Abstract
Ferroptosis is a form of iron-dependent programmed cell death, which is distinct from apoptosis, necrosis, and autophagy. Mitochondria play a critical role in initiating and amplifying ferroptosis in cancer cells. Voltage-Dependent Anion Channel 1 (VDAC1) embedded in the mitochondrial outer membrane, exerts roles in regulation of ferroptosis. However, the mechanisms of VDAC1 oligomerization in regulating ferroptosis are not well elucidated. Here, we identify that a VDAC1 binding protein V-Set and Transmembrane Domain Containing 2 Like (VSTM2L), mainly localized to mitochondria, is positively associated with prostate cancer (PCa) progression, and a key regulator of ferroptosis. Moreover, VSTM2L knockdown in PCa cells enhances the sensitivity of RSL3-induced ferroptosis. Mechanistically, VSTM2L forms complex with VDAC1 and hexokinase 2 (HK2), enhancing their binding affinity and preventing VDAC1 oligomerization, thereby inhibiting ferroptosis and maintaining mitochondria homeostasis in vitro and in vivo. Collectively, our findings reveal a pivotal role for mitochondria-localized VSTM2L in driving ferroptosis resistance and highlight its potential as a ferroptosis-inducing therapeutic target for the treatment of PCa.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is one of the most common cancer types threatening the health of men globally1,2,3. While radiation, surgery, and androgen deprivation therapy could improve the survival rate and time of the patients, many of them eventually developed into castration-resistant and metastatic prostate cancer and portends a poor prognosis4. At present, published evidences have indicated that ferroptosis could enhance the sensitivity of advanced PCa cells to therapeutic agents5,6, exploring the key factors in regulating ferroptosis is imperative to find effective targets for PCa treatment.
Accumulated evidences indicated that mitochondria play a critical role in maintaining the biosynthetic and metabolic activities of cancer cells, and serves as key regulatory nodes in initiating and amplifying ferroptosis7,8,9,10. Voltage-dependent anion Channel (VDAC), predominantly embedded in the mitochondrial outer membrane (MOM), governs mitochondrial homeostasis and distinct modes of cell death, including apoptosis, autophagy, and ferroptosis11,12,13,14. VDAC1, the most abundant among the three VDAC isoforms, has been well-documented as a crucial regulator of cell death through interactions with various proteins, including anti-apoptotic proteins (B-cell lymphoma 2 (Bcl-2), Bcl-extra large (Bcl-xL) and hexokinase (HK1 and HK2))15,16,17,18, proapoptotic proteins (BH3-interacting domain death agonist (BID) and Bcl-2-associated X protein (Bax))19,20, mitophagy-related proteins (Translocator Protein (TSPO) and Parkin)21,22,23 et al. It was reported that HK2 plays an important role in keeping the monomeric status of VDAC1 in cancer cells24. Moreover, the oligomeric form of VDAC1, rather than its monomeric counterpart, is involved in the regulation of ferroptosis25,26. However, the regulatory mechanism underlying VDAC1 oligomerization in the modulation of ferroptosis in prostate cancer is still elusive.
In this study, we explored the binding proteins of VDAC1 in prostate cancer cells using immunoprecipitation coupled to mass spectrometry (IP/MS) analysis, and found an interacting protein of VDAC1, VSTM2L. VSTM2L, also known as C20orf102, was initially found to interact with Humanin (HN), and was selectively expressed in the central nervous system. VSTM2L is a secreted antagonist of HN and could play a role in regulating HN biological functions27. Recent studies suggested that VSTM2L is recognized as a potential biomarker closely associated with cancer cell metastasis and prognosis28,29,30. Thus far, the biological roles and the underlying molecular mechanisms of VSTM2L in PCa cells are still blurry.
Here, we show that the VDAC1 binding protein VSTM2L, mainly localized to mitochondria, is an oncogene and ferroptosis suppressor in PCa cells. VSTM2L knockdown impedes cell growth and migration by facilitating ferroptosis of PCa. Molecular biology studies uncover that VSTM2L is a crucial factor in keeping the interaction between VDAC1 and HK2. Decreased VSTM2L leads to the dissociation of HK2 from VDAC1 and the increased oligomerization of VDAC1, which disturbs the homeostasis of mitochondria and triggers the ferroptosis of PCa cells. Our findings not only reveal a link between VSTM2L and ferroptosis but also disclose the regulatory mechanism underlying VDAC1 oligomerization in the modulation of ferroptosis, suggesting that VSTM2L is a predictive factor with therapeutic potential for PCa treatment.
Results
VSTM2L is a VDAC1 binding protein in PCa Cells
Considering the crucial role of VDAC1 in maintaining the homeostasis of mitochondria, we explored the interacted proteins with VDAC1 in PCa cells by IP-MS analysis. Among the top ten scored proteins (VDAC1, VSTM2L, PHB2, CSTA, ALB, HSPD1, DDX1, HNRNPM, RPS3 and PHB1) (Fig. 1A, Fig. S1) (Supplementary Data 1), Mitochondrial inner membrane receptor protein PHB1 and PHB2 were regulators of mitophagy in cancer, ischemia-reperfusion (I/R) injury, cardiomyopathy and other diseases31,32,33. CSTA, a cysteine endopeptidase inhibitor with previously shown antinutritional effect on mites, was widely studied in cancer and immune defense34,35. Albumin (ALB) as a serum biomarker, the level is important to cardiovascular36. HSPD1, DDX1, and HNRNPM were widely reported in cancer. RPS3, had been well studied in DNA repair and apoptosis, was identified as a non-Rel subunit of NF-kappaB and located in nucle37,38. VSTM2L, as top one-scored protein, was reported related to few types of cancer29,30, but the biological functions of VSTM2L are not clear. Moreover, the functional role of VSTM2L in PCa cells has never been reported. So, we focus on the investigation for the function of VSTM2L in PCa. To confirm the IP-MS results, co-immunoprecipitation (co-IP) assay was performed in VDAC1 and VSTM2L co-transfected HEK293T cells, the results showed VDAC1 interacted with VSTM2L (Fig. 1B, C). Moreover, the semi-endogenous interaction between VSTM2L and VDAC1 was determined in Myc-VDAC1 overexpressed DU145 or 22Rv1 cell lines by Co-IP assay (Fig. 1D, E). The results illustrated that the interaction between VDAC1 and VSTM2L were observed in both androgen-sensitive (LNCaP, 22Rv1) and androgen-insensitive (DU145) cell lines. To further evaluate whether there is direct interaction between VSTM2L and VDAC1, we performed the Glutathione S-transferase (GST) pull-down assay and confirmed the direct binding of VSTM2L to VDAC1 (Fig. 1F). In addition, Coomassie Brilliant Blue staining of the IP cell lysates revealed that VSTM2L was successfully pulled down and further to LC-MS (Fig. 1G). Venn diagram revealed that 574 proteins were overlapped in VSTM2L and VDAC1 binding proteins (Fig. 1H) (Supplementary Data 2). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways analysis of these 574 proteins further revealed that these proteins were highly enriched in the Citrate cycle (TCA cycle), Glycolysis, and Fatty acid metabolism pathways (Fig. 1I). These results indicated that VSTM2L might play an important role in regulating mitochondria metabolism. In order to clarify the subcellular localization of VSTM2L in the PCa cells, we have separately extracted the mitochondria and cytoplasmic proteins from three prostate cancer cell lines (PC3, DU145, 22Rv1), respectively, and detected the protein levels of VSTM2L. As shown in Fig. 1J, VSTM2L mainly localized to the mitochondria rather than the cytoplasm in PC3, Du145, and 22Rv1 cells. Moreover, Immunofluorescence (IF) staining also showed that endogenous VSTM2L was localized to mitochondria in PCa cells (PC3, DU145, and 22Rv1) (Fig. 1K).
A The top ten proteins, identified by co-IP/MS, was elucidated in association with VDAC1 in LNCaP cells. B, C HEK293T cells were transfected with Myc-VDAC1 and 3 × Flag-VSTM2L expression plasmids as indicated, whole cell lysates were extracted and immunoprecipitated with anti-Flag (B) or anti-Myc (C) antibodies. D, E DU145 (D) and 22Rv1 (E) cells overexpressed Myc-VDAC1-pLVX or control cells were subjected to immunoprecipitation with anti-Myc antibody and followed by western blot analysis. F The direct interaction between VSTM2L and VDAC1 was confirmed by GST pull-down assay. GST-VSTM2L fusion proteins were purified and incubated with PC3 cell lysates and analyzed by western blotting. Purified GST proteins served as negative controls. G A representative image of an SDS-PAGE gel stained with Coomassie blue. LNCaP cells transfected with 3 × Flag-VSTM2L were collected for immunoprecipitation with anti-Flag antibodies, and separated by SDS-PAGE gel. The gel was subsequently stained with Coomassie blue to visualize the distinct binding bands. H Venn diagram showing the 574 proteins obtained by intersecting the IP-MS data of VDAC1 and VSTM2L. I The top 15 positively enriched KEGG pathways of the 574 proteins are shown. Hypergeometric test was used for P-value calculation. J Western blotting analysis of the expression of VSTM2L in mitochondria and cytoplasm of PC3, DU145 and 22Rv1 cells, respectively. Mitochondria and cytoplasmic protein levels were normalized to COX IV and α-Tubulin, respectively. The samples were derived from the same experiment and the gels/blots were processed in parallel. K Representative images of immunofluorescence staining for VSTM2L and MitoTracker in PC3, DU145, and 22Rv1 cells. The nucleus was stained with Hoechst. Scale bars, 8 μm. Source data are provided as a Source Data file.
Taken together, these results declared that an VDAC1 binding protein VSTM2L is localized to mitochondrial in PCa cells and might play a pivotal role in regulating mitochondria metabolism.
VSTM2L is positively associated with prostate cancer aggressiveness
To investigate the functional role of VSTM2L in prostate cancer, we analyzed the top 25 over-expression genes in prostate adenocarcinoma (PRAD) using the published cohort from TCGA. As shown in Fig. 2A, VSTM2L ranked as the 24th over-expression gene in PRAD. Further, we observed a marked elevation of VSTM2L mRNA levels in tumor specimens as compared to normal controls based on the TCGA PRAD cohort, GENT239 and UALCAN40 prostate patient cohorts, respectively (Fig. 2B, C; Fig. S2 A). Then, we measured the mRNA levels of VSTM2L in different PCa cell lines, and observed that VSTM2L was highly expressed in multiple PCa cell lines (22Rv1, DU145, and PC3) in comparison with normal prostate epithelial RWPE-1 cells (Fig. S2 B). Moreover, we collected 78 pairs of prostate tumor and adjacent tissues (the key clinicopathological features of the 78 paired samples were shown in Supplementary Data 3) and examined the protein levels of VSTM2L using immunohistochemistry (IHC) staining assay. The results indicated that the expression levels of VSTM2L were up-regulated in the tumor tissues compared to the adjacent tissues (Fig. 2D-E). In addition, a higher expression of VSTM2L referred to a poor prognosis of PRAD patients according to the Kaplan-Meier progression-free survival (PFS) and disease-free survival (DFS) analysis with the cohort from cBioPortal (Fig. 2F, G; Fig. S2 C–E). Overall, these results suggested that the expression levels of VSTM2L were apparently elevated in prostate cancer samples and associated with shorter survival of patients.
A The top (1-25) over-expressed genes in Prostate adenocarcinoma (PRAD) were analyzed based on the TCGA cohort by using the UALCAN database (Normal, n = 52; Tumor, n = 497). B The mRNA levels of VSTM2L in PRAD and normal tissues of TCGA PRAD cohort and GTEX prostate normal data. P = 7.168E-52. C The mRNA levels of VSTM2L in PRAD and normal tissues from GENT2 database. P = 3.2305E-7. D, E The protein levels of VSTM2L from 78 paired clinical prostate cancer specimens. The IHC staining score was used to quantify the protein levels of VSTM2L. Scale bar =250 μm. P = 2.7147E-17. F, G Association between Progression-free-survival (F) or Disease-free survival (G) of prostate cancer patients and VSTM2L mRNA expression from the TCGA database. H, I The cell proliferation was measured in VSTM2L knockdown 22Rv1 (Control VS shVSTM2L-1 P = 0.000045, Control VS shVSTM2L-2 P = 2.1828E-8) (H) or DU145 (Control VS shVSTM2L-1 P = 0.000002, Control VS shVSTM2L-2 P = 0.000011) (I) cells by CCK8 assay. Data are shown as the mean ± SD (n = 5 biological replicates). J, K The effects of VSTM2L knockdown on the growth of 22Rv1 (Control VS shVSTM2L-1 P = 4.3015E-7, Control VS shVSTM2L-2 P = 4.1099E-7) or DU145 (Control VS shVSTM2L-1 P = 2.9544E-7, Control VS shVSTM2L-2 P = 8.3674E-8) cells, as detected using the colony formation assay. Data are shown as the mean ± SD (n = 4 biological replicates). L-N Wound healing analysis for assessing migration of the indicated cell strains at 0 h and indicated endpoint. Representative images (L, M) and quantification (N) are shown as indicated. Data are presented as the mean ± SD (n = 3 biological replicates). Scale bar = 200 and 250 μm. O, P Representative pictures (O) and quantification analysis (P) of migration assays in control and VSTM2L knockdown 22Rv1 cells (Control VS shVSTM2L-1 P = 0.000008, Control VS shVSTM2L-2 P = 0.000007) or DU145 cells. Data are plotted as mean ± SD (n = 3 biological replicates). Scale bar = 250 μm. P value was determined by unpaired two-tailed Student’s t-test without adjustments. (B, C), paired two-tailed Student’s t-test without adjustments (E), log-rank test (F, G), two-way ANOVA (H, I) and one-way ANOVA (K, N, P). Source data are provided as a Source Data file.
We next explore the biological function of VSTM2L in PCa cells. Prostate cancer cell lines (22Rv1, DU145 and PC3 cells) were infected with lentivirus-based VSTM2L shRNA or scramble shRNA (Control) to obtain the stable strains of VSTM2L knockdown. The knockdown efficiency was detected by real-time PCR and western blot. As shown in Fig. S2 F, G, the VSTM2L shRNA lentivirus significantly decreased the mRNA and protein levels of endogenous VSTM2L compared with the control group. Then, the effect of VSTM2L knockdown on cell proliferation was investigated by CCK-8 assay. The results showed that knockdown of VSTM2L in PCa cell lines (22Rv1, DU145 and PC3 cells) significantly decreased cell proliferation ability compared with control group (Fig. 2H–I; Fig. S2 H). Suppression of VSTM2L expression in PCa cell lines also weakened cell colony formation ability (Fig. 2J, K; Fig. S2 I). The weakened clonogenic ability further demonstrates the significance of VSTM2L in the proliferation ability of prostate cancer cells and tumor formation in vitro. In addition, inhibition of VSTM2L expression restrained the migration abilities of PCa cell lines such as 22Rv1, DU145 and PC3 cells (Fig. 2L-P; Fig. S2 J-M). Taken together, these results indicated that VSTM2L deletion inhibited cell growth and migration in PCa cells, and VSTM2L might be a therapeutic target for PCa treatment in clinical.
VSTM2L suppression promotes ferroptosis of PCa Cells
After diminishing the expression of VSTM2L in 22Rv1 or Du145 cells, we observed the cells with less cellular antenna and the shape of the cells turned round (Fig. 3A). This phenotype suggested that the subcellular structure of VSTM2L depleted 22Rv1 or Du145 cells might have changed. To investigate the impact of VSTM2L on the subcellular structure of PCa cells, the transmission electron microscopy assay was performed after VSTM2L knockdown in 22Rv1 cells. At the cellular level, we observed a remarkable shrinkage of the cells with shRNA targeting VSTM2L in comparison with control cells (Fig. S3 A). At the subcellular level, we observed remarkable alterations of the morphology of mitochondria in VSTM2L knocked down 22Rv1 cells, mainly characterized by a general shrink in size, increase in membrane density, and diminution in cristae (Fig. 3B). In addition, living mitochondria imaging showed a reduction in mitochondria number, mean mitochondria area, mean mitochondria perimeter, mean mitochondria form factor and mean mitochondria aspect ratio in VSTM2L inhibited DU145 and 22Rv1 cells compared with control group. (Fig. 3C–E; Fig. S3 B). Furthermore, the network of mitochondria significantly changed in these cells as well (Fig. 3F; Fig. S3 C). These alterations induced by suppression of VSTM2L expression in prostate cancer cells closely resemble the morphological characteristics observed in cells undergoing ferroptosis41.
A The cell morphology of 22Rv1 and Du145 cells with VSTM2L shRNA (shVSTM2L) or control shRNA (control) were observed by inverted light microscopy. Scale bars = 200 μm. B Representative transmission electron microscopy (TEM) images of 22Rv1 cells with control or shVSTM2L. Scale bars = 1 μm / 500 nm. C–F Representative images (C) and quantification analysis (D–F) of high resolution laser confocal microscopy for the morphology of mitochondria in DU145 cells transfected with control or shVSTM2L. Data shown as mean ± SD, n = 3 for each group, Scale bars = 10 μm. G–I The 22Rv1(G) and DU145 (H) cells with VSTM2L shRNA (shVSTM2L-1, shVSTM2L-2) or control shRNA (Control) were stained with propidium iodide (PI, 3 μg/mL) and analyzed by flow cytometry to evaluate the cell death rate. Data is shown as mean ± SD of n = 3 biological replicates. 22Rv1 (Control VS shVSTM2L-1 P = 6.1418E-7, Control VS shVSTM2L-2 P = 1.1758E-7), DU145 (Control VS shVSTM2L-2 P = 0.00002). J Lipid peroxidation was measured by C11-BODIPY (5 μM) in 22Rv1 (Control VS shVSTM2L-1 P = 0.000019, Control VS shVSTM2L-2 P = 5.9221E-8) and Du145 cells with control or VSTM2L shRNAs. Data shown as mean ± SD of n = 3 biological replicates. K The level of glutathione (GSH) in 22Rv1 and Du145 cells with control or VSTM2L shRNAs. Data shown as mean ± SD of n = 3 biological replicates. L Western blot analysis of GPX4 and VSTM2L protein levels in VSTM2L knockdown PCa cells or control cells. Protein levels were normalized to total GAPDH. P value was determined by unpaired two-tailed Student’s t-test without adjustments (D, E, F) and one-way ANOVA (I, J, K). Source data are provided as a Source Data file.
Ferroptosis is an iron-dependent, non-apoptotic form of cell death triggered by extensive lipid peroxidation that overwhelms lipid protection mechanisms41,42. To determine whether VSTM2L plays roles in regulating ferroptosis, we performed propidium iodide (PI), Annexin V and BODIPY™ 581/591 C11 (C11-BODIPY) staining experiments to test the effect of VSTM2L suppression on the cell death and lipid peroxidation, respectively. The results indicated that reduced VSTM2L protein levels in 22Rv1 or Du145 cells could increase cell death rate (Fig. 3G–I) and lipid peroxide accumulation (Fig. 3J), but have no obvious effect on phosphatidylserine exposure of PCa cells (Fig. S3 D, E), suggesting that VSTM2L knockdown in PCa cells induced ferroptosis not apoptosis. To further confirm the regulation of VSTM2L in ferroptosis, we measured intracellular glutathione (GSH) levels in VSTM2L knocked down 22Rv1 or Du145 cells. As expected, down-regulation of VSTM2L in PCa cell lines attenuated GSH levels (Fig. 3K). We also checked the protein levels of the glutathione peroxidase 4 (GPX4), Bcl-2 associated X protein (Bax), B-cell lymphoma-2 protein (Bcl-2), Caspase3 and Cleaved-Caspase3(C-Caspase3) in VSTM2L knocked down 22Rv1 or Du145 cells by western blot. The results showed that inhibition of VSTM2L expression diminished the protein levels of GPX4 as expected in these two cell lines (Fig. 3L), but have no obvious effect on the level of Bax, Bcl-2, Caspase3 and C-Caspase3 (Fig. S3 F). Moreover, ferroptosis inhibitor ferrostatin-1(Fer-1) can rescue the cell death caused by knockdown of VSTM2L in PCa cells (Fig. S3 G–I). Accordingly, overexpression of VSTM2L in PCa cell lines could reverse cell death and the levels of GSH and GPX4 (Fig. S4 A–F). Collectively, these data suggested that VSTM2L acts as a potential suppressor of ferroptosis by GPX4 inhibition in prostate cancer cells.
VSTM2L inhibition sensitizes PCa cells to ferroptosis inducer, RSL3
To advance our understanding of the role and mechanism of VSTM2L in regulating ferroptosis, we investigated the effect of VSTM2L suppression on ferroptosis sensitivity to RSL3, a small molecule inhibitor target GPX443. Then, cell viability was checked by CCK-8 assay. After 24 h of RSL3 treatment, the viability of PCa cells with VSTM2L silencing, as indicated by the IC50 values, was significantly reduced compared to that of control cells (Fig. 4A). To further evaluate whether VSTM2L-knockdown promoted RSL3-induced ferroptosis in vivo, we constructed a xenograft model with nude mice using 22Rv1 cell lines. A schematic of the in vivo experiment, including tumor inoculation, RSL3 injection and the experimental timeline, was shown in Fig. 4B. All experimental mice maintained a good appetite and behavioral status after tumor inoculation and RSL3 administration until they were humanely euthanized and isolated the subcutaneous tumors. As expected, the VSTM2L suppression groups exhibited tumors with reduced volume and weight compared with the control groups, while there was an enhancement in the anti-tumor effects once VSTM2L suppression and RSL3 treatment were combined (Fig. 4C, D). The results suggested that inhibition of VSTM2L expression enhanced the in vivo therapeutic efficacy of RSL3. Meanwhile, we also tested the in vivo effects of the combined treatment on GSH level. Consistent with the in vitro cell results, VSTM2L knockdown in resected tumors weakened the levels of GSH, which was further reduced by RSL3 administration (Fig. 4E). Moreover, we also observed that tetrahydrobiopterin (BH4), an endogenous lipid antioxidant, changes in the same trend in vivo (Fig. 4F). These results indicated that the intervention of RSL3 indeed induces ferroptosis in vivo.
A Viability of 22Rv1 or DU145 cells transfected with VSTM2L shRNA or control shRNA was measured by CCK-8 in the presence of varying concentrations of RSL3 (0–100 μM) for 24 h. Data shown as mean ± SD of n = 3 biological replicates. B The schematic diagram of the in vivo experimental process. 1 × 106 22Rv1 shVSTM2L cells and control cells were inoculated trans-subcutaneous into nude mice. RSL3 (5 mg/kg) was intraperitoneally injected into the mice on alternate days for a total of six injections. The tumor tissues were isolated on 27th days post-inoculation. C, D General view of tumor weight (Control+RSL3 VS shVSTM2L + RSL3 P = 6.6076E-7) (C) and tumor volume (D) (examined every 3 days) of each indicated group at 27 days after cell injection. Data shown as mean ± SD, n = 5 tumors, ns, not significant. E, F The levels of GSH (E) and BH4 (Control VS shVSTM2L P = 0.000018) (F) in the tumor tissues at the indicated endpoint. Data shown as mean ± SD, n = 5 tumors. G-K Immunohistochemistry (IHC) and hematoxylin and eosin (H & E) staining for Ki-67 (Control VS shVSTM2L P = 0.000005, Control+RSL3 VS shVSTM2L + RSL3 P = 4.1427E-11) (G, H), VSTM2L (Control VS shVSTM2L P = 0.000014, Control+RSL3 VS shVSTM2L + RSL3 P = 0.000011) (G, I), GPX4 (Control VS shVSTM2L P = 0.000063, Control+RSL3 VS shVSTM2L + RSL3 P = 5.0408E-11) (G, J) and C-Caspase3 (G, K) were performed in isolated tumor tissues. Data shown as mean ± SD, n = 5 tumors, and each tumor slice randomly selected 5 magnification fields, Scale bar =20 μm. P value was determined by two-way ANOVA (A, D) and unpaired two-tailed Student’s t-test without adjustments (C, E, F, H-K). Source data are provided as a Source Data file.
Next, hematoxylin and eosin (H&E) staining was applied to evaluate the histomorphological changes within the tumor tissues. The results revealed that VSTM2L suppression induced cell death and administration of RSL3 further exacerbated the phenotype (Fig. 4G). Then, immunohistochemical (IHC) staining was utilized to assess the protein levels of VSTM2L, Ki67, GPX4, and C-Caspase3 in xenograft samples. The results showed that tumors from VSTM2L knockdown group exhibited reduced expression of Ki67, which was lower in the combination group with VSTM2L attenuation and RSL3 treatment, as expected (Fig. 4G, H). Furthermore, the down-regulation of VSTM2L expression was associated with a substantial decrease in GPX4 protein levels in the tumor tissues and the administration of RSL3 further suppressed the expression levels of GPX4 (Fig. 4G, I, J). However, no obvious changes were observed for C-Caspase3 levels both in VSTM2L suppression group and RSL3 treatment group, compared to Control groups (Fig. 4G, K). These results implied that the knockdown of VSTM2L and the intervention of RSL3 do not induce apoptosis but ferroptosis instead, in vivo. Thus, these results provided evidence that targeting VSTM2L and inducing ferroptosis may act as an efficient strategy in PCa treatment.
Fer-1 reverses VSTM2L suppression induced ferroptosis of PCa cells in the presence of RSL3
To further investigate the relationship between VSTM2L and ferroptosis, we performed CCK-8 assay to detect cell viability. The results showed that RSL3-induced cell death in DU145 shVSTM2L cells was abolished by the ferroptosis inhibitor ferrostatin-1(Fer-1), rather than a panel of apoptosis inhibitors (Z-VAD-FMK, Z-IETD-FMK, and Z-DEVD-FMK), as well as autophagy inhibitors 3-Methyladenine (3MA) and Chloroquine (CQ) (Fig. 5A). Moreover, VSTM2L knockdown markedly increased RSL3-induced cell death in 22Rv1 cells, and this effect was blocked by Fer-1 (Fig. 5B). We also observed the morphologic changes in VSTM2L depletion groups under the microscope. The cells have shorter antennae and tighter cell-cell contacts than control cells. RSL3 treatment increased the number of rounded cells, while Fer-1 could reverse this outcome, compared with the control groups (Fig. 5C). In addition, knockdown of VSTM2L in PCa cells accelerated the increase in RSL3-induced cell death, which was inhibited by Fer-1 (Fig. 5D–F). We also observed that the increased accumulation of lipid peroxidation triggered by RSL3 treatment in VSTM2L suppressed PCa cells could be fully reversed by Fer-1(Fig. 5G, H).
A Viability of DU145 cells transfected with VSTM2L shRNA (shVSTM2L-1, shVSTM2L-2) or control shRNA (Control) treated with or without RSL3 (2 μM), Fer-1 (5 μM), 3MA (60 μM), CQ (25 μM), Z-VAD-FMK (10 μM), Z-IETD-FMK (10 μM), Z-DEVD-FMK (10 μM) for 24 h. Data shown as mean ± SD (n = 3 biological replicates). ns, not significant. B Viability of 22Rv1 cells transfected with VSTM2L shRNAs or control shRNA treated with or without RSL3 (1 μM), Fer-1(10 μM). Data shown as mean ± SD (n = 3 biological replicates). ns, not significant. C Bright-field images of 22Rv1 cells with VSTM2L or control shRNA treated with or without RSL3 (1 μM) or Fer-1 (10 μM) for 24 h. D-F Representative flow cytometry histograms show the percentage of cell death in 22Rv1 (D) and DU145 (E) cells transfected with VSTM2L shRNAs or control shRNA, stained by PI (3 μg/mL). Quantification of the percentage of cell death rate in different cell lines (F). Cells were treated with or without RSL3 (DU145, 2 μM; 22Rv1, 1 μM) and Fer-1 (DU145, 5 μM; 22Rv1, 10 μM) for 24 h. Data shown as mean ± SD (n = 3 biological replicates). ns not significant. G Relative C11-BODIPY fluorescence measured by flow cytometry of PCa cells treated with VSTM2L shRNA or control shRNA and cultured with either RSL3 (DU145, 2 μM; 22Rv1, 1 μM), Fer-1 (DU145, 5 μM; 22Rv1, 10 μM) or both for 24 h. H The percentages of lipid peroxidation are presented as the means ± SD (n = 3 biological replicates). ns, not significant. P value was determined by two-way ANOVA. Source data are provided as a Source Data file.
In summary, VSTM2L ablation promotes RSL3 induced ferroptosis of PCa cells, which was blocked by Fer-1. These findings provided robust support for VSTM2L as a potential target to ferroptosis in prostate cancer.
VSTM2L suppresses VDAC1 oligomerization by facilitating the interaction between VDAC1 and HK2
VDAC1, located in the outer membrane of mitochondria (OMM), serves as a crucial gene for mitochondria quality control44. The oligomers of VDAC1 plays an important role in maintaining mitochondrial function and overall cell viability, including import phospholipids into mitochondria45. A recent published study indicates that phospholipids promote ferroptosis in mammalian cells7. To further investigate the mechanism of VSTM2L in regulating ferroptosis via interacting with VDAC1, we co-transfected distinct tagged versions of VDAC1 (Myc- and Flag-tagged) and different amounts of 3 × Flag-VSTM2L vectors into HEK293T cells. Subsequently, we conducted co-IP assay using anti-Myc antibody. The results suggested that the increased amount of VSTM2L did not affect the protein levels of VDAC1, but disturbed the oligomerization between Myc-VDAC1 and Flag-VDAC1, indicating that forced expression of VSTM2L could inhibit VDAC1 oligomerization (Fig. 6A). To further confirm this finding, we performed a cross-linking assay using ethylene glycol bis-(succinimidyl succinate) (EGS). Notably, the suppression of VSTM2L promotes the formation of VDAC1 oligomers; accordingly, VSTM2L overexpression inhibits the oligomerization of VDAC1 in prostate cancer cells (Fig. 6B).
A Co-transfected Myc-VDAC1, Flag-VDAC1, and different amounts of 3 × Flag-VSTM2L into HEK293T cells, the oligomerization of VDAC1 was analyzed by co-IP/WB. The samples derived from the same experiment and the gels/blots were processed in parallel. B VSTM2L suppressed or overexpressed PCa cells were harvested, respectively, and incubated with ethylene glycol bis-(succinimidyl succinate) to cross-link proteins, then subjected to western blot to evaluate VDAC1 oligomeric status. The samples were derived from the same experiment and that gels/blots were processed in parallel. C Schematic representation of the full-length VSTM2L and its truncated mutants was shown. D HEK293T cells were transfected with indicated full-length VSTM2L or its truncated forms, as well as full-length VDAC1. Cell lysates were harvested and subjected to immunoprecipitation with anti-Flag antibodies to map the binding region of VDAC1 with VSTM2L. E 22Rv1 cells were transfected with full-length VSTM2L or its truncated mutant as indicated. Cell lysates were collected and analyzed using a cross-linking assay to identify the functional region of VSTM2L responsible for inhibiting VDAC1 oligomerization. The samples derived from the same experiment and that gels/blots were processed in parallel. F A schematic of the full-length VDAC1 and its N-terminus truncated mutant was presented. G, H HEK293T cells were co-transfected with indicated full-length or N-terminus truncated mutant of VDAC1 and full-length VSTM2L. Cell lysates were collected and subjected to immunoprecipitation with anti-Myc (G) or anti-Flag (H) antibodies. Immunoprecipitants were analyzed with anti-Flag antibody and anti-Myc antibody. I A partial list of binding proteins of VSTM2L in LNCaP cells identified by mass spectrometry analysis. J, K HEK293T cells were co-transfected with V5-HK2, Myc-VDAC1 and 3 × Flag VSTM2L. Cell lysates were harvested and subjected to immunoprecipitation with anti-Flag (J) and anti-Myc (K) antibodies. Immunoprecipitants were analyzed with anti-Flag antibody, anti-V5 antibody and anti-Myc antibody. L DU145 cells overexpressed Myc-VDAC1-pLVX or control cells were subjected to immunoprecipitation with anti-Myc antibody and followed by western blot analysis. M The whole cell lysates were prepared from DU145 cells transfected with VSTM2L shRNA lentivirus or control shRNA lentivirus, and immunoprecipitations were performed with anti-HK2 antibodies, followed by western blot with indicated antibodies. The samples were derived from the same experiment and the gels/blots were processed in parallel. N Representative images of immunofluorescence staining for VDAC1 and HK2 in VSTM2L knockdown DU145 cells or control cells. The nucleus was stained with Hoechst. Scale bar 25 μm and 10 μm. Source data are provided as a Source Data file.
To explore the mechanism of VSTM2L in inhibiting the oligomerization of VDAC1, we established a series of functional domain deletions of VSTM2L (Fig. 6C). Mapping the binding domains revealed that the C-terminal domain of VSTM2L was essential for the binding to VDAC1 (Fig. 6D). Afterwards, we transfected the full-length (FL) and variant of functional domain deletions of VSTM2L into 22Rv1 cells. Cross-linking assay confirmed that full-length VSTM2L and its two domain deletions (S2, S3) indeed apparently inhibited the oligomerization of VDAC1, instead of C-terminal deletion (S1) of VSTM2L (Fig. 6E).
It was reported that the N-terminus of VDAC1 is the functional domain of VDAC146. And the N-terminus of VDAC1 is crucial for VDAC1 oligomerization47,48. Thus, the truncated mutant of N-terminus of VDAC1 (S1) was constructed to check whether VSTM2L could bind to N-terminus of VDAC1 by immunoprecipitation (Fig. 6F). The results showed that VSTM2L can bind at the C-terminus of VDAC1 (Fig. 6G, H). These findings prompted us to hypothesize that the inhibition of VDAC1 oligomer formation by VSTM2L may involve the participation of other proteins. To confirm this hypothesis, we checked the proteins interacted with VSTM2L by IP/MS analysis, and found that Hexokinase 2 (HK2) might be the potential protein connecting these two factors(Fig. 1G; Fig. 6I), as HK2 has been well-documented for the interaction with the N-terminus of VDAC1 to constrain VDAC1 oligomerization47,49,50. Importantly, our exogenous, semi-endogenous and endogenous co-IP assay suggested that a tripartite complex comprising VSTM2L, VDAC1, and HK2 indeed exists (Fig. 6J–M). This observation raised the question that whether VSTM2L inhibited VDAC1 oligomerization through facilitating the binding affinity between VDAC1 and HK2. To address this question, we utilized DU145 cell lysates with VSTM2L knockdown and performed immunoprecipitation with HK2-specific antibodies in parallel with IgG control antibodies to capture VDAC1 protein. The results showed that knockdown of VSTM2L weakened the interaction between VDAC1 and HK2 (Fig. 6M). Moreover, immunofluorescence staining showed that compared with the control group, HK2 was dissociated from VDAC1 after knockdown of VSTM2L in DU145 cells (Fig. 6N). Collectively, the results mentioned above indicated that VSTM2L was essential for mediating the interaction between VDAC1 and HK2.
In conclusion, these findings substantiated our hypothesis that VSTM2L enhances the binding affinity between VDAC1 and HK2, thereby inhibiting the oligomerization of VDAC1.
VSTM2L suppresses ferroptosis and maintains mitochondria homeostasis via inhibiting VDAC1 oligomerization in PCa cells
Accumulated evidences indicated that mitochondria homeostasis is crucial for suppression of ferroptosis in cancer cells7,10,51,52, VDAC1 oligomerization play a pivotal role in regulation of ferroptosis via maintaining the balance of mitochondrial reactive oxygen species (mtROS)26. Mitochondiral ROS was reported to induce ferroptosis51,53,54, while the mechanism by which ferroptosis is triggered in mitochondria is still unknown. Our quantification of mtROS test using MitoSOX probe, revealed that suppression of VSTM2L markedly increased mtROS levels in PCa cells compared with control group (Fig. 7A-B). Accordingly, overexpression of VSTM2L in PCa cells led to the decreased mtROS levels (Fig. S5 A).
A, B The levels of mtROS in 22Rv1 (Control VS shVSTM2L-1 P = 0.000001, Control VS shVSTM2L-2 P = 5.4234E-7) (A) and DU145 (Control VS shVSTM2L-1 P = 0.000001, Control VS shVSTM2L-2 P = 4.6453E-10) cells (B) transfected with VSTM2L shRNA (shVSTM2L-1, shVSTM2L-2) or control shRNA (Control). Data shown as mean ± SD of n = 3 biological replicates. C-F VSTM2L knockdown DU145 cells were cultured with or without VBIT4 (5 μM) for 24 h, then, cell death (C, D), lipid peroxidation (E) and mtROS (F) were assessed by PI (3 μg/μL), C11-BODIPY (5 μM) and MitoSOX (5 μM) staining, respectively. Data shown as mean ± SD of n = 3 biological replicates. ns not significant. G The level of GSH in VSTM2L inhibited DU145 cells treated with or without VBIT4 (5 μM) for 24 h. Data shown as mean ± SD of n = 3 biological replicates. ns not significant. H The level of lipid peroxidation in VSTM2L suppressed DU145 cells cultured with or without MitoQ (10 nM) for 24 h and assessed by C11-BODIPY (5 μM) staining. Data shown as mean ± SD of n = 3 biological replicates. ns not significant. I The level of MMP in VSTM2L knockdown DU145 cells cultured with or without VBIT4 (5 μM) for 24 h. Data shown as mean ± SD of n = 3 biological replicates. ns not significant. J, K Representative images of mitochondrial in VSTM2L depletion DU145 cells cultured with or without VBIT4 (5 μM) by MitoPeDPP (0.5 μM, Scale bars = 25 μm) (J) and Mito-Tracker (200 nM, Scale bars = 10 μm) (K) staining. L–N Mitochondria counts mean mitochondria area, mean mitochondria perimeter (L), mitochondria network (branch counts) (M), mitochondria morphology (mean mitochondria form factor and mean mitochondria aspect ratio) (N) for images shown in K by ImageJ were determined. Data shown as mean ± SD, n = 3 for each group. ns not significant. P value was determined by one-way (A) and two-way ANOVA (D- I, L-N). Source data are provided as a Source Data file.
To explore whether VSTM2L suppression contributed to the ferroptosis of PCa cells through regulating oligomerization of VDAC1, we employed a VDAC1 oligomerization inhibitor, VBIT455,56, in our subsequent studies. The increased cell death, massive lipid peroxidation accumulation, elevated mtROS levels and diminished GSH concentrations, caused by VSTM2L knockdown were dramatically reversed by VBIT-4 (Fig. 7C–G; Fig. S5 B, C). Further, the shVSTM2L cells were cultured with a mitochondira-targeted antioxidant, Mitoquinone (MitoQ), which significantly reduced the accumulation of lipid peroxidation and mtROS (Fig. 7H; Fig. S5 D). These findings suggested that VDAC1 oligomerization inhibitor prevented PCa cells from VSTM2L knockdown-induced VDAC1 oligomerization and ferroptosis. And the accumulation of mtROS, resultant from increased formation of VDAC1 oligomers, is the principal cause of ferroptosis in VSTM2L depletion PCa cells.
To further confirm the notion that the promotion of VDAC1 oligomerization, consequent to VSTM2L knockdown, triggers a comprehensive collapse of mitochondrial homeostasis in PCa cell lines, the JC-1 probe was applied to assess MMP. The results illustrated the decreased MMP levels following the suppression of VSTM2L, and this effect was counteracted by VBIT4 (Fig. 7I). It has been established that mitochondrial lipid peroxidation, driven by accumulated mtROS, plays a pivotal role in the initiation of ferroptosis57. Herein, we employed MitoPeDPP58 probe to check mitochondrial lipid peroxidation detected by using a confocal microscopy. The results revealed that VSTM2L knockdown in DU145 cells showed pronounced bright green fluorescence compared with the control cells, and the MitoPeDPP signal caused by VSTM2L suppression was virtually abolished in the presence of VBIT4 (Fig. 7J). Notably, living imaging of subcellular mitochondria by confocal microscopy demonstrated that VSTM2L knockdown in DU145 cells led to a dramatic mitochondrial morphology and network changes, including reductions in mitochondria number, mean mitochondria area, mean mitochondria perimeter, and branch counts, which were reversed by VBIT4 (Fig. 7K-M; Fig. S6). However, VBIT4 did not show any significant improvement on the reduction of mean mitochondria form factor and mean mitochondria aspect ratio, which were induced by the downregulation of VSTM2L in DU145 cells (Fig. 7N). The results explained the reason that VBIT4 cannot fully reverse the various changes brought about by the knockdown of VSTM2L in PCa cells.
Collectively, the aforementioned results provided ample evidences that the enhanced VDAC1 oligomerization caused by VSTM2L knockdown led to a disruption of mitochondrial homeostasis, thereby contributing to the ferroptosis of PCa cells.
VSTM2L plays oncogenic roles in PCa by inhibiting VDAC1 oligomerization
To explore whether the oligomerization of VDAC1 suppressed by VSTM2L contribute to regulating PCa cells growth, we conducted experiments both in vitro and in vivo with the intervention of VBIT4. We cultured the shVSTM2L DU145 cells or control cells under treatment with VBIT4 for 48 h. EGS-based cross-linking assay suggested that VBIT4 successfully reversed the oligomerization of VDAC1 promoted by the suppression of VSTM2L (Fig. 8A). Then, CCK-8 assay declared that VBIT4 could obviously rescue the diminished proliferation and colony formation abilities caused by VSTM2L knockdown in DU145 cells (Fig. 8B–D). Additionally, VBIT4 reversed the reduced migration rate in shVSTM2L cells as well (Fig. 8E–H). Moreover, 3.5 × 106 DU145 shVSTM2L cells and the control cells were inoculated trans-subcutaneous into nude mice to establish a xenograft model, respectively, the schematic of the experiment was shown in Fig. 8I. VBIT4 (20 mg/kg) was exposed to the mice by gavage each other day until the endpoint. Importantly, VBIT4 had demonstrated the capacity to ameliorate the decelerated growth of tumor tissues resulting from VSTM2L knockdown within the tumor tissues (Fig. 8J-L). Further, H&E staining was performed to assess the histomorphological changes within the tumor tissues. The results showed that VSTM2L depletion induced cell death, and administration of VBIT4 reversed the phenotype (Fig. 8M). Then, immunohistochemical (IHC) staining was utilized to evaluate the protein levels of VSTM2L, Ki67, GPX4 and C-Caspase3 in xenograft samples, respectively. The data indicated that tumors from VSTM2L knockdown group exhibited reduced expression of Ki67, which was rescued by VBIT4 treatment (Fig. 8M, N). Moreover, VSTM2L suppression was associated with a substantial decrease in GPX4 protein levels in the tumor tissues and the administration of VBIT4 reversed the expression of GPX4 (Fig. 8M, O, P). However, no obvious changes were observed for C-Caspase3 levels both in VSTM2L depletion group and VBIT4 treatment group, compared to Control groups (Fig. 8M, Q).
A VSTM2L knockdown DU145 cells cultured with or without VBIT4 (5 μM) for 48 h were harvested for the subsequent cross-link assay, then subject to western blot to assess VDAC1 oligomeric status. The samples derived from the same experiment and that gels/blots were processed in parallel. B–D VSTM2L suppressed DU145 cells were cultured with or without VBIT4 (5 μM) for the indicated time, then the cell viability (n = 5 biological replicates) (B) and colony formation ability (n = 4 biological replicates) (C, D) were analyzed by CCK8 assay and Crystal Violet Aqueous Solution staining, respectively. Data shown as mean ± SD. ns not significant. E, F Wound healing analysis of VSTM2L inhibited DU145 cells in the absence or presence of VBIT4 (5 μM). Representative images (E) and quantification (F) are shown as indicated. Data shown as mean ± SD of n = 3 biological replicates. ns not significant, Scale bars = 200 μm. G, H Representative pictures (G) and quantification analysis (H) of migration assays in VSTM2L knockdown DU145 cells cultured with or without VBIT4 (5 μM). Data shown as mean ± SD of n = 3 biological replicates. ns not significant, scale bars = 250 μm. I A schematic diagram of the in vivo experimental process. 3.5 × 106 DU145 shVSTM2L cells and control cells were inoculated trans-subcutaneous into nude mice. The mice were administrated with VBIT4 (20 mg/kg) by gavage on alternate days until the tumor tissues were isolated on 31th days post-inoculation. J, K General view of tumor weight of each indicated groups at the endpoint. Data shown as mean ± SD, n = 5 tumors, ns not significant. L Tumor growth curves were shown. Data shown as mean ± SD, n = 5 tumors, ns not significant. M–Q Immunohistochemistry (IHC) and hematoxylin and eosin (H & E) staining for Ki-67 (M, N), VSTM2L (M, O), GPX4 (M, P) and C-Caspase3 (M, Q) were performed in isolated tumor tissues. Data shown as mean ± SD, n = 5 tumors and each tumor slice was randomly selected 5 magnification fields, Scale bars =20 μm. P value was determined by two-way ANOVA (B, D, F, H, L) and unpaired two-tailed Student’s t-test without adjustments (K, N–Q). Source data are provided as a Source Data file.
Taken together, these results indicated that VSTM2L exerts its oncogenic roles in prostate cancer by impeding the assembly of VDAC1 oligomers both in vitro and in vivo.
Discussion
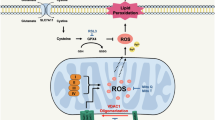
In the present study, we found that a VDAC1 binding partner VSTM2L, mainly localized to mitochondria, plays an oncogenic role in PCa cells. Suppression of VSTM2L led to the dissociation of HK2 from VDAC1 and the increased oligomerization of VDAC1, which disturbed the homeostasis of mitochondria, triggered the ferroptosis, and eventually restrained PCa progression (Fig. 9).
In recent years, the roles of VSTM2L in different cancers have already been reported in the literature. It was reported that VSTM2L is recognized as a potential biomarker associated with ovarian cancer metastasis and prognosis28. High expression of VSTM2L induced resistance to chemoradiotherapy in rectal cancer and might be a potential resistant predictable biomarker for advanced rectal cancer patients receiving preoperative chemo radiotherapy29. Recently, VSTM2L was reported to be a secreted protein related to the viability and survival of cholangiocarcinoma cells30. So far, the subcellular localization and function of VSTM2L in PCa has never been reported. Our study reveals a previously unrecognized localization to mitochondrial for VSTM2L in PCa cells. We also found that VSTM2L was highly expressed in prostate tumors and positively associated with the poor prognosis of PCa patients. Suppression of VSTM2L weakened cell growth and migration ability in PCa. Our findings indicated that VSTM2L plays an oncogenic role in PCa progression.
Ferroptosis is a form of programmed cell death dependent on iron and reactive oxygen species, is mainly characterized by mitochondrial shrinkage, increased density of mitochondrial membranes, reduction or vanishing of mitochondria crista, the accumulation of lipid peroxidation and GSH depletion59,60,61. In our study, we observed a remarkable shrinkage of the cells with shRNA targeting VSTM2L. We also found significant alterations of the morphology of mitochondria in VSTM2L knocked down 22Rv1 cells, mainly characterized by a general shrinkage in size, increased membrane density, and diminution in cristae. Reduction of VSTM2L levels in PCa cells led to increased cell death, lipid peroxide accumulation and decreased GSH levels. Further, VSTM2L knockdown in PCa cells restrained the protein levels of GPX4 not C-Caspase3. In addition, VSTM2L suppression weakened BH4 level, which was exacerbated by RSL3 treatment in vivo. The GTP cyclohydrolase 1 (GCH1) - BH4 system is one of the four reported ferroptosis defense systems at present. The other three are GPX4 - GSH system, ferroptosis suppressor protein 1 (FSP1) - ubiquinol (CoQH2) system, and dihydroorotate dehydrogenase (DHODH) - CoQH2 system62,63. These findings suggested that VSTM2L is a modulator of ferroptosis and protects PCa cells against ferroptosis not apoptosis. However, how VSTM2L regulates the GPX4 expression and BH4 level needs to be further investigated.
Accumulating evidences indicated that RSL3 can induce ferroptosis by inhibiting GPX4 and suppress tumor growth64,65. Ferroptosis inducers (Erastin or RSL3) and in combination with standard-of-care second-generation antiandrogens are therapeutic strategies for advanced prostate cancer66. Ferroptosis has been confirmed to enhance the sensitivity of castration-resistant prostate cancer cells to therapeutic agents5,6. In this study, we discovered that VSTM2L knockdown enhanced the sensibility of RSL3-induced ferroptosis in vitro and in vivo, the discovery holds significant implications for VSTM2L as a prognostic target for prostate cancer.
VDAC1 plays a crucial role in regulation of ferroptosis, and this regulation is achieved through the oligomeric form of VDAC1 rather than its monomeric form. It was reported that protecting mitochondria via inhibiting VDAC1 oligomerization weakened hepatocyte ferroptosis26. Zhou et al. reported that BABP31 could directly bind to VDAC1 and regulate gastric cancer cells ferroptosis through affecting VDAC1 stability and oligomerization25. However, the mechanisms of VDAC1 oligomerization in regulating ferroptosis are not well characterized. Our study found that VSTM2L knockdown in PCa cells promoted VDAC1 oligomerization and inhibited cell growth and migration, which were reversed by VBIT4, an inhibitor of VDAC1 oligomerization, in vitro and in vivo. These findings indicated that VSTM2L regulated prostate cancer progression and ferroptosis via directly binding to VDAC1 and affecting VDAC1 oligomerization.
The three-dimensional structure of VDAC1 shows a channel composed of 19 β-strands with an N-terminal α-helix located near the midpoint of the pore, and the majority of models involve significant conformational shifts of this N-terminal region44,46. The mobility and disorder of the N-terminal domain were confirmed in a crowd of biophysical studies, suggesting that it could exist in a dynamic equilibrium between random coil and α-helix67. The N-terminus of VDAC1 has been identified as the functional domain and response for the oligomerization of VDAC146,47,48. Interestingly, we discovered a binding protein of VSTM2L, HK2, which is well-documented in the literature for its interaction with the N-terminus of VDAC147,49,50, thereby restricting it from freely diffusing out of the pore and constraining VDAC1 oligomerization24,68. In our study, we found that mitochondria-localized VSTM2L plays vital role in maintaining mitochondria homeostasis by stabilize mitochondrial ROS and maintain mitochondrial membrane potential (MMP) in prostate cancer cells. MMP is of crucial significance to the conformation of VDAC1. After the MMP rises, the pore of VDAC1 closes and its conformation changes69,70. Our finding indicated that reduced VSTM2L expression in prostate cancer cells led to the decreased MMP, and the pore of VDAC1 became larger. Further, based on the three-dimensional conformation of VDAC144, we can discover that the β-helix structure of VDAC1 did not make its C-terminal and N-terminal keep in exactly opposite directions. Considering that HK2 binds to the N-terminal of VDAC171, VSTM2L binds to the C-terminal of VDAC1 and VSTM2L depletion led to the dissociation of HK2 from VDAC1, we speculate that the narrowed pore of VDAC1 can shorten the physical distance between VSTM2L and HK2 on the mitochondria and maintain the stability of the HK2/VSTM2L/VDAC1 complex. Thus, we speculated that HK2/VSTM2L/VDAC1 complex makes VDAC1 keeps a stable three-dimensional structure to maintain mitochondrial homeostasis, protects prostate cancer cells against ferroptosis, thereby promoting the development of prostate cancer. However, the detailed conformation of HK2/VSTM2L/VDAC1 complex on mitochondria needs to be further investigated in PCa.
Mitochondrial homeostasis is increasingly acknowledged as essential players in initiating and amplifying ferroptosis7,8. Shoshan-Barmatz et al. reported that VDAC1 acted as a key role in regulating mitochondrial homeostasis and cell death13,49,56, mainly relies on the interactions with the relative proteins. Yagoda et al. reported that erastin, the ferroptosis activator identified in 2003, binds to VDAC2/3 to change mitochondrial membrane permeability and reduce NADH oxidation rates, a mechanism distinctly different from how VDAC1 regulates ferroptosis72. Herein, we found that VSTM2L suppression led to the increased cell death, diminished GSH levels, the decreased MMP levels, mitochondrial lipid peroxidation accumulation, elevated mtROS levels and changed morphology of mitochondria, which were all blocked by VBIT4. Importantly, MitoQ reduced the accumulation of lipid peroxidation and mtROS caused by VSTM2L knockdown. These results suggested that the enhanced VDAC1 oligomerization by VSTM2L knockdown led to a disruption of mitochondrial homeostasis. And mtROS burst induced by the upregulated VDAC1 oligomerization, contributed to mitochondrial homeostasis disruption and then onset of PCa ferroptosis. These findings were consistent with the previous study that mtROS imbalance is accountable for disrupting mitochondrial homeostasis and triggering ferroptosis51,53,54.
In summary, VDAC1 binding partner VSTM2L, was identified as an oncogene and a ferroptosis suppressor in prostate cancer, and VSTM2L regulated PCa progression and ferroptosis via affecting oligomerization of VDAC1. Thus, VSTM2L could serve as a prognostic biomarker for PCa progression and a therapeutic target for PCa treatment.
Methods
Cell culture
Human normal prostate epithelial cell line RWPE1, prostate cancer cell lines (DU145, PC3, 22Rv1 and LNCaP) and HEK293T cells were purchased from the American Type Culture Collection (ATCC). All cell lines were confirmed to be mycoplasma free during our study. The prostate cancer cell lines were authenticated by the commercial suppliers before purchase using short tandem repeat (STR) profiling. All the cells were cultured at 37 °C, with 95 % air and 5 % CO2. RWPE-1 cells were cultured with keratinocyte serum-free medium (Gibco, # 10724-011) with bovine pituitary extract and human recombinant epidermal growth factor (EGF); HEK293T and DU145 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, # 12800017), PC3 cells were cultured in Ham’s F-12 medium (HyClone, # SH30526.01), 22Rv1 and LNCaP cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, # 31800022). 10% (Volume/Volume, v/v) fetal bovine serum (FBS, MCE, # HY-T1000) and 1% (Volume/Volume, v/v) penicillin-streptomycin (PS, Solarbio, # P1400) were supplied to the base medium.
Plasmid constructs and transfection
VSTM2L, VDAC1, HK2 cDNAs were generated in our lab and subcloned into pFLAG-CMV-2, pcDNA3.1 Myc-His A, p3 × FLAG-CMV-8, pcDNA3.1 V5-His A, pEGFP-N1, pLVX-mCNV-Zsgreen1-puro, as indicated in the corresponding figures. The cDNA of VSTM2L was subcloned into the pCDH-CMV-MCS-EF1-Puro vector. Using full-length pEGFP-N1-VSTM2L and p3 × FLAG-CMV-8-VDAC1 cDNAs as templates, a series of functional domain deletions of VSTM2L and VDAC1 were generated.
For lentiviral construct, the shRNA constructs targeting VSTM2L were designed and inserted into the lentiviral vector pLKO.1, the Myc-VDAC1 cDNA was subcloned to lentiviral vector pLVX-mCNV-Zsgreen1-puro. For lentiviral production, HEK293T cells were co-transfected with indicated shRNA constructs or overexpression constructs (1.5 µg each), pMD2G (envelope plasmid, 0.375 µg) and psPAX2 (packaging plasmid, 1.125 µg). In all transfection experiments, Lipofectamine 3000 reagents (Invitrogen, # L3000015) or PEI (MCE, # HY-K2014) were utilized according to the manufacturer’s instructions. The primer and shRNA sequences mentioned previously were comprehensively enumerated in Supplementary Data 4.
Xenograft models
All animal experiments in this project were approved by the Animal Care and Use Committee of Shaanxi Normal University (Approval No. 2024-072). The maximal tumor size permitted by the Animal Care and Use Committee of Shaanxi Normal University is 2000 mm3 in volume for subcutaneous tumor and the tumors of all animals in this study didn’t exceed the criteria. 4-week-old male BALB/C-nu/nu mice were obtained from HFK Bioscience CO., LTD (Beijing, PR China). All mice were maintained under a 12-h light/dark cycle with free access to water and standard mouse chow and housed in a specific-pathogen-free (SPF) facility. All mice were randomized, with each group consisting of five mice, subsequently, one week of adaptive feeding was implemented.
PCa cell lines were suspended and counted in cold phosphate-buffered saline (PBS), and 1 × 106 22Rv1 cells (150 μL, containing 30% matrigel) or 3.5 × 106 DU145 cells (150 μL) were injected into mice subcutaneously. When the tumors reached a minimum size of 1 × 1 mm, the mice were assigned randomly into different treatment groups. RSL3 and VBIT4 were dissolved in DMSO and diluted in corn oil. RSL3 (5 mg/kg) was intraperitoneally injected into the mice each other day, within 10 days. VBIT4 (20 mg/kg) was exposed to the mice by gavage each other day until the endpoint. Tumor size was measured by vernier caliper every 3 days and the volume was calculated according to the following formula: volume=length × width2 × 1/2. Mice were sacrificed at the endpoint as indicated in the corresponding figures, meanwhile, the tumors were isolated and saved in 4% paraformaldehyde until use.
Western blot
Total protein of PCa cell lines was extracted with lysis buffer (500 mM NaCl, 20 mM Tris-HCL pH 8.0, 1% NP-40, 5 mM EDTA, 1 mM DTT, 1 × Protease Inhibitor), the protein concentration was measured using Bicinchoninic Acid Protein Assay Kit (Thermo Scientific, # 23227). Then, 80 μg protein samples mixed with loading buffer were loaded and separated by 15% SDS-PAGE gel (Epizyme Biotech, # PG214). Finally, the protein bands were exposed on a film using the ECL kit in a darkened environment. All primary anti-bodies and concentrations used in this study were listed in the Supplementary Data 5.
GST Pull-down assay
GST Pull-down assay was conducted as previously described by our team73. Briefly, VSTM2L cDNA was cloned to pGEX-6P-1 (GST fusion tag), and the recombinant GST-VSTM2L was expressed in E.coli BL 21, induced by isopropyl-β-D-thiogalactopyranoside (IPTG, 0.5 mM final concentration) at 30 °Covernight on a constant temperature shaker. Subsequently, the bacteria solution was centrifugated at 4000 g for 3 min to collect the pellets at the bottom of the tube. After washing the pellets twice with cold PBS, the pellets were then resuspended and lysed in lysis buffer described above. The expression of recombinant GST-VSTM2Lprotein was confirmed by SDS-PAGE, followed by Coomassie Brilliant Blue staining. Furthermore, glutathione-Sepharose 4B beads (Thermo Scientific, # 16101) were washed three times using 1 mL cold PBS, before incubated with GST or GST-fusion protein at 4 °C. The combined beads were collected and washed as above and then incubated with PC3 cell lysis at 4 °C overnight on a rotor. Finally, beads were harvested and washed as above, resuspended in 2 × Loading buffer and probed by western blotting.
Co-Immunoprecipitation coupled with mass spectrometry (CO-IP/MS)
Cell pellets were harvested and resuspended in 600 μL lysis buffer by sonication for 1 min, and then centrifuged at 12000 g for 15 min at 4 °C. For coimmunoprecipitation, the supernatant was incubated with 3 μg indicated antibodies for 8-10 h at 4 °C on a rotor. Subsequently, the combined protein lysis was incubated with 45 μL of protein A/G-agarose beads (Thermo Scientific, # 20422) at 4 °C on a rotor overnight. The combined beads were washed three times with cold lysis buffer, and mixed with 2 × loading buffer for western blot analysis.
For immunoprecipitation/mass spectrometry (IP/MS), LNCaP cells overexpressing Flag-VDAC1 or 3 × Flag-VSTM2L were harvested, resuspended in 700 μL of lysis buffer, and centrifuged at 12000 g for 15 min at 4 °C. The supernatant was incubated with 7 μg anti-Flag antibody for 8–10 h at 4 °C, followed by incubating with 70 μL of protein A/G-agarose beads at 4 °C overnight. After extensive washes, the immunoprecipitates were resolved with 2 × loading buffer for 15 % SDS- PAGE gel separation. After the gel was stained with Coomassie Brilliant Blue, only the differential bands of the IP groups (n = 1) were excised for mass spectrometry experiments.
Following the protein quality test and trypsin treatment, LC-MS/MS analysis was conducted. The UHPLC-MS/MS analyses were performed using an EASY-nLCTM 1200 UHPLC system (Thermo Fisher, Germany) coupled with an Q ExactiveTM HF-X mass spectrometer (Thermo Fisher, Germany). For LC analysis, the lyophilized powder was dissolved in mobile phase (100% water, 0.1 % formic acid), centrifuged at 14000 g for 20 min at 4 °C, and 1 μg of sample was injected into EASY-nLCTM 1200 nano-upgraded UHPLE system with a home-made C18 Nano-Trap column (4.5 cm × 75 μm, 3 μm). Peptides were separated in a homemade analytical column (15 cm × 150 μm, 1.9 μm). For MS analysis, the separated peptides were analyzed by Q ExactiveTM HF-X mass spectrometer to get the raw data. The resulting spectra from each fraction were searched separately against Uniport database (homo_sapiens_uniprot_2023_10_18_Swissprot.fasta) by the search engines: Proteome Discoverer. The search parameters are set as follows: mass tolerance for precursor ion was 10 ppm and mass tolerance for product ion was 0.02 Da. Carbamidomethyl was specified as fixed modifications, Oxidation of methionine (M) was specified as dynamic modification, and loss of methionine at the N-Terminal. A maximum of 2 missed cleavage sites were allowed. In order to improve the quality of analysis results, the software PD further filtered the retrieval results: Peptide Spectrum Matches (PSMs) with a credibility of more than 99% was identified PSMs. The identified protein contains at least 1 unique peptide. The identified PSMs and protein were retained and performed with FDR no more than 1.0%. KEGG (Kyoto Encyclopedia of Genes and Genomes) were used to analyze the protein family and pathway. The specific conditions for Coomassie Brilliant Blue staining and LC-MS are displayed in Supplementary Fig. 1, Supplementary Data 1, and Supplementary Data 2.
RNA extraction and reverse-transcription quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using RNAiso Plus (Takara, # 9109) according to the manufacturer’s instructions. RNA concentration and purity was analyzed with QubitRNA BR kit (Invitrogen, # Q10211). cDNA was synthesized using the ABScript II cDNA first strand synthesis kit (ABC clone, # RK20400). qPCR reaction was performed by using SYBR Green qPCR Master Mix (TargetMol, # C0006), β-actin was used as the internal reference gene. All primer sequences were listed in Supplementary Data 4.
Cell proliferation and viability assay
1 × 103 PC3 or DU145 cells, 2 × 103 22Rv1 cells per well were seeded in 96-cell plates for cell proliferation. 2 × 103 DU145 cells or 2 × 104 22Rv1 cells per well were seeded in 96-cell plates for cell viability test under different drug stimulations. 10 μL/well CCK-8 regent (CCK-8, TargetMol, # C0005) was added for 4 h before the indicated time points, and then the data were collected by measuring the absorbance at 450 nm. Values were obtained from triplicate wells.
Trans-well assay
Cells were trypsinized and rinsed twice with PBS, followed by resuspended in serum-free medium at concentrations of 2 × 105 for 22Rv1, 3 × 105 cells / mL for DU145 and PC3 cell lines. A 200 μL aliquot of the single-cell suspension was carefully pipetted into Trans-well inserts, either with or without VBIT4 (TargetMol, # T13287). Concurrently, the lower chambers were filled with 750 μL complete culture medium containing 10% FBS. After incubating for the indicated durations, the inserts were rinsed twice with cold fresh PBS, then fixed with 3.7% formaldehyde solution for 5 min at room temperature, followed by another twice rinse. Permeabilization was achieved using cold methanol at -20 °C for 20 min, again followed by double rinse. The cells were ultimately stained with 0.1% Crystal Violet Aqueous Solution (Solarbio, # G1064) for 30 min at room temperature, after which excess dye was wiped off with a cotton swab. The migrated cells were observed and photographed using an Inverted Microscope (Leica, DMi8 automated) or Stereo Microscope (Carl Zeiss Microscopy GmbH). The migration rate, in terms of number or area, was quantitatively analyzed using ImageJ software.
Colony formation assay
500 cells for DU145 or PC3 cells, 1000 cells for 22Rv1 per well were seeded on 24-well plates for 24 h before relevant treatment. The medium was changed every three days. At the end time point, cells were stained with 0.1% Crystal Violet Aqueous Solution. The colony (with >50 cells) number was counted by ImageJ software.
Wound healing assay
Cells were seeded onto 24-well plates at a density of 2 × 105 for DU145 or PC3 cells and 2 × 106 for 22Rv1 cells until confluence. The plates were covered with three evenly and straight location lines across the back by a mark-pen ahead of the scratch. A 200 μL micropipette tip was used to create three evenly distributed scratches, perpendicular to the location line, then washing the wells three times with PBS to remove the floating cells. Then, the complete medium with or without VBIT4 was added into the well. The plates were observed and photographed using an inverted microscope (Leica, DMi8 automated) initially at 0 h and subsequently at the designated endpoint. The wound area was calculated using ImageJ software. The relative migration rate was calculated using the following formula: (wound area at 0 h - wound area at endpoint) / wound area at 0 h × 100%. Three replicates were set for each experiment, and a minimum of three paired pictures were collected per replicate to ensure reproducibility and reliability of the data.
Transmission electron microscope (TEM)
For the analysis of mitochondrial ultrastructure, 22Rv1 cells were fixed with 2.5% glutaraldehyde (resolved in 10 mM PBS (pH 7.0–7.5)) for 2 to 4 h at 4 °C. Then the cells were pre-embedded in 1% agarose and post-fixed with 2% ferrocyanide-reduced osmium tetroxide for 2 h at room temperature in the dark, followed by washing three times with PBS (100 mM, pH 7.4). Subsequently, the dehydrated cells were embedded in resin and sectioned to a thickness of 60–80 nm. The sections were stained with 2% uranium acetate saturated alcohol solution in the dark for 8 min, followed by three washes in 70% alcohol and ultra-water, respectively. Additionally, the sections were stained with 2.6% lead citrate solution for 8 min in a CO2-free environment. The samples were then photographed using a transmission electron microscope.
Mitochondria analysis
Mito-Tracker Deep Red (Beyotime, # C1032) was used to stain the mitochondria, and the nuclei was stained with Hoechst 33342 (TargetMol, # T5840). Briefly, cells were seeded onto the laser confocal dishes for 48 h. The cells were washed twice with warm, serum-free medium and covered with 200 nM Mito-Tracker Deep Red solution (diluted with warm, serum-free medium), then the cells were incubated at 37 °C for 30 minutes in the dark. The labeled cells were washed twice as above, and covered with 3 μg/mL Hoechst solution at 37 °C for 15 minutes in the incubator. After another two times washing procedure, 1 mL warm, complete culture medium with or without VBIT4 was added onto the cells. A high-resolution laser confocal microscope (Leica) with 63x/1.40 Oil DIC objective was used to observe and the labeled cells were photographed, which were lied at 37 °C with humidified atmosphere of 5% CO2.
Quantification of Mitochondrial membrane potential (MMP, Δψm)
Mitochondrial membrane potential was assessed using JC-1 probe (Solarbio, # M8650), as previously described with modifications74. A total of 2 × 105 DU145 cells were seeded onto 24-cell plates overnight and treated with or without 5 μM VBIT4 for 24 h. After discarding the culture medium, the adherent cells were washed twice with fresh PBS. Subsequently, 300 μL of JC-1 working solution was added into the well. The cells were then incubated in the dark at 37 °C for 20 minutes. The labeled cells were washed twice with the cold washing buffer, then trypsinized and resuspended in PBS with 2% FBS for quantification using a flow cytometer (Beckman). JC-1 monomers and aggregates were detected at PE and FITC channels, respectively. The positive control treated with 10 mM CCCP for 24 h was used to adjust compensation, detailed in Supplementary Fig. 7. 30,000 cells were collected per sample.
Cell death assay
Propidium iodide (Beyotime, # ST511) staining was performed to assess cell death, as previously described8,75. Briefly, 2 × 105 cells were seeded on 24-cell plates for 24 h before treatment. After relevant stimuli, cells were collected (including floating dead cells) and washed twice with PBS (Solarbio, # P1010), then incubated with 500 μL PI staining solution (3 μg/ml) in PBS for 30 min at 37 °C. Next, cells were resuspended in 200 μL PBS with 2% FBS, and the cell death rate was measured using flow cytometer. At least, 1 × 104 single cells were collected per well, and three replicates were set for each experiment.
Quantification of lipid peroxidation
To quantify the level of lipid peroxidation of various treatment cells, BODIPY™ 581/591 C11 (Thermo Scientific, # D3861) was used as previously described7,9. A total of 2 × 105 cells were seeded onto 24-well plates overnight, and treated with or without RSL3 (TargetMol, # T3646), Ferrostatin-1 (TargetMol, # T6500) or VBIT4 (TargetMol, # T13287) for 24 h. Cells were then washed twice with PBS and incubated with culture medium (without FBS and PS) containing 5 μM BODIPY™ 581/591 C11 at 37 °C for 30 min in dark. Labeled cells were washed twice with PBS to remove the excess dye, and finally resuspended in cold PBS containing 2% FBS for flow cytometer analysis. A minimum 10,000 events were collected to detect the oxidized C11-BODIPY at FITC channel.
Quantification of mitochondria ROS
As previously reported, MitoSOX (MCE, # HY-D1055) was used to assess the level of mitochondrial superoxide in the study7. Briefly, 2 × 105 cells were seeded on 24-well plates overnight, then treated with or without VBIT4 (1 μM for 22Rv1, 5 μM for DU145) for 24 h. The cells were washed twice with fresh PBS and stained in serum-free medium containing MitoSOX at 37 °C for 30 min in dark. The labeled cells were washed and resuspended in PBS, then detected using flow cytometry at PE channel. All flow cytometry experiments were performed at least in triplicate, and the data was processed with FlowJo (version 10.8).
Quantification of mitochondria lipid peroxidation
MitoPeDPP (Dojindo, # M466) was employed to quantify the level of mitochondria lipid peroxidation in prostate cancer cells, according to the manufacturer’s protocols. Briefly, 5 × 104 DU145 cells were seeded onto a 35 mm laser confocal dish (Biosharp) and cultured for 48 h. The cells were washed twice with a warm, serum-free medium to remove any debris. Subsequently, the cells were cultured with 0.5 μM MitoPeDPP in conjunction with 200 nM Mito-Tracker Deep Red solution (diluted with warm and serum-free medium) in the dark at 37 °C for 30 min. After that, the cells underwent another two rinses with the same medium. Then 1 mL warm and complete culture medium with or without VBIT4 was added to the cells. Finally, the labeled cells were imaged using a high-resolution laser confocal microscope (Leica) with 63x/1.40 Oil DIC objective, at 37 °C with humidified atmosphere of 5% CO2.
Human prostate cancer tissue chip
A prostate cancer tissue microarray (Lot No. PRC1601) containing 78 paired tumor and adjacent tissues was obtained from Guilin Fanpu Biotechnology Co., The information of the patients was in Supplementary Data 3. The study using the tissue microarray was approved by the Ethics Committee of Guilin Fanpu Biotechnology Co., Ltd. (Fanpu [2018] No. 23).
Immunohistochemistry (IHC) and hematoxylin-eosin staining (H & E)
After the prostate tissue chip passed through the standard procedure of immunohistochemistry of our laboratory73, the staining index of VSTM2L was calculated as the following formula: the staining intensity × the scope of stained-positive cells. For staining intensity, the score was evaluated on a 4-point scale: 0 (no appreciable staining, negative), 1 (faint yellow, weak intensity), 2 (yellow-brown, moderate intensity), 3 (brown, strong intensity). The scope of the stained-positive cells was divided into four categories: 1 (< 25% positive cells), 2 (26–50% positive cells), 3 (51–75% positive cells), and 4 (> 75% positive cells). Finally, the expression of VSTM2L was divided into high and low levels according to their mean scores.
For the analysis of immunohistochemistry and H & E staining for xenograft, the fixed tumor tissues were paraffin-embedded subjected to the standard procedures. The primary antibodies utilized were anti-Ki67, anti-VSTM2L and anti-GPX4. The images were captured by an upright microscope (Zeiss). The staining index of VSTM2L/Ki67/GPX4 were calculated according to the microarray avoiding the area of necrosis/stroma. The staining index of Cleaved-Caspase 3 was calculated according to the staining positive cells. 5 regions with the same number of stained cells were selected randomly, in each slice, for quantitative analysis.
VDAC1 cross-linking assay
The VDAC1 cross-linking assay was performed as previously reported with modifications24,56. Briefly, cells were harvested and washed twice with cold PBS (pH 8.0), followed by centrifuging at 3000 g for 5 min at 4 °C. The collected pellets were resuspended in 740 μL of 0.5 mM Ethylene glycol-bis (succinic acid N-hydroxysuccinimide ester) (EGS, Thermo, # 21565) solution diluted with cold PBS (pH 8.0), and incubated for 30 min at room temperature. To quench the reaction, 10 μL of 1.5 M Tris-HCL (pH 7.8) was added, and the reaction mixture was cultured at room temperature for another 15 min. Finally, the cultured cells were harvested in lysis buffer and the protein concentration was measured using BCA assay. 50 μg of the protein samples were subjected to 7.5% SDS-PAGE and immunoblotting using an anti-VDAC1 antibody.
Measurement of glutathione (GSH)
For the purpose of assessing glutathione (GSH) concentrations in cellular and tissue samples, we utilized the Glutathione Assay Kit (Nanjing Jiancheng, # A006-2), following the manufacturer’s guidelines. All experiments were normalized based on the total protein concentration and were performed in triplicate.
Immunofluorescence (IF) assay
A total of 5 × 104 DU145 and PC3 or 1 × 105 22Rv1 cells per well were seeded onto 14 mm glass slide in 24-well plates and cultured for at least 24 h. The cells were washed twice with 1 × PBS to remove any debris. Subsequently, the cells were cultured with 200 nM Mito-Tracker Deep Red solution (diluted with serum-free medium) in the dark at 37 °C for 30 min. After that, the cells underwent another two rinses with 1 × PBS. Then the cells were fixed with cold methanol in the dark at 4 °C for 15 min. Another 3 times washing with 1 × PBS was following. The fixed cells were blocked with 5% BSA for 1 h and incubated with ideal primary antibodies (anti-HK2, anti-VSTM2L, and anti-VDAC1) in the dark at 4 °C overnight. The cells were washed 3 times with cold 1 × PBS and incubated with the fluorescent secondary antibody (diluted with 5% BSA) in the dark at room-temperature for 1 h. After washing 3 times with 1 × PBS, hoechst (5 μg/mL) was applied to stain the cell nucleus. Finally, the stained cells were imaged using a high-resolution laser confocal microscope (Leica) with 63x/1.40 Oil DIC objective. All the primary and secondary antibody details including dilution used are listed in Supplementary Data 5.
Mitochondria and cytoplasmic proteins extraction assay
In order to separately extract the mitochondria and cytoplasmic proteins from prostate cancer cell lines, a commercial Cell Mitochondria Isolation Kit (Byotime, # C3601) was used according to the manufacturer. Briefly, about 2 × 107 22Rv1 cells and 1 × 107 DU145 and PC3 cells were collected and washed with cold 1 × PBS once. 1 mL separation reagent was added to the tube, which was incubated with rotation at 4 °C for 45 min. The cells were homogenized on the ice by a glass homogenizer until more than 50 % of the cells are stained by trypan blue and centrifuged 10 min at 4 °C with 1000 g. The supernatant was collected and centrifuged again at 4 °C with 8000 g for 10 min. The precipitate and supernatant were the mitochondria lysates and cytoplasm lysis, respectively. At last, the collected mitochondria lysates and cytoplasm lysis were subjected to WB procedure. All experiments were performed in triplicate.
ELISA analysis for BH4
BH4 level were analyzed via a commercial ELISA kit (EIAAB, #E2152Ge)76, following the manufacturer’s guidelines. Briefly, 0.05 g xenograft tumors were homogenized in 500 μL ice-cold 1 × PBS (containing protease inhibitor) and stored overnight at –80 °C. After two freeze-thaw cycles, the homogenates were centrifuged for 5 min at 5000 g. The supernatant was collected for ELISA procedure. All experiments were normalized based on the total protein concentration.
Statistics and reproducibility
All experiments were conducted at least in triplicate biological replicates with similar results. GraphPad Prism 9 and SPSS 27 was used to obtain statistical p-values by application of student’s t-test or ANOVA for group comparison. Kaplan-Meier method was used to plot the PFS and DFS curves, compared by log-rank test. GraphPad Prism 9 was used to plot the Kaplan-Meier plots and z-score threshold parameters were used to generate the Kaplan-Meier plots in c-BioPortal. FlowJo (version 10.8) was employed to quantify the data acquired using flow cytometer. ImageJ software was utilized to analyze the morphological parameters of mitochondria. Data were presented as mean ± standard deviation (SD). For all tests, p < 0.05 was considered significant (ns: not significant).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The processed VSTM2L gene expression in Prostate adenocarcinoma (PRAD) data in this study are available at UALCAN Browser (https://ualcan.path.uab.edu/analysis.html), TCGA PRAD cohort (https://portal.gdc.cancer.gov/), UCSC Xena Browser (https://xena.ucsc.edu/) and GENT2 databases. The raw data of the survival curves of VSTM2L were available in cBioPortal (https://www.cbioportal.org/). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE77 partner repository with the dataset identifier PXD052173 (http://www.ebi.ac.uk/pride). The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clinicians 71, 209–249 (2021).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA: A Cancer J. Clinicians 74, 12–49 (2024).
Han, B. et al. Cancer incidence and mortality in China, 2022. J. Natl Cancer Cent. 4, 47–53 (2024).
Pernigoni, N. et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 374, 216–224 (2021).
Zhou, X. et al. Abrogation of HnRNP L enhances anti-PD-1 therapy efficacy via diminishing PD-L1 and promoting CD8(+) T cell-mediated ferroptosis in castration-resistant prostate cancer. Acta pharmaceutica Sin. B 12, 692–707 (2022).
Wang, J. et al. Inhibition of phosphoglycerate dehydrogenase induces ferroptosis and overcomes enzalutamide resistance in castration-resistant prostate cancer cells. Drug resistance updates: Rev. commentaries antimicrobial anticancer Chemother. 70, 100985 (2023).
Qiu, B. et al. Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell 187, 1177–1190.e1118 (2024).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748–2764.e2722 (2023).
Bock, F. J. & Tait, S. W. G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 21, 85–100 (2019).
Flores-Romero, H., Dadsena, S. & García-Sáez, A. J. Mitochondrial pores at the crossroad between cell death and inflammatory signaling. Mol. cell 83, 843–856 (2023).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Shoshan-Barmatz, V., Krelin, Y. & Shteinfer-Kuzmine, A. VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease. Cell Calcium 69, 81–100 (2018).
Yang, Y. et al. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat. Commun. 11, 433 (2020).
Shimizu, S., Narita, M. & Tsujimoto, Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399, 483–487 (1999).
Shimizu, S., Konishi, A., Kodama, T. & Tsujimoto, Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl Acad. Sci. USA 97, 3100–3105 (2000).
Pastorino, J. G., Shulga, N. & Hoek, J. B. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 277, 7610–7618 (2002).
Abu-Hamad, S., Zaid, H., Israelson, A., Nahon, E. & Shoshan-Barmatz, V. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J. Biol. Chem. 283, 13482–13490 (2008).
Shimizu, S., Ide, T., Yanagida, T. & Tsujimoto, Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J. Biol. Chem. 275, 12321–12325 (2000).
Shimizu, S., Matsuoka, Y., Shinohara, Y., Yoneda, Y. & Tsujimoto, Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J. cell Biol. 152, 237–250 (2001).
Liu, Y. et al. Hypoxia-induced GPCPD1 depalmitoylation triggers mitophagy via regulating PRKN-mediated ubiquitination of VDAC1. Autophagy 19, 2443–2463 (2023).
Geisler, S. et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. cell Biol. 12, 119–131 (2010).
Gatliff, J. et al. TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy 10, 2279–2296 (2014).
Baik, S. H. et al. Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation. Sci. Immunol. 8, eade7652 (2023).
Zhou, Q. et al. HNF4A-BAP31-VDAC1 axis synchronously regulates cell proliferation and ferroptosis in gastric cancer. Cell death Dis. 14, 356 (2023).
Niu, B. et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol. Toxicol. 38, 505–530 (2021).
Rossini, L. et al. VSTM2L is a novel secreted antagonist of the neuroprotective peptide Humanin. FASEB J.: Off. Publ. Federation Am. Societies Exp. Biol. 25, 1983–2000 (2011).
Li, Y. et al. VSTM2L contributes to anoikis resistance and acts as a novel biomarker for metastasis and clinical outcome in ovarian cancer. Biochemical biophysical Res. Commun. 658, 107–115 (2023).
Liu, H., Zhang, Z., Zhen, P. & Zhou, M. High expression of VSTM2L induced resistance to chemoradiotherapy in rectal cancer through downstream il-4 signaling. J. Immunol. Res. 2021, 6657012 (2021).
Lee, J., Sim, W., Lee, J. & Kim, J. H. VSTM2L is a promising therapeutic target and a prognostic soluble-biomarker in cholangiocarcinoma. BMB Rep. 57, 324–329 (2024).
García-Chávez, D. et al. Prohibitins, Phb1 and Phb2, function as Atg8 receptors to support yeast mitophagy and also play a negative regulatory role in Atg32 processing. Autophagy 20, 2478–2489 (2024).
Zhang, J. et al. Corosolic acid attenuates cardiac ischemia/reperfusion injury through the PHB2/PINK1/parkin/mitophagy pathway. iScience 27, 110448 (2024).
Du, Y. et al. Pyruvate kinase M2 sustains cardiac mitochondrial quality surveillance in septic cardiomyopathy by regulating prohibitin 2 abundance via S91 phosphorylation. Cell. Mol. life Sci.: CMLS 81, 254 (2024).
Huang, Q. et al. Bilirubin inhibits lung carcinogenesis by up-regulating cystatin A expression in tumor-infiltrating macrophages. Genes Dis. 10, 2222–2225 (2023).
Xu, S. et al. Functional analysis of the cystatin A gene response to SGIV infection in orange-spotted grouper, Epinephelus coioides. Developmental Comp. Immunol. 136, 104502 (2022).
Sato, Y. et al. Malnutrition stratified by marasmus and kwashiorkor in adult patients with heart failure. Sci. Rep. 14, 19722 (2024).
Chen, G. et al. Ribosomal protein S3 mediates drug resistance of proteasome inhibitor: potential therapeutic application in multiple myeloma. Haematologica 109, 1206–1219 (2024).
Park, Y. J. et al. Ribosomal protein S3 associates with the TFIIH complex and positively regulates nucleotide excision repair. Cell. Mol. life Sci.: CMLS 78, 3591–3606 (2021).
Park, S. J., Yoon, B. H., Kim, S. K. & Kim, S. Y. GENT2: an updated gene expression database for normal and tumor tissues. BMC Med. genomics 12, 101 (2019).
Chandrashekar, D. S. et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia (N. Y., NY) 25, 18–27 (2022).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Yang, W. S. & Stockwell, B. R. Ferroptosis: death by lipid peroxidation. Trends cell Biol. 26, 165–176 (2016).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Bayrhuber, M. et al. Structure of the human voltage-dependent anion channel. Proc. Natl Acad. Sci. USA 105, 15370–15375 (2008).
Jahn, H. et al. Phospholipids are imported into mitochondria by VDAC, a dimeric beta barrel scramblase. Nat. Commun. 14, 8115 (2023).
MacKerell, A., Preto, J. & Krimm, I. The intrinsically disordered N-terminus of the voltage-dependent anion channel. PLOS Comput. Biol. 17, e1008750 (2021).
Abu-Hamad, S. et al. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 122, 1906–1916 (2009).
Keinan, N., Tyomkin, D. & Shoshan-Barmatz, V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol. Cell. Biol. 30, 5698–5709 (2010).
Camara, A. K. S., Zhou, Y., Wen, P. C., Tajkhorshid, E. & Kwok, W. M. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front. Physiol. 8, 460 (2017).
Tapia-Abellán, A. et al. Sensing low intracellular potassium by NLRP3 results in a stable open structure that promotes inflammasome activation. Sci. Adv. 7, eabf4468 (2021).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. cell 73, 354–363.e353 (2019).
Stockwell, B. R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Organelle-specific regulation of ferroptosis. Cell Death Differ. 28, 2843–2856 (2021).
Oh, S. J., Ikeda, M., Ide, T., Hur, K. Y. & Lee, M. S. Mitochondrial event as an ultimate step in ferroptosis. Cell death Discov. 8, 414 (2022).
Zhang, E. et al. Preserving insulin secretion in diabetes by inhibiting VDAC1 overexpression and surface translocation in β cells. Cell Metab. 29, 64–77.e66 (2019).
Kim, J. et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366, 1531–1536 (2019).
Lyamzaev, K. G., Panteleeva, A. A., Simonyan, R. A., Avetisyan, A. V. & Chernyak, B. V. Mitochondrial lipid peroxidation is responsible for ferroptosis. Cells 12, 611 (2023).
Zhang, P., Chen, Y., Zhang, S. & Chen, G. Mitochondria-related ferroptosis drives cognitive deficits in neonatal mice following sevoflurane administration. Front. Med. 9, 887062 (2022).
Xie, Y. et al. Ferroptosis: process and function. Cell Death Differ. 23, 369–379 (2016).
Yang, Y. et al. Interaction between macrophages and ferroptosis. Cell Death Dis. 13, 355 (2022).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22, 381–396 (2022).
Fang, X., Ardehali, H., Min, J. & Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 20, 7–23 (2022).
Costa, I. et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Therapeutics 244, 108373 (2023).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18, 280–296 (2021).
Ghoochani, A. et al. Ferroptosis inducers are a novel therapeutic approach for advanced prostate cancer. Cancer Res. 81, 1583–1594 (2021).
Preto, J. & Krimm, I. The intrinsically disordered N-terminus of the voltage-dependent anion channel. PLoS Comput Biol. 17, e1008750 (2021).
Shangguan, X. et al. SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat. Commun. 12, 1812 (2021).
Hodge, T. & Colombini, M. Regulation of metabolite flux through voltage-gating of VDAC channels. J. Membr. Biol. 157, 271–279 (1997).
Gincel, D., Silberberg, S. D. & Shoshan-Barmatz, V. Modulation of the voltage-dependent anion channel (VDAC) by glutamate. J. Bioenerg. Biomembranes 32, 571–583 (2000).
Haloi, N. et al. Structural basis of complex formation between mitochondrial anion channel VDAC1 and Hexokinase-II. Commun. Biol. 4, 667 (2021).
Yagoda, N. et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447, 864–868 (2007).
Gao, P. et al. PELO facilitates PLK1- induced the ubiquitination and degradation of smad4 and promotes the progression of prostate cancer. Oncogene 41, 2945–2957 (2021).
Perelman, A. et al. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 3, e430–e430 (2012).
Koppula, P. et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 13, 2206 (2022).
Chen, Y. et al. Inhibition of ferroptosis by mesenchymal stem cell-derived exosomes in acute spinal cord injury: role of Nrf2/GCH1/BH4 axis. Neurospine 21, 642–655 (2024).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic acids Res. 50, D543–d552 (2022).
Acknowledgements
We thank Zhaoqiang Qian, Qiangqiang Wei, and Lifang Zheng from the Laboratory Animal Center of Shaanxi Normal University for their support and assistance in animal feeding, management and experiment. P.G. was supported by the National Natural Science Foundation of China (82472665) and Fundamental Research Funds for the Central Universities (GK202201004), X.M.D. was supported by the Natural Science Foundation of Shaanxi Province (2023-JC-YB-716), J.L.H. was supported by Excellent Graduate Training Program of Shaanxi Normal University (LHRCTS23091).
Author information
Authors and Affiliations
Contributions
P.G. and X.M.D. conceived and designed the project. J.Y., X.L. assistant by J.L.H., L.L., Y.T.R., and X.N.A. performed the experiments. J.Y., Q.L.H., and X.M.D. performed data analysis. P.G., X.M.D., and J.Y. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jay H Chung and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, J., Lu, X., Hao, JL. et al. VSTM2L protects prostate cancer cells against ferroptosis via inhibiting VDAC1 oligomerization and maintaining mitochondria homeostasis. Nat Commun 16, 1160 (2025). https://doi.org/10.1038/s41467-025-56494-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56494-6