, we used seven mouse strains that are well-established disease models [i.e., obesity/diabetes models, C57BL/6J HamSlc-ob/ob (Ob/Ob) mice and KK-Ay/Ta Jcl mice; a type 1 diabetes model, Streptozotocin (STZ)-induced diabetic C57BL/6J mice; aged models, SAMP8/TaSlc mice and SAMP10-ΔSglt2 mice; a hyperlipidemia model, B6. B6.KOR/StmSlc-Apoeshl mice; and a hepatitis model, B6-nonalcoholic steato-hepatitis (NASH) mice]. These mice were intranasally infected with 103 PFU of MA-SARS-CoV-2 and their survival was assessed. MA-SARS-CoV-2-infected Ob/Ob mice showed significantly reduced survival compared to wild-type mice (Fig. 3a). The other six mouse strains did not show any symptoms after SARS-CoV-2 infection (Fig. 3a). We did not perform any additional studies on these other mouse comorbidity models; therefore, we cannot explain why we did not see severe outcomes with them. MA-SARS-CoV-2 exhibited high pathogenicity with an MLD50 value of 102.5 PFU and caused virus dose-dependent weight loss in Ob/Ob mice (Fig. 3b). MA-SARS-CoV-2 and MA-SARS-CoV-2-Venus showed comparable virulence in Ob/Ob mice (MLD50; 102.5 PFU) (Supplementary Fig. 2).
a Five mice per group of Ob/Ob mice, Streptozotocin (STZ)-induced diabetic C57BL/6J mice, KK-Ay/Ta Jcl mice, SAMP8/TaSlc mice, SAMP10-ΔSglt2 mice, B6.KOR/StmSlc-Apoeshl mice and B6-NASH mice were infected with 103 PFU of MA-SARS-CoV-2, and survival was monitored daily for the indicated timepoints. Age- and sex-matched C57BL/6J mice served as controls. SAMR1/TaSlc mice were used as controls for SAMP8/TaSlc mice and SAMP10-ΔSglt2 mice. n = 5 biologically independent animals. b Five mice per group of Ob/Ob mice were infected with 101–104 PFU of MA-SARS-CoV-2 and survival and body weight were monitored daily for 10 days. The results are expressed as the mean ± SD. n = 5 biologically independent animals. Source data are provided as a Source Data file.
Infection of Ob/Ob mice with MA-SARS-CoV-2 resulted in high virus titers in the lungs and nasal turbinates at 2 dpi, and no substantial difference in viral titers in the respiratory organs at this timepoint was observed between Ob/Ob mice and their control groups (Fig. 4a). Viruses were also recovered from the brain and liver of the infected Ob/Ob mice albeit at low titers (note: the brain samples collected for virus titration included the olfactory bulb). In the lungs of infected Ob/Ob mice at 5 dpi, viral replication was significantly higher than that in the control groups, with a titer of more than 1.0 × 107 PFU/g. In contrast, no difference in viral titers in the nasal turbinates was observed between Ob/Ob mice and their control groups, and the virus was not detected in any other organs tested at 5 dpi. Micro-CT analysis revealed lung abnormalities in MA-SARS-CoV-2-infected Ob/Ob mice, including patchy and ill-defined regions consistent with COVID-19 pneumonia at 4 dpi (Fig. 4b).
a Virus titers in organs of MA-SARS-CoV-2-infected mice. Four mice per group were euthanized and virus titers in the lungs, nasal turbinate, brain, heart, liver, spleen, kidney, and intestine were determined by using plaque assays in VeroE6/TMPRSS2 cells. Dashed lines in the panels indicate the detection limit of the assay for each organ. n = 4 biologically independent animals. b Micro-CT imaging of the lungs of infected mice. Axial CT images of the thorax in MA-SARS-CoV-2-infected C57BL/6J mice and Ob/Ob mice. On day 4 post-infection, the MA-SARS-CoV-2-infected Ob/Ob mice had a higher degree of lung abnormalities than the infected C57BL/J mice, highlighted by the red circle. c Pathological features of MA-SARS-CoV-2-infected Ob/Ob mice. Representative images of lungs are shown. Left and middle columns show hematoxylin and eosin staining. Arrowheads indicate areas of inflammatory cell infiltration. Right column shows immunohistochemistry using a rabbit polyclonal antibody that detects SARS-CoV-2 nucleocapsid protein, with brown staining indicating virus-positive cells. Scale bars, 500 µm in the left column; 100 µm in the middle and right columns. d Histopathological scores for inflammation in the alveoli. Ns not significant. n = 3 biologically independent animals. e Population of immune cells in the lungs of MA-SARS-CoV-2-infected mice. Lung immune cell numbers were determined in the whole lung at 4 dpi in C57BL/6J mice and Ob/Ob mice by flow cytometry. n = 5 biologically independent animals. a, d, e Each dot represents data from an individual animal and the horizontal bars represent means ± SD. Source data are provided as a Source Data file.
We then examined the histopathologic changes in the organs of Ob/Ob mice after MA-SARS-CoV-2 infection. Histopathological analysis revealed infiltration of inflammatory cells such as neutrophils and mononuclear cells into the alveolar regions in the lungs of both Ob/Ob and their control groups at 2 dpi with MA-SARS-CoV-2 (Fig. 4c). At the same timepoint, viral antigen was immunohistochemically detected mainly on alveolar and bronchiolar epithelial cells in both groups. In addition, the histopathological scores for pneumonia were comparable between the Ob/Ob and control groups (Fig. 4d). On day 5 p.i., the inflammation improved in the control group, but persisted in the Ob/Ob mice. In the control group, there was a clear reduction in the number of virus-positive cells. Conversely, there was no notable reduction in the number of virus-positive cells in the Ob/Ob group. These findings were supported by the histopathological scores for pneumonia, which showed an obvious decline in the control group but no obvious reduction in the Ob/Ob group (Fig. 4d). Consistent with our histopathologic analysis, flow cytometry showed that the number of neutrophils and monocytes infiltrating the lungs was significantly increased in the MA-SARS-CoV-2 infected lungs compared to the control group (Fig. 4e).
These results indicate that Ob/Ob mice infected with MA-SARS-CoV-2 or MA-SARS-CoV-2-Venus are a useful model that reflects the pathogenesis of severe COVID-19.
Neutrophils cause impaired pulmonary perfusion in SARS-CoV-2-infected lungs
To analyze the pathobiological changes in MA-SARS-CoV-2-infected Ob/Ob mice, the mice were intranasally infected with 104 PFU of MA-SARS-CoV-2-Venus. Large numbers of infected cells were observed in the lungs of Ob/Ob mice on day 4 p.i. (Fig. 5a and Supplementary Movie 4). Similar to the pathophysiological changes in the infected K18 hACE2 Tg mice, there was a significant increase in the number of neutrophils, platelet aggregates, thrombi, and the ratio of neutrophils in contact with platelets in the pulmonary vasculature of MA-SARS-CoV-2-Venus-infected Ob/Ob mice (Fig. 5b–d). To investigate the functional changes in the neutrophils, we quantified the motility of the vascular neutrophils of the infected mice. Ob/Ob mice infected with MA-SARS-CoV-2-Venus showed significantly increased slow neutrophil movement in pulmonary vessels compared to control groups, indicating increased adhesion time of neutrophils to the pulmonary vascular wall (Fig. 5e, f). Hemodynamics of the lungs of MA-SARS-CoV-2-Venus-infected Ob/Ob mice showed that pulmonary perfusion was obviously impaired compared with that in infected wild-type mice, with limited capillaries available for oxygen exchange (Fig. 6a and Supplementary Movie 5).
a Pathophysiological changes in SARS-CoV-2-infected Ob/Ob mouse lungs. Ob/Ob mice were intranasally infected with 104 PFU of MA-SARS-CoV-2-Venus and observed at 4 days post-infection (dpi). Green indicates virus-infected cells. Fluorescent dextran (white), fluorochrome-conjugated anti-mouse Ly-6G antibody (red), and anti-mouse CD41 (cyan) were i.v. administered to visualize lung architecture, neutrophils, and platelets, respectively. b Infiltrating and vascular neutrophils in MA-SARS-CoV-2-infected lungs. Ob/Ob mice were infected intranasally with 103 PFU of MA-SARS-CoV-2 and infected lungs were analyzed by flow cytometry at 4 dpi. n = 4 biologically independent animals. c Quantitative assessment of platelet aggregates in MA-SARS-CoV-2-Venus-infected lungs. Histograms were plotted based on the size of the detected platelet (CD41-positive objects) signals (leftmost panel). n = 4 biologically independent animals. d The ratio of neutrophils complexed with platelets in the lungs of MA-SARS-COV-2-Venus-infected Ob/Ob mice. The number of neutrophils complexed with platelets in the pulmonary capillaries in the microscopic field of view was counted, and the ratio to the total number of neutrophils was plotted. n = 4 biologically independent animals. e Motility of pulmonary intravascular neutrophils in MA-SARS-CoV-2-infected mice. To track neutrophil movement, a fluorochrome-conjugated anti-mouse Ly-6G antibody was injected i.v. into the MA-SARS-CoV-2-Venus-infected Ob/Ob mice. Tracking analyses were performed to obtain the trajectories of individual neutrophils (left panel, vascular structures are shown in white, and individual trajectories are coded in different colors). n = 5 biologically independent animals. f Assessment of neutrophil behaviors in (e) in terms of slow and rapid motion. The average time for which neutrophils engaged in slow movement (left panel) and the average percentage of neutrophils with rapid movement (right panel) in MA-SARS-CoV-2-Venus-infected lung. b–d, f Each dot represents data from an individual animal and the horizontal bars represent means ± SD. Source data are provided as a Source Data file.
a Pulmonary blood perfusion in MA-SARS-CoV-2-Venus-infected mice. To visualize blood vessels through which erythrocytes passed, MA-SARS-CoV-2-Venus-infected Ob/Ob mice were i.v. inoculated with fluorescently labeled erythrocytes (red) at the indicated timepoint. The mice were also i.v. inoculated with fluorescent dextran (blue) to visualize the lung architecture. White circles indicate vessels in which the transferred erythrocytes did not pass during the observation period in the lungs of MA-SARS-CoV-2-Venus-infected Ob/Ob mice. b Representative images of impaired pulmonary microcirculation caused by neutrophil-platelet complexes in MA-SARS-CoV-2-Venus-infected Ob/Ob mice. Ob/Ob mice were intranasally infected with MA-SARS-CoV-2-Venus and observed at 4 dpi. At this timepoint, fluorescent dextran (white), fluorochrome-conjugated anti-mouse Ly-6G antibody (red), and anti-mouse CD41 (cyan) were i.v. administered to visualize the lung architecture, neutrophils, and platelets, respectively. The mice were also i.v. inoculated with fluorescently labeled erythrocytes (red). White arrows indicate a neutrophil (fuchsia) adhering to a pulmonary vessel wall. Yellow arrowheads indicate platelet (cyan) aggregation and white arrowheads indicate erythrocytes that have been blocked from passing through the vessels. c Quantitative assessment of platelet aggregates in neutrophil-depleted Ob/Ob mice infected with MA-SARS-CoV-2. Ob/Ob mice were injected with anti-Kappa immunoglobulin antibody and anti-Ly-6G antibody to deplete neutrophils. The mice were infected with 104 PFU of SARS-CoV-2-Venus, and then, on day 4 after infection, a fluorochrome-conjugated anti-mouse CD41 antibody was injected i.v. into the mice to visualize platelets. Histograms were plotted based on the size of the detected platelet (CD41-positive) signals (leftmost panel). Each dot represents data from an individual animal and the horizontal bars represent means ± SD. n = 5 biologically independent animals. d Survival and body weight changes of neutrophil-depleted Ob/Ob mice infected with MA-SARS-CoV-2. Ob/Ob mice were injected with anti-Kappa immunoglobulin antibody and anti-Ly-6G antibody to deplete neutrophils. The mice were infected with 104 PFU of SARS-CoV-2-Venus, and survival and body weight were monitored daily for 14 days. The results are expressed as the mean ± SD. n = 7 biologically independent animals. Source data are provided as a Source Data file.
To clarify whether aggregates of neutrophils and platelets contribute to the impaired pulmonary perfusion observed in SARS-CoV-2-infected lungs, we performed a five-color multiple labeling analysis using our in vivo imaging method11. Ob/Ob mice were intranasally infected with MA-SARS-CoV-2-Venus and observed at 4 dpi. At this timepoint, fluorescent dextran, two fluorochrome-conjugated antibodies (Ly-6G and CD41), and fluorescently labeled erythrocytes were i.v. administered and then observed by using our imaging system. In the lungs of Ob/Ob mice infected with MA-SARS-CoV-2-Venus, neutrophil, and platelet aggregates obstructed the flow of the transferred erythrocytes, suggesting that these aggregates may be responsible for the impaired pulmonary perfusion (Fig. 6b and Supplementary Movie 6). In addition, in SARS-CoV-2-infected lungs, neutrophils adhered to the pulmonary vasculature; this was followed by platelet aggregation and stasis of the transferred erythrocytes, suggesting that neutrophil adhesion to the vessel wall is the point of origin for thrombus formation and subsequent impaired pulmonary perfusion.
To assess whether neutrophils are involved in the pathogenesis of MA-SARS-CoV-2, we removed the neutrophils in vivo by using antibodies and analyzed the effect on virus susceptibility. Removal of neutrophils from Ob/Ob mice led to a slight increase in the number of single platelets and a decrease in the number of aggregated platelets and thrombi in the pulmonary vasculature after MA-SARS-CoV-2 infection (Fig. 6c). In addition, the survival period for neutrophil-depleted Ob/Ob mice infected with MA-SARS-CoV-2 infection was prolonged (Fig. 6d). These results suggest that thrombi formed upon neutrophil stagnation may exacerbate MA-SARS-CoV-2-Venus pneumonia in Ob/Ob mice.
Altered expression of adhesion molecules in pulmonary neutrophils in relation to COVID-19 severity
Neutrophils infiltrate the site of infection by interacting with vascular endothelial cells via adhesion molecules21. We therefore analyzed the expression levels of molecules (i.e., CD62L, CD44, E-selectin, Selplg, and Pecam-1)22 linked to the interaction with vascular endothelial cells during neutrophil infiltration in SARS-CoV-2-infected mice by flow cytometry. Neutrophils in the pulmonary vasculature of Ob/Ob mice infected with MA-SARS-CoV-2 exhibited upregulated expression of CD62L, CD44, and E-selectin (Fig. 7). Analysis in this mouse model suggested that SARS-CoV-2 increases neutrophil adhesion to the vessel wall and induces platelet aggregation, which result in impaired pulmonary perfusion in the lungs.
Positive rates of the indicated adhesion molecules on pulmonary vascular neutrophils were determined in the lungs of C57BL/6J mice and Ob/Ob mice at 4 dpi. Bars in each panel indicate expression levels of adhesion factors determined to be positive. For naive, n = 4. For 4 dpi, CD62L (WT, n = 6; Ob/Ob, n = 7), CD44 and E-selectin (WT, n = 6; Ob/Ob, n = 6), Selplg (WT, n = 3; Ob/Ob, n = 3), and Pecam-1 (WT, n = 7; Ob/Ob, n = 7). Each dot represents data from an individual animal and the horizontal bars represent means ± SD. Source data are provided as a Source Data file.
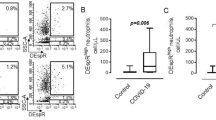
Finally, to determine whether the increased expression levels of adhesion molecules on the surface of vascular neutrophils in severe infections observed in SARS-CoV-2-infected mice is relevant to the pathogenesis of COVID-19 pneumonia in humans, we analyzed published data obtained with clinical samples from COVID-19 patients for adhesion-related gene expression. Using two published datasets of scRNA-seq analyses9,23 from peripheral blood mononuclear cells (PBMCs) from healthy volunteers, mild, and severe cases of COVID-19, we analyzed 474 adhesion-related gene24 expression changes and found that the levels of expression of four genes (CD44, SELL, ICAM3, and CD93) in the two datasets frequently differed between the healthy and severe groups (Fig. 8a, b). In the subset corresponding to neutrophils, the expression levels of the CD44 and SELL (which encodes CD62L) genes were significantly increased in COVID-19 severe cases compared to healthy individuals (Fig. 8c). These findings indicate that the expression of adhesion molecules is also increased in the vascular neutrophils in the lungs of severe COVID-19 cases, supporting the relevance of the data obtained in mice. In contrast, the gene expression of ICAM3 and CD93 was significantly decreased in the severe group compared to that in the healthy group. The expression levels of the SELL gene showed a positive correlation with the severity of COVID-19 in both the Wilk et al.9 and Xu et al.23 datasets and the CD44 gene expression level also positively correlated with COVID-19 severity in the Xu et al.23 dataset; the expression level of the CD44 gene was positively correlated when comparisons were made between healthy and nonventilated patients or ventilated patients in the Wilk et al.9. dataset. In contrast, the expression levels of the ICAM3 and CD93 genes negatively correlated with severity, suggesting that they could be molecular markers for the severity of COVID-19. Finally, blocking the function of the adhesion molecules by using anti-CD44 and anti-CD62L antibodies decreased the contact time between neutrophils and the vessel wall in the lungs of Ob/Ob mice infected with SARS-CoV-2-Venus, and increased the percentage of neutrophils flowing into the bloodstream (Fig. 9a, b, and Supplementary Movie 7). In contrast, inhibition of CD44 and CD62L function by using anti-CD44 and anti-CD62L antibodies did not affect the size of the platelet aggregates after SARS-CoV-2 infection (Fig. 9c).
a–c Analysis of published sc-RNA-seq datasets from COVID-19 clinical samples [Wilk et al. 9 and Xu et al. 23]. a Peripheral blood mononuclear cells from COVID-19 patients were clustered based on the gene expression of each cell and plotted as UMAP (upper panel). Comparison of UMAP between healthy individuals and COVID-19 patients (bottom panel). b Volcano plots of adhesion-related genes in PBMC neutrophils from COVID-19 patients. A total of 474 adhesion-related genes were analyzed in clusters corresponding to the neutrophils in (a). Purple plots indicate genes with significantly altered expression between healthy and severe cases and the dashed gray line indicates that the BH-corrected p-value is 0.05. c Violin plots of gene expression in PBMC neutrophils for four adhesion-related genes whose expression was significantly frequently altered in the two datasets between healthy and severe cases. NonVent (nonventilated) and Vent (ventilated) indicate mild and severe cases of COVID-19, respectively9. Each dot represents data from an individual cell. Source data are provided as a Source Data file.
a Ob/Ob mice were infected intranasally with 104 PFU MA-SARS-CoV-2-Venus, and on days 1, 2, and 3 after infection, anti-CD44 and anti-CD62L antibodies were administered intravenously. On day 4 after infection, a fluorochrome-conjugated anti-Ly-6G antibody was injected i.v. into the mice to track neutrophil movement. Tracking analyses were performed to obtain the trajectories of individual neutrophils (left panel, vascular structures are shown in white, and individual trajectories are coded in different colors). n = 5 biologically independent animals. b Assessment of neutrophil behaviors in (a) in terms of slow and rapid motion. c On day 4 after infection, a fluorochrome-conjugated anti-CD41 antibody was injected i.v. into the mice to visualize platelets. Histograms were plotted based on the size of the detected platelet (CD41-positive) signals (leftmost panel). n = 3 biologically independent animals. b, c Each dot represents data from an individual animal and the horizontal bars represent means ± SD. Source data are provided as a Source Data file.
Discussion
In this study, our in vivo imaging system revealed that the number of neutrophils in pulmonary vessels increases and neutrophil motility decreases in SARS-CoV-2-infected lungs. We also found that platelet aggregation is enhanced in infected lungs, with frequent formation of neutrophil-platelet complexes. The number of blood vessels through which erythrocytes flow for oxygen exchange was reduced in the infected mouse lungs, suggesting that this may contribute to SARS-CoV-2 pathogenesis. Moreover, we found that the expression of adhesion molecules is increased in pulmonary neutrophils in COVID-19 patients and mouse models, suggesting the existence of a common mechanism of SARS-CoV-2 virulence in humans and mice. Our in vivo imaging analysis thus revealed a novel mechanism of SARS-CoV-2 pathogenesis that could not be found using conventional histopathological and histochemical analyses, and flow cytometry.
Analysis of mouse models of severe or lethal SARS-CoV-2 infection showed that platelet aggregation is induced by neutrophil adhesion to the pulmonary vascular wall, resulting in impaired pulmonary perfusion. A major mechanism of thrombus formation in COVID-19 patients is thought to be the induction of platelet aggregation and thrombus formation by NETs released from neutrophils25. Complexes of neutrophils, platelets, and citrullinated histone H3, a marker of NETs, have been found during lung autopsies of COVID-19 patients26. Consistent with these findings, an analysis of human scRNA-seq data revealed a difference in the expression of the PADI4 gene (Supplementary Fig. 3), which is responsible for NET release25, suggesting that NET release was induced in severe COVID-19 cases. However, intravascular neutrophils in the infected lungs had a distinct cell membrane shape and slow motility and did not exhibit the apoptotic morphology that is associated with NET release. This discrepancy may reflect the multistep nature of thrombogenesis that has been postulated in vivo27; our findings of thrombus formation induced by neutrophil adhesion to the vessel wall may be an earlier phenomenon than the thrombus formation involving NETs. Since NET release by neutrophils is induced in response to PAMPs and DAMPs27, it is likely that NETs are released in response to the DAMPs that are released upon tissue damage and induce thrombus formation in the late stages of infection when the infection is more advanced. Our in vivo imaging analysis likely revealed an early stage of thrombus formation that was not available in the autopsy cases of COVID-19.
The expression of the adhesion molecules CD44 and CD62L was upregulated in the pulmonary intravascular neutrophils of the SARS-CoV-2-infected mice, and the expression levels of the genes encoding these factors were also upregulated in pulmonary neutrophils from severe COVID-19 cases. CD62L is expressed on leukocytes and is involved in intercellular adhesion; it has been widely implicated in immune cell responses, from cell migration to antigen presentation28. In neutrophils, CD62L is expressed on the membrane surface from the early stage of differentiation from progenitor cells and is involved in adhesion to vascular endothelial cells during migration21,28. The half-life of mature neutrophils is approximately 6–12 h in the bloodstream, and CD62L expression decreases with neutrophil aging, which is characterized by plasma membrane instability, activation of apoptotic signaling, and other events29. Neutrophil infiltration has been observed in the lungs of patients with COVID-19 and increases in the numbers of both mature and immature neutrophils have been reported14,30. In addition, it has been reported that immature aberrant neutrophils infiltrate the lungs of SARS-CoV-2-infected mice via the S100A8/A9-TLR4 pathway, and are involved in the pathogenesis31. COVID-19 patients have also been reported to have neutropenia, an increase in the number of neutrophils in the blood27, and in our mouse model, SARS-CoV-2 infection increased the number of neutrophils in the pulmonary vessels. Recently, Castanheira et al.32 reported that SARS-CoV-2 infection of a mouse model ubiquitously expressing hACE2 (CAG-AC-70) increased neutrophil numbers in pulmonary vessels. These findings suggest that SARS-CoV-2 infection promotes neutrophil proliferation in the bone marrow and migration to the infected lungs and that immature neutrophils expressing high levels of CD62L are recruited in large numbers to infected pulmonary vessels, leading to susceptibility to thrombus formation and impaired pulmonary perfusion. Consistent with this hypothesis, in the present study, we found that the expression levels of ICAM3, one of the membrane molecules induced by apoptotic signaling33, are significantly decreased in neutrophils from patients with severe COVID-19 compared to healthy controls.
CD44 is also an adhesion factor expressed on leukocytes and is involved in the adhesion and infiltration of neutrophils into the hepatic sinusoids34. It has been reported that neutrophil adhesion is suppressed in the hepatic sinusoids of Cd44-deficient mice with LPS-induced liver injury35. Although the mechanism of Cd44 gene expression in neutrophils remains unclear, we do know that Cd44 expression is increased in monocytes in response to inflammatory cytokines36. In severe cases of COVID-19, inflammatory mediators induced by SARS-CoV-2 infection may also increase CD44 gene expression in neutrophils, contributing to increased adhesion to pulmonary vessels. In addition, it has been reported that neutrophil adhesion to pulmonary blood vessels increases in the LPS-induced septic mouse model, leading to impaired blood flow and the increased expression of adhesion molecules (CD11b and CD18) in neutrophils37. Therefore, the pathological mechanism by which adhesion molecules that are upregulated in neutrophils induce pulmonary blood flow impairment might be a mechanism common to certain inflammatory conditions.
Our study has several limitations that require further investigation. Changes in the expression levels of membrane molecules in vascular endothelial cells in SARS-CoV-2-infected lungs were not examined in this study, and it is unclear how vascular endothelial cells are involved in neutrophil adhesion to the pulmonary vessel walls. It has been hypothesized that thrombus formation proceeds because of dysfunctional endothelial responses to SARS-CoV-2 infection38,39. However, at least in our mouse model, neutrophil adhesion to the vessel wall occurred not only in the proximity of the infected vascular endothelial cells but also away from SARS-CoV-2-infected cells (Figs. 1b and 5a). Another limitation is that it is unclear whether the upregulation of the CD44 and SELL genes in the SARS-CoV-2-infected mouse model and in COVID-19 patients occurs in the entire neutrophil populations or in some subpopulations of neutrophils. There have been many reports of neutrophil heterogeneity23,40,41, suggesting that SARS-CoV-2 infection might induce a subpopulation of neutrophils that express the CD44 or SELL genes at high levels. In addition, the extent to which impaired pulmonary perfusion due to neutrophil adhesion to the pulmonary vascular wall contributes to the lethality of SARS-COV-2 pneumonia is unknown. Finally, blocking the function of adhesion molecules with neutralizing antibodies decreased the contact time between neutrophils and the vessel wall in the lungs of Ob/Ob mice infected with SARS-CoV-2-Venus; however, inhibition of CD44 and CD62L function did not significantly affect platelet aggregate size (Fig. 9a–c). Therefore, it may be possible to improve the pathological condition more significantly by inhibiting the function of adhesion factors in addition to CD44 and CD62L. Since adhesion molecules are involved in the migration and infiltration of neutrophils in response to SARS-CoV-2 infection, dysfunction of adhesion molecules by neutralizing antibodies may also interfere with the protective function of neutrophils against SARS-CoV-2 infection, which has not been fully explored.
Beyond the acute respiratory infection, symptoms such as shortness of breath, cough, arthralgia, myalgia, fatigue, headache, odor/taste disturbance, and palpitations have been reported as COVID-19 sequelae (also called long COVID) even after the infection has resolved42. It has been shown that prolonged post-ischemic symptoms are more likely to occur in groups at high risk for severe COVID-19, particularly the elderly and those with a high BMI (obesity)43. It has also been suggested that microembolization-induced tissue damage may contribute to COVID-19 sequelae44. In the present study, using in vivo imaging of mouse models, we found that the formation of microthrombi composed of neutrophils and platelets impairs pulmonary blood flow and exacerbates COVID-19 pneumonitis. The pathological mechanisms of COVID-19 revealed by this study will lead to the development of more effective treatments for patients with severe COVID-19 and long COVID.
Methods
Ethics statement. All experiments were performed in enhanced biosafety level 3 (BSL3) containment laboratories at the University of Tokyo, which are approved for such use by the Ministry of Agriculture, Forestry, and Fisheries, Japan. The protocols for the animal studies were approved by the University of Tokyo (approval numbers PA19-72 and PA21-07). The ambient conditions of the animal facilities were set at 25 °C and 50% humidity. Animals were acclimated to the facilities prior to the start of the experiments, maintained on a 12-h on/off light cycle, and given access to food and water ad libitum.
Viruses. We generated SARS-CoV-2-Venus, in which the fluorescent reporter gene Venus was inserted, by using reverse genetics. For the construction of SARS-CoV-2-Venus, we replaced the ORF 8 genes of pBAC SARS2 wk521 with the Venus gene by use of recombination and designated the infectious cDNA clone pBAC SARS-CoV-2-Venus. Mouse-adapted (MA)-SARS-CoV-2 and MA-SARS-CoV-2-Venus were also generated by using reverse genetics previously described20. Virus strains were propagated in VeroE6/TMPRSS2 (JCRB 1819) cells45.
Cells. VeroE6/TMPRSS2 (JCRB 1819) cells45 were propagated in 1 mg/ml geneticin (G418; Invitrogen) and 5 μg/ml plasmocin prophylactic (Invitrogen) in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% Fetal Calf Serum (FCS). The cells were regularly tested for mycoplasma contamination by using PCR and confirmed to be mycoplasma-free.
Mice. Eight-week-old hemizygous K18-hACE2 C57BL/6J female mice (strain 2B6.Cg-Tg(K18-ACE-2)2Prlmn/J), 24-week-old C57BL/6J HamSlc-ob/ob female mice, and five-week-old Streptozotocin (STZ)-induced diabetic C57BL/6J male mice were purchased from the Jackson Laboratory Japan. In addition, 24-week-old B6-NASH male mice, B6.KOR/StmSlc-Apoeshl female mice, SAMR1/TaSlc [Senescence-Accelerated Mouse (SAM); senescence-Resistant inbred strains (R)] male mice, SAMP8/TaSlc [Senescence-Accelerated Mouse (SAM); senescence-Prone inbred strains (P)] male mice, and SAMP10-ΔSglt2 male mice were purchased from Japan SLC Inc. KK-Ay/Ta Jcl female mice (24-week-old) were purchased from CLEA Japan Inc. Age- and sex-matched C57BL/6J mice, which served as controls, were purchased from the same vendor as each disease model mouse strain. Eight-week-old k18-hACE2 Tg mice were used in the analyses because they exhibited severe SARS-CoV-2 pneumonia compared to wild-type mice of the same age. The genetically modified mouse strains with underlying diseases were all matched at 24 weeks of age, except for the STZ-induced type 1 diabetes mouse model. STZ-induced type 1 diabetes model mice die within weeks without therapeutic treatment; therefore, we had to use them for the infection experiments immediately after STZ treatment (at 5 weeks of age). In this study, we used female mice to be consistent with our previous studies20,46. For STZ-induced diabetic mice and SAMR1/P8/P10 mice, we used male mice available from the suppliers.
Experimental infection of mice. Mice were intranasally inoculated with 101–105 PFU of SARS-CoV-2-Venus, MA-SARS-CoV-2, or MA-SARS-CoV-2-Venus. Body weights were measured before infection and then daily. C57BL/6J HamSlc-ob/ob mice were infected intranasally with 104 PFU of MA-SARS-CoV-2-Venus, and on days 1, 2, and 3 after infection, 100 µg of anti-mouse CD44 (IM7, Bioxcell) and anti-mouse CD62L (Mel-14, Bioxcell) antibodies, or the corresponding isotype control (2A3 and LTF-2, Bioxcell), was administered intravenously.
Pathological examination. Excised lung tissues were fixed in a 4% paraformaldehyde phosphate buffer solution and processed for paraffin embedding. The paraffin blocks were cut into 3-µm-thick sections and then the sections were stained using a standard hematoxylin and eosin procedure. In addition, tissue sections were stained with a rabbit polyclonal antibody for SARS-CoV nucleocapsid protein (ProSpec; ANT-180, 1:500 dilution, Rehovot) for immunohistochemical analyses. Specific antigen-antibody reactions were visualized by means of 3,3′-diaminobenzidine tetrahydrochloride staining using the Dako Envision system (Dako Cytomation; K4001, 1:1 dilution). Histopathological scores for pneumonia were determined based on the percentage of alveolar inflammation in a given area of a pulmonary section (area score), inflammatory cell density (density score), and the presence of pulmonary edema and/or alveolar hemorrhage (severity score) by using the following scoring system: Area score 0, no inflammation; 1, affected area (≤50%); and 2, affected area (50%). Density score 0, inflammation observed only in the high-power field; 1, inflammation observed in low-power fields; and 2, alveoli filled with inflammatory cells. Severity score 0, absence of pulmonary edema and/or alveolar hemorrhage; and 1, presence of pulmonary edema and/or alveolar hemorrhage. The total score for the five lobes was calculated for each animal; therefore, the scores for individual animals ranged from 0 to 25.
Virus titration assay. C57BL/6J mice and HamSlc-ob/ob mice were intranasally inoculated with 103 PFU of MA-SARS-CoV-2. Two and five days post-infection (dpi), the animals were euthanized and their organs (lungs, nasal turbinate, brain, heart, liver, spleen, kidneys, and intestine) were collected. Confluent VeroE6/TMPRSS2 cells in 12-well plates were infected with 100 μl of a dilution of the organ homogenate. The virus inoculum was removed after incubation for 1 h at 37 °C, and then 1% agarose solution in DMEM was overlaid on the cells. After incubation for 48 h, the agar-covered cells were fixed with 10% neutral buffered formalin. The plaques were counted after the removal of the agar.
Micro-CT imaging. C57BL/6J mice and HamSlc-ob/ob mice were inoculated intranasally with 103 PFU of MA-SARS-CoV-2. Lungs of infected mice were imaged by using an in vivo micro-CT scanner (CosmoScan FX; Rigaku). Under ketamine-xylazine anesthesia, the animals were placed in the imaging chamber and scanned for 2 min at 90 kV, 88 μA, FOV 45 mm, and pixel size 90.0 μm. After scanning, the lung images were reconstructed by using the CosmoScan Database software of the micro-CT version 3.5.5.100 (Rigaku Corporation) and analyzed using the manufacturer-supplied software.
In vivo imaging of mouse lung. The in vivo imaging was performed by using an LSM 980 NLO (Carl Zeiss) equipped with an infrared laser (Chameleon Vision II, Coherent)10,11. K18-hACE2 mice, C57BL/6J mice, and C57BL/6J HamSlc-ob/ob mice were infected with 105 PFU of SARS-CoV-2-Venus or 104 PFU of MA-SARS-CoV-2-Venus. The infected mice were intubated under anesthesia and ventilated at a respiratory rate of 120 breaths per minute. Isoflurane was continuously delivered at 2% to maintain anesthesia. The left lung lobe of the mice was exposed and gently immobilized with a custom-made thoracic suction window. In all experiments, Texas red dextran (70,000 Da, Invitrogen), 5 µg of Phycoerythrin (PE)-conjugated rat anti-mouse CD41 antibody (MWReg30; BD Biosciences), and 5 µg of Alexa Fluor 594-conjugated rat anti-mouse Ly-6G antibody (1A8, Biolegend) were injected i.v. before imaging to visualize the lung vascular structures, platelets, and vascular neutrophils, respectively. For the analyses of pulmonary perfusion, mice infected with SARS-CoV-2-Venus or MA-SARS-CoV-2-Venus were i.v. inoculated with Dio-labeled erythrocytes. A maximal intensity projection of the indicated frames (0–10 min) was generated to show the functional capillary perfused by the erythrocytes, as described previously37. To acquire images in spectral imaging mode, lasers at wavelengths 488 nm, 543 nm, and 910 nm were used for simultaneous excitation of fluorochromes and Venus. All emitted light between 490- and 695-nm wavelengths was detected by using a 20× water-immersion lens (Carl Zeiss). Spectral separation of the acquired lambda stacks was achieved by using the linear unmixing function of the LSM software ZEN blue 3.3 (Carl Zeiss). Processing, assays, and data visualization were performed using CellProfiler 4.2.4 (Broad Institute), and in-house MATLAB (2022a) scripts (MathWorks). Tracking of the neutrophils in the denoised movies was performed by TrackMate (ImageJ, NIH).
Flow cytometry. Mouse lungs were dissociated using a Lung Dissociation Kit (Miltenyi) and gentleMACS Dissociator (Miltenyi) according to the manufacturer’s instructions for flow cytometry (FCM). Samples were then filtered through a 70-μm filter (Miltenyi) after red blood cell lysis and resuspended for subsequent FCM staining. For experiments staining intravascular neutrophils, mice were injected i.v. with 5 µg of PE-conjugated rat anti-mouse Ly-6G antibody (1A8, Biolegend) 5 min before lung collection. For surface staining, cells were stained for 10 min with antibodies in PBS containing 0.5% BSA and 2 mM EDTA. The following antibody clones were diluted 1:100 and used in these studies: Vio-Green-CD45 (REA737, Miltenyi), PE-NK1.1 (REA1162, Miltenyi), PE-Vio615-CD4 (REA604, Miltenyi), PE-Vio770-B220 (REA755, Miltenyi), APC-CD3 (REA641, Miltenyi), APC-Vio770-CD8a (REA601, Miltenyi), Vio-Blue-MHC class II (REA813, Miltenyi), FITC-Ly-6C (REA796, Miltenyi), PE-Vio615-Ly-6G (REA526, Miltenyi), PE-Vio770-CD11c (REA754, Miltenyi), APC-Siglec-F (REA798, Miltenyi), APC-Vio770-CD11b (REA592, Miltenyi), APC-CD44 (IM7 Biolegend), APC- Pecam-1 (W18222B, Biolegend), APC-CD62L (MEL-14, Proteintech), APC-CD62E (P2H3, Invitorogen), and APC-CD162 (4RA10, Elabscience). APC conjugation to the anti-CD62E mouse IgG1 antibody was performed using the APC Labeling Kit-NH2 (Wako) according to the manufacturer’s protocol.
Populations of immune cells were defined as follows: B cells (CD45+ CD3− B220+), NK cells (CD45+ CD3− B220− NK1.1+), CD4 T cells (CD45+ CD3+ B220− CD4+ CD8−), CD8 T cells (CD45+ CD3+ B220− CD4− CD8+), alveolar macrophages (CD45+ CD11bdim Siglec-F+ CD11c+ MHC class II+), dendritic cells (CD45+ CD11b− Siglec-F− CD11c+), neutrophils (CD45+ CD11bhigh Ly-6G+), eosinophils (CD45+ CD11bhigh Siglec-F+ Ly-6G− CD11c−), and monocytes (CD45+ CD11bhigh Siglec-F− Ly-6G− MHC class II− Ly-6Chigh). Samples were analyzed on a flow cytometer (MACSQuant Tyto, Miltenyi).
Neutrophil motility analysis. To track the movement of neutrophils, 5 µg of PE-conjugated rat anti-mouse Ly-6G antibody was injected i.v. into the mice. Neutrophils were imaged at approximately 4 fps for 230 s. All movies were corrected for respiratory motion artifacts and denoised. Single object tracking was performed by using TrackMate (ImageJ, NIH) to obtain the trajectories of individual neutrophils. For each neutrophil, speeds were measured for individual steps in its trajectory and subsequently defined as slow (≤50 µm/s) or rapid (>50 µm/s). We then examined whether a neutrophil performed rapid movement and calculated the durations it engaged in continuous slow movements without being interrupted by the rapid movement.
Quantification of platelet aggregates. CD41 signals were detected in a semi-automated manner by using CellProfiler (Broad Institute), and then divided into three populations according to their sizes: signals covering <8.57 µm2 (50 pixels) were defined as a “single platelet”, signals ≥8.57 µm2 and <34.28 µm2 (200 pixels) as “aggregated platelets”, and signals ≥34.28 µm2 as “thrombocytes”. The frequency analysis of the CD41 signals was conducted using in-house MATLAB scripts (MathWorks).
In vivo depletion of neutrophils. C57BL/6J HamSlc-ob/ob were administered 100 µg of anti-rat Kappa immunoglobulin (MAR18.5, Bioxcell) daily for two days prior to infection as described previously47. In addition, 50 µg of Anti-Ly-6G (1A8, Bioxcell) and corresponding isotype control (2A3, Bioxcell) were administered every other day from one day prior to infection. When mice were sequentially injected with two antibodies, an interval of more than 2 h was set between injections.
scRNA-seq data re-analysis. We reanalyzed two published PBMC scRNA-seq datasets for healthy and SARS-CoV-2-infected humans9,23. For the published data from Wilk et al.9 preprocessed scRNA-seq count data with embedding, clustering, and cell-type assignment were obtained as an RDS file from the COVID-19 Cell Atlas (https://www.covid19cellatlas.org/#wilk20) hosted by the Wellcome Sanger Institute. For the published data from Xu et al.23 we downloaded transcript-by-cell matrices from NCBI (GSE216020) and preprocessed using the Seurat v448 package and DoubletFinder49, which identifies and removes potential doublets23. Cells with less than 500 UMI counts, less than 200 detected genes, and more than 20% mitochondrial gene counts were removed as low-quality cells, as well as potential doublets. Data integration of all samples was performed using the FindIntegrationAnchors and IntegrateData functions in Seurat with the top 3000 most variable genes selected by the FindVariableFeatures function. Cell-type annotation was based on marker genes of each cluster defined by FindAllMarkers functions. The subset corresponding to neutrophils in both datasets (Wilk et al. and Xu et al.) was used for differential gene expression analysis related to cell adhesion24 and neutrophil extracellular trap formation (KEGG: hsa04613), respectively.
Statistics and reproducibility. All animals were randomly allocated to experimental groups. No blinding was performed in any experiment. Sample sizes were based on our previous work. GraphPad Prism 10.3.1, R 4.1.0, and Rstudio 2023.06.1+524 were used to analyze all data, and differences were considered to be statistically significant when the p-value was less than 0.05. No adjustment for multiple comparisons was performed except for the differential gene expression analysis of the scRNA-seq data (Fig. 8c and Supplementary Fig. 3). For Figs. 1d, 2b, d, 5c, d, f, and 9b, data were analyzed by using non-parametric, two-tailed Student’s t-test and p-values are shown in each figure. For Fig. 2c, data were analyzed by using Dunnett’s test and p-values are shown in the figure. For Figs. 4a, e, 5b, and 7, data were analyzed by using Sidak’s test and p-values are shown in each figure. For Fig. 4d, data were analyzed by using the Mann-Whitney test. For Fig. 8c and Supplementary Fig. 3, data were analyzed by using the Wilcoxon signed rank test and the p-values were corrected by using the Benjamini-Hochberg Procedure and are shown in each figure. For Figs. 1b–d, 2d, e, 4b, 5a, and 6a, b, at least three experiments were performed with biologically independent animals and similar results were obtained. Figure 2d is a microphotograph cropped from Fig. 1b.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The scRNA-seq data used in this study are available in the Gene Expression Omnibus database under accession code GSE150728 and GSE216020. Source data are provided with this paper.
Code availability
The code used in this study is available at: https://github.com/tken18/2024NatCom_Neutrophil.
References
Jackson, C. B., Farzan, M., Chen, B. & Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 23, 3–20 (2022).
Lan, J. et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581, 215–220 (2020).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020).
Yang, L. et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 5, 128 (2020).
Merad, M., Blish, C. A., Sallusto, F. & Iwasaki, A. The immunology and immunopathology of COVID-19. Science 375, 1122–1127 (2022).
Paludan, S. R. & Mogensen, T. H. Innate immunological pathways in COVID-19 pathogenesis. Sci. Immunol. 7, eabm5505 (2022).
Sette, A. & Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184, 861–880 (2021).
Wilk, A. J. et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 26, 1070–1076 (2020).
Ueki, H. et al. In vivo imaging of the pathophysiological changes and neutrophil dynamics in influenza virus-infected mouse lungs. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1806265115 (2018).
Ueki, H., Wang, I. H., Zhao, D., Gunzer, M. & Kawaoka, Y. Multicolor two-photon imaging of in vivo cellular pathophysiology upon influenza virus infection using the two-photon IMPRESS. Nat. Protoc. https://doi.org/10.1038/s41596-019-0275-y (2020).
Bao, L. et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583, 830–833 (2020).
Rizvi, Z. A. et al. Omicron sub-lineage BA.5 infection results in attenuated pathology in hACE2 transgenic mice. Commun. Biol. 6, 935 (2023).
Yang, L. et al. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 6, 255 (2021).
Blair, T. A., Michelson, A. D. & Frelinger, A. L. Mass cytometry reveals distinct platelet subtypes in healthy subjects and novel alterations in surface glycoproteins in Glanzmann Thrombasthenia. Sci. Rep. 8, 10300 (2018).
McCray, P. B. Jr et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 81, 813–821 (2007).
Winkler, E. S. et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 21, 1327–1335 (2020).
Chu, H., Chan, J. F. & Yuen, K. Y. Animal models in SARS-CoV-2 research. Nat. Methods 19, 392–394 (2022).
Leist, S. R., Schäfer, A. & Martinez, D. R. Cell and animal models of SARS-CoV-2 pathogenesis and immunity. Dis. Models Mech. https://doi.org/10.1242/dmm.046581 (2020).
Ueki, H. et al. Development of a mouse-adapted reporter SARS-CoV-2 as a tool for two-photon in vivo imaging. Viruses https://doi.org/10.3390/v16040537 (2024).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Chu, D., Dong, X., Shi, X., Zhang, C. & Wang, Z. Neutrophil-based drug delivery systems. Adv. Mater. 30, 1706245 (2018).
Xu, J. et al. Heterogeneity of neutrophils and inflammatory responses in patients with COVID-19 and healthy controls. Front. Immunol. https://doi.org/10.3389/fimmu.2022.970287 (2022).
Zhong, X., Drgonova, J., Li, C. Y. & Uhl, G. R. Human cell adhesion molecules: annotated functional subtypes and overrepresentation of addiction-associated genes. Ann. N. Y. Acad. Sci. 1349, 83–95 (2015).
Ackermann, M. et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 28, 3125–3139 (2021).
Middleton, E. A. et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136, 1169–1179 (2020).
Gorog, D. A. et al. Current and novel biomarkers of thrombotic risk in COVID-19: a consensus statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 19, 475–495 (2022).
Ivetic, A., Hoskins Green, H. L. & Hart, S. J. L-selectin: a major regulator of leukocyte adhesion, migration and signaling. Front. Immunol. 10, 1068 (2019).
Adrover, J. M., Nicolás-Ávila, J. A. & Hidalgo, A. Aging: a temporal dimension for neutrophils. Trends Immunol. 37, 334–345 (2016).
Schulte-Schrepping, J. et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 182, 1419–1440.e1423 (2020).
Guo, Q. et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe 29, 222–235.e224 (2021).
Castanheira, F. V. S. et al. Intravital imaging of three different microvascular beds in SARS-CoV-2–infected mice. Blood Adv. 7, 4170–4181 (2023).
Kaplanski, G., Marin, V., Montero-Julian, F., Mantovani, A. & Farnarier, C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 24, 25–29 (2003).
Khan, A. I. et al. Role of CD44 and hyaluronan in neutrophil recruitment1. J. Immunol. 173, 7594–7601 (2004).
McDonald, B. et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J. Exp. Med. 205, 915–927 (2008).
Brown, K. L., Maiti, A. & Johnson, P. Role of sulfation in CD44-mediated hyaluronan binding induced by inflammatory mediators in human CD14+ peripheral blood monocytes1. J. Immunol. 167, 5367–5374 (2001).
Park, I. et al. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur. Respir. J. 53, 1800786 (2019).
Jin, Y. et al. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 5, 293 (2020).
Xu, S.-W., Ilyas, I. & Weng, J.-P. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 44, 695–709 (2023).
Beyrau, M., Bodkin, J. V. & Nourshargh, S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2, 120134 (2012).
Silvestre-Roig, C., Hidalgo, A. & Soehnlein, O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 127, 2173–2181 (2016).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Sudre, C. H. et al. Attributes and predictors of long COVID. Nat. Med. 27, 626–631 (2021).
Katsoularis, I. et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ 377, e069590 (2022).
Matsuyama, S. et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl Acad. Sci. USA 117, 7001–7003 (2020).
Halfmann, P. J. et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 603, 687–692 (2022).
Boivin, G. et al. Durable and controlled depletion of neutrophils in mice. Nat. Commun. 11, 2762 (2020).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337.e324 (2019).
Acknowledgements
We thank S. Watson for editing the manuscript. We also thank Yuko Sato and Seiya Ozono for their technical assistance. This research was supported by a Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (AMED) (JP19fk0108113, JP20fk0108412, JP21fk0108552, JP243fa627001) by a Japan Program for Infectious Diseases Research and Infrastructure from AMED (JP24wm0125002), the Japan Society for the Promotion of Science (JSPS) (21K14984), the Japan Science and Technology Agency (JST) (Moonshot R&D) (JPMJMS2025), and by the NIAID-funded Center for Research on Influenza Pathogenesis (CRIP; HHSN272201400008C). H.U. was supported by GSK Japan Research Grant 2020, the Astellas Foundation for Research on Metabolic Disorders, the Naito Foundation, the Sumitomo Foundation, the Ichiro Kanehara Foundation, the Uehara Memorial Foundation, the Okinaka Memorial Institute for Medical Research, a Japanese Respiratory Foundation Grant, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the SENSHIN Medical Research Foundation, the Takeda Science Foundation and Kao Research Council for the Study of Healthcare Science. IH.W., HW.H., and CH.W. were supported by the National Science and Technology Council, Taiwan (MOST-110-2320-B-001-005-MY3). M.I. was supported by the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
H.U., M.K., S.I., S.M., and T.S. performed the mouse infection experiments, titrated the virus in organs, and analyzed pathology. M.U. analyzed the micro-CT images. H.U. performed the in vivo imaging analysis and flow cytometry. H.U., IH.W., HW. H., and CH.W. performed image data analyses. W.K. generated the Venus-expressing SARS-CoV-2. H.U., K.H., and E.K. analyzed the single-cell RNA-seq data. H.U., M.I, T.S., and Y.K. obtained funding. H.U. and Y.K. conceived the study and supervised the research. H.U. and Y.K. wrote the initial draft, with all other authors providing editorial comments.
Corresponding authors
Ethics declarations
Competing interests
Y.K. has ongoing unrelated collaborations and/or sponsored research agreements with Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Shionogi & Co. Ltd, Otsuka Pharmaceutical, and KM Biologics and has received royalties from MedImmune and Integrated Biotherapeutics. The remaining authors have no competing interests to declare.
Peer review
Peer review information
Nature Communications thanks Zhichao Ai, Pradeep Uchil, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ueki, H., Wang, IH., Kiso, M. et al. Neutrophil adhesion to vessel walls impairs pulmonary circulation in COVID-19 pathology. Nat Commun 16, 455 (2025). https://doi.org/10.1038/s41467-024-55272-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55272-0