Abstract
Calorie restriction (CR) provides anti-aging benefits through diverse processes, such as reduced metabolism and growth and increased mitochondrial activity. Although controversy still exists regarding CR-mediated lifespan effects, many researchers are seeking interventions that mimic the effects of CR. Yeast has proven to be a useful model system for aging studies, including CR effects. We report here that yeast adapted through in vitro evolution to the severe cellular stress caused by loss of the Ulp2 SUMO-specific protease exhibit both enhanced growth rates and replicative lifespan, and they have altered gene expression profiles similar to those observed in CR. Notably, in certain evolved ulp2Δ lines, an increase in the auto-sumoylation of Ubc9 E2 SUMO-conjugating enzyme results in altered regulation of multiple targets involved in energy metabolism and translation at both transcriptional and post-translational levels. This increase is essential for the survival of aged cells and CR-mediated lifespan extension. Thus, we suggest that high Ubc9 auto-sumoylation exerts potent anti-aging effects by promoting efficient energy metabolism-driven improvements in cell replication abilities. This potential could be therapeutically explored for the development of promising CR-mimetic strategies.
Similar content being viewed by others
Introduction
All organisms transmit heritable characteristics across successive generations in response to environmental changes, including climate fluctuations, habitat modifications, and food scarcity1. At the cellular level, both external and internal environmental changes, such as oxidative stress, nutrient deprivation, or genotoxic stress, typically trigger a range of immediate and long-term adaptive responses to counterbalance the stress stimuli2,3. During acute stress, initial cellular responses (such as in metabolism, protein homeostasis, or enzyme activities) work to swiftly reestablish equilibrium and sustain viability. When these protective responses are not sufficient to mitigate the effects of stress, secondary adaptive mechanisms are implemented that primarily include genetic changes to resist stress. One useful approach for studying such adaptive mechanisms is in vitro laboratory evolution, which includes the artificial induction of cellular or genetic alterations under specific growth conditions that are difficult to produce in nature and offers insights into molecular adaptations in response to environmental change4.
The small ubiquitin-like modifier (SUMO) protein is a crucial regulator of cell homeostasis, especially during environmental stress5. In humans, five genes encode SUMO paralogs6,7, while the budding yeast Saccharomyces cerevisiae expresses a single SUMO ortholog, Smt3, which shares 48% identity with human SUMO16. SUMO is initially synthesized as a C-terminally extended precursor and later processed into its mature form by SUMO-specific proteases (Ulp1 in S. cerevisiae). The mature SUMO is then conjugated to lysine (K) side chains of target proteins via an enzyme cascade similar to the ubiquitin conjugation process8. Initially, a heterodimeric E1 SUMO-activating enzyme (Aos1/Uba2 in S. cerevisiae) forms a thioester linkage between Uba2 and the carboxy terminus of SUMO. The SUMO moiety is then transferred to the active-site cysteine of the Ubc9 E2 SUMO-conjugating enzyme. SUMO can form polymeric chains on substrates that are disassembled by site-specific SUMO proteases. Humans have nine known SUMO proteases, while S. cerevisiae has two (Ulp1 and Ulp2)9.
The SUMO stress response involves rapid changes in SUMO conjugates in response to various stressors and is not yet fully understood. In S. cerevisiae, the Siz1 E3 ligase and Ulp2 SUMO protease play crucial roles in the SUMO stress response10. The primary activity of Ulp2 is as a SUMO chain editor11. Loss of the ULP2 gene leads to many severe cellular defects, including defects in growth, DNA damage and mitotic checkpoint recovery, chromosome stability, and meiosis12.
SUMO system dysregulation after Ulp2 protease loss triggers both short-term and long-term adaptive mechanisms, depending on the continuous culture duration. Short-term adaptation includes the development of a specific multi-chromosome aneuploidy and significant changes in the transcription of ribosomal genes, which promotes compensatory mechanisms for rapid adaptation to Ulp2 loss13,14. Because aneuploidy is typically detrimental to cellular fitness15, long-term adaptation restores euploidy and growth, adjusts transcription, and often leads to countervailing mutations in SUMO-conjugation enzymes14. This stepwise utilization of adaptive mechanisms is relevant to the robust adaptive fitness gains observed in ulp2Δ cells following extended serial culturing.
Aging is a process characterized by the gradual accumulation of molecular, cellular, and organ damage during sexual maturity that ultimately increases illness and mortality susceptibility16. S. cerevisiae serves as a valuable model organism for studying the aging process using two distinct approaches based on either replicative lifespan (RLS) or chronological lifespan (CLS)17. In RLS, the number of mitotic cycles completed by an originating cell before cell senescence is analyzed and used to help understand lifespan determinants of proliferating cells, such as stem cells. In contrast, CLS focuses on understanding the aging process in nondividing yeast cells over time, which simulates postmitotic cells in multicellular organisms. Lifespan studies on yeast have revealed diverse conserved genetic pathways that influence aging, including the sirtuin (Sir2) histone deacetylase (HDAC)-mediated maintenance of chromatin stability and the target of rapamycin (TOR) signaling pathway for nutrient sensing and growth control18.
Calorie restriction (CR), which promotes longevity in a wide range of species, including mammals, is closely linked to both the Sir2 and TOR pathways. While Sir2 activity is enhanced by the altered NAD/NADH ratio under starvation, CR also suppresses the TOR pathway, resulting in increased mitochondrial respiration and reduced oxidative damage18. Our understanding of these mechanisms remains incomplete, in part due to conflicting experimental results on questions such as whether a prolonged lifespan is ensured by respiratory chain dysfunction in Caenorhabditis elegans and the Sir2-independent CR pathway in S. cerevisiae19,20. At the same time, the SUMO pathway regulates diverse cellular processes involved in aging, including telomere function, autophagy, oxidative stress responses, and growth factor signaling21. Thus, a better understanding is needed of the mechanisms connecting sumoylation to the regulation of energy metabolism and other biological events in aging.
In our previous work, we had found that different yeast lines that evolved in vitro from the same founding nascent ulp2Δ colony followed distinct evolutionary paths to restore euploidy, robust growth, and stress resistance14. Most commonly, mutations in SUMO conjugation-pathway enzymes were identified and these correlated with a strong drop in high molecular weight (HMW) polysumoylated proteins. In several of the evolved lines, however, SUMO-conjugate levels remained abnormally high, and no additional mutations to SUMO pathway enzymes were identified.
We have therefore undertaken genome-wide gene expression studies to investigate how such in vitro evolved ulp2Δ cell lines could nevertheless overcome the defects caused by the loss of the Ulp2 protease. Genes related to energy metabolism and protein translation underwent clear changes, with the former being upregulated and the latter being down-regulated in the evolved SUMO conjugate-replete ulp2Δ cells. These changes correlated with increases in growth rate and stress resistance, and RLS extension depended on mitochondrial respiration.
Strikingly, these evolved ulp2Δ cells experienced strongly increased levels of auto-sumoylated Ubc9 enzyme (whether this occurs in cis or in trans was not determined, but we use “auto-sumoylation” following prior usage22). Auto-sumoylation of Ubc9 specifically altered expression and SUMO conjugation of proteins involved in energy metabolism and translation. Interestingly, preventing Ubc9 auto-sumoylation blocked the marked accumulation of SUMO conjugates normally seen in replicatively aged wild-type (WT) cells and also eliminated the prolonged RLS induced by CR. Ubc9 auto-sumoylation was previously shown to alter the substrate preferences of the SUMO-conjugation pathway22,23. Our data suggest that enhanced Ubc9 auto-sumoylation adjusts energy metabolism via gene expression and protein sumoylation changes during aging. This remarkable metabolic malleability enhances replication capacity and the survival of aged cells and may mimic the effects of CR on lifespan extension.
Results
Gene expression pattern in ulp2Δ cells is altered after multiple generations
We recently reported that growth and cell-cycle defects in ulp2Δ cells were restored after ~500 generations (G) of continuous culture with repeated cycles of dilution and outgrowth in fresh medium13,14 (Fig. 1a). Missense mutations in essential SUMO-conjugation-pathway components (Uba2, Aos1, or Ubc9) were observed in all of the laboratory-evolved ulp2Δ cells. In one set of 10 lines evolved in parallel, a single Uba2 cysteine-to-serine point mutation at position 162 (C162S) was observed in all the lines and is presumed to have arisen in the founder colony. However, this did not suppress the growth and cell-cycle impairments and had only a mild suppressive effect on the accumulation of HMW polySUMO-protein conjugates in nascent ulp2Δ cells. In contrast, the other mutants had additional mutations in either Uba2 or Aos1, including uba2C162S, A414P (alanine 414 to proline), and these additional mutations strongly reduced HMW polySUMO-conjugate accumulation in the evolved ulp2Δ strains, which mitigated the defects in growth and cell-cycle progression14. Thus, the mechanism of cell adaptation to Ulp2 loss in evolved ulp2Δ cells with only the Uba2-C162S mutation, has remained unclear.
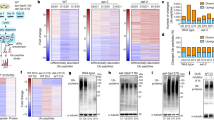
a Scheme for the creation of nascent ulp2Δ cells and subsequent laboratory evolution steps. The ulp2Δ cells containing YCplac33-ULP2 (MHY1379) were sequentially streaked on SD + FOA plates twice to evict the YCplac33-ULP2 plasmid and then transformed with either YCplac33 or YCplac33-ULP2. Cells grew for ~50 generations (G) during these procedures. The cells were grown until saturation, then diluted 1:120 in fresh YPD (6.9 generations per dilution) for long-term passaging. This process was repeated daily for 65 days (~500 G), which corrected the growth defects of ulp2Δ cells. b, c Venn diagram and Gene Ontology (GO) enrichment analysis of genes with significantly decreased (<2-fold, b) or increased (>2-fold, c) expression in nascent (low passage) ulp2Δ, ulp2Δ 500 G (Uba2C162S), and ulp2Δ 500 G (Uba2C162S, A414P) strains, compared to WT strain MHY1379 (ulp2Δ + YCplac33-ULP2). The numbers below the strain names represent the total number of genes whose expression levels were altered by more than two-fold compared to WT. Bars indicate the fold-enrichment of each GO biological process in PANTHER. The genes used for GO analysis are listed in Supplementary Data 1. d Schematic summary of the expression changes in ulp2Δ 500 G (Uba2C162S) compared to nascent ulp2Δ cells depicted in (b, c). e Mutations in ulp2Δ 500 G (Uba2C162S) and ulp2Δ 500 G (Uba2C162S, A414P). Missense or silent represents alteration of amino acids by point mutation or not, respectively.
To begin analyzing potential adaptive mechanisms in the ulp2Δ 500 G (Uba2C162S) strain, gene expression profiles were analyzed by genomic RNA sequencing (RNA-seq; Fig. 1b, c, Supplementary Fig. 1, and Supplementary Data 1). Levels of many transcripts were substantially different (two-fold or more) in the indicated strains compared to expression in the WT. In nascent ulp2Δ cells, the number of genes with increased transcript levels (601) (Fig. 1c) was more than double that of genes with reduced transcript levels (294) (Fig. 1b). Interestingly, the overall changes in transcript levels relative to WT were much more muted in the two evolved ulp2Δ strains tested; specifically, the ratio of the total number of significantly up- and down-regulated genes in nascent ulp2Δ cells was more than double that of the two evolved strains (ulp2Δ 500 G (Uba2C162S; 909 vs. 385) and ulp2Δ 500 G (Uba2C162S, A414P; 566 vs. 201)). This indicates a general return to a WT gene expression balance in the evolved ulp2Δ strains despite the irreversible loss of Ulp2.
Energy metabolism genes, including tricarboxylic acid (TCA) cycle, ATP biosynthesis, and respiratory electron transport chain-related genes, were specifically down-regulated in nascent ulp2Δ (Fig. 1b), whereas ribosomal proteins and SUMO-regulated ribosome biogenesis factors24 were heavily enriched among the genes with a strong increase in expression (Fig. 1c). In direct contrast to this pattern, genes required for translation, including those involved in rRNA maturation, ribosome assembly and nuclear export, and translation factors, were down-regulated in evolved ulp2Δ 500 G (Uba2C162S) cells, while genes for energy reserve and cellular carbohydrate metabolic processes were upregulated in this line. In particular, a significant increase was seen in the mRNA levels for enzymes involved in converting glucose to glycogen and, conversely, for enzymes that catalyze catabolic reactions that breakdown carbohydrates, including hexokinase (Hxk1) in glycolysis, citrate synthase (Cit2) in the TCA cycle, and the glycogen debranching enzyme (Gdb1) required for mobilizing glucose reserves from glycogen deposits25,26,27. These data implied that both the energy storage and consumption processes were markedly activated in ulp2Δ 500 G (Uba2C162S) cells (Fig. 1c and Supplementary Data 1). Notably, no significant feature was observed in the Gene Ontology (GO) analysis for the ulp2Δ 500 G (Uba2C162S, A414P) line. In short, our RNA-seq data revealed that the decrease in enzymes for energy metabolism and increase in translation capacity seen in nascent ulp2Δ cells were reversed in the ulp2Δ 500 G (Uba2C162S) strain (Fig. 1d) but not in the ulp2Δ 500 G (Uba2C162S, A414P) line, suggesting distinct long-term adaptation mechanisms.
We previously reported several adaptive mutations that provide a selective advantage to evolved ulp2Δ cells14. We searched for other potential gene mutations in ulp2Δ 500 G (Uba2C162S) and ulp2Δ 500 G (Uba2C162S, A414P) by genome sequencing but did not find any alterations that would lead to changes in protein sequence except for the noted Uba2 mutations (Fig. 1e). To test whether the enhanced expression of the energy metabolism genes observed in ulp2Δ 500 G (Uba2C162S) could influence the slow growth of nascent ulp2Δ cells, we transformed the WT strain (ulp2Δ + pULP2) with each of 33 different library plasmids containing identified energy metabolism genes and then analyzed cell growth via serial dilution analysis after evicting the ULP2 cover plasmid (Supplementary Fig. 2). None of the tested high-copy genes by themselves suppressed the growth impairment in the ulp2Δ cells. Altogether, these results imply that the growth recovery of ulp2Δ 500 G (Uba2C162S) results from an as yet unidentified (possibly nongenetic) adaptive mechanism that likely requires altered expression of more than a single metabolic enzyme.
Growth rate and RLS are increased in high-passage ulp2Δ cells
Metabolic energy balance is important for cell growth and function; its malfunction is implicated in many complex diseases and aging19. To determine whether the elevated energy metabolism transcripts affect the growth rate of ulp2Δ 500 G (Uba2C162S), we analyzed the growth curves of the indicated cells at different temperatures and using different concentrations and sources of carbon (Fig. 2 and Supplementary Fig. 3). With optimal temperature and carbon source (30 °C and 2% glucose), typical S-shaped growth curves were observed for both the low passage WT and WT 500 G cells (Fig. 2, top left). By 12 h, all strains except low passage ulp2Δ cells appeared to have slowed their doubling rate (diaxic shift). Cell density at saturation was slightly decreased in nascent ulp2Δ cells but had increased relative to WT in the evolved ulp2Δ cells. When the culture conditions were changed to low (0.2%) or very high (20%) glucose concentrations, a high temperature (37 °C), or a nonfermentable carbon source (2% glycerol), the time needed to reach saturation was delayed, and the growth rate changes in the mutants were much greater than under optimal conditions. Remarkably, the two evolved ulp2Δ lines had a clear growth advantage relative to WT cells, especially at 37 °C. These findings indicate that laboratory evolution can induce beneficial effects beyond the correction of vegetative growth defects in ulp2Δ cells.
Cells of the indicated strains pregrown in yeast extract-peptone-dextrose (YPD; 2% glucose) at 30 °C were adjusted to an optical density (OD)600 value of 0.1, then cultured in YP with 0.2%, 2%, or 20% glucose or 2% glycerol at either 30 °C or 37 °C. Growth curves were derived from OD600 measurements every 6 h for 36 h.
It is commonly assumed that growth rate is negatively coupled with lifespan, but some evidence indicates a positive correlation28,29. The SUMO pathway has been linked with cellular senescence and the organismal aging process. Its substrates include several factors that control cellular senescence, such as p53, RB, and SIRT121. Thus, we next examined the change in RLS caused by mutation or deletion of several central SUMO pathway proteins (Ubc9, Ulp1, and Ulp2; Fig. 3a). We observed that normal lifespan was significantly shortened in all the tested mutant strains (ubc9-1, ulp1-ts, and nascent ulp2Δ) suggesting that the dynamic regulation of SUMO conjugation and deconjugation is required for maintaining a normal lifespan.
a–f RLS analysis of indicated strains (a–e) and of uba2Δ ulp2Δ cells with indicated plasmids (f). RLS was measured on yeast extract-peptone-dextrose (YPD) (a–d, f) and YPD supplemented with 3 μg/mL Antimycin A (e), respectively. Rho0 represents cells lacking mitochondrial DNA. The RLS means are shown after the strain names. Asterisks indicate statistically significant differences (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; two-sided Wilcoxon Rank Sum Test).
Because Sir2 is a well-known factor that modulates RLS30,31,32, we investigated whether Sir2 affects RLS in the ulp2Δ strain (Fig. 3b). Consistent with previous results, RLS was decreased in sir2Δ, and the absence of Ulp2 further reduced sir2Δ lifespan; the latter result implies that Ulp2 plays a role in lifespan modulation separate from that of Sir2. In particular, this finding reveals an alternative Sir2-independent pathway that sustains RLS by controlling sumoylation levels.
Remarkably, lifespan was greatly extended in both evolved ulp2Δ strains, with RLSs as much as 80% above WT (Fig. 3c). As noted above, these same strains also exhibited higher growth rates relative to WT (Fig. 2). High rates of cell growth generally correlate with a shorter lifespan, but there are many exceptions. For instance, the loss of the global transcriptional regulator Sus1 or ubiquitin-specific protease Ubp10 simultaneously reduces growth and RLS33,34. Previously, we reported that several Uba2 double mutants, which included the C162S point mutation, suppressed the growth and cell-cycle defects seen in nascent ulp2Δ cells by reducing HMW SUMO-conjugated protein levels14. The C162S mutation by itself did not. To determine whether this reduction caused by Uba2 mutation affects RLS and growth, we analyzed the RLS and growth changes in uba2Δ ulp2Δ cells expressing Uba2, Uba2C162S, A414P, Uba2C162S, or Uba2A414P (Fig. 3d and Supplementary Fig. 4). The expression of Uba2C162S, A414P eliminated the shortened lifespan and proportionally rescued the growth defects in the (nascent) ulp2Δ cells; these effects were not seen with ulp2Δ cells carrying the singly mutated Uba2C162S or Uba2A414P. These findings suggest that Uba2 activity must be reduced to below a certain threshold, in this case requiring two Uba2 mutations, before RLS or growth impairment in ulp2Δ is rescued. The Uba2 double mutation may also be crucial for the RLS change in ulp2Δ 500 G (Uba2C162S, A414P).
Taken together, the growth curve and RLS analyses showed that a normal limitation on cellular replication ability was overcome in both of the tested laboratory-evolved ulp2Δ strains, suggesting that adaptive mechanisms for overcoming Ulp2 loss might extend cellular lifespan.
Extended RLS linked to altered energy metabolism in ulp2Δ 500 G (Uba2C162S)
Based on the RNA-seq experiments (Fig. 1b, c), we next addressed the impact the apparent elevated energy metabolism had on the lifespan of ulp2Δ 500 G (Uba2C162S) (Fig. 3e and Supplementary Fig. 5a). Mitochondrial DNA (mtDNA) is necessary for mitochondrial respiration and energy metabolism35, leading many researchers to investigate RLS in cells lacking mtDNA (rho0 cells) to determine whether mitochondrial respiration affects RLS. Previous results show that the impact of mtDNA loss on cell longevity depends on the yeast strain36,37,38.
We found that the RLS of WT MHY1379 cells, which had been rendered rho0 was decreased by about 20% compared to that of the parental WT, which is consistent with previous results37. Notably, the extended RLS of ulp2Δ 500 G (Uba2C162S) was abolished by loss of mtDNA, whereas ulp2Δ 500 G (Uba2C162S, A414P) rho0 cells retained a significantly higher RLS, suggesting that mitochondrial function is a key element to prolonged RLS in ulp2Δ 500 G (Uba2C162S) and that a distinct pathway is required for the extended lifespan of ulp2Δ 500 G (Uba2C162S, A414P). The addition of antimycin A, which specifically blocks mitochondrial respiration and reduces RLS37, also more significantly impaired RLS in ulp2Δ 500 G (Uba2C162S) than in ulp2Δ 500 G (Uba2C162S, A414P cells) (Fig. 3f and Supplementary Fig. 5b). Thus, our results suggest that extended RLS in ulp2Δ 500 G (Uba2C162S) cells results from a shift in energy metabolism.
Ubc9 auto-sumoylation is increased in ulp2Δ 500 G (Uba2C162S)
Because the global profile of sumoylated proteins was altered in the evolved ulp2Δ strains (Supplementary Fig. 5c), we speculated that changes in SUMO substrates may contribute to cellular transcriptome changes relevant to RLS increase. We performed affinity purification of sumoylated proteins in evolved ulp2Δ strains expressing N-terminal 6His-Flag (HF)-tagged Smt3 followed by mass spectrometry analyses to identify SUMO-modified proteins (Fig. 4, Supplementary Fig. 6, and Supplementary Data 2). In agreement with previous findings39,40,41, HF-Smt3 was linked with multiple proteins involved in cytoplasmic translation, glycolysis/gluconeogenesis, nucleosome core, sumoylation pathway, and septin complex in the WT (Fig. 4a). As expected, the initial Ulp2 loss led to broad changes in the SUMO-conjugate profile (Fig. 4a), which supports the conclusion that the Ulp2 SUMO protease has many target proteins required for diverse pathways, including transcription, the cell cycle, and ribosome biogenesis9. Both evolved ulp2Δ strains tended to deplete rather than increase SUMO-conjugated proteins; among them were proteins involved in energy metabolism and translation, including key enzymes needed for glycolysis/fermentation and gluconeogenesis (e.g., Adh1, Pdc1, Pgk1, and Eno242,43,44), several ribosome subunits, translation control factors (Gis2 and Tef1)45,46, and Ksp1, a kinase involved in TOR signaling47 (Fig. 4b, c).
a–c Sumoylated proteins were purified under denaturing conditions from WT, ulp2Δ, ulp2Δ 500 G (Uba2C162S), and ulp2Δ 500 G (Uba2C162S) cells expressing 6His-Flag–tagged SUMO and were identified via mass spectrometry. Sumoylated proteins were compared between the indicated groups (a WT and ulp2Δ; b ulp2Δ and ulp2Δ 500 G (Uba2C162S); and c ulp2Δ and ulp2Δ 500 G (Uba2C162S, A414P)). The protein interaction network was constructed via STRING. Each node and the distance between the nodes indicate identified proteins and their relatedness, respectively. The colored circles located at the nodes, which indicate the clusters identified by DBSCAN clustering, represent specific biological processes: cytoplasmic translation, glycolysis/gluconeogenesis, nucleosome core, sumoylation pathway, or septin complex. Magenta and green circles next to the protein names denote the SUMO-modified proteins in each strain only.
Among the proteins characterized by changes in SUMO modification in the evolved strains, the SUMO modification of Ubc9, which was not apparent in the nascent ulp2Δ strain, was readily detected in ulp2Δ 500 G (Uba2C162S) cells (Fig. 4b). By contrast, this modification was not observed in the ulp2Δ 500 G (Uba2C162S, A414P) strain (Fig. 4c). Using immunoblot analysis, we detected a large increase in SUMO-conjugated Ubc9 accompanied by a corresponding decrease in its unconjugated state in the ulp2Δ 500 G (Uba2C162S) cells (Fig. 5a). Conversely, SUMO-modified Ubc9 almost disappeared in the ulp2Δ 500 G (Uba2C162S, A414P) strain, presumably due to the low levels of SUMO conjugates in this line.
a Immunoprecipitation (IP) of Ubc9-Flag with anti-Flag agarose from denatured yeast extracts in the indicated strains expressing Flag-tagged Ubc9 followed by immunoblot analysis with anti-SUMO or anti-Flag antibodies. Anti-Pgk1 was used as a loading control for protein input. Arrowheads, open circles, and asterisks indicate sumoylated Ubc9, unsumoylated Ubc9, and nonspecific bands, respectively. b Growth of the ubc9-K153/157 R (ubc9-RR) mutants from two different strain backgrounds (W303 and MHY500). After spotting cells in fivefold serial dilutions, the YPD plates were incubated for 2 days at 30 °C. c, f, g Immunoblot assay of sumoylated proteins in extracts prepared from the indicated strains. The plus and minus symbols represent the presence and absence of YCplac33-ULP2, respectively, in the indicated uba2, ubc9, and ulp2 mutants. Anti-Pgk1 blotting was used to verify equal loading. The stacking gel (bracket) and molecular size standards (in kDa) are indicated. d, e, h RLS analysis of indicated strains, as shown in Fig. 3a.
Previous work demonstrated that the sumoylation of human Ubc9-Lys14 (K14) inhibits its ability to sumoylate the well-known target RanGAP1 but promotes modification of another substrate, Sp10023. In S. cerevisiae, Ubc9 auto-sumoylation is also observed, in this case on two residues, K153 and K157; this negatively regulates septin sumoylation, which is required for maintaining normal cell morphology22. Neither evolved ulp2Δ strain showed any obvious change in Ubc9 cellular localization (Supplementary Fig. 7). Thus, the unusually high levels of Ubc9 auto-sumoylation seen in the in vitro evolved ulp2Δ 500 G (Uba2C162S) strain and potentially the reduced levels of such modification in ulp2Δ 500 G (Uba2C162S, A414P) cells are predicted to rewire cellular SUMO target discrimination through a mechanism not involving changes in the intracellular localization of Ubc9.
Ubc9 auto-sumoylation increases RLS in a Sir2-independent manner
It was previously reported that Ubc9 auto-sumoylation was nearly abolished in a ubc9-K153/157 R (ubc9-RR) mutant; this did not strongly affect vegetative growth or general SUMO conjugation but caused a severe reduction of global cellular sumoylation during meiosis48. We confirmed that cell growth did not differ between the WT and ubc9-RR strains in two different genetic backgrounds (W303 and MHY500; Fig. 5b); however, global SUMO conjugates were comparatively reduced in the ubc9-RR cells (Fig. 5c). Because the ulp2Δ 500 G (Uba2C162S) strain displayed enhanced Ubc9 auto-sumoylation and extended RLS, we tested the effect of Ubc9 auto-sumoylation on RLS (Fig. 5d). The RLS of the ubc9-RR mutant was indeed shorter than that of WT cells, suggesting that Ubc9 auto-sumoylation is required for maintaining a normal lifespan. Moreover, the lifespan of a sir2Δ ubc9-RR double mutant was significantly shorter than that of either single mutant, indicating an additive effect on lifespan when these mutations are combined (Fig. 5e). As noted in Fig. 3b, Ulp2 loss shortens RLS in a Sir2-independent manner, and the results in Fig. 5e suggest that Ubc9 auto-sumoylation also contributes to a normal RLS through a Sir2-independent mechanism. Thus, our data indicate that the involvement of the SUMO pathway in yeast lifespan is distinct from the contribution of Sir2.
Ubc9 auto-sumoylation is required for RLS extension in ulp2Δ 500 G (Uba2C162S)
To assess the effect of Ubc9 auto-sumoylation on global sumoylation in ulp2Δ 500 G (Uba2C162S) cells, we measured SUMO-conjugate levels by anti-SUMO immunoblotting of extracts from ulp2Δ 500 G (Uba2C162S) cells also bearing the ubc9-RR mutant allele (Fig. 5f). The ubc9-RR allele led to a sharp decline in HMW SUMO conjugates in the ulp2Δ 500 G (Uba2C162S) background, while a more moderate reduction was seen in nascent ulp2Δ cells, suggesting that Ubc9 auto-sumoylation is an important factor in the maintenance of cellular SUMO conjugates in evolved ulp2Δ (Uba2C162S) cells.
While Ubc9K153/157R (Ubc9-RR) expression only weakly reduced polySUMO-conjugate accumulation in ulp2Δ cells expressing WT Uba2 (Fig. 5g, lanes 3–4), Ubc9-RR expression in the ulp2Δ strain expressing Uba2C162S efficiently suppressed accumulation of the excess HMW polySUMO conjugates (Fig. 5g, lanes 7-8). This finding implies that the C162S mutation of Uba2 enhances suppression of the ulp2Δ defect by the auto-sumoylation-resistant Ubc9-RR protein. To determine whether Ubc9 auto-sumoylation influences long-lived ulp2Δ 500 G (Uba2C162S) cells, we examined RLS in evolved ulp2Δ cells into which the ubc9-RR allele was introduced (as the only source of Ubc9) (Fig. 5h). The ubc9-RR allele dramatically reduced RLS in the ulp2Δ 500 G (Uba2C162S) strain but not in ulp2Δ 500 G (Uba2C162S, A414P) cells. This implies that Ubc9 auto-sumoylation is necessary for extended RLS in ulp2Δ 500 G (Uba2C162S).
Taken together, our results suggest that Ubc9 auto-sumoylation-dependent SUMO target discrimination is a fundamental aspect of lifespan extension in ulp2Δ 500 G (Uba2C162S). This may be connected to changes in energy metabolism, as suggested by our RNA-seq and mitochondrial DNA elimination data, while ulp2Δ 500 G (Uba2C162S, A414P) increases RLS via a distinct route.
Ubc9 auto-sumoylation affects genome-wide SUMO binding to chromatin
SUMO has both positive and negative impacts on transcription49,50, and its level in chromatin is dynamically regulated by the Ulp2 protease51. Transcription factors provide abundant SUMO substrates39,41, and SUMO is enriched in numerous loci, primarily the promoter regions of constitutively activated genes and genes involved in translation, such as ribosomal protein and tRNA genes50,52,53. Because the ubc9-1 mutation strongly diminishes the association of SUMO with constitutive genes, ribosomal protein genes, and tRNA promoters50,52, we examined the role of Ubc9 auto-sumoylation in SUMO occupancy on chromatin via chromatin immunoprecipitation (ChIP)-seq analysis with strains expressing HF-tagged Smt3 (Fig. 6 and Supplementary Data 3).
a Venn diagram showing overlapping ChIP-seq peaks of HF-Smt3 (SUMO) in wild-type (WT; magenta) and ubc9-RR (green) strains. The ChIP-seq data were obtained from duplicate samples. b, c Pie chart depicting the distribution of HF-Smt3 peaks at promoter and other regions (b) and ORF and ncRNA loci (c) in (a). The numbers before and within the parentheses indicate the number of identified peaks and their percentages, respectively, at gene promoters. d Volcano plots displaying the distinct HF-Smt3 peaks in a. The y axis is the mean of the negative logarithm of P value. The x axis corresponds the log2-fold change value. Red and blue dots denote the significantly increased and decreased peaks in ubc9-RR, respectively, compared to the WT. e ChIP read-density plot for levels of HF-Smt3 from the significantly increased (left panel) and decreased (right panel) peaks in ubc9-RR in (d). SUMO peaks did not exhibit significant changes in heterochromatin regions, rDNA, telomere-proximal regions, HM loci, and centromere regions and are therefore not discussed further here. A ± 3 kb window relative to the center of the peaks is shown. Bottom panels indicate heatmaps of SUMO occupancy in WT and ubc9-RR. f Integrative Genomics Viewer (IGV) screenshot of ChIP-seq data in a, showing HF-Smt3 peaks in WT and ubc9-RR. SUMO occupancy changes correspond to enriched peaks in d, e visualized by subtracting the normalized IP/INPUT peak ratio in WT from ubc9-RR to illustrate the changes at indicated genes. Increased (red) or decreased (blue) HF-Smt3 peaks are shown. g KEGG analysis of the significantly increased (left panel) and decreased (right panel) peaks displayed in d. Bar diagrams indicate the fold-enrichment in each pathway in DAVID. Statistical significance and enrichment scores for each pathway were cacluated by DAVID. Source data are provided as a Source Data file.
The SUMO ChIP-seq data sets contained 813 peaks in the WT and 714 peaks in the ubc9-RR mutant. The majority of the SUMO peaks in the two strains overlapped (Fig. 6a). SUMO was almost always located near the promoter regions (99.7% in both WT and ubc9-RR; Fig. 6b), and of the SUMO-occupied genes, ~70% are protein-coding genes and ~30% are noncoding RNA genes (Fig. 6c), in agreement with previous results52,53. In particular, the ubc9-RR mutation contributed both positively and negatively to SUMO localization at diverse gene promoters, including CIT2, RPS6A, RPL19A, and RPS4B (Fig. 6d–f). KEGG pathway analysis revealed significantly increased and decreased SUMO enrichments in the ubc9-RR strain in genes involved in various pathways, including glycolysis, gluconeogenesis, aminoacyl-tRNA biosynthesis, ribosome, and some metabolic pathways (Fig. 6g). This finding suggests that Ubc9 auto-sumoylation dynamically regulates chromatin sumoylation at multiple genes related to metabolic and translation pathways.
Consistent with our RNA-seq data (Fig. 1b, c), the general chromatin targets of auto-sumoylated Ubc9 included many genes involved in energy metabolism and translation. In cells lacking Ubc9 sumoylation, SUMO modification of the glycolysis and gluconeogenesis gene loci was enhanced, while that of ribosomal protein genes was reduced (Fig. 6g). Therefore, we anticipate that the highly auto-sumoylated Ubc9 in the ulp2Δ 500 G (Uba2C162S) strain may result in lower levels of SUMO modifications on energy metabolism-related genes and elevated levels of SUMO modifications on translation-related genes. Although SUMO modifications can both activate and repress transcription, previous studies suggest that sumoylation is typically associated with transcriptional repression49,50. Thus, the altered gene expression levels in the ulp2Δ 500 G (Uba2C162S) are closely correlated with the anticipated changes in chromatin-SUMO modifications.
Ubc9 auto-sumoylation influences sumoylation of proteins involved in translation and energy metabolism
Ubc9 auto-sumoylation can alter substrate selection by the SUMO-conjugation pathway23. To determine whether Ubc9 auto-sumoylation facilitates a broad transition in substrate targeting that might be connected to the genome-wide changes of SUMO-chromatin association (Fig. 6), we compared SUMO-conjugated substrates between the WT and ubc9-RR strains expressing HF-Smt3 using affinity purification and mass spectrometry (Fig. 7 and Supplementary Data 4). From cells grown in rich media to mid-exponential phase, we identified 245 SUMO-modified proteins only in WT cells, 34 substrates in both the WT and ubc9-RR strains, and none that were exclusively seen in ubc9-RR cells. This indicated that the overall number of sumoylated proteins had greatly declined in the auto-sumoylation mutant (Fig. 7a). KEGG enrichment analysis showed that proteins functioning in translation and metabolism constituted the largest classes of proteins that were detected only in the WT sumoylome (Fig. 7b); these categories were also enriched among the sumoylated proteins found in both WT and ubc9-RR cells (Fig. 7c).
a Pie chart showing the distribution of SUMO-modified proteins in only wild-type (WT; magenta) and in both WT and ubc9-RR (green); no sumoylation proteins were identified only in ubc9-RR. SUMO-modified proteins were identified via mass spectrometry, as shown in Fig. 4a. b, c KEGG analysis of the SUMO-modified proteins found in only WT (b) and in both WT and ubc9-RR (c), as shown in Fig. 6g. Numbers indicate the number of proteins identified in each pathway. d, e Schematic diagram showing the list of Ubc9 auto-sumoylation-governed targets in the translation (d) and energy metabolism (e) pathways. Proteins in the two pathways depicted in b, c are marked as red and blue, respectively. Solid and dashed arrows denote the direction of fluxes and pathways in which intermediate molecules are not depicted, respectively. Metabolites in metabolic reactions are represented as yellow hexagons. The pie graph within d shows the distribution of SUMO targets among ribosomal subunits, and are classified by the indicated groups. PPP pentose phosphate pathway, DHAP dihydroxyacetone phosphate, PDH complex pyruvate dehydrogenase complex, Ac-CoA Acetyl-CoA, OAA oxaloacetate, ETC electron transport chain. Source data are provided as a Source Data file.
Translation and metabolism are complex processes. For 80S initiation complex formation in the initiation of protein synthesis, the eIF4F complex facilitates mRNA association with the 43S preinitiation complex, which leads to the formation of the 48S initiation complex, to which the 60S subunit binds following start codon selection (Fig. 7d)54. For the metabolic breakdown of glucose, glycolysis produces pyruvate in the cytoplasm, which is either converted to ethanol during fermentation or imported into mitochondria for respiration via the TCA cycle (Fig. 7e). SUMO is known to act as a key regulatory factor in both of these pathways24. Our data reveal that multiple translation initiation and termination factors, as well as ribosomal proteins, are only detectably sumoylated when Ubc9 can be auto-sumoylated, and the same is true for multiple glycolytic and TCA enzymes. In immunoblotting experiments, we observed a decrease in mono-sumoylated Lat1, a component of pyruvate dehydrogenase complex, in the ubc9-RR mutant, but conversely, we found an increase in an apparent di-SUMO-conjugated form of the translation initiation factor eIF4B (Tif3), (Supplementary Fig. 8). This supports a previous report that Ubc9 auto-sumoylation can affect the target specificity of sumoylation22. Altogether, these results suggest Ubc9 auto-sumoylation provides a mechanism to control the sumoylation and presumably levels or activity of proteins directly involved in protein synthesis and energy metabolism.
Ubc9 auto-sumoylation is increased in aged cells
Aging induces markedly increased sumoylation levels, which contribute to changes in mitochondrial dynamics and mitophagy in C. elegans55. To look for possible changes in yeast Ubc9 auto-sumoylation in response to aging, we first measured the SUMO conjugate levels in replicatively aged cells (Fig. 8a). Old cells were obtained by isolating biotin-labeled mother cells after three rounds of sorting. We found that the average bud scar number was more than twenty per cell (Fig. 8a, right panel). Similar to C. elegans, global SUMO conjugates were greatly increased in replicatively aged S. cerevisiae cells. By contrast, the ubc9-RR mutation blocked much of the age-linked accumulation of SUMO conjugates. This indicates that Ubc9 auto-sumoylation is needed to sustain enhanced sumoylation as yeast age. Notably, Ubc9 auto-sumoylation also increased substantially in aged cells compared to younger cells, while its unconjugated form was strongly depleted (Fig. 8b).
a, b Immunoblot assay of sumoylated proteins (a) and Ubc9-Flag (b) before and after enrichment for replicatively aged cells; blotting done as in Fig. 5a. Calcofluor stain images of young and old cells are shown on the right in each panel. The number outside of and within the parentheses represents the average number of bud scars (i.e., the replicative age or number of cell divisions) and the number of cells measured for counting, respectively. Scale bar: 5 μm. c, d RLS analysis of WT (W303) (c) and ulp2Δ (d) cells with indicated plasmids on SD - Leu plates, as was done in Fig. 3a. e RLS analysis of the indicated strains on the SD plates supplemented with 2.0% or 0.5% glucose, as in the experiments shown in Fig. 3a. f Quantification of RLS ratio of 0.5% to 2.0% in e and Supplementary Fig. 10. Dot blots represent individual data points obtained from three independent experiments. The error bars represent the SD from three independent experiments. Asterisks indicate statistically significant differences (*P < 0.05, two-tailed Student’s t test). g Schematic diagram depicting the role of Ubc9 auto-sumoylation in longevity.
Next, we investigated whether increased Ubc9 auto-sumoylation could affect yeast RLS (Fig. 8c, d). To enhance Ubc9 auto-sumoylation, we used an in vivo Ubc9 fusion–directed sumoylation approach, which strongly increases the sumoylation of the protein to which Ubc9 is fused56. We cloned the Ubc9 coding sequence downstream of the UBC9 ORF. The expression of the resulting Ubc9-Ubc9 fusion protein, rather than just Ubc9 alone, led to elevated SUMO-conjugated forms of Ubc9 (Supplementary Fig. 9) and significantly extended RLS of WT cells (Fig. 8c). It also significantly rescued the shortened RLS observed in nascent ulp2Δ strain (Fig. 8d). These data strongly suggest that Ubc9 auto-sumoylation is a critical factor for regulating lifespan and promoting longevity.
Ubc9 auto-sumoylation is required for RLS extension by CR
In several organisms, CR leads to increased mitochondrial function to boost endogenous energy production and repress ribosome biogenesis and translation via the downregulation of TOR signaling, resulting in a prolonged lifespan57,58,59,60. Our results (Fig. 1b, c) showed changes in cellular physiological and metabolic characteristics typical of CR in ulp2Δ 500 G (Uba2C162S) cells that had not undergone CR. Additionally, Ubc9 auto-sumoylation dramatically modulates the sumoylation of proteins in pathways known to be affected by CR (Figs. 6 and 7). To investigate the relationship between Ubc9 auto-sumoylation and CR, we measured RLS in a ubc9-RR strain in media with normal or reduced glucose levels (Fig. 8e). The RLS of WT cells grown on SC plates was slightly lower than those grown on YPD-rich medium. As expected from previous work61, RLS was clearly extended by reducing glucose content in the media from 2% to 0.5%. This starvation-induced RLS enhancement was lost in ubc9-RR cells. We repeated the RLS analyses three times and found that the increased RLS observed by lowering glucose concentration from 2.0% to 0.5% was significantly decreased by the inhibition of Ubc9 auto-sumoylation (Fig. 8f and Supplementary Fig. 10). We suggest that Ubc9 auto-sumoylation regulates both transcriptional and post-translational targets of the SUMO pathway, particularly those that are involved in energy metabolism and protein translation, to create a longevity-enhancing state that can also be reached through CR (Fig. 8g).
Discussion
Here we have characterized an unusual mechanism of long-term adaptation to the loss of a key enzyme of the yeast SUMO pathway, the Ulp2 SUMO protease. After sustaining an initial weakly inhibitory point mutation (C162S) in the Uba2 SUMO-activating enzyme, slow-growing and stress-sensitive ulp2Δ cells evolved over ~500 generations to grow faster and with greater stress resistance than WT cells despite the absence of any detectable missense mutations elsewhere in the genome14. The resulting ulp2Δ 500 G (Uba2C162S) strain continued to display abnormally high bulk SUMO-conjugate levels. Unexpectedly, the strain also exhibited enhanced Ubc9 auto-sumoylation, and this adaptation to chronic Ulp2 deficiency led to a greatly extended lifespan. A similarly enhanced RLS was seen in a ulp2Δ 500 G (Uba2C162S, A414P) strain that had evolved in parallel with ulp2Δ 500 G (Uba2C162S); however, the double mutation in Uba2 strongly reduced SUMO-conjugate levels and the increase in lifespan was mechanistically distinct as well.
Previous analyses of yeast Ubc9 mutants defective for auto-sumoylation indicated only modest overall changes in the modification of most SUMO substrates during vegetative growth and little effect on growth, although cell cycle-dependent sumoylation of septins was enhanced22,48. By contrast, in meiosis, the ubc9-RR mutant suffered a strong defect in polySUMO conjugation and had striking defects in meiosis48. Our data indicate a significant positive impact of Ubc9 auto-sumoylation on SUMO-protein conjugate levels in vegetatively growing cells as well; the affected conjugates likely include polySUMO-modified proteins since most are HMW conjugates (Fig. 5c, e, f). Multiple components of the translation machinery and energy metabolism were only detectably sumoylated when Ubc9 could be auto-sumoylated (Fig. 7); because sumoylation generally promotes translation24, this could explain the enhanced growth of ulp2Δ 500 G (Uba2C162S) cells despite the downregulation of translation genes (Fig. 1b).
Klug et al. had previously shown that auto-sumoylated Ubc9 by itself is far less active, at least in vitro, but that it stimulates polySUMO chain formation by unmodified Ubc948. We propose that auto-sumoylated Ubc9, working with the unmodified E2, enhances polySUMO formation on specific proteins in ulp2Δ 500 G (Uba2C162S) cells, causing a redistribution of cellular SUMO among substrates to promote both growth rate and longevity (Fig. 7). Exactly how Ubc9 auto-sumoylation is stimulated in ulp2Δ 500 G (Uba2C162S) remains to be determined. It might result from reduced Ulp1 SUMO protease activity as ulp1 mutations are known to suppress many defects of ulp2Δ mutants12.
Because SUMO plays a crucial role in maintaining cell homeostasis under various stresses5, the SUMO stress response triggered by Ulp2 loss may regulate the proteins necessary for overcoming this stress by targeting specific SUMO targets. Ubc9 auto-sumoylation is critical for the discrimination of target proteins and modulates sumoylation of various targets related to energy metabolism and protein translation at the transcription and post-translational levels. Increased auto-sumoylation contributes to the higher survival of aged cells and prolonged RLS in response to CR. Thus, SUMO target selection and/or sumoylation activity levels likely play a key role in regulating cellular aging by altering core metabolism and protein synthesis.
Various CR regimens have been shown to increase longevity in different model organisms; this is accompanied by increased mitochondrial activity and numbers, resulting in enhanced ATP production19. Although the control of energy metabolism is fundamental to cell growth, decreased growth rate under CR is closely correlated with lifespan extension and slowed aging62. In contrast to CR, in vitro evolved ulp2Δ 500 G (Uba2C162S) cells display increased energy metabolism (as inferred from RNA-seq data) and growth rates yet also have longer lifespans. This is likely mediated by enhanced Ubc9 auto-sumoylation. Ubc9 auto-sumoylation may both promote greater ATP generation and enhance cell replication, regardless of whether cells are subject to CR, but it appears to be required for the CR effect on longevity.
Although CR can improve the health and lifespan of many organisms, food intake reduction may not be easy to implement or sustain in humans. Thus, many researchers are seeking drugs that can mimic the effects of CR without reducing food intake. These drugs are called calorie restriction mimetics (CRMs) and aim to modulate the same metabolic pathways involved in CR, such as glycolysis inhibition (2-deoxyglucose), enhanced insulin action (metformin), or altered stress signaling pathways (resveratrol)63,64,65. While most CRMs have focused on limiting or activating a specific step in the metabolic pathway that results in the restriction of energy source utilization, Ubc9 auto-sumoylation can simultaneously affect multiple targets linked to various pathways without limiting nutrient consumption. Because the anti-aging effects of CR involve diverse pathways, interventions intended to provide control to multiple systems could be more beneficial. Thus, pharmacological interventions that maintain or enhance Ubc9 auto-sumoylation could potentially offer a distinct way to limit aging and age-related diseases.
Methods
Yeast strains and plasmids
Yeast strains and plasmids used in this study are listed in Supplementary Data 5. Standard techniques were used for strain construction. To generate the HYS332, HYS452, HYS453, HYS454, and HYS592 strains, the C-terminal tagging cassette from pFA6a-6xGly-3xFlag::kanMX6 plasmid66 was amplified using PCR and inserted into the UBC9 locus in HYS69, HYS72, HYS88, HYS114, and HYS216 strains. For the construction of 6xHis-Flag-SMT3 strains, the N-terminal tagging cassette was amplified from the HYS40 strain67. The sir2Δ strains were generated by replacing the SIR2 ORF in HYS88, HYS567, and HYS568 strains with kanMX4 modules obtained from Euroscarf and constructed using PCR amplification from HYS209. To make HYS575 and HYS1009, the HIS3MX6 markers in HYS568 and HYS618 were swapped with the TRP1 marker via the transformation of NotI-digested M4757 (kanMX::TRP1 converter plasmid68) and with the LEU2 marker via the transformation of NotI-digested M4755 (kanMX::LEU2 converter plasmid68), respectively. The UBC9-GFP(S65T)::HIS3MX6::LEU2 cassette from HYS1009 was PCR-amplified and subsequently integrated into the native UBC9 locus of HYS69, HYS72, and HYS88 strains.
The ubc9-RR strains were generated by replacing UBC9 ORF with the ubc9-K153/157 R::HIS3MX6::TRP1 cassette from HYS575. The genomic tiling library plasmids containing the appropriate genes were transformed into the HYS88 strain to overexpress the genes of interest. To create yeast strains lacking ULP2, ulp2Δ + YCplac33-ULP2 haploid strains (HYS79, HYS88, HYS126, HYS332, HYS576, HYS596, and HYS604) with or without a LEU2-marked plasmid carrying a gene(s) of interest were twice streaked on 5-FOA plates with or without leucine to evict the ULP2 cover plasmid. The rho0 strains were generated by growth in YPD medium supplemented with ethidium bromide (25 μg/mL)69.
To create pRS315-UBC9-FLAG, a cassette containing UBC9-6xGly-3xFlag and including 1 kb of upstream and downstream sequences of the UBC9 ORF from HYS332, was PCR-amplified and then cloned into the NotI/HindIII sites of pRS315. To generate pRS315-UBC9-UBC9-FLAG and pRS315-UBC9-UBC9, the amplified UBC9 ORF with 1 kb of upstream sequence but without the stop codon was cloned into the NotI/BamHI sites of pRS315. Subsequently, the PCR-amplified UBC9-6xGly-3xFlag cassette from HYS332 and the UBC9 ORF with 1 kb of downstream sequence was cloned into the BamHI/HindIII sites of the modified pRS135, respectively. All strains and plasmids were verified using PCR and/or immunoblot analysis.
Yeast growth conditions
Cells were grown at 30 °C in YPD or synthetic defined (SD) medium using appropriate supplements. Cultures in exponential growth were normalized to an OD600 of 0.1 for growth analysis on plates and were subject to fivefold serial dilutions. Dilution series were spotted onto the appropriate media, and the plates were incubated at 30 °C and 37 °C for 1 to 3 days. To measure the growth rates in liquid cultures, we diluted overnight cultures to an OD600 of 0.1 and then measured the cell density with a spectrophotometer every 6 h. During the in vitro laboratory evolution experiments, the cells were grown to stationary phase in YPD, then diluted at a ratio of 1:120 into fresh YPD medium, as previously described70. This process was repeated daily until 500 cell generations were reached (i.e., at a rate of 6.9 generations per day).
RLS analysis
The RLS of the yeast strains were measured on YPD plates as previously described71 unless otherwise indicated. A total of about 50 virgin daughter cells were subjected to RLS analysis using a microscope equipped with a micro-dissection apparatus (SporePlay, Singer Instruments). The Mann-Whitney test was performed with a cutoff of P = 0.05 to assess lifespan differences. The average lifespan was considered significantly different when P < 0.05 (Supplementary Data 5).
RNA-seq
One OD600 equivalent of exponentially growing cells in YPD medium were used for RNA isolation using the RNeasy Mini Kit (74104, Qiagen). Contaminating DNA was removed from the samples via the DNA-free™ DNA Removal Kit (AM1906, Ambion).
The RNA-seq libraries were constructed according to the Yale Center for Genome Analysis Guidelines. The libraries underwent 101 bp paired-end sequencing using an Illumina HiSeq 2500. The low-quality reads were trimmed and adaptor contamination was removed using Trim Galore (v0.5.0). The trimmed reads were mapped to the S. cerevisiae genome (sacCer3) using HISAT2 (v2.1.0)72. Gene expression levels were quantified using StringTie (v 1.3.3b)73 with gene models from SGD34,35. Differentially expressed genes were identified using DESeq2 (v 1.22.1)74.
DNA-seq
Genomic DNA was extracted from 1 OD600 of cells as previously described14. Whole-genome sequencing was then performed according to the Yale Center for Genome Analysis Guidelines, using 2 × 100 bp sequencing on an Illumina HiSeq 2500 to an average depth of 500× per sample. The reads were aligned to the S. cerevisiae genome using BWA MEM v0.7.15, and a joint calling of variants was performed using FreeBayes v0.9.14 with the following options: -P 0.5; -E 0; -q 10; -C 5.
ChIP-seq
ChIP experiments were performed as previously described75,76. Briefly, formaldehyde was added to mid-log phase cells at a final concentration of 1% for 20 min. Cross-linking was quenched by adding 240 mM glycine. The cross-linked cells were collected via centrifugation, washed twice in ice-cold Tris-buffered saline (TBS), then lysed by ten 30 s pulses of vortex mixing with glass beads in an FA lysis buffer (50 mM HEPES–KOH; pH 7.5; 150 mM NaCl; 1 mM EDTA; 1% Triton X-100; 0.1% Na deoxycholate; 0.1% SDS; protease inhibitor cocktail (4693132001, Roche), 1 mM PMSF (11359061001, Roche)). Chromatin sheared via sonication was incubated with anti-Flag M2 agarose beads (F3165, Sigma-Aldrich) or Protein A-Sepharose beads (mock control; GE17-0780-01, Cytiva) at 4 °C for 14 h. The precipitates were then sequentially washed with FA lysis buffer and 275 mM NaCl, FA lysis buffer with 500 mM NaCl, LiCl washing buffer (10 mM Tris–HCl; pH 8.0; 0.1 mM EDTA; 250 mM LiCl; 0.5% NP-40; 0.5% sodium deoxycholate), and TE (10 mM Tris–HCl; pH 8.0; 1 mM EDTA), then eluted with elution buffer (10 mM Tris–HCl; pH 7.5; 10 mM EDTA; 1% SDS) at 65 °C. Eluted chromatin fragments were treated with protease (P5147, Sigma-Aldrich), then DNA was extracted using a standard phenol/chloroform extraction method.
The ChIP-seq libraries were constructed using a TruSeq DNA Sample prep kit and sequenced on an Illumina NovaSeq 6000 according to the manufacturer’s protocols (TruSeq ChIP Sample preparation guide 15023092 Rev. B). Reads were trimmed using a Trimmomatic (v0.38) and aligned to the yeast reference genome (sacCer3) using BowTie (v1.1.2). Peaks were called utilizing MACS (v2.1.1.20160309) and duplicate reads were processed using Picard (v0.118). Background signal was removed using the mock signal to ensure accurate peak calling. Called peaks were annotated using ChIPSeeker (v1.16.1) with gene models from SGD (https://www.yeastgenome.org/). Comparative data analyses were performed using csaw (v1.34.0). The normalized bedGraph files were generated using MACS2 (‘-B –SPMR’), then converted to bigWig files using the bedGraphToBigWig program. A genome-wide profile was generated using the ‘computeMatrix scale-regions’ and plotProfile tools of the deepTools package (v3.1.3).
Immunoblotting
Preparation of yeast whole-cell extracts and immunoblotting preparations were carried out as described previously77. The levels of Flag-tagged Ubc9, SUMO-conjugate profiles, and Pgk1 were analyzed via immunoblot assay using anti-Flag (F3165, Sigma-Aldrich, 1:2000 dilution), anti-SUMO (Mark Hochstrasser Laboratory78 and ab14405, Abcam; both at 1:2000 dilution), and anti-Pgk1 (459250, Molecular Probes, 1:10,000 dilution), respectively. Sumoylated Ubc9 was detected as previously described with little modification14,79. Briefly, TCA-extracted proteins from 50 OD600 cells were resuspended in 0.6 mL 1× Laemmli sample buffer (60 mM Tris–HCl; pH 6.8; 2% SDS; 10% Glycerol; 5% β-mercaptoethanol; 0.008% Bromophenol blue) with 100 μL unbuffered 2 M Tris, then heated at 100 °C for 5 min. The solution was centrifuged twice at 21,000 × g for 10 min, and then the protein concentration of the supernatant was determined via a Bradford protein assay. Next, 2 mg of the protein was incubated with prewashed 40 μL anti-Flag M2 agarose beads (A2220, Sigma-Aldrich) in IP buffer (50 mM Tris; pH 7.4; 150 mM NaCl; 0.5% NP-40; 20 mM NEM (E3876, Sigma-Aldrich)) at 4 °C for 4 h. After washing three times with IP buffer, the bound protein was eluted with 40 μL of 5× Laemmli sample buffer by heating at 100 °C for 5 min. Both the IP and INPUT samples were analyzed via immunoblot assays as described above. All experiments were independently repeated at least twice with consistent results.
Tandem affinity purification of sumoylated proteins and mass spectrometry
Purification of sumoylated proteins was performed as previously described with little modification67. Cells expressing 6xHis-Flag-tagged Smt3 were grown to OD600 1.0 in 4 L YPD and harvested via centrifugation at 2500 × g for 10 min. The cell pellets were lysed by ten 30 s pulses of vortex mixing with glass beads in a denatured lysis buffer (100 mM NaH2PO4; 10 mM Tris–HCl; pH 8.0; 0.1% SDS; 8 M urea; protease inhibitor cocktail (4693132001, Roche), 1 mM PMSF (11359061001, Roche)). Cell debris were removed by centrifuging twice at 37,000 × g for 30 min each. Protein concentration was measured using the Bradford protein assay, and then 50 mg of the proteins were incubated with 2 mL of prewashed Ni-NTA agarose resin (R90115, Invitrogen) at 4 °C for 4 h and loaded into a column. The resin was sequentially washed with 20 mL denatured wash buffer (100 mM NaH2PO4; 10 mM Tris–HCl; pH 8.0; 6 M urea), cold native wash buffer (50 mM NaH2PO4; 10 mM Tris–HCl; pH 8.0; 150 mM NaCl; 0.02% Tween 20; 20 mM imidazole) with 4 M urea, cold native wash buffer with 2 M urea, and cold native wash buffer. Proteins were eluted with elution buffer (50 mM NaH2PO4; 10 mM Tris–HCl; pH 8.0; 150 mM NaCl; 0.02% Tween 20; 300 mM imidazole) and then incubated with 200 μL anti-Flag M2 agarose beads at 4 °C for 4 h. The beads were washed three times with cold native wash buffer without imidazole, then once with cold water to remove the salts. Finally, the sumoylated proteins were eluted using 0.1 M glycine (pH 2.5) and were concentrated via an Amicon Ultra-4 centrifugal filter (UFC803008, Millipore).
Identification of sumoylated proteins in ulp2Δ 500 G (Uba2C162S) and ulp2Δ 500 G (Uba2C162S) cells was performed using a Q-Exactive plus spectrometer (ThermoFisher) following the Keck MS & Proteomics Resource Guidelines. Proteins were reduced with DTT and alkylated with MMTS prior to extraction using a cold acetone protein precipitation procedure. Precipitated proteins were dissolved in an acid labile detergent (Rapigest™, Waters Inc.) at a 0.1% concentration in 50 mM NH4HCO3. 20 µL of a 1:5 dilution of stock trypsin (1 µg/µL; Promega) was added to the protein mixture, and digestion was carried out with 4 µl of 0.1 mg/ml trypsin (Promega) was added, and the samples were digested at 37 °C for 16 h. The digest was quenched, and Rapigest™ was removed by the addition of 20% trifluoracetic acid at 37 °C for 45 min. Samples were stored at −20 °C until just prior to analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS). LC-MS/MS data were analyzed utilizing Proteome Discoverer 2.4 (Thermo Fisher Scientific) with Mascot search engine (v. 2.7 Matrix Science LLC) using the Saccharomyces cerevisiae protein database. The resulting PD analyses were imported into Scaffold (v. 4.1, Proteome Software) for further interrogation and data filtration. Positive protein identifications were based on hits with two or more unique peptides per protein, and peptides were considered significant if the Mascot Score was better than the 95% confidence level and achieved more than 90% coverage. The protein database searched in-house using the Mascot algorithm (v. 2.2.0) for un-interpreted MS/MS spectra. The data was searched against the SWISSPROT Saccharomyces cerevisiae protein database.
Sumoylated proteins in ubc9-RR were identified using a Q-Exactive plus spectrometer (ThermoFisher) through a nano-electrospray ion source. LC-MS/MS identification of the purified sumoylated proteins were conducted at the New Drug Development Center, KBIO, Osong, Korea. Following the same general procedures as previously described80, 100 μg of purified protein samples were reduced, alkylated, digested with Trypsin/Lys-C mix (V5073, Promega), desalted using OASIS SPE Cartridge (1860000383, Waters), then dried before being run. The dried peptides were examined via online nanoflow LC-MS/MS using a UPLC system (Waters, Millford), which was connected to a Q-Exactive spectrometer through a nano-electrospray ion source to gather particular MS/MS spectra. Proteome Discoverer (SEQUEST, Thermo Fischer Scientific, ver. 1.4.0.288) and Scaffold (X! Tandem, version Scaffold_4.4.6, Proteome Software, Portland, OR) were used to search the databases. The MS/MS data was analyzed against an S. cerevisiae database (S288C). Low-quality identified proteins were removed when they fell below the unique peptide score 1, number of peptides 1, and coverage of 7%. The KEGG pathway database (https://www.genome.jp/kegg/pathway.html) was used for functional classification of the identified SUMO target proteins.
Microscopic analysis
Fluorescence microscopy experiments were performed as previously described81. Cells expressing Ubc9-GFP were grown to mid-log phase in SD medium and then fixed with 1% formaldehyde for 20 min. Following centrifugation, cells were washed with 1 mL PBS to eliminate residual formaldehyde and then stained with 1 μg of 4’,6-diamidino-2-phenylindole (DAPI) to visualize nuclei. Images were acquired using a Leica STELLARIS 8 confocal microscope (Leica Microsystems) with a ×100 oil-immersion objective. GFP, DAPI, and differential interference contrast (DIC) images were captured and processed using the Leica Application Suite X (LAS X) Life Science Microscope Software.
Isolation of aged yeast mother cells
The aged mother cells were isolated as previously described with some modifications82. Thirty OD600 equivalents of cells grown to exponential phase in YPD were harvested, then labeled with 24 mg of EZ-Link™ Sulfo-NHS-LC-Biotin (21335, Thermo Fisher) in 1 mL phosphate-buffered saline (PBS) followed by gentle rotation at room temperature for 20 min. Cells were then washed with 1 mL PBS containing 0.1 M Glycine three times, and the biotin-labeled cells were resuspended in 1 L YPD and incubated at 30 °C for 12–14 h. For the first round of sorting, the harvested cells were incubated with Dynabeads™ Biotin Binder (11407, Thermo Fisher) at 4 °C for 1 h. The biotin-labeled cells were collected with a DynaMag™-15 magnet (12301D, Thermo Fisher) and subsequently washed 9 times with 10 mL cold PBS. Older cells obtained from the second and third rounds of sorting were also inoculated into YPD at 108 cells per 1 L and grown at 30 °C for 12–14 h. The sorting procedure was then repeated, and about 2 × 105 aged cells were saved for immunoblot analysis as described above. To estimate the average number of bud scars, which indicates the number of generations, cells were stained with 10 μM of Calcofluor White M2R (Sigma-Aldrich, 910090), and then the bud scar numbers were counted in composite images created using an IX83 confocal microscope (Olympus).
Statistics and reproducibility
Statistical analyses were performed using GraphPad Prism for experimental data, with specific tests applied as appropriate for each dataset, as indicated in the figure legends and Methods section. Data were assessed for normality, and statistical significance was determined using Student’s t test and multiple testing corrections where applicable. Sample sizes were determined based on standard practices in the field, and no statistical method was used to predetermine sample size. No data was excluded from the analyses. The experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data generated or used in this publication have been deposited in Gene Expression Omnibus and Proteomics Identifications Database, and the Sequence Read Archive with accession numbers: RNA-seq data: GSE254981. ChIP-seq data: GSE278286. Proteome data: PXD048944 and PXD049427. DNA-seq data: PRJNA1238522, SRX28069050 {[accn]}, SRX28069051{[accn]} and SRX28069052 {[accn]}. Figshare [https://doi.org/10.6084/m9.figshare.25340581] Source data are provided with this paper.
References
MacColl, A. D. The ecological causes of evolution. Trends Ecol. Evol. 26, 514–522 (2011).
Ryu, H. Y., Ahn, S. H. & Hochstrasser, M. SUMO and cellular adaptive mechanisms. Exp. Mol. Med. 52, 931–939 (2020).
Fulda, S., Gorman, A. M., Hori, O. & Samali, A. Cellular stress responses: cell survival and cell death. Int. J. Cell Biol. 2010, 214074 (2010).
Mans, R., Daran, J. M. G. & Pronk, J. T. Under pressure: evolutionary engineering of yeast strains for improved performance in fuels and chemicals production. Curr. Opin. Biotechnol. 50, 47–56 (2018).
Enserink, J. M. Sumo and the cellular stress response. Cell Div. 10, 4 (2015).
Huang, W. C., Ko, T. P., Li, S. S. & Wang, A. H. Crystal structures of the human SUMO-2 protein at 1.6 A and 1.2 A resolution: implication on the functional differences of SUMO proteins. Eur. J. Biochem. 271, 4114–4122 (2004).
Liang, Y. C. et al. SUMO5, a novel poly-SUMO isoform, regulates PML nuclear bodies. Sci. Rep. 6, 26509 (2016).
Hendriks, I. A. & Vertegaal, A. C. A comprehensive compilation of SUMO proteomics. Nat. Rev. Mol. Cell Biol. 17, 581–595 (2016).
Hickey, C. M., Wilson, N. R. & Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 13, 755–766 (2012).
Lewicki, M. C., Srikumar, T., Johnson, E. & Raught, B. The S. cerevisiae SUMO stress response is a conjugation-deconjugation cycle that targets the transcription machinery. J. Proteom. 118, 39–48 (2015).
Bylebyl, G. R., Belichenko, I. & Johnson, E. S. The SUMO isopeptidase Ulp2 prevents accumulation of SUMO chains in yeast. J. Biol. Chem. 278, 44113–44120 (2003).
Li, S. J. & Hochstrasser, M. The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol. Cell Biol. 20, 2367–2377 (2000).
Ryu, H. Y., Wilson, N. R., Mehta, S., Hwang, S. S. & Hochstrasser, M. Loss of the SUMO protease Ulp2 triggers a specific multichromosome aneuploidy. Genes Dev. 30, 1881–1894 (2016).
Ryu, H. Y. et al. Distinct adaptive mechanisms drive recovery from aneuploidy caused by loss of the Ulp2 SUMO protease. Nat. Commun. 9, 5417 (2018).
Sheltzer, J. M. & Amon, A. The aneuploidy paradox: costs and benefits of an incorrect karyotype. Trends Genet. 27, 446–453 (2011).
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span—from yeast to humans. Science 328, 321–326 (2010).
Longo, V. D., Shadel, G. S., Kaeberlein, M. & Kennedy, B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 16, 18–31 (2012).
Schieke, S. M. & Finkel, T. Mitochondrial signaling, TOR, and life span. Biol. Chem. 387, 1357–1361 (2006).
Bratic, I. & Trifunovic, A. Mitochondrial energy metabolism and ageing. Biochim. Biophys. Acta 1797, 961–967 (2010).
Kaeberlein, M. & Powers, R. W. 3rd. Sir2 and calorie restriction in yeast: a skeptical perspective. Ageing Res. Rev. 6, 128–140 (2007).
Gong, L., Sun, Q. & Li, D. W. Sumoylation in cellular senescence and aging. Curr. Mol. Med. 16, 871–876 (2017).
Ho, C. W., Chen, H. T. & Hwang, J. UBC9 autosumoylation negatively regulates sumoylation of septins in Saccharomyces cerevisiae. J. Biol. Chem. 286, 21826–21834 (2011).
Knipscheer, P. et al. Ubc9 sumoylation regulates SUMO target discrimination. Mol. Cell 31, 371–382 (2008).
Ryu, H. Y. SUMO pathway is required for ribosome biogenesis. BMB Rep. 55, 535–540 (2022).
Lobo, Z. & Maitra, P. K. Physiological role of glucose-phosphorylating enzymes in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 182, 639–645 (1977).
Kim, K. S., Rosenkrantz, M. S. & Guarente, L. Saccharomyces cerevisiae contains two functional citrate synthase genes. Mol. Cell Biol. 6, 1936–1942 (1986).
Teste, M. A., Enjalbert, B., Parrou, J. L. & Francois, J. M. The Saccharomyces cerevisiae YPR184w gene encodes the glycogen debranching enzyme. FEMS Microbiol. Lett. 193, 105–110 (2000).
Wolf, S. E. & Rosvall, K. A. A multi-tissue view on telomere dynamics and postnatal growth. J. Exp. Zool. A Ecol. Integr. Physiol. 337, 346–355 (2022).
Ingram, D. K., Reynolds, M. A. & Les, E. P. The relationship of genotype, sex, body weight, and growth parameters to lifespan in inbred and hybrid mice. Mech. Ageing Dev. 20, 253–266 (1982).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999).
Dang, W. et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 459, 802–807 (2009).
Sinclair, D. A. & Guarente, L. Extrachromosomal rDNA circles–a cause of aging in yeast. Cell 91, 1033–1042 (1997).
Rhie, B. H., Song, Y. H., Ryu, H. Y. & Ahn, S. H. Cellular aging is associated with increased ubiquitylation of histone H2B in yeast telomeric heterochromatin. Biochem. Biophys. Res. Commun. 439, 570–575 (2013).
Lim, S. et al. Sus1 maintains a normal lifespan through regulation of TREX-2 complex-mediated mRNA export. bioRxiv, https://doi.org/10.1101/2022.04.07.487427 (2022).
Rocher, C. et al. Influence of mitochondrial DNA level on cellular energy metabolism: implications for mitochondrial diseases. J. Bioenerg. Biomembr. 40, 59–67 (2008).
Kirchman, P. A., Kim, S., Lai, C. Y. & Jazwinski, S. M. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics 152, 179–190 (1999).
Yi, D. G., Hong, S. & Huh, W. K. Mitochondrial dysfunction reduces yeast replicative lifespan by elevating RAS-dependent ROS production by the ER-localized NADPH oxidase Yno1. PLoS One 13, e0198619 (2018).
Kaya, A. et al. Evolution of natural lifespan variation and molecular strategies of extended lifespan in yeast. Elife 10, e64860 (2021).
Wohlschlegel, J. A., Johnson, E. S., Reed, S. I. & Yates, J. R. Global analysis of protein sumoylation in Saccharomyces cerevisiae. J. Biol. Chem. 279, 45662–45668 (2004).
Wykoff, D. D. & O’Shea, E. K. Identification of sumoylated proteins by systematic immunoprecipitation of the budding yeast proteome. Mol. Cell Proteom. 4, 73–83 (2005).
Hannich, J. T. et al. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J. Biol. Chem. 280, 4102–4110 (2005).
Bennetzen, J. L. & Hall, B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J. Biol. Chem. 257, 3018–3025 (1982).
Heinisch, J., von Borstel, R. C. & Rodicio, R. Sequence and localization of the gene encoding yeast phosphoglycerate mutase. Curr. Genet. 20, 167–171 (1991).
Goncalves, P. & Planta, R. J. Starting up yeast glycolysis. Trends Microbiol. 6, 314–319 (1998).
Sammons, M. A., Samir, P. & Link, A. J. Saccharomyces cerevisiae Gis2 interacts with the translation machinery and is orthogonal to myotonic dystrophy type 2 protein ZNF9. Biochem. Biophys. Res Commun. 406, 13–19 (2011).
Schirmaier, F. & Philippsen, P. Identification of two genes coding for the translation elongation factor EF-1 alpha of S. cerevisiae. EMBO J. 3, 3311–3315 (1984).
Umekawa, M. & Klionsky, D. J. Ksp1 kinase regulates autophagy via the target of rapamycin complex 1 (TORC1) pathway. J. Biol. Chem. 287, 16300–16310 (2012).
Klug, H. et al. Ubc9 sumoylation controls SUMO chain formation and meiotic synapsis in Saccharomyces cerevisiae. Mol. Cell 50, 625–636 (2013).
Gill, G. Something about SUMO inhibits transcription. Curr. Opin. Genet. Dev. 15, 536–541 (2005).
Rosonina, E., Duncan, S. M. & Manley, J. L. SUMO functions in constitutive transcription and during activation of inducible genes in yeast. Genes Dev. 24, 1242–1252 (2010).
Ryu, H. Y. et al. The Ulp2 SUMO protease promotes transcription elongation through regulation of histone sumoylation. EMBO J. 38, e102003 (2019).
Chymkowitch, P. et al. Sumoylation of Rap1 mediates the recruitment of TFIID to promote transcription of ribosomal protein genes. Genome Res. 25, 897–906 (2015).
Chymkowitch, P. et al. TORC1-dependent sumoylation of Rpc82 promotes RNA polymerase III assembly and activity. Proc. Natl. Acad. Sci. USA 114, 1039–1044 (2017).
Jackson, R. J., Hellen, C. U. & Pestova, T. V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 (2010).
Princz, A., Pelisch, F. & Tavernarakis, N. SUMO promotes longevity and maintains mitochondrial homeostasis during ageing in Caenorhabditis elegans. Sci. Rep. 10, 15513 (2020).
Jakobs, A. et al. Ubc9 fusion-directed SUMOylation (UFDS): a method to analyze function of protein SUMOylation. Nat. Methods 4, 245–250 (2007).
Guarente, L. Mitochondria–a nexus for aging, calorie restriction, and sirtuins? Cell 132, 171–176 (2008).
Kaeberlein, M. et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Sci. Signal. 310, 1193 (2005).
Steffen, K. K. et al. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell 133, 292–302 (2008).
Johnson, S. C., Rabinovitch, P. S. & Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345 (2013).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000).
Masoro, E. J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 126, 913–922 (2005).
Ingram, D. K. et al. Calorie restriction mimetics: an emerging research field. Aging Cell 5, 97–108 (2006).
Kim, J. et al. Metformin ameliorates olanzapine-induced disturbances in POMC neuron number, axonal projection, and hypothalamic leptin resistance. BMB Rep. 55, 293–298 (2022).
Lee, S. et al. Melatonin inhibits glycolysis in hepatocellular carcinoma cells by downregulating mitochondrial respiration and mTORC1 activity. BMB Rep. 55, 459–464 (2022).
Funakoshi, M. & Hochstrasser, M. Small epitope-linker modules for PCR-based C-terminal tagging in Saccharomyces cerevisiae. Yeast 26, 185–192 (2009).
Zhou, W. D., Ryan, J. J. & Zhou, H. L. Global analyses of sumoylated proteins in Saccharomyces cerevisiae - induction of protein sumoylation by cellular stresses. J. Biol. Chem. 279, 32262–32268 (2004).
Voth, W. P., Wei Jiang, Y. & Stillman, D. J. New ‘marker swap' plasmids for converting selectable markers on budding yeast gene disruptions and plasmids. Yeast 20, 985–993 (2003).
Graef, M. & Nunnari, J. Mitochondria regulate autophagy by conserved signalling pathways. EMBO J. 30, 2101–2114 (2011).
Yona, A. H. et al. Chromosomal duplication is a transient evolutionary solution to stress. Proc. Natl. Acad. Sci. USA 109, 21010–21015 (2012).
Steffen, K. K., Kennedy, B. K. & Kaeberlein, M. Measuring replicative life span in the budding yeast. J. Vis. Exp. https://doi.org/10.3791/1209 (2009).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Ryu, H. Y., Zhao, D., Li, J., Su, D. & Hochstrasser, M. Histone sumoylation promotes Set3 histone-deacetylase complex-mediated transcriptional regulation. Nucleic Acids Res. https://doi.org/10.1093/nar/gkaa1093 (2020).
Liu, Y. et al. Tho2-mediated escort of Nrd1 regulates the expression of aging-related genes. Aging Cell 23, e14203 (2024).
Kroetz, M. B., Su, D. & Hochstrasser, M. Essential role of nuclear localization for yeast Ulp2 SUMO protease function. Mol. Biol. Cell 20, 2196–2206 (2009).
Li, S. J. & Hochstrasser, M. A new protease required for cell-cycle progression in yeast. Nature 398, 246–251 (1999).
Gillies, J. et al. SUMO pathway modulation of regulatory protein binding at the ribosomal DNA locus in saccharomyces cerevisiae. Genetics 202, 1377–1394 (2016).
Park, J. H. et al. Proteomic analysis of host cell protein dynamics in the culture supernatants of antibody-producing CHO Cells. Sci Rep-Uk 7, ARTN 44246 (2017).
Ryu, H. Y. & Ahn, S. Yeast histone H3 lysine 4 demethylase Jhd2 regulates mitotic rDNA condensation. BMC Biol. 12, 75 (2014).
Park, P. U., Mcvey, M. & Guarente, L. Separation of mother and daughter cells. Methods Enzymol. 351, 468–477 (2002).
Acknowledgements
We thank Andrea Pichler, Huilin Zhou, S.H. Ahn, and David J. Stillman for supplying strains and plasmids. We also thank Dr. Pichler for helpful discussions on Ubc9 auto-sumoylation. This study was supported by the NIH [GM136325 to M.H.], National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [RS-2023-00212894 to H.Y.R.], the Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Korea government (MSIT) [RS-2024-00404285 to H.Y.R.], Korean Environment Industry & Technology Institute (KEITI) through the Core Technology Development Project for Environmental Diseases Prevention and Management funded by the Korean Ministry of Environment (MOE) [No. 2022003310001 to H.Y.R.], and Learning & Academic research institution for Master’s·PhD students, and Postdocs(LAMP) Program of the National Research Foundation of Korea(NRF) grant funded by the Ministry of Education [No. RS-2023-00301914 to H.Y.R.].
Author information
Authors and Affiliations
Contributions
D.W.J. and H.Y.R. organized and performed most of the experiments. D.Y.L., S.Y.K., and S.W.J carried out parts of RLS and ChIP-seq analyses. D.Z. and J.K. analyzed the RNA-seq and DNA-seq, respectively. T.T.L. and J.H.J. performed proteome analyses. H.S.L. carried out review and editing. M.H. and H.Y.R. designed the study, analyzed the data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jeong, DW., Lee, D.Y., Kim, S.Y. et al. Auto-sumoylation of the yeast Ubc9 E2 SUMO-conjugating enzyme extends cellular lifespan. Nat Commun 16, 3735 (2025). https://doi.org/10.1038/s41467-025-58925-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58925-w