Abstract
Electrochemical alcohol oxidation (EAO) represents an effective method for the production of high-value carbonyl products. However, its industrial viability is hindered by suboptimal efficiency stemming from low reaction rates. Here, we present a synergistic electrocatalysis approach that integrates an active electrode and aminoxyl radical to enhance the performance of EAO. The optimal aminoxyl radical (4-acetamido-2,2,6,6-tetramethylpiperidine 1-oxyl) and Ni0.67V0.33-layered double hydroxide (LDH) are screen as cooperative electrocatalysts by integrating theoretical predictions and experiments. The Ni0.67V0.33-LDH facilitates the adsorption and activation of N-(1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl)acetamide (ACTH) via interactions with ketonic oxygen, thereby improving selectivity and yield at high current densities. The electrolysis process is scaled up to produce 200 g of the steroid carbonyl product 8b (19-Aldoandrostenedione), achieving a yield of 91% and a productivity of 243 g h-1. These results represent a promising method for accelerating electron transfer to enhance alcohol oxidation, highlighting its potential for practical electrosynthesis applications.
Similar content being viewed by others
Introduction
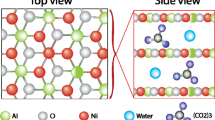
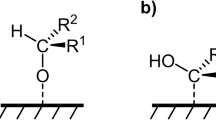
Electrochemical alcohol oxidation (EAO) has garnered increasing attention in recent years due to its capacity to facilitate efficient alcohol oxidation for pharmaceutical synthesis and biomass conversion1,2,3,4,5. In general, EAO can be performed either directly at the electrode surface or indirectly in solution using a mediator6,7,8. In the direct EAO process, the reacting molecules are oxidized at the electrode surface, and metal oxides, which act as heterogeneous electrocatalysts, have been extensively studied (Fig. 1a)9,10,11,12,13,14. Previous studies have systematically investigated the electrocatalytic mechanism of nickel-based oxides/(oxy)hydroxides for EAO, demonstrating that the Ni2+/Ni3+ redox couple can enhance the EAO process15,16,17,18,19. Particularly, the incorporation of various metallic elements with differing valence states into Ni-based hydroxides facilitates the formation of flexible redox centers, thereby boosting the activity of electrocatalysts20,21,22,23. However, this approach is susceptible to unforeseen side reactions or over-oxidation problems at high current densities, resulting in lower selectivity or yield of the target product. In the indirect (mediated) EAO process, aminoxyl radicals have been studied in detail as homogeneous electrocatalysts (Fig. 1b), such as TEMPO (2,2,6,6-tetramethyl-1-piperidine-N-oxyl) and ACT (4-acetamido-TEMPO)24,25,26. The mechanism of electrochemical aminoxyl radical-mediated alcohol oxidation under basic conditions has also been investigated2,7,27,28. The oxoammonium cation forms through the electrochemical oxidation of the aminoxyl radical at the electrode surface, which subsequently reacts with alcohol to produce the corresponding hydroxylamine and carbonyl product28. The indirect EAO process operates at moderate potentials and can tolerate diverse functional groups, but the low current density of the oxidation reaction is due to the slow electron transfer rate of the reaction intermediates. Therefore, it is imperative to enhance the optimization of the reaction kinetics in the EAO process to meet the prerequisites for industrial application.
To achieve rapid electrochemical kinetics in the EAO process, it is essential to facilitate electron transfer between the electrocatalysts and the organic intermediates. The regeneration of the oxoammonium cation from hydroxylamine in the indirect EAO process can proceed via two different pathways: the comproportionation of oxoammonium and hydroxylamine, or the direct 2e−/1H+ proton-coupled electron transfer (PCET) oxidation of hydroxylamine27,29. Efficient PCET oxidation of hydroxylamine could provide a means to enable alcohol oxidation with reduced overpotential and potentially could lead to increased EAO reaction rates30,31. This concept has been demonstrated through an “integrated cooperativity” mechanism using a Cu/aminoxyl catalyst, in which CuII and the aminoxyl participate together to mediate alcohol oxidation31. The reaction rate and turnover frequency of the (bpy)Cu/TEMPO catalytic system was significantly higher than those of TEMPO alone. Hydroxylamine oxidation also may be accomplished through a “serial cooperativity” mechanism, identified with an iron(III) nitrate/aminoxyl co-catalyst system. This pathway features a redox cascade wherein the alcohol is oxidized by an in situ-generated oxoammonium species32. Recently, several studies have demonstrated synergistic effects between metal oxides or other heterogeneous electrocatalysts and aminoxyl radicals in alcohol oxidation33,34,35,36. The complexity of the reaction has, however, resulted in significant challenges in the development process, largely due to the intricate nature of the cooperative catalyst-mediated EAO, which involves heterogeneous electrocatalysts and aminoxyl radicals. Despite considerable efforts, the mechanism of EAO remains elusive, making it particularly challenging to develop EAO systems. Consequently, the search for a synergistic electrocatalyst-mediated EAO reaction mechanism is of paramount importance for the resolution of the aforementioned development dilemma.
Herein, we introduce an alternative strategy to enhance EAO through a synergistic electrocatalytic system using Ni-based layered double-hydroxide (LDH) electrocatalysts combined with aminoxyl radicals (Fig. 1c). A series of different heterogeneous Ni-based LDH electrocatalysts, including Ni alone, NiV, NiMn, NiFe, NiCo, NiCu materials, were evaluated with different aminoxyls, including TEMPO, 4-HO-TEMPO, 4-MeO-TEMPO, ACT, 4-oxo-TEMPO. The results indicate, which are also analyzed using density-functional theory (DFT) calculations, reveal that the NiV electrocatalyst in combination with ACT exhibits excellent performance. Additionally, the results suggest that N-(1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl)acetamide (ACTH) adsorbs onto the NiV surface. A Ni0.67V0.33-LDH/ACT electrochemical co-catalyst system demonstrates excellent EAO performance at high current densities in a continuous flow electrolyzer, with benzaldehyde selectivity and yield reaching up to 99%. This cooperative electrocatalyst system demonstrates a broad substrate scope and functional-group tolerance. The electrolyzer was scaled up to enable the synthesis of a high-value steroid carbonyl product with a productivity of 243 g h−1 at a large current electrolysis of 60 A. This work highlights the utility of synergistic heterogeneous and homogeneous electrocatalysts and provides mechanistic insights into the basis for this behavior.
Results
Preliminary reactivity studies
TEMPO and related aminoxyl radicals have been widely employed for electrochemical alcohol oxidation (EAO)24,25,26,28,29,37,38. In this study, we selected five aminoxyl radicals as model electrocatalysts (Table 1) due to their varying oxoammonium/aminoxyl redox potentials resulting from differences in their R-functional groups. The cyclic voltammograms (CVs) of TEMPO (a), AcNH-TEMPO (ACT, b), and 4-MeO-TEMPO (c) reveal reversible electron transfer (Supplementary Fig. 1), demonstrated good stability in 0.5 M Na2CO3 electrolyte (pH 11.5). In contrast, 4-HO-TEMPO (d) and 4-oxo-TEMPO (e) exhibit irreversible behavior under the same conditions, reflecting the instability of the corresponding oxoammonium species (4-HO-TEMPO+, 4-oxo-TEMPO+), which are susceptible to base-promoted ring opening2,26. Among them, ACT exhibits a mid-point potential. Nickel oxyhydroxide materials (NiOOH) are also promising heterogeneous electrocatalysts for EAO10,11,15,33,34,39,40,41. The resting state of NiOOH is Ni(OH)2 during the open-circuit conditions. We wondered whether the two electrocatalysts could be combined to achieve synergistic benefits for EAO. Benzyl alcohol (BA) was chosen as a model substrate for EAO, and the BA oxidation reactions were conducted under constant-potential conditions (1.45 V vs. RHE) in a continuous-flow parallel-plate electrolyzer with an electrode area of 10 cm2, in which Ni-based materials as the anode and nickel foam as the cathode (see the Methods section for details). Gas chromatography (GC) was utilized to monitor and quantify the concentration changes of the organic compounds. The synthesis and characterization of the NiM-LDH materials are elaborated below and in the Supporting Information (see the Methods section for details). Attempts to perform the direct oxidation of BA at 1.45 V vs. RHE using a Ni(OH)2-impregnated graphite-felt (GF) electrode resulted in a low yield of the target product benzaldehyde (PhCHO) (5%, Table 1, entry 1). Better results were obtained with 5 mol% AcNH-TEMPO (ACT) rather than with a heterogeneous electrocatalyst, increasing the yield of PhCHO to 38% (entry 2). The yield further increased when both Ni(OH)2 and ACT were used together (52%, entry 3), raising the prospect of a synergistic effect that exceeds the sum of the two individual catalysts.
Analysis of heterogeneous Ni(OH)2-based materials that feature other metals within the lattice were only marginally better as heterogeneous electrocatalysts (e.g., entry 4); however, a NiV-LDH material with a Ni0.67V0.33 stoichiometry (described further below) paired with ACT led to a nearly quantitative yield of PhCHO (entry 5). Other NiM-LDH materials combined with ACT were less effective than the NiV-LDH/ACT catalyst system (M = Mn, Fe, Co, Cu; entries 6–9). The low activity of Mn, Fe, Co, and Cu as co-catalysts may result from their limited capacity to promote the generation of NiOOH, leading to insufficient ACT+ regeneration and, consequently, poor EAO performance. Furthermore, ACT is superior to TEMPO (entry 10), 4-HO-TEMPO (entry 11), 4-MeO-TEMPO (entry 12), and 4-oxo-TEMPO (entry 13).
Computational analysis of aminoxyl interactions with NiM-LDH materials
Density-functional theory (DFT) calculations were performed to investigate electrocatalytic reactivity. Fig. 2a displays the reactivity of various aminoxyl radicals on a clean NiOOH electrode surface. The adsorption energies for H-TEMPOH, 4-HO-TEMPOH, 4-MeO-TEMPOH, AcNH-TEMPOH (ACTH), and 4-oxo-TEMPOH were calculated to be −0.44, −0.96, −0.15, −0.73, and −0.68 eV, respectively (Supplementary Fig. 3). The adsorption energy for the above components follows the order of 4-HO-TEMPOH > AcNH-TEMPOH > 4-oxo-TEMPOH > H-TEMPOH > 4-MeO-TEMPOH. Despite the strongest interaction between 4-HO-TEMPOH and NiOOH surface, the oxidative activity of 4-HO-TEMPO is attenuated in alkaline environments, attributed to deprotonation of the HO- group, reduction in the electron density of the aminoxyl radical and impairing its oxidative capability, which is also reflected in the immense instability of 4-HO-TEMPO in the CV curves (supplementary Fig. 1). Therefore, the ACT exhibits good adsorption and superior electrocatalytic activity. Further calculations were performed to evaluate ACTH adsorption onto various NiM-LDH materials, with M = V, Mn, Fe, Co, and Cu. The V-doped NiOOH material exhibited the stronger adsorption of ACTH (Fig. 2b), with adsorption energy calculated to be −2.04 eV, and the NiV-LDH material binds ACTH most strongly among the different aminoxyls (Fig. 2c). The amide carbonyl group of ACT binds to an exposed Ni ion on the NiV-LDH surface, with a calculated Ni-O bond length of 1.98 Å. Additionally, the CVs and UV-Vis analyses were employed to confirm the adsorption of ACT on the NiV-LDH electrode26,30,42,43. As shown in Supplementary Fig. 4a, a pair of redox peaks appeared at pH 11.5 for ACT alone at 1.62 and 1.40 V vs. RHE (green line), corresponding to ACT to ACT+ and ACT+ to ACT, respectively. The Ni0.67V0.33-LDH also exhibited a pair redox peak of Ni2+/Ni3+ (orange line). The ACT/ACT+ peak signal significantly increased after the addition of Ni0.67V0.33-LDH (blue line). Subsequently, the above Ni0.67V0.33-LDH electrode was washed with the MeCN and H2O to remove ACT, after which CV experiments were repeated. The oxidation currents observed were higher than those of the pristine Ni0.67V0.33-LDH, suggesting that ACT had been adsorbed on the surface of the Ni0.67V0.33-LDH electrode. Similarly, the β-Ni(OH)2 electrode demonstrated the ability to adsorb ACT (Supplementary Fig. 4b). To exclude the impact of GF support on ACT adsorption, CVs were conducted following the aforementioned methodology. As illustrated in Supplementary Fig. 5, the impregnated and reacted GF electrode displays a comparable current to that of the initial GF, indicating that ACT was not adsorbed on the surface of the GF support, highlighting the adsorption of ACT by Ni0.67V0.33-LDH.
a Adsorption energy of R-TEMPOH on clean NiOOH surface, inset of (a) is the corresponding DFT optimized structural model of NiOOH surface. The color for each element is pale blue for Ni, red for O, and white for H. b Adsorption energy of AcNH-TEMPOH (ACTH) on the Ni active site of M-NiOOH surface (M = Ni, V, Mn, Fe, Co, Cu). c Adsorption energy of R-TEMPOH on the Ni active site of V-NiOOH surface, inset of (c) is the corresponding DFT optimized structural model of R-TEMPOH on the V-NiOOH surface. The color for each element is light gray for V, pale blue for Ni, red for O, dark gray for C, blue for N, and white for H.
Moreover, UV−visible spectral analysis revealed that the ACT solution exhibited a minimal change in absorbance after the impregnation of the Ni0.67V0.33-LDH (Ni2+, orange line) (Supplementary Fig. 6). Conversely, the solution displayed a pronounced decrease in absorbance after the impregnation of Ni0.67V0.33-LDH (Ni3+, blue line), suggesting that the Ni0.67V0.33-LDH (Ni3+) electrode may have adsorbed or consumed a portion of the ACT. In contrast, the GF (pink line) exhibited no UV−visible absorption, which is consistent with the CV results. Furthermore, the plotted ACT amount-absorbance relationship curves allowed for the estimation of the amount of ACT adsorbed on the NiV-LDH, NiMn-LDH, NiFe-LDH, NiCo-LDH, NiCu-LDH, and β-Ni(OH)2 electrodes (~1 cm × 1 cm), calculated to be 1.20, 0.61, 0.47, 0.33, 0.12, and 0.39 mM, respectively (Supplementary Figs. 7, 8).
Synthesis and characterization of NiM-LDH materials
The heterogeneous NiM-LDH electrocatalysts (M = V, Mn, Fe, Co, Cu) were uniformly distributed on GF using a hydrothermal method44, as schematically illustrated in Fig. 3a. NiV-LDH/GF electrocatalysts with varying Ni/V molar ratios were obtained by controlling the dosage of nickel nitrate and vanadium (III) chloride during the hydrothermal synthesis process. Additionally, Ni(OH)2/GF was synthesized by a similar procedure but without V dopants. The scanning electron microscopy (SEM) displayed that the Ni0.67V0.33-LDH exhibits a three-dimensional (3D) nanosphere morphology composed of ultrathin double hydroxide nanosheets, with a diameter of approximately 600 nm (Fig. 3b, c), sharply contrasting the smooth surface of pristine GF (Supplementary Fig. 9). The Ni(OH)2 also shows that the entire surface of the GF is uniformly coated with the dense nanosheets (Supplementary Fig. 10a, b). The nanosheets undergo a gradual transformation towards a smaller and thicker form with increasing V doping (Supplementary Fig. 10). This transformation is followed by the formation of nanosphere-like structures through the aggregation of nanosheets, beginning with Ni0.75V0.25-LDH. Such porous structure exposes a large number of active sites, facilitating the penetration and diffusion of electrolytes, and increasing the effective contact between the electrode and electrolytes during the EAO process45,46. Similarly, other Ni-based LDHs were evenly distributed on the surface of the GF (Supplementary Fig. 11). Transmission electron microscopy (TEM) images illustrate that the Ni0.67V0.33-LDH has a nanosphere structure composed of thin interconnected nanosheets (Fig. 3d, e), a feature also evidenced by the SEM results.
a Schematic of the preparation method of NiM-LDHs/GF (M=V, Mn, Fe, Co, Cu). b, c SEM images morphology of Ni0.67V0.33-LDH/GF, inset of (b) is the corresponding low-magnification SEM images. d, e TEM, f HRTEM, and g Elemental mapping of Ni0.67V0.33-LDH/GF, inset of (f) is the corresponding selected area electron diffraction (SAED) image. h XRD, XPS spectra of i Ni 2p and j V 2p for Ni0.67V0.33-LDH and β-Ni(OH)2.
The lattice fringe spacing of the Ni0.67V0.33-LDH is ~0.205 nm47,48,49, as revealed by high-resolution TEM (HRTEM) in Fig. 3f. The corresponding selected area electron diffraction (SAED) image, presented in the inset of Fig. 3f, indicates that the Ni0.67V0.33-LDH contains multiple crystal planes in the form of circles of bright dots48,50. Energy dispersive X-ray spectrum (EDX) analysis reveals that the Ni0.67V0.33-LDH contains Ni, V, C and O as the main elemental components, with a V:Ni atomic ratio of ~1:2.38 (Supplementary Fig. 12). Elemental analysis in Fig. 3g further confirms that the elements Ni, V, and O are uniformly distributed within the Ni0.67V0.33-LDH. The mass content of Ni and V was determined using inductively coupled plasma spectroscopy (ICP) to be 28.44 and 13.21%, respectively.
The powder X-ray diffraction (XRD) patterns of Ni0.67V0.33-LDH are shown in Fig. 3h. To avoid interference from the strong signal of the GF, powders synthesized by the same method were characterized. The diffraction peaks of Ni(OH)2 can be assigned to the standard brucite (space group: P3m1) crystal phase of β-Ni(OH)2 (JCPDS No. 73-1520)51,52, without any other peaks. After incorporating V into the structure of Ni(OH)2, the diffraction peaks of Ni0.67V0.33-LDH were centered at 11.46°, 23.26°, 33.46°, 34.41°, 38.76°, and 59.80°, corresponding to the (003), (006), (101), (012), (015), and (110) facets of hexagonal α-Ni(OH)2 (JCPDS No. 38-0715) with larger interlayer spacing20,53,54, respectively. This result indicates that the incorporation of V transforms Ni(OH)2 from β phase to α phase49. In addition, with the exception of Ni0.50V0.50-LDH, which predominantly formed α-Ni(OH)2, a mixed phase of both β-Ni(OH)2 and α-Ni(OH)2 was identified in the NiV-LDH electrocatalysts with other Ni/V ratios (Supplementary Fig. 13a). A similar phenomenon of simultaneous mixed phases of β and α was observed in Mn- and Fe-doped Ni(OH)2, while the structures of Co- and Cu-doped Ni(OH)2 remained as β-Ni(OH)2 (Supplementary Fig. 13b). Moreover, Brunauer–Emmett–Teller (BET) analysis was conducted to determine the specific surface area of Ni0.67V0.33-LDH (Supplementary Fig. 14). The nitrogen adsorption-desorption curves of Ni0.67V0.33-LDH display a type IV isotherm with a hysteresis loop in the relative pressure range of P/P0 = 0.45–1.0, and a pore size distribution between 2 and 8 nm, indicating that Ni0.67V0.33-LDH is a mesoporous material. Besides, Ni0.67V0.33-LDH has a larger BET surface area (9.66 m2 g−1) compared to β-Ni(OH)2 (2.81 m2 g−1), demonstrating that V doping alters the morphology of the nanospheres and is expected to improve the EAO efficiency by facilitating the mass transfer of the reactants and electrolytes. The surface chemical composition and electronic states of the elements in the NiV-LDH electrocatalysts, characterized by varying Ni/V molar ratios, were investigated using X-ray photoelectron spectroscopy (XPS) (Supplementary Fig. 15). The Ni 2p XPS spectrum of Ni0.67V0.33-LDH and β-Ni(OH)2 showed two Ni 2p3/2 peaks (Fig. 3i), corresponding to the dominant Ni2+ species and a Ni3+ species with binding energy of 855.9 and 858.1 eV, respectively53,55. As illustrated in Supplementary Fig. 15b, the content of Ni3+ species exhibited a slight increase following the introduction of V doping. Moreover, the V 2p3/2 signal displays three peaks at 515.8 (V3+), 516.8 eV (V4+), and 517.7 eV (V5+) (Fig. 3j), indicating a significant presence of V4+ species alongside a relatively minor population of V3+ and V5+ species20,56,57,58. This data confirms that V is successfully doped into the Ni0.67V0.33-LDH. The O 1s peaks observed for NiV-LDH at 529.9, 531.1, and 532.9 eV have been attributed to the lattice oxygen (M-O), hydroxyl bonded to metal (M-OH), and adsorbed oxygen on the surface defects (H-O-H), respectively57,59,60. The Ni0.50V0.50-LDH sample demonstrates an increase in lattice oxygen (M-O) content and a concomitant decrease in hydroxyl groups (M-OH) (Supplementary Fig. 15d).
Analysis of synergistic NiM-LDH/ACT electrocatalytic alcohol oxidation
To evaluate the electrochemical performance of NiM-LDH/ACT for EAO, linear sweep voltammetry (LSV) analysis was carried out in a batch reactor using a typical three-electrode system (0.5 M Na2CO3 aqueous electrolyte with pH 11.5). As displayed in Fig. 4a, the LSV of Ni0.67V0.33-LDH electrocatalyst exhibits a higher oxidation peak signal compared to other NiM-LDHs (M = Mn, Fe, Co, Cu). The oxidation peak at 1.53 V vs. RHE was identified as the Ni2+/Ni3+ oxidation peak related to the oxidation of Ni(OH)2 to NiOOH61,62, indicates that the incorporation of V into Ni-based LDH effectively promotes the oxidation of Ni2+ to Ni3+. Similarly, the CV curves of the Ni0.67V0.33-LDH electrocatalyst exhibit a lower onset oxidation potential and a higher current response in comparison to Ni(OH)2 (Supplementary Fig. 16). According to the DFT calculations, the free energy for the dehydrogenation of Ni0.67V0.33-LDH (1.85 eV) is lower than that of Ni(OH)2 (2.51 eV), suggesting that V doping in Ni(OH)2 facilitates the electrooxidation of Ni2+-OH to Ni3+-OH (Supplementary Fig. 17). Fig. 4b demonstrates that the V content in the NiV-LDHs electrocatalysts exhibits a volcano-like relationship with oxidation current density, with a Ni/V atomic ratio of ~2:1 (Ni0.67V0.33-LDH) displaying the higher current density (Supplementary Fig. 18). Use of 5 mol% ACT reveals an oxidation peak at 1.62 V vs. RHE (Fig. 4c), corresponding to oxidation of ACT to the oxoammonium species, ACT+. The intensity of the oxidation peak is significantly enhanced when both Ni0.67V0.33-LDH and ACT are present, reaching up to 130 mA cm−2 at 1.58 V vs. RHE. The corresponding Tafel slope for NiM-LDHs (M = V, Mn, Fe, Co, Cu), NiV-LDHs with different V contents, and Ni0.67V0.33-LDH/ACT as depicted in Supplementary Figs. 19,20.
a LSV analysis (with 80% iR compensation) of NiM-LDHs (M = V, Mn, Fe, Co, Cu) in 0.5 M Na2CO3 electrolyte (pH = 11.5) in batch reactor. b LSV analysis (with 80% iR compensation) of NiV-LDHs with different V contents in 0.5 M Na2CO3 electrolyte in a batch reactor. c LSV analysis (with 80% iR compensation) of Ni0.67V0.33-LDH, ACT (5 mol%), and Ni0.67V0.33-LDH/ACT in 0.5 M Na2CO3 electrolyte in a batch reactor. d LSV analysis (without iR compensation), e current density at 1.45 V vs. RHE and f the corresponding Tafel slope of Ni0.67V0.33-LDH, ACT and Ni0.67V0.33-LDH/ACT in the presence of BA in a continuous-flow electrolyzer. g Relative concentration (con.) of BA, PhCHO, and PhCOOH during the EAO of BA at different potentials from 1.30 to 1.50 V vs. RHE over the cooperative Ni0.67V0.33-LDH/ACT. h Yield and selectivity (Sel.) of PhCHO during the EAO of BA for Ni0.67V0.33-LDH, ACT, and Ni0.67V0.33-LDH/ACT inflow condition. i Selectivity, yield, and Faraday efficiency (FE) of ten stability tests for Ni0.67V0.33-LDH/ACT-catalyzed EAO of BA.
Flow oxidation studies conducted with BA reveal complementary results. The use of 100 mM BA reveals a much higher current density (336 mA cm−2) for the cooperative Ni0.67V0.33-LDH/ACT electrolysis system compared to ACT alone (153 mA cm−2) or Ni0.67V0.33-LDH alone (3 mA cm-2) at 1.45 V vs. RHE in the flow electrolyzer (Fig. 4d, e). The corresponding Tafel slope of Ni0.67V0.33-LDH/ACT is 47 mV dec−1 (Fig. 4f), significantly lower than that of the ACT (64 mV dec−1) or Ni0.67V0.33-LDH (88 mV dec−1), reflecting more favorable reaction kinetics for BA oxidation relative to the two individual catalysts, Ni0.67V0.33-LDH and ACT. Additionally, the electrocatalytic activity of the cooperative Ni0.67V0.33-LDH/ACT electrocatalysts in the oxidation of BA was also investigated in the batch reactor via CV (Supplementary Fig. 21). The current density increased with the incorporation of BA, and the reduction peak of ACT+/ACT disappeared. As illustrated in Supplementary Fig. 22, the oxidation of BA by ACT alone manifested a similar phenomenon, while the Ni0.67V0.33-LDH retained discernible Ni3+/Ni2+ reduction peak current signals after the introduction of BA, indicating that ACT+ was the active species in the oxidation of BA for Ni0.67V0.33-LDH/ACT-catalyzed EAO. Electrochemically active surface area (ECSA) measurements were performed by systematically recording CVs within the non-faradaic potential range to quantify the double-layer capacitance (Cdl, Supplementary Fig. 23). The NiV-LDH shows a bilayer capacitance increase compared to Ni(OH)2, 90.8 vs. 31.2 mF cm−2 (Supplementary Fig. 24), suggesting that increase access to surface sites in the Ni0.67V0.33-LDH material at least partially contributes to its improved electrocatalytic performance. Furthermore, the chronoamperometry (CA) curves of Ni0.67V0.33-LDH/ACT and ACT-catalyzed electrochemical oxidation of BA in the continuous flow cell are illustrated in Supplementary Fig. 25. The turnover frequency (TOF) for Ni0.67V0.33-LDH/ACT-mediated EAO in the continuous flow cell was calculated to be 8447 h−1, significantly higher than that of the ACT-mediated EAO under other conditions (Supplementary Fig. 26).
A series of chronoamperometry experiments were conducted at various potentials (1.30–1.50 V vs. RHE in a 0.5 M Na2CO3 electrolyte) in the continuous-flow electrolyzer to further investigate the origin of the cooperativity between Ni0.67V0.33-LDH and ACT. Fig. 4g shows that the target product, benzaldehyde (PhCHO), was produced with a selectivity (Sel.) of 99% at 1.45 V vs. RHE using the cooperative Ni0.67V0.33-LDH/ACT electrode. Nearly 1930 C (2 F mol−1) of charge is required to completely convert BA into PhCHO (Supplementary Fig. 27). The formation of benzoic acid (PhCOOH) is negligible during this process (Supplementary Fig. 28). Independent testing of Ni0.67V0.33-LDH and ACT for EAO performance at 1.45 V vs. RHE results in only 9 and 38% yields of PhCHO, respectively (Fig. 4h), confirming the synergistic benefits of Ni0.67V0.33-LDH and ACT for alcohol oxidation. Additionally, the oxidation performance of BA with varying Ni/V ratios was further investigated, as shown in Supplementary Fig. 29. The Ni0.67V0.33-LDH displays excellent EAO performance, far exceeding that of Ni0.83V0.17-LDH, Ni0.80V0.20-LDH, Ni0.75V0.25-LDH, and Ni0.50V0.50-LDH. The selectivity and yield of PhCHO remain consistently above 95%, even after 10 consecutive electrochemical oxidation cycles (Fig. 4i). The morphology and structure of Ni0.67V0.33-LDH were not significantly altered after electrochemical oxidation experiments, as shown by SEM, TEM, and elemental mapping (Supplementary Figs. 30, 31). EDX analysis after the reaction revealed a slight loss of Ni and V, and an increase in O content compared to the pre-reaction Ni0.67V0.33-LDH electrocatalyst (Supplementary Fig. 32). However, high-resolution XPS spectra of Ni 2p and V 2p exhibited a slight increase in Ni3+ and V5+ after EAO compared to the as-prepared Ni0.67V0.33-LDH material (Supplementary Fig. 33). These observations are rationalized by partial reconstruction of the LDH material during EAO.
Mechanistic analysis of NiV-LDH/ACT co-catalytic alcohol oxidation
A series of experiments and computations were conducted to investigate the mechanistic features of the EAO reaction. In situ electrochemical impedance spectroscopy (EIS) was performed at various potentials to examine the interfacial behavior of Ni0.67V0.33-LDH/ACT10,15,39. For Ni0.67V0.33-LDH in 0.5 M Na2CO3, the results reveal a shift to lower phase and higher frequencies with increasing applied potentials, reflecting an increase in reaction rates (Supplementary Fig. 34). As shown in Fig. 5a, the phase angle decreases significantly and frequency shifts higher after the addition of ACT and BA (blue line), indicating an accelerated oxidation rate and enhanced kinetics for the EAO of BA. Meanwhile, the corresponding Nyquist plots displayed approximately vertical lines in 0.5 M Na2CO3 (Supplementary Fig. 35), suggesting a high charge transfer resistance for the Ni0.67V0.33-LDH electrode at 1.37 V vs. RHE (Fig. 5b). The Nyquist plots reveal a smaller semicircle when both ACT and BA are present in the electrolytes (blue line), reflecting minor charge transfer resistance that is consistent with efficient Ni0.67V0.33-LDH/ACT-mediated EAO63. In addition, both the Bode plot and Nyquist plot were analyzed for the β-Ni(OH)2/ACT in the batch reactor (Supplementary Figs. 36, 37), the results revealed that the larger semicircle of the Nyquist plot, compared with Ni0.67V0.33-LDH/ACT, implies that transfer resistance can be reduced after the introduction of V.
Comparison of a Bode plots and b Nyquist plots at 1.37 V vs. RHE for Ni0.67V0.33-LDH, Ni0.67V0.33-LDH + BA, Ni0.67V0.33-LDH/ACT, and Ni0.67V0.33-LDH/ACT + BA. In situ Raman spectra (without iR compensation) of c β-Ni(OH)2, d Ni0.67V0.33-LDH, e Ni0.67V0.33-LDH with ACT, and f Ni0.67V0.33-LDH with ACT and BA at various constant potentials (increased from 1.27 to 1.77 V vs. RHE). g Charge density difference of ACTH adsorption for the corresponding structures of V-NiOOH at Ni active site. Cyan and yellow represent the depletion and accumulation of electrons, respectively. h Electron localization function (ELF) for the structure of ACTH adsorption on the V-NiOOH surface. The dark blue regions represent localized electron distribution, the red regions represent electronic delocalized distribution. Electrons localized in the range of 0.0–1.0. i Proposed a mechanism for the Ni0.67V0.33-LDH/ACT-mediated EAO. The color for each element is light gray for V, pale blue for Ni, red for O, dark gray for C, blue for N, and white for H.
In situ Raman experiments were performed at various potentials to monitor the changes in the surface structure of Ni0.67V0.33-LDH and β-Ni(OH)2 electrodes (Fig. 5c–f and Supplementary Figs. 38, 39). For β-Ni(OH)2, no discernible changes was observed upon increasing the potential up to 1.37 V vs. RHE (Fig. 5c), whereas two peaks become evident at 473 and 557 cm−1 upon increasing the potential to 1.47 V vs. RHE10,64. These peaks are assigned to surface Ni3+-O vibrations during the oxidation of Ni(OH)2 to NiOOH. The relatively low sensitivity of the Raman spectrometer and the inactivity of the Ni2+-O vibrational modes resulted in the absence of Ni2+-O vibrational signals in the Raman spectra20,65,66. Ni0.67V0.33-LDH exhibits a Raman signal at ~767 cm−1, which is absent in the β-Ni(OH)2 catalyst and is attributed to a V-O stretch (Fig. 5d). Increasing the applied potential to 1.37 V vs. RHE leads to gradual enhancement of the 767 cm−1 peak and the emergence of NiOOH peaks at 473 and 557 cm−1, indicating that NiOOH forms at a lower potential in Ni0.67V0.33-LDH than in β-Ni(OH)2, thus the introduction of V facilitates the formation of NiOOH, consistent with the CV, LSV and EIS results. The peaks corresponding to NiOOH were attenuated at 1.37 V vs. RHE after the addition of ACT compared to the absence of ACT in situ Raman spectroscopy (Fig. 5e). This observation indicates that Ni3+ was initially formed on the surface of Ni0.67V0.33-LDH, subsequently capturing electrons and partially transforming back into Ni2+67. Because the reduction of the Ni3+ by ACT forms ACT+, which can oxidize BA to PhCHO, these results align with the synergistic benefits of NiV-LDH and ACT for EAO. DFT calculations illuminate the increase in electron density upon electron transfer from an adsorbed ACT molecule to a Ni3+ site (Fig. 5g, h). Taken together, these results raise the possibility that enhanced formation of the more oxidizing and Lewis acidic Ni3+ by the V ions in the NiV-LDH promotes ACT or ACTH adsorption and oxidation to ACT+ species, and the adsorbed ACT+ species could then promote rapid alcohol oxidation (Fig. 5i).
Electrochemical oxidation of other alcohols with the synergistic NiV-LDH/ACT co-catalyst system
The EAO performance was then evaluated using Ni0.67V0.33-LDH, ACT, and Ni0.67V0.33-LDH/ACT system with a variety of different alcohol substrates in a recirculating-flow parallel-plate electrolyzer (Fig. 6). Due to the different solubilities of the substrates, we chose different ratios of Na2CO3 and MeCN to ensure that the substrates completely dissolved and the electrolyte maintained good conductivity.
Substrate scopes of the Ni0.67V0.33-LDH, ACT, and Ni0.67V0.33-LDH/ACT-catalyzed EAOa (A) and flow small-scale up (B). a Anode potential and charge passed for Ni0.67V0.33-LDH/ACT-catalyzed EAO in a small-scale flow electrolyzer. b The relative concentration for the Ni0.67V0.33-LDH/ACT-catalyzed EAO of 9a in a small-scale flow electrolyzer. aReaction conditions: Ni0.67V0.33-LDH anode, nickel foam cathode, electrode surface (10 cm2), constant potential = 1.45 V vs. RHE, flow rate = 200 mL min−1, room temperature, 1a-8a (10 mmol), ACT (5 mol%), 0.5 M Na2CO3, MeCN. Small-scale up: electrode surface (100 cm2), apparent current density = 150 mA cm−2, flow rate = 2000 mL min−1, 9a (50 mmol), ACT (5 mol%). Determined by GC-MS and HPLC analysis. Yield of isolated products.
The cooperative Ni0.67V0.33-LDH/ACT electrocatalyst shows superior performance compared to Ni0.67V0.33-LDH and ACT used independently in the oxidation of primary alcohols (1a-7a) to the corresponding aldehydes, achieving yields of ≥87% for 1b-7b within ≤46 min. The oxidation of the pharmaceutically relevant steroid 19-hydroxyandrost-4-ene-3,17-dione, 8a, with the Ni0.67V0.33-LDH/ACT co-catalyst generated the aldehyde 8b (19-Aldoandrostenedione) in 96% yield, significantly outperforming Ni0.67V0.33-LDH (2%) and ACT alone (30%). Efforts then focused on the synthesis of a precursor of tibolone [(7α,17α)-17-hydroxy-7-methyl-19-norpregn-5(10)-en-20-yn-3-one]. The substrate 9a (7α-methyl-19-aldehyde-4-androstene-3,17-dione) was evaluated on a scale of 50 mmol. Despite its large molecular weight and poor solubility, a current density of 150 mA/cm2 (based on the electrode's two-dimensional surface area) was achieved, maintaining a stable anode potential of 1.4–1.6 V vs. RHE during a 15 A constant current electrolysis with a flow rate of 2000 mL min−1 (Fig. 6B–a). Time-course analysis by high-performance liquid chromatography (HPLC) showed that the concentration of 9a decreases steadily, with parallel formation and conversion of intermediates 9a-1 and 9a-2, ultimately converging into the oxidation product 9b (Fig. 6B–b and Supplementary Fig. 40). High selectivity and yields of 9b (95 and 94%, respectively) were achieve within 25 min.
We then focused in increate the reaction productivity by enhancing the reactor volume and establishing a large-scale electrochemical synthesis system for oxidation of sterol 8a to 8b, accompanied by hydrogen (H2) production at the counter electrode. An anode composed of Ni0.67V0.33-LDH/GF and a Ni foam cathode were integrated within three electrolyzers, with two-dimensional electrode surface areas of 10, 100, and 400 cm2 (Fig. 7a, b). Computational fluid dynamics (CFD) simulations revealed that enlarging the electrolyzer caused non-uniform flow rates within the reactor, particularly at the corners of the flow channels, where the fluid flow is essentially absent (Fig. 7c). Introducing a cavity structure in the filled electrodes enables significantly improved fluid flow, resulting in a more uniform flow rate distribution (Fig. 7d). The LSV curve and the corresponding Tafel slope (Fig. 7e and Supplementary Fig. 41) shows that the onset potential for the oxidation of sterol 8a (200 g) is 1.2 V vs. RHE, significantly lower than that of OER (Fig. 7f). Optimization of the electrolysis method, including the reaction current, electrolyte flow rate and electrolyte temperature, led to high productivity for formation of the steroid carbonyl product 8b from 8a (Fig. 7g). The resulting product exhibited a selectivity of 94% during a constant current electrolysis of 60 A in the 400 cm2 electrolysis cell, with a cell voltage of 3.2–4.0 V (Supplementary Fig. 42). The productivity associated with this reactor was 243 g h−1, with an energy consumption of ~0.71 kWh kg−1. This reaction was repeated on a hectogram scale (182.7 g), affording 8b in 91% isolated yield on the basis of HPLC and 1H NMR analysis (Supplementary Figs. 43–46). Collectively, these results demonstrated the utility of this cooperative Ni0.67V0.33-LDH/ACT-mediated for larger-scale EAO applications.
a Size comparison of the three electrolyzers used in this work, with working areas of 10, 100, and 400 cm2, and an expanded view of the large-scale electrolyzer configurations. b The photograph of large-scale electrolysis setup for Ni0.67V0.33-LDH/ACT-catalyzed EAO. CFD simulation fluid flow traces in the flow channel of continuous flow electrolyzer c without and d with a cavity. e LSV curves (without iR compensation) and (f) potential comparison for OER and EAO of 8a (200 g) in the large-scale flow electrolyzer. g The relative concentration changes of 8a and its oxidation products (8b and 8c) during EAO at 60 A.
Discussion
In summary, we have demonstrated that two previously known electrocatalyst compositions, heterogeneous Ni-based LDH materials, and molecular aminoxyls, show improved performance when used together for electrochemical alcohol oxidation. Experimental and computational analysis suggests that synergistic benefits arise from multiple factors. Vanadium ions in the optimal Ni0.67V0.33-LDH material facilitate the oxidation of Ni2+ to Ni3+ and adsorption of the ACT aminoxyl species to the Ni0.67V0.33-LDH surface promotes electron transfer from the aminoxyl to Ni3+. The adsorbed oxoammonium, formed upon reduction of Ni3+ sites can promote alcohol oxidation. This cooperativity enables the Ni0.67V0.33-LDH/ACT electrocatalyst to achieve high yields and selectivities in the oxidation of primary alcohols to aldehydes (up to 99%, in both cases). The Ni0.67V0.33-LDH/ACT electrocatalyst shows good scope and functional-group tolerance, and its utility is showcased in the oxidation of complex steroid pharmaceutical intermediates. The latter application has been demonstrating in a recirculating parallel-plate flow reactor, exhibiting a productivity of 243 g h−1 and a current density of 150 mA cm−2. This demonstration of synergistic benefits between heterogeneous and homogeneous electrocatalysts provides a foundation for many future studies targeting practical applications in organic electrosynthesis.
Methods
Chemicals and materials
Nickel (II) nitrate hexahydrate (Ni(NO3)2·6H2O, 98%), vanadium (III) chloride (VCl3, 98%), manganese (II) nitrate solution (Mn(NO3)2, 50%), iron (III) nitrate nonahydrate (Fe(NO3)3·9H2O, 98%), cobaltous (II) nitrate hexahydrate (Co(NO3)2·6H2O, 99%), copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, 97%), 2,2,6,6-tetramethyl-1-piperidine-N-oxyl (TEMPO, 98%), 4-acetamido-TEMPO (ACT, 98%), 4-hydroxy-TEMPO (4-HO-TEMPO, 98%), benzyl alcohol (C7H8O, 99%), 4-methylbenzyl alcohol (C8H10O, 98%), 4-chlorobenzyl alcohol (C7H7ClO, 99%), 4-bromobenzyl alcohol (BrC6H4CH2OH, 99%), 4-methoxybenzyl alcohol (C8H10O2, 98%) were purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). 4-Oxo-TEMPO (4-oxo-TEMPO, 98%), 2-naphthalenemethanol (C11H10O, 98%), 4-phenoxybenzyl alcohol (C13H12O2, 98%), and (3,4,5-Trimethoxyphenyl)methanol (C10H14O4, 99%) were purchased from Bide Pharmatech Co., Ltd (Shanghai, China). 4-Methoxy-TEMPO (4-MeO-TEMPO, 98%), urea (CH4N2O, 99.5%), sodium carbonate (Na2CO3, 99.8%), acetonitrile (MeCN, 99%), dichloromethane (CH2Cl2, 99%) was purchased from Macklin Biochemical Co., Ltd (Shanghai, China). Ammonium fluoride (NH4F, GR), hydrochloric acid (HCl, 36.5–38 wt%), nitric acid (HNO3, GR), acetone (CH3COCH3, AR), ethyl alcohol (C2H5OH, AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. Sterols 8a (19-hydroxyandrost-4-ene-3,17-dione) and 9a (7α-methyl-19-aldehyde-4-androstene-3,17-dione) were provided by Zhejiang Xianju Junye Pharmaceutical Co., Ltd. Graphite Felt (thickness 6 mm) was purchased from Jinglong Special Carbon Technology Co., Ltd (Beijing, China). Nafion 417 membrane (thickness 260 μm) was purchased from Thinkre New Material Co., Ltd (Suzhou, China). The Ag/AgCl electrode and Pt wire was purchased from Gaoshi Ruilian Technology Co., Ltd (Wuhan, China). Except noted, all reagents were used without further purification.
Pretreatment of GF
The Graphite Felt (GF 065) was sliced into pieces measuring approximately 3 cm × 3.5 cm × 0.6 cm. It was then subjected to successive ultrasonic treatments in 40 wt% HNO3, acetone, ethanol, and deionized water, each for 30 min. Subsequently, the GF was dried under a vacuum at 60 °C overnight.
Preparation of NiV-LDH
As an example, a NiV-LDH/GF electrode was prepared, which was synthesized using a one-step hydrothermal method. For the preparation of NiV-LDH, a solution with different molar ratios of Ni/V (1:1, 2:1, 3:1, 4:1, 5:1, and 1:0 for the synthesis of Ni0.50V0.50-LDH, Ni0.67V0.33-LDH, Ni0.75V0.25-LDH, Ni0.80V0.20-LDH, Ni0.83V0.17-LDH, and pure Ni(OH)2, respectively) was synthesized by combining Ni(NO3)2·6H2O and VCl3 in 80 mL H2O, keeping the total amount of metal ions (Ni + V) was kept at 2 mmol. After adding 0.360 g urea (6 mmol), 0.148 g NH4F (4 mmol), and the GF, which was uniformly dispersed by ultrasound, the mixture was transferred to a 100 mL stainless steel autoclave. The autoclave was securely sealed, and the mixed solution was maintained at 120 °C for 12 h. Afterward, the autoclave was cooled to room temperature, and the NiV-LDH/GF was washed three times in succession with deionized water and ethanol three times in sequence and subjected to vacuum drying at 60 °C overnight.
Preparation of NiM-LDHs (M = Mn, Fe, Co, Cu)
The preparation of NiMn-LDH, NiFe-LDH, NiCo-LDH, and NiCu-LDH was the same as that of Ni0.67V0.33-LDH except that 50% Mn(NO3)2 (0.66 mmol), Fe(NO3)3·9H2O (0.66 mmol), Co(NO3)2·6H2O (0.66 mmol) and Cu(NO3)2·3H2O (0.66 mmol) were used instead of VCl3.
Preparation of Ni(OH)2
The preparation of Ni(OH)2 is identical to that of NiV-LDH, with the exception of the omission of the V source. The catalysts loading on the GF were calculate about 2 mg cm−2.
Materials characterization
Scanning electron microscopy (SEM, Hitachi FE–SEM S–4700) and scanning transmission electron microscopy (STEM, JEM-ARM300F) were conducted to investigate the morphology and microstructure of the electrocatalyst. X-ray diffraction (XRD) was performed using an X-ray diffractometer with graphite monochromatized Cu Kα radiation (λ = 1.54 Å). The corresponding cell parameters of each sample were then calculated and analyzed using Jade software. X-ray photoelectron spectroscopy (XPS) data were collected using a Thermo Scientific ESCALAB 250Xi X-ray photoelectron spectrometer. Brunauer–Emmett–Teller (BET) specific surface area and pore distribution analysis were conducted using an ASAP2460 analyzer. Inductively coupled plasma atomic emission spectroscopy (ICP–AES) analysis was conducted to accurately measure the weight loading of nickel (Ni) and vanadium (V). In situ Raman spectra were characterized using a confocal Raman imaging microscope (Renishaw InVia, 532 nm laser) under different potentials (1.27 to 1.77 V vs. RHE) using an Ivium-n-Stat electrochemical workstation in a three-electrode system (Supplementary Fig. 38).
Electrochemical measurements
Cyclic voltammetry (CV), linear sweep voltammetry (LSV), chronopotentiometry (CP), chronoamperometry (CA), and electrochemical aminoxyl-mediated oxidation were performed using an Ivium-n-Stat electrochemical workstation in a three-electrode system at room temperature. An Ag/AgCl electrode (in 3.0 M KCl) was employed as the reference electrode (RE), and a Pt wire (1.5 cm × 1.5 cm) was used as the counter electrode (CE). The electrolysis experiments were conducted in a continuous-flow parallel-plate electrolyzer with an electrode area of 10 cm2, where a Nafion 417 membrane (Suzhou Thinkre New Material Co., Ltd.) separated the anode (Ni-based materials, 10 cm2) from the cathode (nickel foam, 10 cm2). The anolyte consisted of 10 mmol benzyl alcohol (BA, 1.08 g) and 5 mol% ACT in 100 mL 0.5 M Na2CO3 solution, with the 0.5 M Na2CO3 solution serving as the catholyte. The 0.5 M Na2CO3 solution is prepared by mixing a specified volume of water with an accurately measured quantity of solid Na2CO3, which has a pH of 11.5 ± 0.2. Prior to each experiment, the solution is freshly prepared to ensure that its chemical properties and conductivity meet the experimental requirements. Moreover, the mixture was stirred to ensure uniformity, and a peristaltic pump was employed to transport the electrolyte into the reaction systems. The electrolysis reaction was conducted at room temperature using a constant potential of 1.45 V vs. RHE. Small-scale and large-scale continuous flow experiments were performed at a constant current, with electrode areas of 100 and 400 cm2, respectively. In the enlarged electrolyzer, the electrodes are not fully distributed across the entire polytetrafluoroethylene (PTFE) chamber (Fig. 7a). Instead, its upper and lower extremities form a cavity structure, which favors the distribution and flow of electrolytes. Moreover, 200 g of sterol 8a (19-hydroxyandrost-4-ene-3,17-dione) and ACT (5% mol) was dissolved in 10 L of a mixed solution (6 L 0.5 M Na2CO3 + 4 L MeCN), and the solution was uniformly mixed using mechanical stirring. After the electrolysis reaction, the electrolyzer was washed three times with CH2Cl2, MeCN, and H2O to ensure the complete removal of any remaining steroid carbonyl products from the surfaces of the electrode and electrolyzer, thereby minimizing product loss.
The CV, LSV, CP, and electrochemical aminoxyl-mediated oxidation were carried out in a one-compartment batch reactor using a three-electrode system. This system consisted of Ni-based materials (~1 cm × 1 cm) as the working electrode, a Pt wire as the counter electrode, and an Ag/AgCl electrode as the reference electrode. Electrochemical performance tests were performed in 100 mL of 0.5 M Na2CO3 solution, both without and with 10 mmol BA. Additionally, the GF (~1 cm × 1 cm) was used as the working electrode to obtain the CV, LSV, and CP curves for ACT. The scan rate for CV was kept at 50 mV s−1, without iR compensation. The scan rate for LSV was kept at 5 mV s−1 ranging from 1.0 to 1.85 VRHE in a batch reactor, with 80% iR compensation. All potentials measured against Ag/AgCl (EAg/AgCl) were converted to the reversible hydrogen electrode (ERHE) scale in this work using E(RHE) = E(Ag/AgCl) + 0.059 pH + 0.197 V, where pH values of the electrolytes were determined with a pH meter. The electrochemical surface area (ECSA) was evaluated in terms of the double-layer capacitance (Cdl). CV was performed in 0.5 M Na2CO3 solution at varying scan rates of 10, 20, 30, 40, 50, and 60 mV s−1 within a potential window where no Faradaic process occurs. All electrochemical experiments were performed at room temperature.
In situ electrochemical impedance spectroscopy (EIS)
In situ EIS measurements were performed using an Ivium-n-Stat electrochemical workstation with a three-electrode system, in which the Ni0.67V0.33-LDH (~1 cm × 1 cm) as the working electrode, a Pt wire as the counter electrode, and an Ag/AgCl electrode as the reference electrode. Electrochemical impedance spectroscopy tests were performed in 100 mL of 0.5 M Na2CO3 solution, with ACT or BA, and both ACT and BA. The frequency range was 0.1 Hz to 100,000 Hz with an amplitude of 5 mV, and the applied potential was varied from 1.27 to 1.67 V vs. RHE with a 0.05 V interval15.
Calculation of turnover frequency (TOF), conversion, selectivity, Faradaic efficiency
The TOF (h−1) of ACT in the presence of BA was calculated as shown in (1)24,25,28:
Here, charge consumption (QT and QA) during the CA experiment was quantified by applying a potential of 0.7 V vs. Ag/AgCl for ACT in the presence and absence of BA, respectively. QB refers to the charge consumed in the electrolyte without either BA or ACT. n represents the number of electrons corresponding to one turnover (n = 2), t is the reaction time (s), and 3600 is the number of seconds per hour, and resulting in a TOF expressed in per hour (h−1). Only the approximate steady-state region, between 3 and 20 s, was used for the calculation.
The Conversion (%), the Selectivity (%), and Faradaic efficiency (F.E.) were calculated as shown in (2), (3) and (4):
Where n and F are the number of electron transfers and the Faraday constant (96485 C mol−1), respectively.
Theoretical calculations
Periodic DFT calculations were performed using the Vienna ab initio simulation package (VASP)68. The exchange-correlation function of Perdew–Burke–Ernzerhof (PBE) and the projected augmented wave (PAW) method69 were used to describe the ion-electron interaction. The cut-off energy for the plane wave basis sets was 400 eV. The O 2s2p and V 3p4s3d electrons are treated as valence states. The convergence criteria of geometry and energy were set to 0.02 eV/Å and 10−6 eV, respectively. In addition, strong correlation effects due to charge localization are modeled by adding a Hubbard U-like term70 to the PBE function. And the effect U value of 5.50 eV for the Ni 3d states was applied Spin polarization was considered for all calculations in this work. For the NiOOH (001) surface, the p-(5 × 5) supercell with a thickness of one layer was constructed based on the bulk phase (space group: P3m1), the Gamma (1 × 1 × 1) k-point mesh was used to reduce the computational cost due to the large cell. The vacuum space of 25 Å was added to the z-direction of the slab modules (Supplementary Data 1).
Data availability
All data that support the findings of this study are present in the paper and the Supplementary Information files. Further information can be acquired from the corresponding authors. Source data are provided in this paper. Source data are provided with this paper.
References
Mo, Y. et al. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions. Science 368, 1352–1357 (2020).
Nutting, J. E., Rafiee, M. & Stahl, S. S. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 118, 4834–4885 (2018).
Ma, C. et al. Recent advances in organic electrosynthesis employing transition metal complexes as electrocatalysts. Sci. Bull. 66, 2412–2429 (2021).
Liu, C., Chen, F., Zhao, B.-H., Wu, Y. & Zhang, B. Electrochemical hydrogenation and oxidation of organic species involving water. Nat. Rev. Chem. 8, 277–293 (2024).
Gao, Y. et al. Electrocatalytic refinery of biomass-based 5-hydroxymethylfurfural to fine chemicals. ACS Catal. 13, 11204–11231 (2023).
Rafiee, M., Mayer, M. N., Punchihewa, B. T. & Mumau, M. R. Constant potential and constant current electrolysis: an introduction and comparison of different techniques for organic electrosynthesis. J. Org. Chem. 86, 15866–15874 (2021).
Wang, F. & Stahl, S. S. Electrochemical oxidation of organic molecules at lower overpotential: accessing broader functional group compatibility with electron−proton transfer mediators. Acc. Chem. Res. 53, 561–574 (2020).
Luo, L. et al. Ultrafine Core@Shell Cu1Au1@Cu1Pd3 nanodots synergized with 3D porous N-doped graphene nanosheets as a high-performance multifunctional electrocatalyst. ACS Nano 17, 2992–3006 (2023).
Ge, R. et al. Selective electrooxidation of biomass-derived alcohols to aldehydes in a neutral medium: promoted water dissociation over a nickel-oxide-supported ruthenium single-atom catalyst. Angew. Chem. Int. Ed. 61, e202200211 (2022).
Chen, W. et al. Activity origins and design principles of nickel-based catalysts for nucleophile electrooxidation. Chem 6, 2974–2993 (2020).
Chen, X. et al. Defect engineering of nickel hydroxide nanosheets by Ostwald ripening for enhanced selective electrocatalytic alcohol oxidation. Green Chem. 21, 578–588 (2019).
Li, S. et al. Biomass valorization via paired electrosynthesis over vanadium nitride-based electrocatalysts. Adv. Funct. Mater. 29, 1904780 (2019).
Zheng, J. et al. Hierarchical porous NC@CuCo nitride nanosheet networks: highly efficient bifunctional electrocatalyst for overall water splitting and selective electrooxidation of benzyl alcohol. Adv. Funct. Mater. 27, 1704169 (2017).
Yan, Y. et al. Electrocatalytic upcycling of biomass and plastic wastes to biodegradable polymer monomers and hydrogen fuel at high current densities. J. Am. Chem. Soc. 145, 6144–6155 (2023).
Zhou, B. et al. Platinum modulates redox properties and 5‐hydroxymethylfurfural adsorption kinetics of Ni(OH)2 for biomass upgrading. Angew. Chem. Int. Ed. 60, 22908–22914 (2021).
Bender, M. T., Lam, Y. C., Hammes-Schiffer, S. & Choi, K.-S. Unraveling two pathways for electrochemical alcohol and aldehyde oxidation on NiOOH. J. Am. Chem. Soc. 142, 21538–21547 (2020).
Zhang, H. et al. On the activity and selectivity of 5-hydroxymethylfurfural electrocatalytic oxidation over cation-defective nickel hydroxides. ACS Catal. 14, 9565–9574 (2024).
Zeng, L. et al. Cooperative Rh-O5/Ni(Fe) site for efficient biomass upgrading coupled with H2 production. J. Am. Chem. Soc. 145, 17577–17587 (2023).
Yan, Y., Zhong, J., Wang, R., Yan, S. & Zou, Z. Trivalent nickel-catalyzing electroconversion of alcohols to carboxylic acids. J. Am. Chem. Soc. 146, 4814–4821 (2024).
Jiang, J. et al. Atomic-level insight into super-efficient electrocatalytic oxygen evolution on iron and vanadium co-doped nickel (oxy)hydroxide. Nat. Commun. 9, 2885 (2018).
Huang, H. et al. Ni, Co hydroxide triggers electrocatalytic production of high-purity benzoic acid over 400 mA cm−2. Energy Environ. Sci. 13, 4990–4999 (2020).
Yang, G. et al. Unraveling the mechanism for paired electrocatalysis of organics with water as a feedstock. Nat. Commun. 13, 3125 (2022).
Wang, C. et al. A novel electrode for value-generating anode reactions in water electrolyzers at industrial current densities. Angew. Chem. Int. Ed. 62, e202215804 (2023).
Rafiee, M., Konz, Z. M., Graaf, M. D., Koolman, H. F. & Stahl, S. S. Electrochemical oxidation of alcohols and aldehydes to carboxylic acids catalyzed by 4-acetamido-TEMPO: an alternative to “Anelli” and “Pinnick” oxidations. ACS Catal. 8, 6738–6744 (2018).
Rafiee, M., Alherech, M., Karlen, S. D. & Stahl, S. S. Electrochemical aminoxyl-mediated oxidation of primary alcohols in lignin to carboxylic acids: polymer modification and depolymerization. J. Am. Chem. Soc. 141, 15266–15276 (2019).
Rafiee, M., Miles, K. C. & Stahl, S. S. Electrocatalytic alcohol oxidation with TEMPO and bicyclic nitroxyl derivatives: driving force trumps steric effects. J. Am. Chem. Soc. 137, 14751–14757 (2015).
Taitt, B. J., Bender, M. T. & Choi, K.-S. Impacts of the regeneration pathways of the oxoammonium cation on electrochemical nitroxyl radical-mediated alcohol oxidation. ACS Catal. 10, 265–275 (2020).
Goes, S. L. et al. Deriving the turnover frequency of aminoxyl-catalyzed alcohol oxidation by chronoamperometry: an introduction to organic electrocatalysis. J. Chem. Educ. 98, 600–606 (2021).
Cardiel, A. C., Taitt, B. J. & Choi, K.-S. Stabilities, regeneration pathways, and electrocatalytic properties of nitroxyl radicals for the electrochemical oxidation of 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 7, 11138–11149 (2019).
Ryan, M. C., Whitmire, L. D., Mccann, S. D. & Stahl, S. S. Copper/TEMPO redox redux: analysis of PCET oxidation of TEMPOH by copper(II) and the reaction of TEMPO with copper(I). Inorg. Chem. 58, 10194–10200 (2019).
Badalyan, A. & Stahl, S. S. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators. Nature 535, 406–410 (2016).
Nutting, J. E., Mao, K. & Stahl, S. S. Iron(III) nitrate/TEMPO-catalyzed aerobic alcohol oxidation: distinguishing between serial versus integrated redox cooperativity. J. Am. Chem. Soc. 143, 10565–10570 (2021).
Li, S. et al. Chromium-doped nickel oxide and nickel nitride mediate selective electrocatalytic oxidation of sterol intermediates coupled with H2 evolution. Angew. Chem. Int. Ed. 62, e202306553 (2023).
He, J. et al. Highly efficient electrocatalytic oxidation of sterol by synergistic effect of aminoxyl radicals and Se-Ni5P4. AIChE J. 69, e18153 (2023).
Wang, M. et al. Process intensification enhanced continuous flow for the effective coupling of sterol electrooxidation with H2 evolution using NiMo-based electrocatalysts. Chem. Eng. Sci. 285, 119589 (2024).
Wang, H. et al. Boosting 5-hydroxymethylfurfural electrooxidation in neutral electrolytes via TEMPO-enhanced dehydrogenation and OH adsorption. Chinese J. Catal. 46, 148–156 (2023).
Chadderdon, X. H., Chadderdon, D. J., Pfennig, T., Shanks, B. H. & Li, W. Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl)furfural for efficient production of biomass-derived monomers. Green Chem. 21, 6210–6219 (2019).
Li, S. et al. Reaction and transport co-intensification enhanced continuous flow electrocatalytic aminoxyl-mediated oxidation of sterol intermediates by 3D porous framework electrode. Chem. Eng. J. 446, 136659 (2022).
Chen, W. et al. Activated Ni–OH bonds in a catalyst facilitates the nucleophile oxidation reaction. Adv. Mater. 34, 2105320 (2022).
Li, S. et al. Doped Mn enhanced NiS electrooxidation performance of HMF into FDCA at industrial-level current density. Adv. Funct. Mater. 33, 2214488 (2023).
Yang, Z., Zhang, B., Yan, C., Xue, Z. & Mu, T. The pivot to achieve high current density for biomass electrooxidation: Accelerating the reduction of Ni3+ to Ni2+. Appl. Catal. B Environ. 330, 122590 (2023).
Gerken, J. B., Pang, Y. Q., Lauber, M. B. & Stahl, S. S. Structural effects on the pH-dependent redox properties of organic nitroxyls: pourbaix diagrams for TEMPO, ABNO, and three TEMPO analogs. J. Org. Chem. 83, 7323–7330 (2018).
Gerken, J. B. & Stahl, S. S. High-potential electrocatalytic O2 reduction with nitroxyl/NOx mediators: implications for fuel cells and aerobic oxidation catalysis. ACS Cent. Sci. 1, 234–243 (2015).
Zhao, T. et al. In situ reconstruction of V-doped Ni2P pre-catalysts with tunable electronic structures for water oxidation. Adv. Funct. Mater. 31, 2100614 (2021).
Ma, L. et al. Self-assembled ultrathin NiCo2S4 nanoflakes grown on Ni foam as high-performance flexible electrodes for hydrogen evolution reaction in alkaline solution. Nano Energy 24, 139–147 (2016).
Paul, R. et al. 3D heteroatom-doped carbon nanomaterials as multifunctional metal-free catalysts for integrated energy devices. Adv. Mater. 31, 1805598 (2019).
Wang, D. et al. Atomic and electronic modulation of self-supported nickel-vanadium layered double hydroxide to accelerate water splitting kinetics. Nat. Commun. 10, 3899 (2019).
He, D. et al. In-situ optimizing the valence configuration of vanadium sites in NiV-LDH nanosheet arrays for enhanced hydrogen evolution reaction. J. Energy Chem. 47, 263–271 (2020).
Tyagi, A., Joshi, M. C., Shah, A., Thakur, V. K. & Gupta, R. K. Hydrothermally tailored three-dimensional Ni–V layered double hydroxide nanosheets as high-performance hybrid supercapacitor applications. ACS Omega 4, 3257–3267 (2019).
Liu, Q. et al. Tuning the coupling interface of ultrathin Ni3S2@NiV-LDH heterogeneous nanosheet electrocatalysts for improved overall water splitting. Nanoscale 11, 8855–8863 (2019).
Gong, M. et al. An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 135, 8452–8455 (2013).
Yang, W. et al. Rapid room-temperature fabrication of ultrathin Ni(OH)2 nanoflakes with abundant edge sites for efficient urea oxidation. Appl. Catal. B Environ. 259, 118020 (2019).
Fan, K. et al. Nickel–vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 7, 11981 (2016).
Dinh, K. N. et al. Ultrathin porous NiFeV ternary layer hydroxide nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting. Small 14, 1703257 (2018).
Sun, X. et al. In situ construction of flexible V-Ni redox centers over Ni-based MOF nanosheet arrays for electrochemical water oxidation. Small Methods 5, 2100573 (2021).
Zeng, K. et al. Atomically dispersed cerium sites immobilized on vanadium vacancies of monolayer nickel-vanadium layered double hydroxide: accelerating water splitting kinetics. Adv. Funct. Mater. 34, 2308533 (2024).
Liu, X. et al. Electrosynthesis of adipic acid with high faradaic efficiency within a wide potential window. Nat. Commun. 15, 7685 (2024).
Sun, H. et al. Highly efficient overall urea electrolysis via single-atomically active centers on layered double hydroxide. Sci. Bull. 67, 1763–1775 (2022).
Jia, Y. et al. Directional electrosynthesis of adipic acid and cyclohexanone by controlling the active sites on NiOOH. J. Am. Chem. Soc. 146, 1282–1293 (2024).
Zeng, K. et al. Optimizing d-p orbital hybridization with abundant unfilled antibonding orbital in multi-metal layered double hydroxide: motivating efficient oxygen evolving. Adv. Funct. Mater. 34, 2315080 (2024).
Luo, R. et al. A dynamic Ni(OH)2-NiOOH/NiFeP heterojunction enabling high-performance E-upgrading of hydroxymethylfurfural. Appl. Catal. B Environ. 311, 121357 (2022).
Sun, H. et al. Porous multishelled Ni2P hollow microspheres as an active electrocatalyst for hydrogen and oxygen evolution. Chem. Mater. 29, 8539–8547 (2017).
Gu, K. et al. Defect‐rich high‐entropy oxide nanosheets for efficient 5‐hydroxymethylfurfural electrooxidation. Angew. Chem. Int. Ed. 133, 20415–20420 (2021).
Gao, L. et al. Operando unraveling photothermal-promoted dynamic active-sites generation in NiFe2O4 for markedly enhanced oxygen evolution. Proc. Nat. Acad. Sci. USA 118, e2023421118 (2021).
Wei, J. et al. Site-specific metal-support interaction to switch the activity of Ir single atoms for oxygen evolution reaction. Nat. Commun. 15, 559 (2024).
Chen, L. et al. Integrated electrochemical and chemical system for ampere-level production of terephthalic acid alternatives and hydrogen. Nat. Commun. 15, 8072 (2024).
Huang, C., Huang, Y., Liu, C., Yu, Y. & Zhang, B. Integrating hydrogen production with aqueous selective semi-dehydrogenation of tetrahydroisoquinolines over a Ni2P bifunctional electrode. Angew. Chem. Int. Ed. 58, 12014–12017 (2019).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys. Rev. B 57, 1505–1509 (1998).
Acknowledgements
Suiqin Li, Shibin Wang, and Yuhang Wang contributed equally to this work. The authors acknowledge the financial support from the National Key R & D Program of China (2022YFA1504200), Zhejiang Provincial Natural Science Foundation of China (No.LR22B060003), National Natural Science Foundation of China (22322810, 22078293, 22141001, and 22008211), the Fundamental Research Funds for the Provincial Universities of Zhejiang (RF-C2023004), China Postdoctoral Science Foundation (2024M752866), Postdoctoral Fellowship Program of CPSF (GZB20230662), and Zhejiang Provincial Postdoctoral Research Project Merit-based Funding (ZJ2023016). The authors acknowledge the sterols support from Zhejiang Xianju Junye Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Contributions
S.L. synthesized the samples and performed the characterizations. S.W. contributed to theoretical calculation. Y.W. performed the partial experiments. J.H. and K.L. contributed to the discussion of the results. J.B.G. and S.S.S. contributed to the discussion of the mechanism. X.Z. and J.W. designed the research, and wrote the paper. All the authors commented on and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Linjiang Wang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Wang, S., Wang, Y. et al. Synergistic enhancement of electrochemical alcohol oxidation by combining NiV-layered double hydroxide with an aminoxyl radical. Nat Commun 16, 266 (2025). https://doi.org/10.1038/s41467-024-55616-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55616-w