Abstract
As global aging accelerates, frailty and depressive symptoms have emerged as critical contributors to cardiovascular disease (CVD) risk among older adults. However, the dynamic interplay between these factors remains underexplored. Here, we examine the associations among frailty, depressive symptoms, and incident CVD using data from five international cohorts (HRS, CHARLS, SHARE, ELSA, MHAS) involving individuals aged 50 and above. Our findings reveal that frailty significantly increases CVD risk, with depressive symptoms partially mediating this relationship. Transitions into frailty elevate CVD risk, while improvements reduce it. Cross-lagged panel network analysis identifies consistent CVD predictors, including hypertension, diabetes, and mobility issues. Subgroups with stronger associations include frail males, older individuals, working or retired people, and those with unhealthy lifestyles. These results underscore the need for integrated interventions targeting frailty and depressive symptoms to prevent CVD in aging populations.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is not only a leading cause of death among individuals aged 45 and older but is also closely associated with disability, reduced quality of life, and high healthcare costs1,2. Globally, CVD accounts for over 17 million deaths annually, constituting 29% of all deaths3. Its prevalence among middle-aged and older adults rises proportionally with age4, and with population aging, the burden of CVD is expected to increase substantially in the coming decades5,6. Thus, investigating the association between frailty and CVD is crucial, as it could elucidate disease mechanisms and inform public health policies aimed at improving health outcomes and quality of life in this population7.
The frailty index (FI) is linked to various adverse health outcomes, including disability, higher mortality, and increased CVD incidence8. Research indicates that frailty often coincides with chronic inflammation, leading to endothelial dysfunction and heightened atherosclerosis risk—a primary pathology of CVD9. Additionally, frailty may impair autonomic function, reducing heart rate variability and elevating risks for cardiovascular events such as myocardial infarction and stroke10,11. At the behavioral level, frailty may also contribute to lifestyle changes such as reduced physical activity, and increased social isolation, which can further elevate CVD risk12.
Studies show that frailty in middle-aged and older adults often coexists with depressive symptoms13, which not only share common risk factors with CVD but may also mediate the frailty-CVD relationship. Depressive symptoms have been associated with increased inflammation, dysregulated autonomic function, and metabolic disruptions14. For instance, depression has been linked to higher levels of inflammatory markers such as C-reactive protein (CRP) and interleukin-6 (IL-6)15, both of which contribute to endothelial dysfunction and CVD progression16. Furthermore, depression can lead to behavioral changes such as physical inactivity, poor nutritional habits, and increased stress responses, which may exacerbate frailty and CVD risk12.
Conversely, frailty itself has been identified as a risk factor for developing depressive symptoms, creating a bidirectional relationship wherein each condition exacerbates the other17. Declining physical function, increased dependency, and social withdrawal associated with frailty may increase psychological distress, contributing to depressive symptoms18. This cyclical interplay suggests that depressive symptoms could act not only as a mediator in the frailty-CVD link but also as a modifiable intervention target for reducing CVD risk in frail individuals19. However, cross-sectional designs typically fail to capture the temporal dynamics and causal pathways between frailty, depressive symptoms, and CVD, often overlooking psychological mechanisms and lacking cultural diversity. Furthermore, previous studies have primarily focused on baseline frailty status and its impact on CVD risk, while limited research has examined the impact of frailty progression or improvement on CVD outcomes20. Recent findings suggest that changes in frailty status may have differential effects on cardiovascular health, but the mechanisms underlying these effects remain unclear21. Given the potential role of depressive symptoms in mediating the frailty-CVD association, understanding this relationship through longitudinal data is critical for developing targeted interventions.
Building on the background discussed, this study aims to further explore the dynamic relationship between frailty, depressive symptoms, and CVD, integrating blood biomarkers such as C-reactive protein (CRP) and lipid metabolism markers to gain deeper insights into the biological mechanisms underlying the frailty-depression-CVD pathway. We utilize five large-scale longitudinal national cohorts (HRS, CHARLS, SHARE, ELSA, and MHAS), which represents diverse ethnic and cultural backgrounds. Compared to prior studies that primarily relied on one or two national cohorts, this cross-national approach enhances the generalizability of our findings and enables us to explore potential differences in the frailty-CVD relationship across various populations. Additionally, we apply cross-lagged panel network analysis to investigate the temporal and dynamic interplay between frailty and CVD, providing a more detailed understanding of their interactions beyond traditional regression models. These approaches provide preliminary insights into the progression of frailty and its clinical implications, which can inform future intervention efforts.
Results
Baseline characteristics
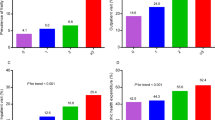
To understand the demographics of the participants, we examined the average age, sex distribution, and frailty levels across cohorts. Table 1 presents baseline characteristics across the five cohorts. The average age of participants in HRS, CHARLS, SHARE, ELSA, and MHAS was 65.72, 61.72, 64.96, 65.35, and 61.67 years, respectively. The proportion of males ranged from 41.92% to 60.44%. Baseline frailty ranged from 13.60% to 28.19%, and CVD incidence during follow-up ranged from 15.73% to 37.73%. National comparisons showed that Switzerland had the lowest levels of baseline frailty (4.29%), and Netherlands had the lowest levels of subsequent CVD incidence (9.18%), whereas Mexico and the U.S. had the highest values for these measures, respectively (Supplementary Table 4). Additionally, Fig. 1 illustrates that, across all five cohorts, participants who developed CVD during follow-up had significantly higher FI scores compared to those who remained non-CVD. Similar trends were observed across different countries. The FI scores of patients with heart disease and stroke were also significantly higher than those of non-patients, as shown in Supplementary Figs. 11-12.
Note: The images A, B, C, D and E illustrated the differences in frailty index across CVD by survey year in the following studies: HRS (Health and Retirement Study), MHAS (Mexican Health and Ageing Study), CHARLS (China Health and Retirement Longitudinal Study), ELSA (English Longitudinal Study of Ageing), and SHARE (Survey of Health, Ageing and Retirement in Europe), respectively. Additionally, image F presented the differences in baseline frailty index across CVD categorized by country. For all countries, the CVD group has a smaller sample size than the non-CVD group; detailed sample sizes are provided in Supplementary Table 49. T test (two-tailed independent t-test) for trend was used to compare the trends in FI and CVD across different survey waves. The years corresponding to the horizontal coordinates in images A, B, C, D, and E represented the FI using this survey period and the incident CVD at follow-up (e.g., The 2000 results in HRS corresponded to the use of FI for 2000 and the prevalence for 2002–2018, and so on). In image F, baseline FI and incident CVD at follow-up were used. Frailty index are presented as mean values, with error bars representing ± SD. The p value indicates the difference in frailty index between CVD and Non-CVD groups. **p < 0.01, ***p < 0.001. Source data are provided with this paper.
Kaplan-Meier curves and Cox models
To evaluate the association between frailty and CVD risk, we used Kaplan-Meier survival curves based on baseline FI classification, indicating that frail participants had a significantly higher risk of CVD (Supplementary Fig. 13). Heart disease and stroke showed similar results (Supplementary Figs. 14-15). Additionally, we conducted Cox proportional hazards models to further explore the relationship between baseline frailty and incident CVD. Table 2 presents the association between baseline FI and incident CVD risk using Cox proportional hazards models. Regardless of covariate adjustment, frail participants at baseline were at significantly higher risk for CVD compared to non-frail participants (e.g., Model 4: HRS: 1.51 [1.40, 1.63]; CHARLS: 1.54 [1.38, 1.71]; SHARE: 1.67 [1.57, 1.78]; ELSA: 1.77 [1.59, 1.98]; MHAS: 1.63 [1.46, 1.82]; Pooled: 1.60 [1.54, 1.66]). Country-specific results also consistently indicated that baseline frailty significantly increased the risk of incident CVD (Supplementary Table 5). The separate effects of baseline frailty status on heart disease and stroke for each cohort and country-specific results were shown in Supplementary Tables 6-9. The results showed frail participants at baseline were at significantly higher risk for heart disease or stroke compared to non-frail participants.
Frailty transitions and nonlinear CVD risk
Next, we investigated the impact of frailty transitions on CVD risk. We compared participants who transitioned from non-frail to frail with those who remained stable in their non-frail status. The analysis revealed that participants transitioning from non-frail to frail status had a significantly higher CVD risk compared to those with stable non-frailty (HRS: 1.16 [1.02, 1.31]; CHARLS: 1.87 [1.50, 2.34]; SHARE: 1.32 [1.15, 1.52]; ELSA: 1.59 [1.20, 2.10]; MHAS: 1.47 [1.22, 1.77]; pooled: 1.34 [1.24, 1.44]) (Supplementary Table 10). Conversely, those who improved from frail to non-frail status had a significantly lower CVD risk compared to participants with stable frailty (HRS: 0.73 [0.61, 0.88]; CHARLS: 0.54 [0.40, 0.74]; SHARE: 0.74 [0.61, 0.88]; ELSA: 0.51 [0.34, 0.76]; MHAS: 0.74 [0.60, 0.92]; Pooled: 0.70 [0.63, 0.77]). Furthermore, participants in the highest quartile for mean FI and FI change had significantly higher CVD risk compared to those in the lowest quartile, indicating a positive association between FI levels and CVD risk (Supplementary Table 11). Restricted cubic spline (RCS) analysis also suggested nonlinear associations between mean FI and FI change with incident CVD (Supplementary Figs. 16-17). Separate analyses of heart disease and stroke showed similar results. For details, saw Supplementary Tables 12-15 and Supplementary Figs. 18-21.
Mediation analysis
We performed mediation analysis to investigate the potential role of depressive symptoms in the relationship between baseline frailty and CVD. Mediation analysis revealed that depressive symptoms partially mediated the association between baseline FI and incident CVD (Fig. 2), with mediation effects as follows: HRS (24.73%), CHARLS (16.67%), SHARE (21.05%), ELSA (37.50%), and MHAS (47.37%). Additionally, depressive symptoms mediated the relationship between baseline non-frailty transitioning to frailty and CVD in all five cohorts (Supplementary Fig. 22). However, depressive symptoms didn’t mediate the association between baseline frailty transitioning to non-frailty and CVD (Supplementary Fig. 23). The results of correlation analyses further indicated that there were significant correlations between baseline frailty, depressive symptoms, blood markers and CVD (Supplementary Fig. 24 and Tables 16-20). That was, there might be a frailty → depressive symptoms → worsening of blood markers → CVD mediated pathway.
Note: FI, frailty index; CVD, cardiovascular disease; (A) Pooled; (B) HRS, Health and Retirement Study; (C) CHARLS, China Health and Retirement Longitudinal Study; (D) SHARE, Survey of Health, Ageing and Retirement in Europe; (E) ELSA, English Longitudinal Study of Ageing; (F) MHAS, Mexican Health and Aging Study. All analyses have been adjusted for age, sex, education, employment, marital status, co-residence with children, smoking, drinking, social activity, and physical activity. In the SHARE and Pooled cohorts, we controlled the different country. In the analyses, HRS used frailty status in 2000, depressive symptoms in 2002, and incident CVD in 2004–2018; CHARLS used frailty status in 2011, depressive symptoms in 2013, and incident CVD in 2015–2018; SHARE used frailty status in 2011, depressive symptoms in 2013, and incident CVD in 2015–2019; ELSA used frailty status in 2004, depressive symptoms in 2006, and incident CVD in 2008–2018; MHAS used frailty status in 2001, depressive symptoms in 2003, and incident CVD in 2012–2018.
Cross-lagged panel network analysis
The cross-lagged panel network analysis examined predictive relationships between FI and CVD indicators over time. Results showed that several common factors across different databases in the predictive relationships where FI indicators at T1 positively predicted CVD indicators at T2 (Fig. 3). Hypertension, diabetes, general health and mobility-related factors (eating, walking, and climbing) consistently appeared in HRS, SHARE, ELSA, and MHAS. Furthermore, arthritis and climbing ability were observed as relevant predictors in CHARLS. The specific edge weights (regularized logistic regression coefficients) were presented in Supplementary Tables 21-25. Additionally, we conducted stability tests for the network models, and the results indicated that the models exhibited strong robustness (Supplementary Figs. 25-26).
Note: (A) HRS: Health and Retirement Study; (B) CHARLS: China Health and Retirement Longitudinal Study; (C) SHARE: Survey of Health, Ageing and Retirement in Europe; (D) ELSA: English Longitudinal Study of Ageing; (E) MHAS: Mexican Health and Aging Study. All analyses were adjusted age, sex, education, employment, marital status, co-residence with children, smoking, drinking, social activity, and physical activity. In the SHARE, we further controlled the different country. The lines in the figure represented odds ratios (OR), with values greater than 1 shown in blue and values less than 1 shown in red. Thicker lines indicate larger OR values. To enhance clarity, autoregressive effects and covariates were not displayed in the figure. Additionally, OR values within the range of 1 ± 0.3 were not presented.
Subgroup analysis
Figure 4 illustrated the association between baseline frailty status and incident CVD across subgroups, showing a stronger association among males, older adults, working or retired persons, unhealthy lifestyle behavior groups (smokers, drinkers, social isolators, and low-frequency exercisers) in all five cohorts and Pooled cohorts.
Note: Subgroup analyses were conducted using Cox proportional hazards models (Model 4). Age was categorized into two groups (50–64 and ≥65 years). All models were adjusted for age, sex, education, employment status, marital status, co-residence with children, smoking, drinking, social activity, and physical activity. In the SHARE and pooled cohorts, country was included as a covariate. For detailed sample sizes, please refer to Table 1. Each circle represents the HR for a specific group, and the horizontal line through the circle denotes the 95% CI. The vertical dashed line represents the null value of HR = 1. Arrows indicate confidence intervals exceeding the plotting range.
Sensitivity analyses
To assess the robustness of our findings, we conducted sensitivity analyses. These analyses remained consistent with the primary findings after excluding participants with chronic comorbidities (Supplementary Tables 26–28), considering the use of medications for chronic diseases (Supplementary Tables 29–31), recalculating FI excluding hypertension, diabetes, and arthritis (Supplementary Tables 32–34), excluding samples lost to follow-up en route (Supplementary Table 35-37), considering death as a competitive risk of CVD (Supplementary Table 38-40), and using PSM method to avoid possible residual confounding bias (Supplementary Tables 41-43 and Figs. 27-29). Baseline frailty status, changes in frailty status, mean FI, and FI change all showed significant associations with incident CVD.
Discussion
Using data from five international longitudinal databases—HRS, CHARLS, SHARE, ELSA, and MHAS—this study reveals that frailty significantly increases the risk of cardiovascular disease (CVD) among middle-aged and older adults, with depressive symptoms acting as a partial mediator. Additionally, a transition from non-frail to frail status notably raises CVD incidence, whereas improvement from frail to non-frail reduces this risk. By confirming the frailty-CVD association across countries and elucidating the mediating role of depression, this study provides evidence to inform tailored public health strategies aimed at improving older adults’ health outcomes. These findings underscore the importance of frailty monitoring and open avenues for depression-focused interventions with significant clinical potential.
This study further highlights the frailty-CVD relationship. For instance, longitudinal studies have shown a substantially higher CVD incidence among frail older adults compared to non-frail peers22, as frailty negatively impacts various bodily functions, accelerating cardiovascular complications23. Through multi-country datasets and extended follow-up, this study reinforces frailty as an independent CVD risk factor, emphasizing the need for ongoing frailty monitoring and management in clinical practice24. The dynamic nature of frailty-CVD risk is evident: individuals transitioning to frailty face an elevated risk of CVD, while recovery from frailty mitigates this risk25, suggesting a complex, nonlinear association influenced by health system compensatory mechanisms. Lower frailty levels may allow cardiovascular systems to counterbalance frailty’s burden26, while surpassing a critical threshold could disrupt this compensatory capacity, steeply raising CVD risk27.
The mediating role of depressive symptoms between frailty and CVD aligns with evidence on emotional disorders and cardiovascular health28. Frailty, characterized by physical weakness, fatigue, and a decline in physical function, can lead to or exacerbate depressive symptoms by increasing dependency and reducing the ability to engage in pleasurable activities, thus creating a sense of hopelessness and isolation29,30. Conversely, depression can accelerate the development of frailty by causing behavioral changes such as reduced physical activity, poor self-care, and diminished social engagement31. This cyclical relationship between frailty and depression may further intensify the risk of CVD, creating a vicious cycle. Therefore, interventions targeting depressive symptoms could serve as an important strategy for improving cardiovascular health and reducing CVD risk in older adults20,32. Evidence suggests depressive symptoms can activate the sympathetic nervous system and trigger chronic inflammation, which accelerates CVD progression in older adults—a mechanism verified in multiple longitudinal studies14,33. Additionally, behavioral medicine research further links depressive tendencies in older adults to unhealthy lifestyles, such as reduced physical activity and irregular diet, significantly elevating CVD risk34. This study builds on these findings by confirming depression’s mediating role in the frailty-CVD pathway, emphasizing that psychological interventions targeting depressive symptoms may offer an approach to improving cardiovascular health in older adults, particularly when traditional physical interventions are less effective. Specifically, depressive symptoms may not only affect mental health but also contribute to the worsening of blood markers such as C-reactive protein and glycated hemoglobin A1c, which can, in turn, exacerbate the negative effects of frailty on CVD15. Furthermore, the relationship between frailty and depression is bi-directional.
The cross-lagged panel network analyses reveal common predictors across datasets in the relationship between FI and CVD, particularly hypertension and diabetes, which are well-established risk factors for CVD35. These chronic conditions impair vascular health and promote inflammation, both of which contribute to the progression of CVD36,37. Hypertension, for instance, leads to increased arterial pressure, damaging the blood vessels and causing structural changes that increase the risk of heart attack and stroke38. Similarly, diabetes accelerates the development of atherosclerosis, a key driver of CVD39. Additionally, factors such as general health, and mobility (including eating, walking, and climbing) are frequently identified in the network, suggesting that these factors influence both frailty and CVD40. Reduced mobility, often a result of frailty, diminishes cardiovascular fitness and exacerbates the strain on the heart, while poor general health can reflect broader underlying conditions that elevate CVD risk18,41. Overall, the network analysis emphasizes the importance of early, cross-domain interventions that target these factors to improve health, mobility, thereby effectively reducing both frailty and CVD risk.
Subgroup analyses reveal that the association between frailty and CVD is stronger for frail males, older adults, working or retired people, and individuals with unhealthy lifestyle behaviors, including smokers, drinkers, social isolators, and low-frequency exercisers. Frail males, in particular, exhibit a stronger association due to factors such as accelerated atherosclerosis and metabolic issues, which exacerbate the cardiovascular burden of frailty42. Older males often experience more severe cardiovascular complications due to these physiological factors. In contrast, females benefit from the protective effects of estrogen, which can slow the progression of atherosclerosis, thereby reducing the strength of the frailty-CVD association43,44,45. Older adults, in general, tend to have higher baseline disease burdens and more significant functional decline, which amplifies the impact of frailty on cardiovascular health46. Compared to their employed or retired counterparts, the association between frailty and CVD appears to be weaker among unemployed older adults. Many older individuals may no longer be suitable for continued employment due to age-related physical decline, diminished energy, and reduced capacity to cope with stress—factors that can exacerbate the health consequences of frailty47,48. For those older adults who remain physically capable of working, unemployment, despite its potential financial strain, may offer certain health-related advantages. Freed from the constraints of sedentary work and rigid occupational schedules, some individuals tend to engage more in routine low-intensity physical activities, such as housework or walking49. These forms of activity are known to support metabolic function and may help attenuate the adverse interplay between frailty and cardiovascular health50,51. Furthermore, unemployment often facilitates the activation of familial and community-based support networks, including spousal companionship52, intergenerational care, and neighborhood assistance53. These forms of social engagement can enhance emotional connectivity and social interaction—both of which have been empirically linked to lower levels of inflammatory biomarkers, such as C-reactive protein54,55. As such, social support may help explain why the observed association between frailty and CVD was weaker in this group56. Not only that, smoking damages vascular health, leading to arteriosclerosis and hypertension, which strengthens the association between frailty and CVD57. Long-term excessive drinking may cause cardiovascular problems such as hypertension, arrhythmias, and cardiomyopathy, further reinforcing the association58. Social isolation increases psychological stress and the lack of social support, potentially accelerating frailty progression and further strengthening the frailty-CVD relationship59. Physical inactivity contributes to weight gain, elevated blood lipids, and poor blood sugar control, thereby enhancing the frailty-CVD connection60. Therefore, these findings highlight the need for targeted frailty management and CVD prevention strategies, especially for frail males, older adults, working or retired people, and those with unhealthy lifestyle behaviors, to improve clinical outcomes61,62.
This study’s insights into frailty and depressive symptoms provide valuable directions for reducing CVD risk in older adults. First, directly addressing frailty is beneficial. For example, Multimodal interventions has effectively reduced risks of heart disease and stroke through strength training and nutrition improvement12. Similarly, cardiovascular disease management in primary care (CONNECT), through customized physical activity, nutrition support, and social interaction, has shown reduced frailty indices and improved cardiovascular health, lowering CVD risk63. Additionally, targeting depressive symptoms as an intervention point for frail older adults has demonstrated efficacy. For instance, in a U.S. Heart Association intervention program, depression treatment with cognitive behavioral therapy (CBT) and healthy lifestyle guidance (e.g., increased physical activity and balanced diet) effectively reduced CVD incidence64. Improving Mood Promoting Access to Collaborative Treatment (IMPACT) has enhanced engagement among older patients, successfully alleviating their depressive symptoms. The psychological support provided through Interpersonal Psychotherapy (IPT) helps to reduce inflammation and improve mental health, potentially lowering the risk of CVD65. Besides, interventions should focus on older adults who are currently employed or retired, as they may face career-related risks that increase the likelihood of cardiovascular disease. Measures include providing psychological support, alleviating work-related stress, and promoting a healthy lifestyle to reduce these risks and improve cardiovascular health66. For individuals with an unhealthy lifestyle, interventions should focus on smoking cessation, reducing alcohol consumption, increasing physical activity, with a recommendation of at least 150 min of moderate-intensity exercise per week67,68. These individualized approaches underscore effective pathways for frailty management and CVD prevention. Multilevel interventions aimed at improving frailty and depression in older adults can yield significant preventive benefits against CVD33,69.
This study’s strengths include using multiple international datasets, enhancing generalizability and reliability. By examining both frailty’s impact on CVD and the mediating role of depressive symptoms, this analysis provides a perspective on mind-body health. However, several limitations remain. As an observational study, causal inference is limited, and unobserved confounders may affect the relationship between frailty, depressive symptoms, and CVD. Despite efforts to account for national differences, different social, economic, cultural contexts, and healthcare systems across countries may influence the results, particularly regarding health management, social support, and cultural attitudes. Additionally, data attrition may affect the sample’s representativeness, especially if dropouts differ systematically from those who remain. Finally, while the study includes multiple cohorts, cross-national comparisons are still challenging due to differences in healthcare infrastructure, access to care, and health behaviors.
In summary, this study reveals a complex relationship between frailty, depressive symptoms, and cardiovascular disease. By emphasizing the mediating role of depression, highlighting the importance of biomarkers, and utilizing cross-lagged panel network analysis, this research provides a direction for clinical interventions, underscoring the importance of comprehensive frailty interventions among older adults to reduce CVD burden.
Methods
Ethics and Inclusion
This study complies with all relevant ethical regulations. Ethical approval for the data used was obtained from the following institutions: HRS (approved by the University of Michigan Institutional Review Board), CHARLS (approved by the Biomedical Ethics Review Committee of Peking University, IRB00001052-11015), SHARE (approved by the Ethics Council of the Max Planck Society), ELSA (approved by the National Research Ethics Service Committee South Central-Berkshire), and MHAS (approved by the Institutional Review Boards and Ethics Committees of the University of Texas Medical Branch in the USA, the National Institute of Statistics and Geography (INEGI), and the National Institute of Public Health (INSP) in Mexico). No additional approvals were required. All participants provided written informed consent. The authors did not conduct direct data collection, and data ownership remains with the original data custodians.
The research team acknowledges the contributions of local researchers involved in data collection and cohort design. Authorship follows the ICMJE criteria, with attribution limited to contributors involved in conceptualization, analysis, interpretation, and manuscript writing. No material transfer, intellectual property concerns, or local restrictions were involved. The study poses no risks of stigmatization or discrimination, and local/regional research relevant to each dataset has been cited appropriately. As only secondary analysis of existing data was performed, no benefit-sharing arrangements or capacity-building plans were required.
Study design and population
Data were derived from five international longitudinal cohorts focused on middle-aged and older adults: the Health and Retirement Study (HRS)70, the China Health and Retirement Longitudinal Study (CHARLS)71, the Survey of Health, Ageing, and Retirement in Europe (SHARE)72, the English Longitudinal Study of Ageing (ELSA), and the Mexican Health and Ageing Study (MHAS)73. These surveys provide globally comparable insights into aging, encompassing information on frailty index, depressive symptoms, blood markers and cardiovascular disease (CVD)74. To fully examine the effects of frailty and changes in frailty status on incident CVD, and the mediating role of depression, this study used long-term findings from five cohorts. The final dataset included survey waves as follows: HRS 2000–2018, CHARLS 2011–2018, SHARE 2011–2019, ELSA 2004–2018, and MHAS 2001–2018.
Sample selection was restricted to participants aged 50 and above, with exclusions for those missing data on baseline frailty index, covariates, and follow-up CVD. The final baseline frailty status analytic sample comprised 12,624 participants from HRS (81,507 observations), 10,288 from CHARLS (37,082 observations), 36,954 from SHARE (143,406 observations), 7173 from ELSA (38,767 observations), and 9855 from MHAS (37,127 observations) (Supplementary Fig. 1). The sample screening processes for analysis of changes in frailty status, mediating role of depression in baseline frailty status and changes in frailty status, cross-lagged panel network analysis, and correlation analysis of blood markers were shown in Supplementary Figs. 2–6, and the processes of heart disease and stroke were shown in Supplementary Figs. 7– 10.
Measures
Assessment of frailty: Frailty was assessed using the Frailty Index (FI), which aggregates age-related health deficits. Based on previous studies, FI construction involved 27 items for HRS, CHARLS, and ELSA, 26 items for SHARE, and 25 items for MHAS21,75, including chronic diseases (excluding heart disease and stroke), self-reported health, functional disabilities, and cognition (Supplementary Table 1). All items, except cognition, were dichotomized (1 = deficit, 0 = no deficit); cognition was treated as a continuous variable, ranging from 0 to 1, with higher scores indicating poorer cognitive function. FI was calculated by summing the item scores and dividing by the total number of items, resulting in a score range from 0 to 1, where higher values indicate greater frailty. In this study, a threshold of 0.25 was used to categorize participants as non-frail (FI < 0.25) or frail (FI ≥ 0.25)76,77.
The measure of changes in frailty status was based on changes in frailty status during the first two periods of the survey. When the participant’s baseline was non-frail, the definition of non-frail in the second period was 0 (i.e., the reference group), and the definition of frail in the second period was 1 (i.e., becoming in worse physical condition). When the participant’s baseline was frail, the definition of frail in the second period was 0 (i.e., the reference group), and the definition of non-frail in the second period was 1 (i.e., in better physical condition). Considering that there was no obvious state change between the two categories of stable non-frail and stable frail in the first two periods, it was difficult to compare them in the same group, so this paper divides the groups based on the baseline frailty status.
Mean FI was calculated as the mean of the frailty index for the first two periods of the survey. FI change was calculated by subtracting the value of the first frailty index from the second frailty index. Mean FI and FI change were divided into quartiles, with the lowest quartile as the reference. To analyze the effects of these three indicators on incident CVD, the frailty index of the first two periods was calculated, and the start time of follow-up was the third period in each cohort (HRS: 2004–2018; CHARLS: 2015–2018; SHARE: 2015–2019; ELSA: 2008–2018; MHAS: 2012–2018).
Assessment of CVD: The outcome event in this study was a self-reported diagnosis of cardiovascular disease (CVD), specifically whether participants were informed by a doctor that they had heart disease (including angina, heart attack, congestive heart failure, and other heart issues) or stroke78,79, and whether took medications for the disease. During sample selection, only participants without CVD at baseline were retained. For follow-up, tracking ended once a participant was diagnosed with CVD; survival time was calculated as the year of CVD onset minus the baseline survey year. For participants who remained non-CVD during the study period, survival time was defined as the last valid follow-up survey or study cutoff date minus the baseline year.
In the HRS, CHARLS, SHARE, ELSA, and MHAS, CVD was ascertained based on the self-reported physician-diagnosed heart disease or stroke, and whether took medications for the disease. In each wave of the five cohorts, participants were asked “Have you been told by a doctor that you have been diagnosed with a heart disease, including angina, heart attack, congestive heart failure, and other heart problems?”, “Are you now taking or carrying medications because of heart disease?”, “Have you been told by a doctor that you have been diagnosed with a stroke?”, and “Are you now taking any medications because of your stroke or its complications?”. Those who reported being diagnosed with heart disease, stroke, or taking medications were considered to have CVD. In the next wave, participants were required to confirm the existence of heart disease and stroke if they reported those in the last wave. If participants disputed self-reported heart disease or stroke from previous waves, they were corrected retrospectively. Our CVD ascertainment was consistent with previous studies using the HRS, CHARLS, SHARE, ELSA, and MHAS cohorts56,78,80. For the accuracy of self-reported heart disease and stroke, Xie et al.81 confirmed that 77.5% of self-reported coronary heart disease (defined as the angina + heart attack) were consistent with their medical records in the ELSA. Glymour et al.82 compared self-reported stroke in the HRS with some classical studies in the USA which had data on the medically verified stroke. They found that the misreporting of stroke was non-systematic, and self-reported stroke could be used to study stroke incidence and risk factors in the HRS.
Depressive symptoms: This study assessed depressive symptoms using the Centre for Epidemiologic Studies Depression Scale (CES-D) and the Euro-Depression Scale (Euro-D), both validated tools for diagnosing depressive symptoms83,84. Specifically, the datasets used were HRS (CESD-8), CHARLS (CESD-10), SHARE (EuroD-12), ELSA (CESD-8), and MHAS (CESD-9), with respective score ranges of 0–8, 0–30, 0–12, 0–8, and 0–9. Diagnostic thresholds for depressive symptoms were 3 for HRS, 10 for CHARLS, 4 for SHARE, 3 for ELSA, and 5 for MHAS85,86; participants scoring at or above these thresholds were classified as having depressive symptoms. The diagnosis of depressive symptoms specific items saw Supplementary Table 2.
Blood markers: In HRS 2006, HbA1c, Total cholesterol and HDL cholesterol were measured in Biosafe Laboratories, and CRP was measured at The University of Vermont. In CHARLS 2015, HbA1c, Total cholesterol, HDL cholesterol, and CRP were measured at the Youanmen Center for Clinical Laboratory of Capital Medical University. In SHARE 2015, HbA1c, Total cholesterol, HDL cholesterol, and CRP were measured at the Department for Laboratory Medicine at the University of Washington (UW) in Seattle, USA. In ELSA 2008, HbA1c, Total cholesterol, HDL cholesterol, and CRP were measured at the Royal Victoria Infirmary Laboratory in Newcastle-upon-Tyne. In MHAS 2012, HbA1c, Total cholesterol, HDL cholesterol, and CRP were measured at the INSP Laboratory.
Covariates: Based on previous research, the covariates selected for this study included demographic factors (sex and age), socioeconomic factors (education and employment), living conditions (marital status and co-residence with children), and behavioral habits (smoking, drinking, social activity, and physical activity). In this study, the participants were divided into two age groups: 50–64, 65 years and above. It should be noted that in this paper, continuous variable of age was used as covariates in the model for analysis, and categorical variable of age was used only for subgroup analysis of age. Education was classified into three categories according to the International Standard Classification of Education (ISCED) 1997: below high school, high school, and above high school. The employment status was coded into two groups: unemployed, currently working or retired, based on questions regarding the participants’ current employment status. Marital status was categorized as married/partnered, unmarried/others, the unmarried/others category included individuals who were separated, divorced, widowed, or never married. Co-residence with children was classified into two categories: no, yes. Smoking behavior was classified into two categories: currently not smoking, currently smoking. Drinking behavior was assessed based on whether or not they had consumed alcohol in the past year and was divided into: not drinking, drinking. Social activity was categorized into two groups (no, yes) based on whether participated in a specific type of activity last month. In CHARLS, SHARE and ELSA, the level of physical activity was categorized into two groups: vigorous (engaging in vigorous activity more than once a week), and others. In HRS and MHAS, the categorization of physical activity was: vigorous (engaging in vigorous activity at least three times a week), and others. A detailed description of the relevant covariates can be found in Supplementary Table 3.
Sex and gender: Sex (male or female) was included as a covariate in all five cohorts, and gender information was not collected. Although not the primary variable of interest, we conducted subgroup analyses stratified by sex.
Statistical analyses
Continuous variables were presented as mean (standard deviation), and categorical variables as number (percent). The characteristics of the sample finally included in the baseline analysis compared with those excluded due to younger age, CVD at baseline, and missing data as shown in Supplementary Tables 44–48.
To analyze the association between baseline frailty status and CVD risk, four Cox proportional hazards models were used to calculate hazard ratios (HR) and their 95% confidence intervals (95% CI), with non-frailty as the reference. Model 1 adjusted for demographic variables; Model 2 further included socioeconomic variables; Model 3 added living conditions; and Model 4 was fully adjusted. Using similar methods, we also analyzed associations between changes in frailty status, mean FI, and FI change with CVD incidence. The measures of these three indicators could be found in Supplementary Methods. Mean FI and FI change were divided into quartiles, with the lowest quartile as the reference. Nonlinear relationships were additionally examined.
In the mediation analysis, we investigated depressive symptoms as a mediator in the relationship between baseline frailty status, frailty changes, and CVD incidence. The effect of baseline frailty status was analyzed using depressive symptoms in the second period and CVD follow-up in the third and beyond period. The effect of frailty changes was analyzed using depressive symptoms in the third period and CVD follow-up in the fourth and beyond period. Cross-lagged panel network analysis was performed using data from the first two surveys to identify complex associations and temporal changes between frailty and CVD indicators. The correlation analysis between frailty, depressive symptoms, blood markers, and incident CVD was intended to explain the physiological mechanism to some extent, and the measurement of blood markers was available in Supplementary Methods. Subgroup analyses were conducted to assess differences in these associations across various populations.
To ensure robustness, we performed several sensitivity analyses of baseline frailty status and frailty changes results. First, participants with chronic comorbidities (two or more of hypertension, diabetes, cancer, arthritis, lung disease, psychiatric disease, memory disease) were excluded to evaluate the frailty-CVD association. Second, FI was recalculated, considering the use of medications for chronic diseases. Third, hypertension, diabetes, and arthritis—CVD risk factors—were excluded from the original FI, and the analysis was repeated. Fourth, the included analysis samples were limited to the selected period of time from baseline follow-up to the end of the study, unless excluded due to CVD, i.e., samples lost to follow-up en route were also excluded. Fifth, death was considered as a competitive risk of CVD, and re-analyzed using a competitive risk model. Lastly, to avoid possible residual confounding bias in analyses, propensity score matching (PSM) method was used. All analyses were conducted using STATA (version 16.0) and R (version 4.4.2), with two-sided p-values below 0.05 considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets analyzed in this study are publicly available from the following sources: the Health and Retirement Study (HRS, https://hrs.isr.umich.edu/), the China Health and Retirement Longitudinal Study (CHARLS, https://charls.pku.edu.cn/), the Survey of Health, Ageing and Retirement in Europe (SHARE, https://share-eric.eu/), the English Longitudinal Study of Ageing (ELSA, https://www.elsa-project.ac.uk/), and the Mexican Health and Aging Study (MHAS, https://mhasweb.org/Home/index.aspx). These datasets are accessible to researchers upon registration and compliance with the respective data use agreements. No proprietary data were used. The subset of processed data generated in this study for replication purposes has been deposited in Zenodo under the accession code https://doi.org/10.5281/zenodo.1528739587. Data access via Zenodo is provided for reproducibility only and in accordance with the original data use agreements. Data supporting the findings of this study are also provided in the Supplementary Information and Source Data files. Source data are provided with this paper.
Code availability
The STATA and R codes used in this study are available for replication purposes at Zenodo (https://doi.org/10.5281/zenodo.15287395)87.
References
Yazdanyar, A. & Newman, A. B. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin. Geriatr. Med. 25, 563–577 (2009).
Tan, M. C. et al. The association of cardiovascular disease with impaired health-related quality of life among patients with type 2 diabetes mellitus. Singap. Med. J. 55, 209 (2014).
Dong, J., Zhang, Z., Sun, J., Yang, X. & Zhang, W. The cardiovascular disease burden attributable to kidney dysfunction from 1990 to 2021: an age-period-cohort analysis of the Global Burden of Disease study. Eur. Heart J.—Qual. Care Clin. Outcomes qcae088 (2024) https://doi.org/10.1093/ehjqcco/qcae088.
Jan, B. et al. Cardiovascular diseases among Indian Older adults: a comprehensive review. Cardiovasc. Ther. 2024, 6894693 (2024).
Khan, M. S. et al. Global epidemiology of heart failure. Nat. Rev. Cardiol. 21, 717–734 (2024).
Lu, W. et al. Worldwide trends in mortality for hypertensive heart disease from 1990 to 2019 with projection to 2034: data from the Global Burden of Disease 2019 study. Eur. J. Prev. Cardiol. 31, 23–37 (2024).
Lu, J. et al. Reducing inequity through tackling social determinants of cardiovascular diseases in China. BMJ 387, e079197 (2024).
Ekram, A. R. M. S. et al. The association between frailty and incident cardiovascular disease events in community-dwelling healthy older adults. Am. Heart J.: Cardiol. Res. Pract. 28, 100289 (2023).
Feng, J., Chen, X., Cai, W., Zhou, X. & Zhang, X. Association between inflammatory bowel disease and frailty: a two-sample Mendelian randomization study. Aging Clin. Exp. Res. 36, 21 (2024).
Arantes, F. S. et al. Heart rate variability: A biomarker of frailty in older adults? Frontiers in Medicine. 9, 1008970 (2022).
Damluji, A. A. et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur. Heart J. 42, 3856–3865 (2021).
Naila et al. Interventions for frailty among older adults with cardiovascular disease: JACC state-of-the-art review. J. Am. College Cardiol. (2022) https://doi.org/10.1016/j.jacc.2021.11.029.
Liu, H. et al. Longitudinal impact of frailty states and sleep duration on subsequent depressive symptoms of older adults. J. Am. Geriatr. Soc. 69, 1003–1011 (2021).
Li, X. et al. Cardiovascular disease and depression: A narrative review. Front. Cardiovasc. Med. 10, 1274595 (2023).
Valkanova, V., Ebmeier, K. P. & Allan, C. L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 150, 736–744 (2013).
Ridker, P. M. & Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular dDisease. Circ. Res. (2021) https://doi.org/10.1161/CIRCRESAHA.121.319077.
Lee, G., Arieli, R., Ryou, Y. J. & Martin, P. The bidirectional relationship between depressive symptoms and functional limitations among centenarian survivors in their 80s: testing bivariate latent change score models. Aging Ment. Health 27, 1720–1728 (2023).
Panza, F. et al. Depressive and biopsychosocial frailty phenotypes: impact on late-life cognitive disorders. J. Alzheimer’s Dis. 94, 879–898 (2023).
Liu, A.-B. et al. Associations of frailty and cognitive impairment with all-cause and cardiovascular mortality in older adults: a prospective cohort study from NHANES 2011–2014. BMC Geriatr. 25, 124 (2025).
Liu, X. et al. Frailty and risk of cardiovascular disease and mortality. PLoS ONE 17, e0272527 (2022).
He, D. et al. Changes in frailty and incident cardiovascular disease in three prospective cohorts. Eur. Heart J. 45, 1058–1068 (2024).
Zhu, X., Ding, L., Zhang, X., Wang, H. & Chen, N. Association between physical frailty, circadian syndrome and cardiovascular disease among middle-aged and older adults: a longitudinal study. BMC Geriatr. 24, 199 (2024).
Davies, K., Maharani, A., Chandola, T., Todd, C. & Pendleton, N. The longitudinal relationship between loneliness, social isolation, and frailty in older adults in England: a prospective analysis. Lancet Healthy Longev. 2, e70–e77 (2021).
Chen, L. et al. Physical frailty, adherence to ideal cardiovascular health and risk of cardiovascular disease: a prospective cohort study. Age Ageing 52, afac311 (2023).
Vazquez-Guajardo, M., Rivas, D. & Duque, G. Exercise as a therapeutic tool in age-related frailty and cardiovascular disease: challenges and strategies. Can. J. Cardiol. 40, 1458–1467 (2024).
Cleary, S., Rosen, S. D., Gilbert, D. C. & Langley, R. E. Cardiovascular health: an important component of cancer survivorship. BMJ Oncol. 2, e000090 (2023).
Perazza, L. R., Brown-Borg, H. M. & Thompson, L. V. Physiological systems in promoting frailty. Compr. Physiol. 12, 3575 (2022).
Zhang, F. et al. Causal influences of neuroticism on mental health and cardiovascular disease. Hum. Genet. 140, 1267–1281 (2021).
Vaughan, L., Corbin, A. L. & Goveas, J. S. Depression and frailty in later life: a systematic review. Clin. Interv. Aging 10, 1947–1958 (2015).
Brown, P. J. et al. Frailty and depression in older adults: a high-risk clinical population. Am. J. Geriatr. Psychiatry 22, 1083–1095 (2014).
McPhee, J. S. et al. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology 17, 567–580 (2016).
Buigues, C. et al. The relationship between depression and frailty syndrome: a systematic review. Aging Ment. Health 19, 762–772 (2015).
Sobolewska-Nowak, J. et al. Exploring the heart–mind connection: unraveling the shared pathways between depression and cardiovascular diseases. Biomedicines 11, 1903 (2023).
Liang, Y. et al. Trends in unhealthy lifestyle factors in US NHANES respondents with cardiovascular disease for the period between 1999 and 2018. Front. Cardiovasc. Med. 10, 1169036 (2023).
Petrie, J. R., Guzik, T. J. & Touyz, R. M. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can. J. Cardiol. 34, 575–584 (2018).
Strain, W. D. & Paldánius, P. M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 17, 57 (2018).
Lopez-Candales, A., Hernández Burgos, P. M., Hernandez-Suarez, D. F. & Harris, D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J. Nat. Sci. 3, e341 (2017).
Faraci, F. M. & Scheer, F. A. J. L. Hypertension: causes and consequences of circadian rhythms in blood pressure. Circ. Res. 134, 810–832 (2024).
Fan, Y. et al. Primordial drivers of diabetes heart disease: comprehensive insights into insulin resistance. Diab. Metab. J. 48, 19–36 (2024).
Afilalo, J. Unsuccessful ageing: from frailty to cardiovascular disease. Eur. Heart J. 45, 1069–1071 (2024).
Sciacchitano, S. et al. To be frail or not to be frail: this is the question—a critical narrative review of frailty. J. Clin. Med. 13, 721 (2024).
Wang, C. et al. Frailty in relation to the risk of carotid atherosclerosis and cardiovascular events in Chinese community-dwelling older adults: a five-year prospective cohort study. Exp. Gerontol. 180, 112266 (2023).
Roeters van Lennep, J. E. et al. Women, lipids, and atherosclerotic cardiovascular disease: a call to action from the European Atherosclerosis Society. Eur. Heart J. 44, 4157–4173 (2023).
Meng, Q. et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor α-mediated autophagy. J. Adv. Res. 28, 149–164 (2021).
Nedkoff, L., Briffa, T., Zemedikun, D., Herrington, S. & Wright, F. L. Global trends in atherosclerotic cardiovascular disease. Clin. Ther. 45, 1087–1091 (2023).
Lynch, D. H. et al. The relationship between multimorbidity, obesity and functional impairment in older adults. J. Am. Geriatr. Soc. 70, 1442–1449 (2022).
Li, J., Yuan, B. & Lan, J. The influence of late retirement on health outcomes among older adults in the policy context of delayed retirement initiative: an empirical attempt of clarifying identification bias. Arch. Public Health 79, 59 (2021).
Andrasfay, T., Fennell, G. & Crimmins, E. Pain, physical demands at work, and future work expectations among older adults in the United States. Innov. Aging 7, igad089 (2023).
Smith, L. P., Ng, S. W. & Popkin, B. M. No time for the gym? Housework and other non-labor market time use patterns are associated with meeting physical activity recommendations among adults in full-time, sedentary jobs. Soc. Sci. Med. 120, 126–134 (2014).
Mishra, M., Wu, J., Kane, A. E. & Howlett, S. E. The intersection of frailty and metabolism. Cell Metab. 36, 893–911 (2024).
Xu, Z. et al. Interacting and joint effects of frailty and inflammation on cardiovascular disease risk and the mediating role of inflammation in middle-aged and elderly populations. BMC Cardiovasc. Disord. 25, 118 (2025).
Ma, J. L. C., Wong, M. M. C. & Cheng, E. W. H. The Efficacy of a Community-Based Project in a Chinese Context. Asian Soc. Work Policy Rev. https://doi.org/10.1111/j.1753-1411.2008.00022.x (2009).
Lee, J. O. et al. Young Adult Unemployment and Later Depression and Anxiety: Does Childhood Neighborhood Matter?. J. Youth Adolesc. 48, 30–42 (2019).
Zagic, D., Wuthrich, V. M., Rapee, R. M. & Wolters, N. Interventions to improve social connections: a systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 57, 885–906 (2022).
Kornienko, O., Riis, J., Davila, M., White, N. S. & Garner, P. W. Preliminary insights into associations between C-reactive protein and social network dynamics. Psychoneuroendocrinology 139, 1–10 (2022).
Teshale, A. B. et al. The relationship between social isolation, social support, and loneliness with cardiovascular disease and shared risk factors: a narrative review. Arch. Gerontol. Geriatr. 111, 105008 (2023).
Fu, M. et al. Advancements in cardiovascular disease research affected by smoking. Rev. Cardiovasc. Med. 25, 298 (2024).
Wong, C. X., Tu, S. J. & Marcus, G. M. Alcohol and arrhythmias. JACC: Clin. Electrophysiol. 9, 266–279 (2023).
Ellwood, A., Quinn, C. & Mountain, G. Psychological and social factors associated with coexisting frailty and cognitive impairment: a systematic review. Res. Aging 44, 448–464 (2022).
Perry, A. S. et al. Physical activity over the lifecourse and cardiovascular disease. Circ. Res. (2023) https://doi.org/10.1161/CIRCRESAHA.123.322121.
Zhang, Z., Xu, H., Zhou, J. & Cao, X. The impact of mold exposure on anxiety symptoms in the older adults: a moderated mediation model based on CLHLS. Ecotoxicol. Environ. Saf. 284, 116967 (2024).
Xu, H., Zhang, Z. & Hua, L. Urban resilience reduces depressive symptoms among middle-aged and elderly adults: a multidimensional analysis based on China longitudinal healthy longevity survey. J. Psychiatr. Res. 178, 250–258 (2024).
Redfern, J. et al. A digital health intervention for cardiovascular disease management in primary care (CONNECT) randomized controlled trial. npj Digit. Med. 3, 1–9 (2020).
Mohseni, M. et al. Improved physical and mental health after a combined lifestyle intervention with cognitive behavioural therapy for obesity. Int. J. Endocrinol. Metab. 21, e129906 (2022).
Baharlou, S. et al. Improving Mood Promoting Access to Collaborative Treatment (IMPACT) program in geriatrics primary care. Innov. Aging 5, 279 (2021).
Yuan, B., Zhang, T. & Li, J. Late-life working participation and mental health risk of retirement-aged workers: how much impact will there be from social security system?. J. Occup. Environ. Med. 64, e409 (2022).
Patnode, C. D., Redmond, N., Iacocca, M. O. & Henninger, M. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: updated evidence report and systematic review for the us preventive services task force. JAMA 328, 375–388 (2022).
Gonçalves, C., Bravo, J., Abreu, A., Pais, J. & Raimundo, A. Comparing high-intensity versus moderate-intensity exercise training in coronary artery disease patients: a randomized controlled trial with 6- and 12-month follow-up. J. Public Health (Berlin) (2024) https://doi.org/10.1007/s10389-024-02224-z.
O’Neill, D. E. & Forman, D. E. Cardiovascular care of older adults. BMJ 374, n1593 (2021).
Cleary, J. L. et al. Polygenic risk and social support in predicting depression under stress. AJP 180, 139–145 (2023).
Xie, Y. et al. Factors associated with depressive symptoms among the elderly in China: structural equation model. Int. Psychogeriatr. 33, 157–167 (2021).
Csajbók, Z. et al. Variation in depressive symptom trajectories in a large sample of couples. Transl. Psychiatry 12, 206 (2022).
Brant, L. C. C., Hamburg, N. M., Barreto, S. M., Benjamin, E. J. & Ribeiro, A. L. P. Relations of digital vascular function, cardiovascular risk factors, and arterial stiffness: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort study. JAHA 3, e001279 (2014).
Howrey, B., Avila, J. C., Downer, B. & Wong, R. Social engagement and cognitive function of older adults in Mexico and the United States: how universal is the interdependence in couples?. J. Gerontol.: Ser. B 76, S41–S50 (2021).
Li, L. Internet use and frailty in middle-aged and older adults: findings from developed and developing countries. Glob. Health 20, 53 (2024).
Hoogendijk, E. O. et al. Frailty: implications for clinical practice and public health. Lancet 394, 1365–1375 (2019).
Fan, J. et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health 5, e650–e660 (2020).
Gao, K. et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. eClinicalMedicine 44, 101264 (2022).
Zhang, Z., Zhao, L., Lu, Y., Xiao, Y. & Zhou, X. Insulin resistance assessed by estimated glucose disposal rate and risk of incident cardiovascular diseases among individuals without diabetes: findings from a nationwide, population based, prospective cohort study. Cardiovasc. Diabetol. 23, 194 (2024).
Skoblow, H. F. & Proulx, C. M. C. - Reactive protein, subjective aging, and incident cardiovascular disease: a mediation model. J. Gerontol. B Psychol. Sci. Soc. Sci. 77, 1654–1658 (2022).
Xie, W., Zheng, F., Yan, L. & Zhong, B. Cognitive decline before and after incident coronary events. J. Am. Coll. Cardiol. 73, 3041–3050 (2019).
Glymour, M. M. & Avendano, M. Can self-reported strokes be used to study stroke incidence and risk factors?: evidence from the health and retirement study. Stroke 40, 873–879 (2009).
Andresen, E. M., Malmgren, J. A., Carter, W. B. & Patrick, D. L. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am. J. Prev. Med. 10, 77–84 (1994).
Braam, A. W. et al. Physical health and depressive symptoms in older Europeans: results from EURODEP. Br. J. Psychiatry 187, 35–42 (2005).
Yan, R. et al. Association between internet exclusion and depressive symptoms among older adults: panel data analysis of five longitudinal cohort studies. eClinicalMedicine 75, 102767 (2024).
Quashie, N. T., Arpino, B., Antczak, R. & Mair, C. A. Childlessness and health among older adults: variation across five outcomes and 20 countries. J. Gerontology: Ser. B 76, 348–359 (2021).
Zhang, Z. et al. Replication Materials for ‘Frailty and Depressive Symptoms in Relation to Cardiovascular Disease Risk in Middle Aged and Older Adults’. Zenodo https://doi.org/10.5281/zenodo.15287395 (2025).
Acknowledgements
We would like to express our sincere gratitude to the individuals and participants who contributed to the construction of the public database used in this study. We sincerely appreciate the YIWANDOU team for their invaluable contribution to data cleaning and processing. We also thank Chuantao Zhou and Ao Liu for their invaluable assistance with data analysis and visualization. This research was supported by the Striving for the First-Class, Improving Weak Links and Highlighting Features (SIH) Key Discipline for Psychology in South China Normal University, GuangDong Basic and Applied Basic Research Foundation (Grant Number: 2025A1515011554), Guangdong Philosophy and Social Science Foundation (Grant Number: GD25YXL09), and the National Natural Science Foundation of China (Grant Number: 82201708).
Author information
Authors and Affiliations
Contributions
Z.Z. and H.J.X. conceptualized the study, conducted data analysis and drafted the manuscript. R.J.Z. and Y.X.Y.Y. were responsible for data validation and visualization. X.L. and Y.M. participated in manuscript editing and critical revision. X.L.Z. and Y.Y.W. supervised the study, provided methodological guidance and approved the final version of the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Z., Xu, H., Zhang, R. et al. Frailty and depressive symptoms in relation to cardiovascular disease risk in middle-aged and older adults. Nat Commun 16, 6008 (2025). https://doi.org/10.1038/s41467-025-61089-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61089-2
This article is cited by
-
Triglyceride-glucose-related indices and cardiovascular outcomes in individuals with pre-frailty or frailty: insights from a prospective cohort analysis using UK Biobank data
Cardiovascular Diabetology (2026)
-
Depression shapes the recall of adverse childhood experiences: evidence from a three-wave longitudinal study of 6,260 Chinese adolescents
Nature Mental Health (2026)
-
Association between estimated cardiorespiratory fitness and arthritis in older adults: evidence from four large cohorts
Clinical Rheumatology (2026)
-
Multi-cohort evidence linking edentulism to frailty among older adults
Scientific Reports (2025)
-
Preserving the Metabolic Engine: Muscle as the Therapeutic Target for Cardiovascular Prevention in Obesity Pharmacotherapy
Current Cardiology Reports (2025)