Abstract
Lung cancer is the leading cause of cancer-related deaths. An enhanced understanding of the immune microenvironments within these tumours may foster more precise and efficient treatment, particularly for immune-targeted therapies. The spatial architectural differences between lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are relatively unexplored. Here, we applied imaging mass cytometry to a balanced cohort of LUAD and LUSC patients, matched for clinical factors such as age, sex, and smoking history, to analyze 204 histopathology images of tumours from 102 individuals with non-small cell lung cancer (NSCLC). By analyzing interactions and broader cellular networks, we interrogate the tumour microenvironment to understand how immune cells are spatially organized in clinically matched adenocarcinoma and squamous cell carcinoma subsets. This spatial analysis revealed distinct patterns of immune cell aggregation, particularly among macrophage populations, that correlated with patient prognosis differentially in adenocarcinoma and squamous cell carcinoma, suggesting potential new strategies for therapeutic intervention. Our findings underscore the importance of analyzing NSCLC histological subtypes separately when investigating the spatial immune landscape, as microenvironmental characteristics and cellular interactions differed by subtype. Recognizing these distinctions is essential for designing precision therapies tailored to each subtype’s unique immune architecture, ultimately enhancing patient outcomes.
Similar content being viewed by others
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide1. Approximately 85% of lung cancers can be classified as non-small-cell lung cancer (NSCLC). NSCLC is further categorized into three primary subtypes: lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and large-cell carcinoma2. LUAD and LUSC, which collectively represent 80-90% of NSCLC, have distinct genetic features, treatments, and prognoses3,4,5. In our previous work, we utilized imaging mass cytometry (IMC) to spatially dissect the tumour immune microenvironment (TIME) of LUAD, identifying features that were highly predictive of recurrence in early-stage disease6. Notably, we discovered that specific interactions between B cells and CD4+ T cells were associated with improved survival in LUAD patients. However, this survival advantage was negated when regulatory T cells were found in close proximity to B cells and CD4+ T cells. Additionally, we observed that many characteristics of the TIME, particularly within the myeloid compartment, were influenced by patient-specific factors such as age, sex, or smoking status. In this follow-up study, we aimed to compare the tumour immune landscape of LUAD with LUSC. Our previous findings underscored the necessity of using clinically matched cohorts when comparing NSCLC patients to avoid confounding variables and ensure accurate interpretation of prognostic features. While distinctions in immunological composition between LUAD and LUSC have been described previously7, understanding differences in their spatial architecture remains relatively unexplored8. To date, no studies have included a balanced comparison of LUAD and LUSC patients with matched clinical features. To address this gap, we employ highly multiplexed IMC to examine the immune characteristics of 204 histopathology images from 102 NSCLC tumours, representing 51 LUAD patients clinically matched to 51 LUSC patients. This approach allowed us to explore differences in the spatial organization of immune cells between LUAD and LUSC while controlling for key clinical variables. Leveraging this controlled cohort, our investigation reveals notable variations in the architectural organization of these tumours, along with complex interactions between immune cells within them, particularly concerning the myeloid compartment. We find that macrophages exhibit distinct organization patterns in LUAD and LUSC which are associated with patient outcomes. We also observe differences in the composition of the vascular niche. LUAD exhibit an enrichment in neutrophil-endothelial interactions in the TIME, which were absent in LUSC, even though neutrophils were twice as frequent in LUSC compared to LUAD. Our study emphasizes the necessity of accounting for histological subtypes when evaluating survival rates using tumour microenvironment classification in NSCLC, as it may not apply universally across all histologies. Our findings elucidate the distinctive immune landscapes and cellular interactions within the most prominent histological subtypes of lung cancer, which could help enhance therapeutic strategies.
Results
Single-cell spatial resolution of NSCLC using imaging mass cytometry
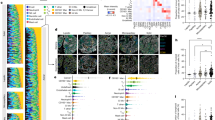
To draw immunological comparisons between the cellular composition of LUAD and LUSC tumours, IMC was performed on a total of 204 tumour samples collected from 102 patients—51 LUAD clinically matched to 51 LUSC. For each patient, a pathologist selected two tumour cores for analysis to account for tumour heterogeneity (Fig. 1A). LUAD and LUSC patients were matched by age, sex, stage, and smoking status (Table 1). We stained tumours with our previously optimized 35-plex antibody panel and applied our established pipeline for segmentation and cell-type assignment6. Due to the inherent differences in LUAD and LUSC biology, we amended our lineage assignment algorithm to better capture the differences in cellular frequency between LUAD and LUSC. In total, we resolved 893,148 cells which were then classified as either tumour, endothelial cells, or one of 14 immune cell types based on canonical lineage markers (Fig. 1B, C, Supplementary Fig. 1A–C, Supplementary Fig. 2, Supplementary Table 1). LUAD tumours had higher levels of immune cell infiltration (45.3% of total cells) compared to LUSC tumours (36.7%) (Fig. 1D). This was primarily attributable to increases in lymphoid cells in LUAD, aligning with prior findings9 (Fig. 1D). Within histological subtypes, we revealed a higher presence of lymphocytes in female LUAD patients (P = 0.0540, Fig. 1E) and in patients older than 75 years (P = 0.0470, Fig. 1F). We next explored differences in specific immune cell frequencies between the two histological subtypes. Reflecting the differences in overall immune cell frequencies, LUSC exhibited a greater proportion of cancer cells (45.1% of total cells) compared to LUAD (36.0%) (Fig. 1G). We noted differences in the prevalence of endothelial cells, with a significantly higher percentage in LUAD, indicative of elevated vascularization (Fig. 1G). Although the overall frequency of total myeloid cells was similar between LUAD and LUSC (Fig. 1D), there were significant differences in nearly all myeloid cell types (Fig. 1G). Most notably, neutrophils were significantly more abundant in LUSC (8.1% of total cells) compared to LUAD (4.1%) (Fig. 1G). Macrophages (including both CD163- and CD163+ subsets) constituted the majority of the myeloid compartment in LUAD (8.6% and 2.8% of total cells, respectively) and were more frequent when compared to macrophage frequencies in LUSC (4.3% and 1.0%) (Fig. 1G). CD14+ CD16- classical monocytes were more abundant in LUSC (2.3%) compared to LUAD (1.2%) (Fig. 1G). We observed the reverse trend for mast cells, which were significantly less frequent in LUSC compared to LUAD (Fig. 1G). This was intriguing given that recent studies suggest that mast cells play a valuable role in determining the prognosis of NSCLC, with LUSC patients having worse outcomes10. Upon characterizing immune lineages underlying the elevation in lymphocytes in LUAD, we found this was driven by an increase in the frequency of CD4+ helper T cells (12.5% in LUAD versus 7.4% in LUSC) and regulatory T cells (Tregs) (0.62% in LUAD versus 0.33% in LUSC) (Fig. 1G). The frequency of CD8+ T cells, B cells and Natural Killer (NK) cells did not significantly differ between LUAD and LUSC (Fig. 1G).
A Schematic depicting the acquisition and analysis of IMC multiplexed images from 102 LUAD and 102 LUSC cores. Created in BioRender. Quail, D. (2025) https://BioRender.com/k14v431. B Distribution of cell types in the tumour microenvironment and corresponding clinical variables (predominant histological pattern, stage, sex, age, body mass index (BMI), smoking status, pack years, and progression status) in LUAD (n = 102) and LUSC (n = 102). Defined cell types are in order of prevalence for each histological subtype, sorted by CD4+ T cell frequency for LUAD and neutrophil frequency for LUSC. C Representative images of multiplex antibody staining and paired segmented images for LUAD and LUSC. Scale bars = 100 μm. Legend for cell types can be seen in B. D Prevalence of total immune, myeloid, and lymphoid cells as % total cells across LUAD (n = 102) and LUSC (n = 102) cores. Two-tailed unpaired multiple comparison adjusted Student’s t tests; mean ± s.e.m. E Lymphoid infiltrate as % of total cells in male (56 cores; 28 patients) and female (46 cores; 23 patients) LUAD patients. Two-tailed unpaired Student’s t test; mean ± s.e.m. F Lymphoid infiltrate as % of total cells in younger (under 75 years, 68 cores; 34 pts) and older (over 75 years, 34 cores; 17 pts) LUAD patients. Two-tailed unpaired Student’s t test; mean ± s.e.m. G Comparison of cell frequencies between LUAD (n = 102) and LUSC (n = 102) cores for stromal cells, neutrophils and macrophages, other myeloid and lymphoid cells. Two-tailed unpaired multiple comparison adjusted Student’s t tests; mean ± s.e.m. H Proportion of each immune cell as % of total immune cells in LUAD (n = 102) cores from most to least prevalent; mean ± s.e.m. I Proportion of each immune cell as % total immune cells in LUSC (n = 102) cores from most to least prevalent; mean ± s.e.m. J Proportion of Ki67+ cancer cells across LUAD (n = 102) and LUSC (n = 102) cores. Two-tailed unpaired Student’s t test; mean ± s.e.m. P value = 0.00000271. K Proportion of pERK+ CD4+ and CD8+ T cells across LUAD (n = 102 and n = 102) and LUSC (n = 99 and n = 101) cores. Two-tailed unpaired Student’s t test; mean ± s.e.m. pERK+ CD4 + : P value = 0.0000248. pERK+ CD8 + : P value = 0.0000177. L Proportion of CD40 + B cells across LUAD (n = 101) and LUSC (n = 93) cores. Two-tailed unpaired Student’s t test; mean ± s.e.m. M Proportion of Arg1+ macrophages across LUAD (n = 102) and LUSC (n = 102) cores. Two-tailed unpaired Student’s t test; mean ± s.e.m. P value = 0.00000733. N Proportion of alpha-cleaved H3+ neutrophils across LUAD (n = 102) and LUSC (n = 102) cores. Two-tailed unpaired Student’s t test; mean ± s.e.m. P value = 0.000000000318. LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, Cl Monocyte classical monocyte, Non-Cl Monocyte non-classical monocyte, Int Monocyte intermediate monocyte, NK cell Natural Killer cell, Treg regulatory T cell, CyTOF cytometry by time of flight, Arg1 Arginase 1.
Within the immune compartment of LUAD tumours, the three most abundant cell types were CD4+ helper T cells (26.2% of immune cells), CD163- macrophages (21.7%) and CD8+ cytotoxic T cells (16.5%) (Fig. 1H, Supplementary Fig. 1D), reflecting our previous findings in an independent cohort6. In contrast, neutrophils were the most abundant cell type in the immune compartment of LUSC tumours, representing 22.5% of all immune cells, followed by CD4+ helper T cells (18.8%) and CD8+ T cells (15.7%) (Fig. 1I, Supplementary Fig. 1D). This comprehensive immune atlas of LUAD and LUSC highlights their distinct immune features across a balanced cohort of patients and enables a more detailed exploration of the spatial immune landscapes in these NSCLC subtypes while controlling for major confounding variables.
We next expanded our analysis from variations in cell lineage frequency to investigate potential differences in cell activation states. Ki67+ cancer cells comprised 4.7% of all cancer cells in LUAD compared to 8.9% in LUSC (Fig. 1J), suggestive of a more proliferative tumour compartment. Regarding lymphoid cells, not only did the LUAD microenvironment show higher T cell infiltration, but it also appeared more conducive to T cell activation. This was evidenced by increased ERK signalling in CD4+ T cells in LUAD compared to those in LUSC (Fig. 1K), known to inhibit the differentiation of these cells into Tregs11. Furthermore, enhanced ERK signalling was also observed in CD8+ T cells in LUAD, which is associated with T cell activation (Fig. 1K)12. In LUAD, there was a greater proportion of CD40+ B cells (Fig. 1L). CD40 activation of B cells is associated with enhanced proliferation, germinal center formation, and the formation of memory B cells13, and has also been explored as a promising immunotherapeutic target in cancer14. Although macrophages were more abundant in LUAD overall (Fig. 1G), they exhibited immunosuppressive features in LUSC (e.g. Arginase 115), versus LUAD (Fig. 1M). Amongst other components of the myeloid compartment, LUSC neutrophils expressed more alpha-cleaved H3 (Fig. 1N)16, a specific marker for neutrophil extracellular traps, which we and others have shown to be associated with immunosuppression and cancer progression17,18,19. These results underscore the complex nature of the immune landscapes in LUSC and LUAD.
Analysis of tumour microenvironments in LUSC and LUAD highlights dynamic pairwise cell–cell interaction patterns and cellular neighbourhoods
Given our findings related to differences in immune cell activation states, we next wanted to examine the spatial distribution of cells within the TIME. We employed a permutation testing strategy to statistically evaluate if specific cell types were likely to interact, and to assess if these spatial relationships differed between LUAD and LUSC. We observed an increased tendency for interactions between B cells and cancer cells in LUAD versus LUSC (Fig. 2A, box i), suggestive of the known associations between specific B cell interactions and improved prognosis in LUAD6, and the association between B cell frequency and improved disease-free survival among LUAD patients in our dataset (Supplementary Table 2). In LUAD, neutrophils, although less common than in LUSC (Fig. 1G), exhibited stronger interaction tendencies with endothelial cells and CD4+ T cells (Fig. 2A, box ii, Fig. 2B, C). In contrast, neutrophils in LUSC were more likely to interact with cancer cells, CD163- macrophages, and classical monocytes (Fig. 2A, box ii, Fig. 2B, C). We observed a similar pattern when looking at the interaction tendencies of CD163- macrophages, which are significantly more abundant in LUAD than LUSC (Fig. 1G). In LUAD, CD163- macrophages tend to interact with B cells, Tregs and CD163+ macrophages (Fig. 2A, box iii, Fig. 2B). In contrast, CD163- macrophages in LUSC show preferential interactions with cancer cells and neutrophils (Fig. 2A, box iii, Fig. 2B). This pattern aligns with the previously noted tendencies for neutrophil interactions, indicating a highly structured cancer-myeloid interaction network in LUSC. This diversity in cellular interactions, underscores the importance of developing subtype-specific therapies that target these unique cellular dynamics. These insights broaden our understanding of the distinct cellular interactions within the tumour microenvironments of LUAD and LUSC, suggesting the presence of further, more intricate cellular engagements beyond simple pairwise interactions that merit further exploration.
A Heat map of pairwise cell–cell interactions (red) or avoidances (blue) across LUAD (upper half of square, n = 102 images) and LUSC cores (lower half of square, n = 102 images) using 10,000 permutations. NK cells and dendritic cells were omitted from the graph given the lack of significant interactions. Black dotted boxes indicate interactions that are referenced in-text. B Plots depicting significant differences in interaction tendencies for neutrophils (left) and CD163- macrophages (right) between LUAD and LUSC. The size of the outer circles corresponds to the P-value comparing LUAD and LUSC interaction scores. Two-tailed unpaired Student’s t test. C Representative segmented images depicting neutrophil-CD4+ T cell and neutrophil-cancer interaction tendencies in LUAD (left) and LUSC (right), respectively. Scale bars = 100 μm. D Heatmap of cell types distributed across 10 neighbourhoods discovered in LUAD (n = 102) and LUSC (n = 102) (10 nearest neighbours, 10 neighbourhoods). E Average distribution of each CN in LUAD (n = 102) and LUSC (n = 102) (10 nearest neighbours,10 neighbourhoods). F Cellular composition of CN1, CN7, and CN8 discovered in LUAD and LUSC (10 nearest neighbours,10 neighbourhoods). G Number of cells belonging to each CN across LUAD (n = 102) and LUSC (n = 102) (10 nearest neighbours, 10 neighbourhoods). Two-tailed unpaired multiple comparison adjusted Student’s t tests; mean ± s.e.m. P value CN1 = 0.0000681, CN3 = 0.000000926, CN7 = 0.000000682, CN8 = 0.0000441. H Kaplan–Meier analysis of overall survival in CN3 high (z-score ≥0, n = 20) and low (z-score <0, n = 31) LUAD patients (10 nearest neighbours, 10 neighbourhoods). Log-rank Mantel–Cox test. I Cellular composition of CN3 discovered in LUAD and LUSC (10 nearest neighbours,10 neighbourhoods). LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, Cl Monocyte classical monocyte, Non-Cl Monocyte non-classical monocyte, Int Monocyte intermediate monocyte, NK cell Natural Killer cell, Treg regulatory T cell.
Given the distinct pairwise interaction landscape between LUAD and LUSC, we were interested in comparing the more complex cellular neighbourhoods (CN) that spatially define these tumours. As previously described6,20,21, we partitioned tumours into cellular neighbourhoods by considering each cell’s ten nearest neighbours. This allowed us to establish ten spatially defined cellular neighbourhoods which we named: tumour boundary (CN1), pan-immune hotspot 1 (CN2), macrophage enriched (CN3), undefined (CN4), B cell enriched (CN5), vascular niche (CN6), tumour compartment (CN7), neutrophil enriched (CN8), lymphoid enriched (CN9) and T cell enriched (CN10) (Fig. 2D). The most abundant cellular neighbourhoods in LUAD and LUSC were CN 1 (tumour boundary) and CN 7 (tumour compartment), respectively (Fig. 2E). CN1 is comprised of 65% cancer cells, with the remaining 35% consisting of immune cells, endothelial cells, and undefined cells (Fig. 2F). Approximately 96% of the cells represented in CN7 were cancer cells, highlighting the more immune-excluded topology of LUSC (Fig. 2F). In line with neutrophils being more abundant in LUSC, we observed a higher proportion of neutrophil-enriched CN8 in LUSC cores (Fig. 2G). While neutrophils were the primary cell type in this neighbourhood (72%), CN8 also contained cancer cells (12%) and CD163- macrophages (6%) (Fig. 2F). This provides further resolution of our pairwise interaction analysis findings, which suggested preferential interactions between neutrophils and cancer cells or macrophages in LUSC (Fig. 2A, B). We next sought to identify spatially defined CNs associated with survival. We found that the only neighbourhood associated with worse survival is CN3, in LUAD specifically (Fig. 2H, Supplementary Table 3). More abundant in LUAD than LUSC, CN3 is primarily comprised of CD163- macrophages but also hosts a variety of other cell types (Fig. 2I). Given that the frequency of CD163- macrophages alone is not sufficient to confer a survival disadvantage (Supplementary Table 2), the spatial dynamics of these macrophages are likely driving this effect. These cellular neighbourhoods offer a comprehensive framework for understanding the spatial relationships within the tumour microenvironments of LUAD and LUSC, reflecting the unique immunological landscapes.
A unique role for macrophages in NSCLC
To further investigate the link between macrophage-enriched neighbourhoods and survival, we refined the concept of neighbourhoods by expanding the total number of defined neighbourhoods from 10 to 30 (Fig. 3A). This facilitated a more nuanced classification of cellular groupings. This led to the discovery of three CD163- macrophage enriched neighbourhoods (CN15, CN23, and CN26) (Fig. 3A, B). Similar to what we observed with a smaller number of neighbourhoods, we found that CN15, containing the highest proportion of CD163- macrophages (62%), was significantly associated with worse outcomes in LUAD patients (Fig. 3C, Supplementary Table 4). CN23 and CN26 contained equal proportions of CD163- macrophages (36%), however only CN23 negatively affected LUAD patient survival (Fig. 3D, E, Supplementary Table 4). The primary difference between these two neighbourhoods is that CN26 contained a significant proportion of neutrophils, while CN23 contained more cancer cells (Fig. 3B). This suggests that the presence of neutrophils in a CD163- macrophage enriched neighbourhood may negate the detrimental effect of CD163- macrophages we are consistently observing in LUAD specifically. We performed a similar neighbourhood analysis, but with just three nearest neighbours, to capture the most detailed level of granularity in interactions beyond just pairwise relationships (Fig. 3F). We found that CD163- macrophages were once again associated with worse survival outcomes, except this time disease-free survival, when found in proximity to cancer cells (CN13) (Supplementary Table 5). Meanwhile, the neighbourhood containing the most CD163- macrophages (CN4), or the neighbourhood containing CD163- macrophages exclusively (CN21), did not exhibit any survival associations (Supplementary Fig. 3A, B).
A Heatmap of cell types distributed across 30 neighbourhoods discovered in LUAD (n = 102) and LUSC (n = 102) (10 nearest neighbours, 30 neighbourhoods). B Distribution of cell types in CN15, CN23, and CN26 discovered in LUAD and LUSC (10 nearest neighbours, 30 neighbourhoods). Legend for other cell types can be seen in J. C Kaplan–Meier analysis of overall survival in CN15 high (z-score ≥0, n = 19) and CN15 low (z-score <0, n = 32) LUAD patients. Log-rank Mantel–Cox test. D Kaplan–Meier analysis of overall survival in CN23 high (z-score ≥0, n = 22) and CN23 low (z-score <0, n = 29) LUAD patients. Log-rank Mantel–Cox test. E Kaplan–Meier analysis of overall survival in CN26 high (z-score ≥0, n = 11) and CN26 low (z-score <0, n = 40) LUAD patients. Log-rank Mantel–Cox test. F Heatmap of cell types distributed across 30 neighbourhoods discovered in LUAD (n = 102) and LUSC (n = 102) (3 nearest neighbours, 30 neighbourhoods). G Distribution of CD163+ macrophages across cellular neighbourhoods discovered in LUAD and LUSC (3 nearest neighbours, 30 neighbourhoods). Neighbourhoods with no CD163+ macrophages excluded from graph. H Kaplan–Meier analysis of overall survival in CN26 high (z-score ≥0, n = 16) and CN26 low (z-score <0, n = 35) LUSC patients. Log-rank Mantel–Cox test. I Kaplan–Meier analysis of disease-free survival in CN26 high (z-score ≥0, n = 9) and CN26 low (z-score <0, n = 42) LUAD patients. Log-rank Mantel–Cox test. J Cellular composition of CN26 and CN19 discovered in LUAD and LUSC (3 nearest neighbours, 30 neighbourhoods). LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, Cl Monocyte classical monocyte, Non-Cl Monocyte non-classical monocyte, Int Monocyte intermediate monocyte, NK cell Natural Killer cell, Treg regulatory T cell.
In our dataset, we subdivided macrophage populations based on the expression of CD163, a scavenger receptor associated with M2-like activation and immunosuppressive activity22,23. We observed widespread distribution of spatially distinct CD163+ macrophages across neighbourhoods, with a notable concentration in CN19 and CN26 (Fig. 3G). By frequency alone, we found that CD163+ macrophages were associated with improved overall survival in LUSC (Supplementary Table 2). Despite CN19 and CN26 containing a comparable number of CD163+ macrophages, it was only CN26 that was associated with better survival in LUSC (Fig. 3H, Supplementary Table 5). Importantly, we observed a divergent impact of CN26 whereby higher concentrations of CN26 correlated with enhanced overall survival in LUSC (Fig. 3H, Supplementary Table 5) but worse disease-free survival in LUAD (Fig. 3I, Supplementary Table 5). We did not observe the same effect in CN19, which contained a lower proportion of macrophages and greater diversity of cell types (Fig. 3J). These findings highlight the complexity of macrophage function within the tumour microenvironment, suggesting that while CD163+ macrophages may generally contribute to improved survival in LUSC, their impact is highly context-dependent and varies according to the specific cellular composition and neighbourhood in which they reside.
LUAD and LUSC have distinct cellular architectures
The concept of pan states within the TIME that could apply across NSCLC subtypes has been previously proposed. Yet, the data from our clinically matched cohort may suggest otherwise. This discrepancy may arise from the common practice of comparing high-dimensional data by selecting only the first two principal components. All images contain greater dimensionality, which likely represent key differences between NSCLC subtypes. This is consistent with current pathological classifications, in which trained pathologists use their expertise to identify distinct patterns that define the subtypes. To investigate this, we first examined whether unsupervised features—specifically the first two principal components derived from either cell frequencies or raw image features—could naturally distinguish adenocarcinoma from squamous cell carcinoma. We began by calculating the two primary principal components of the features using these approaches. This resulted in no clear separation of LUAD and LUSC tumour cores (Fig. 4A, B). Next, we developed a simple multi-layer perceptron (MLP) to capture distinctive features, beyond the first two principal components, to differentiate LUAD from LUSC using raw images. The projected features from this supervised approach demonstrated a clear separation between LUAD and LUSC (Fig. 4C). Together, these results demonstrate that the architecture of these tumours are distinct from each other and should be considered separate.
A PCA based on the frequency of cells in LUAD (n = 102) and LUSC (n = 102). B PCA based on raw image masks in LUAD (n = 102) and LUSC (n = 102). C PCA based on a multi-layer perceptron (MLP) used to capture unique features from raw images in LUAD (n = 102) and LUSC (n = 102). D Heatmap of cell types distributed across 10 neighbourhoods discovered in LUAD (n = 102) (10 nearest neighbours,10 neighbourhoods). E Heatmap of cell types distributed across 10 neighbourhoods discovered in LUSC (n = 102) (10 nearest neighbours, 10 neighbourhoods). F Proportion of cell types in cellular neighbourhoods identified as vascular niche in LUAD and LUSC. G Kaplan–Meier analysis of overall and disease-free survival in LUAD patients with high (z-score ≥0, n = 15) and low (z-score <0, n = 36) proportion of Ki67+ endothelial cells of total endothelial cells. Log-rank Mantel–Cox test. H Kaplan–Meier analysis of overall and disease-free survival in LUSC patients with high (z-score ≥0, n = 19) and low (z-score <0, n = 32) proportion of Ki67+ endothelial cells of total endothelial cells. Log-rank Mantel–Cox test. I Frequency of Ki67+ endothelial cells across LUAD (n = 102) and LUSC (n = 102) cores. Two-tailed unpaired Student’s t test; mean ± s.e.m. J Proportion of Ki67+ endothelial cells interacting with neutrophils in LUAD (n = 99) and LUSC (n = 86). Excludes cores without endothelial cell and neutrophil interactions. Two-tailed unpaired Student’s t test; mean ± s.e.m. LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, Cl Monocyte classical monocyte, Non-Cl Monocyte non-classical monocyte, Int Monocyte intermediate monocyte, NK cell Natural Killer cell, Treg regulatory T cell.
Given these findings, we rediscovered 10 neighbourhoods based on each cell’s 10 nearest neighbourhoods in LUAD and LUSC independently and noticed a distinction in the cellular makeup of the vascular niches (Fig. 4D, E). In LUSC, the vascular niche (CN1) contained 43.6% endothelial cells, and a mix of other immune cell types (Fig. 4F). In contrast, the vascular niche in LUAD contained a notable neutrophil population accounting for 17.2% of all cells in that neighbourhood, compared to just 3.5% neutrophils in the LUSC vascular niche (Fig. 4F). We have previously shown that proliferative Ki67+ endothelial cells are associated with poor overall survival in LUAD, and endothelial cells show preferential interactions with neutrophils in higher grade tumours6. Here, we observe a similar association between the frequency of Ki67+ endothelial cells and worse overall and disease-free survival, which is restricted to LUAD (Fig. 4G, H). In addition to an increase in endothelial cells in LUAD compared to LUSC (Fig. 1G), there was a trend in the proportion of endothelial cells that are Ki67+ being more abundant in LUAD (Fig. 4I). Despite neutrophils being two-times more abundant in LUSC compared to LUAD, the endothelial cells that are interacting with neutrophils are more proliferative (Fig. 4J). Neutrophils can induce endothelial cell proliferation by releasing pro-angiogenic chemokines such as CXCL8 and CXCL124, and in vitro studies have shown that neutrophils stimulate angiogenesis via cell-to-cell interactions with endothelial cells25. While the overall endothelial cell-neutrophil neighbourhood was not associated with survival, this suggests that specific activation states are associated with distinct cellular interactions, highlighting important distinguishing characteristics between LUAD and LUSC.
The only cellular neighbourhood associated with survival was the CD163- macrophage enriched neighbourhood in LUAD (Supplementary Table 6, 7), associated with worse overall survival as previously discussed. To identify cellular neighbourhoods that could be driving survival outcomes in LUSC specifically, we further segmented the tumour microenvironment into 30 distinct neighbourhoods (Supplementary Fig. 4A). Among these, CN29 was notably associated with worse disease-free and overall survival (Supplementary Fig. 4B, C, Supplementary Table 8). CN29 comprised a diverse array of myeloid cell types, particularly macrophages, along with non-immune components such as endothelial cells and undefined cell populations. In the LUSC dataset, we found that undefined cells were 99% CD45 negative and 18% αSMA positive (Supplementary Fig. 4D, E), suggesting that they include a heterogeneous population of cancer-associated fibroblasts. Interestingly, although macrophages were prevalent in other cellular neighbourhoods, none of those macrophage-enriched environments demonstrated the same significant survival associations as CN29 (Supplementary Fig. 4F, Supplementary Table 8). This finding highlights an emerging paradigm in tumour biology: the spatial organization of cells and their interactions within specific cellular niches is a stronger predictor of patient outcomes than the simple frequency of individual cell types. These interactions may create a unique ecosystem that fosters tumour progression, immune evasion, or resistance to treatment, driving poor outcomes. While the frequency of certain immune or stromal cells has traditionally been a prognostic marker, our findings in LUSC suggest that the spatial distribution and interactions of these cells provide a more nuanced understanding of disease progression. Further, examining histological subtypes collectively may lead to an incomplete assessment by obscuring the unique features within each subtype. By analyzing each subtype individually, we can reveal distinct biological characteristics that correlate with clinical outcomes.
Discussion
Our study leverages advanced imaging techniques to investigate the immune environments within the two most common subtypes of non-small cell lung cancer that differ in their anatomical location, association with smoking, genetic aberrations, and prognosis. By using a clinically matched cohort of LUAD and LUSC patients, we can effectively draw biological insights across subtypes and accurately assess prognostic features while minimizing confounding patient characteristics. In line with previous work6,26, LUSC and LUAD tumours were dominated by neutrophils and macrophages, respectively. We demonstrate that apart from variations in immune cell frequencies, tumours of LUSC and LUAD exhibit significant differences in the spatial organization of immune cells. Indeed, our observations revealed notable differences in cell activation states and pairwise interactions between LUSC and LUAD, particularly in the myeloid compartment. Despite the average neutrophil count being nearly twice as high in LUSC compared to LUAD (8.1% in LUSC versus 4.1% in LUAD), we observed a higher proportion of neutrophils interacting with Ki67+ endothelial cells in LUAD, suggestive of angiogenesis. There is extensive literature on the role of tumour-associated macrophages in stimulating angiogenesis22,27,28. While neutrophils play an important role in normal physiological angiogenesis, they likely contribute to blood vessel development in tumours as well29. It has been shown that tumour-associated neutrophils are a major source of the pro-angiogenic factor MMP-9, more so than tumour-associated macrophages30. MMP-9 derived from neutrophils degrades the extracellular matrix, and results in the release of VEGF subsequently stimulating angiogenesis31. In contrast, we found that neutrophils in LUSC expressed more alpha-cleaved H3, a marker of neutrophil extracellular traps. This suggests that neutrophils contribute to tumour progression in diverse ways across different histological subtypes. Importantly, the presence of this neutrophil-enriched vascular niche in LUAD was only identified when we investigated the spatial landscape of LUAD and LUSC as separate subtypes.
The concept of cellular neighbourhoods introduces a critical layer of complexity, revealing that the arrangement and proximity of different cell types may be more important than their isolated abundance, highlighting unique biological features within each histological subtype that correlate with clinical outcomes. While there was a trend in macrophage abundance being associated with poor outcomes in LUAD (Supplementary Table 2), it was not until we evaluated the spatial organization of neighbourhoods in which macrophages were found that a survival association was consistently observed. While the M1-M2 dichotomy is a simplistic view of macrophage phenotypes, CD163- macrophages are generally more M1-like, characterized by the secretion of pro-inflammatory cytokines and ability to kill microorganisms and tumour cells32. In lung cancer, the infiltration of M1-like macrophages is typically associated with better prognosis, with the reverse being true for M2-like tumour-associated macrophages33. However, macrophage phenotypes are highly plastic, and their activity is strongly influenced by their surroundings. Inflammatory damage induced by M1-like macrophages can facilitate a more immunosuppressive environment. For example, we observed that CD163- macrophages had a higher tendency to interact with regulatory T cells in LUAD, which are two cell types more abundant in LUAD compared to LUSC. It has previously been described in NSCLC that macrophages promote Treg expansion in association with tumour progression34. In LUSC, CD163- macrophages were associated with worse outcomes as well, but only in the context of specific cellular neighbourhoods, for example, when in proximity to endothelial cells and undefined cell populations. Importantly, many of these findings were only observed when cellular neighbourhoods were discovered on LUAD and LUSC as distinct histological subtypes.
Certainly, these inconsistencies are made more complex by the evolving landscape of therapeutic options for patients with resectable NSCLC35,36,37,38. Our data provide important context as the routine use of checkpoint inhibition for patients with resectable or resected NSCLC is introduced as new standards of care39,40,41,42.The rather stark difference in the TIME between LUAD and LUSC may corroborate important trends between histologies in the completeness of pathological response and event-free survival after neoadjuvant chemo-immunotherapy43. Indeed, the geographic disposition of immune cells and cellular neighbourhood composition may create scenarios where the application of immune checkpoint blockade provides swift and deep pathological response, while the application of platinum-based chemotherapy could deeply impact the cancer cell compartment as well as myeloid composition. It is interesting to note the heavy preponderance of neutrophil populations within the LUSC cohort, where complete pathological response appears to be somewhat less common after chemo-immunotherapy43. However, data from neoadjuvant trials conducted entirely in Asia suggest high rates of response amongst LUSC patients. Recent data indicate that neutrophils can play key roles not only in terms of immune suppression, but some subsets may in fact support response to checkpoint inhibition44. Our data indicate that deeper phenotypic characterization of tumour-associated neutrophils according to histological subsets of NSCLC are essential to understand how to modulate these cells for improved efficacy of existing and emerging standards of care.
Overall, this study builds on our prior characterization of the TIME in NSCLC6, highlighting the critical influence of the spatial arrangement of immune cells within tumours on clinical outcomes, providing deeper insights into their role in NSCLC progression and prognosis. By extending our previous findings, we have demonstrated how nuanced differences in cellular architecture between LUAD and LUSC can inform our understanding of the TIME. While we sampled two cores from each patient to capture a broader perspective of the tumour immune microenvironment, we acknowledge that the limited size of these regions may introduce some sampling bias. Nonetheless, our findings underscore the importance of considering the complex spatial organization of immune cells in developing more effective cancer treatments and refining prognostic tools.
Methods
Clinical cohort
Patients with primary treatment-naïve LUAD or LUSC seen at the McGill University Health Centre were included in this study. Following nearest neighbour propensity score matching with no replacement in RStudio using a 1:1 ratio, 51 LUSC (102 tumour cores) and 51 LUAD (102 tumour cores) patients were selected. Of note, the TIME characterization of the 51 LUAD patients included in this study was not included in our previous study6. The follow-up time for the LUAD cohort ranged from 0.0054 years (2 days) to 10.06 years for a median follow-up time of 5.59 years. For the LUSC cohort, the median follow-up time was 4.22 years ranging from 0.15 years (56 days) to 9.04 years. All patient tumours were diagnosed by a board-certified thoracic pathologist. Overall survival was defined as the date from diagnosis until death while disease-free survival was defined as the date from diagnosis until recurrence or death. Written informed patient consent was obtained for all samples and clinical information. Detailed clinical information for both cohorts can be found in Table 1. All protocols were approved through the McGill University Health Centre protocol numbers IRB #2014-1119 and 2019-5253.
Sample staining and IMC acquisition
The methods for sample staining, preparation and imaging mass cytometry have been described elsewhere6. The full IMC panel can be found in Supplementary Table 1.
Antibody optimization
All antibodies used for staining have previously been optimized6. Briefly, control tissues (tonsil, appendix, placenta, thymus, normal lung, LUAD, and LUSC) representing positive and negative controls were stained with the antibody panel (with the exception of alpha-cleaved H316). Four markers did not consistently stain the control tissues and were not used for any biological interpretation (GM-CSFR, PD-1, PD-L1 and B7-H3).
Data transformation and normalization
No transformation was performed on the data presented here. Analyses used raw IMC measurements. For the average marker expression heatmaps, a 95th percentile normalization was performed on the expression data. The z-scored cluster means are depicted in the heatmaps (Supplementary Fig. 1B, C). The mean pixel values were calculated based on the expression of every single-cell marker across all channels.
Cell segmentation and lineage assignment
All cells within the IMC images were segmented using a pipeline composed of classical and modern machine-learning-based computer vision algorithms. For lineage assignment, the selected intensities for all markers were identical across the LUAD and LUSC cohorts with the exception of TTF1. For LUAD, TTF1 and pan-cytokeratin were used to call cancer cells whereas for LUSC, pan-cytokeratin was used alone. The extensive code and description of the cell segmentation and lineage assignment pipeline can be found here6,45.
Cell–cell pairwise interaction
To evaluate the significance of pairwise interactions among distinct cell types within individual cores of imaging mass cytometry images, we devised a permutation-based statistical method. For each core, we randomly permuted the locations of cell types within the slide, maintaining the original distribution of cell types, 10,000 times. For each permutation, we quantified the occurrences where the frequency of interactions between any two specific cell types was either greater or less than observed in the actual arrangement. This was done for each of the 10,000 permutations, resulting in a range of counts from 0 to 10,000. These counts represent the number of times the observed interaction was either more frequent (indicating interaction) or less frequent (indicating avoidance) than what was observed in the original image. For each study group, we aggregated these measures to construct distributions of either interaction or avoidance across all cores for each pairwise cell type comparison. Subsequently, we applied a two-sample test to these distributions to compute P values for each pairwise cell type interaction or avoidance, assessing the statistical significance of differences between groups.
Neighbourhood identification
The code and description for cellular neighbourhood (CN) identification was previously described in detail6,20,46. Every cell was subsequently allocated to a CN based on their defining window. The prevalence of each neighbourhood in each core was normalized so that the sum of neighbourhood prevalence for that core was 100%. Within each histological subtype, values of patient-matched cores were averaged. The mean value was then z-scored. Z-score above or equal to 0 and below 0 were compared for survival outcomes.
Statistical analysis and workflow
MATLAB (version 2019b) and Python (version 3.7.12) were used for the image analysis steps and RStudio version 4.2.2 and GraphPad Prism 10.2.0 statistical software for statistical analyses. Data are visualized as mean ± s.e.m; P < 0.05 was considered statistically significant unless otherwise indicated. Survival data were analysed by log-rank (Mantel–Cox) test. All statistical tests are described in the figure legends. For large sample size comparisons, Student’s t test was used47. For pairwise analyses where multiple t tests were performed, all P values are adjusted for False Discovery Rate using the two-stage step-up method of Benjamini, Krieger and Yekutieli.
Multilayer perceptron (MLP) model
Each channel of each image was resized to 1024 × 1024 pixels, then divided into patches of 256 × 256 pixels, resulting in 16 patches per channel per image. We then utilized a pre-trained ResNet-18 model to extract embedding vectors of size 512 for each patch. For each channel, we aggregated the embeddings across all patches by computing their mean, resulting in a signature embedding vector of size 512 for each channel. Given that different channels capture distinct structures within the cells, we concatenated the embedding vectors from all channels. Thus, for an image with N channels, the final embedding vector is of size N x 512. While this approach significantly increases the dimensionality of the data, it is crucial for capturing the complexity of the high-dimensional images.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings in this study, including high-dimensional TIFF images, are available at https://doi.org/10.5281/zenodo.14625562.
Code availability
The original code used to produce the results of this study is available at https://github.com/walsh-quail-labs.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249 (2021).
Gridelli, C. et al. Non-small-cell lung cancer. Nat. Rev. Dis. Prim. 1, 1–16 (2015).
Relli, V., Trerotola, M., Guerra, E. & Alberti, S. Abandoning the Notion of Non-Small Cell Lung Cancer. Trends Mol. Med. 25, 585–594 (2019).
Campbell, J. D. et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet 48, 607–616 (2016).
Chen, J. W. & Dhahbi, J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identification, and gene expression analysis using overlapping feature selection methods. Sci. Rep. 11, 13323 (2021).
Sorin, M. et al. Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature 614, 548–554 (2023).
Wang, C. et al. The heterogeneous immune landscape between lung adenocarcinoma and squamous carcinoma revealed by single-cell RNA sequencing. Sig. Transduct. Target Ther. 7, 289 (2022).
Enfield, K. S. S. et al. Spatial Architecture of Myeloid and T Cells Orchestrates Immune Evasion and Clinical Outcome in Lung Cancer. Cancer Discov. OF1-OF30, https://doi.org/10.1158/2159-8290.Cd-23-1380 (2024).
Zhang, H. et al. Spatial Positioning of Immune Hotspots Reflects the Interplay between B and T Cells in Lung Squamous Cell Carcinoma. Cancer Res. 83, 1410–1425 (2023).
Zhang, P. et al. Mast cell marker gene signature: prognosis and immunotherapy response prediction in lung adenocarcinoma through integrated scRNA-seq and bulk RNA-seq. Front. Immunol. 14, 1189520 (2023).
Chang, C. F. et al. Polar opposites: Erk direction of CD4 T cell subsets. J. Immunol. 189, 721–731 (2012).
Li, D., Molldrem, J. J. & Ma, Q. LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways. J. Biol. Chem. 284, 21001–21010 (2009).
Foy, T. M. et al. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J. Exp. Med. 180, 157–163 (1994).
Kornbluth, R. S., Stempniak, M. & Stone, G. W. Design of CD40 agonists and their use in growing B cells for cancer immunotherapy. Int. Rev. Immunol. 31, 279–288 (2012).
Van de Velde, L. A. et al. T Cells Encountering Myeloid Cells Programmed for Amino Acid-dependent Immunosuppression Use Rictor/mTORC2 Protein for Proliferative Checkpoint Decisions. J. Biol. Chem. 292, 15–30 (2017).
Tilley, D. O. et al. Histone H3 clipping is a novel signature of human neutrophil extracellular traps. Elife 11, https://doi.org/10.7554/eLife.68283 (2022).
Adrover, J. M., McDowell, S. A. C., He, X. Y., Quail, D. F. & Egeblad, M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell 41, 505–526 (2023).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 (2013).
Rayes, R. F. et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis-promoting effects. JCI Insight 4, https://doi.org/10.1172/jci.insight.128008 (2019).
Schürch, C. M. et al. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell 182, 1341–1359.e1319 (2020).
Jackson, H. W. et al. The single-cell pathology landscape of breast cancer. Nature 578, 615–620 (2020).
Mantovani, A., Schioppa, T., Porta, C., Allavena, P. & Sica, A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 25, 315–322 (2006).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Cassatella, M. A. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73, 369–509 (1999).
Yasuda, M. et al. A novel effect of polymorphonuclear leukocytes in the facilitation of angiogenesis. Life Sci. 66, 2113–2121 (2000).
Kargl, J. et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat. Commun. 8, 14381 (2017).
Dirkx, A. E., Oude Egbrink, M. G., Wagstaff, J. & Griffioen, A. W. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J. Leukoc. Biol. 80, 1183–1196 (2006).
Lewis, C. E. & Pollard, J. W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 66, 605–612 (2006).
Tazzyman, S., Lewis, C. E. & Murdoch, C. Neutrophils: key mediators of tumour angiogenesis. Int. J. Exp. Pathol. 90, 222–231 (2009).
Deryugina, E. I. et al. Tissue-Infiltrating Neutrophils Constitute the Major In Vivo Source of Angiogenesis-Inducing MMP-9 in the Tumor Microenvironment. Neoplasia 16, 771–788 (2014).
Christoffersson, G. et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood 120, 4653–4662 (2012).
Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 (2010).
Jackute, J. et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 19, 3 (2018).
La Fleur, L. et al. Targeting MARCO and IL37R on Immunosuppressive Macrophages in Lung Cancer Blocks Regulatory T Cells and Supports Cytotoxic Lymphocyte Function. Cancer Res. 81, 956–967 (2021).
Mountzios, G. et al. Immune-checkpoint inhibition for resectable non-small-cell lung cancer - opportunities and challenges. Nat. Rev. Clin. Oncol. 20, 664–677 (2023).
Dunne, E. G. et al. The Emerging Role of Immunotherapy in Resectable Non-Small Cell Lung Cancer. Ann. Thorac. Surg. https://doi.org/10.1016/j.athoracsur.2024.01.024 (2024).
West, H. J. & Kim, J. Y. Rapid Advances in Resectable Non-Small Cell Lung Cancer: A Narrative Review. JAMA Oncol. 10, 249–255 (2024).
Lee, J. M. et al. Neoadjuvant Targeted Therapy in Resectable NSCLC: Current and Future Perspectives. J. Thorac. Oncol. 18, 1458–1477 (2023).
Forde, P. M. et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 386, 1973–1985 (2022).
Wakelee, H. et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N. Engl. J. Med. 389, 491–503 (2023).
Felip, E. et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398, 1344–1357 (2021).
O’Brien, M. et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 23, 1274–1286 (2022).
Sorin, M. et al. Neoadjuvant Chemoimmunotherapy for NSCLC: A Systematic Review and Meta-Analysis. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2024.0057 (2024).
Gungabeesoon, J. et al. A neutrophil response linked to tumor control in immunotherapy. Cell 186, 1448–1464.e1420 (2023).
Karimi, E. et al. Machine learning meets classical computer vision for accurate cell identification. bioRxiv, https://doi.org/10.1101/2022.02.27.482183 (2022).
Karimi, E. et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 614, 555–563 (2023).
Fagerland, M. W. t-tests, non-parametric tests, and large studies-a paradox of statistical practice? BMC Med. Res. Methodol. 12, 78 (2012).
Acknowledgements
We would like to thank the Thoracic Oncology Clinical Database and Biobank (TOCDB) at the McGill University Health Centre for providing access to tissue and clinical data. The authors are grateful for support from the Single Cell and Imaging Mass Cytometry Platform (SCIMAP, Yuhong Wei) and Histology core facility (Nicole Robinson and Plinio Cruz) at the Rosalind and Morris Goodman Cancer Institute and the Quebec Cancer Consortium, as well as financial support from the Ministère de l’Économie et de l’Innovation du Québec through the Fonds d’accélération des collaborations en santé. The authors acknowledge support from the McGill Interdisciplinary Initiative in Infection and Immunity (Mi4). L.A.W acknowledges funding from the Bachynski Family Foundation, the Canadian Institutes of Health Research (CIHR; PJT-162137), the Canadian Foundation for Innovation, and holds a Rosalind and Morris Goodman Chair in Lung Cancer Research. L.D. is supported by a CIHR Doctoral Award. M.S. is supported by a Fonds de recherche du Québec - Santé Doctoral award and a Vanier Canada Graduate Scholarship. J.D.S is supported by a Fonds de recherche du Québec – Santé Junior 2 Clinician Scientist Award and by the Montreal General Hospital Foundation Dr. Ray Chiu Distinguished Scientist in Surgical Research Award.
Author information
Authors and Affiliations
Contributions
L.D. M.S., D.Q., J.S., and L.A.W. designed the study, reviewed the data, and wrote the manuscript. L.D., M.S., B.L., E.K., M.R., and L.A.W. oversaw and performed all experiments and data analysis. E.K, M.R., and B.L. wrote all the code. D.F.Q., L.D., M.S., Y.W., and L.A.W. designed, optimized, and performed IMC protocols. S.D., M.W.Y., S.H., B.F. and R.R provided additional data analysis and generated schematics. Clinical pathologists P.O.F., S.C.B., B.K., and P.J. curated the human tissue samples and built the patient TMAs. A.M.S., A.A., and M.S. collected and curated all clinical data. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.D.S. - Consulting, advisory role or honoraria: AstraZeneca, Merck, Roche, BMS, Novartis, Chemocentryx, Amgen, Protalix Biotherapeutics, Xenetic Biosciences, Regeneron, Eisai, Peerview, OncLive, Medscape, Pfizer. Grant to institution: AstraZeneca, BMS, Merck, Roche, CLS Therapeutics, Protalix Biotherapeutics, Pfizer, Regeneron. Clinical trial leadership role: BMS, Novartis, Roche, Merck, AstraZeneca. POF has participated in paid consulting services with Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol Meyers Squibb, EMD Serono, Incyte, Merck, Novartis, Pfizer, Precision Rx-Dx and Roche. He has received research support from Astellas, AstraZeneca, Bristol Meyers Squibb and Merck. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Benjamin Izar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Desharnais, L., Sorin, M., Rezanejad, M. et al. Spatially mapping the tumour immune microenvironments of non-small cell lung cancer. Nat Commun 16, 1345 (2025). https://doi.org/10.1038/s41467-025-56546-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56546-x
This article is cited by
-
Spatiotemporal dynamics of tumor-associated neutrophils: bridging the gap between cancer progression and immunotherapy
Molecular Cancer (2026)
-
Rab37-mediated OPN secretion enriches SPP1+ macrophages through autocrine–paracrine signaling to drive lung tumor progression
Oncogenesis (2026)
-
STAT4 mediated modulation of T–B cell interactions profiling: enhances antitumor immunity in non-small cell lung cancer (NSCLC)
Molecular Biology Reports (2026)
-
Overcoming resistance to anti-PD-L1 immunotherapy: mechanisms, combination strategies, and future directions
Molecular Cancer (2025)
-
Integrative analysis of non-small cell lung cancer identifies Jumonji domain-containing 6/ETS homologous factor axis as a target to overcome radioresistance
Signal Transduction and Targeted Therapy (2025)