Abstract
Before assuming its role in the mature brain, serotonin modulates early brain development across phylogenetically diverse species. In mice and humans, early-life SSRI exposure alters the offspring’s brain structure and is associated with anxiety and depression-related behaviors beginning in puberty. However, the impact of early-life SSRI exposure on brain circuit function is unknown. To address this question, we examined how developmental SSRI exposure changes fear-related brain activation and behavior in mice and humans. SSRI-exposed mice showed increased defense responses to a predator odor, and stronger fMRI amygdala and extended fear-circuit activation. Likewise, adolescents exposed to SSRIs in utero exhibited higher anxiety and depression symptoms than unexposed adolescents and also had greater activation of the amygdala and other limbic structures when processing fearful faces. These findings demonstrate that increases in anxiety and fear-related behaviors as well as brain circuit activation following developmental SSRI exposure are conserved between mice and humans. These findings have potential implications for the clinical use of SSRIs during human pregnancy and for designing interventions that protect fetal brain development.
Similar content being viewed by others
Introduction
Maternal anxiety and depression during pregnancy are associated with adverse offspring outcomes ranging from low birth weight to increased risk for psychopathology including anxiety and depression1,2,3. Affected mothers are typically prescribed selective serotonin reuptake inhibitors (SSRIs) to address these symptoms. Currently taken by ~6% of pregnant mothers4, their use during gestation is increasing5. While the rationale for improving maternal mental health is clear, the impact of maternal SSRI use on the developing fetus remains understudied.
For the gestating fetus, SSRIs easily cross the placenta and block fetal serotonin (5-HT) reuptake6. The relative safety of SSRIs on fetal health has largely been assessed in the early postnatal period7. Only a few studies have attempted to assess whether in-utero SSRI exposure impacts offspring health later in life. While SSRI-exposure in utero may have few detrimental effects on human offspring at birth and in early infancy (but see refs. 8,9,10), some studies report cognitive and behavioral abnormalities in later childhood7,11,12. Likewise, several13,14,15 but not all16,17 epidemiological studies find that SSRI use in pregnancy is associated with depression and other psychiatric disorders in adolescent and young adult offspring. The apparently higher rate of offspring mental health issues as a result of gestational SSRI exposure comports with what has been reported in rodents18,19,20,21,22,23. However, the confounding factors complicating human epidemiological studies have cast doubt on whether similar developmental mechanisms are at play in each species.
The late-emerging effects of developmental SSRI exposure on human offspring outcomes are in line with rodent studies which suggest that the sequelae only manifest after sexual maturation24. The peak brain growth (along with peaking axonal and dendritic densities, monoamine levels, neurogenesis and gliogenesis) in rodents occurs in the first two postnatal weeks, which translates to the last trimester of human pregnancy25,26,27,28. Also, in rodents, 5-HT signaling is crucial to fetal brain development and plays a role in cell proliferation, neuronal differentiation, synaptogenesis, and neuronal migration29,30,31. Increases in 5-HT signaling during developmentally sensitive periods lead to altered structure and function throughout the brain in adult mice18,19,20,21,22,23,32. 5-HT is highly conserved across evolution33,34,35, yet it remains unclear whether SSRI exposure during early brain development exerts in humans the same effects seen in rodents. To better bridge between rodent and human studies, mechanistic insight is needed that functionally links developmental SSRI exposure to altered behavior in both mice and humans with the hypothesis that such functional changes should be conserved between species.
To address this hypothesis, we adopted a cross-species approach that harnesses the controlled experimental conditions of animal studies, while directly evaluating clinical relationships through a population study, within a single study framework. To this end, we exposed mice to predator odors while awake in an animal MRI machine and assessed the effect of early-life SSRI exposure on brain circuit activation and behavior. In parallel, we utilized the Adolescent Brain Cognitive Development Study36 data of children who were subjected to emotionally laden (fearful) faces during an fMRI task and assessed the effect of in-utero SSRI exposure on brain circuit activation and behavior. By probing the circuitry of both mouse and human responses to ethologically relevant cues, we sought to determine whether similar brain-circuit effects of early SSRI exposure might be conserved across species. Our results demonstrate that early-life exposure to SSRIs indeed has conserved effects on innate fear circuit activation and behavior across phylogeny, suggesting shared mechanisms that will inform the public health implications of the broad usage of SSRI medications during pregnancy.
Results
PND2-11 fluoxetine increases innate freezing responses to a predator odor in adult mice
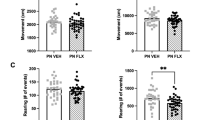
PND2-11 is a sensitive period in mice during which increased 5-HT signaling increases conditioned fear responses and anxiety in adulthood18,20. To increase translatability and establish fMRI compatibility, here we studied the effects of PND2-11 fluoxetine (PNFLX) versus saline (PNSAL) on innate fear responses to a predator odor (mountain lion urine) (Fig. 1A). On day 1 adult mice were habituated to an odor-attenuated chamber. During habituation, we found no significant differences in freezing behavior when comparing PNSAL to PNFLX animals (time x treatment interaction F1,22 = 0.4468, p = 0.5108; time effect F1,22 = 0.5761, p = 0.4559; treatment effect F1,22 = 0.668, p = 0.4225) (Fig. 1B). On day 2 mice were again placed into the testing apparatus and after 2 minutes predator odor was delivered. Two-way ANOVA analysis of freezing behavior revealed a significant interaction between treatment and predator odor (F1,22 = 4.794, p = 0.0395), a significant effect of time (F1,22 = 46.98, p < 0.0001), and a significant effect of treatment (F1,22 = 7.956, p = 0.01) (Fig. 1C). Post-hoc analysis showed that PNSAL and PNFLX animals both increased freezing in response to the odor exposure (p = 0.0023 PNSAL, and p < 0.0001 PNFLX). However, PNFLX animals mounted a significantly higher freezing response when compared to PNSAL animals (post-odor PNSAL vs. PNFLX p = 0.001). On day 2, PNFLX mice also had a lower latency to freeze during odor presentation compared to PNSAL animals (normalized to the latency to freeze on day 1, p = 0.032, Fig. 1C). Using a deep learning algorithm, deeplabcut (DLC) that automates animal pose estimation (Fig. 1E), we detected a significant main effects of predator odor on freezing (F1,22 = 9.783, p = 0.0049, Fig. 1F) and speed (F1,22 = 10.19, p = 0.0042, Fig. 1G). Post-hoc analysis showed that during the predator odor PNFLX animals freeze more than PNSAL (p = 0.0341). Together our data demonstrate that adult PNSAL and PNFLX mice both mount a behavioral response to predator odor, but that the response is exacerbated after PND2-11 FLX exposure. A similar pattern was observed in adolescent PNFLX mice compared to PNSAL mice (Supplemental Fig. 1). Notably, there was no effect of treatment on fear responses to a neutral odor (Supplemental Fig. 2).
A Timeline of the adult mouse behavioral study. Created in BioRender. Zanni, G. (2025) https://BioRender.com/m34p441. B Manual freezing score during habituation early and late phases. C Manual freezing score during context re-exposure and during predator odor. D Latency to freeze expressed as percent change of habituation freezing latency. E Labeling of different animal body parts: nose (purple), tail base (orange), right ear (electric blue), left ear (cyan), right hip (blue), left hip (light blue), mid back (yellow). Top picture shows animals exploring and the bottom picture shows an animal freezing. F Deeplab Cut freezing score during context re-exposure and during predator odor. G Deeplab Cut speed scoring during context re-exposure and during predator odor. Data are presented as mean values +/- SEM. Statistical tests used: ANOVA and Fisher’s LSD posthoc. PNSAL N = 13, PNFLX N = 11. *p < 0.05; **p < 0.01; ***p < 0.001. Source data are provided as a Source Data file.
PND2-11 fluoxetine increases BOLD response to predator odor in fear circuits
To address which brain regions are contributing to the SSRI-induced heightened innate fear response in mice we used fMRI to compare BOLD responses to predator odor in PNFLX mice and PNSAL controls (Fig. 2A). To elicit fear in awake mice we exposed animals to a predator odor in a mouse imaging holding system to which they were previously habituated to reduce motion artifacts37,38, and performed whole-brain imaging (Fig. 2B). The fMRI scans were registered to and analyzed using a 3-dimensional MRI mouse atlas with 134 bilateral segmented and annotated brain areas. Figure 2C and the table in Fig. 2D show the brain areas with a significant increase in positive BOLD volume of activation in response to predator urine odor in awake PNFLX mice compared to PNSAL mice (false discovery rate p = 0.0401) in whole-brain analysis. These areas are ranked in order of their significance and out of the whole brain (134 areas) 28 brain areas showed significantly higher BOLD signals in PNFLX animals. Virtually all these areas belong to the previously described fear neural circuit (e.g., central and medial amygdala, periaqueductal gray, anterior cingulate cortex), that comprise the ascending reticular activating system involved in arousal (e.g., pedunculopontine tegmental area, medullary reticular area, and mesencephalic reticular formation), and regions broadly involved in emotional regulation (e.g., hypothalamus, putamen, and dorsal raphe). Exemplary fMRI time-courses from the central amygdala, mesencephalic reticular formation, and periaqueductal gray demonstrate the robust kinetics of the BOLD response to the odor exposure without an effect of treatment prior to odor administration (Supplemental Fig. 3). Overall, our data demonstrate that PND2-11 fluoxetine treatment leads to an exacerbated functional response to an innate fear stimulus across neural circuits involved in fear, arousal, and emotional regulation.
A Timeline of the adult mouse fMRI study. Created in BioRender. Zanni, G. (2025) https://BioRender.com/s15z123. B Shown are the different components of the mouse imaging system. Below are sagittal and axial views of an awake mouse brain. Note the linearity along the Z-axis. The axial images taken from a 22-slice RARE sequence (0.6 mm thickness) demonstrate complete brain coverage from the olfactory bulbs to the brainstem. C Statistical map displaying relative positive BOLD signal in response to predator urine odor in awake PNFLX compared to PNSAL mice. PNSAL N = 18, PNFLX N = 26. D List of significant brain areas, such as thalamus, putamen, brainstem and cerebellum, central amygdala, substantia nigra, periaqueductal gray, raphe, and habenula ranked in order of their significance in red for change in positive BOLD volume of activation (number of voxels) in PNFLX compared to PNSAL (false discovery rate p = 0.0401). Statistical test used: Wilcox rank sum test corrected for multiple (134) comparisons.
Translation to human adolescent children from the ABCD study
In the rodents, fluoxetine was administered at PND2-11, corresponding to the third trimester in utero in humans27. Because behavioral consequences of prenatal SSRIs have been observed in adolescence10,15,39,40, we leveraged data from the ABCD study to test if our rodent findings translate to humans (Fig. 3A).
A The experimental timeline. At baseline mothers reported on their pregnancy and demographics. Children underwent an MRI scan approximately two years post-baseline, and mothers filled out the Child Behavioral Checklist (CBCL) to assess symptoms at that time and one year post-MRI. Created in BioRender. Zanni, G. (2025) https://BioRender.com/e26b462. B Left. In utero SSRI exposure is associated with increased CBCL symptoms, accounting for maternal lifetime depression and maternal anxiety and depressive symptoms. N = 97 SSRI+, N = 3876 SSRI-. Right. Maternal lifetime depression is also associated with increased offspring symptoms. C Left. In utero SSRI exposure is associated with increased amygdala, hippocampus, insula, putamen and thalamus activation to fearful-neutral faces, depicted as change in standard deviation of the BOLD response accounting for maternal lifetime depression and maternal anxiety and depressive symptoms. Right. Exposure to maternal lifetime depression does not result in significant differences in BOLD response to fearful-neutral faces. D Same analysis as (C) depicted as predicted means by group. N = 70 SSRI+, N = 2989 SSRI-. Left. In utero SSRI exposure is associated with increased BOLD activation to fearful-neutral faces. Right. Exposure to maternal lifetime depression does not result in differential BOLD response. Linear mixed effects models with propensity score weighting, 2-tailed, FDR-corrected. Error bars signify 95% confidence intervals. Precise p-values are reported in the main text. *p < 0.05; ***p < 0.005.
We selected children (Supplemental Table 3 for demographics) for whom 1) the biological mother was the reporter and had reported on medication use during pregnancy, 2) who had child behavior checklist (CBCL) scores at the two year follow-up wave (age 11-13 yo), 3) and complete covariate data (see “Methods”), resulting in N = 97 SSRI+ children exposed to SSRIs in utero and N = 3876 SSRI- unexposed children for analyzes without MRI data. Of this sample, N = 70 SSRI+ children exposed to SSRIs in utero and N = 2989 SSRI- unexposed children had MRI data available and were used for the analyzes containing MRI. All analyzes used propensity score weighting as well as covariates, including maternal lifetime depression and current maternal anxiety and depressive symptoms, to mitigate potential confounding factors between (children of) mothers who were treated with SSRIs and those who were not (see “Methods”).
We first found that in utero SSRI exposure was associated with increased anxiety (b = 0.79, pFDR = 2.06 × 10−18, pbonf = 2.06 × 10−18; standardized betas denote the predicted amount of standard deviations the outcome changes with SSRI exposure), depressive symptomatology (b = 0.43, pFDR = 2.26 × 10−7, pbonf = 6.77 × 10−7), internalizing (b = 0.48, pFDR = 7.86 × 10−10, pbonf = 1.57 × 10−9), and externalizing (b = 0.24, pFDR = 0.002, pbonf = 0.007) behavior at age 11–13 (Fig. 3B). As expected from extensive literature showing that maternal depression increases risk for psychiatric disorders in the offspring41,42, maternal lifetime depression as a covariate in the same model was also significant, but with smaller effect sizes as indicated by the betas, for anxiety (b = 0.13, pFDR = 0.006, pbonf = 0.018), depressive symptoms (b = 0.15, pFDR = 1.43 × 10−3, pbonf = 2.86 × 10−3), internalizing (b = 0.18, pFDR = 1.89 × 10−5, pbonf = 1.89 × 10−5) and externalizing (b = 0.08, pFDR = 0.055, pbonf = 0.22) behavior. Within the same sample, a model with maternal lifetime depression as the predictor and without prenatal SSRI use or maternal anxiety and depressive symptoms at time of child assessment as covariates, also resulted in significant associations with child anxiety (b = 0.33, pFDR = 8.44 × 10−13, pbonf = 2.53 × 10−12), depressive symptoms (b = 0.38, pFDR = 5.43 × 10−17, pbonf = 1.09 × 10−16), internalizing (b = 0.40, pFDR = 5.79 × 10−23, pbonf = 5.79 × 10−23) and externalizing (b = 0.25, pFDR = 5.01 × 10−10, pbonf = 2.01 × 10−9) behavior (Fig. 3B), again showing maternal depression is associated with child outcomes but to a lesser degree than SSRI use according to the standardized coefficients.
To test brain activity associated with an innate fear response, we compared BOLD response to fearful versus neutral faces in SSRI+ versus SSRI- children. Initially, in a hypothesis driven approach, we a priori selected seven regions of interest that have been associated with fear circuit activation43,44, and which we found to have enhanced activation in SSRI-exposed mice during innate fear (Fig. 2: amygdala, rostral and caudal ACC, cuneus, thalamus, caudate and putamen) as well as two regions that were affected in previous rodent or infant developmental SSRI studies (hippocampus and insula)45,46,47. Similar to our findings in mice, SSRI+ children had increased response to fearful-neutral faces in the bilateral amygdala (left: b = 0.76, pFDR = 2.6 × 10−14, pbonf = 2.6 × 10−14; right: b = 0.51, pFDR = 2.07 × 10−6, pbonf = 4.14 × 10−6; Fig. 3C, D), bilateral hippocampus (left: b = 0.45, pFDR = 3.60 × 10−6, pbonf = 1.08 × 10−5; right: b = 0.33, pFDR = 2.82 × 10−4, pbonf = 1.69 × 10−3), bilateral insula (left: b = 0.41, pFDR = 3.20 × 10−5, pbonf = 1.28 × 10−4; right: b = 0.29, pFDR = 0.003, pbonf = 0.021), bilateral putamen (left: b = 0.38, pFDR = 7.02 × 10−5, pbonf = 3.51 × 10−4; right: b = 0.25, pFDR = 0.014, pbonf = 0.121) and left thalamus (b = 0.26, pFDR = 0.012, pbonf = 0.095), but not in ACC, caudate, or cuneus. These findings, except for left thalamus and right putamen, remained significant when accounting for concurrent child CBCL scores (Supplemental Table 4). In an unbiased whole brain analysis, BOLD response to fearful-neutral faces, was again significantly stronger in the amygdala, hippocampus, putamen, and insula after in utero SSRI exposure (Supplemental Table 5). Additionally, the whole brain analysis also revealed increased activation in temporal regions, ventral diencephalon, and brain stem. The latter two may match our rodent findings of increased activity in the hypothalamus and substantia nigra (ventral diencephalon), and periaqueductal gray (brain stem), but differences in brain atlas resolution between mouse and human do not allow us to conclude this matching unequivocally.
Maternal lifetime depression was controlled for as a covariate in these models and was significantly associated with bilateral cuneus and thalamus BOLD responses. However, after FDR correction for multiple comparisons maternal lifetime depression did not remain significantly associated with BOLD response in any region. In a model without covariates for prenatal SSRI exposure and maternal anxiety and depressive symptoms at time of MRI, maternal lifetime depression was not significantly associated with BOLD response to fearful-neutral faces in any region after FDR correction (Fig. 3C,D and Supplemental Table 6). To further isolate SSRI effects from maternal depression effects, we tested whether prenatal SSRI use was also associated with differential BOLD response within the subset of children of mothers who reported having a lifetime history of depression (N = 45 SSRI+ children of mothers with lifetime depression and N = 670 SSRI- children of mothers with lifetime depression). In children of mothers with lifetime depression, prenatal SSRI use remained associated with amygdala response (left: b = 0.74, pFDR = 8.81×10-6, pbonf = 8.81×10-6; right: b = 0.30, p = 0.047, pFDR = 0.28) and marginally left hippocampal response (left: b = 0.32, p = 0.008, pFDR = 0.076). A last sensitivity analysis comparing three groups: 1) children exposed to SSRI in utero, 2) children exposed to maternal lifetime depression who did not take SSRIs during pregnancy, 3) non-exposed children also shows that the SSRI-exposed group had increased BOLD response (Supplemental Table 7), again suggesting specificity of the SSRI exposure effects.
When including interactions with sex into the models to investigate sex differences, we find that increased symptoms and BOLD response to fearful stimuli are predominantly present in SSRI-exposed girls (Supplemental Results and Table S8-12).
Lastly, controlling for prenatal SSRI use and maternal lifetime depression as well as the other covariates, bilateral amygdala response was associated with child anxiety, depressive symptomatology and internalizing, but not externalizing behavior at time of MRI (Table 1). Right amygdala response was associated with future depressive symptoms one year post MRI (N = 64 SSRI+, N = 2664 SSRI- with follow-up data; Table 1). Furthermore, left (but not right) amygdala response to fearful-neutral faces formally mediated the response between SSRI exposure and anxiety symptoms at time of MRI (indirect effect: 0.014, p < 0.0001; total effect: 0.14, p < 0.0001, proportion mediated: 9.3% p < 0.0001), suggesting heightened amygdala activity is part of the mechanism that leads from SSRI exposure to increased anxiety.
These findings show that similar to the rodent findings, in utero SSRI exposure is associated with increased symptomatology, increased fear circuit-related brain activation to innately fearful stimuli and that the amygdala predicts current as well as future depressive symptomatology, suggesting that the amygdala mediates effects by which prenatal SSRI exposure leads to increased risk for psychopathology in the offspring.
Discussion
The present findings demonstrate that elevated 5-HT signaling during early development increases neurobiological and behavioral innate fear responses later in life in both mice and humans. In mice, our behavioral findings add to the wide-ranging consequences observed after PND2-11 SSRI exposure, including reduced motivation, increased anxiety-like behavior, impaired fear extinction, and increased depressive-like behaviors in adulthood18,20,48,49,50. Importantly, these phenotypes in mice align well with the increased anxiety, depression, internalizing as well as externalizing symptoms following in utero SSRI exposure we observed here in human adolescents. The overlap in amplified innate fear circuit activation between mice and humans suggests highly evolutionarily conserved mechanisms of serotonin sensitivity during late gestation and early postnatal brain development.
Insight into possible mechanisms underlying altered behavior and fear circuit activation after developmental SSRI exposure comes predominantly from mouse studies. Mice exposed to early-life SSRIs exhibit reduced innervation of the medial prefrontal cortex (mPFC) by 5-HT fibers18 possibly resulting in reduced mPFC top-down inhibition of the amygdala underlying exaggerated freezing responses in fear conditioning51,52,53. Moreover, we have found in mice that neurons in the infralimbic and prelimbic areas of the PFC exhibit altered firing properties after early-life SSRI exposure, again providing a link to altered top-down control of amygdala function18. Similarly, mice with early-life genetic loss of 5-HT transporter function have less 5-HT innervation of the amygdala and consequently less direct serotonergic inhibitory influence via 5-HT1A receptors in this structure21,54.
We can speculate that similar mechanisms may be operative in the developing human fetus. However, there have been no studies that have attempted to examine the density of 5-HT innervation in human brain structures after in utero SSRI exposure. Furthermore, rodents and higher primates both demonstrate widespread transient expression of the 5-HT transporter in non-serotonergic neurons during the mid-gestation and into early infancy55,56,57,58,59. We have hypothesized that the period of development sensitive to 5-HT blockade in humans also overlaps both the maturation of 5-HT axon arborization and the period of transient expression of 5-HTT in non-serotonergic structures. Hyperactivity in amygdala and insula could be a consequence of early structural changes in amygdala and insula volume and connectivity observed in human neonates45 and childhood39 exposed to in utero SSRIs. Thus, in analogy to our mouse-hypothesis, in utero SSRI exposure in humans may affect fear circuitry function and psychopathology by decreasing top-down control60. Indeed, hyperactivity in the insula-amygdala circuit is associated with (increased risk for61) anxiety disorders62 which comports with the increased internalizing symptoms observed in SSRI-exposed children in our study. Importantly, amygdala reactivity to faces has been associated with future response to antidepressant medication63.
It is important to note that some brain regions were differentially activated in mice and humans, i.e., mouse cuneate, human insula, and human temporal structures. These differences could stem from the olfactory versus visual stimuli used in the mouse and human task, respectively. Alternatively, these differences could be the consequence of divergent brain evolution between mice and humans.
In summary, we have identified an evolutionarily conserved effect of 5-HTT blockade on fear circuit activation in response to innate fear cues later in life. Our experimental mouse model can control for confounding factors, such as maternal depression, comorbidities, and socioeconomic status (that typically confound the interpretation of human observational data), and thereby allows for experimental designs that can establish causality. Mouse to human translation is hardly ever one to one. Yet, parallel translation to humans shows that our findings are clinically relevant and ethologically valid. Our findings across ages furthermore suggest that effects of perinatal SSRI exposure are present in adolescence and persist through adulthood.
Prenatal anxiety and depression adversely affect both mother and child and motivate the public health efforts to identify and treat these disorders. SSRIs are a common therapeutic strategy in perinatal maternal emotional disorders, however the present cross-species data and prior studies on single species4,45,64,65 indicate that we need more mechanistic understanding of how pharmacological factors like SSRIs impact early brain development and later result in maladaptive behaviors. In turn this knowledge will enable the development of more effective treatments that better support the mother and the developing fetus.
Methods
Mouse study
Ethical permission guidelines and subjects
Pet1Cre mice on a 129SvEv/Tac background were bred at Columbia Psychiatry, New York State Psychiatric Institute. All methods were approved by the Animal Care and Use Committee of the New York State Psychiatric Institute and in accordance with the NIH Guide for the Care and Use of Laboratory Animals according to IACUC protocol number 1562. Mice used for experiments were born from litters containing 2–10 pups. Mice were separated by sex and weaned into groups of five mice per cage at P26. Animals were maintained on a 12/12 h light/dark cycle (lights on at 7:00 a.m.) and provided with food and water ad libitum. During the perinatal period, postnatal day 2 to 11 (PND 2-11) mice received daily intraperitoneal injections with either vehicle saline (PNSAL; 0.9% NaCl, 5 ml/kg) or fluoxetine (PNFLX; 10 mg/kg in VEH, 5 ml/kg). This dose paradigm in mice produced therapeutically relevant blood levels 360 ± 123 ng/ml in the pups without gross changes in viability and growth20. Multiple cohorts of mice were created for the various experiments. For innate fear behavioral assessment, we used female and male mice from at least 4 litters and 7 individuals in each treatment group. For adolescent fear behavior we used Pet1Cre mice on a 129SvEv/Tac background (Male PNSAL n = 4, Male PNFLX n = 6, Female PNSAL n = 8, Female PNFLX n = 5). For adult fear behavior we used Pet1Cre mice on a 129SvEv/Tac background (Male PNSAL n = 4, Male PNFLX n = 6, Female PNSAL n = 9, Female PNFLX n = 5. For fMRI experiments we used Male PNSAL n = 8, Male PNFLX n = 13, Female PNSAL n = 10, Female PNFLX n = 13.
Innate fear behavior
Adult mice were handled for a week prior to testing to get acquainted with the experimenter. Innate fear behavior was tested on two consecutive days: habituation and test.
Fear apparatus in adulthood
On the day of testing animals were brought individually into the behavior room in a transport cage. The testing apparatus consisted of an air-tight clear plastic chamber that has a clear lid (12 ½ ‘’L × 8 ½ ‘’W × 8 ½ ‘’H). The animals were tested individually for 5 minutes during habituation and allowed to explore the chamber maze. On the following day the animals were tested for 5 minutes in presence of a predator odor. The predator odor consisted of mountain lion urine (LLC 92012) that was delivered in an odor-attenuated air flow-controlled chamber. Briefly, predator odor air or odor-free air was administered via a stopcock valve connected two Erlenmeyer flasks via different tubing lines. The odor was introduced in the chamber by placing a piece of filter paper scented with predator odor (500 µl) at the top of the flask connected with the odor delivery line. Continuous airflow was maintained through the airtight chamber at 4 psi via calibration columns. The fear odor experiment was performed under a laminar flow hood and airflow was exhausted from the building.
Fear testing in adolescence
On the day of testing animals were brought individually into the behavior room in a transport cage. The testing apparatus was the same as above. A predator-like odor, 2-methyl-2-thiazoline (2MT), was delivered in an odor-attenuated air flow-controlled chamber. We used 2MT because it has consistent formulation and high potency, and this odor has been previously shown to induce measurable levels of fear-like behaviors in mice amounting to 80-90% freezing66,67,68. The testing started with 2 minutes of odor-less air followed by 3 minutes of 2MT, and 10 more minutes of odor-less air (Supplemental Fig. 1).
Manual behavioral scoring and video analysis
A top view camera (Digital USB 2.0 CMOS Camera, DMK 22AUC03) was connected to the ANYmaze software and the animal’s body was tracked throughout habituation and test tasks in both exposures. All videos in adulthood and adolescence were analyzed offline using ANYmaze software. The parameters included in the ANYmaze analysis were total time freezing (3 seconds immobility threshold), total distance traveled, and latency to immobility. For manual scoring we used the key function of ANYmaze and freezing was visually observed as pausing of all mouse activity including head movement that the automated scoring is not able to reliably capture. We analyzed early phases during habituation corresponding to minute 1–2, and late phases from min 3–5. During predator odor we measure freezing responses to the same habituation context re-exposure from minute 1–2 and during predator odor from minutes 3–5.
DeepLabCut tracking analysis
We conducted a more granular analysis of the adult innate fear behavioral response in our animal model using DeepLabCut (DLC)69. This software enables estimation of poses by tracking the animal body parts with high accuracy. Data was recorded at 15 Hz by one camera: the 640 × 480 pixels images were acquired with a Digital USB 2.0 CMOS Camera, DMK 22AUC03. Since our behavior was uniform overtime, we extracted 280 labels using K-Means clustering and two independent human annotators blinded to the treatment were trained to localize the following body parts: nose, left and right ear, left and right hip, tailbase, and midback. The tracking consisted of randomly extracted frames for a total of 20 frames from 14 mice scored separately for habituation and predator odor. We then created a training dataset using a resnet_50 network and default augmentation method. To train the network we used a shuffle=1, training index=0, and a 150k maximum iterations, the point at which the training reached a plateau. To validate the manual scoring of speed and freezing, we used custom Python scripts to analyze the DLC output files (csv array with x/y coordinates and likelihood values for each body part)70. We preprocessed the position data to correct loss of tracking of animal body parts. We removed body parts with unrealistic placements (i.e., with a Euclidean distance larger than the mouse body, ~200 pixels, from the rest of the body parts) and linearly interpolated their position with the positions obtained from contiguous frames. We estimated the animals’ speed using the Euclidean distance of the mid back position between frames. The speed time series was smoothed individually using a moving average filter (15 points).
To estimate freezing time, the 10th percentile of the speed distributions along the entire session was selected as threshold for each individual animal. Any period with at least 15 contiguous samples (i.e., 1 s) under the threshold was set as freezing time. We defined the habituation period as 100 ms–2000 ms and the predator period as 2000 ms – 5000 ms relative to the beginning of the recording. We skipped the early frames since those labels had low likelihood values, indicating that the mouse was not present in the arena yet. If no freezing time was identified between the beginning of the habituation period (100 ms) and the end of the predator period (5000 ms), the speed threshold was individually incremented. The threshold was incremented in steps of 5 percentiles until the freezing time across habituation and predator periods was at least 60 frames (4 seconds).
Functional magnetic resonance imaging (fMRI) during predator odor exposure
Awake Mouse Imaging System
Presented in Fig. 1A are the different components of the mouse imaging system showing a radiofrequency coil and MR compatible restraining system for imaging awake mice (Ekam Imaging, Boston MA USA). As previously described the awake mouse imaging system can reliably detect BOLD signal changes irrespective of baseline changes between unacclimated and acclimated animals38. The quadrature transmit/receive volume coil (ID 38 mm) provides excellent anatomical resolution, signal-noise-ratio (SNR) for voxel-based BOLD fMRI. The unique cushioned helmet design of the holder essentially stabilizes the head in a cushion, minimizing any discomfort normally caused by ear bars and pressure points used to immobilize the head for awake animal imaging71. Odorants are delivered through PE tubing connected to a nose cone built into the tubular bite bar used to secure the front incisors of the mouse in the head holder. A movie showing the set-up of a mouse for awake imaging is available at http://www.youtube.com/watch?v=W5Jup13isqw.
Acclimation
A week prior to the first imaging session, all mice were acclimated to the imaging system with the head restraint and sounds typical of the scanner. Mice were secured into their holding system while anesthetized with 1–2% isoflurane. Following cessation of isoflurane, fully conscious mice were put into a ‘mock scanner’ (a black box with audio of MRI pulses) for 30 minutes for four consecutive days. Acclimation in awake animal imaging significantly reduces physiological effects of the autonomic nervous system including heart rate, respiration, corticosteroid levels, and motor movements helping to improve contrast- to-noise and image quality37,38. For some experiments, acclimation training for 10–14 days is likely ample under certain conditions72. In our experience 4 days has been sufficient37,71 and here we did not detect movement artefacts that would indicate treatment-specific flight or freezing responses.
Imaging acquisition and pulse sequence
Experiments were conducted using a Bruker Biospec 7.0 T/20-cm USR horizontal magnet (Bruker, Billerica, Massachusetts) and a 20-G/cm magnetic field gradient insert (ID = 12 cm) capable of a 120-µs rise time (Bruker)73. At the beginning of each imaging session, a high-resolution anatomical data set was collected using the RARE pulse sequence (20 slice; 0.75 mm; FOV 2.5 cm; data matrix 256 × 256; repetition time (TR) 2.1 sec; echo time (TE) 12.4 msec; Effect TE 48 msec, NEX 6; 6.5 min acquisition time). Functional images were acquired using a multi-slice HASTE pulse sequence (Half Fourier Acquisition Single Shot Turbo Spin Echo). With this sequence it is possible to collect twenty, 0.75 mm thick, axial slices in less than six seconds. With a FOV of 2.5 cm and a data matrix of 96 × 96, the in-plane pixel functional resolution for these studies was 260 µm2.
Provocation paradigm - odor stimulant
Awake adult mice were imaged for changes in BOLD signal intensity in response to the odor of mountain lion urine (PredatorPee Liquid, www.predatorpee.com). Scans consisted of two epochs: a 4 min “baseline” epoch and an 8 min “stimulus presentation” epoch for a total of 12 min. In the custom-designed head restraint described above, the bar that holds the incisors in place functions simultaneously as an odor delivery tube. The odor delivery tube is connected by polyethylene tubing to an aquarium pump located outside the magnet room. During baseline, all subjects were presented with ambient air at a flow rate of approximately 1.5 lpm. At the start of stimulus presentation, sealed plastic cups containing the odor (15uL mountain lion urine on a cotton ball) were placed into the flow stream. Following each subject’s imaging session, all imaging components were thoroughly cleaned and sanitized, and a minimum 10-minute odor clearance period was allowed between subjects for any residual odor to dissipate. Charcoal odor capture canisters were additionally placed around the room to further aid in clearance of olfactory stimuli.
Image analysis
Images were aligned and registered to a 3D mouse brain atlas, which is segmented and labeled with 134 discrete anatomical regions (Ekam Solutions, Boston MA). The alignment process was facilitated by an interactive graphic user interface. The following parameters were applied: Quality: 0.97, Smoothing: 0.35 mm, Separation: 0.50 mm. Gaussian smoothing was performed with a Full Width at Half Maximum (FWHM) of 0.8 mm. The registration process involved translation, rotation and scaling independently and in all three dimensions. Matrices that transformed each subject’s anatomy were used to embed each slice within the atlas. All pixel locations of anatomy that were transformed were tagged with major and minor regions in the atlas. This combination created a fully segmented representation of each subject within the atlas. The inverse transformation matrix [Ti]-1for each subject (i) was also calculated.
In voxel-based analysis, the BOLD % change of each independent voxel was averaged for all subjects. It is a sensitive and robust analysis method that can detect not only changes in signal intensity but also in number of activated voxels within an ROI, thus differences in spread of activation38,74,75. Statistical t-tests were performed on each voxel (ca. 15,000 in number) of each subject within their original coordinate system with a baseline threshold of 1% BOLD change to account for normal fluctuation of BOLD signal in the awake rodent brain76,77,78. To address the multiple t-tests conducted, a mechanism for controlling false-positive detections was introduced79,80, to ensure that, on average, the false-positive detection rate remained below 0.05. The formula used was:
Where Pi is the p-value from the t-test at the i-th pixel within the region of interest (ROI) containing V pixels, each ranked based on its probability value. The false-positive filter value q was set to 0.2 for our analysis, and the predetermined constant c(V) was set to unity81, providing conservative estimates for significance. Pixels that were statistically significant retained their relative percentage change values, while all other pixel values were set to zero. A 95% confidence level, two-tailed distributions, and heteroscedastic variance assumptions were employed for the t-tests. The median number of activated voxels in each of the 134 regions between the PNSAL group and PNFLX group were compared using a Wilcox rank sum test and results were corrected for multiple comparisons using false discovery rate for 134 comparisons.
Statistical analyzes—mouse study
Data were analyzed using GraphPad Prism (version 8.4.3). Two-way ANOVA were used for analysis of variance of Treatment (vehicle vs perinatal fluoxetine), Exposure (habituation vs test), and Time variables. When interactions of variables were significant a multiple comparison post hoc test was conducted, and significance was reported in the graph. Significant main effects of individual variables were also reported in the graph. Outliers were identified using the ROUT method using a Q coefficient of 1% and the pairwise comparison was removed from the analysis. All data are presented as the mean ± SEM. Significance was *p < 0.05; **p < 0.01; ***p < 0.001.
Human study
ABCD study data and IRB ethical permission
For the human experiments we did secondary analyzes of Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®) data, a large longitudinal nationwide study of children recruited from primary and public-school systems at 21 sites nationwide. ABCD study procedures were approved by the Institutional Review Board at the University of California at San Diego, San Diego, CA. Parents provided written informed consent; children provided verbal assent. The local New York State Psychiatric Institute and Columbia University IRB approved secondary analyzes of ABCD data (ABCD Study ID 2008, https://doi.org/10.15154/1528668). Since phenotypes may only appear in adolescence40, we used the latest MRI data available, the two-year follow up data, when the children were 10.6–13.8 years old. The analysis sample was from the NDA’s two year follow-up release (downloaded through October 2022) and had the following additional inclusion criteria: biological mother reporting, data available about prenatal medication use and other prenatal and birth history as well as full data on other covariates (see below) and two year follow-up data available from the parent-report child behavior checklist (CBCL), which resulted in N = 95 SSRI+ children exposed to SSRIs in utero and N = 3813 SSRI- unexposed children. For analyzes including an association with BOLD response we also required complete data on the N-BACK fMRI task including behavior and that MRI scans passed quality control measures as advised by the ABCD study, resulting in N = 68 SSRI+ children exposed to SSRIs in utero and N = 2928 SSRI- unexposed children.
Procedures and variables of interest
Biological mothers completed reports on demographics, prenatal history, CBCL about their child, adult self-report (the equivalent to CBCL for adults, about themselves), and their own psychiatric history through the family history assessment (see Supplemental Table 12 for detailed variables).
MRI
As reported in detail elsewhere36 children performed the emotional N-back task in the MRI scanner. Briefly, children viewed images of positive (happy), negative (fearful) or neutral emotional faces or pictures of places and indicated if the current image was the same as the previous image (0-back) or as the image they saw two images earlier (2-back). MRI images were acquired on one of three types of 3 T MRI scanners (Siemens Prisma, Phillips, General Electric 750) using multichannel coils (Casey et al., 2018). Image processing was standardized using a preprocessing pipeline implemented by the ABCD study, previously reported82. Briefly, after preprocessing which included motion correction, registration and normalization to the mean across time per voxel, task-related activation strength was estimated through subject-level general linear models using AFNI’s 3dDeconvolve83. A gamma function and its temporal derivative were used to model the hemodynamic response. Beta coefficients and standard errors of the mean were mapped to FreeSurfer generated Regions of Interests (ROIs): cortical surface parcellations and subcortical segmentations. A weighted (a function of remaining images after motion correction) average across two runs was calculated for final mean beta weights and SEMs for each ROI and contrast. We used the mean beta weights for the contrast fearful compared to neutral faces, in a-priori selected regions of interest: amygdala, hippocampus, putamen, insula, rostral and caudal ACC, cuneus and thalamus. This contrast collapses over memory conditions, thus averaging out memory effects, and independently measures BOLD response to innately fearful stimuli compared to neutral stimuli, similar to the mouse study84. The regions were selected to translate the mouse findings (Fig. 2) and/or because in earlier work with neonatal infants45 we had found that the amygdala and the insula were affected. However we also performed an unbiased whole-brain analysis including all available ROIs to ensure findings are similar regardless of approach (Supplemental Table 5)
Measures
Predictor: prenatal SSRI use
Data regarding SSRI use once mothers knew that they were pregnant were used to make a composite SSRI use variable. Children were grouped as exposed to SSRI if mothers reported having used while they knew they were pregnant the following: fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, and/or paroxetine, but did not also use other types of antidepressants such as bupropion, duloxetine, MAO-inhibitors, tricyclic antidepressants, anti-anxiety medication, opioids, antipsychotic medication, anti-convulsant medication, stimulants or sleep aids.
Because the SSRI exposure had the largest effect size on the amygdala response, we then selected bilateral amygdala BOLD response to fearful versus neutral faces as a predictor for symptomatology at time of MRI and at the 1 year time point after MRI (N = 2670 had this follow-up data available).
Potential confounders
All our analyzes controlled for birth weight, child age at the time of MRI, sex assigned at birth, puberty score at time of MRI, maternal lifetime depression, maternal depression and anxiety symptomatology at time of child MRI (ASR), maternal age at birth, maternal race/ethnicity, area deprivation index, maternal education, combined household income, birth complications, doctors visits during pregnancy, pregnancy illnesses, prenatal vitamin use, prematurity, whether the pregnancy was planned, delivery by cesarean, substance use knowing of pregnancy of the following substances: caffeine, tobacco, alcohol, cannabis and cocaine and crack cocaine, oxycodone. In addition, we added in random effects terms for MRI scanner site and family relatedness.
For analyzes concerning the emotional N-back fMRI task we additionally controlled for behavior on the task for neutral and fearful images (rate of correct responses and reaction time). We performed sensitivity analyzes and added in child CBCL anxiety, depressive, internalizing and externalizing symptoms to ensure that effects were not due to potential differences in symptomatology. To further ensure that the findings were not solely due to differences in maternal depression between groups, we selected the subsample of children whose mothers reported they had ever been depressed and repeated analyzes of prenatal SSRI exposure on BOLD response in the subsample. We also performed analyzes directly comparing three groups: in utero SSRI exposed children, children exposed to maternal depression but not SSRIs and unexposed children.
Statistical analyzes—human study
Analyzes were performed using R (version 1.4.1717). For descriptive analyzes, chi-square tests were used for categorical variables, and t-tests for continuous variables. For regression analysis linear mixed effects models were used using R-package “lme4”. Analyzes were two-tailed and adjusted for multiple comparisons (9 brain regions x 2 hemispheres) with FDR corrections with significance set at pFDR < 0.05. All continuous variables were standardized, binary variables were not, thus beta values estimate change in standard deviation of the outcome variable between groups (similar to Cohen’s D). All analyzes were weighted by propensity scores to minimize confounding due to differences between mothers who used SSRIs prenatally and mothers who did not (see below). Individual BOLD response values were winsorized to 3 standard deviations from the mean to diminish the influence of spurious outliers. To investigate sex differences, sex was entered into the models as an interaction term. When there was a significant interaction analyzes were also run stratified by sex. Multicollinearity was of no concern with maximum variance inflation factor quantifying any multicollinearity was 3.5 (for behavioral measures on the N-back task), where 1 is the minimum and values of 10 and higher indicate concerning multicollinearity85. Removing the behavioral N-back variables from the models did not change the results. Mediation analyzes were performed including covariates and propensity score weighting using R-package “mediation” for causal mediation analysis.
Propensity score weighting
We used propensity score weighting to generate balancing weights for causal effect estimation. We used R-package Weightit to balance prenatal covariates between SSRI exposure and control groups using an ATT (average treatment effect on the treated) estimand. The list of covariates we balanced were the following: knowing of pregnancy use of: alcohol, tobacco, cannabis, caffeine, cocaine/crack cocaine, heroin/morphine, oxycodone, prenatal vitamins, pregnancy illnesses, whether the pregnancy was planned, maternal lifetime depression, maternal age at pregnancy, number of doctors’ visits during pregnancy, mother’s race/ethnicity, maternal education. The resulting propensity scores were used to weight participants for all linear mixed models. These variables were also entered into the linear mixed models in a double robust approach86.
Power analysis
For each of the mouse data, we based animal numbers on an alpha = 0.05, power = 0.80, with variability and effect size from prior data or if those data were not available with a medium/large effect size (f = 0.5). In the pharmacological manipulation (perinatal fluoxetine) we assumed at least 30% effect size. Missing data was addressed by appropriate mixed effects models when possible.
For the human data we used data from an existing cohort study. We calculated that we were powered to detect small-medium effect sizes (d ~ 0.35) with a power of 80% and medium and large effect sizes (d > 0.5) with a power of more than 90%.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
ABCD study data is available for download through nda.nih.gov. Source data are provided with this paper.
References
Betts, K. S., Williams, G. M., Najman, J. M. & Alati, R. The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depress Anxiety 32, 82–90 (2015).
O’connor, T. G., Heron, J. & Glover, V. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J. Am. Acad. Child Adolesc. Psychiatry 41, 1470–1477 (2002).
Field, T., Diego, M. & Hernandez-Reif, M. Prenatal depression effects on the fetus and newborn: a review. Infant Behav. Dev. 29, 445–455 (2006).
Andrade, S. E. et al. Use of selective serotonin reuptake inhibitors (SSRIs) in women delivering liveborn infants and other women of child-bearing age within the US food and drug administration’s mini-sentinel program. Arch. Women’s. Ment. Health 19, 969–977 (2016).
Molenaar, N. M. et al. The international prevalence of antidepressant use before, during, and after pregnancy: a systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J. Affect. Disord. 264, 82–89 (2020).
Hendrick, V. et al. Placental passage of antidepressant medications. Am. J. Psychiatry 160, 993–996 (2003).
Hermansen, T. K. & Melinder, A. Prenatal SSRI exposure: Effects on later child development. Child Neuropsychol. 21, 543–569 (2015).
Bérard, A., Zhao, J.-P. & Sheehy, O. Sertraline use during pregnancy and the risk of major malformations. Am. J. Obstet. Gynecol. 212, 795.e1–795.e12 (2015).
Chambers, C. D., Johnson, K. A., Dick, L. M., Felix, R. J. & Jones, K. L. Birth outcomes in pregnant women taking fluoxetine. N. Engl. J. Med. 335, 1010–1015 (1996).
Malm, H., Artama, M., Gissler, M. & Ritvanen, A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet. Gynecol. 118, 111–120 (2011).
Alwan, S. et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N. Engl. J. Med. 356, 2684–2692 (2007).
Singal, D. et al. In utero antidepressants and neurodevelopmental outcomes in kindergarteners. Pediatrics 145, e20191157 (2020).
Liu, X. et al. Antidepressant use during pregnancy and psychiatric disorders in offspring: danish nationwide register based cohort study. BMJ 358, j3668 (2017).
Rommel, A.-S., Bergink, V., Liu, X., Munk-Olsen, T. & Molenaar, N. M. Long-term effects of intrauterine exposure to antidepressants on physical, neurodevelopmental, and psychiatric outcomes: a systematic review. J. Clin. Psychiatry 81, 19r12965 (2020).
Bliddal, M. et al. Prenatal antidepressant exposure and emotional disorders until age 22: a danish register study. Child Adolesc. Psychiatry Ment. Health 17, 73 (2023).
Suarez, E. A. et al. Association of antidepressant use during pregnancy with risk of neurodevelopmental disorders in children. JAMA Intern. Med. 182, 1149–1160 (2022).
Rommel, A.-S. et al. Long-term prenatal effects of antidepressant use on the risk of affective disorders in the offspring: a register-based cohort study. Neuropsychopharmacology 46, 1518–1525 (2021).
Rebello, T. J. et al. Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J. Neurosci. 34, 12379–12393 (2014).
Miceli, S. et al. High serotonin levels during brain development alter the structural input-output connectivity of neural networks in the rat somatosensory layer IV. Front. Cell. Neurosci. 7, 88 (2013).
Ansorge, M. S., Zhou, M., Lira, A., Hen, R. & Gingrich, J. A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306, 879–881 (2004).
Li, Q., Wichems, C., Heils, A., Lesch, K.-P. & Murphy, D. L. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J. Neurosci. 20, 7888–7895 (2000).
Linhares, S. S. G. et al. Prenatal exposure to fluoxetine modulates emotionality and aversive memory in male and female rat offspring. Behav. Pharmacol. 33, 575–588 (2022).
Persico, A. M. et al. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J. Neurosci. 21, 6862–6873 (2001).
Ansorge, M. S., Morelli, E. & Gingrich, J. A. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J. Neurosci. 28, 199–207 (2008).
Dobbing, J. & Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 3, 79–83 (1979).
Bockhorst, K. H. et al. Early postnatal development of rat brain: in vivo diffusion tensor imaging. J. Neurosci. Res. 86, 1520–1528 (2008).
Semple, B. D., Blomgren, K., Gimlin, K., Ferriero, D. M. & Noble-Haeusslein, L. J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 106-107, 1–16 (2013).
Suri, D., Teixeira, C. M., Cagliostro, M. K. C., Mahadevia, D. & Ansorge, M. S. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology 40, 88–112 (2015).
Kindt, K. S., Tam, T., Whiteman, S. & Schafer, W. R. Serotonin promotes go-dependent neuronal migration in caenorhabditis elegans. Curr. Biol. 12, 1738–1747 (2002).
Azmitia, E. C. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 56, 413–424 (2001).
Ranzil, S. et al. The relationship between the placental serotonin pathway and fetal growth restriction. Biochimie 161, 80–87 (2019).
Baudat, M., de Kort, A. R., van den Hove, D. L. A. & Joosten, E. A. Early-life exposure to selective serotonin reuptake inhibitors: Long-term effects on pain and affective comorbidities. Eur. J. Neurosci. 55, 295–317 (2022).
Wollesen, T., Degnan, B. M. & Wanninger, A. Expression of serotonin (5-HT) during CNS development of the cephalopod mollusk, Idiosepius notoides. Cell Tissue Res 342, 161–178 (2010).
Edsinger, E. & Dölen, G. A conserved role for serotonergic neurotransmission in mediating social behavior in octopus. Curr. Biol. 28, 3136–3142.e4 (2018).
Mohammad-Zadeh, L. F., Moses, L. & Gwaltney-Brant, S. M. Serotonin: a review. J. Vet. Pharmacol. Ther. 31, 187–199 (2008).
Casey, B. J. et al. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 32, 43–54 (2018).
King, J. A. et al. Procedure for minimizing stress for fMRI studies in conscious rats. J. Neurosci. Methods 148, 154–160 (2005).
Ferris, C. F. et al. Studies on the Q175 knock-in model of huntington’s disease using functional imaging in awake mice: evidence of olfactory dysfunction. Front. Neurol. 5, 94 (2014).
Koc, D. et al. Prenatal antidepressant exposure and offspring brain morphologic trajectory. JAMA Psychiatry 80, 1208–1217 (2023).
Malm, H. et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J. Am. Acad. Child Adolesc. Psychiatry 55, 359–366 (2016).
Hammen, C. & Brennan, P. A. Severity, chronicity, and timing of maternal depression and risk for adolescent offspring diagnoses in a community sample. Arch. Gen. Psychiatry 60, 253–258 (2003).
van Dijk, M. T., Murphy, E., Posner, J. E., Talati, A. & Weissman, M. M. Association of multigenerational family history of depression with lifetime depressive and other psychiatric disorders in children: results from the Adolescent Brain Cognitive Development (ABCD) study. JAMA Psychiatry 78, 778–787 (2021).
Silva, B. A., Gross, C. T. & Gräff, J. The neural circuits of innate fear: detection, integration, action, and memorization. Learn. Mem. 23, 544–555 (2016).
Staples, L. G. Predator odor avoidance as a rodent model of anxiety: learning-mediated consequences beyond the initial exposure. Neurobiol. Learn. Mem. 94, 435–445 (2010).
Lugo-Candelas, C. et al. Associations between brain structure and connectivity in infants and exposure to selective serotonin reuptake inhibitors during pregnancy. JAMA Pediatr. 172, 525–533 (2018).
Mukhopadhyay, S., Chatterjee, A., Tiwari, P., Ghai, U. & Vaidya, V. A. Postnatal fluoxetine treatment alters perineuronal net formation and maintenance in the hippocampus. eNeuro 8 https://doi.org/10.1523/eneuro.0424-20.2021 (2021).
Karpova, N. N., Lindholm, J., Pruunsild, P., Timmusk, T. & Castrén, E. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur. Neuropsychopharmacol. 19, 97–108 (2009).
Unroe, K. A., Glover, M. E., Shupe, E. A., Feng, N. & Clinton, S. M. Perinatal SSRI exposure disrupts G protein-coupled receptor BAI3 in developing dentate gyrus and adult emotional behavior: relevance to psychiatric disorders. Neuroscience 471, 32–50 (2021).
Menezes, E. C. et al. Reduced motivation in perinatal fluoxetine-treated mice: a hypodopaminergic phenotype. J. Neurosci. 41, 2723–2732 (2021).
Hutchison, S. M., Mâsse, L. C., Pawluski, J. L. & Oberlander, T. F. Perinatal selective serotonin reuptake inhibitor (SSRI) and other antidepressant exposure effects on anxiety and depressive behaviors in offspring: A review of findings in humans and rodent models. Reprod. Toxicol. 99, 80–95 (2021).
Bloodgood, D. W., Sugam, J. A., Holmes, A. & Kash, T. L. Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl. Psychiatry 8, 60 (2018).
Yu, W. et al. Prenatal selective serotonin reuptake inhibitor (SSRI) exposure induces working memory and social recognition deficits by disrupting inhibitory synaptic networks in male mice. Mol. Brain 12, 29 (2019).
Giustino, T. F. & Maren, S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front. Behav. Neurosci. 9, 298 (2015).
Gross, C., Santarelli, L., Brunner, D., Zhuang, X. & Hen, R. Altered fear circuits in 5-HT1A receptor KO mice. Biol. Psychiatry 48, 1157–1163 (2000).
Gaspar, P., Cases, O. & Maroteaux, L. The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012 (2003).
Okaty, B. W., Commons, K. G. & Dymecki, S. M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 20, 397–424 (2019).
Hale, M. W. & Lowry, C. A. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology 213, 243–264 (2011).
Jacobs, B. L. & Azmitia, E. C. Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229 (1992).
Kiyasova, V. et al. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. 31, 2756–2768 (2011).
Kredlow, M. A. et al. Prefrontal cortex, amygdala, and threat processing: implications for PTSD. Neuropsychopharmacology 47, 247–259 (2022).
Stein, M. B., Simmons, A. N., Feinstein, J. S. & Paulus, M. P. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am. J. Psychiatry 164, 318–327 (2007).
Liu, W.-J. et al. Abnormal functional connectivity of the amygdala-based network in resting-state FMRI in adolescents with generalized anxiety disorder. Med. Sci. Monit. 21, 459–467 (2015).
Williams, L. M. et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology 40, 2398–2408 (2015).
Rotem-Kohavi, N., Williams, L. J. & Oberlander, T. F. Advanced neuroimaging: a window into the neural correlates of fetal programming related to prenatal exposure to maternal depression and SSRIs. Semin. Perinatol. 44, 151223 (2020).
Rotem-Kohavi, N. et al. Alterations in resting-state networks following in utero selective serotonin reuptake inhibitor exposure in the neonatal brain. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 39–49 (2019).
Isosaka, T. et al. Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell 163, 1153–1164 (2015).
Matsuo, T. et al. Artificial hibernation/life-protective state induced by thiazoline-related innate fear odors. Commun. Biol. 4, 101 (2021).
Wang, Y. et al. Large-scale forward genetics screening identifies Trpa1 as a chemosensor for predator odor-evoked innate fear behaviors. Nat. Commun. 9, 2041 (2018).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Akiti, K. et al. Striatal dopamine explains novelty-induced behavioral dynamics and individual variability in threat prediction. Neuron 110, 3789–3804.e9 (2022).
Ferris, C. F. Applications in awake animal magnetic resonance imaging. Front. Neurosci. 16, 854377 (2022).
Mandino, F., Vujic, S., Grandjean, J. & Lake, E. M. R. Where do we stand on fMRI in awake mice? Cereb. Cortex 34 (2024).
Sadaka, A. H. et al. Cannabidiol has a unique effect on global brain activity: a pharmacological, functional MRI study in awake mice. J. Transl. Med. 19, 220 (2021).
Bhattacharyya, P. K., Phillips, M. D., Stone, L. A. & Lowe, M. J. Activation volume vs BOLD signal change as measures of fMRI activation – Its impact on GABA – fMRI activation correlation. Magn. Reson. Imaging 42, 123–129 (2017).
Kenkel, W. M. et al. Functional magnetic resonance imaging in awake transgenic fragile X rats: evidence of dysregulation in reward processing in the mesolimbic/habenular neural circuit. Transl. Psychiatry 6, e763 (2016).
Brevard, M. E., Duong, T. Q., King, J. A. & Ferris, C. F. Changes in MRI signal intensity during hypercapnic challenge under conscious and anesthetized conditions. Magn. Reson. Imaging 21, 995–1001 (2003).
Friston, K. J. et al. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 2, 189–210 (1994).
Buxton, R. B., Wong, E. C. & Frank, L. R. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn. Reson. Med. 39, 855–864 (1998).
Genovese, C. R., Lazar, N. A. & Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878 (2002).
Genovese, C. & Wasserman, L. Operating characteristics and extensions of the false discovery rate procedure. J. R. Stat. Soc. (2002).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300 (1995).
Hagler, D. J. et al. Image processing and analysis methods for the adolescent brain cognitive development study. Neuroimage 202, 116091 (2019).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Chaarani, B. et al. Baseline brain function in the preadolescents of the ABCD Study. Nat. Neurosci. 24, 1176–1186 (2021).
Alin, A. et al. Multicollinearity. Wiley Interdisciplinary Rev.: Comput. Stat. 2, 370–374 (2010).
Funk, M. J. et al. Doubly robust estimation of causal effects. Am. J. Epidemiol. 173, 761–767 (2011).
Acknowledgements
We would like to thank members of the Ansorge’s lab for methodological and conceptual discussions. This work was supported by an AFSP Young Investigator Award YIG-R-001-19 (MvD), Depression Center Pilot Award from the Columbia Department of Psychiatry (AT), Sackler Institute for Developmental Psychobiology (GZ), 1K01MH131895-01A1 (GZ). R01MH099118 (MSA), Sackler Institute for Developmental Psychobiology (MSA), R01MH036197 (MMW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of any other sponsor.
Author information
Authors and Affiliations
Contributions
G.Z., M.S.A, M.C.C., M.T.v.D., A.T., J.A.G., C.F., and M.M.W. conceived and supervised all aspects of the study. G.Z. and M.C.C. performed behavioral and imaging experiments in mice. A.S.M. assisted with mouse behavior. M.T.v.D. performed behavioral and imaging assessment of human ABCD data. G.Z. and M.T.v.D. wrote the manuscript. A.L.K. and N.P. assisted with Matlab script for Deeplabcut analysis of behavior. A.L.R. and A.L.K. assisted with data curation and analysis. C.F., M.C.C., and P.K. performed and analyzed brain imaging experiments in mice. P.S. and K.I. performed Deeplabcut analysis of behavior. C.L.C. and P.D.G. contributed conceptually to the human study design. G.Z. and M.T.v.D. contributed equally and have the right to list their name first in their C.V., M.S.A., and J.A.G. share last authorship. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
MMW has received royalties from Oxford University Press, Perseus Books Group, American Psychiatric Association Publishing, and Multi-Health Systems. CFF has a financial interest in Animal Imaging Research, a company that makes radiofrequency electronics and holders for awake animal imaging. CFF and PK have a partnership interest in Ekam Solutions, a company that develops 3D MRI atlases for animal research. None of these present any conflict with the present work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zanni, G., van Dijk, M.T., Cagliostro, M.C. et al. Perinatal SSRI exposure impacts innate fear circuit activation and behavior in mice and humans. Nat Commun 16, 4002 (2025). https://doi.org/10.1038/s41467-025-58785-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58785-4
This article is cited by
-
Progress and opportunity in developmental neurobiology and youth mental health
Neuropsychopharmacology (2026)
-
Potential risks and benefits of prenatal selective serotonin reuptake inhibitor medications for maternal mental health and child development
Nature Mental Health (2025)