Abstract
The development of an efficient and durable photoelectrode is critical for achieving large-scale applications in photoelectrochemical water splitting. Here, we report a unique photoelectrode composed of reconfigured gallium nitride nanowire-on-silicon wafer loaded with Au nanoparticles as cocatalyst that achieved an impressive applied bias photon-to-current efficiency of 10.36% under AM 1.5G one sun illumination while exhibiting stable PEC hydrogen evolution over 800 h at a high current density. Specifically, by tailoring the GaN nanowires via a simple alkaline-etching step to expose the inner (10\(\bar{1}\bar{1}\)) facets, we achieve a highly coupled semiconductor nanowire-cocatalyst heterointerface with strong electron interaction. The strongly coupled reconfigured GaN nanowire/Au heterointerface not only optimizes the electronic structure of Au nanoparticles to form abundant highly active interfacial regions, eventually realizing superior hydrogen evolution activity but also enables GaN nanowires to provide a stronger anchoring effect for Au nanoparticles, preventing the detachment of Au nanoparticles during the intense hydrogen evolution process. The proposed photoelectrode offers a feasible structure for overcoming the efficiency-reliability bottleneck of PEC devices for producing clean hydrogen fuel.
Similar content being viewed by others
Introduction
Solar-to-hydrogen evolution directly from sunlight and water, two of the richest natural resources on our planet, offers us the most viable and sustainable pathway for generating clean hydrogen fuel1,2. Photoelectrochemical (PEC) water splitting has emerged as one of the environmentally friendly and sustainable approaches for clean energy production3,4. The development of photoelectrodes with excellent catalytic activity and long-term stability is key to achieving a highly efficient and stable PEC system. However, for those photoelectrodes that consist of pure semiconductors with suitable bandgaps (e.g., Si, metal-oxides, III-V compounds.), they are frequently susceptible to photocorrosion and chemical corrosion5,6. Moreover, such intrinsic sunlight-absorbing semiconductors often suffer from sluggish reaction kinetics and rely heavily on loading cocatalysts to boost their catalytic activity7,8. Thus, to simultaneously address the issues of both stability and activity in photoelectrodes, the adoption of protective layers and cocatalyst strategies has been widely implemented in the establishment of PEC systems.
Although various protective layers have been proposed to enhance the stability of semiconductor materials, these protective layers cannot permanently remain bound on semiconductors due to self-reduction and dissolution in aqueous environments9,10. For the cocatalyst loading approach, the loose cocatalyst/semiconductor interface induced by the weak interactions between the semiconductor and the cocatalyst severely limits the lifespan of the photoelectrodes during PEC operation, especially due to cocatalyst detachment11,12,13,14,15. To date, the mainstream strategies aimed at preventing cocatalyst detachment have predominantly focused on introducing (1) a protective layer (such as solid oxides, hydrogels, or organic layers) to encapsulate the entire cocatalyst/semiconductor system16,17,18,19 and (2) a functional interlayer between the cocatalyst and semiconductor to provide additional anchoring sites, thereby strengthening their binding20. However, all the above approaches require additional complex processes to introduce new materials while they may block catalytic active sites or impede the mass transfer of reactants and products. Therefore, ideally, photoelectrodes should posscess21: (1) favorable bandgap and band structure with an optimized geometrical configuration for efficient light harvesting and fast charge transfer; (2) outstanding catalytic activity for fast reaction kinetics; and (3) long-term stability without suffering corrosion or physical detachment of the cocatalysts to achieve efficient and durable PEC operation. However, such a photoelectrode that fully meets all the above requirements has rarely been reported for use in PEC water splitting.
Notably, by epitaxial growing N-polar GaN nanostructure on silicon, GaN nanowires can effectively protect the underlying silicon photocathode against degradation in acidic electrolytes22,23. Notably, the GaN nanowire-on-Si wafer photocathodes can exhibit notable PEC HER performance by loading the cocatalysts (such as Pt24 and MoS225) onto the GaN nanowires. Unfortunately, the low adhesion of cocatalysts on GaN nanowires allows them to be easily separated from the nanowires, resulting in a substantial decrease in the GaN/Si photoelectrode stability. Recent research has shown that the long-term stability of PEC hydrogen evolution can be achieved through the repeated deposition of cocatalysts26.
Here, we report a chemically robust gallium-nitride (GaN) semiconductor in the form of a nanowire array that is spontaneously grown on a silicon substrate to create a GaN nanowire-on-Si wafer architecture, forming a one-dimension on three-dimension (1D/3D) configured photoelectrode. By further tailoring the GaN nanowires through a simple selective chemical-etching step to expose the inner GaN (10\(\bar{1}\bar{1}\)) active facets, followed by loading Au nanoparticles as cocatalysts, the constructed Au/Faceted-GaN/Si photocathodes can exhibit a high onset potential (Von) of 0.52 V vs. RHE and a large applied bias photon-to-current efficiency (ABPE) of 10.36% under AM 1.5G one sun illumination, and stabilize hydrogen production at a high current density for more than 800 h. This superior PEC performance can be mainly attributed to facet-induced strong electronic interactions at the GaN(10\(\bar{1}\bar{1}\))/Au interface, forming strongly coupled reconfigured GaN nanowire/Au heterointerface, which not only induces the GaN (10\(\bar{1}\bar{1}\)) facet to have greater adsorption energy for Au nanoparticles, providing a stronger anchoring effect that prevents Au nanoparticle detachment during the intense PEC hydrogen evolution process but also regulates the electronic structure of Au nanoparticles to optimize the hydrogen adsorption free energy, achieving abundant highly active interfacial regions. Therefore, our study demonstrated an effective strategy for achieving both highly efficient and stable PEC water splitting for hydrogen generation, providing an exciting opportunity for the future development of an advanced hydrogen production platform.
Results
Tailoring the GaN nanowire facet
First, we fabricate a GaN/Si photocathode consisting of a planar n+-p-p+ Si substrate and an array of n-GaN nanowires (NWs). Nitrogen-polar (N-polar) n-type GaN nanowires were epitaxially grown on a n+-p-p+ Si (100) wafer under nitrogen-rich conditions using plasma-assisted molecular beam epitaxy (MBE), where the Si wafer served as an efficient solar-light absorption layer and the photogenerated electrons transfer to the surface of the GaN nanowire array for the hydrogen evolution reaction (HER) (Fig. 1a and Supplementary Fig. S1). Details of the growth process of the GaN nanowires are provided in the Methods section. The self-assembled GaN nanowires exhibit a wurtzite crystal structure, with the (000\(\bar{1}\)) facet exposed on the top surface27. Generally, the N-polar (000\(\bar{1}\)) facet of GaN exhibits high reactivity with \({{{{\rm{OH}}}}}^{-}\) ions, which is attributed to the fact that OH- ions can attack the back bonds of the Ga atoms to break the Ga-N bond28 (Supplementary Fig. S2). Specifically, \({{{{\rm{OH}}}}}^{-}\) ions are adsorbed to the surface of GaN (000\(\bar{1}\)) facet, and then, Ga atoms react with \({{{{\rm{OH}}}}}^{-}\) ions to form Ga2O3, which subsequently dissolves in high-pH environments28,29. The overall reaction is as follows:\(\,2{{{\rm{GaN}}}}+3{{{{\rm{H}}}}}_{2}{{{\rm{O}}}}{\to }^{{{{{\rm{OH}}}}}^{-}}{{{{\rm{Ga}}}}}_{2}{{{{\rm{O}}}}}_{3}+2{{{\rm{N}}}}{{{{\rm{H}}}}}_{3}\). This structure etching process will preferentially terminate at the semi-polar (10\(\bar{1}\bar{1}\)) facet of the GaN. This preference is linked to changes in the states of surface bonding (Supplementary Fig. S3), resulting in a slow etching rate for the (10\(\bar{1}\bar{1}\)) facet30,31. Thus, the selective exposure of the semi-polar (10\(\bar{1}\bar{1}\)) facet in GaN NWs can be achieved through alkaline etching. In the crystal model diagram of ideal wurtzite GaN (Fig. 1b), the angle between the (000\(\bar{1}\)) facet and the (10\(\bar{1}\bar{1}\)) facet is observed to be 62°. Furthermore, the atomic arrangements on both the (000\(\bar{1}\)) and (10\(\bar{1}\bar{1}\)) facets are illustrated in Fig. 1c. On the (000\(\bar{1}\)) facet, the N atoms display a 3-fold coordinated structure, whereas on the (10\(\bar{1}\bar{1}\)) facet, coordinatively undersaturated sites with 2-fold coordinated N atoms are presented. These coordinatively undersaturated sites contribute to activating surface properties, providing additional adsorption sites to stabilize the cocatalyst32,33.
a Schematic diagram of the GaN/Si photoelectrode structure and the alkaline etching process of GaN NWs. b The crystal model diagram of GaN with wurtzite crystal structure. c The atomic arrangement of the (000\(\bar{1}\)) and (10\(\bar{1}\bar{1}\)) facet. N2f and N3f represent 2-fold coordinated N sites and 3-fold coordinated N sites, respectively. d SEM image of as-grown GaN NWs and faceted GaN NWs. e TEM image of as-grown GaN NWs and faceted GaN NWs. f HAADF-STEM image of the faceted GaN NWs (enlarged view of the pink box in e). g The high-resolution atom image of the faceted GaN NWs (enlarged view of the blue box in f).
Therefore, we employed a NaOH solution to etch the GaN nanowires, successfully etching the (000\(\bar{1}\)) facet to expose an inner (10\(\bar{1}\bar{1}\)) facet (Fig. 1a). The morphological characteristics of the GaN nanowire arrays were investigated using scanning electron microscopy (SEM). The GaN nanowire arrays, vertically aligned on a planar silicon substrate, exhibit a relatively uniform size with a length of approximately 350 nm and a diameter of approximately 35 nm (Supplementary Fig. S4). Notably, after etching, the top surfaces of the nanowires exhibit a hexagonal pyramid, whereas the as-grown nanowires showcase relatively smooth top surfaces (Fig. 1d). This morphological transition was further confirmed by transmission electron microscope (TEM) analysis, which demonstrated that the exposure of the inner facet induced a noticeable change in the top surface morphology of the nanowires (Fig. 1e). Aberration-corrected high-angle annular dark-field scanning TEM (HAADF-STEM) clearly revealed the pyramidal triangle profile and high crystal quality of the faceted GaN nanowires (Fig. 1f and Supplementary Fig. S5). Due to the slower etching rate and better chemical stability of the nonpolar (10\(\bar{1}\)0) facets on the sidewalls in comparison to the polar (000\(\bar{1}\)) facets, the sidewalls remain minimally affected during the etching process on the (000\(\bar{1}\)) facet28,34. Further, a high-resolution atomic image at the top of the faceted GaN nanowires was obtained through aberration-corrected HAADF-STEM measurements (Fig. 1g). The interplanar distance along the growth direction (c-plane) of the GaN nanowires was measured to be 2.60 Å, corresponding to the (0 0 2) facet, while the inclined plane formed post-etching showed an interplanar distance of 2.40 Å, with an angle of 62° with the (0 0 2) facet, which can be attributed to the (1 0\(\,\bar{1}\)) facet. This observation suggested that the pyramids on the top of the GaN NWs are enclosed by the facet family of (10\(\bar{1}\bar{1}\)). Consequently, through alkaline etching, the GaN nanowires were selectively tailored to expose the inner (10\(\bar{1}\bar{1}\)) facets.
Revealing intrinsic properties of the GaN facets
Further, we explored the intrinsic differences in properties between the GaN (000\(\bar{1}\)) and (10\(\bar{1}\bar{1}\)) facets. Notably, the surface states of the nanowires usually can induce surface Fermi level pinning, which plays a critical role in determining the final position of the Fermi level at the GaN nanowire surface. The Fermi level pinning can hinder the change of surface Fermi level, undermining the effective charge transfer between the surface and the loaded metal, which leads to a weakened interaction between the surface and the loaded metal35,36. Therefore, to analyze the potential effects of facet evolution on the charge transfer properties of nanowires, Kelvin probe force microscopy (KPFM) was employed to study the changes in the surface state by measuring the surface potential. As illustrated in Fig. 2a, b, the overlaid topography-potential three-dimensional spatial maps reflect the sharper profiles of the faceted GaN nanowires compared to those of as-grown GaN nanowires, which is attributed to the formation of pyramidal morphology on the top of the faceted GaN NWs. The corresponding individual topography and contact potential difference (CPD) images are shown in Supplementary Figs. S6 and S7. After selective etching, the faceted GaN nanowires exhibit more negative CPD values, signifying an upward shift in the Fermi level of the faceted GaN nanowires37 (detailed numerical analysis is shown in Supplementary Fig. S8). The valence band XPS spectrum further confirmed the upward shift in the Fermi level of the GaN nanowires after etching38 (Supplementary Fig. S9). These findings suggest that the Fermi level in faceted GaN nanowires is closer to the conduction band, signifying an improvement in the surface states, thereby alleviating Fermi level pinning39. Thus, the surface of faceted GaN nanowires is expected to be more prone to charge transfer with the loaded metal, potentially inducing strong electronic interactions.
Overlaid topography-potential three-dimensional spatial maps of a as-grown GaN NWs and b Faceted-GaN NWs. c PEIS curves of GaN/Si and the Faceted-GaN/Si photoelectrode in 0.5 M H2SO4 (pH = 0). d Comparison of the ECSA of GaN/Si and Faceted-GaN/Si estimated from the Cdl values. e Density of states of GaN (10\(\bar{1}\bar{1}\)) and GaN (000\(\bar{1}\)). f Electron localization function analysis of GaN (10\(\bar{1}\bar{1}\)) and GaN (000\(\bar{1}\)).
Subsequently, we performed electrochemical characterization of both the as-grown and faceted GaN nanowires in 0.5 M H2SO4 (pH = 0). Initially, the chopped linear sweep voltammetry (LSV) was conducted to evaluate the photoelectrochemical water splitting performance of the GaN/Si photoelectrode. To get an optimal level of faceting, etching studies were performed systematically as a function of time and KOH concentration (detailed analysis is shown in Supplementary Fig. S10). Notably, the Faceted-GaN/Si photoelectrode demonstrated a substantial improvement in performance in comparison to the GaN/Si photoelectrode (Supplementary Fig. S11). Further, the charge transfer resistance (Rct) at the nanowire/electrolyte interface was analyzed via photoelectrochemical impedance spectroscopy (PEIS) tests. The Nyquist plots of both GaN/Si and Faceted-GaN/Si are depicted in Fig. 2c, with larger circle diameters indicating a greater Rct. As expected, Faceted-GaN/Si shows a notable lower Rct than GaN/Si, which means a faster charge transfer at the faceted GaN/electrolyte interface during the reaction process. This optimization can be attributed primarily to the exposure of the (10\(\bar{1}\bar{1}\)) facet, with additional contribution from the reduced surface states of the nanowires (Supplementary Fig. S12). Meanwhile, cyclic voltammetry (CV) was used to measure the double-layer capacitance (Cdl) to evaluate the electrochemical surface area (ECSA) of the photoelectrodes (Supplementary Fig. S13). As illustrated in Fig. 2d, Faceted-GaN/Si (41.24 uF/cm2) displays a larger Cdl value than GaN/Si (24.27 uF/cm2), suggesting a greater intrinsic active area in the GaN (10\(\bar{1}\bar{1}\)) facets, which are expected to form more adsorption sites. In addition, the GaN nanowires still maintain high chemical stability in harsh electrolyte conditions after alkaline etching (Supplementary Fig. S14).
Next, complementing the experimental analysis, we carried out first-principles calculations based on density functional theory (DFT). Specifically, we obtain the surface energy of the (000\(\bar{1}\)) and (10\(\bar{1}\bar{1}\)) facets, respectively, where the facet activity is closely linked to the surface energy (Supplementary Fig. S15). The (10\(\bar{1}\bar{1}\)) facet demonstrates a higher surface energy than the (000\(\bar{1}\)) facet, potentially facilitating an increased exposure ratio of active sites to promote adsorption40. Moreover, since the exposure of different crystal faces will lead to distinct surface atomic arrangements and structures, a facet-induced alteration of the surface electronic structure is anticipated. The alterations in the electronic structure of two facets were investigated by the density of states (DOS). The calculation structure models of the (000\(\bar{1}\)) and (10\(\bar{1}\bar{1}\)) crystal faces are shown in Supplementary Fig. S16. As shown in Fig. 2e, the (10\(\bar{1}\bar{1}\)) facets, in comparison to the (000\(\bar{1}\)) facets, exhibit a pronounced intermediate electronic state in the forbidden band gap. The facet alteration results in the generation of new hybrid states, as indicated by the projected DOS (Supplementary Fig. S17), with the primary contribution to these new hybrid states originating from N 2p states, which can be proved by the N K-edge XAS spectra (Supplementary Figs. S18 and S19). Meanwhile, electron localization function (ELF) analysis revealed that the ELF values at the 2-fold coordinated N sites on the (10\(\bar{1}\bar{1}\)) facet were notable larger than those at the 3-fold coordinated N sites on the (000\(\bar{1}\)) facet (Fig. 2f), suggesting a more localized electron distribution41. Therefore, the uniquely undersaturated 2-fold coordinated N sites in the (10\(\bar{1}\bar{1}\)) facet not only tune the electronic state of the surface, leading to more active surface properties but also offer additional adsorption active sites, which are expected to subsequently construct GaN(10\(\bar{1}\bar{1}\))/cocatalyst interfaces with strong electronic interactions.
Constructing highly coupled cocatalyst/GaN heterointerface
Afterward, we construct a GaN nanowire/cocatalyst system to investigate the interaction between GaN NWs and cocatalysts. Among these cocatalysts, noble metal particles, for example, gold (Au), as a plasmonic-catalyst, are currently effectively used in hydrogen evolution processes42,43. Specifically, the Au nanoparticles can exhibit localized surface plasmon resonance (LSPR) when illuminated by light in the visible and/or near-infrared spectral regions, resulting in the generation of localized electromagnetic fields, localized heating, and the excitation of hot charge carriers in the vicinity of nanoparticles, these properties can be utilized to trigger and accelerate numerous reactions44,45. Here, we employed a light-assisted electrodeposition method to decorate Au nanoparticles on the GaN nanowires (detailed experimental procedures are provided in the Methods section) (Fig. 3a). To delve deeper into the role of LSPR effects in PEC water splitting, we obtained the spatial distribution of the electric field intensity at the interface of Au nanoparticles within an aqueous solution via finite-difference time-domain (FDTD) simulations. First, we establish two FDTD simulation models (Supplementary Fig. S20). Under visible light irradiation, the modification of Au nanoparticles effectively induced an increase in the localized electromagnetic field, and highly intensive “hot spots” were observed in the nanogap region between the particles, suggesting strong LSPR intensity46 (Fig. 3b, Supplementary Figs. S21 and S22). Simultaneously, we performed the steady-state photoluminescence (PL) spectra of the GaN nanowires before and after Au decoration (Fig. 3c). The center of the PL emission peak in the spectrum is at 360 nm, corresponding to GaN nanowires with a band gap of 3.44 eV. Interestingly, the center of the PL emission peaks redshifts by approximately 1 nm after Au decoration, which may be related to the reduction of the effective band gap caused by the Franz–Keldysh effect, owing to the enhanced local electric field induced by the LSPR effect47,48. This observed electromagnetic near-field enhancement is anticipated to boost the hydrogen evolution catalytic activity at the Au-GaN/electrolyte interface43.
a Schematic diagram of the synthesis of Au nanoparticle/GaN NW system via the photoelectron deposition method. b FDTD simulations of the electric field intensity (|E|) over Au/GaN NWs at 655 nm. c PL spectra of GaN/Si, Au/GaN/Si, and Au/Faceted-GaN/Si. d TEM light-field image of Au/Faceted-GaN/Si. e The TEM dark-field image of Au/Faceted-GaN/Si. f The aberration-corrected HAADF-STEM image of Au/Faceted-GaN/Si. g EDS elemental mapping of Au/Faceted-GaN/Si. h N K-edge spectrum of GaN/Si, Au/GaN/Si, Faceted-GaN/Si, and Au/Faceted-GaN/Si. i High-resolution N 1s XPS spectrum of GaN/Si, Au/GaN/Si, Faceted-GaN/Si, and Au/Faceted-GaN/Si. j High-resolution Au 4f XPS spectrum of Au/GaN/Si and Au/Faceted-GaN/Si.
The top-view and 30°-view SEM images show that Au nanoparticles tend to be located at the top of the nanowires (Supplementary Fig. S23). The TEM images display the entire GaN nanowire, where the GaN nanowire grows along the [000\(\bar{1}\)] direction (Fig. 3d). The TEM dark-field image further reflects that the Au nanoparticles were mainly distributed on the faceted surface at the top of the GaN nanowires (Fig. 3e). According to the lattice fringes measured by aberration-corrected HAADF-STEM (Fig. 3f), the lattice fringe spacings d = 2.4 Å and d = 2.6 Å correspond well to the crystallographic facets of GaN (1 0 \(\bar{1}\)) and GaN (0 0 2), respectively, and d = 2.35 Å can be attributed to the (1 1 1) facet of Au, which proves the formation of the Au/GaN(10\(\bar{1}\bar{1}\)) interface. Moreover, Energy-dispersive spectroscopy (EDS) elemental mapping confirms the even distribution of Ga (blue) and N (green) elements within the GaN nanowires and the successful loading of Au nanoparticles (Fig. 3g). Similarly, the formation of the Au/GaN(000\(\bar{1}\)) interface in Au/GaN/Si was proven (Supplementary Fig. S24).
To gain insight into the detailed surface chemical states and electronic interactions of the GaN nanowires, surface sensitivity X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS) were conducted. As shown in Fig. 3h, the N K-edge spectrum exhibits four feature peaks, attributed to the presence of two different types of Ga-N bonds in wurtzite-structured GaN49. Compared to that of Faceted-GaN/Si, the N K-edge spectrum of Au/Faceted-GaN/Si displays a notable decrease in the intensity of feature peaks with an obvious blueshift, which suggests the aggregation of electrons around the N sites50. Moreover, as illustrated in Fig. 3i, after loading with Au nanoparticles, the energy positions of the N 1s XPS spectra exhibit a negative shift, further confirming the formation of an electron-rich region of N sites51. In contrast, for the GaN/Si, the N K-edge spectrum remained almost constant before and after loading with Au nanoparticles, indicating no significant alteration in the electron distribution around the N sites. The energy positions of the N 1s XPS spectra also remained nearly unchanged after depositing the Au nanoparticles. Moreover, a clear positive shift in the Au 4f spectra of Au/Faceted-GaN/Si and an increase in the Auσ+ content (Auσ+: Au0) are observed compared to those of Au/GaN/Si (Fig. 3j), which confirms the formation of an electron-deficient region around Au in Au/Faceted-GaN/Si52. The XPS and XAS results indicate that in Faceted-GaN/Si, the coupling of Au nanoparticles with the GaN (10\(\bar{1}\bar{1}\)) facet leads to electron redistribution in the interfacial region, where Au transfers electrons to GaN, forming strong electronic interactions between the Au nanoparticles and GaN NWs. In contrast, in pristine GaN/Si, only weak electronic interactions are observed between Au and the GaN NWs. This enhanced electronic interaction between the Au nanoparticles and the GaN (10\(\bar{1}\bar{1}\)) facet could lead to stronger Au/GaN NW interface effects, which can be advantageous for improving the water splitting activity of Au nanoparticles while enhancing the anchoring effect of the GaN NWs on the Au nanoparticles53. Moreover, a decrease in the surface electron density of Au nanoparticles is expected to lower the surface energy, contributing to enhanced structure stability54,55. In addition, the spectral features in the high-resolution Ga 3d and N 1s XPS spectra remain similar before and after Au nanoparticle loading, suggesting that the GaN nanowires remain stable during the light-assisted electrodeposition process (Fig. 3i and Supplementary Fig. S25). In summary, the exposure of the inner (10\(\bar{1}\bar{1}\)) facet of the GaN nanowires facilitated the formation of strong electronic interactions with the loaded Au nanoparticle cocatalyst. This reconfigured GaN/cocatalyst interface is expected to stabilize the cocatalyst, ultimately achieving high PEC water decomposition performance.
Demonstrating efficient and stable photoelectrochemical water splitting
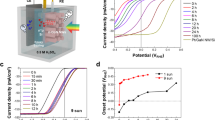
Further, to verify the PEC water splitting performance of the Au/GaN NW system, we prepared the above samples as photocathodes. These prepared photocathodes were evaluated in a standard three-electrode system in a 0.5 M sulfuric acid solution environment (Fig. 4a). The n+-p-p+ Si substrate can serve as an efficient solar-light absorption layer to produce photogenerated carriers in the PEC hydrogen evolution process (Supplementary Fig. S26). Figure 4b illustrates the LSV curves of Au/GaN/Si and Au/Faceted-GaN/Si under AM 1.5G one sun illumination. The deposition time and potential of Au nanoparticles on the photoelectrodes were optimized (Supplementary Fig. S27). In comparison to that of Au/GaN/Si (Fig. 4c), the hydrogen evolution performance of Au/Faceted-GaN/Si notable improved, with the initial potential Von shifting from 0.29 V vs. RHE to 0.52 V vs. RHE (Supplementary Fig. S28) and the photocurrent density increasing from 8.8 mA·cm−2 to 36.4 mA·cm−2 at 0.2 V vs. RHE. Here, we specify the voltage corresponding to the photocurrent density reaching 1 mA/cm2 as the onset potential. The corresponding applied bias photon-to-current efficiency (ABPE) plots were subsequently generated based on LSV curves. In Fig. 4e, the maximum ABPE value of Au/GaN/Si is only 2.27% at 0.13 V vs. RHE, whereas the maximum ABPE value of Au/Faceted-GaN/Si is 10.36% at 0.33 V vs. RHE, an increase of approximately 4.56 times. This measured ABPE value surpasses the majority of silicon-based photocathodes reported in the literature to date (Supplementary Table S1). Further, as shown in Fig. 4e, the incident photon current efficiency (IPCE) of both Au/GaN/Si and Au/Faceted-GaN/Si were measured at 0 V vs. RHE (the IPCE of the Faceted-GaN/Si and GaN/Si photoelectrodes are shown in Supplementary Fig. S29). It can be observed that both show similar trends and the IPCE values at all wavelengths are greater for Au/Faceted-GaN/Si than for Au/GaN/Si. We obtained the calculated photocurrent density by integrating IPCE curves with the standard solar spectrum (Supplementary Fig. S30), which is close to the measured photocurrent density, indicating that the simulated AM 1.5G sunlight matches the standard solar spectrum. In addition, for the Au/Faceted-GaN/Si photoelectrode, we further measured the hydrogen production and Faradaic efficiency of H2 at 0 V vs. RHE. As illustrated in Fig. 4f, the hydrogen production remains nearly constant for each 10-minute interval within 60 min, demonstrating high stability during hydrogen evolution. Meanwhile, the Faradaic efficiency of H2 is close to 100%, confirming the efficient utilization of photogenerated electrons during the reaction process.
a Schematic diagram of the PEC hydrogen evolution reaction in a three-electrode configuration. CE is the counter electrode and RE is the reference electrode. b LSV curves of Au/GaN/Si and Au/Faceted-GaN/Si under AM 1.5G one sun illumination in 0.5 M H2SO4 (pH = 0) (Details of the light intensity calibration in the Methods section). c The current density and onset potential of Au/GaN/Si and Au/Faceted-GaN/Si at 0.2 V vs. RHE. d The ABPE curves of Au/GaN/Si and Au/Faceted-GaN/Si. e IPCE curves of Au/GaN/Si and Au/Faceted-GaN/Si. f The Faradic efficiency and amount of H2 production of Au/Faceted-GaN/Si in 0.5 M H2SO4 (pH = 0) at 0 V vs. RHE. g Long-term stability evaluation of Au/GaN/Si and Au/Faceted-GaN/Si in 0.5 M H2SO4 (pH = 0) under AM 1.5G 1.14 suns illumination at 0 V vs. RHE. h PEC water splitting performance of Au/Faceted-GaN/Si compared with Au/GaN/Si under AM 1.5G 1.14 suns illumination. All the test curves were measured without iR compensation.
Subsequently, the photoactivity of the prepared photoelectrode was verified (Supplementary Fig. S31), and the long-term working stability of the photoelectrodes was evaluated using the chronoamperometry (CA) technique at 0 V vs. RHE under AM 1.5G 1.14 suns illumination (Fig. 4g). The photocurrent density of the Au/GaN/Si photoelectrode gradually decreased during operation at a rate of 0.04 mA·cm−2·h−1 and started to decline sharply at a large rate of 0.33 mA·cm−2·h−1 after approximately 100 h of operation. Interestingly, the photocurrent density of the Au/Faceted-GaN/Si photoelectrode remains stable even after running for more than 800 h, at a decline rate of only 0.0045 mA·cm−2·h−1. Further, by comparing the LSV curves after different reaction times, it can be observed that the PEC performance of Au/Faceted-GaN/Si only experiences slight degradation after a long reaction time of 768 h (Supplementary Fig. S32). Therefore, the Au/Faceted-GaN/Si photoelectrode exhibits comparable stability during H2 evolution under a high current density. However, for the Au/GaN/Si photoelectrode, the LSV curves after 96 h of operation show a significant attenuation in PEC performance, and the ABPE decreases by more than 93%, reaching only 0.19% (Supplementary Fig. S33). To further investigate the reasons for the difference of photoelectrode stability, SEM analysis was conducted on Au/GaN/Si and Au/Faceted-GaN/Si after different working times. The morphology of the GaN nanowires in these photoelectrodes all remained intact with minimal corrosion, demonstrating the high stability of the GaN nanowires in the photoelectrochemical environment (Supplementary Figs. S34 and S36), which was further proved by TEM images (Supplementary Fig. S37). However, after 96 h of operation, the surface coverage of Au nanoparticles on top of the GaN nanowires decreased notable in Au/GaN/Si (Supplementary Fig. S34), indicating that the Au nanoparticles fell off the top of the GaN nanowires during operation. Notably, by reloading the Au nanoparticles onto the old Au/GaN/Si photoelectrode, the PEC hydrogen evolution performance was restored, proving that the attenuation of the performance was caused by the detachment of Au nanoparticles (Supplementary Fig. S35). This weak interaction between the Au nanoparticles and the GaN(000\(\bar{1}\)) facet makes Au nanoparticles easily fall off, leading to serious damage to the PEC performance of the Au/GaN/Si photoelectrode. Notable, for Au/Faceted-GaN/Si, there was almost no significant detachment of Au nanoparticles at the top of the faceted GaN nanowires after 288 h of operation, and after 768 h of operation, a large distribution of Au nanoparticles was still observed at the top surface of the GaN nanowires (Supplementary Figs. S36 and S37). This could be attributed to the fact that the strong electronic interaction between the Au nanoparticles and the GaN(10\(\bar{1}\bar{1}\)) facet creates a robust anchoring effect that keeps the Au nanoparticles in place, successfully preventing the detachment of the Au cocatalyst. Therefore, the Au/Faceted-GaN/Si photoelectrode exhibit higher PEC hydrogen evolution performance compared to Au/GaN/Si photoelectrode (Fig. 4h), and to our knowledge, the PEC water splitting performance of the Au/Faceted-GaN/Si photoelectrode is comparable to those of previously reported advanced photocathodes, particularly in terms of long-term stability at a high current density (Supplementary Tables S1 and S2).
Uncovering the Facet-induced enhancement of PEC performance
To explore the reasons behind the high HER performance of the Au/GaN(10\(\bar{1}\bar{1}\)) interface in Au/Faceted-GaN/Si, we first performed PEIS tests on Au/GaN/Si and Au/Faceted-GaN/Si to investigate the charge transfer kinetics during the HER process. As shown in Fig. 5a, the Nyquist plots of both photoelectrodes all exhibit two arcs, and the corresponding equivalent circuit model fitted to the Nyquist plots is shown in Fig. 5b. Specifically, Rs represents the resistance of the silicon substrate and copper back contact interface, while according to the Mott–Schottky curves exhibiting the characteristics of a p-type semiconductor (p-Si) (Supplementary Fig. S38), Cbulk and Rct, bulk should be attributed to the capacitance and corresponding charge transport resistance inside Si, respectively. Csurf and Rct, surf represent the capacitance and charge transfer resistance of the Au/GaN interface to the electrolyte, respectively. The fitted results show quite similar Rs values for both photoelectrodes, indicating the reliability of the back contact (Supplementary Table S3). Simultaneously, the remarkably smaller Rct, surf in the Au/Faceted-GaN/Si photoelectrode imply more efficient charge transfer from the Au/GaN(10\(\bar{1}\bar{1}\)) interface to the electrolyte during the reaction process, enhancing carrier transport at the interface. Additionally, the KPFM test demonstrated that loading Au nanoparticles further lowered the Fermi level position of the GaN nanowires (Fig. 5c, Supplementary Figs. S39 and S40). Notably, compared to the GaN/Si, a more pronounced shift in the Fermi level position of Faceted-GaN/Si is observed after the loading of Au nanoparticles (Supplementary Table S4), further underscoring the enhanced electron transfer between the Au nanoparticles and the GaN(10\(\bar{1}\bar{1}\)) facet56. The existence of surface states on the as-grown GaN NW surface leads to Fermi level pinning, impeding electron transfer with Au, corresponding to the above results.
a PEIS curves of Au/GaN/Si and Au/Faceted-GaN/Si in 0.5 M H2SO4 (pH = 0). b The equivalent circuit model to fit the Nyquist plots. c The contact potential difference (CPD) mapping of Au/GaN/Si and Au/Faceted-GaN/Si. d The adsorption energy of Au nanoparticles on the GaN(00\(0\bar{1}\)) facet and GaN(10\(\bar{1}\bar{1}\)) facet. e Charge density difference distributions and Bader charge analysis of Au/GaN(00\(0\bar{1}\)) and Au/GaN(10\(\bar{1}\bar{1}\)). The yellow and green contours represent the charge accumulation and depletion, respectively. f Gibbs free energy of hydrogen adsorption by Au/GaN(00\(0\bar{1}\)) and Au/GaN(10\(\bar{1}\bar{1}\)) during the HER process. g The density of states (DOS) of Au 5d orbital on the Au/GaN(00\(0\bar{1}\)) and Au/GaN(10\(\bar{1}\bar{1}\)), where the dotted line represents the position of the d-band center. h Crystal orbital Hamilton population (COHP) analysis for the Au-H bonds in Au/GaN (00\(0\bar{1}\)) and Au/GaN(10\(\bar{1}\bar{1}\)).
Then, to gain a deeper understanding of the structural-reactivity relationship between different structures and HER performance, we carried out DFT calculations for the Au/GaN(00\(0\bar{1}\)) and Au/GaN(10\(\bar{1}\bar{1}\)) models to analyze their associated electronic structures and catalytic activity. First, we calculated the adsorption energy of Au nanoparticles on the GaN(00\(0\bar{1}\)) facet and the GaN(10\(\bar{1}\bar{1}\)) facet, respectively. As depicted in Fig. 5d, the adsorption energy for Au nanoparticles on the GaN(10\(\bar{1}\bar{1}\)) facet was −34 eV, surpassing that on the GaN(00\(0\bar{1}\)) facet (−18 eV), indicating a pronounced preference for the adsorbing of Au nanoparticles on the GaN(10\(\bar{1}\bar{1}\)) facet57. Meanwhile, the charge density difference distributions, along with Bader charge analysis, reveal more charge transfer between Au and GaN(10\(\bar{1}\bar{1}\)) facet. In the Au/GaN(00\(0\bar{1}\)) configuration, Au transfers 1.58 electrons to GaN(00\(0\bar{1}\)), whereas in Au/GaN(10\(\bar{1}\bar{1}\)), the transfer is 1.95 electrons to GaN(10\(\bar{1}\bar{1}\)), suggesting a stronger electronic interaction between Au and GaN(10\(\bar{1}\bar{1}\)) facet, consistent with the above results from XPS, XAS, and KPFM analyses. These conclusions further support the superior anchoring effect of the GaN(10\(\bar{1}\bar{1}\)) facet to the Au nanoparticles, reducing the likelihood of detachment during the reaction process. In addition, the density of states (DOS) was conducted to enhance the understanding of electronic structure modulation (Supplementary Fig. S41). The coupling between Au and the GaN(10\(\bar{1}\bar{1}\)) facet gives rise to a higher density of hybrid states around the Fermi level, facilitating efficient charge transfer during the HER process58,59. Further, the Gibbs free energy of hydrogen adsorption (ΔGH*) was calculated to determine the HER reactivity of Au/GaN(10\(\bar{1}\bar{1}\)) and Au/GaN(00\(0\bar{1}\)) (Fig. 5f and Supplementary Fig. S42). According to Sabatier’s principle, the optimal hydrogen adsorption free energy should be maintained at around 0 eV, which is conducive to both the adsorption of H* and the desorption of H260,61. The Au/GaN(00\(0\bar{1}\)) exhibits a ΔGH* of −0.43 eV, suggesting a strong hydrogen adsorption capacity that may hinder hydrogen desorption. In contrast, Au/GaN(10\(\bar{1}\bar{1}\)) corresponds to a ΔGH* of −0.04 eV, which is closer to 0 eV, indicating weaker hydrogen adsorption and facile desorption of H2, resulting in better HER activity. Interestingly, we can observe that the GaN(10\(\bar{1}\bar{1}\)) facet successfully regulates the d-band center of Au atoms (Fig. 5g), and in comparison to that of the Au/GaN(00\(0\bar{1}\)), the d-band center of Au atoms in Au/GaN(10\(\bar{1}\bar{1}\)) is further away from the Fermi level, which leads to a reduced hydrogen binding energy (HBE) for Au atoms62. Additionally, this can be directly demonstrated by analyzing crystal orbital Hamilton population (COHP) for the Au-H bonds in both Au/GaN(00\(0\bar{1}\)) and Au/GaN(10\(\bar{1}\bar{1}\))63. As depicted in Fig. 5h, the antibonding states of the Au-H bonds in Au/GaN(10\(\bar{1}\bar{1}\)) notably shift toward the Fermi level. The corresponding integral of COHP (ICOHP) for the Au-H bonds shows a decreasing negative value, changing from −0.704 eV to −0.351 eV, indicating a weaker interaction between Au and H, which explains the improvement of the hydrogen adsorption free energy64. In short, the above results indicate that the coupling of Au and the GaN(10\(\bar{1}\bar{1}\)) facet results in stronger electronic interactions, which provides an enhanced anchoring effect of GaN(10\(\bar{1}\bar{1}\)) facet to the Au cocatalyst. Moreover, these strong electronic interactions also modulated the Au 5d orbital states, optimizing the hydrogen adsorption free energy. Ultimately, this dual optimization achieves the simultaneous enhancement of both PEC activity and stability.
Interestingly, to further demonstrate the widespread applicability of this optimization strategy, we extended the approach by loading the classic HER cocatalyst platinum (Pt) on GaN NWs. Both theoretical calculations and experimental results indicate that the electronic interaction between Pt and GaN(10\(\bar{1}\bar{1}\)) facet is more pronounced than that between Pt and GaN(00\(0\bar{1}\)) facet (Supplementary Figs. S43–S46). Similarly, these enhanced electronic interactions not only optimize the hydrogen adsorption free energy of Pt atoms, thereby improving the HER activity but also enhance the anchoring effect of GaN NWs on Pt nanoparticles, resulting in prolonged stability. This result is consistent with the above-mentioned findings regarding the Au nanoparticle-loaded photoelectrode. In summary, the exposure of the (10\(\bar{1}\bar{1}\)) facet enhances the electronic interaction between GaN and the cocatalyst, activating the cocatalyst and thereby increasing its catalytic activity. Concurrently, these strong electronic interactions can further stabilize the cocatalyst to prevent their detachment from GaN NWs, greatly improving the stability of the reaction. Therefore, exciting, we can infer that loading cocatalysts with different catalytic activities onto the proposed photoelectrode, which is composed of a nitride nanowire-on-silicon architecture with optimized configurations, presents an exciting opportunity and viable routes for the development of a variety of efficient and stable artificial photosynthesis reactions, such as the CO2 reduction reaction, N2 reduction reaction, nitrate reduction reaction et al., promoting the advancement of sustainable and clean energy production. Meanwhile, it is noteworthy that this unique GaN nanowire-on-Si 1D/3D architecture is expected to achieve large-scale production and application owing to the GaN and Si are two of the most extensively produced semiconductors globally and can be fabricated using mature traditional processes in silicon foundries.
Discussion
In conclusion, we achieved outstanding catalytic activity and long-term stability in a reconfigured GaN nanowire-on-Si photocathode decorated with the Au nanoparticle cocatalyst for PEC hydrogen evolution. Specifically, by tailoring the GaN nanowires via a simple alkaline-etching step to expose the inner (10\(\bar{1}\bar{1}\)) facet of GaN, we establish a GaN (10\(\bar{1}\bar{1}\)) facet/Au nanoparticle interface with strong electronic interaction. The photoelectrode, built with this reconfigured GaN/Au interface, exhibits a high onset potential of 0.52 V vs. RHE and an impressive ABPE value of 10.36% under AM 1.5G one sun illumination, and it can stably operate for more than 800 h at a high current density (−40 ± 1 mA/cm2 under AM 1.5G 1.14 suns illumination). As proven by the experimental results and first principles analysis, this facet-induced strong electronic interaction enhances the anchoring capability of GaN nanowires to Au nanoparticles, while also regulating the electronic structure of Au nanoparticles to improve both the hydrogen evolution activity and structural stability. Our research provides a straightforward and efficient way to improve the nitride semiconductor/cocatalyst interface, laying a foundation for the subsequent extensive application of nitride semiconductors in various artificial photosynthetic reactions, promoting the development of economic and large-scale artificial photosynthesis.
Methods
Materials
Gold chloride trihydrate (HAuCl4·3H2O, AR), Chloroplatinic acid solution (H2PtCl6·xH2O, AR) were purchased from Aladdin Co., Ltd. Ethanol (AR) were purchased from Shanghai Research Institute of Chemical Industry. The electrolyte uses dilute sulfuric acid standard titration solution (0.5 mol/L, executive standard: GB/T601-2016), which is purchased from Codow Co., Ltd. Sodium hydroxide standard solution (5 mol/L, GB/T601-2016) was purchased from HubioPharm Co., Ltd. All chemicals were used without further purification. The Pt mesh and Ag/AgCl electrode were purchased from Tianjin Aida Hengsheng Technology Development Co., Ltd.
GaN nanowire-on Si wafer fabrication
The n+-p-p+ Si substrate was prepared by doping a 2-inch double-sided p-type silicon wafer (100) (thickness: 290\(\pm\)10 μm; resistivity: 1–10 Ω·cm) with the thermal diffusion method. First, the silicon side is spin-coated with liquid phosphorus for n-type doping, and the other side is spin-coated with liquid boron for p-type doping. Then, the silicon wafers were placed at 950 °C for 240 min of thermal diffusion in an argon atmosphere, which formed n+-Si and p+-Si on both sides of the silicon wafer. Subsequently, n-GaN nanowire arrays were synthesized on n+-p-p+ Si substrates using plasma-assisted molecular beam epitaxy (MBE) under nitrogen-rich growth conditions. First, before the n+-p-p+ Si substrate was placed in the growth chamber, acetone, methanol, and DI water were used to remove contaminants from the n+-p-p+ Si substrate surface and the surface impurity oxide was removed in a 10% buffered hydrofluoric acid solution. Then, heat the Si substrate in the growth chamber at 200–250 °C for 40–60 min to remove water and organic contaminants. In the GaN nanowire growth process, the temperature of the Si substrate is maintained at 700–750 °C, the temperature of the Ga cell is set to 1010 °C, and the N2 flow rate of the plasma excitation is set to 1.5 standard cubic centimeter per minute (SCCM), where the plasma power of 400 W. The growth of the nanowire array lasted for 3–4 h. No catalysts were used during the GaN nanowires growth, and GaN nanowires could be spontaneously formed and vertically grown on silicon substrates.
Etching processes
First, the GaN nanowire/Si wafers were cleaned with acetone and deionized water to remove organic pollutants. The clean GaN nanowire/Si wafers were then soaked in the 3.5 M NaOH solution for 40 s. After wet etching, the nanowires were cleaned with deionized water to remove any residual NaOH solution, followed by drying with an N2 gas flow. Additionally, we studied etching with different concentrations of NaOH solution (2 M, 3.5 M, and 5 M) and different treatment times (10 s, 25 s, 40 s, and 55 s), while maintaining all other conditions constant.
Photoelectrode preparation
The fabrication process of photoelectrodes involves several steps. First, the GaN nanowire/Si wafers was diced into pieces (0.05–0.1 cm2) using diamond pen, and then the surface oxide on the back of the Si substrate was removed. To establish an ohmic contact, Ga-In eutectic (Alfa Aesar) was applied to the back of the Si substrate. Subsequently, the wafer was affixed to a copper sheet using silver paste (SPI Supplies). To ensure proper insulation, the entire wafer, except for the nanowire surface in contact with the electrolyte, was covered with insulating epoxy. The photoelectrode was subsequently air-dried for 24 h before conducting measurements.
Light-assisted electrodeposition of Au nanoparticles
An Au/GaN/Si photoelectrode was synthesized by a light-assisted electrodeposition method. Specifically, 150 μL of 20 mg/ml HAuCl4 was dissolved in 10 mL of 0.5 M sodium sulfate solution as the electrolyte, which was added to a three-electrode cell composed of a GaN/Si photoelectrode working electrode, a Pt counter electrode, and an Ag/AgCl reference electrode. A 655 nm light-emitting diode (LED) was used as the light source and the light intensity was adjusted to 2 mW·cm−2, the light intensity was calibrated using a Newport Model No. 2936 R optical power meter. Using the I-t mode for deposition, the applied potential was −0.5 V vs. RHE, and the deposition time was 600 s to obtain Au nanoparticles. All the methods used for Au nanoparticle deposition in this article are consistent with those above. The deposition method used for Pt nanoparticles was consistent with that used for the Au nanoparticles, and the precursor solution HAuCl4 was replaced by H2PtCl6. We perform the PEC performance of photocathode optimization studies by depositing Au NPs under different deposition potentials (−0.3 VRHE, −0.5 VRHE, and −0.7 VRHE) and different deposition durations (400 s, 600 s, and 800 s).
Material characterization
Scanning electron microscopy (SEM) on Hitachi SU8220 systems. Morphological analysis of the synthesized GaN nanowires was conducted using high-resolution transmission electron microscopy (HRTEM) on JEOL-2100F systems operating at 200 kV. The aberration-corrected high-angle annular dark-field scanning TEM (HAADF-STEM) images were carried out using Spherical Aberration Corrected Transmission Electron Microscope (Themis Z) and Energy-dispersive X-ray spectroscopy (EDS) was carried out using a Themis Z device operating. Photoluminescence (PL) measurements were performed at room temperature using a 266 nm excitation pulse laser, with the PL signal collected by an ultraviolet objective and analyzed using an OceanOptics QE Pro spectrometer. Synchrotron X-ray absorption spectroscopy (XAS) was acquired on the Photoemission End station at the MCD-A and MCD-B beamline in the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China. X-ray photoelectron spectroscopy (XPS) measurements were conducted using a Thermo Scientific K-Alpha XPS instrument equipped with an Al Kα source (hν = 1486.6 eV). Spectral positions were corrected using adventitious carbon, by shifting the C 1s core level position to 284.8 eV. The KPFM measurements were performed using an atomic force microscope (AFM) (NTEGRA SPECTRA, NT-MDT). A Pt-coated tip was employed as the probe for the measurements. KPFM measurements are carried out in an atmospheric environment.
Electrochemical measurements of PEC water splitting
PEC experiments were conducted within a three-electrode photoelectrochemical reaction cell using a CHI760E (CH instruments) electrochemical workstation, including a working electrode (GaN/Si photoelectrode), a counter electrode (Pt mesh), a reference electrode (Ag/AgCl), and a 0.5 M H2SO4 electrolyte (pH = 0). Use the dilute sulfuric acid standard titration solution as the electrolyte, ensuring it was freshly purchased and promptly used. All final potentials were converted to a reversible hydrogen electrode (RHE) with the following conversion: \({E}\,\left({{{\rm{vs}}}}.{{{\rm{RHE}}}}\right)=E\,\left({{{\rm{vs}}}}.{{{\rm{Ag}}}}/{{{\rm{AgCl}}}}\right)+0.197{{{\rm{V}}}}+0.059\times {{{\rm{PH}}}}\). All the curves were measured without iR compensation at room temperature. The calibration of the reference electrode was conducted as follows: Platinum wires were used as the working and counter electrodes, while an Ag/AgCl electrode was calibrated to serve as the reference. A H2-saturated 0.5 M H2SO4 solution was used as the electrolyte. The corrected potential of the reference electrode was determined by performing cyclic voltammetry (CV) near the open circuit voltage. The light source is a 300 W xenon lamp (PLS–SXE300D, Beijing Perfect Light Technology Company) equipped with an AM 1.5G filter (Beijing Perfect Light Technology Company). We use a silicon photodiode solar power meter to calibrate the light intensity to 80 mW/cm2 to simulate AM 1.5G one sun intensity. This is due to integrating the power from 280 to 1100 nm in the standard AM 1.5G one sun spectrum, yielding a total of ~80 mW/cm2. Since the operational range of the AM 1.5G filter is 400–1100 nm, thus we used this calibrated approach to evaluate the LSV curves of the photoelectrodes, ensuring the accuracy of the maximum saturation photocurrent intensity of the photoelectrodes under simulated AM 1.5G one sun illumination. When using a full-spectrum light power meter (range 200–11000 nm) to calibrate the light intensity to 100 mW/cm2, according to the test results from the silicon photodiode solar power meter, the light intensity reached 90.8 mW/cm2 within the 400–1100 nm range, which is ~14% higher than 80 mW/cm2, thus it can be considered as AM 1.5G 1.14 suns. All tests use the above 300 W xenon lamp equipped with an AM 1.5G filter as the light source. The sample was illuminated through a quartz window of the cell. Linear sweep voltammetry (LSV) data was recorded at a scan rate of 20 mV/s. The ABPE of the photocathode was obtained based on the LSV curves using the following equation24: \({{{\rm{ABPE}}}}\left(\%\right)=\frac{J\left({V}_{{RHE}}-{E}_{{rev}}\right)}{{P}_{{in}}}\times 100\), where J refers to the photocurrent density, Erev = 0 V vs. RHE, VRHE refers to the applied voltage vs. RHE, and Pin = 100 mW/cm2, refers to the incident light intensity. The electrochemical double-layer capacitance (Cdl) was calculated by cyclic voltammetry (CV) curves in the region of between 0.36 and 0.46 V vs. RHE with different scanning rates of 120, 140, 160, 180, and 200 mV/s in 0.5 M H2SO4 (pH = 0). The ECSA was proportional to the Cdl. The measured potential ranges of the photoelectrochemical impedance spectroscopy (PEIS) were 0 V vs. RHE in the frequency range of 0.1–200,000 Hz with an amplitude of 5 mV. During the working stability test, to exclude the influence of Pt deposition, we used the graphite rod as the counter electrode instead of Pt foil. Meanwhile, the photoelectrode back contact is re-fabricated every 96 h to prevent damage to ohmic contact. For the IPCE test, we used a monochromator with a 300 W xenon lamp equipped with an AM 1.5G filter as the simulated light source, and the light intensity was calibrated using a Newport Model No. 2936 R optical power meter. The IPCE of the photocathode was derived using the following equation24: \({{{\rm{IPCE}}}}\left(\%\right)=\frac{\left(1240\times I\right)}{\lambda \times {P}_{{in}}}\times 100\), where I refers to the photocurrent density (mA/cm2), λ refers to the incident light wavelength (nm), and Pin is the incident light intensity (mW/cm2). The Faraday efficiency tests were performed at 0 V vs. RHE in 0.5 M H2SO4 (pH = 0). A gas chromatograph (GC, Shimadzu 2014) was equipped with a thermal conducting detector (TCD) for the H2 product detection, where using N2 (\(\ge\) 99.995%) as a carrier gas. The Faraday efficiency (ηFaraday) of the photocathode was calculated using the following equation: \({\eta }_{{{{\rm{Faraday}}}}}\,\left(\%\right)=\frac{2\times {n}_{{H}_{2}}\left(t={T}_{0}\right)\times F}{{\int }_{0}^{T0}I{t\; dt}}\times 100\), where \({n}_{{H}_{2}}\) (mol) refers to the amount of hydrogen produced in T0 time (s), F refers to the Faradaic constant (96485 C/mol), I (A) refers to the photocurrent value during the test, and t (s) refers to the test time. The calculated photocurrent density was obtained by integrating IPCE curves with the standard solar spectrum (ASTM G173-03), using the following equation: \({J}_{c}={\int }_{340}^{1100}\frac{\lambda \times {IPCE}\left(\lambda \right)\times E\left(\lambda \right)}{1240}d\lambda\), where λ (nm) refers to the incident light wavelength.
Finite-difference time-domain (FDTD) simulations
FDTD calculations with commercial software (FDTD solution, Lumerical Inc., Vancouver, Canada) were used to simulate the optical properties of GaN nanostructures and GaN/Au nanostructures. Perfectly-Matched Layer (PML) boundaries enclose the spatial domain of FDTD. The field source was total-field scattered-field and propagating from the water into the GaN nanostructure. To ensure the accuracy of electric field calculations, a uniform mesh size of 0.1 nm (x, y, and z) was used to cover the Au NPs. Optical data from Palik’s Handbook of Optical Constants were used for Au and H2O. The refractive index of the n-GaN nanostructure was taken from the reported values65.
DFT calculation details
The Vienna Ab initio Simulation Package66,67 is used to conduct the spin-polarized DFT calculations. A generalized gradient approximation is adopted based on the Perdew–Burke–Ernzerhof exchange-correlation functional68 to perform the exchange-correlation energies calculations. The plane wave energy cutoff is set as 400 eV and the van der Waals interaction between the adsorbates and substrate is further treated by the DFT-D3 method69.
All atoms are allowed to relax until the forces are less than 0.04 eV/Å and the convergence criteria of total energies is set to 10−5 eV. The structure optimization and electronic structure calculations are performed with 1 × 1 × 1 and 3 × 3 × 1 k-point grids, respectively. The GaN (00\(0\bar{1}\)) and (10\(\bar{1}\bar{1}\)) model consists of six layers of Ga-N, the bottom three of which are fixed at the corresponding bulk structure. Meanwhile, hydrogen atoms are used to passivate the dangling bonds at the bottom of the slab. The Au20 cluster is used to decorate the GaN surface in the calculations. The Au20 cluster with tetrahedral structure, has been widely used as a representative model system for investigating the catalytic characteristics of gold nanoclusters70. The vacuum regions are kept at least 15 Å between the periodically repeated slabs along the c-axis to eliminate the effect of interlayer interaction. The surface energies of GaN (00\(0\bar{1}\)) and (10\(\bar{1}\bar{1}\)) models are calculated according to the formula as follows70: \(\,{E}_{{surface}}=\frac{1}{2A}({E}_{{slab}}-n\times {E}_{{bulk}}-m\times {E}_{{H}_{2}})\), where \({E}_{{surface}}\) is the surface energy of the GaN models, \({E}_{{slab}}\) is the total energy of the relaxed GaN surface slab, \({E}_{{bulk}}\) is the total energy of the GaN bulk unit cell, n is the number of bulk GaN units in this slab, \({E}_{{H}_{2}}\) is the total energy of one hydrogen molecule, m is half the number of hydrogen atoms in the slab, and A represents the surface area of the slab. The HER process can be described as:\(\,{H}^{*}+{H}^{+}+{e}^{-}\to {H}_{2}\), where \({H}^{*}\) represents the H atom adsorbed on the Au/GaN surface, The Gibbs free energy of this adsorbed intermediate state is obtained as follows71: \(\,\triangle {G}_{{H}^{*}}=\triangle {E}_{{H}^{*}}+\triangle {E}_{{ZPE}}-T\triangle {S}_{{H}^{*}}\), where \(\triangle {E}_{{H}^{*}}\), \(\triangle {G}_{{ZPE}}\), and \(\triangle {S}_{{H}^{*}}\) denotes the difference of adsorption energy, zero point energy, and entropy, respectively. The temperature T is set to 298.15 K in the calculations.
Data availability
The data that support the findings of this study are available in the article, supplementary information file, source data file or from the corresponding authors upon request. Source data are provided with this paper.
References
Lewis, N. S. Research opportunities to advance solar energy utilization. Science 351, aad1920 (2016).
Walter, M. G. et al. Solar Water Splitting Cells. Chem. Rev. 110, 6446–6473 (2010).
Yang, W., Prabhakar, R. R., Tan, J., Tilley, S. D. & Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 48, 4979–5015 (2019).
Jiang, C. R., Moniz, S. J. A., Wang, A. Q., Zhang, T. & Tang, J. W. Photoelectrochemical devices for solar water splitting - materials and challenges. Chem. Soc. Rev. 46, 4645–4660 (2017).
Chen, S. Y. & Wang, L. W. Thermodynamic Oxidation and Reduction Potentials of Photocatalytic Semiconductors in Aqueous Solution. Chem. Mater. 24, 3659–3666 (2012).
Khaselev, O. & Turner, J. A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 280, 425–427 (1998).
Andrei, V., Roh, I. & Yang, P. D. Nanowire photochemical diodes for artificial photosynthesis. Sci. Adv. 9, eade9044 (2023).
Li, Y. Y. et al. Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting. Nat. Catal. 7, 77–88 (2024).
Tan, J. et al. Fullerene as a Photoelectron Transfer Promoter Enabling Stable TiO2‐Protected Sb2Se3 Photocathodes for Photo‐Electrochemical Water Splitting. Adv. Energy Mater. 9, 1900179 (2019).
Bae, D., Seger, B., Vesborg, P. C. K., Hansen, O. & Chorkendorff, I. Strategies for stable water splitting via protected photoelectrodes. Chem. Soc. Rev. 46, 1933–1954 (2017).
Arunachalam, M., Kanase, R. S., Zhu, K. & Kang, S. H. Reliable bi-functional nickel-phosphate /TiO2 integration enables stable n-GaAs photoanode for water oxidation under alkaline condition. Nat. Commun. 14, 5429 (2023).
Rojas, H. C. et al. Polymer-based photocathodes with a solution-processable cuprous iodide anode layer and a polyethyleneimine protective coating. Energy Environ 9, 3710–3723 (2016).
Tan, J. et al. Hydrogel protection strategy to stabilize water-splitting photoelectrodes. Nat. Energy 7, 537–547 (2022).
Yang, W. et al. Benchmark performance of low-cost SbSe photocathodes for unassisted solar overall water splitting. Nat. Commun. 11, 861 (2020).
Dong, W. J. & Mi, Z. T. One-dimensional III-nitrides: towards ultrahigh efficiency, ultrahigh stability artificial photosynthesis. J. Mater. Chem. A 11, 5427–5459 (2023).
Wu, B. et al. Stable solar water splitting with wettable organic-layer-protected silicon photocathodes. Nat. Commun. 13, 4460 (2022).
Wang, S. J. et al. Spatial decoupling of light absorption and reaction sites in n-Si photocathodes for solar water splitting. Natl. Sci. Rev. 8, nwaa293 (2021).
Jeon, D. et al. Superaerophobic hydrogels for enhanced electrochemical and photoelectrochemical hydrogen production. Sci. Adv. 6, eaaz3944 (2020).
Labrador, N. Y. et al. Enhanced Performance of Si MIS Photocathodes Containing Oxide Coated Nanoparticle Electrocatalysts. Nano Lett. 16, 6452–6459 (2016).
Koo, B. et al. Reduced Graphene Oxide as a Catalyst Binder: Greatly Enhanced Photoelectrochemical Stability of Cu(In,Ga)Se Photocathode for Solar Water Splitting. Adv. Funct. Mater. 28, 1705136 (2018).
Wang, S., Liu, G. & Wang, L. Crystal Facet Engineering of Photoelectrodes for Photoelectrochemical Water Splitting. Chem. Rev. 119, 5192–5247 (2019).
Xiao, Y. et al. Oxynitrides enabled photoelectrochemical water splitting with over 3,000 hrs stable operation in practical two-electrode configuration. Nat. Commun. 14, 2047 (2023).
Zeng, G. S. et al. Development of a photoelectrochemically self-improving Si/GaN photocathode for efficient and durable Hproduction. Nat. Mater. 20, 1130–1135 (2021).
Vanka, S. et al. High Efficiency Si Photocathode Protected by Multifunctional GaN Nanostructures. Nano Lett. 18, 6530–6537 (2018).
Zhou, B. et al. Gallium nitride nanowire as a linker of molybdenum sulfides and silicon for photoelectrocatalytic water splitting. Nat. Commun. 9, 3856 (2018).
Vanka, S. et al. Long-term stability studies of a semiconductor photoelectrode in three-electrode configuration. J. Mater. Chem. A 7, 27612–27619 (2019).
Kibria, M. G. et al. Atomic-Scale Origin of Long-Term Stability and High Performance of p-GaN Nanowire Arrays for Photocatalytic Overall Pure Water Splitting. Adv. Mater. 28, 8388–8397 (2016).
Tautz, M. et al. Anisotropy and Mechanistic Elucidation of Wet‐Chemical Gallium Nitride Etching at the Atomic Level. Phys. Status Solidi A 217, 2070060 (2020).
Jung, S., Song, K. R., Lee, S. N. & Kim, H. Wet Chemical Etching of Semipolar GaN Planes to Obtain Brighter and Cost‐Competitive Light Emitters. Adv. Mater. 25, 4470–4476 (2013).
Wang, L. et al. N-polar GaN etching and approaches to quasi-perfect micro-scale pyramid vertical light-emitting diodes array. J. Appl. Phys. 114, 133101 (2013).
Li, D. S. et al. Selective etching of GaN polar surface in potassium hydroxide solution studied by x-ray photoelectron spectroscopy. J. Appl. Phys. 90, 4219–4223 (2001).
Trovarelli, A. & Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 7, 4716–4735 (2017).
Li, C. et al. Facet-Dependent Photoelectrochemical Performance of TiO2 Nanostructures: An Experimental and Computational Study. J. Am. Chem. Soc. 137, 1520–1529 (2015).
Kang, Y. et al. Light‐Induced Adaptive Structural Evolution in Gallium Nitride Nanowire/Nickel Hydroxide Symbiotic System in Photoelectrochemical Environment. Adv. Funct. Mater. 34, 2311223 (2023).
Wang, J. et al. Subsurface Engineering Induced Fermi Level De‐pinning in Metal Oxide Semiconductors for Photoelectrochemical Water Splitting. Angew. Chem. Int. Edit. 135, e202217026 (2023).
Chen, R. S. et al. MoS2 Transistor with Weak Fermi Level Pinning via MXene Contacts. Adv. Funct. Mater. 32, 2204288 (2022).
Chen, W. et al. Manipulating Surface Band Bending of III-Nitride Nanowires with Ambipolar Charge-Transfer Characteristics: A Pathway Toward Advanced Photoswitching Logic Gates and Encrypted Optical Communication. Adv. Mater. 36, 2307779 (2024).
Kibria, M. G. et al. Visible light-driven efficient overall water splitting using p-type metal-nitride nanowire arrays. Nat. Commun. 6, 6797 (2015).
Grodzicki, M., Moszak, K., Hommel, D. & Bell, G. R. Bistable Fermi level pinning and surface photovoltage in GaN. Appl. Sur. Sci. 533, 147416 (2020).
Zhang, C. et al. Structure-catalytic functionality of size-facet-performance in pentlandite nanoparticles. J. Energy Chem. 78, 438–446 (2023).
Wang, K. et al. Intentional corrosion-induced reconstruction of defective NiFe layered double hydroxide boosts electrocatalytic nitrate reduction to ammonia. Nat. Water 1, 1068–1078 (2023).
Wang, S.-S. et al. Boosting Electrocatalytic Hydrogen Evolution over Metal–Organic Frameworks by Plasmon-Induced Hot-Electron Injection. Angew. Chem. Int. Edit. 58, 10713–10717 (2019).
Zhao, J. et al. Localized surface plasmon resonance for enhanced electrocatalysis. Chem. Soc. Rev. 50, 12070–12097 (2021).
Sheng, H. et al. Strong synergy between gold nanoparticles and cobalt porphyrin induces highly efficient photocatalytic hydrogen evolution. Nat Commun 14, 1528 (2023).
Zhang, Y. et al. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 118, 2927–2954 (2017).
Halas, N. J., Lal, S., Chang, W.-S., Link, S. & Nordlander, P. Plasmons in Strongly Coupled Metallic Nanostructures. Chem. Rev. 111, 3913–3961 (2011).
Cavallini, A. et al. Franz-Keldysh effect in GaN nanowires. Nano Lett. 7, 2166–2170 (2007).
Shang, Q. et al. Surface Plasmon Enhanced Strong Exciton–Photon Coupling in Hybrid Inorganic–Organic Perovskite Nanowires. Nano Lett. 18, 3335–3343 (2018).
Chiou, J. W. et al. Electronic structure of GaN nanowire studied by x-ray-absorption spectroscopy and scanning photoelectron microscopy. Appl. Phys. Lett. 82, 3949–3951 (2003).
Ye, C. et al. Electron-State Confinement of Polysulfides for Highly Stable Sodium-Sulfur Batteries. Adv. Mater. 32, e1907557 (2020).
Duan, Y. et al. Interfacial Engineering of Ni/V2O3 Heterostructure Catalyst for Boosting Hydrogen Oxidation Reaction in Alkaline Electrolytes. Angew. Chem. Int. Edit. 135, e202217275 (2023).
Gao, M. L., Li, L., Sun, Z. X., Li, J. R. & Jiang, H. L. Facet Engineering of a Metal–Organic Framework Support Modulates the Microenvironment of Palladium Nanoparticles for Selective Hydrogenation. Angew. Chem. Int. Edit. 134, e202211216 (2022).
Chen, Y. et al. Facet-engineered TiO2 drives photocatalytic activity and stability of supported noble metal clusters during H2 evolution. Nat. Commun. 14, 6165 (2023).
Dong, T. et al. Tunable Interfacial Electronic Pd–Si Interaction Boosts Catalysis via Accelerating O2 and H2O Activation. JACS Au. 3, 1230–1240 (2023).
Lin, G. et al. Suppressing Dissolution of Pt‐Based Electrocatalysts through the Electronic Metal–Support Interaction. Adv. Energy Mater. 11, 2101050 (2021).
Li, Y., Zhang, Y., Qian, K. & Huang, W. Metal–Support Interactions in Metal/Oxide Catalysts and Oxide–Metal Interactions in Oxide/Metal Inverse Catalysts. ACS Catal. 12, 1268–1287 (2022).
Zhang, B. et al. Atomically Dispersed Pt1–Polyoxometalate Catalysts: How Does Metal–Support Interaction Affect Stability and Hydrogenation Activity? J. Am. Chem. Soc. 141, 8185–8197 (2019).
Wang, D. H. et al. Bidirectional photocurrent in p-n heterojunction nanowires. Nat. Electron. 4, 645–652 (2021).
Fang, S. et al. Light-Induced Bipolar Photoresponse with Amplified Photocurrents in an Electrolyte-Assisted Bipolar p-n Junction. Adv. Mater. 35, e2300911 (2023).
Mahmood, J. et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nano. 12, 441–446 (2017).
Fang, S. et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 11, 1029 (2020).
Liu, Y. et al. Modulating Hydrogen Adsorption via Charge Transfer at the Semiconductor–Metal Heterointerface for Highly Efficient Hydrogen Evolution Catalysis. Adv. Mater. 35, 2207114 (2022).
Nelson, R. et al. LOBSTER: Local orbital projections, atomic charges, and chemical‐bonding analysis from projector‐augmented‐wave‐based density‐functional theory. J. Comput. Chem. 41, 1931–1940 (2020).
Ai, X. et al. Transition‐Metal–Boron Intermetallics with Strong Interatomic d–sp Orbital Hybridization for High‐Performance Electrocatalysis. Angew. Chem. Int. Edit. 59, 3961–3965 (2020).
Kawashima, T., Yoshikawa, H., Adachi, S., Fuke, S. & Ohtsuka, K. Optical properties of hexagonal GaN. J. Appl. Phys. 82, 3528–3535 (1997).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Morgan, B. J., Carrasco, J. & Teobaldi, G. Variation in surface energy and reduction drive of a metal oxide lithium-ion anode with stoichiometry: a DFT study of lithium titanate spinel surfaces. J. Mater. Chem. A 4, 17180–17192 (2016).
Wang, W. et al. Thgraphene: a novel two-dimensional carbon allotrope as a potential multifunctional material for electrochemical water splitting and potassium-ion batteries. J. Mater. Chem. A 10, 9848–9857 (2022).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Grant no. 62322410, 51727901). We also thank Dr. ChengJie Zuo, Dr. Yi Kang, and Dr. Lin Cheng for providing private funding to support this work. The work was partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication. Additionally, this work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China, thank Dr. Mei Sun from the HRTEM group for her help in TEM experiments. We would like to thank Solarever at Mexico for providing us the silicon substrates. Hefei Advanced Computing Center is acknowledged for computational support. We would like to thank BL12B at National Synchrotron Radiation Laboratory (NSRL) for the synchrotron beamtime.
Author information
Authors and Affiliations
Contributions
W.C. and D.H.W. contributed equally to this work. H.D.S. conceived the idea and designed the experiments. H.D.S., W.C., and D.H.W. discussed the experiment details. Z.T.Q. prepared and fabricated silicon substrate. S.L. and K.L. performed the MBE growth of the gallium nitride nanowires. W.C., X.L., Yang K., Y.M.L., W.G.G., and S.F. performed the sample preparation and characterizations. W.C., Y.Y.L., D.Y.L., and X.D.Y. carried out the PEC hydrogen evolution performance measurements, collected and analyzed the experimental data. W.Y.W. and W.H. conducted the theoretical calculation. Y.M.L. completed the FDTD simulation. W.C., D.H.W., and H.D.S. wrote the manuscript. Y.Y.L., C.W., and W.S.Y. performed the XAS measurement. W.C., D.H.W., Yang K., Y.Y.L., R.L., Y.J.X., J.H.H., C.J.Z, Yi K., L.C., and H.D.S. discussed the manuscript details. W.C., D.H.W., R.L., J.H.H., C.J.Z., Yi K., L.C., S.L., and Y.J.X. revised the manuscript. All authors discussed the results and participated in final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, W., Wang, D., Wang, W. et al. Enhanced solar hydrogen production via reconfigured semi-polar facet/cocatalyst heterointerfaces in GaN/Si photocathodes. Nat Commun 16, 879 (2025). https://doi.org/10.1038/s41467-024-55743-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55743-4