Abstract
Alterations in myocardial energy substrate metabolism and mitochondrial injury following myocardial infarction (MI) lead to structural and functional abnormalities of the heart. The fatty acid translocase CD36 (CD36) plays a pivotal role in regulating lipid homeostasis and mitochondrial metabolism. Here, we demonstrate that inhibiting the palmitoylation of CD36 and the resulting alteration in its subcellular localization alleviates lipid metabolism disorders and mitochondrial dysfunction in cardiomyocytes of male mice post-MI. Mechanistically, the inhibition of CD36 palmitoylation enhances cardiac function through a dual mechanism: first, by alleviating fatty acid overload mediated by plasma membrane CD36, thereby restoring lipid metabolic balance; second, by augmenting the activity of the mitochondrial CD36-PGAM5 signaling axis and modulating Fundc1 and Drp1 Dephosphorylation, which subsequently improves mitophagy efficiency. Overall, our study highlights the significant role of CD36 palmitoylation in preserving heart function by regulating downstream metabolic signaling pathways, suggesting that targeting CD36 palmitoylation could be a promising therapeutic strategy for MI.
Similar content being viewed by others
Introduction
Myocardial infarction (MI) typically arises from an acute blockage in a coronary artery. Despite a decline in acute-phase mortality after MI in recent decades, it remains the leading cause of global mortality and morbidity1. The aftermath of tissue ischemia post-MI can result in cardiac contractile dysfunction, scar formation, and structural remodeling. However, it is crucial to recognize that the effects of MI extend beyond structural alterations2. Ischemic tissues also endure metabolic stress due to inadequate oxygen and nutrients, leading to a decrease in adenosine triphosphate (ATP) synthesis. This, in turn, heightens the vulnerability of the damaged heart, creating a vicious cycle. Mounting evidence suggest that disruptions in myocardial fuel metabolism and bioenergetics contribute to the development of various heart diseases3,4,5. Therefore, it is crucial to identify changes in energy metabolism following MI and to optimize the metabolic remodeling responses, as this approach can provide insights into the underlying pathophysiology and offer innovative therapeutic opportunities for patients with MI.
The heart utilizes various fuels, such as fatty acid (FA), glucose, lactate, ketones, pyruvate, and amino acids, to sustain normal cardiac function. FA is a primary energy source for the adult heart, playing a pivotal role in cardiac energy supply by contributing to approximately 70% of cardiac ATP through oxidation6,7. In addition, PA provides essential coenzyme factors for oxidative phosphorylation of other metabolic substrates in the mitochondria. Disruptions in FA metabolism have been strongly linked to multiple heart diseases8. Current evidence indicates that patients with diabetes mellitus often exhibit increased levels of DAG and ceramide in their skeletal muscle and myocardium, harmful lipid metabolism intermediates directly associated with mitochondrial dysfunction and impaired glucose and FA oxidation9,10. The accumulation of these lipids, termed lipotoxicity, can induce apoptosis, inflammation, and insulin resistance through protein kinase C family members. This lipotoxicity is also implicated in heart dysfunction, making the maintenance of FA metabolite homeostasis crucial for proper cardiac energy supply and overall heart function11.
One underlying mechanism for lipotoxic damage caused by elevated levels of incomplete lipid metabolites is the excessive accumulation of lipids surpassing FA oxidation12,13. Mitochondria, constituting 30% of cardiomyocytes, serve as the primary site for FA oxidative phosphorylation14. The uptake and oxidation of FA in a normal myocardium are meticulously regulated to maintain a dynamic balance. However, following MI, the heart often undergoes severe mitochondrial dysfunction and impaired overall oxidative metabolism, potentially rendering it more susceptible to lipotoxic damage induced by the accumulation of toxic lipids. Cellular uptake of FA across the cell membrane is the initial step in FA metabolism and a crucial factor in regulating lipid homeostasis15. The transmembrane glycoprotein CD36 (CD36), also known as FA translocase, is widely distributed in various tissues, participating in numerous physiological processes, including the uptake of long-chain fatty acids (LCFA), lipid metabolism, and regulation of chronic metabolic inflammation. Notably, CD36 plays a significant role in maintaining myocardial lipid homeostasis, facilitating 70% of the uptake of long-chain FAs in myocardial tissue. Studies have confirmed that impaired synthesis and abnormal distribution of CD36 can result in reduced energy supply to the myocardium, leading to impaired myocardial contractile function16,17,18,19,20. However, downregulation of CD36 in diabetic cardiomyopathy reduces toxic lipids and improves cardiac contractile function21. Therefore, the impact of CD36 on the myocardium may not be consistent and may vary depending on the pathological background.
The function of CD36 is well-established to be highly contingent on its subcellular membrane localization22,23. However, the molecular mechanisms governing the subcellular distribution of CD36 remain incompletely understood. Protein palmitoylation, a post-translational modification involving the covalent attachment of palmitic acid (PA) to a cysteine residue in a protein, plays a crucial role in regulating the subcellular distribution and function of proteins24. It has been verified that human CD36 undergoes palmitoylation at both its N- and C-terminal cysteine residues25,26. Inhibiting CD36 palmitoylation has demonstrated efficacy in improving tissue inflammation and hepatic steatosis in non-alcoholic steatohepatitis by balancing FA metabolism27. The inhibition of CD36 palmitoylation has also been shown to impede the trafficking of CD36 to the plasma membrane, resulting in decreased FA uptake in adipocytes28. However, no research has explored the functional consequences of CD36 palmitoylation in MI. In the present study, we have established that CD36 palmitoylation was markedly upregulated in cardiomyocytes following MI. We therefore explored the regulation of CD36 palmitoylation in the development of MI and the underlying molecular mechanisms. Our findings reveal that inhibiting CD36 palmitoylation reduces its localization to the plasma membrane, which partially restores the balance between fatty acid (FA) uptake and oxidation, thereby preventing harmful liposome accumulation after MI. Additionally, the inhibition of CD36 palmitoylation promotes the relocalization of CD36 to mitochondria and enhances autophagy through its interaction with PGAM5, thereby sustaining mitochondrial homeostasis and cardiac energy supply. The findings provide the evidence that CD36 palmitoylation as a potential therapeutic target for mitigating MI injury.
Results
Proteomics analysis reveals that metabolic reprogramming and mitochondrial dysfunction after MI
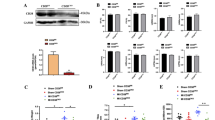
A mouse model of MI was established by ligating the left coronary artery for 4 weeks, following the previously described method29. In this model, cardiac function was impaired, as indicated by reduced ejection fraction (EF) and fractional shortening (FS) measurements (Supplementary Fig. 1a). To have comprehensive understanding of protein expression and dynamics in response to MI, we performed proteome sequencing on heart tissue isolated from control mice and ischemic injury mice. Hierarchical clustering analyses demonstrated marked alterations in cardiac protein levels post-MI (Fig. 1a, b). A total of 3490 proteins were identified, with 307 significantly different between the sham and MI groups, including 112 up-regulated protein and 195 down-regulated proteins (FC > 1.2 or FC < 0.83, p < 0.05) (Fig. 1c). Gene ontology (GO) biological process enrichment analysis of the differentially expressed proteins (DEPs) indicated that the downregulated proteins were primarily associated with energy generation-related processes, such as “ATP metabolic process”, “oxidative phosphorylation” and “electron transport chain” (Fig. 1d). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses revealed a significant reduction in metabolic pathways in MI, including “the citrate cycle (TCA cycle)”, “carbon metabolism”, “metabolic pathways”, and “oxidative phosphorylation”. Conversely, the upregulated genes were associated with cardiac pathological pathways, specifically the “NF-kappa B signaling pathway” and “ferroptosis” (Fig. 1e). Notably, further analysis as illustrated in Fig. 1f revealed FA metabolism was greatly affected among the substance metabolism pathways, with almost all proteins associated with “fatty acid metabolism”, “fatty acid degradation” and “fatty acid elongation” being approximately twofold less abundant in MI. The subcellular localization analysis was performed on the differentially expressed proteins of the two groups. As depicted in Fig. 1g, the predominant subcellular localization of these differential proteins was at mitochondria, which accounted for 30% of the total differential proteins. Almost all proteins involved in oxidative phosphorylation, TCA cycle and ribosomal were significantly downregulated (Fig. 1h). According to the above analysis, MI is accompanied by energy metabolism disorder and multiple metabolic pathways in myocardial tissue are altered after MI.
a Schematic of the study design of Proteome-sequencing, n = 3 mice per group. b Hierarchical clustering of differentially expressed proteins in MI vs. Sham hearts. c Volcano plot displays the fold changes of all detected proteins and p-values in the hearts of mice between the Sham and MI groups, numbers indicate the proteins with expression differences exceeding 1.2-fold. P-values were assessed by a two-sided Student's test. d Gene Ontology (GO) BP terms analysis of differentially expressed proteins in MI vs. Sham hearts, Red, pathways upregulated in MI hearts. Blue, pathways downregulated in the MI heart (Significance was calculated by the hypergeometric test). e Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of differentially expressed proteins in MI vs. Sham hearts, Red, pathways upregulated in MI heart; Blue, pathways downregulated in MI heart (Significance was calculated by hypergeometric test). f Heat map of differentially expressed proteins enriched in pathways related to fatty acid (FA). Each box represents a different animal, with color indicating log2 fold-change. g Pie charts indicating the main subcellular localization of the differentially expressed proteins in MI vs. Sham hearts. h Heat maps of mitochondrial-related protein levels. Each box represents a different animal, with color indicating log2 fold-change.
To further investigate alterations in the energy metabolism post-MI, in parallel to the proteome sequencing, we also harnessed the same batch of myocardial tissues for a full-spectrum metabolome detection based on widely targeted metabolomics. PCA analysis showed that there was a significant difference between the two groups (Supplementary Fig. 2a). Hierarchical clustering analyses and a volcano map of the myocardial metabolites highlighted differences in myocardial metabolite levels between the sham and MI groups (Supplementary Fig. 2b, c). Subsequent pathway enrichment analysis of distinct metabolites revealed significantly enriched FA-related metabolic pathways, as illustrated in Supplementary Fig. 2d. In line with the findings from proteome sequencing, metabolome sequencing results also indicated notable alterations in multiple pathways associated with FA metabolism following MI. These collective results suggested that in MI, cardiac tissue exhibits abnormal lipid metabolism, coupled with severe mitochondrial dysfunction and impaired overall oxidative metabolism.
Lipotoxic metabolites accumulation in the myocardium after MI
To corroborate the findings from proteomic analysis, we conducted experimental confirmation. qRT-PCR results revealed impaired mitochondrial function and disrupted energy metabolism post-MI, which aligns with the findings from the sequencing analysis. Specifically, the expression of genes related to mitochondrial complexes and the levels of adenosine triphosphate (ATP) in the myocardium were significantly reduced at the infarct border zone following MI (Fig. 2a, b). To further investigate whether impaired mitochondrial function following MI is associated with a reduction in the number of mitochondria linked to cardiomyocyte death, we isolated heart mitochondria from both Sham and MI mice. We subsequently assessed the expression changes of mitochondrial electron transport chain complexes using Western blotting. As illustrated in Fig. 2c, the expression levels of OXPHOS complex proteins in the mitochondria of myocardial tissue within the infarct border zone of the MI group were reduced compared to those in the Sham group. Furthermore, we explored physiological markers of dysregulated lipid metabolism to assess changes in cardiac FA metabolism post-infarction. The results showed a clear trend towards increased levels of plasma free fatty acids (FFAs), although not statistically significant (Supplementary Fig. 3a). Notably, triglyceride (TG) levels in the infarct border zone showed a downward trend compared to the sham group (Supplementary Fig. 3b). In contrast, MI resulted in a significant rise in levels of ceramide and DAG in the myocardium, compared to control samples (Fig. 2d, e). Elevated levels of these lipid species can contribute to cardiac stress and lipotoxicity. These findings indicated the accumulation of harmful lipid intermediates in the heart during MI, while neutral lipids and ATP production remained reduced. The levels of malondialdehyde (MDA) were significantly increased in the MI group, indicating an elevated degree of lipid peroxidation in MI hearts (Fig. 2f). In addition, the content of superoxide dismutase (SOD) was decreased, further suggesting an increased oxidative stress in the myocardium after MI (Fig. 2g). To understand the factors driving these phenotypic changes, we examined the levels of key enzymes involved in FA metabolism. Our data revealed a reduction in gene expression of CPT1 and CPT2, the crucial enzymes responsible for transporting FA across the mitochondrial membrane to the matrix for the initiation of β-oxidation. Furthermore, the MI group exhibited a significant decrease in the expression of diacylglycerol acyltransferase 1 (DGAT1) and diacylglycerol acyltransferase 2 (DGAT2), which are crucial enzymes involved in the final step of triglyceride (TG) synthesis, compared to the control subjects. Reduced PGC1α mRNA levels further suggest impaired mitochondrial oxidative metabolism (Fig. 2h). FAs, in addition to serving as important energy substrates for the heart, provide essential coenzyme factors for the oxidative phosphorylation of other metabolic substrates in the mitochondria. Glucose, another vital substrate for heart metabolism, is known to be influenced by changes in FA metabolism according to numerous studies11,12,30. The accumulation of specific bioactive lipids, such as ceramide and DAG, may mediate insulin resistance31. In our study, the results of a Micro-PET/CT scan results revealed that, four weeks after MI, the uptake of glucose in the myocardium was significantly reduced compared to the mice in the sham group (Fig. 2i). These changes were observed alongside a decrease in the plasma membrane localization of Glut4, while the total protein levels remained unchanged (Fig. 2j). The purity of cell membrane protein was confirmed by the lack of intracellular protein GAPDH expression (Supplementary Fig. 3c). Glut4 is a crucial protein responsible for facilitating insulin-dependent glucose transport in cardiac tissue. In summary, these results demonstrated that glucose and lipid metabolism in cardiac tissue are disrupted following MI, and this alteration may be attributed to an overall decrease in mitochondrial oxidative capacity, resulting in elevated levels of harmful lipids.
a qRT-PCR for quantifying the expression of mitochondrial-related genes between Sham and MI. Beta actin served as a reference gene. n = 8. b Myocardial ATP content in mice. n = 9. c Western blotting analysis of OXPHOS complexes in mitochondria of mouse cardiac tissue. Data are representative of six independent experiments. d, e Analyses of myocardial ceramide (d) and diacylglycerol (DAG) levels (e) in Sham and MI mice. n = 5. f, g Analyses of myocardial malondialdehyde (MDA) (f) and superoxide dismutase (SOD) (g) levels. n = 12 (MDA), n = 7 (SOD). Statistical significance was assessed by a two-tailed unpaired Student t test. h Changes in the expression of myocardial metabolism-related genes expression. Beta-actin as a reference gene. n = 6 (CPT1 and DGAT1), n = 9 (CPT2) and n = 10 (DGAT2 and PGC1α). i Representative 18F-FDG images with microPET in the coronal and axial planes in Sham and MI mice 4 weeks post-LAD. Data are representative of five independent experiments. j Total protein level and plasma membrane distribution of Glut4 in cardiac tissue, as determined by Western blot analysis. n = 4. Data are presented as means ± SEM. Statistical significance was assessed by a two-tailed unpaired Student t test (a, b, d–h and j). Source data are provided as a Source Data file.
Single-cell RNA sequencing identifies CD36 as a key regulator of cardiomyocytes metabolism
To gain insights into potential mechanisms underlying metabolic remodeling in the myocardium after MI and to explore molecular insights across cell types, we conducted single-cell RNA sequencing (scRNA-seq) using droplet-based encapsulation using the 10X Genomics platform (Fig. 3a). Cluster analysis, performed with uniform manifold approximation and projection (UMAP) dimensionality reduction after integration with Seurat, categorized cardiac cells into 9 distinct clusters based on marker genes. The proteomic study indicated changes in mitochondrial protein expression, suggesting a significant decline in mitochondrial oxidative phosphorylation capacity and ATP production following MI. Considering that cardiomyocytes, with the highest mitochondrial content, belong to the predominant cell type in the heart, we focused our subsequent analyses on differentially expressed genes (DEGs) in distinct groups of cardiomyocytes. Cardiomyocytes were identified based on the marker genes Pln and Slc8a1 (Fig. 3b). In this way, we identified a set of 452 genes that were differentially expressed in cardiomyocytes between the sham and MI groups with 214 upregulated and 238 downregulated genes (Fig. 3c). Consistent with proteomic analyses, Gene ontology (GO) analyses revealed that significant alterations in electron transport chain and mitochondrial related genes in cardiomyocytes between the two groups (Supplementary Fig. 4a). KEGG pathway analyses confirmed enrichment in oxidative phosphorylation, thermogenesis, fatty acid degradation, cardiac muscle contraction, and citrate cycle (TCA cycle) in cardiomyocytes DEGs (Supplementary Fig. 4b). Combining the proteomic and cardiomyocytes-transcript sequencing studies allowed us to assess the correlation between the two datasets. Further correlation analysis was conducted, and a Venn diagram was generated to illustrate the significant overlap between DEPs identified in the proteome and DEGs identified in the transcriptome of cardiomyocytes. Specifically, 34 genes that displayed enrichment in both data types (Fig. 3d). GO and KEGG enrichment analyses showed that the overlapping downregulated genes were enriched for electron transport chain and oxidative phosphorylation pathways, suggesting that mitochondrial function was suppressed not only post-translationally manner but also transcriptionally (Fig. 3e, f). Consistent with the overall changes observed in myocardial tissue through proteome sequencing results, the single-cell data analysis revealed that cardiomyocytes, the primary cell type responsible for cardiac contractility, exhibited pronounced mitochondrial dysfunction and alterations in metabolic pathways following MI.
a Schematic workflow of the cardiac tissues single-cell sequencing. Cells were isolated by mechanical and enzymatic dissociation from the ventricular tissues of adult mice. Live cells were used for scRNA-seq using the 10X Genomics platform. b Uniform Manifold Approximation and Projection (UMAP) plot of the aggregate of all sequenced cardiac cells identified 9 broad cell types after unsupervised clustering. Violin plot showing the expression levels of cardiomyocyte genes Acta2 and Atp2a2 signature in all identified cell types. c Volcano plot of differentially expressed genes in MI vs. Sham cardiomyocytes (fold change > 1.2, P-value < 0.05). P-values were assessed by the Wilcoxon rank-sum test. d A Venn diagram showing the overlap of detected cardiac DEPs and DEGs of cardiomyocytes. e GO pathway enrichment and (f) KEGG pathway enrichment in the overlapping data (Significance was calculated by a hypergeometric test). g Gene expression visualized in UMAP plots for CD36, Fabp4 and Fabp5 in cardiomyocytes of Sham and MI mice.
Interestingly, further analysis of the volcano plot revealed that among the upregulated genes, at least three genes related to FA metabolism were identified, namely CD36, Fabp4, and Fabp5 as show in UMAP plot (Fig. 3g). Among these, CD36 exhibited the most significant increase in cardiomyocyte following MI, suggesting a more pronounced role in the context of MI (Fig. 3c). The entry of fatty acids into cells is a crucial initial step in their utilization by cardiomyocytes, with CD36 serving as the key protein that mediates this transport17,32. Furthermore, as a member of the scavenger receptor family, CD36 is involved in various cellular functions, widely distributed in different tissues, and playing a role in numerous physiological processes, including lipid homeostasis and regulation of chronic metabolic inflammation. However, its specific role in MI remains unclear. To gain further insights, we conducted immunoprecipitation of CD36 and performed proteomic analysis to identify its binding partners in cardiomyocytes (Supplementary Fig. 5a). We identified 386 proteins, and biological process. KEGG analysis revealed that the enriched proteins were predominantly involved in metabolic processes (Supplementary Fig. 5b, c). Subcellular localization analysis of the CD36 interactome revealed that a high percentage of interacting partners are mitochondrial proteins (Supplementary Fig. 5d). These findings indicate that CD36 and its interactome may play a crucial role in metabolic processes and mitochondrial function in cardiomyocytes. To further validate the findings from single-cell sequencing, we assessed the expression of CD36 protein in the total protein extracted from myocardial tissue. The results indicated that, unlike the single cell sequencing outcomes, the levels of CD36 in total myocardial tissue protein remained relatively unchanged between the sham and MI group (Fig. 4a). We propose that the myocardial tissue comprises multiple cell types, and variations in CD36 expression within cardiomyocytes may be obscured by the gene expression profiles of other cell types present in the sample. To test this hypothesis, we isolated adult mouse cardiomyocytes from both the Sham and MI groups and evaluated changes in CD36 levels using PCR and Western Blot analysis. The results indicated a significant increase in CD36 mRNA expression in the MI group (Fig. 4b); however, the total CD36 protein levels did not correspondingly increase with mRNA expression (Fig. 4c). This discrepancy may be attributed to post-transcriptional regulatory mechanisms, such as the influence of microRNAs (miRNAs)33,34. The subcellular localization of the CD36 protein is crucial for its function22,23. Therefore, we conducted an analysis of CD36 protein expression in subcellular protein fractions from the heart and cardiomyocytes. The results indicated noticeable changes in CD36 localization following MI. Specifically, plasma membrane CD36 levels, which mediate LCFA uptake, were found to be increased, whereas subcellular CD36 (located in the cytoplasm containing organelles) levels were decreased (Fig. 4a, c). These findings were further corroborated by confocal microscopy analysis, which revealed elevated levels of CD36 on the plasma membrane in cardiomyocytes from MI mice (Fig. 4d).
a Western-blot analysis of CD36 in different fractions of myocardium. n = 9 for total CD36; n = 5 for plasma membrane CD36; n = 8 for subcellular CD36. b CD36 mRNA level in isolated adult cardiomyocytes from Sham and MI mice. n = 6. c Western-blot analysis of CD36 in different fractions of isolated adult cardiomyocytes from Sham and MI mice. n = 6 for total CD36 and plasma membrane CD36; n = 8 for subcellular CD36. d Immunostaining assay was used to analyze the colocalization of CD36 (green) and Na+/K+-ATPase (red) in isolated adult cardiomyocytes. Trace outline is used for line-scan analysis of relative fluorescence intensities of CD36 with Na+/K+-ATPase signals. Scale bar = 100 µm. Data are presented as means ± SEM. Statistical significance was assessed by a two-tailed unpaired Student t test (a–c). Source data are provided as a Source Data file.
In summary, the integration of orthogonal multiomics data offers a more comprehensive understanding of post-transcriptional and post-translational regulations in MI. Our comparative analyses of single cell sequencing and proteomic consistently highlight the importance of defects in fuel/energy metabolism and mitochondrial derangements in cardiac dysfunction following MI. Furthermore, CD36 emerges as a potential molecular target for regulating cardiomyocyte metabolic processes and mitochondrial function following MI.
Inhibition of CD36-mediated FA uptake improves cardiac function by reducing infarction size and modifying metabolism
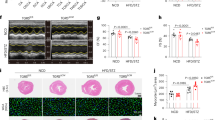
One significant cause of lipotoxic damage is the excessive accumulation of lipids that surpasses the capacity for FA oxidation. Based on our findings of altered CD36 localization and elevated levels of incomplete lipid metabolism products post-MI, we hypothesize that increased plasma membrane localization of CD36 might worsen the imbalance of lipid uptake and oxidation, promote the accumulation of lipotoxic metabolites, and exacerbate cardiac dysfunction following MI. To test this hypothesis, we utilized sulfosuccinimidyl oleate sodium (SSO), a CD36-specific inhibitor that binds to CD36 receptors on the cell surface to inhibit the transport of FAs into the cells (Supplementary Fig. 6a). Firstly, we evaluated the inhibitory efficacy of SSO on FA uptake at both in vivo and in vitro levels. As illustrated in Supplementary Fig. 6b–d, our flow cytometry analysis demonstrated that SSO effectively reduced the binding and uptake of FAs in cardiomyocytes. Concurrently, SSO exhibited a relatively sustained inhibitory effect on FA uptake in mouse hearts. To further determine whether controlled inhibition of CD36 could protect the heart against ischemic injuries, we surgically induced MI (permanent left anterior descending coronary artery ligation) in C57BL/6 mice and administered either SSO or solvent once a week intraperitoneally at 40 mg/kg for 4 weeks (Fig. 5a). Echocardiographic examination showed that treatment with SSO exerted significant beneficial effects on LV ejection fraction and LV fractional shortening, mitigating the negative impact of MI (Fig. 5b). This was further supported by the staining of mouse hearts using 2,3,5-triphenyltetrazolium chloride (TTC) revealing a significant decrease in infarct size with SSO treatment (Fig. 5c).
a Schematic diagram of the study design, CD36-specific inhibitor Sulfosuccinimidyl oleate sodium (SSO) or solvent once a week intraperitoneally for 4 weeks after MI. Echocardiography was performed at 4 weeks post-MI. b Ejection fraction (EF) and fractional shortening (FS) values were determined to evaluate cardiac function by echocardiography in mice of each group. n = 8 per group. c Averaged infarct size by 2,3,5-triphenyltetrazolium chloride (TTC) staining. n = 5. d, e Histological analysis of myocardial tissue sections with Masson staining (d) and hematoxylin-eosin (H&E) staining (e). n = 6 per group. f Representative transmission electron microscopy images of myocardium from mice with or without SSO treatment after MI. n = 5 per group. g, h Cardiac ceramide (g) and DAG levels (h) after treatment with SSO detected by ELISA assay. n = 8 for ceramide, n = 7 for DAG. i, j Quantitation analysis of Cardiac tissue malondialdehyde (MDA) (i) and superoxide dismutase (SOD) (j) levels. n = 9 per group. Data are presented as means ± SEM. Statistical significance was assessed by Kruskal-Wallis, followed by the false discovery rate [FDR] method of Benjamini and Hochberg test (b, i) and One-way ANOVA, followed by Tukey post hoc multicomparisons test (c, d, g, h and j). Source data are provided as a Source Data file.
Furthermore, hematoxylin and eosin (H&E) staining, as well as Masson staining, confirmed the protective effect of SSO on cardiac tissue to some extent. In comparison with the sham operation group, the arrangement of myocardial fibers was disrupted after MI, accompanied by the appearance of fibrous tissue and collagen deposition. However, SSO treatment alleviated these abnormalities (Fig. 5d, e). The transmission electron microscopy (TEM) analyses revealed myofilament lysis and sparse presence, disarrayed mitochondrial cristae structures in MI mice. In addition, mice treated with SSO exhibited mild damage to the myocardial ultrastructure, but with reduced cellular edema and preservation of the mitochondrial matrices (Fig. 5f). Based on the observed improvement of mitochondrial structure by SSO, as determined by TEM results, we conducted further analysis to investigate the effect of SSO on ATP production. The results demonstrated that SSO treatment ameliorated the inhibition of ATP generation induced by MI (Supplementary Fig. 7a). These findings suggest that SSO treatment has the potential to enhance mitochondrial function in the context of MI.
Next, to determine whether SSO improved lipid profile, we proceeded to measure the levels of intracellular lipids and the extent of lipid peroxidation in each experimental group. The MI-induced elevation in ceramide and DAG was significantly reduced in the myocardium of SSO-treated mice as compared to solvent-treated mice (Fig. 5g, h). In addition, we assessed lipid peroxidation levels by quantifying the content of MDA and SOD. The results demonstrated that SSO administration led to a significant decrease in MDA content and an increase in SOD levels (Fig. 5i, j). Given that ceramides likely contribute to the inhibition of insulin signaling31, we investigated the effect of SSO on the plasma membrane localization of Glut4. Interestingly, treatment with SSO increased the levels of Glut4 proteins in the plasma membrane, while the total Glut4 protein remained constant (Supplementary Fig. 7b–d). Overall, lipid peroxidation and lipid peroxide-induced injury may worsen oxidative stress by impairing mitochondrial function, creating a vicious cycle. However, our findings demonstrate that inhibiting CD36 with SSO can break this vicious circle to reduce the excessive accumulation of toxic liposomes and correct mitochondrial abnormalities.
Palmitoylation is required for both the plasma membrane localization and cellular FA-uptaking activity of CD36
We then went on to investigate the molecular mechanisms underlying the enhanced localization of CD36 on the plasma membrane following MI. As protein palmitoylation plays a crucial role in regulating protein trafficking and localization, the effects of the palmitoylation of CD36 in the cardiovascular system remained unclear. The palmitoylation levels of CD36 in MI mice were estimated using the immunoprecipitation and acyl-biotin exchange (IP-ABE) method35. The results showed a significant increase in the level of palmitoylated CD36 proteins in MI hearts compared to sham controls (Fig. 6a). We then explored whether palmitoylation regulates the plasma membrane localization and cellular FA-uptake activity of CD36 in cardiomyocytes. Cardiomyocytes were treated with PA to induce palmitoylation, and 2-bromopalmitate (2 bp), a potent inhibitor of palmitoyl transferase, was used to inhibit palmitoylation. The results demonstrated that the presence of PA significantly increased CD36 palmitoylation, while 2 bp decreased CD36 palmitoylation in cardiomyocytes (Fig. 6b). Subcellular fractions were isolated to verify the impact of palmitoylation on CD36 distribution on the cell plasma membrane. The results demonstrated that PA significantly enhanced the CD36 presence in the plasma membrane, while 2 bp notably reduced it. No significant change was observed in the total CD36 protein levels (Fig. 6c). These findings indicated that palmitoylation modification regulates the subcellular translocation of CD36 in cardiomyocytes. We then continued to investigate whether bidirectional alterations in the palmitoylation of CD36 might contribute to the observed changes in the physiological function of CD36. Previous studies have established CD36 as a crucial factor in LCFA uptake17,32,36,37. To elucidate the impact of CD36 palmitoylation on the FA binding and uptake activity in cardiomyocytes, we utilized a bivariate flow cytometric assay, a technique developed specifically to address bias arising from differences in receptor expression in cell-based ligand binding assays. Our findings demonstrated a prominent enhancement in the binding and uptake of Biodipy FL-16 in cardiomyocytes that were pretreated with PA. This enhancement was found to be contingent on the surface expression of CD36. Conversely, when CD36 palmitoylation was inhibited by 2 bp, there was a noticeable decrease in Biodipy FL-16 binding and uptake in a manner dependent on the surface expression of CD36 (Fig. 6d, e and Supplementary Fig. 8a, b). Together, these data provided evidence that the palmitoylation of CD36 plays a crucial role in modulating the FA binding and uptaking activity in cardiomyocytes, through regulating the subcellular localization of CD36. Next, we experimentally explore the specific function of CD36 palmitoylation in hypoxic injury. Western blot assay showed that hypoxia induces an increase in CD36 palmitoylation and plasma membrane CD36 levels in cardiomyocytes. This effect is further exacerbated by PA. Conversely, treatment with 2 bp reverses the increases in CD36 palmitoylation and plasma membrane CD36 levels induced by hypoxic stimulation (Fig. 6f–h). In addition, Hypoxic stimulation increased the uptake and binding of FAs as well as the levels of toxic lipid metabolites like ceramides and DAG in neonatal mouse ventricular cardiomyocytes (NMVCs), which was significantly amplified by PA treatment but was mitigated by 2 bp treatment (Fig. 6i–l). Collectively, these results indicate that the inhibition of CD36 palmitoylation can ameliorate the hypoxia-induced accumulation of toxic liposomes in cardiomyocytes by altering the plasma membrane localization of CD36.
a Protein levels of palmitoylated CD36 in hearts of Sham and MI mice. n = 6 per group. b Palmitoylated CD36 protein levels in PA (160 µM, 24 h) or 2 bp (25 µM, 24 h) treated cardiomyocytes (NMVCs). n = 9 per group. c Western blotting analysis of plasma membrane and total CD36 protein in PA or 2 bp treated cardiomyocytes (NMVCs). n = 6 per group. d, e Bivariate flow cytometry analysis was conducted to investigate the impact of CD36 palmitoylation on FA uptaking in cardiomyocytes (NMVCs). d Mean Fluorescence Intensity of Bodipy FL-C16. n = 6. e The values provided are in arbitrary fluorescence units. In cases where there were 3 cells or fewer for a given PE fluorescence channel, the point is plotted at 0 on the y-axis. The blue dots plotted along the x-axis indicate significant differences in FA uptaking values between Control and PA or 2 bp treatment cells with similar CD36 expression levels (determined by Student’s t-tests reaching the 99.9% confidence value)79. f–h Palmitoylated CD36 protein and plasma membrane CD36 protein levels in PA or 2 bp treated cardiomyocytes (NMVCs) after 24 h of hypoxia induction. n = 5 for palmitoylated CD36; n = 6 for surface CD36. i, j FA uptake (i) and binding (j) analyses were conducted on cardiomyocytes (NMVCs) treated with PA or 2 bp following 24 h of hypoxia induction. n = 7 for cell uptake; n = 5 for cell binding. k, l Ceramide (k) and DAG (l) levels in cardiomyocytes (NMVCs) in each group. n = 8 for ceramide; n = 7 for DAG. Data are presented as means ± SEM. Statistical significance was assessed by a two-tailed unpaired Student t test (a), One-way ANOVA, followed by Tukey post hoc multicomparisons test (b, c [Surface CD36], d and f–l) and Kruskal-Wallis, followed by false discovery rate [FDR] method of Benjamini and Hochberg test (c [Total CD36]). Source data are provided as a Source Data file.
To delve further into the impact of palmitoylation on CD36, we employed site-directed mutagenesis to construct a mutant plasmid denoted as AA-SS25, where the four cysteine residues were substituted with alanine and serine residues. Next, we transfected both WT-CD36 and AA-SS-CD36 plasmids into cardiomyocytes to increase in CD36 expression in both groups (Fig. 7a). However, the plasma membranes levels of CD36 were significantly lower in the AA-SS-CD36 group compared to the WT-CD36 group, accompanied by a significant upregulation of subcellular CD36 levels (Fig. 7b). Two-color flow cytometry results, similar to 2 bp, demonstrated a notable reduction in FA binding and uptake in the AA-SS-CD36 group compared to the WT-CD36 group. This decrease was dependent on the surface expression of CD36 (Fig. 7c, d and Supplementary Fig. 8c, d). Our previous findings have shown that inhibiting CD36-mediated FA uptake can decrease toxic lipid metabolites and oxidative stress levels following MI. Consistently, we observed that hypoxic stimulation increased ceramide and DAG levels in cardiomyocytes, while mutations at the CD36 palmitoylation sites restored the impairments induced by hypoxic treatment (Fig. 7e, f). In addition, we assessed the impact of CD36 palmitoylation on mitochondrial function by measuring the Mitochondrial respiration (oxygen consumption rate; OCR) of cardiomyocytes. As illustrated in Fig. 7g, we observed that hypoxic treatment suppressed oxidative phosphorylation in cardiomyocytes. However, the inhibition of CD36 palmitoylation partially mitigated the mitochondrial damage caused by hypoxia treatment, which was manifested by a certain degree of improvement in basal respiration, ATP production, and the coupling efficiency of energy production. Reactive oxygen species (ROS) are predominantly generated in the mitochondria and serve various functions within cells. In conditions of low oxygen levels (hypoxia), mitochondria produce elevated levels of ROS as a result of a disrupted electron transport chain. Therefore, we examined the impact of CD36 palmitoylation on the production of mitochondrial ROS (mtROS). The MitoSOX staining assay revealed that mutation of the CD36 palmitoylation site led to a reduction in mtROS levels during hypoxia compared with overexpression WT-CD36 group (Fig. 7h). In summary, these findings suggest that palmitoylation is necessary for the FA-uptake activity of CD36, as it is crucial for directing and ensuring the subcellular localization of CD36. Furthermore, Strategies that limit oxidative stress generally reduce cell death, smaller infarct size, and preserve cardiac function. Our results indicate that CD36 palmitoylation site mutations effectively mitigate mitochondrial damage and decrease mitochondrial ROS generation during hypoxia. This outcome highlights the potential of inhibiting CD36 palmitoylation in improving cardiac infarct size following MI.
a Total CD36 protein expression in cardiomyocyte (NMVCs) was detected by FACS. n = 6 per group. b The plasma membrane and cytoplasm fraction were isolated from cardiomyocytes (NMVCs), and the relative distribution of CD36 protein in different fractions was analyzed by western blot. n = 6 for surface CD36; n = 10 for subcellular CD36. c, d (c) The dual-parameter contour plot correlating FL-C16 uptaking against CD36 expression, as well as a histogram showing the Mean Fluorescence Intensity (MFI) for each group. n = 7. d Cysteine mutation in the palmitoylation site of CD36 reduced FA transport. The blue points plotted along the x-axis indicate significant differences in FA uptaking values between WT-CD36 and AA-SS-CD36 cells with similar CD36 expression levels (determined by Student’s t tests reaching the 99.9% confidence value). e, f Ceramide (e) and DAG (f) levels in cardiomyocytes (NMVCs) transfected with WT-CD36 and AA-SS-CD36 after 24 h of hypoxia induction. n = 5 for ceramide; n = 7 for DAG. g The mitochondrial oxygen consumption rate (OCR) in cardiomyocytes (NMVCs) transfected with WT-CD36 and AA-SS-CD36 after 24 h of hypoxia induction. n = 5 per group. h Mean fluorescence intensity of MitoSOX in cardiomyocytes (NMVCs) transfected with WT-CD36 and AA-SS-CD36 after 24 h of hypoxia induction. n = 6 per group. Data are presented as means ± SEM. Statistical significance was assessed by One-way ANOVA, followed by Tukey post hoc multicomparisons test (a and e–h), two-tailed Mann-Whitney U test (b [surface CD36], c) and two-tailed unpaired t test (b [subcellular CD36]). Source data are provided as a Source Data file.
Inhibition of CD36 palmitoylation alleviates cardiac injury induced by MI
To ascertain the role of CD36 palmitoylation in post-MI cardiac injury, we generated CD36 conventional knockout mice using the CRISPR/Cas9 technique. Employing an AAV9-mediated delivery system with the cTNT promoter, we achieved cardiomyocyte-specific overexpression of WT-CD36 and AA-SS-CD36 in vivo (Fig. 8a). These constructs were then injected into CD36KO mice. As endogenous CD36 was absent in this model, its effects were excluded. As illustrated in Supplementary Fig. 9a, loss of expression of CD36 protein in the myocardium of CD36KO mice and elevated CD36 protein level in cardiomyocytes of CD36KO-WT-CD36 and CD36KO-AA-SS mice were confirmed. Western blot results indicated no significant post-MI alteration in the expression of total CD36 protein among the various groups. However, CD36KO-AA-SS mice exhibited notably lower CD36 expression on the cell membrane compared to CD36KO-WT-CD36 mice, accompanied by an increase in subcellular CD36 levels (Fig. 8b). Echocardiography revealed that the suppressing of CD36 palmitoylation had a prominently improved post-MI cardiac function, evident in increased EF and FS compared to WT-CD36 mice (Fig. 8c–e). In addition, mutation of CD36 palmitoylation sites resulted in a significant reduction in myocardial fibrosis, as indicated by decreased collagen volume (Fig. 8f, g). Moreover, the HE staining and TEM analyses unveiled that mutating the CD36 palmitoylation site protected myocardial ultrastructure and mitigated mitochondrial damage (Fig. 8h, i). Furthermore, CD36KO-AA-SS-CD36 mice exhibited significantly decreased levels of myocardium ceramide, DAG, and MDA, with remarkably increased SOD levels compared to CD36KO-WT-CD36 mice following MI (Fig. 8j–m).
a Schematic diagram of the study design, CD36 knockout (CD36KO) mice were injected with adenoassociated virus 9 (AAV9)–cTnT (cardiac troponin T2)-WT-CD36 (wildtype CD36) or AAV9-cTNT-AA-SS-CD36 (CD36 with mutated palmitoylation sites) via the tail vein. After 4 weeks, the mice underwent left anterior descending coronary artery ligation surgery, followed by echocardiographic measurements on cardiac function. b Protein levels of CD36 in total homogenates and different fractions of WT-CD36 and AA-SS-CD36 mice. n = 6 for total CD36; n = 4 for surface membrane CD36.; n = 8 for subcellular CD36. c–e Echocardiography determined EF and FS values to assess cardiac function after sham or LAD surgery in WT-CD36 and AA-SS-CD36 mice. n = 7 per group. f–h Histological sections with hematoxylin-eosin (H&E) staining and Masson staining of myocardial tissues from WT-CD36 and AA-SS-CD36 mice. n = 5 per group. i Representative transmission electron microscopy images of cardiac tissues from WT-CD36 and AA-SS-CD36 mice (yellow arrow indicates autophagosome). n = 5 per group. j–m Changes in cardiac biomarker levels: cardiac ceramide (j), DAG (k), malondialdehyde (MDA) (l) and superoxide dismutase (SOD) (m) levels in WT-CD36 and AA-SS-CD36 mice. n = 10 for ceramide; n = 8 for DAG; n = 9 for MDA and SOD. Data are presented as means ± SEM. Statistical significance was assessed by One-way ANOVA, followed by Tukey post hoc multicomparisons test (b–g and k) and Kruskal-Wallis, followed by the false discovery rate [FDR] method of Benjamini and Hochberg test (j, l and m). Source data are provided as a Source Data file.
To gain further insight into the impact of inhibiting CD36 palmitoylation on mitochondrial function and ATP production, we conducted additional experiments. Our findings revealed that, following MI, the hearts of CD36KO-AA-SS mice exhibited increased ATP production and enhanced activities of mitochondrial complexes I and II. Furthermore, there was a partial improvement in mitochondrial complexes III and IV, as compared to CD36KO-WT-CD36 mice, whilst the complexes V remained unchanged (Supplementary Fig. 9b–g). In addition, following MI, the plasma membrane distribution of Glut4 was increased in cardiomyocytes of CD36KO-AA-SS mice than CD36KO-WT-CD36, whilst the total protein levels of Glut4 remained unchanged (Supplementary Fig. 9h). In summary, our results demonstrated that inhibiting CD36 palmitoylation enhances cardiac function by reducing infarction size and lipotoxic metabolites, normalizing mitochondria function and preventing lipid overload following impaired mitochondrial oxidative capacity.
Autophagy was required for CD36 palmitoylation inhibition-mediated myocardial functional recovery
Previous studies have established that autophagy acts as a self-degradative mechanism, pivotal in controlling cellular damage and providing protection against MI injury. This process involves the elimination of damaged proteins and organelles38. Numerous studies have reported a reduction in autophagy within myocardial tissue following MI39,40. Consistent with these findings, our research also observed increased levels of SQSTM1 protein and decreased levels of LC3B-II protein in myocardial tissue after MI (Supplementary Fig. 10a–c). Notably, as depicted in Fig. 8i, transmission electron microscopy results revealed a greater accumulation of autophagic structures in CD36KO-AA-SS-CD36 mice compared to CD36KO-WT-CD36 mice after MI. This observation suggests that inhibiting CD36 palmitoylation may induce autophagy in cardiomyocytes following MI. To validate this hypothesis, we further examined the protein levels of autophagosome-bound LC3B-II protein and the abundance of the autophagy substrate SQSTM1/p62. The results showed an increase in autophagy level in CD36KO-AA-SS-CD36 mice compared to CD36KO-WT-CD36 mice, particularly in the heart following MI, as indicated by elevated levels of LC3B-II and reduced levels of SQSTM1 (Fig. 9a). Similar results were observed in cardiomyocytes, where inhibiting CD36 palmitoylation led to increased levels of LC3B-II protein and decreased levels of SQSTM1 protein (Fig. 9b). To further elucidate the underlying molecular mechanisms by which CD36 regulates autophagy, we analyzed the regulation of autophagosome formation using inducers and autophagic flux inhibitors in cardiomyocytes. The mTOR inhibitor rapamycin (Rapa) was employed to induce autophagic flux, while chloroquine (CQ) was utilized to inhibit autophagic flux by blocking the fusion of autophagosomes with lysosomes. In addition, we utilized a dual-fluorescence autophagy system (mRFP-GFP-LC3) and a Cyto-ID immunofluorescence staining that selectively labeled autophagy-related vesicles to evaluate autophagosome formation. The results consistently showed that, regardless of the treatment (rapamycin or chloroquine), inhibiting CD36 palmitoylation led to an increase in the formation of autophagosomes in cardiomyocytes (Supplementary Fig. 11a, b). To investigate whether autophagy regulation is necessary for the myocardial functional recovery post-MI induced by CD36 palmitoylation inhibition in vivo, we utilized 3-methyladenine (3-MA), an autophagy antagonist. The results demonstrate that CD36 palmitoylation inhibition enhances cardiac function following MI, as evidenced by the increases in LVEF and LVFS levels. However, the therapeutic effects of CD36 palmitoylation inhibition were diminished in the presence of 3-MA (Supplementary Fig. 12a–c). In addition, western blot results indicate that following the administration of 3-MA treatment, the level of LC3B-II protein in mouse myocardial tissue is reduced (Supplementary Fig. 12d). These findings suggest that inhibiting CD36 palmitoylation could partially safeguard post-MI cardiac function by promoting autophagy in cardiomyocytes.
a Western blotting analysis of LC3II and SQSTM1 protein levels in myocardium from WT-CD36 and AA-SS-CD36 mice. n = 7. b Western blotting analysis of LC3II and SQSTM1 protein levels in cardiomyocytes transfected with WT-CD36 or AA-SS-CD36 for 48 h. n = 10. c Overlap between CD36 binding proteins, as assessed by CoIP-MS analysis and proteins in the Autophagy Database (http://autophagy.info/). d Endogenous CD36 immunoprecipitation followed by anti-CD36 and anti-PGAM5 western blot analysis of cardiomyocytes (NMVCs). n = 4. e Quantification of Cyto-ID staining in cardiomyocytes (NMVCs) transfected with WT-CD36 or AA-SS-CD36 for 48 h, and then treated with 500 nM rapamycin (Rapa) and 60 µM CQ, the combination for 16 h. n = 6 per group. f–h Western blotting analysis of the mitochondria expression of CD36 and PGAM5 in cardiomyocytes (NMVCs) transfected with WT-CD36 or AA-SS-CD36. n = 7 per group. i Relative PGAM5 mRNA levels in cardiomyocytes transfected with WT-CD36 or AA-SS-CD36 for 48 h. n = 5. j Time course of PGAM5 protein stability in cardiomyocytes (NMVCs) transfected with WT-CD36 or AA-SS-CD36 after treatment with CHX (10 µg/ml). n = 6. k Ubiquitination of exogenous PGAM5 in cardiomyocytes (NMVCs) transfected with WT-CD36 or AA-SS-CD36. Data are representative of six independent experiments. l The mitochondria expression of phosphorylation Fundc1 and total Fundc1 protein in cardiomyocytes (NMVCs) transfected with WT-CD36 or AA-SS-CD36. n = 6 for phosphorylation Fundc1; n = 7 for total Fundc1. Data are presented as means ± SEM. Statistical significance was assessed by Kruskal-Wallis, followed by false discovery rate [FDR] method of Benjamini and Hochberg test (a), two-tailed unpaired Student t test (b, d, g, i and l), two-way ANOVA, followed by Tukey post hoc multicomparisons test (e, j) and two-tailed Mann-Whitney U test (h). Source data are provided as a Source Data file.
PGAM5 playing a crucial role in CD36 palmitoylation inhibition-mediated autophagy in cardiomyocytes
To explore potential downstream mediators of CD36 palmitoylation inhibition-regulated autophagy, we examined whether the amino acid sequence of the CD36 protein for a functional LC3 interaction region (LIR) motif through the iLIR Autophagy Database (https://ilir.warwick.ac.uk/index.php). In this way, we identified a LIR between the 21–28 amino acid sequence (Supplementary Fig. 13a). However, the Co-IP assay results indicated that CD36 protein did not coimmunoprecipitate with LC3B protein in cardiomyocytes (Supplementary Fig. 13b). Next, we intersected the 386 identified CD36 interacting proteins through coimmunoprecipitation coupled with mass spectrometry (CoIP-MS) with 783 predicted autophagy-related genes from the Autophagy Database (http://autophagy.info/) to obtain 25 candidate genes (Fig. 9c). We conducted an additional Reactome pathway analysis (https://www.omicshare.com/) of these 25 candidate genes to further identify genes significantly enriched in specific biological processes, particularly autophagy. Pathways with a maximum of False Discovery Rate (FDR) of 0.05 are retained. As shown in Supplementary Table 1, PGAM5 is one of two genes enriched in the mitophagy signaling pathway. Validation results from co-immunoprecipitation assays demonstrated that CD36 interacts with PGAM5 in cardiomyocytes, with this interaction being enhanced when CD36 palmitoylation is inhibited (Fig. 9d). We further experimentally verified the role of PGAM5 in CD36 palmitoylation inhibition-induced cardiomyocyte autophagy. shRNAs were employed to suppress the expression of PGAM5, utilized in subsequent experiments (Supplementary Fig. 14a). The results indicated that the overexpression of AA-SS-CD36 enhanced the formation of autophagosomes in cardiomyocytes from both the Control group and the group treated with Rapa and CQ together, while this effect was markedly abolished by cotransfection of PGAM5 shRNA (Fig. 9e). These findings suggested that PGAM5 plays a crucial role in CD36 palmitoylation inhibition-mediated autophagy in cardiomyocytes.
CD36 palmitoylation inhibition prevents the ubiquitination and degradation of PGAM5 and facilitates mitophagy
PGAM5 primarily localized in mitochondria, is closely associated with mitochondrial quality control through multiple pathways41. This leads us to speculate whether the changes in the localization of CD36, mediated by palmitoylation, facilitate its relocalization to the mitochondria, subsequently enhancing its interaction with PGAM5 and ultimately affecting autophagy. To test this hypothesis, we initially isolated cardiomyocyte mitochondria and assessed the expression levels of CD36 and PGAM5. The results indicated that the inhibition of CD36 palmitoylation resulted in a significant increase in both CD36 and PGAM5 levels within the mitochondria (Fig. 9f–h). Furthermore, following MI, the expression of CD36 and PGAM5 in the mitochondria of myocardial tissue located in the infarct border area was found to be decreased (Supplementary Fig. 14b). We subsequently further investigated how the inhibition of CD36 palmitoylation stimulated the expression of mitochondrial PGAM5 protein. Intriguingly, PGAM5 mRNA levels did not exhibit significant changes in cardiomyocytes from both the OE-WT and OE-AA-SS groups (Fig. 9i). This observation suggests that PGAM5 may have post-translational regulation. Consequently, we assessed the protein stability of PGAM5 by treating the cells with cycloheximide to inhibit new protein synthesis. The results demonstrated that PGAM5 protein decayed more slowly in the cardiomyocytes of the OE-AA-SS group compared to those in the OE-WT group, indicating that CD36 palmitoylation inhibition enhances PGAM5 protein stability (Fig. 9j). Ubiquitination is a significant form of posttranslational modification that plays a crucial role in regulating the stability of various proteins. Recent studies have indicated that ubiquitination is instrumental in modulating both the levels and functions of the PGAM5 protein42,43. Consequently, we sought to investigate whether the inhibition of CD36 palmitoylation is involved in the modulation of PGAM5 ubiquitination. We conducted a protein docking analysis, and the results indicated that CD36 can directly interact with PGAM5 (Supplementary Fig. 15a). Notably, the ubiquitination modification sites of PGAM5, specifically the Lys-140 and Lys-143 residues, are situated within the binding site of CD36 (Supplementary Fig. 15b, c). This interaction may influence the ubiquitination modification of PGAM5. Ultimately, the results of co-immunoprecipitation further demonstrate that the ubiquitination of the PGAM5 protein is reduced when CD36 palmitoylation is inhibited (Fig. 9k). PGAM5, a mitochondrial serine/threonine phosphatase, regulates the phosphorylation of the Fundc1 protein and participates in mitophagy44. To further elucidate the mechanism by which the inhibition of CD36 palmitoylation regulates autophagy in cardiomyocytes, we measured the phosphorylation levels of Fundc1 in cardiomyocytes. Our results indicate a decrease in the phosphorylation level of Fundc1 in mitochondria of cardiomyocytes from the OE-AA-SS group, while the total protein levels remained unchanged (Fig. 9l). These findings suggest that the inhibition of CD36 palmitoylation may activate the mitophagy pathway by mediating the dephosphorylation of Fundc1. To elucidate the critical role of mitophagy in the beneficial effects of CD36 palmitoylation inhibition post-MI, we utilized the mitophagy antagonist Mdivi-1. The results indicated that inhibition of CD36 palmitoylation significantly enhanced post-MI cardiac functional parameters, as evidenced by the increases in LVEF, LVFS and ATP levels. Strikingly, this functional improvement was substantially attenuated following Mdivi-1 treatment (Supplementary Fig. 16a–d), underscoring the mechanistic dependence of therapeutic efficacy on the activation of mitophagy. Complementary western blot analysis confirmed the pharmacological efficacy of Mdivi-1, showing a reduction in myocardial LC3B-II levels (Supplementary Fig. 16e). The initiation of mitophagy is accompanied by mitochondrial fission, which is essential for the degradation of damaged mitochondria45. DRP1 is a regulator of mitochondrial fission, and studies have indicated that PGAM5 can modulate Drp1 phosphorylation to influence mitochondrial fission46,47. Our results demonstrate that inhibiting CD36 palmitoylation reduces the ubiquitination and degradation of PGAM5. Therefore, we further to investigate whether DRP1 also participates in mitophagy mediated by CD36 palmitoylation inhibition together with FUNDC1. To this end, we examined the phosphorylation levels and the mitochondria expression of Drp1 in cardiomyocytes. Western blot results indicate a decrease in the S637 phosphorylation of Drp1 in cardiomyocytes of the OE-AA-SS group, while the S616 phosphorylation and total protein levels remained unchanged. In addition, Drp1 recruitment to mitochondria was significantly increased in cardiomyocytes from the OE-AA-SS group, which is essential for the occurrence of mitochondrial fission (Supplementary Fig. 16f). Combining these experimental findings, we demonstrate that the inhibition of CD36 palmitoylation facilitates the relocalization of CD36 to the mitochondria, subsequently enhancing its interaction with PGAM5. This interaction inhibits the ubiquitination and degradation of PGAM5, leading to an increase in mitochondrial PGAM5 levels, which affects the phosphorylation levels of Fundc1 and Drp1, ultimately influencing mitophagy in cardiomyocytes.
Discussion
Nutrient deficiency and metabolic disorders are common characteristics in the cardiomyocyte environment during severe ischemia. Optimizing mitochondrial function and regulating substrate metabolism are crucial for enhancing myocardial energy metabolism, preserving or improving myocardial mechanical function, and ultimately enhancing cardiac function and increasing survival rates. However, the key mediators and mechanisms regulating cardiac energy metabolism after MI remain unknown. This study utilized multi-omics sequencing combined analysis to unveil the significant regulatory role of CD36 in cardiac metabolism and mitochondrial function post-MI. Moreover, our comprehensive analysis integrating molecular and physiological approaches demonstrated that enhanced CD36 palmitoylation post-MI heightens it’s the plasma membrane localization. Inhibiting CD36 FA uptake or palmitoylation protects against cardiac dysfunction. Post-MI, mitochondrial dysfunction, including reduced oxidative phosphorylation and ATP production, contributes to imbalances in FA uptake and oxidation, exacerbated by increased plasma membrane localization facilitated by CD36 palmitoylation. Consequently, lipotoxic metabolites are generated and accumulate, causing damage to myocardial cells. Inhibiting CD36 palmitoylation prevents harmful liposome accumulation, enhances autophagy via PGAM5, and improves mitochondrial and cardiac function. These findings identify CD36 palmitoylation as a potential therapeutic target for MI injury.
The heart’s high ATP turnover is vital for continuous blood pumping48, given its limited energy storage. Prolonged ATP depletion leads to contractile failure in seconds. Mitochondrial oxidative phosphorylation drives 95% ATP production in the normal adult heart49, making disruptions impactful. Cardiometabolic characteristics connect ATP production to contractile function50,51. Altered metabolic substrates, pathways, and mitochondrial dysfunction contribute to impaired cardiac energy production in heart diseases like MI52,53,54. Post-MI, the heart undergoes dynamic and complex alterations in energy metabolism, which are likely integral to the heart’s broader response to the stress induced by the MI and the subsequent remodeling process. This underscores the necessity of understanding these variations to facilitate the development of effective treatment strategies. This study employs multi-omics approaches, including Proteome, Metabolome, and single-cell sequencing, to comprehensively analyze remodeled myocardial tissue 28 days after MI, uncovering metabolic remodeling from altered FA use to severe mitochondrial dysfunction.
Alterations in cardiac metabolism after MI extend beyond ATP deficiency, impacting cardiac function through varied mechanisms. The heart’s ability to metabolize diverse fuels—FA, glucose, lactate, ketones, pyruvate, and amino acids—for ATP generation is crucial. FA is the preferred substrate for energy production in the heart when the oxygen supply is not limited55,56. Under normal conditions, 60–70% of cardiomyocyte ATP comes from FA oxidation57. Excessive non-adipocyte FA accumulation induces lipotoxic damage58. exacerbates insulin resistance and contributes to conditions like diabetic cardiomyopathy. These damages are primarily caused by disturbances in glucose and lipid metabolism, which are characteristic of diabetic cardiomyopathy12,59,60. Interestingly, in our study on MI mice, we identified severe myocardial lipotoxicity, evidenced by elevated DAG and ceramide levels. Unlike diabetic cardiomyopathy, MI hearts exhibited decreased expression of genes related to FA oxidation and safe lipid storage in triglycerides. This led to FA accumulation surpassing mitochondrial FA oxidation capacity, generating potentially harmful lipids like DAG and ceramides. Both DAG and ceramide can exacerbate mitochondrial dysfunction and impact insulin signaling pathways, a notion that is substantiated by our findings. In addition, heightened mitochondrial oxidative stress was confirmed by increased malondialdehyde concentration and decreased superoxide dismutase levels31,61.
Cardiomyocytes, being rich in mitochondria, constitute ~ 0% of cellular volume62. Analysis of single-cell RNA sequencing datasets from mouse hearts revealed a significant increase in CD36 mRNA levels in MI cardiomyocytes compared to sham. Subsequent experiments confirmed this finding; however, the total CD36 protein levels did not show a corresponding increase with mRNA expression. This discrepancy may be attributed to complex post-transcriptional regulatory mechanisms63,64. Interestingly, further analysis demonstrated a shift in CD36 protein localization post-MI, notably increasing plasma membrane CD36 responsible for mediating FA uptake. FA uptake is a critical regulatory point in lipid homeostasis, with CD36 facilitating about 70% of the process. Abnormal CD36 distribution on the cell membrane is linked to excessive FA uptake and subsequent insulin resistance13,65. This increased membrane localization of CD36 protein after MI appears to be an adaptive response, aiming to sustain fuel supplies for oxidative ATP production amid reduced mitochondrial oxidative phosphorylation capacity. However, given the compromised oxidative phosphorylation during MI, this change may result in lipid accumulation surpassing the oxidation capacity, exacerbating the imbalance between lipid uptake and oxidation. This, in turn, can result in the production of lipotoxic metabolites such as DAG and ceramide, intensifying mitochondrial oxidative stress in a detrimental cycle59,66. The hypothesis that inhibiting CD36-mediated FA uptake could disrupt the vicious circle, mitigate mitochondrial abnormalities, and reduce harmful lipid production was tested using pharmacological CD36 inhibitors in mice after MI67. Our results demonstrated that inhibiting CD36-mediated FA uptake resulted in a reduction in lipotoxic metabolite production and accumulation, an improvement in bioenergetics by improving glucose and lipid metabolism and a restoration of heart function post-MI.
Further exploration into the molecular mechanisms underlying the enhanced plasma membrane localization of CD36 following MI. Protein palmitoylation is known to play a pivotal role in modulating protein functions, being implicated in a variety of diseases. Previous studies have indicated that palmitoylation is essential for the localization of CD36 on the plasma membrane in hepatocytes and adipocytes15,27. However, the role of CD36 palmitoylation in MI remained unclear. The present study explored the levels and function of palmitoylated CD36 in the heart. Our results reveal a notable elevation in palmitoylated CD36 in MI mice. Moreover, PA, a palmitoylation inducer, enhanced the membrane localization of CD36 in cardiomyocytes. Notably, the presence of palmitoylated CD36 on the plasma membrane enhanced FA binding and uptake in cardiomyocytes. The inhibition of CD36 palmitoylation, achieved through mutations of palmitoylation sites (AA-SS) or pharmacological agents, resulted in decreased plasma membrane CD36, subsequently inhibiting FA binding and uptake in cardiomyocytes. This coupling of FA binding and uptake to CD36 palmitoylation prompted an investigation into the effects of cardiomyocyte-specific mutations of CD36 palmitoylation sites on cardiac metabolism and function post-MI.
Under systemic CD36 knockout conditions, our study demonstrates that mutations in CD36 palmitoylation sites significantly reduce the infarct area and improve cardiac function after MI. The shared phenotypes observed in mice with CD36 palmitoylation site mutations and those treated with a pharmacological CD36 inhibitor strongly support the existence of common regulatory mechanisms in cardiac metabolism. These mechanisms include reductions in cardiotoxic liposomes, inhibition of mitochondrial oxidative stress, improvement in myocardial mitochondrial function and enhancement of myocardial glucose uptake. An interesting observation in our study is that inhibiting CD36 palmitoylation through site mutations significantly promotes autophagy in cardiomyocytes after MI, potentially mediated by the altered intracellular localization of CD36 due to palmitoylation inhibition. While previous studies have shown the involvement of CD36 in autophagy in macrophages and B cells68,69, our study uncovers the regulatory effect of CD36 palmitoylation on autophagy in cardiomyocytes. In addition, we identified PGAM5 as a downstream target of CD36 through coimmunoprecipitation coupled with mass spectrometry (CoIP-MS). PGAM5 is a multifunctional protein that plays a critical role in the regulation of mitochondrial quality control through various pathways. It facilitates mitochondrial fission, which may contribute to the process of mitophagy70. PGAM5 dephosphorylates Drp1 at Ser-637, triggering its GTPase activity, leading to mitochondrial fragmentation46. Therefore, abnormalities in PGAM5 function are closely linked to mitochondrial damage. Yang et al. reported a significant down-regulation of myocardial PGAM5 expression during ischemia-reperfusion (I/R) injury71. Here, we demonstrate that PGAM5 expression in the mitochondria of myocardial tissue was notably decreased following MI, and that PGAM5 is essential for the inhibition of CD36 palmitoylation-mediated autophagy. Mechanistically, our study demonstrates that the inhibition of CD36 palmitoylation promotes CD36 translocation to the mitochondria and enhances its interaction with PGAM5, leading to increased mitochondrial PGAM5 levels independent of mRNA changes. Furthermore, the stability experiments reveal that inhibiting CD36 palmitoylation enhances the stability of PGAM5 protein. These observations suggest that the regulation of PGAM5 may involve post-translational mechanisms. Ubiquitination, a crucial post-translational modification, is known to significantly influence the stability of various proteins. As illustrated by Zhong et al., S100A9 can act as a scaffold, recruiting ubiquitin-specific peptidase 10 and PGAM5 to form a complex. This interaction facilitates the deubiquitylation and stabilization of PGAM5, thereby promoting mitochondrial fission, which subsequently fuels the growth and metastasis of hepatocellular carcinoma (HCC)42. In our study, we have established a direct interaction between CD36 and PGAM5. Protein molecular docking predictions indicate that the ubiquitination modification sites of PGAM5 are partially located within the CD36 binding domain, and inhibition of CD36 palmitoylation decreases the ubiquitination level of the PGAM5 protein. Therefore, it is plausible that alterations in ubiquitination status are the primary reason for the observed increase in PGAM5 levels within the mitochondria. Finally, while we could not definitively elucidate the exact molecular mechanism by which PGAM5 accumulation leads to cardiomyocyte autophagy, our findings indicate that this process involves Fundc1 and Drp1-mediated mitophagy and is dependent on the phosphatase activity of PGAM5. Our data provide partial support for this hypothesis, as we observed a decrease in the phosphorylation level of Fundc1 and Drp1 in cardiomyocytes with mutations at the CD36 palmitoylation site, which coincided with an increase in mitochondrial PGAM5.
Based on the above content, inhibiting CD36 palmitoylation not only improves cardiac function in mice by reducing lipotoxic damage and improving energy metabolism, but also controls mitochondrial quality and enhances the metabolic adaptability of cardiomyocytes by enhancing autophagy. Our findings strongly suggest that inhibiting CD36 palmitoylation holds therapeutic potential for clinical application in MI in the future. However, further rigorous investigations are required to test this notion. Despite our valuable findings, there are several limitations in our study. First, palmitoylation can be catalyzed by palmitoyl transferases, also known as DHHC. Recent studies have found that the palmitoyl transferases DHHC4 and DHHC5 are required for mediating CD36 palmitoylation in adipocytes15,28. Our study demonstrates that levels of CD36 palmitoylation are elevated in cardiomyocytes following MI. However, the specific palmitoyl transferase involved in this process remains unclear. Although the primary aim of our research is to investigate the mechanism of CD36 palmitoylation in regulating cardiac function after MI and the associated downstream regulatory network, exploring the specific palmitoyl transferase involved in this process is also a research direction of significant scientific importance. Therefore, we plan to explore this aspect further in our future research. Second, although our data demonstrate that CD36 can regulate the plasma membrane localization of Glut4, it is important to note that these findings are observational in nature. The precise molecular mechanisms behind this phenomenon remain unknown. We speculate that altering the levels of detrimental lipid metabolites, such as ceramide and DAG, may influence the insulin signaling pathway, thereby serving as a potential mechanism through which CD36 regulates Glut4 localization at the plasma membrane. However, given the complex interplay between CD36 and Glut4, it is likely that additional regulatory mechanisms are involved. For instance, FAs lead to increased acetyl-CoA levels, which in turn result in higher protein acetylation that inhibits Glut4 translocation to the plasma membrane and subsequently reduces glucose uptake72. Furthermore, FAs can induce the disassembly of v-ATPase and cause de-acidification of the endosomes, resulting in altered Glut4 localization73,74,75. Further research is needed to investigate into these issues. Third, only male mice were analyzed in this study, and generalizability to female mice is not known.
In conclusion, cardiac energy supply relies on the close coupling of substrate uptake and oxidation. Our study discovered that the mitochondrial oxidative phosphorylation capacity of cardiomyocytes is significantly impaired following MI. This impairment leads to an imbalance in lipid uptake and oxidation, resulting in the accumulation of toxic lipid metabolites and further damage to the structure and function of mitochondria. However, inhibiting CD36 palmitoylation can break this vicious cycle and protect cardiac function following MI. On one hand, inhibition of CD36 palmitoylation results in a reduced localization of CD36 to the plasma membrane, which partially restores the balance between FA uptake and oxidation following MI. This process diminishes the accumulation of detrimental lipid metabolites and mitigates further mitochondrial damage. On the other hand, inhibiting CD36 palmitoylation facilitates the relocalization of CD36 to mitochondria and enhances autophagy through its interaction with PGAM5. This interaction is crucial for regulating mitochondrial quality, sustaining mitochondrial homeostasis and cardiac energy supply. Our study revealed the effects of the palmitoylation of CD36 in the heart and indicates that modulation of CD36 palmitoylation represents a strategy for the treatment of MI injury.
Methods
Animals and animal models
The adult male C57BL/6 mice (8–10 weeks, 22–25 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Vital River, Beijing, China). Mice were housed in sterile cages with a 12/12 h light/dark cycle, a constant temperature of 23 ± 3 °C, and a humidity of 55% to 60%. They received autoclaved food (Keao Xieli, Beijing, China) and water. All animal experimental procedures and protocols were approved by the Animal Ethics Committee at Harbin Medical University (IRB3039723) and performed in accordance with Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23; revised 1996). The experimenters were blinded to the treatment/genotype grouping information throughout the experiment and quantification. Mice were only excluded from the study if they died. The group sizes were determined based on our previous experience in establishing a mouse model of MI (MI). For Sulfosuccinimidyl oleate sodium (SSO) treatment, administered SSO (ab145039, abcam, Cambridge, UK) once a week intraperitoneally at 40 mg/kg for 4 weeks after MI. Mock-control mice received a solvent of equivalent volume.
Sex as a biological variable
Our study exclusively examined male mice. To eliminate the influence of gender as a confounding factor, we chose male mice for this study, considering the periodic changes in estrogen levels in female mice. It is unknown whether the findings are relevant for female mice.
Mouse model of MI
Male mice (8–10 weeks old, 22–25 g) were anesthetized with avertin (200 mg/kg) administered intraperitoneally (i.p.). They were then intubated and ventilated using a rodent ventilator with a tidal volume of 225 µl and a frequency of 130 breaths per minute. Following the shaving and cleaning of the chest with 75% alcohol, a thoracotomy was performed in the 3rd and 4th intercostal area to expose the heart. The pericardium was opened, and the left anterior descending coronary artery (LAD) was ligated with a 7-0 nylon suture, approximately 1–2 mm from the lower edge of the left atrium. Mice in the sham-operated group underwent the same surgical procedure, but without ligation of the artery.
Generation of CD36 knockout mice
CD36 knockout (KO) mice were generated using the CRISPR/Cas9 technique by Cyagen Biosciences Co., Ltd (Cyagen, Jiangsu, China). Cas9 mRNA and gRNAs were produced through in vitro transcription. Genomic PCR of tail DNA was conducted to determine the homozygote filial generation. (Forward1: 5’-GTATGTCACTTCTCCCTCTCCTTT-3’; Reverse1: 5’-AACAGTGTGTACTTTGCTTCTCCT-3’; Reverse2: 5’-GCATCACTAGTTCTCAAAGAGCAAA-3’).
Infection of adeno-associated virus (AAV9) carrying WT-CD36 and AA-SS-CD36 gene
To induce cardia-specific overexpression of WT-CD36 and AA-SS-CD36, the adeno-associated virus serotype 9 (AAV9) vector carrying WT-CD36 gene and AA-SS-CD36 were constructed by Genechem biotechnology Co., Ltd (shanghai, China). The AAV9 virus was administered to mice through the tail vein at a dose of 5 × 1011 PFU per animal. Four weeks after injection, MI model was established.
Echocardiography measurements
Echocardiographic measurements were performed to assess the cardiac function of mice using the Vevo2100 High-Resolution Imaging System (Visual Sonics, Toronto, ON, Canada) equipped with a 10 MH2 phased-array transducer for M-mode recording. Briefly, the mice were anaesthetized with Avertin (Sigma-Aldrich, St Louis, USA) and the anterior chest area was depilated using NairTM depilatory cream (Church & Dwight Co., Inc., Princeton, NJ, USA). Medical ultrasonic couplant (Tianjin Yajie Medical Material Co., Ltd., Tianjin, China) was applied to the depilated area. Two-dimensional targeted M-mode traces were recorded from the parasternal short-axis view at the level of the mid-papillary muscles and from the parasternal long-axis view just below the papillary muscle. Measurements were taken for left ventricular (LV) dimensions, including diastolic (LVPW; d) and systolic (LVPW; s) wall thickness, as well as the left ventricular internal diameter at end-diastole (LVID; d) and end-systole (LVID; s). Finally, the ejection fraction (EF) was calculated using the formula EF = (LVEDV - LVESV)/LVEDV × 100% and the fractional shortening (FS) as (LVIDd - LVIDs)/LVIDd × 100%. The data represent the average of measurements from three consecutive beats.
TTC staining
To determine the infarct size in MI. we froze and sliced the heart tissue into 1 mm thick slices. Then, the slices were quickly removed into 2% 2,3,5-triphenyltetrazolium chloride (TTC, Solarbio, China) incubated in the dark for 15 min at 37 °C. The slices were imaged by a stereomicroscope (Zeiss Stemi 508, Germany) and calculated by Image J software.
Neonatal mouse cardiomyocytes culture and treatment
Isolation and culture of neonatal mouse ventricular cardiomyocytes (NMVCs) were performed using previously reported procedures76. Briefly, neonatal mice aged 1–3 days were rinsed in 75% alcohol, and their hearts were isolated and collected in a clean petri dish. The hearts were then cut into pieces and digested using 0.25% trypsin (Gibco, USA). The resulting cells were passed through a 200-mesh sieve and centrifuged at 200 x g for 5 min. Following centrifugation, the cells were resuspended in DMEM (Hyclone Laboratories, Utah, USA) supplemented with 10% fetal bovine serum (Biological Industries, Israel) and 1% penicillin/streptomycin (Solarbio, Beijing, China). The cells were plated in culture bottles to facilitate the adherence of cardiac fibroblasts. The suspended cells were collected as cardiomyocytes and cultured in DMEM containing 10% FBS at 37 °C in a humidified incubator with 5% CO2 for further experiments. Subsequently, to induce hypoxic injury, NMVCs were incubated under hypoxic conditions (5% CO2, 95% N2, 37 °C) in medium that was free of FBS and antibiotics for 24 h after transfection or drug treatment (PA, 160 µM for 24 h, KC004, Kunchuang, Xi’an, China; or 2 bp, 25 µM for 24 h, 238422, Sigma-Aldrich, MO, USA). For autophagy study, cells were cultured in normal medium following transfection and subsequently treated with the autophagy modulators rapamycin (500 nM, ENZ-51031-K200, ENZO, New York, USA) or chloroquine (CQ) (60 µM, ENZ-51031-K200, ENZO, New York, USA), either alone or in combination, for a duration of 16 h.
Isolation of adult mouse cardiomyocytes
Adult male mice (8–10 weeks old, 22–25 g) were anesthetized with avertin (200 mg/kg) administered intraperitoneally (i.p.). The chest was opened to expose the heart, which was then rapidly separated, and the aorta was cannulated on a constant-flow Langendorf apparatus at 37 °C. The heart was digested by perfusion with Tyrode’s solution containing NaCl (123 mM), KCl (5.4 mM), HEPES (10 mM), NaH2PO4 (0.33 mM), MgCl2 (1.0 mM), glucose (10 mM), Type II collagenase (1 mg/ml), protease (0.02 mg/ml), and bovine serum albumin (BSA, 1 mg/ml), at pH 7.4. Perfusion was stopped when the tissue turned softening, and the left ventricle was then separated and gently dispersed to obtain isolated cardiomyocytes. The cell suspension was passed through a 100 µm filter, and the cells underwent 4 sequential rounds of gravity settling to enrich cardiomyocytes.
Synthesis and transfection of overexpression plasmids and shRNAs
AA-SS-CD36 refers to a mutated form of CD36, where the cysteine pair (Cys-3, 7) in the wild-type CD36 is replaced with alanine, and the residues towards the carboxyl terminus (Cys-464, 466) are replaced with serine. The cDNAs of both the wild-type CD36 (WT-CD36) and the mutated form (AA-SS-CD36) were inserted into pcDNA3.1 (+) with T7 promoter and EcoRI/XhoI using IBSBIO in Shanghai, China. To achieve knockdown of the PGAM5 gene, specific shRNAs were designed and cloned into hU6-MCS-CMV-Puromycin, also by Genechem biotechnology in Shanghai, China. Transfection of plasmids was performed by Lipofectamine 2000 reagent (Invitrogen, Carlsbad, California, USA).
Co-immunoprecipitation
To investigate the interaction between proteins, the Universal IP/Co-IP Toolkit (Magnetic Beads) (Abbkine, Wuhan, China) was utilized. Cultured cells were lysed in a lysis buffer containing 1% protease inhibitor (Roche, Switzerland) for 20 min in an ice bath. Subsequently, ultrasonic crushing was performed three times. Protein samples were obtained by centrifugation at 14,000 × g for 15 min at 4 °C. Afterwards, 30 µl of magnetic beads were added to the cell lysate for pre-clarification. The mixture was then rotated and incubated at room temperature for 20 min, followed by separation of the magnetic beads from the lysate using a magnetic separation stand. For the formation of immune complexes, protein samples were incubated overnight at 4 °C with 5 µg of antibodies. Subsequently, 30 µl of magnetic beads were added to the immune complex sample and incubated at 4 °C with rotary agitation for 4 h. After washing the samples three times with PBST, the co-immunoprecipitation products were obtained and analyzed by Western blot. The antibodies CD36 (sc-7309, Santa Cruz, California, USA), LC3B (ab192890, abcam, Cambridge, UK), Ubiquitin (58395 s, CST, Danvers, Massachusetts, USA) and PGAM5 (ab308447, abcam, Cambridge, UK; sc-515880, Santa Cruz, California, USA) were used for the co-immunoprecipitation.
Detection of palmitoylated CD36 proteins
Protein of CD36 palmitoylation was assessed using immunoprecipitation and acyl-biotin exchange (IP-ABE) as previously reported procedures35. Briefly, total protein was extracted using a lysis buffer containing 1% IGEPALCA-630, 50 mM Tris-HCl pH 7.5, 150 mM NaCl, and 10% glycerol, along with protease inhibitors and 50 mM N-ethylmaleimide (NEM). The protein concentration was measured using the BCA Protein Assay Kit. Each lysate sample, containing 1000 µg total protein, was supplemented with 5 µg of primary antibody directed against CD36 and the antibody-lysate mixed samples were incubated overnight at 4 °C with gentle agitation. After precipitating the antibody-lysate mixed samples and magnetic beads, the samples were re-suspended in a stringent buffer containing 1% IGEPALCA-630, 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 10% glycerol, and 10 mM MEM 0.1% SDS, and incubated for 10 min on ice. For the HAM samples, 500 µl of lysis buffer pH 7.2 was added, while for the + HAM samples, 500 µl of HAM buffer was added. All samples were rotated at room temperature for 55 min. Subsequently, the samples were treated with Biotin-BMCC buffer and rotated for 50 min at 4 °C. Finally, the samples were analyzed by western blot. To detect palmitoylated CD36, the membranes were incubated with HRP-conjugated anti-streptavidin (1:10000; ab7403, abcam, Cambridge, UK) for 1 h at 37 °C.
Protein extraction and western blot
Subcellular protein fractions (plasma membrane and cytoplasm) from the infarction border zone heart and cardiomyocytes were isolated using the Minute Plasma Membrane Isolation Kit (Invent Biotechnologies) and ProteoExtract® Transmembrane Protein Extraction Kit (Merck, Darmstadt, Germany) in accordance with the provided instructions. In the first step, cells or homogenized tissues are permeabilized, and their soluble (cytosol) fraction is separated from the insoluble (membrane) fraction through centrifugation. Briefly, the cells were sensitized with Buffer prior to being processed through a proprietary filter under high-speed centrifugal force77,78. This procedure yielded a cell lysate that contained ruptured cell membranes and intact nuclei. Subsequently, the sample was centrifuged at 700 × g for one minute (the pellet contains intact nuclei). The supernatant was then transferred to a fresh 1.5 ml microcentrifuge tube and centrifuged at 4 °C for 10–30 min at 16,000 × g. The supernatant (cytosol fraction) and the pellet (total membrane protein fraction, which includes organelles and plasma membranes) were preserved. The total membrane protein fraction was resuspended in 200 µl of Buffer by vortexing. Following this, it was centrifuged at 7800 × g for 5 min at 4 °C, allowing for the collection of the pellet (organelle membrane proteins fraction). The supernatant was then carefully transferred to a fresh 2.0 ml microcentrifuge tube, to which 1.6 ml of cold PBS was added. After centrifugation at 16,000 × g for 30 min, the supernatant was discarded, and the pellet (isolated plasma membrane) was retained. In the second step, membrane proteins are extracted from the lipid bilayer using a novel extraction buffer from the ProteoExtract® Transmembrane Protein Extraction Kit. The extraction buffer is added to the fractions of plasma membrane or organelle membrane proteins, respectively. The mixture is then incubated for 45 min at room temperature with gentle agitation. Following incubation, the sample is centrifuged at 16,000 × g for 15 min at 4 °C. The supernatant is collected, which is enriched in plasma membrane proteins or organelle membrane proteins. Finally, the organelle membrane proteins are mixed with the cytosolic fraction obtained from the first step to yield cytoplasmic proteins. Mitochondria were purified following the user protocol in the mitochondria extraction kit (KTP4003, KTP4004, Abbkine, Wuhan, China). Total protein was extracted from cardiomyocytes or the myocardium in the infarct margin area with RIPA lysis buffer (Solarbio, Beijing, China) containing 1% protease inhibitor (Roche, Switzerland). Protein concentration of all samples was determined and quantified by BCA Protein Assay Kit (Beyotime, Shanghai, China). Protein lysates were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7.5% − 15%) and then with a range of 7.5% − 15% and then transferred onto polyvinylidene fluoride membrane (PVDF) (Millipore, Bedford, MA, USA) for subsequent studies. To block nonspecific binding, the membrane was incubated with 5% non-fat milk at room temperature for 2 h. The primary antibody was then added and incubated overnight at 4 °C. Following this, the membrane was incubated with an anti-rabbit or anti-mouse HRP-conjugated secondary antibody (Abbkine, Wuhan, China) at room temperature for 2 h. The membranes were washed with TBST (0.05% Tween in Tris-buffered saline) and visualized using enhanced chemiluminescence (ECL) chromogenic substrate (Abbkine, Wuhan, China). Protein bands were visualized using the Bio-Rad ChemiDoc XRS + chemiluminescence imaging system (Bio-Rad, Hercules, CA, USA). The primary antibodies were used as follows: anti-CD36 (1:1000; 14347 s, CST, Danvers, Massachusetts, USA; 1:200; sc-7309, Santa Cruz, California, USA), anti-LC3B (1:1000; ab192890, abcam, Cambridge, UK), anti-Ubiquitin (1:1000; 58395 s, CST, Danvers, Massachusetts, USA), anti-PGAM5 (1:1000; ab308447, abcam, Cambridge, UK; sc-515880, Santa Cruz, California, USA), anti-Glut4 (1:500; AF5386, Affinity, USA; 1:400, sc-53566, Santa Cruz, California, USA), Anti-SQSTM1 (1:5000; ab109012, abcam, Cambridge, UK), anti-Na+/K+-ATPase (1:500; 3010 s, CST, Danvers, Massachusetts, USA), anti-Vdac1 (1:1000; DF6140, Affinity, OH, USA), anti-phospho-Fundc1 (1:500; AF0001, Affinity, OH, USA), anti-Fundc1(1:500; AF0002, Affinity, OH, USA), anti-Total OXPHOS Rodent (1:1000; ab110413, abcam, Cambridge, UK), anti-Drp1 (1:1000; ab184247, abcam, Cambridge, UK), anti-Phospho-Drp1(S637) (1:500; DF2980, Affinity, OH, USA), anti-Phospho-Drp1(S616) (1:500; AF8470, Affinity, OH, USA), anti-GAPDH (1:1000; TA309157, ZSGB-BIO, Beijing, China) and anti-Beta actin (1:1000; AB0035, Abways, Shanghai, China).
Real-time quantitative PCR
Total RNA was extracted from cardiomyocytes or the myocardium in the infarct margin area using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Total RNA was reverse transcribed into cDNA using PrimeScript™ RT reagent kit (Takara, Otsu City, Shiga Prefecture, Japan). Quantitative RT-PCR was performed with SYBR Green (Roche, Basel, Switzerland) using an ABI 7500 fast Real-Time PCR instrument (Applied Biosystems, Foster City, USA). Relative mRNA levels were quantified using the 2−ΔΔCT method with Beta-actin as a reference gene. A list of primer sequences in this study is provided as Supplementary Table 2.
ATP content of myocardium detection
Myocardial ATP was extracted from fresh myocardial tissue in the infarct border zone using the Enhanced ATP Assay Kit (Beyotime, Shanghai, China), following the provided instructions. The concentration of ATP in the sample was determined by measuring it with a luminometer, using a standard curve generated with standard ATP samples. To normalize ATP contents, the total amount of tissue protein was utilized.
Cardiac lipid analyses
The levels of triglycerides (TG), superoxide dismutase (SOD), and malondialdehyde (MDA) in the myocardial tissue of the infarct border zone were measured using commercial reagent kits (Nanjing Jiancheng, Jiangsu, China), following the provided instructions. The diacylglycerol (DAG) and ceramide levels were examined using an enzyme-linked immunosorbent assay (J&Lbio, Shanghai, China) according to the instructions.
Immunostaining
Cells were fixed in 4% formaldehyde for 15 min at room temperature. Subsequently, 0.25% Triton X-100 in PBS was added and allowed to stand for 10 min at room temperature. To block nonspecific antigen, ready-to-use normal goat serum was applied and incubated at 37 °C for 1 h. Following a wash with PBS, the cells were incubated with primary antibodies overnight at 4 °C, followed by incubation with the corresponding secondary antibodies. Nuclei were stained with DAPI. Finally, the cells were examined under a laser scanning confocal microscope (Nikon, Tokyo, Japan). The antibodies were used as follows: anti-CD36 (1:50; sc-7309, Santa Cruz, California, USA), anti-Na+/K+-ATPase (1:200; 3010 s, CST, Danvers, Massachusetts, USA), DyLight 488, Goat Anti-Mouse IgG (1: 200; Abbkine, Wuhan, China) and Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 594 (1:200; Invitrogen, Carlsbad, California, USA).
Myocardial glucose uptake Micro-PET/CT Imaging
18F-FDG was routinely produced in a GE TracerLab using a commercially available module, following standard protocols with quality control measures to ensure a radiochemical purity of 99%. Cardiac uptake of 18F-FDG was assessed using previously reported procedures. Briefly, the mice were fasted for 6 h before the imaging session, with ad libitum access to water. The mice were then anesthetized using isoflurane (R510-22-10, RWD, Shenzhen, China). An injection of approximately 200 µCi of 18F-FDG was administered via the tail vein. A total of 15 min of static scanning was performed at 1 h after injection, with the mice maintained under anesthesia (1.5% isoflurane), using a SuperArgus small-animal PET/CT scanner (Sedecal, Madrid, Spain).
Histology
Cardiac tissue was washed and fixed in 4% buffered formaldehyde for H&E and Masson’s trichrome staining. The tissue was then embedded in paraffin and 2 µm sections were prepared using a Leica RM2245 (Leica, Weztlar, Germany). Staining of the sections was performed following standard protocols. Image analysis procedures were performed with Image J software.
Two-color flow cytometry assay of fatty acid binding or uptake and bivariate analysis
FL-C16 (Invitrogen, Carlsbad, California, USA) was incubated with cardiomyocytes at 4 °C for 1 h for fatty acid (FA) binding or incubation at 37 °C for 1 h for FA uptake. Subsequently, 200 µL of PBS containing 0.5% Triton was added to each sample, followed by an incubation at room temperature in the dark for 15 min. The cells were then immunostained with anti-CD36-PE (102605, Biolegend, California, USA) for 30 min. Data acquisition was carried out using a FACScan flow cytometer. The resulting data were exported and analyzed using the 2D tabulation method mentioned earlier79. These data were then imported into Excel 2019, and a student’s t test was performed to compare FA binding/absorption.
Proteomics
Proteomics was performed by MetWare Biotechnology Co., Ltd (Wuhan, China) based on a previously described protocol80. Briefly, heart tissue was isolated from Sham or MI mice (peri-infarct zone) and prepared for LC-MS/MS analysis. To begin, proteins were homogenized in a lysis buffer containing 2.5% SDS and 100 mM Tris-HCl (pH 8.0). The proteins were then subjected to reduction/alkylation with dithiothreitol/iodoacetamide. Trypsin (T575-31N-10, Signalchem, British Columbia, Canada) was added at a ratio of 1:50 (enzyme: protein, w/w) for overnight digestion at 37 °C. The next day, TFA was used to adjust the pH to 6.0 and stop the digestion process. After centrifugation at 12000 × g for 15 min, the supernatant was purified using a Sep-Pak C18 desalting column to isolate the peptides. The peptide eluate was then vacuum dried and stored at − 20 °C for future use. Prior to analysis, the peptides were reconstituted in TMT reagent buffer and labeled with different TMT labeling reagents. The labeled samples were mixed and subjected to another round of desalting using a Sep-Pak C18 column. LC-MS/MS data acquisition was performed using a Q Exactive plus mass spectrometer coupled with an Easy-nLC 1200 system. Peptides were loaded through an autosampler and separated using a C18 analytical column (50 µm × 15 cm, C18, 2 µm, 100 Å, Thermo Fisher). The separation gradient was established using mobile phase A (0.1% formic acid) and mobile phase B (80% ACN, 0.1% formic acid), with a constant flow rate of 300 nL/min. For DDA mode analysis, each scan cycle consisted of one full-scan mass spectrum (R = 70 K, AGC = 3e6, max IT = 20 ms, scan range = 350–1800 m/z) followed by 15 MS/MS events (R = 35 K, AGC = 1e5, max IT = 100 ms). The HCD collision energy was set to 32, and the isolation window for precursor selection was set to 1.2 Da. Former target ion exclusion was set for 35 s.
Database search
The MS raw data were analyzed using MaxQuant (V1.6.6) and the Andromeda database search algorithm. The spectra files were searched against the Swissprot.Mouse.20200826 proteome database with the following parameters: TMT mode was enabled for quantification, and variable modifications included Oxidation (M), Acetyl (Protein N-term), and Deamidation (NQ). Fixed modifications included Carbamidomethyl (C), and digestion was performed using Trypsin/P. The MS1 match tolerance was set at 20 ppm for the first search and 4.5 ppm for the main search, while the MS2 tolerance was set at 20 ppm. The amino acid length range of unique peptides for protein identification (7−40). To enhance the quality of the analysis results, the software PD further filtered the retrieved results. Only Peptide Spectrum Matches (PSMs) with a credibility of more than 99% were considered as identified PSMs. The identified protein had to contain at least 1 unique peptide. The identified PSMs and proteins were retained and subjected to a false discovery rate (FDR) of no more than 1.0%. Proteins identified as decoy hits, contaminants, or only identified by sites were removed, and the remaining identifications were used for further quantification analysis. Pathway enrichment analysis was performed by Gene Ontology and Reactome database analysis using the R package, clusterProfiler.
Single-cell RNA sequencing
Cardiac tissue was obtained from Sham and MI mice (peri-infarct zone). The tissue was transferred into an RNase-free culture dish containing an appropriate amount of calcium-free and magnesium-free 1 × PBS on ice. Then, it was cut into 0.5 mm2 pieces. The tissue pieces were dissociated into single cells using a dissociation solution consisting of 0.35% collagenase IV, 2 mg/ml papain, and 120 Units/ml DNase I. This dissociation process was carried out in a 37 °C water bath with shaking for 20 min at 100 rpm. After digestion, the process was terminated by adding 1 × PBS containing 10% fetal bovine serum. The resulting cell pellet was resuspended in 100 µl of 1 × PBS (0.04% BSA), and any remaining red blood cells were lysed using a red blood cell lysis buffer. To increase the overall cell viability to greater than 85%, dead cells were removed using the Miltenyi® Dead Cell Removal Kit. Subsequently, single-cell suspensions were loaded onto the 10x Chromium system to capture 7000 single cells, following the manufacturer’s instructions of the 10X Genomics Chromium Single-Cell 3’ kit (V3). The cDNA amplification and library construction steps were performed according to the standard protocol. Finally, the libraries were sequenced on an Illumina NovaSeq 6000 sequencing system (paired-end multiplexing run, 150 bp) by LC-Biotechnology Co. Ltd. (Hangzhou, China) at a minimum depth of 20,000 reads per cell.
Single-cell RNA sequencing data analysis
Sequencing results were demultiplexed and converted to FASTQ format using Illumina bcl2fastq software (version 2.20). Sample demultiplexing, barcode processing, and single-cell 3’ gene counting were performed using the Cell Ranger pipeline ((https://support.10xgenomics.com/single-cell-geneexpression/ software/pipelines/latest/what-is-cell-ranger, version 6.1.1) from 10x Genomics. The Cell Ranger output was loaded into Seurat (version 3.1.1) for dimensional reduction, clustering, and analysis of scRNA-seq data. Overall, 16,000 cells passed the quality control threshold: all genes expressed in less than 1 cell were removed, the number of genes expressed per cell > 500 as low and < 5000 as high cut-off, UMI counts less than 500, the percent of mitochondrial-DNA derived gene-expression < 25%. To visualize the data, we further reduced the dimensionality of all 16,000 cells using Seurat and mapped them into 2D space. The steps involved: (1) calculating the expression value of genes using the LogNormalize method of the ‘Normalization’ function in Seurat software, (2) performing PCA analysis using the normalized expression values and selecting the top 10 principal components for clustering and UMAP analysis, and (3) identifying cluster-specific marker genes using the weighted Shared Nearest Neighbor (SNN) graph-based clustering method. Marker genes were identified using the Wilcoxon rank-sum test (default parameters: “bimod”: Likelihood-ratio test) with the FindAllMarkers function in Seurat. Marker genes were defined as those expressed in more than 10% of the cells in a cluster and with an average log (Fold Change) greater than 0.26.
Statistical analysis
Data are expressed as the mean ± SEM. Data were analyzed using GraphPad Prism 8.0 software. Prior to statistical analysis, normality of the data was assessed using the D’Agostino & Pearson test (for n ≥ 8) and the Shapiro-Wilk test (for n < 8). For normally distributed data with equal variance, a two-tailed unpaired t test (Student’s t test) was performed for two-group comparisons, while a one-way ANOVA with Tukey’s multiple comparisons test was used for comparing the effect of treatment in more than two groups. Non-normally distributed data were analyzed using the two-tailed Mann-Whitney test for two groups, and the Kruskal-Wallis test followed by the false discovery rate (FDR) method of Benjamini and Hochberg for multiple groups. A significance level of P < 0.05 was considered statistically significant. No experiment-wide or across-test multiple test correction was applied, and only within-test corrections were made. The representative image was selected from one of the repeated experiments that best represented the mean value. The ‘N’ or ‘n’ in figure legends indicates biological replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD053227 and PXD056711. Single-cell sequencing data have been deposited to the Genome Sequence Archive with dataset identifier CRA017131. The mass spectrometry Metabolomics data have been deposited to MetaboLights with dataset identifier MTBLS12600. All other data generated or analyzed in this study are available within the article and its supplementary information files. Source data are provided in this paper.
References
Roth, G. A. et al. Global, regional, and National Burden of Cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 70, 1–25 (2017).
Liu, N. et al. LncRNA LncHrt preserves cardiac metabolic homeostasis and heart function by modulating the LKB1-AMPK signaling pathway. Basic Res. Cardiol. 116, 48 (2021).
He, Y. et al. Energy metabolism disorders and potential therapeutic drugs in heart failure. Acta Pharm. Sin. B 11, 1098–1116 (2021).
Noordali, H., Loudon, B. L., Frenneaux, M. P. & Madhani, M. Cardiac metabolism - A promising therapeutic target for heart failure. Pharm. Ther. 182, 95–114 (2018).
Oeing, C. U. et al. MTORC1-Regulated metabolism controlled by TSC2 limits cardiac reperfusion injury. Circ. Res. 128, 639–651 (2021).
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res 128, 1487–1513 (2021).
Huss, J. M. & Kelly, D. P. Mitochondrial energy metabolism in heart failure: a question of balance. J. Clin. Invest. 115, 547–555 (2005).
Lopaschuk, G. D., Ussher, J. R., Folmes, C. D., Jaswal, J. S. & Stanley, W. C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258 (2010).
Kolleritsch, S. et al. Low cardiac lipolysis reduces mitochondrial fission and prevents lipotoxic heart dysfunction in Perilipin 5 mutant mice. Cardiovasc. Res. 116, 339–352 (2020).
Chae, S. et al. A mitochondrial proteome profile indicative of type 2 diabetes mellitus in skeletal muscles. Exp. Mol. Med. 50, 1–14 (2018).
Chokshi, A. et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 125, 2844–2853 (2012).
Li, X. et al. Distinct cardiac energy metabolism and oxidative stress adaptations between obese and non-obese type 2 diabetes mellitus. Theranostics 10, 2675–2695 (2020).
Zhu, B. et al. Lipid oversupply induces CD36 sarcolemmal translocation via dual modulation of PKCzeta and TBC1D1: an early event prior to insulin resistance. Theranostics 10, 1332–1354 (2020).
Tang, X. et al. Mitochondrial GSNOR Alleviates Cardiac Dysfunction via ANT1 Denitrosylation. Circ. Res. 133, 220–236 (2023).
Wang, J. et al. DHHC4 and DHHC5 Facilitate fatty acid uptake by palmitoylating and targeting CD36 to the Plasma Membrane. Cell Rep. 26, 209–221 (2019).
Coort, S. L. et al. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes 53, 1655–1663 (2004).
Ouwens, D. M. et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 50, 1938–1948 (2007).
Glatz, J. F. C., Heather, L. C. & Luiken, J. CD36 as a gatekeeper of myocardial lipid metabolism and therapeutic target for metabolic disease. Physiol. Rev. 104, 727–764 (2024).
Wang, H. et al. Inhibition of fatty acid uptake by TGR5 prevents diabetic cardiomyopathy. Nat. Metab. 6, 1161–1177 (2024).
Sung, M. M. et al. Cardiomyocyte-specific ablation of CD36 accelerates the progression from compensated cardiac hypertrophy to heart failure. Am. J. Physiol. Heart Circ. Physiol. 312, H552–H560 (2017).
Shu, H. et al. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 118, 115–129 (2022).
Luiken, J. J. et al. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 51, 3113–3119 (2002).
Zeng, S. et al. Inhibition of fatty acid translocase (FAT/CD36) palmitoylation enhances hepatic fatty acid beta-oxidation by increasing its localization to mitochondria and interaction with long-chain acyl-CoA synthetase 1. Antioxid. Redox Signal. 36, 1081–1100 (2022).
Xu, M. et al. Palmitoyltransferase ZDHHC3 aggravates nonalcoholic steatohepatitis by targeting S-Palmitoylated IRHOM2. Adv. Sci. 10, e2302130 (2023).
Tao, N., Wagner, S. J. & Lublin, D. M. CD36 is palmitoylated on both N- and C-terminal cytoplasmic tails. J. Biol. Chem. 271, 22315–22320 (1996).
Thorne, R. F. et al. Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum. Biochim. Biophys. Acta 1803, 1298–1307 (2010).
Zhao, L. et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J. Hepatol. 69, 705–717 (2018).
Hao, J. W. et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 11, 4765 (2020).
Li, J. M. et al. CPAL, as a New mediator of cardiomyocyte metabolic alterations and pyroptosis, regulates myocardial infarction injury in mice. Engineering 20, 49–62 (2023).
Fan, L. et al. Muscle Kruppel-like factor 15 regulates lipid flux and systemic metabolic homeostasis. J. Clin. Invest. 131, https://doi.org/10.1172/jci139496 (2021).
Chaurasia, B. et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365, 386–392 (2019).
Kim, T. T. & Dyck, J. R. The role of CD36 in the regulation of myocardial lipid metabolism. Biochim. Biophys. Acta 1861, 1450–1460 (2016).
Miscianinov, V. et al. MicroRNA-148b targets the TGF-beta pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol. Ther. 26, 1996–2007 (2018).
Yan, Z. et al. The antimicrobial peptide YD attenuates inflammation via miR-155 targeting CASP12 during liver fibrosis. Acta Pharm. Sin. B 11, 100–111 (2021).
Brigidi, G. S. & Bamji, S. X. Detection of protein palmitoylation in cultured hippocampal neurons by immunoprecipitation and acyl-biotin exchange (ABE). J. Vis. Exp. 72, https://doi.org/10.3791/50031 (2013).
Schwenk, R. W., Luiken, J. J., Bonen, A. & Glatz, J. F. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc. Res. 79, 249–258 (2008).
Angin, Y. et al. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem. J. 448, 43–53 (2012).
Ghafouri-Fard, S. et al. Exploring the role of non-coding RNAs in autophagy. Autophagy 18, 949–970 (2022).
Sciarretta, S. et al. Trehalose-induced activation of autophagy improves cardiac remodeling after Myocardial Infarction. J. Am. Coll. Cardiol. 71, 1999–2010 (2018).
Guan, Z. et al. Nuanxinkang prevents the development of myocardial infarction-induced chronic heart failure by promoting PINK1/Parkin-mediated mitophagy. Phytomedicine 108, 154494 (2023).
Wang, Y. et al. Mechanisms involved in the regulation of mitochondrial quality control by PGAM5 in heart failure. Cell Stress Chaperones 29, 510–518 (2024).
Zhong, C. et al. S100A9 Derived from Chemoembolization-Induced Hypoxia Governs Mitochondrial Function in Hepatocellular Carcinoma Progression. Adv. Sci 9, e2202206 (2022).
Liu, S. et al. The E3 ubiquitin ligase MARCH2 protects against myocardial ischemia-reperfusion injury through inhibiting pyroptosis via negative regulation of PGAM5/MAVS/NLRP3 axis. Cell Discov. 10, 24 (2024).
Chen, G. et al. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol. Cell 54, 362–377 (2014).
Ni, H. M., Williams, J. A. & Ding, W. X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 4, 6–13 (2015).
Yu, B. et al. Mitochondrial phosphatase PGAM5 modulates cellular senescence by regulating mitochondrial dynamics. Nat. Commun. 11, 2549 (2020).
Chen, Y. et al. Phosphoglycerate mutase 5 knockdown alleviates neuronal injury after traumatic brain injury through Drp1-mediated mitochondrial Dysfunction. Antioxid. Redox Signal 34, 154–170 (2021).
Ritterhoff, J. & Tian, R. Metabolic mechanisms in physiological and pathological cardiac hypertrophy: new paradigms and challenges. Nat. Rev. Cardiol. 20, 812–829 (2023).
Gao, F. et al. A defect in mitochondrial protein translation influences mitonuclear communication in the heart. Nat. Commun. 14, 1595 (2023).
Sun, S. et al. TBC1D15-Drp1 interaction-mediated mitochondrial homeostasis confers cardioprotection against myocardial ischemia/reperfusion injury. Metabolism 134, 155239 (2022).
Bartman, C. R., TeSlaa, T. & Rabinowitz, J. D. Quantitative flux analysis in mammals. Nat. Metab. 3, 896–908 (2021).
Fillmore, N., Mori, J. & Lopaschuk, G. D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharm. 171, 2080–2090 (2014).
Peoples, J. N., Saraf, A., Ghazal, N., Pham, T. T. & Kwong, J. Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 51, 1–13 (2019).
Wang, Z. Y., Liu, X. X. & Deng, Y. F. Negative feedback of SNRK to circ-SNRK regulates cardiac function post-myocardial infarction. Cell Death Differ. 29, 709–721 (2022).
Edwards, K. S. et al. Uncoupling protein 3 deficiency impairs myocardial fatty acid oxidation and contractile recovery following ischemia/reperfusion. Basic Res. Cardiol. 113, 47 (2018).
Cardoso, A. C. et al. Mitochondrial Substrate Utilization Regulates Cardiomyocyte Cell Cycle Progression. Nat. Metab. 2, 167–178 (2020).
Han, L. et al. Lipid droplet-associated lncRNA LIPTER preserves cardiac lipid metabolism. Nat. Cell Biol. 25, 1033–1046 (2023).
Wu, X. et al. SIRT6 Mitigates Heart Failure with preserved ejection fraction in diabetes. Circ. Res. 131, 926–943 (2022).
Shen, S., Liao, Q., Zhang, T., Pan, R. & Lin, L. Myricanol modulates skeletal muscle-adipose tissue crosstalk to alleviate high-fat diet-induced obesity and insulin resistance. Br. J. Pharm. 176, 3983–4001 (2019).
Stefan, N. & Haring, H. U. Circulating fetuin-A and free fatty acids interact to predict insulin resistance in humans. Nat. Med. 19, 394–395 (2013).
Holland, W. L. et al. Lipid mediators of insulin resistance. Nutr. Rev. 65, S39–S46 (2007).
Cui, M. et al. Dynamic transcriptional responses to injury of regenerative and non-regenerative cardiomyocytes revealed by single-nucleus RNA sequencing. Dev. Cell 53, 102–116.e108 (2020).
Liu, Y. et al. Transcription factor Meis1 act as a new regulator of ischemic arrhythmias in mice. J. Adv. Res. 39, 275–289 (2022).
Kim, T. Y. et al. SK channel enhancers attenuate Ca2+-dependent arrhythmia in hypertrophic hearts by regulating mito-ROS-dependent oxidation and activity of RyR. Cardiovasc. Res. 113, 343–353 (2017).
Talati, M. H. et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am. J. Respir. Crit. Care Med. 194, 719–728 (2016).
Kinghorn, K. J. et al. Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction. Brain 138, 1801–1816 (2015).
Mansor, L. S. et al. Inhibition of sarcolemmal FAT/CD36 by sulfo-N-succinimidyl oleate rapidly corrects metabolism and restores function in the diabetic heart following hypoxia/reoxygenation. Cardiovasc. Res. 113, 737–748 (2017).
Sanjurjo, L. et al. The human CD5L/AIM-CD36 axis: A novel autophagy inducer in macrophages that modulates inflammatory responses. Autophagy 11, 487–502 (2015).
He, C. et al. CD36 and LC3B initiated autophagy in B cells regulates the humoral immune response. Autophagy 17, 3577–3591 (2021).
Cheng, M. et al. PGAM5: A crucial role in mitochondrial dynamics and programmed cell death. Eur. J. Cell Biol. 100, 151144 (2021).
Yang, C. et al. Mitochondrial phosphatase PGAM5 regulates Keap1-mediated Bcl-xL degradation and controls cardiomyocyte apoptosis driven by myocardial ischemia/reperfusion injury. Vitr. Cell Dev. Biol. Anim. 53, 248–257 (2017).
De Loof, M. et al. Enhanced protein acetylation initiates fatty acid-mediated inhibition of cardiac glucose transport. Am. J. Physiol. Heart Circ. Physiol. 324, H305–H317 (2023).
Luiken, J., Nabben, M., Neumann, D. & Glatz, J. F. C. Understanding the distinct subcellular trafficking of CD36 and GLUT4 during the development of myocardial insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165775 (2020).
Schwenk, R. W. et al. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia 53, 2209–2219 (2010).
Liu, Y. et al. Palmitate-induced vacuolar-type H(+)-ATPase inhibition feeds forward into insulin resistance and contractile dysfunction. Diabetes 66, 1521–1534 (2017).
Xiao, H. et al. CIRKIL Exacerbates cardiac ischemia/reperfusion injury by interacting with Ku70. Circ. Res. 130, e3–e17 (2022).
Georgiou, D. K. et al. Ca2 + Binding/permeation via calcium Channel, CaV1.1, regulates the intracellular distribution of the fatty acid transport protein, CD36, and fatty acid metabolism. J. Biol. Chem. 290, 23751–23765 (2015).
Zhang, Y. et al. Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Res. 29, 609–627 (2019).
Tzircotis, G., Thorne, R. F. & Isacke, C. M. A new spreadsheet method for the analysis of bivariate flow cytometric data. BMC Cell Biol. 5, 10 (2004).
Zhang, L. et al. Aptamer proteomics for biomarker discovery in heart failure with reduced ejection fraction. Circulation 146, 1411–1414 (2022).
Acknowledgements
This study was funded by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (2024ZD0537908 to Y.Z.), National Natural Science Foundation of China (82273919 and 81961138018 to Y.Z.; 82370328 to J.L.; 82404619 to Q.Z.) and the HMU Marshal Initiative Funding (HMUMIF-21022 to Y.Z.).
Author information
Authors and Affiliations
Contributions
Q.Z., B.Y., and Y.Z. designed the study. Q.Z., J.L., and X.L. arranged experiments and analyzed the results. Q.Z., X.C., L.Z., Z.Z., X.D., H.L., T.Z., and Y.H. performed cellular experiments. Q.Z., X.C., H.X., W.Z., Z.S., and X.S. conducted animal experiments. Q.Z., J.L., B.Y., and Y.Z. wrote and finalized the article. All persons have made contributions to this work. Q.Z. and J.L. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhan Gao, Ying Ge, and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Q., Li, J., Liu, X. et al. Inhibiting CD36 palmitoylation improves cardiac function post-infarction by regulating lipid metabolic homeostasis and autophagy. Nat Commun 16, 6602 (2025). https://doi.org/10.1038/s41467-025-61875-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61875-y
This article is cited by
-
Lipid overload meets S-palmitoylation: a metabolic signalling nexus driving cardiovascular and heart disease
Cell Communication and Signaling (2025)