Abstract
Synchronous neuronal activity is a hallmark of the developing mouse primary somatosensory cortex. While the patterns of synchronous neuronal activity in cortical layer 2/3 have been well described, the source of the robust layer 2/3 activity is still unknown. Using a novel microprism preparation and in vivo 2-photon imaging in neonatal mice, we show that synchronous neuronal activity is organized in barrel columns across layers. Monosynaptic rabies tracing and slice electrophysiology experiments reveal that layer 2/3 pyramidal neurons receive significant layer 5 inputs during the first postnatal week, and silencing layer 5 synaptic outputs results in a significant reduction in spontaneous activity, abnormal sensory-evoked activity and disrupted layer 4-layer 2/3 connectivity. Our results demonstrate that translaminar layer 5-layer 2/3 connectivity plays an important role in synchronizing the developing barrel column to ensure the strengthening of layer 4-layer 2/3 connections, supporting the formation of the canonical cortical organization in barrel cortex.
Similar content being viewed by others
Introduction
The cerebral cortex processes external signals it receives, and integrates them with internally generated information, such as memories and expectations, to make percepts and guide behaviors. During the early postnatal period, the mouse primary somatosensory cortex (S1) receives sensory stimuli at a time when neural networks are dominated by intrinsically generated synchronous activity, providing an excellent model for studying how early experience interacts with intrinsic programs to shape the topographic representation of external stimuli. In S1, each functional barrel receives sensory inputs from one corresponding whisker on the snout, forming a sensory map in the cortex to encode the animal’s tactile experience1. The development of this precise mapping is largely attributed to sensory stimuli and feedback from the sensory periphery via thalamocortical afferents2,3,4,5. The thalamocortical somatotopic map exists embryonically, and disruption in thalamic activation during an early critical period leads to the loss of columnar and barrel organization6,7. While thalamocortical inputs are thought to orchestrate the early synchronous neuronal activity (SNA) required for circuit maturation7,8,9, cortico-cortical synaptic connectivity also plays a crucial role in shaping the patterns of SNA10,11,12,13,14. Despite well-characterized developmental and experience-dependent synaptic plasticity in cortico-cortical connections during the first three postnatal weeks (PNWs)15,16,17,18,19,20,21, how in vivo SNA is influenced by the rapid synaptic remodeling during this critical period is not known.
SNA is a hallmark of early cortical networks and is essential for cell and circuit maturation22,23. The precise pattern of SNA not only reflects the maturation of cell properties and synaptic connections, but also regulates developmental programs such as sensory map refinement and cell apoptosis14,24,25,26. Large-scale SNA driven by synaptic events has been described extensively both in vitro and in vivo in S1 during the first two PNWs. However, most of these studies described SNA patterns in two-dimensional space—either on a brain slice or on one plane at a single cortical depth11,12,13,27. Therefore, it remains to be elucidated how these patterns map onto barrel columns as they take form concurrently. While columnar organization of early SNA across cortical layers has been described by in vivo electrophysiological and imaging studies, it is unclear if it reflects synchronized activity at the somatic level in the supragranular layers, or if the SNA measured is dominated by axonal and dendritic activity of deep layer neurons and thalamic afferents. In addition, there is still considerable debate on whether columnar activation reflects cortical columns consisting of neurons derived from the same radial glia lineage9,28,29, or whether it is organized in barrel columns as early as the 1st PNW7,30.
What drives SNA in layer (L) 2/3 of the barrel cortex is also not well understood. Studies using in vivo 2-photon imaging indicate that L2/3 SNA is organized in synchronous local assemblies that are spatially restricted by GABAergic interneurons from P6-P1414,25,31, and they may not be confined by barrel boundaries12. Rabies viral tracing experiments show very limited direct synaptic inputs from the thalamus to L2/3 pyramidal neurons at P725, and L4-L2/3 synaptic connections are not well-established until P1416,21,32,33. In addition, subplate inputs, a major excitatory drive to relay thalamic activation to L4 (and to a lesser extent L5a and L1) in the 1st PNW, do not directly innervate L2/334,35,36. Taken together, the abundant SNA observed in L2/3 during the first two PNWs is either intrinsically and independently generated, or is supported by other developmentally transient synaptic inputs until L4-L2/3 inputs mature at P14. Clarifying the mechanisms by which L2/3 SNA is generated is crucial towards understanding the construction of functional barrel columns and cortical maps.
In this study, we used a novel 2-photon microprism imaging technique in neonatal mice to directly visualize the spatial organization of SNA across cortical layers and assessed the laminar inputs to L2/3 pyramidal neurons throughout the first three PNWs. We show that SNAs in S1 are organized in columns across cortical layers at the end of the 1st PNW, and these SNA columns are indeed barrel columns as defined by aggregates of thalamocortical afferents in L4. In addition, we show that L2/3 pyramidal neurons are synchronized within the columnar SNA by L5 inputs at P7—rabies synaptic tracing and patch clamp recording indicate significant direct L5-L2/3 inputs in the 1st PNW before the strengthening of L4-L2/3 inputs in the 3rd PNW. Reducing L5 synaptic inputs by expressing tetanus toxin light chain (TeLC) or inhibitory Designer Receptors Exclusively Activated by Designer Drugs (Gi-DREADD) specifically in L5 pyramidal neurons leads to significant reduction in L2/3 SNAs, lower number of sensory stimulus-responsive neurons in L2/3, as well as decreased L4-L2/3 synaptic strength. In summary, L5 plays an essential role in providing excitatory inputs to L2/3 during the early postnatal stage prior to the maturation of L4-L2/3 connectivity. This transient arrangement synchronizes the activation of L2/3 with deeper cortical layers in the same barrel column, enabling synaptic plasticity and facilitating translaminar connectivity. Results from this study uncover a basic developmental strategy to ensure synchronization and cohesiveness in circuit assembly across all cortical layers.
Results
Early SNA is spatially organized in barrel columns
In neonates, cortical neurons display robust spontaneous activity, and their activation is organized in synchronized assemblies11,12,14,25,26,37,38. However, how the assembly activity is synchronized across cortical layers is unclear. To directly visualize patterns of spontaneous activity simultaneously across layers, we adapted and optimized microprism implantation in neonatal mice for 2-photon imaging (Fig. 1). In adult mice, prism implant has been extensively used and validated, and shows minimal brain damage incurred beyond 50 μm from the prism-brain interface39,40,41. To capture neuronal activity simultaneously from L5 to L1, we implanted a 1 mm by 1 mm microprism in Emx1Cre:ROSALSL.GCaMP6s (Emx1:GCaMP6s) mice at P6 (Fig. 1a). We have previously used this genetic cross to measure SNA in vivo, which allows GCaMP6s to be expressed early and uniformly for measuring network activity14. Stable neuronal activity can be imaged after a recovery period as short as 24 h. Similar to adult studies, we did not observe signs of morphological defects, extensive cell damage, or atypical microglia activation following recovery (Fig. S1a-d). To maximally avoid imaging any potentially damaged tissue, imaging data were collected at least 200 μm from the prism-brain interface. We performed longitudinal imaging from P7 to P14 (Fig. S1e, f, Videos S1–3). Single-cell calcium event frequency, amplitude, and duration from P7 to P14 were consistent with previously described developmental trends (Fig. S1g–i)25. In addition, cell activity decorrelated significantly by the end of the 2nd PNW, characteristic of the developing barrel cortex (Fig. S1j, Video S3)12,25. Taken together, these results confirm that the implant and presence of the microprism did not disrupt neuronal activity or cause developmental delay.
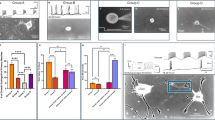
a Schematic depicting the placement of microprism in the barrel cortex. b Image of S1 at P14 after microprism was removed. Barrels are indicated by VGLUT2 staining. Arrow indicates microprism placement. Scale bar = 1 mm. c Example ROIs from the same (magenta, violet and orange) and different columns (blue and green) marked on a 2-photon FOV at P7. ROIs are 25 × 25 μm squares from dividing the FOV by a grid. Scale bar = 50 μm. d dF/F transients measured in the corresponding ROIs shown in (c). e Example SNA at 4 distinct time points during one video. Scale bar = 50 μm. f ROIs are color-coded using a k-means clustering algorithm. Best clustering (highest silhouette values) corresponds to clusters organized in columns as shown. g Silhouette values of the ROIs organized in columns versus randomized ROIs (0.12 ± 0.011 vs. -0.0047 ± 0.0035, two-sided paired t-test, p = 0.0008). h Pairwise correlation between ROIs, sorted by columns. The three corresponding clusters in (f) are labeled. i The percentage of pairs that are significantly correlated within the same column, compared to the pairs in the entire FOV (80.03 ± 6.22 vs. 38.41 ± 4.75, two-sided paired t-test, p = 0.0004). n = 5 videos from 5 mice. ***p < 0.001. Box plot (g, i) whiskers indicate the minimum and maximum values. Bounds of the box are the 1st and 3rd quartile and the center line indicates the median. Source data are provided as a Source Data file.
We next analyzed SNA patterns across layers observed through microprism at P7 (Fig. 1c). It was visually apparent that SNAs were organized vertically in columns, and each field of view (FOV, 509 × 509 μm) contained 2–3 of such columns (Figs. 1e, Video S1). These SNAs occurred at an average rate of 3.5 ± 1.1 events per minute. To measure the patterns of these SNAs, we divided the FOV into a 25 × 25 μm grid. We used a k-means clustering algorithm based on pairwise correlation between region of interests (ROIs)26,42. The clusters were determined by the highest silhouette values, which indeed corresponded to columns organized vertically (Fig. 1f, g). In addition, we calculated the percentages of correlated pairs of ROIs within the columns versus pairs within the entire FOV, and within-column percentages were significantly higher (Fig. 1h, i). These results demonstrated that SNAs at the end of the 1st PNW were synchronized in vertical columns across cortical layers. Most of the SNAs were restricted to a single column, while about 20% of SNAs involved multiple columns (Figs. S2a–c). Of these multi-column events, the majority propagated in a single direction in the FOV (unidirectional, left-to-right or right-to-left), while a smaller proportion propagated in both directions (bidirectional, Fig. S2d).
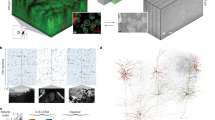
The pattern of early spontaneous activity in the upper cortical layers is thought to either reflect the spatial organization of columns derived from clonally related neurons12,37,43,44, or stimulus-selective barrel columns activated by peripheral inputs8,9,45,46. In order to determine whether the coactive columns were barrel columns, we injected the AAV1.hsyn.GCaMP6s virus at P0 into Olig3Cre:ROSALSL.tdTomato (Olig3:Ai9) mice, in which barrels were labeled by thalamic axons expressing tdTomato, and imaged spontaneous activity through implanted prism at P6-9 (Fig. 2a, b). In some animals, we observed sparse tdTomato-expressing soma in the cortex, however the soma labeling did not affect the identification of the barrel structure labeled by dense axonal terminals (Fig. 2c). Overlaying calcium activity onto the barrel columns revealed that spontaneous columnar activity was confined within the barrel boundaries (Fig. 2d). The percentage of correlated ROI pairs was significantly higher within the same barrel columns compared to that of the whole FOV (Fig. 2e). In order to rule out that activity in the ascending dendrites from L5 and L4 neurons might influence the correlation within the columns, we repeated the analyses using cells (with neuropil subtraction) instead of grid ROIs and found similar results (Fig. 2f). To further confirm that barrel structures identified in Olig3:Ai9 mice were functionally activated by whisker stimulation, we applied air puffs to contralateral whiskers at P12-14 in the same animals that had been imaged for spontaneous activity earlier, although only L4 and L2/3 could be consistently imaged through the prism as the brains grew (Fig. 2g, h). We found that a subset of L4 neurons were reliably activated by whisker stimulation, and a significantly larger proportion of L4 neurons than L2/3 neurons responded as expected (Fig. 2i). Together, these results support the idea that early SNAs during the 1st to 2nd PNW correspond to barrel columns, consistent with previous electrophysiological findings9,30.
a Schematic depicting experimental design. b Immunohistochemical staining of barrel cortex from a P8 Olig3:Ai9 mouse injected with GCaMP6s virus at P0. Barrel structure can be identified by thalamocortical axons expressing tdTomato. Scale bar = 100 μm. c 2-photon FOV through a microprism (top) of the barrel cortex in a P8 Olig3:Ai9 mouse injected with GCaMP6s virus. Barrel structure of the same FOV, labeled by tdTomato expression, is illustrated with red ovals and barrel columns are delineated with dotted lines (bottom). d Example SNA at 5 time points from the same video. Each time point is represented by a different color in the merged FOV. e The percentage of ROI pairs that are significantly correlated within the same barrel column, compared to the pairs in the entire FOV (93.74 ± 1.32 vs. 49.58 ± 7.94, two-sided paired t-test, p = 0.014). ROIs are 25 × 25 μm squares from dividing the FOV by a grid. f The same percentage of pairs correlated analysis using cell soma instead of ROIs from a grid (80.39 ± 4.61 vs. 43.59 ± 3.64, two-sided paired t-test, p = 0.013). n = 4 videos from 4 mice. g Schematic depicting whisker stimulation during 2-photon imaging in the same microprism implanted mice at P12-14. h Example 2-photon FOVs at P8 versus P12 in the same mouse. i The percentage of cells activated from whisker stimulation in barrels in L4 versus L2/3 (19.40 ± 4.47 vs. 10.99 ± 4.67, two-sided paired t-test, p = 0.0045). n = 4 videos from 4 mice. Scale bar = 50 μm. *p < 0.05, **p < 0.01. Box plot (e–f, i) whiskers indicate the minimum and maximum values. Bounds of the box are the 1st and 3rd quartile and the center line indicates the median. Source data are provided as a Source Data file.
Distribution of translaminar inputs to L2/3 is developmentally regulated
During the 1st PNW, L2/3 pyramidal neurons receive limited long-range synaptic inputs, such as those from the thalamus25, suggesting that the spontaneous activity in L2/3 could be driven instead by intracortical connections. To identify the cortical synaptic inputs driving L2/3 pyramidal neurons and to understand how these inputs evolve across development, we compared monosynaptic inputs onto L2/3 pyramidal neurons during the first three consecutive PNWs using an approach we previously established for developing mice25,31 (Fig. 3). We first electroporated Emx1Cre mouse embryos with a pAAV-EF1a-flex-GFP-TVA-B plasmid at embryonic day (E) 15.5 to target L2/3 pyramidal neurons. Next, we injected a recombinant rabies virus, SAD ΔG_BFP(EnvA), into the barrel cortex at P0-1 or P14, and analyzed the number of presynaptic input cells distributed in different cortical layers at P7 or P21 respectively. We subsequently compared these connectivity patterns with our previous P7-P14 mapping data31 (Fig. 3a). As expected, electroporated cells (green) and starter cells (yellow) were located within L2/3, while presynaptic cells (red) were distributed throughout all cortical layers (Fig. 3b, c). Notably, we observed a number of clusters of presynaptic cells labeled during the 1st PNW, reminiscent of clonally related sister cells thought to underlie spontaneously active, previously termed “neuronal domains” during the early postnatal period29,47,48,49,50 (Fig. 3d). This suggests that while in vivo SNAs are organized in barrel columns at the end of the 1st PNW, they may contain smaller coactive units of clonally related sister cells during the very early postnatal stage.
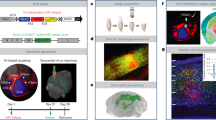
a Schematic depicting experimental design. b Tracing inputs to L2/3 pyramidal neurons in the 1st, 2nd, and 3rd PNWs. Ctip2 staining and neurite arborization of electroporated L2/3 neurons (GFP) are also shown to demonstrate layer delineation for 1st PNW (left). Red: rabies virus (RbV) infected presynaptic cells; green: electroporated neurons in L2/3; yellow: starter cells. Blue: Ctip2 staining for L5 identification in 1st PNW. c Confocal image of example starter cells in the 2nd PNW, indicated by arrows. d Example of clusters of presynaptic cells labeled in the 1st PNW, indicated by arrows. e Relationship between number of starters versus presynaptic inputs. Data fitted with linear regression, and R2 is indicated on the plots. f Number of input cells per starter cell across development (One-way ANOVA with Bonferroni’s test; 1st vs. 2nd, 88.05 ± 14.10 vs. 44.00 ± 9.69, p = 0.018; 1st vs. 3rd, 25.76 ± 1.04, p = 0.0010; 2nd vs. 3rd, p = 0.54). Box plot whiskers indicate the minimum and maximum values. Bounds of the box are the 1st and 3rd quartile and the center line indicates the median. g–i Input distributions across cortical layers for 1st (g), 2nd (h), and 3rd (i) PNW (One-way ANOVA with Bonferroni’s test for L4 vs. L5: 1st, 19.38 ± 2.03 vs. 29.26 ± 2.26, p = 0.048; 2nd, 30.98 ± 1.25 vs. 31.93 ± 1.07, p = 0.54; 3rd, 34.23 ± 2.01 vs. 24.67 ± 1.84, p = 0.0011). Same 2nd PNW dataset for age comparison here was used as a control dataset in a previous publication31. n = 5 brains for 1st PNW, 5 brains for 2nd PNW, and 6 brains for 3rd PNW. Scale bar = 100 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant, p > 0.05. Bars represent the means ± SEM. Source data are provided as a Source Data file.
We quantified the total number of starter cells and presynaptic input cells in the ipsilateral cortex for every brain. Good linear correlations between the number of starter cells and input cells were observed for all three PNWs, indicating low variability and sufficient range of starter cell numbers for proper sampling51 (Fig. 3e). The number of input cells per starter was significantly greater during the 1st PNW compared to the 2nd and 3rd PNWs (Fig. 3f). This developmental reduction of presynaptic inputs is consistent with the enhanced topographic precision and axonal refinement of cortical and thalamic afferents during the early postanal stage33,52. We then quantified the percentage of presynaptic inputs from each cortical layer for the first three PNWs. During the 1st PNW, we found a largest proportion of inputs to L2/3 neurons were from L5, significantly higher than L4-L2/3 inputs (Fig. 3g). Notably, we also observed significant deep-layer presynaptic inputs including subplate cells in the 1st PNW, while there was little subplate labeling in the 2nd and 3rd PNW (Fig. 3b). This observation is consistent with previous reports that the subplate can activate upper-layer neurons early postnatally but largely disappear by P335,53,54. During the 2nd PNW, the percentages of L5- versus L4-L2/3 inputs became comparable31 (Fig. 3h), while L4-L2/3 inputs were the largest in the 3rd PNW, significantly higher than L5 (Fig. 3i). In the adult barrel cortex, L4 is the major excitatory input layer to L2/355,56,57, while L5-L2/3 connections are sparse58,59,60. Our data suggest that developmentally transient connections between L5 and L2/3 may be robust before the maturation of L4-L2/3 inputs in the 3rd PNW. This is also supported by our observation that L5 activation preceded L2/3 in most of the columnar activation we observed in P7-8 in vivo microprism imaging experiments, suggesting that L5 is a major synaptic input layer to L2/3 that drives columnar SNA during the 1st PNW (Fig. S2e).
L5 provides strong synaptic inputs to L2/3 prior to the maturation of L4-L2/3 connectivity
To directly assess the translaminar synaptic strength across barrel columns and developmental stages, we next performed a series of slice electrophysiological experiments. First, to confirm that L5-L2/3 connections were organized in barrel columns as indicated by SNA patterns, we placed an electric stimulating electrode in L5 and recorded the excitatory postsynaptic currents (EPSCs) in a L2/3 neuron in the same barrel column as the stimulating electrode and subsequently one in the adjacent barrel, or vice versa (Fig. 4a, b). To achieve similar stimulation intensity, we selected L2/3 neurons that were located at similar distances from the stimulating electrode by choosing neighboring-column neurons close to barrel septa. Our results showed that L2/3 neurons in the same column received significantly larger L5 inputs compared to those in the neighboring column, confirming that translaminar L5-L2/3 inputs are preferentially organized in barrel columns (Fig. 4c, d).
a Schematic depicting experimental setup for comparing the strength of L5 inputs to L2/3 pyramidal neurons in the same versus neighboring column in 1st PNW. b Example DIC image of L4 barrels. Patch pipette is positioned in L2/3. M: medial, L: lateral. Scale bar = 100 μm. c Example EPSC traces elicited by electrically stimulating L5 neurons and recorded in L2/3 pyramidal neurons in the same (right) versus neighboring (left) barrel column. d EPSC response amplitudes in L2/3 pyramidal neurons in the two locations (same vs. neighbor, 128.61 ± 12.67 vs. 55.07 ± 9.98, two-sided paired t-test, p = 0.0074). n = 7 cells for each location. e Schematic depicting experimental setup for comparing the strength of L4 versus L5 inputs to L2/3 neurons in the same barrel column. f Example EPSCs recorded in the same L2/3 neuron while stimulating L4 (top) versus L5 (bottom) at the end of 1st, 2nd, and 3rd PNW. g Quantification of peak amplitudes of the first responses of the paired pulse. 1st and 2nd PNWs: n = 16 pairs, 3rd PNWs: n = 15 pairs. (1st and 3rd PNWs: two-sided Wilcoxon test, 105.87 ± 20.72 vs. 29.79 ± 7.28, p = 0.0063, and 104.47 ± 14.82 vs. 254.13 ± 48.25, p = 0.0099 respectively; 2nd PNW: two-sided paired t-test, 94.83 ± 10.70 vs. 227.04 ± 44.70, p = 0.0099). h Contribution of L5 in total L4 and L5 inputs. 1st and 2nd PNWs: n = 16 cells, 3rd PNWs: n = 15 cells. (One-way ANOVA with Bonferroni’s test: 1st vs. 2nd, 71.26 ± 6.60 vs. 37.29 ± 5.34, p = 0.0004; 1st vs. 3rd, 35.39 ± 5.44, p = 0.0003.) Bars represent means ± SEM. i Paired-pulse ratio (PPR) across the three PNWs for L5 (left) versus L4 (right) stimulation (Kruskal-Wallis test: L5 1st to 3rd PNWs: 0.99 ± 0.095 vs. 0.98 ± 0.055 vs. 0.96 ± 0.28, p = 0.33; L4 1st to 3rd PNWs, 0.85 ± 0.12 vs. 0.9932 ± 0.038 vs. 1.01 ± 0.045, p = 0.12). 1st PNW, n = 20 cells; 2nd PNW, n = 19 cells; 3rd PNW, n = 17 cells. **p < 0.01, ***p < 0.001. Box plot (d, g, i) whiskers indicate the minimum and maximum values. Bounds of the box are the 1st and 3rd quartile and the center line indicates the median. Source data are provided as a Source Data file.
Next, to assess the synaptic strengths of L5- versus L4-L2/3 neurons across development, we recorded EPSCs in the same L2/3 pyramidal neuron while electrically stimulating L5 or L4 in the same barrel column, across the three postnatal stages same as the rabies tracing experiments – P6-P7 for the 1st PNW, P12-P14 for the 2nd PNW, and P19-P21 for the 3rd PNW (Fig. 4e, f). At 1st PNW, L2/3 pyramidal neurons received significantly larger EPSCs from L5 than L4 inputs. In contrast, EPSC amplitudes elicited by L4 simulation increased markedly in the 2nd PNW and 3rd PNW to become significantly larger than those by L5 (Fig. 4g, h), thus reversing the large 1st PNW L5 contribution at 2nd and 3rd PNW (Fig. 4h). To confirm that our electric stimulation was largely restricted to stimulating somas in the intended layer and not ascending fibers from deeper layers or the thalamus, we performed an optogenetic experiment by selectively stimulating L5 using a L5 pyramidal neuron-targeting Rbp4-Cre mouse line61,62,63 injected with a Cre-dependent ChR2 virus (AAV5-Ef1a-DIO-hChR2-EYFP) at P0 (Fig. S2f). At steady-state light intensity, similar EPSC amplitude was observed using light stimulation compared to electric stimulation (Fig. S2h, i). Lastly, the average paired-pulse ratios (PPRs) of L4- and L5-evoked responses remained unchanged throughout the first three PNWs (Fig. 4h). This suggests that the larger L4-L2/3 inputs over the 2nd and 3rd PNWs are not a result of higher release probability at the level of the synapse, but a result of increased number of L4 presynaptic inputs, consistent with the anatomical data from our rabies tracing experiments (Fig. 3g-i).
Acute suppression of L5-L2/3 inputs reduces SNAs in L2/3 at 1st but not 2nd PNW
Our data from in vivo imaging, rabies tracing, and electrophysiological recording experiments all point to substantial L5 inputs to L2/3 pyramidal neurons in the 1st PNW, before L4 inputs are established during the 2nd and 3rd PNW. These results suggest that L5 synaptic inputs may play a developmentally transient yet critical role to drive L2/3 SNA. To directly compare the contribution ofL5-L2/3 inputs to SNA during the 1st versus 2nd PNW in vivo, we used a chemogenetic approach to acutely reduce L5 activation (Fig. 5). We crossed the L5 pyramidal neuron-targeting Rbp4-Cre line to a mouse line expressing Cre-dependent Gi DREADD and mCitrine (ROSALSL.hM4Di-mCitrine)64, and injected the red-shifted calcium indicator AAV1-Syn-jRGECO1a at P0 (Fig. 5a, b). In brain slices, DREADD activation with CNO (10 μM) led to an outward current and a moderate reduction of spiking, but not elimination of activity, in L5 pyramidal neurons (Fig. S3a, b). We implanted cranial windows at P6, and imaged SNA in L2/3 after injecting with saline or CNO (1 mg/kg) at either P7-8 or P14 (Fig. 5a). Compared to saline injection, CNO significantly reduced L2/3 SNA frequency and amplitude at P7-8 (Fig. 5c, d). As a control, CNO injection did not reduce SNA frequency or amplitude in Cre-negative mice (Fig. S3c, d). At P14, CNO had no effect on SNA frequency, and unexpectedly increased SNA amplitude (Fig. 5e, f). Since L5 neurons also provide the majority of cortical outputs to subcortical regions, including the thalamic reticular nucleus (TRN), increased SNA amplitude may reflect changes in cortico-thalamic feedback in these circuits due to reduced L5 outputs65. Alternatively, increased SNA amplitude could be a result of dampened disynaptic inhibition given that L5-to-upper-layer-interneuron connections have been reported at least in the mature S166,67,68.
a Schematic depicting experimental setup for 1st versus 2nd PNW acute L5 suppression. b Example image of mCitrine (hM4Di) expression in S1 of an Rbp4-Cre:hM4Di mouse. Scale bar = 100 μm. c Example calcium transients from single cells in the same P7 mouse after saline (left) versus CNO (right) injection (1 mg/kg). d Calcium transient frequency (left: 25.63 ± 2.74 vs. 20.88 ± 2.54, two-sided Wilcoxon test, p = 0.0014) and amplitude (right: 97.48 ± 11.73 vs. 89.04 ± 9.85, two-sided paired t-test, p = 0.038) comparison at P7-8 after saline versus CNO injection imaged from the same FOV. e Example calcium transients from single cells in the same P14 mouse after saline (left) versus CNO (right) injection (1 mg/kg). f Calcium transient frequency (left: 41.89 ± 2.70 vs. 42.89 ± 4.31, two-sided paired t-test, p = 0.75) and amplitude (right: 50.17 ± 2.17 vs. 66.03 ± 7.42, two-sided paired t-test, p = 0.043) comparison at P14 after saline versus CNO injection imaged from the same FOV. P7-8, n = 16 video pairs from 8 mice; P14, n = 9 video pairs from 4 mice. *p < 0.05, **p < 0.01. Box plot (d, f) whiskers indicate the minimum and maximum values. Bounds of the box are the 1st and 3rd quartile and the center line indicates the median. Source data are provided as a Source Data file.
L5-driven SNA in L2/3 is required for the maturation of whisker-dependent sensory processing and L4-L2/3 synaptic connection
While early SNA has long been recognized as an important feature of developing cortical circuits, only until recently did accumulating evidence support that SNA plays a functionally instructive role in circuit assembly14,24,25,69. Given that our data showed L4-L2/3 connections were established later than L5-L2/3, we tested if whisker-evoked sensory responses and the maturation of L4-L2/3 inputs would be affected if SNAs in L2/3 were disrupted by reducing L5-L2/3 inputs. We selectively reduced L5-L2/3 inputs by expressing TeLC in L5 neurons14,70,71. We targeted L5 pyramidal neurons using the Rbp4-Cre line, and co-injected the red-shifted calcium indicator AAV1-Syn-jRGECO1a with AAV1-CMV-flex-TeLC-eGFP or AAV1-hSyn-DIO-eGFP as a control at P0 (Fig. 6a–c). We implanted cranial windows at P6, and imaged L2/3 SNA in control and TeLC conditions at P7-8, and whisker-evoked response at P14 (Fig. 6a). Similar to the acute L5 silencing experiments with Gi-DREADD at 1st PNW (Fig. 5c, d), we observed a significant reduction in L2/3 calcium event frequency and amplitude as well as the percentage of cells that participate in network events in the TeLC condition at P7-8 (Fig. 6d–f), supporting that diminishing L5 synaptic outputs during the 1st PNW can substantially reduce SNA in developing L2/3 neurons. Given that thalamocortical inputs have been shown to provide substantial excitatory drive to deep layers and L1 interneurons in the 1st PNW, we also examined the impact of reduced thalamocortical inputs on L2/3 SNA by expressing TeLC in the thalamus using the Olig3Cre line crossed with the RosaLSL.TeLC mouse line71,72 (Fig. S4). We observed a horizontal spatial expansion and loss of columnar organization in SNA, but calcium event frequency and amplitude were maintained (Fig. S4d, e). This is consistent with previous reports that disrupted thalamic activation leads to broadening of SNA in upper cortical layers7, suggesting that while thalamocortical projections instruct the patterns of SNAs, intracortical connections, including those initiated by L5, may act as a crucial source for generating or amplifying early SNA.
a Schematic depicting experimental setup. b Example image of TeLC-eGFP and jRGECO1a expression in an Rbp4-Cre mouse. Scale bar = 500 μm. c Expression in S1 across layers. Scale bar = 100 μm. d Calcium transient frequency at P7-8 for control (Con) versus TeLC condition (17.87 ± 1.69 vs. 13.00 ± 1.17, two-sided unpaired t-test, p = 0.023). e Calcium transient amplitude at P7-8 (98.90 ± 7.82 vs. 55.18 ± 4.84, two-sided unpaired t-test, p < 0.0001). f Percentage of cells that participate in network events at P7-8 (26.27 ± 2.79 vs. 19.64 ± 1.82, two-sided Mann-Whitney test, p = 0.041). d–f Con: n = 15 videos from 7 mice, TeLC: n = 16 videos from 7 mice. g Percentage of L2/3 neurons activated by air-puff whisker stimulation (17.61 ± 1.95 vs. 13.92 ± 1.92, two-sided Mann-Whitney test, p = 0.14). h Average amplitude of calcium transients evoked by whisker stimulation (40.14 ± 1.38 vs. 48.86 ± 2.059, two-sided Mann-Whitney test, p < 0.0001). g–h Con: n = 9 videos from 4 mice, TeLC: n = 15 videos from 5 mice. i Schematic depicting experimental timeline. j Placement of stimulating and recording electrodes. k Example evoked EPSC traces recorded at P14 in a L2/3 pyramidal neuron from control hemisphere (Con) versus TeLC-infected hemisphere. l Average EPSC amplitude for neurons from Con versus TeLC hemisphere at P14 (115.88 ± 24.61 vs. 13.26 ± 10.59, two-sided paired t-test, p = 0.033). n = 5 cells from each side matched in the same section. m Example EPSC traces recorded at P21 in a L2/3 pyramidal neuron from Con versus TeLC hemisphere. n Average EPSC amplitude for neurons from Con versus TeLC hemisphere at P21 (218.72 ± 48.42 vs. 76.23 ± 13.23, two-sided paired t-test, p = 0.014, n = 6 cells from each side matched in the same section.) *p < 0.05, ****p < 0.0001. Box plot (d–h, l, n) whiskers indicate the minimum and maximum values. Bounds of the box are the 1st and 3rd quartile and the center line indicates the median. Source data are provided as a Source Data file.
Next, we evaluated whisker evoked responses in L2/3 at P14 in the same mice expressing TeLC in L5, and found while the percentage of L2/3 neurons activated by air puff stimulation remained similar between control and TeLC conditions, amplitudes of evoked calcium events were significantly reduced in the TeLC condition (Fig. 6g, h). These results suggest that reduced L5-L2/3 inputs during development may have a particularly large impact on the strength of synaptic inputs L2/3 neurons receive during sensory input, namely from L4 neurons. To directly test this, we assessed the strength of L4-L2/3 synaptic connections in Rbp4-Cre mice expressing AAV1-CMV-flex-TeLC-eGFP at P14, as well as at P21 when the critical period for circuit maturation in the barrel cortex was completed (Fig. 6i). As a comparison, we recorded the strength of L4-L2/3 inputs on the contralateral hemisphere with no TeLC expression in the same brain slice. A stimulating electrode was placed in L4, and EPSCs were recorded in pyramidal neurons in L2/3 directly above in the same barrel column (Fig. 6j). The same stimulation intensity was used on TeLC and control side. At P14, average EPSC amplitude on the TeLC side was significantly lower than the contralateral control side, supporting that reduced SNA in L2/3 driven by L5 can lead to weakened L4-L2/3 connectivity (Fig. 6k, l). The reduction in EPSC amplitude remained to be significant at P21, indicating that the reduction in synaptic connectivity persisted through development (Fig. 6m, n).
Discussion
While development of thalamocortical afferents and deep layer circuits in the barrel cortex have been extensively described, how superficial layer circuits are established during the early postnatal stage is not well understood. In this study, we directly examined the patterns of SNA in L2/3 in relation to deep layers and barrel columns, and identified the source of early L2/3 activation. By directly imaging SNA across cortical layers through the use of a microprism, we found SNA was organized translaminarly, but largely confined within the boundaries of barrel columns. Rabies synaptic tracing and slice patch clamp experiments showed that L2/3 pyramidal neurons received significant levels of direct L5 synaptic inputs during the 1st PNW, which were gradually overtaken by L4 inputs in the 2nd and 3rd PNW. This was a surprising finding given that L5-L2/3 connectivity was previously described as sparse or weak in the adult brain58,59,60. While strong L5-L2/3 connectivity has been recently identified in adult primary visual cortex, these projections are rare and highly selective73. Suppression of L5 synaptic outputs in the 1st PNW diminished L2/3 SNA and resulted in smaller sensory-evoked responses in L2/3 and weaker L4-L2/3 synaptic connections in the 3rd PNW. Our data clarified a long-standing question on whether early SNA in L2/3 is independently orchestrated or is organized in unison with the functional barrel columns. In addition, we uncovered a transient excitatory circuit that supports SNA in the superficial layers of barrel cortex, unifying them with activity in deep layers to form coherent translaminar connectivity in the developing barrel columns.
We identified strong L5-L2/3 connections that support L2/3 SNA during the 1st PNW and early 2nd PNW, but what drives the early L5 activation? Subplate neurons have been shown to innervate L5a36, and direct thalamic inputs onto L5 pyramidal neurons are present during this stage (although weaker compared to those onto L5 interneurons) as well as in adulthood74,75,76. Therefore, early SNA in the thalamus, generated by central sources and/or sensory periphery77, likely activates L5 either directly through thalamocortical afferents or indirectly through subplate relay. Since L4 is activated concurrently by the same thalamic and subplate inputs36,45,78, the robust L5-L2/3 connection during the 1st PNW keeps L2/3 synchronized with the sensory recipient layers. This creates the condition for concurrent morphological development and spike-time dependent plasticity required for the establishment and strengthening of L4-L2/3 connection during the first three PNWs. We present a proposed model to summarize the development of this translaminar circuit (Fig. S5). The coactivation of L4 and L2/3 may transition from relying on thalamic-driven (directly or via L5) SNAs in the 1st PNW, to sensory-evoked activation during the 2nd and 3rd PNWs, when SNAs become broader and more complex due to the strengthening of lateral intracortical connectivity9,78. Experience-dependent strengthening of L4-L2/3 connection has been well-characterized previously to occur during a short window at the end of the 2nd PNW21. Our data uncover an earlier, intracortical source of activation for L2/3 by L5 that may enable the initiation of this critical period for sensory experience. Indeed, reducing L5 synaptic output not only disrupted L2/3 SNAs at the end of the 1st PNW, but also impaired sensory-evoked activation as well as L4-L2/3 synaptic strength in the 2nd and 3rd PNWs (Fig. 6).
It is worth noting that our rabies tracing experiments also revealed significant presynaptic inputs from L6 and subplate to L2/3 during the 1st PNW, which decreased significantly during 2nd and 3rd PNWs. The low L6-L2/3 connectivity in later stages of development is consistent with reports in the mature brain73,79. We also used a larger prism (1.5 mm) in attempts to capture L6 and subplate activity. While we did observe SNA in L6, we feared that the larger microprism penetrating corpus callosum may disrupt thalamocortical projections and interfere with activation (Fig. S6). Therefore, it remains to be determined whether L6 and L5 are always activated simultaneously during SNA, or if L6 and/or subplate are involved in distinct SNA patterns. In addition, the subplate in S1 is required for spontaneous spindle bursts, an electrophysiological hallmark of the developing cortex both in vitro and in vivo54,80. While calcium imaging provides single-cell spatial resolution, it does not have the temporal resolution afforded by in vivo electrophysiology to resolve network oscillatory frequency. Nevertheless, the frequency of SNA occurrence in this study is in agreement with previously reported occurrence of spindle bursts and gamma oscillations in neonates9.
While evidence suggests that developing thalamocortical afferents provide substantial excitatory inputs in S1, disrupting SNA in the thalamus resulted in not reduced, but overactive, hypersynchronous SNA in S1 of neonatal mice that spreads horizontally instead of vertically in columns7. We saw similar horizontally synchronized SNA within each layer in our in vivo experiments after diminishing thalamic synaptic outputs in Olig3:TeLC mice (Fig. S4). Interestingly, in this condition, while L2/3 and L5 showed correlated activity, L4 appeared to be in dyssynchrony with the other layers (Fig. S4c). This suggests that while the emergence of functional columns relies on thalamic control and entrainment of cortical excitability, there are likely separate cortical sources of spontaneous SNA as well, or at least means to amplify SNA within the cortex when thalamocortical inputs are insufficient to support activity-dependent maturation7,81. Given that SNA is indispensable for neuronal and network maturation, it is not surprising that multiple mechanisms are present to maintain a source of correlated activity during crucial stages of development22. The origin of the centrally generated SNA is unclear, but cortical traveling waves originated from ventral regions amplified by mGluR activation have been implicated7,82,83. In addition, a recent study also implicated odor-driven activity originated from the olfactory bulb as yet another source of SNA that propagates across S1 during the 1st PNW84.
SNA is a general feature of all developing networks, but the underlying mechanisms vary greatly across different systems. In addition, the patterns of SNA can undergo profound changes during specific developmental stages due to maturation of cell intrinsic properties and the presence of developmentally transient circuits77. Accumulating evidence suggests that these transient circuits coexist with circuits that will prevail into adulthood, and increasing influence of inputs from sensory periphery and long-range projections trigger the eventual disappearance of the transient circuits25,36,75,76,85,86. In S1, transient thalamic inputs to somatostatin (SST) interneurons during the 1st PNW are required for the maturation of parvalbumin (PV) interneurons75, while thalamic inputs to L1 reelin (Re) interneurons during the same time function to spatially restrict the activation of L2/3 during sensory stimuli and shape the functional barrel columns25. Furthermore, recent evidence indicates that thalamic inputs regulate the maturation of translaminar L1 single bouquet cells which sharpen barrel map in L487. Interestingly, embryonic L5 pyramidal neurons have recently been shown to form transient, multilayered circuit before cortical lamination begins88. The L5-L2/3 excitatory connection identified in this study further supports that transient circuits are a fundamental mechanism that the developing brain utilizes to construct complex networks.
In summary, our study demonstrates that early SNA in the developing S1 is organized in barrel columns, and that the translaminar activation is largely driven by L5 pyramidal neurons. This transient L5-L2/3 connectivity plays an instrumental role in orchestrating the columnar organization. These findings provide insight into circuit mechanisms of neurodevelopmental disorders, implicating that alterations in a single cell type/connection can have long-term effects on subsequent circuit assembly in the developing brain.
Methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee of Yale University School of Medicine (protocol 2024-20430) or the Weill Cornell Medicine Research Animal Resource Center (protocol 2013-0122). All mice were provided with food and water ad libitum and housed in a room maintained on a 12-hour light/dark cycle (7 am to 7 pm) at 72 °F with 45% humidity. All mouse lines have been described previously: Emx1Cre (JAX 005628), Rbp4-Cre (MMRRC_037128-UCD), ROSALSL.hM4Di-mCitrine (JAX 026219), ROSALSL.GCaMP6s (Ai96, JAX 024106), ROSALSL.TdTomato (Ai9, JAX 007909), Olig3Cre (A gift from Y. Nakagawa, University of Minnesota), ROSALSL.TeLC (a gift from M. Goulding, Salk Institute for Biological Studies). Information about mouse strains including genotyping protocols can be found at http://www.jax.org and elsewhere72,89. A total of 130 mouse pups, aged from P7 to P21, were used for experiments. Pups of both sexes were included in the analysis of all experiments. Sex was not considered a factor in experimental design as only young animals (<p21) prior to sexual maturity were used in experiments. Random animal assignments to experimental groups were also not influenced by sex. For timed pregnancies, noon on the day of the vaginal plug was counted as E0.5. Mice not requiring tissue collection were euthanized by decapitation after anesthesia or exposure to carbon dioxide (>P10).
Viral injections
Viral injections were performed in S1 at P0-P1 with glass micropipettes attached to a Drummond Nanoject II. Mice were anesthetized by hypothermia. The following viruses were used: AAV1-Syn-jRGECO1a (Addgene 100854), AAV1-hsyn-GCaMP6s (UPenn viral core), AAV1-CMV-flex-TeLC-eGFP (gift from Peter Wulff), AAV1-hsyn-DIO-eGFP (Addgene 50457), AAV5-Ef1a-DIO-hChR2-EYFP (Addgene 35509). The following coordinates were used: 1.4 mm rostral from lamda, 1.5 mm lateral from the sagittal suture, at 0.25 mm depth for L2/3 and 0.45 mm for L514,25,31. For single-virus injections, 100 nl virus was delivered, for two-virus injections, 150 nl of viral cocktail at 1:1 ratio was delivered.
Rabies viral tracing
In utero electroporation was performed as previously described25,31. In brief, timed pregnant mice were anaesthetized and embryos were injected through the uterine wall in the lateral ventricle with DNA plasmids at E15.5. After square pulses were delivered for electroporation, the uterus was placed back in the abdominal cavity and the mouse was sutured. A pAAV-EF1a-flex-GFP-TVA-B plasmid (Addgene 26197) was electroporated in pregnant Emx1Cre dams. After the electroporated pups were born, modified rabies virus SAD-ΔG-BFP (EnvA, Salk Institute) was injected at P0-1, P7-8 or P14 using a Drummond Nanoject II. The following coordinates were used: for P0-1, 1.4 mm rostral from lamda, 1.5 mm lateral from the sagittal suture, 0.25 mm deep from pia; for P7-8, 2.5 mm rostral from lamda, 2.5 mm lateral from sagittal suture, and 0.4 mm deep from pia; for P14, 3.3 rostral from lamda, 2.6 mm lateral from sagittal suture, at 0.5 mm deep from pia. Pups were returned to the dam after injection and perfused for immunohistochemistry a week later. Processing of rabies-infected brains was performed as previously described25,31. Briefly, consecutive 20 μm sections were collected on a cryostat (Leica CM3050S). Confocal images were taken using an Olympus IX81 confocal microscope with Fluoview FV1000 software (P7-8 injection dataset, same dataset as published previously31), or with an Olympus Complete FV3000RS confocal system (P0 and P14 injection datasets). In a subset of experiments, particularly 1st PNW brains with P0-1 injections, layer-specific marker Ctip2 was used along with the location and the arborization of electroporated L2/3 pyramidal neurons to aid cortical layer identification. All starter neurons (BFP + , GFP + ) and presynaptic rabies infected neurons (BFP + ) were identified and counted from each brain. The numbers of presynaptic neurons from different layers of the ipsilateral S1 were quantified and used in the analyses.
Immunohistochemistry
Mice were deeply anesthetized with hypothermia ( < or equal to P7) or euthasol (>P7). After toe pinch reflex disappeared, mice were transcardially perfused with 10-20 ml of phosphate buffered saline (PBS) and 10–20 ml of 4% paraformaldehyde (PFA) in PBS. Brains were post-fixed with 4% PFA for 45 minutes (young mice) or overnight (older mice) at 4 °C, and equilibrated in 30% sucrose for >16 h at 4 °C before mounting in Tissue-tek (Sakura). 20 μm coronal sections were used for rabies tracing experiment cell counting, and 50 μm sections were used for all other experiments. After 3×5-minute PBS washes, sections were blocked with 5% normal goat serum, 0.5% Triton in PBS at room temperature for 1 h. Sections were then incubated with appropriate primary antibodies overnight (>16 h) at 4 °C. The following primary antibodies were used: 1:1000 rabbit anti-Iba1 (FUJIFILM Wako Shibayagi Corp Cat #019-19741, RRID:AB_839504); rabbit anti-Hsp70/72 (Enzo Life Sciences Cat#ADI-SPA-812, RRID:AB_10013742); guinea pig anti-VGlut2 (Synaptic Systems Cat#135404, RRID:AB_887884); goat anti-GFP (Rockland Cat#600-101-215, RRID:AB_218182); chicken anti-RFP (Rockland Cat#600-901-379S, RRID:AB_10703148). After 5×10-minute PBS washes, sections were incubated at room temperature for 2 h with species-appropriate Alexa Fluoro-488, -568, -647 conjugated secondary antibody (1:500) in blocking buffer, followed by 3×10-minutes PBS washes and DAPI stain before mounting with ProLong Glass Antifade Mountant (Invitrogen P36984).
Images were acquired with either a Leica M165FC fluorescent microscope, or an Echo Revolution microscope and analyzed with ImagePro-10 software. To calculate the immunoreactivity of Iba1 and Hsp70/72, regions of interest with ~1 × 0.25 mm across cortical layers were selected adjacent to the prism lens injury site, and the mirror position in the contralateral hemisphere. Four sections were used for the calculation in each animal. The mean fluorescence intensity on the ipsilateral side of the prism site was normalized to the average mean intensity of the contralateral side of the injury site. Mean intensity is reported as fold change of the prism site compared to the contralateral side.
Cranial window surgery and image acquisition
Cranial window surgery and 2-photon imaging were performed according to previously published procedures14,25,90. Briefly, P6-7 pups were anesthetized by ice-induced hypothermia and given Ethiqa XR® (3.25 mg/kg, sc). Then a head plate was attached, and a piece of skull was removed. For microprism implant, a 1 mm × 1 mm right-angle microprism (Tower Optics) was attached to a 3 mm glass coverslip using NOA63 optical adhesive (Thorlabs). A 1 mm slit in the dura was then made without disturbing the pia, adjacent to the area of the barrel cortex. The prism was then carefully inserted into the brain, with the right-angle prism facing the barrel cortex towards the posterior-lateral direction. Care was taken to avoid large surface blood vessels. Post hoc histology was performed on all prism implants to confirm localization. For cranial window imaging experiments without microprism, a 2 mm or 3 mm glass coverslip was placed over the cranial window and secured. Following recovery from cranial window, pups were given Meloxicam (2 mg/kg, sc) and then returned to the cage with dam. Imaging sessions were performed starting on the following day. During imaging, pups were head-fixed, un-anaesthetized, and remained in quiet resting state. A FluoView FVMPE-RS Multiphoton Imaging System with a 25x (1.05 NA) water immersion lens (Olympus) was used for experiments with microprisms. A Thorlabs Bergamo II Multiphoton Microscope and ThorImageLS software was used for all TeLC and Dreadd experiments. For Emx1:GCaMP6s and AAV1-hsyn-GCaMP6s experiments, imaging was performed at 2-photon excitation of 920 nm and detected with a 495/540 nm filter. For experiments with jRGECO1a, 2-photon excitation of 1040 nm and 561/635 nm filter were used. Laser scanning was delivered using a resonance scanner at the rate of 5 Hz, Image frames were 509 × 509 μm (512 × 512 pixels). Each video consisted of 2000 frames.
Whisker stimulation
Experiments measuring neuronal responses to whisker stimulation were performed as previously described25,31. Briefly,100 ms air-puff stimulation to the contralateral whiskers to the imaging hemisphere was delivered using a picospritzer (Parker Hannifin). The pressure of the air puff was adjusted so that all whiskers on the whisker pad were displaced, but the mouse was not startled and did not display any signs of discomfort. Airpuff was delivered every 300 frames ( ~ 60 s) to minimize adaptation.
In vivo chemogenetic manipulation
Acute chemogenetic dampening of L5 pyramidal neuron activation was performed in Rbp4-Cre:hM4Di mice, at either P7-8, or P14. On imaging day, mice were first injected with saline (volume equal to what was calculated for CNO, i.p.), and baseline calcium activity was recorded 30 minutes after saline injection. Mice were then removed from head fixation, allowed to rest in home cage for 30 minutes, then injected with equal volume of CNO (1 mg/kg). A second imaging session was performed 30 minutes after CNO injection, in a FOV closely matching the baseline imaging after saline injection.
Calcium imaging analysis
Movies were first motion corrected using the Stabilizer plugin for NIH ImageJ91 (http://www.cs.cmu.edu/~kangli/code/Image_Stabilizer.html) to account for small xy displacement. Calcium signals were then processed and analyzed using CalciumDX package92, similar as previously described25. For grid-based analyses in prism experiments, 25 μm × 25 μm ROIs divided by a grid and calcium signal was calculated using the average intensity inside each ROI grid. For columnar analysis, we used a k-means clustering algorithm based on pairwise correlation between region of interests (ROIs)14,26,42. Briefly, pair-wise correlation between ROIs was determined, and clustered them into 1, 2, …7, or 8 clusters (k). The silhouette value, which measures how well points within a cluster matches its own cluster compared to other clusters, was calculated for each k value (i.e. 1 to 8 clusters), and the clustering with the highest silhouette value was selected. We then randomized the location of the ROIs and calculated the highest silhouette values again for k = 1–8. The highest silhouette values for each video were then averaged and compared. For cell-based analyses, image processing and calcium signal detection were performed using the Suite2p package93, available open source in GitHub repository (https://github.com/MouseLand/suite2phttps://github.com/MouseLand/suite2p). Movies were pre-processed using automated motion correction to account for small xy displacement. Automated cell detection was performed, followed by manual curation. ROIs with distinct cell body morphology were added, and non-somatic ROIs were excluded. To remove neuropil contamination (Fneu), the surrounding neuropil signal was subtracted from each somatic ROI signal, scaled by a factor of 0.793. The average calcium fluorescence of each cell was calculated as F = Fraw – 0.7Fneu. Traces from cells were then processed and analyzed as previously described, using a combination of CalciumDX and custom-written analysis routines written in MATLAB (Mathworks)14,25.
Electrophysiology
Brain slices were prepared as previously described94. Briefly, mice were first anesthetized with chloral hydrate (400 mg/kg, i.p.). After decapitation, brains were removed rapidly and placed in ice-cold ( ~ 4°C) artificial cerebrospinal fluid (ACSF) in which sucrose (252 mM) was substituted for NaCl (sucrose-ACSF) to prevent cell swelling. Coronal slices (400 μm) were cut in sucrose-ACSF with an oscillating-blade vibratome (Leica VT1000S). After placing slices in a submerged recording chamber, the bath temperature was raised to 32°C. The standard ACSF (PH ~ 7.35) was equilibrated with 95% O2/5% CO2 and contained (in mM): 128 NaCl, 3 KCl, 2 CaCl2, 2 MgSO4, 24 NaHCO3, 1.25 NaH2PO4, and 10 D-glucose. A recovery period of ~1–2 hr was allowed before commencement of recording.
Pyramidal neurons were visualized by using an Olympus BX50WI microscope (40x IR objective) with infrared differential interference contrast (IR/DIC) video microscopy. A CMOS camera (ORCA-Flash4.0 LT, Hamamatsu) was used to visualize neurons in slice. Low-resistance patch pipette (3-5 MΩ) were pulled from patch-clamp glass (Warner Instrument) using a horizontal micropipette puller (P-1000, Sutter Instrument). The pipettes were filled with the internal solution containing (in mM): 115 K gluconate, 5 KCl, 2 MgCl2, 2 Mg-ATP, 2 Na2ATP, 10 mM Na2-phosphocreatine, 0.4 mM Na2GTP, and 10 mM Hepes, calibrated to pH 7.33. Whole-cell recordings were performed with a Multiclamp 700B amplifier (Axon Instruments). The output signal was low-pass filtered at 3 kHz, amplified x100 and digitized at 15 kHZ and acquired using Clampex 10.5/Digidata 1550 A software. Series resistance, monitored throughout the experiment, was usually between 4-8 MΩ. To minimize series resistance errors, cells were discarded if series resistance rose above 10 MΩ. Liquid junction potential was not corrected.
In experiments measuring evoked EPSCs in L2/3 pyramidal neurons in a barrel column, barrels were visualized with transmitted light95,96. Whole-cell recordings were made from L2/3 pyramidal neurons above the geometric center of the barrel, and a tungsten bipolar stimulator (Harvard apparatus) was placed in a L4 barrel to deliver electric stimulation using an isolated pulse stimulator unit (ISO-Flex A.M.P.I). The stimulator was then carefully moved to L5, directly below the barrel, to deliver stimulation of the same intensity, while maintaining whole-cell recording in the same L2/3 pyramidal neuron. In half of the L2/3 pyramidal neurons recorded, L5 was stimulated first followed by L4 stimulation. A pair of extracellular stimulation was delivered with an inter-stimulus interval of 50 ms every 10 s, and 20 responses were averaged. In experiments recording L4-L2/3 connectivity when TeLC was expressed in L5 pyramidal neurons, evoked EPSCs were first recorded in L2/3 pyramidal neurons in the hemisphere with TeLC expression as indicated by GFP+ somas in L5 and axons in L2/3. Evoked EPSCs were then recorded on the contralateral side in the mirror barrel column on the same brain slice. In half of the experiments, the contralateral side was stimulated and recorded first. Data were analyzed using Clampfit software (Molecular Devices).
Statistical methods
Statistical analyses were performed and graphs were generated using MATLAB algorithms or Graphpad Prism 10 software. Sample size, statistical tests, p-values and significance for each experiment are indicated in the legends. For all data analyses, normality tests were first performed to determine the use of parametric versus non-parametric analyses. Significance was determined at 95% confidence level (p < 0.05). All statistical tests were two-sided. Data were presented as mean ± SEM. Box plots whiskers indicate the minimum and maximum values. Bounds of the box are the 1st and 3rd quartile and the center line indicates the median.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All numerical source data supporting the discoveries are available as a Source Data file provided with the article. Raw data from electrophysiology, immunohistochemistry and imaging experiments are too large to deposit; however, data are available upon request for all readers without any restriction. Source data are provided with this paper.
Code availability
Codes used to perform calcium imaging analysis, Suite2P and CalciumDX, have been previously published and are publicly available on GitHub (https://github.com/MouseLand/suite2p; https://github.com/ackman678/CalciumDX).
References
Woolsey, T. A. & der Loos, H. V. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 17, 205–242 (1970).
Woolsey, T. A. & Wann, J. R. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. J. Comp. Neurol. 170, 53–66 (1976).
Fox, K. Barrel Cortex. https://doi.org/10.1017/cbo9780511541636 (Cambridge University Press, 2008).
Li, H. et al. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron 79, 970–986 (2013).
Dooley, J. C., Sokoloff, G. & Blumberg, M. S. Movements during sleep reveal the developmental emergence of a cerebellar-dependent internal model in motor thalamus. Curr. Biol. 31, 5501–5511.e5 (2021).
Fox, K., Schlaggar, B. L., Glazewski, S. & O’Leary, D. D. Glutamate receptor blockade at cortical synapses disrupts development of thalamocortical and columnar organization in somatosensory cortex. Proc. Natl Acad. Sci. 93, 5584–5589 (1996).
Antón-Bolaños, N. et al. Prenatal activity from thalamic neurons governs the emergence of functional cortical maps in mice. Science 364, eaav7617 (2019).
Minlebaev, M., Colonnese, M., Tsintsadze, T., Sirota, A. & Khazipov, R. Early gamma oscillations synchronize developing thalamus and cortex. Science 334, 226–229 (2011).
Yang, J. W. et al. Thalamic network oscillations synchronize ontogenetic columns in the newborn rat barrel cortex. Cereb. Cortex 23, 1299–1316 (2013).
Minlebaev, M., Ben-Ari, Y. & Khazipov, R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J. Neurophysiol. 97, 692–700 (2007).
Allene, C. et al. Sequential Generation of two distinct synapse-driven network patterns in developing neocortex. J. Neurosci. 28, 12851–12863 (2008).
Golshani, P. et al. Internally mediated developmental desynchronization of neocortical network activity. J. Neurosci. 29, 10890–10899 (2009).
Kirmse, K. et al. GABA depolarizes immature neurons and inhibits network activity in the neonatal neocortex in vivo. Nat. Commun. 6, 7750 (2015).
Duan, Z. R. S. et al. GABAergic restriction of network dynamics regulates interneuron survival in the developing cortex. Neuron 105, 75–92.e5 (2020).
Fox, K. et al. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J. Neurosci. https://doi.org/10.1523/jneurosci.12-05-01826.1992 (1992).
Lendvai, B., Stern, E. A., Chen, B. & Svoboda, K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881 (2000).
Maravall, M., Stern, E. A. & Svoboda, K. Development of intrinsic properties and excitability of layer 2/3 pyramidal neurons during a critical period for sensory maps in rat barrel cortex. J. Neurophysiol. 92, 144–156 (2004).
Bender, V. A., Bender, K. J., Brasier, D. J. & Feldman, D. E. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J. Neurosci. 26, 4166–4177 (2006).
Clem, R. L. & Barth, A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron 49, 663–670 (2006).
Benedetti, B. L., Glazewski, S. & Barth, A. L. Reliable and precise neuronal firing during sensory plasticity in superficial layers of primary somatosensory cortex. J. Neurosci. 29, 11817–11827 (2009).
Wen, J. A. & Barth, A. L. Input-specific critical periods for experience-dependent plasticity in layer 2/3 pyramidal neurons. J. Neurosci. 31, 4456–4465 (2011).
Blankenship, A. G. & Feller, M. B. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Publ. Group 11, 18–29 (2009).
Martini, F. J., Guillamón-Vivancos, T., Moreno-Juan, V., Valdeolmillos, M. & López-Bendito, G. Spontaneous activity in developing thalamic and cortical sensory networks. Neuron 109, 2519–2534 (2021).
Burbridge, T. J. et al. Visual circuit development requires patterned activity mediated by retinal acetylcholine receptors. Neuron 84, 1049–1064 (2014).
Che, A. et al. Layer I interneurons sharpen sensory maps during neonatal development. Neuron 99, 98–116.e7 (2018).
Modol, L. et al. Assemblies of perisomatic GABAergic neurons in the developing barrel cortex. Neuron 105, 93–105.e4 (2020).
Easton, C. R. et al. Distinct calcium signals in developing cortical interneurons persist despite disorganization of cortex by Tbr1 KO. Dev. Neurobiol. https://doi.org/10.1002/dneu.22354 (2015).
Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S. & Kriegstein, A. R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001).
Yu, Y.-C., Bultje, R. S., Wang, X. & Shi, S.-H. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458, 501–504 (2009).
Yang, J.-W., Hanganu-Opatz, I. L., Sun, J.-J. & Luhmann, H. J. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J. Neurosci. 29, 9011–9025 (2009).
Babij, R. et al. Gabrb3 is required for the functional integration of pyramidal neuron subtypes in the somatosensory cortex. Neuron https://doi.org/10.1016/j.neuron.2022.10.037 (2022).
Stern, E. A., Maravall, M. & Svoboda, K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex In Vivo. Neuron 31, 305–315 (2001).
Bender, K. J., Rangel, J. & Feldman, D. E. Development of columnar topography in the excitatory layer 4 to layer 2/3 projection in rat barrel cortex. J. Neurosci. 23, 8759–8770 (2003).
Zhao, C., Kao, J. P. Y. & Kanold, P. O. Functional excitatory microcircuits in neonatal cortex connect thalamus and layer 4. J. Neurosci. 29, 15479–15488 (2009).
Tolner, E. A., Sheikh, A., Yukin, A. Y., Kaila, K. & Kanold, P. O. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J. Neurosci. 32, 692–702 (2012).
Viswanathan, S., Sheikh, A., Looger, L. L. & Kanold, P. O. Molecularly defined subplate neurons project both to thalamocortical recipient layers and thalamus. Cereb. Cortex 27, 4759–4768 (2016).
Yuste, R., Nelson, D. A., Rubin, W. W. & Katz, L. C. Neuronal domains in developing neocortex: mechanisms of coactivation. Neuron 14, 7–17 (1995).
Adelsberger, H., Garaschuk, O. & Konnerth, A. Cortical calcium waves in resting newborn mice. Nat. Neurosci. 8, 988–990 (2005).
Andermann, M. L. et al. Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron 80, 900–913 (2013).
Moda-Sava, R. N. et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364, eaat8078 (2019).
Spellman, T., Svei, M., Kaminsky, J., Manzano-Nieves, G. & Liston, C. Prefrontal deep projection neurons enable cognitive flexibility via persistent feedback monitoring. Cell 184, 2750–2766.e17 (2021).
Malvache, A., Reichinnek, S., Villette, V., Haimerl, C. & Cossart, R. Awake hippocampal reactivations project onto orthogonal neuronal assemblies. Science 353, 1280–1283 (2016).
Sun, J.-J. & Luhmann, H. J. Spatio-temporal dynamics of oscillatory network activity in the neonatal mouse cerebral cortex. Eur. J. Neurosci. 26, 1995–2004 (2007).
Elias, L. A. B., Wang, D. D. & Kriegstein, A. R. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448, 901–907 (2007).
Mizuno, H. et al. Patchwork-type spontaneous activity in neonatal barrel cortex layer 4 transmitted via thalamocortical projections. Cell Rep. 22, 123–135 (2018).
Dooley, J. C., Glanz, R. M., Sokoloff, G. & Blumberg, M. S. Self-generated whisker movements drive state-dependent sensory input to developing barrel cortex. Curr. Biol. 30, 2404–2410.e4 (2020).
Yuste, R., Peinado, A. & Katz, L. C. Neuronal domains in developing neocortex. Science 257, 665–669 (1992).
Peinado, A., Yuste, R. & Katz, L. C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron 10, 103–114 (1993).
Yu, Y.-C. et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature 486, 113–117 (2012).
Gutman-Wei, A. Y. & Brown, S. P. Mechanisms underlying target selectivity for cell types and subcellular domains in developing neocortical circuits. Front Neural Circuit 15, 728832 (2021).
Tran-Van-Minh, A., Ye, Z. & Rancz, E. Quantitative analysis of rabies virus-based synaptic connectivity tracing. Plos One 18, e0278053 (2023).
Agmon, A., Yang, L. & Jones, E. & O’Dowd, D. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J. Neurosci. 15, 549–561 (1995).
Price, D. J., Aslam, S., Tasker, L. & Gillies, K. Fates of the earliest generated cells in the developing murine neocortex. J. Comp. Neurol. 377, 414–422 (1997).
Luhmann, H. Subplate cells: amplifiers of neuronal activity in the developing cerebral cortex. Front. Neuroanat. 3, https://doi.org/10.3389/neuro.05.019.2009 (2009).
Armstrong-James, M., Fox, K. & Das-Gupta, A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J. Neurophysiol. 68, 1345–1358 (1992).
Petersen, C. C. H. & Sakmann, B. Functionally independent columns of rat somatosensory barrel cortex revealed with voltage-sensitive dye imaging. J. Neurosci. 21, 8435–8446 (2001).
Petersen, C. C. H., Grinvald, A. & Sakmann, B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J. Neurosci. 23, 1298–1309 (2003).
Thomson, A. M. & Bannister, A. P. Postsynaptic pyramidal target selection by descending layer III pyramidal axons: dual intracellular recordings and biocytin filling in slices of rat neocortex. Neuroscience 84, 669–683 (1998).
Lefort, S., Tomm, C., Sarria, J. C. F. & Petersen, C. C. H. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316 (2009).
Jiang, X. et al. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350, aac9462–aac9462 (2015).
DeNardo, L. A., Berns, D. S., DeLoach, K. & Luo, L. Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat. Neurosci. 18, 1687–1697 (2015).
Graybuck, L. T. et al. Enhancer viruses for combinatorial cell-subclass-specific labeling. Neuron 109, 1449-1464 (2021).
Krone, L. B. et al. A role for the cortex in sleep–wake regulation. Nat. Neurosci. 24, 1210–1215 (2021).
Zhu, H. et al. Cre‐dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. genesis 54, 439–446 (2016).
Carroll, B. J., Sampathkumar, V., Kasthuri, N. & Sherman, S. M. Layer 5 of cortex innervates the thalamic reticular nucleus in mice. Proc. Natl Acad. Sci. 119, e2205209119 (2022).
Dantzker, J. L. & Callaway, E. M. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat. Neurosci. 3, 701–707 (2000).
Jiang, X., Wang, G., Lee, A. J., Stornetta, R. L. & Zhu, J. J. The organization of two new cortical interneuronal circuits. Nat. Publ. Group 16, 210–218 (2013).
Vecchia, D. et al. Temporal sharpening of sensory responses by layer V in the mouse primary somatosensory cortex. Curr. Biol. 30, 1589–1599.e10 (2020).
Bitzenhofer, S. H., Pöpplau, J. A., Chini, M., Marquardt, A. & Hanganu-Opatz, I. L. A transient developmental increase in prefrontal activity alters network maturation and causes cognitive dysfunction in adult mice. Neuron 109, 1350–1364.e6 (2021).
Murray, A. J. et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat. Neurosci. 14, 297–299 (2011).
García, N. V. D. M., Priya, R., Tuncdemir, S. N., Fishell, G. & Karayannis, T. Sensory inputs control the integration of neurogliaform interneurons into cortical circuits. Nat. Neurosci. 18, 393–401 (2015).
Zhang, Y. et al. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60, 84–96 (2008).
Hage, T. A. et al. Synaptic connectivity to L2/3 of primary visual cortex measured by two-photon optogenetic stimulation. Elife 11, e71103 (2022).
Constantinople, C. M. & Bruno, R. M. Deep cortical layers are activated directly by thalamus. Science 340, 1591–1594 (2013).
Tuncdemir, S. N. et al. Early somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron 89, 521–535 (2016).
Marques-Smith, A. et al. A transient translaminar GABAergic interneuron circuit connects thalamocortical recipient layers in neonatal somatosensory cortex. Neuron 89, 536–549 (2016).
Molnár, Z., Luhmann, H. J. & Kanold, P. O. Transient cortical circuits match spontaneous and sensory-driven activity during development. Science 370, eabb2153 (2020).
Nakazawa, S., Yoshimura, Y., Takagi, M., Mizuno, H. & Iwasato, T. Developmental phase transitions in spatial organization of spontaneous activity in postnatal barrel cortex layer 4. J. Neurosci. 40, 7637–7650 (2020).
Xu, X. et al. Primary visual cortex shows laminar‐specific and balanced circuit organization of excitatory and inhibitory synaptic connectivity. J. Physiol. 594, 1891–1910 (2016).
Yang, J.-W., Reyes-Puerta, V., Kilb, W. & Luhmann, H. J. Spindle bursts in neonatal rat cerebral cortex. Neural Plast. 2016, 3467832 (2016).
Siegel, F., Heimel, J. A., Peters, J. & Lohmann, C. Peripheral and central inputs shape network dynamics in the developing visual cortex in vivo. Curr. Biol. 22, 253–258 (2012).
Conhaim, J. et al. Developmental changes in propagation patterns and transmitter dependence of waves of spontaneous activity in the mouse cerebral cortex. J. Physiol. 589, 2529–2541 (2011).
Easton, C. R. et al. Genetic elimination of GABAergic neurotransmission reveals two distinct pacemakers for spontaneous waves of activity in the developing mouse cortex. J. Neurosci. 34, 3854–3863 (2014).
Cai, L., Argunşah, A. Ö., Damilou, A. & Karayannis, T. A nasal chemosensation–dependent critical window for somatosensory development. Science 384, 652–660 (2024).
Reyes, N. S. L. et al. Transient callosal projections of L4 neurons are eliminated for the acquisition of local connectivity. Nat. Commun. 10, 4549 (2019).
Ibrahim, L. A. et al. Bottom-up inputs are required for establishment of top-down connectivity onto cortical layer 1 neurogliaform cells. Neuron 109, 3473–3485.e5 (2021).
Iannone, A. F. et al. The chemokine Cxcl14 regulates interneuron differentiation in layer I of the somatosensory cortex. Cell Rep. 43, 114531 (2024).
Munz, M. et al. Pyramidal neurons form active, transient, multilayered circuits perturbed by autism-associated mutations at the inception of neocortex. Cell 186, 1930–1949 (2023).
Vue, T. Y. et al. Thalamic Control of Neocortical Area Formation in Mice. J. Neurosci. 33, 8442–8453 (2013).
Che, A. & García, N. V. D. M. An in vivo calcium imaging approach for the identification of cell-type specific patterns in the developing cortex. Front Neural Circuit 15, 747724 (2021).
K. Li, “The image stabilizer plugin for ImageJ,” http://www.cs.cmu.edu/~kangli/code/Image_Stabilizer.html (2008).
Ackman, J. B., Burbridge, T. J. & Crair, M. C. Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490, 219–225 (2012).
Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at bioRxiv https://doi.org/10.1101/061507 (2017).
Liu, R.-J. & Aghajanian, G. K. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl Acad. Sci. 105, 359–364 (2008).
Allen, C. B., Celikel, T. & Feldman, D. E. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 6, 291–299 (2003).
Bender, K. J., Allen, C. B., Bender, V. A. & Feldman, D. E. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J. Neurosci. 26, 4155–4165 (2006).
Acknowledgements
We would like to thank Dr. Robert Fetcho for helping with MATLAB scripts. This work was supported by NINDS Grant 1K99-4R00NS114166 (to A.C.); 1R01NS133434 (to A.C.); 1R01DA0593578 (to A.C.), BBRF Young Investigator Award (to A.C.); 2R01MH110553 (to N.V.D.G.), 1R01NS116137 (to N.V.D.G), 1R01MH125006 (to N.V.D.G.), Irma Hirschl/Monique Weill-Caulier Career Scientist (to N.V.D.G.), 5F30MH117939 (to R.B.); NIGMS Grant T32GM007739 to Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program (To Z.S.D and R.B). This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors.
Author information
Authors and Affiliations
Contributions
A.C. and N.V.D.M.G designed the microprism imaging experiments and rabies tracing experiments. A.C. designed the electrophysiology experiments and layer-specific silencing imaging experiments. A.C. and J.V. performed and analyzed microprism imaging experiments. A.C., L.L. and S.K. analyzed microprism imaging data. A.C., R.B., Z.S.D. and S.W. performed the rabies tracing experiments. A.C., J.V., A.W., J. L.S., L.L., R.B. and Z.S.D. analyzed rabies tracing data. J.V., V.K.M. and L.L. performed layer-specific silencing imaging experiments. V.K.M., A.C. and L.L. analyzed data from these experiments. J.V. and V.K.M. performed validation immunohistochemistry experiments. R.L. and L.L. performed and analyzed all electrophysiology experiments, except the optogenetic experiment, which was performed and analyzed by L.S.. A.C. and N.V.D.M.G. secured funding and supervised the research. A.C. wrote the manuscript with the help of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Daniele Linaro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vargas-Ortiz, J., Lin, L., Martinez, V.K. et al. Translaminar synchronous neuronal activity is required for columnar synaptic strengthening in the mouse neocortex. Nat Commun 16, 1296 (2025). https://doi.org/10.1038/s41467-024-55783-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55783-w