Abstract
The efficient electrochemical CO2 reduction to C2+ products at high current densities remains a significant challenge. Here we show inherently hydrophobic and hierarchically porous Cu supraparticles comprising sub-10 nm Cu constituent particles for ampere-level CO2-to-C2+ electrosynthesis. These supraparticles feature abundant grain boundaries for high C2+ selectivity, coupled with interconnected mesopores and interparticle macropore cavities to enhance the accessibility of the active sites and mass transfer, breaking the trade-off between activity and mass transfer in Cu-based catalysts. Moreover, the intrinsic hydrophobicity of the supraparticles mitigates the water-flooding issue of catalytic layer in flow cells, improving the stability at high current densities. Consequently, the Cu supraparticles achieve ampere-level CO2 electrolysis up to 3.2 A cm-2 with a C2+ Faradaic efficiency of 74.9% (compared to 1.21 A cm-2 and 55.4% for Cu nanoparticles) and maintain stability at 1 A cm-2 for over 100 h. This work provides profound insights into the effect of the coupling of mass transfer and catalytic reaction under a high current and presents a corresponding solution by superstructure design.
Similar content being viewed by others
Introduction
The electrocatalytic CO2 reduction reaction (ECO2RR), powered by the renewable electricity, holds great potential not only for producing valuable fuels and chemicals but also for contributing to a sustainable carbon cycle1,2,3. Among the various electrocatalysts developed CO2RR4,5,6, Cu-based materials have emerged as the most effective for producing valuable multicarbon products (C2+), such as ethylene (C2H4) and ethanol (C2H5OH), ascribe to the moderate CO* binding energy, which facilitates the C–C coupling7,8,9,10,11,12,13. Moreover, the ECO2RR in the electrolytic cell equipped with the gas diffusion electrode (GDE) is predominantly a gas-liquid-solid triple-phase interface reaction (TPIR), with gas diffusion limitation14,15,16. Therefore, for practical applications, ECO2RR catalysts must exhibit both superb activity and excellent mass transfer capabilities to ensure efficient operation at high current densities for the production of C2+ products17,18,19,20.
Grain boundary sites have been recognized as efficient sites for the formation of C2+ product21,22,23,24. However, in conventional nanoparticle (NPs)-based catalyst layers, smaller particle sizes promote the formation of the grain boundary sites and improve active site exposure, while larger interparticle voids, resulting from larger particle sizes, facilitate mass transfer. As a result, a trade-off exists between optimizing mass transfer and maximizing catalytic activity25,26,27. To address this issue, the design of porous Cu-based materials has been identified as an effective strategy to integrate interfacial mass transportation and active site modulation for achieving CO2-to-C2+ conversion at high current densities25,28,29,30.
However, the fabrication of well-defined porous Cu based materials often involves complex and time-consuming processes, such as chemical etching or sacrificial template methods25,30,31,32. Moreover, the resulting catalysts often exhibit large particle or crystallite sizes, ranging from tens to hundreds of nanometers25,30,31, which compromises the activity for C2+ products and limits active site exposure. Therefore, addressing the trade-off between mass transfer and the catalytic activity in Cu-based materials remains a significant challenge.
Herein, we prepared Cu2O supraparticles composed of sub-10 nm-sized Cu2O constituent particles via the spontaneous evolution of copper hydride (CuH) supraparticles, which possess abundant inherent mesopores (3–10 nm) and large interparticle voids (>100 nm). During the ECO2RR process, Cu2O supraparticles are in situ reduced to metallic Cu while maintaining a similar supraparticle structure, and abundant grain boundaries form between the supraparticle interfaces. The maintained interconnected porous suprastructure significantly enhances the active site exposure and mass transfer, while the formation of abundant grain boundary sites greatly facilitates the *OC–CHO coupling pathway, effectively breaking the trade-off between efficient mass transfer and the superior activity. Additionally, the intrinsic hydrophobicity of the supraparticles alleviates water-flooding in the catalytic layer of flow cell, enhancing stability, especially under high current densities. Benefiting from this effective coupling of mass transfer and catalytic reaction, the well-defined metallic Cu supraparticles demonstrate remarkable capability for ampere-level CO2 electrolysis, achieving a high C2+ current density of 2.40 A cm−2 with corresponding Faradic efficiency (FE) of 74.9% (1.21 A cm−2 and 55.4% for Cu nanoparticles counterpart), and demonstrating a satisfactory stability at 1 A cm−2 for over 100 h.
Results and discussion
Design and characterization of Cu2O supraparticles
Interface-connected Cu2O supraparticles (SP-Cu2O) were synthesized via the spontaneous evolution of assembled CuH supraparticles under ambient condition (Fig. 1a; the detailed synthesis process is described in the “Methods” section). The dynamic structure evolution of CuH was tracked by X-ray diffraction (XRD) and X-ray absorption spectroscopy (XAS). Based on the XRD spectra (Fig. 1b), the initial sample undergoes a transformation into a mixed phase of CuH and Cu2O within the first 10 h, followed by complete conversion to pure Cu2O phase after 30 h, which remains stable in air. According to Scherrer equation, the crystalline diameters of initial CuH and derived Cu2O are estimated to be 5.0 and 8.5 nm (Supplementary Table 1), respectively. Moreover, the Cu K-edge X-ray absorption near-edge structure (XANES) spectra reveal that both CuH and its spontaneously-derived Cu2O exhibit oxidation states similar to that of standard Cu2O (Supplementary Fig. 1). The FT k3χ(k) functions of extended X-ray absorption fine structure (EXAFS) data in R-space show gradually intensified Cu-O bond signal strength, indicating the transformation of CuH to Cu2O (Fig. 1c).
a Schematic demonstration for the synthesis of CuH-derived SP-Cu2O. XRD patterns (b) and Cu K-edge EXAFS spectra (c) for CuH evolving process under ambient condition. SEM (d, e) and TEM (f) images, inset in (d) is the size distribution diagram. g pore-size distribution curve obtained from N2 adsorption-desorption measurements. Finite element simulation of triple-phase reaction interfaces: the hydroxide concentration flux for the models without (h) and with (i) mesopores (10 nm in diameter); the current distribution at the triple-phase reaction interfaces based on simplified 2D catalyst models without (j) and with (k) mesopores. Source data are provided as a Source Data file.
Scanning electron microscopy (SEM) images (Fig. 1d, e) show that the derived Cu2O supraparticles (SP-Cu2O) are assembled from smaller NPs and exhibit an open porous structure with an average diameter of 468 ± 78 nm. Transmission electron microscopy (TEM) and high resolution (HR)-TEM images of SP-Cu2O further demonstrate that the average diameter of the constituent NPs is 8.3 ± 0.9 nm (Fig. 1f and Supplementary Fig. 2), consistent with the XRD results. Moreover, these smaller constituent NPs are interconnected, giving the assembled SP-Cu2O an inherent porous structure (Supplementary Fig. 3). As confirmed by the N2 adsorption-desorption measurements (Fig. 1g and Supplementary Fig. 4), SP-Cu2O possess abundant mesopores (pore diameters primarily from 3 to 10 nm), which could facilitate the exposure of interior active sites.
As for the ECO2RR, the effective diffusion of in situ produced OH− is essential for mitigating alkalinity, which obstructs CO2 transfer to active sites33,34,35. To reveal the diffusion behavior of OH− within pores of supraparticles, we conducted the finite element simulation of the triple-phase interface using simplified two-dimensional (2D) catalyst models with/without mesopores (ca. 10 nm in diameter; Fig. 1h–k). Clearly, the model without mesopores shows severe OH− accumulation in the catalyst, as indicated by the OH− concentration distribution (Fig. 1h). For the model with mesopores, the porous channels provide efficient transport path for OH− flux (Fig. 1i), facilitating CO2 transfer to the interior pores of supraparticles for subsequent conversion. The mesopore-intensified reaction interfaces generate much higher current density compared to that without mesopores (Fig. 1j, k), highlighting the potential of suprastructure in achieving the efficient ECO2RR performance.
Characterization and finite element simulation of catalyst layer
The ECO2RR performance of SP-Cu2O relies not only on the accessibility of active sites provided by their intrinsic mesopores but also the efficient gas-liquid transport through the packing pore network of catalyst layer. To elucidate the effect of packing pores on mass transfer, we carried out the structure characterization and gas-liquid transport simulation of catalytic layer, comparing SP-Cu2O with conventional Cu nanoparticles (NP-Cu) of similar particle sizes but lacking supraparticle structure (Supplementary Fig. 5; see the synthesis process for NP-Cu in “Methods” section). From the results of mercury intrusion porosimetry (Supplementary Fig. 6), SP-Cu2O possesses a bimodal pore system comprising intrinsic mesopore and interparticle macropores centered at ~250 nm, whereas NP-Cu exhibits monomodal packing macropores centered at ~116 nm. Notably, SP-Cu2O demonstrates the higher geometric tortuosity (τ = 38.4 vs. 25 for NP-Cu) yet paradoxically shows the superior permeability (191.2 mDarcy vs. 176.2 mDarcy). This apparent deviation from classical tortuosity-permeability correlation suggests the presence of interconnected macroporous “highways” in SP-Cu2O, which enables low-resistance mass transport.
Moreover, focused ion beam (FIB)-SEM tomography was performed to directly visualize the packing pore structure of SP-Cu2O and NP-Cu (Fig. 2a–d). Evidently, SP-Cu2O presents a homogeneous distribution of interconnected macropores (Fig. 2a, c), forming continuous transport routes. In contrast, NP-Cu features dispersed macropores with limited interconnectivity (Fig. 2b, d). To quantitatively analyze these findings, we reconstructed 2D pore networks from the high-resolution FIB-SEM images (Fig. 2c, d) using a validated contrast correction algorithm36. The resultant models clearly confirm that the SP-Cu2O catalyst layer forms the interconnected macropore pathways, whereas NP-Cu only has fragmented macropores and interconnected narrow pore channels.
FIB-SEM images of SP-Cu2O (a) and NP-Cu (b) catalyst layers. Extract process of packing pore models for (c) SP-Cu2O and (d) NP-Cu. Finite element simulation of gas-liquid transport within the packing pores: diffusion velocity of (e) OH− and (f) CO2 for SP-Cu2O; diffusion velocity of (g) OH− and (h) CO2 for NP-Cu. For (e–h), the electrolyte (1 M KOH) and CO2 flow in opposite directions through the upper and lower boundaries of the porous model, respectively.
Beyond the hierarchical porosity, the supraparticle architecture endows SP-Cu2O with intrinsic hydrophobicity as evidenced by a water contact angle of 145° (Supplementary Fig. 7), in sharp contrast to the hydrophilic NP-Cu with a value of 14° (Supplementary Fig. 8). The strong hydrophobicity has the potential to alleviate the water flooding phenomenon prevalent in conventional catalytic layers, thereby enhancing the three-phase boundary dynamics in GDEs37. To factor in this critical property during our mass-transfer analyses, we incorporated the hydrophilicity/hydrophobicity characteristics into the subsequent simulations, enabling the more precise assessment of the gas-liquid transport behavior within the catalytic layer.
Figure 2e–h shows the results of finite element simulations of coupled gas-liquid transport. Under counter-current flow conditions (the electrolyte flowing downward and CO2 flowing upward), the interconnected macroporous network substantially increases the OH− diffusion velocity (Fig. 2e) compared to the narrow pore pathways in NP-Cu (Fig. 2g). This accelerated OH− transport in turn facilitates CO2 mass transfer, as indicated by the significantly enhanced CO2 diffusion velocity within the macropore transport pathway in SP-Cu2O (Fig. 2f, h). The simplification of disregarding the contribution of intrinsic mesopores results in an underestimation of the overall mass transport capacity of SP-Cu2O. Nevertheless, compared with conventional nanoparticle assemblies, the characteristic macroporous channels in the supraparticle catalytic layer fundamentally enhance gas-liquid transport during ECO2RR. In the context, multiscale characterization and simulations have established that the supraparticle structure enables the synergistic optimization of active site accessibility (through intrinsic mesopores) and mass transport efficiency (via macroporous networks), providing a paradigm for designing high-performance catalytic systems.
Monitoring the evolution of SP-Cu2O during ECO2RR
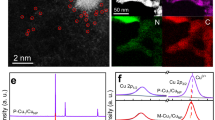
Due to the tendency of non-zero-valent Cu-based catalysts to undergo reduction during the ECO2RR, identifying their valence state changes during is crucial for determining the true active sites. This understanding is essential for the design and synthesis of effective Cu-based CO2RR catalyst8,38,39. However, the oxidation of low-valence Cu in air complicates the investigation of the surface chemical information of post-reaction samples. To address this issue, we conducted flow-cell tests in an argon-filled glovebox and the catalysts were then transferred to a vacuum suitcase before being moved to the XPS chamber. These quasi-in-situ XPS measurements allowed us to exclude the influence of ambient gas and accurately capture the chemical states of the Cu surfaces after reaction. Clearly, the Cu LMM Auger spectra (Fig. 3a) and XPS valence spectra (Fig. 3b) exhibit major peaks at 918.6 and 2.9 eV, respectively, evidencing the chemical state of Cu040,41. Moreover, XPS depth profiles for the post-reaction SP-Cu2O shows no difference between its surface and inner chemical states (Fig. 3a, b), indicating that SP-Cu2O was fully transformed into metallic Cu.
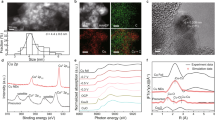
Quasi-in-situ XPS characterization and depth profiles at −0.6 V: Cu LMM Auger spectra (a) and band spectra (b). In situ XRD characterization: c spectra at different applied potentials, d time-evolved XRD heatmap at −1.0 V and e time-dependent crystal size of reduced SP-Cu2O. In situ XAFS at −1.0 V: f XANES spectra and g the corresponding first derivative spectra, h EXAFS spectra and i wavelet transforms of the Cu K-edge EXAFS. a–i Potentials are not iR corrected. Source data are provided as a Source Data file.
Furthermore, time-evolved in situ XRD characterization was employed to track the dynamic structure evolution of SP-Cu2O during ECO2RR (Fig. 3c–e). As depicted in Fig. 3c, SP-Cu2O partially transforms into metallic Cu after just 10 min at −0.4 V vs. RHE (unless otherwise stated, all potentials are referenced to the reversible hydrogen electrode, RHE), with almost no Cu2O remaining after 30 min at the same potential. At higher overpotentials (−0.6, −0.8, −1.0 V), only metallic Cu is observed, which is consistent with the results of quasi-in-situ XPS characterizations. The time-evolved XRD patterns of SP-Cu2O at −1.0 V (Fig. 3d) indicate that SP-Cu2O is reduced to metallic Cu within 300 s. According to the Scherrer equation, the time-dependent crystalline diameters of the constituent NPs of SP-Cu2O after 300 s at −1.0 V are estimated (Fig. 3e). The initial size of the constituent NPs is ca. 6.7 nm, gradually increasing to ca. 9.0 nm (Supplementary Table 2) before stabilizing, demonstrating good size stability during ECO2RR.
In situ XAS was further employed to track the changes in the average chemical states and atomic structures of SP-Cu2O during ECO2RR (Fig. 3f-i). According to the XANES spectra of Cu K-edge and their first derivative spectra (Fig. 3f, g), SP-Cu2O is reduced to metallic Cu within the first 10 min at −1.0 V and remain stable during the subsequent reaction process. In addition, the corresponding k3-weighted FT-EXAFS show that Cu-Cu peak of metallic Cu at 2.23 Å emerges and Cu-O bond at 1.54 Å disappears concurrently in the SP-Cu2O (Fig. 3h and Supplementary Table 3) as the reaction progresses, indicating the reduction of SP-Cu2O and the formation of metallic Cu. Moreover, the coordination number of Cu for the sample after 60 min of reaction is determined to be ca. 9.2, much lower than that of bulk Cu foil, suggesting the presence of abundant low-coordination sites (such as step or grain boundary sites) within the reduced SP-Cu2O. We further applied the wavelet transforms of the Cu K-edge EXAFS to visually examine the changes in coordination features during ECO2RR. Two regions are observed at ca. 1.54 and 2.23 Å (Fig. 3i), corresponding to the Cu-O and Cu-Cu coordination of initial Cu2O and the Cu-Cu coordination of metallic Cu, respectively. As the reaction progresses, the peak at 1.54 Å gradually diminishes, while the peak at 2.23 Å progressively intensifies, further confirming that SP-Cu2O is gradually reduced to metallic Cu during the ECO2RR. Furthermore, SEM (Supplementary Fig. 9), TEM and HAADF-STEM (Supplementary Fig. 10) were conducted on reduced SP-Cu2O collected in an argon-filled glovebox. The mesoporous structure of the post-reduction samples is well preserved (Supplementary Fig. 10a, b) with rich grain boundaries containing Cu(111) and Cu(100). The above results indicate that the prepared SP-Cu2O is in situ reduced to metallic Cu during the ECO2RR, featuring abundant defects and grain boundary sites, while the size of its constituent particles remains largely unchanged.
To disentangle structural and chemical state effects, a CuH-derived Cu supraparticle catalyst with primary particle size of 22.1 nm (SP-Cu) was synthesized (Supplementary Figs. 11–13 and Supplementary Table 1 in the Supplementary Information). In situ XRD (Supplementary Fig. 14 and Supplementary Table 2) and quasi-in situ XPS (Supplementary Fig. 15) confirm identical metallic states for SP-Cu and conventional NP-Cu, eliminating chemical state variability as a performance determinant. This controlled comparison isolates structural advantages (e.g., grain boundaries, pore structure) as the origin of the disparity in the ECO2RR performance. In addition, from the hydroxide electrosorption (OHad) studies (Supplementary Fig. 16), the three-investigated catalysts have the similar distribution of exposed Cu(100), Cu(110), and Cu(111) facets, further excluding the crystal face effect on the ECO2RR.
ECO2RR performance evaluation
The ECO2RR performances of SP-Cu2O, SP-Cu, and NP-Cu were evaluated in a home-made flow cell (Supplementary Fig. 17) with CO2-saturated 1 M KOH as the electrolyte. To maintain consistency throughout the text, we will continue to refer to the prepared catalyst as SP-Cu2O in this section, even though it was converted to metallic Cu during the ECO2RR. Linear scanning voltammogram (LSV) curves (Fig. 4a) show that the instantaneous current densities of the SP-Cu2O reach 3.48 A cm−2 at −0.9 V, higher than that of both SP-Cu (2.62 A cm−2) and NP-Cu (1.21 A cm−2), indicating the combination of supraparticle structure and abundant grain boundary sites endows SP-Cu2O with higher ECO2RR activity. To further evaluate product selectivity, the gas and liquid products were determined and quantified by gas chromatography (Supplementary Fig. 18) and 1H NMR spectrometer (Supplementary Fig. 19), respectively. The Faradaic efficiencies (FE) of the products on SP-Cu2O, SP-Cu, and NP-Cu at different applied current densities are depicted in Fig. 4b and Supplementary Figs. 20–22. As the current density increases, the C2+ selectivity of all catalysts gradually rises while the FE of CO decreases, suggesting that adsorbed CO (CO*) acts as a key intermediate for C–C coupling42,43. Consequently, the increased C2+ formation suppresses the desorption of CO* into CO.
a LSV curves of SP-Cu2O, SP-Cu, and NP-Cu in 1 M KOH solution fed with CO2 gas. b FE of all detected products at different applied current densities on SP-Cu2O. c FE of C2+ products; d C2H4 partial current densities, e C2H5OH partial current densities and f C2+ partial current densities of SP-Cu2O, SP-Cu, and NP-Cu. g full-cell energy conversion efficiency (ECEfull-cell) for C2H4, C2H5OH, and C2+ production of SP-Cu2O in 3 M KOH solution. h Stability test on SP-Cu2O at 1 A cm−2 in 3 M KOH solution. a, c–f The 90% iR compensation was conducted to calculate the applied potential. The error bars represent standard deviations from at least three independent measurements. Source data are provided as a Source Data file.
As for the potential-dependent FE (Fig. 4c), SP-Cu2O achieves a high FE 74.9% for C2+ at −0.94 V, which is significantly higher than those of SP-Cu (63.1%) and NP-Cu (55.4%). Specifically, C2H4 and C2H5OH are the main C2+ products and their partial current densities are shown in Fig. 4d and Fig. 4e, respectively. At −0.94 V, the SP-Cu2O exhibits partial current densities of 1.23 and 0.79 A cm−2 for C2H4 and C2H5OH, respectively, which are 1.95 and 1.40 times higher than those of SP-Cu, and 4.03 and 3.21 times than those of NP-Cu. These values also exceed most previously reported results (Supplementary Table 4)44,45,46, indicating the decent C2+ product selectivity of SP-Cu2O. Overall, SP-Cu2O exhibits significantly higher total C2+ partial current densities (2.40 A cm−2) than that of SP-Cu (1.52 A cm−2) and NP-Cu (0.66 A cm−2) in 1 M KOH electrolyte (Fig. 4f). These results underscore the importance of hierarchically porous suprastructure and abundant in situ derived grain boundary sites in achieving high C2+ activity and selectivity. To evaluate the intrinsic CO2RR activity, Pb underpotential deposition (UPD) experiments21 (Supplementary Fig. 23) were conducted to determine the ECSAs of SP-Cu2O, SP-Cu, and NP-Cu for the calculation of ECSA-normalized C2+ partial current densities. As shown in Supplementary Table 5, SP-Cu2O exhibits a higher ECSA-normalized C2+ partial current densities than that of SP-Cu and NP-Cu, indicating a high intrinsic activity of SP-Cu2O. The C2+ selectivity of SP-Cu2O can be further optimized by tuning the electrolyte concentration47,48,49 and the FE of C2+ can reach as high as 83.4% at 1.6 A cm−2 in 3 M KOH electrolyte (Supplementary Fig. 24).
Subsequently, a full electrolyzer system (Supplementary Table 6) was constructed, incorporating a cost-effective NiFe/Ni foam anode3 (Supplementary Fig. 25) with the anode’s geometric surface area intentionally scaled to fourfold that of the cathode. This design strategy serves dual purposes: (1) mitigating the oxygen evolution reaction overpotential by reducing anodic current density through enhanced active site availability, and (2) minimizing mass transport limitations at high current densities. The resultant configuration establishes well-defined kinetic conditions that enables the evaluation of the ultimate performance of SP-Cu2O within the specific full-electrolyzer setup. As a result, the system achieves a total current density of 1.24 A cm−2 with 79.4% C2+ Faradaic efficiency at 3.0 V without iR compensation (Supplementary Fig. 26). Notably, it attains 30.0% full-system energy-conversion efficiency at a C2+ partial current density of 1.04 A cm−2 (Fig. 4g). Moreover, SP-Cu2O maintains the total ECO2RR FEs and C2+ FEs around 90.0 % and 70.0 % over 108-h electrolysis at 1 A cm−2 in 3 M KOH solution (Fig. 4h and Supplementary Fig. 27), demonstrating the significant stability of SP-Cu2O.
Additionally, Pb-UPD curves (Supplementary Fig. 23) show that SP-Cu2O presents a Pb-stripping peak at −0.55 V vs. Hg/Hg2SO4, which is correlated to the undercoordinated and defective Cu sites, probably as grain boundaries21. Clearly, the intensity of such a Pb-stripping peak on SP-Cu2O is significantly higher than that on SP-Cu, proving more grain boundaries on SP-Cu2O. The enriched grain boundaries probably act as highly-active sites and the well-reserved mesoporous structure of supraparticles promotes the mass transfer, thus enabling SP-Cu2O with high activity, selectivity, and stability for ECO2RR.
Operando probing ECO2RR intermediates and interfacial sites
It is known that triple-phase reaction region spans tens to hundreds of nanometers and is nearly impossible to quantify for evaluating the GDE quality. In this case, the electrolysis performance serves as an indicator to draw conclusions, but a deep understanding is lacking. To address this, we performed operando Raman tests with a flow cell to probe the evolution of reaction intermediates and interfacial sites on GDE during ECO2RR (Fig. 5a). It should be noted that the laser beam of Raman spectrometer can reach the surface or near-surface of GDE-electrolyte interface through the pore channels of catalytic layer. As a result, the reaction interface containing information about intermediates and catalytic sites information can be inferred from the collected Raman signals (Fig. 5a).
a Schematic illustration of operando Raman tests for probing the reaction intermediates and interfacial sites. Raman spectra collected at −0.6 V vs. RHE in 1 M KOH (pH = 13.5) on b SP-Cu2O, c SP-Cu and d NP-Cu. e, f Continues Raman spectra collected at different applied potentials on SP-Cu2O. g In situ FTIR spectra in CO2-saturated 0.1 M KHCO3 (pH = 6.8) at different applied potentials on SP-Cu2O. b–g Potentials are not iR corrected. Source data are provided as a Source Data file.
Figure 5b-d show the Raman spectra collected at −0.6 V for SP-Cu2O, SP-Cu, and NP-Cu, respectively. No characteristic peak assigned to Cu2O is observed for all three catalysts during ECO2RR, indicating the metallic Cu serve as the active site, in accordance with above in situ structural analyses of SP-Cu2O. The detailed assignments of Raman peaks are listed in Supplementary Table 7. Typically, two noticeable Raman peaks at ~2045 cm−1 and ~2088 cm−1 appear on the surface of SP-Cu2O and SP-Cu (Fig. 5b, c), which are attributed to the low frequency band (LFB) from *CO on terrace sites and the high frequency band (HFB) from CO* on step sites50,51, respectively. Analysis of the ratio of HFB/(HFB + LFB) reveals that much more step sites are present on the SP-Cu2O, further verifying that the SP-Cu2O possess more grain boundaries. Additionally, SP-Cu2O presents a dominated Cu-CO stretching vibration peak at 364 cm−1 and a greatly suppressed frustrated rotational mode at ca. 280–300 cm−1, indicating a higher CO coverage on SP-Cu2O than SP-Cu (Fig. 5c) and NP-Cu (Fig. 5d)52,53,54,55. This implies that the enriched grain boundaries serve as effective sites for concentrating CO* and thereby promote subsequent C-C coupling.
Raman spectra of SP-Cu2O at different applied potentials are shown in Figs. 5e, f. Similar peaks to those in Fig. 5b are observed at −0.1 to −0.5 V. Nevertheless, as the overpotential increases, the Raman signals correspond to ECO2RR intermediates disappear and peaks located at 384 and 615 cm-1 (Fig. 5e and Supplementary Fig. 28) that attributed to adsorbed oxygen (Cu-Oad) emerge56,57. We speculated that the continuous Raman laser beam of SP-Cu2O GDE leads to the infiltration of surface and near-surface pore channels, preventing effective triple-phase reactions from occurring in these regions and resulting in the disappear of corresponding signal. To investigate the evolution of observable metallic Cu sites on GDE, we conducted deconvolution analyses of Raman signals at 2000–2100 cm−1, as shown in Fig. 5f. As the overpotential increases, the peak intensity associated with terrace and defect sites presents an increasing trend, both originating from the reduction of Cu oxides. Moreover, the ratio of HFB/(HFB + LFB) increases with the increasing overpotential, suggesting that the evolution of Cu2O supraparticles preferentially generates defect sites, likely in the form of grain boundaries at the supraparticle interfaces. Additionally, in situ FTIR measurements on SP-Cu2O shows the obvious *COOH, *CO, and OCCHO* signals (Fig. 5g and Supplementary Fig. 29)58,59, demonstrating that the in situ-produced grain boundaries sites facilitate the C1 intermediates formation and the subsequent C–C coupling reaction.
Density functional theory simulations
To elucidate the enhanced C2+ product selectivity at grain boundary sites, we performed density functional theory (DFT) calculations to evaluate free energy profiles for three C–C coupling pathways (CO*-CO*, CO*-CHO*, and CO*-COH*) on Cu(111), Cu(100), and Cu(100)/Cu(111) boundary surfaces (Fig. 6a, Supplementary Figs. 30–32 and Supplementary Data 1). Following our established methodology60, we calculated effective free energy barriers to compare pathway preferences (see “Computational Methods”). Figure 6b and Supplementary Fig. 33 present the potential-dependent effective free energy barriers for these pathways across the three surfaces. Since CO-CO dimerization is a non-electrochemical step, the systematic comparison of the optimal pathways (Fig. 6c) suggests a critical potential-dependent transition: the dominant C-C coupling mechanism shifts from CO*-CO* to CO*-CHO* as the applied potential becomes more negative. Under optimized operational conditions, the CO*-CHO* pathway dominates across all surfaces and presents a negative ∆G of the CO* transformation to CHO* step. In this case, the effective free energy barrier of the CO*-CHO* pathway is solely determined by CO* and CHO* dimerization. As shown in Fig. 6d, the Cu(100)/Cu(111) boundary exhibits the lowest C-C formation barrier of 0.63 eV, outperforming 0.92 eV for Cu(111) and 0.75 eV for Cu(100). This hierarchy directly correlates with experimental C2+ selectivity trends, confirming grain boundaries as kinetically favorable sites for the C-C coupling.
a Top view of slabs of Cu(111), Cu(100), and Cu(100)/Cu(111) grain boundary, the yellow spheres represent the atoms on the surface, and the orange spheres represent bulk Cu atoms. b, c The effective free energy barriers of three C2 production process under varied potentials. d Free energy profiles of the preferred C–C coupling step, i.e., CO*-CHO*, over Cu(111), Cu(100), and grain boundary (GB)-enriched surface. e Reaction network of C2H4 and C2H5OH formation following the formation of OCCHO. Source data are provided as a Source Data file.
We further investigated the reaction pathways of C2H4 and C2H5OH formation on Cu(100) and Cu(100)/Cu(111) boundary surfaces. A reaction network consisting of 45 intermediates, 106 elementary steps, and 799 possible reaction pathways was generated from OCCHO* to C2H4 and C2H5OH (Fig. 6e). More details related to all elementary steps are provided in Supplementary Table 8. The C2H4 formation is slightly preferred than C2H5OH formation on both Cu(100) and Cu(100)/Cu(111) boundary. The grain boundary-enriched surface is energetically favored for C2 formation, which is consistent with our experimental observation that the grain boundary-enriched SP-Cu2O catalyst can achieve an enhanced C2+ selectivity and production compared to SP-Cu.
Discussion
In conclusion, hierarchically-porous and inherently-hydrophobic Cu supraparticles, composed of sub-10 nm constituent nanoparticles, were prepared by in situ electrochemical reduction of SP-Cu2O for efficient ECO2RR. The as synthesized catalyst possesses rich grain boundaries for high C2+ selectivity, abundant mesopores, and interparticle voids for enhanced mass transfer, and hydrophobicity for alleviated water-flooding. Consequently, the metallic Cu supraparticles achieve efficient C2+ product preparation at 3.2 A cm−2 with a high FE of 74.9%. Moreover, the as-assembled full-cell CO2 electrolysis operates stably at 1 A cm−2 in 3 M KOH solution for over 108 h, demonstrating the application potential of the prepared Cu supraparticles. Furthermore, the assembled full-cell with SP-Cu2O as cathode, reaches an energy-conversion efficiency of 30.0% at C2+ current density of 1.04 A cm−2 (and total CO2RR current density of 1.24 A cm−2). This work highlights the importance and efficiency of coupling mass transfer and activity for application-oriented catalyst design.
Methods
Chemicals
Potassium bicarbonate (KHCO3, ≥99.5%), ethanol (C2H5OH, ≥99.8%), perchloric acid and sodium perchlorate monohydrate (NaClO4·H2O, ≥98.0%) were purchased from Sinopharm Chemical Reagent Co., Ltd. Tert-butylamine borane (TBAB, ≥97%) was purchased from Shanghai Excellent Chemical Co., Ltd. Deuterium Oxide (D2O, ≥99.9%), dimethyl sulfoxide (DMSO, ≥99.9%), cupric chloride anhydrous (CuCl2, ≥99.0%), potassium hydroxide (KOH, ≥90.0%) and lead perchlorate trihydrate [Pb(ClO4)2·3H2O, ≥97%] were purchased from Adamas. All materials were used as received without further purification. Ultrapure water from a Millipore autopure system was used in all our experiments.
Synthesis of CuH
1 mmol of CuCl2 and 5 mL of 1 mol L−1 TBAB solution (C2H5OH as the solvent) were added into 200 mL of C2H5OH. After the reaction under ultrasonic water bath at a temperature of 30 ± 2 °C for 30 min, the CuH product was then filtered and washed with C2H5OH at least three times.
Synthesis of CuH-derived catalysts
The freshly prepared CuH was exposed under ambient conditions (temperature: 25 ± 2 °C, humidity: 50 ± 10%) for >30 h to obtain the spontaneously-derived catalyst with Cu2O crystal phase (designated as SP-Cu2O). For a comparison, the freshly prepared CuH was treated by Ar-saturated water to obtain the derived catalyst with Cu crystal phase (designated as SP-Cu).
Synthesis of Cu nanoparticles catalyst
10 mmol of CuCl2 dissolved in 200 mL of H2O was quickly reduced by 20 mL of 1 mol L−1 TBAB solution to form the Cu nanoparticles catalyst (designated as NP-Cu).
Characterization
Powder X-ray diffraction (XRD) patterns were collected on a Bruker AXS D8 ADVANCE powder X-ray diffractometer with a Cu Kα (λ = 1.5418 Å) radiation source, operating at 40 kV and 40 mA. SEM images were obtained on a field-emission scanning electron microscopy (Zeiss Gemini 300, Germany). TEM, HRTEM, HAADF-STEM images, and EDX mapping images were collected on an FEI talos F200x G2 operated at 200 kV. X-ray photoelectron spectroscopy (XPS) was carried out on a ThermoFisher ESCA 250XI with a monochromatic excitation source of Al Kα radiation (hν = 1486.6 eV) performed under 12 kV and 4 mA. Brunauer-Emmett-Teller (BET) surface area and Barrett-Joyner-Halenda (BJH) pore volume (desorption branch) were calculated from N2-physisorption measurements on ASAP2460. The pore size distribution curves were calculated by the nonlocal density functional theory. The water contact angles of the samples were measured using a Contact Angle Meter (Model OCA15EC).
Simulation of triple-phase reaction interface within a mesoporous particle
Finite element method (FEM) simulation was employed with COMSOL Multiphysics to simulate the triple-phase reaction interface, surface reaction and OH− concentration distribution by combining the modules of “Transport of Diluted Species”, “Electric Current” “Laminar Flow” and “Phase Field”. Two-dimensional (2D) models with and without mesoporous (10 nm in diameter) were constructed. In the module of “Transport of Diluted Species”, a reaction interface that electrolyte and CO2 gas come cross at the catalyst model from two different directions was specified. Since the reaction rate of triple-phase interface is controlled by the local OH− concentration (cOH−), as evidenced in previous work, a simplified pseudo surface reaction (CO2 + H2O + e− → CO + 2OH−, kOH- = 1/cOH−) was added for simulating current density at the triple-phase reaction interface. The OH− diffusion constant is 5.273 • 10−9 m2 s−161. The initial OH− concentration in the electrolyte is 1 mol L−1 and a constant 0.0001 mol L−1 OH− boundary condition was set on the top of the simulation box. In the “Electric Current” module, the potentials of the upper and lower boundaries were set to be 0 and 1 V, respectively. In the “Laminar Flow” module, we added two-point constraints with zero pressure on the upper boundary to ensure convergence of the model. In the “Phase Field” module, a wetting wall with a contact angle of pi/4 was used on the surface of the catalyst particles to simulate the electrolyte infiltrating.
Simulation of gas-liquid transport in the packing pores of catalyst layer
The simulation framework integrates advanced image processing techniques with multiphysics modeling in COMSOL Multiphysics. High-resolution FIB-SEM images were processed and imported for geometric reconstruction, where machine learning algorithms were employed to capture void edge features, and the Edge Difference method was applied to enhance pore boundary definition through contrast optimization between pores and particles. This approach enables precise extraction of pore-defining particle morphology and subsequent inverse selection for pore model generation. The model establishes experimentally validated boundary conditions, with the upper surface configured as the electrolyte inlet and gas outlet, and the lower surface as the electrolyte outlet and gas inlet, with other specific parameters keeping consistency with the above simulation. The computational framework incorporates the Creeping Flow module for solving Stokes equations under isothermal conditions, coupled with the Electrochemistry module to simulate the CO2 electroreduction process, utilizing reaction parameters derived from the aforementioned simulations. Furthermore, to account for material-specific interfacial properties, the Phase Field Method was implemented to resolve wettability-dependent fluid dynamics, enabling detailed analysis of liquid and gas flow behaviors under varying hydrophilic and hydrophobic conditions.
Preparation of gas diffusion electrode
To enhance the waterproofness of gas diffusion electrode (GDE) specially at the ampere-level current density, a porous PTFE membrane with 8 μm of thickness was closely attached onto the carbon fiber of the gas diffusion layer (GDL, YLS-30T, Toray) assisted by the PTFE emulsion pasting and subsequent high-temperature treatment in air at 350 °C for 2 h. To construct the catalytic layer of GDE, 20 mg of catalyst and 50 uL of Nafion solution (Dupont, 5 wt%) were ultrasonically dispersed into 0.95 mL of C2H5OH. Afterward, the catalyst ink was sprayed onto the microporous carbon layer of GDL on a hot plate at a temperature of 80 °C, to form the catalytic layer with an active area of 0.5 × 0.5 cm2 and the catalyst loading of 1.0 ± 0.1 mg cm−2.
Evaluation of Electrochemical Active Surface Area (ECSA)
ECSAs of all-investigated Cu-based catalysts were evaluated by Pb underpotential deposition (UPD) in a H-cell, with Hg/Hg2SO4 (saturated K2SO4 solution as the filling solution) and Pt foil as the reference electrode and counter electrode, respectively. Before Pb UPD, oxides within Cu electrodes were reduced at −0.4 V vs. RHE in Ar-saturated 1 M KOH solution. Afterward, the obtained electrodes were cleaned by Ar-saturated ultrapure water and then quickly transferred into an Ar-saturated solution of 0.1 M NaClO4, 10 mM HClO4, and 3 mM Pb(II)(ClO4)221. Subsequently, cyclic voltammetry (CV) with a scan rate of 10 mV s−1 was performed in a potential range of −0.50 to −0.85 V vs. Hg/Hg2SO4 to obtain the Pb UPD curves. The Cu ECSA calculation is based on the integrated charge values of Pb UPD and a conversion factor of 357.5 μC cm−2 from the benchmark of polished Cu foil62. The tests were conducted at room temperature (25 ± 2 °C) and pressure (1.01 × 105 Pa).
Electrosorption of hydroxide (OHad)
Investigations of OHad on the reduced Cu GDEs were carried out by flowing Ar through the flow-cell electrolyzer. To prevent bulk oxidation of Cu, a potential window of -0.2 to 0.55 V (vs. RHE) was employed63. Prior to testing, Cu oxides were electrochemically reduced at −0.6 V (vs. RHE) for 30 min in the flow cell under a CO2 atmosphere. Immediately following electrolysis, the gas feed was switched back to Ar, the electrolyte flow was halted, and cyclic voltammetry was subsequently performed. The test was carried out at room temperature (25 ± 2 °C) and pressure (1.01 × 105 Pa).
Electrochemical measurements and products analyses
Electrochemical measurements were carried out in a home-made flow cell (separated by Alkymer® anion exchange membrane) with three electrodes connected to a CHI 660e electrochemical workstation equipped with a high current amplifier CHI 680c. The anion exchange membrane (3.0 × 3.0 cm2) was soaked in 1.0 M KOH for 24 h and then rinsed with deionized water before use. Pt foil and Ag/AgCl electrode (with saturated KCl as the filling solution) were used as the counter and reference electrodes, respectively. The electrolyte used is 1 M KOH (pH = 13.5) and was freshly prepared for each test. Constant current electrolysis at room temperature (25 ± 2 °C) and pressure (1.01 × 105 Pa) was conducted for the performance evaluation. All potentials were converted to the RHE scale the following formulas:
\({{\rm{E}}}({{\rm{RHE}}})={{\rm{E}}}({{\rm{Ag}}}/{{\rm{AgCl}}})+0.197+0.0591 \times {{\rm{pH}}}+0.9 \times {iR}\)
The 90% iR compensation was conducted in flow cell experiment at each potential, where the resistance was determined by extrapolating high-frequency impedance data. The potential of the reference electrode (vs. RHE) in 1 M KOH solution was calibrated to 1.00 ± 0.01 V using a reversible hydrogen electrode purchased from Phychemi Co., Ltd., which was in close agreement with the theoretical calculation of 0.99 V (vs. RHE). As for the full-cell electrolysis, the used electrolyte is 3 M KOH solution, and a high-performance NiFeOx catalyst with the area of 1 cm2 was adopted as the anode, and the distance between anode and cathode is 1.0 cm. All electrolytes mentioned above were stored under airtight conditions in volumetric flasks at room temperature (25 ± 2 °C) and used within 1 week of preparation. For all performance tests, the electrolyte was circulated through the electrolyte chambers of flow cell with a flow rate of 5 mL min−1 via a peristaltic pump. High-purity CO2 was supplied to the gas chamber of flow cell with a constant flow rate of 80 mL min−1. The volumetric flowrate of outlet gas was monitored by the electronic soap-film flowmeter (BL100, Beijing Ke’an Labor Insurance New Technology Co., Ltd.). Meanwhile, the outlet gas was collected in a gas sampling bag (300 mL) during the ECO2RR and then quantified by gas chromatography (Agilent 7890B) equipped with FID and TCD detectors for analyzing the gas phase composition. The GC was calibrated by standard gases (H2, CO, CH4, and C2H4 in CO2) before use.
Faradaic efficiencies of gas products can be calculated as below:
Wherein, \({Q}_{x}\) is the spent charge for product (x) production; \({Q}_{{total}}\) is the total charge passed during the electrolysis; i is the applied current (A); t is the electrolysis time (s); zx is the number of transferred electrons for producing a molecule of product (x); \({v}_{x}\) is the volume concentration of product (x) measured by gas chromatography; \({G}_{{out}}\) is the volumetric flowrate of outlet gas; F = 96485 C/mol, P0 = 1.01 × 105 Pa, R = 8.314 J mol–1 K–1, T0 = 298 K.
Liquid products from both anolyte and catholyte, along with those carried by the outlet gas, were collected and analyzed using a Bruker Avance-III 400 MHz 1H NMR spectrometer with dimethyl sulfoxide (DMSO) as the internal standard. Sample preparation involved mixing 700 µL of electrolyte with 35 µL of 5 mM DMSO in D2O. Faradaic efficiencies of liquid products can be calculated as below:
Wherein, \({Q}_{x}\) is the spent charge for product (x) production; \({Q}_{{total}}\) is the total charge passed during the electrolysis; zx is the number of transferred electrons for producing a molecule of product (x); \({n}_{x}\) is the quantity (mol) for the products (x); i is the applied current (A); t is the electrolysis time (s); F = 96485 C/mol.
Full-cell energy conversion efficiencies of C2H4, C2H5OH and C2+ can be calculated as below:
Wherein, E0 is the thermodynamic potential (vs. RHE) for CO2RR to species x, which is 0.06 V for C2H4, 0.12 V for CH3COO-, 0.08 V for C2H5OH and 0.10 V for n-C3H7OH. E is the cell voltage in two-electrode system.
Operando Raman spectra measurements
Operando Raman spectra tests were performed using a Horiba LabRAM HR Evolution Raman spectroscopy with 633 nm excitation laser. A home-made operando cell was used for Raman signal collection. Similar to the used flow cell for performance evaluation, the GDE was equipped between the electrolyte chamber of cathodic side and gas chamber. The electrolyte chambers of cathodic side and anodic side were also separated by Alkymer® anion exchange membrane to avoid any cross-contamination.
In situ FTIR measurements
In situ FTIR spectra tests were performed using a custom-built three-electrode H-cell on a Zolix Foli20-Z-B FTIR spectrometer. The working electrode was prepared by drop-casting the catalyst ink onto a polished Au-coated Si wafer with an active area of 1 cm2. The electrolyte was CO2-saturated 0.1 M KHCO3 (pH = 6.8). To simulate the gas flow behavior within GDE, continuous CO2 gas was bubbled at a flow rate of 10 sccm onto the surface of the catalytic layer.
Ex Situ and in situ XAFS measurements
Ex situ and in situ XAFS measurements were conducted at the BL14W1 beamline in Shanghai Synchrotron Radiation Facility (SSRF) with the electron beam energy of 3.5 GeV and stored currents of 180 mA (decay)64. A 38-pole wiggler with the maximum magnetic field of 1.2 T inserted in the straight section of the storage ring was used. XAFS data were collected using a fixed-exit double-crystal Si(111) monochromator. For the in situ XAFS measurements, a custom-designed in situ electrolytic cell was utilized. The catalyst ink was dropped onto the hydrophobic carbon paper to prepare the working electrode. During the measurements, CO2 was bubbled into 0.1 M KOH. Lytle detector was used to collect the fluorescence signal of the Cu K-edge (8979 eV) XAFS spectra, and the energy was calibrated using Cu foil. The photon flux at the sample position was 2.6 × 1012 photons per second.
The raw data analysis was carried out using IFEFFIT software package in light of the standard data analysis procedure65. The spectra were calibrated, averaged, pre-edge background subtracted, and post-edge normalized, and then Cu-K edge XANES spectra and their first derivative spectra were exported using Athena program in IFEFFIT software package. The Fourier transformation of the k3-weighted EXAFS oscillations, k3·χ(k), from k space to R space was carried out to obtain a radial distribution function and data fitting was done by Artemis program in IFEFFIT.
In situ XRD measurements
In situ synchrotron XRD characterizations were conducted at the BL02U2 beamline in Shanghai Synchrotron Radiation Facility (SSRF). The XRD data were collected using a custom-designed three-electrode cell, with Ag/AgCl electrode and Pt wire as reference and counter electrodes, respectively. The working electrode was obtained as the preparation procedure of GDE, with an active area of 1 cm−2. During the measurement, the cell was filled with CO2-saturated 0.1 M KHCO3 (8 mL, pH = 6.8) and meanwhile CO2 was bubbled into the electrolyte (0.1 M KHCO3) with a flow rate of 20 mL min−1. Continuous data collection (10 s per record) was carried out in transmission geometry throughout the cell during the ECO2RR at −1.0 V/RHE. To present the comparable results, the value of 2-theta (2θsynchrotron) corresponding to the synchrotron X-ray wavelength of 0.6887 Å (λsynchrotron) was transformed into the data (2θCu Kα) corresponding to the routine Cu Kα X-ray wavelength of 1.5418 Å (λCu Kα)66.
Computational methods
DFT calculations were performed employing the Vienna Ab initio Simulation Package (VASP) code67 with projector augmented wave (PAW) method68. All the calculations were based on the generalized gradient approximation (GGA) with revised Perdew-Burke-Ernzerhof (RPBE) exchange-correlation functional69. Four-layer slabs with 4 × 4 supercells were used for Cu(111) and Cu(100) surfaces. The top-view and side-view of of Cu(111), Cu(100) and Cu(100)/Cu(111) surfaces are shown in Supplementary Fig. 30. Specifically, the Cu(100)/Cu(111) interface was constructed by removing two rows of surface Cu atoms from a 2 × 4 Cu(211) slab, as illustrated in Supplementary Fig. 31. The most favorable transition-state coupling structures for the three surfaces are shown in Supplementary Fig. 32. The Cu atoms of bottom two layers were fixed during the optimizations and the vacuum region were higher than 12 Å. The Monkhorst–Pack mesh k-point grids was 2 × 2 × 1 for Cu(100) and grain boundary-enriched surface, 3 × 3 × 1 for Cu(111) surface. The plane wave cutoff was 500 eV and the convergence criteria was 0.05 eV/Å in force and 1 × 10−4 eV in energy. The transition states were obtained by constrained minimization method70,71. As for solvent effect, we used the VASPsol method for an implicit model with the relative permittivity of 8072. To compare the preference of different C-C coupling pathways, effective free energy barriers of these pathways have been calculated as suggested in our recent work60. Supplementary Figs. 34–37 and Supplementary Note 1 illustrate the method used in the current work to calculate the effective free energy barriers for the C-C bond formation through the coupling of CO* and CHO*/COH*, with the example of OCCHO* formation. All transition state structures are provided in Supplementary Data 1.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and Supplementary Information files. Source data are provided with this paper.
References
Belsa, B. et al. Materials challenges on the path to gigatonne CO2 electrolysis. Nat. Rev. Mater. 9, 535–549 (2024).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Liu, B. et al. Photovoltaic-powered electrochemical CO2 reduction: benchmarking against the theoretical limit. ACS Energy Lett. 8, 981–987 (2023).
Jiang, M. et al. Review on strategies for improving the added value and expanding the scope of CO2 electroreduction products. Chem. Soc. Rev. 53, 5149–5189 (2024).
Ross, M. B. et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2, 648–658 (2019).
Wang, G. X. et al. Electrocatalysis for CO2 conversion: from fundamentals to value-added products. Chem. Soc. Rev. 50, 4993–5061 (2021).
Song, Z., Wang, X., Ren, Z. & Fu, H. Relationships between structural design and synthesis engineering of Cu-based catalysts for CO2 to C2 electroreduction. Chem. Eng. J. 479, 147606 (2024).
Jun, M. et al. Strategies to modulate the copper oxidation state toward selective C2+ production in the electrochemical CO2 reduction reaction. Adv. Mater. 36, 2313028 (2024).
Lu, T. et al. Electrocatalytic CO2 reduction to ethylene: from advanced catalyst design to industrial applications. Adv. Mater. 35, e2310433 (2023).
Zheng, Y. et al. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 141, 7646–7659 (2019).
Chen, C. et al. Oxidation of metallic Cu by supercritical CO2 and control synthesis of amorphous nano-metal catalysts for CO2 electroreduction. Nat. Commun. 14, 1092 (2023).
Deng, T. et al. Electrochemical CO2 reduction to C2+ products over Cu/Zn intermetallic catalysts synthesized by electrodeposition. Front. Energy 18, 80–88 (2023).
Zang, Y., Wei, P., Li, H., Gao, D. & Wang, G. Catalyst design for electrolytic CO2 reduction toward low-carbon fuels and chemicals. Electrochem. Energy Rev. 5, 29 (2022).
Chen, Q. et al. Electrocatalytic CO2 reduction to C2+ products in flow cells. Adv. Mater. 36, e2303902 (2024).
Lin, Z. et al. Recent progress on electrode design for efficient electrochemical valorisation of CO2, O2, and N2. Angew. Chem. Int. Ed. 62, e202301435 (2023).
Wang, Y. et al. Rational design of three-phase interfaces for electrocatalysis. Nano Res. 12, 2055–2066 (2019).
Weng, C.-C. et al. Engineering gas-solid-liquid triple-phase interfaces for electrochemical energy conversion reactions. Electrochem. Energy Rev. 5, 19 (2022).
Rabiee, H. et al. Regulating the reaction zone of electrochemical CO2 reduction on gas-diffusion electrodes by distinctive hydrophilic-hydrophobic catalyst layers. Appl. Catal. B Environ. 310, 121362 (2022).
Moore, T. et al. Elucidating mass transport regimes in gas diffusion electrodes for CO2 electroreduction. ACS Energy Lett. 6, 3600–3606 (2021).
Johnson, E. F. et al. Pathways to enhance electrochemical CO2 reduction identified through direct pore-level modeling. EES Catal. 1, 704–719 (2023).
Yang, Y. et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023).
Xu, X. et al. Grain boundary engineering: an emerging pathway toward efficient electrocatalysis. InfoMat 6, e12608 (2024).
Ding, J. et al. Unconventional grain fragmentation creates high-density boundaries for efffcient CO2-to-C2+ electro-conversion at ampere-level current density. Nano Energy 128, 109945 (2024).
Choi, W. et al. Exploring the influence of cell configurations on Cu catalyst reconstruction during CO2 electroreduction. Nat. Commun. 15, 8345 (2024).
Geng, Q. et al. Revolutionizing CO2 electrolysis: fluent gas transportation within hydrophobic porous Cu2O. J. Am. Chem. Soc. 146, 10599–10607 (2024).
Fu, X. et al. Evolution of atomic-scale dispersion of FeNx in hierarchically porous 3D air electrode to boost the interfacial electrocatalysis of oxygen reduction in PEMFC. Nano Energy 83, 105734 (2021).
Hou, M. et al. Manipulating and optimizing the hierarchically porous electrode structures for rapid mass transport in solid oxide cells. Adv. Funct. Mater. 32, 2203722 (2022).
Zou, C. Q. et al. Porous copper microspheres for selective production of multicarbon fuels via CO2 electroreduction. Small 15, 1902582 (2019).
Liu, L. X. et al. Enriching the local concentration of CO intermediates on Cu cavities for the electrocatalytic reduction of CO2 to C2+ Products. ACS Appl. Mater. Interfaces 15, 16673–16679 (2023).
Yang, P. P. et al. Protecting copper oxidation state via intermediate confinement for selective CO2 electroreduction to C2+ Fuels. J. Am. Chem. Soc. 142, 6400–6408 (2020).
Zheng, X. Z. et al. Highly efficient CO2 reduction on ordered porous Cu electrode derived from Cu2O inverse opals. Nano Energy 48, 93–100 (2018).
Chen, L.-Y. et al. Nanoporous copper with tunable nanoporosity for SERS applications. Adv. Funct. Mater. 19, 1221–1226 (2009).
Lim, C. et al. Breaking the current limitation of electrochemical CO2 reduction via a silica-hydroxide cycle. Energy Environ. Sci. 17, 6215–6224 (2024).
Chen, C. B. et al. Address the “alkalinity problem” in CO2 electrolysis with catalyst design and translation. Joule 5, 737–742 (2021).
Lees, E. W. et al. Gas diffusion electrodes and membranes for CO2 reduction electrolysers. Nat. Rev. Mater. 7, 55–64 (2022).
Schettini, R. et al. Contrast image correction method. J. Electron. Imaging 19, 023005 (2010).
Niu, Z. Z. et al. Hierarchical copper with inherent hydrophobicity mitigates electrode flooding for high-rate CO2 electroreduction to multicarbon products. J. Am. Chem. Soc. 143, 8011–8021 (2021).
Deng, B. et al. Active site identification and engineering during the dynamic evolution of copper-based catalysts for electrocatalytic CO2 reduction. Sci. China Chem. 66, 78–95 (2023).
Wu, Z.-Z., Gao, F.-Y. & Gao, M.-R. Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction. Energy Environ. Sci. 14, 1121–1139 (2021).
Biesinger, M. C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 49, 1325–1334 (2017).
Hüfner, S. et al. X-ray photoelectron spectra of the valence bands of some transition metals and alloys. Phys. Rev. B 8, 4511–4524 (1973).
Wang, X., Hu, Q., Li, G., Yang, H. & He, C. Recent advances and perspectives of electrochemical CO2 reduction toward C2+ products on Cu-based catalysts. Electrochem. Energy Rev. 5, 28 (2022).
Xu, L., Trogadas, P. & Coppens, M. O. Nature‐inspired electrocatalysts for CO2 reduction to C2+ products. Adv. Energy Mater. 13, 202302974 (2023).
Zheng, M. et al. Electrocatalytic CO2-to-C2+ with ampere-level current on heteroatom-engineered copper via tuning *CO intermediate coverage. J. Am. Chem. Soc. 144, 14936–14944 (2022).
Inoue, A., Harada, T., Nakanishi, S. & Kamiya, K. Ultra-high-rate CO2 reduction reactions to multicarbon products with a current density of 1.7 A cm−2 in neutral electrolytes. EES Catal. 1, 9–16 (2023).
Zhu, C. et al. Ampere-level CO2 reduction to multicarbon products over a copper gas penetration electrode. Energy Environ. Sci. 15, 5391–5404 (2022).
Dinh, C. T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Ma, W. C. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C-C coupling over fluorine-modified copper. Nat. Catal. 3, 478–487 (2020).
Li, J. et al. Hydroxide is not a promoter of C2+ product formation in the electrochemical reduction of CO on copper. Angew. Chem. Int. Ed. 59, 4464–4469 (2020).
An, H. Y. et al. Sub-second time-resolved surface-enhanced raman spectroscopy reveals dynamic CO intermediates during electrochemical CO2 reduction on copper. Angew. Chem. Int. Ed. 60, 16576–16584 (2021).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C. 121, 12337–12344 (2017).
Akemann, W. et al. Vibrational modes of CO adsorbed on disordered copper films. J. Raman Spectrosc. 22, 797–803 (1991).
Zhan, C. et al. Revealing the CO coverage-driven C-C coupling mechanism for electrochemical CO2 reduction on Cu2O nanocubes via operando raman spectroscopy. ACS Catal. 11, 7694–7701 (2021).
Feng, J. et al. Modulating adsorbed hydrogen drives electrochemical CO2-to-C2 products. Nat. Commun. 14, 4615 (2023).
Guo, W. et al. Asymmetric Cu sites for enhanced CO2 electroreduction to C2+ products. CCS Chem. 6, 1231–1239 (2024).
Zhao, Y. R. et al. Speciation of Cu surfaces during the electrochemical CO reduction reaction. J. Am. Chem. Soc. 142, 9735–9743 (2020).
Chang, X. X. et al. pH Dependence of Cu surface speciation in the electrochemical CO reduction reaction. ACS Catal. 10, 13737–13747 (2020).
Wang, J. et al. Atomically dispersed Ga synergy lewis acid-base pairs in F-doped mesoporous Cu2O for efficient eletroreduction of CO2 to C2+ products. Adv. Funct. Mater. 34, 2404274 (2024).
Zhu, H. L. et al. A porous π-π stacking framework with dicopper(I) sites and adjacent proton relays for electroreduction of CO2 to C2+ products. J. Am. Chem. Soc. 144, 13319–13326 (2022).
Liu, H. & Yang, B. Potential-dependent C-C coupling mechanism and activity of C2 formation in the electrocatalytic reduction of CO2 on defective Cu(100) surfaces. Chem. Commun. 58, 709–712 (2022).
Garcia de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm-2. Science 367, 661–666 (2020).
Sebastián-Pascual, P. et al. Surface characterization of copper electrocatalysts by lead underpotential deposition. J. Electroanal. Chem. 896, 115446 (2021).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Yu, H. et al. The XAFS beamline of SSRF. Nucl. Sci. Tech. 26, 050102 (2015).
Newville, M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 8, 322–324 (2001).
Ma, L. et al. Operando unraveling the dynamic correlation between structure evolution and performance shift during CO2 electroreduction on In2O3 electrodes. Appl. Catal. B Environ. 369, 125153 (2025).
Hafner, J. Ab-initio simulations of materials using VASP: density-functional theory and beyond. J. Comput. Chem. 29, 2044–2078 (2008).
Blochl Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Hammer, B., Hansen, L. B. & Norskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Liu, Z. P. & Hu, P. General rules for predicting where a catalytic reaction should occur on metal surfaces: a density functional theory study of C-H and C-O bond breaking/making on flat, stepped, and kinked metal surfaces. J. Am. Chem. Soc. 125, 1958–1967 (2003).
Michaelides, A. et al. Identification of general linear relationships between activation energies and enthalpy changes for dissociation reactions at surfaces. J. Am. Chem. Soc. 125, 3704–3705 (2003).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Acknowledgements
This work was partially supported by the National Key Research and Development Programs of China (2022YFA1504600 H.Y.), the Basic Science Center Program for Ordered Energy Conversion of the National Natural Science Foundation of China (51888103 Q.L.), the National Natural Science Foundation of China (52106248 L.M., 22279029 J.C.), the Innovative Funds Plan of Henan University of technology (2022ZKCJ01 J.C.), the Doctor Foundation of Henan University of Technology (2023BS010 L.M.) and the BL14W1, BL02U2 and BL03HB beamlines at Shanghai Synchrotron Radiation Facility. We gratefully acknowledge the research groups led by Prof. Chuangang Hu (Beijing University of Chemical Technology) and Profs. Zhi Liu/Fan Yang (ShanghaiTech University) for assisting with in situ characterization and insightful discussions. Special thanks are extended to Prof. Lijun Yang (Nanjing University), Beijing Wangtu Technology Co., LTD and Dr. Fu Qiang for their expert guidance in finite-element simulations. We also thank the HPC Platform of ShanghaiTech University for computing time.
Author information
Authors and Affiliations
Contributions
H.Y. and Q.L. conceived and supervised the project. L.M. carried out the catalyst’s synthesis, characterization, and performance test. B.M. and J.M. carried out the XAFS measurements and data fitting. H.L. and B.Y. performed the theoretical calculations. J.C. and Q.C. helped in material characterization and data analysis. H.Y. and L.M. wrote the manuscript. All authors discussed, commented on and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yafei Li, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, L., Liu, H., Mei, B. et al. Cu supraparticles with enhanced mass transfer and abundant C-C coupling sites achieving ampere-level CO2-to-C2+ electrosynthesis. Nat Commun 16, 3421 (2025). https://doi.org/10.1038/s41467-025-58755-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58755-w