Abstract
The endocardium plays a pivotal role in governing myocardial development, and understanding the intrinsic regulatory insights will help apprehend pathological cardiomyopathy. Glycerol-3-phosphate acyltransferase 4 (GPAT4) is an endoplasmic reticulum (ER) membrane anchored protein. While the role of GPAT4 in glycerophospholipid biosynthesis is well established, its function in the ER is less explored. Here, we generate Gpat4 global and tissue-specific knockout mice and identify the essential role of GPAT4 in endocardial development. Deficiency of GPAT4 provokes endocardial ER stress response and enhances ER-mitochondrial (ER-mito) communications, leading to mitochondrial DNA (mtDNA) escape. As a result, the cGAS-STING pathway is triggered to stimulate type-I-interferon response, which affects heart development. Finally, abolishment of the cGAS-STING-type-I-interferon pathway rescues the heart defects of Gpat4 deletion mice. These findings uncover the pivotal role of GPAT4 in the maintenance of ER homeostasis during endocardial and heart development. Meanwhile, this study highlights the importance of the cGAS-STING pathway in cardiac organogenesis.
Similar content being viewed by others
Introduction
The endocardium is a specialized endothelial tissue covering the inner layer of the heart1,2. The endocardium plays a crucial role in cardiac development because it participates in myocardial trabeculation and compaction, and contributes to coronary vascular formation3,4,5. These developmental processes are modulated by interactions and reciprocal regulations between the endocardium and the myocardium through signaling molecules6,7,8,9. The precise coordination between endocardial cells and cardiomyocytes has been demonstrated as crucial for ventricular growth and chamber maturation1,9,10,11. Abnormal endocardial development often results in excessive trabeculation and poor myocardial formation. Understanding endocardial development will help decipher the pathogenesis of defective ventricular myocardium, such as the left ventricular non-compaction cardiomyopathy (LVNC), illustrated with enormous trabeculae and poorly developed myocardium that frequently leads to heart failure5,12,13.
GPATs (glycerol-3-phosphate acyltransferases) function in the Kennedy pathway of glycerophospholipid biosynthesis, and are rate-limiting enzymes for triacylglycerol (TAG) synthesis14,15. GPATs consist of four members: GPAT1-4. While GPAT1 and 2 are localized in the mitochondria, GPAT3 and 4 are membrane-anchored proteins in the endoplasmic reticulum (ER)16.
The ER forms a vast membrane network in the cells, which extends from the nuclear envelope outward to the cellular membrane17,18. The ER is responsible for protein translocation, protein folding, and protein post-translational modification. It is also a place to store calcium and for lipid and carbohydrate metabolism. Perturbation of ER functions triggers ER stress response, which further prompts a series of adaptive mechanisms including ER-associated degradation (ERAD) and the unfolded protein response (UPR), to counteract the elevated ER stress19,20. The UPR in the ER is mediated by three transcriptional programs: 1. PERK-p-eIF2α-ATF4-CHOP; 2. ATF6; and 3. IRE1-XBP1. Activation of UPR frequently exerts impact on mitochondrial functions21,22. For instance, maladaptive UPR affects the ER-mitochondrial (ER-Mito) communications/contacts and a large amount of Ca2+ are transferred from ER to mitochondria, causing mitochondrial Ca2+ overloading and escape of mitochondrial DNA (mtDNA) to intracellular compartments23,24. Cytosolic mtDNA provokes cGAS-STING pathway leading to type I interferon response, which may initiate cytotoxic effects, such as induction of cell apoptosis25.
Although the role of GPAT4 in glycerophospholipid biosynthesis (Kennedy pathway) in the central tissues for de novo lipogenesis (adipose tissues and the liver) is well established, its function in ER biology is still elusive. Here, we show the pivotal function of GPAT4 in the maintenance of ER homeostasis and in fine-tuning ER-Mito communication, which plays an essential role in endocardial and heart development. Meanwhile, we pinpointed the importance of cGAS-STING pathway in cardiac organogenesis.
Results
Defective heart development in Gpat4 global knockout mice
To understand the physiological role of GPAT4, we generated Gpat4 global knockout (KO) mice via CRISPR–Cas9 technology (Figure S1A). Gpat4 KO mice were found embryonic lethal at around E18.5 (Figure S1B). Dissection of embryos at embryonic day 14.5 (E14.5) and E15.5 revealed obvious subcutaneous edema of Gpat4 KO mice, suggesting of impaired cardiovascular development (Fig. 1A). Morphological and histological examination of the hearts detected dilated ventricles with thinned myocardial wall (Myo) and excessive trabeculae (Tra) in the heart of Gpat4 KO mice (Fig. 1B–E, S1C). Cell proliferation was prominently decreased in the KO heart (Fig. 1F, G). Western blotting analysis confirmed absence of GPAT4 protein in the KO mice, and uncovered a significant increase of the pro-apoptotic protein BAX but a profound reduction of the anti-apoptotic protein BCL2 in the heart tissues of KO mice (Fig. 1H, I). Whole mount immunofluorescence staining displayed severely disrupted coronary vascular formation in the KO mice (Fig. 1J–N).
A Gross analysis of WT and Gpat4 KO embryos and hearts at E14.5. The red arrows indicate subcutaneous edema. Scale bars, 500 µm. B Immuno-staining of heart sections at E14.5. Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). Scale bars, 500 µm. C Quantification of heart width, n = 3. **P = 0.0066. D Higher magnification of the boxed areas in (B). Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). Tra, trabecular layer; Myo, myocardial layer. E Quantification of the Tra and Myo thickness of the heart sections in (D). Note the substantially reduced thickness of the Myo but markedly increased thickness of the Tra in the heart of KO mice. LV, left ventricle; RV, right ventricle, n = 3. Scale bars, 100 µm. Left-Right, **P = 0.0024, **P = 0.0083, **P = 0.0040, ***P < 0.0001. F EdU staining. Scale bars, 100 µm. Edu (in green), Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). G Quantification of EdU+ Cells in Tra and Myo, n = 3. Left-Right, ***P < 0.0001, **P = 0.0084. H, I Western blotting analysis and quantification (heart tissues from E14.5 WT and KO mice), n = 3. Left-Right, ***P < 0.0001, **P = 0.0013, **P = 0.0014. J–N Heart whole mount staining of the coronary vasculature and quantification of the vessels, n = 3. J displays the coronary veins. J1–J4 are higher magnification of the boxed areas in (J) Endomucin (EMCN) stains coronary veins (in green). K Quantification of vein diameter, n = 3, ***P = 0.0004. L Quantification of Vascular density, n = 3, ***P = 0.0009. M manifests the coronary arteries that was stained by JAG1 antibody (in green). Scale bars, 100 µm. N Quantification of JAG1+ diameter, n = 3, ***P = 0.0002. Data are mean ± s.e.m. Two-tailed unpaired Student’s t-test.
Taken together, these findings define the crucial function of GPAT4 in embryonic heart development.
Essential role of GPAT4 in endocardial development
Next, we generated Gpat4 conditional (Gpat4 flox) mice for tissue-specific deletion. The excessive trabeculation and thinned myocardial layer in the Gpat4 global knockout mice suggests of endocardial defects. Endocardial cells are specialized endothelial cells, and we therefore, deleted Gpat4 using endothelial-specific Cre mice (Tie2-Cre). Similar to the global knockout mice, endothelial-specific deletion of Gpat4 (Tie2-Cre; Gpat4F/F) mice were unable to survive and exhibited prominent subcutaneous edema and enlarged heart at E14.5 (Fig. 2A, S2A, B). Histological analysis and immuno-fluorescence staining revealed heart dilation and confirmed successful removal of Gpat4 in the endocardial cells of knockout mice (Fig. 2B–E). It was noticed that the trabecular layer was exaggerated but the myocardial layer appeared thinner in the heart of knockout mice compared to control (Fig. 2D, E). EdU staining demonstrated reduced cell proliferation in the heart of Gpat4-deficient mice (Fig. 2F, G). Western blotting analysis confirmed effective deletion of Gpat4, and indicated increased cell apoptosis in the heart of knockout mice (Fig. 2H, I). In addition, the cardiac vasculature in the knockout mice was found abnormal (Fig. 2J–N). These results were consistent with those observed in the global knockout mice.
A Gross analysis of Gpat4F/F and Tie2-Cre; Gpat4F/F embryos (E14.5) and hearts. The red arrows indicate subcutaneous edema. Scale bars, 500 µm. B Immuno-staining of heart sections. Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). Scale bars, 500 µm. C Quantification of heart width, n = 3, **P = 0.0019. D Higher magnification of the boxed areas in (B). GPAT4 (in green), Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). Tra, trabecular layer; Myo, myocardial layer. E Quantification of the Tra and Myo thickness of the heart sections in (D). Note the substantially reduced thickness of the Myo but markedly increased thickness of the Tra in the heart of KO mice, n = 3. Scale bars, 100 µm. Left-Right, ***P < 0.0001, ***P < 0.0001, ***P < 0.0001, ***P = 0.0002. F EdU staining. Scale bars, 100 µm. Edu (in green), Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). G Quantification of EdU+ Cells in Tra and Myo, n = 3. Left-Right, ***P < 0.0001, **P = 0.0045. H, I Western blotting analysis and quantification, n = 3. Left-Right, **P = 0.0019, **P = 0.0022, ***P < 0.0001. J–N Heart whole mount staining of the coronary vasculature and quantification of the vessels, n = 3. J displays the coronary veins. (J1–J4) are higher magnification of the boxed areas in (J). Endomucin (EMCN) stains coronary veins (in gray). K Quantification of vein diameter, n = 3, ***P = 0.0003. L Quantification of Vascular density, n = 3, **P < 0.0015. M manifests the coronary arteries that was stained by JAG1 antibody (in red). N Quantification of JAG1+ diameter, n = 3, ***P < 0.0001. Scale bars, 100 µm. O Gross analysis of Gpat4F/F and Nfatc1-Cre; Gpat4F/F embryos (E14.5) and hearts. The red arrows indicate subcutaneous edema. Scale bars, 500 µm. P Quantification of heart width, n = 3, **P = 0.0055). Immuno-staining to display the trabeculae (Tra) and myocardium (Myo) layers of hearts from E14.5 embryos. Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). Scale bars, 100 µm. R Quantification of the Tra and Myo thickness of the heart sections in (Q), n = 3. Left-Right, **P = 0.0013, ***P = 0.0003, **P = 0.0024, **P = 0.0074. Data are mean ± s.e.m. Two-tailed unpaired Student’s t-test.
Furthermore, we deleted Gpat4 particularly in the endocardial cells using Nfatc1-Cre mice and detected similar phenotypes to those seen in the whole and endothelial-specific knockout mice (Fig. 2O–R).
Finally, ex vivo heart explant culture using ventricular tissues from Tie2-Cre; Gpat4F/F mice, unveiled profoundly reduced outward migration of endocardial cells compared to control (Fig. S2C–G). Scratch assays in HUVECs demonstrated that knockdown of Gpat4 impaired cell migration (Fig. S2H, I).
Collectively, these results have proved the essential role of GPAT4 in endocardial development.
GPAT4 deficiency triggered ER stress response and enhanced ER-mitochondrial communications
GPAT4 is a rate-limiting enzyme of TAG synthesis in the adipocytes and hepatocytes, and the GPAT family consists of four members: GPAT1-4. The levels of TAG, together with the other phospholipids including phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylinositol (PI) were examined in the hearts and whole embryos but no big differences were detected between control and Gpat4 deletion mice (Figure S3A–C). RNA-seq and qPCR analyses of heart tissues demonstrated unchanged mRNA levels of Gpat1, Gpat2, and Gpat3 upon Gpat4 deletion (Figure S3D–G). These findings exclude the possibility that Gpat4 affects cardiac development through regulation of phospholipid synthesis.
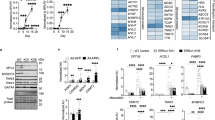
RNA-seq analysis using heart tissues from E14.5 embryos uncovered significant upregulation in the expression of genes involved in ER-stress response, such as Eif2ak3 (encoding the protein kinase RNA-like ER kinase, PERK), heat shock protein 90 beta family member 1 (Hsp90b1), Atf4, Atf6 and Xbp1 (Fig. 3A). These changes were validated by qPCR and Western blotting analysis (Fig. 3B–D).
A RNA-seq analysis of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts, Heatmap of dysregulated genes. Red and blue colors represent upregulated and downregulated genes, n = 3. B qPCR analysis of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts, n = 3. Left-Right, ***P = 0.0005, **P = 0.0010, ***P = 0.0002, ***P = 0.0007, ***P = 0.0002, ***P = 0.0006, ***P = 0.0007. C, D Western blotting analysis and quantification of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts, n = 3. Left-Right, **P = 0.0014, ***P = 0.0004, **P = 0.0015, **P = 0.0013, *P = 0.0106. E, F Immunoprecipitation-mass spectrometry (GPAT4 antibody) analysis with KEGG/GO (HUVECs). G The association between GPAT4 protein and EIF2S1/eIF2α protein predicted by AF3. H Immuno-precipitation (GPAT4 and EIF2S1) and Western blotting analysis (HUVECs). I AF3 modeling of the association relationship among the three proteins of PERK, GPAT4 and EIF2S1/eIF2α. J AF3 modeling of association between PERK protein and EIF2S1/eIF2α protein. K Immuno-precipitation (PERK and EIF2S1) and Western blotting analysis in Ctrl and siGPAT4 groups (HUVECs). L Immuno-precipitation (PERK and EIF2S1) and Western blotting analysis in Ctrl and GPAT4 OE groups (HUVECs). M, N Ctrl and GPAT4 OE groups, Western blotting analysis and quantification (HUVECs), n = 3. Left-Right, **P = 0.0081, **P = 0.0012, *P = 0.0181, **P = 0.0016, **P = 0.0013. O, P TEM analysis of Gpat4F/F and Tie2-Cre; Gpat4F/F endocardial cells in E11.5 hearts and quantification of ER-Mito contacts, n = 3. Scale bars, 1 µm. Left-Right, *P = 0.0161, *P = 0.0158, *P = 0.0495, **P = 0.0078. Q, R Western blotting analysis and quantification of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts, n = 3. Left-Right, *P = 0.0168, **P = 0.0042, ***P = 0.0004, **P = 0.0013, **P = 0.0036. Data are mean ± s.e.m. Two-tailed unpaired Student’s t-test.
The above results suggest of a critical function of GPAT4 in maintaining ER homeostasis. To understand this, we performed immuno-precipitation (IP) using GPAT4 antibody, and searched for GPAT4-binding proteins through mass spectrometry analysis in the endothelial cell line of HUVEC (human umbilical vein endothelial cell). This experiment led to identification of about 30 potential GPAT4 binding proteins. We then performed bioinformatical analysis of the biological processes (BP) in which these proteins participate, of the cellular compartments (CC) where these proteins are located, and of molecular functions (MF) that these proteins perform. The results uncovered that these proteins were involved in protein folding and refolding, are mainly located in the ER, and function to bind misfolded or unfolded proteins, and to process proteins in the ER (Fig. 3E, F). Among these proteins, some belong to the heat shock protein 70 family (HSPA1A, HSPA1B, HSPA1L and HSPA6) and some are important regulators of ER stress response (EIF2S1/eIF2α, PDIA4 and P4HB) (Fig. 3F).
Next, we investigated how GPAT4 modulates UPR. Using AlphaFold 3 (AF3)26, an accurate modeling to predict complex formation containing nearly all molecular types, we recognized multiple binding sites between EIF2S1/eIF2α and GPAT4 (Fig. 3G). A subsequent IP and Western blotting experiment confirmed the binding between these two proteins (Fig. 3H).
It has been widely accepted that PERK phosphorylation of EIF2S1/eIF2α regulates UPR to ER stress27,28,29. Upon putting together the three proteins of PERK, GPAT4 and EIF2S1/eIF2α, AF3 revealed simultaneous association of PERK with EIF2S1/eIF2α as well as GPAT4 with EIF2S1/eIF2α. Nonetheless, no direct association between PERK and GPAT4 was predicted by AF3 (Fig. 3I). Once leaving GPAT4 out of the three proteins assembly, AF3 predicted a substantial increase in the binding ability between PERK and EIF2S1/eIF2α (Fig. 3J) when compared to the presence of GPAT4 (Fig. 3I). Similarly, AF3 predicted a much stronger association of GPAT4 with EIF2S1/eIF2α compared to the presence of PERK (Fig. 3G). These results suggest a competition between GPAT4 and PERK in binding to EIF2S1/eIF2α.
IP-Western blotting analysis demonstrated a stronger binding of PERK to EIF2S1/eIF2α upon GPAT4 knockdown compared to control (Fig. 3K). Consistently, GPAT4 overexpression (OE) markedly reduced the association between PERK to EIF2S1 (Fig. 3L). As a result, activation of the UPR pathways were greatly reduced upon GPAT4 OE and the levels of phospho- EIF2S1/eIF2α, CHOP and ATF4 were remarkably dropped (Fig. 3M, N).
Further investigation of endocardial cells using transmission electron microscopy (TEM) displayed evident ER swelling and abnormal mitochondria in the Tie2-Cre; Gpat4F/F mice (Fig. 3O). Additionally, profoundly enhanced ER-mitochondrial (ER-Mito) contacts (MAMs) were observed in the endocardial cell of the Tie2-Cre; Gpat4F/F mice (Fig. 3O, P). The ER-Mito contacts are tethered by the calcium transport channel IP3R-GRP75-VDAC1-MCU and MFN230,31. Western blotting analysis demonstrated a significantly increased level of these proteins in the heart tissues of Gpat4 mutant mice compared to control (Fig. 3Q, R).
Taken together, these results indicate that GPAT4 is critical in maintaining ER homeostasis, whose disruption causes ER stress response leading to enhanced UPR-ER and ER-Mito communications.
Gpat4 deletion elicited cGAS-STING and type I interferon response
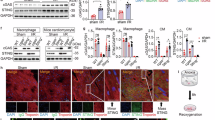
KEGG and GO analyses of the RNA-seq datasets using heart tissues from the E14.5 mice, indicated a significant enrichment of pathways related to the type I interferon response in the Tie2-Cre; Gpat4F/F mice (Fig. 4A, B). Among the upregulated genes, many were interferon-stimulated genes (ISGs), including Usp18, Isg15, Ifit1, Ifna4, Stat1 and DDX5825. This changed pattern of gene expression profile was also defined in the heart tissues (at both E12.5 and E14.5) of Gpat4 global knockout mice (Figure S4A–F). qPCR analysis confirmed these findings (Figs. 4C, S4G, H).
A RNA-seq analysis of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts, Heatmap of dysregulated genes. Red and blue colors represent upregulated and downregulated genes, n = 3. B KEGG analysis of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts. C qPCR analysis of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts, n = 3. Left-Right, ***P = 0.0003, ***P = 0.0004, ***P = 0.0002, ***P < 0.0001, ***P = 0.0003, ***P = 0.0009, ***P = 0.0007, ***P = 0.0002, ***P = 0.0009, **P = 0.0057. D, E Western blotting analysis and quantification of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts, n = 3. Left-Right, **P = 0.0050, **P = 0.0086, **P = 0.0022, *P = 0.0453. F cGAS staining of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts. Scale bars, 100 µm. cGAS (in green), Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). G co-localization analysis of cGAS and endocardium. H STING staining of Gpat4F/F and Tie2-Cre; Gpat4F/F E14.5 hearts. Scale bars, 100 µm. STING (in green), Endomucin (EMCN) stains endocardium (in red) and DAPI stains the nuclei (in blue). I co-localization analysis of STING and endocardium. Data are mean ± s.e.m. Two-tailed unpaired Student’s t-test.
One important trigger of type I interferon response is activation of the cGAS-STING-TBK1-IRF3 signaling pathway32. We tested this signaling pathway by Western blotting and the results demonstrated that the protein levels of cGAS, STING, phospho-TBK1 and phospho-IRF3 were all significantly elevated in the heart tissues from either endothelial-specific or global Gpat4 knockout mice (Figs. 4D, E, S4I, J). Immunofluorescence staining revealed augmented cGAS and STING proteins were localized to the endocardium (Fig. 4F–I).
We noticed that activation of the ER response occurred earlier than that of the cGAS-STING pathway (Figure S4K–N). While ATF4 showed markedly enhanced presence in the endocardium of Gpat4 mutant mice at E11.5 (Figure S4K, L), cGAS was nearly undetectable in these mice (Figure S4M, N). In addition, the numbers of genes with substantially enhanced expression levels in E14. 5 heart tissues were much bigger than those in E12.5 heart (Figure S4B, C, E, F), which was confirmed by fold changes (Figure S4G, H). The fold changes in E14.5 heart tissues were similarly much more than in E12.5 heart tissues.
STING is localized in the membrane of ER as GPAT433. In the IP-MS results of GPAT4, the Sting protein was not detected. Immuno-precipitation test did not find a direct interaction between GPAT4 and STING (Figure S5A).
In addition, we examined the cGAS-STING pathway in the heart tissues from cardiomyocyte-specific Gpat4 deletion mice (cTnt-Cre mediated deletion, viable) and failed to detected a difference between the control and knockout mice (Figure S5B–D).
Put together, these findings suggest that disruption of Gpat4 triggered the cGAS-STING signaling pathway, resulting in activation of type I interferon response in the endocardium. These events occurred after the activation of ER stress response.
Endothelial loss of GPAT4 resulted in augmented ER-Mito communication, mitochondrial dysfunction, and activation of cGAS-STING pathway
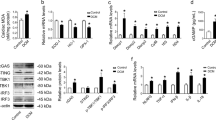
Next, we performed experiments in HUVEC cells to study the relation of Gpat4 knockdown and activation of cGAS-STING pathway. Small interfering RNA (siRNA) technology was applied to achieve Gpat4 knockdown. Loss of Gpat4 impaired cell survival (Fig. 5A). Western blotting analysis demonstrated substantially increased protein levels of pro-apoptotic BAX, Cytochrome-C (Cyto-c) and cleaved Caspase-3 but markedly reduced anti-apoptotic BCL2 upon Gpat4 knockdown (Fig. 5B, C). qPCR and Western blotting analysis indicated enhanced ER stress response in SiGpat4 cells (Fig. 5D–F). The protein levels of the ER-Mito contacts components (IP3R, GRP75, VDAC1, MCU and MFN2) were profoundly augmented in Gpat4 knockdown cells (Fig. 5G, H). Consistently, immuno-fluorescence staining revealed closer proximity between the ER and mitochondria (Fig. 5I), along with an increase in the number of ER-Mito contacts (MAMs) that was measured with a fluorescence MERCs (Mito-ER Contact Sites) reporter to detect ER-Mito contacts (approaching of ER with Mito produces functional GFP)34 (Fig. 5J). Disruption of Gpat4 also gave rise to hyper-activation of the cGAS-STING signaling pathway and type I interferon response (Fig. 5K–M).
A Cell survival was assessed in Ctrl and siGPAT4 groups by Cell Counting Kit-8 (CCK8), n = 6, ***P < 0.0001. B, C Western blotting analysis and quantification of Ctrl and siGPAT4 HUVECs, n = 3. Left-Right, **P = 0.0015, ***P = 0.0008, ***P = 0.0002, ***P = 0.0003, ***P = 0.0003. D qPCR analysis of Ctrl and siGPAT4 HUVECs, n = 3. Left-Right, **P = 0.0026, ***P = 0.0003, *P = 0.0349, **P = 0.0083, *P = 0.0104, **P = 0.0069, *P = 0.0420. E–H Western blotting analysis and quantification of Ctrl and siGPAT4 HUVECs, n = 3. F Left-Right, **P = 0.0044, ***P = 0.0003, ***P = 0.0007, **P = 0.0017, *P = 0.0181. H Left-Right, **P = 0.0070, ***P = 0.0003, **P = 0.0011, **P = 0.0026, ***P = 0.0007. I Proximity/overlapping analysis of mitochondria (Tom20 in red) and ER (Pdi in green). The left down boxed area is higher magnification of the right up boxed area. Note enhanced ER-Mito proximity in the siGPAT4 cells. Scale bars, 10 µm. J ER tracker (in red) and ER-Mito contact (in green) test using a fluorescence reporter to detect the contact. The left down boxed area is higher magnification of the right up boxed area. Note increased ER-Mito contacts in siGPAT4 cells, n = 3. ***P = 0.0003. Scale bars, 10 µm. K qPCR analysis of Ctrl and siGPAT4 HUVECs, n = 3. Left-Right, **P = 0.0043, *P = 0.0166, ***P = 0.0004, ***P = 0.0007, ***P = 0.0008, *P = 0.0151, **P = 0.0046, ***P = 0.0002, ***P = 0.0007, ***P = 0.0008. L, M Western blotting analysis and quantification of Ctrl and siGPAT4 HUVECs, n = 3. Left-Right, *P = 0.0106, **P = 0.0017, **P = 0.0098, **P = 0.0058. N, O Ca2+ probe signals. N Rhod-2 probe to display mitochondrial Ca2+ and Rhod-2 (%) quantification, Rhod-2 in red and MitoTracker in green, n = 3. ***P = 0.0002. Scale bars, 10 µm. O Ca2+ probe to detect Ca2+ in the ER-Mito contacts and positive contacts (%) quantification, OMM-NEMOs for Ca2+ in green and mKate-ER for ER-Mito contacts in red, n = 3. ***P = 0.0003. Scale bars, 10 µm. P Representative curves of the time scan of MAMs Ca2+ after His treatment in Ctrl and siGPAT4 HUVECs, n = 6. Q, R Measurement of mitochondrial membrane potential. Q TMRE probe signal and TMRE MFI quantification of Ctrl and siGPAT4 HUVECs, n = 3. ***P < 0.0001. Scale bars, 10 µm. R JC-1 probe signal and JC-1 monomers MFI quantification of Ctrl and siGPAT4 HUVECs, n = 3. ***P = 0.0003. Scale bars, 10 µm. S MitoSox probe signal and MitoSOX MFI quantification of Ctrl and siGPAT4 HUVECs, n = 3. **P = 0.0010. Scale bars, 10 µm. T, U Mitochondria (Tom20 in red) and dsDNA staining (in green), and co-localization analysis of Ctrl and siGPAT4 HUVECs. Scale bars, 10 µm. V qPCR analysis of cytoplasmic mtDNA of Ctrl and siGPAT4 HUVECs, n = 3. *P = 0.0135. W ISG gene expression of Ctrl and siGPAT4 HUVECs, n = 3. Left-Right, ***P < 0.0001, *P = 0.0132, ***P = 0.0002, ***P = 0.0006, *** P < 0.0001, ***P = 0.0001. Data are mean±s.e.m. Two-tailed unpaired Student’s t-test. n represents three independent cell experiments.
Fluorescent Ca2+ probes (Rhod-2, monitoring mitochondrial Ca2+) and OMM-NEMOs-mKate-ER (a recently developed Ca2+ indicator for measurement of Ca2+ in the ER-Mito contacts) analysis revealed apparently more Ca2+ signals in the mitochondria and in the ER-Mito contacts in SiGpat4 cells than that of control (Fig. 5N, O)35. Mitochondrial membrane potential (measured using TMRE and JC-1 probes) was reduced while MitoSox signal (superoxide generated in the mitochondria) was prominently enhanced upon Gpat4 knockdown (Fig. 5P–S). Co-staining of dsDNA and TOM20 revealed an increased presence of leaked mtDNA in the cytoplasm after Gpat4 knockdown (Fig. 5T). Additionally, measurement of cytoplasmic mtDNA content displayed a significant increase in the GPAT4 knockdow cells (Fig. 5V). VDAC is a mitochondrial membrane protein and constitutes the channel for mtDNA release. The VDAC oligomerization inhibitor VBIT-4 can suppress mtDNA release36. We observed a remarkable reduction of expression levels of interferon stimulated genes (ISGs) after VBIT-4 treatment compared to untreated cells (Fig. 5W), which further supports the conclusion that the release of mtDNA activates the cGAS-STING signaling pathway.
In the myocardial cell line H9c2, reducing GPAT4 levels showed little impact on pro- and anti- apoptotic proteins (Figure S6A, B). ER stress response and cGAS-STING were comparable between control and SiGpat4 cells (Figure S6C, D).
Collectively, these in vitro studies demonstrate that loss of GPAT4 caused augmented ER-Mito communications, mitochondrial dysfunction, and activation of cGAS-STING pathway specifically in the endothelial cells.
Blockage of the cGAS-STING pathway rescued the endocardial defects of Gpat4-deficient mice
To test whether blockage the cGAS-STING pathway would improve endocardial development in Gpat4 knockout mice, we generated double knockout mice for Gpat4/Ifnar1. Ifnar1 encodes interferon a and b receptor 1 (IFNAR1) that mediates type I interferon response. At E14.5, Gpat4-/-; Ifnar1-/- (DKO) mice exhibited no subcutaneous edema or cardiac dilation (Fig. 6A, B). Moreover, endocardial and myocardial development in the DKO mice was comparable to that of control. (Fig. 6C, D). ER stress response in the DKO mice was similar to that of Gpat4 KO mice (Fig. 6E). However, the genes downstream of type I interferon signaling demonstrated a significant decrease in the DKO mice compared to the Gpat4-/- mice (Fig. 6F). At the protein level, IFNAR1 in the hearts of E14.5 DKO mice was markedly reduced, and apoptosis-related proteins BAX and BCL2 exhibited reversed changes compared to Gpat4-/- mice (Fig. 6G, H). In addition, DKO mice for Gpat4/Sting (Gpat4-/-; Sting-/-) were produced and these mice showed no edema or cardiac dilation, and the heart development was similar to control (Fig. 6I–L). At the mRNA level, ER stress response in the DKO mice was similar to that of Gpat4 KO mice, while the genes downstream of type I interferon signaling showed significantly decreased levels (Fig. 6M, N). At the protein level, apoptosis-related proteins manifested reversed changes compared to Gpat4-/- mice (Fig. 6O, P).
A, B Gross analysis of embryos and hearts and quantification analysis of WT, Gpat4-/- and Gpat4-/-; Ifnar1-/- E14.5 hearts, n = 3. **P = 0.0046, **P = 0.0020. Scale bars, 500 µm. C, D Histological analysis of trabecular and myocardial layers, and quantification of WT, Gpat4-/- and Gpat4-/-; Ifnar1-/- E14.5 hearts, n = 3. ***P = 0.0006, ***P = 0.0005. Scale bars, 100 µm. E, F qPCR analysis of WT, Gpat4-/- and Gpat4-/-; Ifnar1-/- E14.5 hearts, n = 3. E Left-Right, **P = 0.0045, ***P < 0.0001, ***P = 0.0001, ns P = 0.1205, ***P < 0.0001, ns P = 0.5378, ***P = 0.0002, ns P = 0.6501, ***P = 0.0004, ns P = 0.6854, **P = 0.0011, ns P = 0.9643, **P = 0.0014, ns P = 0.0563, ***P = 0.0003, ns P = 0.6969. F Left-Right, ***P < 0.0001, **P = 0.0019, ***P = 0.0003, **P = 0.0025, **P = 0.0048, *P = 0.0175, **P = 0.0068, **P = 0.0048, **P = 0.0067, *P = 0.0325, ***P = 0.0007, **P = 0.0035, ***P = 0.0001, *P = 0.0113, **P = 0.0067, **P = 0.0063. G, H Western blotting analysis and quantification of WT, Gpat4-/- and Gpat4-/-; Sting-/- E14.5 hearts, n = 3. Left-Right, ***P < 0.0001, ns P = 0.1314, ***P < 0.0001, ***P < 0.0001, ***P = 0.0001, *P = 0.0178, *P = 0.0152, *P = 0.0269. I, J Gross analysis of embryos and hearts and quantification analysis of WT, Gpat4-/- and Gpat4-/-; Sting-/- E14.5 hearts, n = 3. *P = 0.0103, **P = 0.0352. Scale bars, 500 µm. K, L Histological analysis of trabecular and myocardial layers, and quantification of WT, Gpat4-/- and Gpat4-/-; Sting-/- E14.5 hearts, n = 3. *** P < 0.0001, *** P < 0.0001. Scale bars, 100 µm. M, N qPCR analysis of WT, Gpat4-/- and Gpat4-/-; Sting-/- E14.5 hearts, n = 3. M Left-Right, **P = 0.0038, ***P < 0.0001, ***P = 0.0003, ns P = 0.5043, ***P < 0.0001, ns P = 0.8883, ***P = 0.0002, ns P = 0.8013, ***P < 0.0001, ns P = 0.9270, ***P = 0.0006, ns P = 0.1899, ***P = 0.0003, ns P = 0.8434, ***P = 0.0001, ns P = 0.6958. N Left-Right, ***P = 0.0004, *P = 0.0207, ***P < 0.0001, ***P = 0.0004, **P = 0.0037, **P = 0.0084, **P = 0.0026, **P = 0.0050, **P = 0.0034, *P = 0.0208, **P = 0.0018, **P = 0.0095, ***P = 0.0001, *P = 0.0126, *P = 0.0139, *P = 0.0495. O, P Western blotting analysis and quantification of WT, Gpat4-/- and Gpat4-/-; Sting-/- E14.5 hearts, n = 3. Left-Right, ***P < 0.0001, ns P = 0.9582, ***P = 0.0009, ***P < 0.0001, ***P = 0.0002, ***P < 0.0001, ***P = 0.0009, *P = 0.0306. Data are mean ± s.e.m. Two-tailed unpaired Student’s t-test.
Collectively, these findings demonstrated that blockage of the cGAS-STING pathway is beneficial to heart development in the Gpat4 KO mice, and further confirm that endocardial defects are a result of hyper-activation of type I interferon response.
Discussion
In this study, we defined an essential function of GPAT4 in endocardial and heart development through sustaining ER homeostasis in the endocardial cells. Disruption of GPAT4 evokes ER stress response and enhances ER-Mito communications, leading to mitochondrial Ca2+ overloading and mtDNA escape (Fig. 7A, B). As a result, the cGAS-STING pathway is triggered to elicit type I interferon response, which impairs endocardial survival and heart development (Fig. 7A, B). Thus, we unraveled unique regulatory insights of GPAT4-mediated ER-Mito communication and cGAS-STING pathway on endocardial and heart development.
(NORMAL) Under the normal condition, GPAT4 in the endocardial cells binding with EIF2S1/EIF2α to sustain ER homeostasis and fine-tunes ER-Mito communications to safeguard endocardial development. (GPAT4 LOSS) In the absence of GPAT4, ER homeostasis is disrupted and ER stress response is triggered by phosphorylating EIF2S1/EIF2α, leading to enhanced ER-Mito communications, which in turn, causes Mito Ca2+ overloading and mtDNA escape. As a result, cGAS-STING-type I interferon response is elicited, which induces endocardial apoptosis. In the end, this leads to defective trabecular compaction (poor myocardial development) and excessive trabeculation. (Created in BioRender. zhao, t. (2025) https://BioRender.com/m79n092).
Our work highlights a unique role of GPAT4 in endocardial ER homeostatic maintenance, which is crucial for endocardial and heart development. GPAT4 demonstrates a competitive association with EIF2S1/eIF2a against PERK to hinder UPR to ER stress response, stabilizing EIF2S1/eIF2a in the ER.
In the central lipogenic cells for glycerophospholipid biosynthesis, such as adipocytes and hepatocytes, GPAT4 plays a critical role as one of the rate-limiting enzymes in the Kennedy pathway. However, the involvement of GPAT4 in endocardial development seems independent from lipid biosynthesis because the endocardial cells are not responsible for lipid storage and we detected very low level of TAG in these cells. In addition, no big differences in the levels of phospholipids including TAG, PC, PE and PI between Gpat4 KO and control mice support this assumption.
Our study demonstrated the fine-tuning of ER-Mito communications and cGAS-STING pathway in heart development. These biological processes have been reported in pathological heart disease in the adults but their involvement in heart development has never been reported.
Organelle biology is an emerging new frontier for biomedical study to address questions in cell biology and developmental biology, and to understand diseases from novel aspects. We and others observed the critical role of mitochondria in myocardial development in mouse models and our recent studies indicate severe consequences of mitochondrial dysfunction in myocardial formation37,38. On the other hand, the current work uncovered a pivotal role of GPAT4-mediated ER homeostasis in endocardial development, which pinpoints the importance of ER in sustaining endocardial and heart development. However, the current study suggests that GPAT4 is dispensable for cardiomyocyte ER homeostasis and for myocardial development. A plausible argument lies in that the ER in cardiomyocytes are sarcoplasmic reticulum (SR) that functions to regulate Ca2+ transport and excitation-contraction coupling, while ER plays a regulatory role in protein synthesis, modification and secretion, and lipid synthesis39. There was a previous study demonstrating that pregnant hyperglycemia (diabetes) could provoke ER stress in the embryonic heart and affect heart development40. Whether is GPAT4 participating this pathological process is unknown.
In the future, we are interested in exploring the function of GPAT4 in the cardiomyocytes of adult mice to understand whether it plays an important role in cardiac physiology and pathology.
In conclusion, our study elucidates the intricate regulatory roles of Gpat4 in endocardial cells during embryonic heart development. The findings contribute to a better understanding of the molecular mechanisms underlying cardiac development and provide a foundation for potential therapeutic strategies aimed at addressing cardiac developmental abnormalities.
Methods
Mice
Gpat4+/- mice were generated using CRISPR-Cas9 technology. Gpat4 flox, Ifnar1+/- and Sting+/- mice were obtained from Gempharmatech Co. Ltd. (Nanjing, China). Tie2-Cre, Nfatc1-Cre, and cTnT-Cre mice were as previously reported4,41,42. All mouse strains were maintained on a C57BL/6 genetic background. Mice were group-housed in accordance with the regulations on mouse welfare and ethics of Nanjing University, with 12 h/12 h light–dark cycles and ad libitum access to food and water. The Institutional Animal Care and Use Committee (IACUC) of Nanjing University approved all animal procedures used in this study. All primers used in genotyping these mice are listed in Table S1.
Immunofluorescence (IF) staining
Embryos and hearts fixed in 4% PFA were dehydrated and embedded in OCT medium (Sukura, 4583). Eight-micrometer-thick sections were cut using a Leica CM1950 automated cryostat. For IF staining, sections were placed at room temperature for 20 min and washed in PBS. Goat serum (Beyotime, C0265) was used to block the sections, which were then incubated with primary antibodies overnight at 4 °C. The next day, the sections were washed with PBS and incubated with secondary antibodies for 2 h. Finally, the sections were washed again in PBS and sealed with 50% glycerol before confocal imaging. Detailed information of the antibodies used are shown in Table S2.
In vivo EdU labelling
To assess proliferation rates in embryonic hearts, pregnant mice were intraperitoneally injected with 50 mg/kg body weight of EdU (RIBIO, C10371) 48 hours prior to dissection. Embryonic hearts were then isolated for cryosectioning analysis. EdU incorporation was detected using the Apollo EdU 567 reagent.
Whole-mount immunofluorescence and imaging of embryonic hearts
Whole embryos at E15.5 were dissected, and their chests were fixed in 4% paraformaldehyde (PFA, Aladdin C104188) on ice for 5 h with shaking. The chests were then washed with shaking for 2 × 8 min in PBS at room temperature. The hearts were excised and cut on the ventral side for artery morphological analysis and on the dorsal side for venous morphological analysis. For immunostaining, hearts were blocked and permeabilized in Goat Serum (BOSTER, AR0009) for 2 h at room temperature with shaking. Primary antibodies were added to 0.5% PBST solution, and hearts were incubated at 4 °C overnight with shaking. The following day, hearts were washed in 0.5% PBST for 5 × 12 min to remove unbound primary antibodies. Secondary antibodies were diluted in PBT, and samples were incubated at 4 °C overnight with shaking, followed by 5 × 12 min washed in PBT the next day. Large Z-volumes of the samples were imaged using different objectives and tile scanning on Leica SP8 Navigator microscopes.
Protein interaction prediction
AlphaFold 3, an accurate modeling to predict complex formation containing nearly all molecular types. (https://alphafoldserver.com). Search the sequence of the target proteins on the UNIPROT and copy the sequence into AlphaFold 3 for prediction. Analysis of the interaction structure using Pymol software.
Explant culture, immunostaining
The left ventricles without atria were isolated from E11.5 embryos, rinsed with PBS to remove blood cells and placed in the Matrigel (Yeasen, 40183 s) with culture media in 35 mm confocal dishes (Beyotime, FCFC020). Explants were cultured in 5% CO2 at 37 °C for 2 days. Hearts were fixed in 4% PFA for 60 mins, washing the explants with 0.5% PBST three times. Explants were blocked and permeabilized in Goat Serum (BOSTER, AR0009) for 2 hours at room temperature with shaking. Primary antibodies were added to fresh 0.5% PBST solution, and explants were incubated at 4 °C overnight. The following day, hearts were washed in 0.5% PBST for 5 × 20 min to remove unbound primary antibodies and then incubated with corresponding secondary antibodies in 0.5% PBST 2 hours at room temperature. After three washes with PBS for 15 min, the explants were placed in PBS and photographed using a confocal microscope (Olympus, FV3000).
Cell culture and transfection
Human Umbilical Vein Endothelial Cells (HUVECs) were maintained in Gibco DMEM basic (1X) medium, supplemented with 10% fetal calf serum and 1% penicillin–streptomycin, in the presence of 5% CO2 at 37 °C incubators. Transient transfection was performed using Lipofectamine 2000 (Life Technologies; 11668-027) following the manufacturer’s instructions. Fresh medium was added to the cells 4 to 6 h after transfection. The cells were collected 48 to 72 h after transfection for further analysis. The siRNA sequences were showed in Table S3. The two plasmids (Mitot and ERt) of the MERCs (Mito-ER Contacts) reporter and the construct of OMM-NEMOs-mKate-ER were as reported34,35. For GPAT4 overexpression (OE) construct, the sequence of GPAT4 was synthesized by GenePharma Co. Ltd. (Shanghai, China) and then cloned into pcDNA3.1 vector between the HindIII and EcoRI sites to create the pcDNA3.1-GPAT4 plasmid.
Cell survival assay
After trypsinization and centrifugation, the harvested cells were seeded into a 96-well plate (1000 cells/well). A Cell Counting Kit-8 (Yeasen, 40203ES) was used to measure cell viability. In brief, a mixture of medium and CCK-8 reagent was added to the wells, and the plate was incubated at 37 °C for 2 h. The absorbance of the wells was measured at 450 nm using a Bio-Rad multimode plate reader (Hercules).
Measurement of mitochondrial Ca2+ uptake
The fluorescent dye Rhod-2/AM (Yeasen, 40776ES) and Mito-Tracker Green (Beyotime, C1048) were used to measure cells mitochondrial Ca2+ uptake. Briefly, HUVECs cells were incubated with Rhod-2/AM and Mito-Tracker Green to allow the cells to load the dyes according to the protocol. Cells were viewed with a spinning disk confocal super-resolution microscope (Olympus, FV3000) at the following wavelengths: 549 nm (excitation) and 578 nm (emission), and the frame rate was 2 frames/s.
Mitochondrial membrane potential
TMRE: For Mitochondrial membrane potential assay, treated cells were loaded with the potentiometric dye 500 nM TMRE (Beyotime, C2001S) at 37 °C for 30 min and the staining was viewed by a confocal scanning microscope after washing. The fluorescence intensity of the HUVECs was detected with confocal microscopy (Olympus, FV3000) and quantified using Image J software.
JC-1: Enhanced mitochondrial membrane potential assay kit with JC-1 (Beyotime, C2003S) was used to detect mitochondrial membrane potential. HUVECs were transferred to JC-1 staining solution at 37 °C in 5% CO2 for 30 min the staining was viewed by a confocal scanning microscope after washing. The fluorescence intensity of the HUVECs was detected with confocal microscopy (Olympus, FV3000) and quantified using Image J software.
Measurement of mitochondrial oxidative stress
Mitochondrial ROS (mtROS) was detected by MitoSOX red mitochondrial superoxide indicator (Yeasen, 40778ES) according to the instructions of the manufacturer. Next, HUVECs were loaded with MitoSOX reagent (5 μmol/L) in the dark at 37 °C for 30 min and the staining was viewed by a confocal scanning microscope after washing. The fluorescence intensity of the HUVECs was detected with confocal microscopy (Olympus, FV3000) and quantified using Image J software.
Quantification of endocardial complexity
To measure endocardial branch points, the total area covered by endocardial network and total length of the endocardial network, the transverse heart sections of E14.5 control and Gpat4 knockout embryonic hearts were stained with EMCN for visualization of endocardial networks.
Western blot analysis
Tissues and cells were washed with cold PBS and lysed on ice with RIPA buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% TritonX-100, 0.1% SDS, 1% Na-deoxycholate, 1 mM EDTA] containing protease inhibitor cocktail (Roche) and PhosSTOP (Roche) for 30 min. Supernatant fractions were collected after centrifugation at 13,500 g for 10 min at 4 °C, and protein concentration was quantified by BCA Protein Assay Kit (Beyotime, P0012). After separation via SDS-PAGE, proteins were transferred to PVDF membranes (Millipore), blocked in 5% non-fat milk or bovine serum albumin and incubated with appropriate primary antibodies in Table S2. Membranes were cut and sections probed separately to reduce wastage of samples.
Quantitative real-time PCR
Total RNA from cells or heart tissues was isolated using TRIzol reagent (Invitrogen), and reversely translated into cDNA using HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, R312-01). Real-Time PCR was performed in MicroAmp™ Optical 96-Well Reaction Plate with Barcode & Optical Caps (Applied Biosystems) using Hieff UNICON® qPCR SYBR Green Master Mix (Yeasen, 11200ES). Reactions were carried out on the QuantStudio™ 5 Real-Time PCR System (Applied Biosystems). All primers used in qRT-PCR are listed in Table S4. Triplicate amplifications were carried out for each target gene and the housekeeping genes Actb and relative expression values were calculated using the ΔΔCt analysis method.
Cytoplasm mitochondrial DNA isolation
HUVECs are collected and suspended using a mitochondrial isolation reagent (MCE, HY-K1060). Then, transfer the cell suspension to a glass homogenizer for homogenization. Afterwards, perform low-speed (600 × g, 4 °C, 10 min) centrifugation to separate the nuclei, and subject the supernatant to high-speed (11,000 × g, 4°C, 10 min) centrifugation to separate the mitochondria and cytoplasm. Finally, extract RNA from the cytoplasm and use it for reverse transcription and qPCR to assess the expression levels of mtDNA.
RNA-seq analysis
RNA quality was determined using the 2100 Bioanalyser (Agilent) and quantified using the ND-2000 (NanoDrop Technologies). Only a highquality RNA sample (OD260/280 = 1.8∼2.2, OD260/230 ≥ 2.0, RIN ≥ 6.5, 28S:18S ≥ 1.0, >2 μg) was used to construct the sequencing library. The RNAseq transcriptome library was prepared using the TruSeq™ RNA sample preparation Kit from Illumina using 1 μg of total RNA and sequenced with the Illumina HiSeq xten/NovaSeq 6000 sequencer (2 × 150 bp read length). The raw paired end reads were trimmed and quality controlled by SeqPrep and Sickle with default parameters. Then clean reads were separately aligned to reference genome with orientation mode using TopHat software. The mapping criteria of bowtie was as follows: sequencing reads should be uniquely matched to the genome allowing up to two mismatches, without insertions or deletions. The expression level of each transcript was calculated according to the fragments per kilobase of exon per million mapped reads (FPKM) method. R statistical package software EdgeR was used for differential expression analysis.
LC–MS/MS for protein identification
Protein immune-precipitation was performed using GPAT4 antibody in the HUVEC cells and precipitated proteins were in-gel digested by adding 1% trypsin and incubating at 37 °C overnight to peptides. The AB SCIEX Zeno TOF 7600 mass spectrometer and Waters MicroLC system were used for IDA(MS) analysis. The LC gradient were prepared with Buffer A (0.1% formic acid and 2% acetonitrile in MS water) and Buffer B (0.1% formic acid and 2% water in MS acetonitrile). The analytical column is loaded with peptide samples at a concentration of 0.1 μg/μL, with 4 μL per sample. Separation is performed using a linear gradient from 3% to 80% Buffer B over 46 min at a flow rate of 5 μL/min. The column is then washed with Buffer B for 2 min and re-equilibrated with 3% buffer for 7 minutes. The resulting raw wiff files are subjected to ProteinPilotTM software searching, with a false discovery rate (FDR) controlled below 1%. We utilized ProteinPilotTM software for the database search of raw mass spectrometry data, employing a stringent FDR cutoff of less than 1%. Proteins identified with a minimum of two unique peptides were considered as validly identified proteins
Statistical analysis
All data analyses were performed with GraphPad Prism software, version 8.0 (GraphPad Software). ImageJ (National Institutes of Health) was applied for quantification study. Statistical comparisons were carried out using the two-tailed Student’s t tests. A value of P < 0.05 (*) was considered statistically significant; P < 0.01 (**), and P < 0.001 (***) were considered very statistically significant. ns represented no significant difference. In all the figures, measurements were reported as the mean ± SEM. The representative data shown as IP-WB, IF staining images and Proximity/overlapping analysis were obtained from at least three independent experiments (for Figs. 3 H, K, L, 4F, H, 5 I, T).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the conclusion of this study are available within the paper and the Supplemental information. The RNA-seq data have been deposited in the NCBl Sequence Read Archive under accession number PRJNA1170849. The protein mass spectrometry data have been deposited in ProteomeXchange under accession number PXD061136. Source data are provided with this paper.
References
Zhang, H., Lui, K. O. & Zhou, B. Endocardial Cell Plasticity in Cardiac Development, Diseases and Regeneration. Circ. Res 122, 774–789 (2018).
Luxan, G., D’Amato, G., MacGrogan, D. & de la Pompa, J. L. Endocardial Notch Signaling in Cardiac Development and Disease. Circ. Res 118, e1–e18 (2016).
D’Amato, G. et al. Endocardium-to-coronary artery differentiation during heart development and regeneration involves sequential roles of Bmp2 and Cxcl12/Cxcr4. Dev. Cell 57, 2517–2532 e2516 (2022).
Wu, B. et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 151, 1083–1096 (2012).
Del Monte-Nieto, G. et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 557, 439–445 (2018).
de la Pompa, J. L. & Epstein, J. A. Coordinating tissue interactions: Notch signaling in cardiac development and disease. Dev. Cell 22, 244–254 (2012).
Bressan, M. et al. Reciprocal myocardial-endocardial interactions pattern the delay in atrioventricular junction conduction. Development 141, 4149–4157 (2014).
Saint-Jean, L. et al. Myocardial differentiation is dependent upon endocardial signaling during early cardiogenesis in vitro. Development 146, https://doi.org/10.1242/dev.172619 (2019).
Miao, Y. et al. Intrinsic Endocardial Defects Contribute to Hypoplastic Left Heart Syndrome. Cell Stem Cell 27, 574–589 e578 (2020).
Tian, Y. & Morrisey, E. E. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ. Res. 110, 1023–1034 (2012).
Lu, P. et al. Prerequisite endocardial-mesenchymal transition for murine cardiac trabecular angiogenesis. Dev. Cell 58, 791–805 e794 (2023).
Garcia-Pavia, P. & de la Pompa, J. L. Left ventricular noncompaction: a genetic cardiomyopathy looking for diagnostic criteria. J. Am. Coll. Cardiol. 64, 1981–1983 (2014).
Luxan, G. et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med 19, 193–201 (2013).
Valentine, W. J. et al. Update and nomenclature proposal for mammalian lysophospholipid acyltransferases, which create membrane phospholipid diversity. J. Biol. Chem. 298, 101470 (2022).
Yu, J. et al. Update on glycerol-3-phosphate acyltransferases: the roles in the development of insulin resistance. Nutr. Diab. 8, 34 (2018).
Nagle, C. A. et al. Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6-/- mice. J. Lipid Res 49, 823–831 (2008).
Phillips, M. J. & Voeltz, G. K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69–82 (2016).
Cohen, S., Valm, A. M. & Lippincott-Schwartz, J. Interacting organelles. Curr. Opin. Cell Biol. 53, 84–91 (2018).
Senft, D. & Ronai, Z. A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 40, 141–148 (2015).
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
van Vliet, A. R. & Agostinis, P. When under pressure, get closer: PERKing up membrane contact sites during ER stress. Biochem Soc. Trans. 44, 499–504 (2016).
Haynes, C. M. & Ron, D. The mitochondrial UPR - protecting organelle protein homeostasis. J. Cell Sci. 123, 3849–3855 (2010).
Vannuvel, K., Renard, P., Raes, M. & Arnould, T. Functional and morphological impact of ER stress on mitochondria. J. Cell Physiol. 228, 1802–1818 (2013).
Perez-Trevino, P., Velasquez, M. & Garcia, N. Mechanisms of mitochondrial DNA escape and its relationship with different metabolic diseases. Biochim Biophys. Acta Mol. Basis Dis. 1866, 165761 (2020).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Schroder, M. & Kaufman, R. J. The mammalian unfolded protein response. Annu Rev. Biochem 74, 739–789 (2005).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Katona, M. et al. Capture at the ER-mitochondrial contacts licenses IP(3) receptors to stimulate local Ca(2+) transfer and oxidative metabolism. Nat. Commun. 13, 6779 (2022).
Yuan, M. et al. IP3R1/GRP75/VDAC1 complex mediates endoplasmic reticulum stress-mitochondrial oxidative stress in diabetic atrial remodeling. Redox Biol. 52, 102289 (2022).
Zhang, X., Bai, X. C. & Chen, Z. J. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity 53, 43–53 (2020).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Yang, Z., Zhao, X., Xu, J., Shang, W. & Tong, C. A novel fluorescent reporter detects plastic remodeling of mitochondria-ER contact sites. J Cell Sci 131, https://doi.org/10.1242/jcs.208686 (2018).
Zhang, Z. et al. CGI1746 targets sigma(1)R to modulate ferroptosis through mitochondria-associated membranes. Nat. Chem. Biol. 20, 699–709 (2024).
Kim, J. et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366, 1531–1536 (2019).
Xu, M. et al. The SRCAP chromatin remodeling complex promotes oxidative metabolism during prenatal heart development. Development 148, https://doi.org/10.1242/dev.199026 (2021).
Zhao, K., Huang, X., Zhao, W., Lu, B. & Yang, Z. LONP1-mediated mitochondrial quality control safeguards metabolic shifts in heart development. Development 149, https://doi.org/10.1242/dev.200458 (2022).
Michalak, M. & Opas, M. Endoplasmic and sarcoplasmic reticulum in the heart. Trends Cell Biol. 19, 253–259 (2009).
Zhao, Z. Endoplasmic reticulum stress in maternal diabetes-induced cardiac malformations during critical cardiogenesis period. Birth Defects Res B Dev. Reprod. Toxicol. 95, 1–6 (2012).
Feng, Q. et al. PDK1 regulates vascular remodeling and promotes epithelial-mesenchymal transition in cardiac development. Mol. Cell Biol. 30, 3711–3721 (2010).
Jiao, K. et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 17, 2362–2367 (2003).
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (2019YFA0801601) and from the National Natural Science Foundation of China (91854111, 31930029 and 92468103) to Zhongzhou Yang, and from the National Natural Science Foundation of China (3247122 and 32170786) to Xiong Su, and from the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX23_0140) to Tianyang Zhao. We thank Dr. Xiao Yang (Beijing Institute of Lifeomics, Beijing) for providing the Tie2-Cre mice, and thank Bin Zhou (Albert Einstein College of Medicine, New York) for providing the cTnT-Cre mice. We are indebted to Gempharmatech Co. Ltd. (Nanjing) for providing the Infar1 and Sting knockout mice. We are grateful to Dr. Chao Tong (Zhejiang University) for providing the Mito-ER contacts (MERCs) reporter plasmids.
Author information
Authors and Affiliations
Contributions
Conceptualization: Yang Z., Yang H., and Gao M. Methodology: Zhao T., Wang Y., and Su X. Investigation: Zhao T., Jin K. and Wang X. Supervision: Yang Z., Luo W. and Gao M. Writing – original draft: Zhao T. Writing – review & editing: Yang Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks A Phillip West, Sergio Lavandero, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, T., Jin, K., Wang, X. et al. GPAT4 sustains endoplasmic reticulum homeostasis in endocardial cells and safeguards heart development. Nat Commun 16, 3345 (2025). https://doi.org/10.1038/s41467-025-58722-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58722-5