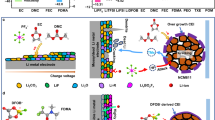
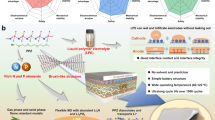
Abstract
Safe electrolytes operable over a wide temperature range are essential for lithium metal batteries, offering high redox interfacial stability, fast ion transport kinetics, and inherent safety. However, conventional electrolytes rarely achieve these characteristics simultaneously, typically sacrificing one to improve another. In this work, an ether-based thermoresponsive electrolyte is developed, in which the Li+ solvation structure varies with temperature and facilitates the formation of a polycrystalline electrode/electrolyte interface, enabling the desired properties under conventional salt concentrations. The solvation sheath is reconfigured by 1,3,5-trioxane, which promotes anion dissociation and enhances charge-transfer kinetics. Additionally, 1,3,5-trioxane triggers a cationic ring-opening polymerization of tetrahydrofuran at 60 °C, yielding oxidation-resistant ether-based polymers that improve high-temperature stability and safety. As a result, Li||LiNi0.8Co0.1Mn0.1O2 cells utilizing this electrolyte operate reliably across a broad temperature window (−60 to 60 °C). Furthermore, a practical 1.5 Ah Li|| Ni0.8Co0.1Mn0.1O2 pouch cell delivers a capacity retention of 74.7% after 60 cycles at −40 °C and 0.05 C (20 h charge/discharge), with a specific energy of 317.1 Wh kg−1(including packaging foil).
Similar content being viewed by others
Introduction
With the global push toward carbon neutrality, there is an urgent demand for high-specific-energy batteries to improve the efficiency of energy storage and conversion1,2,3. Advanced lithium metal batteries (LMBs), with specific energies exceeding 400 Wh kg−1, have emerged as strong candidates for next-generation secondary batteries4,5. However, their practical deployment is hindered by persistent parasitic reactions, uncontrolled dendrite formation, and low ionic conductivity, which together lead to rapid capacity degradation and eventual cell failure6,7,8,9. Theoretically, uniform and stable solid electrolyte interface (SEI) and cathode electrolyte interface (CEI) can significantly enhance the electrochemical performance of LMBs10,11. In conventional electrolytes, unstable organic components such as lithium dicarbonate ((ROCO2Li)2), semicarbonates (ROCO2Li), and alcohol salts (ROLi) contribute to sluggish Li+ transport and undesirable side reactions3,12. Moreover, high electrolyte viscosity can induce local anion aggregation under low-temperature conditions (below −40 °C)13,14. Therefore, electrolyte engineering strategies aimed at constructing robust interfacial layers and adaptive solvation structures are beneficial for facilitating rapid desolvation and ensuring stable LMB operation over a wide temperature range15.
Recently, various electrolyte strategies have been developed to enhance the cycling stability, temperature adaptability, and safety of LMBs. Liquefied gas electrolytes16,17, (locally) highly concentrated electrolytes18,19, and mixed-salt systems20,21 have demonstrated promising performance at low temperatures. However, the thermodynamic instability of the SEI and the presence of low-flash-point free-solvent molecules still result in thermal runaway. Cyclophosphazene-based flame-retardant electrolyte systems22 can ensure safe and stable battery operation under high-temperature conditions. Nevertheless, the increased viscosity of these electrolytes at low temperatures hinders Li+ desolvation, ultimately leading to battery failure. Achieving stable low-temperature performance (≤−40 °C) under lean electrolyte conditions (≤2.5 g Ah−1) with conventional salt concentrations remains challenging6,23. Among various solvents (Supplementary Table 1), tetrahydrofuran (THF) stands out as a preferred solvent for low-temperature LMBs due to its low freezing point (−108.4 °C), high Li+ transport kinetics, and low viscosity24,25. Additionally, 1,3,5-trioxanean (TO) can form a polymer layer on the Li metal negative electrode surface, enhancing interfacial stability and safety3,26,27. The combination of THF and TO offers a promising route for achieving safe operation of LMBs across a wide-temperature range.
In this work, we developed a thermoresponsive electrolyte comprising THF, TO, and lithium bis(fluorosulfonyl)imide (LiFSI) to ensure safe and stable operation of LMBs across a wide temperature range (−60 to 60 °C). The addition of TO reduces the binding energy between Li+ and THF, thereby improving ionic transport kinetics at low temperatures, and promotes the formation of Li-polyoxymethylene (LiPOM) in both the CEI and SEI. This LiPOM layer facilitates uniform Li+ flux and inhibits electrolyte-induced electrochemical corrosion, enabling compatibility with high-voltage positive electrodes at conventional salt concentrations. Moreover, at elevated temperatures, TO trigger cationic ring-opening polymerization of THF, resulting in an ion-aggregate-dominated solvation structure and the in situ generation of polyethers, which enhance high-temperature performance and safety. As a result, a 1.5 Ah pouch cell delivers a specific energy of 386.8 Wh kg−1 at 25 °C and maintains 317.1 Wh kg−1 at −40 °C, with 74.7% capacity retention after 60 cycles.
Results
Design of the thermoresponsive electrolyte and temperature-dependent solvated structures
The stable operation of LMBs at low temperatures requires electrolytes with high ionic conductivity, low viscosity, and low freezing point28. THF exhibits favorable characteristics in all these aspects and is thus considered the optimal solvent for low-temperature LMBs (Supplementary Table 1). Quantum chemical calculations reveal that THF possesses the lowest unoccupied molecular orbital (LUMO) energy level, indicating strong electron-donating capability and favorable reductive stability toward the Li negative electrode. In contrast, LiFSI shows the lowest LUMO and the highest Occupied Molecular Orbital (HOMO) energy levels, suggesting it preferentially undergoes redox reactions, leading to the formation of inorganic-rich inner SEI and CEI layers. The additive TO displays a lower LUMO and a higher HOMO than THF, enabling preferential redox activity to generate LiPOM, which suppresses THF decomposition (Fig. 1a, Supplementary Fig. 1, and Supplementary Data 1). Reduction potentials of the three species confirm these observations: TO (0.57 V) exhibits a higher reduction potential than THF (0.38 V), while both are significantly lower than that of FSI− (1.64 V) (Fig. 1d) 3,29. Furthermore, electrostatic potential (ESP) simulations indicate that, upon TO addition, ESP is redistributed from FSI− to the solvent molecules. The resulting decrease in solvent ESP suggests that TO effectively balances the energy distribution within the solvated sheath, thereby mitigating continuous decomposition of the lithium salt. This is expected to enhance low-temperature electrochemical performance at conventional Li-salt concentrations (Supplementary Fig. 2). Based on this rationale, 1 M LiFSI in THF was selected as the reference electrolyte (designated as THF-based electrolyte), and 20% molar ratios were introduced as an additive to obtain the THF-based electrolyte.
a Comparison of the HOMO and LUMO energy levels for various commonly used Li salts and ether-based solvents. b, c RDF plots of Li–OTHF, Li–OFSI−, Li–NFSI− and Li‒OTO pairs in THF- (b) and TO-based (c) electrolytes. d The calculated reduction potential of THF, TO and FSI− based on DFT. e, f Fitted Raman spectra in THF- (e) and TO-based (f) electrolytes at different temperatures. g, h 7Li-NMR (g) and 1H-NMR (h) spectra of THF- and TO-based electrolytes. Colors of elements: H, white; C, green; O, red. i Gel permeation chromatography (GPC) test results of TO-based electrolyte at 60 °C. j Schematic diagram of temperature-dependent solvation structure of TO-based electrolyte.
Molecular dynamics (MD) simulations, along with the corresponding radial distribution functions (RDFs), were utilized to assess the coordination structures of solvents, cations, and anions in THF-based and TO-based electrolytes8,30. The results reveal that the average coordination in THF- and TO-based electrolytes is Li+(THF-O)2.8(FSI-O)0.9 and Li+(THF-O)1.9(TO-O)0.7(FSI-O)1.3, respectively. These findings indicate that TO participates in the solvation of the Li+ solvation shell within the electrolyte (Li-OTO peaks observed at ~2.1 Å), reducing the coordination of Li+-THF, while enhancing the coordination of Li+-FSI− (Fig. 1b, c and Supplementary Data 2). The blue shift of the S-N-S peaks in the Raman spectra, coupled with the downfield shift in the 7Li NMR spectra, further supports this conclusion (Fig. 1e–g). The fitted curves of the Raman bands in the 700–780 cm−1 range show that the TO-based electrolyte forms a solvated structure predominantly composed of contact ion pairs (CIPs) at −40 °C. This enhanced anionic interfacial chemistry ensures high charge transfer kinetics at low temperatures, thereby improving electrochemical performance (Fig. 1f).
Variable-temperature Raman and NMR spectroscopy provide direct insights into changes in the solvated structure with temperature. In the TO-based electrolyte, the concentrations of AGG I (one FSI− coordinating with two Li+) and AGG II (one FSI− coordinating with more than two Li+)31 increased with temperature, indicating a significant reduction in free solvent molecules and a greater involvement of anions in the formation of the solvated sheath at elevated temperature (Fig. 1f). Furthermore, the shift of the variable-temperature 7Li NMR spectra to higher fields offers additional evidence for the enhanced coordination of Li+ with anions (Fig. 1g). Additionally, MD snapshots demonstrate strong anion aggregation and the formation of ionic clusters at 60 °C, while the corresponding RDFs show a weakening of Li-OTHF coordination and a strengthening of Li-OFSI− coordination with increasing temperature (Supplementary Fig. 3). These results suggest that the thermal motion of the polar THF solvent molecules increases with temperature, altering the original ion-dipole interactions and prompting the formation of temperature-dependent solvated structures.
In general, the optimal electrolyte for high-temperature LMBs should exhibit a stable solvated structure, a robust redox-stable interface, and a solvent with high safety32. MD simulations conducted at 60 °C were used to extract three representative solvated structures (with a proportion exceeding 75%) from each electrolyte, and their binding energies were computed using density functional theory (DFT). The results indicate that the solvated structures of the TO-based electrolyte exhibit higher binding energies compared to the THF-based electrolyte, suggesting superior thermal and oxidative stability (Supplementary Fig. 4 and Supplementary Data 3). As shown in Fig. 1h and Supplementary Fig. 5, the characteristic THF peaks in the TO-based electrolyte were significantly diminished, while new NMR peaks for hydrogen and carbon were observed, resulting in the formation of a polyether (PTHF) with enhanced thermal stability. GPC analysis reveals that PTHF has a high number average molecular weight (Mn) of 1.82 × 105 and a high weight average molecular weight (Mw) of 3.47 × 105 g mol−1. The polymer dispersity index is approximately 2, indicating a uniform molecular weight distribution, which contributes to the improved high-temperature performance and safety of the batteries (Fig. 1i).
The thermal polymerization mechanism and the evolution of the solvated structure of the TO-based electrolyte are described in detail in Fig. 1j, Supplementary Fig. 6–9, and Supplementary Note 1. At −40 °C, the thermal motion of the molecules is minimal, and THF, TO maintain relatively strong ionic dipole interactions with Li+, resulting in a CIP-dominated solvated structure. This structure accelerates the Li+ desolvation process and facilitates fast charge transfer kinetics at low temperatures. As the temperature increases, the thermal motion and kinetic energy of THF molecules rise, leading to cationic ring-opening polymerization in the presence of primary TO-oxonium ions, resulting in the formation of PTHF (Supplementary Fig. 9). This process significantly reduces the number of free solvent molecules in the electrolyte and decouples the solvents within the solvated structure, thereby forming a more thermally stable, anion-rich solvated sheath (i.e., the solvated structure transitions from CIP to AGG I and AGG II). Notably, unlike the polymerization mechanism of THF at high temperatures, TO undergoes a ring-opening polymerization reaction when in contact with Li-metal, initiated by a small amount of H+(FSIOH)−, leading to the formation of a robust and compact LiPOM layer33. This layer significantly enhances the redox stability of the SEI and CEI (Supplementary Figs. 7 and 8). Consequently, the TO-based electrolyte demonstrates high safety and wide-temperature performance, overcoming the limitations of conventional electrolytes and achieving compatibility between thermodynamically stable interfaces, high safety, and fast charge transfer kinetics at low temperatures.
Electrolyte properties and interfacial dynamics
Although the increase in TO content enhances the polymerization degree of the electrolyte at high temperatures, it also leads to the formation of an excessively thick LiPOM layer at the interface, which hinders the interfacial charge transfer kinetics. To achieve an optimal LiPOM layer thickness, we prepared TO-based electrolytes with varying molar ratios. Figure 2a and Supplementary Fig. 10 illustrate the variation of ionic conductivity with temperature for the five electrolytes. The TO-based (20%) electrolyte exhibits the highest ionic conductivity of 3.928 mS cm−1 at −40 °C. The activation energy (Ea) of the electrolyte was calculated using the Arrhenius equation (Fig. 2b)34, revealing the lowest Ea value of 12.1 kJ mol−1 for the TO-based (20%) electrolyte among the designed formulations. A lower Ea indicates accelerated ion diffusion kinetics, contributing to the highest Li+ transference number of 0.629 (Supplementary Fig. 11). Notably, when the TO-based (20%) electrolyte is polymerized into a gel form, it still maintains high ionic conductivity (0.89 mS cm−1 at −20 °C) and a Li+ transference number of 0.752, which is comparable to that of advanced gel electrolytes reported in the literature (Supplementary Fig. 12 and Supplementary Table 2). The enhanced Li+ transference number is attributed to the assisted Li+ diffusion mechanism, where Li+ is transferred from one anion of Li salts to another via binding sites35. The results of linear scanning voltammetry (LSV) also show a positive shift in the oxidation onset potential to 4.73 V as the TO content increases (Fig. 2c). Although this could significantly enhance the oxidation window of the electrolyte, it also causes a deterioration in the wettability of the electrolyte with the separator (Supplementary Fig. 13). Overall, the TO-based (20%) electrolyte effectively improves ionic conductivity, ion transference number, and oxidative decomposition potential, while maintaining moderate viscosity at low temperatures.
a Ionic conductivity, b Arrhenius curves and corresponding calculated Ea, c LSV measurements of the five designed electrolytes at a scan rate of 1 mV s−1. d–f In situ raw FTIR spectra and differential spectra of TO-based electrolyte during potentiostatic plating/stripping. g, h Temperature-dependent distribution of relaxation times (DRT) plot derived from EIS data for the TO- (g) and THF-based (h) electrolytes in Li||NCM811 coin cells.
An in situ IR cell was designed to monitor the dynamic changes in the interfacial electrolyte structure of copper electrodes during the plating/stripping processes (Supplementary Fig. 14 and Supplementary Note 2) 36,37. This setup aimed to provide insight into the changes in solvation structure in THF-based and TO-based electrolytes during charging/discharging, and to elucidate the effects of these changes on SEI formation. The raw IR transmittance spectra from in situ FTIR and the differential spectra are presented in Fig. 2d–f. The data reveal that the characteristic peaks of ROLi (835 ~ 845 cm−1) and C-S (~800 cm−1, lithium salt decomposition) are less intense, and a distinct C = O peak (1725 ~ 1825 cm−1) is observed in the TO-based electrolyte38,39. This indicates that the organic SEI formed is a robust and dense LiPOM layer derived from TO, rather than an unstable ROLi layer originating from THF. In contrast, the THF-based electrolyte showed six times stronger ROLi and C-S signals, with no C = O signal detected (Supplementary Fig. 15). These findings suggest that the TO-derived LiPOM layer acts as a barrier, hindering the inward diffusion of LiFSI to the negative electrode, thereby slowing the subsequent decomposition of LiFSI and reducing side reactions.
The electrode interface kinetics of THF- and TO-based electrolytes were further investigated using distribution of relaxation times (DRT) analysis in Li||NCM811 coin cells (Fig. 2g, h and Supplementary Fig. 16). Four distinct processes can be identified based on their corresponding time constants (τ): Li deposition (102 > τ > 101)40, interfacial desolvation of Li+ solvates (101 > τ > 10−1)41, Li+ conduction in the SEI (10−1 > τ > 10−3)42, and polymer ionic conduction (10−4)43,44. Compared to the cell with the THF-based electrolyte, the impedance of Li plating and Rct in the TO-based electrolyte is reduced to one-tenth. This indicates that the addition of TO significantly reduces the Li+ desolvation process and enhances lithium deposition efficiency at low temperatures. Furthermore, the negligible RSEI in the TO-based electrolyte suggests easier Li+ migration through the SEI at low temperatures. It is also noteworthy that the additional impedance due to polymer ionic conduction in the TO-based electrolyte at high temperatures further supports the idea that TO can trigger cationic ring-opening polymerization of THF.
Effect of the designed electrolyte on Li plating/stripping at low temperatures
To evaluate the impact of the designed LiPOM layer on Li plating/stripping, the Coulombic efficiency (CE) of THF-based and TO-based electrolytes in Li||Cu cells was initially assessed using the Aurbach method45. As shown in Fig. 3a, the TO-based electrolyte demonstrates a CE of 99.06% at −40 °C and 0.5 mA cm−2, significantly outperforming the THF-based electrolyte (76.03%). Additionally, its initial lithium nucleation overpotential is only 278 mV, indicating that the inclusion of TO effectively enhances the Li plating/stripping efficiency and kinetics. Long-term cycling tests were then conducted at a current density of 0.5 mA cm−2 with a cutoff areal capacity of 1 mAh cm−2. After 250-cycles, the average CE of the Li||Cu cell with the TO-based electrolyte reached 98.56% (Fig. 3b), accompanied by a comparatively lower and more stable polarization potential in the corresponding potential profile (Supplementary Fig. 17a). Furthermore, the Li||Li symmetric cell operated stably for 450 h under these conditions (Fig. 3c). In contrast, the CE of the THF-based electrolyte exhibited significant fluctuations during the initial stages, and a notable short-circuit phenomenon was observed after 20 cycles. This demonstrates that the TO-derived LiPOM layer effectively protects the Li negative electrode from solvent molecule erosion, inhibits the growth of lithium dendrites, prevents the formation of dead lithium, and ensures high Li plating/stripping reversibility and long-term stability at low temperatures. As shown in Fig. 3d, the Li||Cu cell with the TO-based electrolyte exhibits remarkable rate performance at −40 °C, with a CE of 95.97% at a high current density of 1 mA cm−2. The cell maintains stability when the current density is returned to 0.1 mA cm−2, with the corresponding potential profile remaining consistent with the initial state (Supplementary Fig. 17b). In contrast, the THF-based electrolyte shows a notable decline in CE at 0.5 mA cm−2, resulting in short-circuiting and failure at 1 mA cm−2. These results conclusively demonstrate that the addition of TO enhances the interfacial charge transfer kinetics of the cell at low temperatures.
a, b CE (a) and Long-term CE (b) of Li||Cu cells with designed electrolytes under a current density of 0.5 mA cm−2 and a cutoff areal capacity 1 mAh cm−2 at −40 °C. c Long-term cycling performance of Li||Li cells with designed electrolytes at 0.5 mA cm−2 and 1 mAh cm−2. d Rate performance of Li||Cu cells with designed electrolytes at different current densities. e–j SEM images (e, h), surface topography (f, i) and Gaussian statistical distribution of surface potentials (g, j) of lithium deposition on Cu foil with THF- (e–g) and TO-based (h–j) electrolytes at 0.5 mA cm−2 with a cutoff areal capacity of 1 mAh cm−2 at −40 °C.
To elucidate the significant discrepancies in CE and cycling stability across different electrolytes, scanning electron microscopy (SEM) and Kelvin probe force microscopy (KPFM) were employed to investigate the morphology and surface potential of Li deposits, providing initial insights into the underlying causes of these discrepancies from a morphological perspective. As shown in Fig. 3e–g and Supplementary Fig. 17c, d, the amount of Li plating on the Cu collector was significantly reduced in the THF-based electrolyte, and the formation of whisker-like Li structures was prominently observed under SEM, leading to a considerable increase in Li plating porosity, surface roughness, and surface potential. This behavior is attributed to the slow Li+ desolvation and interfacial charge transfer processes of the THF-based electrolyte at low temperatures, ultimately resulting in tip-driven Li deposition, which causes severe short-circuiting and failure of the cell8. In contrast, following the introduction of TO, the Li deposits exhibited a dense and uniform bulk deposition morphology, with surface roughness and surface potential values of only 823.1 nm and 115 mV, respectively (Fig. 3h–j and Supplementary Fig. 17e,f). This improvement contributes to the achievement of higher CE and longer cycle life, indicating a significant enhancement of the solvation process and SEI kinetics in the TO-based electrolyte.
The Li||Cu and Li||Li cells assembled with the TO-based electrolyte also demonstrate high CE and long-cycle stability at 25 °C. Specifically, the Li||Cu cell cycled for 400 cycles with an average CE of 99.38%, while the Li||Li cell cycled stably for 1400 h at a current density of 1 mA cm−2 and a cutoff areal capacity of 2 mAh cm−2. Moreover, the L||Cu cell maintained a CE of 96.36% even at a high current density of 5 mA cm−2 (Supplementary Fig. 18). The Li deposits formed in the TO-based electrolyte were also more homogeneous and flat, forming larger bulk deposits with a surface roughness and surface potential of 504.7 nm and 11.9 mV, respectively, as observed via SEM and KPFM (Supplementary Fig. 19). This was further corroborated by in-situ observations using polarized light microscopy (Supplementary Fig. 20). The TO-based electrolyte formed a denser and more uniform Li layer, enhancing the efficiency of Li deposition and effectively suppressing the growth of lithium dendrites. In contrast, the Li deposition in the THF-based electrolyte was sparse, leading to the formation of lithium dendrites and dead lithium.
Interfacial chemistry study
To gain a deeper understanding of the composition and relative content of the SEI in both electrolytes, depth-profiling XPS measurements were performed (Fig. 4a–c and Supplementary Fig. 21). The fine fitting results of the C 1 s spectra revealed additional O-C-O and C = O signals in the TO-based electrolyte, providing insights into the pathways and products of TO decomposition3. The O-C-O signal was assigned to the TO-derived LiPOM layer, while the C-O signal was attributed to the THF derivative, which contributed to the formation of a short-chain organic SEI. As Ar+ sputtering depth increased, a notable decrease in the relative content of organic species was observed, accompanied by a significant rise in the relative content of inorganic components, including LiF, Li2O, and Li3N. This increase in inorganic components is expected to enhance the ionic conductivity of the SEI and reduce the kinetic barriers of Li+ transport through the SEI. Figure 4c shows the percent composition of each species in the SEI derived from both electrolytes at low temperatures. The SEI derived from the TO-based electrolyte exhibited a higher concentration of O-C-O (19%) in the surface layer, along with a greater abundance of LiF (64%) and LiO2 (8%) in the internal layer. This suggests that the dense LiPOM layer effectively mitigates the solvent-induced erosion of the lithium metal.
a, b C 1 s, F 1 s and Li 1 s spectra of SEI in TO- (a) and THF-based (b) electrolytes at the sputtering times of 0, 25, and 50 s. c Relative compositions of Li-containing and C-containing species in SEI formed in designed electrolytes. d Depth sputtering profiles and 3D renders of the Li SEI in Li||Li cells with TO-based electrolytes. Normalized intensity is obtained through 1000/(intensity) conversion. e, f Cryo-TEM images of deposited Li in the cell with TO- (e) and THF-based (f) electrolytes.
Time-of-flight secondary ion mass spectrometry (TOF-SIMS) analysis further provided reliable evidence supporting the SEI formation from TO-based electrolytes46. The ionic fragments LiF2− and C2HO− are indicative of LiF and organic species, respectively. The homogeneous distribution of organic species in the surface layer suggests that TO decomposes to form LiPOM, contributing to SEI formation. As the sputtering time increases, the proportion of the organic component decreases, resulting in a gradual shift of the SEI structure towards a LiF-dominant composition. (Fig. 4d and Supplementary Fig. 22).
Cryo-transmission electron microscopy (cryo-TEM) was employed to further investigate the dual-layered SEI structure in both electrolytes. As shown in Fig. 4e, the SEI derived from the TO-based electrolyte consists of a homogeneous organic outer layer, measuring 1.94 nm, and an inner layer composed of inorganic phases such as LiF, Li2O, and Li2CO3, which aligns with the XPS and ToF-SIMS results. The schematic of the SEI structure is provided in Supplementary Fig. 23a. This SEI structure exhibits high strength and elasticity, effectively inhibiting the electron tunneling effect and homogenizing the Li+ flux47. As a result, the electrolyte can achieve high CE and promote dense and uniform lithium deposition at low temperatures. In contrast, in THF-based electrolytes, the SEI fragile and inhomogeneous, unable to prevent solvent-induced degradation of the Li negative electrode, leading to the growth of lithium dendrites (Fig. 4f and Supplementary Fig. 23b). Based on evidence from XPS, TOF-SIMS, and cryo-TEM, we conclude that TO in the electrolyte facilitates the formation of a dense LiPOM outer layer on the conventional anion-derived SEI, which protects the Li negative electrode and enables the cell to exhibit high CE and long-cycle stability.
In situ X-ray diffraction (XRD) was employed to investigate the effects of two electrolyte-derived CEIs on the structural evolution of the NCM811 positive electrode during charge/discharge cycles (Fig. 5a, b). Within the voltage range of 3.0 ~ 4.5 V, the NCM811 positive electrode underwent a series of phase transitions: H1 → M → H2 → H3a, corresponding to expansion and contraction of the lattice volume (Supplementary Fig. 24a, b)48,49. Notably, the intensity and Brag angles of the (003) and (101) peaks of the NCM811 positive electrode exhibited significant variations during the phase transitions when using the THF-based electrolyte, with the H1 → M transition being particularly pronounced. In contrast, the volume expansion and peak trailing were considerably suppressed when the TO-based electrolyte was used, indicating a reversible phase transition and enhanced structural stability. The fully charged Li||NCM811 cell underwent accelerated degradation tests at 4.5 V (Supplementary Fig. 24c). The results showed that the leakage current density of the cell with the TO-based electrolyte was as low as 5.44 μA cm−2, indicating that the TO-derived CEI effectively mitigated interfacial side reactions and passivated the positive electrode. Additionally, to assess the protection provided by the CEIs formed by the two electrolytes against structural changes in the positive electrode at −40 °C, XRD analysis was performed on the NCM811-positive electrode after 100 cycles (Supplementary Fig. 25). The separation of the (006)/(102) peaks was more distinct in the TO-based electrolyte, suggesting that the transition from the layered to the rock salt phase was effectively suppressed50. As shown in Fig. 5c, ex-situ TEM analysis of cycled positive electrodes revealed that the TO-based electrolyte formed a uniform amorphous CEI ~3.71 nm thick on the surface of NCM811, which was thinner and more homogeneous than the CEI formed with the THF-based electrolyte (6.15 nm). Moreover, the layered structure was well preserved from the surface to the bulk, with negligible formation of the rock salt phase. In contrast, a thicker rock salt phase of 3.6 nm was observed in the THF-based electrolyte (Fig. 5d). Furthermore, SEM analysis of the positive electrode cycled in the TO-based electrolyte showed no development of intergranular cracks, indicating structural stability (Supplementary Fig. 26). Collectively, these results suggest that the TO-based electrolyte is effective in forming a dense and robust CEI, which protects the NCM811 electrode from structural degradation during phase transitions.
a, b In-situ XRD characterization of the NCM811 positive electrode during the initial charge/discharge in TO- (a) and THF-based (b) electrolytes. c, d HRTEM and FFT images of cycled NCM811 positive electrodes with TO- (c) and THF-based (d) electrolytes after 100 cycles at −40 °C. e Depth sputtering profiles and 3D renders of the CEI in Li||NCM811 cells with TO-based electrolytes. Normalized intensity is obtained through 1000/(intensity) conversion. f Schematic diagram of CEI formation and positive electrode particles cycled in THF- and TO-based electrolytes.
To gain a deeper understanding of the chemical state and compositional characteristics of the CEI, the NCM811 positive electrode was subjected to a depth-profiling XPS analysis after 100 cycles. As shown in supplementary Fig. 27, the CEI derived from the TO-based electrolyte exhibits a greater abundance of O-C-O and C = O signals, indicating the successful formation of a LiPOM surface layer. With ongoing Ar+ sputtering, the relative content of organic species decreases significantly, while the relative content of LiF increases markedly. In contrast, the CEI derived from the THF-based electrolyte shows a prominent C-O signal, suggesting the presence of short-chained, unstable organic compounds on the surface. This is the primary reason for its poor cathodic reversibility and structural degradation. The 3D structure of the CEI and the spatial distribution of its components were further elucidated by TOF-SIMS depth profiling (Fig. 5e and Supplementary Fig. 28). The TO-based electrolyte-derived CEI is characterized by a dense organic layer on the surface and a LiF-dominated inorganic layer beneath, with a low concentration of Li2CO3 in the surface layer. This composition effectively inhibits side reactions10. The schematic diagram of the CEI formation and the cycling of positive electrode particles in both electrolytes is shown in Fig. 5f. The CEI formed by the TO-based electrolyte consists of a dense, homogeneous LiPOM outer layer and a LiF-rich inner layer. This structure prevents interfacial side reactions and the dissolution of TMs, preserves the initial structure of NCM811, and enhances interfacial stability. In contrast, in THF-based electrolytes, the positive electrode undergoes solvent-induced corrosion, leading to the dissolution of TMs and the rupture of NCM particles.
Performance of practical Li-metal cells at wide temperature
To demonstrate the effectiveness of the designed electrolyte operating over a broad temperature range, Li||NMC811 full cells were assembled to thoroughly evaluate the TO-based electrolyte system. As shown in Fig. 6a, b, the TO-based electrolyte full cell exhibits stable long-cycle performance, retaining 87.3% of its capacity after 500 cycles at 25 °C and 0.2 A g−1. Additionally, the dQ/dV curves show a high degree of overlap, with only a minor potential change of 16 mV, indicating the phase transition reversibility and structural stability of the positive electrode (Fig. 6c). More importantly, the Li||NMC811 full cell with the TO-based electrolyte retains a specific capacity of 176.8 mAh g−1 after 200 cycles at −40 °C, with a capacity retention of 90% and an average CE of 99.4% at a low current rate of 0.04 A g−1 (charge/discharge) (Fig. 6d, e). It also demonstrates impressive low-temperature rate performance, achieving a high discharge capacity of 173 mAh g−1 at 0.2 A g−1 (0.02 A g−1 charge), which outperforms previously reported advanced electrolyte systems (Supplementary Fig. 29 and Supplementary Table 3). This performance can be attributed to the improved solvation structure of TO and the formation of a dual-layered CEI and SEI, which effectively enhance the Li+ diffusion and interfacial charge transfer kinetics at low temperatures. Thus, a reversible phase transition of the positive electrode is achieved (notably, the H1 → M phase transition process, where the potential increases by only 48 mV after 200 cycles), reducing polarization and capacity loss (Fig. 6f). In contrast, the initial specific capacity of the cell with the THF-based electrolyte was only 120 mAh g−1, which decayed to 41% of the initial capacity after 100 cycles. Furthermore, the TO-based electrolyte demonstrates a capacity retention of up to 88.9% after 400 cycles at 60 °C (Fig. 6g, h), due to the solvation structure transformation and solvent molecule reconfiguration at elevated temperatures. Notably, the polymerization of the electrolyte into a gel state does not affect the subsequent performance of the cell. The Li||NCM811 coin cell using the TO-based gel polymer electrolyte achieved a high specific capacity of nearly 163 mAh g−1 with 85% capacity retention after 300 cycles at 0.2 A g−1 and 25 °C. It also demonstrated a specific capacity of 154 mAh g−1 at the 200th cycle with 84% capacity retention at 0.04 A g−1 and −20 °C (Supplementary Figs. 30 and 31).
a Long-term cycling performance of Li||NCM811 cells with different electrolytes at 0.2 A g−1 at 25 °C. b Corresponding charge/discharge curves with TO-based electrolyte, and c the dQ/dV curves for different cycles. d Long-term cycling performance of Li||NCM811 cells with different electrolytes at 0.04 A g−1 and −40 °C. e Corresponding charge/discharge curves with TO-based electrolyte, and f the dQ/dV curves for different cycles. g Long-term cycling performance of Li||NCM811 cells with different electrolytes at 0.1 A g−1and 60 °C. h Corresponding charge/discharge curves with TO-based electrolyte. i Cycling performance of Li||NCM811 pouch cell at 0.01 A g−1 and −40 °C. j Discharge profiles (0.02 A g−1) of Li||NMC811pouch cell using TO-based electrolyte at different temperatures (the cell was charged at 0.04 A g−1 under 25 °C). k Comparison of the state-of-the-art performance of Li metal pouch cells in terms of specific energy, temperature and electrode areal capacity as reported in the literature. The source of the literature data shown in this figure can be found in Supplementary Information, Table 5.
The outstanding wide-temperature performance and long cycle stability of advanced TO-based electrolytes make them highly promising for practical use in LMBs. Li||NCM811 pouch cells (1.5 Ah) with lean electrolytes (2 g Ah−1) and an N/P ratio of 2.2 were assembled, achieving an initial specific energy of 386.8 Wh kg−1 (Supplementary Fig. 32). The specific energy of the cell was calculated by considering the masses of all components within the pouch cells (Supplementary Table 4). Remarkably, the LMB pouch cell demonstrated an initial specific energy of 317.1 Wh kg−1 at −40 °C and exhibited exceptional cycling stability, retaining 74.7% of its capacity after 60 cycles (Fig. 6i). In contrast, the discharge capacity of cells with THF-based electrolytes dropped to 0 after 10 cycles. The disassembly of the cycled Li||NCM811 pouch cells was performed to investigate the underlying cause of the significant difference in cycling performance at low temperatures between the two electrolytes. The Li negative electrode cycled in the THF-based electrolyte exhibited substantial depletion and showed considerable amounts of dead lithium and whisker-like formations (Supplementary Fig. 33a, b). In contrast, the lithium in the TO-based electrolyte remained in the form of lumpy deposits, although the distribution of lithium on the Cu collector displayed slight inhomogeneity after repeated cycling (Supplementary Fig. 33c, d). Furthermore, the temperature adaptability of the Li||NCM811 pouch cells was assessed. The cells operated stably within the temperature range of −60 to 60 °C, with capacity retention values of 81.5% at −40 °C and 61.7% at −60 °C (Fig. 6j). Notably, these results are reproducible and are not limited to a specific selected cell (Supplementary Fig. 34). Additionally, the TO-based electrolyte designed in this study exhibits the most optimal overall performance in terms of wide-temperature stability, electrode areal capacity, and specific energy when compared to advanced electrolytes reported in the literature (Fig. 6k and Supplementary Table 5).
To further demonstrate the practical application potential of the TO-based electrolyte, it was incorporated into a pouch cell and mounted on a robot, which operated stably under the extreme temperature condition of −40 °C (Supplementary Fig. 35 and Supplementary Movie 1). The safety of LMBs is generally determined by both the intrinsic safety of the electrolyte and the thermal runaway temperature of the positive electrode material in a fully charged state51,52. As shown in Supplementary Fig. 36a, b, the polymerization of products of the TO-based electrolyte did not exhibit combustion during the ignition test, and the thermal decomposition temperature of the electrolyte reached 211 °C with minimal heat release. In situ variothermal XRD revealed that the formation of the dual-layered CEI effectively suppressed oxygen release from the positive electrode during charging and enhanced thermodynamic stability (Supplementary Fig. 36c, d). As a result, the thermal safety of LMBs utilizing the TO-based electrolyte was significantly improved.
Discussion
In summary, we introduce a thermoresponsive electrolyte design strategy that enables the safe and stable operation of LMBs across a temperature range of −60 to 60 °C. Both theoretical and experimental data confirm that the additive TO can interact with the Li+ solvation structure, thus enhancing Li+ transport and charge transfer kinetics. The findings from HRTEM, XPS, TOF-SIMS, and in-situ XRD reveal that the SEI and CEI formed by the TO-based electrolyte consist of a uniform, thin, and durable outer layer of LiPOM, with an inner layer rich in LiF and Li2O, effectively preventing degradation of both electrodes. Notably, as temperature increases, the solvation structure of the electrolyte undergoes a transformation, with solvent molecules reorganizing into a polyether structure that exhibits improved oxidation resistance, significantly enhancing battery safety. Consequently, the practical 1.5 Ah Li||NCM811 pouch cell with the optimized electrolyte operates stably between −60 to 60 °C. Specifically, at 25 °C, the pouch cell achieves 100 cycles with an initial specific energy of 386.8 Wh kg−1. Surprisingly, at −60 °C, it retains 61.7% of its room temperature discharge capacity, while at −40 °C, it reaches an initial specific energy of 317.1 Wh kg−1 and maintains 74.7% capacity retention over 60 cycles.
Methods
Materials
Active materials (LiNi0.8Co0.1Mn0.1O2 (NCM811) powders), polyvinylidene difluoride (PVDF, Mw = 1,200,000 Da, 99.5%), Super P (particle size: 40–50 nm, 99.5%), carbon-coated aluminum foil (Al, 15 μm-thick), separator (Celgard 2325, thickness: 25 μm, PP/PE/PP three-layer membrane, pore size: average diameter 0.028 μm, porosity: 39 ± 5%,) were sourced from Guangdong Canrd New Energy Technology Co., Ltd. Prior to assembling the cell, a manual slicer (MSK-T10) was used to cut the separator into discs with a diameter of 18 mm. Coin cell components (CR-2032, positive electrode case: 18 × 2.91 mm, negative electrode case: 16 × 2.77 mm, spacer: 15.8 × 1 mm, spring: 15.6 × 1.1 × 0.25 mm) were purchased from Nanjing Mojiesi Energy Technology. Lithium bis(fluorosulfonyl)imide (LiFSI, >98%), tetrahydrofuran (THF, ≥ 99.9%), N-methyl-2-pyrrolidone (NMP, ≥99.5%) were obtained from Shanghai Aladdin Bio-Chem Technology Co., Ltd. 1,3,5-Trioxane (TO, >99.0% (GC)) was purchased from Tokyo Chemical Industry Co., Ltd. Li foil (15.6 mm diameter and 250 μm thick) and double-sided Cu foil (50 μm of Li per side, Cu thickness: 8 μm, total thickness of Li negative electrode: 108 μm) were acquired from Beijing InnoChem Science & Technology Co., Ltd. The double-layer NCM811 positive electrode (22 mg cm−2 on each side) was sourced from Guangdong Canrd New Energy Technology Co., Ltd. All materials were stored in an argon-filled glove box (storage time: less than three months, storage temperature: 30°C)
Positive electrode preparation
The NCM811 positive electrodes were prepared following a series of steps in a dry room with a dew point of −50 °C. A mixture consisting of 85 wt% NCM 811 powders (as active materials), 10 wt% Super P (as an electron conductive additive), and 5 wt% PVDF (as a binder) was wet-mixed with 5 wt% NMP solvent for 12 h using a stirrer. Subsequently, the slurry was cast onto a carbon-coated Al foil using an automatic coating machine and dried under vacuum at 100 °C for 6 h. Once the solvents were evaporated, the positive electrode was rolled using a heated electric vertical rolling machine (GRS-JS400L) and then punched into discs with a diameter of 14 mm using a manual slicer (MSK-T10). Prior to full-cell assembly, the electrode underwent further vacuum drying at 100 °C overnight. The mass loading of active materials on the positive electrode laminates was ~12.5 or 22 mg cm−2, and the thickness was around 0.05 or 0.08 mm.
Preparation of electrolyte
The THF solvent was dried over 4 Å molecular sieves (Aladdin) prior to use. LiFSI and TO were used as received. A THF-based electrolyte was prepared by dissolving 1 M LiFSI (0.187 g) in 1 mL of THF. Specifically, LiFSI was added to Schlenk tubes, and THF was transferred using a glass syringe (tetrafluoroethylene push head, stainless steel needle). Subsequently, 0.018 g of TO was dissolved in the THF-based electrolyte to prepare the TO-based electrolyte, achieving a LiFSI:TO molar ratio of 1:0.2. All preparation steps were carried out at room temperature (~25 °C) within an argon-filled glove box (H2O < 0.1 ppm, O2 < 0.1 ppm, temperature: 30 °C, pressure: 1 atm). The entire process proceeded without the need for stirring, and the electrolyte must be prepared and used immediately before cell assembly.
Coin cells and pouch cells assembling
All coin cells were assembled in an Argon-filled glove box with H2O < 0.1 ppm and O2 < 0.1 ppm. The Li||Li CR-2032 coin cells were assembled in the following sequence: positive electrode case, Li foil, Celgard 2325 separator (18 mm), Li foil, spacer, spring, and negative electrode case. For the Li||Cu CR-2032 coin cells, the assembly sequence was as follows: positive electrode case, Cu foil (14 mm), Celgard 2325 separator, Li foil, spacer, spring, and negative electrode case. The Li||NCM811 full cells were assembled in the following order: positive electrode case, aluminum disk (19 mm), NCM811 electrode (12.5 mg cm−2, single-side coated, areal capacity: 2.5 mAh cm−2), Celgard 2325 separator, Li foil (250 μm, areal capacity: 48.25 mAh cm−2), spacer, spring, and negative electrode case. The corresponding N/P ratio was 19. All coin cells were filled with 75 μL of electrolyte. Specifically, 40 μL of electrolyte was pipetted onto the positive electrode (model: Discovery-H, pipette tips: 100 μL), followed by placement of the separator, and then 35 μL of electrolyte was added to fully wet the separator. To prevent corrosion of the stainless-steel case at high voltages, an additional piece of Al foil was placed between the positive electrode case and the NCM positive electrode disk.
The Li‖NCM811 pouch cell (10.0 × 7.0 cm2) was assembled in a dry room with a dew point of −50 °C. The cell structure comprised 4 positive electrode layers and 5 negative electrode layers, resulting in an N/P ratio of 2.2, indicating a lithium-rich configuration. The negative electrode, consisting of Cu current collector and double-layer 50 μm Li foil, and the positive electrode, composed of double-layer active material (22 mg cm−2 per side), were stacked sequentially and separated by a Celgard 2325 separator. Electrolyte (3.0 g) was injected after cell stacking but prior to heat sealing, followed by a 12 h soaking period under vacuum conditions to ensure complete wetting of the electrodes and separator. Any residual gas was evacuated under vacuum before final pouch sealing to prevent gas accumulation and ensure cell compactness. The electrolyte was added by weight in a controlled manner, resulting in an electrolyte-to-capacity ratio of 2 g Ah−1. The final cell exhibited a discharge capacity of 1.5 Ah, a mid-point voltage of 3.80 V, and a discharge energy of 5.7 Wh. The total cell mass was 14.738 g, corresponding to a specific energy of 386.8 Wh kg−1. The tab materials used for the current collection were Al for the positive electrode and Cu for the negative electrode. The cycling performance of the pouch cells was tested under a fixed device that applied 1.0 MPa external pressure to maintain interfacial contact and suppress lithium dendrite growth.
Electrochemical testing
All electrochemical data presented in this work were obtained from CR-2032 coin cells or pouch cells. Galvanostatic tests at high or low temperatures were performed using a Neware BTS 4000 system, while room temperature (25 ± 3 °C) galvanostatic tests were carried out with a LANHE-CT 2001A system. High or low temperature measurements were conducted in a JHY-H-50L temperature chamber (gas environment: air) to maintain the cell at a constant temperature. For Li||NCM811 coin cells, galvanostatic cycling tests were performed within a voltage window of 2.8–4.3 V (no constant voltage charging was applied during the measurements). The specific current and specific capacity for all coin cells were calculated based on the mass of the positive electrode active material.
All potentiostatic tests were conducted using a CHI-760E. Electrochemical impedance spectroscopy (EIS) was performed over a frequency range from 1 MHz to 100 mHz with an amplitude of 5 mV using a Chenhua electrochemical workstation (perturbation type: potentiostatic, number of data points: 12 points per decade frequency). Prior to measurements, a 1000 s open-circuit potential period was applied to stabilize the potential. LSV measurements were carried out from 3.0 V to 5.5 V with a scan rate of 1 mV s−1 using Li||c-Al cells. The electrochemical floating test was performed on Li||NCM811 cells with different electrolytes. The cells were initially charged to 4.5 V at a specific current of 20 mA g−1 and then held for 12 h while the current was monitored using a LANHE-CT 2001A system.
The ionic conductivity of the electrolyte was evaluated using a custom-designed two-electrode coin cell. Two stainless steel electrodes (diameter: 16 mm, thickness: 1 mm) were symmetrically positioned between a polytetrafluoroethylene (PTFE) disc (thickness: 0.027 inches, approximately 0.686 mm). A glass-fiber separator (diameter: 18 mm) saturated with electrolyte was placed within the washer, restricting its surface area to a predefined value. The electrolyte conductivity was then determined using EIS with the following equation:
where R represents the measured ionic resistance, and A and L denote the area of the electrodes and the distance between them, respectively. Data were collected over a temperature range of 40 °C to −60 °C using a JHY-H-50L temperature chamber, which maintained the cell at a specified temperature for 2 h prior to each measurement.
The transfer numbers of the electrolytes were determined using a widely recognized potentiostatic polarization technique with a CHI-760E instrument. The transfer coefficient was then calculated using the following equation:
where ΔV represents the applied bias, R0 is the initial cell impedance, and RSS is the steady-state cell impedance.
To evaluate the CE of Li||Cu cells, Aurbach’s method was applied. The overall CE was calculated using the following equation45
Qc represents the deposition or dissolution capacity over n cycles, with a fixed value of 1 mAh cm−2. Qt is the initial Li reservoir capacity deposited on the Cu foil, while Qs denotes the capacity finally stripped from the Cu foil.
Pouch cells were cycled within a voltage range of 3.0–4.3 V at a rate of 0.1 C at 25 °C. Additionally, pouch cells were cycled within a voltage range of 2.8–4.3 V at a rate of 0.05 C at −40 °C. The cycling performance of the pouch cells was evaluated using a fixed device that applied an external pressure of 1.0 MPa.
Material characterizations
In this study, nuclear magnetic resonance (NMR) spectroscopy was employed to characterize the organic solvents and electrolytes, with each analysis involving the dissolution of a 20 μL sample in deuterated chloroform1.H and 7Li NMR spectra were obtained using Bruker 400 MHz and Bruker AVANCE NEO 600 MHz spectrometers, respectively. Gel permeation chromatography (GPC) measurements (1260 Infinity II, Agilent Technologies) were conducted by dissolving the polymerization product in THF. The wettability of the four electrolytes under investigation was characterized using a contact angle (CA) measuring instrument (JC2000DS2), with the electrolyte volume controlled at 10 μL per measurement. Raman spectroscopy data of the electrolytes were collected using a LabRAM HR Evolution Raman micro-spectrometer equipped with a 633 nm laser. The characterization of electrolyte solvents was directly performed in the air environment. The morphologies of Li deposition in different electrolytes were observed by FESEM (JSM-7800F, JEOL), while HR-TEM (JEM-2100F, JEOL) was used to examine the thickness and morphology of the CEI. Cryo-TEM images were obtained using a transmission electron microscope (Talos F200X G2) under liquid nitrogen at 200 kV. The roughness and potential of the lithium-embedded negative electrode surface were measured by KPFM (SPM-9700HT). XPS data were collected using a Thermo Scientific K-Alpha+. The analysis chamber had a vacuum of ~2 × 10−7 mbar, with an X-ray source: monochromatic Al Kα source, energy: 1486.6 eV, voltage: 12 kV, beam current: 6 mA, and an analyzer scanning mode of CAE. The work function was 4.2 eV. Depth-profiling XPS was performed at an etching rate of 0.2 nm s−1. ToF-SIMS (PHI nano ToF II, ULVAC-PHI) was also employed to investigate the components of SEI and CEI, with an analysis area of 100 μm × 100 μm and a sputtering area of 400 μm × 400 μm. The negative electrode was removed from cycled cells and completely sealed before being taking out of the argon-filled glove box. There was a brief exposure to air (<30 s) during the transfer to the instrument for testing. When conducting Cryo-TEM, a cryogenic vacuum sample stage (Manufacturer: Fischione, model: 2550) was used to prevent the sample from being exposed to the air environment. KPFM measurements were performed in an argon-filled atmosphere chamber.
XRD data were obtained using a Bruker D8 Advance X-ray diffractometer, equipped with a LynxEye one-dimensional detector, and operated with Cu-Ka radiation at 40 kV and 40 mA (λ = 1.5418 Å). The measurements were carried out with a step size of 0.02° and a counting time of 0.1 s per step. Operando XRD experiments were conducted during the charging and discharging cycles at a current density of 0.5 mA cm−2, maintained at 25 °C, with diffraction patterns collected every 8 min. The assembly and materials used for the in situ battery are consistent with these of the Li||NCM 811 coin cells described earlier, with only the positive and negative electrodes replaced by in-situ devices. The LIB-MS-R device, provided by Beijing Scistar Technology Co. Ltd, simulates a Li+ coin cell to monitor lithium dendrite growth and its associated changes. This device consists of two platinum electrodes, each with a diameter of 8 mm, and a 1 × 1 cm PTFE separator.
Density functional theory calculations
DFT calculations were performed using the Gaussian 16 quantum chemistry software. Initially, all molecules were pre-optimization at the B3LYP/6-31 G* level. Subsequently, the Molclus program was used to explore various configurations of the complex. The optimized geometry was obtained by applying the DFT-D3 van der Waals (vdW) correction proposed by Grimme. During this process, all atoms were allowed to relax until the atomic force on each atom fell below 0.005 eV Å−1, and the energy was minimized to less than 1.0 × 10−6 eV. A total of 200 initial configurations were generated, each of which was optimized at the B3LYP/3-21 G* level. The configuration with the lowest energy was then further optimized at the B3LYP/6-311 G(d) level. Intermolecular interactions were characterized using the Grimme d3bj dispersion model. The binding energy was calculated according to the following equation53.
MD simulations
All MD simulations were performed using the GROMACS 2023 simulation package54,55. The systems were modeled with the OPLS-AA force field56. Parameters for THF and TO molecules were obtained from the LigParGen web server, while those for Li+ and FSI− were taken from the works of Jensen et al.57 and Lopes et al.58, respectively. The Lorentz-Berthelot mixing rules were applied to compute the Lennard-Jones (L-J) parameters for cross-interactions. The molar ratios of the electrolytes were determined based on the experimental component of this study. Periodic boundary conditions were imposed in all three dimensions. The Particle Mesh Ewald (PME) method was used to calculate long-range electrostatic interactions, with a cutoff distance of 1.2 nm for real-space interactions. Additionally, a short-range vdW cutoff of 1.2 nm was applied.
The initial simulation boxes were constructed using Packmol59. After energy minimization, all systems were equilibrated for 20 ns in the NPT ensemble and 35 ns in the NVT ensemble, respectively. Each production run was performed for 5 ns in the NVT ensemble, with a time step of 2 fs and data saved every 0.2 ps. The system’s temperature was controlled using the Nosé-Hoover thermostat, maintaining a simulated temperature of 233.15 K. Pressure coupling was applied using the Berendsen method, ensuring that the pressure was maintained at 1 bar60. Visualization of the results was carried out using the VMD software package. The binding energy of Li+ was calculated by including the internal energy term (ΔEint), vdW interactions(ΔEvdW), and electrostatic interactions (ΔEele), employing the Generalized Born model through the gmx_MMPBSA61.
Data availability
All data supporting the findings of this study are presented in the manuscript and Supplementary Information, or are available from the corresponding author upon request. Source data are provided with this paper.
References
Zhou, G., Chen, H. & Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 7, 312–319 (2022).
Piao, Z. et al. Stable operation of lithium metal batteries with aggressive cathode chemistries at 4.9 V. Angew. Chem. Int. Ed. 62, e202300966 (2023).
Zhang, Q.-K. et al. Homogeneous and mechanically stable solid–electrolyte interphase enabled by trioxane-modulated electrolytes for lithium metal batteries. Nat. Energy 8, 725–735 (2023).
Cheng, X.-B., Zhang, R., Zhao, C.-Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Yoon, M. et al. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 6, 362–371 (2021).
Xia, Y. et al. Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries. Nat. Energy 8, 934–945 (2023).
Feng, Y. et al. Challenges and advances in wide-temperature rechargeable lithium batteries. Energy Environ. Sci. 15, 1711–1759 (2022).
Holoubek, J. et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 6, 303–313 (2021).
Zhang, W. et al. A reversible self-assembled molecular layer for lithium metal batteries with high energy/power densities at ultra-low temperatures. Energy Environ. Sci. 17, 4531–4543 (2024).
Li, Z. et al. Tailoring polymer electrolyte ionic conductivity for production of low- temperature operating quasi-all-solid-state lithium metal batteries. Nat. Commun. 14, 482 (2023).
Xian, J.-J. et al. Spin mapping of intralayer antiferromagnetism and field-induced spin reorientation in monolayer CrTe2. Nat. Commun. 13, 257 (2022).
Wang, W.-W. et al. Evaluating solid-electrolyte interphases for lithium and lithium-free anodes from nanoindentation features. Chem 6, 2728–2745 (2020).
Zhang, Z. et al. Capturing the swelling of solid-electrolyte interphase in lithium metal batteries. Science 375, 66–70 (2022).
Yao, Y.-X. et al. Ethylene-carbonate-free electrolytes for rechargeable li-ion pouch cells at sub-freezing temperatures. Adv. Mater. 34, 2206448 (2022).
Lu, D. et al. Ligand-channel-enabled ultrafast Li-ion conduction. Nature 627, 101–107 (2024).
Rustomji, C. S. et al. Liquefied gas electrolytes for electrochemical energy storage devices. Science 356, eaal4263 (2017).
Yang, Y. et al. High-efficiency lithium-metal anode enabled by liquefied gas electrolytes. Joule 3, 1986–2000 (2019).
Yamada, Y., Wang, J., Ko, S., Watanabe, E. & Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Gu, R. et al. An ether-based electrolyte solvation strategy for long-term stability and ultra-low temperature Li-metal batteries. Adv. Funct. Mater. 34, 2310747 (2024).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Zhou, P. et al. Rationally designed fluorinated amide additive enables the stable operation of lithium metal batteries by regulating the interfacial chemistry. Nano Lett. 22, 5936–5943 (2022).
Meng, Y. et al. Designing phosphazene-derivative electrolyte matrices to enable high-voltage lithium metal batteries for extreme working conditions. Nat. Energy 8, 1023–1033 (2023).
Xue, W. et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 6, 495–505 (2021).
Liu, J. et al. A comparison of carbonate-based and ether-based electrolyte systems for lithium metal batteries. J. Electrochem. Soc. 170, 010535 (2023).
Li, X. et al. Fast interfacial defluorination kinetics enables stable cycling of low-temperature lithium metal batteries. J. Am. Chem. Soc. 146, 17023–17031 (2024).
Wu, H. et al. LiDFOB initiated in situ polymerization of novel eutectic solution enables room-temperature solid lithium metal batteries. Adv. Sci. 7, 2003370 (2020).
Zhang, J. et al. Smart deep eutectic electrolyte enabling thermally induced shutdown toward high-safety lithium metal batteries. Adv. Energy Mater. 13, 2202529 (2023).
Jin, C.-B. et al. Taming solvent–solute interaction accelerates interfacial kinetics in low-temperature lithium-metal batteries. Adv. Mater. 35, 2208340 (2023).
Chen, Y. et al. Steric effect tuned ion solvation enabling stable cycling of high-voltage lithium metal battery. J. Am. Chem. Soc. 143, 18703–18713 (2021).
Yao, N., Chen, X., Fu, Z.-H. & Zhang, Q. Applying classical, ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries. Chem. Rev. 122, 10970–11021 (2022).
Ruan, D. et al. Solvent versus anion chemistry: unveiling the structure-dependent reactivity in tailoring electrochemical interphases for lithium-metal batteries. JACS Au 3, 953–963 (2023).
Fang, M. et al. A temperature-dependent solvating electrolyte for wide-temperature and fast-charging lithium metal batteries. Joule 8, 91–103 (2024).
Liu, F. et al. Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries. Sci. Adv. 4, eaat5383 (2018).
Luo, L. et al. Enabling ultralow-temperature (−70 °C) lithium-ion batteries: advanced electrolytes utilizing weak-solvation and low-viscosity nitrile cosolvent. Adv. Mater. 36, 2308881 (2024).
Fu, J. et al. Lithium nitrate regulated sulfone electrolytes for lithium metal batteries. Angew. Chem. Int. Ed. 59, 22194–22201 (2020).
Yamada, Y. et al. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 136, 5039–5046 (2014).
Wang, J. et al. Visualizing and regulating dynamic evolution of interfacial electrolyte configuration during de-solvation process on lithium-metal anode. Angew. Chem. Int. Ed. 63, e202400254 (2024).
Marino, C. et al. Solvation and dynamics of lithium ions in carbonate-based electrolytes during cycling followed by operando infrared spectroscopy: the example of NiSb2, a typical negative conversion-type electrode material for lithium batteries. J. Phys. Chem. C. 121, 26598–26606 (2017).
Wu, L. et al. Lithium nitrate mediated dynamic formation of solid electrolyte interphase revealed by in situ Fourier transform infrared spectroscopy. Electrochim. Acta 466, 142973 (2023).
Zhang, S. et al. Oscillatory solvation chemistry for a 500 Wh kg−1 Li-metal pouch cell. Nat. Energy 9, 1285–1296 (2024).
Ma, B. et al. Molecular-docking electrolytes enable high-voltage lithium battery chemistries. Nat. Chem. 16, 1427–1435 (2024).
Chen, Y. et al. Breaking solvation dominance of ethylene carbonate via molecular charge engineering enables lower temperature battery. Nat. Commun. 14, 8326 (2023).
Hahn, M. et al. Investigating solid polymer and ceramic electrolytes for lithium-ion batteries by means of an extended distribution of relaxation times analysis. Electrochim. Acta 344, 136060 (2020).
Lu, Y., Zhao, C.-Z., Huang, J.-Q. & Zhang, Q. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 6, 1172–1198 (2022).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J.-G. Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Chen, Y. et al. Armoring LiNi1/3Co1/3Mn1/3O2 cathode with reliable fluorinated organic–inorganic hybrid interphase layer toward durable high rate battery. Adv. Funct. Mater. 30, 2000396 (2020).
Zhao, Q., Stalin, S. & Archer, L. A. Stabilizing metal battery anodes through the design of solid electrolyte interphases. Joule 5, 1119–1142 (2021).
Zhang, D. et al. Lithium hexamethyldisilazide as electrolyte additive for efficient cycling of high-voltage non-aqueous lithium metal batteries. Nat. Commun. 13, 6966 (2022).
Sun, J. et al. The origin of high-voltage stability in single-crystal layered Ni-rich cathode materials. Angew. Chem. Int. Ed. 61, e202207225 (2022).
Wei, Y. et al. Kinetics tuning of Li-ion diffusion in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 137, 8364–8367 (2015).
Chu, Y. et al. Thermodynamically stable dual-modified LiF&FeF3 layer empowering Ni-rich cathodes with superior cyclabilities. Adv. Mater. 35, 2212308 (2023).
Jiang, F.-N. et al. Thermoresponsive electrolytes for safe lithium-metal batteries. Adv. Mater. 35, 2209114 (2023).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Kaminski, G. A., Friesner, R. A., Rives, J. T. & Jorgensen, W. L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 105, 6474–6487 (2001).
Jorgensen, W. L., Maxwell, D. S. & Rives, J. T. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225 (1996).
Vilseck, J. Z., Rives, J. T. & Jorgensen, W. L. Evaluation of CM5 charges for condensed-phase modeling. J. Chem. Theory Comput. 10, 2802–2812 (2014).
Jensen, K. P. & Jorgensen, I. L. Halide, ammonium, and alkali metal ion parameters for modeling aqueous solutions. J. Chem. Theory Comput. 2, 1499–1509 (2006).
Lopes, J. N. C. & Pádua, G. A. H. Molecular force field for ionic liquids composed of triflate or bistriflylimide anions. J. Phys. Chem. B 108, 16893–16898 (2004).
Martinez, M., Andrade, R., Birgin, E. G. & Martinez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157 (2009).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182 (1981).
Tresanco, M. S. V., Tresanco, M. E. V., Valiente, P. A. & Moreno, E. gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Comput. 17, 6281–6291 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22479094, 22075174), the Science and Technology Commission of Shanghai Municipality (20520740900 and 19DZ2271100), and the International Joint Laboratory on Resource Chemistry.
Author information
Authors and Affiliations
Contributions
R.G. conceived the idea, performed the experiment, and wrote the manuscript. D.Z. performed MD simulations. S.X. and X. G. contributed to the results discussion and data analysis. Y.X. and Z.S. carried out some electrochemical experiments and analysis. Q.X. and S.G. conducted DFT calculations and analysis. K.L. contributed to the DRT analysis. J.X., S.Z., P.S., and Y.M. supervised this work, discussed the results, and revised this manuscript. All the authors discussed the results and assisted with the manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kyung-Koo Lee and the other, anonymous, reviewers for their contribution to the peer review of this work. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, R., Zhang, D., Xu, S. et al. Thermoresponsive ether-based electrolyte for wide temperature operating lithium metal batteries. Nat Commun 16, 5474 (2025). https://doi.org/10.1038/s41467-025-60524-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60524-8