Abstract
Remnant cholesterol, identified by triglyceride-rich lipoprotein, is a significant causal risk factor for ischemic heart diseases. The association of triglyceride levels with all-cause and cause-specific outcomes in heart failure (HF) remains unexplored. Using a previously validated territory-wide clinical information registry, all eligible patients diagnosed with HF (N = 127124) from 2000 to 2020 were included. In this population-based cohort (mean age: 71.4 ± 12.2 years, 51.8% male), the association between triglyceride levels and risk of all-cause mortality and cardiovascular disease was a U-shapedḍ curve. High triglyceride levels (≥3.0 mmol/L) were associated with atherosclerotic cardiovascular disease admission or death; conversely, lower triglyceride levels (<1.2 mmol/L) were associated with higher risks of HF readmission or death. The risk of adjusted all-cause mortality reached a nadir between triglyceride levels of 1.2 mmol/L and 3.0 mmol/L. Results were externally validated in BIOSTAT-CHF. Our findings have important implications for defining the role of triglyceride levels in contributing to the diverse outcomes in patients with HF.
Similar content being viewed by others
Introduction
Plasma lipid is a well-established causal risk factor for the development of atherosclerosis and cardiovascular disease1,2,3. Although it was generally acknowledged that higher plasma cholesterol was related to increased cardiovascular risks1,4, others have shown conflicting results where lower cholesterol levels contributed to higher risks of cardiovascular death (CVD), non-CVD4, or all-cause mortality5. Further, recent epidemiologic studies have demonstrated U-shaped associations where low and high levels (vs. intermediate level) of total cholesterol (TC)6, low-density lipoprotein-cholesterol (LDL-C)7 or high-density lipoprotein-cholesterol (HDL-C)8 conferred a greater risk of all-cause mortality and CVD in the general population or in patients with risk factors such as diabetes9.
Remnant cholesterol was calculated as total cholesterol minus LDL cholesterol minus HDL cholesterol. Thus, remnant cholesterol mathematically equals serum triglycerides in mg/dL divided by 5 (or triglyceride in mmol/L divided by 2.2) in individuals who had LDL cholesterol calculated using Friedewald’s equation10. As the substantial content of remnant cholesterol, the association between triglyceride and mortality, however, has long been controversial. Studies on the general population11, or patients with established coronary heart disease12 or atherosclerotic cardiovascular disease (ASCVD)13 have shown that hypertriglyceridemia was associated with a higher risk of mortality or cardiovascular events, while others provided inconsistent evidence showing an inverse association where lower triglyceride levels were linked to a higher risk of mortality14,15 and stroke16, or non-significant association after adjusting for cardiovascular risk factors17. Despite the crucial role of metabolic processes, especially triglyceride metabolism, in the development of heart failure (HF)18, the differential risk prediction for triglyceride and specific causes for different cardiovascular outcomes in HF remains unexplored.
We therefore investigate the association between triglyceride levels with the risk of all-cause mortality and distinct cardiovascular outcomes (including both ASCVD and HF) in a population-based HF cohort. Using a validated population-based cohort of patients with incident HF, we demonstrate that triglyceride was associated with all-cause and cardiovascular mortality in a U-shaped manner. Specifically, the two ends of the U-shape curve can be attributed to distinct causes of cardiovascular events, including ASCVD and HF-related outcomes. Additionally, the findings are externally validated by the BIOSTAT CHF cohort, underscoring the clinical significance of defining the role of triglyceride levels in contributing to the diverse adverse outcomes in patients with HF.
Results
A total of 1,27,124 HF patients were included in this study with a median follow-up of 11.3 years (IQR 6.2–17.9 years); during this period, 83,615 died, of which 27,095 were due to cardiovascular causes. The flow chart of this study cohort is shown in Fig. 1. Table 1 shows the baseline characteristics of the overall population and by triglyceride categories. The mean age was 71.4 ± 12.2 years old and 51.8% were male; 44.7% had hypertension and 37.0% had coronary artery disease (CAD), while 30.3% had diabetes and nearly a quarter had atrial fibrillation (AF). Patients with higher triglyceride concentrations were younger, more frequently female, and had more hypertension, diabetes, CAD, dyslipidemia, and chronic renal diseases, but with less AF. The TC, LDL-C, non-HDL, and TyG levels increased whereas HDL-C declined with increasing triglyceride levels. (Table 1) Detailed ICD-9 and ICD-10 codes were provided in Supplementary Table 1.
Triglyceride levels with ASCVD or HF-related death or admission
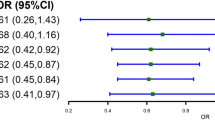
A differential trend was observed when we divided causes of cardiovascular events into ASCVD-related or HF-related outcomes. A positive relationship was found between high triglyceride levels with ASCVD-related death or admission as compared with intermediate triglyceride levels (Table 2, Fig. 2). Conversely, increased risks for HF death were seen for those with low triglyceride levels compared with intermediate triglyceride levels (Table 2, Fig. 3). With regards to the incidence of HF readmission, an inverse relationship between triglyceride levels and HF readmission was found where a low triglyceride level was associated with a higher risk of HF and a high triglyceride level was associated with a lower risk of HF compared to intermediate triglyceride levels (Table 2, Fig. 3).
P-values for non-linear model tests were <0.001 and 0.001, respectively. ASCVD atherosclerotic cardiovascular disease, CI confidence interval, HR hazard ratio, N number. The variables adjusted are listed in Table 1.
P-values for non-linear model tests were <0.001. CI confidence interval, HR hazard ratio, HF heart failure, N number. The variables adjusted are listed in Table 1.
Triglyceride levels with all-cause mortality or cardiovascular death
The association between triglyceride levels on a continuous scale and the risk of all-cause mortality was a U-shaped curve. The risks of all-cause mortality reached a nadir between triglyceride levels of 1.2 mmol/L and 3.0 mmol/L and were lowest at 1.95 mmol/L (Fig. 4). A low (triglyceride <1.2 mmol/L) or high level (triglyceride >3.0 mmol/L) of triglyceride was associated with increased risks of all-cause mortality (Fig. 4, Table 2). Compared with patients with triglyceride concentrations of 1.2–3.0 mmol/L, the multivariable-adjusted HR for all-cause mortality was 1.11 (95% CI, 1.09 to 1.13) for patients with triglyceride concentrations less than 0.8 mmol/L, 1.07 (1.05–1.09) for 0.8 ≤ triglyceride < 1.0 mmol/L, 1.06 (1.03–1.08) for 1.0 ≤ triglyceride < 1.2 mmol/L, 1.05 (1.00–1.10) for 3.0 ≤ triglyceride < 5.7 mmol/L, and 1.22 (1.03–1.45) for patients with triglyceride ≥ 5.7 mmol/L (p < 0.05 for all). This association remained consistent when CVD was considered as the endpoint (Fig. 4, Table 2). The crude incidence rates of outcomes for each subgroup were listed in Supplementary Table 2.
P-values for non-linear model tests were 0.123 and 0.046, respectively. CI confidence interval, HR hazard ratio, N number. The variables adjusted are listed in Table 1.
External validation using BIOSTAT-CHF
The prognostic role of triglyceride level was externally validated using the BIOSTAT-CHF cohort. Among 2516 patients with HF, 1309 patients had baseline lipid records. Low triglyceride levels were associated with a higher risk of all-cause mortality while the risk was neutral in high triglyceride levels. (Supplementary Fig. 1)
Sensitivity analyses
Sensitivity analyses showed minor differences in the estimated associations between triglyceride and mortality. Exclusion of death within 2 years after HF diagnosis gave similar results to the whole population (Table 3). When modeling triglyceride as a time-weighted covariate, the effects of triglyceride on all-cause mortality or CVD were slightly enhanced in HR for risks of mortality were found in low and high triglyceride levels (Table 3). When limiting the analysis to subgroups with BMI records, the associations persisted independent of low or high BMI status. (Supplementary Table 3) Moreover, a similar U-shaped curve between triglyceride levels and all-cause mortality after adding BMI was found in the subgroup with a nadir of 1.22–3.15 mmol/L (Supplementary Fig. 2). After adjusting NRI in the Cox regression model, the results remained consistent, demonstrating that the U-shape associations between triglyceride and all-cause mortality and cardiovascular death were independent of nutritional status (Supplementary Table). After excluding patients with MI, unstable angina, arterial revascularization, or ischemic stroke prior to HF diagnosis, new-onset ASCVD was modeled in the new cohort (N = 71,181). The results consistently showed that high triglyceride levels were positively associated with an increasing risk of death or admission for incident ASCVD while no significant relationship was found in low triglyceride levels (Supplementary Fig. 3). The U-shaped relationship between LDL-C, HDL-C, TC, TyG, and all-cause mortality were further confirmed in our large-scale HF cohort, consistent with previous studies7,8, providing substantial support for the validity of our database.(Supplementary Fig. 4A–D) Additionally, different (five, six, and seven) knots for drawing restricted cubic spline curves were used to test the robustness of the intermediate range of triglycerides level. Furthermore, the association between triglyceride levels and all-cause mortality and cardiovascular death was consistent after adjusting only for CAD (Supplementary Table 5). The findings showed little variation, confirming the robustness of the intermediate range (1.2–3.0 mmol/L) as the reference for the triglycerides level (Supplementary Fig. 5).
Subgroup analyses
Stratified by age and sex, the U-shaped associations of triglyceride levels with all-cause mortality were consistent (Fig. 5, Supplementary Fig. 6). Subgroups with or without CAD, DM, and AF yielded similar results (Fig. 5, Supplementary Fig. 7). Likewise, we found that the association between triglyceride and all-cause mortality remained U-shape curve in those not receiving lipid-lowering treatment, whereas for individuals receiving lipid-lowering treatment, low triglyceride levels were still significantly associated with increased risks of all-cause mortality while neutral in high triglyceride levels (Supplementary Fig. 8).
Among patients with BMI records (n = 28,086), a similar U-shaped relationship of triglyceride levels with all-cause or CV mortality was observed, independent of the BMI category (Supplementary Table 3). Given the potential mediation role of LDL-C which might attenuate the effects, we further evaluated the associations between triglyceride categories and all-cause mortality or CVD within low (≤1.7 mmol/L) or high LDL-C levels (>1.7 mmol/L) using Cox proportional hazards model which once again showed results similar to the primary analysis (Supplementary Table 6).
Discussion
In this large population cohort of 1,27,124 patients with HF from a previously validated database of the Hong Kong population, triglyceride level exhibited a U-shaped association with all-cause mortality and overall cardiovascular mortality19. The triglyceride level with the lowest risk of all-cause mortality was between 1.2 and 3.0 mmol/L in patients with HF, the higher range being slightly higher than the optimal triglyceride concentration defined in the general population. Notably, the two ends of the U-shaped curve were attributed to differential causes of cardiovascular events. As expected, a higher triglyceride level (triglyceride > 3.0 mmol/L) was associated with increased risks of ASCVD admission or related death, whereas a lower triglyceride level (triglyceride < 1.2 mmol/L) was predominantly associated with higher risks of adjusted HF readmission or related death. The associations were also independent of BMI, LDL-C, or NRI. Although previous studies have demonstrated the positive association between triglyceride levels and cardiovascular outcomes11,20, emphasis was mainly on higher triglyceride levels, and the clinical association of lower triglyceride levels remains unclear. This study demonstrated that both low and high levels of triglyceride were associated with increased risks of all-cause mortality and CVD in patients with HF in a U-shaped manner. Further, this study delineated the differential association between triglyceride and HF or ASCVD-related outcomes in a large population-based HF cohort. While previous studies have shown that elevated triglyceride levels are linked to adverse cardiovascular outcomes21,22,23, none have revealed a U-shaped relationship. Our study’s unique contribution further lies in uncovering a distinct pattern of outcomes, where low triglyceride levels are associated with HF outcomes, while high triglyceride levels are linked to ASCVD outcomes. This differential association sets our study apart from others that did not focus on specific outcomes.
High triglyceride level with ASCVD-related outcome
Plasma triglyceride level is a biomarker for triglyceride-rich lipoproteins and their remnants, which has become an important contributor to ASCVD24. Hypertriglyceridaemia is very common (~10% in the population) with a similar burden to other metabolic risk factors, such as diabetes and obesity. Several retrospective analyses of randomized clinical trials, including TNT, IDEAL, PROVE-IT, dal-OUTCOMES, and MIRACL, have demonstrated that triglyceride is positively associated with ASCVD in patients with established CAD21,22,23. Likewise, two recent observational studies restricted to patients with established DM or ASCVD who were on statin have also observed significantly higher ASCVD risks in those with triglyceride ≥1.69 mmol/L9,25. In a study that included only healthy young men showed that a high triglyceride level obtained 5 years apart predicted risk of coronary heart disease26. A meta-analysis that included 2,62,525 participants in the Western population demonstrated that triglyceride concentration was moderate-highly associated with incident CAD20. The association between triglyceride levels with ASCVD is further established in the Asian population, including both Chinese27 as well as Korean28. Accordingly, a recent expert consensus decision pathway has recommended that hypertriglyceridemia should be addressed and managed in patients at risk of ASCVD29. In patients with HF, especially those with concomitant CAD, the subsequent risk of ischemic events is clinically substantial30. In a study that evaluated 171 autopsy results of patients with HF, up to 56% of patients who died suddenly had evidence of an acute ischemic event31. This is further validated in our study, where 11.5% of the population experienced an ASCVD-related admission and 4.5% developed ASCVD-related death. In the context of HF, it is possible that even a small MI may present as sudden death rather than a nonfatal MI32. Identifying risk factors that may provide meaningful risk prediction to subsequent ischemic events is therefore relevant to reducing deterioration and sudden cardiac death in patients with HF. Our current study validates the role of hypertriglyceridemia with ASCVD in a large cohort of patients with HF. Further, the current results highlight the substantial residual risk of ASCVD, probably due to shared risk factors as well as underlying CAD that is common in patients with HF, which could possibly be mitigated by managing hypertriglyceridemia.
Low triglyceride level with HF-related outcome
While some studies in patients with CAD and the current guideline recommendation may suggest that lipid profile is positively associated with adverse outcomes and many have adopted a “lower the better” theory19,23, others have shown contrary findings. Rather than a positive association, a prospective study that included patients with ischemic stroke demonstrated that low serum triglyceride was associated with a higher mortality33. Among 108243 individuals recruited in a general population study, a low LDL cholesterol was associated with all-cause mortality7 which was partly explained by higher mortality from cancer. A post-hoc analysis of the EVEREST study that included 3957 patients with HF with reduced EF demonstrated that low triglyceride level was positively related to all-cause mortality15. The relation between low triglyceride levels with all-cause mortality was further illustrated in a cohort of 833 outpatients with HF34. Our study verified such observations, by including a large population of HF accounting for potential confounders as well as concomitant drugs, and showed that low triglyceride level, compared with intermediate triglyceride level, was associated with higher risks of readmission or death from HF. This association could be explained by reverse causation with triglyceride levels characterizing a poor general health status and wasting before mortality occur35. In this study, those with low triglyceride levels are likely to be older with a greater incidence of AF. This is consistent with the theory that low levels of triglyceride are an indirect marker of underlying frailty. The BIOSTAT-CHF cohort externally validated our observation of low triglyceride levels associated with mortality. An obesity paradox has been well explored, indicating that debilitation and malnutrition, which a low triglyceride level could reflect, are crucial among the HF population. Intriguingly, after limiting the analysis to a subgroup with BMI records (Supplementary Table 3), the relation between a low triglyceride level and all-cause mortality remained, suggesting that the “triglyceride paradox”36 may better reflect underlying frailty, malnourishment as well as inflammatory burden that could provide independent prognostic information in addition to body habitus.
U-shape association with mortality
Given the discrepancy regarding dyslipidemia on differential causes of mortality, it is possible that lipid profile exerts distinct effects on patients’ outcomes according to the type of events. To elucidate the mechanisms underlying such U-shape association, our study conducted a cause-specific analysis, where we divided the events into ASCVD-related outcomes or HF-related outcomes. The results demonstrated that differential outcomes contribute to two ends of the U-shape curve. As expected, high triglyceride levels were positively associated with ASCVD and related mortality. On the other hand, this study revealed the finding that low triglyceride levels were associated with HF rehospitalization and related mortality, highlighting the complex interplay between lipid profiles and cardiovascular outcomes. Hence, triglycerides may have different prognostic values specific to the HF population rather than the general population. Specifically, the association between low triglyceride levels and HF outcomes observed in this study may not be evident in the general population, warranting further investigation in diverse populations.
A multicenter cohort study in 14,478 CAD patients consistently showed a U-shaped curve between HDL-C levels and all-cause mortality or CVD8. A recent prospective cohort study of 1,08,243 general population from Denmark has found a U-shaped association between LDL-C levels with all-cause mortality7. With regards to cardiovascular mortality, any increase in LDL-C was associated with an increased risk of fatal myocardial infarction, whereas low levels of LDL-C were associated with an increased risk of fatal heart failure, although with a large 95% confidence interval. Extending these observations, our study shows that a low triglyceride was associated with HF-related mortality whereas a high triglyceride was associated with ASCVD-related outcome. As such, rather than a single directional relation, we have demonstrated that both low and high levels of triglyceride were associated with increased risks of all-cause mortality and CVD in HF patients in a U-shaped manner.
Nonetheless, in the BIOSTAT-CHF study, a high triglyceride level was found to be neutral in relation to all-cause mortality which might be attributed to the small sample size as well as a highly selected population that was predominantly heart failure with reduced ejection fraction or preserved ejection fraction with high N-terminal pro-brain natriuretic peptide (NT-proBNP) levels. Moreover, this U-shaped relation of triglyceride was not found in a study involving 87,192 individuals from the Copenhagen General Population Study, where an elevated remnant cholesterol (or plasma triglycerides, a commonly used marker for remnant cholesterol) was shown to be associated with two-fold mortality from cardiovascular, infectious and endocrinological causes11. The different study populations could explain the inability to demonstrate a U-shape relation with triglyceride. The Copenhagen Population Study was conducted in the general population, of which the mean age was younger (55years vs. 71years) with a much lower prevalence of cardiovascular risk factors, such as diabetes (2% vs. 30%) and kidney disease (3% vs. 22%) compared to the present study. Further, their investigation on outcomes of all-cause mortality (4252 vs. 83,615) and cardiovascular mortality (687 vs. 27,095), were considerably lower in magnitude compared to our study. Moreover, our recruited population exhibited a higher disease burden, with 37% presenting with CAD (vs. 3%), allowing for the assessment of the association of low triglyceride levels in individuals with underlying HF who are frailer and more malnourished. These differences suggested that a U-shape relation with triglyceride could be explained by the U-shape association unique to the HF population.
Clinical implication
By using clinical demographics, biomarkers, and advanced cardiovascular imaging, risk stratification for patients with HF has been improved in recent years. Nonetheless, the prognostic role of lipid profiles in this population has not been addressed. Our results, by using a large validated population-based cohort, have shown the U-shaped prognostic association of triglyceride with adverse outcomes in patients with HF. In addition to being an inexpensive, non-invasive, and readily available biomarker, a high triglyceride level may further direct specific treatment, such as icosapent37 and other classes of investigational drugs38, to reduce major ischemic events that deserve verification in patients with HF. However, as shown in our studies and others, a low triglyceride level may not always represent a lower risk of subsequent adverse events. Rather than considering merely the low risk of ASCVD, clinicians should be alert when encountering a low triglyceride level in patients with HF and should consider a comprehensive assessment, with the evaluation of possible underlying frailty and debilitation contributing to future risk of HF-related adverse outcomes. Future studies are needed to evaluate the optimal monitoring and thresholds for intervening on triglyceride levels with lipid-lowering or nutritional treatment.
Strength and limitation
This is a large population-based database of HF patients with comprehensive profiles, including but not limited to demographics, history of diseases, medication prescription, laboratory tests, detailed causes of death, with long duration and more than 90% completed follow-up39 which was well-validated by our previous work40. It is worth noting that approximately 5%–10% of follow-up data was missing, attributed to patients seeking care in private hospitals or being outside of the hospital setting. Secondly, the similar U-shaped association between TC, LDL-C, HDL-C, and all-cause mortality further validated this database. Thirdly, sensitivity and subgroup analyses were performed to reduce potential bias and further verify the robustness of the results. External validation with the BIOSTAT-CHF cohort showed consistent results of which a low triglyceride level was associated with an increased risk of all-cause mortality. Nonetheless, the neutral relation between high triglyceride levels and adverse outcomes in the BIOSTAT-CHF cohort could possibly be explained by a smaller number of study population with a limited follow-up period.
One limitation of our study is the lack of information on left ventricular ejection fraction and NT-proBNP, leading to failure in stratifying HF severity and its phenotypes. However, a recent study in individuals with established diabetes and/or atherosclerotic CV disease showed that elevated triglyceride was significantly associated with incident HF diagnosis, irrespective of the severity of HF41. The residual confounding bias of unmeasured variables, e.g., frailty, might remain even after multivariable adjustment and subgroup analyses. Although the BIOSTAT-CHF cohort externally validates our observation of low triglyceride levels in association with mortality, the results should be interpreted with caution because of the relatively limited number of events (N = 345). Finally, a causal relationship cannot be ascertained because of the observational nature of this study. Future genetic instruments and Mendelian randomization analyses are warranted to further investigate the causal relationship between triglyceride levels and adverse outcomes in the HF population.
To conclude, in this population-based study of 1,27,124 patients with HF, triglyceride concentrations exhibited a U-shaped association with all-cause mortality. A differential cause-specific outcome was observed where a high triglyceride level was associated with ASCVD-related outcome and a low triglyceride level was associated with HF-related outcome. Our findings have important clinical implications for defining the role of triglyceride levels in contributing to the diverse adverse outcomes in patients with HF. Future randomized-controlled trials in optimizing triglyceride levels in HF settings and other diseased populations are warranted.
Methods
Study population
This study included all eligible in-patients diagnosed with HF between 2000 and 2020 (N = 1,27,124) from a previously validated territory-wide electronic database, the Clinical Data Analysis and Reporting System (CDARS) established by the Hospital Authority in Hong Kong since 1993 which covers approximately 90% of the Hong Kong population39. Patients with at least one triglyceride record measured within one year before HF diagnosis were included in this study. Patients aged < 18 years old, diagnosed with HF before 2000, with missing data on triglyceride, total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C) or high-density lipoprotein-cholesterol (HDL-C), with extreme triglyceride values (triglyceride < 0.2 or >20 mmol/L)17, with severe renal failure (eGFR < 30 ml/min/1.73 m2) or severe hepatic dysfunction (serum bilirubin > 51.3 umol/L and serum albumin < 28 g/L) were excluded from the cohort (N = 1,26,234). The index date was defined as the date of the first diagnosis of HF. This retrospective cohort study was approved by the institutional review board of the University of Hong Kong and the West Cluster of the Hong Kong Hospital Authority. (IRB number: UW 20-817) As patient data was de-identified in CDARS, the need for individual consent was waived.
Exposure
All triglyceride values were measured with fasting blood samples. Triglyceride levels measured within one year prior to the index date were used as the primary exposure. For patients who had more than one measurement, the value closest to the index date was used (58.7% with triglyceride measurements <1 month, 14.6% <3 months, 12.1% <6 months from the index date). Further, during the follow-up period, more than one measurement of triglyceride for each patient was derived to model the time-weighted triglyceride as a proxy of triglyceride variations over time to test the robustness of the study results.
Covariates
Demographic variables (age, sex, smoking, and alcohol use status), comorbidities (hypertension [HT], diabetes mellitus [DM], obesity, stroke, anemia, coronary artery disease [CAD], peripheral vascular disease [PVD], dyslipidemia, atrial fibrillation [AF], cirrhosis, chronic kidney disease [CKD], cancer, rheumatism, Parkinson’s disease, depression, sleep apnea) and medication prescriptions (aspirin, angiotensin-converting enzyme inhibiter [ACEI], angiotensin receptor blockers [ARB], beta blocker, calcium channel antagonist [CCB], diuretics, statin, insulin, and other anti-diabetes drugs) were derived up to three years before the index date. Baseline drug use was defined as consecutive drug use of more than 90 days40. Other lipid profiles, including TC, LDL-C, and HDL-C were collected within one year before the index date. Non-HDL was calculated as Total cholesterol − HDL. Triglyceride-glucose index (TyG) was calculated as ln[fasting triglyceride (mg/dl) × fasting blood glucose (mg/dl)/2]42. The value closest to the index date for each patient was used.
Endpoints
Death and cardiovascular events records were retrieved from CDARS. Causes of death were labeled by the International Classification of Diseases code (ICD) where both the ninth version (ICD-9) and the tenth version (ICD-10) codes were used. If a cardiovascular event/disease was recorded as the primary or secondary diagnosis (ICD-9: 390-459; ICD-10: I00-I99), death was classified as cardiovascular death (CVD). Other causes of death/events were as follows: ASCVD-related death or admission, and HF-related death or readmission. ASCVD-related death or admission was defined as death or admission for a composite of myocardial infarction (MI), unstable angina, arterial revascularization, or ischemic stroke. When examining the ASCVD admission, patients were followed up until the incidence of MI, unstable angina, arterial revascularization, ischemic stroke, or death, 31st December 2021, whichever came earlier. When examining HF readmission, patients were followed up until the incidence of HF readmission, death, or 31st December 2021, whichever came earlier. When evaluating all-cause death, CVD, ASCVD, or HF death, patients were followed up until death or 31st December 2021.
Statistical analyses
Continuous variables were summarized as mean ± standard deviation (SD), and categorical variables as numbers and percentages. ANOVA or χ2 tests were used to estimate differences among groups as appropriate. Post-hoc analyses were provided to examine pairwise differences between each group. (Supplementary Table 7) A correlation matrix was applied to examine the multicollinearity between variables. (Supplementary Fig. 9) Associations between concentrations of triglyceride on a continuous scale and the risks of all-cause mortality, CVD, HF, and ASCVD death or admission were evaluated by cubic spline curves based on multivariable-adjusted Cox proportional hazards models with all the variables being adjusted listed in Table 1. To balance the best fit and avoid overfitting, the number of knots (between three and seven) with the lowest Akaike information criterion was chosen (knots = 4). The same number of knots (four knots) from the splines for all-cause mortality was also applied in splines for CVD, HF, and ASCVD death or admission to allow direct comparison of all analyses.
As the concentrations of triglyceride associated with the lowest risk of adverse outcomes lay in the lowest hazard ratio (HR) of the spline curve, the range between the intersection of the spline curve for all-cause mortality with an HR of 1.0 was defined as the intermediate range (1.2–3.0 mmol/L) by using the optimal equal-HR method43. Further, in this study, the cut-off values under 1.2 mmol/L were considered to be a low triglyceride level since there is currently a lack of consensus on lower cut-offs of triglyceride levels. Those with low triglyceride levels were further stratified into three groups according to the lower two quintiles (0.8 mmol/L for 20% cut-off point and 1.0 mmol/L for 40% cut-off point) cut-off values of the whole cohort. As such, three groups of low triglyceride level were constructed accordingly (triglyceride < 0.8, 0.8 ≤ triglyceride < 1.0, 1.0 ≤ triglyceride < 1.2 mmol/L). Triglyceride concentrations above 3.0 mmol/L were divided into two groups 3.0 ≤ triglyceride < 5.7, triglyceride ≥ 5.7 mmol/L44. Associations between categories of triglyceride concentrations and all-cause mortality, CVD, HF, and ASCVD death or admission were evaluated by Cox proportional hazards regression model with competing risks where appropriate. Further, the associations between triglyceride categories and all-cause mortality were investigated within subgroups including old or young, female or male, with or without comorbid CAD, DM, AF, low or high LDL-C levels, with or without lipid-lowering treatment. P for interaction was checked before stratified analysis where P < 0.05 was considered to be significant. An external validation of the relation between triglyceride and mortality was performed using the BIOSTAT-CHF cohort, a multicentre, prospective observational study consisting of 2516 patients with HF from Europe45,46. The BIOSTAT cohort did not register lipid-lowering treatments and we could therefore not correct for that.
In sensitivity analyses, we excluded death within 2 years (N = 49,886) after HF diagnosis to minimize immortal time bias47. Second, we collected triglyceride values during the follow-up period and modeled triglyceride as a time-weighted covariate. The associations between time-weighted triglyceride and all-cause mortality or CVD were analyzed. Third, we limited the analysis to the subgroup with BMI recordings (N = 28,086) to evaluate whether the association between triglyceride and mortality was independent of BMI48. Additionally, we added the Nutritional Risk Index (NRI) as a surrogate for nutritional status48. Fourth, to test the robustness of the “intermediate range”, we drew the restricted cubic spline curve within the subgroup with BMI records and determined cut-points using the optimal equal-HR method43. Fifth, the associations between LDL-C, HDL-C, TC, TyG, and all-cause mortality were also examined to compare with previous studies. Further, different knots were used to determine the “intermediate range”. In addition, we adjusted only for CAD in the Cox regression model with competing risk as appropriate. Finally, we excluded patients with MI, unstable angina, arterial revascularization, or ischemic stroke prior to the index date (N = 55,943) to investigate the associations between triglyceride levels and new-onset ASCVD admission or death to minimize reverse causality. P < 0.05 was significant for all. Statistical analyses were performed using R.V.4.2.1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available in the article and its Supplementary information. The data underlying this article is under restricted access due to the inclusion of confidential patient information. For strictly research purposes, the data may be accessed by contacting the corresponding author (khkyiu@hku.hk). Eligible parties, including local academic institutions, government departments, or non-governmental organizations, can apply for access to CDARS data through the Hong Kong Hospital Authority Data Sharing Portal (https://www3.ha.org.hk/data). More information regarding the application process can be found at https://www3.ha.org.hk/data/Provision/ApplicationProcedure.
Code availability
The analytic codes used in this study are available at https://github.com/u3007493/Codes-for-Triglyceride-and-HF/tree/main.
References
Prospective Studies Collaboration; Lewington, S. et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370, 1829–1839 (2007). https://doi.org/10.1016/S0140-6736(07)61778-4. Erratum in: Lancet. 2008 Jul 26;372(9635):292.
Jorgensen, A. B. et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 34, 1826–1833 (2013).
Varbo, A. et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 61, 427–436 (2013).
Casiglia, E. et al. Total cholesterol and mortality in the elderly. J. Intern. Med. 254, 353–362 (2003).
Ravnskov, U. et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open 6, e010401 (2016).
Kwon, D., Yi, J. J., Ohrr, H. & Yi, S. W. Total cholesterol and mortality from ischemic heart disease and overall cardiovascular disease in Korean adults. Medicine 98, e17013 (2019).
Johannesen, C. D. L., Langsted, A., Mortensen, M. B. & Nordestgaard, B. G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ 371, m4266 (2020).
Liu, C. et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. 7, 672–680 (2022).
Nichols, G. A., Philip, S., Reynolds, K., Granowitz, C. B. & Fazio, S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diab. Obes. Metab. 21, 366–371 (2019).
Wadstrom, B. N., Pedersen, K. M., Wulff, A. B. & Nordestgaard, B. G. Remnant cholesterol, not LDL cholesterol, explains peripheral artery disease risk conferred by apoB: a cohort study. Arterioscler. Thromb. Vasc. Biol. 44, 1144–1155 (2024).
Wadstrom, B. N., Pedersen, K. M., Wulff, A. B. & Nordestgaard B. G. Elevated remnant cholesterol, plasma triglycerides, and cardiovascular and non-cardiovascular mortality. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehac822 (2023).
Klempfner, R. et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the bezafibrate infarction prevention study and registry. Circ. Cardiovasc, Qual. Outcomes 9, 100–108 (2016).
Lawler, P. R. et al. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur. Heart J. 41, 86–94 (2020).
Kozdag, G. et al. Low serum triglyceride levels as predictors of cardiac death in heart failure patients. Tex. Heart Inst. J. 40, 521–528 (2013).
Greene, S. J. et al. Prognostic significance of serum total cholesterol and triglyceride levels in patients hospitalized for heart failure with reduced ejection fraction (from the EVEREST Trial). Am. J. Cardiol. 111, 574–581 (2013).
Dziedzic, T., Slowik, A., Gryz, E. A. & Szczudlik, A. Lower serum triglyceride level is associated with increased stroke severity. Stroke 35, e151–e152 (2004).
Lindman, A. S., Veierod, M. B., Tverdal, A., Pedersen, J. I. & Selmer, R. Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian counties study. Eur. J. Epidemiol. 25, 789–798 (2010).
Kintscher, U., Foryst-Ludwig, A., Haemmerle, G. & Zechner, R. The role of adipose triglyceride lipase and cytosolic lipolysis in cardiac function and heart failure. Cell Rep. Med. 1, 100001 (2020).
Huang, Y. Q., Liu, X. C., Lo, K., Feng, Y. Q. & Zhang, B. A dose-independent association of triglyceride levels with all-cause mortality among adults population. Lipids Health Dis. 19, 225 (2020).
Sarwar, N. et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 115, 450–458 (2007).
Faergeman, O. et al. Plasma triglycerides and cardiovascular events in the treating to new targets and incremental decrease in end-points through aggressive lipid lowering trials of statins in patients with coronary artery disease. Am. J. Cardiol. 104, 459–463 (2009).
Schwartz, G. G. et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 65, 2267–2275 (2015).
Miller, M. et al. Investigators PI-T. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J. Am. Coll. Cardiol. 51, 724–730 (2008).
Laufs, U., Parhofer, K. G., Ginsberg, H. N. & Hegele, R. A. Clinical review on triglycerides. Eur. Heart J. 41, 99–109c (2020).
Toth, P. P. et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J. Am. Heart Assoc. 7, e008740 (2018).
Tirosh, A. et al. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann. Intern. Med. 147, 377–385 (2007). Sep 18.
Liu, J. et al. Impact of diabetes, high triglycerides and low HDL cholesterol on risk for ischemic cardiovascular disease varies by LDL cholesterol level: a 15-year follow-up of the Chinese Multi-provincial Cohort Study. Diabetes Res. Clin. Pract. 96, 217–224 (2012).
Kim, E. H. et al. Serum triglyceride levels and cardiovascular disease events in Koreans. Cardiology 131, 228–235 (2015).
Virani, S. S. et al. 2021 ACC expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia: a report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 78, 960–993 (2021).
Gheorghiade, M. et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation 114, 1202–1213 (2006).
Uretsky, B. F. et al. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation 102, 611–616 (2000).
Adabag, A. S., Therneau, T. M., Gersh, B. J., Weston, S. A. & Roger, V. L. Sudden death after myocardial infarction. JAMA 300, 2022–2029 (2008).
Ryu, W. S., Lee, S. H., Kim, C. K., Kim, B. J. & Yoon, B. W. Effects of low serum triglyceride on stroke mortality: a prospective follow-up study. Atherosclerosis 212, 299–304 (2010).
Freitas, H. F., Barbosa, E. A., Rosa, F. H., Lima, A. C. & Mansur, A. J. Association of HDL cholesterol and triglycerides with mortality in patients with heart failure. Braz. J. Med Biol. Res. 42, 420–425 (2009).
Liu, Y. et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 291, 451–459 (2004).
Lv, Y. B. et al. Triglycerides paradox among the oldest old: “the lower the better? J. Am. Geriatr. Soc. 67, 741–748 (2019).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2019).
Lewis, G. F. & Hegele, R. A. Effective, disease-modifying, clinical approaches to patients with mild-to-moderate hypertriglyceridaemia. Lancet Diab. Endocrinol. 10, 142–148 (2022).
Li, B., Cheung, K. S., Wong, I. Y., Leung, W. K. & Law, S. Nonaspirin nonsteroidal anti-inflammatory drugs and gastric cancer risk after Helicobacter pylori eradication: a territory-wide study. Cancer 127, 1805–1815 (2021).
Ren, Q. W. et al. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur. Heart J. 42, 3049–3059 (2021).
Toth, P. P., Philip, S., Hull, M. & Granowitz, C. Elevated triglycerides (>/=150 mg/dL) and high triglycerides (200-499 mg/dL) are significant predictors of new heart failure diagnosis: a real-world analysis of high-risk statin-treated patients. Vasc. Health Risk Manag. 15, 533–538 (2019).
Wang, D. et al. Association of the triglyceride-glucose index variability with blood pressure and hypertension: a cohort study. QJM 117, 277–282 (2024).
Chen, Y. et al. A novel approach to determine two optimal cut-points of a continuous predictor with a U-shaped relationship to hazard ratio in survival data: simulation and application. BMC Med. Res. Methodol. 19, 96 (2019).
Simha, V. Management of hypertriglyceridemia. BMJ 371, m3109 (2020).
Ouwerkerk, W. et al. Effects of combined renin-angiotensin-aldosterone system inhibitor and beta-blocker treatment on outcomes in heart failure with reduced ejection fraction: insights from BIOSTAT-CHF and ASIAN-HF registries. Eur. J. Heart Fail 22, 1472–1482 (2020).
Santema, B. T. et al. Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet 394, 1254–1263 (2019).
Bhaskaran, K., dos-Santos-Silva, I., Leon, D. A., Douglas, I. J. & Smeeth, L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diab. Endocrinol. 6, 944–953 (2018).
Caccialanza, R. et al. Nutritional parameters associated with prolonged hospital stay among ambulatory adult patients. CMAJ 182, 1843–1849 (2010).
Acknowledgements
Thanks to the Hong Kong Hospital Authority for building CDARS which facilitates extensive clinical data for research. Thanks to Ang Li, an associate professor from SJTU, for providing the supercomputer to conduct the big data analysis. Thanks to Tiew Hwa Katherine Teng, Hung-Fat Tse, Adriaan A. Voors, Jasper Tromp, and Carolyn S.P. Lam for their contribution to reviewing and editing the manuscript. This study was supported by the Sanming Project of Medicine in Shenzhen, China (No. SZSM201911020) & supported by HKU-SZH Fund for Shenzhen Key Medical Discipline (No. SZXK2020081). The funders [Sanming Project of Medicine in Shenzhen, China (No. SZSM201911020) and HKU-SZH Fund for Shenzhen Key Medical Discipline (No. SZXK2020081)] had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: R.Q.W., Y.K.H.; Methodology: R.Q.W., W.O., T.H.K.T., J.T.; Investigation: R.Q.W., Y.K.H.; Visualization: R.Q.W., W.O., Y.K.H.; Project administration: Y.K.H., C.S.P.L.; Supervision: Y.K.H., C.S.P.L., H.F.T.; Writing original draft: R.Q.W., Y.K.H., Y.K.T., C.T.W.T.; Writing review and editing: R.Q.W., T.H.K.T., Y.K.H., M.Z.W., A.A.V.
Corresponding author
Ethics declarations
Competing interests
C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from AstraZeneca, Bayer, Boston Scientific and Roche Diagnostics. has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Actelion, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., Us2.ai, Janssen Research & Development LLC, Medscape, Merck, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Sanofi and WebMD Global LLC; and serves as co-founder & non-executive director of Us2.ai. JT received speaker or consultancy fees from Roche Diagnostics, Daiichi Sankyo, Boehringer Ingelheim, and Us.2ai. He is supported by the National University of Singapore start-up grant and a Ministry of Education tier 1 grant. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Vito Bruno, Laura Loehr, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, QW., Teng, TH.K., Ouwerkerk, W. et al. Triglyceride levels and its association with all-cause mortality and cardiovascular outcomes among patients with heart failure. Nat Commun 16, 1408 (2025). https://doi.org/10.1038/s41467-025-56790-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56790-1
This article is cited by
-
Dual clinical utility of cholesterol, high density lipoprotein, and glucose index: from advanced cardiovascular-kidney-metabolic syndrome pathogenesis to inflammatory-mediated mortality across early-transitional stages
Diabetology & Metabolic Syndrome (2025)
-
Spatial analysis of air pollutant exposure and its association with metabolic diseases using machine learning
BMC Public Health (2025)
-
Associations of the Zhejiang University (ZJU) index with cardiovascular diseases and mortality among US adults: a national cohort study
Lipids in Health and Disease (2025)
-
Association of lipid accumulation product with mortality in patients with diabetes mellitus
Scientific Reports (2025)