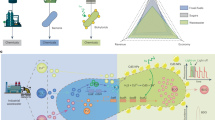
Abstract
Bio-denitrification is vital in wastewater treatment plants (WWTPs), yet its integration with naturally abundant thermal energy remains unexplored. Here, we introduce a biohybrid-based pyroelectric bio-denitrification (BHPD) process that harnesses thermoelectric energy from ambient temperature fluctuations. By integrating Thiobacillus denitrificans with tungsten disulfide (WS2), we develop a biohybrid system that achieves complete denitrification over three 5-day cycles under 5 °C temperature fluctuations. WS2 either precipitates on the cellular surface or is internalized by cells, generating pyroelectric charges that serve as reducing equivalents to drive bio-denitrification. In real wastewater, the BHPD process enhances nitrate removal by up to 8.09-fold under natural temperature fluctuations compared to stable-temperature conditions. Life-cycle assessment demonstrates that the BHPD process has significantly lower environmental impacts than the conventional anaerobic-anoxic-oxic process, and cost analysis confirms its economic feasibility. Our findings highlight the potential of the pyroelectric effect in enhancing bio-denitrification, offering valuable insights for a paradigm shift in WWTPs.
Similar content being viewed by others
Introduction
Global urbanization is driving an increasing demand for more effective infrastructure, particularly wastewater treatment plants (WWTPs), to manage the escalating volumes of sewage while complying with ever-stricter regulations1,2,3. Denitrification plays a crucial role in WWTPs by addressing the growing challenge of nitrogen removal and facilitating the conversion of approximately half of the reactive nitrogen into dinitrogen gas4. Traditional denitrification in these plants heavily relies on the anaerobic-anoxic-oxic (A-A-O) process5. However, the low ratio of influent chemical oxygen demand (COD) to nitrogen (COD/N) usually seen in wastewater6, necessitates the addition of supplementary organic carbon sources such as glucose, acetate, and methanol as electron donors to facilitate heterotrophic denitrification. This practice consumes substantial energy and chemicals, leading not only to an increase in operational costs of 3.5–8.5% per m3 6, but also contributing over 20% to the carbon footprint of wastewater treatment7. Although hydrogen gas (H2) can serve as an effective inorganic electron donor for autotrophic denitrification, its low solubility, coupled with challenges related to transportation and storage, has constrained its use in wastewater treatment. This restricted application necessitates the adoption of complex auxiliary processes, such as membrane-aerated biofilm reactors (MABRs), to minimize wastage8. Thus, it’s urgent to develop new technologies to address these problems.
More than 60% of the primary energy produced globally each year is lost as heat9. For instance, solar energy, the most abundant renewable resource on Earth, provides as much energy in just one hour (4.3 × 1020 J) as the entire world consumes in a year (4.1 × 1020 J)10,11. However, this energy is often wasted as heat, with daily solar exposure causing temperature variations of 10–15 °C, depending on the physical properties of water systems12,13. In addition to solar energy, thermal energy can also be derived from other sources, such as sewage in WWTPs. Approximately 40% of the heat generated in residential buildings is discharged into the sewage system14. Lowing the temperature of 1 m3 of wastewater by 1 °C can generate ~1.16 kWh of heat, making sewage a cost-effective and compelling source of thermal energy15. This clean and abundant thermal energy can induce temperature fluctuations that trigger pyro-catalysis with pyroelectric materials. In this approach, the oscillation of electric dipoles within pyroelectric materials generates polarized charge carriers, enabling reactions such as H2 evolution with 2D few-layer black phosphorene (15–65 °C)16 or BaTiO3 particles (40–70 °C)17, CO2 reduction to methanol with Bi2WO6 nanosheets (15–70 °C)18, and degradation of Rhodamine B with NaNbO3 nanorods (23–50 °C)19 or ZnO nanorods (22–62 °C)20. Therefore, pyro-catalysis driven by thermal energy has garnered increasing attention in recent years as a promising method for efficient redox processes.
However, the connection between pyro-catalysis and microbial metabolism, such as bio-denitrification in WWTPs, has received limited attention. This oversight may stem from the fact that the pyro-catalysis efficiency is directly proportional to both the rate and intensity of temperature fluctuation21. In contrast, the traditional view holds that microbial activity is highly temperature-dependent, with even small temperature fluctuations potentially affecting community stability and diversity22,23. This discrepancy poses challenges to the potential synergy between pyro-catalysis and bio-denitrification using abundant thermal energy, a process known as pyroelectric bio-denitrification. However, recent studies have demonstrated that bacteria can alter their cell morphology and composition in response to stressful environments24, suggesting that denitrifying bacteria may be capable of self-adjusting to specific temperature fluctuations. This is further supported by the successful isolation of denitrifying bacteria from extreme environments, demonstrating their remarkable capacity to denitrify effectively beyond their optimal temperature range25, indicating potential compatibility with pyroelectric systems. In light of these insights, tungsten disulfide (WS2), a member of the tungsten family distinguished by its high surface-to-volume ratio, excellent pyroelectric properties, and exceptional carrier mobility26,27, emerges as a promising pyroelectric material. Meanwhile, Thiobacillus denitrificans (T. d), a facultative autotrophic and electrotrophic denitrifier prevalent in diverse aquatic ecosystems28, serves as an ideal microbial counterpart. The complementary properties of WS2 and T. d render them promising candidates for constructing a biohybrid system that couples thermal energy harvesting with efficient bio-denitrification.
Here, we present a biohybrid-based pyroelectric bio-denitrification (BHPD) process by incorporating WS2 and T. d (Fig. 1a). The constructed T. d-WS2 biohybrids enable complete bio-denitrification over three successive 5-day cycles in synthetic wastewater at near room temperature (25–30 °C). Temperature variations of just 5 °C significantly reduce energy consumption for temperature control while demonstrating tolerance to fluctuations during heating-cooling cycles. Furthermore, we reveal that WS2 can either precipitate on the cellular surface or be internalized by T. d, supplying sustainable pyroelectric charges as efficient reducing equivalents for denitrification (Fig. 1b). Implementing in real wastewater treatment, we achieve a maximum 8.09-fold increase of nitrate removal with the BHPD process under natural temperature fluctuations, comparing to conditions without temperature variation. Life cycle assessment (LCA) and cost analysis indicate the advantage of pyroelectric bio-denitrification over conventional processes.
a Depiction of T. d-WS2, proceeding pyroelectric bio-denitrification via temperature fluctuations during heating-cooling cycle. b Construction of T. d-WS2 biohybrids. c Proposed pathways for pyroelectric bio-denitrification by T. d-WS2. The electrons generated from both intracellular and extracellular WS2 under temperature variation were utilized by T. d for NO3− reduction to N2/N2O.
Results and discussion
Pyroelectric bio-denitrification performance with T. d-WS2
Thiobacillus denitrificans (T. d, DSM 12475) bacteria were inoculated and cultured in the thiosulfate-containing medium (Medium I, Supplementary Table 1) for 2 days. Based on the results of preliminary experiments (Supplementary Fig. 1), 7.5 mg of WS2 was then added. Comprising nanosheets and nanoparticles (Supplementary Fig. 2, Supplementary Note 1), WS2 exhibited excellent biocompatibility (Supplementary Fig. 3). The mixture was then cultured at 30 °C and 120 rpm until the growth of T. d reached an exponential growth phase (optical density at 600 nm (OD600) ≈ 0.2). Subsequently, the well-constructed T. d-WS2 biohybrids were centrifuged, separated from the supernatant, and redispersed in the thiosulfate-free medium (Medium II, Supplementary Table 1) for pyroelectric bio-denitrification experiments over a period of 5 days via heating-cooling cycling (3/30 min). These experiments were carried out under infrared radiation with an optical power density of 400 mW cm−2 and a temperature fluctuation range of 5 °C, starting from an initial temperature of 25 °C (Supplementary Figs. 4 and 5). As shown in Fig. 2a, during the heating period (∆T > 0), temperature variations are speculated to cause the oscillation and alignment of internal electric dipoles, reducing the polarization magnitude and disrupting the electrical balance between screening charges and bound polarization charges. As a result, some screening charges turn into pyroelectric free charges. Conversely, during the cooling period (∆T < 0), the polarization intensity increased, and free charges are obliged to redistribute to compensate for the dipole change. Simultaneously, charges opposite to screening charges are generated on the surfaces. As the selected heating period (3 min) is significantly shorter than the natural cooling period (30 min), WS2 theoretically generates approximately ten times more charges during the heating process than during the cooling process, as the pyroelectric charge is proportional to the rate of temperature change (dT/dt)29. These pyroelectric charges are hypothesized to act as effective reducing equivalents for T. d, thereby facilitating the bio-denitrification process. This hypothesis was confirmed by the pyroelectric bio-denitrification experiments, in which T. d-WS2 achieved nearly 100% NO3− reduction over 5 days (Fig. 2b), employing sodium lactate as sacrificial agents according to the preliminary experiments (Supplementary Fig. 6) and methyl viologen (MV2+) as electron shuttles. In sharp contrast, the removal of T. d, WS2, sodium lactate, and MV2+ led to a significant decrease in NO3− reduction rate (Fig. 2b, Supplementary Fig. 7), demonstrating the synergistic cooperation of these components. Approximately 20% of NO3− removal by T. d-WS2 without a temperature change can primarily be attributed to the release of a small amount of sulfur from WS2, which serves as an electron donor for T. d30. This interpretation is substantiated by the significant decrease in sulfur concentration observed during the first 3 days (Supplementary Fig. 8). Further investigations confirmed that the superior NO3− reduction performance of T. d-WS2 was independent of light irradiation, as comparable results were achieved using a water bath for temperature control instead of infrared radiation (Supplementary Fig. 9). This finding was further corroborated by the UV–vis spectrum of WS2, which revealed a direct band gap (Eg) of 3.12 eV (λadsorption = 397 nm, Supplementary Fig. 10a, b). This value exceeds the energy range of infrared radiation (wavelength range: 600–1000 nm, Supplementary Fig. 4), indicating that infrared illumination cannot induce the generation of photogenerated electrons. As a result, the influence of photoelectrons is eliminated during pyroelectric bio-denitrification. In addition, the NO3− reduction performance with T. d-WS2 showed a linear correlation with the temperature variation rate, and the dosages of T. d (Supplementary Fig. 11), attributed to the improved efficiencies of pyroelectric charge production and utilization for denitrification. Analysis of nitrogenous products revealed that N2O-N was the primary denitrification product (78.4%) during pyroelectric bio-denitrification (Supplementary Table 2). This may be due to the inhibition of N2O reductase by reactive oxygen species (ROS), which were confirmed in the reaction system through the electron paramagnetic resonance (EPR) spectra (Supplementary Fig. 12). Similar results were also observed in the NO3− reduction facilitated by photoelectric and piezoelectric bio-denitrification5,31. Remarkably, the pyroelectric bio-denitrification with T. d-WS2 proceeded continuously and completely over three successive 5-day cycles (i.e., a total of 15 days, Fig. 2c). The isotope labeling experiment with K15NO3 as electron acceptors further demonstrated that the produced N2/N2O stemmed from NO3− reduction, as evidenced by the peaks at m/z = 30 (15N2) and m/z = 46 (15N2O) (Fig. 2d).
a Schematic illustration for the pyroelectric bio-denitrification process by T. d-WS2 via heating-cooling cycling (3/30 min). b NO3− reduction by T. d-WS2 and deletional controls. c Typical time course of NO3− reduction by T. d-WS2 over three successive 5-day cycles. d Labeled 15N-KNO3 reduction by T. d-WS2. Signal intensity is presented in arbitrary units (arb. units). e, f ToF-SIMS imaging of 15N (e) and 13C (f) in T. d-WS2 (the inset images show T. d-WS2 without temperature variation); representative of 10 images. g Variation of the cell biomass in T. d-WS2 over three successive 5-day cycles. h CLSM images of T. d-WS2 (Red and green colors represent the dead and live cells, respectively); representative of 10 images. Data are presented as mean values ± SD derived from n = 3 independent experiments. Scale bars: 50 μm in (e, f) 50 μm in the insert images, and 20 μm in (h). Source data are provided as a Source Data file.
Autotrophic denitrification by denitrifying bacteria can stimulate their own growth and biomass enrichment32. Time-of-flight secondary ion mass spectrometry (TOF-SIMS) was employed to illustrate carbon (C) and nitrogen (N) assimilation by T. d utilizing pyroelectric charges as electron donors. A substantial enrichment of 15N (Fig. 2e) and 13C (Fig. 2f) was observed compared to the constant-temperature control, with δ 13C level of 2873‰ and δ 15N level of 31317‰ (Supplementary Fig. 13). The minor 13C signal detected in the control (Supplementary Fig. 14) is consistent with the fact that all living organisms naturally contain small amounts of 13C in their carbon atoms33. Further research showed higher cell biomass (Fig. 2g) and increased cell viability and count in T. d-WS2 in response to temperature fluctuations (Fig. 2h, Supplementary Fig. 15), providing direct evidence of cellular uptake during pyroelectric bio-denitrification. Three-dimensional excitation-emission matrix (3D-EEM) spectra revealed that the intensities of components in cellular polymeric substances (EPS), including tyrosine-like (regions I), protein-like (regions II), fulvic acid-like (regions III and IV) substances, became stronger in T. d-WS2 during pyroelectric bio-denitrification, comparing to conditions without temperature variation (Supplementary Fig. 16). These substances served as transient media to enhance microbial extracellular electron transfer34,35.
Surface precipitation of WS2 nanosheets in biohybrid systems
Although WS2 is typically considered hydrophobic, its exfoliation in aqueous media using ultrasonic methods (i.e., ultrasound-mediated shear forces) imparts substantial negative surface charges to the WS2 nanosheets36. This leads to the compression of the electrical double layer around the negatively charged nanosheets, allowing the layers to remain bound together through classical Derjaguin–Landau–Verwey–Overbeek interactions37. This inference was confirmed by the SEM image (Fig. 3a), which showed nanosheet aggregates composed of S and W elements, providing a larger surface area for the close and abundant accumulation of T. d (Supplementary Fig. 17). The composition of W-S aggregates in biohybrids was further ascertained by Raman spectra with peaks at 351 cm−1 (E12g) and 415 cm−1 (A1g) (Supplementary Fig. 18a), which were identified as distinct signals of WS238. Consistent results were obtained by X-ray diffraction (XRD) patterns (Supplementary Fig. 18b), where a standard diffraction peak at 14.36o was observed (JCPDS #08-0237), corresponding to the 2H phase (trigonal prismatic D3h) of WS2 with an S-W-S interplanar distance of 6.18 Å (Supplementary Fig. 18c)39. A new (002) characteristic peak at 9.44o with the interplanar distance of 9.20 Å was detected, demonstrated the co-existence of 1 T phase (octahedral Oh) WS240. The presence of both 1 T and 2H phases of WS2 in biohybrids was further confirmed by the selected area electron diffraction (SAED) pattern (Supplementary Fig. 18c), might contribute to the unique pyroelectric properties of biohybrids.
a SEM image of T. d-WS2 (the inset image shows bare T. d); representative of 10 images. b, c Temperature (b) and potential (c) distribution in WS2 via finite element analysis with COMSOL. d SECM topographic images of WS2; representative of 10 images. e I-t curves under a heating/cooling cycle (30/30 s). f Correlation analysis of pyro-current intensity with temperature variation rate and WS2 dosage. Data are presented as mean values ± SD derived from n = 3 independent experiments. Statistical analysis was conducted with paired two-tailed t test. Scale bars: 2 μm in a, and 1 μm in the insert image. Source data are provided as a Source Data file.
Pyroelectric materials may exhibit ferroelectric properties, indicating their potential to generate an electric field via the imbalanced polarization charges21. Therefore, piezoelectric force microscope (PFM) was used to explore the ferroelectric behavior of WS2 in biohybrids. A distinct contrast between bright and dark regions in the vertical piezoresponse amplitude and phase images (Supplementary Fig. 19), indicative of ferroelectric domains oriented upwards and backwards, demonstrated varying polarization in the WS2 nanosheet aggregates. This was corroborated by the local piezoelectric hysteresis loop, showing a full 180° change in phase angle and a butterfly-like shape of the amplitude loop (Supplementary Fig. 20), thereby verifying the robust ferroelectric response of WS2 aggregates. Finite element (FE) analysis with COMSOL was further conducted, providing deeper insights into the underlying fluid dynamics, heat transfer, and electrochemical reactions of WS2 that are involved in the pyroelectric bio-denitrification process. The FE simulation used a simplified structural model with dimensions of 60 × 60 × 9 nm (detailed parameters in Supplementary Table 3). The results illustrated significant pyro-potential differences on the WS2 nanosheets in response to temperature variations (Fig. 3b, c). This analysis was validated through the atomic force microscopy (AFM) imaging, detecting higher potentials under temperature fluctuations (Supplementary Fig. 21). This was because the alternating cooling/heating excitation induced a net change of the electric dipole moment of the WS2 nanosheets, consequently leading to the generation of free charges. The distinct pyro-current response was confirmed by scanning electrochemical microscopy (SECM) topographic images (Fig. 3d), where higher currents were observed under infrared radiation. Consistent results were obtained from I-t curves under infrared radiation, showing a sharp increase in current intensity in WS2 when subjected to a rapid temperature rise from 25 °C to 30 °C in 30 s (Fig. 3e and Supplementary Fig. 22). Furthermore, upon the instantaneous temperature decrease resulting from turning off the infrared radiation, a reverse current was produced due to redistribution of the pyroelectric charges in WS2. This current was independent of possible light energy, as evidenced by comparable values under dark temperature cycling conditions with a water bath (Supplementary Fig. 23a) and negligible pyro-current signals under xenon lamp irradiation (Supplementary Fig. 23b). The introduction of T. d resulted in a significant reduction of peak pyro-current intensity, possibly due to the utilization of electrons by T. d for metabolism (see detailed discussion below). In addition, a correlation between pyro-current intensity and temperature variation rate was observed (Fig. 3f), possibly owing to the increased movement of pyroelectric charges under higher temperature variation rates. Furthermore, pyro-current intensity was found to be proportional to the WS2 dosage (Fig. 3f), likely due to the larger surface area facilitating enhanced thermal exchange, resulting in a greater pyroelectric current output.
Cellular internalization of WS2 nanoparticles in biohybrid systems
Nanoparticles can be internalized by living cells and significantly influence microbial metabolisms41. To investigate the potential internalization of WS2 nanoparticles into T. d, the constructed T. d-WS2 biohybrids were subjected to ultrasonication and centrifugation to remove surface-bound WS2 from the T. d cells. This process resulted in a significantly lower cell roughness (Supplementary Fig. 24a, b), approaching a similar condition observed in bare T. d (Supplementary Fig. 24c), along with a smoother cell surface (Supplementary Fig. 24d–f). Subsequent TEM cross-section observation revealed intracellular presence of nanomaterials, typically sized between 20 and 50 nm (Fig. 4a). EDS mapping further demonstrated that these nanomaterials and their clusters consisted of S and W elements (Supplementary Fig. 25), verifying the successful transportation of WS2 into the cytoplasm of T. d. In contrast to the bare T. d, which showed no luminescence emission in a completely dark image (Supplementary Fig. 26), yellow WS2 fluorescence was detected after its internalization by T. d, and it perfectly matched the shape of the cell (Supplementary Fig. 27). Figure 4b displayed a detailed presentation of the emission intensity profile from the bottom through the top cross-sections of T. d with a step increment of 0.04 μm along the Z direction. Such cellular internalization of WS2 is possibly attributed to its attachment to biorecognition molecules such as peptides, antibodies, nucleic acids or small-molecule ligands (energy-dependent pathways), as well as passive diffusion (energy-independent pathways)42,43,44.
a Representative TEM images of cross-section slices of the WS2-fed cells, where the dark clusters in the solid-line circles are assigned to the accumulated WS2 nanoparticles (the inset image shows bare T. d)); representative of 10 images. b Stacked confocal images of T. d cells at different heights Z show the distribution of WS2 throughout the cell; representative of 10 images. c–e Variation of NO3− reduction rate (P1 = 0.0269, P2 = 3.5738 × 10−9) (c), ATP concentration (P3 = 4.7999 × 10−6, P4 = 8.6249 × 10−5) (d), and NADPH/NADP+ ratio (P5 = 0.0005, P6 = 0.0008) (e) in T. d-WS2. Data are presented as mean values ± SD derived from n = 3 independent experiments. Statistical analysis was conducted with paired two-tailed t test: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Scale bars: 300 nm in (a), and 300 nm in the insert images. Source data are provided as a Source Data file.
After confirming the successful internalization of WS2 by T. d, we proceeded to evaluate its contribution to the pyroelectric bio-denitrification performance of WS2-T. d. Following a 5-day period, the NO3− concentration with the WS2-internalized T. d was reduced by 25%, indicating its approximate contribution to the pyroelectric bio-denitrification performance of WS2-T. d (Fig. 4c). Ammonia (NH4+) was simultaneously produced via the assimilatory nitrate reduction pathway during the pyroelectric bio-denitrification (Supplementary Table 2), and T. d can utilize NH4+ to synthesize reductive organic nitrogen compounds, such as amino acids45. These compounds, coupled with another cellular components (such as NADH and ATP), can subsequently serve as sacrificial reagents (i.e., electron donors), helping to main an electron balance in the biohybrid system during endogenous denitrification46. This was confirmed by a higher ATP concentration (37.46% increase, Fig. 4d) and an improved NADPH/NADP+ ratio (46.50% increase, Fig. 4e) under temperature fluctuations, providing more energy for bio-denitrification47. In addition, electrochemical impedance spectroscopy (EIS) revealed reduced electron transfer resistance with WS2-internalized T. d under temperature fluctuations, promoting intracellular electron transfer and utilization (Supplementary Fig. 28a). This inference was further proven by differential pulse voltammetry (DPV) analysis, where higher peaks of electrochemical active sites were detected (Supplementary Fig. 28b). These electrochemical active sites probably include cytochromes34, as evidenced by their increased abundance demonstrated in heme-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (Supplementary Fig. 29, Supplementary Note 2). The findings were consistent with previous research showing that intracellular nanomaterials could enhance charge transfer and microbial metabolism through electrostatic or coordination interaction with cytochromes or enzymes48.
Metabolic responses of T. d during pyroelectric bio-denitrification
To comprehensively understand the pyroelectric bio-denitrification process with T. d-WS2, the metabolic responses of T. d for selective energy transfer under temperature fluctuations were evaluated using transcriptomic analyses. Figure 5a illustrates the general metabolic pathways of T. d, including the stress response, electron transport chain (ETC), denitrification, Calvin-Benson-Bassham (CBB) cycle, and gluconeogenesis (GNG) pathway. It was observed that genes coding the chaperones and stress proteins, such as clpB, grpE, dnaJ, and groES, were significantly upregulated during temperature fluctuations (Fig. 5b), facilitating the creation of more conducive microenvironments to sustain microbial viability under the heat shock and stress conditions49. Cytochromes were identified as the crucial electron carriers for energy transduction processes, speculated to be involved in the ETC process during pyroelectric bio-denitrification, as evidenced by their high-level expression. For instance, the expression of membrane-bound cytochrome b561, increased by 0.80-fold (P < 0.01) under temperature fluctuations. Subsequently, these gene regulators control another within a gene regulatory network. For instance, electrons were transferred to NADH dehydrogenase complex (complex I/II) to catalyze the transfer of two electrons from NADH to quinone (nuo, ~2.26-fold, P < 0.001), coupled with providing the proton resource required for ATP synthesis (atp, ~3.7-fold, P < 0.001)50,51,52. The high-energy reducing equivalents produced could be then used for bio-denitrification process (NO3− → NO2− → NO → N2O → N2), demonstrated by the significant upregulation of denitrification operons nar, nir, nor and nos by 0.55–2.58 times, 0.89–2.49 times, 1.83–6.98 times and 0.34 times, respectively. The higher expression of nor enabled T. d to efficiently convert NO into N2O, consistent with the product selectivity analysis discussion above53. In addition, the CBB cycle also serves a crucial electron sink for electron uptake by T. d54. Compared with the control group, the transcriptional levels of genes encoding the CBB cycle were upregulated during heating and cooling cycles. For instance, genes encoding aldehyde-3-phosphate dehydrogenase (gap2, ~1.2-fold, P < 0.001), fructose-1,6-bisphosphatase (fbp, ~1.0-fold, P < 0.001), transketolase (tkt, ~0.8-fold, P < 0.001), and phosphoribulokinase (cbbP, ~1.04-fold, P < 0.001) were highly expressed, facilitating CO2 fixation during pyroelectric bio-denitrification. T. d can convert organic intermediates produced in the CBB cycle into glucose or precursors for glycogen synthesis, thereby sustaining energy supply and metabolic homeostasis under oligotrophic conditions (i.e., in the absence of exogenous glucose)55 and facilitating environmental adaptation (e.g., to temperature fluctuations)56. This was supported by the upregulation of key genes encoding enzymes in the GNG pathway, including glucose-6-phosphate isomerase (pgi, ~1.29-fold, P < 0.001) and 2, 3-Bisphosphoglycerate-independent phosphoglycerate mutase (gpml, ~0.66-fold, P < 0.001).
a Schematic illustration for the metabolic response circuits of T. d for selective energy transfer under temperature fluctuations. The purple section represents the stress response process, the pink section represents denitrification process, the orange section represents electron transport chain (ETC), the green section represents Calvin-Benson-Bassham (CBB) cycle, and the blue section represents gluconeogenesis (GNG) pathway. The full names of the genes and products involved in the corresponding processes can be found in Supplementary Data 1. OM and IM denote the outer and inner membranes, respectively. b Heat maps for the expression levels of key genes with T. d-WS2 under temperature fluctuations and control (n = 3), and the log2 (Fold Change) values of genes with T. d-WS2 under temperature fluctuations. c Gene Ontology (GO) functional enrichment analysis of the key processes with T. d-WS2 during pyroelectric bio-denitrification. The size of the dots indicates the amount of the functionally enriched genes, and the color of the dots indicates the significance of the enriched genes. Statistical analysis was conducted with paired two-tailed t test and DESeq2 (1.20.0), with P-values adjusted using the Benjamini-Hochberg method. d Correlation analysis of gene expression by Pearson method and correlation analysis of cytochrome with other key genes by Mantel test analysis. The line color represents the value of the Mantel’s p, and the line thickness represents the value of Mantel’s r. Source data are provided as a Source Data file.
Gene ontology (GO) functional enrichment analysis was conducted to further validate the biological significance of the enriched genes, particularly focusing on nitrogen metabolism, carbon fixation, oxidative phosphprylation, carbon metabolism, and stress response (Fig. 5c). A total of 10 functional enrichments with 830 highly significant genes related to nitrogen metabolism was observed, encompassing various processes such as organonitrogen compound metabolic process, cellular nitrogen compound biosynthetic process, and cellular nitrogen compound metabolic process. Furthermore, the enriched genes also contributed to other crucial metabolic pathways during pyroelectric bio-denitrification, such as the oxidative phosphorylation process for ATP synthesis and electron transfer activity. Pearson analysis illustrated that some upregulated genes in denitrification, carbon fixation, and oxidative phosphorylation were interdependent. Cytochrome was positively correlated with nar and nir (Fig. 5d), highlighting its important role in bio-denitrification, particularly the outer membrane cytochrome, which may be involved in extracellular electron transfer (Fig. 5d). The results suggested that microbial metabolic processes were highly coordinated, and these interactions were critical for microbial survival and adaptation in adverse environments57,58.
To further confirm the role of outer membrane cytochromes in pyroelectric bio-denitrification, as suggested by transcriptomic analyses indicating their involvement in electron transfer from WS2 to T. d, proteinase K was employed to digest outer membrane proteins59. Flow cytometric analysis (Supplementary Fig. 30a) and cell permeability measurements (Supplementary Fig. 30b, c) indicated that the activity of T. d was not significantly affected at a concentration of 2.5 U mL−1 compared to the untreated control, suggesting that this dosage effectively digested outer membrane proteins without disrupting periplasmic and intracellular proteins (Supplementary Note 3). The intensity of the heme-containing proteins significantly decreased following proteinase K digestion (Supplementary Fig. 30d), providing further evidence for the removal of outer membrane cytochromes. The proteinase K-treated T. d was subsequently combined with WS2 to construct T. d-WS2 biohybrids for pyroelectric bio-denitrification. The results showed that the NO3− concentration was only decreased by 28% (Supplementary Fig. 31), which is consistent with the 25% reduction observed upon the removal of the surficial WS2 nanosheets acting as the electron source. Therefore, the findings from both the elimination of extracellular electron source (removal of extracellular WS2) and the disruption of extracellular electron transfer (digestion of outer membrane cytochromes) demonstrated that both extracellular and intracellular electron transfer contributed to pyroelectric bio-denitrification, with contributions of 25–28% and 72–75%, respectively.
Potential of pyroelectric bio-denitrification for actual wastewater treatment
To assess the effectiveness of the BHPD process for actual wastewater treatment, we collected three types of wastewater samples: swine wastewater, kitchen wastewater, and stainless-steel wastewater (the main compositions are detailed in Supplementary Table 4). The biohybrid system was constructed using 24 mL of these actual wastewater samples, 1 mL of inoculum sludge sourced from a denitrification tank, and 7.5 mg of WS2 (Supplementary Fig. 32). Compared to the WS2 control, which primarily utilized the organic matter in wastewater as an electron donor for bio-denitrification, the additional natural temperature fluctuations induced by the presence or absence of light significantly enhanced NO3− reduction. This resulted in a 0.75–0.85-fold increase in swine wastewater (Fig. 6a) and a 0.75–1.13-fold increase in kitchen wastewater (Fig. 6b) over two consecutive cycles. Additionally, the generation of nitrite and nitrous oxide was reduced by 28–43% and 31–53%, respectively. This reduction may be attributed to the presence of various coexisting organic compounds (e.g., acetate, methanol, ethanol, and ethylene glycol), low-valence ions (e.g., As3+ and Sb3+), and potential emerging contaminants (e.g., microplastics), which can serve as alternative sacrificial agents to rapidly quench ROS and enhance the activity of denitrifying microorganisms60,61,62. The enhancement was even more pronounced in stainless-steel wastewater (6.96–8.09-fold, Fig. 6c), which can be attributed to its lower organic matter content and higher NO3− concentration. Specifically, the higher organic matter in kitchen and swine wastewaters serves as an available substrate, supporting heterotrophic denitrification by denitrifying bacteria. Even when some denitrifying bacteria cannot directly utilize organic matter as a carbon source, they may still benefit through syntrophic metabolism63, wherein electron transfer from co-metabolizing microorganisms facilitates the bio-denitrification process, even without temperature fluctuations. In contrast, stainless-steel wastewater contains substantially less organic matter, rendering pyroelectric electrons the primary electron source for bio-denitrification. Consequently, temperature fluctuations effectively stimulate pyroelectric electron production, thereby enhancing denitrification, particularly given the higher NO3− concentration as the primary electron acceptor. This is an interpretation supported by the significantly higher biomass growth observed in the group with temperature fluctuations, which showed a 3.14-fold increase compared to the control. These results demonstrated that the bio-denitrification performance in actual wastewater treatment can be markedly improved by employing the denitrifiers-WS2 biohybrid system under natural temperature fluctuations.
a–c Typical time course of NO3− reduction in swine wastewater (a), kitchen wastewater (b), and stainless-steel wastewater (c) over two successive cycles. d Schematic diagram of the A-A-O, H2-MABR, and BHPD processes. e, f Impact scores of all environmentally relevant descriptors, as assessed through the LCA (e), and economic cost (f) of these three routes with a membrane life of 15 years in H2-MABR process. Data are presented as box-and-whisker plots derived from 10,000 Monte Carlo simulation trials. In each plot, the central line within the box represents the median, while individual data points indicate the mean. The upper and lower edges of the box correspond to the 90th and 10th percentiles, respectively, and the whiskers indicate the minimum and maximum values. Source data are provided as a Source Data file.
Building on the laboratory experimental results, we further employed LCA to comprehensively evaluate the environmental sustainability of the BHPD process across its entire life cycle, compared to traditional methods, such as the A-A-O and H2-MABR processes (Fig. 6d, Supplementary Tables 5–13, and Supplementary Data 2). The system boundary for the life cycle of wastewater treatment primarily focused on the secondary wastewater treatment process (Supplementary Fig. 33). Similar to the H2-MABR, the abundant CO2 present in biogas from anaerobic tanks can serve as a cost-free carbon source for microbial growth and metabolism in the BHPD process. Environmental impacts associated with construction were not included, as they contributed less than 2% to the overall assessment64. It was evident that the BHPD process was more ecofriendly than the conventional A-A-O process, as reflected in the significantly lower impact scores across most assessed descriptors (Fig. 6e, Supplementary Tables 14–21). While the environmental impacts of the BHPD process were comparable to or slightly exceed those of the H2-MABR process, several key points warrant attention: 1) The membrane lifespan in the H2-MABR process was assigned a maximum of 15 years in the LCA. However, MABR membranes typically last between 3 and 5 years in practice, with some lasting as little as 0.5 years64,65. The LCA results indicated that the environmental impacts of the H2-MABR process increased with a shorter membrane lifespan, with some impacts surpassing those of the BHPD process and even the A-A-O process under a membrane lifespan of 0.5 years (Supplementary Fig. 34); 2) Since the LCA calculations for the BHPD process are based on the laboratory data, there is significant potential to further reduce its environmental impact when produced on an industrial scale. Overall, the LCA results suggest that BHPD process is a promising approach for bio-denitrification in WWTPs, owing to its environmental benefits.
Furthermore, based on the laboratory experimental results and LCA findings, a cost analysis was conducted to assess the economic feasibility of implementing the BHPD process for large-scale wastewater treatment. Using average prices for raw materials and electricity (Supplementary Tables 22–27), the analysis revealed that the cost of treating 1 m3 of wastewater using the BHPD process (0.424 US$) was significantly lower than that of the conventional A-A-O process (0.537 US$) and the H2-MABR process (0.596 US$), highlighting the economic advantages of the BHPD process (Fig. 6f, Supplementary Tables 28–30). The expenses associated with the preparation of WS2 are expected to decrease significantly as production scales up beyond laboratory conditions66. Moreover, considering the greater convenience and efficiency of WS2 transportation and storage compared to H2, further research and large-scale implementation of the BHPD process are highly encouraged.
Discussion
Biohybrid systems combine the unique properties of nanomaterials with the precise specificity of microorganisms67, creating an innovative platform with significant potential for wastewater treatment. A key advantage of these systems is their ability to form nanomaterials in situ by harnessing multiple pollutants from wastewater61, thereby delivering dual benefits when exposed to various external stimuli. For instance, semiconductor nanomaterials, which generate photoelectrons upon light irradiation, have been integrated with microorganisms to form biohybrids68. These systems have shown promise in applications such as wastewater bio-denitrification69, the treatment of heavy metal-contaminated wastewater70, and the scalable solar-to-chemical conversion of pollutants60. However, their efficiency in real wastewater treatment is largely dependent on the light transmittance of the wastewater. The presence of significant suspended particles and organic matter often obstructs light penetration, thereby limiting the generation of photoelectrons by the semiconductor nanomaterials. In addition, piezoelectric nanomaterials have also been incorporated into biohybrids to harness mechanical energy and enhance wastewater bio-denitrification5. However, the primary sources of mechanical energy in WWTPs, such as aeration and mechanical mixing, are typically concentrated in the aerobic compartments. In contrast, the anaerobic compartments, such as the anaerobic section in the A-A-O process, remain largely undisturbed to avoid the O2 introduction and maintain microbial stability, limiting their potential to utilize mechanical energy throughout the entire system.
In this study, we develop a cost-competitive and environmentally friendly approach to enhance bio-denitrification by employing pyroelectric nanomaterials. Particularly, this pyroelectric bio-denitrification process can be effectively promoted even with a narrow temperature gradient (5 °C), in contrast to traditional pyroelectric processes that typically require large temperature gradients to generate and separate electrons. This advancement is primarily attributed to the interaction between pyroelectric materials and microorganisms. Previous studies have shown that microorganisms, particularly those forming microbial biofilms, can modify the local electrostatic environment at the surface of nanomaterials71. This modification can lead to the localized accumulation of electrons, lower the activation energy for electron transfer across the biotic-abiotic interface, and, in some cases, suppress charge carrier recombination by acting as electron sinks72. As a result, the temperature gradient requirement for pyroelectric bio-denitrification is significantly reduced. Based on the information gathered, the electron transfer pathways involved in pyroelectric bio-denitrification within the BHPD system are proposed (Supplementary Fig. 35). As the reduction potential of WS2 (−3.15 V, Supplementary Fig. 10c, d) is significantly more negative than that of the abundant membrane-bound cytochromes in T. d (e.g., −0.02 to 0.06 V for cytochrome b73,74), electrons are initially transferred from WS2 to the widely distributed cytochromes at the abiotic-biotic interface during temperature fluctuations. This transfer channel effectively prevents the backflow of pyroelectric electrons. Subsequently, the electron flow from cytochromes to ubiquinone and denitrifying enzymes is also thermodynamically feasible, as evidenced by the higher redox potentials of Q/QH2 (0.113 V75) and NO3−/NO2− (0.43 V76), thereby driving pyroelectric bio-denitrification.
WWTPs are evolving into Nutrient + Energy + Water (NEW) factories, driving a paradigm shift from pollution control to resource recovery within a circular economy framework77. Significant quantities of recoverable heat energy, six to eight times greater than chemical energy78, can be harnessed through processes such as water source heat pumps. The intermittent recovery of thermal energy from sewage may enable simultaneous temperature variations, creating conditions conducive to pyroelectric bio-denitrification. Furthermore, this recovered thermal energy can be stored to maintain a narrow temperature gradient for pyroelectric bio-denitrification during periods of thermal deficit, alongside other thermal sources such as solar thermal energy. Compared with conventional processes, pyroelectric bio-denitrification offers unique advantages by reducing reliance on mechanical equipment and chemical additives. A significant challenge for bio-denitrification in WWTPs is the drop in temperature, which can occur due to geographic factors (such as higher latitudes or hilly regions) and seasonal shifts (such as transitions into winter or spring)79. Therefore, unlike light or mechanical vibration for photoelectric and piezoelectric denitrification, thermal energy not only serves as an energy source but also supports bio-denitrification by maintaining cellular physiology, microbial growth rates, and community structure through optimal temperature control. Furthermore, the rapid temperature fluctuations in low-temperature wastewater can accelerate the oscillation of electric dipoles and the subsequent generation of pyroelectrons, which, in turn, provide an electron flow for microbial metabolisms. In addition, besides WS2, a well-known pyroelectric material, other materials also show considerable promise for pyroelectric bio-denitrification. These include single crystals with excellent stability and controllability (e.g., triglycine sulfate, ZnO), ceramics that are easy to prepare and offer excellent mechanical properties (e.g., BaTiO3 (BTO)), inorganic films with low thermal capacity and manufacture cost (e.g., BiFeO3), and polymer and composites that offer flexibility, low cost, and ease of mass production (e.g., polyvinylidene fluoride (PVDF))29. In situ synthesized nanoparticles, such as hexagonal cadmium sulfide (CdS) nanorods that can be produced directly from metal ions and sulfate in wastewater60, also exhibit remarkable pyroelectric properties80. These materials not only reduce the ecological and environmental risks associated with the introduction of external pyroelectric materials but also enhance the practicality of pyroelectric bio-denitrification. As a result, pyroelectric bio-denitrification holds significant application potential in WWTPs.
However, it is important to acknowledge that nitrifying bacteria are highly sensitive to temperature fluctuations, with lower temperatures significantly reducing their activity and nitrification rates81. Consequently, appropriate strategies should be explored to ensure a sufficient nitrate supply for the BHPD process. These may include optimizing operational parameters82, such as hydraulic retention time (HRT), sludge retention time (SRT), and dissolved oxygen (DO). Furthermore, bioaugmentation through the direct introduction of pure strains or mixed cultures (e.g., psychrophilic bacteria), as well as immobilization techniques such as attachment, containment, and encapsulation79, can also be considered. In addition, several other challenges must be addressed when scaling up from laboratory scale to larger production scales. These include thermal management issues, such as preventing thermal hotspots and ensuring efficient heat transfer, identifying effective pyroelectric materials with long-term stability, and designing modular, flexible reactors. Moreover, wastewater contains a complex mix of organic compounds, heavy metal ions, and sacrificial agents, with variations in water quality altering the interactions among these pollutants60. This dynamic interplay can have both beneficial and detrimental effects on the generation, transfer, and utilization of pyroelectrons, ultimately impacting pyroelectric bio-denitrification. Furthermore, wastewater functions as a complex ecosystem, where shifts in water quality can profoundly affect microbial community structure and function, particularly the ecological niche of nitrogen-cycling microorganisms83. Understanding electron distribution and ROS production mechanisms during pyroelectric bio-denitrification is also crucial for future technological optimization, aiming to achieve highly efficient NO3− reduction while minimizing the generation of nitrite and nitrous oxide. Therefore, a deeper understanding of the energy-microbe-element nexus, coupled with the development of targeted strategies, is essential for large-scale practical application.
Methods
Construction and characterization of T. d-WS2
T. d was acquired from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures. After a 2-day inoculation in thiosulfate-containing medium (Supplementary Table 1), 7.5 mg of WS2, purchased from Nanjing XFNANO Materials (Nanjing, China) that synthesized through a chemical exfoliation method of lithium-ion intercalation84, was introduced. The mixture was cultivated at 30 °C and 120 rpm until T. d reached an exponential growth phase. Subsequently, the suspension was centrifuged at 5400 × g for 5 min and washed three times with a 0.9% NaCl solution for further use.
The constructed T. d-WS2 biohybrids were fixed with 2.5% glutaraldehyde at 4 °C for 24 h, followed by gradient dehydration using ethanol at 5400 × g for 5 min85. Subsequently, the samples were metalized with a chromium layer using a sputter coater (Quorum SC7620, USA) to facilitate visualization of surface morphology using a scanning electron microscope (SEM, Hitachi Regulus 8100, Japan), which was also equipped with an energy dispersive X-ray spectrometer (EDS) for elemental composition analysis. Raman spectra were acquired with a Raman spectrometer (Renishaw inVia, UK) equipped with a 532-nm laser. XRD patterns were obtained using a XRD diffractometer (SmartLab Rigaku, Japan) with Cu–Kα radiation, covering a diffraction angular 2θ range of 5–70° at an increment of 1° min−1. High-resolution transmission electron microscopy (HRTEM) image and corresponding SAED pattern of the sample were obtained with a TEM (FEI Talos F200X G2, USA). The Tauc plots were obtained using a UV–Vis spectrophotometer (UV-2600, Shimadzu, Japan), and the valence band spectra were measured using a valence band X-ray photoelectron spectroscopy (VB XPS) system (K-Alpha, Thermo Scientific, USA).
Cell surface roughness and topography were evaluated with an AFM (Bruker MM8, Germany). After the sequential fixation, dehydration, and resin penetration of WS2-internalized T. d, the resin blocks were sectioned to a thickness of 70–90 nm using an ultra-microtome (Leica EM UC7, Germany) and placed onto the copper (Cu) TEM grids for imaging and element analysis with the Hitachi HT7800 TEM86. Images of WS2 confocal fluorescence were obtained by a confocal laser scanning microscope (CLSM, Zeiss LSM880, Germany). To further characterize the internalization of WS2 by T. d, the stacked confocal images of T. d cells at different heights (Z) were obtained using the Zeiss LSM880 CLSM, where Z = 0 corresponds to the lowest contrast of the cell in a brightfield image. The excitation wavelength was 395 nm, and the detection wavelength range was 605–800 nm.
PFM measurements were conducted utilizing an AFM system (Bruker Dimension Icon, USA), which was equipped with a platinum-iridium coated AFM tip (SCM-PIT-V2) and operated in dual AC resonance tracking (DART) mode. The surface potential was measured by scanning Kelvin probe force microscopy (SKPFM, Bruker Dimension Icon, USA, Supplementary Method 1). SECM experiments were performed using an SECM instrument (AMETEK VersaSCAN, USA). A 25-μm-diameter Pt microelectrode was chosen as the SECM tip (Eprobe = + 300 mV vs Ag/AgCl), and the electrolyte solution consisted of 100 mM K3Fe(CN)6 in 0.1 M NaOH87. Current-time (I-t) curves under temperature fluctuations were obtained in a single-chamber cell connected to the electrochemical workstation (Chenhua CHI 760E, China)5.
Pyroelectric bio-denitrification experiments with T. d-WS2
The mixture containing T. d-WS2 biohybrids in the thiosulfate-containing medium was centrifuged at 5400 × g for 5 min, washed three times with a 0.9% NaCl solution, and redispersed in the thiosulfate-free medium, following the removal of NH4Cl and Na2S2O3·5H2O (Supplementary Table 1). Subsequently, 0.1% sodium lactate and 300 μM MV2+ were added to serum bottles as sacrificial donor and electron shuttle, respectively. The headspace of serum bottles was then purged with a filter-sterilized nitrogen gas for 30 min at a rate of 1 mL min−1 to remove oxygen, and immediately sealed with sealed with Teflon®-coated rubber and aluminum crimp caps to maintain a strict anaerobic environment. Pyroelectric bio-denitrification experiments were conducted under infrared radiation with a 3/30 min turn-on/off cycle over 5 days. The optical power density was set at 400 mW cm−2 (wavelength range: 600–1000 nm, Supplementary Fig. 4), with a temperature fluctuation range of 5 °C, starting from an initial temperature of 25 °C (Supplementary Fig. 5). After that, the mixture was centrifuged and fresh sterilized thiosulfate-free medium was added to start a new cycle (for a total of three cycles). As a control, a series of deletional control experiments were conducted, in which T. d, WS2, infrared radiation, sodium lactate, and MV2+ were individually removed. In addition, the pyroelectric bio-denitrification performance was also evaluated at different temperature variation rates (0.0, 0.5, 1.0, and 1.5 °C min−1) and with varying dosages of T. d (0-, 1-, 2-, and 3-fold) and WS2 (0, 2.5, 5.0, 7.5, and 10.0 mg). To exclude the influence of light irradiation (i.e., photoelectrons) on pyroelectric bio-denitrification performance, the experiment was also conducted under identical conditions but using a water bath (Aohua HH-501, China) for temperature control instead of infrared radiation. ROS in the biohybrid systems were characterized using a Bruker ESR5000 EPR spectrometer, with DMPO employed as the spin-trapping agent for •H, •OH and •O2− radicals. An isotope labeling experiment with T. d-WS2 biohybrids was performed in an thiosulfate-free medium containing K15NO3 with high purity helium gas as the headspace gas.
To investigate the potential internalization of WS2 nanoparticles into T. d, the constructed T. d-WS2 biohybrids were subjected to ultrasonication 4 kHz for 5 min, based on preliminary experiments (Supplementary Fig. 36, Supplementary Note 4), and then centrifugation at 1200 × g for 1 min to remove surface-bound WS2 from the T. d cells. The resulting biohybrid system was then employed for pyroelectric bio-denitrification under the previously described experimental conditions. Furthermore, to elucidate the role of outer membrane cytochromes in pyroelectric bio-denitrification, proteinase K was employed to digest outer membrane proteins of T. d59. Briefly, T. d cells in the exponential growth phase were harvested, washed twice, and resuspended in an osmotically balanced wash buffer. They were then treated with varying concentrations (1, 2.5, 5.0 U mL−1) of proteinase K (Sigma, MO) for 30 min, Subsequently, the proteinase K reaction was halted by the addition of a protease inhibitor (Roche, NJ) to the samples. The cells were then washed twice more and used to reconstruct the T. d-WS2 biohybrids for pyroelectric bio-denitrification experiments under the previously described experimental conditions.
To evaluate the effectiveness of the biohybrid system for actual wastewater treatment, three types of wastewater samples, including swine wastewater, kitchen wastewater, and stainless-steel wastewater, were collected from Fuzhou, Fujian Province, China. The main compositions of these three wastewaters are detailed in Supplementary Table 4. Pyroelectric bio-denitrification experiments were carried out on the roof of the Research Center for Water Resources and Security Building at Fujian Agriculture and Forestry University in Fuzhou, China (latitude: 26.05°N, longitude: 119.14°E) under natural sunlight with an average light intensity of 700 W m−2. The experiments consisted of two phases: from 07:00 to 10:00 and 16:00 to 18:00, with 10 min of irradiation for temperature increase followed by 30 min indoors for temperature decrease; and from 11:00 to 16:00, with 5 min of irradiation for temperature increase and 30 min indoors for temperature decrease. The biohybrid system contains 24 mL of these actual wastewater samples, 1 mL of inoculum sludge sourced from a denitrification tank of WWTP (Fuzhou, Fujian Province, China), and 7.5 mg of WS2.
Characterization methods
Cell biomass was characterized using the BCA Protein Assay Kit (Thermo Scientific Pierce, USA) after protein extraction with the One Step Bacterial Active Protein Extraction Kit (Sangon Bioengineering, China). Cellular ATP level was determined using an enhanced ATP Assay Kit (Beyotime, China), while NADPH/NADP+ conversion ratio was measured with Coenzyme II NADP(H) Content Assay Kit (BC1100, Solarbio, China), according to the manufacturer’s instructions. Cell number and cell viability were visualized using Zeiss LSM880 CLSM with Live/Dead® BacLight Bacterial Viability Kit (Molecular Probes, Invitrogen, USA), which consists of a mixture of SYTO 9 and propidium iodide fluorescent dyes, and then analyzed with ImageJ software (NIH, version 1.41). Cell viability was also measured by flow cytometry (Attune NxT, USA)88. EIS and differential pulse voltammetry (DPV) analyses were conducted using a Chenhua CHI 760E electrochemical workstation. Specifically, 50 µL of the T. d-WS2 suspension was gently drop-cast onto a 1 cm × 1 cm indium tin oxide glass substrate and allowed to air-dry naturally, forming a thin-film working electrode. This electrode, together with a platinum sheet counter electrode, a saturated calomel reference electrode, and 0.1 M phosphate-buffered saline as the electrolyte, constituted the three-electrode system89. To analyze the concentration of c-type cytochromes in T. d-WS2, the samples were suspended in 1× LDS (lithium dodecyl sulfate) sample buffer (GenScript, Nanjing, China), boiled, and separated by heme-stained SDS-PAGE90. Bacterial membrane permeability of T. d was assessed using the N-Phenyl-1-naphthylamine assay and the o-nitrophenyl-β-d-galactopyranoside hydrolysis assay91.
Product measurements
The concentrations of NO3− and NO2− were analyzed using an ion chromatography system (DionexTM ICS900, USA), equipped with a 4 × 250 mm IonPac AS19 column (Dionex), an EGC III KOH eluent generator cartridge, and a conductivity detector. N2O and N2 concentrations were determined by a gas chromatograph (GC, Agilent 7890, USA) equipped with an electron capture detector. Gas products from the isotope labeling experiment were assessed using a gas chromatography-mass spectrometry (GC-MS, Agilent 7890-5975c, USA).
TOF-SIMS analysis
TOF-SIMS measurements were conducted in an autotrophic medium containing K15NO3 and NaH13CO3, utilizing a ToF-SIMS 5 instrument5. The mass difference resulting from the isotopic substitution allows for reliable identification of fragments involving labeling atoms. Each obtained data frame was generated after 10 scans over the analysis area with one single shot per each pixel. Data treatment was conducted using SurfaceLab 6.7 software (IONTOF GmbH).
Transcriptomic analysis
The mixtures containing T. d-WS2 biohybrids after pyroelectric bio-denitrification were collected via centrifugation at 5400 × g for 5 min at 4 °C. Total RNA extraction was conducted using TRIzol reagent (Invitrogen, USA), following established protocols. Then the quality of the samples was assessed using the RNA Nano 6000 Assay Kit on the Bioanalyzer 2100 system (Agilent, USA). PCR amplification was then carried out using Phusion High-Fidelity DNA Polymerase and the Index X primer. The P5 adapter sequence was 5’-AATGATACGGCGACCACCGAGA-3’, with its reverse complement being 5’-TTACTATGCCGCTGGTGGCTCT-3’. Similarly, the P7 adapter sequence was 5’-CGTATGCCGTCTTCTGCTTG-3’, and its reverse complement was 5’-CAAGCAGAAGACGGCATACG-3’. The resulting PCR products were purified (AMPure XP system), and the library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumia), following the manufacturer’s instructions. Subsequently, the library preparations were sequenced on an Illumina Novaseq platform, generating 150 bp paired-end reads. HTSeq v0.6.1 was used to count the reads numbers mapped to each gene. Fragments per kilobase of transcript per million mapped reads of each gene were calculated based on the length of the gene and read count mapped to this gene. Differential expression analysis was performed using the DESeq R package (1.18.0).
Finite element simulation with COMSOL
To analyze the temperature and electric field characteristics of WS2, the finite element simulation was conducted using the COMSOL Multiphysics software. The simulated geometry of WS2 was selected as 60 nm × 60 nm × 9 nm, aligning with the dimensions of the corresponding experimental samples. Throughout the simulation, WS2 is assumed to be homogeneous and isotropic, with its physical and optical properties remaining affected by temperature variations. The initial temperature is set at 298 K (room temperature), while the incident wavelength and power are specified as 0.5 microns and 250 W, respectively. Meanwhile, the spatial distribution follows a Gaussian model. The detailed simulation parameters are provided in Supplementary Table 3.
LCA and cost analysis
The LCA was conducted in accordance with the guidance of the International Organization for Standardization standards 14040 and 14044 series. The associated environmental impacts were simulated using Simapro 9.5 software with the Ecoinvent (v.3.9.1) database as the inventory. Supplementary Fig. 33 and Supplementary Method 2 show the system boundaries for the A-A-O, H2-MABR, and BHPD processes, focusing primarily on the secondary wastewater treatment process. Environmental impacts associated with construction were excluded, as they contributed <2% to the overall assessment. The inventories for A-A-O, H2-MABR, and BHPD processes, concerning the treatment of 10,000 cm3 of wastewater, were shown in Supplementary Tables 5–13 and Supplementary Data 2. Based on these data, the ReCiPe 2016 Midpoint (H) V1.07/World (2010) H method was employed to assess 18 environmental impacts, which were obtained at the midpoint level (Supplementary Tables 14–21). In addition, cost analysis of raw materials and electricity for the A-A-O, H2-MABR, and BHPD processes was conducted, and the detailed data were summarized in Supplementary Tables 22–30. The uncertainty of environmental impactors in the LCA and the economic costs of different materials and electricity during bio-denitrification via various routes were evaluated using a Monte Carlo simulation with Latin hypercube sampling (10,000 trials).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. The RNA-seq data generated in this study have been deposited in the NCBI under accession PRJNA1195236. Source data are provided with this paper.
References
Ward, B. et al. Denitrification as the dominant nitrogen loss process in the Arabian sea. Nature 461, 78–81 (2009).
Qu, J. et al. Emerging trends and prospects for municipal wastewater management in China. ACS Est. Eng. 2, 323–336 (2022).
Lu, L. et al. Wastewater treatment for carbon capture and utilization. Nat. Sustain. 1, 750–758 (2018).
Bian, J. et al. Synergy of cyano groups and cobalt single atoms in graphitic carbon nitride for enhanced bio-denitrification. Water Res. 218, 118465 (2022).
Ye, J. et al. Wastewater denitrification driven by mechanical energy through cellular piezo-sensitization. Nat. Water 2, 531–540 (2024).
Wang, T. et al. Sulfur autotrophic denitrification as an efficient nitrogen removals method for wastewater treatment towards lower organic requirement: a review. Water Res. 245, 120569 (2023).
Qu, S. et al. Carbon footprint drivers in China’s municipal wastewater treatment plants and mitigation opportunities through electricity and chemical efficiency. Engineering Per-press at https://doi.org/10.1016/j.eng.2024.01.021 (2024).
Syron, E. & Casey, E. Membrane-aerated biofilms for high rate biotreatment: performance appraisal, engineering principles, scale-up, and development requirements. Environ. Sci. Technol. 42, 1833–1844 (2008).
Pandya, S. et al. Pyroelectric energy conversion with large energy and power density in relaxor ferroelectric thin films. Nat. Mater. 17, 432–438 (2018).
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Tansu, A., Ogungbemi, A. T. & Hocanın, F. T. The challenges and serviceability of solar power: suggestion on solving the Nigeria energy crisis. Int. J. Energ. Stud. 7, 127–141 (2022).
Lynch, J., Rishel, G. & Corbett, E. Thermal alteration of streams draining clearcut watersheds: quantification and biological implications. Hydrobiologia 111, 161–169 (1984).
Malcolm, I. A., Hannah, D. M., Donaghy, M. J., Soulsby, C. & Youngson, A. F. The influence of riparian woodland on the spatial and temporal variability of stream water temperatures in an upland salmon stream. Hydrol. Earth Syst. Sci. 8, 449–459 (2004).
Hepbasli, A., Biyik, E., Ekren, O., Gunerhan, H. & Araz, M. A key review of wastewater source heat pump (WWSHP) systems. Energ. Convers. Manag. 88, 700–722 (2014).
Dürrenmatt, D. J. & Wanner, O. A mathematical model to predict the effect of heat recovery on the wastewater temperature in sewers. Water Res. 48, 548–558 (2014).
You, H. et al. Room-temperature pyro-catalytic hydrogen generation of 2D few-layer black phosphorene under cold-hot alternation. Nat. Commun. 9, 2889 (2018).
Belitz, R. et al. Waste heat energy harvesting by use of BaTiO3 for pyroelectric hydrogen generation. Energ. Harvest. Syst. 4, 107–113 (2017).
Xiao, L. et al. Pyroelectric nanoplates for reduction of CO2 to methanol driven by temperature-variation. Nat. Commun. 12, 318 (2021).
You, H. et al. Highly efficient pyrocatalysis of pyroelectric NaNbO3 shape-controllable nanoparticles for room-temperature dye decomposition. Chemosphere 199, 531–537 (2018).
Qian, W. et al. Thermo-electrochemical coupling for room temperature thermocatalysis in pyroelectric ZnO nanorods. Electrochem. Commun. 81, 124–127 (2017).
Wang, C., Tian, N., Ma, T., Zhang, Y. & Huang, H. Pyroelectric catalysis. Nano Energy 78, 105371 (2020).
García, F. C., Bestion, E., Warfield, R. & Yvon-Durocher, G. Changes in temperature alter the relationship between biodiversity and ecosystem functioning. Proc. Natl Acad. Sci. USA 115, 10989–10994 (2018).
Burman, E. & Bengtsson-Palme, J. Microbial community interactions are sensitive to small changes in temperature. Front. Microbiol. 12, 672910 (2021).
Schimel, J., Balser, T. C. & Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 88, 1386–1394 (2007).
Cabezas, A. et al. Ubiquity and diversity of cold adapted denitrifying bacteria isolated from diverse Antarctic ecosystems. Front. Microbiol. 13, 827228 (2022).
Kashyap, D. K., Srivastava, A. K. & Gupta, M. K. Lightweight, self-poled, flexible piezoelectric tungsten disulfide quantum dots-reinforced PVDF-HFP-based nanogenerator. ACS Appl. Electron. Mater. 6, 862–874 (2024).
Kumar, R. et al. Surfactant-assisted WS2 nanostructures for enhanced NO2 sensing performance. IEEE Sens. J. 24, 5813–5820 (2024).
Chen, X. et al. Mn3O4 nanozyme coating accelerates nitrate reduction and decreases N2O emission during photoelectrotrophic denitrification by Thiobacillus denitrificans-CdS. Environ. Sci. Technol. 54, 10820–10830 (2020).
Zhang, D., Wu, H., Bowen, C. R. & Yang, Y. Recent advances in pyroelectric materials and applications. Small 17, 2103960 (2021).
Fan, C., Zhou, W., He, S. & Huang, J. Sulfur transformation in sulfur autotrophic denitrification using thiosulfate as electron donor. Environ. Pollut. 268, 115708 (2021).
Huang, S. et al. Dissolved organic matter acting as a microbial photosensitizer drives photoelectrotrophic denitrification. Environ. Sci. Technol. 56, 4632–4641 (2022).
Ren, G. et al. Growth of electroautotrophic microorganisms using hydrovoltaic energy through natural water evaporation. Nat. Commun. 15, 1–10 (2024).
Šantrůčková, H., Birdc, M., Frouzb, J., Šustr, V. & Tajovský, K. Natural abundance of 13C in leaf litter as related to feeding activity of soil invertebrates and microbial mineralisation. Soil Biol. Biochem. 32, 1793–1797 (2000).
Xiao, Y. et al. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 3, e1700623 (2017).
Yang, G. et al. Bidirectional extracellular electron transfer pathways of Geobacter sulfurreducens biofilms: molecular insights into extracellular polymeric substances. Environ. Res. 245, 118038 (2024).
Mohona, T. M. et al. Aggregation behavior of inorganic 2D nanomaterials beyond graphene: insights from molecular modeling and modified DLVO theory. Environ. Sci. Technol. 53, 4161–4172 (2019).
Derjaguin, B. & Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 43, 30–59 (1993).
Lee, C. et al. Unveiling defect-related raman mode of monolayer WS2 via tip-enhanced resonance raman scattering. ACS Nano 12, 9982–9990 (2018).
Liu, Q. et al. Stable metallic 1T-WS2 nanoribbons intercalated with ammonia ions: the correlation between structure and electrical/optical properties. Adv. Mater. 27, 4837–4844 (2015).
Li, X., Liu, Z., Zhu, D., Yan, Y. & Chen, Y. Controllable synthesis of few-layer ammoniated 1T′-phase WS2 as an anode material for lithium-ion batteries. Nanoscale 14, 5869–5875 (2022).
Zhang, H. et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production. Nat. Nanotechnol. 13, 900–905 (2018).
Yuan, P., Zhou, Q. & Hu, X. The phases of WS2 nanosheets influence uptake, oxidative stress, lipid peroxidation, membrane damage, and metabolism in algae. Environ. Sci. Technol. 52, 13543–13552 (2018).
Yuan, P., Zhou, Q. & Hu, X. WS2 nanosheets at noncytotoxic concentrations enhance the cytotoxicity of organic pollutants by disturbing the plasma membrane and efflux pumps. Environ. Sci. Technol. 54, 1698–1709 (2020).
Yang, W., Thordarson, P., Gooding, J. J., Ringer, S. P. & Braet, F. Carbon nanotubes for biological and biomedical applications. Nanotechnology 18, 412001 (2007).
Zeng, J.-Y. et al. Light-driven biohybrid system utilizes N2 for photochemical CO2 reduction. Natl. Sci. Rev. 10, nwad142 (2023).
Beller, H. R. et al. The genome sequence of the obligately chemolithoautotrophic, facultatively anaerobic bacterium Thiobacillus denitrificans. J. Bacteriol. 188, 1473–1488 (2006).
Zhang, J. et al. Determination of the cytosolic NADPH/NADP ratio in saccharomyces cerevisiae using shikimate dehydrogenase as sensor reaction. Sci. Rep. 5, 1–12 (2015).
Yang, C. et al. Carbon dots-fed Shewanella oneidensis MR-1 for bioelectricity enhancement. Nat. Commun. 11, 1379 (2020).
Motohashi, K., Watanabe, Y., Yohda, M. & Yoshida, M. Heat-inactivated proteins are rescued by the DnaK⋅ J-GrpE set and ClpB chaperones. Proc. Natl Acad. Sci. USA 96, 7184–7189 (1999).
Cross, R. L. & Müller, V. The evolution of A-, F-, and V-type ATP synthases and ATPases: reversals in function and changes in the H+/ATP coupling ratio. FEBS Lett. 576, 1–4 (2004).
Sazanov, L. A. & Hinchliffe, P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311, 1430–1436 (2006).
Steffens, L. et al. High CO2 levels drive the TCA cycle backwards towards autotrophy. Nature 592, 784–788 (2021).
Zhang, I. H. et al. Partitioning of the denitrification pathway and other nitrite metabolisms within global oxygen deficient zones. ISME Commun. 3, 76 (2023).
Lin, S. et al. Sulfur bacteria-reinforced microbial electrochemical denitrification. Bioresour. Technol. 393, 130121 (2024).
Wilson, W. A. et al. Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol. Rev. 34, 952–985 (2010).
Goh, Y. J. & Klaenhammer, T. R. Insights into glycogen metabolism in Lactobacillus acidophilus: impact on carbohydrate metabolism, stress tolerance and gut retention. Microb. Cell Fact. 13, 1–12 (2014).
Bueno, E., Mesa, S., Bedmar, E. J., Richardson, D. J. & Delgado, M. J. Bacterial adaptation of respiration from oxic to microoxic and anoxic conditions: redox control. Antioxid. Redox Signal. 16, 819–852 (2012).
Mooshammer, M. et al. Decoupling of microbial carbon, nitrogen, and phosphorus cycling in response to extreme temperature events. Sci. Adv. 3, e1602781 (2017).
Beller, H. R. et al. Whole-genome transcriptional analysis of chemolithoautotrophic thiosulfate oxidation by Thiobacillus denitrificans under aerobic versus denitrifying conditions. J. Bacteriol. 188, 7005–7015 (2006).
Pi, S. et al. Solar-driven waste-to-chemical conversion by wastewater-derived semiconductor biohybrids. Nat. Sustain. 6, 1673–1684 (2023).
Zuo, W., Yu, Y. & Huang, H. Making waves: microbe-photocatalyst hybrids may provide new opportunities for treating heavy metal polluted wastewater. Water Res.195, 116984 (2021).
Ye, J. et al. Sustainable conversion of microplastics to methane with ultrahigh selectivity by a biotic–abiotic hybrid photocatalytic system. Angew. Chem. 134, e202213244 (2022).
Morris, B. E., Henneberger, R., Huber, H. & Moissl-Eichinger, C. Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev. 37, 384–406 (2013).
Li, R. et al. Life cycle assessment of hydrogenotrophic denitrification in membrane aerated biofilm reactors for sustainable wastewater treatment. Water Res. 267, 122529 (2024).
Li, J., Wang, Z. & Wang, Y. Integrating membrane aerated biofilm reactors with biological nitrogen removal processes: a new paradigm for achieving sustainable wastewater treatment plants. Chem. Eng. J. 475, 146025 (2023).
Li, X. et al. Porous organic polycarbene nanotrap for efficient and selective gold stripping from electronic waste. Nat. Commun. 14, 263 (2023).
Stauber, R. H. et al. Small meets smaller: effects of nanomaterials on microbial biology, pathology, and ecology. ACS Nano 12, 6351–6359 (2018).
Hu, J. et al. Biophotoelectrochemistry for the treatment of emerging contaminants. Sci. China Technol. Sci. 67, 3051–3066 (2024).
Cui, S. et al. Intracellularly-photosensitized bio-hybrid with biogenic quantum dots for enhanced wastewater denitrification. Chem. Eng. J. 457, 141237 (2023).
Zhang, Y. et al. Semiconductor biohybrids for enhanced bifunctional wastewater sulfur and heavy metal removal. Green. Chem. 26, 3940–3948 (2024).
Liang, J. et al. Revisiting solar energy flow in nanomaterial-microorganism hybrid systems. Chem. Rev. 124, 9081–9112 (2024).
Yang, Y., Liu, L.-N., Tian, H., Cooper, A. I. & Sprick, R. S. Making the connections: physical and electric interactions in biohybrid photosynthetic systems. Energ. Environ. Sci. 16, 4305–4319 (2023).
Asard, H., Barbaro, R., Trost, P. & Bérczi, A. Cytochromes b561: ascorbate-mediated trans-membrane electron transport. Antioxid. Redox Signal. 19, 1026–1035 (2013).
Takeuchi, F., Hori, H., Obayashi, E., Shiro, Y. & Tsubaki, M. Properties of two distinct heme centers of cytochrome b561 from bovine chromaffin vesicles studied by EPR, resonance Raman, and ascorbate reduction assay. J. Biochem. 135, 53–64 (2004).
Zhou, X. et al. Mechanisms of extracellular photoelectron uptake by a Thiobacillus denitrificans-cadmium sulfide biosemiconductor system. Chem. Eng. J. 468, 143667 (2023).
Beller, H. R. Anaerobic, nitrate-dependent oxidation of U (IV) oxide minerals by the chemolithoautotrophic bacterium Thiobacillus denitrificans. Appl. Environ. Microbiol. 71, 2170–2174 (2005).
Zheng, M. et al. Pathways to advanced resource recovery from sewage. Nat. Sustain. 7, 1–10 (2024).
Hao, X., Li, J., van Loosdrecht, M. C., Jiang, H. & Liu, R. Energy recovery from wastewater: heat over organics. Water Res. 161, 74–77 (2019).
Zhou, H., Li, X., Xu, G. & Yu, H. Overview of strategies for enhanced treatment of municipal/domestic wastewater at low temperature. Sci. Total Environ. 643, 225–237 (2018).
Zhang, M., Hu, Q., Ma, K., Ding, Y. & Li, C. Pyroelectric effect in CdS nanorods decorated with a molecular co-catalyst for hydrogen evolution. Nano Energy 73, 104810 (2020).
Ducey, T. F., Vanotti, M. B., Shriner, A. D., Szogi, A. A. & Ellison, A. Q. Characterization of a microbial community capable of nitrification at cold temperature. Bioresour. Technol. 101, 491–500 (2010).
Yang, Q. et al. Nitrogen removal via nitrite from municipal wastewater at low temperatures using real-time control to optimize nitrifying communities. Environ. Sci. Technol. 41, 8159–8164 (2007).
Liu, T. et al. Sustainable wastewater management through nitrogen-cycling microorganisms. Nat. Water 2, 936–952 (2024).
Chen, M. et al. Light-driven nitrous oxide production via autotrophic denitrification by self-photosensitized Thiobacillus denitrificans. Environ. Int. 127, 353–360 (2019).
Jiang, K. et al. STC2 activates PRMT5 to induce radioresistance through DNA damage repair and ferroptosis pathways in esophageal squamous cell carcinoma. Redox Biol. 60, 102626 (2023).
Rohnke, B. A., Singh, S. P., Pattanaik, B. & Montgomery, B. L. RcaE-dependent regulation of carboxysome structural proteins has a central role in environmental determination of carboxysome morphology and abundance in Fremyella diplosiphon. mSphere 3, e00617–00617 (2018).
Hu, A. et al. Metal-free semiconductor-based bio-nano hybrids for sustainable CO2-to-CH4 conversion with high quantum yield. Angew. Chem. Int. Ed. 61, e202206508 (2022).
Emerson, J. B. et al. Schrödinger’s microbes: tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 5, 1–23 (2017).
Ye, J. et al. Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid. Appl. Catal. B: Environ. 257, 117916 (2019).
Liu, X. et al. Bacterial vesicles mediate extracellular electron transfer. Environ. Sci. Technol. Lett. 7, 27–34 (2019).
Wu, J. et al. Self-supply of H2O2 and O2 by a composite nanogenerator for chemodynamic therapy/hypoxia improvement and rapid therapy of biofilm-infected wounds. Chem. Eng. J. 459, 141507 (2023).
Acknowledgements
This work was supported by the National Science Fund for Excellent Young Scholars (42322706 to J.Y.) and the National Natural Science Foundation of China (42177206 to J.Y. and 62305058 to S.W.). We also thank Prof. Shan-fei Fu and Dr. Rui Li (Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences) for their assistance with the LCA.
Author information
Authors and Affiliations
Contributions
J.Y., S.W., B.Z. and S.Z. conceived the idea and designed the experiments. J.Y., C.Y., Z.Z. and W.G. performed the experiments. J.Y., S.W., C.Y. and W.G. analyzed the data. J.Y., S.W., B.Z. and S.Z. wrote and revised the manuscript. All the authors contributed to the interpretation of the data and preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xiang Gao, Devendra Saroj and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, J., Wang, S., Yang, C. et al. Biohybrid-based pyroelectric bio-denitrification driven by temperature fluctuations. Nat Commun 16, 5877 (2025). https://doi.org/10.1038/s41467-025-60908-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60908-w
This article is cited by
-
Microbiologically Influenced Corrosion in Magnesium Implants: Mechanisms, Challenges, and Opportunities for Biomedical Innovation
Journal of Bio- and Tribo-Corrosion (2026)