Abstract
The prognosis of metastatic endometrial carcinoma (EC), one of the most common gynecological malignancies worldwide, remains poor, and the underlying driver of metastases is poorly understood. Dysregulation in estrogen-related signaling and inactivation of tumor suppressor PTEN are two essential risk factors of EC. However, whether and how they are interconnected during EC development remains unclear. Here, we demonstrate that the deacetylase SIRT7 is upregulated in EC patients and mouse models, facilitating EC progression in vitro and in vivo. Mechanistically, in an estrogen-dependent fashion, SIRT7 mediates PTEN deacetylation at K260, promoting PTEN ubiquitination by the E3 ligase NEDD4L, accelerating PTEN degradation and, consequently, expediting EC metastasis. Additionally, SIRT7 expression strongly correlates with poor survival in EC patients with wild-type PTEN, though no significant correlation is observed in PTEN mutation patients. These results lay the foundation for the study of targeting estrogen-SIRT7-PTEN axis, to restore PTEN abundance, offering potential avenues for EC therapy.
Similar content being viewed by others
Introduction
Endometrial cancer (EC) is the most frequent gynecological malignancy in developed countries, with continuously increasing incidence and mortality rates in recent 10 years1,2. Surgery is the main initial management of the primary EC tumor, with or without post-operative radio- or chemotherapy. EC is broadly divided into two subtypes, endometrioid and non-endometrioid carcinoma. The endometrioid type contributes to up to 80% of EC and is known as a hormone-dependent disease, with exposure to estrogen unopposed to progesterone as one of the major risk factors3. Overall, the outcome of the early-stage patients after surgery and radio- or chemotherapy is favorable, however, for patients who have more advanced diseases with metastasis to lymph nodes or other organs, available treatments have limited effects, and the 5-year survival rate is reported to be less than 20%4. Meanwhile, no therapeutic regimen has been globally accepted5,6. Hence, identifying the metastasis drivers of EC and understanding the EC metastatic mechanisms are critical to benefit the patients.
The phosphatase and tensin homolog deleted on chromosome ten (PTEN) is described as a haplo-insufficient tumor suppressor and studies have demonstrated that partial loss of PTEN is sufficient to promote several types of malignancies, including breast cancer, prostate cancer, and uterine cancer, etc.7,8,9,10. As a dual phosphatase, PTEN exerts tumor suppressor function to modulate PIP3 concentration on membrane11, negatively mediating PI3K-AKT pathway. Meanwhile, PTEN participates in a variety of biological processes through its substrates such as FAK12, IRS113, CREB14 , etc. For endometrial cancer, PTEN is one of the most frequently altered and inactivated genes15. Intriguingly, a study has shown the loss of PTEN protein without mRNA level alterations in endometrioid endometrial cancer16. In poor prognosis EC subtypes, serous carcinoma, 45% of patients have PTEN protein level loss by immunohistochemistry (IHC) while the PTEN mutation rate is only about 7%17. More importantly, the negative PTEN IHC staining is strongly related to a poor survival rate of patients with advanced EC18. Collectively, these clues have suggested that PTEN loss at protein level is a crucial and independent cancer-promoting event in EC. Furthermore, these findings indicate that the post-translational regulation of PTEN may play a critical role in the progression of EC, especially under the PTEN intact genetic context. However, how PTEN protein is regulated in EC process remains poorly investigated.
Lysine acetylation and deacetylation have emerged as essential post-translational modifications involved in various biological functions19. Studies have revealed that the acetylation levels of non-histone proteins can influence the protein function, abundance, localization, and affinity to other proteins or DNA20. Notably, recent studies have shown that crosstalk of the acetylation or deacetylation with proteomic pathways could precisely tune the cellular protein abundance. For instance, the acetylation of MOB1, a protein involved in the Hippo pathway, could promote its stability by limiting its binding affinity to E3 ligase Praja2 and affecting the subsequent ubiquitination21. SIRT2 could deacetylate and stabilize FGL1 protein in a ubiquitination-dependent manner in liver cancer to promote immune evasion22. In addition, accumulating evidence has shown that changes in acetylation level could serve as one of the underlying mechanisms of cancer development through modifying its oncogenic or tumor suppressing substrates20. Thus, acetyltransferases and deacetylases are recognized as critical potential targets in cancer therapy. In EC, previous proteogenomic characterization23 has pointed out the heterogeneity of the acetylome and suggested that acetylation regulators play critical roles in the occurrence and development of endometrial carcinoma. Characterizing such acetyltransferases and deacetylases will help us to understand the tumor biology of EC and more importantly, develop promising therapeutic approaches.
In mammals, sirtuins (SIRT1-7) are a family of nicotinamide adenine dinucleotide (NAD+) dependent, evolutionarily conserved deacetylases. Sirtuin family has seven members sharing similar catalytic cores yet having various substrates and different subcellular localizations. They are involved in numerous processes during ageing, cancer, and other diseases24,25. Previous quantitative mass spectrometry analysis implied that in mouse embryonic fibroblasts cells, SIRT7, the least studied sirtuin protein, might be a potential deacetylase of PTEN26, which is the key tumor suppressor in EC described above. Studies have manifested that, as a mainly nucleolus localized protein, SIRT7 exhibits deacetylation, desuccinylation and deglutarylation activities27. SIRT7 deacetylates H3K18 to drive oncogenic transformation28 and its deacetylating function on H3K36 is associated with heterochromatin silencing and genomic stability29. It is also reported that SIRT7 is involved in chromatin remodeling by desuccinylating H3K12230. The non-histone substrates of SIRT7 include ATM31, SMAD432, NPM33 , etc., which makes SIRT7 a critical modulator in genome stability, cellular stress response, as well as tumor development34,35. It is lately demonstrated by several studies that SIRT7 could promote tumorigenesis or aggravate tumor progression in different cancer types such as pancreatic ductal adenocarcinoma36, prostate cancer37, thyroid cancer38 and colorectal cancer39,40. However, the effect of SIRT7 in EC development is currently unclear.
Herein, we identify the tumor suppressor PTEN as a substrate of SIRT7 in endometrial cancer, and elaborate SIRT7 facilitating endometrial cancer metastasis. Utilizing human patient samples and two established spontaneous EC murine models, we find that SIRT7 exhibits higher expression level in tumor samples. In detail, SIRT7 deacetylates PTEN, promoting its degradation mediated by E3 ligase NEDD4L in an estrogen-dependent manner. More importantly, we identify that K260 is a deacetylation site of PTEN, and the acetylation level of PTEN-K260 is significantly associated with EC progression both in human and mice. Our study illustrates one key node of PTEN stability regulation network and its deacetylation in tumor progression process. We elucidate the basic pathogenesis of high expressed SIRT7 endometrial cancer and the possibility of achieving PTEN expression recovery to slow down cancer progression by suggesting SIRT7 as a promising therapeutic target for endometrial cancer.
Results
SIRT7 high expression is positively associated with EC progression
Aging is an important and independent risk factor of EC, with increasing incidence, mortality, and recurrence rate41. Sirtuins, known as longevity proteins, yet the links between these longevity proteins and endometrial cancer are still poorly understood42. Since clues suggested that the level of tumor suppressor PTEN was strongly associated with the development of EC, initially, we aim to screen that whether the members of Sirtuins could regulate PTEN. The co-IP results suggested that SIRT1, 6, 7 could interact with PTEN (Supplementary Fig. 1a). Moreover, from the The Cancer Genome Atlas (TCGA) database of Uterine Corpus Endometrial Carcinoma (UCEC), we found that the expression level of SIRT6 and SIRT7 were upregulated while other Sirtuin family members such as SIRT1, SIRT2, SIRT3, SIRT4 were downregulated in UCEC tumor tissue compared with normal uterus tissue, and SIRT5 showed no difference (Fig. 1A and Supplementary Fig. 1b). Of note, SIRT7 was relatively high expressed in high grade EC samples, a more aggressive subset of ECs with relatively unfavorable clinical outcome43, compared to grade 1/2 EC tumors. The similar pattern was seen in database GSE11581044, an expression profiling of EC in different grades carried out previously (Fig. 1B). Moreover, further analysis of TCGA revealed that EC patients with high SIRT7 expression were associated with poor overall survival (Supplementary Fig. 1c), indicating that SIRT7 expression might be related with the malignancy and poor prognosis of endometrial cancer, while EC patients with SIRT6 high expression, oppositely, having a relatively good survival condition (Supplementary Fig. 1d). These all suggest that among Sirtuin members, SIRT7 might be a PTEN regulator and its high expression might be associated with EC poor progression.
A SIRT7 expression analysis of normal endometrium tissue (n = 35) and endometrial cancer tissue (n = 544), data from TCGA. B SIRT7 expression analysis of endometrial cancer in grade 1–2 and high grade, data from TCGA (left, G1/G2 n = 218, High Grade n = 326), data from GSE115810 (right, G1/G2 n = 18, High Grade n = 6). C SIRT7 expression analysis of human EC tissue by IHC. Endometrial cancer and paired para-tumor tissues (tumor n = 21, para-tumor n = 18) were stained with SIRT7 and quantified by IHC score. Scale bar = 50 μm. D SIRT7 expression analysis by Western blot. Tissues of paired endometrial cancer with adjacent normal tissue (n = 10) were analyzed for SIRT7 expression and quantified. The relative expression level was calculated by SIRT7/GAPDH. ImageJ software was used. E SIRT7 expression analysis of human EC tissue by IHC. Primary tumor tissue of EC and paired metastatic tissues in ovary or fallopian tube (n = 13) were stained with SIRT7 and quantified by IHC score. Scale bar = 50 μm. F SIRT7 expression analysis of human EC tissue by IHC. Primary tumor tissue of EC and paired metastasis tissues in lymph nodes (n = 15) were stained with SIRT7 and quantified by IHC score. Scale bar = 50 μm. G The diagram of Lkb1f/f mice crossed with Pgr-Cre mice. H The representative photograph, H&E staining and immunohistochemistry staining of uterus tissue of Lkb1 CKO (Lkb1f/f Pgr-Cre) mice and control mice (Lkb1f/f) at different weeks post birth (n = 5). Scale bar = 50 μm. I Western blot analysis of the uterus tissue of Lkb1 CKO mice and control mice at six weeks post birth. Different EC tumor nodules of Lkb1 CKO mice were isolated and analyzed (n = 6). Relative Sirt7 expression was quantified by Sirt7/Tubulin. The P value was calculated with two tailed unpaired t test for (A–C, I) and calculated with two tailed paired t test for (D–F). Data are presented as mean values ± s.e.m.
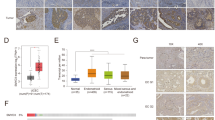
We then evaluated SIRT7 protein level in tumor versus para-tumor tissues from EC patients in different stages and different pathological subtypes. Tumor (n = 21) and para-tumor (n = 18) tissues of EC patients were collected and examined by immunohistochemistry (IHC), significant higher levels of SIRT7 were detected in tumor samples (Fig. 1C) compared to para-tumor samples. In addition, the western blot analysis manifested that, in 9 out of 10 patients, SIRT7 was upregulated in tumor tissues (Fig. 1D), collectively suggesting a potential tumor-promoting role of SIRT7 in endometrial cancer. To further investigate SIRT7’s role in cancer development in EC patients, metastatic tumor tissues were collected, including metastasis in ovaries, fallopian tubes (n = 13), and in lymph nodes (n = 15). SIRT7 expression level, detected by immunohistochemistry, showed a remarkable increase in metastatic tumor tissues compared to paired primary tumor tissues (Fig. 1E, F). These results imply that SIRT7 has the potential to facilitate tumor progression and may contribute to tumor metastasis in EC.
To further investigate the oncogenic function of SIRT7 in EC in vivo, two spontaneous EC murine models previously validated were utilized. Given that Lkb1 knockout in uterus by the Cre recombinase could lead to invasive endometrial malignancies, and low expression levels of Lkb1 in endometrial cancers correlate with invasiveness45,46, we crossed the Lkb1-flox mice with Pgr-cre mice47,48 to obtain ablation of Lkb1 in mice uterus (Fig. 1G and Supplementary Fig. 1e). The Lkb1-CKO (Lkb1f/f Pgr-cre) mice developed spontaneous tumors consistent to what was previously described45 (Fig. 1H), and Sirt7 level was detected at 6 weeks post birth or 12 weeks post birth. As examined by immunohistochemistry and Western blot, we found that the Sirt7 levels were preferentially upregulated in uterus tumor tissue of Lkb1 CKO mice compared with normal uterus tissue in control mice (Fig. 1H, I). Moreover, by the time when the Lkb1 CKO mice reached the age of 12 weeks, the uterus carcinoma had invaded and infiltrated nearly all layers of the uterine muscle, accompanied by adhesions to surrounding tissues (Fig. 1H). At this point, we observed a significant increase in the Sirt7 staining in the tumor tissues compared to that in 6-week-old Lkb1 CKO mice, suggesting Sirt7’s potential role in promoting tumor progression within the murine model. Furthermore, we verified our findings in another EC mouse model, Ptenf/f Pgr-Cre spontaneous model (PC model)49,50. This PC model could develop uterus carcinoma spontaneously as early as 30 days post birth and myometrial invasion in 90 days post birth51. Sirt7 level was detected by Western blot and immunohistochemistry at 10 weeks post birth, and consequently, upregulation of Sirt7 was also exhibited in the uterus tumor tissue in Pten CKO mice compared with normal uterus tissue in control mice (Supplementary Fig. 1f, g). Taken together, these results illustrate that SIRT7 facilitates the progression of endometrial cancer.
SIRT7 accelerates endometrial cancer metastasis
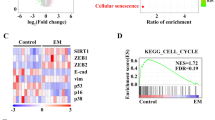
In order to explore the mechanism of SIRT7-mediated EC tumor progression, we analyzed the transcriptional profiles of parental endometrial cancer cells HEC-1B cells or SIRT7 knockdown HEC-1B cells by RNA-sequencing (Fig. 2A). With pathway enrichment analysis, we found that several tumor migration related pathways were remarkably downregulated in SIRT7 KD HEC-1B cells (Fig. 2B), such as ‘ECM receptor interaction’, and ‘Focal adhesion’. Consistently, gene set enrichment analysis (GSEA) also demonstrated that the signatures of tumor migration ‘epithelial–mesenchymal transition’ (EMT) is one of the most altered pathways in SIRT7 KD cells compared to control cells (Fig. 2C). Similarly, in TCGA database, when we compare the differentially expressed genes between SIRT7 high expression and low expression groups, the epithelial mesenchymal transition (EMT) gene set is significantly enriched in GSEA analysis (Supplementary Fig. 2a). These findings indicated that SIRT7 might play a crucial role in EC metastasis. Hence, we separately generated SIRT7 KD cell lines with two non-overlapping shRNAs in two EC cell lines, HEC-1B and KLE (Supplementary Fig. 2b). As shown in Fig. 2D–G, SIRT7 knockdown reduced the migratory and invasive capacity of HEC-1B and KLE cells by wound healing and transwell assays. Particularly, SIRT7 knockdown downregulated more than 85% of the invasion rate of HEC-1B cells (Fig. 2E). However, no significant changes were observed in cell growth and viability in vivo and in vitro (Supplementary Fig. 2c–g). We also did not see obvious morphological changes in HEC-1B cells after SIRT7 knockdown (Supplementary Fig. 2h). Also, some EMT markers were examined in HEC-1B shRFP/shSIRT7 cells, and we found the downregulation of N-cadherin, ZEB1 and upregulation of ZO-1 after SIRT7 knockdown, no changes were observed in the protein levels of Vimentin, β-catenin or SLUG (Supplementary Fig. 2i).
A The schematical process of RNA-Sequencing. Differentially expressed genes based on the RNA-Seq results were analyzed by KEGG enrichment (B) and GSEA enrichment (C). D Representative images of wound healing assay for HEC-1B cells with or without SIRT7 knockdown. The area of migration was calculated in six random views and normalized by shRFP group, scale bar = 500 μm, n = 6, cell cultures from three independent experiments. E Representative images of transwell assay and invasion assay for HEC-1B cells with or without SIRT7 knockdown. Cell number of six random views and normalized to control for migration or invasion ability quantification, scale bar = 200 μm, n = 6, cell cultures from three independent experiments. F Wound healing assay for KLE cells with or without SIRT7 knockdown, n = 3, cell cultures from three independent experiments, scale bar = 500 μm. G Transwell assay and invasion assay for KLE cells with or without SIRT7 knockdown, n = 4, cell cultures from four independent experiments, scale bar = 200 μm. H Diagram of the construction of a liver metastasis mouse model. Created in BioRender. Hu, Z. (2025) https://BioRender.com/f78p401. I Representative images of the mice liver, spleen and H&E-stained liver demonstrating the normal liver tissue and metastasis nodules in spleen injection assay. The metastasis area was marked with asterisks and arrows, scale bar = 100 μm. Tumor burden analysis was calculated by tumor area/liver area identified by H&E staining, n = 6. J Diagram of the uterus orthotopic injection mouse model. Created in BioRender. Hu, Z. (2025) https://BioRender.com/m69e532. K Representative images of the mice liver, uterus and H&E-stained liver in uterus orthotopic injection assay. The incidence of metastasis nodules occurred on liver was shown in the table and the tumor burden was calculated. shRFP group n = 11, shSIRT7-1 group n = 9, shSIRT7-2 group n = 9, scale bar = 100 μm. Data are presented as mean values ± s.d. for (D–G), and ±s.e.m. for (I, K). The P value was calculated with two tailed unpaired t test.
Next, to explore the in vivo function of SIRT7 in tumor metastasis, we utilized the hepatic metastasis model by intrasplenic injection52,53 in nude mice. SIRT7 proficient (WT) and deficient (KD) HEC-1B cells were injected under the spleen capsule after the mice were anesthetized. Eight weeks post inoculation, the metastasis nodules on liver were photographed and measured by H&E staining (Fig. 2H). Astonishingly, SIRT7 knockdown could eliminate the tumor metastasis process to liver in mice, with reduction of tumor burden from 8% to less than 1% (Fig. 2I). To better simulate the metastasis context and pattern of endometrial cancer in situ, we also established the orthotopic tumor model in nude mice. HEC-1B cells with or without SIRT7 knockdown were injected into the uterus lumen of nude mice after anesthesia. The surgical orthotopic injection was performed as indicated54,55,56 and particular attention was paid to ensure no visible leakage occurred (Fig. 2J). It is manifested that in the shRFP group, seven out of eleven mice developed diffused liver metastases following in utero injection, whereas only 1/9 and 0/9 mice in the SIRT7 knockdown group exhibited liver metastasis (Fig. 2K). The significant decrease in tumor burden observed after SIRT7 knockdown in the orthotopic tumor model suggests the effective inhibition of EC cell metastasis in nude mice by SIRT7 deletion. Collectively, these results demonstrate that SIRT7 knockdown could abolish tumor metastasis ability in endometrial cancer both in vivo and in vitro.
SIRT7 interacts with and directly deacetylates PTEN
As mentioned above, PTEN, the key tumor suppressor in endometrial cancer, is closely associated with metastasis and could interact with SIRT7. To investigate the relationship and connection of PTEN and SIRT7 in EC, we hypothesized that SIRT7 might functionally regulate PTEN via deacetylation. Thus, further co-immunoprecipitation assays were performed in cell lysates of HEK293FT cells ectopically expressing FLAG-SIRT7 and GFP-HA-PTEN. The western blot analysis showed the interaction between the ectopically expressed SIRT7 and PTEN, validated with anti-HA or anti-FLAG antibodies (Fig. 3A, B). Moreover, endogenous interactions were further verified in HEK293FT and HEC-1B cell lines, both precipitated wild type PTEN (Fig. 3C). To further confirm the direct interaction between SIRT7 and PTEN, GST pull-down assay was conducted with HIS-SIRT7 and GST-PTEN proteins purified from E. coli, and correspondingly, purified SIRT7 and PTEN showed a direct interaction in vitro (Fig. 3D). Furthermore, binding domain of the two proteins were investigated with the aim of detailing the interplay pattern of SIRT7 and PTEN. We fragmented SIRT7 based on its functional domains, and found out both SIRT7-FL and its enzymatic core fragment (amino acid 90-331) interacted with PTEN (Fig. 3E). We also fragmented PTEN into PTEN- N terminus (amino acid 1-185) and C terminus (amino acid 186-403), and as a result, the N terminus, including the dual-specificity phosphatase (DUSP) domain and a PIP2-binding motif (PBM)57, was identified to interact with SIRT7 (Fig. 3F).
A, B Co-IP assays of the interaction of GFP-HA-PTEN and FLAG-SIRT7 in HEK293FT cells. GFP-HA-PTEN and FLAG-SIRT7 were overexpressed in HEK293FT cells and immunoprecipitants were enriched with anti-HA (A) antibodies or anti-FLAG (B) antibodies. C Endogenous Co-IP assays of the interaction between SIRT7 and PTEN using antibodies against PTEN in HEK293FT cells (upper) and HEC-1B cells (lower). D GST-pulldown assay. Purified GST-PTEN, GST proteins were harvest with purified HIS-SIRT7 and precipitated with anti-GST antibodies then subjected to immunoblots. E Truncations of GST-SIRT7 were constructed and the truncation proteins were harvested with FLAG-PTEN and precipitated with anti-GST antibodies. F Truncations of GST-PTEN were constructed and the truncation proteins were harvested with FLAG-SIRT7 and precipitated with anti-GST antibodies. G Acetylation level of PTEN in HEK293FT cells while SIRT7-WT or SIRT7-H187Y was overexpressed. The pan-Acetyl antibodies were used to detect the acetylation level of PTEN after immunoprecipitation. H Acetylation level of PTEN in HEK293FT cells with or without SIRT7 knockdown in HEK293FT cells. I Acetylation level of PTEN in HEK293FT cells. For HEK293FT cells, SIRT7 were knockdown and then FLAG-SIRT7 was re-expressed. J, K The immunoblots showing the acetylation level of PTEN in vitro. FLAG-SIRT7 and HA-PTEN was purified by antibodies from cell lysates. PTEN acetylation level was detected by pan-acetylation antibodies after harvesting with or without SIRT7, NAD+ and NAM as indicated. L Immunoblots showing acetylation level of WT-PTEN and KR mutants (K60R, K223R, K260R, K344R, 4KR), detected by pan-acetyl antibodies after immunoprecipitation. To ensure similar expression levels among various PTEN-mut-plasmids, plasmids of different volumes are transfected. M Amino acid sequences of PTEN (aa251-264) of human, mouse, rat, and dog. N Immunoblots showing acetylation levels of WT PTEN and PTEN-K260R in vitro after incubation with or without SIRT7 and NAD+, detected by pan-AcK antibodies. O Immunoblots showing PTEN-K260 acetylation levels of PTEN-WT/K260R/K260Q after immunoprecipitation. The PTEN-K260 acetylation level was detected by AcK260-PTEN specific antibody after immunoprecipitation. P Immunoblots showing the K260 acetylation level of endogenous PTEN after immunoprecipitation in sgSIRT7 (pool) or sgScramble HEK293FT cells. The PTEN-K260 acetylation level was detected by AcK260-PTEN specific antibody.
To gain further insights into how SIRT7 regulates PTEN, we hypothesized that SIRT7 could deacetylate PTEN. The acetylation level of ectopic PTEN was decreased when wild type SIRT7 (SIRT7-WT) is overexpressed in HEK293FT cells but not the enzymatic dead mutants, SIRT7-H187Y (Fig. 3G). Consistently, knockdown of SIRT7 by two independent short hairpin RNAs led to a marked increase in the ectopic PTEN acetylation level (Fig. 3H), and such increase could be blocked by reintroduction of the SIRT7 construct (Fig. 3I). Moreover, for the purpose to identify the direct role of SIRT7 in deacetylating PTEN, in vitro deacetylation assays were performed. The acetylation level of PTEN almost vanished in the presence of SIRT7 along with NAD+ (Fig. 3J), while remained unchanged if the reaction was inhibited by nicotinamide (NAM) (Fig. 3K), which is a general sirtuin inhibitor28. Collectively, these results demonstrate that SIRT7 directly interacts and deacetylates PTEN.
To further map the deacetylation sites of PTEN by SIRT7, we performed a mass spectroscopy analysis (Supplementary Fig. 3a), and 4 sites were identified as the potential deacetylation sites, K60, K223, K260, K344 (Supplementary Fig. 3b). To determine the PTEN deacetylation sites, we replaced each lysine site with amino arginine separately (K60R, K223R, K260R, K344R), or together (4KR). WT-PTEN and the mutant PTEN plasmids were transiently transfected into HEK293FT cells, and acetylation levels of WT and mutant PTEN were determined by Western blot (Fig. 3L). The acetylation level was faint only when K260R- or 4KR- PTEN was transfected, indicating K260, an evolutionarily conserved lysine residue (Fig. 3M), might be the predominant acetylation site of PTEN. On top of that, in vitro deacetylation assays showed that FLAG-SIRT7 purified from cell lysates could lead to the significantly decreased acetylation level of WT-PTEN in the presence of NAD+, but little alteration was observed when expressing the K260R-PTEN mutant (Fig. 3N), which suggested that K260-PTEN could be the deacetylation site regulated by SIRT7.
To precisely monitor the acetylation level at PTEN-K260 and to ascertain the deacetylation of PTEN modulated by SIRT7, we generated a specific antibody for PTEN-K260 acetylation (AcK260-PTEN). The specificity and efficiency of this antibody were demonstrated by dot blot experiments with modified or unmodified peptides (Supplementary Fig. 3c). As a result, the acetylation levels of PTEN-K260 mutant and WT-PTEN were evaluated by AcK260-PTEN antibody, which furthermore indicated that PTEN was deacetylated at K260 site (Fig. 3O). Furthermore, the acetylation level of endogenous PTEN was examined using AcK260-PTEN antibody and consequently, the acetylation level of PTEN-K260 remarkably elevated after SIRT7 depletion (Fig. 3P). In in vitro assay, the acetylation level of PTEN-K260 almost abolished after incubating PTEN with SIRT7 and NAD+ (Supplementary Fig. 3d). These results suggest that SIRT7 could regulate PTEN deacetylation at K260. Of note, the KQ mutation of PTEN-K260 did not change the SIRT7-PTEN interaction (Supplementary Fig. 3e). In addition, we assessed the acetylation status of Pten-K260 in mouse uterine tissue following immunoprecipitation and the findings revealed that in EC tumor tissues from Lkb1 CKO mice, the acetylation level of Pten-K260 was significantly reduced compared to that in the uterine tissue of control mice (Supplementary Fig. 3f), which was consistent with the elevation of Sirt7 level in EC tumor mice as demonstrated in Fig. 1. Altogether, these results suggest that PTEN is a bona fide substrate of SIRT7 and it can be deacetylated by SIRT7 at lysine 260.
SIRT7-mediated PTEN deacetylation is associated with PTEN instability
We then proceeded to further explore the function of PTEN deacetylation by SIRT7. Astonishingly, we noticed that in SIRT7 knockdown HEC-1B cells, endogenous protein level of PTEN was significantly higher than that in control cells (Fig. 4A) and the phenomenon was confirmed in another EC cell line KLE (Supplementary Fig. 4a). Also, similar pattern was exhibited when SIRT7 was knocked out in HEC-1B cells with three different sgRNAs (Supplementary Fig. 4b). Correspondingly, the level of PTEN showed a dose-dependent decrease while overexpressing SIRT7-WT (Fig. 4B and Supplementary Fig. 4c), however, overexpressing enzymatic dead mutant SIRT7-H187Y abolished the PTEN decrease (Fig. 4C) in HEC-1B cells. To determine whether SIRT7 regulates PTEN expression by impeding its transcription, we examined the mRNA level and the promoter activity of PTEN, and there were no significant changes when we overexpressed or knocked down SIRT7 (Supplementary Fig. 4d–g), suggesting that SIRT7 could regulate PTEN expression through a post-transcriptional manner.
A–C PTEN protein level shown by immunoblots and quantification analysis of three independent repeats (n = 3). SIRT7 was knocked down (A), otherwise SIRT7 (B) or SIRT7-WT/H187Y (C) was overexpressed in HEC-1B cells. The relative expression level was analyzed in Image J, calculated by PTEN/Tubulin, and normalized by the control lane. The P value was calculated with two tailed unpaired t test. D The immunoblots showing the half-life of protein PTEN in HEC-1B cells with or without SIRT7 knockdown and the quantitative analysis of three independent repeats (n = 3). HEC-1B cells were treated with CHX (100 μg/ml) for indicated time and subjected for immunoblot analysis. HEC-1B shSIRT7 stable cell line was used and relative PTEN protein level was analyzed in Image J, calculated by PTEN/Tubulin, and normalized by the lane ‘0 h’. E Immunoblots showing the half-life of protein PTEN in HEC-1B cells over-expressing SIRT7-WT or SIRT7-H187Y and quantitative analysis (n = 3). F Immunoblots showing the half-life of protein PTEN-WT or PTEN-K260Q overexpressed in HEC-1B cells and quantitative analysis (n = 3). Two-way ANOVA was used for the P value calculation for (D–F). G Representative images of the mice uterus in immunofluorescence assay. The frozen sections of mice uterus tissue from control mice (Sirt7f/f) and Sirt7 uterus CKO mice (Sirt7f/f Pgr-cre) (n = 3) were stained with SIRT7, PTEN and DAPI. Scale bar = 50 μm. H Representative images of IHC staining of the uterus tissue from control mice (Sirt7f/f) and Sirt7 uterus CKO mice (Sirt7f/f Pgr-cre) (n = 5). The uterus tissue of the indicated mice was stained with SIRT7, PTEN, AcK260-PTEN antibodies. Scale bar = 50 μm. Data are presented as mean values ± s.e.m.
Next, we wonder how SIRT7 effects on the turnover of PTEN protein. We assayed the half-life of PTEN under the treatment of the protein synthesis inhibitor cycloheximide (CHX). The half-life of PTEN extended from ~20 hrs in control cells to more than 36 hrs in SIRT7 knockdown cells (Fig. 4D and Supplementary Fig. 4h). Moreover, overexpression of SIRT7 could expedite PTEN turnover with CHX inhibition while the SIRT7-H187Y enzymatically dead mutants could not (Fig. 4E and Supplementary Fig. 4i), which implied that SIRT7 accelerated the degradation of PTEN via its deacetylase activity. Hence, we deduced that SIRT7 might regulate the instability of PTEN by regulating PTEN deacetylation. To further confirm the association of stability and acetylation level of PTEN, the turnover of PTEN mutants at K260 was examined. As a result, the half-life of PTEN proteins was remarkably extended when PTEN-K260Q was overexpressed, mimicking a constant acetylated PTEN version (Fig. 4F). Moreover, the half-life of PTEN-K260Q did not change as SIRT7 was overexpressed in HEC-1B cells (Supplementary Fig. 4j). These data strongly suggest that SIRT7 mediates PTEN deacetylation at K260 and accelerates the degradation of PTEN.
To further explore SIRT7-modulated function of PTEN in vivo, we established the conditional SIRT7 knockout mice in uterus (Supplementary Fig. 4k). The immunofluorescence staining of the cross section of mouse uterus showed that SIRT7 was knocked out in most uterus tissues due to Pgr expressing and relatively strong PTEN staining was observed in SIRT7 knocked out samples (Fig. 4G), indicating a negative correlation between SIRT7 and PTEN expression. Such correlation was also seen in IHC staining of Sirt7 CKO and WT mice. Most importantly, the acetylation level of K260-PTEN was manifested to significantly arise in Sirt7 CKO mice (Fig. 4H), highlighting that the SIRT7-mediated PTEN downregulation was dependent on the deacetylation of PTEN by SIRT7. In all, these findings present a SIRT7-PTEN modulation pattern that SIRT7 deacetylates PTEN at K260, consequently favoring PTEN degradation in vitro and in vivo.
PTEN-K260 deacetylation by SIRT7 leads to NEDD4L-mediated ubiquitination
We next sought out to explore whether proteasomes or lysosome pathways are involved in SIRT7 mediated PTEN degradation. HEC-1B cells overexpressing SIRT7 or empty vector were treated by proteasome inhibitor MG132, lysosome inhibitor chloroquine (CHQ), or DMSO respectively. Consequently, PTEN level reduced by ~40% after SIRT7 overexpression, and such effect was blocked by MG132 treatment (Fig. 5A), while lysosome inhibition only exerted faint changes on PTEN level, indicating that SIRT7 mediated PTEN degradation was mainly dependent on proteasome. We next investigated how SIRT7 affects the ubiquitination levels of PTEN. It was shown by the immunoprecipitation assays that the ubiquitination levels of PTEN were substantially elevated in cells with GFP-SIRT7 overexpression (Fig. 5B). Conversely, the ubiquitination level of PTEN dramatically declined in SIRT7 deficient HEK293FT cells, when PTEN was expressed both ectopically and endogenously (Fig. 5C and Supplementary Fig. 5a). These pieces of evidence pointed out that SIRT7 could be involved in promoting PTEN ubiquitin-proteasomes dependent degradation. Furthermore, in line with the state of SIRT7 downregulation, the ubiquitination of PTEN-K260Q mutant maintained a lower level compared to PTEN WT, and conversely, the ubiquitination level of PTEN-K260R mutant was higher than PTEN WT (Fig. 5D), indicating that the PTEN-K260 acetylation level regulated by SIRT7 was crucial for its ubiquitination.
A Immunoblot manifesting PTEN protein level and the quantitative analysis for three repeats (n = 3). SIRT7 was overexpressed in HEC-1B cells, and the cells were treated with DMSO or MG132 (10 μM, 8 h) or CHQ (20 μM, 16 h) before being collected. The error bars indicate the s.e.m. values. The P value was calculated with two tailed unpaired t test. B Ubiquitination level of PTEN with or without SIRT7 overexpression in HEK293FT cells. C Ubiquitination level of PTEN in sgSIRT7 (pool) or sgScramble HEK293FT cells. D Ubiquitination level of PTEN-WT/ K260Q/ K260R. For B–D, the HA-Ub and FLAG-EV, FLAG-PTEN-WT/K260Q/K260R were overexpressed in HEK293FT cells as indicated, and the ubiquitination level was detected after immunoprecipitation. E Mass spectrum results suggesting the E3 ligase interacting with PTEN and ranked by number of unique peptides. F PTEN protein level shown by immunoblots after NEDD4L knockdown. NEDD4L was deleted in HEC-1B cells by short hairpin RNAs. G Ubiquitination level of FLAG-PTEN after NEDD4L knockdown. HA-Ub and FLAG-PTEN were over expressed, and the ubiquitination level was detected after immunoprecipitation. H Ubiquitination level of FLAG-PTEN after NEDD4L knockdown and SIRT7-Myc overexpression. HA-Ub and FLAG-PTEN were over expressed, and the ubiquitin level was detected after immunoprecipitation. I Co-IP manifesting the interaction between endogenous NEDD4L and PTEN after Myc-SIRT7 overexpression in HEK293FT cells. J Co-IP manifesting the interaction between endogenous NEDD4L and PTEN-WT/K260Q after Myc-SIRT7 overexpression in HEK293FT cells.
We then sought out to figure out the corresponding E3 ligase in this SIRT7-PTEN axis. With Co-IP and mass spectrum, we identified several E3 ligases associated with PTEN (Fig. 5E). However, only NEDD4L and TRIM25 were negatively correlated with PTEN in endometrial cancer at the protein levels as analyzed in CPTAC database (Supplementary Fig. 5b), inferring that they might be the potential E3 ligases of PTEN in endometrial cancer. To investigate whether NEDD4L or TRIM25 is involved in the SIRT7-mediated PTEN degradation, NEDD4L and TRIM25 were knocked down respectively with short hairpin RNAs in EC cell line HEC-1B. Our results showed that PTEN level was significantly increased by NEDD4L depletion while TRIM25 knockdown only brought about a slight change (Fig. 5F and Supplementary Fig. 5c). In addition, ubiquitination assay confirmed that ubiquitination level of exogenous and endogenous PTEN both declined in NEDD4L knock down HEK293FT cells (Fig. 5G and Supplementary Fig. 5d), and SIRT7 overexpression would no longer elevate PTEN ubiquitination in NEDD4L-deficient condition (Fig. 5H and Supplementary Fig. 5e). Moreover, NEDD4L or SIRT7 overexpression could increase the ubiquitination level of PTEN-WT, however, could not change the ubiquitination of PTEN-K260Q (Supplementary Fig. 5f, g). Taken together, these results demonstrates that NEDD4L is responsible for PTEN ubiquitination, following its deacetylation by SIRT7 in endometrial cancer.
Next, we stepped deeper to investigate the regulation pattern of SIRT7 in NEDD4L mediated PTEN ubiquitination and found out that SIRT7 overexpression could enhance the interaction between PTEN and NEDD4L (Fig. 5I and Supplementary Fig. 5h). Notably, the interaction between PTEN and NEDD4L almost vanished when PTEN was mutated to PTEN-K260Q, while SIRT7 overexpression could no longer enhance the interaction between PTEN-K260Q and NEDD4L (Fig. 5J). These results, collectively, imply that SIRT7-mediated PTEN deacetylation could facilitate PTEN ubiquitination by promoting the interaction between NEDD4L and PTEN. In summary, these findings suggest that PTEN-K260 deacetylation mediated by SIRT7 promotes PTEN degradation by favoring its ubiquitination, which could be mediated by the E3 ligase NEDD4L in endometrial cancer.
PTEN-K260 deacetylation by SIRT7 promotes endometrial cancer metastasis
To further explore the biological and functional relevance of SIRT7 mediated PTEN deacetylation and degradation, we sought to decipher whether SIRT7 facilitates tumor metastasis via promoting PTEN-K260 deacetylation. To assess whether SIRT7’s role in tumor migration is PTEN related, the transwell assay was performed in ISHIKAWA cells, an EC cell line with PTEN deletion58, and it turned out that SIRT7 had minimal effect on migratory and invasive abilities of ISHIKAWA cells because of the depletion of PTEN (Supplementary Fig. 6a). Furthermore, we established PTEN depleted HEC-1B cells with two separated short hairpin RNAs and found that SIRT7 knockdown did not give impetus to the migration or invasion in HEC-1B cells with the absence of PTEN (Supplementary Fig. 6b, c), whereas there was a drastic downregulation in HEC-1B cells with adequate WT PTEN expression as we previously shown (Fig. 2D–G), which demonstrates that SIRT7 promotes endometrial cancer metastasis through a PTEN-dependent manner.
To further explore the effect of the SIRT7-mediated PTEN-K260 deacetylation during endometrial cancer metastasis, we re-expressed shRNA target sequence-modified plasmids rPTEN-WT or rPTEN-K260Q in HEC-1B PTEN knocked down cells (Supplementary Fig. 6d). With wound healing and transwell assays, we found that rPTEN-WT inhibited cell migration and invasion (Fig. 6A, B), corresponding to the previous report that PTEN modulates cell migration negatively59,60,61,62. More importantly, rPTEN-K260Q exerted an even stronger migration-inhibiting activity than rPTEN-WT, while rPTEN-K260R had a less inhibitory effect on migration and invasion compared to rPTEN-WT (Fig. 6A, B), suggesting that the K260 acetylation level of PTEN regulated by SIRT7 could modulate cell migratory ability in EC. In addition, cell migration and invasion were exacerbated when overexpressing SIRT7, which could be rescued by the expression of PTEN-WT, and the expression of PTEN- K260Q further abolished the cell migration and invasion activity (Fig. 6C, D). To further explain the SIRT7-PTEN axis in vivo, we then injected HEC-1B cells expressing rPTEN-WT/ 260Q using the spleen injection metastasis mouse model. The results showed that cells with rPTEN-WT expressing developed less liver metastasis tumors in mice than PTEN deficient HEC-1B cells. Meanwhile, the metastasis process was further blocked in mice that were injected with HEC-1B rPTEN-K260Q cells (Fig. 6E). Moreover, in uterus orthotopic injection model, HEC-1B cells expressing empty vector, rPTEN-WT, or rPTEN-260Q were injected into the uterus lumen of nude mice. It was manifested that all the mice in the EV group develop metastatic nodules in the liver after injection, however, only four out of six mice injected with HEC-1B cells expressing rPTEN-WT afflicted with liver metastasis. More importantly, only one out of six mice injected with HEC-1B cells expressing rPTEN-K260Q mutant emerged disseminated masses on liver, and the tumor burden of K260Q group significantly decreased as well, compared to rPTEN-WT group (Fig. 6F), all inferring that the high acetylation status of PTEN-K260 could exhibit a more metastasis inhibitory role than WT-PTEN both in vitro and in vivo.
A Wound healing assay in HEC-1B-rescue cells. For HEC-1B-rescue cells, PTEN was knocked down and empty vector, rPTEN-WT, rPTEN-K260Q or rPTEN-K260R was expressed. Relative area of migration was measured and normalized to the ‘EV’ group, n = 6, cell cultures from three independent experiments, scale bar = 500 μm. B Transwell and invasion assay in HEC-1B-rescue cells. Relative cell number was quantified, n = 8, cell cultures from four independent experiments, scale bar = 200 μm. C Wound healing assay in HEC-1B cells. SIRT7 was overexpressed and then PTEN-WT, PTEN-K260Q or empty vector was then expressed, n = 6, cell cultures from three independent experiments, scale bar = 500 μm. D Transwell and invasion assay in HEC-1B cells. SIRT7 was overexpressed and then PTEN-WT, PTEN-K260Q or empty vector was then expressed, n = 8, cell cultures from three independent experiments, scale bar = 200 μm. E Representative images of the mice liver, spleen, and H&E-stained liver after the injection of HEC-1B-rescue cells into the spleen. Tumor burden was calculated, EV group n = 12, rPTEN-K260Q/rPTEN-K260R group n = 10, scale bar = 100 μm, metastasis nodules marked with asterisks. F Representative images of the mice liver, uterus, and H&E-stained liver, n = 6, scale bar = 100 μm. The incidence of liver metastasis nodules was shown and tumor burden was calculated. G AcK260-PTEN level analysis of human EC tissue by IHC. Primary tumor and paired metastasis tissue (in ovary/fallopian, n = 13) or metastatic lymph nodes (n = 15) were stained with AcK260-PTEN and quantified by IHC score. Scale bar = 50 μm H AcK260-PTEN level analysis of human EC tissue by IHC. Tumor (n = 21) and paired para-tumor tissue (n = 18) were stained with AcK260-PTEN, and quantified by IHC score. Scale bar = 50 μm. The P value was calculated with two tailed unpaired t test for (A–F, H), and was calculated with two tailed paired t test for (G). I Kaplan–Meier overall survival analysis of EC patients with or without PTEN mutation, data from the TCGA, n = 175 for PTEN-WT (SIRT7 high = 117; SIRT7 low = 58), n = 332 for PTEN-mut (SIRT7 high = 222; SIRT7 low = 110). Cut-off = 0.33. Data are presented as mean values ± s.d. for (A–D), and ± s.e.m. for (E–H).
Next, we stepped to explore how NEDD4L was involved in the SIRT7-PTEN regulated cell migratory function. It was shown in transwell assay that NEDD4L deficiency cast a negative influence on cell migration and invasion in HEC-1B cells, however such effect was abolished in PTEN deficient cells (Supplementary Fig. 7a), implying NEDD4L could promote EC cell migration in a PTEN dependent manner. SIRT7 overexpression could promote cell migration and invasion in control cells, detected by transwell assay, however, in cells with NEDD4L depletion, such significant change was not observed. Moreover, further knockdown of PTEN on this basis could once again enhance the cells’ migration and invasion abilities, indicating SIRT7’s role in promoting cell migration is PTEN and NEDD4L dependent. (Supplementary Fig. 7b). Furthermore, in transwell assays, we observed that NEDD4L overexpression could lead to an increase of cell migration and invasion with PTEN-K260 in a low acetylation status (mimicked by K260R), while could no longer cause significant alterations to cell migration while PTEN-K260 is in a high acetylation level (mimicked by KQ mutant) (Supplementary Fig. 7c), emphasizing that deacetylation of PTEN-K260 regulated by SIRT7 is crucial to NEDD4L involved cell migration regulation process.
Furthermore, to explore whether the SIRT7-mediated PTEN deacetylation correlated with EC progression in patients, we detected PTEN-K260 acetylation level with the AcK260-PTEN antibody in human tissues. The IHC staining showed that AcK260-PTEN was remarkably decreased in metastatic EC tumor tissues in ovary, fallopian tube and lymph node compared to primary tumor tissues (Fig. 6G) and was also diminished in tumor tissues compared to para-tumor tissues (Fig. 6H). These results were corresponding to the findings above that SIRT7 expression was elevated in EC tumor and metastatic tumor tissues (Fig. 1). In addition, the PTEN staining also showed a significant decrease in metastatic tumor tissues compared to primary tumor tissues, and showed reduced expression in tumor tissues compared to para-tumor tissues (Supplementary Fig. 7d, e), indicating a negative correlation between SIRT7 and PTEN, which could be due to the deacetylation of PTEN-K260. Collectively, these results suggest that SIRT7-mediated PTEN-K260 deacetylation is functional in cell migration and invasion and the acetylation level at K260 of PTEN could serve as a prognosis marker for endometrial cancer metastasis.
As mentioned before, we have shown that SIRT7’s role in promoting tumor migration is PTEN-dependent. Considering PTEN is the most frequent gene mutated in EC63, we next decided to examine whether SIRT7 expression has different influence on PTEN mutated or PTEN wild type EC patients. It is demonstrated in TCGA database that, SIRT7 overexpression has been remarkably related to a poorer overall survival condition of PTEN gene wild type EC patients (p = 0.012), however, in patients with PTEN gene mutated, no significant relevance was observed (p = 0.77) (Fig. 6H). Though study showed that PTEN mutation took place in about 65.5% of EC samples, these patients were reported to have relatively favorable prognosis64. Correspondingly, TCGA data manifested that EC patients with wild type PTEN expression have a worse overall survival compared to PTEN mutated patients (Supplementary Fig. 7f). Thus, our findings of the important oncogenic role of SIRT7 in EC may emerge SIRT7 as a potential target for the treatment of PTEN non-mutated EC patients, which, especially, provides insights to improve the prognosis of EC patients without PTEN mutation.
Estrogen acts as the upstream regulator of SIRT7-PTEN axis
As shown above, SIRT7 acts as an oncogene in EC metastasis process, and relies on an intact PTEN context. Next, we aimed to explore what is the pathological regulator for this SIRT7-PTEN axis. It is well known that endometrial cancer is a hormone dependent disease and that estrogen exposure in the long term without a progesterone antagonism is one of the most important risk factors of endometrial cancer65,66,67. We then wondered whether estrogen is involved in the upstream switch of SIRT7-mediated PTEN deacetylation-ubiquitination process. As shown in Fig. 7A, we observed that along with 17β-estradiol stimulation, PTEN protein level was declined in a concentration dependent manner, without any significant change of SIRT7 levels in HEC-1B cells (Fig. 7A). However, in SIRT7 deficient HEC-1B cells, the decline of PTEN level under estrogen stimulation vanished (Fig. 7B), which suggested that estrogen could influence PTEN expression in a SIRT7-dependent fashion. To investigate the molecular mechanisms by which estrogen stimulation induces PTEN decaying by SIRT7, we explored whether the interplay between SIRT7 and PTEN is affected. Astonishingly, Proximity Ligation Assay (PLA) assay showed a significant increase of SIRT7-PTEN association when HEC-1B cells were treated with 17β-estradiol (Fig. 7C). Co-immunoprecipitation assay further confirmed that 17β-estradiol could reinforce the interaction between SIRT7 and PTEN in a dose-dependent manner, both in HEC-1B (Fig. 7D) and HEK293FT cells (Fig. 7E). These suggest that estrogen stimulation could promote the association between SIRT7 and PTEN. However, we noted that overexpression of estrogen-receptor ESR1 did not change SIRT7-PTEN interaction (Fig. 7F). Next, we stepped to investigate the deacetylation process under estrogen stimulation and found out that 17β-estradiol stimulation abated the acetylation level of PTEN to a lower level when SIRT7 was overexpressed (Fig. 7G). Notably, in HEC-1B cells, estrogen stimulation could decrease the K260 acetylation level of endogenous PTEN, detected by the AcK260-PTEN antibody (Fig. 7H), suggesting that estrogen stimulation promotes the deacetylation of PTEN regulated by SIRT7. Moreover, 17β-estradiol treatment also increased PTEN-NEDD4L interaction, as well as the ubiquitination level of PTEN (Fig. 7I, J). Altogether, these clues indicate that estrogen stimulation could promote the SIRT7-PTEN axis in endometrial cancer.
A Immunoblots showing PTEN level after the cells being treated with 17 beta-Estradiol (E2) for 48 hours with indicated concentration in HEC-1B cells. B Immunoblots showing PTEN level after estrogen treatment for 48 hours with indicated concentration in HEC-1B cells with or without SIRT7 knockdown. C Representative pictures and quantitative analysis of PLA assay demonstrating the interaction of endogenous PTEN and SIRT7 protein with or without E2 stimulation. HEC-1B cells were treated with E2 (100 nM, 48 h) and MG132 (10 μM, 6 h) as indicated. 100 cells were quantified. Scale bar = 100 μm. D Co-IP assay manifesting the interaction between endogenous SIRT7 and PTEN in HEC-1B cells with E2 stimulation for 48 hours with indicated concentration. E Co-IP assay manifesting the interaction between SIRT7 and PTEN in HEK293FT with E2 stimulation for 48 hours with indicated concentration. F Co-IP assay manifesting the interaction between SIRT7 and PTEN after ESR1 overexpression in HEK293FT cells. G The acetylation level of PTEN in HEK293FT cells with stimulation of E2 (50 nM, 48 h) with or without SIRT7 overexpression. H The AcK260 level of endogenous PTEN in HEC-1B cells with or without stimulation of E2 (100 nM, 48 h) detected by AcK260-PTEN antibody. I Co-IP assay manifesting the interaction between endogenous NEDD4L and PTEN in HEK293FT with SIRT7 overexpression and E2 stimulation as indicated in HEK293FT cells. J The ubiquitination level of PTEN in HEK293FT cells with stimulation of E2 of indicated concentration. Cells were treated with MG132 (10 μm) for 6 hours before lysis. AcK260-PTEN (K) and SIRT7 (L) analysis of human uterus endometrium tissue during menstrual cycle by IHC. Endometrium in secretory phase (n = 12) and proliferative phase (n = 17) were stained with AcK260-PTEN and SIRT7 and quantified by IHC score. Scale bar = 50 μm. M Schematic diagram showing the mechanisms that SIRT7 upregulation facilitates EC progression through PTEN-K260 deacetylation and subsequent ubiquitination and degradation under long-term stimulation of estrogen. Created in BioRender. Hu, Z. (2025) https://BioRender.com/d55g597. The P value was calculated with two tailed unpaired t test for (C, K, L). Data are presented as mean values ± s.e.m.
Notably, estrogen has been explicated to periodically fluctuate during female menstrual cycle, causing several cyclic biological changes, including the renovation and thickness change of endometrium68 and studies have pointed out that the estrogen takes part in pathways facilitating the growing of the endometrium69,70. Given that PTEN expression also alters during the menstrual71, we then ask if the estrogen-SIRT7-PTEN axis is involved in the renovation in the menstrual cycle. We collected the endometrium samples of patients with no hyperplasia or cancer, who were in the proliferative and secretory period of menstrual. As a result, IHC staining revealed that during the secretory phase, the acetylation level of PTEN-K260 in the endometrium was significantly higher compared to the proliferative phase, corresponding to the dominant status of estrogen in the proliferative phase, and there was no significant change of SIRT7 staining (Fig. 7K, L). These provided clues that the AcK260-PTEN could be fluctuated during the menstrual cycle, responding to the estrogen regulation.
In summary, these findings indicate that estrogen could act as the upstream regulator of SIRT7-PTEN axis during menstrual cycle in physiological state. In endometrial cancer, when SIRT7 is hyper-expressed, prolonged estrogen exposure leads to the exacerbation of PTEN-K260 deacetylation by SIRT7. The significant low acetylation level at K260 results in extensive ubiquitination of PTEN mediated by NEDD4L, subsequently leading to PTEN degradation. Low PTEN protein level prompt the migratory and invasive potential of endometrial cancer and result in poor prognosis of the tumor (Fig. 7M).
Discussion
Cellular protein level of tumor suppressor PTEN is tightly correlated with the progression of endometrial cancer. Current studies have pointed out that PTEN protein loss is related to its dysregulation of post-translational modification, such as ubiquitination and phosphorylation72,73,74,75. In this study, we demonstrate deacetylation of PTEN at K260 is responsible for promoting its subsequent ubiquitination and degradation. In endometrial cancer, we identify SIRT7, one of the mammalians sirtuins, as the major regulator of such deacetylation-ubiquitination axis of PTEN. Anomalous SIRT7 overexpression is associated with PTEN loss, tumor migration and invasion in endometrial cancer.
Increased expression and a potential oncogenic role of SIRT7 in tumor is reported in various cancer types. For instance, SIRT7 is overexpressed in prostate cancer and SIRT7 depletion inhibits cell proliferation by the androgen receptor signal pathway37; SIRT7 exhibits higher expression and is associated with a poor prognosis, promoting tumor development in hepatocellular carcinoma76,77,78; SIRT7 upregulation exhibits an oncogenic property and could serve as a prognostic factor in colorectal cancer39; Increased SIRT7 expression promotes thyroid tumorigenesis through activation of AKT and p70S6K138. On the other hand, some studies have also manifested that SIRT7 achieves anti-tumor activities under other contexts. SIRT7 hyperphosphorylation inhibits tumor progression by preventing K63-linked AKT polyubiquitination and activation in breast cancer79, and SIRT7 is also reported to suppress breast cancer migration via TGF-β signaling32. In endometrial cancer, data from TCGA indicates that SIRT7 is upregulated in EC and SIRT7 high expression is correlated to poor prognosis. Here, in our study, we have shown that SIRT7 is overexpressed in endometrial cancer both in human tissues and two established transgenic EC mouse models, presenting prominent evidence of the potential tumor-facilitating role of SIRT7 in endometrial cancer.
Mutant PTEN is an oncogenic driver for EC initiation. However, as to PTEN wild type and intact genetic context, people have little idea about how this subtype of EC tumor develops and what is the specific context-dependent role of PTEN. Post-translational modifications (PTMs) of PTEN are critical for controlling its activity, abundance, and subcellular localizations, so PTEN modulating pattern regulated by PTMs presents great complexity and has received substantial concern in recent years. For example, ubiquitin ligase MARCH8 promotes the hepatocellular carcinoma progression through modulating PTEN ubiquitination and degradation80. Phosphorylation of PTEN on tyrosine 240 by FGFR2 could promote DNA repair in glioblastoma, and is tightly associated with the therapeutic resistance to ionizing radiation81. Ge et al.82 identified FBXO22 involved in selective nuclear PTEN ubiquitination and degradation in colon cancer, and loss of nuclear PTEN is related to comparatively more advanced carcinoma82,83,84. Here, we have identified the NAD+-dependent deacetylase SIRT7 as an upstream negative modulator of PTEN in EC and SIRT7 mediated PTEN deacetylation leads to PTEN abatement and tumor progression. Our work provides a missing piece of the function of post-translational modifications of PTEN and uncovers the critical role of SIRT7 in PTEN regulation, especially in endometrial cancer with PTEN wild type context. The conception of “tumor suppressor reactivation” of PTEN was proposed in prostate cancer years ago and it is suggested that PTEN reactivation by targeting its critical modifying enzyme could be sufficient to mitigate PTEN down-regulated tumor process85. It was also reported that down-regulation of PTEN expression at protein level is tightly associated with poor progression in advanced EC18. Thus, our findings provided an insight of treatment for EC patients that targeting SIRT7 might be able to restore PTEN expression and restrain the EC advancement.
Previous studies have suggested that acetylation level of PTEN is responsible of adjusting its affinity to proteins and membrane, as well as regulating its enzymatic function directly. In detail, acetylation at K125/K128 of PTEN by PCAF negatively regulates PTEN lipid phosphatase activity86. Deacetylation at K163 by HDAC6 is relevant to PTEN membrane affinity87. Acetylation level on K402 affects PTEN interaction with PDZ-domain-containing proteins, which was reported to be regulated mainly by CBP and SIRT188. Our study identified SIRT7 as a deacetylase of PTEN, deacetylating PTEN at lysine 260. We also showed the acetylation level regulated by SIRT7 was tightly associated with the ubiquitination and stability of PTEN. Lysine 260 locates in the C2 domain of PTEN, which is the functional domain responsible for the association with membrane, lipid vehicles, and C terminal tail89,90,91. Nguyen et al. demonstrated that K to A mutations at lysine in the CBR3 loop in C2 domain would decrease PTEN membrane localization in Dictyostelium cells92. Nevertheless, the role of the acetylation level of K260 remains vague. In our study, we have shown that PTEN with a higher acetylation level at K260, mimicked by K260Q, is less ubiquitinated and remains more stable. Meanwhile, further studies are needed to explore whether other lysine sites in this region are acetylated and what are the corresponding functions. In addition, whether the deacetylation of PTEN-K260 will influence its conformation is also uninvestigated, considering that the intramolecular interplay between C2 domain and C terminal tail of PTEN is associated with its stability and accessibility to ubiquitination93.
PTEN inhibits tumor migration in AKT-dependent or independent pathways. It is well-established that PTEN could limit cancer metastasis by PI3K-AKT pathway61,94, as well as regulate cell polarity and chemotaxis by sustaining a PIP2-PIP3 gradient95,96. As a dual phosphatase, PTEN could also alter cell adhesion and focal adhesion process by targeting FAK59,97. Recent studies have addressed the novel gatekeeper roles of PTEN in tumor metastasis. Jiang et al.98 have reported that loss of PTEN enhances cholangiocarcinoma cell migration by disrupting TFEB-regulated lysosome formation. Chang et al.99 illustrated that PTEN knockdown could favor the invasiveness of pancreatic neuroendocrine tumors via DUSP19-mediated VEGFR3 dephosphorylation. In addition, another recent work has pointed out that PTEN loss expedite cell migration through AMPK activity100. In our study, we have established that SIRT7-regulated PTEN downregulation leads to more aggressive EC migration both in EC cells and mouse models. However, our experiment showed that phosphorylation level of AKT at Thr308 and Ser473 are not significantly altered by SIRT7 knockdown (Supplementary Fig. 4l). We supposed that it is due to SIRT7’s sophisticated role in PI3K-AKT signaling, which could explain that cell proliferation is not distinctly inhibited, under SIRT7 mediated PTEN downregulation condition, in HEC-1B cells (Supplementary Fig. 2b–g). It has been reported that SIRT7 favors AKT dephosphorylation by targeting FKBP51 and depletion of SIRT7 significantly increases AKT activity in mice101. On the other hand, SIRT7 promotes AKT phosphorylation and activation in a DBC1/SIRT1 dependent manner in thyroid cancer38. Nevertheless, our results, collectively, hint that SIRT7-PTEN axis presents the major effect on tumor migration in EC with an AKT-independent manner.
Continuous exposure to estrogen and dysregulation of the hormone pathways is one of the major risk factors of endometrial cancer102. It is well biologically and etiologically demonstrated that rising and falling of estrogen levels across the menstrual cycle is one of the key factor dominating series of alterations in menstrual cycle, including the proliferation, shedding and remodeling of endometrium68. The upregulation of estrogen helps renovate the functional layer of the endometrium. Our data have suggested that estrogen stimulation augments the interaction between SIRT7 and PTEN, and downregulates PTEN expression in a SIRT7-dependent manner, which implies that estrogen could be the upstream switch of SIRT7-PTEN axis. Intriguingly, IHC results unraveled that PTEN-K260 level was altered along menstrual cycles, indicating a biological function of SIRT7-mediated PTEN deacetylation during menstrual cycles. Though to further ascertain whether estrogen-SIRT7-PTEN axis exert any functions in menstrual cycle, more examinations focused on it are urged.
In summary, our study demonstrates that SIRT7 mediates deacetylation of PTEN and diminishes its expression, unveiling a mechanism of SIRT7 involved in PTEN deacetylation-ubiquitination pathway. We show that SIRT7 facilitates the EC metastasis by promoting PTEN degradation in a deacetylation-dependent fashion, which provides a insight to target SIRT7, subsequently restore PTEN level, as potential therapeutic strategy, for patients with advanced endometrial cancer.
Methods
Ethical statement
All animal studies were conducted under the NIH Guide for the Care and Use of Laboratory Animals and approved by Tongji University School of Medicine Animal Care and Use Committee (Approval Number: TJBG02524101). The immunohistochemistry and western blot studies of human EC tissue were approved by Ethics Committee of Shanghai First Maternity and Infant Hospital (Approval Number: KS22356). Clinical samples were collected from Shanghai First Maternity and Infant Hospital, with informed consent obtained from each participant.
Cell lines and cell culture
The human EC cell line HEC-1B (Cat: TCHu115) and human embryonic kidney cell line HEK293FT (Cat: SCSP-5212) were purchased from Chinese National Collection of Authenticated Cell Cultures. Human EC cell lines Ishikawa (Cat: FH0305) and KLE (Cat: FH0304) were purchased from Shanghai Fuheng Biotechnology and certificated by STR analysis. ISK, HEC-1B cells were cultured in DMEM/F12 medium (BasalMedia, #L310KJ). KLE and HEK293FT cells were cultured in DMEM medium (BasalMedia, #L110KJ). The medium was supplemented with 10% FBS (Gibco, #16000-044) and 1% penicillin/streptomycin (Gibco, #15140-122). All cells were cultured in a 37 °C incubator with 5% CO2 and mycoplasma test was regularly conducted.
Transgenic mouse models
The Sirt7f/f mouse was a gift from professor Baohua Liu of Shenzhen University Health Science Center, Shenzhen, China. The Lkb1f/f (C57BL/6J-Stk11em1(flox)Cya) mouse was purchased from Cyagen, Guangzhou, China. The Ptenf/f mouse (B6.129S4-Ptentm1Hwu/J, #006440) and Pgr-cre (B6.129S(Cg)-Pgrtm1.1(cre)Shah/AndJ, #017915) mouse were purchased from JAX lab. Sirt7f/f, Lkb1 f/f or Ptenf/f mice were crossed with Pgr-cre mice to get the uterus conditional KO mice of indicated genes and the mice were sacrificed for analysis at indicated birth weeks. All mice were housed under specific-pathogen-free conditions and with a 12-hour light/12-hour dark cycle. The ambient temperature was kept at 22 °C ± 2 °C with a 45% humidity. All mice were fed with standard Irradiated Diet (Jiangsu Xietong Company, #XTI01ZJ). Animal studies were conducted under the NIH Guide for the Care and Use of Laboratory Animals. In all studies, the tumor burden did not exceed 10% of body weight and the maximal tumor size was less than 2 cm³, as permitted by Tongji University School of Medicine Animal Care and Use Committee.
Plasmids, antibodies, and reagents
The information of antibodies is provided in Supplementary Table 1.
The SIRT7 plasmids were gifts from Professor Wei-Guo Zhu of Shenzhen University Health Science Center; The HA-PTEN plasmids were purchased from Addgene (Cat. #78776) and the CDS was cloned into a pEGFP vector to get the GFP-HA-PTEN plasmids. The FLAG-PTEN plasmids in a pQCXIN vector were a gift from Professor Shao-Ming Shen of Shanghai Jiao Tong University School of Medicine; The mutant and WT PTEN plasmids were generated based on a PLVX-IRES-Puro backbone and mutants were generated by the site-specific mutagenesis method (Cat. #FM111-02, Transgen) following sequencing confirmation. The GST-tagged plasmids were cloned into a pGEX4T1 vector. The FLIG-NEDD4L plasmids were provided by professor Jian-Wei Zhou from Nanjing Medical University.
shRNA expressing plasmids was constructed in a pLKO.1 vector and sequences are as follows:
shSIRT7-1: CCCTGAAGCTACATGGGAA,
shSIRT7-2: AGCCATTTGTCCTTGAGGAA,
shPTEN-1: CAGCATACACAAATTACAAAAGT,
shPTEN-2: TAGAGTTCTTCCACAAACAGAAC,
shNEDD4L-1: TGAGGATCATTTGTCCTAC,
shNEDD4L-2: GCTAGACTGTGGATTGAGT,
shTRIM25-1: ACAACAAGAATACACGGAAAT,
shTRIM25-2: GTGCCCGATTCCTCTTAGAGA.
The information of reagents used is as follows:
Cycloheximide (Biovision, Cat. #1041), MG132 (MCE. Cat. #HY-13259), Chloroquine (MCE, Cat. #17589 A), β-Estradiol (Sigma, Cat. #E2758).
Cell transfection and RNA interference
HEK293FT cells were transfected with plasmids using PEI (Polysciences, USA). For endometrial cancer cells, the transient transfection of plasmids and siRNA was conducted with the EN130 program in a Lonza 4D instrument (Lonza, Cologne, Germany). Lentivirus infection was used to obtain stable cell line. 6 μg lentiviral constructions, 4 μg pSPAX2, 2 μg pMD2G were co-transfected into HEK293FT cells. 72 hours after transfection, the supernatants were collected, and filtered through 0.45μm membrane (Millipore, USA) for cell infection. Puromycin in appropriate concentration was used for cell selection.
siRNAs sequences used are as follows:
SIRT7 siRNA 1: GGGAGTACGTGCGGGTGTT;
SIRT7 siRNA 2: CCCTGAAGCTACATGGGAA.
CRISPR-Cas9-based gene editing
LentiCRISPRv2 vectors were used in CRISPR-Cas9-based gene editing. The plasmids were constructed as described103,104.
gRNA sequences used are as follows:
sgSIRT7-1: GAGCCGCTCCGAGCGCAAAG,
sgSIRT7-2: GCGTCTATCCCAGACTACCG,
sgSIRT7-3: CGAGAGCGCGGACCTGGTAA.
RNA extraction and real-time quantitative PCR
Total RNA was extracted with kit (TIANGEN, #DP419) according to the instructions. mRNA was reverse transcribed into cDNA with Transgen EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen, #AE311-03). RT-qPCR was performed with SYBR Green qPCR Mix (Roche, #04913914001). The primers used are as follows:
PTEN-F: TGGATTCGACTTAGACTTGACCT,
PTEN-R: GGTGGGTTATGGTCTTCAAAAGG,
SIRT7-F: ATGAGCAGAAGCTGGTGC,
SIRT7-R: CTGTCTGGTGTCTGTGGA.
RNA sequencing and GSEA analysis
RNA Seq was performed by Majorbio, Shanghai, China. Significant differentially expressed genes were analyzed by DEseq2 according with a significance level P adjust <0.05, |log2FC | >=1. Data from RNA-Sequencing were subjected to GSEA. GSEA was performed by the GSEA v4.2.3 program comparing shRFP vs shSIRT7 HEC-1B cells. The gene sets were downloaded from MSigDB.
TCGA data analysis
The transcriptome, simple nucleotide variation and clinical data of TCGA-UCEC was downloaded from the TCGA websites. Transcript per million was used to present SIRT7 expression. For the survival rate of PTEN mutant/wild-type UCEC patients (Fig. 6), samples were classified based on PTEN mutation status, and then were classified as either SIRT7 high or SIRT7 low based on SIRT7 expression level using a 33% cutoff. Kaplan-Meier overall survival analysis was conducted by survival (v 3.5.8) and survminer (v 0.4.9) packages in R studio. The P value was calculated using the two-sided log-rank test.
The survival rate of TCGA-UCEC dataset in Supplementary Fig. 1c, d was analyzed by GEPIA 2.0105 (http://gepia2.cancer-pku.cn/#survival) with a 50% cutoff. P value was calculated by log-rank method. Overall survival rate was analyzed.
Mass spectrum
The mass spectrum analysis was conducted at the School of Life Sciences, Fudan University, Shanghai, China. For sample preparation, FLAG-PTEN plasmids were overexpressed in HEK293FT cells. No control sample was analyzed. One protein sample (number of replicates =1) was prepared after enrichment with anti-FLAG antibodies and subjected to SDS-PAGE. Gel bands of interest were excised and subjected to 10 mM dithiothreitol (DTT) for 30 minutes at 56 °C, followed by alkylation with 50 mM iodoacetamide (IAA) for 45 minutes in the dark at room temperature. Then, the gel lane was excised and treated with 5 ng/μl of sequencing-grade modified trypsin (Promega) overnight at 37 °C for protein digestion. The digestion was terminated by adding 10% formic acid (FA). The supernatants were collected, and the peptides were extracted using a solution of 30% acetonitrile (ACN). The resulting peptide mixtures were dried and reconstituted in 0.1% formic acid for mass spectrometry analysis.
For LC-MS analysis, a nanoflow EASYnLC 1200 system (Thermo Fisher Scientific, Odense, Denmark) coupled with an Orbitrap Exploris480 mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) was employed. A one-column system was adopted for all analyses. Samples were analyzed on a home-made C18 analytical column (75 µm i.d. × 25 cm, ReproSil-Pur 120 C18-AQ, 1.9 µm (Dr. Maisch GmbH, Germany))106. The mobile phases consisted of Solution A (0.1% formic acid) and Solution B (0.1% formic acid in 80%ACN).The derivatized peptides were eluted using the following gradients: 5–8% B in 2 min, 8–44% B in 38 min, 44–70% B in 8 min, 70–100% B in 2 min, 100% B for 10 min, at a flow rate of 200 nL min. High-field asymmetric-waveform ion mobility spectrometry (FAIMS) was enabled during data acquisition with compensation voltages set as −40 and −60 V.MS1 data were collected in the Orbitrap (60,000 resolution). Charge states between 2 and 7 were required for MS2 analysis, and a 45 s dynamic exclusion window was used. Cycle time was set at 1 s. MS2 scans were performed in the Orbitrap with HCD fragmentation (isolation window 1.6; 15,000 resolutions; NCE 30%).
The data were processed with UniProt human protein database (22,045 entries, download in 02/02/2020) and the using Protein Discoverer (version 2.4, thermo Fisher Scientific) with Mascot (version 2.7.0, Matrix Science)107. The mass tolerances were 10 ppm for precursor and fragment Mass Tolerance 0.05 Da. Up to two missed cleavages were allowed. Minimum number of unique peptides for protein identification was 1. The search engine set cysteine carbamidomethylation as a fixed modification, and set N-acetylation in the proteins and oxidation of methionine as variable modifications.
Cell viability and colony formation assay
Cells were seeded with indicated number in 6-well-plates and cell number was calculated every 24 hours. For colony formation assay, 1000 cells were seeded, incubated for 2 weeks, fixed with paraformaldehyde then stained with crystal violet. Colonies with at least 50 cells were counted.
Wound healing assay
Endometrial cancer cells were seeded in 6-well plates at approximately 90% confluence. A wound was created at the well center using a pipette tip, and any debris was removed with PBS washes. Cells were then incubated in serum-free medium for the specified time period before imaging. Image J software was used to measure migration area. Views from three independent experiment were quantified.
Migration and invasion assay
For migration assays, 50,000 cells were seeded in the upper chamber of a transwell plate (Cat. #3422, Corning, USA) with serum-free medium. For invasion assays, 250,000 cells were seeded after pre-coating the membrane with 1:10 diluted Matrigel (Cat. #356234, Corning, USA) and allowing it to solidify. The lower chamber was filled with complete medium containing 10% FBS. After the indicated incubation period, the membrane was washed twice with PBS and any remaining cells on the upper surface were removed with a cotton swab. Cells were then fixed with paraformaldehyde and stained using crystal violet. Views from three/four independent experiment were quantified. Image J software was used to quantify cell number.
Luciferase assay
For promoter-firefly luciferase plasmid construction, a 2 kb DNA segment upstream of transcription start site of PTEN was cloned into the pGL3-Basic vector and the genomic DNA template was extracted from HEC-1B cells. HEC-1B cells were transfected with 2 μg PTEN promoter-firefly luciferase plasmids along with 3 ng Renilla luciferase plasmids for normalization of the transfection efficiency. For overexpressing situation, empty vectors or FLAG-SIRT7 plasmids were co-transfected. Cells were collected and lysed 72 hours post transfection. Luciferase activity was measured with a Dual-Luciferase Reporter System (Cat. #E1910, Promega, USA) and a GloMax luminometer (Cat. #E5311, Promega, USA).
GST pull-down
GST pull down assays were conducted as previously described108. GST and GST-fusion proteins were induced in Escherichia coli using 0.1 mM isopropyl-β-D-thiogalactopyranoside and incubated overnight at 16 °C. These proteins were then purified with glutathione Sepharose 4B beads (GE Healthcare, USA). The beads and GST proteins were combined in reaction buffer [10 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 100 mM NaCl] and co-incubated at 4 °C with either His-tagged proteins isolated from bacteria or Flag-tagged proteins extracted from cell lysates. Following overnight incubation, the beads were washed three times using the specified buffer and subsequently analyzed by western blotting.
Immunoprecipitation
Cells transfected with indicated plasmids were collected and lysed in IP buffer [20 mM HEPES pH = 8.0, 0.2 mM EDTA pH = 8.0, 5% glycerol, 150 mM NaCl, 1% NP40 and protease inhibitor] on ice for 30 min, and then the cell lysates were sonicated 10 times on ice at 35% amplitude, 1 s per time, centrifuged at 18000 g (13500 rpm) for 15 min at 4 °C. The supernatant was afterwards incubated with indicated antibodies or IgG overnight at 4 °C on a rotation. Same amounts protein A/G beads (Cat. #A10001M, Abmart, China) were added and the incubation was continued for another 2 hours. The beads were washed with IP buffer for 3 times (centrifuged at 100 g) before being boiled and subjected to western blotting.
In vitro deacetylation assay
In vitro deacetylation assay was performed as described31. Briefly, HEK293FT cells transfected with HA-PTEN or FLAG-SIRT7, respectively, were collected and lysed with IP buffer. For in vitro deacetylation reaction, HA-PTEN or FLAG-SIRT7 proteins were enriched from the cell lysates by antibodies and protein A/G. The protein components were incubated in the reaction buffer [100 mM KCl, 20 mM Tris-HCl (pH 7.9), 5 mM MgCl2, 0.2 mM EDTA, 10% glycerol, 0.5 mM DTT] at 30 °C for 1 h in the presence or absence of 5 mM NAD+. The acetylation of PTEN was analyzed by western blotting.
In situ Proximity Ligation Assay assay
HEC-1B cells were seeded on confocal dishes and treated for estrogen (100 nM, 48 h) and MG132(10 μL, 6 h) as indicated. Cells were washed with PBS and followed by fixation with 4% paraformaldehyde (PFA) for 15 minutes. Then, HEC-1B cells were washed with PBS twice and underwent a subsequent permeabilization with 0.5% Triton X-100 for 15 minutes and were then blocked using 3% BSA for 1 hour at room temperature. Subsequently, cells were co-stained with anti-PTEN (mouse) and anti-SIRT7 (rabbit) primary antibodies. Proximity ligation assay (PLA) staining was conducted utilizing the Duolink In Situ Red Starter Kit (mouse/rabbit) in strict accordance with the instructions provided. In short, cells were stained with Duolink In Situ PLA Probe Anti-Rabbit PLUS and Duolink In Situ PLA Probe Anti-Mouse MINUS for 1 hour at 37 °C. Following washed by wash buffer A for 2 times, samples were treated with ligation solution added with ligase for 30 minutes at 37 °C for ligation and followed by wash. Then, the amplification polymerase solution was applied for 100 minutes at 37 °C. At last, the dishes were sealed with Duolink In Situ Mounting Medium containing DAPI. Fluorescent images were captured and PLA signals were quantified using ImageJ software.
Xenograft and metastasis mouse model
Five-week-old female BALB/c Nude mice were divided into three group randomly for both xenograft assay and spleen injection. HEC-1B cells were injected subcutaneously at day 0 (2\(\times\)106 cells in 100 μl PBS). Tumor volume was recorded every two or three days by measuring diameters, and the volume was calculated as volume = width2 × length/2.
The mouse liver metastasis model was established as previously described53. In brief, the female BALB/c Nude mice at 6-week post birth and similar body weight were grouped randomly and anesthetized by Avertin (0.1 ml/10 g) before injection. A superficial incision about 1 centimeter was made at the middle left of abdomen to expose the spleen. HEC-1B cells were injected gently into the splenic capsule at the middle of the spleen (2\(\times\)105 cells in 50 μl PBS), and after that, the injection sites were pressed for 30 s to avoid the leakage and intraperitoneal dissemination. After the incision was closed, the mice were warmed on a warming plate with close attention until they were awake. The mice were sacrificed 8 weeks post inoculation for measurements of the tumor nodules on the liver.
Orthotopic injection mouse model
Female nude mice in 8 weeks old were applied for orthotopic injection. The orthotopic injection was conducted as previously described55,56 with some modifications. The nude mice were divided randomly into different groups and anesthetized by Avertin (0.1 ml/10 g) before injection. a 0.5–1 cm incision was made in the lower abdomen for exposure of the uterine horn. The distal portion of the uterine horn was identified and pulled out. HEC-1B cells (2\(\times\)106 cells in 25 μl PBS) were injected into the lumen of uterine horn. The uterine was carefully returned to the abdomen and the injection site was closely monitored, ensuring no visible spillage taken place and then the incision was closed with staples. Mice were monitored every day and were sacrificed at 4 weeks after injection or they exhibited signs of severe distress or morbidity, including significant weight loss (>20% of initial body weight), severe lethargy, hunched posture, restricted mobility, or severe ascites.
Immunohistochemistry
This study was approved by Ethics Committee of Shanghai First Maternity and Infant Hospital. Clinical samples were collected from Shanghai First Maternity and Infant Hospital, with informed consent obtained from each participant. None of the patients had received radiotherapy, endocrine therapy, or chemotherapy before the surgery. The IHC staining were performed using methods described in previous study109. Briefly, tissue slides were deparaffinized, rehydrated, and treated with 3% H2O2 to quench endogenous peroxidase activity. This was followed by antigen retrieval and BSA blocking. Slides were then incubated with diluted primary antibodies overnight at 4 °C. Subsequently, the DAB detection kit (Cat. #KIT9710, MXB Biotechnologies, Fuzhou, China) was utilized. The stained images were scored by the intensity (1: low; 2: weak; 3, moderate; 4, strong) and the percentage of positive cells (1: 0–25%; 2: 26–50%; 3: 51–75%; 4: 76–100%) and under a double-blind protocol. The final score = intensity × percentage.
Generation of polyclonal rabbit anti-human AcK260-PTEN antibody
The polyclonal antibody specific for the acetylated PTEN at K260 was produced by PTM Biolab, Hangzhou, China. Rabbits were immunized with the acetylated PTEN-K260 peptide (EFFH-(Acetyl)K-QNKMLKKD C) as well as a non-acetylated peptide (EFFH-K-QNKMLKKD C). Prior to immunization, both peptides were synthesized and analyzed using MS analysis. The sera obtained from immunized rabbits was then filtered with the unmodified peptide, followed by affinity purification using the corresponding acetyl-peptide.
Statistics and reproducibility
Statistical significances were carried out in Prism 8 by Student’s two tailed t test for comparison between two groups, Pearson’s χ2 test for correlation analysis, and two-way ANOVA followed by Bonferroni’s multiple comparisons test for comparison among groups at multiple time points. Error bars indicate s.e.m. or s.d. as indicated in figure legends. Sample sizes were estimated based on our experience with similar experiments and reference to previously published studies. For RNA-seq data, three biological replicates were set for each group. For animal and clinical sample experiments, more than 5 biological replicates were used. For Western blot assays, experiments were repeated three times independently with similar results and representative images were given.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw LC-MS data generated in this study have been deposited in the iproX website under a PXD number PXD046043 (https://www.iprox.cn//page/project.html?id=IPX0007270000). RNA-seq data have been deposited at the NCBI Sequence Read Archive under accession number PRJNA1026067. Data from GSE115810 were used. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 (2012).
Lu, K. H. & Broaddus, R. R. Endometrial cancer. N. Engl. J. Med. 383, 2053–2064 (2020).
O’Malley, D. M. et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J. Clin. Oncol. 40, 752–761 (2022).
Makker, V. et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N. Engl. J. Med. 386, 437–448 (2022).
Arend, R. C., Jones, B. A., Martinez, A. & Goodfellow, P. Endometrial cancer: molecular markers and management of advanced stage disease. Gynecol. Oncol. 150, 569–580 (2018).
Berger, A. H., Knudson, A. G. & Pandolfi, P. P. A continuum model for tumour suppression. Nature 476, 163–169 (2011).
Lee, Y. R., Chen, M. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol. 19, 547–562 (2018).
Alimonti, A. et al. Subtle variations in Pten dose determine cancer susceptibility. Nat. Genet. 42, 454–458 (2010).
Álvarez-Garcia, V., Tawil, Y., Wise, H. M. & Leslie, N. R. Mechanisms of PTEN loss in cancer: it’s all about diversity. Semin. Cancer Biol. 59, 66–79 (2019).
Papa, A. & Pandolfi, P. P. The PTEN(-)PI3K axis in cancer. Biomolecules 9, 153 (2019).
Tamura, M., Gu, J., Takino, T. & Yamada, K. M. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 59, 442–449 (1999).
Shi, Y. J. et al. PTEN is a protein tyrosine phosphatase for IRS1. Nat. Struct. Mol. Biol. 21, 522–527 (2014).
Gu, T. T. et al. CREB is a novel nuclear target of PTEN phosphatase. Cancer Res. 71, 2821–2825 (2011).
Bell, D. W. & Ellenson, L. H. Molecular genetics of endometrial carcinoma. Annu. Rev. Pathol. 14, 339–367 (2019).
Clements, A. E., Bravo, V., Koivisto, C., Cohn, D. E. & Leone, G. WWP2 and its association with PTEN in endometrial cancer. Gynecol. Oncol. Rep. 13, 26–29 (2015).
Jones, N. L. et al. Identification of potential therapeutic targets by molecular profiling of 628 cases of uterine serous carcinoma. Gynecol. Oncol. 138, 620–626 (2015).
Kanamori, Y. et al. PTEN expression is associated with prognosis for patients with advanced endometrial carcinoma undergoing postoperative chemotherapy. Int. J. Cancer 100, 686–689 (2002).
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009).
Dang, F. & Wei, W. Targeting the acetylation signaling pathway in cancer therapy. Semin. Cancer Biol. 85, 209–218 (2022).
Jin, J. et al. Oxidative stress-CBP axis modulates MOB1 acetylation and activates the Hippo signaling pathway. Nucleic Acids Res. 50, 3817–3834 (2022).
Lin, M. et al. Targeting fibrinogen-like protein 1 enhances immunotherapy in hepatocellular carcinoma. J. Clin. Invest. 133, e164528 (2023).
Dou, Y. et al. Proteogenomic characterization of endometrial carcinoma. Cell 180, 729–748.e726 (2020).
Sauve, A. A., Wolberger, C., Schramm, V. L. & Boeke, J. D. The biochemistry of sirtuins. Annu. Rev. Biochem. 75, 435–465 (2006).
Wu, Q. J. et al. The sirtuin family in health and disease. Signal Transduct. Target Ther. 7, 402 (2022).
Zhang, C. et al. Quantitative proteome-based systematic identification of SIRT7 substrates. Proteomics 17, 13–14 (2017).
Tang, M., Tang, H., Tu, B. & Zhu, W. G. SIRT7: a sentinel of genome stability. Open Biol. 11, 210047 (2021).
Barber, M. F. et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 487, 114–118 (2012).
Wang, W. W. et al. A click chemistry approach reveals the chromatin-dependent histone H3K36 deacylase nature of SIRT7. J. Am. Chem. Soc. 141, 2462–2473 (2019).
Li, L. et al. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat. Commun. 7, 12235 (2016).
Tang, M. et al. SIRT7-mediated ATM deacetylation is essential for its deactivation and DNA damage repair. Sci. Adv. 5, eaav1118 (2019).
Tang, X. et al. SIRT7 antagonizes TGF-beta signaling and inhibits breast cancer metastasis. Nat. Commun. 8, 318 (2017).
Ianni, A. et al. SIRT7-dependent deacetylation of NPM promotes p53 stabilization following UV-induced genotoxic stress. Proc. Natl Acad. Sci. USA 118, e2015339118 (2021).
Vazquez, B. N. et al. SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair. EMBO J. 35, 1488–1503 (2016).
Singh, C. K. et al. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal 28, 643–661 (2018).
He, X. et al. O-GlcNAcylation and stablization of SIRT7 promote pancreatic cancer progression by blocking the SIRT7-REGγ interaction. Cell Death Differ. 29, 1970–1981 (2022).
Ding, M. et al. SIRT7 depletion inhibits cell proliferation and androgen-induced autophagy by suppressing the AR signaling in prostate cancer. J. Exp. Clin. Cancer Res. 39, 28 (2020).
Li, H. et al. SIRT7 promotes thyroid tumorigenesis through phosphorylation and activation of Akt and p70S6K1 via DBC1/SIRT1 axis. Oncogene 38, 345–359 (2019).
Yu, H. et al. Overexpression of sirt7 exhibits oncogenic property and serves as a prognostic factor in colorectal cancer. Clin. Cancer Res. 20, 3434–3445 (2014).
Tang, M. et al. Downregulation of SIRT7 by 5-fluorouracil induces radiosensitivity in human colorectal cancer. Theranostics 7, 1346–1359 (2017).
Bourgin, C. et al. Endometrial cancer in elderly women: which disease, which surgical management? A systematic review of the literature. Eur. J. Surg. Oncol. 42, 166–175 (2016).
Jung-Hynes, B., Reiter, R. J. & Ahmad, N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J. Pineal Res. 48, 9–19 (2010).
Morotti, M., Soleymani Majd, H., Casarin, J., Alazzam, M. & Damato, S. Histomolecular features of high-grade endometrial cancers. Minerva Med. 112, 20–30 (2021).
Hermyt, E. et al. Interplay between miRNAs and genes associated with cell proliferation in endometrial cancer. Int. J. Mol. Sci. 20, 6011 (2019).
Contreras, C. M. et al. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis. Model Mech. 3, 181–193 (2010).
Contreras, C. M. et al. Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas. Cancer Res. 68, 759–766 (2008).
Monsivais, D. et al. Uterine ALK3 is essential during the window of implantation. Proc. Natl Acad. Sci. USA 113, E387–E395 (2016).
Jeong, J. W. et al. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 28, 31–40 (2009).
Wang, X. et al. Pten and Dicer1 loss in the mouse uterus causes poorly differentiated endometrial adenocarcinoma. Oncogene 39, 6286–6299 (2020).
Blaisdell, A. et al. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell 28, 785–799 (2015).
Daikoku, T. et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 68, 5619–5627 (2008).
Yu, H. K. et al. Suppression of colorectal cancer liver metastasis and extension of survival by expression of apolipoprotein(a) kringles. Cancer Res. 64, 7092–7098 (2004).
Zhuo, W. et al. Long noncoding RNA GMAN, up-regulated in gastric cancer tissues, Is associated with metastasis in patients and promotes translation of Ephrin A1 by competitively binding GMAN-AS. Gastroenterology 156, 676–691.e611 (2019).
Winship, A. L., Van Sinderen, M., Donoghue, J., Rainczuk, K. & Dimitriadis, E. Targeting interleukin-11 receptor-alpha impairs human endometrial cancer cell proliferation and invasion in vitro and reduces tumor growth and metastasis In vivo. Mol. Cancer Ther. 15, 720–730 (2016).
Lee, J. W. et al. EphA2 targeted chemotherapy using an antibody drug conjugate in endometrial carcinoma. Clin. Cancer Res. 16, 2562–2570 (2010).
Kamat, A. A. et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin. Cancer Res. 13, 7487–7495 (2007).
Masson, G. R. & Williams, R. L. Structural mechanisms of PTEN regulation. Cold Spring Harb. Perspect. Med. 10, a036152 (2020).
Van Nyen, T., Moiola, C. P., Colas, E., Annibali, D. & Amant, F. Modeling endometrial cancer: past, present, and future. Int. J. Mol. Sci. 19, 2348 (2018).
Tamura, M. et al. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617 (1998).
Xu, J. et al. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 36, 19 (2017).
Raftopoulou, M., Etienne-Manneville, S., Self, A., Nicholls, S. & Hall, A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303, 1179–1181 (2004).
Alfieri, R., Giovannetti, E., Bonelli, M. & Cavazzoni, A. New treatment opportunities in phosphatase and tensin homolog (PTEN)-deficient tumors: focus on PTEN/focal adhesion kinase pathway. Front. Oncol. 7, 170 (2017).
Urick, M. E. & Bell, D. W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 19, 510–521 (2019).
Tao, Y. & Liang, B. PTEN mutation: a potential prognostic factor associated with immune infiltration in endometrial carcinoma. Pathol. Res. Pr. 216, 152943 (2020).
Janzen, D. M. et al. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res. 73, 4697–4710 (2013).
Jick, S. S., Walker, A. M. & Jick, H. Estrogens, progesterone, and endometrial cancer. Epidemiology 4, 20–24 (1993).
Smith, D. C., Prentice, R., Thompson, D. J. & Herrmann, W. L. Association of exogenous estrogen and endometrial carcinoma. N. Engl. J. Med. 293, 1164–1167 (1975).
Abraham, G. E., Odell, W. D., Swerdloff, R. S. & Hopper, K. Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone, and estradiol-17 beta during the menstrual cycle. J. Clin. Endocrinol. Metab. 34, 312–318 (1972).
Huhtinen, K., Stahle, M., Perheentupa, A. & Poutanen, M. Estrogen biosynthesis and signaling in endometriosis. Mol. Cell Endocrinol. 358, 146–154 (2012).
Shang, Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat. Rev. Cancer 6, 360–368 (2006).
Guzeloglu-Kayisli, O. et al. Regulation of PTEN (phosphatase and tensin homolog deleted on chromosome 10) expression by estradiol and progesterone in human endometrium. J. Clin. Endocrinol. Metab. 88, 5017–5026 (2003).
Wang, X. et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128, 129–139 (2007).
Maddika, S. et al. WWP2 is an E3 ubiquitin ligase for PTEN. Nat. Cell Biol. 13, 728–733 (2011).
Torres, J. & Pulido, R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 276, 993–998 (2001).
Vazquez, F., Ramaswamy, S., Nakamura, N. & Sellers, W. R. Phosphorylation of the PTEN tail regulates protein stability and function. Mol. Cell Biol. 20, 5010–5018 (2000).
Zhao, J. et al. SIRT7 regulates hepatocellular carcinoma response to therapy by altering the p53-dependent cell death pathway. J. Exp. Clin. Cancer Res. 38, 252 (2019).
Kim, J. K. et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology 57, 1055–1067 (2013).
Dong, L. et al. An NAD(+)-dependent deacetylase SIRT7 promotes HCC development through deacetylation of USP39. iScience 23, 101351 (2020).
Tang, X. et al. Combined intermittent fasting and ERK inhibition enhance the anti-tumor effects of chemotherapy via the GSK3beta-SIRT7 axis. Nat. Commun. 12, 5058 (2021).
Xu, Y., Zhang, D., Ji, J. & Zhang, L. Ubiquitin ligase MARCH8 promotes the malignant progression of hepatocellular carcinoma through PTEN ubiquitination and degradation. Mol. Carcinog. 62, 1062–1072 (2023).
Ma, J. et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell 35, 504–518.e507 (2019).
Ge, M. K. et al. FBXO22 degrades nuclear PTEN to promote tumorigenesis. Nat. Commun. 11, 1720 (2020).
Gimm, O. et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am. J. Pathol. 156, 1693–1700 (2000).
Whiteman, D. C. et al. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int. J. Cancer 99, 63–67 (2002).
Lee, Y. R. et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 364, eaau0159 (2019).
Okumura, K. et al. PCAF modulates PTEN activity. J. Biol. Chem. 281, 26562–26568 (2006).
Meng, Z., Jia, L. F. & Gan, Y. H. PTEN activation through K163 acetylation by inhibiting HDAC6 contributes to tumour inhibition. Oncogene 35, 2333–2344 (2016).
Ikenoue, T., Inoki, K., Zhao, B. & Guan, K. L. PTEN acetylation modulates its interaction with PDZ domain. Cancer Res. 68, 6908–6912 (2008).
Naguib, A. et al. PTEN functions by recruitment to cytoplasmic vesicles. Mol. Cell 58, 255–268 (2015).
Huang, J. et al. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat. Commun. 3, 911 (2012).
Rahdar, M. et al. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc. Natl Acad. Sci. USA 106, 480–485 (2009).
Nguyen, H. N. et al. Mechanism of human PTEN localization revealed by heterologous expression in Dictyostelium. Oncogene 33, 5688–5696 (2014).
Yang, J. M. et al. Characterization of PTEN mutations in brain cancer reveals that pten mono-ubiquitination promotes protein stability and nuclear localization. Oncogene 36, 3673–3685 (2017).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Comer, F. I. & Parent, C. A. PI 3-kinases and PTEN: how opposites chemoattract. Cell 109, 541–544 (2002).
Funamoto, S., Meili, R., Lee, S., Parry, L. & Firtel, R. A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109, 611–623 (2002).
Song, M. S., Salmena, L. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 13, 283–296 (2012).
Jiang, T. Y. et al. PTEN deficiency facilitates exosome secretion and metastasis in cholangiocarcinoma by impairing TFEB-mediated lysosome biogenesis. Gastroenterology 164, 424–438 (2023).
Chang, T. M. et al. PTEN regulates invasiveness in pancreatic neuroendocrine tumors through DUSP19-mediated VEGFR3 dephosphorylation. J. Biomed. Sci. 29, 92 (2022).
Peglion, F. et al. PTEN inhibits AMPK to control collective migration. Nat. Commun. 13, 4528 (2022).
Yu, J. et al. Regulation of serine-threonine kinase Akt activation by NAD(+)-dependent deacetylase SIRT7. Cell Rep. 18, 1229–1240 (2017).
Liang, J. & Shang, Y. Estrogen and cancer. Annu. Rev. Physiol. 75, 225–240 (2013).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Tang, Z., Kang, B., Li, C., Chen, T. & Zhang, Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W560 (2019).
Kovalchuk, S. I., Jensen, O. N. & Rogowska-Wrzesinska, A. FlashPack: fast and simple preparation of ultrahigh-performance capillary columns for LC-MS. Mol. Cell Proteom. 18, 383–390 (2019).
Perkins, D. N., Pappin, D. J. C., Creasy, D. M. & Cottrell, J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
Tang, M. et al. SMYD2 inhibition-mediated hypomethylation of Ku70 contributes to impaired nonhomologous end joining repair and antitumor immunity. Sci. Adv. 9, eade6624 (2023).
Ma, A. et al. USP1 inhibition destabilizes KPNA2 and suppresses breast cancer metastasis. Oncogene 38, 2405–2419 (2019).
Acknowledgements
We thank Professor Baohua Liu (Shenzhen University, Guangdong, China) for generous share of the Sirt7f/f mice. We thank Professor Wei-Guo Zhu (Shenzhen University, Guangdong, China), Professor Shaoming Shen (Shanghai Jiao Tong University, Shanghai, China), Professor Jianwei Zhou (Nanjing Medical University, Nanjing, China) for kindly providing plasmids. We thank CPTAC and TCGA for providing high quality, high-throughput data for public analysis. We thank BioRender.com for providing professional platform and icons for creating flowchart diagrams. The work was supported by the Shanghai Science and Technology Development Funds-Shanghai Rising-Star Program to M.T. (22QA1407400), National Natural Science Foundation of China (82172975, 81972438 to X.W., 81971338 to W.L., 32170602 to M.T.), Natural Science Foundation of Shanghai to W.L. (grant numbers: 22ZR1449400), and Special Health Industry Project of Pudong New Area Health Commission to X.W. (PW2021D-06).
Author information
Authors and Affiliations
Contributions
ZY.H., M.T., YJ.H., and XP.W. conceived, designed, performed the experiments and wrote the manuscript. ZY.H., M.T., YJ.H., BL.C., XX.S., GF.C., A.H., XQ.L., A.R.S., LJ.J., Q.L., and XH.X. analyzed the data and performed material preparation. M.T., W.L., ZY.M., and XP.W. discussed the results and commented on the manuscript. XP.W. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Berta Vazquez and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, Z., Tang, M., Huang, Y. et al. SIRT7 facilitates endometrial cancer progression by regulating PTEN stability in an estrogen-dependent manner. Nat Commun 16, 2989 (2025). https://doi.org/10.1038/s41467-025-58317-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58317-0