Abstract
Cytokinins are adenine derivatives serving as phytohormones, which are essential regulators of plant growth, development, and response to environmental factors. The transport process of cytokinins determines their spatial distributions and is critical to their functions. The AZA-GUANINE RESISTANT (AZG) family member AZG2 in Arabidopsis thaliana has been identified as a cytokinin and purine transporter. Here, we characterize the binding and transport of AZG2 towards the natural cytokinin, trans-zeatin (tZ), as well as adenine. AZG2 structures are determined in both the substrate-unbound, adenine-bound, and tZ-bound states, under both acidic and neutral pH. Key residues involved in substrate binding are identified. Two distinct conformations are observed in the tZ-bound state of AZG2 in the neutral pH. Structural analysis reveals the structural dynamics of AZG2 during cytokinin transport, which fit into the elevator-type transport model. These results provide insights into the molecular mechanism of cytokinin transport in plants.
Similar content being viewed by others
Introduction
Cytokinins regulates multiple aspects of plant life processes, like shoot and root growth, leaf development and senescence, seed germination, nutrient metabolism, and responses to different environment stresses including drought, salinity, and temperature1,2,3,4. The major cytokinins in plants are adenine derivatives with N6-isoprenoid or aromatic modifications, of which the isoprenoid derivatives isopentenyladenine (iP) and trans-zeatin (tZ) are the two most abundant ones4,5. The cytokinin signaling perception is initiated by cytokinin binding to the transmembrane ARABIDOPSIS HISTIDINE KINASE (AHK) family receptors located in both the plasma and endoplasmic reticulum (ER) membrane, and then transduced to the nucleus through a phosphorelay pathway1,6,7,8,9,10,11,12,13. Like most plant hormones, the spatial distributions of cytokinins fine-tune their signaling and are generated synergistically through the biosynthesis, metabolism, and transport processes14,15,16,17,18,19. Different cytokinins have been shown to have specific biosynthesis sites and transport directions in the long-distance. tZ is mainly synthesized in the root and transported acropetally to the shoot through the xylem, while iP is produced in the shoot and transported basipetally to the root20,21,22,23,24. Besides the long-distance transport, the short-distance transport of cytokinins including both inter- and intra-cell movements also plays important roles in their signaling19,25, specially given that the cytokinin binding domain of the AHA receptors is located in the extracellular space or the ER lumen side while the key enzymes generating active cytokinins, the LONELY GUY (LOG) family proteins, are localized in the cytosol or nuclei26,27. Multiple transporter families have been suggested to be involved in cytokinin transport25,28, including the ATP-binding cassette (ABC) family29,30,31,32, the equilibrative nucleoside (ENT) family33,34, the purine permease (PUP) family35,36,37,38,39, the Sugars Will Eventually be Exported Transporter (SWEET) family40, and the AZG family transporters41,42. These transporters may function in different aspects of cytokinin transport, like the Arabidopsis ABCG14 and Oryza ABCG18 have been suggested to be cytokinin exporters and mediate the long-distance root-to-shoot transport of cytokinin29,30,31, while the Arabidopsis AZGs are importers and regulate local cytokinin distributions41,42.
AZG2 in Arabidopsis thaliana is the first identified member of the AZG family that is involved in cytokinin transport41. It was at first characterized as adenine and guanine transporters, together with its homolog AZG143. Arabidopsis AZG1 and AZG2 share a sequence identity of 53% (Supplementary Fig. 1a). Though both serving as purine and cytokinin transporters, they also show variations in the cellular or subcellular localization and the transport mechanism. While AZG1 is more widely expressed in the root apical meristem42, expression of AZG2 is restricted to a few cells around the lateral root primordia41. Whereas AZG1 is localized in the plasma membrane42, AZG2 has been shown to be present in both the plasma and ER membrane, suggesting a role of AZG2 in the intracellular cytokinin transport and signaling41. AZG1 transports substrates in a more proton gradient-dependent manner and may function as a proton/substrate symporter41. AZG2 is less dependent on the proton gradient, consistent with its ER membrane localization where lacks a cross-membrane pH difference41. However, by abolishing the proton gradient using protonophore, the substrate transport activity was also reduced for AZG2, though less pronounced than AZG1, suggesting that the proton gradient can further facilitate the substrate transport of AZG241. Recently, structures of AZG1 have been determined in the apo state and in complex with adenine or three different cytokinins, including tZ, 6-benzyleaminopurine, and kinetin44. However, all determined AZG1 structures exhibit the same inward-facing conformation44. Together with an AlphaFold2 predicted structure, an elevator transport mechanism has been proposed for AZG1, similar to the nucleobase/ascorbate transporter (NAT) family homologs with which AZG1 shares the same transmembrane fold44,45. It awaits further characterizations of the AZG2-mediated purine and cytokinin transport process, and the molecular basis for the different transport mechanisms with AZG1.
In this study, we characterized the substrate binding and transport activity of AZG2 towards adenine and cytokinin, and determined the structures of AZG2 in the substrate-unbound (apo) state, the adenine-bound state, and tZ-bound state, under both acidic and neutral pH conditions. Notably, two distinct conformations were observed in the tZ-bound state under neutral pH, providing direct structural evidences for the elevator-type transport mechanism proposed for transporters of the AZG family.
Results
Substrate binding and transport characterizations of AZG2
We first characterized the purine and cytokinin transport of Arabidopsis AZG1 and AZG2 using the [3H]-labeled adenine and tZ, respectively, in a human embryonic kidney 293 F (HEK293F) cell-based transport assay system. Successful protein expression and plasma membrane-localization of the transporters were verified by fluorescence microscopy and Western blotting (Supplementary Fig. 2a,b). As the transport results showed, at an extracellular buffer of pH 5.5, cells expressing AZG1 or AZG2 accumulated more [3H]-adenine or [3H]-tZ compared to the control cells expressing empty vector (Fig. 1a,b). Notably, cells expressing AZG2 accumulated more radio-labeled substrates than cells expressing AZG1 (Fig. 1a,b), although the protein expression level of AZG1 was higher than that of AZG2 as detected by Western blotting (Supplementary Fig. 2b). The relatively low transport activity of AZG1 in HEK293F cells was also observed by Xu et al.44, indicating that the fully active transport of AZG1 may require other factors or regulations missing in the HEK293F cell system. We then determined the substrate-saturating transport kinetics for AZG2. The Vmax for the transport of [3H]-adenine and [3H]-tZ by AZG2 were determined to be 259.4 ± 12.6 and 88.0 ± 3.6 pmol min−1 per 106 cells, and the Km values for adenine and tZ were 1.02 ± 0.16 and 0.73 ± 0.09 μM, respectively (Fig. 1c,d). The Km value of tZ transport is almost the same as that in the previous report which was determined in the endogenous Arabidopsis cells (0.8 ± 0.6 μM)41, suggesting capability of active transport of AZG2 in the heterologous HEK293F system. We also tested the substrate transport of AZG2 at a neutral buffer pH (pH 7.4). The results showed that both [3H]-adenine and [3H]-tZ accumulations mediated by AZG2 at pH 5.5 were higher than those at pH7.4, suggesting that the proton gradient can facilitate the adenine and tZ transport (Supplementary Fig. 2c). This is consistent with the previous report that in yeast cells, the transport of hypoxanthine, another purine substrate of AZG2, was reduced in the absence of a proton gradient compared to that in the presence41. Due to the low readings of the substrate transport mediated by AZG1 in HEK293F cells, we could not get conclusive results of its pH dependency, which is not further analysed and discussed here. To investigate the substrate specificity of AZG2, we measured the [3H]-tZ uptake in the presence of the excess unlabeled potential ligands, including adenine, two natural cytokinins tZ and iP, two synthetic cytokinins benzyladenine (BA) and kinetin, and a cytokinin precursor trans-zeatin riboside (tZR), respectively (Supplementary Fig. 1b). The results showed that both adenine and the tested cytokinins strongly inhibited the [3H]-tZ uptake while the precursor tZR not (Supplementary Fig. 2g). It suggests that AZG2 can recognize multiple types of cytokinins, consistent with previous reports of AZG2 and AZG141,42,44.
a Cell-based transport assay result showing that the uptake of [3H]-adenine was increased in HEK293F cells expressing AZG1 or AZG2, compared with control cells (transfected with the empty vector). b Cell-based transport assay result showing that the uptake of [3H]-trans-zeatin was increased in HEK293F cells expressing AZG1 or AZG2, compared with control cells. c Concentration-dependent [3H]-adenine uptake by AZG2. d Concentration-dependent [3H]-trans-zeatin uptake by AZG2. Data points in (c) and (d) were nonlinear-fitted using the Michaelis–Menten equation. All data points in (a–d) represent three (n = 3) independent measurements. Data are mean ± s.d. Significant differences in (a) and (b) were determined by one-way ANOVA with Dunnett’s multiple comparisons of the experimental groups against the control group. ns, not significant; * P < 0.0332; ** P = 0.0025; *** P < 0.0005; **** P < 0.0001. Exact P values are labeled. e ITC results for adenine binding to AZG2 at pH 5.5. The inset panel shows the original heat-change recordings. f ITC results for trans-zeatin binding to AZG2 at pH 5.5. The ITC experiments in (e) and (f) were each repeated three times with similar results. Source data is provided as a Source Data file.
We then purified AZG2 protein from the HEK293F cells and determined its binding affinity towards adenine or tZ at both acidic pH (pH 5.5) and neutral pH (pH 7.4) using isothermal titration calorimetry (ITC). At pH 5.5, the dissociation constant (Kd) was 0.77 ± 0.09 μM for adenine (Fig. 1e), and 0.39 ± 0.09 μM for tZ (Fig. 1f), respectively, consistent with the Km values determined in the transport assays. We noticed that although the Kd value for tZ is lower than adenine which indicates a higher binding of tZ, the uptake rate for adenine is higher than tZ (Supplementary Fig. 2c). However, since we are measuring the apparent dissociation constant value using the ITC method, which is a weighted average value of the dissociation constants under different transport conformations, this value does not always correlate with the transport rate46. It suggests an unresolved relationship between the binding affinity (the transport thermodynamics) and the transport kinetics for membrane transporters, which needs further investigations47. At pH 7.4, the binding affinities were both reduced for adenine and tZ, with Kd values of 6.67 ± 2.78 μM and 13.40 ± 71.00 μM, respectively (Supplementary Fig. 2d). We also purified AZG1 and determined its binding towards adenine and tZ under different pH conditions. At pH 5.5, the Kd values were 0.62 ± 0.11 μM for adenine (Supplementary Fig. 2e) and 0.64 ± 0.10 μM for tZ (Supplementary Fig. 2e), in the same level as AZG2. While at pH 7.4, the Kd values were significantly increased, with 5.03 ± 2.29 μM for adenine (Supplementary Fig. 2f) and 10.60 ± 6.28 μM for tZ (Supplementary Fig. 2f), indicating reduced binding affinities. Altogether, the results suggest that the binding of both adenine and tZ to AZG1 and AZG2 is influenced by pH levels, with acidic pH enhancing binding and higher pH decreasing it. Besides adenine and tZ, we tested the binding affinity of AZG2 towards other cytokinins including iP, BA, and kinetin, and the precursor tZR. Consistent with the competition assay results, iP, BA, and kinetin can also bind to AZG2, with the Kd values of 0.71 ± 0.08 μM, 2.09 ± 0.47 μM, and 0.71 ± 0.09 μM, respectively, in a similar level to adenine or tZ (Supplementary Fig. 2h–j). By contrast, no binding was detected for the precursor tZR (Supplementary Fig. 2k). Together, it suggests that the substrate binding pocket of AZG2 may tolerate certain variations of the side chains modified at the C6 position of adenine in different cytokinins, but not the riboside modification at the N7 position of adenine in the cytokinin precursor tZR (Supplementary Fig. 1b).
With the purified proteins, we tried to establish a proteoliposome-based transport assay for AZG1 and AZG2 and characterize the substrate transport under different pH conditions. In the presence of the transmembrane pH, Na+ and K+ gradients which mimics the conditions of the planta cell (Supplementary Fig. 3a), active transport was observed for proteoliposomes loaded with wild-type (WT) AZG1 or AZG2 protein, compared to the control liposomes with no protein loaded (Supplementary Fig. 3b). Like the cell-based transport assay result, AZG2 also had a higher accumulation of [3H]-adenine than AZG1 (Supplementary Fig. 3b). By adding the sodium or potassium ionophore, sodium ionophore III or valinomycin, to eliminate the cross-membrane sodium or potassium gradient, no significant differences were observed for either AZG1 or AZG2 mediated adenine transport (Supplementary Fig. 3b). When the pH gradient was removed in the system (Supplementary Fig. 3c), active transport was still observed for AZG2, either in the absence or presence of the ionophores (Supplementary Fig. 3d). However, both AZG1 and AZG2 transport was reduced compared to that in the presence of the proton gradient (Supplementary Fig. 3e), consistent with the cell-based transport assay results. This further supports that the proton gradient facilitates both AZG1 and AZG2-mediated transport. Similar to the cell-based transport results, AZG2 also had a higher accumulation of [3H]-tZ than AZG1 in the proteoliposomes (Supplementary Fig. 3f). The fitted Km value for tZ was 0.38 ± 0.04 μM in the proteoliposome-based transport system (Supplementary Fig. 3g), at the same level to the Km value (0.73 ± 0.09 μM) obtained in the cell-based assay system.
Architecture of AZG2 in the apo state
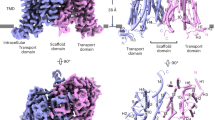
With the suspension HEK293F cell cultures and transient expression system, AZG2 protein was purified in large scale using the detergent glyco-diosgenin (GDN) at pH 7.4 (Supplementary Fig. 4a). We then determined its structure using cryo-electron microscopy (cryo-EM) single particle analysis (Supplementary Figs. 4b–d and 5a–d). The overall resolution of the EM map was 3.4 Å (Fig. 2a, Supplementary Fig. 5a). Like AZG1, AZG2 forms a homodimer (Fig. 2b). An intracellularly-facing cavity was formed in the transmembrane region of AZG2, as shown by the electrostatic potential surface analysis (Supplementary Fig. 6a). It suggests that the captured AZG2 structure is in an inward-facing conformation as AZG1 in the apo state44. Each subunit of AZG2 contains 14 transmembrane segments (TMs), which can be divided into two domains like the homologous structures of the NAT family it belongs to45,48, the transport domain and the scaffold domain (Fig. 2c–e). Besides, the first 7 TMs and the last 7 TMs are inverted repeats, and have a two-fold pseudo-symmetry with which the two halves can be superposed by an axis along the membrane plane (Supplementary Fig. 7a). The scaffold domain of AZG2 consists of TM5-TM7 and TM12-TM14, and mediates the dimer formation (Fig. 2c,e). The transport domain consists of TM1-TM4 and TM8-TM11 (Fig. 2c,e). As a signature of the NAT family48, TM3 and TM10 of AZG2 each contains three parts, an amino (N)-terminal loop followed by a β-strand and an α-helix, and form a crossover structure with the anti-parallel β-sheet in the middle (Fig. 2e and Supplementary Fig. 7b). Besides the transmembrane segments, AZG2 has an extracellular or ER luminal domain formed by the linkers between TMs, including the linkers of TM1-TM2, TM5-TM6, and TM7-TM8 (Fig. 2d,e). The linker between TM5 and TM6 forms an anti-parallel β-sheet, and is part of the dimer interface (Fig. 2c–e). In the cytosolic side, sequence prior to TM1 forms two short a-helices (Fig. 2d,e). The TM4-TM5 linker and the sequence following TM14 also form α-helix structure (Fig. 2d,e).
a Overview of the EM density for AZG2 at pH 7.4. Densities corresponding to the two protomers are colored dark magenta and blue, respectively. b Cartoon representation of the AZG2 structure in the apo state at pH 7.4. The two protomers are colored dark magenta and blue, respectively. c Cartoon representation of the scaffold domain and transport domain of AZG2 at different views. The scaffold domain is colored gray, and the transport domain is colored dark red. d Overall structure of the AZG2 protomer. The transmembrane helices are colored in the rainbow style. e A topological diagram of AZG2.
Structural alignments of the dimeric AZG1 and AZG2 in the apo state reveal a root-mean-square deviation (RMSD) of 1.2 Å (896 Cα atoms aligned) (Fig. 3a), supporting that they share the same dimer organization and protein fold. Compared with AZG1, the extracellular domain of AZG2 is relatively smaller (Fig. 3b). Notably, the TM5-TM6 linker of AZG2 which is involved in the dimer formation is much shorter than that of AZG1 (Fig. 3b). Consistently, the dimer interface of AZG2 differs from that of AZG1 in the extracellular region. In AZG2, the TM5-TM6 linker mediates the dimer formation mainly through hydrophobic interactions between V219, L226, and V227 from each monomer (Fig. 3c). In AZG1, besides the hydrophobic interactions, the dimer interface is mediated by extensive hydrogen bonds between the extra anti-parallel β-sheets in the TM5-TM6 linker from each monomer (Fig. 3d). The TM5-TM6 linker also interacts with the TM1-TM2 linker of the opposing subunit in AZG144. Thus, AZG1 has a larger and stronger dimer interface than AZG2.
a Structural alignment of AZG2 and AZG1 (PDB code: 8IRL) in the apo state. AZG2 and AZG1 are colored purple and cyan, respectively. b Structural alignment of AZG2 and AZG1 protomer. Structural variations in the linker domain are indicated by dashed circles. c Cartoon representation of AZG2 in the side-view and top-view. A zoomed-in view of dimer interface in the extracellular domain of AZG2 is shown. The two protomers of AZG2 are colored red and blue, respectively. Residues at the interface are shown in sticks and colored yellow. d Cartoon representation of AZG1 (Protein Data Bank (PDB) accession code: 8IRL) in the side-view and top-view. A zoomed-in view of dimer interface in the extracellular domain of AZG1 is shown. The two protomers of AZG1 are colored cyan and orange, respectively. Residues at the interface are shown in sticks and colored yellow. Hydrogen bonds are shown in red dashed lines.
To identify possible conformational changes, we also determined the structure of AZG2 with protein samples prepared at a buffer pH of 5.5 (Supplementary Figs. 8a–d and 9a–c). The overall map resolution was 3.9 Å with the C1 symmetry applied (Supplementary Fig. 9a). Structure alignment of the AZG2 structures at pH 5.5 and pH 7.4 reveals a RMSD of 0.9 Å (715 Cα atoms aligned), showing that while one subunit of the dimer merges well, another subunit has a conformational change in the transport domain (Supplementary Fig. 7c). We focused on the analysis of the subunit which undergoes conformational changes. Compared to the structure at pH 7.4, the scaffold domain of this subunit remains unchanged (Supplementary Fig. 7d), and the transport domain rotates around the pivotal point at the amino (N)-terminus of TM2, by about 10° (Supplementary Fig. 7e). Consequently, the other TMs have both lateral rotations and vertical translations towards the membrane plane, specifically for TM1, TM3, TM4, TM8-TM10 which have a vertical translation by about two helical turns (~8 Å) towards the cytosolic side (Supplementary Fig. 7e,f). The movement of the transport domain is in a rigid manner since the transport domains merge well when aligned separately (Supplementary Fig. 7g). Surface analysis of the mobile subunit determined at pH 5.5 showed that the binding site formed by the crossover of TM3 and TM10 is neither accessible from the extracellular (or luminal) side nor the cytosolic side (Supplementary Fig. 6b), thus indicating that this captured structure is in an occluded conformation. The observed structural transitions of this subunit from pH 5.5 to pH 7.4 suggest that AZG2 adopts the elevator transport mechanism that the scaffold domain remains unmoved, and the transport domain undergoes up-and-down movement to load the cargo on one side of the membrane and unload on the other side during substrate transport49.
Adenine and tZ coordination by AZG2
Unlike the apo state structure of AZG144, no obvious density corresponding to endogenous adenine was observed in the binding site of AZG2 structures in the apo state (Supplementary Fig. 9j). To reveal the substrate recognition mechanism, we determined the adenine-bound and tZ-bound structures of AZG2 at pH 5.5, by incubating the protein samples with substrate prior to cryo-sample preparations (Supplementary Figs. 8e,f and 9d-i). The overall resolution was 3.3 Å and 3.4 Å for the adenine-bound and tZ-bound structures, respectively (Supplementary Fig. 9d,g). Clear densities were observed in the conserved substrate binding site (Supplementary Fig. 9n,o), which can be docked well with adenine or tZ, separately. In the adenine-bound structure, adenine is located at the pocket formed by the crossover of TM3 and TM10 (Fig. 4a,b). The binding pocket is mainly negative in electrostatic potential (Supplementary Fig. 6c). The phenyl ring of F396 forms π-π interaction with the purine ring (Fig. 4b). The side chains of Y56 and E398, as well as the main chain carbonyl or amine groups of M138, T395 and V397, form hydrogen bonding with the nitrogen atoms in the purine ring (Fig. 4b, Supplementary Fig. 1b). In the tZ-bound structure, the purine ring has a similar binding mode with the adenine-bound structure, through both hydrophobic stacking and hydrogen bonds (Fig. 4d,e). Besides, the hydroxyl group of the isoprenoid side chain of tZ also forms hydrogen bonding with D345 and T392 (Fig. 4e, Supplementary Fig. 1b).
a Cartoon representation of the AZG2 structure in the adenine-bound state at pH 5.5. The two protomers are colored dark magenta and blue, respectively. The adenine molecules are shown in spheres, indicated by red dashed circles, with carbon atoms colored yellow and nitrogen atoms colored blue. b Coordination of adenine by AZG2 at pH 5.5. Side chains of residues surrounding adenine are shown in sticks, with carbon atoms colored purple. Hydrogen bonds are shown as red dashed lines. c Characterization of [3H]-adenine transport for the wild-type (WT) AZG2 and the mutants in the cell-based transport assay. d Cartoon representation of the AZG2 structure in the trans-zeatin-bound state at pH 5.5. The tZ molecules are shown in spheres, with carbon colored cyan, nitrogen colored blue, and oxygen colored red. e Coordination of trans-zeatin by AZG2 at pH 5.5. Side chains of residues surrounding trans-zeatin are shown in sticks, with carbon atoms colored purple. f Characterization of [3H]-trans-zeatin transport for the wild-type AZG2 and the mutants in the cell-based transport assay. All data points in (c) and (f) represent three (n = 3) independent measurements. Data are mean ± s.d. Significant differences were determined by one-way ANOVA with Dunnett’s multiple comparisons tests. ns, not significant; * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001. Exact P values are labeled. Source data is provided as a Source Data file.
Structure alignments of the adenine-bound or tZ-bound structures of AZG1 and AZG2 revealed a highly conserved substrate binding site (Supplementary Fig. 6g,h). Residues corresponding to Y56, M138, D345, F396, and E398 of AZG2 are invariant in AZG1 (Supplementary Fig. 6g,h). V352 and V397 of AZG2 are substituted by a threonine and isoleucine residue in AZG1, respectively (Supplementary Fig. 6g,h). The largest difference is that the G348 of AZG2 is replaced by an aspartic acid residue in AZG1 (D393) (Supplementary Fig. 6g,h). Notably, the tZ molecule adopts distinct conformations in the AZG1 and AZG2 structures (Supplementary Fig. 6h). While the side chain of tZ points to D393 in AZG1 (corresponding to G348 in AZG2) (Supplementary Fig. 6i), it rotates at the C11 position, and points to D345 instead (corresponding to D390 in AZG1) (Supplementary Figs. 1b and 6j). Since the EM densities for tZ are well resolved in the AZG1 and AZG2 structures, each conformation should be reasonable in the corresponding protein.
To check the binding sites of adenine and tZ, we carried out an all-atom molecular dynamics simulation based on the adenine-bound and tZ-bound structures of AZG2. Similar to the simulations of the tZ-bound structure of AZG144, the bound adenine and tZ molecules were all released after a certain period of simulation (Supplementary Movies 1 and 2, and Supplementary Data 1), consistent with that the transporter is in the inward-facing conformation to release the substrate. However, in the initial period, we noticed that the bound tZ molecule also had configurational changes (Supplementary Movie 2 and Supplementary Data 1), suggesting a flexibility of the side chain of tZ in the binding pocket. Meanwhile, in the simulation of the tZ-bound structure of AZG144, both configurations were also captured. The observed conformations of tZ in the structures could be the more energy-favorable state of each transporter. Whether such a variation is induced by the unconserved D393 (AZG1)/G348 (AZG2) position or other factors, and the effect of such differences to the tZ transport by each transporter awaits further investigations.
To test the roles of the identified residues involved in adenine and tZ binding, we generated a series of AZG2 variants by mutating each of the residues to alanine or other residues, and then examined their substrate transport activities using the cell-based transport assay. Western blotting of the mutants showed that the mutations did not affect their protein expression (Supplementary Figs. 6k and 10h). As shown by the transport assay results, the accumulations of both [3H]-adenine and [3H]-tZ were significantly reduced for the Y56A, M138A, and F396A mutants, compared to that of the WT AZG2 (Fig. 4c,f). The Y56F and F396Y mutants retained about 50% of the [3H]-tZ transport activity of the WT, while M138R, M138I, and F396L had much decreased activities to less than 50% of the WT (Supplementary Fig. 10a). It further supports that the hydrogen bonding and π-π interaction between the purine ring of adenine or tZ and AZG2 are critical to the substrate recognition and transport. Though the side chain of M138 is not directly involved in substrate binding, it may pack with the surrounding hydrophobic residues, including I57, V60, I64, I205 and L442 (Supplementary Fig. 10b), and stabilize the local conformation of the binding pocket. Adenine or tZ transport was not affected for the T392A or E398A mutant (Fig. 4c,f). While the accumulation of [3H]-adenine was increased by 50% for the D345A mutant (Fig. 4c), the [3H]-tZ transport was reduced for this mutant (Fig. 4f), consistent with the structural observation that D345 is involved in the interaction with tZ. The carboxyl group of the side chain of D345 is critical to the [3H]-tZ transport, based on the results that the D345E mutant had a similar transport activity to the WT whereas the D345N had a decreased activity (Supplementary Fig. 10a). We also tested the tZ binding affinities for the mutants using ITC. The results showed that the Y56A, M138A, F396A and V397A mutants all had abolished or largely reduced binding affinities towards tZ (Supplementary Fig. 10c-g), while the control mutant, M449A, which residue is not directly involved in substrate binding, had a comparable binding affinity to the WT protein (Supplementary Fig. 10g), further supporting the structural observations.
Structural dynamics of AZG2 during tZ transport
We also determined the adenine- and tZ-bound structures of AZG2 at pH 7.4 (Supplementary Figs. 4e,f and 5e-m). For the adenine-bound state at 7.4, an EM map with an overall resolution of 3.4 Å was obtained (Supplementary Figs. 5e). Structure alignment of this structure with the apo state structure at pH 7.4, the adenine-bound state at pH 5.5, or the tZ-bound state at pH 5.5 revealed an almost identical structure, all in the inward-facing conformation, with RMSD values around 0.5-0.6 Å (Supplementary Fig. 6f). Notably, for the tZ-bound AZG2 protein at pH 7.4, two distinct conformations were identified during data processing, yielding two EM maps with an overall resolution of 3.4 Å and 3.5 Å, respectively (Supplementary Figs. 4f and 5h,k). One of the conformations aligned well with the tZ-bound structure at pH 5.5, also in the inward-facing conformation, named state-1 (Supplementary Fig. 4g). In the other conformation, both of the two subunits undergo structural changes similar to that observed in one of the subunits in the apo state structure at pH 5.5, named state-2 (Supplementary Fig. 4h). Alignment of the dimeric state-1 and state-2 structures revealed a RMSD of 3.1 Å (831 Cα atoms aligned) (Fig. 5a). The two scaffold domains merge well (Fig. 5a,b), while each transport domain undergoes a rotational shift around the pivotal point at the N-terminus of TM2 (Fig. 5c). As such, the transport state changes from the occluded conformation of state-2 to the inward-facing conformation of state-1. Similar to the conformational changes occur in the apo state structures under different pH conditions, TM1, TM3, TM4, TM8-TM10 have a vertical translation by about two helical turns towards the cytosolic side (Fig. 5c,d). The substrate-binding pocket also shifts downwards from state-2 to state-1, carrying the loaded tZ molecule towards the cytoplasm (Fig. 5d,e). The vertical shift for the tZ molecule was about 6 Å (Fig. 5e). The binding between tZ and AZG2 remained largely the same during state transitions (Figs. 4e and 5f). Hydrogen bonding between the purine ring with the side chain of Y56 or main chain of M138 and V397, and the π-π interaction between F396 and the purine ring were also observed in the state-2 conformation, as well as the hydrogen bonding between the side chain of D345 and the hydroxyl group of tZ (Fig. 5f). We also carried out an all-atom molecular dynamics simulation of the tZ-bound state-2 structure of AZG2. In this simulation, the tZ molecule remained bound in the binding pocket (Supplementary Movie 3 and Supplementary Data 1), unlike that in the inward-facing conformation. Configuration flexibilities were also observed for the side chain of tZ (Supplementary Movie 3 and Supplementary Data 1), further supporting its dynamics in the binding pocket. Taken together, it further confirms that the transport process of AZG2 adopts the elevator model that the cargo is loaded to the binding pocket, and the transport domain slides along the scaffold domain in the lipid bilayer to unload to another side of the membrane49.
a Structural alignment of the two different states of the trans-zeatin-bound AZG2 at pH 7.4. State-1 and state-2 are colored light blue and dark blue, respectively. The tZ molecules are shown in spheres. b Structural alignment of the monomeric AZG2 structures in the two different states at pH 7.4. c Structural changes of the transport domain of AZG2 in the two different states at pH 7.4. The tZ molecules are shown in spheres, with carbon atoms colored marine in state-2 and cyan in state-1. d View of the transmembrane segments of the AZG2 transport domain in the two different states. The tZ molecules are shown in sticks. Vertical shift of transmembrane segment is indicated by red dashed lines. e Vertical shift of the tZ molecule in the two different states. f Coordination of trans-zeatin by AZG2 in the state-2 structure at pH 7.4. Hydrogen bonds are shown as red dashed lines.
Discussion
In this study, we characterized the adenine and the representative cytokinin, trans-zeatin, binding and transport of AZG2 under different pH conditions. As the results showed, both the substrate binding affinity and transport activity of AZG2 at acidic pH (pH 5.5) were higher than those at neutral pH (pH 7.4), suggesting that AZG2 can also utilize the proton gradient for substrate transport, though may be less dependent on it than AZG141. For the representative NAT family members with known structures, the bacterial uracil transporter UraA50,51 and fungal uric acid/xanthine transporter UapA52 also function as proton symporters, while the mammalian vitamin C transporter SVCT153,54 are sodium-coupled transporter. Acidic residues around the substrate binding site have been suggested to play critical roles in the proton or sodium coordination and coupling44,50,51,53,54. Sequence and structure alignments between AZG1 and AZG2 indicate the substitution of an aspartic acid residue (D393) by a glycine (G348) may contribute to the different degree of pH dependency of the two transporters44. We generated a G348D mutant and tested its tZ transport activity under different pH in the cell-based assay. The result showed that the accumulation of tZ was reduced for G348D compared to the WT, either under pH 5.5 or pH 7.4, and the transport did not show an increased pH dependency (Supplementary Fig. 3h). Thus, the proton-driven substrate transport mechanism of AZG1 and AZG2, and the molecular basis for the different level of proton-gradient dependency awaits further investigations. Notably, the AZG1-mediated adenine and cytokinin transport was much lower than AZG2 in the HEK293F cell-based or in the proteoliposome-based transport assay, different as reported by Tessi et al. in the yeast or planta-cell based transport assays41,42. The missing factors or possible regulation mechanisms of AZG1 transport remain an intriguing question. Meanwhile, the subcellular localization of AZG2 remains to be further investigated. AZG2 has been suggested to localize to both the plasma and ER membrane41. However, these assays were carried out with expressing AZG2 under strong constitutive promoters, which may lead to unpredictable artifacts. Further investigations are needed to confirm the localization of AZG2 under native promoter in plants.
Structural changes were captured for the AZG2 in the apo state and tZ-bound state under different pH conditions. Specifically, in the apo state AZG2 structure determined at pH 5.5, the two protomers displayed distinct conformations, with one in the inward-facing conformation and the other in the occluded conformation. In the tZ-bound AZG2 protein at pH 7.4, two states were captured, with both protomers changing from the inward-facing conformation (state-1) to the occluded conformation (state-2). In the structure, the two scaffold domains mediate the dimerization and the two transport domains are separated with no direct contacts. The conformational changes were identical as observed in the apo and tZ-bound states, with the scaffold remaining unchanged and the transport domain rotating along the scaffold domain. Taken together, the two protomers may function independently during substrate transport, consistent with the biochemical analysis results of UraA51. Such a transport process follows the elevator transport mechanism proposed for the homologous transporters with similar topology and fold (Supplementary Fig. 11)49. For UapA, mouse and human SVCT1, the structures have been determined in the inward-facing conformation52,53,54. For UraA, both substrate-bound occluded and inward-facing conformations have been captured50,51. In a recent report of human SVCT1, a substrate-free occluded conformation was also captured, in which the scaffold domain (or the gate domain named in SVCT1) undergoes rotational shift around the transport domain (or the core domain named in SVCT1), and the dimer interface was rearranged, compared to the inward-facing conformation54. It suggests that the dimer formation and reassembly are important to the transport process of this family transporters. To fully reveal the transport cycle of AZG2 and the NAT family transporters, more conformations await captured, particularly an outward-facing conformation (Supplementary Fig. 11). Taken together, our functional and structural characterizations of AZG2 facilitate our understanding of the cytokinin transport and signaling mechanisms in plants, and provide a basis for further functional comparisons of the AZG1 and AZG2 in substrate transport.
Methods
Chemical treatments
Chemicals including adenine (MedChemExpress, CAS:73-24-5) and trans-zeatin (tZ, MedChemExpress, CAS:1637-39-4) were dissolved in ddH2O to a concentration of 15 mM and 12.5 mM, respectively. Chemicals including trans-Zeatin riboside (tZR, MedChemExpress, CAS:6025-53-2), N6-(Δ2-isopentenyl)adenine (iP, Sigma, CAS: 2365-40-4), kinetin (MedChemExpress, CAS:525-79-1), benzyladenine (BA, MedChemExpress, CAS: 1214-39-7), were dissolved in DMSO to a concentration of 10 mM. For radiolabeled transport assay, [3H]-adenine (specific activity 28 Ci mmol−1, American Radiolabeled Chemicals) and [3H]-trans-zeatin (specific activity 1.5 Ci mmol−1, American Radiolabeled Chemicals) were dissolved in ddH2O and ethyl alcohol to a concentration of 35.7 μM and 666.7 μM, respectively. Sodium ionophore III (MedChemExpress, CAS:81686-22-8) and valinomycin (Sigma, CAS:2001-95-8) were dissolved in DMSO to a concentration of 100 μM, respectively.
Plasmid construction and generation
The full-length Arabidopsis AZG1 (At3g10960) or AZG2 (At5g50300) cDNA was amplified from the Arabidopsis thaliana cDNA library. Site-directed mutagenesis was performed with a standard two-step PCR and verified by DNA sequencing. The wild-type and mutant sequences of AZG2 were subcloned into the pCAG vector with a C-terminal FLAG tag (DYKDDDDK) and a His8 tag for protein expression and transport assay. The wild-type sequences of AZG1 and AZG2 were subcloned into the pCAG vector with a N-terminal EGFP tag for fluorescence imaging. Primers used in this study are listed in Supplementary Table 1.
Protein expression and purification
For AZG1 and AZG2 expression, plasmids were transfected into the HEK293F cells (Gibco, catalog number: A14635) cultured in SMM 293T-II medium (M293TII, Sino Biological Inc.) at 37 °C, 130 rpm, and 5% CO2. When the cell density reached ~2 × 106 cells per ml, for 800 ml cells, 1.5 mg plasmid containing AZG1 or AZG2 gene and 4 mg polyethylenimines (PEIs) (Polysciences) were pre-incubated in 45 ml fresh medium for 15 min. The mixture and a further 50 ml fresh medium were then added into the cell culture, followed by a static incubation of 15 min. Transfected cells were further cultured at 37 °C, 130 rpm, and 5% CO2 for 12 h. After that, 10 mM sodium butyrate (Sigma-Aldrich) was added to the culture, and cells were cultured for additional 48 h at 30 °C before harvested by centrifugation at 2100 × g. Cell pellets were resuspended in lysis buffer containing 25 mM Tris-HCl, pH 7.4, and 150 mM NaCl. After addition of 1.5% (w/v) dodecyl maltopyranoside (DDM, Anatrace) supplemented with 0.3% (w/v) cholesteryl hemisuccinate (CHS, Sigma-Aldrich) and the protease inhibitor cocktail containing 1 mM phenylmethylsulfonyl fluoride (PMSF), aprotinin (1.3 mg ml−1), pepstatin A (0.7 mg ml-1) and leupeptin (5 mg ml−1), the cell suspension was incubated at 4 °C, 20 rpm for 2 hours. Then the insoluble fraction was precipitated by centrifugation at 12,000 × g for 60 min (Beckman, Type 70 Ti), and the supernatant was collected and incubated with anti-FLAG M2 affinity resin (Sigma-Aldrich) at 4 °C for 40 min.
To prepare protein samples subjected to the cryo-EM analysis, the M2 resin was rinsed three times with buffer A containing 25 mM Tris-HCl (pH 7.4), 150 mM NaCl and 0.02% (w/v) GDN (Anatrace), and then eluted with buffer B containing 25 mM Tris-HCl, pH 7.4, 150 mM NaCl and 0.02% (w/v) GDN plus 200 µg ml−1 FLAG peptide. The protein eluent was concentrated with a 50 kDa cut-off Centricon (Millipore), and then applied to the Superose-6 column in buffer C containing 25 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.01% (w/v) GDN. Peak fractions were pooled together and further concentrated to 10 mg ml−1 before cryo-EM sample preparation. All purification procedures were carried out at 4 °C. For protein sample at pH 5.5, the procedures remained unchanged except that Tris-HCl in the buffer was replaced by MES-NaOH (pH 5.5).
To prepare samples for isothermal titration calorimetry (ITC) assay and proteoliposome reconstitution, wild-type and mutant AZG1 or AZG2 proteins were purified in the same way as the protein sample at pH 7.4. Peak fractions were pooled and concentrated to ~2 mg ml−1 for the following experiments.
Proteoliposome reconstitution
Soybean phospholipids (Sigma-Aldrich) were dissolved in a mixture of chloroform:methanol 3:1 (v/v) (Sigma-Aldrich) at 50 mg ml−1, then dried under nitrogen gas and washed in pentane (Sigma-Aldrich). Lipids were resuspended to 20 mg ml-1 by the inside-buffer (20 mM HEPES, pH 7.4, 145 mM KCl, 5 mM NaCl), and frozen and thawed 10 times in liquid nitrogen. For subsequent reconstitution, liposomes were extruded 31 times through the 0.4 mm membrane filters (Cytiva) and incubated with 1% (w/v) DDM at 4 °C for 1 h. The detergent-solubilized protein was added into the liposomes at a protein:lipid ratio of 1:100 (w/w) and incubated at 4 °C for 1 hour. DDM was removed by adding 100 mg ml-1 Bio-Beads (BioRad) in four batches. The proteoliposomes were then homogenized by five freeze-thaw cycles followed by another round of extrusion. Proteoliposomes were harvested by ultracentrifugation at 160,000 × g for 1 h, and resuspended by the inside buffer to a final concentration of 100 mg ml−1.
Proteoliposome-based [3H]-adenine and [3H]-trans-zeatin transport assay
The proteoliposome-based transport assay was performed using a rapid filtration method55. Briefly, the reaction was initiated by the addition of 2 μl proteoliposomes to 48 μl reaction buffer (the outside-buffer, containing 145 mM NaCl, 5 mM KCl, 20 mM MES, pH 5.5 or 20 mM HEPES, pH 7.4), 0.2 μl [3H]-adenine or [3H]-trans-zeatin at room temperature. DMSO, 1 μM sodium ionophore III, 1 μM valinomycin, and 1 μM sodium ionophore III plus 1 μM valinomycin were added to the reaction buffer, respectively. Transport was stopped by transferring the samples to the glass fiber filters (0.3 mm, Advantec) after a 5 min incubation. The filters were then washed rapidly with 8 ml ice-cold outside-buffer. The filters were incubated with 3 ml scintillation solution for 2 h, and the radioactivity was measured by liquid scintillation counting (Tri-Carb 2910TR, Perkin-Elmer). For the time course assay, the reaction was initiated by the addition of 2 μl proteoliposomes to 48 μl reaction buffer (the outside-buffer, containing 145 mM NaCl, 5 mM KCl, 20 mM MES, pH 5.5) containing 0.2 μl [3H]-trans-zeatin at room temperature and the accumulation of [3H]-trans-zeatin were stopped at indicated time points. For the concentration-dependent transport assay, the reaction was initiated by adding 2 μl proteoliposomes to 48 μl reaction buffer (the outside-buffer, 145 mM NaCl, 5 mM KCl, 20 mM MES, pH 5.5) that contained variable concentrations of [3H]-trans-zeatin at room temperature and the accumulation of [3H]-trans-zeatin were stopped after a 5 min incubation.
Cell-based [3H]-adenine and [3H]-trans-zeatin transport assay
HEK293F cells at a density of 2 × 106 cells per ml were transfected with the plasmid containing the coding sequence for AZG1 or AZG2, or the empty vector at ratio the same as protein expression. After 24 h of cultivation at 37 °C, 130 rpm, and 5% CO2, cells were collected by centrifugation for the uptake assay. 3 × 106 cells were resuspended with 250 μl PBS citrate buffer, pH 5.5 (10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl, pH adjusted by citric acid anhydrous) containing 0.1 µl [3H]-adenine or 0.2 µl [3H]-trans-zeatin. The uptake process was terminated by centrifugation, and cells were then washed twice with the ice-cold PBS buffer (pH 7.4) and resuspended with the same buffer plus 1% Triton X-100 for cell lysis. Radioactivity was measured by liquid scintillation counting (Tri-Carb 2910TR, Perkin-Elmer) after incubation with 2 ml scintillation solution for 2 hours. For the time course assay, the accumulation of [3H]-adenine or [3H]-trans-zeatin was terminated at indicated time points. For the substrate competitive transport assay, 50 μM extra non-radiolabeled chemicals were added to 250 μl PBS citrate buffer, pH 5.5, containing 0.2 µl [3H]-trans-zeatin, and the uptake was terminated by centrifugation at 2 min. For the mutants, the [3H]-adenine or [3H]-trans-zeatin accumulation at 2 min was calculated as the relative difference compared to the control group normalized to wild type. To measure the transport kinetics, 3 × 106 cells were resuspended by 250 μl PBS citrate buffer (pH 5.5) containing 0.1 µl [3H]-adenine and unlabeled adenine at indicated concentrations, or [3H]-trans-zeatin at indicated concentrations. The uptake process was terminated at 2 min by centrifugation and followed by the same experimental procedure as mentioned above.
Isothermal titration calorimetry
The binding affinities were measured using a MicroCal iTC200 microcalorimeter (MicroCal). Proteins in buffer containing 25 mM MES, pH 5.5 (or 25 mM HEPES, pH 7.4), 150 mM NaCl, and 0.01% (w/v) GDN were concentrated to 0.03 mM, and titrated by 0.1 mM adenine, 0.15 mM trans-zeatin, 0.15 mM iP, 0.15 mM BA, 0.15 mM kinetin, or 0.15 mM tZR dissolved in buffer the same as for protein at 25 °C, respectively. Binding isotherms were plotted, analyzed, and fitted based on the one-set-of-sites binding model with the n value fixed to 1.0, using MicroCal PEAQ-ITC Analysis Software (v.1.22, Malvern Panalytical).
Cryo-EM sample preparation
For the apo state AZG2 sample preparation, aliquots of 4 µl purified wild-type AZG2 protein were applied to the holey carbon grid (Quantifoil Au R1.2/1.3, 300 mesh), glow-discharged for 10 s with SOLARUS 950 Plasma Cleaner (Gatan) using H2 and O2. The grid was then blotted with a Vitrobot Mark IV (Thermo Fisher Scientific) operating at 8 °C and 100% humidity with a blotting time of 3 s. The filter paper used was Grade 597 Filter Paper (Cytiva Whatman™). The grid was then immersed into liquid nitrogen-cooled liquid ethane. For the adenine-bound state AZG2 sample, aliquots of 4 μl purified protein were incubated with 1 mM adenine on ice for 30 min, and the sample was then prepared as above. For the trans-zeatin-bound state AZG2 sample, aliquots of 4 μl purified protein were incubated with 1 mM trans-zeatin on ice for 30 min, and then applied to cryo-sample preparation. Proteins at different pHs were prepared following the same procedure.
Cryo-EM data collection and processing
All data were collected by the EPU software in the super-resolution mode on a 300-kV Titan Krios microscope (Thermo Fisher Scientific) equipped with a K3 Summit direct electron detector (Gatan) and a GIF Quantum energy filter (Gatan), at a nominal magnification of 81,000 × with defocus values ranging from −1.0 to −2.0 μm and a calibrated pixel size of 0.55 Å. Each movie stack was acquired with an exposure time of 3 s and dose-fractioned into 32 frames, yielding a total accumulated dose of 50 e− Å−2. Data collected were then imported to RELION 4.0, motion corrected, and dose weighted by MotionCor256,57. The resulting micrographs were binned twofold, yielding a pixel size of 1.1 Å, and then imported into cryoSPARC (v.3.2.0)58. The defocus values of each image were determined by CTFFIND459. Data processing of apo state AZG2, adenine-bound state AZG2, and trans-zeatin-bound state AZG2 at pH 7.4 or pH 5.5 followed a similar scheme, and a detailed procedure was described below as an example for apo state AZG2 at pH 7.4. Detailed flowcharts for all data processing can be found in Supplementary Figs. 4d–f and 8d–f. For the apo state AZG2 at pH 7.4, 3,765 micrograph stacks were collected. 4,345,976 particles were automatically picked by the template picker in cryoSPARC, and then four maps served as a reference for further heterogeneous refinement were generated by volume tools at different lowpass filter. After several rounds of heterogeneous refinement and ab-initio reconstruction, 65,805 particles were selected for non-uniform refinement, and finally yielded a reconstruction map at 3.4 Å resolution. The application of symmetry in processing can be found in the Supplementary Fig. 4d. The overall resolution of the final map was determined by the gold-standard Fourier shell correlation (FSC) at a 0.143 criterion60, and the local resolutions were estimated using implementations in cryoSPARC.
Model building and refinement
The initial protein model was built by fitting the predicted model of AZG2 using AlphaFold2 into the cryo-EM maps with UCSF Chimera61,62. Models were then adjusted and built based on the cryo-EM density features using Coot63. The adenine and trans-zeatin molecules were incorporated into the density and refined in PHENIX with restraints generated by eLBOW64. Structure refinements were carried out by PHENIX using the default phenix.real_space_refine parameters with no further constraints applied64. Overfitting of the model was monitored by refining the model in one of the two independent maps from the gold-standard refinement approach and by testing the refined model against the other map. Statistics of the 3D reconstruction and model refinement can be found in Supplementary Tables 2 and 3.
Molecular dynamics simulation
The adenine-bound (in pH 5.5), tZ-bound (in pH 5.5), and tZ-bound state-2 (in pH 7.4) structures of AZG2 were incorporated into a planar lipid bilayer constituted by 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphorylcholine using the CHARMM-GUI software65, respectively. Each system was then hydrated using the TIP3P water model and neutralized with 150 mM NaCl. A periodic rectangular box with dimensions of 138 × 138 × 118 Å3 was generated, which has approximately 229,000 atoms. The CHARMM36m force field was applied for the protein and lipids, while the force field parameters for tZ were obtained from the CGenFF program. To initiate the relaxation of the system, the energy minimization was performed using the steepest descent algorithm. The simulation system was then pre-equilibrated under multistep isothermal-isovolumetric (NVT) and isothermal-isobaric (NPT) conditions, with the restraints progressively reduced at each step. Unrestrained production simulations were conducted with a 2-fs time step at 310 K. Each structure was simulated for three repeats with different initial velocities. The simulations were carried out using the GROMACS 2021.4 package66, and analyzed using the visual molecular dynamics (VMD) software67.
Fluorescence imaging
To confirm the expression of AZG1 and AZG2 in HEK293F cells, cells were grown on coverslips in 35 nm plates overnight for fluorescence imaging after transfection for 24 h at 37 °C. The bright-field images were collected using a halogen lamp, and the GFP fluorescence was excited by the mercury lamp light source with a 488 nm excitation filter on an inverted fluorescence microscope. The emission was monitored at 510 nm using a CCD camera. All fluorescence images were captured via the LSM880 confocal laser scanning microscope (Zeiss) and analyzed with ZEN (Zeiss).
Western blotting analysis
Cells used for transport assay were lysed by incubating with 1.5% DDM for 2 h, followed by centrifugation for 1 h at 12,000 × g. Whole cell lysate samples were subsequently run on SDS-PAGE for 120 min at 100 V. The gel was transferred to a polyvinylidene fluoride membrane (Merck Millipore) with the Trans-Blot SD semi-dry electrophoretic transfer system (BioRad). The membrane was blocked with 5% (w/v) non-fat milk (Sangon Biotech) for 1 h at room temperature, followed by incubation with the primary antibody (anti-FLAG tag mouse monoclonal antibody, 1:3,000, CWBIO, catalog number: CW0287, lot number: 01222/12422) for 1 h. Then the membrane was washed 5 times with TBST buffer (20 mM Tris-HCl, pH 7.0, 150 mM NaCl, 0.1% (w/v) Tween-20) before incubation with the secondary antibody (HRP-conjugated goat-anti-mouse IgG, 1:5,000, CWBIO, catalog number: CW0102S, lot number: 01325/33621) for 1 h at room temperature. The membrane was washed 5 times and imaged after incubation with SuperSignal West Pico PLUS chemiluminescent substrate (Thermo Scientific).
Statistics and reproducibility
Data were analyzed with GraphPad Prism 8. Data were considered significant if P < 0.05 with the unpaired two-tailed Student’s t test, or one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison tests. No statistical method was used to predetermine sample size. All of the functional assays were performed with at least three replicates and described in the figure legends. The sample sizes were chosen to ensure the reproducibility of the experiments and to obtain meaningful results. No data were excluded from the analyses. For cryo-EM 3D refinement, all particles were randomly split into two groups. The Investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Model coordinates and cryo-EM density maps for the AZG2 apo state, adenine-bound state, trans-zeatin-bound state-1 and trans-zeatin-bound state-2 structures at pH7.4 have been deposited in the Protein Data Bank under accession codes 9J13, 9J16, 9J17, and 9J18, respectively, and in the Electron Microscopy Data Bank under accession codes EMD-61066, EMD-61069, EMD-61070, and EMD-61071, respectively. Model coordinates and cryo-EM density maps for the AZG2 apo state, adenine-bound state, trans-zeatin-bound state structures at pH5.5 have been deposited in the Protein Data Bank under accession codes 9J12, 9J15, and 9J14, respectively, and in the Electron Microscopy Data Bank under accession codes EMD-61065, EMD-61068, and EMD-61067, respectively. Source data are provided with this paper.
References
Kieber, J. J. & Schaller, G. E. Cytokinin signaling in plant development. Development 145, dev149344 (2018).
Li, S. M., Zheng, H. X., Zhang, X. S. & Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 40, 271–282 (2021).
Werner, T. & Schmülling, T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 12, 527–538 (2009).
Sakakibara, H. Cytokinins: activity, biosynthesis, and translocation. Annu Rev. Plant Biol. 57, 431–449 (2006).
Mok, D. W. S. & Mok, M. C. Cytokinin metabolism and action. Annu Rev. Plant Phys. 52, 89–118 (2001).
Inoue, T. et al. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409, 1060–1063 (2001).
Ueguchi, C., Koizumi, H., Suzuki, T. & Mizuno, T. Novel family of sensor histidine kinase genes in Arabidopsis thaliana. Plant Cell Physiol. 42, 231–235 (2001).
Kim, H. J. et al. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc. Natl Acad. Sci. USA 103, 814–819 (2006).
Caesar, K. et al. Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum. J. Exp. Bot. 62, 5571–5580 (2011).
Wulfetange, K. et al. The cytokinin receptors of Arabidopsis are located mainly to the endoplasmic reticulum. Plant Physiol. 156, 1808–1818 (2011).
Lomin, S. N. et al. Studies of cytokinin receptor-phosphotransmitter interaction provide evidences for the initiation of cytokinin signalling in the endoplasmic reticulum. Funct. Plant Biol. 45, 192–202 (2018).
Antoniadi, I. et al. Cell-surface receptors enable perception of extracellular cytokinins. Nat. Commun. 11, 4284 (2020).
Kubiasová, K. et al. Cytokinin fluoroprobe reveals multiple sites of cytokinin perception at plasma membrane and endoplasmic reticulum. Nat. Commun. 11, 4285 (2020).
Aloni, R., Langhans, M., Aloni, E., Dreieicher, E. & Ullrich, C. I. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J. Exp. Bot. 56, 1535–1544 (2005).
Zürcher, E. et al. A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol. 161, 1066–1075 (2013).
Müller, B. & Sheen, J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453, 1094–1097 (2008).
Anfang, M. & Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 63, 102055 (2021).
Nedved, D., Hosek, P., Klíma, P. & Hoyerova, K. Differential subcellular distribution of cytokinins: How does membrane transport fit into the big picture? Int. J. Mol. Sci. 22, 3428 (2021).
Zhao, J. Z. et al. Spatially distributed cytokinins: metabolism, signaling, and transport. Plant Commun. 5, 100936 (2024).
Zhang, Y. Q., Berman, A. & Shani, E. Plant hormone transport and localization: signaling molecules on the move. Annu. Rev. Plant Biol. 74, 453–479 (2023).
Hirose, N. et al. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 59, 75–83 (2008).
Kudo, T., Kiba, T. & Sakakibara, H. Metabolism and long-distance translocation of cytokinins. J. Integr. Plant Biol. 52, 53–60 (2010).
Matsumoto-Kitano, M. et al. Cytokinins are central regulators of cambial activity. Proc. Natl. Acad. Sci. USA 105, 20027–20031 (2008).
Osugi, A. et al. Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nat. Plants 3, 17112 (2017).
Hu, Y. J. & Shani, E. Cytokinin activity - transport and homeostasis at the whole plant, cell, and subcellular levels. N. Phytol. 239, 1603–1608 (2023).
Kurakawa, T. et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445, 652–655 (2007).
Kuroha, T. et al. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell 21, 3152–3169 (2009).
Liu, C. J., Zhao, Y. J. & Zhang, K. W. Cytokinin transporters: multisite players in cytokinin homeostasis and signal distribution. Front. Plant Sci. 10, 693 (2019).
Ko, D. et al. ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl Acad. Sci. USA 111, 7150–7155 (2014).
Zhang, K. W. et al. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat. Commun. 5, 3274 (2014).
Zhao, J. Z. et al. ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J. Exp. Bot. 70, 6277–6291 (2019).
Kim, A. et al. Non-intrinsic ATP-binding cassette proteins ABCI19, ABCI20 and ABCI21 modulate cytokinin response at the endoplasmic reticulum in Arabidopsis thaliana. Plant Cell Rep. 39, 473–487 (2020).
Hirose, N., Makita, N., Yamaya, T. & Sakakibara, H. Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport. Plant Physiol. 138, 196–206 (2005).
Sun, J. P. et al. Arabidopsis SOI33/AtENT8 gene encodes a putative equilibrative nucleoside transporter that is involved in cytokinin transport in planta. J. Integr. Plant Biol. 47, 588–603 (2005).
Zürcher, E., Liu, J. C., di Donato, M., Geisler, M. & Müller, B. Plant development regulated by cytokinin sinks. Science 353, 1027–1030 (2016).
Gillissen, B. et al. A new family of high-affinity transporters for adenine, cytosine, and purine derivatives in Arabidopsis. Plant Cell 12, 291–300 (2000).
Bürkle, L. et al. Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J. 34, 13–26 (2003).
Hu, Y. J. et al. Multi-Knock-a multi-targeted genome-scale CRISPR toolbox to overcome functional redundancy in plants. Nat. Plants 9, 572–587 (2023).
Xiao, Y. H. et al. Endoplasmic reticulum-localized PURINE PERMEASE1 regulates plant height and grain weight by modulating cytokinin distribution in rice. Front Plant Sci. 11, 618560 (2020).
Radchuk, V. et al. SWEET11b transports both sugar and cytokinin in developing barley grains. Plant Cell 35, 2186–2207 (2023).
Tessi, T. M. et al. Arabidopsis AZG2 transports cytokinins in vivo and regulates lateral root emergence. N. Phytol. 229, 979–993 (2021).
Tessi, T. M. et al. AZG1 is a cytokinin transporter that interacts with auxin transporter PIN1 and regulates the root stress response. N. Phytol. 238, 1924–1941 (2023).
Mansfield, T. A., Schultes, N. P. & Mourad, G. S. AtAzg1 and AtAzg2 comprise a novel family of purine transporters in Arabidopsis. Febs Lett. 583, 481–486 (2009).
Xu, L. Y. et al. Structures and mechanisms of the Arabidopsis cytokinin transporter AZG1. Nat. Plants 10, 180–191 (2024).
Gournas, C., Papageorgiou, I. & Diallinas, G. The nucleobase-ascorbate transporter (NAT) family: genomics, evolution, structure-function relationships and physiological role. Mol. Biosyst. 4, 404–416 (2008).
Zhang, X. J. C., Zhao, Y., Heng, J. & Jiang, D. H. Energy coupling mechanisms of MFS transporters. Protein Sci. 24, 1560–1579 (2015).
Zhang, X. C. & Han, L. Uniporter substrate binding and transport: reformulating mechanistic questions. Biophys. Rep. 2, 45–54 (2016).
Diallinas, G. Dissection of transporter function: from genetics to structure. Trends Genet. 32, 576–590 (2016).
Drew, D. & Boudker, O. Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem 85, 543–572 (2016).
Lu, F. R. et al. Structure and mechanism of the uracil transporter UraA. Nature 472, 243–246 (2011).
Yu, X. Z. et al. Dimeric structure of the uracil: proton symporter UraA provides mechanistic insights into the SLC4/23/26 transporters. Cell Res 27, 1020–1033 (2017).
Alguel, Y. et al. Structure of eukaryotic purine/H+ symporter UapA suggests a role for homodimerization in transport activity. Nat. Commun. 7, 11336 (2016).
Wang, M. X. et al. Structural basis of vitamin C recognition and transport by mammalian SVCT1 transporter. Nat. Commun. 14, 1361 (2023).
Kobayashi, T. A. et al. Dimeric transport mechanism of human vitamin C transporter SVCT1. Nat. Commun. 15, 5569 (2024).
Ying, W. et al. Structure and function of the Arabidopsis ABC transporter ABCB19 in brassinosteroid export. Science 383, eadj4591 (2024).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Chen, S. X. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Pettersen, E. F. et al. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 66, 213–221 (2010).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph Model 14, 33–38 (1996).
Acknowledgements
We thank the Cryo-EM Center of the University of Science and Technology of China for the EM facility support. This work was supported by the National Natural Science Foundation of China (32322041, W2412029 and 32321001 to L.S., and 32471279 to X.L.), Natural Science Foundation of Anhui Province (2408085JX005 to L.S.), Center for Advanced Interdisciplinary Science and Biomedicine of IHM (QYPY20230034 to L.S.), Strategic Priority Research Program of Chinese Academy of Sciences (XDB37020103 to L.S.), Fundamental Research Funds for the Central Universities (WK9100000031 to L.S.), USTC Research Funds of the Double First-Class Initiative (YD9100002004 to L.S., and YD9100002020 to X.L.), Science, Technology, and Innovation Commission of Shenzhen Municipality Projects (JCYJ-2020010915-0003938, RCYX20200714114645019 and GXWD2020123115722002-20200831175432002 to L.Z.). L.S. is supported by an Outstanding Young Scholar Award from the Qiu Shi Science and Technologies Foundation. L.S. and X.L. are supported by the Young Scholar Awards from the Cyrus Tang Foundation. L.Z. is supported by a Presidential Fellowship at the Chinese University of Hong Kong, Shenzhen.
Author information
Authors and Affiliations
Contributions
X.L. and L.S. conceived the project. X.L. and L.S. designed the experiments and analysed data. H.W. and W.Y. performed the molecular cloning, protein purification work. H.W., Z.Y., and W.Y. performed the binding and transport assays. H.W., Z.Y., and Y.G. performed the cryo-EM data collection. H.W. and Z.Y. performed the cryo-EM data processing and model-building work. H.L. and L.Z. performed the simulation work. H.W., X.L., and L.S. wrote the manuscript. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, H., Yang, Z., Liu, H. et al. Structural basis of cytokinin transport by the Arabidopsis AZG2. Nat Commun 16, 3475 (2025). https://doi.org/10.1038/s41467-025-58802-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58802-6