Abstract
Metabolic reprogramming of amino acids represents a vulnerability in cancer cells, yet the mechanisms underlying serine metabolism in acute myeloid leukemia (AML) and leukemia stem/initiating cells (LSCs/LICs) remain unclear. Here, we identify RNA N6-methyladenosine (m6A) modification as a key regulator of serine biosynthesis in AML. Using a CRISPR/Cas9 screen, we find that depletion of m6A regulators IGF2BP3 or METTL14 sensitizes AML cells to serine and glycine (SG) deprivation. IGF2BP3 recognizies m6A on mRNAs of key serine synthesis pathway (SSP) genes (e.g., ATF4, PHGDH, PSAT1), stabilizing these transcripts and sustaining serine production to meet the high metabolic demand of AML cells and LSCs/LICs. IGF2BP3 silencing combined with dietary SG restriction potently inhibits AML in vitro and in vivo, while its deletion spares normal hematopoiesis. Our findings reveal the critical role of m6A modification in the serine metabolic vulnerability of AML and highlight the IGF2BP3/m6A/SSP axis as a promising therapeutic target.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a rapidly progressing hematopoietic malignancy characterized by the uncontrolled clonal expansion of immature myeloid cells and the impaired differentiation of myeloid progenitor cells1,2. Despite the advances in new therapies available in recent years, outcomes of AML patients remain poor, with a five-year survival rate of approximately 30%. This is primarily due to the existence of therapy-resistant leukemia cells that have been characterized as leukemia stem/initiating cells (LSCs/LICs) and thought to be the origin of leukemia initiation, relapse, and drug resistance1,3,4,5,6. Thus, there is an urgent need to identify the molecular mechanisms underlying the maintenance of LSCs/LICs stemness in order to develop more efficacious therapeutic strategies for AML patients.
Metabolic reprogramming is a hallmark of cancer, which sustains rapid growth and proliferation of cancer cells7,8. Evidence is emerging that deregulation of amino acids metabolism, such as serine metabolism, plays a key role in the development of AML, especially in the maintenance of LSCs/LICs9,10. Serine participates in numerous fundamental metabolic processes, including one-carbon metabolism, de novo synthesis of nucleotides (i.e., purines and deoxythymidines), and the generation of other non-essential amino acids, such as glycine, therefore maintaining high proliferation rates of cancer cells both in vitro and in vivo11,12,13,14. As a non-essential amino acid, serine can be imported into cells from the extracellular environment or synthesized de novo through the serine synthesis pathway (SSP)12,15. The SSP converts the glycolytic intermediate 3-phosphoglycerate (3-PG) into serine through three consecutive enzymatic reactions catalyzed sequentially by 3-phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase (PSAT1), and phosphoserine phosphatase (PSPH). Although transcriptional regulatory mechanisms have been discovered to control serine level and cell fate in AML10,16,17, epigenetic regulation of serine metabolism in AML remains to be explored.
N6-methyladenosine (m6A) is the most abundant internal mRNA modification in mammalian cells and has been closely linked to leukemogenesis18,19,20,21,22,23,24,25. This reversible modification is co-transcriptionally catalyzed by the m6A methyltransferase (writer) complex composed of core subunits METTL3/METTL14/WTAP and other cofactors26,27,28,29 and could be removed by m6A demethylases, FTO and ALKBH518,19,30,31. Reader proteins, such as YTH domain-containing protein 1/2 (YTHDC1/2)32,33,34,35, YTH domain-containing family member 1/2/3 (YTHDF1/2/3)20,36, and insulin-like growth factor 2 mRNA-binding protein 1/2/3 (IGF2BP1/2/3)37, can recognize m6A modifications in mRNAs and determine RNA fate by regulating mRNA processing, splicing, export, translation, and stability38,39. We and others have reported that RNA m6A modification functions as a key player in various metabolic pathways of AML, including glycolysis, glutaminolysis, and synthesis of branched-chain amino acids40,41,42,43. Considering the critical roles of serine metabolism in AML, it is of great importance to investigate whether RNA modification, especially m6A modification, is also involved in the regulation of serine metabolism in AML.
Here, by utilizing CRISPR/Cas9-based screening, we uncover a key function of m6A modification in controlling biosynthesis of serine and promoting survival and stemness of AML cells. Knockdown (KD) of METTL14 or IGF2BP3 could sensitize AML cells to serine and glycine (SG) deprivation-induced growth arrest. Mechanistically, IGF2BP3 recognizes m6A modification on ATF4, PHGDH and PSAT1 mRNAs and thereby promotes their stability/expression, leading to the sustained production of serine and its downstream metabolites to fulfill the high metabolic demand of AML cells and LSCs/LICs. Inhibition of SSP by genetic silencing or acute degradation of IGF2BP3 exhibits a synergistic effect with SG deprivation in vitro and in vivo. In contrast to the high expression and oncogenic role of IGF2BP3 in AML cells and especially in LSCs, Igf2bp3 deletion shows little effect on normal hematopoiesis, highlighting that targeting IGF2BP3 (alone or together with SG deprivation) is a promising and safe strategy for AML therapy.
Results
PHGDH inhibition synergizes with SG deprivation to suppress AML
It has been reported that many cancer cells, including AML cells, depend on serine for optimal growth44,45,46. To verify the dependency of AML cells on serine, we first removed SG from the culture medium, because serine can also be converted from cellular glycine by serine hydroxymethyltransferase (SHMT)11,12. Indeed, the growth of human AML cells, as well as the colony-forming ability of MLL-AF9 (MA9)-transduced mouse hematopoietic stem/progenitor cells (HSPCs), was significantly inhibited in SG-free medium (Fig. 1A, B). On the contrary, when added back to the medium, SG promotes cell growth in a concentration-dependent manner (Fig. S1A). These results indicate that exogenous serine promotes AML cell survival/growth.
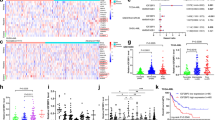
A Growth curves of AML cell lines grown in complete medium (CM) or equivalent medium lacking serine and glycine (-SG). B Mouse HSPCs were transduced with MLL-AF9 (MA9) retroviruses, followed by cultured in CM or -SG progenitor medium for 2 days. The cells were then plated into mouse methylcellulose medium for CFA. Colony numbers and representative photos of colonies are shown. Bar = 200 μm. C A schematic diagram, created from GDP (https://BioGDP.com, Agreement number: GDP2025UWV74M), depicting the CRISPR/Cas9-based screening strategy. Lentivirus-transduced Molm13 Cas9 cells were selected with puromycin for 5 days and collected for an initial gDNA sample (denoted as T0). Cells were further grown in CM or -SG medium for 8, 12, or 17 population doublings, and were denoted as P8, P12, or P17, respectively. For each gene, we calculated its gene score as the mean log2 fold change (LFC) in the abundance of all the sgRNAs targeting the corresponding gene in different population doublings compared to T0. The SG-dependent score refers to the difference in gene scores in the absence versus presence of SG at the same population doubling. D The SG-dependent scores of the top 30 genes from the screening in (C), with the m6A regulatory genes IGF2BP3 and METTL14 being highlighted in red and purple, respectively. E, F The normalized abundance of the 12 sgRNAs targeting IGF2BP3 (E) or METTL14 (F) from cells cultured in CM or -SG medium and collected at P8, P12, and P17. The definition of data points is same as in (C). n = 12 sgRNAs. The boxes extend from the 25th to the 75th percentiles, with a distinct line marking the median. Whiskers extend to the maximum and minimum data points. G Growth curves of Molm13 Cas9 transduced with control sgRNA (sgNS) or IGF2BP3 sgRNA (sgBP3) and grown in CM or -SG medium. H Growth curves of control or IGF2BP3 KD cells in CM or -SG medium. I Growth curves of control or METTL14 KD Molm13 cells in CM or -SG medium. J Schematic illustration showing the AID2 system in inducing rapid degradation of IGF2BP3 protein. K Relative growth of IGF2BP3-mAID (clone BP3-mAID cells) cells in CM or -SG medium with or without treatment with 5’Ph-IAA (IAA) for 24 h. Mean ± SD values are shown. n = 2 biologically independent repeats in (A, B, G, H, I, and K). Source data are provided as a Source Data file.
Nonetheless, SG deprivation only exhibited a moderate inhibition on AML cell survival/growth (Fig. 1A, B), suggesting that serine produced inside cells is also critical for AML cells. Indeed, shRNA-mediated KD of PHGDH, the first rate-limiting enzyme in the SSP pathway, resulted in growth inhibition in human AML cells (Fig. S1B, C). Consistently, cell growth inhibition was also observed when AML cells were treated with NCT-503 (Fig. S1D, E), a well-established small-molecule inhibitor targeting PHGDH47,48,49,50. Notably, NCT-503 further sensitized AML cells to SG deprivation, as shown by the remarkable decline in the growth of human AML cells compared to either treatment alone (Fig. S1F, G). These results together demonstrate that both uptake and biosynthesis of serine are essential for AML cell survival/growth and suggest that simultaneous inhibition of SSP and deprivation of SG supply can exhibit a synergistic (or at least a cooperative) effect in inhibiting AML.
Epigenetic-focused CRISPR/Cas9 screen reveals that inhibition of m6A pathway sensitizes AML cells to SG deprivation
Based on these findings, we performed a CRISPR/Cas9 knockout (KO) screen to identify epigenetic genes involved in the regulation of SSP by discovering the genes whose loss sensitizes AML cells to SG deprivation. The AML cell line Molm13 stably expressing Cas9 (namely Molm13-Cas9) was constructed and infected with a pool of lentiviruses targeting 423 epigenetic genes as well as positive and negative controls at a multiplicity of infection (MOI) < 0.3 (Fig. 1C). After puromycin selection, the pool of KO cells was maintained in complete medium (CM) or medium deprived of SG (-SG) and collected after 0, 8, 12, and 17 population doublings for massively parallel sequencing of all sgRNAs. Through negative selection screen (Figs. 1D and S1H), we identified 30 epigenetic genes whose depletion inhibits AML growth in CM and confers more disadvantage for cell survival in SG-free medium in a time-dependent manner (Figs. 1D; S1I, J and Table S1). Notably, among them, there were two RNA m6A-related proteins, IGF2BP3 and METTL14 (Fig. 1D–F). The sgRNAs for IGF2BP3 and METTL14 were gradually dropped out after passaging cells in SG-free medium (Fig. 1E, F), suggesting that m6A modification is critical for survival of AML cells when exogenous SG is limited. When we analyzed the protein level of IGF2BP3, we found that the expression of IGF2BP3 was higher in AML cell lines compared to primary MNC from healthy controls (Fig. S1K). We therefore selected Molm13 and NB4 as representatives of cell lines with high IGF2BP3 expression, and U937 and MM6 as representatives of those with relatively low levels of IGF2BP3, for further studies. To validate the results from screening, we constructed sgRNAs/shRNAs specifically targeting IGF2BP3 or METTL14 (Fig. S1L–O). Survival/growth of AML cells was suppressed in the absence of SG, and loss of IGF2BP3 or METTL14 further exacerbated the defect (Fig. 1G–I), consistent with the results from CRISPR/Cas9 screening.
The abundance of RNA m6A methylation is dynamically regulated by m6A writers and erasers, while its effect of RNA fate determination relies on m6A readers32,36,38,39. Thus, we focused on the m6A reader IGF2BP3 for further study. We further utilized the auxin-inducible degron version 2 (AID2) system51 to induce rapid depletion of the IGF2BP3 protein (Fig. 1J), in which DNA sequence encoding a 7-kD degron from Arabidopsis IAA17 termed mini-AID (mAID) was tagged to the 3’ end of IGF2BP3 locus in U937 cells stably expressing the Oryza sativa F-box protein OsTIR1 (F74G mutant) (Fig. S1P). The resulting IGF2BP3-mAID fusion protein in the U937 single clone cells (named BP3-mAID cells) can be recognized by the SKP1-CUL1-F-box (SCF) E3 ligase complex in the presence of 5’Ph-IAA (an auxin analog) to trigger rapid degradation of the mAID-tagged IGF2BP3 protein (Figs. 1J and S1Q), and thus would avoid potential secondary effects caused by genetic knockdown/knockout. Consistent with sgRNA/shRNA-mediated genetic KO/KD of IGF2BP3, acute degradation of IGF2BP3 protein in BP3-mAID cells also significantly sensitized AML cells to SG deprivation (Fig. 1K). Together, our data imply that RNA m6A modification and its reader IGF2BP3 may play important roles in regulating serine metabolism.
Metabolomics and transcriptomics analyses reveal that IGF2BP3 regulates de novo serine biosynthesis in AML
To understand whether and how m6A is involved in serine metabolism regulation in AML, we performed metabolite profiling in the control and IGF2BP3 KD Molm13 cells incubated with U-[13C]-glucose overnight by liquid chromatography-mass spectrometry (LC-MS). Enrichment analysis revealed that metabolites downregulated after IGF2BP3 KD were associated with various metabolic pathways, including the purine metabolism, methionine metabolism, gluconeogenesis, glycine and serine metabolism, and the tricarboxylic acid cycle (TCA cycle) (Fig. 2A), and the abundance of most of the 13C-labeled metabolites in these pathways were reduced in IGF2BP3 KD cells (Figs. 2B, C and S2A). Among these pathways, serine metabolism was noted for its connections with other key metabolic pathways (e.g., the TCA cycle) and its critical role in the synthesis of purine, glutathione (GSH), and amino acids12,13,52 (Figs. 2D and S2A), underscoring a potential central role of serine metabolism in mediating the function of IGF2BP3. Importantly, level of 13C-labeled (M + 3) serine significantly decreased upon IGF2BP3 KD (Fig. 2E), suggesting that biosynthesis of serine was regulated by IGF2BP3. Interestingly, the percentage of M + 6 ~ M + 10 (derived from serine) of purines decreased remarkably upon IGF2BP3 knockdown, while that of M + 5 (derived from the pentose phosphate pathway) remained largely unchanged (Fig. 2C), suggesting a relatively specific impact of IGF2BP3 on serine synthesis and metabolism. Consistent with the observed changes from LC-MS, a significant reduction in intracellular serine abundance after IGF2BP3 KO or KD was confirmed by fluorescence-based quantification assays (Fig. 2F, G). Moreover, METTL14 KD also led to a significant decrease of the intracellular serine level (Fig. 2H). Such data suggest that m6A regulators control the biosynthesis and catabolism of serine to sustain the one-carbon metabolism and energy supply of AML cells.
A Bubble diagram showing the enrichment of metabolic pathways by the C13-labeled metabolites with reduced levels after IGF2BP3 KD in Molm13 cells. B Heatmaps showing levels of representative C13-labeled metabolites after IGF2BP3 KD in Molm13 cells. C The total levels and isotopolog distribution (M + n, n refers to numbers of 13C) of purines measured by LC-MS in Molm13 cells transduced with IGF2BP3 shRNAs or shNS and grown in medium containing U-[13C]-glucose. D Schematic of the serine synthesis pathway and its downstream pathways. E The levels of C13-labeled serine (M + 3) were measured by LC-MS in Molm13 cells transduced with IGF2BP3 shRNAs or shNS and grown in medium containing U-[13C]-glucose. F–H Intracellular serine level measured by fluorescence-based serine detection assays in Molm13 cells transduced with shRNAs (F) or sgRNAs (G) targeting IGF2BP3 or shRNAs targeting METTL14 (H), compared to those with negative control (shNS). I The Venn diagram showing the overlap of significantly downregulated genes (adjusted P < 0.05, fold change <0.67) in Molm13 cells with IGF2BP3 KD, as well as in U937 BP3-mAID cells treated with 5’Ph-IAA for 6 or 24 h to induce degradation of the IGF2BP3 protein. J Pie chart showing numbers of transcripts with or without m6A modifications among the 61 overlapping genes in (H), and heatmap showing expression changes of the 48 m6A-containing transcripts in IGF2BP3 KD Molm13 cells or IGF2BP3-depleted U937 cells. K GO enrichment analysis of the 48 candidate targets of IGF2BP3 in (I). The degrees of interactions were generated by STRING. Mean ± SD values are shown. n = 2 biologically independent repeats in (C) and (E), while n = 3 biologically independent repeats in (F–H). P-values were calculated with one-sided Hypergeometric test (A, K); two-tailed Wald test adjusted with Benjamini-Hochberg Procedure (I); two-tailed student’s t-test (F–H); Source data are provided as a Source Data file.
To uncover the underlying mechanism of IGF2BP3 in regulating serine biosynthesis in AML cells, we performed RNA sequencing (RNA-seq) in IGF2BP3 KD Molm13 cells and BP3-mAID cells, along with their controls (Fig. S2B, C). To obtain early responding target genes, we included a short-term treatment (6 h) of BP3-mAID cells with 5’ Ph-IAA in addition to the 24-h treatment. There were 61 genes significantly downregulated (adjusted P < 0.05, fold change <0.67) in all the groups with IGF2BP3 depletion (Fig. 2I). Among them, mRNAs of 48 genes contain at least one m6A modification site according to our published m6A-seq data53 (Figs. 2J; S2D and E). Gene ontology (GO) analysis of these 48 genes revealed an enrichment in cellular metabolic pathways, including the L-serine biosynthetic process (Fig. 2K). The decreased expression of genes involved in the L-serine biosynthetic process after IGF2BP3 KD or acute IGF2BP3 degradation was further confirmed by qRT-PCR (Fig. S2F, G). These data indicate that IGF2BP3 augments serine biosynthesis in AML.
IGF2BP3 promotes SSP through directly increasing target gene expression in an m6A-dependent manner
PHGDH and PSAT1, two rate-limiting enzymes of SSP12, as well as the transcription factor ATF4 that has been reported to positively regulate the expression of PHGDH and PSAT154, are in the list of potential IGF2BP3 target genes involved in serine biosynthesis (Fig. 2K). It should be noted that SHMT2, an enzyme that plays an important role in serine catabolism, is also a potential target of IGF2BP3. However, as we focus on serine biosynthesis, we did not include SHMT2 for further study. Consistent with the qRT-PCR results (Fig. S2F, G), western blotting showed a decrease of ATF4, PHGDH and PSAT1 protein levels upon IGF2BP3 depletion in AML cells (Figs. 3A, B; S3A and S3B), and the opposite is true when wild-type (WT) IGF2BP3 but not IGF2BP3 with KH3-4 mutation that hinders m6A recognition was forced expressed (Figs. 3C and S3C). In addition, METTL14 KD also suppressed the mRNA and protein levels of ATF4, PHGDH and PSAT1 (Figs. 3D; S3D–S3F). These data indicate that SSP-related genes ATF4, PHGDH and PSAT1 are regulated by IGF2BP3 in an m6A-dependent manner. Consistent with previous reports, knockdown of ATF4 resulted in reduced mRNA levels of PHGDH and PSAT1 (Fig. S3G), suggesting that ATF4 may also mediate the regulation of IGF2BP3 on the expression of PHGDH and PSAT1.
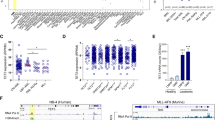
A Western blot after IGF2BP3 KD in various AML cell lines. GAPDH was used as a loading control. B Western blot of U937 BP3-mAID cells after treatment with 5’Ph-IAA for the indicated time periods. ACTB was used as a loading control. C Western blot of Molm13 cells after overexpression of wild-type (BP3-WT) or KH3-4 mutated (BP3-KH34) IGF2BP3. D Western blot of Molm13 cells after METTL14 KD. E IGV tracks showing the distribution of m6A (GEO: GSE97408) or IGF2BP3 binding sites (GEO: GSE90639) in target mRNAs. Gray shadow depicts high-confidence m6A regions for qPCR validation in (G) and (H). F RIP assays using an IGF2BP3 antibody were performed in Molm13 cells, followed by qPCR to detect direct binding of IGF2BP3 to target mRNAs at regions indicated in (E). G MeRIP-qPCR was performed in control and METTL14 KD Molm13 cells to detect the change of m6A abundance at indicated regions of ATF4, PHGDH, and PSAT1 transcripts. H Bst DNA polymerase-mediated cDNA extension and qPCR assays to evaluate relative m6A abundance changes at specific sites within ATF4, PHGDH, and PSAT1 transcripts in control and METTL14 KD Molm13 cells. I Control and IGF2BP3 KD Molm13 cells were treated with actinomycin D for indicated time periods and the RNA level of target genes was examined by qPCR. 18S rRNA was used as a loading control. The mRNA half-life (t1/2) was calculated and shown. (J, K) Live cell counting (J) and cytometric analysis of Annexin V+ apoptotic cells (K) in Molm13 cells upon IGF2BP3 KD and ATF4, PHGDH, or PSAT1 overexpression. EV, empty vector. (L, M) Molm13 cells were co-transduced with control or IGF2BP3 shRNAs and ATF4/PHGDH/PSAT1 overexpression vectors and subjected to colony-forming assays (L) and serine level detection (M). Representative results from one of the two independent experiments were shown (A–D). Mean ± SD values are shown. n = 3 biologically independent repeats in (F, G, H, I, K, and M), while n = 2 biologically independent repeats in (J) and (L). Two-tailed student’s t-test (F–H), two-way ANOVA (K, M). Source data are provided as a Source Data file.
To determine whether ATF4, PHGDH and PSAT1 were direct targets of IGF2BP3, we analyzed the m6A peaks distribution and IGF2BP3 binding on ATF4, PHGDH and PSAT1 mRNAs using our published m6A-seq and IGF2BP3 RIP-seq data37,53. ATF4, PHGDH and PSAT1 transcripts all displayed high abundance of m6A modification, which decreased when METTL14 was knocked down, and most of these m6A peaks are associated with IGF2BP3 enrichment/binding (Fig. 3E). A peak region with both high m6A abundance and IGF2BP3 enrichment was selected for each transcript for validation. As expected, RIP assays using anti-IGF2BP3 antibody confirmed that the m6A-modified regions in these transcripts could be bound by IGF2BP3 (Fig. 3F), and m6A-qPCR showed a decrease of m6A modification in these regions upon METTL14 KD (Fig. 3G). In addition, we utilized Bst DNA polymerase-mediated cDNA extension and qPCR assays to confirm the exact m6A modification sites in these regions, which also suggests a decrease of m6A abundance in the examined sites upon METTL14 KD (Figs. 3H and S3H). Given that IGF2BP3 can stabilize m6A-modified target mRNAs through interacting with mRNA stabilizers such as huR and PABPC140, we examined the mRNA half-life of these two targets. Indeed, IGF2BP3 KD significantly promoted the decay of ATF4, PHGDH and PSAT1 mRNAs (Fig. 3I). Taken together, these results demonstrate that IGF2BP3 directly binds to m6A-modified ATF4, PHGDH and PSAT1 mRNA transcripts and enhance their stability in AML cells.
In line with the regulation of the SSP by IGF2BP3 and the critical role of serine in AML cells, we observed a substantial growth inhibition (Fig. 1G, H) and a significant induction of apoptosis (Fig. S3I) and cell cycle arrest (Fig. S3J) in AML cell lines and PDX samples upon IGF2BP3 KD. Notably, apoptosis induced by IGF2BP3 KD could be prevented by ectopic expression of WT but not KH3-4 mutated IGF2BP3 (Fig. S3K), indicating an m6A-dependent regulation. In addition, IGF2BP3 KD resulted in a remarkable suppression of the colony-forming ability of AML cells (Fig. S3L). In consistence, KD of ATF4, PHGDH or PSAT1 exhibited a growth inhibitory effect on AML cells (Figs. S1B, C and S3M–O). Furthermore, ectopic expression of ATF4, PHGDH, or PSAT1 could partially rescue IGF2BP3 KD-induced cell growth inhibition (Fig. 3J), apoptosis induction (Fig. 3K), and inhibition of colony-forming ability of AML cells (Figs. 3L; S3P and Q). Importantly, IGF2BP3 depletion-induced decrease of cellular serine level could also be partially rescued by forced expression of ATF4, PHGDH, or PSAT1 (Fig. 3M), suggesting that ATF4, PHGDH, and PSAT1 are functionally important targets of IGF2BP3 and that SSP is a critical target pathway of IGF2BP3 in AML.
IGF2BP3 promotes AML leukemogenesis as an m6A reader
The above data demonstrate the critical oncogenic role of IGF2BP3 in vitro as a regulator of the SSP. To elucidate the role of IGF2BP3 in AML leukemogenesis, mouse c-kit+ HSPCs were isolated from bone marrow (BM) and used for colony-forming/replating assay (CFA) and BMT (Fig. 4A). shRNA-mediated KD of Igf2bp3 inhibited MA9-mediated leukemogenesis in vivo, as showed by the delayed leukemia onset and the longer survival duration of the recipients (Figs. 4B and S4A). Histological analysis revealed a notable decrease of immature blast cells in peripheral blood (PB) and BM, as well as less leukemia cell infiltration in spleens and livers in mice transplanted with Igf2bp3 KD donor cells relative to the wild-type control group (Fig. 4C). Consistently, KD of Igf2bp3 significantly inhibited cell transformation and colony formation mediated by MA9, PML-RARa or FLT3-ITD/NPM1-mut (Figs. 4D–F and S4B), as well as cell growth (Fig. S4C). Conversely, ectopic expression of IGF2BP3 significantly enhanced MA9-mediated colony-forming ability in mouse HSPCs (Fig. 4G). However, this promoting effect was largely eliminated when Mettl14 was knocked out (Fig. 4G), indicating that the function of IGF2BP3 in AML cell transformation is dependent on m6A modification.
A Schematic illustration of the in vivo primary BMT assay and in vitro CFA with HSPCs co-transduced with MA9 retroviruses and Igf2bp3 shRNA (or shNS) lentiviruses. B Kaplan–Meier curves showing the effect of Igf2bp3 KD on MA9-induced leukemogenesis. C Wright-Giemsa staining of BM cells and PB blood smear, and hematoxylin and eosin (H&E) staining of livers and spleens of the representative primary BMT mice from (B) at the endpoint. Scale bars from left to right: 20 μm, 50 μm, 200 μm, 300 μm. D, E CFA using mouse HSPCs transduced with MA9 (D) or PML-RARa (E) plus shNS, Bp3-sh1, or Bp3-sh2 viruses. F HSPCs from FLT3-ITD/NPM1-mut mice were transduced with shNS, Bp3-sh1, or Bp3-sh2 viruses and seeded for CFA. Bar = 200 μm. G HSPCs from Mettl14fl/fl-CREERT mice were transduced with MA9 retroviruses and seeded for CFA. Mettl14 KO was induced by addition of 4-Hydroxytamoxifen (4-OHT, 1 mM) during plating. H Schematic illustration of the in vivo primary BMT assay and in vitro CFA with HSPCs from Igf2bp3 WT and KO mice. I Colony numbers and representative photos of colonies in the CFA assays illustrated in (H). Bar = 200 μm. J Flow cytometric analysis of CD45.2+ donor cell percentages in PB of recipient mice 8 weeks after BMT. n = 7 mice. K Kaplan–Meier curves showing the effect of Igf2bp3 KO on MA9-induced leukemogenesis. L, M Representative images of liver (L) and spleen (M) tissues from the primary BMT mice in (K) at the endpoint. N Wright-Giemsa staining of BM cells and PB blood smear, and H&E staining of livers and spleens of representative primary BMT recipient mice from (K) at the endpoint. Scale bars are same as in (C). O HSPCs from WT or Igf2bp3 KO mice were co-transduced with MA9 and ATF4-, PHGDH-, or PSAT1-overexpressing viruses and seeded for CFA. P Schematic illustration of the secondary BMT assay and CFA with MA9-induced leukemic cells transduced with Igf2bp3 shRNA (or shNS) lentiviruses. Q CFA assays showing the effect of Igf2bp3 KD on BM cells from MA9 leukemia mice. Bar = 200 μm. R Kaplan–Meier curves showing the result of secondary BMT using cells collected from the first plating of (O). Representative results from one of at least two mice were shown (C, N). Mean ± SD values are shown. n = 2 biologically independent repeats in (D, E, F, G, I, O, and Q). Statistical analysis: Two-tailed student’s t-test (J); log-rank test (B, K, and R). Source data are provided as a Source Data file.
We also constructed Igf2bp3 KO mice by deleting a 161nt DNA fragment of exon 6 using CRISPR/Cas9 technology to further investigate the role of Igf2bp3 in AML initiation/development (Figs. 4H and S4D). Efficient deletion of Igf2bp3 was confirmed through genotyping, western blot analysis, and Sanger sequencing (Fig. S4E–G). Consistent with the results of Igf2bp3 KD, homozygous deletion of Igf2bp3 significantly suppressed MA9-mediated colony formation (Fig. 4I). Moreover, Igf2bp3 KO remarkably inhibited engraftment of donor cells in the recipient mice (Fig. 4J), leading to the significant delay of AML onset and substantially elongated survival in the recipient mice (Fig. 4K). Consistently, mice transplanted with Igf2bp3 KO donor cells displayed much lower leukemia burden in the BM, PB, livers and spleens (Fig. 4L–N). Consistent to Igf2bp3 KD or KO, KD of Atf4, Phgdh or Psat1 remarkably suppressed MA9-mediated colony-forming (Fig. S4H, I). Similar to what has been found in human AML cells (Fig. 3L), ectopic expression of ATF4, PHGDH, or PSAT1 partially rescued Igf2bp3 KO-induced reduction in the colony-forming ability of MA9-transduced HSPCs (Figs. 4O and S4J), further confirming Atf4, Phgdh, and Psat1 as bona fide targets of Igf2bp3. These data together suggest that Igf2bp3 promotes the initiation/development of AML.
CFA and secondary BMT assays were then conducted to determine the role of Igf2bp3 and its target genes in AML maintenance (Fig. 4P). As expected, Igf2bp3 KD dramatically inhibited colony-forming ability of leukemic BM blast cells from MA9 leukemia mice (Fig. 4Q) and significantly prolonged the survival in the secondary BMT recipient mice (Fig. 4R). In agreement, KD of Atf4, Phgdh, or Psat1 also significantly impaired the colony-forming of MA9 leukemic BM cells (Fig. S4K, L). These data imply that Igf2bp3 and the SSP are critical not only for the initiation/development but also for the maintenance of AML.
IGF2BP3 is essential for the self-renewal of AML LSCs/LICs
Given the critical role of IGF2BP3 in AML, we analyzed the TNMplot database (https://tnmplot.com/analysis/) and observed that the mRNA level of IGF2BP3 is significantly higher in AML patients compared to healthy controls (Fig. 5A). We further checked its expression in LSCs/LICs because they are responsible for chemoresistance, relapse, and unfavorable outcomes of AML patients. Expression level of IGF2BP3 was significantly higher in CD34+ immature AML cells than that in CD34- blast cells, while both are significantly higher than that in normal HSCs (Fig. 5B, A). Importantly, functionally defined LSCs expressed higher levels of IGF2BP3 than their non-LSC counterparts (Fig. 5C). Based on these data, we speculated that IGF2BP3 may also play a role in the stemness maintenance of LSCs/LICs. We then sorted CD34+ cells from patient-derived AML cells and conducted loss-of-function studies (Fig. 5D). As expected, KD of IGF2BP3 resulted in dramatic inhibition of cell proliferation, induction of cell apoptosis, and a remarkable suppression of colony formation of CD34+ AML cells (Figs. 5E–G and S5B).
A Expression of IGF2BP3 mRNA in AML patient samples and healthy donors from the TNMplot database. Box plot, center line, median; box limits, upper and lower quartiles; whiskers, 1.5× interquartile range. n = 407 healthy samples; n = 151 AML samples. B Expression of IGF2BP3 in CD34+ and CD34- cells from BM of healthy donors (normal) or AML patients as detected by microarray (GSE30029). n = 31 CD34+ normal samples; n = 46 CD34+ AML samples; n = 44 CD34- AML samples. C Expression of IGF2BP3 in blast (LSC−, non-engrafting) or LSC (LSC+, engrafting) cells from AML patients (GSE199452). n = 40 LSC− samples; n = 70 LSC+ samples. D Experimental scheme for (E–G). Patient-derived CD34+ cells were transduced with shNS or shRNAs targeting IGF2BP3 and subjected to different assays. E–G Growth curves (E), percentages of Annexin V+ apoptotic cells (F), and colony numbers (G) of patient-derived CD34+ leukemia cells with or without IGF2BP3 KD. Bar = 200 μm. H The percentage of L-GMP population (CD45.2+Lin-c-kit+Sca1-CD34+CD16/32+) in BM of the primary BMT mice. n = 6 mice per group. I, J Statistics of the percentage of Annexin V+ (I) or Ki67+ (J) L-GMPs of mice in (H). n = 6 mice. K In vitro LDA assays using MA9-transduced HSPCs from Igf2bp3 WT and KO mice. Logarithmic plots show the percentage of nonresponding wells (those without any colonies) at different doses of cells seeded. The estimated LSC/LIC frequency is calculated by ELDA and shown. L, M In vitro LDA assays of mouse HSPCs co-transduced with MA9 plus shRNA targeting Igf2bp3 (L) or its target genes (M). N HSPCs collected from Igf2bp3 WT and HO mice were co-transduced with MA9 and target gene-overexpressing viruses and selected in methylcellulose medium with G418 and puromycin for 7 days before seeded for LDA assays. Mean ± SD values are shown. n = 2 biologically independent repeats in E, G while n = 3 biologically independent repeats in (F). Statistical analysis: Two-tailed student’s t-test (A, C, F, H–J); two-way ANOVA (B); One-sided Chi-squared test (K–N). Source data are provided as a Source Data file.
We next examined the population of leukemic granulocyte-monocyte progenitors (L-GMPs), which have been widely recognized as mouse LSCs of MA9-induced AML, in the BM of our primary BMT recipients shown in Fig. 4H. KO of Igf2bp3 caused a markedly decrease in the percentage of L-GMPs (Figs. 5H and S5C), likely attributed to the increased apoptosis and reduced proliferation of L-GMPs (Figs. 5I, J and S5D, E). An in vitro limiting dilution assay (LDA) was further conducted to evaluate the effect of Igf2bp3 depletion on the in vitro stemness. In line with the observed changes in the L-GMP population, KO of Igf2bp3 significantly decreased cell stemness (Fig. 5K). Consistent with Igf2bp3 KO, KD of Igf2bp3 or its target gene Atf4, Phgdh, or Psat1 also markedly reduced stemness in MA9-transformed mouse HSPCs (Fig. 5L, M). Furthermore, ectopic expression of Atf4, Phgdh, or Psat1 could partially reverse the reduction of stemness caused by Igf2bp3 KO (Fig. 5N). Therefore, these results demonstrate that IGF2BP3 is essential for the maintenance and function of leukemia stemness through regulating SSP.
Igf2bp3 is dispensable for normal hematopoiesis
To evaluate the safety of IGF2BP3 as a potential therapeutic target for AML, we examined the impact of Igf2bp3 deletion on normal hematopoiesis (Fig. 6A). Compared to their wild-type (WT) siblings, mice with homozygous deletion of Igf2bp3 showed no significant difference on the cell number of all mature blood cell types in PB (Fig. 6B–D). In addition, the percentages of HSPCs, including Lin--Sca-1+-Kit+ (LSK) cells, long-term HSC (LT-HSC), short-term HSC (ST-HSC), and different types of progenitor cells, as well as lineage committed cells including myeloid cells, B cells, and T cells in the BM of Igf2bp3 KO mice were also similar to those in their WT siblings (Fig. 6E–I). Furthermore, we performed competitive BMT assays (Fig. 6J) and found that Igf2bp3 KO has little effect on the BM repopulation ability of HSCs (Fig. 6K–N). In line with these findings, IGF2BP3 and its SSP-related target genes are expressed at low levels across various tissues and organs, particularly in whole blood, in adult humans (Fig. S6). Collectively, these data demonstrate that deletion of Igf2bp3 does not affect HSC and multilineage development during steady-state hematopoiesis and under the stress of transplantation, highlighting Igf2bp3 as a safe therapeutic target.
A Schematic outline of experimental strategy testing the effect of Igf2bp3 KO on mouse static normal hematopoiesis. n = 7 mice per group. B–D PB analysis of Igf2bp3 WT and KO mice. The density of white blood cells (WBC), lymphoma cells (LYM), red blood cells (RBC) (C), palates (PLT) (D), neutrophils (NEUT), monocytes (MONO), and eosinophils (EO) (E) are shown. E Flow cytometric gating strategies for HSC and progenitors. F, G Frequencies of various hematopoietic progenitors in the BM of Igf2bp3 WT and KO mice as examined by flow cytometry. LSK, Lin-Sca1+cKit+ cells; MPP, multipotent progenitor (Lin-Sca1+cKit+CD48+CD150-); LT-HSC, long-term HSC (Lin-Sca1+cKit+CD48-CD150+); ST-HSC, short-term HSC (Lin-Sca1+cKit+CD48-CD150-); LMPP, lymphoid-primed multipotent progenitor (Lin-Sca1highcKithighCD127+CD135+); CLP, common lymphoid progenitor (Lin-Sca1lowcKitlowCD127+CD135+); CMP, common myeloid progenitor (Lin-Sca1-cKit+CD34intCD16/32low); GMP, granulocyte-monocyte progenitor (Lin-Sca1-cKit+CD34+CD16/32high); MEP, megakaryocyte–erythroid progenitor (Lin-Sca1-cKit+CD34-CD16/32-). H Flow cytometric gating strategies for mature cells. I Frequencies of various mature cells in the BM of Igf2bp3 WT and KO mice as examined by flow cytometry. J Schematic outline of competitive repopulation assay. n = 7 mice per group. K Flow cytometric gating strategy for BM cells derived from Igf2bp3 WT or KO donor mice in the competitive repopulation assay. L Flow cytometry analysis for different donor-derived cells (CD45+) in PB of recipient mice 4, 8, 12, and 16 weeks after BMT. M, N Percentage of donor-derived stem cell (M) and progenitor (N) compartments in the bone marrow of recipients 16 weeks after BMT. Mean ± SD values are shown. Statistical analysis: Two-tailed student’s t-test (B–D, F, G, I, L–N); n.s., not significant. Source data are provided as a Source Data file.
IGF2BP3 suppression in combination with SG restriction exhibits potent anti-AML efficacy in vitro and in vivo
The afore-mentioned data have suggested the potential of IGF2BP3 as a therapeutic target for AML treatment, which is also supported by the finding that higher expression of IGF2BP3 is associated with poor outcomes in AML patients (Fig. S7A, B). Based on our findings that PHGDH inhibition sensitized AML cells to SG deprivation and that IGF2BP3 acts as a regulator of the SSP, we reasoned that suppression of IGF2BP3 would achieve potent anti-leukemia effects when combined with SG deprivation. Indeed, IGF2BP3 KD in patient-derived AML cells resulted in a more substantial inhibition of cell growth and the colony-forming ability in vitro when combined with SG deprivation (Figs. 7A, B and S7C–E), and led to a more prolonged survival of recipient mice when the SG were restricted from the diet (Fig. 7C). In addition, KD of Igf2bp3 showed a synergistic effect with SG restriction in inhibiting the colony-forming capability of BM cells from MA9-induced leukemic mice (Figs. 7D, E and S7F). In line with the in vitro results, recipient mice transplanted with Igf2bp3 KD cells had prolonged survival than those transplanted with control cells, and the survival was further improved when fed with SG-free diet than with normal diet (Fig. 7F). We also performed xenotransplantation assays by injecting the U937 BP3-mAID cells into immunodeficient mice through tail vein and induced degradation of IGF2BP3 in vivo with 5’Ph-IAA treatment (Fig. 7G). Consistently, SG starvation or 5’Ph-IAA treatment significantly prolonged the survival of recipient mice, while their combination further delayed leukemia onset and improved mice survival (Fig. 7H). Overall, these results provide proof-of-concept evidence that exogenous SG limitation in combination with IGF2BP3 depletion/inhibition represents a promising therapeutic approach for AML treatment.
A Experimental scheme for (B) and (C). M-NSG immunodeficient recipient mice were transplanted with AML PDX cells (FLT3-TKD mutation) transduced with shNS or shRNA targeting Igf2bp3, and fed with a regular (control) or SG-free (-SG) diet. B Colony numbers of AML PDX cells (FLT3-TKD) are shown to reflect the effect of SG deprivation (-SG) on control (NS) or Igf2bp3 KD (shBp3) cells compared to complete medium (CM). C Kaplan–Meier curves showing survival of recipient mice fed with a regular (control) diet or an equivalent diet lacking SG (-SG). n = 7 mice per group. D Experimental scheme for (E) and (F). BM cells from MA9 leukemic mice were transduced with shNS or shRNA targeting Igf2bp3 and selected with puromycin before subjected to CFA assays (E) or BMT assays (F). E Colony numbers in CFA assays are shown to reflect the effect of SG deprivation (-SG) on control (NS) or Igf2bp3 KD (shBp3) cells compared to complete medium (CM). F Kaplan–Meier curves showing survival of recipient mice fed with a regular (control) diet or an equivalent diet lacking SG (-SG). n = 8 mice per group. G Experimental scheme of the xenotransplantation assays in (H). M-NSG immunodeficient recipient mice were transplanted with U937 BP3-mAID cells and fed with a regular (control) or SG-free (-SG) diet. 5’Ph-IAA (3 mg/kg) or vehicle control (DMSO) were given via intraperitoneal injection (i.p.) every day for 7 consecutive days starting from the 8th day after transplantation. H Kaplan–Meier curves showing survival of the M-NSG recipient mice in (G). n = 7 mice per group. I Schematic illustration of the working model and therapeutic strategy proposed in the study, created with the assistance of GDP (Agreement number: GDP2025KABQ7W). Mean ± SD values are shown. n = 2 biologically independent repeats in (B) and (E). Statistical analysis: log-rank test (C, F, and H). Source data are provided as a Source Data file.
Discussion
Accumulating evidence has established serine metabolism as one of the central hubs for cancer metabolism; however, its regulatory mechanism in AML remains incomplete. By performing CRISPR screening in SG-depleted medium versus complete medium, we demonstrated that loss of m6A-related proteins IGF2BP3 and METTL14 sensitized AML cells to SG deprivation. Through metabolomics, transcriptomics, and integrative analyses of m6A-seq and RIP-seq data, we uncovered that IGF2BP3 promotes SSP and serine metabolism through enhancing the mRNA stability and expression of ATF4, PHGDH, and PSAT1 as an m6A reader in AML. Furthermore, we showed that IGF2BP3 deficiency augments the therapeutic efficacy of SG limitation in AML mice, highlighting the great potential of combining SG deprivation with IGF2BP3 inhibition for AML treatment (Fig. 7I). It should be noted that besides IGF2BP3, other genes may also play roles in regulating serine metabolism, such as SIRT4 that affects glutamine metabolism55 and YBX1 that was shown to be a cofactor of IGF2BP proteins and plays a role in the growth and clonogenic potential of AML cells56,57.
Given the critical role of serine for cancer cell survival, dietary SG deprivation or inhibition of serine synthesis have been tested in both cultured cancer cells and tumor-bearing mice for their anti-cancer efficacies46,58. Because serine taken up from the extracellular milieu may be able to support cell survival, we combined IGF2BP3 KD/depletion with SG deprivation and demonstrated that IGF2BP3 KD/depletion could sensitize AML cells to SG deprivation in vitro and in vivo, provides proof-of-concept evidence that targeting IGF2BP3 is a potent therapeutic strategy for AML, especially when combined with dietary SG deprivation. Further studies are warranted to develop effective inhibitors specifically targeting IGF2BP3 for potential combined therapies.
The oncogenic roles of IGF2BP3 have been reported in several types of cancers, including breast cancer, colorectal carcinoma, and lung cancer59. In AML, it was shown that KD of IGF2BP3 resulted in inhibition of cell proliferation and induction of apoptosis in AML cells lines60,61. However, whether IGF2BP3 acts as an m6A reader and what the role and mechanism of IGF2BP3 in LSCs/LICs self-renewal and in vivo leukemogenesis remain to be addressed. In the present study, we utilized genetic KO/KD as well as acute degradation of IGF2BP3 to study its function and mechanism in AML. By using mutated IGF2BP3 that loses m6A binding ability and modulating METTL14 expression to adjust cellular m6A level, we demonstrated that the oncogenic role of IGF2BP3 relies on its activity as an m6A reader. Our mechanistic studies revealed that ATF4, PHGDH, and PSAT1 are functionally important targets of IGF2BP3 and could be stabilized (and likely more actively translated) via binding of IGF2BP3 to the m6A sites in their mRNAs, thereby promoting serine biosynthesis and leukemogenesis. It is possible that inhibition of SSP by IGF2BP3 KD leads to inactivation of mTORC1 signaling and global suppression of protein synthesis62. Noticeable, our data prove that IGF2BP3 is critical for the maintenance of LSCs/LICs while its depletion does not affect normal hematopoiesis, consistent with previous reports that IGF2BP3 is an oncofetal protein59 and further suggesting it as an attractive target for AML therapy.
Recently, we and others have reported the direct or indirect participation of m6A modification in the regulation of glycolysis, glutaminolysis, branched-chain amino acids metabolism, and fatty acids metabolism in AML40,41,42,43. Our present study that m6A also plays a role in controlling serine biosynthesis by regulating the expression of key rate-limiting enzymes in this process provides further evidence that m6A modification has been extensively utilized to reprogram cancer cell metabolism. Because the metabolic processes are intertwined, it is of interest to investigate whether other metabolic events are controlled by m6A in AML and other cancer types.
Overall, our study uncovers an m6A-related mechanism on the regulation of the SSP and serine biosynthesis in AML, in which IGF2BP3 plays a critical oncogenic role to promote AML leukemogenesis and LSCs/LICs self-renewal. In addition, we provide proof-of-concept evidence that targeting the SSP, either through suppressing IGF2BP3 or PHGDH, exhibits potent anti-leukemia efficacies when combined with dietary SG restriction. Further studies are needed to identify IGF2BP3 inhibitors with the hope of developing combined therapeutic approaches for AML therapies. IGF2BP3 depletion impairs serine biosynthesis and dampens AML cells, including LSCs/LICs, while having no significant influence on normal hematopoiesis, making it an attractive target for AML therapy.
Methods
Mice and animal housing
The Igf2bp3 knockout (Igf2bp3−/−) mice in the C57BL/6 background were generated in the animal facility of Cincinnati Children’s Hospital Medical Center, and the Mettl14 inducible knockout mice (Mettl14fl/flCreERT mice) in the C57BL/6 background was kindly provided by Dr. Chuan He at the University of Chicago. C57BL/6 (CD45.2) mice were purchased from GemPharmatech Co. Ltd (Nanjing, China), and B6.SJL (CD45.1) mice were purchased from The Jackson Laboratory. All laboratory mice were maintained in the animal facilities at City of Hope (COH), Ruiye Bio-tech Guangzhou Co.Ltd (Ruiye), or Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences (GIBH). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of COH, Ruiye, or GIBH.
Mouse bone marrow transplantation (BMT)
The BMT assays were conducted as previously described with minor modifications40. Briefly, MA9-transformed colony cells were transplanted via tail vein injection into lethally irradiated (5 Gy plus 5 Gy) 7- to 9-week-old male or female B6.SJL (CD45.1) or C57BL/6 (CD45.2) recipient mice. For each recipient mouse, 0.1–0.2 × 106 donor cells from CFA assays and a whole bone marrow cell (1 × 106) freshly harvested from a B6.SJL (CD45.1) or C57BL/6 (CD45.2) mouse were transplanted together as helper cells. For secondary BMT, BM cells from primary leukemic mice were transduced with shRNAs targeting Igf2bp3 or control shRNA (shNS). The cells were then subjected to colony-forming assays (CFAs) and harvested for transplantation into sublethally irradiated (4.5 Gy) 7- to 9-week-old B6.SJL (CD45.2) recipient mice. Leukemic mice were euthanized by CO2 inhalation when they exhibited signs of systemic illness (e.g., weight loss > 15%, reduced mobility, abnormal posture, respiratory distress, and bleeding tendencies). BM cells were isolated from both the tibia and femur, and 50,000 cells were loaded for cytospin preparation after lysis of red blood cells. BM cytospin and blood smear slides were stained with Wright-Giemsa (Polysciences). Portions of the spleen and liver from leukemic mice were collected, fixed in formalin, and embedded in paraffin before being sectioned and stained with hematoxylin and eosin (H&E).
Leukemic patient samples and samples of normal mononuclear cells (MNCs)
The leukemic samples from patients with FLT3-TKD mutation or MLL-rearranged AML were obtained at the time of diagnosis or relapse, with informed consent, from the First Affiliated Hospital of Jinan University and Sun Yat-sen University Cancer Center (SYSUCC). These samples were approved by the institutional review boards of the hospitals. Leukemia mononuclear cells (MNCs) were purified using Ficoll-Paque PLUS (GE Healthcare Life Sciences). CD34+ leukemia cells were purified using CD34+ beads (Miltenyi Biotec). Normal MNCs were purified from cord blood of healthy donors from the First Affiliated Hospital of Jinan University using Ficoll-Paque PLUS.
Cell culture
Molm13, NB4, and U937 cells were maintained in regular RPMI-1640 medium (Invitrogen, Carlsbad, CA) containing 10% FBS, 2 mM L-Glutamine, and 1% penicillin–streptomycin. HEK293T cells were maintained in regular DMEM medium (Invitrogen, Carlsbad, CA) containing 10% FBS, 2 mM L-Glutamine, and 1% penicillin–streptomycin. MM6 cells were cultured in regular RPMI-1640 medium containing 2 mM L-Glutamine, 1×Non-Essential Amino Acid, 1 mM sodium pyruvate, and 9 mg/ml insulin (Invitrogen). Primary CD34+ cells derived from patient-derived xenograft (PDX) AML models were cultured in IMDM medium supplemented with 20% FBS, 1% penicillin–streptomycin and 10 ng/ml each of SCF, TPO, Flt-3L, IL-3, and IL-6. HEK293T and U937 were obtained from American Type Culture Collection (ATCC). Molm13, MM6, and NB4 were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ).
Plasmid construction
Wild-type and KH3-4 domain mutant human IGF2BP3 sequences were PCR amplified from corresponding pcDNA3-based vectors, which were gifted by Hüttelmaier (Martin Luther University, Germany). The amplified sequences were then subcloned into the cl20c-N-2×FLAG-GFP lentiviral vector using homologous recombination. Human ATF4, PHGDH and PSAT1 coding sequences were reverse-transcribed and PCR amplified from total RNA of Molm13, and subcloned into the cl20c-N-2×FLAG-GFP lentiviral vector. shRNA vectors targeting human or mouse IGF2BP3, ATF4, PHGDH, and PSAT1 were constructed by synthesizing shRNA-encoded DNA oligos and cloning them into the pLKO.1 vector (Addgene). Mature antisense sequences of shRNAs were listed in Table S2 in the Supplementary Data 1 file. IGF2BP3 sgRNA oligonucleotides were synthesized and cloned into the ipUSEPR lentiviral sgRNA vector. All gRNA sequences were listed in Table S2 in the Supplementary Data 1 file.
The sequences coding OsTRI(F74G) were synthesized and subcloned into the cl20c-N-2×FLAG-puro lentiviral vector. sgRNA targeting IGF2BP3 were synthesized and cloned into Px330 (Addgene). The donor plasmids contain C-terminal (last exon) IGF2BP3, mAID2-tag and T2A-neomycin or T2A-blasticidin coding sequence. Homology sequences to the C-terminal end of IGF2BP3 was PCR amplified from U937 genomic DNA and ligated to pUC57, which was used to generate the pUC57-mAID-NeoR/BSD donor template.
Cell growth and apoptosis assays
The cell proliferation/growth was assessed by counting cells using AO/PI staining. For apoptosis assays, we used the FITC Annexin V Apoptosis Detection Kit 1 (BD Biosciences, San Diego, CA) following the manufacturer’s instructions. In brief, cells were washed twice with cold PBS, lysed with 1× binding buffer, stained with anti-Annexin V and propidium iodide (PI) for 15 min at room temperature (RT), and subjected to flow cytometric analysis on a BD LSRFortessa X-20 analyzer (BD Biosciences).
Virus preparation and infection
Virus preparation and infection were performed as previously described with some modifications40,53,63. Briefly, retroviruses or lentiviruses were produced in HEK293T cells by co-transfection of individual expression construct with the pCL-Eco packaging vector (IMGENEX, San Diego, CA) or the pMD2.G:pPAX2 packaging mix (individually purchased from Addgene), respectively. The virus particles were harvested at 48 h after transfection and added to cells, with or without concentration. Spinoculation was performed to allow the infection of viruses. In some cases, when co-infecting AML cells with overexpression and shRNA viruses, overexpression viruses were spined down first in RetroNectin-coating plates before cells and shRNA viruses were added for spinoculation.
CRISPR/Cas9-based screening
Guide RNA Oligos were synthesized by synbio-tech company and cloned into the ipUSEPR lentiviral sgRNA vector. Molm13 Cas9 cells were infected with the sgRNA pool lentiviruses at an MOI of <0.3, puromycin selected for 5 days, and an initial gDNA sample was collected. Cells were further grown in CM or -SG medium for 8, 12, or 17 population doublings. 5–10 million cells were collected for each population followed by genomic DNA (gDNA) extraction using the Quick-DNA Midiprep Plus Kit (Zymo Research) according to manufacturer’s protocol. For library construction, gDNA was amplified for 25 cycles using VAHTS HiFi polymerase (Vazyme) and primer pairs that contain barcodes. PCR product was run on 2% agarose gel, and the ~260- to 270-bp product was cut out for DNA recovery using the QIAquick Gel Extraction Kit (Qiagen). Barcoded libraries were then sequenced using the DNBSEQ-T7 instrument (MGI). The raw sequencing reads were trimmed using cutadapt v.4.1 to remove the constant flanking sequences of sgRNA sequences64. Read counts of the sgRNAs were measured with mageck-vispr v.0.5.9.565 and then normalized using the control sgRNAs in the sgRNA libraries. The per-gene log2-fold changes of sgRNAs abundance and P-values between cell populations were calculated using mageck-vispr with the null distribution of RRA. The SG-dependent scores were calculated as log2(-SG/T0) - log2(CM/T0), with smaller values indicating a greater dependence on SG. Genes with SG-dependent scores less than −0.1 were used for further studies.
Generation of the IGF2BP3-mAID cell line
The generation of the IGF2BP3-mAID cell line was conducted as previously described51. In brief, U937 cells stably expressing OsTRI(F74G) were established. Then, U937 OsTRI(F74G) cells were electro-transfected with Cas9 encoding plasmid, IGF2BP3 sgRNA, and donor plasmids using the Neon transfection system (MPK5000, Invitrogen). Cells were cultured for 48–72 h with medium containing puromycin, neomycin, and blasticidin at 48 h post transfection. Single cells were plated into 96-well plates and neomycin/blasticidin resistant clones were eventually sub-cultured into 6-well plates and collected for Western blotting to detect mini-AID (mAID)-tag IGF2BP3 and endogenous IGF2BP3 expression levels, and genomic DNA PCR to detect homologous recombination.
Auxin‑induced degradation
5-Phenyl-1H-indole-3-acetic acid (5’Ph-IAA, HY-134653, MedChem Express) was dissolved in DMSO to prepare 10 mM stocks for cell culture or 6 mg/ml stocks for in vivo experiment. The stocks were then further diluted with DMSO to the desired concentration before the experiment. For inducing degradation of endogenous IGF2BP3 fused with mAID in vitro, 5’Ph-IAA was added directly to the culture medium at an appropriate concentration (1 μM) for indicated times. For inducing degradation of endogenous IGF2BP3 fused with mAID in vivo, 5’Ph-IAA was diluted with PBS to 0.6 mg/ml and injected intraperitoneal (i.p.) every day for 7 consecutive days.
In vitro colony-forming and replating (CFA) assay
Bone marrow (BM) cells were collected from 5- to 7-week-old wild-type, Mettl14fl/flCreERT, or Igf2bp3−/− mice, and BM progenitor (HSPC, i.e., Lin- or c-kit+) cells were enriched with the Mouse Lineage Cell Depletion Kit or the CD117 microbeads (Miltenyi Biotec). BM progenitor cells were co-transduced with various combinations of retroviruses or lentiviruses using “spinoculation”. The cells were then plated into mouse methylcellulose medium (1201, ReachBio or HSC006, R&D) supplemented with 10 ng/ml of human recombinant IL-6 (PeproTech), murine recombinant IL-3 (PeproTech), GM-CSF (PeproTech), and 50 ng/ml of murine recombinant SCF (PeproTech). For HSPCs from Mettl14fl/flCreERT, Cre-ERT2-IRES-GFP (Cre) or ERT2-IRES-GFP (EV; as a negative control) lentiviruses were used additionally to transduce cells. 4-OHT (1 mg/ml, Sigma-Aldrich), G418 (1.0 mg/ml, GIBCO BRL, Gaithersburg, MD) and/or puromycin (1.5 μg/ml, Sigma-Aldrich) were added when necessary. Cultures were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 5 to 6 days and serial replating was performed by collecting colony cells and replating them in methylcellulose medium every 5–6 days. Colonies were counted for each passage.
For CFA assays using human primary leukemic cells, PDX cells transduced with lentivirus were seeded into MethoCult H4434 Classic medium (StemCell Technologies) with the addition of 1.5 μg/ml puromycin when necessary. Cultures were incubated at 37 °C in a humidified atmosphere with 5% CO2 for 7–10 days before counting.
In vitro limiting dilution assay (LDA)
HSPCs from wild-type C57BL/6 mouse transduced with MLL-AF9 retroviruses together with relative lentiviruses as indicated were seeded into Mouse Methylcellulose Base Medium (HSC006, R&D) supplied with 10 ng/ml of human recombinant IL-6 (PeproTech), murine recombinant IL-3 (PeproTech), GM-CSF (PeproTech) and 50 ng/ml of murine recombinant SCF (PeproTech), along with 1.5 mg/ml of puromycin (Sigma-Aldrich). Five days later, the colony cells were collected and replated into 96-well plates with six different cell number doses for each group. The number of wells with MLL-AF9 colonies was counted 7 to 10 days later, and the estimated frequencies of LSCs/LICs were calculated using the ELDA software.
Flow cytometric analysis
Flow cytometry analysis of mouse BM cells was conducted as previously described with some modifications19,40. Cells from BM of transplanted mice were harvested for analysis. After blocking nonspecific binding with affinity-purified anti-mouse CD16/32 (1:1000, ThermoFisher Scientific), cells were stained at RT with various antibodies diluted in Flow Cytometry Staining Buffer (ThermoFisher Scientific) for 20 min at dark and resuspended in IC Fixation Buffer (ThermoFisher Scientific) before being loaded for flow cytometry analysis in BD FACS FortessaX-20.
For the hematopoietic and leukemia stem cell and mature cell analysis, suspended single cells were prepared from bone marrow, spleen and peripheral blood. Red cells were lysed using ACK LYSING Buffer (BD) before staining for all FACS analysis. Cells were incubated with antibodies in FACS buffer at room temperature (RT) for 20 min in the dark. All antibodies were purchased from Biolegend. FITC anti-mouse Lineage Cocktail (5 μl/test), anti-Sca1-PerCP/Cy5.5 (1 μl/test), c-Kit-APC/Cy7 (1 μl/test), CD34-APC (1 μl/test), and CD16/32-PE (1 μl/test) antibodies are used for the analysis of the leukemic stem cell L-GMP population or hematopoietic progenitor cell (HPC) population. Pacific-blue Annexin V was used to examine the apoptosis of L-GMPs, while Brilliant Violet 650-Ki67 was used for evaluation of the cell proliferation of L-GMPs. For hematopoietic stem cell (HSC) analysis, anti-Sca1-PerCP/Cy5.5 (1 μl/test), c-Kit-APC/Cy7 (1 μl/test), CD48-APC (1 μl/test), and CD150-PE (1 μl/test) antibodies were employed. All cells were analyzed by flow cytometry on the LSR FortessaX-20 (BD).
RNA extraction and real-time qPCR (RT-qPCR) analysis
Total RNA was isolated using the TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. For cDNA synthesis, 500–1000 ng of total RNA was reverse-transcribed into cDNA in a total reaction volume of 20 μl using HiScript III RT Super-Mix for qPCR (+gDNA wiper) (Vazyme, China) according to the manufacturer’s instructions. Quantitative real-time PCR (qPCR) was then performed in a 10 μl reaction volume system containing 0.5 μl diluted cDNA using Applied Biosystems PowerUp SYBR Green Master Mix (Thermo Fisher) on the QuantStudio 7 Flex PCR system (Thermo Fisher Scientific). ACTB was used as an internal control for gene expression evaluation, while 18S rRNA was used as an internal control for RNA stability assays.
RNA-seq and data analysis
Total RNA from Molm13 cells with or without IGF2BP3 KD or from U937 cells with or without IGF2BP3 degradation (BP3-mAID cells cells treated with 5’Ph-IAA) was isolated using the TRIzol reagent (Thermo fisher Scientific). Library construction of 1 μg RNA per sample was made using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, USA) following manufacturer’s recommendations and sequenced on an Illumina NovaSeq platform in pair-end read mode with 150 bp per read as previously described40. Briefly, PCR products were purified (AMPure XP system) and the quality of library was assessed on the Agilent Bioanalyzer 2100 system. The raw RNA-seq reads were quality checked by FastQC v.0.11.566 and trimmed by cutadapt v.4.164 to remove low quality bases and adaptor sequences. The clean reads were then mapped to the human genome (hg38) with HISAT2 v.2.1.167 supplying the GENCODE Release 39 gene annotation. A post-alignment filter was preformed to remove alignments to ribosomal RNA (rRNA) and non-concordant mate pairs. Read counts on genes were calculated by HTSeq v.2.0.268 against the GENCODE Release 39 gene annotation. Normalization and differential expression analysis were performed using DESeq2 v.1.40.269 with an absolute fold change >1.5 or <0.67, and adjusted P-value < 0.05. Gene ontology (GO) analysis of designated gene set was carried out with Metascape v.3.5.2023050170. Protein-Protein Interaction (PPI) Networks Functional Enrichment Analysis was performed by STRING v.11.571.
Metabolite extraction and mass-spectrometry-based metabolomics analysis
Molm13 cells with or without IGF2BP3 KD were cultured in their regular culture medium without glucose but supplemented with U-[13C6]glucose (25 mM, Cambridge Isotope Laboratories, Inc.). 24 h later, 1 × 106 cells were collected and rinsed with 150 mM NH4Ac solution (pH 7.3), and 100 μl of cold 80% methanol (Optima* LC/MS, ThermoFisher Scientific) was added to the cells. 1 nmol norvaline (Sigma-Aldrich) was added to each sample as internal standard. Samples were then vortexed for 30 sec and incubate the tubes for 30 min at −80 °C. Place the samples on ice to warm up, then vortex the samples again, and spin the samples in a microcentrifuge at top speed for 10 min at 4 °C. The supernatant was transferred to a new tube, and the pellet was resuspended in 50 μl cold 80% methanol for second extraction. The combined 150 μl supernatant was dried using the Vacufuge Plus (Eppendorf) at 30 °C. Extracted metabolite samples were stored at −80 °C.
The mass spectrometry-based analysis of extracted metabolites was conducted at Metabolomics Facility of Guangzhou National Laboratory. Metabolites were resuspended in 50 μl of “extraction solution” (Acetonitrile/Methanol/Aqueous = 4:4:2). The mixture was kept in ice to sonicate for 5 min followed by centrifugation at 12,000 × g for 5 min and one-10th of the sample was analyzed with an Agilent 1290II ultra-high-pressure liquid chromatography (UHPLC) system equipped with 6546 quadrupole time-of-flight (QTOF) mass spectrometry. A Waters ACQUITY UPLC BEH Amide column (2.1 mm × 100 mm × 1.7 μm) and guard column (2.1 mm × 5 mm × 1.7 μm) at 35 °C was used to separate metabolites with mobile phase A: 100% aqueous containing 15 mM ammonium acetate and 0.3% ammonium hydroxy and mobile phase B: 90% acetonitrile (v/v) aqueous containing 15 mM ammonium acetate and 0.3% ammonium hydroxy. The linear gradient was set as follows: 10% A (0.0–8.0 min), 50% A (8.0–10.0 min), 50% A (10.0–11.0 min) and 10% A (11.0–20.0 min). The total running time was 20 min and flow rate was 0.3 ml/min. The mass spectrometer was equipped with Agilent Jet-stream source operating in negative and positive ion mode with source parameters set as follow: Nebulizer gas, 45 psi; Sheath gas temperature, 325 °C; Sheath gas flow, 10 L/min; Dry gas temperature, 280 °C; Dry gas flow, 8 l/min; Capillary voltage, 3500 v for two ion modes and nozzle voltage, 500 v for positive and 1000 v for negative mode. The QTOF scan parameters were set as follows: Scan speed, 1.5 scan/s; scan range, 50–1700 m/z and ion fragmentor voltage, 140 v. The acquired data quality was monitored by Amino Acid standards (Merck, Sigma-Aldrich Production GmbH, Switzerland), mix-samples quality controls and blanks. Peak integration and metabolite isotopologue identification was accomplished using Profinder 10.0 (Agilent). The variance of samples was analyzed with F-test and the significance of abundance changes of metabolites between control and knockdown groups was estimated by two-tailed student’s t-test. Compounds with p-value < 0.05 were defined as differential metabolites and kept for downstream analysis. MetaboAnalyst v.5.072 was then used to analyze the enriched metabolic pathways of significantly changed metabolites with default parameters.
Intracellular serine abundance detection
The intracellular serine level was measured using the DL-serine assay kit (abcam) according to the manufacturer’s instructions. In brief, cells were cultured with serine- and glycine depleted medium. One million cells were collected followed by homogenized on ice with 100 μl ice cold Assay Buffer LII/Serine Assay Buffer. Samples were centrifuge at 15,000 × g for 10 min at 4 °C, and the supernatant was collected and pretreated with Sample Cleanup Mix and deproteinized. 10 μl of each pretreated sample was used for final detection. The fluorescence of all sample wells was measured at Ex/Em = 535/587 nm in endpoint mode in a Cytation 5 luminometer (BioTek Instruments, Inc).
Western blot assay
Cells were counted and washed twice with PBS, followed by lysis in 1×SDS buffer (100 μl for 1 × 106 cells) at 95 °C for 10 min. Equal volumes of cell lysates were separated by SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with 5% non-fat milk, incubated sequentially with primary and secondary antibodies, and detected by immunoblotting with the ECL Western Blotting Substrate (Thermo Fisher Scientific). Antibodies used for western blotting were as follows: IGF2BP3 (1:1000, A303-426A, Bethyl Laboratories, or 14642-1-AP, Proteintech), METTL14 (1:1000, HPA038002, Sigma-Aldrich), ATF4 (1:1000, 11815S, Cell Signaling Technology), PHGDH (1:1000, 14719-1-AP, Proteintech), PSAT1 (1:1000, 10501-1-AP, Proteintech), FLAG (1:1000, 14793S, Cell Signaling Technology), GAPDH (1:1000, M171-3, MBL International), β-Actin (1:1000, 3700, Cell Signaling Technology). GAPDH or β-Actin (ACTB) was used as a loading control. Statistical analysis of western blotting was analyzed using the ImageJ software. Uncropped scans were provided in the Source Data file and the Supplementary Information file.
RNA immunoprecipitation (RIP)
RNA immunoprecipitation was performed as previously described with some modifications40. In brief, 10 million cells were harvested and washed with cold PBS, followed by two rounds of 254 nm UV crosslinking. Cells were lysed with 1 ml of RIP buffer (150 mM KCl, 25 mM Tris, pH7.4, 5 mM EDTA, 0.5 mM DTT, 0.5% IGEPAL, 100 U/ml RNAase inhibitor SUPERasein, and cocktail proteinase inhibitors), and sonicated for 10 cycles with 30 s on and 30 s off for each cycle using Bioruptor (Diagenode). 10% of lysate was saved as input. The remaining lysate was pre-cleared with protein A/G magnetic beads, and then subjected to immunoprecipitation using anti-IGF2BP3 antibodies at 4 °C for 2–4 h, followed by incubation with protein A/G magnet beads at 4 °C overnight with rotation. Beads were washed with RIP buffer for three times. Immunoprecipitated samples were subjected to DNase and Proteinase K digestion, followed by total RNA isolation (in parallel with the input samples) using the TRIzol reagent. The RNAs were further analyzed by qPCR using primers listed in Table S2 in the Supplementary Data 1 file.
Gene-specific m6A qPCR
Gene-specific m6A was tested begin with low input m6A-MeRIP. 20 μg of DNase-treated total RNA was sheared to approximately 200 nt in length by metal-ion induced fragmentation and subsequently purified. The fragmented RNA was incubated with m6A antibody (ab151230-100ug, Abcam)- or rabbit IgG-conjugated Protein A/G Magnetic beads in 500 μl 1× IP buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.5, 0.1% IGEPAL CA-630) supplemented with RNase inhibitors at 4 °C overnight. Methylated RNAs were washed, eluted with TRIzol, and purified with kit (Zymo, Direct-zol RNA Miniprep Plus, R2072). One tenth of fragmented RNA was saved as input control, and further analyzed by qPCR along with the MeRIPed RNAs using primers listed in Table S2 in the Supplementary Data 1 file. The enrichment of m6A in each sample was calculated by normalizing the amplification cycle (Cq) value of the m6A-IP portion to the Cq value of the corresponding input portion.
Bst DNA polymerase-mediated cDNA extension and qPCR (BST-qPCR)
The experiment was performed as previously described40. Briefly, total RNA was isolated from Molm13 cells with or without METTL14 depletion. BST DNA polymerase-mediated cDNA extension was performed at 60 °C for 40 min in a 25 μl reaction containing 200 ng of total RNA, 2.5 μl of 10× Isothermal Amplification Buffer II, 1.5 μl of MgSO4 (100 mM), 3.5 μl of dNTP Mix (10 mM), 1 μl of site-specific reverse primer, and 1.0 μl of Bst 3.0 DNA polymerase (8000 U/ml, New England Biolabs). The cDNAs were used for qPCR detection. The cDNAs reverse-transcribed from non-m6A site primers were used as internal controls. All the primers used in the qPCR are listed in Table S2 in the Supplementary Data 1 file.
RNA stability assays
Molm13 cells with or without IGF2BP3 KD were treated with actinomycin D (A9415, Sigma-Aldrich) at a final concentration of 5 μg/ml and collected at the indicated time points. Total RNA was extracted using the EastepTM Super Total RNA Extraction Kit (Promega) and analyzed using qPCR assays. The half-life of mRNA was calculated as previously reported40.
Serine and glycine deprivation
For all serine and glycine-deprivation experiments, cells were cultured in RPMI-1640 without serine and glycine (DZPYG0062, BOSTER) supplemented with 10% FBS, 1% penicillin–streptomycin.
Drug treatment of AML mouse model
For serine/glycine-restriction experiments, animals were fed either with a specialized diet devoid of serine and glycine, which was custom-formulated by Trophic Animal Feed High-Tech, or a control diet that contains all essential amino acids. Mice were maintained on each diet after transplantation and maintained on their respective diets until experimental end point.
Xenotransplantation experiments
M-NSG female mice (8–10-week-old) were used as recipient mice for xenotransplantation experiments. When PDX cells (FLT3-TKD mutation) were used as donor cells, each M-NSG mouse was injected intraperitoneal with 20 mg/kg busulfan once, followed by intravenous injection with 1 × 106 PDX cells transduced with shRNAs targeting Igf2bp3 or control shRNA (shNS) the next day. Mice were maintained on a diet lacking serine and glycine (-SG) or the regular diet after transplantation.
In addition, U937 BP3-mAID cells were used for transplanted via tail vein injection into M-NSG recipient mice. For each recipient mouse, 1 × 106 BP3-mAID cells were transplanted. Eight days post-transplantation, 5’Ph-IAA (3 mg/kg) was injected intraperitoneally to induce IGF2BP3 degradation every day for 7 consecutive days. Mice were maintained on a diet lacking serine and glycine (-SG) or the regular diet after transplantation.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data of RNA-seq and CRISPR/Cas9-based screening obtained in this study have been deposited in the NCBI BioProject under the accession number PRJNA1114628. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Dohner, H., Weisdorf, D. J. & Bloomfield, C. D. Acute myeloid leukemia. N. Engl. J. Med. 373, 1136–1152 (2015)
Kantarjian, H. et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 11, 41 (2021).
Chen, J., Odenike, O. & Rowley, J. D. Leukaemogenesis: more than mutant genes. Nat. Rev. Cancer 10, 23–36 (2010).
Shlush, L. I. et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 547, 104–108 (2017).
Hou, S., Liu, J. & Zhu, Y. Multi-omics advances for molecular characterization, precision medicine, and prognostic implications in leukemia. Cell Investig. 1, 100007 (2025).
Zeng, C. Advances in cancer treatment: the role of new technologies and research. Cell Investig. 1, 100001 (2025).
Faubert, B., Solmonson, A. & DeBerardinis, R. J. Metabolic reprogramming and cancer progression. Science 368, https://doi.org/10.1126/science.aaw5473 (2020).
Pavlova, N. N., Zhu, J. & Thompson, C. B. The hallmarks of cancer metabolism: still emerging. Cell Metab. 34, 355–377 (2022).
Jones, C. L. et al. Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell 34, 724–740.e724 (2018).
He, X. et al. Bone marrow niche ATP levels determine leukemia-initiating cell activity via P2X7 in leukemic models. J. Clin. Invest. 131, https://doi.org/10.1172/JCI140242 (2021).
Mattaini, K. R., Sullivan, M. R. & Vander Heiden, M. G. The importance of serine metabolism in cancer. J. Cell Biol. 214, 249–257 (2016).
Geeraerts, S. L., Heylen, E., De Keersmaecker, K. & Kampen, K. R. The ins and outs of serine and glycine metabolism in cancer. Nat. Metab. 3, 131–141 (2021).
Newman, A. C. & Maddocks, O. D. K. Serine and functional metabolites in cancer. Trends Cell Biol. 27, 645–657 (2017).
Jeong, S. et al. High fructose drives the serine synthesis pathway in acute myeloid leukemic cells. Cell Metab. 33, 145–159.e146 (2021).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376 (2017).
Bjelosevic, S. et al. Serine biosynthesis is a metabolic vulnerability in FLT3-ITD–driven acute myeloid leukemia. Cancer Discov. 11, 1582–1599 (2021).
Di Marcantonio, D. et al. ATF3 coordinates serine and nucleotide metabolism to drive cell cycle progression in acute myeloid leukemia. Mol. Cell 81, 2752–2764.e2756 (2021).
Li, Z. et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell 31, 127–141 (2017).
Shen, C. et al. RNA demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell 27, 64–80.e69 (2020).
Paris, J. et al. Targeting the RNA m(6)A Reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell 25, 137–148.e136 (2019).
Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012).
Meyer, K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149, 1635–1646 (2012).
Shi, H., Wei, J. & He, C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell 74, 640–650 (2019).
Yang, Y., Hsu, P. J., Chen, Y. S. & Yang, Y. G. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 28, 616–624 (2018).
Vu, L. P. et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 23, 1369–1376 (2017).
Liu, J. et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95 (2014).
Ping, X. L. et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 24, 177–189 (2014).
Schwartz, S. et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 8, 284–296 (2014).
Huang, H. et al. Histone H3 trimethylation at lysine 36 guides m(6)A RNA modification co-transcriptionally. Nature 567, 414–419 (2019).
Jia, G. et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887 (2011).
Zheng, G. et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 (2013).
Cheng, Y. et al. N(6)-Methyladenosine on mRNA facilitates a phase-separated nuclear body that suppresses myeloid leukemic differentiation. Cancer Cell 39, 958–972.e958 (2021).
Hsu, P. J. et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 27, 1115–1127 (2017).
Roundtree, I. A. & He, C. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Trends Genet. 32, 320–321 (2016).
Sheng, Y. et al. A critical role of nuclear m6A reader YTHDC1 in leukemogenesis by regulating MCM complex-mediated DNA replication. Blood 138, 2838–2852 (2021).
Zaccara, S. & Jaffrey, S. R. A unified model for the function of YTHDF proteins in regulating m(6)A-modified mRNA. Cell 181, 1582–1595.e1518 (2020).
Huang, H. et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20, 285–295 (2018).
Huang, H., Weng, H. & Chen, J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell 37, 270–288 (2020).
Deng, X., Qing, Y., Horne, D., Huang, H. & Chen, J. The roles and implications of RNA m(6)A modification in cancer. Nat. Rev. Clin. Oncol. 20, 507–526 (2023).
Weng, H. et al. The m(6)A reader IGF2BP2 regulates glutamine metabolism and represents a therapeutic target in acute myeloid leukemia. Cancer Cell 40, 1566–1582.e1510 (2022).
Han, L. et al. METTL16 drives leukemogenesis and leukemia stem cell self-renewal by reprogramming BCAA metabolism. Cell Stem Cell 30, 52–68.e13 (2023).
Qing, Y. et al. R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m(6)A/PFKP/LDHB axis. Mol. Cell 81, 922–939.e929 (2021).
Cheng, Y. et al. Decoding m(6)A RNA methylome identifies PRMT6-regulated lipid transport promoting AML stem cell maintenance. Cell Stem Cell 30, 69–85.e67 (2023).
Muthusamy, T. et al. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature 586, 790–795 (2020).
Maddocks, O. D. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Tajan, M. et al. Serine synthesis pathway inhibition cooperates with dietary serine and glycine limitation for cancer therapy. Nat. Commun. 12, 366 (2021).
Rossi, M. et al. PHGDH heterogeneity potentiates cancer cell dissemination and metastasis. Nature 605, 747–753 (2022).
Choi, B. H. et al. Lineage-specific silencing of PSAT1 induces serine auxotrophy and sensitivity to dietary serine starvation in luminal breast tumors. Cell Rep. 38, 110278 (2022).
Luo, M. Y. et al. Metabolic and nonmetabolic functions of PSAT1 coordinate signaling cascades to confer EGFR inhibitor resistance and drive progression in lung adenocarcinoma. Cancer Res. 82, 3516–3531 (2022).
Mullarky, E. et al. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl Acad. Sci. USA 113, 1778–1783 (2016).
Yesbolatova, A. et al. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat. Commun. 11, 5701 (2020).
Yang, M. & Vousden, K. H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016).
Weng, H. et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell 22, 191–205.e199 (2018).
DeNicola, G. M. et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat. Genet. 47, 1475–1481 (2015).
Jeong, S. M. et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23, 450–463 (2013).
Feng, M. et al. YBX1 is required for maintaining myeloid leukemia cell survival by regulating BCL2 stability in an m6A-dependent manner. Blood 138, 71–85 (2021).
Perner, F. et al. YBX1 mediates translation of oncogenic transcripts to control cell competition in AML. Leukemia 36, 426–437 (2022).
Wang, K. et al. PHGDH arginine methylation by PRMT1 promotes serine synthesis and represents a therapeutic vulnerability in hepatocellular carcinoma. Nat. Commun. 14, 1011 (2023).
Lederer, M., Bley, N., Schleifer, C. & Huttelmaier, S. The role of the oncofetal IGF2 mRNA-binding protein 3 (IGF2BP3) in cancer. Semin. Cancer Biol. 29, 3–12 (2014).
Zhang, N. et al. The m6A reader IGF2BP3 promotes acute myeloid leukemia progression by enhancing RCC2 stability. Exp. Mol. Med. 54, 194–205 (2022).
Fan, J., Zhuang, M., Fan, W. & Hou, M. RNA N6-methyladenosine reader IGF2BP3 promotes acute myeloid leukemia progression by controlling stabilization of EPOR mRNA. PeerJ 11, e15706 (2023).
Park, Y., Reyna-Neyra, A., Philippe, L. & Thoreen, C. C. mTORC1 balances cellular amino acid supply with demand for protein synthesis through post-transcriptional control of ATF4. Cell Rep. 19, 1083–1090 (2017).
Zhang, S. et al. NAT10-mediated mRNA N(4)-acetylcytidine reprograms serine metabolism to drive leukaemogenesis and stemness in acute myeloid leukaemia. Nat. Cell Biol. 26, 2168–2182 (2024).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12 (2011).
Li, W. et al. Quality control, modeling, and visualization of CRISPR screens with MAGeCK-VISPR. Genome Biol. 16, 281 (2015).
Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Putri, G. H., Anders, S., Pyl, P. T., Pimanda, J. E. & Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 38, 2943–2945 (2022).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Szklarczyk, D. et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Pang, Z. et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 49, W388–W396 (2021).
Acknowledgements
We would like to thank Nannan Wang in the Proteomics and Metabolomics Platform at Guangzhou National Laboratory for help with LC-MS data acquisition and analysis, and Dan Meng in the Advanced Cell Technology Core Facility, Guangzhou National Laboratory for assistance during flow cytometric analyses. This work was supported in part by the National Key R&D Program of China 2020YFA0112403 (H.W.), National Natural Science Foundation of China 82270168 (H.W.), Major Project of Guangzhou National Laboratory GZNL2023A02009 (H.W.) and GZNL2023A02010 (H.W.), the Pearl River Talent Recruitment Program 2021ZT09Y233 (H.W.), Science and Technology Program of Guangzhou 2024A04J6321 (H.H.), Guangdong Basic and Applied Basic Research Foundation 2021A1515010425 (H.H.), Young Talents Program of Sun Yat-sen University Cancer Center YTP-SYSUCC-0044 (H.H.), and the National Institutes of Health (NIH) R01 CA280389 (J.C.), R01 CA243386 (J.C.), R01 CA271497 (J.C.), R01 CA236399 (J.C.), and R01 DK124116 (J.C.), as well as Simms/Mann Family Foundation (J.C.).
Author information
Authors and Affiliations
Contributions
H.W., H.H. and J.C. conceived the project. H.W. and H.H. designed and supervised experiments conducted in the laboratories. F.H., X.Z., W.G., J.L., Y.Y., H.M., E.P., Y.L., J.H., C.J, Y.K. and Z.C., performed experiments; Y.W. performed data analyses; H.W., H.H., Y.H., C.Z., L.Y., C.C. and J.C. contributed reagents/analytic tools and/or grant support; F.H., H.W., Y.W., H.H. and J.C. wrote and/or revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kim Kampen, Francois Mercier and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, F., Wang, Y., Zhang, X. et al. m6A/IGF2BP3-driven serine biosynthesis fuels AML stemness and metabolic vulnerability. Nat Commun 16, 4214 (2025). https://doi.org/10.1038/s41467-025-58966-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58966-1