Abstract
Recent evidence suggests short-term exposure to particulate matter (PM) air pollution can impact brain function after a delay period. It is unknown whether effects are predominantly due to the olfactory or lung-brain pathways. In this study 26 adults (Mage = 27.7, SDage = 10.6) participated in four conditions. They were exposed to either high PM concentrations or clean air for one hour, using normal inhalation or restricted nasal inhalation and olfaction with a nose clip. Participants completed four cognitive tests before and four hours after exposure, assessing working memory, selective attention, emotion expression discrimination, and psychomotor vigilance. Results showed significant reductions in selective attention and emotion expression discrimination after enhanced PM versus clean air exposure. Air quality did not significantly impact psychomotor vigilance or working memory performance. Inhalation method did not significantly mediate effects, suggesting that short-term PM pollution affects cognitive function through lung-brain mechanisms, either directly or indirectly.
Similar content being viewed by others
Introduction
Globally, air pollution is the leading environmental risk factor to human health, increasing premature mortality1. The detrimental impacts of poor air quality on cardiovascular and respiratory systems are widely acknowledged2,3. More recently a growing body of literature suggests a relationship between lifetime exposure to low-quality air with altered neurodevelopment4 and incidence of neurodegenerative diseases5,6, such as multiple sclerosis and Alzheimer’s disease7, Parkinson’s disease8, as well as neuropsychological illnesses9. Critically, these diseases are characterised by decline in cognitive functioning due to the death of cortical neurons.
Particulate matter (PM) air pollution in the PM2.5 size range, PM with diameters ≤ 2.5 μm, is the class of air pollutants most responsible for human health effects. In 2015 ~4.2 million deaths were attributed to PM2.5 alone10. Currently, the World Health Organization (WHO) recommend that 24-h and annual limits of PM2.5 should not exceed 15 and 5 μgm−3, respectively11. Recent evidence suggests that short-term exposure to particulate PM air pollution can temporarily impair several key cognitive functions, including selective attention12,13,14, switch costs (which are relevant to multitasking)15, decision-making16, processing speed12,15, functional connectivity17, and even global cognitive functioning as assessed by the Mini-Mental State Examination (MMSE)18,19.
Cognitive functioning encompasses a diverse array of mental processes crucial for everyday tasks. Supermarket shopping illustrates the independence and interdependence of different cognitive functions. Executive function, especially selective attention, aids in decision-making and goal-directed behaviour20, such as prioritising items on your shopping list while filtering out distractions from other products and avoiding impulse buys. Working memory serves as a temporary workspace for holding and manipulating information, vital for tasks requiring simultaneous processing and storage21, like comparing prices and/or brands to make informed purchases. Socio-emotional cognition involves the ability to detect and interpret emotions in oneself and others, facilitating social interactions and empathy22, enabling socially acceptable behaviour during shopping. Although these skills are separate cognitive facets, they work together to enable the successful completion of everyday tasks. Considering each facet separately allows investigation into the cognitive processes most vulnerable and susceptible to disruption from air pollution exposure, essential for targeted interventions and promoting resilience.
While the precise mechanism by which air pollution exerts acute and chronic effects on the brain remains a topic of ongoing debate and research, two main mechanistic models which are not mutually exclusive, have been proposed. The first proposed model stipulates that these effects stem from the direct impact of nasally inhaled particles or chemical constituents with both neurons and non-neuronal glial cells in the brain. This impact is thought to occur via translocation along olfactory neurons to the olfactory bulb23,24,25. Olfactory sensory neurons have a diameter of ~200 nm26, potentially allowing ultrafine particles (≤ 100 nm)27 to translocate through this process. However, direct impact could also occur via the exchange of soluble particle components in the lungs28 and for particles fine enough, entry to the blood stream29, leading to eventual translocation across the blood-brain barrier from circulation30,31. The ‘direct’ model gains support from evidence of combustion-like nanoparticles found in the brains of animals and humans residing in polluted environments32, with biomarkers indicating features associated with early dementia, microglia immune defence activation, and neuronal injury33. The observation that the olfactory bulb is a ‘hot spot’ for this type of neuronal damage34 support the notion of a nasal route of impact. This suggests that blocking nasal inhalation might lessen the direct effect of air pollution on brain function. In summary, the direct model proposes that the inhalation of air pollution via the olfactory and respiratory pathways together converges toward cognitive impairments through direct processes.
The second proposed mechanism, the ‘indirect’ model, stipulates that neuronal damage arises from adverse effects of systemic inflammation caused by the presence of air pollution in the lungs. It is well known that both chronic and acute systemic inflammation can lead to deleterious effects on central nervous system functions, such as cognition and motivation, via glial-neurovascular activity23,35. In this view, neuronal impacts are considered an indirect result of inflammatory mediators activated in the lungs, which trigger an immune response that subsequently induces oxidative stress and neuroinflammation36,37,38,39. The effect of inflammation on cognition is widely observed within the literature40,41,42,43,44,45,46. As with other instigators of systemic inflammation, this view suggests that air pollution exposure should take several hours to induce effects on neuronal activity and cognition. It further suggests that any effects would be unaffected by the presence or absence of nasal inhalation.
Whilst the two models propose distinct processes, a time-lag is expected between inhalation and the manifestation of cognitive or behavioural effects resulting from an inflammatory response. This expectation aligns with evidence from studies where inflammation is induced via vaccination47,48. In these studies, a transient decline in cognitive functioning becomes apparent several hours after the vaccination process41,42.
Indeed, there is evidence that exposure to PM air pollution requires a delay of several hours before cognitive, behavioural, or neurophysiological changes become evident. One cognitive behavioural study identified an association between recent PM air quality and Visual Information Processing in school children12. Electroencephalography research reveals changes in frontal lobe brain activity within 30–60 min of exposure to PM pollution from cooking and diesel exhaust49,50. Although activity was not measured beyond this time frame, a separate follow-up study demonstrated that frontal lobe brainwave alterations persist for up to 4 h after exposure51. Another behavioural study found that anti-inflammatory medication protected against an identified association between modelled recent PM air pollution exposure and cognitive impairment19. Individuals taking anti-inflammatory medication showed no association, but others showed impaired cognitive function from recent PM pollution exposure. Integrating across cognitive, behavioural, and neurophysiological evidence, there is support for the concept that short-term exposure to PM air pollution triggers inflammation, subsequently leading to measurable cognitive impairment after a delay period.
Regardless of the model, isolating the respiratory tract may result in a reduction of cognitive effects across various cognitive domains. This reduction could arise from the impact on different brain areas through distinct routes of exposure, or the mediation of cognitive effects via a global dose reduction following the suppression of the olfactory pathway. Although direct study of this remains challenging, a behavioural experimental paradigm can still offer valuable insights into the investigation of acute PM air pollution exposure on crucial cognitive functions, and of the comparative negative consequences of the olfactory pathway.
This study aims to identify if exposure to high PM concentrations negatively impacts cognitive functioning after 4 h, comparative to cleaner air. Furthermore, the study aims to identify whether inhalation route mediates an effect of air quality on cognitive function. It is the first study to our knowledge to experimentally manipulate inhalation routes of particulate matter air pollution, investigating pathway-dependent effects on key cognitive functions.
Results
All participants completed four sessions comprised of (1) a round of four cognitive tests, (2) a 1-h period of air exposure, (3) a 4-h delay period, and (4) another round of cognitive tests. Half the sessions had clean air during the participant exposure period and half had PM pollution induced by burning and extinguishing a candle in the exposure room prior to the start of the exposure period. Pollutant concentration was monitored through the exposure period in all conditions. In a fully crossed, within-subjects design, half of each participant’s sessions had nasal inhalation restricted (using a swimming nose clip) during the air exposure period and half did not. Cognitive tests assessed selective attention, emotion expression recognition, working memory, and psychomotor vigilance.
Cognitive function
Selective attention
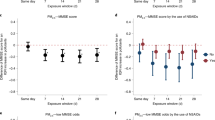
Exposure to PM pollution significantly reduced participant selective attention performance comparative to clean air exposure, as evidenced by a significant two-way interaction between pollution exposure and session time [F(1, 25) = 6.446, p = 0.018, ηp2 = 0.205, 1-β = 0.685]. See Fig. 1a. Participants demonstrated an increase in cognitive control score (ΔRT) between the PM pollution pre-exposure (Mean ΔRT = 0 ms, s.d., = 21) and post-exposure (Mean ΔRT = 13 ms, s.d., = 19) measures, indicating a decline in selective attention performance, as evidenced by an increase in cognitive control scores; the opposite pattern was identified pre- (Mean ΔRT = 5 ms, s.d., = 15) and post- (Mean ΔRT = 1 ms, s.d., = 12) clean air exposure. T-tests revealed no significant performance differences pre- versus post- clean air exposure [t(25) = 1.252, p = 0.111], whereas selective attention performance significantly declined between pre- and post- PM pollution exposure [t(25) = -2.082, p = 0.024].
Changes in selective attention performance pre- and post-exposure (a) for the two pollution exposure conditions (averaged across both inhalation conditions); and (b) across all four exposure/inhalation condition combinations. Selective attention performance deteriorated following PM pollution exposure, indicated by an increase in ΔRT values (a: red line). In contrast, performance improved after clean air exposure, reflected by a decrease in ΔRT (a: blue line). This interaction was statistically significant, as determined by a repeated measures ANOVA with Bonferroni corrections for multiple comparisons [F(1, 25) = 6.446, p = 0.018]. Inhalation pathway did not mediate any changes in selective attention performance (b), as no significant three-way interaction was identified using a repeated measures ANOVA with Bonferroni corrections [F(1, 25) = 0.526, p = 0.475]. n = 26 independent human participants (biological replicates), within a fully repeated measures design. a Data are presented as mean values +/- SEM; (b) Median represented by the horizontal line, box indicates interquartile range (25th-75th percentiles), whiskers show full data range between minimum and maximum; white circles indicate individual data points; black diamonds indicate condition means; *p < 0.05. Source data are provided as a Source Data file.
There was no significant three-way interaction identified between inhalation pathway, pollution exposure, and session time (F < 1). See Fig. 1b. No other significant main effects or interactions were identified: main effect of session time [F(1, 25) = 1.064, p = 0.312]; inhalation*pollution interaction [F(1, 25) = 1.118, p = 0.300]; other F’s < 1.
This indicates that exposure to air pollution via candle burning reduced participant selective attention performance comparative to after exposure to clean air, regardless of inhalation pathway. This provides evidence that control over selective visual attention, a critical cognitive function, is impaired in clinically healthy adults 4 h after acute PM air pollution exposure.
Emotion discrimination
Exposure to PM pollution significantly reduced participant emotion expression discrimination performance comparative to clean air, as evidenced by a significant two-way interaction between pollution exposure and session time [F(1, 25) = 5.552, p = 0.027, ηp2 = 0.182, 1-β = 0.620]. Whilst emotion expression recognition performance was reduced in the clean air condition between pre- (Mean d\({}^{{\prime} }=\) 2.75, s.d., = 0.50) and post-exposure (Mean d\({}^{{\prime} }=\) 2.66, s.d., = 0.54), there was a more severe reduction between pre- (Mean d\({}^{{\prime} }=\) 2.78, s.d., = 0.47) and post- (Mean d\({}^{{\prime} }=\) 2.56, s.d., = 0.56) PM pollution exposure. See Fig. 2a. This provides possible evidence that emotion expression recognition is impaired in clinically healthy adults 4 h after acute PM air pollution exposure. T-tests revealed no significant performance differences pre- versus post- clean air exposure [t(25) = 1.550, p = 0.067], whereas expression recognition performance significantly declined between pre- and post- PM pollution exposure [t(25) = 3.271, p = 0.002]. There was no three-way interaction between inhalation pathway, pollution exposure, and session time (All F’s < 1). See Fig. 2b. However, there was a main effect of session time identified [F(1, 25) = 8.167, p = 0.008, ηp2 = 0.246, 1-β = 0.784], such that participants performed better pre-exposure (Mean d\({}^{{\prime} }=\) 2.77, s.d., = 0.46) compared to post-exposure (Mean d\({}^{{\prime} }=\) 2.61, s.d., = 0.53) irrespective of emotion, pollution, or inhalation condition.
Changes in expression discrimination performance pre- and post-exposure (a) for the two pollution exposure conditions (averaged across both inhalation conditions); and (b) across all four exposure/inhalation condition combinations. Average expression discrimination performance, measured as \({{{{\rm{d}}}}}^{{\prime} }\), declined significantly after exposure compared to before exposure (a). A repeated measures ANOVA with Bonferroni corrections revealed a statistically significant main effect of time [F(1, 25) = 8.167, p = 0.008]. Critically a greater decline in expression discrimination performance was identified following PM pollution exposure (a: red line) compared to clean air exposure (a: blue line). The interaction was statistically significant, as determined by a repeated measures ANOVA with Bonferroni corrections for multiple comparisons [F(1, 25) = 5.552, p = 0.027]. Inhalation pathway did not mediate changes in expression discrimination performance (b), as no significant three-way interaction was found using a repeated measures ANOVA with Bonferroni corrections [F(1, 25) = 0.868, p = 0.360]. n = 26 independent human participants (biological replicates), within a fully repeated measures design. a Data are presented as mean values +/- SEM; (b) Median represented by the horizontal line, box indicates interquartile range (25th-75th percentiles), whiskers show full data range between minimum and maximum; white circles indicate individual data points; black diamonds indicate condition means; *p < 0.05; **p < 0.01. Source data are provided as a Source Data file.
No three-way or four-way interactions with the inclusion of emotion expression, to assess approach bias, were identified (All F’s < 1). Approach bias refers to participants’ natural inclination to favour positive-affective stimuli (happy faces) over negative-affective stimuli (fearful faces), driven by emotion regulation processes. Please refer to the methodology section for further explanation. We attribute the inability to detect changes in approach bias to two primary reasons. Firstly, the significant higher-order effect of discrimination ability obscures that of bias; changes in bias cannot be observed, as discrimination ability was not consistent between sessions. Secondly, there is an interaction between session time and emotion expression, without pollution exposure mediation [F(1, 25) = 5.817, p = 0.024, ηp2 = 0.189, 1-β = 0.640]. Bias towards fearful stimuli was largely similar between pre- (Mean d\({}^{{\prime} }=\) 2.576, s.d., = 0.54) and post-exposure (Mean d\({}^{{\prime} }=\) 2.517, s.d., = 0.55), whereas bias towards positive-affective stimuli decreased between pre- (Mean d\({}^{{\prime} }=\) 2.957, s.d., = 0.43) and post-exposure (Mean d\({}^{{\prime} }=\) 2.710, s.d., = 0.55). This implies a possible role of fatigue in approach bias changes, also decreasing the possibility of observing a pollution-mediated effect.
A main effect of emotion expression was also identified [F(1, 25) = 35.018, p < 0.001, ηp2 = 0.583, 1-β = 1], such that participants showed generalised bias towards positive-affective (happy) faces (Mean d\({}^{{\prime} }=\) 2.83, s.d., = 0.46) compared to negative-affective (fearful) faces (Mean d\({}^{{\prime} }=\) 2.55, s.d., = 0.52) regardless of session, exposure, or inhalation condition. See Table 1 for details of ANOVA main effects and interactions of interest.
Response time (RT) for correct ‘Hit’ trials was also analysed to identify if the significant pollution-mediated variations in emotion expression discrimination stemmed from strategic processes, such as trade-offs between speed and accuracy. Results of the ANOVA identified a significant main effect of emotion expression [F(1, 25) = 16.606, p < 0.001, ηp2 = 0.399, 1-β = 0.975], whereby participants responded faster to positive-affective (Mean RT = 535 ms, s.d., = 56) comparative to negative-affective (Mean RT = 544 ms, s.d., = 55) stimuli. There was also a significant main effect of session time identified [F(1, 25) = 4.636, p = 0.041, ηp2 = 0.156, 1-β = 0.544], such that participants responded faster post-exposure (Mean RT = 536 ms, s.d., = 59) compared to pre-exposure (Mean RT = 543 ms, s.d., = 52). Faster response times between sessions may explain the global decline in performance (indexed by \({{{{\rm{d}}}}}^{{\prime} }\)) between sessions.
Critically, no other main effects or interactions were identified, leading us to confirm that pollution-mediated changes to expression discrimination performance were not a result of compensatory speed-accuracy mechanisms.
Psychomotor speed and sustained attention
PM pollution exposure did not significantly impact simple response time or sustained attention measures, as assessed by the Psychomotor Vigilance Task. This expected outcome allows for a confident conclusion that the previously described declines in selective attention and emotion expression recognition are not due to a global decline in cognitive speed, but rather are attributable to the complex cognitive functions outlined.
The stability of both psychomotor speed, reflecting global processing speed, and sustained attention, indicative of continued concentration over relatively long periods, is outlined below.
Psychomotor Speed: There was no significant effect of inhalation pathway [F(1, 25) = 1.691, p = 0.205], pollution exposure [F(1, 25) = 1.070, p = 0.311], session time (F < 1), or subsequent interactions (F’s < 1) on psychomotor speed. See Table 2.
Sustained Attention: There was no significant effect of inhalation pathway (F < 1), pollution exposure (F < 1), session time [F(1, 25) = 1.199, p = 0.284], or subsequent interactions on sustained attention: inhalation*pollution [F(1, 25) = 4.121, p = 0.053]; pollution*session [F(1, 25) = 3.437, p = 0.076]; inhalation*pollution*session [F(1, 25) = 2.046, p = 0.165]; inhalation*session (F < 1). See Table 2.
Working memory
PM pollution exposure did not significantly impact working memory performance as measured with the Spatial n-back Task. An individual ANOVA was conducted for each level of difficulty (n-value).
1-back: A significant interaction between inhalation pathway and session time was identified [F(1, 25) = 4.766, p = 0.039, ηp2 = 0.160, 1-β = 0.555], such that regardless of pollution exposure, in the normal inhalation condition, participant performance improved between pre- (Mean d\({}^{{\prime} }=\) 3.899, s.d., = 0.66) and post-exposure (Mean d\({}^{{\prime} }=\) 3.997, s.d., = 0.60), whereas in the restricted inhalation condition performance deteriorated between pre- (Mean d\({}^{{\prime} }=\) 4.023, s.d., = 0.65) and post-exposure (Mean d\({}^{{\prime} }=\) 3.810, s.d., = 0.60). T-tests revealed no significant performance differences pre- versus post-exposure in either the normal inhalation [t(25) = 0.955, p = 0.174] or restricted inhalation conditions [t(25) = 1.529, p = 0.069]. Therefore, while a significant interaction effect was identified, neither inhalation pathway significantly influenced performance between pre- and post-exposure.
No other significant main effects or interactions were identified: session time [F(1, 25) = 2.996, p = 0.096]; all other main effects and interactions (F’s < 1). See Table 3 for condition means.
2-back: No significant main effects or interactions were identified: session time [F(1, 25) = 2.996, p = 0.096]; all other main effects and interactions (F’s < 1). See Table 3 for condition means.
3-back: No significant main effects or interactions were identified: inhalation pathway [F(1, 25) = 1.772, p = 0.195]; inhalation*pollution [F(1, 25) = 1.247, p = 0.275]; all other main effects and interactions (F’s < 1). See Table 3 for condition means.
4-back: No significant main effects or interactions were identified: pollution*session [F(1, 25) = 3.956, p = 0.058]; inhalation*session [F(1, 25) = 1.606, p = 0.217]; all other main effects and interactions (F’s < 1). See Table 3 for condition means. Results of the 4-back were nearing random chance, indicating a lack of meaningful pattern or reliability in the results. Given this observation, it would be advisable to avoid utilising this test in future assessments, as its validity is questionable based on the proximity to chance-level outcomes.
Air quality
PM concentrations during the exposure period (1 h) were significantly higher in the PM pollution condition (PM2.5 mean = 28.54 μg m−3, s.d., = 6.85; PM10 mean = 37.85 μg m−3, s.d., = 7.32), compared to the clean air condition, where no candle was burned (PM2.5 mean = 3.99 μg m−3, s.d., = 1.20; PM10 mean = 9.00 μg m−3, s.d., = 2.81). PM2.5 [F(1, 25) = 326.012, p < 0.001] and PM10 [F(1, 25) = 418.035, p < 0.001]. No significant main effects of inhalation pathway condition or interaction were identified (F’s < 1). See Fig. 3a, b. This indicated that burning and extinguishing candles prior to participant exposure led to significantly higher PM pollution during the hour exposure compared to no manipulation of air quality (clean air condition).
Average air pollutant concentrations across each exposure session for (a) PM2.5; (b) PM10; and (c) CO2. Significantly higher concentrations of all pollutants were identified during particulate matter (PM) pollution exposure conditions compared to clean air, as determined by repeated measures ANOVAs with Bonferroni corrections: PM2.5 [F(1, 25) = 326.012, p < 0.001]; PM10 [F(1, 25) = 418.035, p < 0.001]; and CO2 [F(1, 25) = 292.685, p < 0.001]. CO2 concentrations were significantly higher in the normal inhalation condition compared to the restricted inhalation condition, as evidenced by a significant two-way interaction using repeated measures ANOVA with Bonferroni corrections [F(1, 25) = 6.819, p = 0.015]. n = 26 independent human participants (biological replicates), within a fully repeated measures design. Median represented by the horizontal line, box indicates interquartile range (25th-75th percentiles), whiskers show full data range between minimum and maximum; white circles indicate individual data points; black diamonds indicate condition means; **p < 0.01; ***p < 0.001. Source data are provided as a Source Data file.
For Carbon Monoxide (CO), 99.87% of the recorded samples showed no detectable carbon monoxide [0 parts per million (ppm)]. The highest observed values were 3.5 ppm in the clean air condition and 3 ppm in the PM pollution condition.
Results of the ANOVA investigating CO2 concentrations indicated that there was a significant main effect of pollution condition [F(1, 25) = 292.685, p < 0.001], such that CO2 concentrations during exposure were significantly higher in the PM pollution condition (mean = 712 ppm, s.d., = 48) compared to clean air condition (mean = 598 ppm, s.d., = 42). A significant main effect of inhalation pathway was also identified [F(1, 25) = 6.819, p = 0.015, ηp2 = 0.214, 1-β = 0.709], such that CO2 concentrations were significantly higher under normal inhalation (mean = 670 ppm, s.d., = 49) compared to restricted inhalation conditions (mean = 640 ppm, s.d., = 51). No significant interaction was present (F < 1). See Fig. 3c. The observed higher CO2 concentrations during normal inhalation, as opposed to restricted inhalation, may be attributed to inevitable differences in respiratory processes of participants during the two conditions. It is suggested that when blocking the nasal pathway, less CO2 is retained in the body and subsequently exhaled, compared to unrestricted breathing through the nose and mouth52. This difference in respiratory behaviour could explain the observed significant differences between CO2 concentrations between the two inhalation conditions.
Exposure awareness
At the end of each session, participants reported the pollution condition (clean air or candle) they believed they had experienced. A χ2 analysis showed no significant association between the actual pollution exposure condition experienced and participant-reported exposure [χ2(1, N = 104) = 1.943, p = 0.163]. This suggests that the study blinding was successful and the awareness of air condition would not significantly contribute to explaining the identified cognitive changes, thereby enhancing the validity of the study. See Table 4 for frequencies and confidence judgement values.
Discussion
In this study, participants were exposed to either high concentrations of particulate matter (PM) air pollution, using candle burning as the PM source, or clean air during conditions of either normal or nasally restricted inhalation. Before, and 4 h after exposure, participants completed four computer tasks to assess working memory, selective attention, emotion expression discrimination, psychomotor speed, and sustained attention. Exposure to PM air pollution led to a decline in selective attention performance and emotion expression discrimination ability, subsets of executive functioning. Working memory performance and psychomotor vigilance were unaffected by PM exposure. Inhalation method did not mediate any identified results.
These findings support prior research on the impact of PM pollution exposure on executive processing. The deficits in selective attention closely corroborate previous evidence showing a reduction in selective attention performance following short-term PM exposure12,13,14. Additionally, research on longer-term exposure reveals similar impacts on executive function performance53,54. The identified deficits in expression discrimination mediated by PM corroborate evidence from a vaccination study, which demonstrated a decline in emotion recognition 6.5 h after typhoid vaccination, accompanied by upregulation of inflammatory biomarkers42. This suggests that PM pollution-induced inflammation may lead to socio-cognitive impairments. A critical component of emotion expression recognition is the engagement of ‘theory of mind’55, a social-cognitive skill wherein individuals interpret their own and others’ mental states to predict and explain behaviour56. Previous studies have identified connections between air pollution and interpersonal attraction57, a notion partially substantiated by research linking recent ambient concentrations of PM to the incidence of violent crime in US cities58. These findings may validate the concept that PM exposure leads to socio-emotional dysfunction, aligning with previous research that links inflammation and air pollution to deficits in emotional recognition and a rise in antisocial behaviour.
Interestingly, functional similarities exist between selective attention and expression discrimination processes59, and a link is present between the neural mechanisms of reactive and proactive cognitive control and social anxiety60, potentially further supporting the connection between these neural constructs. Moreover, there is growing evidence highlighting the impact of systemic inflammation on the attenuation or functional alterations of prefrontal cortical neurons61, which strengthens the link between acute inflammatory processes and executive cognitive dysfunction. These studies may suggest that PM pollution exposure leads to executive and socio-cognitive dysfunction due to the impact of air pollution-mediated inflammation on brain regions that share functional similarities between selective attention and emotion recognition processes.
In light of the significant effects of particulate matter exposure on selective attention and emotion expression discrimination, it may appear unexpected that there was no identified impact of PM pollution on working memory performance. Notably, existing research12,13 indicates the resilience of working memory against short-term pollution exposure episodes, despite the susceptibility to pollution-induced effects of other facets of cognition. Nevertheless, there is documented evidence of chronic exposure to high particulate concentrations negatively affecting both working memory62,63,64,65,66 and episodic memory67. Moreover, there is evidence suggesting that working memory can be affected following inflammation induced by vaccination43,46. This growing body of evidence suggests a potential divergence in the impact of PM pollution on executive, higher order cognitive processing, and working memory. Further insights into these differences could be investigated through non-invasive brain imaging studies. One interesting outcome of this study was an interaction between session time (pre- versus post-exposure) and inhalation pathway (normal versus restricted inhalation) on immediate recall of spatial locations (1-back of the Spatial n-back task). Although further analysis revealed no significant changes from inhalation method between pre- and post-exposure, the potential impact of disrupting breathing patterns with a nose clip (albeit not during cognitive tasks) merits additional investigation. This effect was unique to this specific circumstance as it was not observed in any other tasks or in more challenging n-back blocks.
It is not believed that the effects observed in this study can be attributed to differences in CO2 concentration. Recent research examining the impact of CO2 exposure in office environments found no significant effect on cognitive functioning with concentrations up to 2100 ppm68; the maximum average hourly CO2 concentration in this study was reported as 845 ppm. Additionally, while some studies indicate cognitive changes at concentrations as low as 1000 ppm69, it is noteworthy that these effects are typically observed only while exposure is ongoing and when it lasts longer than 1 h70, with an expectation of rapid recovery immediately following exposure. Given the 4-h delay between exposure end and post-exposure testing in our study, we find it unlikely that CO2 exposure serves as a causal factor for the identified changes in cognitive function.
Blocking the orthonasal pathway (the nose) to the olfactory bulb did not significantly mediate changes in cognitive function related to air pollution. This suggests that the identified effects may be mediated through the respiratory system, either directly or indirectly. However, it is challenging to draw definitive conclusions without further investigation. Although it is not feasible to entirely block the entry of odourants to the olfactory epithelium via the retronasal route (the pathway from the mouth to the nose), the mechanisms of odour perception differ considerably between nasal and oral breathing71. Research indicates that oral-only breathing does not activate the neural pathways associated with olfactory processing to the same extent as nasal breathing, which can hinder the ability to learn olfactory cues72. Thus, while some odour perception may still occur through alternative routes, obstructing the nasal pathway significantly reduces the intensity of olfactory stimulation and, consequently, the entry of pollutants.
A condition isolating the olfactory pathway (nasal-only breathing) would provide further clarity on this unanswered query, although it would be experimentally very difficult to block particle entry into the lungs. Whilst not significant, there was a trend identified in the Face Identification Task suggesting a more pronounced effect of particulate matter air pollution on selective attention performance in the normal inhalation condition, where both the respiratory and olfactory pathway were unrestricted. This could suggest an important role of the olfactory pathway in selective attention ability, but not other cognitive functions such as emotion expression recognition. Although, it may also be reasonable to assume that any worsening of effects could simply be due to a higher pollution dosage, possibly evidenced by the significantly elevated average CO2 concentrations during exposures when inhalation was not restricted. Similarly, it is conceivable that, assuming a similar composition, particles of different sizes may be key to understanding a possible differing role of the olfactory or respiratory pathways. The nasal mucosa, being highly vascularised, facilitates swift absorption of chemicals and drugs73; therefore both soluble and insoluble particles could potentially reach the brain through this route. Indeed, evidence suggests that ultrafine particles demonstrate increased effectiveness in traversing the alveolar/capillary barrier74. There is a clear necessity for further research to confirm the potential role of the olfactory pathway in selective attention ability and to better understand the effects of PM air pollution on cognitive function.
In conclusion, this study showed a reduction in higher-order cognitive processing 4 h after exposure to high concentrations of PM2.5 in healthy individuals, while spatial working memory function is robust against short-term exposure episodes. Blocking of the olfactory pathway did not significantly mediate particulate pollution-related cognitive function changes, however further research is strongly encouraged to investigate pathway-related differences, allowing for a more nuanced understanding of the distinctive factors and mechanisms that contribute to the identified trends within this study. Identifying cognitive effects within a healthy population suggests that the impact of air pollution exposure on brain function may be more significant in more vulnerable groups. Future research could focus on populations at higher risk of cognitive problems, such as older adults.
Methods
This project received ethical approval from the University of Birmingham Science, Technology, Engineering and Mathematics (STEM) ethics committee, number ERN_21-1188. Written informed consent was obtained from each participant, and we confirm that the research complied with all relevant ethical regulations.
Briefly, the methodology of the study was a single-blind within-participants design. Participants took part in four cognitive tasks (total time 60 min) prior to, and 4 h following, a 1-h exposure session. Air quality was manipulated using candle burning as previously described in Shehab and Pope (2019)18. This has been shown to be a low-cost, controlled method to increase PM2.5 concentrations to levels that might be experienced in an urban area75. PM2.5, PM10, Carbon Dioxide (CO2), and Carbon Monoxide (CO) were monitored throughout exposure to all air quality conditions. Inhalation pathway was manipulated by using a swimming nose clip, blocking the olfactory pathway, thereby decreasing the concentration of particulate pollution entering the body via this route. Participants took part in both PM pollution and clean air conditions whilst wearing the nose clip (allowing for ‘restricted’ oral-only inhalation) or without nose clip (’normal’ inhalation through both nose and mouth).
Participants
Thirty-nine staff and students at the University of Birmingham, Birmingham, UK were recruited through on-campus advertisements and offered cash on completion of each study visit. Individuals who reported current neurological, psychiatric, inflammatory, or respiratory disorders (e.g., multiple sclerosis, depression, rheumatoid arthritis, asthma), or current smoking (including e-cigarettes) were excluded. See Supplementary Methods for full study exclusion criteria. A full dataset was collected from 30 participants. All data was removed from four participants due to scores on the Depression, Anxiety, and Stress Scale (DASS) indicative of undisclosed or diagnosed mental health conditions; a pattern of random responses during cognitive tasks, suggesting potential interference or disruption (referred to as Sabotage); and self-reported non-compliance or adherence to study instructions.
The resultant data set for analysis contained 26 participants. Mean age = 27.7 years, s.d., = 10.6, range 19–67; 57.7% female (reported gender and biological sex).
Power analyses were conducted based on unpublished data from previous experiments, using conservative estimates to ensure adequate sensitivity. For the Expression Recognition Task, using effect size and variance from prior data on approach bias before and after exposure to low-quality air (via candle burning) or ambient air (ΔApproach bias = 0.27, s.d., = 0.45), a sample size of 30 participants was conservatively estimated to provide 90% power to detect a significant change in socio-emotional processing at p < 0.05. Similarly, for the Face Identification Task, based on previous data on cognitive load and attention after diesel exhaust exposure (ΔRT = 22 ms, s.d., = 25 ms), 30 participants were estimated to provide 90% power to detect a 15 ms change in response time (p < 0.05). Although the final sample size was 26 participants, the conservative nature of the power analyses ensures that the study retains sufficient sensitivity despite the slightly smaller sample.
Design
This single-blind study used a repeated measures experimental design across 4 days. The within-subject factors were inhalation pathway, (normal inhalation, restricted inhalation); pollution exposure, (clean air, PM pollution); and session time (pre-exposure, post-exposure). The order of inhalation and exposure conditions were counterbalanced across the four sessions. Condition orders were randomly assigned to participant ID numbers (1–32) before data collection began; note that inhalation pathway condition was always the same for two subsequent exposures. Participants were subsequently assigned unique ID numbers sequentially based on successful recruitment. To address unequal distribution of conditions following participant attrition, a new participant ID was generated for the same condition. This aimed to control for potential psychological biases such as learning and fatigue effects in the experimental design. See Supplementary Table 1 for the condition order details of the 26 participants included in the data analysis.
Materials
A Windows 10 computer running Matrix Laboratory (MATLAB) version R2022a (9.4; MATLAB, 2022)76 was used to run the cognitive tasks. All tasks were in the format of a MATLAB script utilising the Psychophysics Toolbox version 3.0.1477. Task scripts and instructions are available on GitHub. We opted not to use assessments of general cognition, such as the Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Wechsler Adult Intelligence Scale (WAIS) as these were primarily derived to index severe damage or failure of cognition, as occurs in stroke or brain damage. As such they are insensitive to more subtle but nonetheless important cognitive degradation. TSI Optical Particle Sizer (OPS) 3330 was used to measure concentrations of PM2.5 and PM10 throughout participant exposure. LI-COR LI-820 CO2 Gas Analyzer was used to measure concentrations of Carbon Dioxide. LASCAR EL-USB-CO Data Logger was used to measure concentrations of Carbon Monoxide. To provide PM (particulate matter) pollution, 100% stearin candles made from animal fat were burned. These unscented candles, measuring 190 mm in height and 22 mm in radius, were purchased from a major hypermarket. A swimming nose clip was used to reduce pollution concentration entering the olfactory pathway in the restricted inhalation condition.
Air quality monitoring
TSI OPS 3330: This instrument uses single particle counting technology to measure particles from 0.3 to 10 micrometres (μm) across 16 size channels. These channels were set to the default values of sample weighting factors defined by European Standard EN481 for PM2.5 and PM10. The instrument was set to record particle concentration and size distribution in 10 second intervals. The authors note there was no means of measuring the relative number of ultrafine particles. We use PM2.5 mass concentration as our metric of PM pollution, following standard public health protocols.
LI-COR LI-820 CO2 Gas Analyzer: This sensor is a non-dispersive infrared gas analyser measuring CO2 concentrations. The instrument was set to record in 1 second intervals, with resultant values measured as particles per million (ppm).
LASCAR EL-USB-CO Data Logger: This standalone instrument was set to record in 10 second intervals, with CO values measured as particles per million (ppm).
Subjective measures
The Depression, Anxiety, and Stress Scale (DASS)78 was used to identify recent participant depression, anxiety, and stress. Participants rated to what extent 42 statements applied to them over the past week on a scale of 0 to 4 (Did not apply at all—Applied to me very much, or most of the time). Higher scores indicate higher levels of psychological distress, with a maximum of 42 available for each metric (depression, anxiety, and stress).
Participants responded to a question about their awareness of the pollution exposure condition: “This morning, you spent 60 min in a room. Before entering, the room either had a burning candle or not. Please indicate which air condition you believe that you experienced today” responding to one of two options: Candle or No candle. Participants were also asked of their confidence in their answer: “How confident are you in your answer?” responding to one of the following five options: Not confident at all (0); Slightly confident (1); Somewhat confident (2); Fairly confident (3); or Completely confident (4).
Cognitive tasks
These tasks, originally developed for this study, have subsequently been detailed in a published protocol for a different experiment, HIPTox79.
Spatial n-back task
The n-back task is a continuous performance assessment employed to evaluate working memory capacity80. In this widely used cognitive task, participants are presented with sequences of stimuli, in this case, spatial locations, and must determine whether the currently displayed stimulus matches the one presented presented ‘n’ trials earlier. As ‘n’ increases, the volume of information retained in working memory also grows. It is broadly recognised that working memory has a limited capacity, with Miller (1956)81 proposing that the number of items that can be held is ~7 ± 2. Consequently, as ‘n’ increases, both the task’s difficulty and the proportion of errors tend to rise. Poor performance on the task suggests deficits in encoding, maintaining, and/or retrieving information.
The stimuli comprised a centrally positioned 3 × 3 grid with white grid lines (red-green-blue coordinate, RGB [255, 255, 255]) displayed on a grey background (RGB [128, 128, 128]). The grid measured 16.5 cm in both height and width, subtending a visual angle of ~15.78∘ in both dimensions when viewed from a distance of 60 cm. The grid was situated centrally on the screen to ensure focused stimulus presentation.
During each trial, a blank 3 × 3 grid was displayed for 600 ms, followed by a single white square appearing in one of the nine possible grid locations for 1000 ms. Subsequently, the blank grid was shown once more. Participants were required to remember the sequence of grid positions and indicate for each trial whether the square occupied the same location as it had ‘n’ trials earlier, or different location. This was a two-alternative forced choice task, where participants used the ‘m’ and ‘z’ keyboard keys to respond ‘same’ and ‘different’ respectively; these keys were reversed for left-handed participants. Responses were expected within 10 seconds of the prompt. See Figure 4a for an illustration of the sequence of displays in each trial. The task consisted of four blocks of 45 trials, each containing eight matches (i.e., ‘same’ locations). The value of ‘n’ increased with each block: block 1, n = 1; block 4, n = 4.
a Spatial n-back Task; (b) Face Identification Task, which used face images sourced from Set A of the Karolinska Directed Emotional Faces dataset86 (placeholder images are displayed in the figure rather than the actual stimuli); (c) Expression Recognition Task, which used face images sourced from the RADIATE database91,92 (placeholder images are displayed in the figure rather than the actual stimuli); (d) Psychomotor Vigilance Task—Psychomotor speed trials; and (e) Psychomotor Vigilance Task—sustained attention trials. The screens shown are for illustrative purposes only; refer to the text for the actual stimuli sizes and on-screen locations.
Face identification task
This task evaluates executive function by assessing selective attention, akin to established cognitive assessments such as the Stroop82 and Flanker83 tasks. Participants must disregard distractions and focus on the primary task objective.
Given the limited capacity of high-level cognitive systems, the brain employs two complementary mechanisms for attentional control. Proactive control is used to plan strategically and engage selectively with anticipated relevant information while avoiding foreseeable distractions. Simultaneously, reactive control is activated to adjust behaviour in response to unexpected events. For example, preparing coffee involves proactive control to locate and approach the kettle while ignoring the biscuit tin (particularly if on a diet). Conversely, reactive control is required to avoid a colleague who unexpectedly steps into your path. These mechanisms compete for attentional resources; successful task execution depends on sustained proactive control84,85, while distraction caused by unforeseen, irrelevant events reflect reactive control.
The stimuli included a white (RGB [255, 255, 255]) spatial cue arrow pointing left or right and a centrally presented white fixation cross, all displayed on a black background (RGB [0, 0, 0]). Faces were sourced from the A set of the Karolinska Directed Emotional Faces86, encompassing all available emotional expressions: fearful, angry, disgusted, happy, neutral, and surprised. Scrambled images were generated by dividing face images into 13,984 squares and randomising their positions. Each image subtended 8.6∘ × 11.0∘, with two images displayed simultaneously, each laterally offset by approximately ± 8.1∘ of visual angle for symmetrical presentation.
Participants were instructed to respond as quickly as possible to identify the gender presentation of the target face using either the ‘A’ and ‘Z’ keyboard keys (for left-handed participants) or the ‘L’ and ‘P’ keys (for right-handed participants), using the index and middle fingers of their dominant hand. Key assignment to ‘male’ and ‘female’ was counterbalanced across participants. The task consisted of four blocks of 60 trials each, totalling 240 trials.
Each trial began with a fixation cross presented for 500 milliseconds (ms), followed by a spatial cue for 400 ms that reliably indicated the location of the upcoming target. This was followed by another fixation cross (350–850 ms, determined by a random integer between 20 and 50 sampled at a 17 Hz frame rate). The face array then appeared for 75 ms, followed by a final fixation cross presented for 1500 ms or until participants responded. The face array comprised a central fixation cross, a distractor image (either scrambled or a face), and a target image (face). Gender and emotional expression congruency were balanced across trials, ensuring all stimulus combinations were equally likely. Participants were instructed to identify the gender presentation of the target face as quickly and accurately as possible following the short (75 ms) presentation of the face array. See Fig. 4b for the sequence of displays in each trial.
In this task, participants were required to identify the gender presentation of a target face while either another face (in two-face trials) or a non-face (in one-face trials) distractor was present. The presence of a face distractor typically increases response time87, reflecting reactive selective attention capture. Studies on cognitive control consistently show that response slowing caused by distractors is more pronounced when the preceding trial lacked a compelling distractor (one-face trials) compared to trials with distractors (two-face trials)88. This phenomenon is attributed to prior distractor suppression (in trial n-1), which enhances proactive processing of the target in the current trial n. In contrast, the absence of prior distractor suppression weakens proactive control, increasing vulnerability to distractor capture89. Differences in response times (RTs) between two-face trials following two-face trials (repeat sequences) and those following one-face trials (change sequences) inversely reflect proactive control (ΔRT). Thus, greater response times in change sequences compared to repeat sequences indicate reduced proactive control.
Expression recognition task
This Go/No-go task utilised happy and fearful facial expressions as target stimuli to assess decision-making performance between positive-affective and negative-affective expressions. The task examined both approach bias and the ability to discriminate between emotional expressions.
Approach bias
This aspect of the task explores the tendency to engage with positive-affective stimuli (e.g., happy faces) more readily than with negative-affective stimuli (e.g., fearful faces). This preference is thought to reflect underlying emotion regulation processes90. A natural inclination to approach positive stimuli and avoid negative stimuli is expected to produce a bias in accuracy, with better performance for happy targets and poorer performance for fearful targets.
Expression discrimination
This aspect constitutes the perceptual component of the task. Participant accuracy, regardless of the target expression, indicates the ability to distinguish among different emotion expressions. A higher overall accuracy demonstrates an improved capacity to promptly perceive and categorise happy and fearful facial expressions, which is a pivotal concept in theory of mind56, a critical social-cognitive skill.
The expressive faces used in this task were sourced from the RADIATE database91,92. Each face image was displayed centrally against a white background (RGB [255, 255, 255]), with explanatory text and fixation crosses presented in black (RGB [0, 0, 0]). Stimuli consisted of a centrally positioned face image measuring 8 cm in both height and width, subtending a visual angle of ~7.68∘ in both dimensions when viewed from a distance of 60 cm.
Participants were instructed to respond to faces displaying the target expression (happy or fearful) and to withhold responses to non-target expressions. Participant responses were made as quickly as possible by pressing the keyboard spacebar with their dominant hand. The target expression alternated between blocks, beginning with ‘happy’. Each trial began with a fixation cross presented for 550–950 ms, followed by an expressive face image displayed for 100 ms, and then a blank screen lasting 700 ms or until a response was made. See Fig. 4c for the sequence of displays in each trial. The task consisted of six blocks of 44 trials each, with 28 target (Go) images and 16 non-target (No-go) images per block. All face stimuli featured either open mouths (more expressive) or closed mouths (less expressive), with these conditions evenly distributed between Go and No-go trials within each block.
Psychomotor vigilance task
This basic response time task assessed simple reaction time, providing a measure of global processing (psychomotor speed) and the ability to maintain concentration over extended periods (sustained attention)93.
The target stimulus was a centrally presented red circle with a diameter of 0.5 cm (RGB [255, 60, 0]), displayed against a black background (RGB [0, 0, 0]). The circle subtended a visual angle of ~0.48∘ when viewed from a distance of 60 cm. Explanatory text and fixation crosses were shown in white (RGB [255, 255, 255]).
Participants were instructed to focus on a centrally positioned fixation cross and respond as quickly as possible when the target, a small red circle, appeared. Each trial began with a fixation cross displayed for 400–800 ms in psychomotor speed trials, or for 25–35 s in sustained attention trials. This was followed by the appearance of the red circle target for 400 ms or until the participant responded. If the keyboard spacebar was pressed during the target presentation, a white circle was shown for 400 ms as feedback. See Fig. 4d and e for a visual representation of both trial sequence types. The task consisted of one block of 85 trials, including 10 sustained attention trials with long fixation intervals and 75 psychomotor speed trials with shorter fixation intervals.
Procedure
Upon arrival, participant suitability was checked against exclusion criteria and after informed consent participants completed the four cognitive tasks in the cognitive testing room: Spatial n-back Task; Face Identification Task; Expression Recognition Task; and Psychomotor Vigilance Task. Twenty minutes prior to participants completing the tasks, two candles were lit (PM pollution condition only) in an adjacent ‘exposure’ room (4.15 × 2.95 × 3.40 m = 41.6 m3) and windows closed (all conditions). One minute prior to task completion, the candles were extinguished and the air quality sensors were activated to record Particulate Matter (PM), Carbon Dioxide (CO2), and Carbon Monoxide (CO) concentrations. Participants, wearing a nose clip in the restricted inhalation condition, were taken into the exposure room and seated close to the air quality sensors. A fan circulated air in the room. Participants remained in the exposure room for 60 min, during which they completed the DASS questionnaire. After 60 min of exposure to either elevated PM concentrations (PM pollution condition) or room air (clean air condition), participants left the lab.
Four hours later, participants returned to the cognitive testing room. The cognitive tasks were repeated in the same order as previously described. Participants were then asked to indicate whether they believed they were exposed to the clean air or PM pollution condition earlier that day, and they received monetary compensation for their time. This procedure was repeated, after a minimum two-week washout period, until all four conditions were completed. See Fig. 5.
Data processing
Spatial n-back task
Each trial has four possible outcomes94: a hit (correctly identifying a ‘same’ location), a correct rejection (correctly identifying a ‘different’ location), a miss (failing to identify a ‘same’ location), or a false alarm (incorrectly responding ‘same’ to a ‘different’ location).
Working memory ability was quantified using \({{{{\rm{d}}}}}^{{\prime} }\), which represents the standardised difference between the signal-present (same location) and signal-absent (different location) distributions. It was calculated as the Z-score of the hit rate [#Hits / (#Hits + #Misses)] minus false alarm rate [#False Alarms / (#False Alarms + #Correct Rejections)]. This calculation was performed separately for each n-back value, with lower \({{{{\rm{d}}}}}^{{\prime} }\) scores indicating poorer working memory performance. As ‘n’ increases, performance is expected to decline, consistent with the increased working memory load. Response time (RT) was not considered in this task, as participants were not instructed to respond quickly and were given a relatively long response window of 10 seconds.
Face identification task
The primary metric of interest in this task is cognitive control, measured using ΔRT (the difference in response time between repeat sequences and change sequences). Accuracy is not considered in the analyses, as it was only necessary to establish a task goal for participants, but is not relevant for calculating cognitive control.
Trials were excluded from statistical analyses if there was no response on the current or previous trial, or if the response on the current trial (n) was too fast (RT < 200 ms). Additionally, individual RTs were trimmed if they deviated by more than ± 2 standard deviations from the mean RT for repeat and change sequences, respectively.
Expression recognition task
As with the Spatial n-back Task, there are four possible outcomes for each trial94: a hit (correct ‘Go’ response to the target expression), a correct rejection (correct ‘No-go’ response to the non-target expression), a miss (incorrect ‘No-go’ response to the target expression), and a false alarm (incorrect ‘Go’ response to the non-target expression).
Trials were excluded if response times (RT) were below 200 ms, indicating an anticipation error. The metric \({{{{\rm{d}}}}}^{{\prime} }\), which measures expression sensitivity, was calculated as the Z-score of the hit rate [#Hits / (#Hits + #Misses)] minus false alarm rate [#False Alarms / (#False Alarms + #Correct Rejections)]. This was computed separately for each emotion expression type. A lower \({{{{\rm{d}}}}}^{{\prime} }\) score indicates reduced sensitivity to the stimulus signal, reflecting greater difficulty in distinguishing between expressions.
Psychomotor vigilance task
The primary metric of interest in this task was Response Time (RT), irrespective of whether participants respond within the 400 ms window required for ‘correct’ response feedback. RT is defined as the time elapsed between perceiving the target visual stimulus and pressing the response button.
Sustained attention was measured by RT following long fixation pauses (ten trials), with a shorter RT indicating better sustained attention. Psychomotor speed was assessed by RT following short fixation pauses (75 trials), with a shorter RT reflecting faster psychomotor speed.
It is expected that average RTs will not differ significantly between the pre-exposure and post-exposure test sessions. This would suggest that exposure to low-quality air does not affect sustained attention or basic psychomotor functioning. If significant RT differences are observed, they may reflect fatigue rather than more complex mechanisms linking air pollution exposure to diminished higher-order cognitive functioning.
Data analysis
Cognitive Tasks: Approach bias (Expression Recognition Task) was analysed using a four-way (2 × 2 × 2 × 2) repeated measures analysis of variance (ANOVA), with inhalation pathway (normal, restricted); pollution exposure (clean air, PM pollution); session time (pre-exposure, post-exposure); and emotion expression (happy, fearful) as the factors. All other cognitive metrics of interest were analysed using a three-way (2 × 2 × 2) repeated measures ANOVA, with inhalation pathway; pollution exposure; and session time as the factors. Bonferroni corrections for multiple comparisons were applied to each analysis. To further investigate interaction effects, one-tailed paired samples T-tests were conducted as necessary. The authors note that the statistical significance of all reported T-tests would be the same if conducted as two-tailed tests.
Air Quality: Pollutant concentrations were averaged across the 1-h exposure period. PM2.5, PM10, and CO2 were analysed separately using a two-way (2 × 2) repeated measures analysis of variance (ANOVA), with inhalation pathway (normal, restricted) and pollution exposure (clean air, PM pollution) as the factors. Bonferroni corrections for multiple comparisons were applied to each analysis.
Exposure Awareness: The frequencies of the actual air pollution exposure condition and the self-reported exposure condition were compared using a χ2 test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available in the article, its Supplementary information and Source data file. Raw and processed demographic, cognitive, and air quality data generated in this study and used for analysis have been deposited in the University of Birmingham eData Repository95. Task instructions and MATLAB scripts used for cognitive testing are available on GitHub. Source data are provided with this paper.
Change history
17 February 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-025-57017-z
References
World Health Organization. Ambient air pollution: A global assessment of exposure and burden of disease. https://www.who.int/publications/i/item/9789241511353 (2016).
Landrigan, P. J. et al. The lancet commission on pollution and health. Lancet 391, 462–512 (2018).
Pope, C. A. & Dockery, D. W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 56, 709–742 (2006).
Basagaña, X. et al. Neurodevelopmental deceleration by urban fine particles from different emission sources: A longitudinal observational study. Environ. Health Perspect. 124, 1630–1636 (2016).
Delgado-Saborit, J. M. et al. A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci. Total Environ. 757, 143734 (2021).
Castellani, B. et al. Mitigating the impact of air pollution on dementia and brain health: Setting the policy agenda. Environ. Res. 215, 114362 (2022).
Calderón-Garcidueñas, L. et al. Air pollution and brain damage. Toxicol. Pathol. 30, 373–389 (2002).
Levesque, S., Surace, M. J., McDonald, J. & Block, M. L. Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J. Neuroinflammation 8, 105 (2011).
Wang, Y., Xiong, L. & Tang, M. Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease: Inhaled PM on the CNS. J. Appl. Toxicol. 37, 644–667 (2017).
Cohen, A. J. et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet 389, 1907–1918 (2017).
World Health Organization. WHO global air quality guidelines. https://iris.who.int/bitstream/handle/10665/345329/9789240034228-eng.pdf?sequence=1 (2021).
Saenen, N. D. et al. Recent versus chronic exposure to particulate matter air pollution in association with neurobehavioral performance in a panel study of primary schoolchildren. Environ. Int. 95, 112–119 (2016).
Bos, I. et al. Subclinical effects of aerobic training in urban environment. Med. Sci. Sports Exerc. 45, 439–447 (2013).
Faherty, T. et al. Acute diesel exhaust exposure causes a delayed reduction in cognitive control. https://doi.org/10.21203/rs.3.rs-1064957/v1 (2021).
Mallach, G. et al. Randomized cross-over study of in-vehicle cabin air filtration, air pollution exposure, and acute changes to heart rate variability, saliva cortisol, and cognitive function. Environ. Sci. Technol. 57, 3238–3247 (2023).
Künn, S., Palacios, J. & Pestel, N. Indoor air quality and strategic decision making. Manag. Sci. 69, 5354–5377 (2023).
Gawryluk, J. R., Palombo, D. J., Curran, J., Parker, A. & Carlsten, C. Brief diesel exhaust exposure acutely impairs functional brain connectivity in humans: a randomized controlled crossover study. Environ. Health 22, 7 (2023).
Shehab, M. A. & Pope, F. D. Effects of short-term exposure to particulate matter air pollution on cognitive performance. Sci. Rep. 9, 8237 (2019).
Gao, X. et al. Short-term air pollution, cognitive performance and nonsteroidal anti-inflammatory drug use in the veterans affairs normative aging study. Nat. Aging 1, 430–437 (2021).
Diamond, A. Executive functions. Annu. Rev. Psychol. 64, 135–168 (2013).
Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 63, 1–29 (2012).
Adolphs, R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 12, 169–177 (2002).
Shou, Y. et al. Ambient PM2.5 chronic exposure leads to cognitive decline in mice: From pulmonary to neuronal inflammation. Toxicol. Lett. 331, 208–217 (2020).
Oberdörster, G. et al. Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicol. 16, 437–445 (2004).
Elder, A. et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 114, 1172–1178 (2006).
Hatt, H. Olfactory Sense, 3018–3023 (Springer Berlin Heidelberg, Berlin, Heidelberg, 2008).
Kwon, H.-S., Ryu, M. H. & Carlsten, C. Ultrafine particles: unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 52, 318–328 (2020).
Miller, F. J. et al. Size considerations for establishing a standard for inhalable particles. J. Air Pollut. Control Assoc. 29, 610–615 (1979).
Du, Y., Xu, X., Chu, M., Guo, Y. & Wang, J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 8, E8–E19 (2016).
Adivi, A., Lucero, J., Simpson, N., McDonald, J. D. & Lund, A. K. Exposure to traffic-generated air pollution promotes alterations in the integrity of the brain microvasculature and inflammation in female ApoE−/− mice. Toxicol. Lett. 339, 39–50 (2021).
Block, M. L. & Calderón-Garcidueñas, L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32, 506–516 (2009).
Maher, B. A. Airborne magnetite- and iron-rich pollution nanoparticles: Potential neurotoxicants and environmental risk factors for neurodegenerative disease, including alzheimer’s disease. J. Alzheimer’s. Dis. 71, 361–375 (2019).
Calderón-Garcidueñas, L., Reynoso-Robles, R. & González-Maciel, A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: The culprit of alzheimer and parkinson’s diseases. Environ. Res. 176, 108574 (2019).
Calderón-Garcidueñas, L. et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cognition 77, 345–355 (2011).
Seaton, A., Tran, L., Chen, R., Maynard, R. L. & Whalley, L. J. Pollution, particles, and dementia: A hypothetical causative pathway. Int. J. Environ. Res. Public Health 17, 862 (2020).
Leszek, J. et al. The links between cardiovascular diseases and alzheimer’s disease. Curr. Neuropharmacol. 19, 152–169 (2020).
Liu, Y. et al. Association of cigarette smoking with cerebrospinal fluid biomarkers of neurodegeneration, neuroinflammation, and oxidation. JAMA Netw. Open 3, e2018777 (2020).
Ly, M. et al. Obesity and white matter neuroinflammation related edema in alzheimer’s disease dementia biomarker negative cognitively normal individuals. J. Alzheimer’s. Dis. 79, 1801–1811 (2021).
Ebert, S. E. et al. Molecular imaging of neuroinflammation in patients after mild traumatic brain injury: a longitudinal 123I-CLINDE single photon emission computed tomography study. Eur. J. Neurol. 26, 1426–1432 (2019).
Ashraf-Ganjouei, A., Moradi, K., Bagheri, S. & Aarabi, M. H. The association between systemic inflammation and cognitive performance in healthy adults. J. Neuroimmunol. 345, 577272 (2020).
Balter, L. J. et al. Low-grade inflammation decreases emotion recognition - evidence from the vaccination model of inflammation. Brain, Behav., Immun. 73, 216–221 (2018).
Balter, L. J. et al. Selective effects of acute low-grade inflammation on human visual attention. NeuroImage 202, 116098 (2019).
Harrison, N. A., Doeller, C. F., Voon, V., Burgess, N. & Critchley, H. D. Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biol. Psychiatry 76, 585–593 (2014).
Krabbe, K. S. et al. Low-dose endotoxemia and human neuropsychological functions. Brain, Behav., Immun. 19, 453–460 (2005).
Lasselin, J. et al. Lipopolysaccharide alters motivated behavior in a monetary reward task: A randomized trial. Neuropsychopharmacology 42, 801–810 (2017).
Reichenberg, A. et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58, 445–452 (2001).
Bahador, M. & Cross, A. S. Review: From therapy to experimental model: a hundred years of endotoxin administration to human subjects. J. Endotoxin Res. 13, 251–279 (2007).
Murray, C. L., Skelly, D. T. & Cunningham, C. Exacerbation of cns inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1β and IL-6. J. Neuroinflammation 8, 50 (2011).
Naseri, M. et al. The impact of frying aerosol on human brain activity. NeuroToxicology 74, 149–161 (2019).
Crüts, B. et al. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part. Fibre Toxicol. 5, 4 (2008).
Driessen, A. et al. Short-term Exposure to Nanoparticle-rich Diesel Engine Exhaust Causes Changes in Brain Activity but not in Cognitive Performance in Human Volunteers, 243-255 (The Royal Society of Chemistry, 2012). https://doi.org/10.1039/9781849735247-00243
Tanaka, Y., Morikawa, T. & Honda, Y. An assessment of nasal functions in control of breathing. J. Appl. Physiol. 65, 1520–1524 (1988).
Gui, Z. et al. Exposure to ambient air pollution and executive function among chinese primary schoolchildren. Int. J. Hyg. Environ. Health 229, 113583 (2020).
Harris, M. H. et al. Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicology Teratol. 57, 60–70 (2016).
Mier, D. et al. The involvement of emotion recognition in affective theory of mind: Emotion recognition and affective ToM. Psychophysiology 47, 1028–1039 (2010).
Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y. & Plumb, I. The “reading the mind in the eyes” test revised version: A study with normal adults, and adults with asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry 42, 241–251 (2001).
Rotton, J., Barry, T., Frey, J. & Soler, E. Air pollution and interpersonal attraction. J. Appl. Soc. Psychol. 8, 57–71 (1978).
Burkhardt, J. et al. The relationship between monthly air pollution and violent crime across the United States. J. Environ. Econ. Policy 9, 188–205 (2019).
Albert, J., López-Martín, S., Tapia, M., Montoya, D. & Carretié, L. The role of the anterior cingulate cortex in emotional response inhibition. Hum. Brain Mapp. 33, 2147–2160 (2012).
Schmid, P. C., Kleiman, T. & Amodio, D. M. Neural mechanisms of proactive and reactive cognitive control in social anxiety. Cortex 70, 137–145 (2015).
Mittli, D. Inflammatory processes in the prefrontal cortex induced by systemic immune challenge: Focusing on neurons. Brain Behav. Immun. Health 34, 100703 (2023).
Ailshire, J. A. & Clarke, P. Fine particulate matter air pollution and cognitive function among U.S. older adults. J. Gerontol. Ser. B: Psychological Sci. Soc. Sci. 70, 322–328 (2015).
Schikowski, T. et al. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ. Res. 142, 10–16 (2015).
Tonne, C., Elbaz, A., Beevers, S. & Singh-Manoux, A. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 25, 674–681 (2014).
Rivas, I. et al. Association between early life exposure to air pollution and working memory and attention. Environ. Health Perspect. 127, 5 (2019).
Sunyer, J. et al. Traffic-related air pollution and attention in primary school children: Short-term association. Epidemiology 28, 181–189 (2017).
Ailshire, J. A. & Crimmins, E. M. Fine particulate matter air pollution and cognitive function among older US adults. Am. J. Epidemiol. 180, 359–366 (2014).
Chen, D., Huebner, G., Bagkeris, E., Ucci, M. & Mumovic, D. Effects of short-term exposure to moderate pure carbon dioxide levels on cognitive performance, health symptoms and perceived indoor environment quality. Build. Environ. 245, 110967 (2023).
Azuma, K., Kagi, N., Yanagi, U. & Osawa, H. Effects of low-level inhalation exposure to carbon dioxide in indoor environments: A short review on human health and psychomotor performance. Environ. Int. 121, 51–56 (2018).
Jacobson, T. A. et al. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustainability 2, 691–701 (2019).
Jaime-Lara, R. B., To, L. & Joseph, P. V. Anatomy, Physiology, and Neurobiology of Olfaction, Gustation, and Chemesthesis, 3-20 (Springer International Publishing, 2021). https://doi.org/10.1007/978-3-030-86282-4_1
Pause, B. M., Krauel, K., Sojka, B. & Ferstl, R. Is odor processing related to oral breathing? Int. J. Psychophysiol. 32, 251–260 (1999).
Amponsah, S. K. & Adams, I. Drug Absorption via the Nasal Route: Opportunities and Challenges, 25–42 (Springer International Publishing, 2023). https://doi.org/10.1007/978-3-031-23112-4_3
McMartin, C., Hutchinson, L. E., Hyde, R. & Peters, G. E. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J. Pharm. Sci. 76, 535–540 (1987).
Laursen, K. R. et al. Airway and systemic biomarkers of health effects after short-term exposure to indoor ultrafine particles from cooking and candles - a randomized controlled double-blind crossover study among mild asthmatic subjects. Part. Fibre Toxicol. 20, 26 (2023).
The MathWorks Inc. Matlab version: 9.12 (r2022a), Natick, Massachusetts: The MathWorks Inc. 2022. http://www.mathworks.com (n.d.).
Brainard, D. H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
Lovibond, P. & Lovibond, S. The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behav. Res. Ther. 33, 335–343 (1995).
Faherty, T. et al. Hiptox—hazard identification platform to assess the health impacts from indoor and outdoor air pollutant exposures, through mechanistic toxicology: A single-centre double-blind human exposure trial protocol. Int. J. Environ. Res. Public Health 21, 284 (2024).
Kirchner, W. K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 55, 352–358 (1958).
Miller, G. A. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychol. Rev. 63, 81–97 (1956).
Stroop, J. R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662 (1935).
Eriksen, B. A. & Eriksen, C. W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 16, 143–149 (1974).
Botvinick, M., Nystrom, L. E., Fissell, K., Carter, C. S. & Cohen, J. D. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402, 179–181 (1999).
Braver, T. S. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci. 16, 106–113 (2012).
Lundqvist, D., Flykt, A. & Öhman, A. The Karolinska directed emotional faces (KDEF). CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet (1998).
Langton, S. R., Law, A. S., Burton, A. M. & Schweinberger, S. R. Attention capture by faces. Cognition 107, 330–342 (2008).
Gratton, G., Coles, M. G. H. & Donchin, E. Optimizing the use of information: Strategic control of activation of responses. J. Exp. Psychol.: Gen. 121, 480–506 (1992).
Egner, T. & Hirsch, J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 8, 1784–1790 (2005).
Elliot, A. J. The hierarchical model of approach-avoidance motivation. Motiv. Emot. 30, 111–116 (2006).
Conley, M. I. et al. The racially diverse affective expression (RADIATE) face stimulus set. Psychiatry Res. 270, 1059–1067 (2018).
Tottenham, N. et al. The nimstim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 168, 242–249 (2009).
Dinges, D. F. & Powell, J. W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods, Instrum. Computers 17, 652–655 (1985).
Harvey, L. O. The critical operating characteristic and the evaluation of expert judgment. Organ. Behav. Hum. Decis. Process. 53, 229–251 (1992).
Faherty, T., Raymond, J. E., McFiggans, G. & Pope, F. D. Research data supporting the publication “acute particulate matter exposure diminishes executive cognitive functioning after four hours regardless of inhalation pathway: raw and processed data" (2024). https://doi.org/10.25500/edata.bham.00001145
Acknowledgements
The authors would like to take this opportunity to thank all volunteer participants for their involvement in the project. The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Environment Research Council [grant number NE/W002213/1], awarded to T.F., G.M., and F.D.P.; and the University of Birmingham Institute for Global Innovation Clean Air Theme [grant number 5054], awarded to T.F., J.E.R., and F.D.P.
Author information
Authors and Affiliations
Contributions
T.F., G.M., and F.D.P. secured funding for the study; T.F. and F.D.P. formulated the research question; T.F., J.E.R., and F.D.P. conceived and designed the experiment; T.F. conducted the experiment, analysed the results, and wrote the initial manuscript; T.F., J.E.R., G.M., and F.D.P. reviewed the manuscript; and all authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Anna Oudin, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Faherty, T., Raymond, J.E., McFiggans, G. et al. Acute particulate matter exposure diminishes executive cognitive functioning after four hours regardless of inhalation pathway. Nat Commun 16, 1339 (2025). https://doi.org/10.1038/s41467-025-56508-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56508-3
This article is cited by
-
Parameterising the effect of human occupancy and kinetic energy on indoor air pollution
npj Climate and Atmospheric Science (2026)
-
Reactive nitrogen-driven atmospheric multiphase buffering induces unintended concomitant pollution
Nature Communications (2025)