Abstract
Temporomandibular disorders (TMD) intelligent diagnosis promises to elevate clinical efficiency and facilitate timely TMD management for patients. However, development of TMD intelligent diagnostic tools with high accuracy and sensitivity presents challenges, particularly in sensing minute deformations and ensuring rapid self-recovery. Here we report a biocompatible hydrogel electronic sensor with instantaneous self-recovery (within 2.1 s) and ultra-low detection limit (0.005% strain). It could efficiently diagnose disc displacement with reduction (DDwR) with satisfactory accuracy of 90.00%, and also had a clear indication of the typical clinical manifestations of DDwR and the timing of temporomandibular joint (TMJ) clicking, with a sensitivity of up to 100% in human compared to the diagnostic criteria for TMD (DC/TMD). Furthermore, a predictive model based on waveform features achieved 84.4% accuracy and 86% sensitivity, reducing dependence on physicians. In summary, the hydrogel sensor is expected to become a radiation-free, non-invasive, practical and effective tool for future TMD diagnosis.
Similar content being viewed by others
Introduction
Temporomandibular disorders (TMD) are the most common diseases occurring in the temporomandibular joint (TMJ), with a prevalence of 5%–12%1, and it affects up to 31% of young individuals2,3, resulting in annual costs exceeding $4 billion4,5. In the early stages, TMD symptoms are relatively mild or even asymptomatic, mainly manifested as joint clicking, which is often neglected6. As the disease progresses, it can lead to serious damage, including perforation of the articular disks, severe dysfunction of the orofacial system (such as facial asymmetry, abnormal jaw movements, and restricted mouth opening affecting feeding), and pain, which is the second most common cause of maxillofacial pain after dental pain7. These significantly impact patients’ physical and mental health, as well as their quality of life. Additionally, TMD poses challenges for dentists, as it can interfere with the treatment of other conditions, such as orthodontics8, implants, and prosthetics9. Unfortunately, most people have difficulty recognizing TMD in a timely manner and managing it appropriately. Therefore, there is a pressing need for TMD diagnosis. Typically, TMD diagnosis relies on patients' symptoms, clinical examination, cone beam computed tomography (CBCT), magnetic resonance imaging (MRI), and arthrography10. It is widely known that TMJ specialists are in short supply globally, only available in a handful of major hospitals11 (Supplementary Fig. 1). CBCT involves radiation, MRI exposes patients to a claustrophobic environment, and arthrography is an invasive procedure. More importantly, these monitoring tools are still dependent on TMJ physicians for the adjudication of results. These limitations hinder TMD diagnosis and treatment. To address this challenge, new diagnostic tools for TMJ diseases have been developed, including surface electromyography (sEMG)12, vibroarthrography (VAG)13,14, and many others (Supplementary Table 1). However, these new tools often struggle to meet clinical requirements due to poor validity15, complex operating procedures16, and stringent testing environments14. Therefore, there is an urgent need for the development of radiation-free, non-invasive, expedited, and effective methods to aid TMD diagnosis.
TMJ, one of the most complex joints in the body17, consists of the condyle, articular fossa, articular node, and fibrocartilaginous disc between them. It is the only diarthrodial joint in the human head18 and plays a crucial role in activities such as chewing and speaking. TMJs function through the rotation and translation of the condyles within the articular fossa, subjecting them to compression, shear, and tension7,19. These actions manifest as deformation of the facial skin overlying the preauricular area, which corresponds to the condyles’ location. Some studies have successfully predicted condylar movement envelope surface based on facial morphology20, indicating that monitoring facial skin deformation at the condyle’s position can reflect the TMJ’s functional status. In addition, in the clinical examination of TMD, manual palpation is the most common and basic method21. During this procedure, the physicians place their hands over the preauricular region while the patient performs movements such as mouth opening, closing, lateral movement, protrusion, and retrusion, so that the state of TMJ can be felt comprehensively. Inspired by clinical joint palpation, we wondered whether a sensor that can indicate TMD by monitoring facial skin deformation could be developed, thus simulating physician palpation. Hydrogel strain sensors stand as an attractive alternative due to their similarity with biological tissue, including three-dimensional (3D) network structure, and adjustable elastic modulus22. However, although a large amount of hydrogel strain sensors has been developed to monitor human physiological data23,24,25,26,27, including joint activities28, none of them have been used for diagnosis of TMD due to the fact that the intricate and highly dynamic TMJ poses higher demands on the sensor’s minimal detection limits and rapid self-recovery capabilities. This study aims to develop and validate a biocompatible hydrogel electronic sensor specifically for diagnosing disc displacement with reduction (DDwR).
Previously, we designed and developed a series of supramolecular hydrogels with good self-recovery properties29,30, biocompatibility31, and adhesion properties32 based on dynamic boron ester bonds. Inspired by these previous endeavors, in this study, according to the 3D analysis of facial morphology during TMJ movements, a joint palpation-inspired diagnostic tool based on hydrogel electronic sensor (named PVA-EBPD hydrogel sensor) with ultra-low detection limit and instantaneous self-recovery capability was successfully developed (Fig. 1), by the strategy of dynamic covalent bonding, which performs satisfactory biocompatibility, favorable skin adhesion properties and high accuracy and sensitivity for DDwR diagnosis. The PVA-EBPD hydrogel, featuring multiple o-diol structures and multiple boron ester bonds, is synthesized from polyvinyl alcohol (PVA) and (E)-4,4′-(1,2-ethenediyl) bis (1,2-phenylene diol) (EBPD) through a green and simple procedure in water, which contributes to its promising biocompatibility in vivo and in vitro. The presence of multiple boron ester bonds enables it to achieve favorable skin adhesion properties, instantaneous self-recovery within 2.1 s and rapid response to strain changes within 0.12 s. In addition, the ultra-low detection limit (0.005% strain) and the high gauge factor (GF) of up to 3.098 within a strain range of 0–40% make the PVA-EBPD hydrogel sensor suitable for monitoring limb joint activities such as walking, turning, and lying down, as well as finer activities of the TMJ such as tapping and chewing in test animals like pigs. Moreover, the study with human research participants revealed that the PVA-EBPD hydrogel sensor can sensitively and accurately indicate DDwR (sensitivity up to 100%, accuracy up to 90.00% compared to diagnostic criteria for TMD (DC/TMD)) (Supplementary Table 1).By building predictive models with an accuracy of up to 84.40%, this hydrogel sensor facilitates intelligent assisted diagnosis, highlighting its potential for TMD diagnosis in clinical settings.
Results and discussion
3D analysis of facial morphology on the condylar region during TMJ movements
To precisely quantify the skin deformation at the corresponding position of the condyle during TMJ movement, we collected photographs of the preauricular area (body surface projection position of TMJ) from 18 volunteers during mouth opening and closing, lateral movements to left and right, as well as protrusion and retrusion movements (TMJ palpation related movements). Initially, the photographs were reconstructed into 3D photographs, followed by a comparative analysis of the deformation parameters in the preauricular region of the face reflecting TMJ movements during various actions (Fig. 2a and Supplementary Fig. 2). Then, the deformation in different directions was compared. As shown in Supplementary Fig. 2, Direction 3 yields the largest deformation, potentially allowing us to gather more relevant signals. In addition, Supplementary Fig. 2b illustrates that in the area around the condyle, other directions tend to be interfered with by hair and beard, and the red dashed box is optimal. Further analysis in this region on Direction 3 was performed. As shown in Supplementary Table 2 and Fig. 2b, c, the movements of the TMJ can be reflected in the deformation of the facial skin at the corresponding position of the condyle, with different TMJ activities exhibiting distinct deformation patterns and parameters. Among these, the most pronounced deformation occurs during mouth opening, with a maximum facial skin depression of 3.26 mm and an average depression of 1.62 mm. This is due to the condyle rotating slightly forward and then sliding along the posterior oblique surface of the articular eminence to below the articular tuberosity, resulting in the hollowing of the joint fossa33. During the lateral movement to the left, the maximum change of the left TMJ is 1.34 mm, with an average of 0.32 mm, while the maximum depression of the right TMJ is 1.96 mm, with an average of 0.55 mm. Similar, albeit opposite, results are observed during lateral movement to the right. This occurs because, during left lateral movement, the condyle of the left TMJ rotates laterally, while the right TMJ slides anteriorly, medially, and inferiorly34, leading to prominence on the left side and depression on the right side. The reverse is true for movements in the opposite direction. In protrusion movements, as the condyle slides forward with the disc below the tuberosity, TMJ exhibits a maximum depression of 1.55 mm and an average depression of 0.38 mm. Therefore, TMJ movements can reflect changes in facial morphology, indicating the potential to detect TMJ disorders by monitoring facial deformations. However, as indicated by the above results, the average deformation caused by TMJ activity ranges only from 0.31 mm to 1.62 mm, highlighting the need for highly sensitive sensors to monitor such small deformations.
a 3D reconstructed images of volunteer TMJ while performing mouth opening and closing, lateral movements to the left and right, and protrusion and retrusion movements. b Deformation analysis on the condylar region after overlaying the 3D images during various TMJ movements with those during closing mouth (red indicating depression, blue indicating prominent). c Maximum morphometric variables of comparative analysis regions from 18 volunteers (All data are Mean ± SEM). Source data are provided as a Source Data file.
PVA-EBPD hydrogel crosslinked by multiple boron ester bonds with instantaneous self-recovery properties
The PVA-EBPD hydrogel, crosslinked by multiple boron ester bonds, exhibits instantaneous self-recovery properties. It is prepared through a straightforward one-pot synthesis method involving PVA, EBPD, and NaB(OH)4 in ultrapure water (Fig. 3a). Initially, 6 mg of EBPD powder is dissolved in a 0.25 M NaB(OH)4 solution (200 µL) and ultrasonicated to obtain a clarified EBPD solution. This solution is then thoroughly mixed with a PVA aqueous solution (0.15 g/mL, 1 mL) to form the PVA-EBPD hydrogel. The chemical raw materials and products involved in the synthesis process are harmless, and the synthesis process does not involve heating, aligning with the principles of green chemistry35. Although a classical PVA hydrogel can be obtained by directly mixing PVA with a NaB(OH)4 solution (Supplementary Fig. 3), the inverting-vial test reveals that the PVA-EBPD hydrogel demonstrates more satisfactory stability compared to the PVA hydrogel, without collapsing up to 20 days. This enhanced stability is attributed to the formation of various types of boron ester bonds in the PVA-EBPD hydrogel system (Fig. 3b). The results of 11B nuclear magnetic resonance (NMR) spectroscopy confirm this conclusion. As shown in Fig. 3c, compared to the signal peak of the NaB(OH)4 solution at 3.06 ppm, the peak of the EBPD solution shifted to 7.96 ppm, indicating the formation of boron ester bonds between EBPD molecules (EBPD-EBPD). In addition, the PVA hydrogel exhibited a broad peak at –4.76 ppm and a sharp peak at 19.59 ppm, suggesting the formation of boron ester bonds between PVA with PVA (PVA-PVA). Notably, three additional peaks appeared in the PVA-EBPD hydrogel system compared to the PVA hydrogel, which was attributed to the formation of boron ester bonds between EBPD molecules (7.35 ppm), EBPD and PVA (1.13 ppm), and PVA and EBPD-EBPD (13.97 ppm). In summary, the multiple peaks in the PVA-EBPD hydrogel imply the presence of multiple boron ester bonds. Such conclusion is further strengthened by Fourier transform infrared (FT-IR) spectra (Fig. 3d). The FT-IR spectrum of the dried PVA-EBPD hydrogel, PVA hydrogel, and EBPD solution exhibits a new absorption band at around 1100 cm–1, corresponding to the B–O–C bond stretching frequency29. However, the displacement of this absorption band differs slightly between the PVA-EBPD hydrogel, PVA hydrogel, and EBPD solution, with the PVA-EBPD hydrogel at 1107 cm⁻1 and the EBPD solution at 1117 cm⁻1. This discrepancy may be attributed to the different molecules connected by boron ester bonds. Additionally, the C=C bending vibration displacement of the PVA-EBPD hydrogel and EBPD solution is at 1507 cm⁻1 compared to the EBPD powder at 1523 cm⁻1, which may be due to the impact of the boron ester bond formation between EBPD molecules on their bending vibration, whereas it was not observed in the PVA hydrogel. Then, to visualize the microscopic morphology of the PVA-EBPD hydrogel, scanning electron microscopy (SEM) is conducted. Figure 3e illustrates that the lyophilized PVA-EBPD hydrogel presents a loose honeycomb-like structure with porous holes that may be more conducive to ion migration. In contrast, the PVA hydrogel exhibits a structure resembling bone trabeculae, and the freeze-dried PVA solution behaved more densely in its microstructure due to the absence of a hydrogel structure.
a Schematic diagram of the preparation process of PVA-EBPD hydrogel. b Schematic diagram of the reaction process of PVA-EBPD hydrogel. c 11B NMR spectra of PVA-EBPD hydrogel, PVA hydrogel, EBPD solution, and NaB(OH)4 solution in D2O. d FT-IR spectra of lyophilized PVA-EBPD hydrogel, PVA hydrogel, EBPD solution, PVA solution, and NaB(OH)4 solution. e SEM image of lyophilized PVA-EBPD hydrogel, PVA hydrogel, and PVA solution (2 samples per group). f Formability of PVA-EBPD hydrogel. g Strain sweep test of PVA-EBPD hydrogel and PVA hydrogel at a constant angular frequency rate of 10 rad/s. h Frequency sweep of PVA-EBPD hydrogel and PVA hydrogel at a constant strain of 1%. i Schematic diagram of the self-recovery property of PVA-EBPD hydrogel. j, k Self-recovery properties of PVA-EBPD hydrogel (j) and PVA hydrogel (k) by dynamic step-strain sweep experiments at a constant angular frequency of 10 rad s−1 in the oscillating mode of the rheometer. l Viscosity and recovery time of PVA-EBPD hydrogel in the rotational mode of the rheometer. m Cyclic stability testing of PVA-EBPD hydrogel at the shear rate of 11/s. Source data are provided as a Source Data file.
After characterizing the cross-linking mechanism of PVA-EBPD hydrogel, we proceeded to investigate its fundamental properties. Firstly, as depicted in Fig. 3f, it is evident that PVA-EBPD hydrogel possesses favorable formability, allowing it to be shaped into various forms, such as circles, triangles, rectangles, hearts, pentagrams, dumbbells, and stars. To assess the mechanical strength of the PVA-EBPD hydrogel, we conducted rheological measurements. The stress sweep tests (Fig. 3g) revealed that the storage moduli (G′, measurement of elastic properties) consistently exceeded the loss moduli (G′′, measurement of viscous properties) across a range of strain variations from 0.1% to 100%, indicating the formation of a viscoelastic PVA-EBPD hydrogel with the capacity to withstand strains of less than 100%. Furthermore, the values of G′ and G″ remained stable within the strain range of 0.1%–10%, signifying the linear viscoelastic region (LVR) of the PVA-EBPD hydrogel. Consequently, a 1% strain was selected for subsequent frequency sweep experiments to avoid perturbing the hydrogel network. Frequency sweep results (Fig. 3h) showed that the PVA-EBPD hydrogel behaves as a fluid at low frequencies (G″ > G′), and a solid-like behavior (G′ > G″) at higher sweep frequencies (>1 Hz), which is likely due to both chemical cross-linking through dynamic covalent bonds and physical cross-linking of PVA long chains within the system36. PVA hydrogel displayed similar rheological properties to PVA-EBPD hydrogel, albeit with a slightly higher modulus. The energy storage modulus and loss modulus of the PVA-EBPD hydrogel are between 103 and 104 Pa at a sweep frequency of 1 Hz, which is in general agreement with that of soft tissues37, as well as with that of porcine skin and rabbit skin (Supplementary Fig. 4). This indicates that the PVA-EBPD hydrogel has the skin tissue-like flexibility, and it can match well with the skin in terms of mechanical properties.
Given the high mobility of joints, materials for joint monitoring should possess promising self-recovery properties, ensuring adaptability to joint movements and rapid recovery following changes in strain to avoid disrupting the detection of subsequent movements. Fortunately, the presence of multiple dynamic boron ester bonds imparts notable self-recovery properties to PVA-EBPD hydrogel (Fig. 3i). PVA-EBPD hydrogel withstood stretching of over 1 meter without breaking, in contrast to PVA hydrogel, which readily fractured (Supplementary Fig. 5). Additionally, Fig. 3j illustrates that the PVA-EBPD hydrogel remained in a gel state under an initial strain (γ) of 1% (within LVR). When the strain was increased from 1% to 300% at ω = 10 rad s–1, G′ became lower than G″, signifying that the gel was disrupted. Impressively, the PVA-EBPD hydrogel promptly recovered to a solid state (G′ > G″), with its modulus completely restored to the initial level when the strain reverted to 1%. In contrast, PVA hydrogel, while capable of self-recovery, required 69 s for its modulus to stabilize after being damaged at 300% strain, and the final modulus only reached 49% of the initial modulus (Fig. 3k), highlighting the superior self-recovery performance of PVA-EBPD hydrogel. Results in Supplementary Fig. 6 further substantiate this conclusion. While both PVA-EBPD hydrogel and PVA hydrogel specimens could self-heal after physical contact (Supplementary Fig. 6a), PVA-EBPD hydrogel achieved scarless healing within 1 min (Supplementary Fig. 6b), with no observable healing traces under a microscope, whereas healed cracks were still visible in PVA hydrogel at 3 min (Supplementary Fig. 6c). In addition, during TMJ activity monitoring, the PVA-EBPD hydrogel may not experience complete fragmentation but rather undergo some perturbations. Therefore, we evaluated its ability to recover quickly after perturbations through rheological measurements in rotational mode. As shown in Fig. 3l, the viscosity of the PVA-EBPD hydrogel could reach over 106 at a shear rate of 0.25 1/s. When the shear rate increased to 0.5 1/s, the viscosity slightly decreased, but when the shear rate returned to 0.25 1/s, the viscosity quickly reverted to its initial state within 2.1 s. As the applied shear rate gradually increased to 1 1/s, 5 1/s, and 10 1/s, the corresponding recovery times also increased gradually to 3.4 s, 5.5 s, and 6.7 s, respectively. Nonetheless, the PVA-EBPD hydrogel consistently restored its initial viscosity within a few seconds. Its self-recovery time is much faster than other self-recovery hydrogels (Supplementary Table 3). Furthermore, the PVA-EBPD hydrogel demonstrated instantaneous and complete recovery for at least 16 cycles (Fig. 3m). These findings indicate that the PVA-EBPD hydrogel possesses instantaneous and repeatable self-recovery ability, making it ideal for continuous monitoring the multi-activity TMJs subjected to compressive, shear, and tensile loads7.
Ultra-low detection limit of PVA-EBPD hydrogel
Based on the 3D analysis above, it is clear that the movement of the TMJ condyle in the articular fossa can be reflected as a change in facial morphology over the preauricular region20. However, TMJ is a very delicate and precise joint, distinct from the large joints in the limbs of the body, the strain is altered only by a few millimeters during TMJ activity38. This requires that the sensor must be sensitive enough to minute strains to capture differences between normal and abnormal TMJ activity. Therefore, we explored the strain-sensing properties of the PVA-EBPD hydrogel sensor. Initially, the PVA-EBPD hydrogel was connected to a simple series circuit (Supplementary Fig. 7), and the light bulb noticeably dimmed with an increase in strain applied to the PVA-EBPD hydrogel, indicating that the resistance of the PVA-EBPD hydrogel is significantly affected by deformation (Fig. 4a and Supplementary Movie 1). Typically, the quantitative response to deformation is investigated through the relative resistance change (∆R/R0) signal39. The gauge factor (GF), which is defined as the slope of the (ΔR/R0) versus applied strain, is an indispensable parameter for investigating the sensitivity of strain sensors40,41. As shown in Supplementary Fig. 8, the tensile fracture strain for rabbit skin is around 160%, while pig skin is less than 90%, and human epidermis has been reported to experience a strain variation in the range of 0–70%42. In contrast, for the fine and delicate joint of the TMJ, the strain range is even smaller. Therefore, the response of the PVA-EBPD hydrogel to small strains is of greater interest. The GF of the PVA-EBPD hydrogel consisted of three distinct stages (3.098, 5.133, and 8.294), representing its sensitivity at low strain level (0–40%), moderate strain level (40%–70%), and high strain level (70%–100%), respectively (Fig. 4b). Under small strains (0–100%), the sensitivity of the PVA-EBPD hydrogel sensor is higher than that of other ion-conductive hydrogel sensors reported in the literature (Supplementary Fig. 9), highlighting the high sensitivity of the PVA-EBPD hydrogel strain sensor. Furthermore, the PVA-EBPD hydrogel demonstrated rapid response and recovery characteristics, both occuring within 0.12 s (Fig. 4c). These attributes make it highly suitable for TMJ movement18,43, and these qualities can be partly attributed to the presence of intense dynamic covalent bonds (multiple boron ester bonds) within the PVA-EBPD hydrogel. Furthermore, Fig. 4d–g and Supplementary Fig. 10 illustrate the response to 0.005%–10% strain, and it can be noted that the response increases progressively with an increase in strain. Most importantly, the PVA-EBPD hydrogel exhibits stable and precise sensing to small strains (0.1%) over 200 cycles (Fig. 4h), which is helpful in monitoring the subtle and intricate movements of the TMJ.
a Light bulb brightness changes with PVA-EBPD hydrogel elongation. b The real-time relative resistance variation (∆R/R0) of the PVA-EBPD hydrogel strain sensor over a strain region of 0–100%. c Response time and recovery time of resistance of PVA-EBPD hydrogel under strain altering. d–g The ∆R/R0 of the PVA-EBPD hydrogel in response to different levels of tensile strain. h Stability of PVA-EBPD hydrogel sensor in 200 tensile cycles (0.1% strain). i Schematic diagram of joint motion detection by adhering PVA-EBPD hydrogel sensor to finger, wrist, and elbow joints. j–o Relative resistance changes of the PVA-EBPD hydrogel sensor in response to repeated bending/unbending movements of the finger joint at 45° (j) and 90° (k), repeated bending/unbending movements of the wrist joint at 60° (l) and 90° (m), rapid (n) and slow (o) repeated flexion and extension of the elbow joint. Source data are provided as a Source Data file.
To validate the PVA-EBPD hydrogel’s ability to monitor joint motions, we initially adhered it to the finger, wrist, and elbow joints for preliminary testing (Fig. 4i). As shown in Fig. 4j, when the finger bent at 45°, the ∆R/R0 signal was approximately 100%, while at 90° of bending, the ∆R/R0 signal exceeded 150% (Fig. 4k). This indicates that the PVA-EBPD hydrogel sensor can accurately and reliably detect movements of the finger joint. Similarly, an increase in the ∆R/R0 signal was observed as the wrist joint bent at different angles (Fig. 4l, m). Additionally, the PVA-EBPD hydrogel is capable of providing sensitive and stable sensing, whether the elbow joint was bent rapidly (Fig. 4n) or slowly (Fig. 4o). In brief, the PVA-EBPD hydrogel enables highly reversible and stable strain sensing for simple flexion and extension activities of extremity joints, making it a promising candidate for monitoring minor and delicate TMJ conditions.
Good skin adhesion properties and biocompatibility of PVA-EBPD hydrogel
To effectively monitor TMD, it is crucial for the PVA-EBPD hydrogel to adhere securely to the skin during TMJ movement, and this requires favorable skin adhesion properties44. We conducted tack tests at 10 mm/s, the recommended speed for medical adhesives45, using a PP25 rotor. As shown in Fig. 5a, the adhesion force of the PVA-EBPD hydrogel reached 40 N, which is significantly stronger than that of PVA hydrogel (30 N). Furthermore, unlike the PVA hydrogel, which separated from the PP25 rotor, the PVA-EBPD hydrogel remained securely attached at the conclusion of the test at this high speed (Fig. 5b), demonstrating its notable adhesion performance. Moreover, the adhesion force increased with higher separation speeds, making it suitable for highly active TMJs (Fig. 5c and Supplementary Fig. 11a). The favorable adhesion of the PVA-EBPD hydrogel is attributed to its multiple hydroxyl and multiple boron ester bonds, enabling hydrophilic interactions46 with the interface (including hydrogen and boron ester bonds), and hydrophobic interactions47 facilitated by the benzene ring of EBPD (Fig. 5d). Given the adherence of the PVA-EBPD hydrogel to the facial skin corresponding to the TMJ condyle for sensing, we subsequently investigated its adhesion to the skin. The classical theory of adhesion outlines three commonly utilized assays to evaluate adhesion properties: tensile, shear, and peeling48. As shown in Fig. 5e–g and Supplementary Fig. 11b, c, the adhesion strength of PVA-EBPD hydrogel was better than that of PVA hydrogel both on rabbit skin and pig skin, and the shear strength on rabbit skin could reach 17.7 kPa. The adhesion strength to the skin was essentially as robust as that of previously reported44,49,50,51 adhesive hydrogels, which is suitable and does not cause skin damage during removal, as it takes approximately 10 MPa and 30 MPa of force to detach from rabbit skin and pig skin, respectively (Supplementary Fig. 8). Furthermore, the PVA-EBPD hydrogel exhibited the capability to adhere to various material interfaces, such as a glass slide, plastic wrap, A4 paper, and sponge (Supplementary Fig. 11d), with adhesion at the kPa level as confirmed by the lap-shear test (Supplementary Fig. 11e–g). In summary, the PVA-EBPD hydrogel demonstrates favorable adhesion properties, especially on the skin. It can be affixed to joints without the need for adhesive tape, offering convenience while preventing the tape from restricting joint movement, making it an optimal choice for TMD monitoring.
a Tack test curves of PVA-EBPD hydrogel and PVA hydrogel at a test speed of 10 mm/s. b Tack test device and status of PVA-EBPD hydrogel and PVA hydrogel at the test end point. (The thick red arrow represents the unbroken PVA-EBPD hydrogel.) c The adhesion force of PVA-EBPD hydrogel and PVA hydrogel at different test speeds in tack test (All data are Mean ± SEM, n = 5 independent samples, ** for P < 0.01, *** for P < 0.001, Statistics was calculated by two-way ANOVA with Sidak’s multiple comparisons). d Schematic diagram of the adhesion mechanism of the PVA-EBPD hydrogel. e–g Comparison of adhesion strength of PVA-EBPD hydrogel and PVA hydrogel on rabbit skin by tack test (e), lap-shear tests (f), and peel tests (g) (All data are Mean ± SEM, n = 3 independent samples, ** for P < 0.01, *** for P < 0.001, Statistics was calculated by t-test). h Live-dead cell staining of Hacat cells incubated with PVA-EBPD hydrogel for 12 h and 24 h. Scale bar: 300 μm. i Live Hacat cell count in 12 h and 24 h (All data are Mean ± SEM, n = 5 independent samples). j CCK8 assays on 12 h and 24 h (All data are Mean ± SEM, n = 3 independent samples). k Skin status of rabbits after application of PVA-EBPD hydrogel and saline gauze on the back for 4 h. Scale bar: 5 mm. l H&E staining of rabbit skin after application of PVA-EBPD hydrogel and saline for 4 h. Scale bar: 200 μm and 100 μm. m Skin of pigs after application of PVA-EBPD hydrogel and saline gauze on the back for 4 h (3 animals per group). n The blood counts of pigs in WBC (red blood cell count), RBC (white blood cell count), HGB (hemoglobin), and PLT (platelet count) after applying PVA-EBPD hydrogel on the backs of pigs (All data are Mean ± SEM, n = 3 animals per group). o Blood biochemistry examination of pigs in ALT (alanine transferase), AST (aspartate transferase), Urea, and CREA (creatinine) after applying PVA-EBPD hydrogel on the backs of pigs (All data are Mean ± SEM, n = 3 animals per group). Source data are provided as a Source Data file.
Given that the PVA-EBPD hydrogel must adhere to the facial skin during monitoring, its notable biocompatibility is of great significance for patient compliance in clinical applications. Thus, a series of biocompatibility studies were carried out, including cytotoxicity assessments, skin irritation evaluations, histopathological analysis of the skin and major organs, hematological examinations, and biochemical tests. Live/dead staining and CCK8 assays demonstrated that human normal skin immortalized keratinocytes (Hacat cells) and human oral keratinocytes (HOK cells) maintained good viability and growth after co-incubation with the PVA-EBPD hydrogel for 12 and 24 h (Fig. 5h–j and Supplementary Fig. 12), indicating negligible cytotoxicity. In addition to in vitro cytotoxicity assessments, in vivo biosafety was examined in New Zealand white rabbits and Bama miniature pigs. Firstly, the skin irritation potential of the PVA-EBPD hydrogel was evaluated according to the criteria for biological evaluation of medical devices (test for skin irritation)52. Figure 5k–m demonstrates that, in comparison with a saline control dressing, the PVA-EBPD hydrogel did not induce significant skin erythema or edema in rabbits and pigs after dressing removal, and the skin irritation index score was 0 (Supplementary Tables 4 and 5), signifying a lack of skin irritation. Hematoxylin and eosin (H&E) staining further confirmed that the PVA-EBPD hydrogel did not cause visible damage to skin tissues, as the epithelium at the PVA-EBPD hydrogel site remained intact, without leukocyte infiltration, vascular congestion and edema (Fig. 5l and Supplementary Fig. 13). The microscopic irritation index scores were minimal, measuring only 0.07 and 0.13 at 1 h and 24 h after dressing removal, respectively (Supplementary Table 6). This reinforced the absence of skin irritation associated with the PVA-EBPD hydrogel. Furthermore, histocompatibility of the PVA-EBPD hydrogel with major organs (the heart, liver, spleen, lung, and kidney) was assessed, revealing normal tissue structures without evident inflammatory cell infiltration, bleeding, or organ damage (Supplementary Fig. 14), suggesting good biocompatibility. Moreover, hematological analysis revealed that the blood counts and blood biochemistry of pigs did not significantly change after PVA-EBPD hydrogel administration compared to the normal control (Fig. 5n, o and Supplementary Fig. 15). Therefore, the PVA-EBPD hydrogel exhibited satisfactory biocompatibility in various aspects, including cytology, histology, and hematology, indicating promising clinical translation prospects.
PVA-EBPD hydrogel for monitoring limb joint and TMJ activity in pigs
The PVA-EBPD hydrogel exhibits noteworthy properties, including instantaneous self-recovery, well-perceived functions to minute strains, favorable skin adhesion, and good biocompatibility. These attributes render it a promising candidate for wearable soft sensors designed to detect a wide range of joint movements. To evaluate its performance, the PVA-EBPD hydrogel was adhered to the elbow, knee, and ankle joints of pigs (Fig. 6a), and the joints were manipulated passively. Each joint exhibited distinctive movement patterns (Fig. 6b–e and Supplementary Movie 2), underscoring the PVA-EBPD hydrogel’s capacity to accurately monitor passive limb activities. Furthermore, the PVA-EBPD hydrogel sensor was attached to the right ankle joint of the pig to monitor its free movement (Supplementary Movie 3). Figure 6f demonstrates its sensing traces during walking, exhibiting an upward narrow peak and a downward sharp peak immediately following, which corresponds to the pronation and supination of the ankle joint during walking53. In addition, when the pig is lying down, the ankle joint is in excessive dorsiflexion54, so the sensing line shows an upward broad peak, of course, the width of this peak depends on its squatting duration, and when the pig is standing up, the ankle joint changes from dorsiflexion to plantarflexion, and the trajectory of the sensing line decreases. However, due to the complexity of the squatting and rising process, the activities involved in the process are not only dorsiflexion and plantarflexion of the ankle joint55, so there are many small and miscellaneous peaks in the monitoring curves to be analyzed further (Fig. 6g). Of note, when the pig turned to the right, the sensor recorded a broad downward peak, reflecting the substantial supination movement of the right ankle joint during the turn55. Subsequent walking activity following the turn was also clearly captured (Fig. 6h). These demonstrate the PVA-EBPD hydrogel’s ability to sensitively and accurately detect and record joint activities in real-life scenarios, and it does not need any adhesive to be fixed (the tape appearing in the figures was used to secure the wires), signifying its significant potential for clinical applications.
a Schematic diagram of the PVA-EBPD hydrogel sensor adhered to the elbow, knee, ankle joints, and TMJ of a pig to monitor the joint movements. b–e Passive motion sensing lines of the elbow (b), knee (c and d), and ankle (e) joints. f–h Active motion, including walk (f), squat (g), and turn round (h) sensing lines of the right ankle joint in real scenarios. i–k Bilateral TMJ passive opening and closing mouth (i), lateral movement to the left and right sides (j), protrusion and retrusion (k) sensing map lines. l–n Sensorimotor maps for active possible tapping movement (l), eating (m), and eating with head-turning (n). (The red arrows in f represent the position of PVA-EBPD hydrogel sensor adhesion). Source data are provided as a Source Data file.
Although PVA-EBPD hydrogel sensor can accurately monitor limb joint activities in pigs, TMJ activities are more delicate and complex than limb joint activities56, and it is not yet known whether PVA-EBPD hydrogel sensor can be applied to monitor TMJ activities. Hence, we applied PVA-EBPD hydrogel to the facial skin area corresponding to the pig’s TMJ to monitor TMJ activities. TMJ clinical examination typically involves three primary movements: opening/closing, lateral left/right, and protrusion/retrusion57, so during TMJ sensing monitoring, we also evaluate TMJ from these three dimensions (Supplementary Movie 4). During mouth opening, the condyle initially undergoes a slight forward rotation, subsequently sliding along the posterior oblique surface of the articular eminence to below the articular tuberosity33. This movement results in a temporary void or hollow depression within the articular fossa, which is visually represented as a downward peak on the sensorimotor map line. As the mouth closes, the condyle’s path returns from below the articular tuberosity to the articular fossa, and the sensorimotor map line reverts to its initial position (Fig. 6i). The sensory mapping for lateral movements presents an intriguing pattern. In this context, the left TMJ displays a downward peak when moving to the right and an upward peak during lateral movement to the left, whereas the right TMJ exhibits the opposite behavior (Fig. 6j). This is a consequence of the fact that the left TMJ condyle will glide anteriorly, medially, and inferiorly during right lateral movements, while it will rotate laterally by 3 mm when moving to the left34, and therefore shows an alternating up and down peak pattern on the sensorgram. In protrusion and retrusion movements, the condyle slides forward with the disc below the tuberosity, and it subsequently returns to the intercuspal position (ICP), which is expressed as a downward peak in Fig. 6k. The fundamental movements of the TMJ along the three dimensions, including opening and closing, lateral movement to the left and right, as well as protrusion and retrusion, are effectively detected by the PVA-EBPD hydrogel sensor. They exhibit distinctive peaks consistent with the actual movements, suggesting that the PVA-EBPD hydrogel sensor is a suitable tool for monitoring TMJ activities.
Moreover, the functional movements of TMJ, like tapping and eating, were subjected to monitoring (Supplementary Movie 5). Tapping movements, the rapid repetition of mandibular opening and closing movements, are fundamental jaw movements that elicit the jaw position for occlusal adjustment and registration of interocclusal relation58. These tapping actions are significantly relevant in daily dental clinical practice. This is because the occlusal position, derived from mandibular tapping movements from the mandibular resting position (commonly known as the tapping point), is acknowledged to align with the maximal ICP59. Fortunately, we monitored possible tapping movements in pigs in the absence of food using the PVA-EBPD hydrogel sensor (Fig. 6l). It exhibited high reproducibility similar to real tapping. However, unlike real tapping, it was accompanied by lateral movements because the peaks of the left TMJ corresponded to the troughs of the right TMJ, which might be caused by muscle memory of the masticatory muscles. In addition, masticatory is a complex and asymmetrical sequential lateral movement. During mastication, the condyle on the working side undergoes rotational motion along the vertical axis of the posterior margin of the condylar-mandibular ascending branch. Simultaneously, the condyle on the non-working side experiences a sliding movement along the posterior slope of the articular tuberosity in an anterior downward and inward direction60. As illustrated in Fig. 6m, when a pig chews on the right side, its right TMJ (the working side) engages in rotational motion, whereas the left TMJ (the non-working side) presents a more substantial sliding motion. Intriguingly, the thick subcutaneous fat and the anatomical features of the pig, where the neck is not distinctly demarcated from the face, lead to bilateral baseline mirror-symmetric sensorimotor maps during feeding when accompanied by head-turning movements, as portrayed in Fig. 6n. In our examination of the pig’s TMJ, we observed that, in contrast to the left TMJ, the sensing lines of the pig’s right TMJ consistently exhibited numerous abnormal and irregular peaks, which might be attributed to the uneven articular surface of the pig’s right TMJ, potentially stemming from prolonged consumption of coarse food. In summary, despite the considerably thicker subcutaneous fat in pigs compared to humans, we have ascertained that the PVA-EBPD hydrogel sensor is capable of accurately monitoring not only the basic activities of the TMJ, including mouth opening and closing, lateral movement, and protrusion and retrusion but also the intricate functional activities like tapping and eating. This capability holds promise for its potential utilization in clinical settings for the sensitive monitoring of TMD in humans.
PVA-EBPD hydrogel for human DDwR monitoring
The notable sensitivity of the PVA-EBPD hydrogel sensor in monitoring limb joint and TMJ activity in pigs suggests its potential utility in detecting human TMJ activity and serving as a diagnostic tool for TMD. To evaluate the feasibility of using the PVA-EBPD hydrogel sensor for monitoring TMJ activity and assisting in DDwR diagnosis in humans, 120 volunteers (median age: 24 years; range: 19–54 years) were enrolled in this study based on the inclusion and exclusion criteria (Supplementary Table 7). No participants withdrew from the study. The baseline characteristics of participants are summarized in Supplementary Table 8. All volunteers were previously diagnosed with either normal TMJ or DDwR by TMJ specialists at West China Hospital of Stomatology, Sichuan University. TMJ-related histories and clinical examination findings were documented in accordance with Supplementary Table 9. According to the clinical diagnosis, the volunteers were divided into a normal TMJ group (n = 58) and a DDwR group (n = 62). Subsequently, the PVA-EBPD hydrogel sensor adhered to the facial skin at the corresponding location of the condylar process on the volunteers. The volunteers then cooperated in performing mouth opening and closing, lateral movements, and protrusion and retrusion movements ten times while the TMJ motion sensing map lines were recorded (Fig. 7a and Supplementary Movies 6–8).
a Schematic diagram of the trajectory of the condyles of the human TMJ during opening and closing of the mouth, lateral movement to the left and right sides, and protrusion and retrusion. b–g Sensorimotor maps of opening and closing mouth in six normal TMJ volunteers respectively. h–m Sensorimotor maps of opening and closing mouth in six DDwR volunteers respectively. (Purple arrows represent abnormal TMJ activity.) Source data are provided as a Source Data file.
The results demonstrated the sensorimotor lines of normal TMJ volunteers over five cycles of mouth opening and closing, which exhibited bilaterally synchronized, symmetrical, and smooth downward single peaks (Fig. 7b–g and Supplementary Fig. 16). In contrast, volunteers with DDwR displayed unsmooth sensorgram lines accompanied by many sharp stray peaks (Fig. 7h–m and Supplementary Fig. 17), which could be originated from the abnormal TMJ activity in the DDwR volunteers. According to DC/TMD, which is well recommended for use in clinical and research applications, abnormalities such as clicking, popping, and/or snapping noise occur at least one of three repetitions during TMJ movements, combined with medical history, a diagnosis of DDwR can be made61. Therefore, according to DC/TMD, the occurrence of unsmooth sharp stray peaks at least one of three repetitions during monitoring was defined as TMJ abnormality in the sensing atlas. First, we described the agreement between the two tests for the 30 participants by the Cohen′s Kappa. The results revealed a Kappa coefficient of 0.86, indicating almost complete agreement and repeatability between the two tests. Then we expanded the sample size for subsequent analysis. As illustrated in Table 1, the PVA-EBPD hydrogel sensor provided favorable accuracy (90.00%). In addition, all volunteers with a clinical diagnosis of DDwR presented TMJ abnormality in the sensorimotor line, with a sensitivity of up to 100%, a specificity of 79.31%, and Youden Index of 0.79, which was better than other auxiliary diagnostic tools such as VAG13 and surface electromyography (sEMG)12 (Supplementary Table 1). The limited specificity of the PVA-EBPD hydrogel sensor was mainly attributed to uncoordinated TMJ movements in some volunteers, which despite causing abnormalities in the sensorgram lines, did not meet the clinical diagnostic criteria for DDwR. In particular, clicking, abnormal opening patterns, and inconsistent bilateral joint motility are common clinical manifestations of DDwR. Therefore, the sensitivity of the PVA-EBPD hydrogel sensor to these three abnormal clinical manifestations was counted (Supplementary Table 10). The results revealed that the sensitivities for clicking, abnormal opening pattern, and inconsistent joint mobility were 98.41%, 70.49%, and 79.14%, respectively, which indicated that the PVA-EBPD hydrogel sensor was most responsive to TMJ clicking.
Furthermore, the emergence of clicking at three different stages was analyzed: opening and closing the mouth, lateral movement to the left and right sides, and protrusion and retrusion. As depicted in Table 2, the PVA-EBPD hydrogel sensor achieved high sensitivity for all three stages of clicking, especially in the opening and closing mouth stage with a sensitivity of 98.28%. Subsequently, we compared the sensorgrams of DDwR volunteers who manifest only joint clicking in clinical examination without significant other disturbances (such as abnormal opening patterns) with those of normal TMJ volunteers (Supplementary Fig. 18). Supplementary Fig. 18b, e illustrate that the volunteers with DDwR displayed a distinct peak at the middle stage of mouth closing, coinciding with the characteristic click at the middle of mouth closure in right TMJ. In the case of normal TMJ lateral movements, the action involves stretching on one side while contracting on the other, and the sensorimotor lines exhibit a smooth curve with bilateral mirror symmetry (as illustrated in Supplementary Fig. 18c). Conversely, when the left TMJ experienced clicking at leftward movement (Supplementary Fig. 18f), abnormal peaks were evident in the sensory map line at the corresponding periods. During protrusion, where the condyle only slides forward with the articular disc below the articular tuberosity without rotation, the sensorgrams displayed downward peaks, albeit smaller in amplitude than during mouth opening and closing (as shown in Supplementary Fig. 18d). When clicking incidents occurred during both protrusion and retrusion, the sensory map line exhibited two distinctive abnormal peaks (Supplementary Fig. 18g). In conclusion, the PVA-EBPD hydrogel sensor accurately captures abnormal TMJ activity, in agreement with clinical examination. Notably, it can not only identify abnormal manifestations but also pinpoint the specific timeframes during which these manifestations occur, making it a valuable tool for the DDwR diagnosis.
Intelligent assisted TMD efficient diagnosis
Although DDwR can be recognized with high accuracy and sensitivity by the PVA-EBPD hydrogel sensor, the judgment of the sensorgram is still dependent on physician interpretation, which is not only subjective but also does not really reduce the burden on the physician. In order to realize a more efficient diagnosis of DDwR without physician dependence, the waveform data from volunteers with normal TMJ and DDwR was further analyzed. Firstly, since mouth opening and closing was the most representative activity, the Continuous Wavelet Transform (CWT) with the Morlet wavelet basis was employed to observe oscillatory patterns in the time domain of sensorimotor maps during the opening and closing mouth. The magnitude scalograms obtained from the CWT showed the local time and frequency characteristics of different frequency components, and Fig. 8a, b showed the magnitude scalograms derived from the waveform data of normal TMJ and DDwR, which reflected their significant differences. Then, we extracted the waveform data features of the sensorgrams based on wavelet time scattering network (WTSN) and then constructed a prediction model using the long short-term memory network (LSTM) with 5-fold cross-validation, the schematic diagram was showed in Fig. 8c. As the results in Fig. 8d showed, the constructed model has an accuracy of 84.40% (95% confidence interval (CI): 79.96%, 88.84%), a sensitivity of 0.86 (95% CI: 0.79, 0.94), and a specificity of 0.80 (95% CI: 0.70, 0.91). And the Area Under the Receiver Operating Characteristic Curve (AUC) of the constructed prediction model was 0.88, and the 95% CI was from 0.85 to 0.92 (Fig. 8e). The high accuracy indicated that the optimal model could be used as an auxiliary diagnostic tool for TMJ movement, and the sensitivity of the constructed model was higher than the specificity, which indicated that it was suited for the initial screening of DDwR. Taken together, the auxiliary diagnostic tool built above could assist DDwR diagnosis more objectively, efficiently, and conveniently.
a Magnitude scalograms derived from the waveform data of normal TMJ with CWT. b Magnitude scalograms derived from the waveform data of DDwR with CWT. c Schematic diagram of the prediction model construction for the auxiliary diagnostic tool. d Accuracy, sensitivity, specificity, AUC of prediction model (All data are Mean ± SEM, n = 5 predictive models). e Receiver Operating Characteristic Curve (ROC) of the prediction model. The 5-fold cross-validation was utilized for model construction, and the mean AUC was 0.88 (95% CI: 0.85, 0.92). Source data are provided as a Source Data file.
In summary, according to the specific clinical requirements for TMD diagnosis, we have designed and successfully developed a sensitive PVA-EBPD hydrogel sensor using an eco-friendly and straightforward approach, which can accurately monitor intricate TMJ activity and identify DDwR. The PVA-EBPD hydrogel possesses dermatoid tissue-like flexibility, instantaneous self-recovery properties, and favorable skin adhesion, allowing it to conform seamlessly to the skin without requiring external fixation and preventing detachment from the skin during TMJ movement. Furthermore, its appropriate sensitivity and stability in detecting slight strains, coupled with rapid response and recovery times, make it an essential prerequisite for DDwR detection. Its good biocompatibility ensures safe use on facial skin, paving the way for clinical application. Notably, this hydrogel sensor is not limited to monitoring limb joint activities in pigs, such as walking, squatting, and turning, but can also effectively track TMJ activities like mouth opening and closing, lateral movement to both sides, and protrusion and retrusion, in addition to functional activities like tapping and feeding. Moreover, the PVA-EBPD hydrogel sensor can identify abnormal TMJ activity in humans (sensitivity up to 100% compared to DC/TMD) and pinpoint the timing of these abnormalities in volunteers with DDwR, thereby providing accurate indications of TMD. In addition, based on the waveform data features extracted by WTSN and the prediction model constructed by LSTM, it was found that this auxiliary diagnostic tool can realize the efficient and rapid diagnosis of DDwR. Consequently, this hydrogel sensor, with instantaneous self-recovery, ultra-low detection limit, favorable skin adhesion, good biocompatibility, and intelligent and efficient diagnosis of DDwR, is expected to offer a fresh approach managing TMD in the future.
Methods
Ethical statement
Our research complies with all relevant ethical regulations and was approved by the West China Hospital of Stomatology Institutional Review Board. We affirm that human research participants provided written informed consent, for publication of the images in Fig. 2 and Supplementary Figs. 2, 18, and 19.
Materials
All chemical reagents were available purchased from commercial sources and of pure analytical grade and used without further purification. Polyvinyl alcohol (Mw 13000-23000, 87-89% hydrolyzed), boric acid, and sodium hydroxide were bought from Sigma-Aldrich (USA). (E)-4,4′-(ethene-1,2-diyl) bis (benzene-1,2-diol) (Cas number: 136273-05-7, molecular weight: 244.25) was purchased from Jiangsu Aikon (Nanjing, China). Dulbecco’s modified eagle medium (DMEM), keratinocyte serum-free medium (K-SFM; Thermo Fisher Scientific), fetal bovine serum (FBS), and penicillin–streptomycin liquid was purchased from Gibco (USA). Trypsin was purchased from Sigma-Aldrich (USA). Cell counting kit 8 (CCK8) was purchased from DOJINDO Molecular Technology (Japan).
Animal experiments
Female New Zealand white rabbit (2–2.5 kg) and Bama miniature pig (365 days) were obtained from CHENGDU DOSSY EXPERIMENTAL ANIMALS Co., Ltd. (Chengdu, China). All the animal experiments were conducted following the guidelines outlined in the “Principles of Laboratory Animal Care” (NIH). All the experiments were approved by the Animal Care and Use Committee of West China Hospital of Stomatology (WCHSIRB-D-2023-552). The animals had free access to sterilized water and food in a temperature-controlled room (22 ± 1 °C) with a 12 h light/dark cycle. They were fed adaptively for one week in this circumstance before the experiments.
3D analysis of facial morphology on the condylar region during TMJ movements
Photographs of the volunteers were captured using a camera (Canon T8i), and 3D reconstruction and analysis of the images were analyzed using a VECTRA H2 system (Canfield Scientific, Inc.). During image analysis, a region measuring 2 cm in length and 1 cm in width was delineated around the projection point of the condyle on the surface of the body. This region was utilized to analyze the deformation parameters of the face during various TMJ movements compared to the closed-mouth position. All identifiable images of participants have obtained the consent.
Preparation of the PVA-EBPD hydrogel
PVA-EBPD hydrogel was synthesized by a simple method in one pot. Firstly, NaB(OH)4 solution (0.25 M) was obtained by mixing equal volumes of boric acid aqueous solution (0.5 M) and sodium hydroxide aqueous solution (0.5 M). Briefly, precisely weighted EBPD powder (6 mg) was dissolved in NaB(OH)4 solution (0.25 M, 200 μL) and ultrasonicated to obtain a clarified EBPD solution. Then it was mixed well with PVA aqueous solution (0.15 g/mL, 1 mL) to form PVA-EBPD hydrogel. PVA hydrogel was synthesized by directly mixing PVA aqueous solution (0.15 g/mL, 1 mL) with NaB(OH)4 aqueous solution (0.25 M, 200 μL). The hydrogel formation was validated using the vial inversion test, in which the vial containing the hydrogel was inverted at room temperature. If no sample flow was detected, the hydrogel formation was determined. And photographs of the samples were taken.
11B nuclear magnetic resonance (NMR) spectroscopy
Firstly, 0.5 mmol of boric acid and 0.5 mmol of sodium hydroxide were dissolved in 1 mL of D2O to obtain 0.5 M of boric acid solution and 0.5 M of sodium hydroxide solution, respectively, and then the two were mixed in equal volumes to obtain NaB(OH)4 solution (0.25 M) in D2O. In addition, precisely weighted 0.15 g PVA was dissolved in 1 mL of D2O to obtain 0.15 g/mL of PVA solution in D2O. Precisely weighted EBPD powder (3 mg) was dissolved in NaB(OH)4 solution (0.25 M, 100 μL) and ultrasonicated to obtain a clarified EBPD solution in D2O, and then it was mixed well with PVA solution in D2O (0.15 g/mL, 500 μL) to form PVA-EBPD hydrogel in NMR tubes. The PVA hydrogel in D2O was obtained by mixing 500 μL of PVA solution (0.15 g/mL) in D2O with 100 μL of NaB(OH)4 solution (0.25 M) in D2O. EBPD+ NaB(OH)4 represents dissolving 15 mg of EBPD powder in 500 μL of NaB(OH)4 solution in D2O. 11B NMR spectra were acquired at 600 MHz using an AV II spectrometer (Bruker, Germany).
Fourier transform infrared (FT-IR) spectra characterization
FT-IR spectra of EBPD powder, lyophilized PVA-EBPD hydrogel, lyophilized PVA hydrogel, lyophilized EBPD solution (EBPD+NaB(OH)4), lyophilized PVA solution, lyophilized NaB(OH)4 solution were compressed with potassium bromide and the spectra were recorded in the 4000–400 cm−1 range using a Bruker spectrophotometer.
Hydrogel morphology characterization
The morphology of the PVA-EBPD hydrogel, PVA hydrogel, and PVA solution was examined with a high-resolution INSPECT F50 scanning electron microscope (SEM). The hydrogels were freeze-dried for 24 h and attached to the silica wafer. Then, the xerogels surfaces were coated with a gold layer before being examined with the SEM. The formability of PVA-EBPD hydrogels was characterized by placing them in polytetrafluoroethylene molds with different shapes.
Rheological measurements of hydrogel
Rheological behaviors of the hydrogel were investigated using an Anton Paar Physica MCR 302 Rheometer (Austria). The hydrogel’s storage modulus (G′) and loss modulus (G′′) were measured to determine its viscoelastic characteristics. The parallel plate with a diameter of 25 mm was chosen for the oscillation mode and rotation mode. The gels were placed on the sampling platform. Next, the parallel plate PP25 was lowered to a gap distance of 0.25 mm, the excess sample was scraped off, and the temperature was set at 25 °C. Strain sweep tests were performed at a constant angular frequency of 10 rad/s within strain from 0.1 to 1000%. The frequency sweep experiment was performed at 1% strain and within the frequency range of 0.01 to 100 Hz. Self-recovery properties were tested by dynamic step-strain sweep experiments at a constant angular frequency of 10 rad/s and alternating 1% and 300% shear strain in oscillating mode. The viscosity and recovery time of PVA-EBPD hydrogels were tested at an initial shear rate of 0.25 1/s in the rotational mode. Cyclic stability testing of PVA-EBPD hydrogel was carried out at alternating 0.25 1/s and 11/s shear rates.
Adhesion performance tests
To assess the adhesion performance of the hydrogel, firstly we used the tack mode of the rheometer (Anton Paar Physica MCR 302 Rheometer, Austria) and placed the hydrogel between the sample stage and the PP25 rotor, applying a normal force of 0.5 N for 60 s to make a close contact, then unloading the normal force to 0 N for 30 s, waiting for the system to stabilize, and finally, the rotor was raised at a speed of 10 mm/s and the displacement versus force curves of the separation were recorded. The adhesion force at different speeds is the maximum separation force value when the rotor rises at different speeds respectively (n = 5). Adhesion work (W) in the supplementary material was calculated based on the adhesion (Fn), displacement (x), and maximum contact area (Amax), as shown in Eq. 1:
where the maximum contact area (Amax) was calculated based on Eq. 2:
where R is the radius of the rotor and H is the maximum distance47. In the skin adhesion tack test, fresh skin tissues from a female New Zealand white rabbit were glued onto the sampling platform of the rheometer and the PP25 rotor, and then the hydrogel was placed between the two, and the test was carried out using the method of the above test (n = 3). The detachment stress was calculated using the following Eq. 3:
where Fnmax was the maximum separation force value and S was the contact area (the area of the PP25 rotor). Then, lap shear and peel strength49 were measured using a universal testing machine (Instron 5567, USA) with a loading speed of 10 mm/s. The contact area was 15 mm × 10 mm. The adhesion values are obtained by dividing the maximum separation force by the lap-shear area.
Sensing properties test
The strain-sensing performance was recorded by a Keithley 2602 B source meter under a constant voltage of 3 V. This instrument is equipped with an NPLC filtering function, where NPLC stands for the Number of Power Line Cycles, with N representing the multiple factors, and PLC (Power Line Cycle) referring to the power line cycle. The AC power noise can be reduced by the NPLC filtering function. The relative resistance change was calculated using Eq. 4:
where R and R0 are the real-time resistance and original resistance of the hydrogel, respectively. The gauge factor (GF) values of the hydrogels were calculated based on the slope of a plot of the relative resistance variation rate vs the strain. During the joint activity sensing monitoring, the PVA-EBPD hydrogel was attached to different areas of a human body or pig and then connected to a source meter via wires to form a circuit to detect signal float under different actions.
Cytotoxicity assessment
The cytotoxicity of PVA-EBPD hydrogel was evaluated by CCK8 experiments and live-dead cell staining. Two keratinocyte lines (Hacat and HOK) were used in this study. Hacat cell line was authenticated by the China Center for Type Culture Collection. HOK cell line was authenticated by Core Facilities of West China Hospital. Each cell line was authenticated using the STR cell line authentication method. Hacat cells and HOK cells were cultured in DMEM with 10% FBS and keratinocyte serum-free medium (K-SFM) in a humidified 37 °C cell incubator containing 5% CO2 and 95% air, respectively. In CCK8 assays, all cells were seeded in a 96-well plate with a density of 1 * 104 cells per well and incubated overnight until fully attached. Then discarding the culture medium, the samples (100 μL) containing fresh medium and various concentrations of PVA-EBPD hydrogel were introduced into each well. The cells were co-incubated with the samples for 12 and 24 h. Afterward, the medium was replaced with 100 μL fresh culture medium with 10% CCK8. 2 h later, the absorbance of the solution was recorded using Varioskan™ LUX multimode microplate reader (Thermo Fisher Scientific, USA) at 450 nm wavelength. The relative cell viability (%) was calculated by Eq. 5:
In live-dead cell staining, cells were seeded in 24-well plates at 4 × 104 cells per well and incubated overnight until fully attached. Then discarding the culture medium, the samples (500 μL) containing fresh medium and various concentrations of PVA-EBPD hydrogel were introduced into each well. After co-culturing 12 and 24 h, cells were stained by a live/dead cell staining kit (KeyGEN BioTECH) for 30 min. Images were taken under a confocal laser scanning microscopy (CLSM). Viable cell counts were calculated as a percentage compared to a blank control.
In vivo biosafety evaluation
The experiment investigated the irritation62,63 of PVA-EBPD hydrogel on the back skin of New Zealand white rabbits and Bama miniature pig. Since the PVA-EBPD hydrogel needs to adhere to the skin for use, the studies on skin irritation have provided a reference for the safety evaluation of its clinical application64. In the New Zealand white rabbits, the animals were divided into two groups: 1 h after dressing removal group and 24 h after dressing removal group (n = 6). Twenty-four hours before the experiment, the hair on the backs of the rabbits was shaved carefully. According to the self-control method, the back skin of the animal was divided into four areas: PVA-EBPD hydrogel sites (A and D areas) and saline control sites (B and C areas). PVA-EBPD hydrogel and saline gauze were applied to the skin, and the test area was washed with warm water after 4 h to remove residual materials. At 1 h and 24 h after removal of the material, the erythema and edema were observed and recorded, and the naked eye scoring was performed according to Supplementary Table 11, and the irritation level was determined according to Supplementary Table 12. The average skin irritation score (M1) was calculated according to Eq. 6:
Then, the animals were euthanized at 1 h and 24 h after removal of the material respectively, and the skin tissue or major organs (the heart, liver, spleen, lungs, and kidneys) were harvested, embedded in paraffin, sectioned, and stained with H&E for skin histological analysis according to Supplementary Table 13, and degree of microscopic irritation response was assessed according to Supplementary Table 14. The average skin irritation microscopic score (M2) was calculated according to Eq. 7:
In the Bama miniature pigs, the animals had only one group for self-control (n = 3), the other similar to the test of rabbits, the back of the animal was shaved, the dressing material was applied, and the dressing was removed after 4 h, and then the skin conditions of the dressing site were observed continuously at 1 h, 24 h, 48 h, and 72 h after the removal of the dressing and scored (Supplementary Table 11) to delineate the grade (Supplementary Table 12). Then, after 72 h, the skin on the back of the pig was cut off, embedded in paraffin, sectioned, and stained with H&E for skin histological analysis. In addition, blood samples were obtained 4 h after the application of PVA-EBPD hydrogel for routine and biochemical analyses.
PVA-EBPD hydrogel sensor for human DDwR monitoring
The study with human research participants' registration number is ChiCTR2300076975. The study complies in full with the STROBE statement. Based on previous studies and the feasibility of the study, the sample size was calculated in this study using the peak frequency of motion graph lines as the primary index. In this case, α was set to 0.05, β was set to 0.1, the overall mean and standard deviation were obtained from the literature, and the sample size formula was as follows Eq. 8:
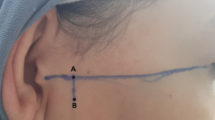
n represents the sample size; g is the number of groups; \({\bar{{{{\boldsymbol{X}}}}}}_{{{{\boldsymbol{i}}}}}\), Si is the mean and standard deviation; φ will be obtained by looking up the table according to α, β. According to the sample size calculation formula and the lost visit rate, the total sample collection of this project is 120 cases. A total of 120 volunteers aged 19–54 years were enrolled in this human study. Inclusion and exclusion criteria are identified in Supplementary Table 7. At the start of the study, allergic history should be taken in detail in all subjects. When the allergic history was uncertain, the volunteers underwent patch testing65. Written informed consent was obtained from all volunteers. This entire study protocol was approved by the West China Hospital of Stomatology Institutional Review Board (WCHSIRB-D-2023-347) and was conducted according to the guidelines put forth in the Declaration of Helsinki. Firstly, the placement direction of the hydrogel sensor was determined by 3D analysis of facial morphology on the condylar region during TMJ movements (Supplementary Fig. 2). Then, following the steps as shown in Supplementary Fig. 19, the PVA-EBPD hydrogel was adhered to the facial skin corresponding to the condyle of the volunteer and connected to the source table through a wire. Finally, the volunteer cooperated with the opening and closing of the mouth, lateral movement to the left and right sides, and protrusion and retrusion movement 10 times, while recording the sensing lines, and the whole process took only 3 min.
Statistical analysis
All the quantitative experiments in this study were performed at least in triplicate. The obtained data were represented as mean value ± SEM (standard error of the mean). Statistical differences among different groups were determined through t-test, one-way ANOVA, two-way ANOVA, Chi-square test, Chi-square with Yates’ correction, and Fisher’s exact test using the GraphPad Prism 6.0 software, which was considered statistically significant when P < 0.05 (* for P < 0.05, ** for P < 0.01, *** for P < 0.001 and **** for P < 0.0001), while P > 0.05 was represented as N.S. meaning no significance. In the study with human research participants, the repeatability of the PVA-EBPD hydrogel sensor was assessed by Cohen′s Kappa. In addition, a true positive (TP) is an outcome where the PVA-EBPD hydrogel sensor correctly predicts the positive class. Similarly, a true negative (TN) is an outcome where the PVA-EBPD hydrogel sensor correctly predicts the negative class. A false positive (FP) is an outcome where the PVA-EBPD hydrogel sensor incorrectly predicts the positive class. And a false negative (FN) is an outcome where the PVA-EBPD hydrogel sensor incorrectly predicts the negative class. The specificity was calculated according to Eq. 9:
The sensitivity was calculated according to Eq. 10:
The accuracy was calculated according to Eq. 11:
Youden Index was calculated according to Eq. 12:
The waveform data features extracted by WTSN and the prediction model constructed by LSTM
Data derived from TMJ sensorimotor data on mouth opening and closing of participants in the study with human research participants. The dataset contains 16 s of TMJ sensorimotor data and was saved as waveform data. Firstly, to analyze waveform data to observe oscillatory patterns in the time domain, the WSTN was utilized to extract the waveform characteristics from the waveform data66. In this study, the WSTN employs CWT, utilizing the Morlet wavelet basis, to perform decomposition operations on waveform signals. CWT enables to analyze signals at multiple scales, and the magnitude scalograms were plotted for the identification of local time and frequency characteristics of different frequency components. The formula for the Morlet wavelet function is as Eq. 13:
\({{{\boldsymbol{\psi }}}}\left({{{\boldsymbol{t}}}}\right)\) is the definition of the Morlet wavelet at time t, ξ0 is the central frequency of the wavelet, \({{{\boldsymbol{i}}}}\) is the is the imaginary unit. \(s\) is the standard deviation, which controls the width of the wavelet in the time domain and affects its time-frequency localization characteristics, in this study, \(s=1\). The CWT convolves the signal with Morlet wavelets of different scales, given the input signal is \({{{\boldsymbol{x}}}}({{{\boldsymbol{t}}}})\), the formula is expressed as Eq. 14:
By taking the modulus of the wavelet transform result, we extract the amplitude information. Subsequently, applying a low-pass filter to the amplitude information yields the scattering coefficients. The WSTN invariance scale of 0.22 s, the signal length is 800, the sampling frequency is 50 Hz, the quality factors are [8, 1], and the over-sampling factor is 0.
The resulting wavelet scattering coefficients could provide a representation of the sensorgrams. The coefficients can reflect the following features, including local amplitude variations, instantaneous frequency changes, local temporal oscillations, signal discontinuities, local modulation information, time translation invariance, the local distribution of different frequency components, and time-frequency localization characteristics. By decomposing the TMJ sensorimotor signal into features from these coefficients using wavelet transforms, WSTN can effectively separate the baseline drift from the underlying TMJ activity67,68. The waveform data features extracted by WTSN were normalized and used for constructing the prediction model with the LSTM.
LSTM is a special type of recurrent neural network that can learn long-term dependencies and capture more information in time series data69. LSTM effectively manages the flow of information by incorporating gating mechanisms, namely the input gate, forget gate, and output gate. Its foundation rests on maintaining a cell state, which is responsible for retaining and forgetting information. At each time step, the forget gate determines how much information to retain from the previous state, the input gate regulates the extent to which the current input is stored, and the output gate decides which information will be output. By progressively updating both the cell state and hidden state, LSTM can generate outputs that are used for predictions.
We built an LSTM network with 512 hidden units for constructing a prediction model. Then set the hyperparameters accordingly, the maximum epochs were 300, the minimum batch size was 14, the learning rate was 0.0001 and the ADMM (Alternating Direction Method of Multipliers) was utilized. The 5-fold cross-validation was used for model construction. To evaluate the performance of the prediction model, the accuracy, sensitivity, specificity, and AUC with 95%CI were computed in the test dataset, and the ROC was plotted.
The model construction and evaluation were performed using MATLAB version R2022a and R version 4.2.1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available in the main text or the supplementary materials. Source data are provided with this paper.
Code availability
All codes used in this study are available git repositories: http://github.com/chxhstatis/TMDprediction.
References
Shrivastava, M., Battaglino, R. & Ye, L. A comprehensive review on biomarkers associated with painful temporomandibular disorders. Int. J. Oral. Sci. 13, 23 (2021).
Valesan, L. F. et al. Prevalence of temporomandibular joint disorders: a systematic review and meta-analysis. Clin. Oral. Investig. 25, 441–453 (2021).
Wu, J. et al. Temporomandibular disorders among medical students in China: prevalence, biological and psychological risk factors. BMC Oral. Health 21, 549 (2021).
Vinayahalingam, S. et al. Deep learning for automated segmentation of the temporomandibular joint. J. Dent. 132, 104475 (2023).
Brosset, S. et al. 3D Auto-Segmentation of Mandibular Condyles. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 1270–1273 (2020).
Maranini, B., Ciancio, G., Mandrioli, S., Galiè, M. & Govoni, M. The role of ultrasound in temporomandibular joint disorders: an update and future perspectives. Front. Med. 9, 926573 (2022).
Bielajew, B. J. et al. Knee orthopedics as a template for the temporomandibular joint. Cell Rep. Med. 2, 100241 (2021).
Mohlin, B. et al. TMD in relation to malocclusion and orthodontic treatment. Angle Orthod. 77, 542–548 (2007).
Ding, L. et al. The effect of functional mandibular advancement for adolescent patients with skeletal class II malocclusion on the TMJ: a systematic review and meta-analysis. BMC Oral. Health 22, 51 (2022).
Tran, C., Ghahreman, K., Huppa, C. & Gallagher, J. E. Management of temporomandibular disorders: a rapid review of systematic reviews and guidelines. Int. J. Oral. Maxillofac. Surg. 51, 1211–1225 (2022).
Momin, M., Miloro, M., Mercuri, L. G., Munaretto, A. & Markiewicz, M. R. Senior oral and maxillofacial surgery resident confidence in performing invasive temporomandibular joint procedures. J. Oral Maxillofac. Surg. 75, 2091.e1–2091.e10.
Szyszka-Sommerfeld, L., Sycińska-Dziarnowska, M., Spagnuolo, G. & Woźniak, K. Surface electromyography in the assessment of masticatory muscle activity in patients with pain-related temporomandibular disorders: a systematic review. Front. Neurol. 14, 1184036 (2023).
Łysiak, A., Marciniak, T. & Bączkowicz, D. Repeatability of the vibroarthrogram in the temporomandibular joints. Sensors https://doi.org/10.3390/s22239542 (2022).
Youssef, H. M. M., Elia, R. Z. & Ali, K. A. M. The accuracy of high resolution ultrasound in the diagnosis of TMJ disc displacement in comparison with MRI. QJM Int. J. Med. 114, hcab106.026 (2021).
Dworkin, S. F. & LeResche, L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J. Craniomandib. Disord. 6, 301–355 (1992).
Han, B. J., Kang, H., Liu, L. K., Yi, X. Z. & Li, X. Q. Comparisons of condylar movements with the functional occlusal clutch and tray clutch recording methods in CADIAX system. Int. J. Oral. Sci. 2, 208–214 (2010).
Wang, Q., Jia, J., Zhou, C., Ye, W. & Bi, R. A Bibliometric analysis of research on temporomandibular joint disc displacement from 1992 to 2022. Healthcare https://doi.org/10.3390/healthcare11142108 (2023).
Zimmerman, B. K. et al. Role of interstitial fluid pressurization in TMJ lubrication. J. Dent. Res. 94, 85–92 (2015).
Allen, K. D. & Athanasiou, K. A. A surface-regional and freeze-thaw characterization of the porcine temporomandibular joint disc. Ann. Biomed. Eng. 33, 951–962 (2005).
Chen, K. et al. Prediction of condylar movement envelope surface based on facial morphology. Heliyon 9, e17769 (2023).
Więckiewicz, W., Woźniak, K., Piątkowska, D., Szyszka-Sommerfeld, L. & Lipski, M. The diagnostic value of pressure algometry for temporomandibular disorders. BioMed. Res. Int. 2015, 575038 (2015).
Zhu, T. et al. Recent advances in conductive hydrogels: classifications, properties, and applications. Chem. Soc. Rev. 52, 473–509 (2023).
Li, J. et al. Engineering smart composite hydrogels for wearable disease monitoring. Nano-micro Lett. 15, 105 (2023).
Li, S. et al. Monitoring blood pressure and cardiac function without positioning via a deep learning–assisted strain sensor array. Sci. Adv. 9, eadh0615 (2023).
Guo, L. et al. A chemically crosslinked hydrogel electrolyte based all-in-one flexible supercapacitor with superior performance. J. Alloy. Compd. 843, 155895 (2020).
Zhang, H., Niu, W. & Zhang, S. Extremely stretchable and self-healable electrical skin with mechanical adaptability, an ultrawide linear response range, and excellent temperature tolerance. ACS Appl. Mater. interfaces 11, 24639–24647 (2019).
Lu, X., Zhang, L., Zhang, J., Wang, C. & Zhang, A. Facile preparation of dual functional wearable devices based on hindered urea bond-integrated reprocessable polyurea and AgNWs. ACS Appl. Mater. interfaces 14, 41421–41432 (2022).
Zhan, W. et al. Carboxymethylcellulose reinforced, double-network hydrogel-based strain sensor with superior sensing stability for long-term monitoring. Int. J. Biol. Macromol. 241, 124536 (2023).
Liu, T. et al. pH-responsive dual-functional hydrogel integrating localized delivery and anti-cancer activities for highly effective therapy in PDX of OSCC. Mater. Today 62, 71–97 (2023).
Qi, J. et al. Inosine-based supramolecular hydrogel for highly efficient PD-L1 blockade therapy via mediating CD8+ T cells. Adv. Funct. Mater. 32, 2204273 (2022).
Wang, Z. et al. High-strength and injectable supramolecular hydrogel self-assembled by monomeric nucleoside for tooth-extraction wound healing. Adv. Mater. 34, e2108300 (2022).
Ding, T. et al. Mucoadhesive nucleoside-based hydrogel delays oral leukoplakia canceration. J. Dent. Res. 101, 921–930 (2022).
Orsini, M. G. et al. Clinical predictability of temporomandibular joint disc displacement. J. Dent. Res. 78, 650–660 (1999).
Işık, G., Kenç, S., Özveri Koyuncu, B., Günbay, S. & Günbay, T. Injectable platelet-rich fibrin as treatment for temporomandibular joint osteoarthritis: a randomized controlled clinical trial. J. Craniomaxillofac. 50, 576–582 (2022).
Kar, S., Sanderson, H., Roy, K., Benfenati, E. & Leszczynski, J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 122, 3637–3710 (2022).
Xu, J. et al. Rheological and microstructural properties of polysaccharide obtained from the gelatinous Tremella fuciformis fungus. Int. J. Biol. Macromol. 228, 153–164 (2023).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Chen, K. N. et al. Comparative study of temporomandibular articular fossa bone surface and the envelope surface of the condyle movement. Chin. J. Dent. Res. 25, 179–187 (2022).
Lu, X., Si, Y., Zhang, S., Yu, J. & Ding, B. In situ synthesis of mechanically robust, transparent nanofiber-reinforced hydrogels for highly sensitive multiple sensing. Adv. Funct. Mater. 31, 2103117 (2021).
Song, J. et al. Mechanically and electronically robust transparent organohydrogel fibers. Adv. Mater. 32, e1906994 (2020).
Kim, C.-C., Lee, H.-H., Oh, K. H. & Sun, J.-Y. Highly stretchable, transparent ionic touch panel. Science 353, 682–687 (2016).
Wang, G., Zhang, Q., Wang, Q., Zhou, L. & Gao, G. Bio-based hydrogel transducer for measuring human motion with stable adhesion and ultrahigh toughness. ACS Appl. Mater. interfaces 13, 24173–24182 (2021).
Krohn, S. et al. Multi-slice real-time MRI of temporomandibular joint dynamics. Dentomaxillofac. Radiol. 48, 20180162 (2019).
Zhu, Z. et al. Blue-ringed octopus-inspired microneedle patch for robust tissue surface adhesion and active injection drug delivery. Sci. Adv. 9, eadh2213 (2023).
Creton, C. Pressure-sensitive adhesives: an introductory course. MRS Bull. 28, 434–439 (2003).
Mo, J. et al. Design of ultra-stretchable, highly adhesive and self-healable hydrogels via tannic acid-enabled dynamic interactions. Mater. Horiz. 8, 3409–3416 (2021).
Wang, C. et al. Supramolecular topology controlled self-healing conformal hydrogels for stable human–machine interfaces. J. Mater. Chem. C. 10, 8077–8088 (2022).
Khutoryanskiy, V. V. & Khutoryanskiy, V. V. Mucoadhesive Materials and Drug Delivery Systems (Wiley, Newark, 2014).
Hu, S. et al. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 12, 1689 (2021).
Liu, X. et al. Multifunctional self-healing dual network hydrogels constructed via host-guest interaction and dynamic covalent bond as wearable strain sensors for monitoring human and organ motions. ACS Appl. Mater. Interfaces 13, 14612–14622 (2021).
Han, L. et al. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano 11, 2561–2574 (2017).
Wolfe, M. K. et al. Seeking Clearer Recommendations for Hand Hygiene in Communities Facing Ebola: A Randomized Trial Investigating the Impact of Six Handwashing Methods on Skin Irritation and Dermatitis. PloS one 11, e0167378 (2016).
Capodaglio, P. et al. Effect of obesity on knee and ankle biomechanics during walking. Sensors https://doi.org/10.3390/s21217114 (2021).
Dill, K. E., Begalle, R. L., Frank, B. S., Zinder, S. M. & Padua, D. A. Altered knee and ankle kinematics during squatting in those with limited weight-bearing-lunge ankle-dorsiflexion range of motion. J. Athl. Train. 49, 723–732 (2014).
Swinton, P. A., Lloyd, R., Keogh, J. W., Agouris, I. & Stewart, A. D. A biomechanical comparison of the traditional squat, powerlifting squat, and box squat. J. Strength Cond. Res. 26, 1805–1816 (2012).
Castaño-Joaqui, O. G., Cano-Sánchez, J., Campo-Trapero, J. & Muñoz-Guerra, M. F. TMJ arthroscopy with hyaluronic acid: A 12-month randomized clinical trial. Oral. Dis. 27, 301–311 (2021).
Scott, A. J. TMJ dysfunction—principles of the clinical examination. J. Prosthet. Dent. 37, 550–558 (1977).
Yamamoto, T., Nishigawa, K., Bando, E. & Hosoki, M. Effect of different head positions on the jaw closing point during tapping movements. J. Oral. Rehabil. 36, 32–38 (2009).
Noguchi, K. et al. Anticipation and motor control on repetitive tooth tapping produced by open–close jaw movements. J. Oral. Rehabil. 35, 20–26 (2008).
Israel, H. A., Diamond, B., Saed-Nejad, F. & Ratcliffe, A. The relationship between parafunctional masticatory activity and arthroscopically diagnosed temporomandibular joint pathology. J. Oral. Maxillofac. Surg. 57, 1034–1039 (1999).
Schiffman, E. et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral. Facial Pain. Headache 28, 6–27 (2014).
Zhang, M. et al. Formulation of aucklandiae radix extract-loaded nanoemulsions and its characterization and evaluations in vitro and in vivo. Appl. Biochem. Biotechnol. 195, 3156–3179 (2023).
Li, H., Peng, Q., Guo, Y., Wang, X. & Zhang, L. Preparation and in vitro and in vivo study of Asiaticoside-loaded nanoemulsions and nanoemulsions-based gels for transdermal delivery. Int. J. Nanomed. 15, 3123–3136 (2020).
Wehkamp, U., Ardigò, M., Papadavid, E., Querfeld, C. & Nikbakht, N. Chlormethine gel for patients with mycosis fungoides cutaneous T cell lymphoma: a review of efficacy and safety in clinical trial and real-world settings. Adv. Ther. 39, 3979–4002 (2022).
Woodruff, C. M. & Botto, N. The role of patch testing in evaluating delayed hypersensitivity reactions to medications. Clin. Rev. Allergy Immunol. 62, 548–561 (2022).
Andén, J. & Mallat, S. Deep scattering spectrum. IEEE Trans. Signal Process. 62, 4114–4128 (2014).
Bruna, J. & Mallat, S. Invariant scattering convolution networks. IEEE Trans. Pattern Anal. Mach. Intell. 35, 1872–1886 (2013).
Lostanlen, V. Scattering.m—a MATLAB toolbox for wavelet scattering. https://github.com/lostanlen/scattering.m (2022).
Hochreiter, S. & Schmidhuber, J. Long short-term memory. Neural Comput. 9, 1735–1780 (1997).
Acknowledgements
The authors gratefully acknowledge support from the National Key R&D Program of China (Grant No. 2022YFC2402900 to H.Z.), the National Natural Science Foundation of China (NSFC) (Grant Nos. 82271035 to H.Z. and 82201074 to J.W. and 82370962 to H.X.), Clinical Research Program, West China Hospital of Stomatology Sichuan University (Grant No. LCYJ-MS-202309 to H.Z), the Research Funding for Talents Developing, West China Hospital of Stomatology Sichuan University (Grant No. RCDWJS2023-21 to H.Z.), the Multidisciplinary Research Program of West China Hospital of Stomatology, Sichuan University (Grant No. RD-03-202109 to H.Z.). The authors also acknowledge Xiaoqiang Xia, Weiqi Li, Lideng Cao, and Yinghui Wen (State Key Laboratory of Oral Diseases, West China Hospital of Stomatology, Sichuan University) for help in evaluating skin irritation response, analyzing data conducting sensory and monitoring in animal test pigs. The authors also acknowledge Xiaoyan Wang and Yani Xie from the Analysis and Testing Center of Sichuan University for helping us perform NMR and IR studies. The authors would like to thank Jialiang Lai (State Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University), Wenjie Yan (College of Traditional Chinese Medicine, Inner Mongolia Medical University) for helping us providing sensing technical support and providing oral photographs.
Author information
Authors and Affiliations
Contributions
H. Zhao, Y. Yan, and L. Yu conceptualized the project. Y. Yan, L. Yu, X. Lu, Z. Yang, H. Xu, X. Zhang, and J. Wang performed the experiments under the supervision of Q. Chen, Q. Han, and H. Zhao. H. Zhao, H. Xu and J. Wang provided funding acquisition. Y. Yan and H. Xu wrote the original draft. Y. Yan, H. Zhao, L. Yu, X. Zhang, and H. Xu reviewed and edited the manuscript. All authors discussed the results and commented on the manuscript. Y. Yan, L. Yu, and X. Zhang contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Eduardo Castrillon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, Y., Yu, L., Zhang, X. et al. Instantaneous self-recovery and ultra-low detection limit hydrogel electronic sensor for temporomandibular disorders intelligent diagnosis. Nat Commun 16, 839 (2025). https://doi.org/10.1038/s41467-025-55996-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-55996-7