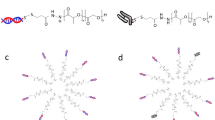
Abstract
Lipid nanoparticles (LNPs) are widely used for nucleic acid delivery but face challenges like limited targeting and accelerated blood clearance (ABC) effect. We design three ionizable oligomers (IOs) that, with polylactide-polyethylene glycol (PLA-PEG), form a potential siRNA delivery system, named Ionizable Polymeric Micelles (IPMs). The siRNA encapsulated IPMs escape from lysosomes upon cellular uptake, and silence the target gene. A fibroblast activation protein inhibitor modified IPMs (FAPi-IPMs) show higher targeting for activated hepatic stellate cells (HSCs) compared to that for hepatocytes, silencing both HSP47 and HMGB1, reducing collagen secretion and liver inflammation, thereby treating fibrosis. Moreover, IPMs and FAPi-IPMs mitigate ABC effect and produce fewer PEG antibodies than LNPs, and show minimal apolipoprotein adsorption in vivo compared with LNPs, differentiating their targeting effects from LNPs. In conclusion, IPMs represent a nucleic acid delivery system with alternative targeting ability and reduced ABC effect.
Similar content being viewed by others
Introduction
Small interfering RNA (siRNA), an effective tool capable of silencing specific gene expression, possesses advantages such as high specificity, efficiency, and duration, and has been widely applied in gene therapy. However, due to the negative charge, easy degradation, and inability to enter cells of siRNA itself, its drug development faces challenges1,2. Therefore, the delivery system for siRNA drugs is essential for their clinical application. In 2018, a siRNA drug, Onpattro (Patisiran) from Alnylam, received approval from the Food and Drug Administration, marking the first clinical use of LNP as a delivery carrier3, which later played a crucial role in the widespread clinical application of mRNA COVID-19 vaccines4,5. LNPs are composed of cationic or ionizable lipids, cholesterol, helper lipids, and PEGylated lipids, encapsulating RNA-based drugs. Upon intravenous injection, rapid detachment of PEG leads to the adsorption of plasma proteins to evolve in protein corona on the LNP surface, among which ApoE facilitates the targeted entry of LNPs into hepatocytes6. On one hand, LNPs are particularly suitable for treating hepatocytes-related diseases. On the other hand, the limited in vivo targeting after intravenous injection severely restricts their scope of clinical use7,8. Meanwhile, LNPs are associated with accelerated blood clearance due to the generation of PEG antibodies, posing a challenge for multiple dosing regimens9. Therefore, overcoming the limitations of LNPs in targeting organs and cells in vivo to broaden the clinical therapeutic scope of RNA-based drugs has attracted significant attention. Currently, many researchers have synthesized alternative ionizable lipids, integrating them into LNPs to confer selective capabilities for different organs, including spleen10, lung10,11,12,13,14,15,16, brain17 and bone marrrow18,19. However, current research is still confined within the scope of LNPs, unable to avoid the inherent problems of LNPs.
Among various types of nanocarriers, polymeric micelle is one of the most used. It is a core-shell nanostructure with hydrophobic inner core and hydrophilic outer shell formed by self-assembly of two or more block amphiphilic polymers in water. Commonly used polymeric materials include PLA-PEG, PLGA-PEG, and poloxamers20,21,22. Paclitaxel polymeric micelles formed by PLA-PEG have been approved for marketing in South Korea and China for targeted cancer therapy, achieving good clinical efficacy23,24. However, the hydrophobic core is typically used to encapsulate hydrophobic drugs, and difficult to encapsulate hydrophilic RNA-based drugs. Some researchers have introduced cationic segments into polymer structures, such as PEI and PAMAM25,26, or added cationic lipids such as DOTAP into formulations27,28, which improved the encapsulation of nucleic acids. However, their positive charge poses safety concerns in vivo, thus hindering their clinical application29. Therefore, current micelle-based nucleic acid delivery system has not made satisfactory progress.
The liver is a vital organ for digestion and detoxification, and liver diseases cause over one million deaths worldwide annually30. Chronic liver damage can lead to liver inflammation and fibrosis, further progressing to cirrhosis and liver cancer31. However, due to the unclear symptoms during hepatic fibrosis, many patients delay treatment32,33. The proliferation and activation of hepatic stellate cells (HSCs) are critical steps in this process34,35. HSCs are multifunctional non-parenchymal cells in the liver, responsible for storing vitamin A, synthesizing, and secreting various collagenases and small amounts of extracellular matrix, releasing transforming growth fact β (TGF-β) and hepatocyte growth factor, and participating in liver metabolism. Upon stimulation by pathological factors, the liver undergoes inflammation, during which inflammatory cells such as lymphocytes and Kupffer cells are activated and release various inflammatory factors, among which HMGB1 plays a crucial role36,37,38. HMGB1, a highly conserved nuclear protein, is secreted by various cells such as damaged hepatocytes and macrophages39. The release of HMGB1 and other inflammatory factors leads to further activation of Kupffer cells and the activation and proliferation of quiescent hepatic stellate cells40,41,42. HSP47 is a collagen-specific molecular chaperone highly expressed in activated hepatic stellate cells, promoting the massive secretion of collagen, leading to collagen deposition, hepatic fibrosis, and cirrhosis development43,44. Therefore, reducing liver inflammation and decreasing collagen deposition are the two main strategies for hepatic fibrosis treatment. In previous studies LNPs have been utilized to encapsulate HSP47 siRNA to alleviate hepatic fibrosis by inhibiting collagen deposition. Our team has also achieved significant efficacy by utilizing LNPs encapsulating HMGB1 siRNA to reduce liver inflammation and alleviate hepatic fibrosis45,46.
Considering the current limitations of LNPs in terms of restricted targeting and accelerated blood clearance, in this study, we design and synthesize three ionizable oligomers, which together with PLA-PEG form a potential nucleic acid drug delivery system, named Ionizable Polymeric Micelles (IPMs) (Fig. 1). The results demonstrate that IPMs effectively encapsulate both siHSP47 and siHMGB1 and exhibit lysosomal escape capability upon cellular uptake (Fig. 1). After introducing the targeting molecule FAPi, IPMs achieve specific delivery to activated HSCs, demonstrating superior anti-hepatic fibrosis effects in vivo (Fig. 1). Following intravenous injection, IPMs show potential neutrophil hitchhiking ability. Meanwhile, the production of anti-PEG antibodies by both IPM formulations is significantly lower than that by LNP, resulting in mitigated accelerated blood clearance (ABC) phenomenon (Fig. 1).
Results
The feasibility of siRNA delivery via IPM
To enable effective encapsulation of nucleic acid drugs by PEG-PLA polymeric micelle, we designed and synthesized three IOs (Fig. 2a, Supplementary Figs. 1–3). They consist of two parts: a tertiary amine head group and a hydrophobic chain segment. The tertiary amine head group serves as the ionizable moiety, capable of capturing H+ protons under acidic conditions to electrostatically adsorb nucleic acid drugs, while the hydrophobic chain can adsorb onto the PLA block of PEG-PLA through hydrophobic interactions, thereby forming ionizable polymeric micelles (IPM) encapsulating nucleic acid drugs. The three IOs were dissolved together with PEG-PLA in ethanol to form an organic phase, and self-assembled into IPM encapsulating siRNA via microfluidic chip assembly (Fig. 2b). To investigate the feasibility of oligomers bearing tertiary amine groups in forming IPMs with PEG-PLA blocks for siRNA delivery, we examined the physicochemical properties of the micelles at four N/P ratios (2.73, 5.45, 8.18, 10.91). The results showed that at N/P = 5.45, the IPM exhibited the highest encapsulation efficiency, reaching 76.18 ± 1.40%, with a particle size of 107.8 ± 2.9 nm, a PDI of 0.138 ± 0.011, and a zeta potential of −0.269 ± 0.298 (Fig. 2d). In the process of designing oligomers, considering their compatibility with PLA and the biodegradability of ester bonds, we designed ionizable oligolactic acid with two different tail chain lengths (n = 4 and n = 8), namely Ionizable Oligo Lactic Acid 4 (IOLA4) and Ionizable Oligo Lactic Acid 8 (IOLA8), and further screening revealed that compared with IPM (IOLA4), IPM (IOLA8) had slightly higher encapsulation efficiency (70.90 ± 1.68%) and smaller, more uniform particle size (145.90 ± 6.26 nm) (Fig. 2e). However, considering that oligolactic acid may degrade too quickly resulting in poor stability, we designed ionizable oligoalanine as the tail chain,namely Ionizable Oligo Alanine 2 (IOA2). Interestingly, compared with IPM (IOLA), IPM (IOA2) had smaller, more uniform particle size (78.57 ± 1.52 nm), a zeta potential closer to 0, and higher encapsulation efficiency (84.12 ± 0.45%) (Fig. 2c, e). Subsequently, different molecular weight PEG-PLA copolymers were screened, including PEG2000-PLA2000, PEG2000-PLA5000, and PEG5000-PLA8000. The results showed that compared with PEG2000-PLA2000 and PEG2000-PLA5000, IPMs formed with PEG5000-PLA8000 exhibited higher encapsulation efficiency and smaller PDI (0.095 ± 0.013) (Fig. 2f). It was also observed that increasing the PLA block molecular weight resulted in larger IPM particle size, while increasing the PEG block molecular weight led to smaller IPM particle size (Fig. 2f). This may be attributed to the ability of PEG to prevent aggregation of IPMs during preparation and storage, thereby reducing particle size. TEM results revealed that all three IPMs displayed a spherical morphology, with IPM (IOA2) having the smallest particle size (Fig. 2g). Furthermore, TEM analysis of RNA staining in IPMs with thionine (0.1 mM) revealed a punctate distribution of RNA within the IPM, with IPM (IOA2) exhibiting a higher RNA concentration (Fig. 2g). These results demonstrate that all three oligomers can form uniform micellar formulations with PEG-PLA blocks and effectively encapsulate siRNA.
a Schematic representation of different ionizable oligomers (IO) structures. b Schematic illustration of IPM structure. c Particle size distribution of IPMs formed by different IOs with PEG5000-PLA8000. d Particle size, PDI, and encapsulation efficiency of IPMs at different N/P ratios (n = 3 samples). IPM achieves the smallest particle size and PDI, along with higher encapsulation efficiency, at N/P = 5.45, with a zeta potential close to 0. e Particle size, PDI, encapsulation efficiency, and zeta potential of IPMs formed by different IOs (PEG5000-PLA8000) (n = 3 samples). IPM (IOA2) exhibits the smallest particle size, PDI, and highest encapsulation efficiency. PEG5000-PLA8000 abbreviated as P5P8, PEG2000-PLA5000 abbreviated as P2P5, PEG2000-PLA2000 abbreviated as P2P2. f Particle size, PDI, encapsulation efficiency, and zeta potential of IPMs formed by different segment molecular weights of PEG-PLA (IOA2) (n = 3 samples). IPM (PEG5000-PLA8000) shows the smallest particle size and highest encapsulation efficiency. g TEM images of IPMs formed by different IOs with PEG5K-PLA8K and nucleic acid stained by thionine. The morphology appears spherical, with IPM (IOA2) having the smallest particle size, around 100 nm, and a higher concentration of RNA with a more punctate distribution. Values represent mean ± standard deviation (SD).
Comparison of in vitro siRNA delivery efficacy among three IPMs
To further explore the potential of each formulation for delivering small interfering RNA (siRNA), we co-incubated IPM/Cy5-siNC with activated hepatic stellate cells (HSCs) for 2 h and observed the uptake of IPM/Cy5-siNC by confocal microscopy, with DAPI labeling the cell nuclei. Results showed successful uptake of IPM/Cy5-siNC after 2 h, with stronger fluorescence observed in cells from the IPM (IOLA8) group compared with the IPM (IOLA4) group, and significantly stronger fluorescence in the IPM (IOA2) group than in the other two groups (Fig. 3a). Flow cytometry was used to assess the efficiency of activated HSCs uptake of IPM (Fig. 3b, Supplementary Fig. 5). The highest cellular uptake efficiency was observed with IPM (IOA2, PEG5000-PLA8000), reaching 75.52 ± 2.80% (Fig. 3b). This indicates that IPM (IOLA8), formed with longer oligo-lactic acid tails, outperforms IPM (IOLA4) formed with shorter tails, and IPM (IOA2) exhibits the strongest capacity for siRNA delivery, possibly due to the structural stability of IO and the ionization capacity of the tertiary amine head group with a shorter alkyl chain. Through lysosomal escape experiments, the Cy5 fluorescence signal carried by siNC and LysoTracker Green DND-26 co-localization diminished over time, demonstrating lysosomal escape of siNC in IPMs (Fig. 3c). Notably, IPM (IOA2) achieved a Pearson’s R < 0.5 at 4 h, indicating reduced co-localization correlation, achieving lysosomal escape the fastest, while IPM (IOLA8) achieved a Pearson’s R < 0.5 at 8 h, and IPM (IOLA4) showed a Pearson’s R < 0.5 at 4 h but with no significant reduction in Overlap R and weak co-localization signal at 2 hours, possibly due to its lower cellular uptake efficiency (Fig. 3d, e).
a Confocal microscopy images of HSCs co-incubated with IPM/Cy5-siNC formed by different IOs for 2 h, with DAPI labeling nucleus. b Flow cytometry assay demonstrating the highest cellular uptake efficiency of IPM (IOA2, PEG5000-PLA8000) (n = 3 cell culture wells). c The confocal images of the lysosomal escape experiments revealing a decline in the co-localization signals from 4 to 6 h, with LysoTracker Green labeling of lysosomes and Hochest 33342 labeling of nuclei. d Statistical analysis of Pearson’s R for co-localization of Cy5 signal with lysosomal signal. At 4 h, the Pearson’s R in the IPM (IOA2) group was <0.5, changing from moderate to low correlation (n = 3 cell culture wells). e Statistical analysis of Overlap R for co-localization of Cy5 signal with lysosomal signal (n = 3 cell culture wells). f TNS assay for the apparent pKa of the three blank carriers (n = 3 independent experiments). g In vitro RNAi using IPM encapsulating siHSP47 and siHMGB1 formed by PEG5000-PLA8000 and IOA2, IOLA4, IOLA8, with LNP serving as a positive control and LNP/siNC serving as a negative control (n = 3 cell culture wells). The qPCR results showing the effective silencing of the HSP47 (h) and HMGB1 (i) genes in vitro, with LNP and Lipo2000 serving as positive controls and LNP/siNC and no siRNA serving as negative controls (n = 3 cell culture wells). Data were analyzed by One-way ANOVA followed by Tukey test. Values represent mean ± standard deviation (SD).
The theoretical pKa values for most ionizable groups fall within the range of 8–9.5. Measurement by the TNS dye-binding method should be within the range of 6–747. One hypothesis is that the positive charge on LNPs forms ion pairs with negatively charged intracellular lipid membranes, leading to membrane rupture and release48. Since the intracellular pH ≈ 5, the degree of protonation at this pH is relevant to lysosomal escape capability49. The lower the pKa value, the stronger the protonation ability. When the pH is lower than the pKa, the degree of ionization of the compound is higher. Therefore, at the same pH, compounds with lower pKa values have higher ionization degrees and more positive charges. Theoretical pKa values for IOA2 are the lowest at 7.99, indicating the strongest protonation ability and the highest number of positive charges, making it more prone to lysosomal escape and more efficient for nucleic acid delivery (Supplementary Table. 1). TNS assay results showed that the minimum pKa for IPM (IOA2) was 5.595, while those for IPM (IOLA4) and IPM (IOLA8) were close, at 6.145 and 6.149, respectively (Fig. 3f). When using the three formulations to deliver siHSP47 and siHMGB1, IPM (IOA2) exhibited the highest gene silencing efficiency at 77.39 ± 0.12% and 88.80 ± 0.38% respectively, better than positive control LNP in vitro (Fig. 3g–I, Supplementary Fig. 6a, b).
In vivo and in vitro targeting effects of FAPi-IPMs
To enhance activated HSCs targeting for the treatment of hepatic fibrosis, we introduced FAPi targeting molecules via maleimide-thiol addition at the end of Mal-PEG5000-PLA8000 (Fig. 4a, Supplementary Figs. 4 and 7), which can target the fibroblast activation protein (FAP) highly expressed by activated HSCs. The synthesized FAPi-PEG5000-PLA8000, along with other materials, was used to prepare FAPi-IPMs. The binding of FAPi to FAP not only mediates the cell-targeting specificity of FAPi-IPMs to activated HSCs but also interferes with fibroblast activation and collagen production. Realtime fluorescence quantitative PCR results showed upregulation of FAP expression in bleomycin-stimulated activated HSCs (Fig. 4b). Flow cytometry results showed approximately a 5% higher Cy5-positive rate in the FAPi-IPM group compared with the IPM group, with an increase of up to 20% after bleomycin stimulation (Fig. 4c, Supplementary Fig. 8). This demonstrates the targeting ability of FAPi-IPM, which is more obvious after bleomycin stimulation. The FAPi-IPM uptake rate of activated HSCs decreased with increasing FAPi concentration in a dose-dependent manner, as evidenced by competitive binding of free FAPi to FAP (Fig. 4d, e). Further in vitro RNAi experiments showed that both IPM/siHMGB1+siHSP47 and FAPi-IPM/siHMGB1+siHSP47 significantly inhibited the translation of their respective target proteins HSP47 and HMGB1, following bleomycin stimulation of HSCs (Fig. 4f, Supplementary Fig. 6c, d). The gene silencing efficiency of FAPi-IPM for HSP47 and HMGB1 was 55.39 ± 1.22% and 42.46 ± 3.49% respectively, better than LNP (Fig. 4g, h).
a GPC molecular weight distribution of FAPi-PEG5000-PLA8000 and PEG5000-PLA8000. b qPCR results demonstrating upregulation of FAP mRNA expression in HSCs following bleomycin stimulation (n = 3 cell culture wells). c Flow cytometry analysis of the cellular uptake efficiency of IPM and FAPi-IPM by HSCs before and after bleomycin stimulation. FAPi-IPM exhibits targeting capability, being more effectively internalized by HSCs, with significant differences observed after bleomycin stimulation (n = 3 cell culture wells). d Competitive inhibition assay with varying concentrations of free FAPi, showing dose-dependent cellular uptake of FAPi-IPM (n = 3 cell culture wells). e Confocal microscopy results indicating increased intracellular fluorescence intensity in FAPi-IPM/Cy5-siNC-treated cells after bleomycin stimulation, with a decrease in intracellular fluorescence intensity as free FAPi concentration increases. f Western blot results of IPM/siHMGB1+siHSP47 and FAPi-IPM/siHMGB1+siHSP47 after bleomycin stimulation of HSCs, with LNP/siHMGB1+siHSP47 serving as a positive control. g, h Effective downregulation of target genes HSP47 and HMGB1 relative expression by IPM and FAPi-IPM (n = 3 cell culture wells). i Schematic representation of hepatic fibrosis modeling. j ROI values in the liver of mice with hepatic fibrosis (n = 3 mice). k Flow cytometry showing FAM positivity of activated hepatic stellate cells (α-SMA labeled) in the livers of mice with hepatic fibrosis (n = 3 mice). l The immunofluorescence results of liver tissues from mice with hepatic fibrosis 24 h after the tail vein injection, with DAPI labeling of the nuclei and α-SMA labeling of activated hepatic stellate cells. m Fluorescence imaging of the liver 24 h after injection in mice with liver fibrosis. Data were analyzed by ANOVA followed by Tukey test. Values represent mean ± standard deviation (SD).
To further verify the targeting specificity of FAPi-IPM in vivo, we established a hepatic fibrosis mouse model by intraperitoneal injection of a 20% CCl4 olive oil solution (2 mL/kg weight, Ip., Tiw., 12 weeks) (Fig. 4i). Twenty-four hours after tail vein injection, the livers from mice with hepatic fibrosis in the FAPi-IPM group exhibited stronger fluorescence intensity, with quantitative results indicating that the liver fluorescence intensity in the FAPi-IPM group was approximately 1.5 times higher than that in the IPM group. (Fig. 4m, j, Supplementary Fig. 9c, f). The rate of FAM positivity of activated HSCs in the livers of the FAPi-IPM group was approximately twice that of the IPM group and three times higher than that of the LNP group (Fig. 4k, S4e). Further immunofluorescence on tissue sections verified the targeting ability of FAPi-IPM, showing that the fluorescence of FAPi-IPM delivering Cy5-siNC was strongest in HSCs, followed by hepatocytes, with minimal presence in liver sinusoidal endothelial cells and Kupffer cells (Fig. 4l, Supplementary Fig. 9a, b, d). In comparison, the IPM and LNP groups exhibited significantly stronger Cy5 fluorescence in Kupffer cells, with Overlap R and Pearson’s R values between 0.3 and 0.5, indicating a low correlation. This suggests that IPM itself is prone to phagocytosis by Kupffer cells, while FAPi modification alters the biological distribution of IPM (Supplementary Fig. 4a, b). Given that LNPs are primarily taken up by hepatocytes after intravenous injection, the result demonstrates the unique properties of the IPMs we prepared, providing the potential for nucleic acid delivery to cells beyond hepatocytes (Supplementary Fig. 9a, b).
Co-delivery of siHSP47/siHMGB1 genes to treat hepatic fibrosis
To assess the therapeutic potential of FAPi-IPM in delivering siHMGB1 and siHSP47 for the treatment of hepatic fibrosis, we aimed to silence excessive HMGB1 and reduce liver inflammation while silencing HSP47 to reduce collagen production by HSCs, thus alleviating hepatic fibrosis (1 mg/kg weight, Iv., Biw., 2 weeks) (Fig. 5a). Histological examination by H&E staining revealed that in the PBS group, liver lobule boundaries were indistinct, with moderate collagen fiber proliferation around central veins and portal areas, bile duct hyperplasia, and hepatocyte vacuolar degeneration. In contrast, treatment groups showed mild collagen fiber proliferation around central veins, occasional pigment deposition, and minimal hepatocyte vacuolar degeneration, with no significant abnormalities in portal areas or inflammatory cell infiltration (Fig. 5b). Masson’s trichrome staining showed that compared with the PBS group, the FAPi-IPM group exhibited a significant reduction in collagen deposition area to 2.37 ± 0.54%, approximately 4% lower than the IPM group (P < 0.0001), with no significant difference compared with the control group (P = 0.1301) (Fig. 5c, d). Immunohistochemical staining for HSP47 demonstrated a decrease in positivity in the FAPi-IPM group by 26.06 ± 2.58% compared with the PBS group, showing significant differences compared with the IPM group (P = 0.0466) and the LNP group (P < 0.0001), but no significant difference compared with the control group (P = 0.6541) (Fig. 5e, f). Immunohistochemical staining for HMGB1 showed a positivity rate of 14.41 ± 1.66% in the FAPi-IPM group, with no significant differences compared with the IPM group (P = 0.1701) and the LNP group (P = 0.0431) (Fig. 5g, h). Western blot analysis confirmed the successful inhibition of the target proteins HSP47 and HMGB1 in the FAPi-IPM group (Fig. 5n–p). Quantitative PCR results further validated the significant downregulation of HSP47 and HMGB1 mRNA levels compared with the PBS group, with reductions of 72.31 ± 5.06% and 58.06 ± 3.95%, respectively, in the FAPi-IPM group, with HSP47 mRNA levels showing no significant difference compared with the negative control group (Fig. 5q, r). Serum analysis showed a decrease in TNF-α levels to 5.83 ± 1.16 pg/mL in the FAPi-IPM group (Fig. 5i). AST and ALT levels were reduced in the treatment groups, with only the FAPi-IPM treatment group showing levels within the normal range (10.06 U/L < ALT < 96.47 U/L, 36.31 U/L < AST < 9235.48 U/L), with no significant difference compared with the control group (Fig. 5j, k). Liver appearance improved in the treatment groups, with the FAPi-IPM group showing smoother liver surfaces and restoration of reddish-brown color (Fig. 5l). Body weight curves showed slower weight gain in mice during modeling compared with the control group, with faster weight gain observed in the treatment groups after administration (Fig. 5m). IPM and FAPi-IPM showed no toxicity to major organs in mice, demonstrating biological safety (Supplementary Fig. 10). These results indicate that FAPi-IPM/siHMGB1+siHSP47 effectively achieves gene knockdown of HMGB1 and HSP47, controls liver inflammation, reduces collagen deposition, and alleviates hepatic fibrosis, nearly restoring liver levels to normal.
a Schematic representation of the dosing regimen for targeting hepatic stellate cells with FAPi-IPM co-delivery of siHSP47 and siHMGB1 for the treatment of hepatic fibrosis (n = 5 mice). b H&E staining results of the liver showing histological changes. c Masson’s trichrome staining results of the liver demonstrating a reduction in collagen deposition area in the FAPi-IPM group. d Quantification of collagen deposition area. e Immunohistochemical staining for HSP47 in the liver showing a decrease in HSP47-positive area and expression in the FAPi-IPM group. f Proportion of HSP47-positive area. g Immunohistochemical staining for HMGB1 in the liver showing a decrease in HMGB1-positive area and expression in the FAPi-IPM group. h Proportion of HMGB1-positive area. i–k Serum levels of TNF-α, AST, and ALT in mice. The FAPi group shows a decrease in ALT and AST levels to the normal range, with no significant difference compared with the negative control group. l Liver appearance showing changes in the PBS group and alleviation in the FAPi-IPM group. m Body weight curve of mice showing weight recovery after administration in the treatment groups. n Western blot results demonstrating inhibition of HSP47 and HMGB1 protein expression in the treatment groups. o, p Significant silencing of HSP47 and HMGB1 protein expression in the FAPi-IPM group in vivo. q, r qPCR results demonstrating significant gene silencing of HSP47 and HMGB1 in the treatment groups. Data were analyzed by ANOVA followed by Tukey’s multiple comparisons test. Values represent mean ± standard deviation (SD).
Pharmacokinetics and biodistribution of IPM and LNP
To further compare the in vivo fate of IPM and LNP, we investigated their biodistribution and pharmacokinetics. In vivo imaging and fluorescence microscopy of liver tissue sections showed siRNA labeled with FAM encapsulated in LNP entered the liver more rapidly, reaching maximum accumulation at 2 h and being rapidly cleared within 4 h. In contrast, FAM-siRNA encapsulated in IPM gradually accumulated between 0.5 and 2 h and was cleared by 8 h, while which in FAPi-IPM exhibited maximal liver fluorescence intensity at 24 h with slower clearance (Fig. 6a, c). Immunofluorescence staining using F4/80 to label liver Kupffer cells (KCs) showed that at 2 and 4 hours, Pearson’s R and Overlap R values were greater than 0.3 in the LNP group, indicating colocalization, while IPM and FAPi-IPM groups showed no colocalization, suggesting that LNP was more readily engulfed by KCs (Fig. 6b, d). To further investigate the effect of FAPi modification on cell uptake of IPM and the rapid liver clearance of LNP, liver perfusion was performed to obtain hepatocytes, non-parenchymal cells, and splenic cells for flow cytometry analysis (Supplementary Fig. 11). The results showed that the uptake rates of IPM and LNP by hepatocytes decreased over time, possibly due to their engulfment by Kupffer cells at 2 h (Fig. 6e). In contrast, FAPi-IPM was less taken up by hepatocytes at 2 h, with subsequent uptake rates gradually increasing to 36.64 ± 1.92% (Fig. 6e). Additionally, at 2 h, IPM, LNP, and FAPi-IPM were mainly taken up by splenic mononuclear cells, with LNP also being engulfed by dendritic cells (DCs) and neutrophils and then cleared, while IPM maintained high mononuclear cell uptake rates at all three time points (Fig. 6f). Fluorescence quantification of splenic tissue at 2 h showed slightly higher average fluorescence intensity in the LNP group compared with IPM (P = 0.1677) (Supplementary Fig. 12h). Colocalization results indicated that at 2 h, LNP was more engulfed by mononuclear cells, while IPM and FAPi-IPM were predominantly taken up by neutrophils (Supplementary Fig. 12). In vivo, LNP was rapidly cleared, with an AUC0-24 of 100.9 µg/mL, significantly greater than that of the IPM group (P = 0.028) (Fig. 6j, Supplementary Table. 2). Serum analysis after four administration showed that all three formulations stimulated the production of anti-PEG IgM, with OD450 ≥ 0.15 considered positive (Fig. 6h). The results showed a rapid increase in anti-PEG production in the LNP group after the third dosing, with the LNP group ultimately exhibiting the highest titer (33,168), followed by the IPM group (12,825), and FAPi- IPM group having the lowest (6617) (Supplementary Fig. 13b, c). After repeated administration, pharmacokinetic results indicated ABC effects for both LNP and IPM. However, compared to the LNP group, which had an AUC0-24 rechallenged/AUC0-24 ratio of 16.4%, the FAPi-IPM group showed a significantly higher ratio of 41.2% (Fig. 6 I, K, L, Supplementary Fig. 13a, Supplementary Table. 2).
a Fluorescence imaging of healthy male C57BL/6 mice (6–8 weeks old) injected with FAPi-IPM, IPM, or LNP/FAM-siNC via the tail vein at 2, 4, 8, and 24 h. LNP enters the liver more rapidly and is rapidly cleared, while FAPi-IPM exhibits prolonged liver distribution. b Immunofluorescence staining of liver tissue (F4/80 labeling KCs) at 24 h post-injection reveals differences in uptake among formulations. c ROI values indicate LNP accumulates most in the liver at 4 h, whereas FAPi-IPM shows delayed liver accumulation at 24 h (n = 3 mice). d Pearson’s R of FAM fluorescence and KCs in liver showing rapid phagocytosis of LNP by KCs at 2–4 h (n = 3 mice). e Cell distribution changes show LNP is captured by hepatocytes and Kupffer cells at 2 h, while FAPi-IPM enters hepatocytes later at 8 h; IPM enters hepatocytes less overall (n = 3 mice). f In the spleen, all formulations are mainly taken up by mononuclear cells, with FAPi-IPM showing the highest uptake (n = 3 mice). g The 2-h FAM colocalization results in the spleen indicate that all three formulations strongly colocalize with neutrophils and are mostly taken up by neutrophils. h Schematic representation of dosing regimen: PEGylated drug injections on days 0, 3, 6, and 9, with pharmacokinetic assays on days 1 and 10, and anti-PEG IgM levels assessed on days 1, 3, 6, 9, and 14 (n = 6 mice). i Pharmacokinetic curves of the three PEGylated drugs and pharmacokinetic curves rechallenged on day 10. AUC0-24 of the three PEGylated drug on day 1 (j) and day 10 (k). l Ratio of AUC0-24 rechallenged and AUC0-24. Data were analyzed using ANOVA, with Sidak multiple comparisons for Fig. 6c, and Tukey multiple comparisons for others. Values represent mean ± standard deviation (SD).
Comparison of protein coronae adsorbed on IPM and LNP
We hypothesized that different behaviors between IPM and LNP in biological distribution may be related to differences in their adsorbed protein coronae. Firstly, after co-incubation with serum for 7 days, the three formulations showed minor changes in particle size, indicating their inherent stability in serum (Supplementary Fig. 14). To validate this hypothesis, we compared protein corona composition of three carriers based on a scFv-based affinity chromatography as previously reported50 (Fig. 7a). Silver staining results indicated that the protein corona of the formulations was similar both in vivo and in vitro, with noticeable differences in bands around 130, 100, 55, 25, and 15 kDa for IPM and LNP (Fig. 7b). During the process of separating the adsorbed protein coronae of the formulations, the peak time of protein corona for LNP and IPM was longer compared with FAPI-IPM. This could be attributed to the longer PEG chains of IPM and FAPI-IPM, which exhibited stronger binding affinity with anti-PEG scFv, resulting in lower competitive elution efficiency with PEG8000 (Fig. 7c–e). To further provide functional information on the protein corona, we identified the protein composition and relative abundance by collecting the two tubes with the highest protein concentrations. The results showed that FAPi-IPM and IPM adsorbed higher concentrations and more diverse types of proteins compared with LNP (Fig. 7f, g). It is noteworthy that, unlike LNP, FAPi-IPM and IPM almost did not adsorb apolipoproteins (Fig. 7h). There were 649 common proteins among the three formulations (Fig. 7i). The top 20 enriched proteins adsorbed by LNP included complement 3 (C3) and ApoE, containing more immunoglobulins and apolipoproteins compared with IPM and FAPi-IPM in all protein compositions (Fig. 7j). This may explain the rapid targeting of LNP to hepatocytes, while both IPM formulations did not. Previous studies have shown that soluble ApoE adsorbed on the surface of LNP51,52 in the circulation promotes binding to low-density lipoprotein receptors (LDLr) highly expressed on the sinusoidal surface of hepatocytes53,54, leading to LNP internalization and subsequent RNA intracellular delivery55. It has also been revealed that the rapid clearance of LNP may be due to the activation of the complement system by LNP, leading to the opsonization effect of complement C3 fragments, which accelerates the uptake of PEGylated nanocarriers by the mononuclear phagocyte system (MPS), resulting in rapid clearance from the bloodstream9,56,57. To further obtain biological functional information of the protein corona, we conducted GO and KEGG analyses. Molecular Function (MF) was sorted by gene count, and the top 8 functions of IPM and FAPi-IPM were basically consistent, with the difference being that the protein corona adsorbed by FAPi-IPM were associated with cation binding and metal ion binding, while those adsorbed by IPM were associated with nucleic acid binding and nucleotide binding (Fig. 7k, Supplementary Fig. 15). It is noteworthy that the top 2 functions of all three formulations were binding and protein binding, but there was a significant difference in the LNP group, which was associated with oxidoreductase activity, possibly accelerating metabolism (Fig. 7k, Supplementary Fig. 15). KEGG results showed significant involvement of proteins adsorbed by LNP and IPM in the complement pathway (Fig. 7, Supplementary Fig. 15).
a Schematic illustration of the process for isolating the in vitro and in vivo protein coronae adsorbed on formulations. b Silver staining results of protein coronae adsorbed on FAPi-IPM (F), IPM (M), and LNP (L) in vitro and in vivo. Three times was repeated independently with similar results. c–e Protein concentrations collected from the three PEGylated drugs via affinity chromatography columns, with tubes 13 to 18 eluted with PEG8000. f The two tubes with the highest protein concentrations collected from each formulation via affinity chromatography columns were defined as the protein coronae adsorbed on the formulations, with FAPi-IPM and IPM exhibiting higher protein corona concentrations than LNP. g The number of protein types adsorbed on the three formulations. h Top 50 proteins adsorbed on the three formulations classified by complement, immunoglobulins, apolipoproteins, etc. i Venn diagram. j Top 20 proteins adsorbed on the three formulations. Molecular function (k) and KEGG analyses (l) of the protein coronae adsorbed on IPM. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. Values represent mean ± standard deviation (SD).
Discussion
In this study, we successfully designed and synthesized three IOs formulations with potential for nucleic acid delivery. Through screening based on physicochemical properties, nucleic acid delivery capability, lysosomal escape ability, and RNAi efficiency, we optimized the formulation and developed a potential nucleic acid delivery system, IPM. During the prescription screening process, we found that IOA2 with oligoalanine tails outperformed IOLA4 and IOLA8 with oligolactic acid tails. This may be attributed, on one hand, to the greater stability of amide bonds compared to ester bonds in vivo, along with the introduction of more nitrogen. On the other hand, it may be due to the shorter alkyl chain between the tertiary amine head group and the hydroxyl group, resulting in stronger protonation ability and enhanced capabilities for electrostatic adsorption of siRNA and escape from lysosomes. To apply this potential delivery system to the treatment of hepatic fibrosis using siRNA therapy targeting activated HSCs overexpressing FAP, we further introduced the targeting molecule FAPi. In vitro, FAPi-IPM exhibited approximately 20% higher uptake by activated hepatic stellate cells compared with IPM, with a dose-dependent effect. Meanwhile, the RNAi efficiency targeting HSP47 and HMGB1 respectively increased to 55.39 ± 1.22% and 42.46 ± 3.49% respectively. In vivo, FAPi-IPM showed significant targeting to hepatic stellate cells in mice with hepatic fibrosis, with reduced phagocytosis by KCs compared with IPM, leading to more effective knockdown of HMGB1 and HSP47, resulting in reduced levels of ALT, AST and TNF-α, thus effectively alleviating hepatic fibrosis.
In the treatment of hepatic fibrosis, we found that IPM achieved similar therapeutic effects compared with the positive control, LNP. To explore the differences in the in vivo fate of IPM compared with traditional LNP, we further investigated the biodistribution and pharmacokinetic performance of the three formulations. The results of biodistribution showed that, in healthy mice, compared with IPM and LNP, FAPi-IPM did not exhibit significant targeting ability, which may be related to the non-activation of HSCs and low expression of FAP in healthy mice. FAPi-IPM was less prone to phagocytosis by KCs and entered HSCs later than IPM. IPM and FAPi-IPM were more likely to be taken up by neutrophils than LNP, whereas LNP was more likely to be taken up by monocytes and DCs. This cellular distribution pattern may lead to faster clearance of LNP compared with IPM and FAPi-IPM. Pharmacokinetic results confirmed our hypothesis. FAPi-IPM and IPM were metabolized more slowly in the liver than LNP, with smaller serum clearance rate constants and longer half-lives. Additionally, after four administrations, the titer of anti-PEG IgM produced by FAPi-IPM was much lower than that of IPM and LNP, and after the first administration, IPM and LNP exhibited significant ABC effects, with LNP showing a more pronounced effect.
To further analyze the reasons for the different in vivo fates, we examined the differences in the composition and relative abundance of soft protein coronas adsorbed by the three formulations in vitro and in vivo. The results showed that FAPi-IPM and IPM adsorbed higher concentrations and more types of proteins in vitro and in vivo than LNP. The top 50 proteins adsorbed by FAPi-IPM and IPM contained fewer complement proteins and almost no apolipoproteins, and most of the adsorbed proteins were functionally related to binding. This explains why they are cleared more slowly in the serum, possibly due to rapid adsorption of proteins in the circulation, camouflaging themselves as non-“foreign” particles, thereby avoiding capture and clearance by the mononuclear phagocyte system. The composition of the top 50 proteins adsorbed by LNP differed significantly from the other two formulations, containing apolipoprotein E and complement component 3, and the function of the adsorbed proteins was related to oxidoreductase activity, with significant involvement in the complement pathway, revealing the rapid distribution in hepatocytes and rapid metabolism in vivo.
In conclusion, we have developed a potential strategy for nucleic acid drug delivery carriers, which exhibit targeting beyond hepatocytes, and significantly mitigated ABC phenomenon. In future, we may take advantage of their higher uptake rates in neutrophils to develop targeted therapies for inflammation-related diseases58,59,60. In addition, ionizable oligomer structure modification is also significant for improving the capability of IPM.
Methods
Ethical statement
All animal experiments were conducted following guidelines approved by the Ethics Committee of East China Normal University (ARXM2023088).
Animals
SPF grade Male C57BL/6 mice aged 6–8 weeks were purchased from the Shanghai Laboratory Animal Center. The animals are kept in the Experimental Animal Center of the Institute of Brain Functional Genomics (license number: SYXK (Shanghai) 2014-0006), with a barrier environment, and IVC independent air supply cages are used inside the barrier environment. Anesthetize mice with isoflurane: Adjust the induction concentration to 3–4%, wait for the anesthetic to fill the induction box, about 1 min, place the animal in the induction box, then close the induction box and wait for the animal to be completely anesthetized (this process takes about 2–3 min). The induction box can be gently shaken to check if the animal is completely anesthetized. If the animal is flipped over to the side and has not attempted to return to its lying position, it indicates that the animal is completely anesthetized. Two weeks after siRNA treatment, mice were anesthetized with 10% chloral hydrate (0.004 ml/g body weight) intraperitoneally and euthanized for cervical dislocation to minimize animal pain. Animals are euthanized, and the bodies are disinfected and placed in body bags, frozen and stored for harmless incineration as soon as possible.
Materials
PEG5000-PLA8000, PEG2000-PLA5000, PEG2000-PLA2000, and mPEG-DSPE were purchased from Ruixi Biological Technology (Xi’an, China). Mal-PEG5000-PLA8000 was obtained from Bonsun Biological Technology Co., Ltd. (Shaanxi, China). 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Fibroblast activation protein inhibitor (FAPi) was synthesized by the research group of Prof. Jiahui Yu at East China Normal University. ALC-0315 (s89624) was obtained from YuanYe (Shanghai, China). 2-(Dimethylamino)-6-naphthalenesulfonic acid (T275812) was purchased from Aladin. Thionine (A602642) was obtained from Sangon Biotech (Shanghai, China). Lysosome Green fluorescent probe (4073ES50) was purchased from Yeason Biotechnology Co., Ltd. (Shanghai, China). Lipofectamine 2000 and Trizol (11668-027) were purchased from Invitrogen (USA). AceQ Universal SYBR qPCR Master Mix (Q511-02) and HiScript® II Q RT SuperMix for qPCR (+g DNA wiper) (R223-01) were obtained from Vazyme (Nanjing, China). Quant-iT RiboGreen RNA assay kit (R11490) was purchased from Thermo (USA). Alanine aminotransferase (ALT) assay kit (UV-LDH method) was obtained from Changchun Huili. Aspartate aminotransferase assay kit (Aspartate substrate method) was purchased from Leadman Life Technology Co., Ltd. Mouse TNF-alpha ELISA Kit (GEM0004) was purchased from Servicebio (Wuhan, China). BCA protein assay kit was purchased from Sigma-Aldrich (St. Louis, MO, USA). HisSep Ni-NTA Agarose 6FF resin (20503ES60) was obtained from Yeasen Biotechnology Co., Ltd. (Shanghai, China). Fast Silver Staining Kit (P0017S) and TMB substrate (P0209) were purchased from Beyotime Biotechnology (Shanghai, China). Percoll (17089109) was purchased from Cytiva Co., Ltd. (Shanghai, China). Collagenase IV (BS165) was obtained from Biosharp (China). MACS Tissue Storage Solution (130-100-008) was purchased from Miltenyi Biotec (Germany). HSCs were obtained from Shanghai YuKadi Pharmaceutical Technology Co., Ltd. Information on the antibodies is provided in Supplementary Data 1.
Two siRNAs targeting mouse HMGB1 mRNA and HSP47 mRNA were synthesized by Guangzhou RiboBio Co., Ltd., and some experiments utilized siRNAs labeled with fluorescent dyes Cy5 or FAM at the 5’ end (Table 1). Primers for qPCR targeting mouse HMGB1, HSP47, fibroblast activation protein (FAP), and GAPDH were synthesized by Guangzhou RiboBio Co., Ltd (Table 2).
Synthesis of IOs
Compound IOA2 was synthesized through esterification reaction of 10-bromodecanol with N-Ac-Ala-Ala-OH, followed by substitution with ethanolamine. Protected lactide dimer and lactide tetramer were obtained via a series of reactions with propyl acetate. After deprotection of the hydroxyl end followed by acetylation and deprotection of the carboxyl end followed by condensation, compounds IOLA4 and IOLA8 were obtained after removal of the silane protecting group. Reagents and instruments related to synthesis are listed in Supplementary Tables 3 and 4.
Preparation of IPM and FAPi-IPM
PEG-PLA and IOs were dissolved in anhydrous ethanol at a ratio of 40.2:59.8 (mol/mol) as the organic phase, while siRNA (N/P = 5.45) was dissolved in pH 5.0 10 mM citrate buffer as the aqueous phase, with twice the volume of the aqueous phase in pH 6.0 10 mM citrate buffer as the buffer phase. The aqueous phase and organic phase were mixed at a flow rate of 3:1 using a microfluidic device (NEXSTAR NANO2, C10-1). The mixture was then centrifuged in ultrafiltration centrifuge tubes (MWCO = 10 kDa) at 2000 × g for 20 min three times, followed by elution with PBS and collection of the final product IPM. FAPi-IPM had a composition of PEG-PLA: ionizable oligomers: FAPi-PEG-PLA = 40.2:59.8:1 (mol/mol), with the rest of the process remaining consistent.
Preparation of LNP
ALC-0315/DSPC/Chol/PEG2000-DSPE was dissolved in ethanol at a ratio of 50:10:38.5:1.5 (mol/mol) as the organic phase, siRNA (N/P = 5.67) was dissolved in pH 5.0 10 mM citrate buffer as the aqueous phase, and two times the volume of the aqueous phase in pH 6.0 10 mM citrate buffer as the buffer phase. C5-3 chips were selected and prepared by microfluidic instrumentation with an aqueous phase: organic phase at a flow rate of 3:1, centrifuged three times at 2000 × g for 20 minutes in ultrafiltration centrifuge tubes (MWCO = 10 kDa), eluted by the addition of PBS and the collection of final products.
Methods of characterization
Encapsulation efficiency was determined using Quant-iT Ribogreen RNA assay. Dynamic light scattering (DLS) technique was employed with Zetasizer Nano ZS (Malvern, UK) to measure the nanoparticle hydrodynamic radius, polydispersity index (PDI), and zeta potential. Transmission electron microscopy (TEM) JEOL 100-CX (JEOL USA Inc., Peabody, MA) was used to observe the morphology of nanoparticles, followed by staining of RNA with thionine (0.1 mM) to observe the inner position of nucleic acid drugs. Samples were prepared at a concentration of 0.5 mg/mL. The prepared formulation was co-incubated with 10% (v/v) serum, and the stability in serum was assessed by measuring the particle size within 7 days.
Determination and calculation of pKa
The pKa of the micelles was determined using the TNS method. Blank nanoparticles were prepared and diluted in a series of citrate buffer solutions with pH ranging from 3 to 10 at a concentration of 6 μM. TNS was dissolved in DMSO at a concentration of 300 μM, and 2 μL of the TNS solution was mixed with 100 μL of blank nanoparticles. Fluorescence intensity was measured under excitation at 325 nm and emission at 435 nm. The fluorescence intensity readings were curve-fitted against the pH values to calculate the pKa.
All quantum chemical calculations were performed using the Gaussian 16 (A.03) software package61. DFT calculations were based on the wb97xd/6-31 g(d)62 functional and included long-range corrections. Geometry optimization and Gibbs free energy (gas-phase and solvation) calculations were performed at the wb97xd/6-31 g(d) level, and pKa values were calculated using the method reported in the literature63. Molecular coordinates are provided in Supplementary Data 2.
In vitro lysosomal escape
IPMs and LNP were co-incubated with HSCs for 2, 4, 6, and 8 h. The cells were stained with Hoechst 33342 for nucleus labeling and LysoTracker Green DND-26 for lysosome labeling. The changes in co-localization signals between Cy5 fluorescence and lysosomes were observed under a laser scanning confocal microscope (LEICA, Germany). Pearson’s R and Overlap R were calculated and analyzed.
In vitro targeting experiment
After co-incubation of micelle-encapsulated Cy5-siNC with activated HSCs for 6 h, flow cytometry was performed to measure Cy5+ cells and calculate the cellular uptake rate. Following a 4-h co-incubation, HSCs were fixed with paraformaldehyde, stained with Hoechst 33342 for nucleus labeling, and sealed with anti-fluorescence quenching mounting medium. The Cy5 fluorescence signal in the cells was observed under a confocal microscope.
In vitro gene silencing efficiency
HSCs activation was induced by culturing cells in medium supplemented with bleomycin (1 µg/mL) for 24 h. The medium was then replaced with opti-MEM, and IPM/siHSP47+siHMGB1 with or without FAPi targeting moiety was added for a 6-hour co-incubation. After 48 h of incubation, RNA was harvested for qPCR analysis, while protein was harvested after 72 h for Western blot analysis to assess RNAi efficiency at both the gene and protein levels.
Establishment of in vivo hepatic fibrosis model
Male C57BL/6 mice aged 6–8 weeks were intraperitoneally injected with a 20% CCl4 solution in mineral oil (2 mL/kg body weight) three times per week for 12 consecutive weeks. Liver sections were evaluated for fibrosis scoring using the Metavir fibrosis scoring system, and the modeling was considered successful when the pathology of the mouse liver reached stage S3.
In vivo targeting experiment
Groups of control and hepatic fibrosis model mice (n = 3) were intravenously injected with FAPi-IPM and IPM which encapsulated Cy5-siNC (1 mg/kg). Live imaging was conducted at 2, 4, 8, and 24 h after the injection. At the final time point, organs including the brain, heart, liver, spleen, lungs, and kidneys were harvested to observe the fluorescence signal intensity. Groups of control and hepatic fibrosis model mice (n = 3) were intravenously injected with LNP, FAPi-IPM or IPM which all encapsulated FAM-siNC (1 mg/kg). Liver tissues were further processed for histological analysis, with α-SMA marking hepatic stellate cells (HSCs), CD146 marking hepatic sinusoidal endothelial cells, F4/80 marking Kupffer cells, and albumin marking hepatocytes. The spleen was also examined for CD45-positive leukocytes and CD68-positive macrophages. The targeting for HSCs in the liver was evaluated. At 24 h post-injection, liver perfusion was performed to separate non-parenchymal cells. Activated HSCs were labeled with α-SMA recombinant mouse mAb, followed by staining with goat anti-mouse AF647 secondary antibody. The uptake rate of activated hepatic stellate cells in fibrosis liver was defined as the proportion of FAM-positive cells, measured by flow cytometry.
In vivo hepatic fibrosis treatment
C57BL/6 male mice with successfully established models were divided into four groups (n = 5): IPM/siHSP47+siHMGB1 with and without FAPi targeting moiety, LNP positive control group, and PBS negative control group. Treatment was administered twice a week for two consecutive weeks (1 mg/kg). On the 14th day, serum levels of alanine transaminase (ALT), aspartate transaminase (AST), and tumor necrosis factor-α (TNF-α) were measured and compared with pre-treatment levels. Liver and other major organs were harvested for H&E and Masson’s trichrome staining to observe collagen fiber proliferation, edema necrosis, inflammatory cell infiltration, and congestion in liver tissues. Immunohistochemistry, Western blot, and qPCR (Bio Rad) were employed to detect the expression levels of HMGB1 and HSP47 proteins and genes in liver tissues.
Distribution in organs of healthy mice
Male C57BL/6 mice aged 6–8 weeks were intravenously injected with LNP, FAPi-IPM or IPM which all encapsulated FAM-siNC (1 mg/kg). At 30 min, 2 h, 4 h, 8 h, and 24 h post-injection, heart, liver, spleen, lung, and kidney were harvested for fluorescence imaging using a small animal live imaging system (Bruker Corporation). Immunofluorescence staining was performed for liver and spleen tissues. In the liver, α-SMA was used to label hepatic stellate cells (HSCs), CD146 for liver sinusoidal endothelial cells, F4/80 for Kupffer cells, and Albumin for hepatocytes. In the spleen, CD45 was used to label leukocytes, CD68 for macrophages, CD11c for dendritic cells, CD3 for T cells, CD19 for B cells, Ly6g for neutrophils, and Ly6c for monocytes. Co-localization analysis with FAM was conducted for each marker.
Cellular distribution
Male C57BL/6 mice aged 6–8 weeks were intravenously injected with LNP, FAPi-IPM or IPM which all encapsulated FAM-siNC RNA (1 mg/kg). At 2 h, 8 h, and 24 h post-injection, organs were collected, and liver perfusion was performed. Liver tissue was minced and incubated at 37 °C for 0.5 h, then ground through a 70 μm cell strainer into a 50 mL centrifuge tube. The sample was washed with 15 mL ice-cold HBSS-CaCl2 and centrifuged at 4 °C, 50 × g, for 3 min. The supernatant was transferred to a new 50 mL tube for non-parenchymal cell isolation, while the pellet was washed twice with 15 mL ice-cold HBSS-CaCl2. The final pellet was resuspended in 1 mL PBS to obtain parenchymal cells. The supernatant for non-parenchymal cell isolation was further centrifuged at 4 °C, 650 × g, for 7 min. The cell pellet was resuspended in 1 mL PBS and gently layered on a 25% (2.5 mL)/50% (2 mL) Percoll gradient. The sample was centrifuged horizontally at 4 °C, 1500 × g, for 20 min. The intermediate cell layer (2 mL) was collected, diluted with 20 mL ice-cold PBS, and centrifuged at 4 °C, 650 × g, for 7 min to disrupt the Percoll gradient. The pellet containing non-parenchymal cells was retained. Hepatocytes were labeled with albumin antibody, followed by staining with goat anti-rabbit BV421 secondary antibody. Liver sinusoidal endothelial cells were labeled with APC-CD146, Kupffer cells with BV421-F4/80. For spleen cells, the spleen was transferred to an EP tube with 1000 μL PBS, minced, and ground through a 70 μm cell strainer into a six-well plate. The strainer was rinsed with 1 mL PBS, and the cell suspension was collected and centrifuged at 4 °C, 700 × g, for 5 min. The supernatant was discarded, and 1 mL red blood cell lysis buffer was added to the cell pellet, incubating on ice for 8 min. Cells were centrifuged at 4 °C, 1500 × g, for 8 min. The lysis step was repeated until all red blood cells were removed. Finally, cells were washed with ice-cold PBS and centrifuged at 4 °C, 700 × g, for 5 min. Spleen tissues were ground over a 70 μm cell strainer, and cell suspensions were collected and lysed. Dendritic cells were labeled by BV421-CD11c, macrophages by APC-F4/80, T cells by APC-CD3, B cells by BV421-CD19, neutrophils by APC-Ly6g, and monocytes by APC/Cy7-Ly6c. Flow cytometry (FACS Vantage, Franklin Lakes, NJ) was used to analyze the percentage of FAM+ cells.
Determination of Anti-PEG IgM antibody titers
Enzyme-linked immunosorbent assay (ELISA) was performed to measure the titers of anti-PEG IgM antibodies. A high-binding 96-well plate was coated with mPEG 2000-DSPE at a concentration of 20 µg/mL in ethanol, with 100 µL per well, and left overnight to dry. The plate was then blocked with 5% BSA for 1 h. Mouse sera, diluted 10-fold with 0.1% BSA-PBS, were added to the plate in a serial dilution manner, starting from 1:10, and incubated at 37 °C for 1 h. After washing, the plate was incubated with Goat Anti-Rat IgM mu chain (HRP) secondary antibody (ab97180) diluted 1:5000 in 0.1% BSA for 1 h at 37 °C. TMB substrate was added to each well, and the plate was incubated for 8 min for color development. The reaction was terminated with 0.18 M sulfuric acid. The optical density (OD) at 450 nm was measured using a microplate reader (Biotek), with OD450 ≥ 0.15 considered positive. The titers were calculated accordingly.
Pharmacokinetics
Male C57BL/6 mice aged 6–8 weeks were intravenously injected with LNP, FAPi-IPM or IPM which all encapsulated FAM-siNC (1 mg/kg). Blood samples were collected at 15 min, 30 min, 2 h, 4 h, 8 h, and 24 h post-injection. The emulsion should be disrupted using methanol, after which the fluorescence intensity of FAM in the serum should be measured. A standard curve should be constructed based on the fluorescence intensity of the FAM-siNC, from which the concentration of siRNA can then be calculated.
Comparison of protein corona adsorption
Using a previously reported method by Zhan’s lab50, 20 µL of FAM-siNC-loaded micelles and LNP (maintained at the same concentration by fluorescence quantification) were separately mixed with 60 µL of His-tagged PEG-scFv (corresponding to 400 µg of antibody for micelles and 100 µg for LNP) and incubated at room temperature for 10 min. Subsequently, the mixtures were combined with 20 µL of C57BL/6 mouse serum and incubated in vitro at 37 °C for 30 min. In vivo, Male C57BL/6 mice aged 6–8 weeks were intravenously injected with LNP, FAPi-IPM or IPM which all encapsulated Cy5-siNC (1 mg/kg) (maintained at the same concentration by fluorescence quantification). Blood samples (20 µL) were collected from the tail vein using EDTA anticoagulant. The samples were directly mixed with 60 µL of His-tagged PEG-scFv (same as before). Ni-NTA was packed into a 3 mL gravity column, washed six times with PBS, and the pre-mixed solutions were slowly applied to enable a complete interaction of Ni and His-tagged PEG-scFv for 30 minut at room temperature. Fractions #1-#12 were collected using PBS containing 5 mM imidazole (to wash out unbound protein components), while fraction #13 onwards was eluted using 100 µL of PEG8000 in PBS (100 mg/mL) and collected into #14 for fluorescence fraction, 50 µL per tube, until #18 (protein corona). The components collected from the three formulations were quantified using BCA assay, and two tubes were combined and denatured by adding 1/4 of 5× loading buffer and heating at 100 °C for 10 min, following SDS-PAGE separation with silver staining. Approximately 200 ng of total peptides from each sample were separated using a nanoUPLC liquid chromatography system (nanoElute2) coupled to a mass spectrometer equipped with a nanospray ion source (timsTOF Pro2). Chromatographic separation was achieved using a 75 μm ID × 15 cm reversed-phase column (IonOpticks C18-CSI, 1.6 µm, Aurora Ultimate). The raw data files were searched against a database using the SpectroMine (4.2.230428.52329, Biognosys AG) software application Pulsar retrieval engine for qualitative analysis. Subsequently, label-free quantification was performed by summing the intensities of all peptides in the sample, and relative protein levels were compared. The top 50 proteins in protein corona on the three nanocarriers were functionally categorized using the DAVID database, followed by Gene Ontology (GO) and Encyclopedia of Genes and Genomes (KEGG) analyses.
Data collection
Flow cytometry data were processed using FlowJo software. Laser confocal microscopy and immunofluorescence results were analyzed for colocalization using the Colocalization Finder plugin in ImageJ. Immunohistochemistry results were analyzed for positivity rates using the IHC plugin in ImageJ. The Split Channels function in ImageJ was used to convert images to single-color channel 8-bit grayscale images, and the average grayscale values were calculated to determine the average immunofluorescence intensity. The Color Threshold function in ImageJ was used to set ranges and calculate the collagen deposition area in Masson’s staining.
Statistics and reproducibility
All data are presented as mean ± standard deviation. Student’s t test (two-sided) was used to analyze data between two groups. For data involving three or more groups, analysis of variance (ANOVA) was conducted followed by Tukey’s multiple comparison test. Statistical significance was defined as ns (p > 0.05), * (p < 0.05), ** (p < 0.01), or *** (p < 0.001), **** (p < 0.0001), with a 95% confidence interval. All data analyses were performed using GraphPad Prism v9.5 software. All samples were run in triplicate.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The relevant raw data for each figure and table in both the main manuscript and the Supplementary Information are available in the Source Data. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with accession code PXD057444. Source data are provided with this paper.
References
Pattipeiluhu, R. et al. Liquid crystalline inverted lipid phases encapsulating siRNA enhance lipid nanoparticle mediated transfection. Nat. Commun. 15, 1303 (2024).
Kim, Y.-K. RNA therapy: rich history, various applications and unlimited future prospects. Exp. Mol. Med. 54, 455–465 (2022).
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Liu, K. et al. Multiomics analysis of naturally efficacious lipid nanoparticle coronas reveals high-density lipoprotein is necessary for their function. Nat. Commun. 14, 4007 (2023).
Rhym, L. H., Manan, R. S., Koller, A., Stephanie, G. & Anderson, D. G. Peptide-encoding mRNA barcodes for the high-throughput in vivo screening of libraries of lipid nanoparticles for mRNA delivery. Nat. Biomed. Eng. 7, 901–910 (2023).
Zhu, Y. et al. Screening for lipid nanoparticles that modulate the immune activity of helper T cells towards enhanced antitumour activity. Nat. Biomed. Eng. 8, 544–560 (2024).
Wang, H. et al. Polyethylene glycol (PEG)-associated immune responses triggered by clinically relevant lipid nanoparticles in rats. NPJ Vaccines 8, 169 (2023).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Sago, C. D. et al. Augmented lipid-nanoparticle-mediated in vivo genome editing in the lungs and spleen by disrupting Cas9 activity in the liver. Nat. Biomed. Eng. 6, 157–167 (2022).
Lokugamage, M. P. et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 5, 1059–1068 (2021).
Li, B. et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat. Biotechnol. 41, 1410–1415 (2023).
Xue, L. et al. High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models. Nat. Commun. 15, 1884 (2024).
Qiu, M. et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl Acad. Sci. USA 119, e2116271119 (2022).
Zhao, G. et al. TGF-βR2 signaling coordinates pulmonary vascular repair after viral injury in mice and human tissue. Sci. Transl. Med. 16, eadg6229 (2024).
Ma, F. et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci. Adv. 6, eabb4429 (2020).
Xue, L. et al. Rational design of bisphosphonate lipid-like materials for mRNA delivery to the bone microenvironment. J. Am. Chem. Soc. 144, 9926–9937 (2022).
Lian, X. et al. Bone-marrow-homing lipid nanoparticles for genome editing in diseased and malignant haematopoietic stem cells. Nat. Nanotechnol. https://doi.org/10.1038/s41565-024-01680-8 (2024).
Guan, S. et al. Self-assembled peptide–poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat. Nanotechnol. 14, 287–297 (2019).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Zhong, R. et al. Hydrogels for RNA delivery. Nat .Mater 22, 818–831 (2023).
Shi, M. et al. Comparing nanoparticle polymeric micellar paclitaxel and solvent-based paclitaxel as first-line treatment of advanced non-small-cell lung cancer: an open-label, randomized, multicenter, phase III trial. Ann. Oncol. 32, 85–96 (2021).
Kim, D.-W. et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 18, 2009–2014 (2007).
Liu, N., Bechinger, B. & Süss, R. The histidine-rich peptide LAH4-L1 strongly promotes PAMAM-mediated transfection at low nitrogen to phosphorus ratios in the presence of serum. Sci. Rep. 7, 9585 (2017).
Casper, J. et al. Polyethylenimine (PEI) in gene therapy: current status and clinical applications. J. Controlled Release 362, 667–691 (2023).
Zhao, G. et al. Nanoparticle-delivered siRNA targeting Bruton’s tyrosine kinase for rheumatoid arthritis therapy. Biomater. Sci. 7, 4698–4707 (2019).
Wang, Y. et al. Dually regulating the proliferation and the immune microenvironment of melanoma: Via nanoparticle-delivered siRNA targeting onco-immunologic CD155. Biomater. Sci. 8, 6683–6694 (2020).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Li, X., Wang, S. & Li, Z. Updated key points of Guidelines for the diagnosis and management of hepatic encephalopathy in cirrhosis (2018). J. Clin. Hepatol. 35, 1485–1488 (2019).
Li, X. et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 21, 541–557 (2021).
Tsuchiya, Y. et al. Fibroblast growth factor 18 stimulates the proliferation of hepatic stellate cells, thereby inducing liver fibrosis. Nat. Commun. 14, 6304 (2023).
Boursier, J. et al. Practical diagnosis of cirrhosis in non-alcoholic fatty liver disease using currently available non-invasive fibrosis tests. Nat. Commun. 14, 5219 (2023).
Wan, S. et al. Activated hepatic stellate cell-derived Bmp-1 induces liver fibrosis via mediating hepatocyte epithelial-mesenchymal transition. Cell Death Dis. 15, 41 (2024).
Zhou, Y. et al. The m6A reader IGF2BP2 regulates glycolytic metabolism and mediates histone lactylation to enhance hepatic stellate cell activation and liver fibrosis. Cell Death Dis. 15, 189 (2024).
Mao, F. et al. Identification of pyroptosis-related gene signature in nonalcoholic steatohepatitis. Sci. Rep. 14, 3175 (2024).
King, K. Y. & Goodell, M. A. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol. 11, 685–692 (2011).
Lu, J.-L., Yu, C.-X. & Song, L.-J. Programmed cell death in hepatic fibrosis: current and perspectives. Cell Death Discov. 9, 449 (2023).
Díaz, L. A., Arab, J. P., Louvet, A., Bataller, R. & Arrese, M. The intersection between alcohol-related liver disease and nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 20, 764–783 (2023).
Li, J. et al. HMGB1-induced autophagy facilitates hepatic stellate cells activation: a new pathway in liver fibrosis. Clin. Sci. 132, 1645–1667 (2018).
Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 66, 1300–1312 (2017).
Borthwick, L. A. & Mann, D. A. Osteopontin and HMGB1: novel regulators of HSC activation. Nat. Rev. Gastroenterol. Hepatol. 13, 320–322 (2016).
Sato, Y. et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 26, 431–442 (2008).
Han, X. et al. Ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis. Nat. Commun. 14, 75 (2023).
Zhou, J. E. et al. Hepatic macrophage targeted siRNA lipid nanoparticles treat non-alcoholic steatohepatitis. J. Controlled Release 343, 175–186 (2022).
Zhang, J. et al. Liver-Targeted siRNA lipid nanoparticles treat hepatic cirrhosis by dual antifibrotic and anti-inflammatory activities. ACS Nano 14, 6305–6322 (2020).
Buschmann, M. D. et al. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 9, 65 (2021)
Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010).
Hajj, K. A. et al. Branched-tail lipid nanoparticles potently deliver mRNA in vivo due to enhanced ionization at endosomal pH. Small 15, 1805097 (2019).
Chu, Y. et al. Deciphering protein corona by scFv-based affinity chromatography. Nano Lett. 21, 2124–2131 (2021).
Allison, S. J. & Milner, J. RNA interference by single- and double-stranded siRNA with a DNA extension containing a 3′ nuclease-resistant mini-hairpin structure. Mol. Ther. Nucleic Acids 3, 141 (2014).
Kumar, V. et al. Shielding of lipid nanoparticles for siRNA delivery: Impact on physicochemical properties, cytokine induction, and efficacy. Mol. Ther. Nucleic Acids 3, 210 (2014).
Miao, L. et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 11, 2424 (2020).
Sato, Y., Kinami, Y., Hashiba, K. & Harashima, H. Different kinetics for the hepatic uptake of lipid nanoparticles between the apolipoprotein E/low density lipoprotein receptor and the N-acetyl-D-galactosamine/asialoglycoprotein receptor pathway. J. Controlled Release 322, 217–226 (2020).
Pattipeiluhu, R. et al. Anionic lipid nanoparticles preferentially deliver mRNA to the hepatic reticuloendothelial system. Adv. Mater. 34,2201095 (2022).
Mendes, B. B. et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Prim. 2, 24 (2022).
Lee, Y., Jeong, M., Park, J., Jung, H. & Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 55, 2085–2096 (2023).
Pan, J. et al. Bacteria-derived outer-membrane vesicles hitchhike neutrophils to enhance ischemic stroke therapy. Adv. Mater. 35, 2301779 (2023).
Luo, Z. et al. Neutrophil hitchhiking for drug delivery to the bone marrow. Nat. Nanotechnol. 18, 647–656 (2023).
Li, M. et al. Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat. Commun. 11, 1126 (2020).
Frisch, M. J. et al. Gaussian 16 Rev. C.01. (2016).
Chai, J. Da & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Liptak, M. D. & Shields, G. C. Accurate pKa calculations for carboxylic acids using Complete Basis Set and Gaussian-n models combined with CPCM continuum solvation methods. J. Am. Chem. Soc. 123, 7314–7319 (2001).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82373813 [Yan], 22077034 [Yu], 32101131 [Jiang], 22273023 [He]), the National Key R&D Program of China (Grant No. 2019YFA0905200), Shanghai Frontiers Science Center of Molecule Intelligent Syntheses.
Author information
Authors and Affiliations
Contributions
Z.Z. and Z.Y.: Conceived and designed the experiments, wrote the paper. Z.Z.: Performed the experiments, analyzed the data. Y.F., C.Z., and K.J.: Performed the experiments, analyzed the data. Y.F., X.S., and J.Y.: Contributed materials. M.J., Z.Y., J.W., F.P., R.F., Y.M., J.Z., and L.S.: Performed the experiments. C.Z., X.H., and K.J.: Contributed analysis tools.
Corresponding authors
Ethics declarations
Competing interests
The authors have no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Z., Feng, Y., Jiang, M. et al. Ionizable polymeric micelles (IPMs) for efficient siRNA delivery. Nat Commun 16, 360 (2025). https://doi.org/10.1038/s41467-024-55721-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55721-w
This article is cited by
-
Zn2+-mediated siRNA-doxorubicin self-assembled nanoparticles amplify Immunogenic cell death via aggravating redox dyshomeostasis for cancer therapy
Journal of Nanobiotechnology (2026)
-
Soft nanoparticles as delivery agents for RNA-based anticancer therapy
Polymer Journal (2026)
-
Nanodynamic therapy for cancer: mechanistic innovations, targeting strategies and multimodal treatments
Journal of Translational Medicine (2025)
-
USP13 promotes hepatic stellate cells activation and aggravates liver fibrosis through deubiquitinating SMAD3
Hepatology International (2025)