Abstract
Acute kidney injury (AKI) frequently arises as a complication of hepatic ischemia-reperfusion injury (HIRI), yet simultaneous optical imaging of both remains challenging due to the lack of unimolecular dual-responsive probes. Herein, we report a library of hemicyanine-based chemiluminophores (HCLs) with tunable emission to second near-infrared window (725 − 1025 nm) achieved by integrating bicyclic dioxetane onto hemicyanine skeletons to develop multiple-responsive chemiluminescent probes. HCL1 and HCL5 respectively emitting at 725 nm and 1025 nm are selected to construct a cascaded activatable reporter CAR for crosstalk-free duplex chemiluminescence imaging of interlinked biomarkers. Following systemic injection to male mice, CAR preferentially accumulates in the liver and reports HIRI-associated superoxide anion (O2•−), which initiates self-fragmentation and liberates the secondary reporter KIR into kidneys to report AKI-associated N-acetyl-β-D-glucosaminidase (NAG). Such mechanism allows CAR to serve as a reservoir for gradual release of AKI reporters, providing a significantly prolonged imaging window compared to co-administering separate probes. CAR further permits remote detection of HIRI-induced AKI via urinalysis. This study not only offers a powerful tool for simultaneous detection of HIRI and HIRI-induced AKI, but also highlights a unimolecular probe design for ultrasensitive detection of deeply-seated intercorrelated diseases.
Similar content being viewed by others
Introduction
Hepatic ischemia reperfusion injury (HIRI), which occurs when the liver experiences inadequate oxygen and nutrient supply, followed by the restoration of blood flow, often leads to oxidative stress and inflammation1,2,3. It is a common complication in various clinical scenarios such as hemorrhagic shock, trauma, liver resection, and transplantation, and stands as a leading cause of acute liver failure (ALF) during the perioperative period4,5. Patients with ALF frequently develop acute kidney injury (AKI) (Fig. 1a), with the incidence varying between 40% and 85%, depending on the etiology6,7,8. Notably, the occurrence of AKI in orthotopic liver transplantation can reach up to ~95%, significantly increasing mortality6,7,8. Timely therapeutic intervention has been shown to improve patient outcomes, highlighting the need for early diagnostic methods that can simultaneously detect HIRI and HIRI-induced AKI, as well as advancing the understanding of elusive pathophysiological mechanisms linking HIRI with AKI9,10.
a The effects of liver ischemia duration on the induction of AKI. The severity of HIRI-induced AKI is highly associated with the duration of liver ischemia. b Schematic illustration of CAR for imaging of O2•− and NAG and urinalysis. After systemic administration of CAR into living mice, it efficiently accumulates into the liver and specifically liberates NIR-II CL signal to report O2•− levels, followed by inducing self-elimination to defragment the backbone of CAR and effectively releasing the NIR-I CL reporter (KIR) to the kidneys for sensing NAG levels, finally excreting out for fluorescently urinalysis. c Chemical structures of CAR and its fragments in response to O2•− and NAG, respectively. Source data are provided as a Source Data file.
Current clinical diagnostic techniques primarily rely on assessing serological biomarkers such as serum alanine aminotransferase (ALT)/aspartate aminotransferase (AST) and serum creatinine (sCr)/blood urea nitrogen (BUN) for liver and kidney dysfunction, respectively. However, these biomarkers are late-stage indicators and lack the sensitivity to detect HIRI and AKI at an early stage11. Biopsy is another routine method for diagnosing organ damage; whereas it is invasive, carries a risk of secondary injury and provides only a limited and static view of the pathological state12. Although non-invasive imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) have been used to reveal anatomic and functional changes in both liver and kidneys, these methods are insensitive to detecting early biomarkers at the molecular level during HIRI-induced injury13.
Chemiluminescence (CL) imaging known for its ultrahigh sensitivity and high signal-to-background ratio (SBR) has become one of the most reliable bioimaging techniques14. In particular, the chemiluminophore Schaap’s dioxane with excellent chemical amenability has been constructed into versatile activatable probes that inspire chemiluminescence imaging of a diverse array of pathological biomarkers including reactive species (superoxide anion, hydrogen peroxide, singlet oxygen, peroxynitrite, etc.) and enzymes (β-galactosidase, alkaline phosphatase, γ-glutamyl transpeptidase, cathepsin B, granzyme B, etc)15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30. However, most of these probes have visible emission with limited tissue penetration depth, and the red shift of emission wavelength remains challenging31,32,33,34,35,36,37,38,39,40. In addition, existing probes mostly respond to merely one biomarker, failing to simultaneously diagnose the primary and the related complication diseases, which requires detecting two interlinked biomarkers. While co-administration of two separate probes may seem like a feasible approach, it demands precise alignment of imaging windows for both probes, which can be challenging due to their differing pharmacokinetic profiles41,42,43,44,45. Unimolecular dual-locked probes might overcome these challenges by using a single probe that sequentially responds to two biomarkers at primary and complication disease sites, with the initial response triggering the liberation of a secondary reporter46,47,48,49. Such sequential logic response also favors the pharmacokinetic conversion of the probe for multiorgan imaging, providing opportunities to investigate the pathophysiological mechanisms between the primary disease and its remote complication50,51,52,53,54,55. However, unimolecular dual-responsive reporters are rarely explored, especially for detecting interlinked diseases such as HIRI and HIRI-induced remote AKI.
Here, we develop a unimolecular cascaded activatable reporter (CAR) with dual responses and pharmacokinetic conversion for non-invasive multiplex chemiluminescence imaging and fluorescent urinalysis of HIRI and associated AKI (Fig. 1b, c). We first synthesize a library of hemicyanine-based chemiluminophores (HCLs) with tunable emission from 725 to 1025 nm and structural flexibility for constructing activatable probes. Unlike conventional chemiluminescence resonance energy transfer (CRET) approaches, HCLs are constructed by respectively integrating a benz[e]indolium, benz[c,d]indolium, naphtho[c,d]indolium and thiophen-benz[c,d]indolium moiety onto xanthonium- based bicyclic dioxetane to expand linear π-conjugation, affording record-long-wavelength emission among reported bicyclic dioxetane chemiluminophores (Fig. 2e and Supplementary Table 1). From this library, HCL1 emitting in the first near-infrared window (NIR-I) and HCL5 in the second NIR window (NIR-II) are selected to construct CAR because of their minimal spectral overlap. HCL1 and HCL5 are respectively caged with cleavable moieties responsive to N-acetyl-β-D-glucosaminidase (NAG), a biomarker of renal tubular damage in AKI, and superoxide anion (O2•−), a biomarker of oxidative stresses in HIRI, and are linked with a renal-clearable unit polyethylene glycol (PEG) (Fig. 1c)28,29. After systemic administration to living mice (Fig. 1b), CAR efficiently accumulates in the liver, where it specifically reports O2•− level via NIR-II CL. This response further triggers its self-elimination to expose and release the NIR-I CL reporter (KIR), which travels to the kidneys and detects NAG, finally getting excreted out and enabling in vitro urinalysis30. Such cascaded activation thus enables crosstalk-free and simultaneous monitoring of two intercorrelated pathological events in the liver-kidney axis during the onset and progression of HIR.
a, b Schematic illustration of the redshift of chemiluminescence via energy-transfer-based indirect chemiluminescence emission pathway and direct chemiluminescence emission pathway. c Activation and chemiexcitation pathway of adamantyl-dioxetanes and bicyclic dioxetanes. d Evaluation of the thermal stability of BD-OTf and AD-OTf. Comparison of the reserved CL intensities of BD-OTf and AD-OTf when stored at 25 °C for different duration time. (n = 3, mean ± s.d.). e Structures of adamantyl-dioxetanes scaffold15,17,23,56 and hemicyanine-based bicyclic dioxetanes and their CL emission wavelengths are indicated by arrows. Their maxima CL emissions are given underneath the corresponding structures. f Absorption, (g) fluorescence spectra and (h) Chemiluminescence spectra of HCLS (10 μM) in dimethyl sulfoxide/H2O (v/v = 1/1). (a.u. means arbitrary units). i Molecular orbitals of HCLS. The HOMO and LUMO energy levels are indicated. j Calculated HOMO/LUMO energy gaps (ΔE) of HCLS. Source data are provided as a Source Data file.
Results
Design, synthesis, and characterization of NIR-II chemiluminophores
To shift the chemiluminescence towards a more biologically transparent NIR window, dioxetane substrates are often conjugated onto fluorophores, forming CRET systems (Fig. 2a). However, this indirect approach significantly reduces chemiluminescent brightness due to low energy transfer efficiencies and additional energy relay processes. A more effective strategy is to directly modify dioxetane onto NIR fluorophores (Fig. 2b). However, due to the challenges in chemical synthesis, dioxetane-based chemiluminophores with the NIR-I emission (660–780 nm) are scarcely studied, and those reaching beyond 900 nm are rarely reported16,56,57,58. Moreover, improved thermal stability is essential for chemiluminophores. To address this, we synthesized the bicyclic dioxetane (BD-1) and adamantyl dioxetane (AD-1) (Fig. 2c) and determined their reserved chemiluminescence after placing on the bench at room temperature for 6 h. As shown in Fig. 2d and Supplementary Fig. 1, BD-OTf demonstrated superior thermal stability, as evidenced by a negligible decrease on the reserved chemiluminescence intensity compared to AD-OTf. Thereafter, we tailored the bicyclic dioxetane scaffold by conjugating xanthonium and indolium derivatives, which feature an extended D-π-A motif and render rigid conjugated structures (Fig. 2e). Such conjugation extension effectively tuned and redshifted the chemiluminescence emission of the HCL family, ranging from 725 nm to a maximum of 1025 nm (Fig. 2e), which was ~ 225 nm longer than the previously reported bicyclic dioxetane probe (~ 800 nm)59,60. The detailed synthetic routes of HCLs were shown in Fig. 3. Briefly, the key precursor 5 was synthesized from commercial 3,5-dimethoxybenzyl bromide through substitution of the benzyl bromide and oxidation reaction to yield compound 1. In the presence of lithium diisopropylamide, compound 1 was converted to compound 2 through a cyclization reaction, which was further converted to aldehyde compound 3 via AgOTf-aided direct formyl transfer. Compound 3 was directly deprotected by BBr3 to afford ortho-hydroxy-formyl 4, followed by cascaded oxa-Michael addition and dehydration to yield aldehyde compound 5. It was then condensed with indoles 11, 12, 14, 16 and 19 and subsequently oxidation via [2 + 2] cycloaddition with singlet oxygen (1O2) using methylene blue as the photosensitizer to yield HCL1-5. The chemical structures of the intermediates and final HCLs were confirmed through 1H NMR, 13C NMR and mass spectrometry. The photophysical properties of HCL1-5 were measured and shown in Fig. 2f–h and Supplementary Fig. 2. The absorption maxima in dimethyl sulfoxide/H2O were at 652, 695, 755, 798, and 822 nm for HCL1-HCL5, respectively. Furthermore, HCL1-5 exhibited strong chemiluminescence across NIR-I and NIR-II regions with maximum emission peaks at 725, 735, 850, 920, and 1025 nm, respectively, which corresponded well with their fluorescence spectra (Fig. 2g and Supplementary Table 1) and implied their abilities for both NIR chemiluminescence and fluorescence imaging. These findings also confirmed that integrating different π-extended indolium derivatives onto the xanthonium moiety successfully induced bathochromic shift in their emission wavelengths. To understand the mechanism of this spectral shift, time-dependent density functional theory (TD-DFT) calculations and electron-hole analysis were performed. Overall, HCL1-HCL5 displayed gradually decreased LUMO levels and increased HOMO levels (Fig. 2i), showing reduced energy gaps from 2.19, 2.08, 1.93, 1.92 to 1.77 eV, respectively (Fig. 2j). Decreased HOMO-LUMO energy gaps and the excitation energy were observed, which in accordance with the red shift of the emission wavelengths from HCL1 to HCL5 (Supplementary Fig. 3). This finding aligned well with the bathochromic shifts in emission and provided the theoretical validation for the strategy of expanding π-conjugation on indolium moieties. Among HCLs, HCL1 and HCL5 respectively emitting at 725 and 1025 nm demonstrate negligible spectral overlap and high potential for crosstalk-free duplex chemiluminescence imaging. We herein selected HCL1 and HCL5 for the construction of unimolecular dual-responsive probes in the following studies.
a Synthetic routes of HCLs. Reagent and conditions: (1) Compound 21, sodium hydride, THF, rt, 6 h. (2) Pyridinium Chlorochromate, DCM, rt, 12 h. (3) Lithium diisopropylamide, THF, − 78 °C, 6 h. (4) Silver trifluoromethanesulfonate, Dichloromethyl methyl ether, DCM, − 78 °C, 8 h. (5) Boron tribromide, DCM, − 78 °C, 3 h. (6) Compound 25, DMF, Cs2CO3, rt, 12 h. (7) AcONa, EtOH, 50 °C, 6 h. (8) Methylene blue, O2, hv, 1 h. b Synthetic routes of probe CAR. (1) Compound 22, Cs2CO3, K2CO3, DCM, rt, 2 h. (2) NaOMe, DCM, rt, 1 h. (3) SH-PEG2000-alkyne, sodium ascorbate, CuSO4, THF, H2O, rt, 2 h. (4) Compound 23, 24, triphosgene, 4-di-methylaminopyridine, 0 °C, 2 h. (5) Triethylamine, THF, rt, 3 h. (6) Methylene blue (MB), MeOH, O2, hv, 2 h. c Synthetic routes of probe LIR. (1) Trifluorosulfonic anhydride, Pyridine, DCM, rt, 2 h. (2) Methylene blue (MB), O2, hv, 1 h. d Synthetic routes of probe KIR. (1) Compound 24, THF, trimethylamine, 3 h. (2) Methylene blue (MB), MeOH, O2, hv, 1 h. Source data are provided as a Source Data file.
Synthesis and in vitro characterization of CAR
Unimolecular dual-responsive chemiluminescence probe CAR was constructed through three main steps (Fig. 3b): (i) Synthesis of the N-acetyl-β-D-glucosamine-caged NIR-I chemiluminescent substrate 8, (ii) synthesis of the trifluoromethanesulfonate-caged NIR-II chemiluminescent substrate 10, and (iii) conjugation of NIRF-I and NIR-II chemiluminescent substrates (8&10) followed by oxidation to afford CAR. Briefly, compound 6 was synthesized similarly to HCL1, then reacted with a bromo-glucose derivative to afford compound 7 after hydrolysis. The azide group on 7 was conjugated with the alkyne-functionalized PEG to produce substrate 8. The trifluoromethanesulfonate-caged self-immolative substrate 23 was synthesized as described in previous studies (Supplementary Fig. 4), enabling conjugation with the precursor 9 and maleimide linker 24 to afford NIR-II chemiluminescent substrate 10. The thiol group on the terminal of substrate 8 was conjugated with the maleimide group of substrate 10 via a click reaction, followed by [2 + 2] cycloaddition with 1O2 using methylene blue to yield CAR. 1H NMR, 13C NMR and mass spectra confirmed the chemical structures of the intermediates and CAR. Two control probes liver injury reporter (LIR) and kidney injury reporter (KIR) were also synthesized and characterized (Fig. 3b, c). Synthesis routes of 11, 12, 14, 16 and 19 were depicted in Supplementary Fig. 4. 1H NMR and mass spectra confirmed their chemical structures.
The amphiphilicity of CAR allowed it to spontaneous assemble into spherical nanoparticles, as shown by transmission electron microscopy (TEM) (Fig. 4l). The average hydrodynamic size of CAR was measured to be ~180 nm (Fig. 4j). No precipitation and obvious change in fluorescence were detected for CAR during the storage in phosphate buffer solution (PBS) or fetal bovine serum (FBS) for 5 days (Supplementary Fig. 5a, b) and in different pH buffer solution (Supplementary Fig. 6). Zeta potential measurement revealed that CAR was negatively charged (Supplementary Fig. 5c). CAR had two absorption peaks at ~650 nm and ~800 nm, corresponding to the absorbance of HCL1 and HCL5 moieties, respectively (Supplementary Fig. 5d). CAR by itself was non-chemiluminescent and non-fluorescent because both HCL1 and HCL5 moieties were in ‘caged’ states that the electron-donating abilities of the aromatic hydroxyl groups were diminished. Upon exposure to KO2 and NAG, CAR demonstrated red-shifted absorption peaks at ~ 700 nm and ~ 820 nm (Supplementary Fig. 5d). In the presence of NAG, NIR-I (~ 720 nm) chemiluminescence and fluorescence from CAR increased by ~ 8500-fold and ~8-fold, respectively (Fig. 4a, b). Similarly, incubation with KO2 increased NIR-II (~ 1025 nm) chemiluminescence and fluorescence increased by ~6500-fold and ~ 7.5-fold, respectively. Such dramatic emission enhancement were ascribed to the cleavage of the glycosidic bond of N-acetyl-β-D-glucosamine moiety by NAG and trifluoromethanesulfonate group by O2•−, which triggered 1,4-elimination and led to the fragmentation into uncaged probes FL1R and FL2R (Fig. 1c), both with a strong electron-donating phenolate group on the luminophore.
a, b NIR-I chemiluminescence and fluorescence spectra of CAR (20 μM) in the absence or presence of NAG (40 mU) in PBS (10 mM, pH 7.4). Inset: The corresponding fluorescence and chemiluminescence images using the IVIS spectrum imaging system. c Michaelis-Menten saturation curve for NAG (10 mU) toward CAR (10, 20, 30, 60 and 90 μM) in PBS (10 mM, pH 7.4). (n = 3, mean ± s.d.). d NIR-I chemiluminescence and fluorescence changes of CAR (10 μM) after incubation with ROS (40 μM), metal ions (40 μM), glutathione (GSH), β-galactosidase (β-Gal), gamma-glutamyl transferase (GGT), and NAG in PBS (10 mM, pH 7.4). (n = 3, mean ± s.d.). e, f NIR-II chemiluminescence and fluorescence spectra of CAR (20 μM) in the absence or presence of KO2 (80 μM) in PBS (10 mM, pH 7.4). g NIR-II chemiluminescence kinetic profiles of CAR in the presence of KO2 in PBS (10 mM, pH 7.4). h NIR-II chemiluminescence and fluorescence changes of CAR (10 μM) after incubation with ROS (40 μM), metal ions (40 μM), GSH, β-Gal, GGT, and KO2 (80 μM) in PBS (10 mM, pH 7.4). (n = 3, mean ± s.d.). i NIR-II chemiluminescence intensities of CAR as a function of the concentration of KO2 (0–50 nM). (n = 3, mean ± s.d.; a.u. means arbitrary units). j, k Dynamic light scattering of CAR before and after incubation with KO2 in buffer solution. l Representative TEM images of CAR before and after incubation with KO2 in buffer solution. Source data are provided as a Source Data file.
This fragmentation was validated by TEM and dynamic light scattering (DLS), which indicated that the size of CAR decreased to ~ 30 nm and ~ 4 nm after exposure to KO2 (Fig. 4j, k). CAR showed minimal change in chemiluminescence and fluorescence in the presence of interfering analytes, including other reactive oxygen species, metal ions, and other enzymes, confirming their excellent stability and specificity (Fig. 4d, h). In addition, the catalytic efficiency (Kcat/Km) of NAG towards CAR was calculated to be 0.85 μM−1S−1 (Fig. 4c). A good linear relationship was observed between the NIR-II chemiluminescent signal and KO2 concentration, with an estimated limit of detection (LOD) of approximately 12 nM (Fig. 4i). The chemiluminescence half-life of CAR was ~ 12 min (Fig. 4g), which is suitable for in vivo imaging. In addition, optical changes of control probes LIR and KIR in response to KO2 and NAG were characterized and shown in Supplementary Fig. 5e–g.
Tissue penetration and clearance pathways
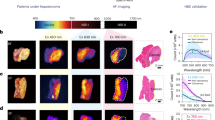
We then compared the penetration depth and imaging sensitivity of chemiluminescence and fluorescence channels from HCL1 and HCL5 (Fig. 5a). To do so, three trifluoromethanesulfonate caged-luminophores, including BD-OTf, HCL1-OTf and HCL5-OTf were synthesized (Fig. 5b). In the presence of KO2, the trifluoromethanesulfonate group was cleaved and resulted in maximum chemiluminescence emission at approximately 450, 725 and 1025 nm for these three probes, respectively (Fig. 5c). The triggered chemiluminescence as well as fluorescence signals were recorded under different depths of chicken breast tissue. Chemiluminescence signals from BD-OTf and HCL1-OTf decreased to the background levels at tissue thickness of 0.5 cm and 2 cm, respectively; whereas that from HCL5-OTf remained discernible even at 3 cm (Fig. 5d). In contrast, fluorescence signals from BD-OTf, HCL1-OTf and HCL5-OTf declined to background levels at tissue thickness of 0.5 cm, 1 cm and 1.5 cm, respectively (Fig. 5d). In addition, the SBRs of chemiluminescence for HCL5-OTf (591 and 124) were 5.8 and 10.3-fold higher than that of BD-OTf (101 and 12) and 1.6 and 8.2-fold higher than that of HCL1-OTf (302 and 15) at chicken tissue thicknesses of 0.5 and 3.0 cm, respectively (Fig. 5e). Although fluorescence SBRs improved with increasing emission wavelengths, they were much lower than the corresponding chemiluminescence SBRs (Fig. 5f). These findings verified that chemiluminescence provides enhanced imaging sensitivities and deeper tissue penetration than fluorescence, highlighting its superiority for in vivo imaging.
a Schematic illustration of tissue-penetration study. b, c Chemical structures and chemiluminescence spectra of BD-OTf, HCL1-OTf and HCL5-OTf. d Representative chemiluminescence and fluorescence images of BD-OTf, HCL1-OTf and HCL5-OTf (40 µM) solutions upon overlaying chicken tissues with the desired thickness on top of the samples. e, f SBRs for chemiluminescence and fluorescence imaging of BD-OTf, HCL1-OTf and HCL5-OTf as a function of chicken tissue depth in panel d. (n = 3, mean ± s.d.). g Schematic illustration of renal and hepatobiliary clearance pathways in living mice. h Blood concentration (% ID g−1) decay of CAR, KIR and LIR after i.v. injection into living mice. (n = 3, mean ± s.d.). i The total renal and fecal clearance efficiency of CAR, KIR and LIR after 7 days of injection. (n = 3, mean ± s.d.). j Fluorescence images of the abdominal cavity of mice after 1 h injection of CAR, LIR, FL2R, PBS, KIR and FL1R (2.5 μmol kg−1 body weight). Bladder (Bl), kidneys (Ki), liver (Li), muscle (Mu), spleen (Sp). k NIR-II fluorescence intensity of liver from mice after 1 h injection of CAR, LIR and FL2R. (n = 3, mean ± s.d.; a.u. means arbitrary units) l NIR-I fluorescence intensity of kidneys after 1 h injection of PBS, KIR and FL1R. (n = 3, mean ± s.d.). Source data are provided as a Source Data file.
To investigate the pharmacokinetics of CAR and the control probes (LIR and KIR), their concentrations in the blood were analyzed by high-performance liquid chromatography (HPLC) after intravenous (i.v.) injection into living mice (Fig. 5g). Blood concentration curves showed that LIR and KIR rapidly decreased to 0% injected doses (ID) g−1 at 2 h post-injection (Fig. 5h), while CAR persisted for up to 24 h. And CAR had a much longer elimination half-life (t1/2β) (~ 5 h) compared to LIR (~ 32 min) and KIR (~ 25 min). Moreover, the renal clearance efficiency of KIR was ~ 92% ± 2% ID at 24 h post-injection in living mice, in sharp contrast to ~ 5% ± 1.3% ID for LIR and ~ 3% ± 1.2% ID for CAR. On the contrary, the fecal clearance efficiency of LIR and CAR were determined to be ~ 70% ± 3% ID and ~ 58% ± 2.2%, respectively, much higher than 2.1% ± 0.5% ID for KIR (Fig. 5i). Furthermore, the biodistributions of CAR, LIR, KIR and their activation forms (FL2R and FL1R) were investigated by NIR-II and NIR-I fluorescence imaging after i.v. injection. NIR-II fluorescence of FL2R mainly located in the liver with minimal signal in other organs; while no obvious NIR-II fluorescence signal of CAR and LIR was found, as they are intrinsically non-fluorescent (Fig. 5j). Similarly, a bright NIR-I fluorescence signal of FL1R was observed in the kidneys (Fig. 5j), whereas minimal signal from KIR was observed. In addition, the optical and chemical profiles of CAR, KIR and their fragments excreted renally from mice were measured. The absorption and HPLC of KIR recovered from urine (CAR recovered from feces) and their as-synthesized forms were almost identical (Supplementary Fig. 7), confirming their structural stability and minimal in vivo metabolism in mice. Moreover, no increment in serological levels of sCr, BUN, ALT and AST were observed between PBS groups and CAR groups in healthy mice (Supplementary Fig. 8). Histological staining revealed that no signs of cell death or necrosis observed in the major organs, implying CAR and its fragments had high biocompatibility (Supplementary Fig. 9). Collectively, these findings indicated that CAR and LIR showed liver-oriented accumulation and were mainly excreted through the hepatobiliary clearance pathway, which should be attributed to the large particle size (~ 180 nm) of CAR that far exceeded the kidney filtration threshold (~ 6 nm) and the hydrophobicity of LIR. In contrast, KIR and FL1R were mainly eliminated through the renal clearance pathway because of its high hydrophilicity (distribution coefficient Log D = − 9.58) and low molecular weight (~ 2.5 kDa) relative to the glomerular filtration molecular weight cutoff (~ 50 kDa).
Cascaded multiplex imaging and urinalysis of HIRI and HIRI-induced AKI using a unimolecular dual-responsive probe CAR
The performance of CAR for real-time non-invasive imaging of HIRI and HIRI-induced AKI was evaluated in living mice. A partial hepatic warm IR model was established according to previous reports28. Briefly, the abdomen was opened to dissect the interlobular ligaments when male mice were anesthetized. A microvascular clamp was then used to interrupt the arterial and portal venous blood supply to the left lateral and median liver lobes for 15, 30, 45, or 60 min (Fig. 6a). Afterwards, the clamp was removed and the abdominal cavity was closed. Such an operation procedure resulted in a segmental (~ 70%) liver ischemia. A sham-operation group was also established using the same procedure, involving only abdominal trauma without vascular occlusion. Healthy mice or mice treated with an antioxidative drug (N-acetylcysteine (NAC)) prior to the operation were used as control groups.
a Schematic illustration of the development of the hepatic ischemia-reperfusion mouse model with different ischemia durations and chemiluminescence imaging after injection of CAR (10 μmol kg−1 body weight). b, c The dynamic NIR-II chemiluminescence intensities of liver and the dynamic NIR-I chemiluminescence intensities of kidney in living mice after injection of CAR. (n = 3, mean ± s.d.). Two-tailed Student’s t test. d, e Representative NIR-II and NIR-I chemiluminescence images of living mice subjected to liver ischemia with different durations (15, 30, 45 and 60 min) after injection of CAR (10 μmol kg−1 body weight). NIR-II chemiluminescence images were acquired with an acquisition time of 30 s. NIR-I chemiluminescence images were acquired under bioluminescence mode of the IVIS spectrum imaging system with the acquisition time of 60 s. f Online urinalysis: representative fluorescence images of excreted FL1R in the urine from living mice subjected to liver ischemia with different durations; Offline urinalysis: representative fluorescence images of CAR after incubation with the urine samples collected from living mice subjected to liver ischemia with different duration. g, i Change fold of NIRF intensity in urinalysis, ALT, AST, sCr, BUN, TNF-α and IL-6. h The cascaded injury scores and the complication index from living mice subjected to liver ischemia after injection of CAR. (n = 3, mean ± s.d.). Two-tailed Student’s t test. Healthy group versus control and experimental groups (**p < 0.01, ***p < 0.001, ****p < 0.0001). N.S.: no statistically significant differences. Source data are provided as a Source Data file.
Longitudinal chemiluminescence imaging was conducted after injection of CAR at 4 h post-reperfusion. For mice subjected to 15 min and 30 min of liver ischemia, the injection of CAR led to a gradual increase of NIR-II chemiluminescence signals in the liver (Fig. 6d), peaking at 30 min of post-injection (Fig. 6b). Such hepatic activation of CAR indicated the upregulation of O2•− during the progression of HIRI. At this time point, NIR-II chemiluminescence signals were 15.2-fold and 17.3-fold higher than that of the control mice, respectively. However, no obvious NIR-I chemiluminescence signal was observed in kidneys from mice subjected to 15 min and 30 min of liver ischemia (Fig. 6d), suggesting that the kidneys were unaffected under these conditions. For mice subjected to 45 min and 60 min of liver ischemia, similar trends in NIR-II chemiluminescence signal changes were observed in the liver, showing a 26.5-fold and 41.2-fold higher than that of the control group at 30 min post-injection of CAR, respectively (Fig. 6b). Notably, bright NIR-I chemiluminescence signals were observed in the kidneys (Fig. 6d), reflecting upregulated NAG detected by liberated KIR, which indicated the occurrence of HIRI-associated AKI. Importantly, the signals in the kidneys remained detectable even at 60 min post-injection of CAR. At 30 min post-injection of CAR, kidney signals from mice subjected to 45 min and 60 min of liver ischemia were 32.4-fold and 42.6-fold higher than that of the control group (Fig. 6c). By contrast, NIR-II chemiluminescence signals in the liver and NIR-I signals in the kidneys decreased by 4.8-fold and 1.2-fold when mice were pre-treated with NAC to protect the liver against IRI insult (Fig. 6b–d). Although O2•− is associated with lesion formation in physical trauma, sham-operated mice with only abdominal trauma showed negligible chemiluminescence increase in the liver and kidneys, showing the high specificity of CAR to report HIRI instead of other injuries. This should be ascribed to the liver-oriented accumulation of CAR, resulting in minimal probe accumulation in other traumatic tissues. Such observation was further confirmed by in vivo fluorescence imaging and ex vivo biodistribution studies (Supplementary Fig. 10–12). In addition, chemiluminescence imaging by CAR showed ~ 4.5 and ~ 4.2-fold higher SBRs at 45- and 60 min post-injection, respectively, compared to fluorescence imaging (Supplementary Fig. 13), due to the absence of tissue autofluorescence.
Moreover, HIRI-induced AKI was detected remotely through urinalysis using two CAR-based methods (Supplementary Fig. 14a): (i) online urinalysis, which involved direct NIR-I fluorescence analysis of urinary FL1R from mice subjected to liver ischemia after CAR injection; and (ii) offline urinalysis, which involved collecting urine samples from mice subjected to liver ischemia, followed by in vitro CAR incubation and fluorescence readout. In the online urinalysis (Fig. 6f, g and Supplementary Fig. 14b, c), no significant fluorescence enhancement was observed in mice subjected to 15 min and 30 min liver ischemia. However, the fluorescence increased by 10.8- and 14.3-fold when liver ischemia duration reached 45 min and 60 min, respectively. These fluorescence signal changes in urinary FL1R correlated well with the abovementioned chemiluminescence imaging results, as the excreted FL1R was activated by renal tubular NAG during the onset and progression of HIRI-induced AKI. The HPLC and mass spectra further confirmed the production of FL1R in urine (Supplementary Fig. 15). A similar trend was found in the offline urinalysis (Fig. 6f, g). CAR had no statistically significant enhancement after incubation with urine from mice subjected to 15- and 30 min liver ischemia, but the fluorescence increased by 4.3- and 6.5-fold after 45 and 60 min of liver ischemia. This occurs because NAG is normally secreted in very low concentrations from renal tubules into urine, but its secretion rises dramatically during the onset and progression of AKI61. In addition, urinary signals were reduced after pre-treatment with NAC or in sham-operation mice, which were consistent with the real-time in vivo imaging results.
To assess the correlation between the severity of HIRI and associated AKI, the kidney-to-liver ratio of signals (defined as cascaded injury score) and urinary-to-liver ratio of signals (termed as complication index) were calculated as a function of liver ischemia duration (Fig. 6h). The cascaded injury score was close to 0 when mice were subjected to 15 and 30 min liver ischemia but sharply increased to 42 and 47 when liver ischemia duration reached 45 min and 60 min, respectively (Fig. 6h). A similar trend was observed for complication index when increasing ischemia duration. These findings indicate that mice subjected to more than 45 min of liver ischemia develop severe AKI, and the severity of kidney damage correlates with the extent of liver injury (Fig. 7a). The diagnosis of HIRI induced-AKI was further confirmed by serum creatinine and BUN results (Fig. 6iand Supplementary Fig. 16).
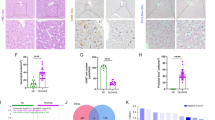
a Schematic illustration of the correlation between liver ischemia duration and the chemiluminescence signals in the liver and kidneys and fluorescence signals in urine. b Schematic illustration for the pathological mechanisms of HIRI-induced AKI. c Mean fluorescence intensity of anti-caspase-3 in the liver. (n = 3, mean ± s.d.). d Mean fluorescence intensity of FL1R in the kidney after injection of CAR. (n = 3, mean ± s.d.; a.u. means arbitrary units). e Representative photomicroscope images of H&E staining in paraffin-embedded liver and kidney sections and confocal fluorescence microscopy images of liver and kidney from mice subjected to liver ischemia with different duration, followed by 4 h reperfusion. The blue and green signals are from 4’,6-diamidino-2-phenylindole (DAPI) and caspase-3 antibody, respectively. The red signal is from FL1R. Two-tailed Student’s t test. Healthy group versus control and experimental groups (**p < 0.01, ***p < 0.001, ****p < 0.0001). NS: no statistically significant differences. Source data are provided as a Source Data file.
Clinical and preclinical assays were used to test the liver function (ALT and AST) and the level of proinflammatory cytokines, including tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) in sera of mice (Fig. 6i and Supplementary Fig. 17, 18). The ALT and AST had a negligible increase in mice subjected to 15 and 30 min liver ischemia relative to the control mice, but they significantly increased by 9.5- and 5.2-fold, 13.2- and 9.5-fold in mice subjected to 45 and 60 min liver ischemia. Elevated levels of serum pro-inflammatory cytokines TNF-α (8.5-, 10.5-, 26.5- and 35.9-fold) and IL-6 (3.5-, 5.6-, 18.6- and 22.5-fold) were observed in mice subjected to 15, 30, 45, and 60 min liver ischemia, implying a heightened inflammatory response. Although these cytokine levels were upregulated in mice subjected to liver ischemia, they also increased in sham-operated mice, suggesting that cytokine measurements have low specificity to diagnose HIRI. In comparison, CAR-based imaging is more sensitive than these assays and achieved the earliest prediction of HIRI. Another conventional assessment by histological analysis revealed noticeable loss of normal liver architecture and severe hepatocyte necrosis in mice subjected to 45 and 60 min liver ischemia followed by 4 h reperfusion (Fig. 7e); while no significant lesion was observed in mice subjected to 15 and 30 min liver ischemia, healthy control mice, NAC-pretreated mice, or sham-operation mice. Hepatocyte necrosis was observed when the reperfusion period was extended to 8 h for mice subjected to 15 and 30 min liver ischemia (Supplementary Fig. 19), while kidneys remained unaffected. Histological abnormalities in the kidneys were only seen in mice subjected to 45 and 60 min liver ischemia followed by 4 h reperfusion, showing marked vascular stasis in the interstitial capillary lumina and focal tubular dilatation with intraluminal shedding of cytoplasmic debris and formation of granular eosinophilic casts (Fig. 7e). While no abnormalities were found in other groups. No obvious histological changes in other major organs, including heart, spleen and lung, were found (Supplementary Fig. 20), consistent with previous studies that the kidneys are the most clinically affected organs in HIR, followed by lung, spleen and heart62. The proposed mechanisms behind this include the initiation of oxidative stress, the recruitment of leukocytes, intrarenal vasoconstriction, induced intrarenal ischemia, as well as renal tubular necrosis (Fig. 7b)1,2,3,4,62. Immunostaining of liver sections from mice subjected to 45 and 60 min ischemia displayed strong green fluorescence for caspase-3, signifying the apoptosis of hepatocytes after liver ischemia (Fig. 7e). The intensities were ~ 4.8-, and 6.6-fold higher than that of control mice (Fig. 7c). NIR-I fluorescence signals of FL1R in the kidney sections from mice subjected to 45 and 60 min liver ischemia were ~ 6.5-fold and 8.8-fold higher than that of control mice (Fig. 7d) while no or slightly enhancement was found in other groups, consistent with the real-time in vivo imaging results.
Multiplex imaging of HIRI and HIRI-induced AKI using two single-channel probes LIR and KIR
We then compared the performance of the unimolecular CAR with the co-injection of two monochromatic probes, LIR and KIR. The latter were simultaneously coadministered into mice subjected to liver ischemia at 4 h post-reperfusion (Fig. 8a), followed by longitudinal chemiluminescence imaging. In mice subjected to 30 min liver ischemia, co-injection of LIR and KIR led to a gradual NIR-II chemiluminescence signals increase in the liver, reaching their peaks at 30 min after LIR injection and decreasing at later time points (Fig. 8b, c). The liver signal was 21.8-fold higher than that in healthy controls at 30 min post-LIR injection (Fig. 8c), indicating O2•− upregulation during HIRI progression. A similar trend was observed in the liver of mice subjected to 45 and 60 min liver ischemia, while the liver signals increased by 29.6- and 41.3-fold relative to that of control mice (Fig. 8c). Ex vivo biodistribution studies indicated consistent results (Supplementary Fig. 21, 22).
a Schematic illustration of the development of the hepatic ischemia-reperfusion mouse model with different ischemia durations and chemiluminescence imaging after simultaneously injection of LIR and KIR (10 μmol kg−1 body weight). b Representative NIR-II and NIR-I chemiluminescence images of living mice subjected to different ischemia durations (30, 45 and 60 min) after injection of LIR and KIR (10 μmol kg−1 body weight). NIR-II chemiluminescence images were acquired with an acquisition time of 30 s. NIR-I chemiluminescence images were acquired under bioluminescence mode of the IVIS spectrum imaging system with the acquisition time of 60 s. c The dynamic NIR-II chemiluminescence intensities of liver (n = 3, mean ± s.d.). d the dynamic NIR-I chemiluminescence intensities of the kidney in living mice after injection of LIR and KIR (10 μmol kg−1 body weight). (n = 3, mean ± s.d.). Two-tailed Student’s t test. Healthy group versus control and experimental groups (**p < 0.01, ***p < 0.001, ****p < 0.0001). N.S: no statistically significant differences. e A scheme diagram of cascade intrahepatic and intrarenal activation of CAR from blood circulation and following excretion. Injected CAR can firstly react with O2•− to undergo in situ fragmentation and release FL2R and KIR. The resulted FL2R would excreted through the hepatobiliary elimination pathway (liver→bile→intestine→feces); released KIR can accumulate in the kidneys and activated by upregulated NAG, liberating FL1R for non-invasive urinalysis (kidney→bladder→urine). f A scheme diagram of simultaneous injection of LIR and KIR and their independent intrahepatic and intrarenal activation from blood circulation and following excretion. Injected LIR can react with O2•− and excrete through the hepatobiliary elimination pathway; injected KIR can accumulate in the kidneys and activated by upregulated NAG, releasing FL1R for non-invasive urinalysis. g Comparison of the entire imaging window period of CAR and co-injection of LIR and KIR. Source data are provided as a Source Data file.
In mice subjected to 30 min liver ischemia, NIR-I chemiluminescence signals in the kidneys were almost undetectable, but they increased dramatically when liver ischemia duration increased to 45 and 60 min (Fig. 8b, d). However, the maximum kidney signals appeared at 15 min post-injection of KIR and rapidly decreased to background levels at 30 min post-injection, which differs from the performance of CAR that the maximum kidney signals were observed at 30 min post-injection and remained detectable even at 60 min (Fig. 6c). The short kidney imaging window for KIR is likely attributed to its short plasma elimination half-life (t1/2β) of ~ 25 min after a bolus injection (Figs. 5h and 8f). In contrast, CAR, with its cascade activation mechanism, acted as a reservoir to release AKI reporters (KIR) upon reaction with O2•−, which circumvented its rapid clearance issue (Fig. 8e) and resulted in an extended imaging window that aligned with O2•− detection (Fig. 8g). Therefore, CAR outperforms coadministration of LIR and KIR for real-time and simultaneous imaging of HIRI and AKI in the liver-kidney axis.
Discussion
AKI is a common complication in the setting of HIRI; however, in vivo simultaneous optical imaging of HIRI and HIRI-induced AKI remains challenging because of the lack of unimolecular dual-responsive probes. Despite substantial progress made recently in developing dual-locked probes, their use for simultaneous detection of two intercorrelated diseases progress remains underexplored. To address this, unimolecular dual-responsive probes with spontaneous pharmacokinetic conversion for probing in multiple organs provide a promising strategy. To this end, we design a library of HCLs with tunable, long-wavelength chemiluminescence emission by incorporating benz[e]indolium, benz[c,d]indolium, naphtho[c,d]indolium and thiophen-benz[c,d]indolium moieties onto xanthonium-based bicyclic dioxetane to enlarged π conjugation (Supplementary Table 1). The representative chemiluminophores HCL1 and HCL5 with crosstalk-free emissions at 725 nm and 1025 nm are then respectively caged with NAG- and O2•− -responsive moieties and hooked together through a renal clearance unit to obtain a unimolecular cascaded activatable reporter CAR. In mouse models, CAR demonstrates liver-oriented accumulation and enables NIR-II CL imaging specific to HIRI-associated O2•−, which in turn initiates the in situ fragmentation of CAR and liberates KIR to enter the kidneys to image AKI-associated NAG via NIR-I CL. Notably, this cascaded activation allows CAR to act as a reservoir to slowly release KIR, which extends the imaging window for AKI detection and enables a synchronized window for both HIRI and associated AKI detections. Such alignment provides a more accurate diagnosis for the onset of complications, which is not possible with the coadministration of two separate probes, LIR and KIR. Furthermore, CAR permits remote detection of HIRI-induced AKI through either directly measuring excreted FL1R or incubating CAR with urine, serving as a ‘urine fingerprint’ to sensitively reflect the severity of HIRI. Thereby, CAR offers a powerful tool for diagnosing HIRI and HIRI-induced AKI in clinics, either through imaging inspections or point-of-care testing.
In summary, this study highlights a unimolecular dual-responsive chemiluminescent probe with crosstalk-free record-long emissions and switchable pharmacokinetics for the simultaneous diagnosis of two intercorrelated diseases. The flexible molecular design can be feasibly tailored to detect a diverse range of biomarkers, not only expanding the probe-box available for detection of intercorrelated diseases beyond the liver-kidney axis (for example, chemotherapy and its associated AKI, or interlinked diseases in the gut-liver axis) but also contributing to more accurate and early diagnosis of these diseases in clinical settings.
Methods
General Information
All chemicals were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai Bide Pharmatech Ltd, Energy Chemical Co., Ltd., Shanghai Macklin Biochemical Co., Ltd., unless otherwise stated. ALT activity assay kit, AST activity assay kit, IL-6 activity assay kit, TNF-α activity assay kit, creatinine assay kit and urea assay kit were purchased from Sigma-Aldrich. Silica gel (200–300 mesh) was used for column chromatography. Cleaved-caspase-3 antibody was purchased from Cell Signaling Technology. TLC was conducted on silica gel HSGF254 glass plates. UV-Vis absorption and fluorescence spectra were measure on a Shimadzu UV2600 spectrometer and a HORIBA Fluoromax-4 fluorescence spectrophotometer, respectively. Chemiluminescence was recorded on SpectraMax i3x (Molecular Devices, USA). HPLC was performed on a Shimadzu LC-16P system equipped with an Ascentis C18 HPLC Column (25 cm × 21.2 mm, 5 μm) using gradient elution. Proton-nuclear magnetic resonance (1H NMR) was conducted with a Bruker 400 MHz NMR instrument. Chemical shifts are reported in ppm relative to residual protic solvent resonances. Mestre Nova LITE v12.0.1.20560 software (Mestre lab Research S.L.) was used to analyze the NMR spectra. All the NMR spectra and Mass spectra are provided in Supplementary Figs. 23–109. Electrospray ionization-mass spectrometry (ESI-MS) spectra were obtained on a Waters SQD2 with Waters H-Class UPLC equipped with a standard ESI source. Tissues were cut into sections using a cryostat (Leica, Germany) and examined using a Nikon ECLIPSE 80i microscope (Nikon Instruments Inc, USA). Confocal microscopy images of tissue sections were acquired on a FV3000 confocal laser scanning microscope (Olympus Corporation, Japan). NIR-I fluorescence and chemiluminescence imaging were performed on an IVIS spectrum imaging system (PerkinElmer, Inc, USA). NIR-II fluorescence and chemiluminescence imaging were carried out on a NIR-II imaging system (Shanghai Didi-United Biotech) at a power of 4 W with the detector of InGaAs, the light filter default to 1000 nm. Blood and urine samples were collected using heparinized capillary tubes and metabolic cages, respectively. MALDI-TOF analysis was performed on a Bruker ultraflex TOF/TOF instrument. All the images and elements in Figs. 1a, b, c, 5a, g, 6a, 7a, 8a, e, f, g and Supplementary Figs. 1a, 14a were created by PowerPoint, and no permission proof or license was required.
Synthesis of compound 1
Compound 1 was prepared according to the literature procedure63. To a solution of 3,5-dimethoxybenzyl bromide (916 mg, 4 mmol) and compound 21 (800 mg, 5 mmol) in dry THF (20 mL) was added sodium hydride (240 mg, 10 mmol) at 0 °C, then the reaction mixture was stirred at room temperature overnight under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the intermediate as a colorless oil (1.18 g, yield of 95%). A solution of the intermediate (620 mg, 2 mmol) and PCC (473 mg, 2.2 mmol) in DCM (20 mL) was stirred at room temperature overnight under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 1 as a yellowish oil (604 mg, yield of 98%). 1H NMR (400 MHz, CDCl3) δ 6.45 (d, J = 2.4 Hz, 2H), 6.36 (t, J = 2.0 Hz, 1H), 4.43 (s, 2H), 3.78 (s, 6H), 3.49 (s, 2H), 1.28 (s, 6H), 1.24 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 170.58, 160.77, 141.22, 105.07, 99.44, 82.51, 77.81, 73.03, 55.25, 41.17, 37.00, 28.61, 23.47, 20.94. ESI-MS (m/z): [M + H]+ Calcd for C18H28O4 :308.20; Found 308.41.
Synthesis of compound 2
Compound 2 was prepared according to literature procedure63. To a solution of compound 1 (308 mg, 1 mmol) in dry THF (10 mL) was added lithium diisopropylammonium (2 M in THF, 1.5 mL) at − 78 °C for 2 h under an Ar atmosphere. The reaction mixture was quenched with cold H2O (15 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 2 as a yellowish oil (227 mg, yield of 74%). 1H NMR (400 MHz, CDCl3) δ 6.71 (d, J = 2.4 Hz, 2H), 6.37 (t, J = 2.4 Hz, 1H), 4.96 (s, 1H), 3.90 (d, J = 8.8 Hz, 1H), 3.78 (s, 6H), 3.69 (d, J = 8.8 Hz, 1H), 1.92 (s, 1H), 1.39 (s, 3H), 1.19 (s, 3H), 0.92 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 160.30, 149.86, 137.97, 125.29, 107.95, 100.50, 83.12, 55.34, 47.15, 32.42, 27.43. ESI-MS (m/z): [M+Na]+ Calcd for C18H28O4 :331.20; Found 331.42.
Synthesis of compound 3
Compound 3 was prepared according to the literature procedure63. To a solution of compound 2 (308 mg, 1 mmol) in dry DCM (10 mL) was added silver trifluoromethanesulfonate (258 mg, 1 mmol) and stirred for 30 min, followed by the addition of 1,1-dichlorodimethyl ether and stirred for 2 h under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 3 as a white solid (210 mg, yield of 68%). 1H NMR (400 MHz, CDCl3) δ 10.27 (s, 1H), 7.15 (d, J = 2.4 Hz, 1H), 6.41 (d, J = 2.4 Hz, 1H), 5.39 (s, 1H), 5.09 (s, 1H), 3.90 (d, J = 4.8 Hz, 6H), 3.68 (dd, J = 20.4, 8.8 Hz, 2H), 1.37 (s, 3H), 1.26 (s, 3H), 0.86 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 189.98, 165.37, 165.24, 146.31, 117.96, 106.93, 97.04, 88.96, 88.59, 79.69, 55.98, 55.63, 49.06, 40.75, 26.44, 23.83. ESI-MS (m/z): [M + Na]+ Calcd for C19H28O5 :359.19; Found 359.50.
Synthesis of compound 4
Compound 4 was prepared according to the literature procedure63. A solution of compound 3 (336 mg, 1 mmol), BBr3 (500 mg, 2 mmol) and dry DCM (5 mL) was stirred at 0 °C for 2 h under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 4 as a yellow solid (226 mg, yield of 78%). 1H NMR (400 MHz, CDCl3) δ 11.97 (s, 1H), 9.83 (s, 1H), 6.35 (d, J = 4.4 Hz, 2H), 3.91 (s, 2H), 1.35 (s, 6H), 1.07 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 159.28, 156.72, 147.76, 138.89, 126.05, 108.17, 99.79, 83.41, 56.34, 55.62, 47.17, 32.56, 31.70, 27.03. ESI-MS (m/z): [M + H]+ Calcd for C17H22O4 :291.15; Found 291.26.
Synthesis of compound 5
A solution of compound 4 (290 mg, 1 mmol), Cs2CO3 (325 mg, 1 mmol), compound 25 (188 mg, 1 mmol) and DMF (3 mL) was stirred at room temperature for 12 h under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 4 as a yellow solid (137 mg, yield of 36%). 1H NMR (400 MHz, CDCl3) δ 10.35 (s, 1H), 6.72 (s, 1H), 6.63 (d, J = 2.0 Hz, 1H), 6.59 (s, 1H), 3.91 (s, 1H), 2.62 – 2.56 (m, 2H), 2.47 (t, J = 5.6 Hz, 2H), 1.74 (dd, J = 6.7, 3.2 Hz, 4H), 1.57 (s, 6H), 1.06 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 200.80, 166.52, 160.75, 141.15, 105.14, 99.45, 83.89, 78.22, 73.01, 55.27, 50.07, 41.15, 37.05, 30.48, 25.66, 23.56, 23.30. HRMS (m/z): [M]+ Calcd for C24H28O4 :380.1988; Found 380.1623.
General procedure for the synthesis of compound HCLs
A solution of compound 5 (380 mg, 1 mmol), EtOH (10 ml), AcONa (82 mg, 1 mmol) and indole derivatives (1 mmol) was stirred at 60 °C for 2 h under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the intermediate. A solution of intermediate (0.1 mmol), methylene blue (5 mg, 0.015 mmol) and methanol (10 mL) was stirred and inserted an air pump needle into the mixed solution to blow O2 in an ice bath for 2 h under white light. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure without purification to afford HCLs.
Compound HCL1
Purplish blue solid (368 mg, yield of 68%). 1H NMR (400 MHz, DMSO-d6) δ 8.60 (d, J = 15.2 Hz, 1H), 7.79 (d, J = 7.2 Hz, 1H), 7.69 (d, J = 16.4 Hz, 1H), 7.55 (s, 2H), 7.49 (d, J = 8.0 Hz, 1H), 6.99 (s, 1H), 6.92 (d, J = 10.4 Hz, 1H), 6.54 (d, J = 14.8 Hz, 1H), 3.89 (s, 3H), 2.72 (d, J = 20.0 Hz, 4H), 1.86 (s, 2H), 1.79 (s, 6H), 0.88 (s, 17H). 13C NMR (101 MHz, DMSO) δ 160.83, 155.18, 143.05, 141.91, 135.29, 132.19, 132.06, 129.82, 129.13, 126.54, 122.96, 116.69, 115.07, 114.41, 112.77, 102.53, 67.88, 50.11, 40.64, 40.43, 40.22, 40.01, 39.80, 39.59, 39.38, 38.56, 32.57, 30.27, 28.83, 28.65, 27.88, 27.88, 24.13, 23.72, 22.86, 20.63, 14.36, 11.27. HRMS (m/z): [M]+ Calcd for C36H42NO5+ :568.3507; Found 568.0858.
Compound HCL2
Purplish blue solid (482 mg, yield of 78%). 1H NMR (400 MHz, DMSO-d6) δ 8.62 (d, J = 8.0 Hz, 1H), 8.44 (d, J = 8.4 Hz, 1H), 8.34 (d, J = 8.8 Hz, 1H), 8.25 (d, J = 11.6 Hz, 2H), 8.10 (d, J = 8.8 Hz, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.81 (s, 2H), 7.74 (d, J = 15.2 Hz, 1H), 7.35 (s, 1H), 3.97 (s, 3H), 2.96 (s, 4H), 2.03 (s, 2H), 1.97 (s, 6H), 0.88 (s, 17H). 13C NMR (101 MHz, DMSO) δ 188.13, 182.37, 148.54, 147.95, 143.97, 139.11, 136.72, 136.58, 135.45, 133.65, 133.09, 132.51, 132.29, 132.17, 131.60, 130.95, 130.00, 129.74, 129.64, 129.48, 129.06, 126.08, 125.93, 124.65, 116.27, 113.09, 105.24, 103.59, 97.26, 57.60, 57.49, 32.63, 26.39, 26.21, 20.49. HRMS (m/z): [M]+ Calcd for C40H44NO5+ :618.3214; Found [M + H]+ 619.3244.
Compound HCL3
Purplish green solid (294 mg, yield of 51%). 1H NMR (400 MHz, DMSO-d6) δ 9.09 (d, J = 14.8 Hz, 1H), 8.75 (d, J = 7.6 Hz, 1H), 8.51 (d, J = 8.0 Hz, 1H), 8.09 (s, 1H), 7.99 (s, 1H), 7.88 (s, 1H), 7.57 (s, 1H), 7.43 – 7.35 (m, 1H), 7.23 (s, 1H), 7.14 – 7.07 (m, 1H), 6.99 (d, J = 14.8 Hz, 1H), 4.57 (s, 3H), 1.87 (s, 4H), 1.56 – 1.50 (m, 2H), 1.23 (s, 17H). 13C NMR (101 MHz, DMSO) δ 152.08, 148.07, 143.45, 141.53, 129.56, 128.90, 128.61, 123.46, 120.28, 114.95, 111.48, 61.49, 53.67, 51.82, 35.49, 35.29, 32.44, 32.05, 29.45, 28.12, 27.64, 27.42, 26.77, 25.55, 24.90, 24.07, 20.06, 12.25. HRMS (m/z): [M]+ Calcd for C37H38NO5+ :576.2477; Found 576.5732.
Compound HCL4
Purplish brown solid (206 mg, yield of 33%). 1H NMR (400 MHz, DMSO-d6) δ 9.19 (d, J = 7.2 Hz, 1H), 9.10 (d, J = 14.8 Hz, 1H), 8.97 (d, J = 8.4 Hz, 1H), 8.63 (s, 1H), 8.44 (d, J = 7.8 Hz, 1H), 8.26 (d, J = 6.4 Hz, 1H), 8.14 (d, J = 7.6 Hz, 1H), 8.09 (d, J = 5.2 Hz, 1H), 7.89 (d, J = 8.4 Hz, 1H), 7.83 (d, J = 3.6 Hz, 1H), 7.61 (s, 1H), 7.56 (m, 1H), 7.31 (d, J = 15.2 Hz, 1H), 3.80 (s, 3H), 2.07 (s, 4H), 1.65 (s, 2H), 1.03 (s, 17H). 13C NMR (101 MHz, DMSO) δ 172.76, 143.56, 142.74, 142.51, 141.56, 141.09, 130.05, 129.11, 126.75, 125.68, 125.03, 123.04, 122.72, 122.54, 116.55, 112.02, 110.90, 102.16, 50.72, 50.66, 49.50, 48.61, 29.32, 28.07, 27.97, 26.35, 26.06, 25.96, 24.78), 24.62, 24.38, 23.85, 21.56. HRMS (m/z): [M]+ Calcd for C41H40NO5+ :626.2744; Found 625.1776.
Compound HCL5
Purplish brown solid (157 mg, yield of 24%). 1H NMR (400 MHz, DMSO-d6) δ 8.53 (d, J = 13.2 Hz, 1H), 8.24 (dd, J = 16.0, 7.8 Hz, 2H), 7.78 (s, 2H), 7.54 (dd, J = 13.6, 6.3 Hz, 2H), 7.36 (d, J = 8.4 Hz, 1H), 7.28 (s, 1H), 7.23 (d, J = 7.6 Hz, 1H), 7.13 – 7.08 (m, 1H), 6.73 (s, 1H), 6.39 (d, J = 12.4 Hz, 1H), 4.06 (s, 3H), 2.32 (s, 4H), 1.27 (s, 2H), 1.05 (s, 17H). 13C NMR (101 MHz, DMSO) δ 178.22, 160.86, 160.79, 153.90, 142.72, 141.77, 133.41, 129.34, 129.24, 127.89, 127.62, 123.29, 116.69, 115.03, 114.50, 113.76, 105.07, 103.91, 101.75, 76.45, 73.80, 70.66, 68.48, 60.90, 50.99, 50.61, 49.03, 44.76, 28.88, 28.03, 27.96, 25.86, 25.21, 23.97, 20.40. HRMS (m/z): [M]+ Calcd for C41H40NO5S+ :658.2622; Found [M + H]+ 659.2445.
Synthesis of compound 8
A solution of compound 6 (62 mg, 0.1 mmol), compound 22 (41 mg, 0.1 mmol), K2CO3 (27 mg, 0.2 mmol), Cs2CO3(33 mg, 0.1 mmol) and DCM (3 mL) was stirred at room temperature for 2 h under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure to afford the intermediate. It could be directly put into the next step of the reaction without purification. A solution of the intermediate (0.1 mmol), NaOMe (27 mg, 0.5 mmol) and DCM (3 mL) was stirred at room temperature for 1 h under an Ar atmosphere. The reaction mixture was quenched with NH4Cl (50 mL) and extracted with DCM for three times. Compound 7 was afforded by reduced-pressure concentration. A solution of compound 7 (41 mg, 0.05 mmol), SH-PEG2000-alkyne (85 mg, 0.06 mg), sodium ascorbate (20 mg, 0.1 mmol), CuSO4 (16 mg, 0.1 mmol), H2O (2 mL) and THF (2 mL) was stirred at room temperature for 2 h. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The crude product was concentrated by cooling under reduced pressure and purified by HPLC to afford compound 8 as a blue solid. 1H NMR (400 MHz, D2O) δ 8.52 (d, J = 13.6 Hz, 1H), 7.65 (d, J = 7.2 Hz, 1H), 7.50 (dd, J = 17.6, 10.2 Hz, 2H), 7.35 (d, J = 8.0 Hz, 1H), 7.15 (s, 1H), 7.02 (d, J = 11.6 Hz, 2H), 6.36 (d, J = 15.2 Hz, 1H), 4.22 (s, 15H), 3.89 (s, 16H), 3.68 (s, 600H), 3.29 (t, J = 6.4 Hz, 4H), 2.70 – 2.58 (m, 12H), 1.81 (d, J = 6.0 Hz, 3H), 1.71 (d, J = 14.4 Hz, 6H), 0.87 (d, J = 15.2 Hz, 17H). 13C NMR (101 MHz, DMSO) δ 177.16, 162.76, 162.20, 161.54, 154.60, 144.99, 142.30, 142.19, 141.93, 141.93, 135.65, 134.97, 134.68, 129.72, 129.32, 127.18, 126.23, 124.87, 123.26, 115.13, 114.88, 114.26 – 114.07, 113.43, 103.98, 102.41, 81.85, 73.39, 69.23, 63.95, 57.77, 53.66, 51.03, 50.63, 36.25, 31.23, 30.22, 28.77, 28.51, 28.55 – 27.84, 28.06, 27.61, 26.31, 26.02, 25.26, 24.08, 20.48, 19.57, 18.21, 13.29, 1.64. MALDI-TOF MS found: ~ 2951.631.
Synthesis of compound 10
A solution of compound 9 (63 mg, 0.1 mmol), compound 23 (30 mg, 0.1 mmol), compound 24 (20 mg, 0.1 mmol), triphosgene (90 mg, 0.3 mmol), DMAP (25 mg, 0.2 mmol) and DCM (5 mL) was stirred at 0 °C for 2 h under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure to afford compound 10 (100 mg, yield of 85%. It could be directly put into the next step of the reaction without purification. 1H NMR (500 MHz, DMSO-d6) δ 8.58 (d, J = 12.5 Hz, 1H), 8.29 (d, J = 7.0 Hz, 1H), 8.24 (d, J = 8.0 Hz, 1H), 8.11 (s, 1H), 7.80 (s, 1H), 7.77 (s, 1H), 7.54 (d, J = 4.5 Hz, 2H), 7.38 (d, J = 8.5 Hz, 1H), 7.27 (s, 1H), 7.25 (s, 1H), 7.23 (s, 1H), 7.10 (s, 1H), 6.73 (d, J = 8.5 Hz, 2H), 6.52 – 6.47 (m, 1H), 6.41 (d, J = 13.5 Hz, 1H), 4.09 (s, 3H), 2.66 – 2.58 (m, 6H), 2.33 (s, 4H), 1.70 (s, 2H), 1.62 (s, 2H), 1.53 (s, 2H), 1.05 (s, 17H). 13C NMR (101 MHz, DMSO) δ 190.75, 184.98, 151.22, 150.59, 146.60, 141.72, 139.35, 139.27, 139.18, 138.09, 136.24, 135.72, 135.14, 134.91, 134.81, 134.22, 133.66 – 133.45, 132.64, 132.27, 132.09, 131.18, 128.68, 128.52, 127.25, 118.87, 115.68, 107.83, 106.20, 43.77, 39.54, 36.48, 35.26, 35.17, 31.70, 31.52, 30.85, 30.13, 29.03, 28.84. HRMS (m/z): [M]+ Calcd for C63H65F3N3O12S2+ :1176.3800; Found [M + H]+ 1177.4300.
Synthesis of compound CAR
A solution of compound 8 (0.1 mmol), compound 10 (0.1 mmol), Et3N (0.5 mL) and THF (3 mL) was stirred at room temperature for 3 h under an Ar atmosphere. The reaction mixture was concentrated under reduced pressure to afford the intermediate. A solution of intermediate (0.1 mmol), methylene blue (5 mg, 0.015 mmol) and methanol (10 mL) was stirred and inserted an air pump needle into the mixed solution to blow O2 in ice bath for 2 h under white light. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined water layer was concentrated under reduced pressure and purified by HPLC to afford compound CAR. 1H NMR (400 MHz, DMSO-d6) δ 8.63 (s, 1H), 8.18 (d, J = 8.0 Hz, 14H), 7.68 (s, 5H), 7.56 (s, 2H), 7.49 (d, J = 11.6 Hz, 1H), 6.97 (s, 2H), 6.55 (d, J = 14.8 Hz, 1H), 4.23 (s, 119H), 3.91 (s, 36H), 3.66 (dd, J = 8.4, 4.8 Hz, 70H), 3.41 (s, 39H), 3.24 (s, 32H), 1.95 (s, 70H), 0.88 (s, 40H). 13C NMR (126 MHz, DMSO) δ 188.12, 183.99, 182.38, 165.71, 158.87, 158.70, 148.60, 147.96, 144.00, 142.71, 139.11, 136.74, 136.58, 135.47, 133.64, 133.11, 132.53, 132.31, 132.27, 132.19, 131.62, 130.95, 130.02, 129.74, 129.66, 129.49, 129.23, 128.91, 126.08, 125.92, 124.65, 116.28, 113.09, 105.23, 103.61, 84.10, 75.60, 71.54, 71.54, 66.23, 65.98, 62.41, 62.18, 58.78, 57.61, 57.56, 47.67, 41.18, 40.15, 37.20, 36.93, 33.87, 32.60, 32.79 – 32.15, 32.47, 29.09, 28.93, 28.64, 28.40, 28.22, 28.17, 28.07, 27.52, 26.41, 26.22, 23.17, 21.27, 20.77, 20.63, 20.39, 20.26. MALDI-TOF MS found: ~4158.
Synthesis of compound LIR
A solution of compound 9 (63 mg, 0.1 mmol), trifluoromethane-sulfonic anhydride (30 mg, 0.1 mmol), pyridine (0.5 mL) and DCM (3 mL) was stirred at room temperature for 2 h under an Ar atmosphere. The reaction mixture was concentrated under reduced pressure to afford the intermediate. A solution of intermediate (0.1 mmol), methylene blue (5 mg, 0.015 mmol) and methanol (10 mL) was stirred and inserted an air pump needle into the mixed solution to blow O2 in an ice bath for 2 h under white light. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The purification using column chromatography on silica gel was performed to afford the compound LIR (52 mg, yield of 66%). 1H NMR (400 MHz, DMSO-d6) δ 9.03 – 8.74 (m, 2H), 8.42 (s, 1H), 8.25 (s, 1H), 8.12 (s, 2H), 7.93 (s, 2H), 7.69 (s, 2H), 7.40 (s, 2H), 7.10 (s, 1H), 4.69 (s, 3H), 3.55 (s, 2H), 2.80 (s, 2H), 1.87 (d, J = 32.4 Hz, 4H), 1.50 (s, 6H), 0.88 (s, 9H). 13C NMR (101 MHz, DMSO) δ 178.22, 160.87, 160.83, 153.90, 145.71, 142.72, 141.77, 133.41, 129.29, 129.29, 127.92, 127.62, 123.28, 116.70, 115.02, 114.51, 113.75, 105.06, 103.93, 101.74, 76.45, 73.77, 70.65, 68.52, 60.93, 50.98, 50.60, 49.04, 44.75, 28.88, 28.02, 27.95, 25.86, 25.20, 23.96, 20.40. HRMS (m/z): [M]+ Calcd for C42H39F3NO7S2+ :790.2115; Found [M + H]+ 791.2142.
Synthesis of compound KIR
A solution of compound 8 (0.1 mmol), compound 24 (20 mg, 0.1 mmol), Et3N (0.5 mL) and THF (5 mL) was stirred at room temperature for 2 h under an Ar atmosphere. The reaction mixture was concentrated under reduced pressure to afford the intermediate. A solution of intermediate (0.1 mmol), methylene blue (5 mg, 0.015 mmol) and methanol (10 mL) was stirred and inserted an air pump needle into the mixed solution to blow O2 in an ice bath for 2 h under white light. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The purification using column chromatography on silica gel was performed to afford the compound KIR. 1H NMR (400 MHz, DMSO-d6) δ 8.68 (d, J = 14.8 Hz, 1H), 7.81 (s, 1H), 7.78 (s, 1H), 7.61 (d, J = 8.8 Hz, 2H), 7.50 (d, J = 7.2 Hz, 1H), 7.23 (s, 1H), 7.12 (d, J = 8.4 Hz, 1H), 6.66 (d, J = 14.8 Hz, 1H), 5.02 (d, J = 7.6 Hz, 1H), 4.51 (t, J = 7.2 Hz, 3H), 4.10 (s, 10H), 3.80 (s, 9H), 3.58 (d, J = 18.4 Hz, 822H), 3.29 (s, 9H), 3.11 (s, 3H), 1.81 (d, J = 6.8 Hz, 13H), 0.88 (s, 18H). 13C NMR (126 MHz, DMSO) δ 178.19, 170.34, 160.85, 160.81, 156.58, 156.43, 153.90, 145.68, 142.71, 141.75, 133.45, 129.34, 129.29, 127.87, 127.62, 123.29, 116.68, 115.04, 114.49, 113.74, 105.05, 103.89, 101.75, 81.84, 76.45, 73.79, 70.64, 69.29, 69.22, 68.47, 63.95, 63.66, 60.89, 50.98, 50.59, 49.03, 44.73, 44.23, 30.19, 27.98, 27.94, 25.85, 25.21, 24.09, 20.40. MALDI-TOF MS found: ~ 3147.782.
Synthesis of compound 11
Compound 11 was prepared according to literature procedure64. A solution of 2,3,3-Trimethylindolenine (160 mg, 1 mmol) and methyl iodide (288 mg, 2 mmol) was stirred at room temperature for 6 h under an Ar atmosphere. The mixture was added to the diethyl ether (50 mL), the solid was filtered and washed with diethyl ether for three times to afford compound 11 (170 mg, yield of 98%). 1H NMR (400 MHz, DMSO-d6) δ 7.92 (dd, J = 6.0, 3.2 Hz, 1H), 7.85 – 7.81 (m, 1H), 7.64 – 7.61 (m, 2H), 3.98 (s, 3H), 2.78 (s, 3H), 1.53 (s, 6H). 13C NMR (101 MHz, DMSO) δ 196.47, 142.09, 129.77, 129.29, 123.81, 115.64, 54.43, 35.41, 22.22, 14.93. ESI-MS (m/z): [M + H]+ Calcd for C12H16N+ :174.13; Found 174.17.
Synthesis of compound 12
A solution of 1,1,2-Trimethyl-1H-benzo[e]indole (209 mg, 1 mmol) and methyl iodide (288 mg, 2 mmol) was stirred at room temperature for 6 h under an Ar atmosphere. The mixture was added to the diethyl ether (50 mL), the solid was filtered and washed with diethyl ether for three times to afford compound 12 (197 mg, yield of 88%). 1H NMR (400 MHz, DMSO-d6) δ 8.37 (d, J = 8.4 Hz, 1H), 8.30 (d, J = 8.8 Hz, 1H), 8.22 (d, J = 8.0 Hz, 1H), 8.10 (d, J = 8.8 Hz, 1H), 7.81 – 7.76 (m, 1H), 7.73 (dd, J = 8.0, 1.2 Hz, 1H), 4.09 (s, 3H), 2.87 (s, 3H), 1.75 (s, 6H). 13C NMR (101 MHz, DMSO) δ 196.38, 139.97, 136.97, 133.48 – 132.68, 130.98, 130.22, 128.86, 127.59, 123.90, 113.67, 55.74, 35.74, 21.78, 14.68. ESI-MS (m/z): [M + H]+ Calcd for C16H18N+ :224.14; Found 224.17.
Synthesis of compound 13
Compound 13 was prepared according to the literature procedure64. A solution of benz[cd]indol-2(1H)-one (169 mg, 1 mmol), methyl iodide (288 mg, 2 mmol), THF (10 mL) and sodium hydride (96 mg, 4 mmol) was stirred at 0 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 13 (168 mg, yield of 92%). 1H NMR (400 MHz, CDCl3) δ 8.06 (d, J = 7.2 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.73 – 7.67 (m, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.47 (dd, J = 8.4, 7.0 Hz, 1H), 6.90 (d, J = 6.8 Hz, 1H), 3.45 (s, 3H). 13C NMR (126 MHz, MeOD) δ 138.89, 134.83, 131.20, 130.88, 129.65, 120.52, 32.96. ESI-MS (m/z): [M + H]+ Calcd for C12H9NO :183.07; Found 184.13.
Synthesis of compound 14
Compound 14 was prepared according to the literature procedure64. A solution of compound 13 (183 mg, 1 mmol), THF (5 mL) and methylmagnesium bromide (2.5 mL) was stirred at 50 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 14 (134 mg, yield of 74%). 1H NMR (400 MHz, DMSO-d6) δ 8.87 (d, J = 76.4 Hz, 2H), 8.47 (d, J = 23.6 Hz, 2H), 8.07 (d, J = 60.4 Hz, 2H), 4.22 (s, 3H), 3.20 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 130.64, 128.91, 128.57, 128.44, 126.70, 125.02, 124.10, 120.25, 104.62, 26.28. ESI-MS (m/z): [M + H]+ Calcd for C13H12N+ :182.10; Found 182.08.
Synthesis of compound 15
Compound 15 was prepared according to the literature procedure65. A solution of indolin-2-one (133 mg, 1 mmol), o-bromobenzaldehyde (368 mg, 2 mmol), Cs2CO3 (495 mg, 1.5 mmol) and DMSO (10 mL) was stirred at 120 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 15 (113 mg, yield of 52%). 1H NMR (400 MHz, DMSO-d6) δ 10.68 (s, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.53 (d, J = 6.0 Hz, 1H), 7.44 (t, J = 8.4 Hz, 1H), 7.24 (t, J = 7.2 Hz, 1H), 7.10 (d, J = 7.6 Hz, 1H), 6.88 (d, J = 7.6 Hz, 1H), 6.80 (t, J = 7.6 Hz, 1H). 13C NMR (101 MHz, DMSO) δ 168.56, 143.59, 135.29, 134.10, 133.43, 131.84, 131.08, 130.85, 129.67, 128.44, 123.61, 123.03, 121.76, 120.99, 110.76. ESI-MS (m/z): [M + H]+ Calcd for C15H9NO :219.07; Found 221.11.
Synthesis of compound 16
Compound 16 was prepared according to the literature procedure65. A solution of compound 15 (219 mg, 1 mmol), THF (5 mL) and methylmagnesium bromide (2.5 mL) was stirred at 50 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the intermediate compound. A solution of the intermediate compound (1 mmol), THF (5 mL) and methylmagnesium bromide (2.5 mL) was stirred at 50 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. It could be directly put into the next step of the reaction without purification. 1H NMR (500 MHz, DMSO) δ 7.82 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.45 (t, J = 7.5 Hz, 1H), 7.34 (t, J = 7.5 Hz, 1H), 7.11 (d, J = 7.5 Hz, 1H), 7.06 (d, J = 7.5 Hz, 1H), 6.88 (t, J = 7.5 Hz, 1H), 3.22 (s, 3H), 1.99 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 130.64, 128.91, 128.57, 128.44, 126.70, 125.02, 124.10, 120.25, 26.28. ESI-MS (m/z): [M + H]+ Calcd for C17H14N+ :232.11; Found [M+Na]+ 255.65.
Synthesis of compound 17
Compound 17 was prepared according to the literature procedure65. A solution of compound 13, N-Bromosuccinimide (178 mg, 1 mmol) and MeCN (5 mL) was stirred at room temperature under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 17 (179 mg, yield of 79%). 1H NMR (400 MHz, CDCl3) δ 8.15 (d, J = 8.4 Hz, 1H), 8.09 (d, J = 7.2 Hz, 1H), 7.80 (t, J = 6.4 Hz, 1H), 7.68 (d, J = 7.6 Hz, 1H), 6.76 (d, J = 7.6 Hz, 1H), 3.43 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 167.45, 139.64, 131.10, 130.11, 129.59, 128.66, 126.86, 125.93, 124.97, 113.98, 105.60, 26.32. ESI-MS (m/z): [M + H]+ Calcd for C12H8BrNO :260.18; Found 261.27.
Synthesis of compound 18
A solution of compound 17 (261 mg, 1 mmol), thiophene (168 mg, 2 mmol), K2CO3 (270 mg, 1 mmol), Pd(OAc)2 (22.4 mg, 0.1 mmol) and DMAC (10 mL) was stirred at 120 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 18 (111 mg, yield of 42%). 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 8.4 Hz, 1H), 8.10 (d, J = 6.8 Hz, 1H), 7.76 (d, J = 7.2 Hz, 1H), 7.59 (d, J = 7.2 Hz, 1H), 7.42 (d, J = 5.2 Hz, 1H), 7.28 (d, J = 2.8 Hz, 1H), 7.20 (dd, J = 5.2, 3.6 Hz, 1H), 6.93 (d, J = 7.2 Hz, 1H), 3.47 (s, 3H). 13C NMR (101 MHz, DMSO) δ 170.16, 167.43, 140.43, 139.09, 130.23, 130.18, 129.82, 128.69, 127.38, 127.10, 127.01, 126.31, 126.17, 125.22, 125.14, 106.69, 41.91. ESI-MS (m/z): [M + H]+ Calcd for C16H11NOS :265.06; Found 266.19.
Synthesis of compound 19
Compound 19 was prepared according to the literature procedure65. A solution of compound 18 (265 mg, 1 mmol), THF (5 mL) and methylmagnesium bromide (2.5 mL) was stirred at 50 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. It could be directly put into the next step of the reaction without purification.
Synthesis of compound 20
Compound 20 was prepared according to literature procedure65. A solution of compound 1-Methoxy-1-(trimethylsilyloxy)-2-methyl-1-propene (870 mg, 5 mmol), trimethylacetyl chloride (660 mg, 5.5 mmol), TiCl4 (1.13 g, 6 mmol) and dry DCM (20 mL) was stirred at 0 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 20 (178 mg, yield of 96%). 1H NMR (400 MHz, CDCl3) δ 3.72 (s, 3H), 1.39 (s, 6H), 1.19 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 52.51, 50.15, 43.50, 26.44, 25.07, 21.60, 19.95. ESI-MS (m/z): [M + H]+ Calcd for C10H18O3 :186.02; Found 187.24.
Synthesis of compound 21
Compound 21 was prepared according to literature procedure63. A solution of compound 20 (744 mg, 4 mmol) and LiAlH4 (444 mg, 12 mmol) in DCM (20 mL) was stirred at room temperature overnight under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure to afford compound 21 as a colorless oil (627 mg, yield of 98%). It could be directly put into the next step of the reaction without purification. 1H NMR (400 MHz, CDCl3) δ 3.52 (d, J = 10.8 Hz, 1H), 3.43 (d, J = 10.4 Hz, 1H), 3.30 (s, 1H), 2.99 (s, 2H), 1.04 (s, 9H), 1.04 (s, 3H), 1.03 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 85.41, 74.70, 40.44, 37.28, 28.48, 26.04, 25.51, 20.68. ESI-MS (m/z): [M + H]+ Calcd for C9H20O2 :160.15; Found 183.15.
Synthesis of compound 22
Compound 22 was prepared according to the literature procedure65. A solution of compound β-D-Glucosamine pentaacetate (389 mg, 1 mmol), HBr/HOAc (3.2 mL) and HOAc was stirred at 0 °C under an Ar atmosphere. The reaction mixture was quenched with sodium bicarbonate solution (50 mL) and extracted with DCM for three times. The crude product was concentrated, and it could be directly put into the next step of reaction without purification to afford compound 22 (359 mg, yield of 88%). 1H NMR (400 MHz, CDCl3) δ 5.85 (s, 1H), 5.71 (d, J = 8.8 Hz, 1H), 5.17 (dd, J = 21.2, 11.2 Hz, 2H), 4.29 (d, J = 9.2 Hz, 1H), 4.14 (d, J = 14.4 Hz, 1H), 3.84 (d, J = 9.6 Hz, 1H), 2.09 (s, 3H), 2.05 (s, 6H), 1.94 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 171.23, 170.68, 170.16, 169.56, 169.29, 92.57, 72.74, 72.64, 67.89, 61.71, 52.94, 23.14, 20.88, 20.64. ESI-MS (m/z): [M + H]+ Calcd for C14H20BrNO8 :409.04; Found 410.29.
Synthesis of compound 23
Compound 23 was prepared according to the literature procedure66. A solution of 2,6-tert-butyldimethylsilyl-4-methylphenyl trifluoro-methanesulfonate (528 mg, 1 mmol), TBAF (574 mg, 2.2 mmol) and THF (5 mL) was stirred at room temperature under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with EtOAc for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 23 (288 mg, yield of 96%). 1H NMR (400 MHz, CDCl3) δ 7.38 (s, 2H), 4.75 (s, 4H), 2.40 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 163.42, 141.19, 135.16, 124.07, 53.29, 21.02. ESI-MS (m/z): [M-H]− Calcd for C10H12F3O5S 300.03; Found [M + K]− 338.43.
Synthesis of compound 24
A solution of tert-butyl (6-aminohexyl) carbamate (216 mg, 1 mmol), maleic anhydride (100 mg, 1.1 mmol), NaOAc (82 mg, 1 mmol) and Ac2O (5 mL) was stirred at 120 °C under an Ar atmosphere. The reaction mixture was quenched with H2O (50 mL) and extracted with DCM for three times. The combined organic layer was concentrated under reduced pressure. The purification using column chromatography on silica gel was performed to afford the compound 24 (133 mg, yield of 68%). 1H NMR (400 MHz, CDCl3) δ 6.71 (s, 2H), 3.52 (t, J = 7.2 Hz, 2H), 2.35 (t, J = 7.2 Hz, 2H), 1.64 (dtd, J = 22.4, 15.1, 7.6 Hz, 6H), 1.35 (dd, J = 15.6, 8.4 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 179.65, 170.91, 134.08, 37.58, 33.79, 28.15, 26.10, 24.08. ESI-MS (m/z): [M + H]+ Calcd for C10H16N2O2 :196.12; Found [M+Na]+ 219.224.
Synthesis of compound 25
A solution of cyclohexanone (98 mg, 1 mmol), PBr3 (1 mL), DCM (5 mL) and DMF (1.5 mL) was stirred at 0 °C under an Ar atmosphere. The reaction mixture was quenched with sodium bicarbonate solution (50 mL) and extracted with DCM for three times. The crude product was concentrated, and it could be directly put into the next step of reaction without purification to afford compound 25 (165 mg, yield of 88%). 1H NMR (400 MHz, CDCl3) δ 10.02 (s, 1H), 2.79 – 2.71 (m, 2H), 2.31 – 2.24 (m, 2H), 1.80 – 1.73 (m, 2H), 1.73 – 1.65 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 193.69, 143.67, 135.23, 38.80, 24.97, 24.24, 21.06. ESI-MS (m/z): [M + H]+ Calcd for C7H9BrO :187.98; Found 188.05.
Preparation of stock solution
CAR (10 µM) was dissolved in PBS (10 mM, pH 7.4) to obtain a stock solution after filtration by using a syringe filter (0.22 μm). H2O2, HClO, and O2− stock solution was prepared by directly diluting H2O2, NaClO, and KO2, respectively. •OH was generated by Fenton reaction between H2O2 and Fe(ClO4)2. Stock solutions of NaCl, KCl, MgSO4, glutathione, β-galcatosidase, gamma-glutamyl transferase and N-acetyl-β-D-glucosaminidase were prepared with distilled water.
Sensing capability
The CAR solution (20 µM) was incubated with KO2 (80 µM) and NAG (40 mU) in PBS (10 mM, pH = 7.4). Absorption and fluorescence spectra of the solution were measured on a UV-VIS and fluorescence spectrophotometer. NIR-I fluorescence images were acquired using the IVIS spectrum imaging system with the excitation at 675 ± 10 nm, the emission at 720 ± 10 nm and the acquisition time of 0.1 s. NIR-II fluorescence images were acquired using the NIR-II imaging system with the excitation at 808 nm, the emission at 1000 ± 10 nm and the acquisition time of 0.1 s. Chemiluminescence images were acquired under bioluminescence mode with an open filter and an acquisition time of 30 s.
Selectivity studies
The CAR solution (20 µM) was incubated ROS (80 µM), metal ions (80 µM) and enzymes including glutathione (1.0 U), β-galcatosidase (1.0 U), gamma-glutamyl transferase (1.0 U) and N-acetyl-β-D-glucosaminidase (40 mU) in HEPES buffer (50 mM, pH 7.4) or PBS (10 mM, pH 7.4) at 37 °C for 120 min. Fluorescence and chemiluminescence enhancements of CAR were measured after incubation.
The limit of detection (LOD)
Chemiluminescence intensities of CAR (20 µM) were determined after the addition of different concentrations of KO2. The LOD (Limit of Detection) was calculated based on the equation: LOD = 3σ/k, where σ is the standard deviation of emission intensity of blank, and k is the slope of the plot of emission intensities against the concentration of KO2.
Chemiluminescence kinetic profiles
KO2 (80 µM) was added into the CAR solution (20 µM) in PBS (10 mM, pH = 7.4). Chemiluminescence intensities were continuously acquired and plotted as a function of time.
Enzymatic kinetics assays
CAR (10, 20, 30, 60 and 90 µM) were incubated with NAG (10 mU) at 37 °C. The cleavage compound was quantified using HPLC after incubation. The initial reaction velocity (µM min−1) was calculated, plotted against the concentration of CAR and fitted to a Michaelis-Menten curve. The kinetic parameters were calculated by the following equation: V = Vmax[S] / (Km + [S]), where V is the initial velocity, and [S] is the substrate concentration.
Thermal stability
BD-OTf or AD-OTf (20 µM) was dissolved in FBS solution and placed in 13 tubes with the same concentration and stored at room temperature in dark. Chemiluminescence intensities were recorded for each sample after the addition of KO2 (80 µM) at different storage time points.
pH Stability
CAR solution (20 µM) was incubated in different buffer solution with a pH range from 4.0 to 8.5 at 37 °C for 3 h. Fluorescence intensity of CAR was measured on IVIS spectrum imaging system or NIR-II imaging system after incubation.
Histological examination
All tissues were fixed with 4% paraformaldehyde (PFA), dehydrated in ethanol solution, embedded in paraffin, and cut into 10 μm thick sections for H&E staining. Paraffin was removed by washing with xylene, and sections were incubated with hematoxylin for 4 min and eosin for 2 min, followed by washing with distilled water. Stained sections were examined using the FL Automated Imaging System + EVOS® on-stage incubator. Mouse peritoneum was snap frozen in OCT (Tissue-Tek) and stored at − 20 °C until sectioning. Frozen samples were cryosected (7-8 μm) using a freezer equipped with a cryotape transfer system and a tungsten blade. Sections were dried at room temperature for 2-4 h and then frozen at − 20 °C until staining. Prior to staining, sections were fixed with 100% acetone at − 20 °C for 10 min, dried at 37 °C for 5 min, and closed with 10% goat serum (Boster 17I27C04) for 90 min at room temperature, followed by washing with PBS. Sections were then first incubated with rat anti-mouse Ly-6G (Gr-1) (Proteintech, 65140-1-lg, 1:200) primary antibody overnight, washing with PBS to remove unbound antibody. Followed by goat anti-rat-lgG H&L (Alexa Fluor®488) (Abcam, ab150157, 1:200) secondary antibody for 1 h and washed three times with PBS for 5 min at room temperature. These washing steps were also performed between each staining step. After staining, all sections were stained with 50 μL DAPI (Solarbio C0065) for 5 min at room temperature and washed 4 times with PBS for 5 min. Slides were mounted with neutral balsam (Sinopharm Chemical Reagent Co, Ltd.). The images were then imaged using an SP5 upright or SP8 inverted confocal microscope (Olympus FV3000) with a 20x objective. Images were processed using Olympus Micro software (FV31S-SW) and analyses with Image J software.
Tissue-penetration studies
BD-OTf solution (40 µM), HCL1-OTf solution (40 µM) and HCL5-OTf solution (40 µM) in PBS (10 mM, pH 7.4) were place into the wells of a black 96-well plate. Chicken tissue with the desired thickness was covered on the top of the wells. After the addition of KO2 (80 µM), chemiluminescence images were acquired using the IVIS spectral imaging system in bioluminescence mode with an open filter and an acquisition time of 30 s. The NIR-II chemiluminescence acquisition of time was 30 s. The SBR was calculated as SBR. The fluorescence images were acquired at an excitation wavelength of 400 ± 10 nm, 670 ± 10 nm or 808 nm and an emission wavelength of 450 ± 10 nm, 720 ± 10 nm or 1000 ± 10 nm with an acquisition time of 0.1 s. The SBR was calculated as SBR = fluorescence intensity (or chemiluminescence intensity)/background, where background is the signal intensity of the adjacent tissue acquired during imaging. The SBRs were plotted as a function of tissue depth.
TEM Sample Preparation
CAR (1 µM) was incubated with KO2 (4 µM) in phosphate-buffered solution (1 × PBS, pH = 7.4), the carbon-coated side of the grid was gently immersed in the dispersion, the photo was taken by a Hitachi TEM system. Magnification = 40000, Accelerating Voltage = 100000.
Computational details
The geometric optimization and vibrational frequency analysis were performed using density functional theory (DFT) at the B3LYP/6-311 G(d,p) level with Grimme’s D3(BJ) dispersion correction (GD3BJ) in the Gaussian 16 program package. The SMD implicit solvation model with DMSO as the solvent was applied during these calculations. Optimized structures were confirmed as true minima by the absence of imaginary frequencies. Excited-state properties of the first excited state were investigated using time-dependent density functional theory (TD-DFT) at the same level. Frontier molecular orbitals were visualized using the IQmol molecular viewer.
Pharmacokinetic studies
All experimental mice were approved by the Institutional Animal Care and Use Committee (IACUC) of Sun Yat-sen University (SYSU) with the approval number SYSU-IACUC-2022-001938. 8-week-old male mice were selected to be injected with CAR, LIR and KIR (2.5 µmol kg−1 body weight) intravenously. Before injection, the tail of the mice was cut and blood was drawn with heparin capillary. After injection of CAR, LIR and KIR, blood was taken at 1, 4, 9, 16, 30, 60, 120, 240, 720 and 1440 min respectively. The collected blood samples were stored in an ice box to prevent coagulation, and then centrifuged at 2247 x g for 15 min. CAR, LIR and KIR in the blood were quantified using HPLC and plotted as a function of time to calculate the elimination half-life value (t1/2β).
Renal clearance studies
Male mice were i.v. injected with CAR (2.5 µmol kg−1 body weight), LIR (2.5 µmol kg−1 body weight) and KIR (2.5 µmol kg−1 body weight) and placed in metabolic cages. Feces was collected at 7 d post-injection, and urine was collected at 24 h post-injection, diluted in PBS and centrifuged 2247 x gfor 10 min and filtered by 0.22 µm syringe filter. CAR, LIR and KIR in the urine were quantified using HPLC. Then, determine the UV absorption and fluorescence spectrum in urine, and then sacrificed the mice and collect the main organs, homogenized in PBS buffer (10 mM, pH = 7.4) and centrifuged 2247 x g for 15 min to remove insoluble components. The supernatant containing extracted molecules were taken for HPLC analysis.
Biocompatibility studies
Major organs including heart, liver, spleen, lung and kidneys were collected from mice after 24 h injection of PBS, CAR, FL1R, FL2R, LIR and KIR (2.5 µmol kg−1 body weight), then placed into 4% paraformaldehyde for histological examination.
Establishment of liver ischemia-reperfusion models in living mice
Male mice were randomly selected and partial hepatic ischemia-reperfusion model was prepared through carefully orchestrated series of three main steps: opening the abdomen after the mice was entirely anesthetized and dissecting the interlobular ligaments with using microvascular clamp to interrupt the arterial and portal venous blood supply to the left lateral and median liver lobes for 15 min, 30 min, 45 min or 60 min then reperfusion for 4 h. The control groups were treated with PBS (0.2 mL) or NAC (10 mg kg−1 day−1 during the study, intraperitoneal injection) or sham-operation.
Real-time in vivo chemiluminescence and fluorescence imaging of HIRI in living mice
Real-time chemiluminescence and fluorescence imaging was conducted at 4 h reperfusion after i.v. injection of CAR (2.5 µmol kg−1 body weight) or LIR (2.5 µmol kg−1 body weight) and KIR (2.5 µmol kg−1 body weight). Chemiluminescence images were acquired under bioluminescence mode with an open filter and an acquisition time of 30 s. Fluorescence images were acquired using the IVIS spectrum imaging system with the excitation at 670 ± 10 nm, the emission at 720 ± 10 nm and the acquisition time of 0.1 s. Fluorescence and chemiluminescence intensities of liver and kidney in living mice were analyzed by the ROI analysis using the Living Image 4.3 Software and Image J. Mice were euthanized after imaging at 60 min post-injection of probes. Major organs were collected and placed into 4% paraformaldehyde for histological examination.
In vivo stability
Collected urine was measured in PBS (10 mM, pH 7.4) by UV-Vis and fluorescence spectrophotometry, imaged with the IVIS spectral imaging system, and analyzed by HPLC as well as MALDI-TOF mass spectrometry.
Statistics analysis
The in vivo and ex vivo chemiluminescence and fluorescence intensities were quantified with ROI analysis using Living Image 4.3 Software. Data are mean ± standard deviation (S. D.) unless stated otherwise. Investigators were blinded to group allocation during experiments. Statistical differences between two groups were tested with a two-tailed Student’s t test, and more than three groups were determined by one-way analysis of variance followed by Tukey’s post hoc test. For all tests, P-values less than 0.05 were considered statistically significant. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. All statistical calculations were performed using GraphPad Prism 6.0, including assumptions of tests used.
Animal handling
All male mouse experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC), Sun Yat-sen University (SYSU), with the approval number SYSU-IACUC-2023-B0504. To determine the clearance pathway and biocompatibility in living male mice, 8 weeks KunMing male mice (7 groups, three mice for each group, 2.5 μmol/kg body weight) were injected with CAR, KIR and LIR intravenously. All male mice were fed with non-fluorescent chow (Xietong Pharmaceutical Bioengineering CO., Ltd, XT19008). Housing conditions: 12 h dark/12 h light cycle, ambient temperature: 20–25 °C, Humidity: 45–55%.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data (including synthetic procedures, spectral data, theoretical calculation, H&E staining results, and in vitro and in vivo data) supporting the findings of this study are available within the article and from the corresponding author(s) upon request. Source data are provided as a Source Data file. Source data are provided in this paper.
References
Li, B. et al. Cell apoptosis and fas gene expression in liver and renal tissues after ischemia-reperfusion injury in liver transplantation. Transplant. Proc. 42, 1550–1556 (2010).
Lee, H. T., Park, S. W., Kim, M. & D’Agati, V. D. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab. Invest. 89, 196–208 (2009).
Kadkhodaee, M., et al. Alteration of renal functional, oxidative stress and inflammatory indices following hepatic ischemia-reperfusion. Gen. Physiol. Biophys. 31, 195–202 (2012).
Miranda, L. E. C. et al. Effects of partial liver ischemia followed by global liver reperfusion on the remote tissue expression of nitric oxide synthase: lungs and kidneys. Transplant. Proc. 42, 1557–1562 (2010).
Park, S. W., Chen, S. W. C., Kim, M., D’Agati, V. D. & Lee, H. T. Human activated protein c attenuates both hepatic and renal injury caused by hepatic ischemia and reperfusion injury in mice. Kidney Int. 76, 739 (2019).
Yoshidome, H., Kato, A., Edwards, M. J. & Lentsch, A. B. Interleukin-10 inhibits pulmonary NF-κb activation and lung injury induced by hepatic ischemia-reperfusion. Am. J. Physiol. 277, 919–923 (1999).
Seifi, B., Kadkhodaee, M., Delavari, F., Mikaeili, S. & Ostad, S. N. Pretreatment with pentoxifylline and n-acetylcysteine in liver ischemia reperfusion-induced renal injury. Ren. Fail. 34, 610 (2012).
Park, S. W., Kim, M., Chen, S. W. C., D’Agati, V. D. & Lee, H. T. Shinganine 1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock 33, 31–42 (2009).
Jaeschke, H. & Woolbright, B. L. Current strategies to minimize hepatic ischemia–reperfusion injury by targeting reactive oxygen species - sciencedirect. Transplant. Rev. 26, 103–114 (2012).
He, L. H., Yao, D. H., Wang, L. Y., Zhang, L. & Bai, X. L. Gut microbiome-mediated alteration of immunity, inflammation, and metabolism involved in the regulation of non-alcoholic fatty liver disease. Front. Microb. 12, 761836 (2021).
Granger, N. D. & Kvietys, P. R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 6, 524 (2015).
Weigand, K., Brost, S., Steinebrunner, N., Schemmer, P. & Müller, M. Ischemia/reperfusion injury in liver surgery and transplantation: Pathophysiology. Hpb. Surg. 3, 176723 (2012).
Graaf, W., Bennink, R. J. & Gulik, T. M. V. Nuclear imaging techniques for the assessment of hepatic function in liver surgery and transplantation. J. Nucl. Med. 51, 742 (2010).
Cao, Y. et al. HClO-activated near-infrared chemiluminescent probes with a malononitrile group for in-vivo imaging. Adv. Mater. 37, 2408941 (2024).
Son, S. et al. Chemiluminescent probe for the invitro and invivo imaging of cancers over-expressing nqo1. Angew. Chem. Int. Ed. 58, 1739–1743 (2019).
Gnaim, S., Gholap, S. P. & Shabat, D. Modular access to diverse chemiluminescent dioxetane-luminophores through convergent synthesis. Angew. Chem. Int. Ed. 61, e202202187 (2022).
David, M., Leirikh, T. & Shabat, D. Chemiexcitation acceleration of 1,2-dioxetanes by spiro-fused six-member rings with electron-withdrawing motifs. Angew. Chem. Int. Ed. 63, e202410057 (2024).
David, M., Gutkin, S. & Shabat, D. Unprecedented photoinduced-electron-transfer probe with a turn-on chemiluminescence mode-of-action. Angew. Chem. Int. Ed. 64, e202417924 (2024).
Yang, D., Ye, S., Hananya, N., Green, O. & Shabat, D. A highly selective and sensitive chemiluminescent probe for real-time monitoring of hydrogen peroxide in cells and animals. Angew. Chem. Int. Ed. 59, 14326–14330 (2020).
Shelef, O., Gnaim, S. & Shabat, D. Self-immolative polymers: an emerging class of degradable materials with distinct disassembly profiles. J. Am. Chem. Soc. 143, 21177–21188 (2021).
Shelef, O., Kopp, T., Tannous, R., Shabat, D. & Fridman, M. Enzymatic activity profiling using an ultrasensitive array of chemiluminescent probes for bacterial classification and characterization. J. Am. Chem. Soc. 146, 5263–5273 (2024).
Huang, J. et al. Chemiluminescent probes with long-lasting high brightness for in vivo imaging of neutrophils. Angew. Chem. Int. Ed. 61, e202203235 (2022).
Huang, J., Jiang, Y., Li, J., Huang, J. & Pu, K. Molecular chemiluminescent probes with a very long near-infrared emission wavelength for in vivo imaging. Angew. Chem. Int. Ed. 60, 3999 (2021).
Wang, X., Liew, S., Huang, J. & Pu, K. Dual-locked enzyme-activatable biorthogonal fluorescence turn-on imaging of senescent cancer cells. J. Am. Chem. Soc. 146, 22689–22698 (2024).
Liu, J., Huang, J., Wei, X., Cheng, P. & Pu, K. Near-infrared chemiluminescence imaging of chemotherapy-induced peripheral neuropathy. Adv. Mater. 36, 2310605 (2024).
Huang, J., Xu, M., Cheng, P. & Pu, K. A tandem-locked chemiluminescent probe for imaging of tumor-associated macrophage polarization. Angew. Chem. Int. Ed. 63, e202319780 (2024).
Huang, J., Su, L., Xu, C. & Pu, K. Molecular radio afterglow probes for cancer radiodynamic theranostics. Nat. Mater. 22, 1421–1429 (2023).
Huang, J. et al. A renally clearable activatable polymeric nanoprobe for early detection of hepatic ischemia-reperfusion injury. Adv. Mater. 34, 2201357 (2022).
Huang, J., Li, J., Lyu, Y., Miao, Q. & Pu, K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 18, 1133–1143 (2019).
Huang, J., Lyu, Y. & Pu, K. A renal-clearable duplex optical reporter for real-time imaging of contrast-induced acute kidney injury. Angew. Chem. Int. Ed. 58, 17796 (2019).
Ruan, B., Yu, M., Zhou, Y. & Huang, J. Size-transformable superoxide-triggered nanoreporters for crosstalk-free dual fluorescence chemiluminescence imaging and urinalysis in living mice. Angew. Chem. Int. Ed. 62, e202305812 (2023).
Zhou, Y., Zhu, L. & Huang, J. Tailored zwitterionic hemicyanine reporters for early diagnosis and prognostic assessment of acute renal failure. Angew. Chem. Int. Ed. 62, e202315457 (2023).
Chen, Z., Li, Q., Wu, Y., Liu, J. & Song, J. Molecular engineering of direct activated NIR-II chemiluminescence platform for in vivo chemiluminescence-fluorescence duplex imaging. Nat. Commun. 16, 238 (2025).
Liu, Y., Teng, L. & Song, G. “Four-In-One” design of a hemicyanine-based modular scaffold for high-contrast activatable molecular afterglow imaging. J. Am. Chem. Soc. 145, 5134–5144 (2023).
Lei, L., Yang, F., Meng, X., Zhang, X. & Song, G. Noninvasive imaging of tumor glycolysis and chemotherapeutic resistance via de novo design of molecular afterglow scaffold. J. Am. Chem. Soc. 145, 24386–24400 (2023).
Yue, R., Li, Z., Liu, H., Zhang, X. & Song, G. Imaging-guided companion diagnostics in radiotherapy by monitoring APE1 activity with afterglow and MRI imaging. Nat. Commun. 15, 6349 (2024).
Su, X., Kong, X., Sun, K. & Li, F. Enhanced blue afterglow through molecular fusion for bio-applications. Angew. Chem. Int. Ed. 61, e202201630 (2022).
Chen, L., Sun, K., Hu, D. & Li, F. Ultra-long near-infrared repeatable photochemical afterglow mediated by reversible storage of singlet oxygen for information encryption. Angew. Chem. Int. Ed. 135, e202218670 (2023).
Chen, C., Zhang, X., Gao, Z. & Ding, D. Preparation of AIEgen-based near-infrared afterglow luminescence nanoprobes for tumor imaging and image-guided tumor resection. Nat. Protoc. 19, 2408–2434 (2024).
Chen, C., Gao, H., Ou, H. & Ding, D. Amplification of activated near-infrared afterglow luminescence by introducing twisted molecular geometry for understanding neutrophil-involved diseases. J. Am. Chem. Soc. 144, 3429–3441 (2022).
Gao, Z., Jia, S., Ou, H., Hong, Y. & Ding, D. An activatable near-infrared afterglow theranostic prodrug with self-sustainable magnification effect of immunogenic cell death. Angew. Chem. Int. Ed. 134, e202209793 (2022).
Lu, L., Li, B., Ding, S., Fan, Y. & Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 11, 4192 (2020).
Pei, P., Chen, Y. & Zhang, F. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 16, 1011–1018 (2021).
Yang, J., Zhu, B., Zhang, J. & Ran, C. Half-curcumin-based chemiluminescence probes and their applications in detecting quasi-stable oxidized oroteins. Angew. Chem. Int. Ed. 63, e202409896 (2024).
Zhang, J., Wickizer, C. & Ran, C. In vivo three-dimensional brain imaging with chemiluminescence probes in Alzheimer’s disease models. Proc. Natl. Acad. Sci. USA 120, e2310131120 (2023).
Kuang, S., Zhu, B., Zhang, J. & Ran, C. A photolabile curcumin-diazirine analogue enables phototherapy with physically and molecularly produced light for alzheimer’s disease treatment. Angew. Chem. Int. Ed. 62, e202312519 (2023).
Zou, J. et al. Singlet oxygen “afterglow” therapy with NIR-II fluorescent molecules. Adv. Mater. 33, 2103627 (2021).
Zhu, J., Chen, W., Yang, L. & Miao, Q. A self-sustaining near-infrared afterglow chemiluminophore for high-contrast activatable imaging. Angew. Chem. Int. Ed. 63, e202318545 (2024).
Jiang, Y., Zhao, M., Miao, J., Chen, W. & Miao, Q. Acidity-activatable upconversion afterglow luminescence cocktail nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 15, 2124 (2024).
Chen, F., Xia, X., Ye, D., Deng, T. & Liu, F. A green-emitting luminol analogue as the next-generation chemiluminescent substrate in biochemical analysis. Anal. Chem. 95, 5773–5779 (2023).
An, R., Wei, S., Huang, Z., Liu, F. & Ye, D. An activatable chemiluminescent probe for sensitive detection of γ-glutamyl transpeptidase activity in vivo. Anal. Chem. 91, 13639–13646 (2019).
Yu, M. et al. Redox-active ferrocene quencher-based supramolecular nanomedicine for NIR-II fluorescence-monitored chemodynamic therapy. Angew. Chem. Int. Ed. 63, e202318155 (2024).
Zhao, X., Liu, J., Fan, J., Cao, H. & Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem. Soc. Rev. 50, 4185–4219 (2021).
Ma, Z. et al. Interface-Mediation-Enabled High-Performance Near-Infrared AgAuSe Quantum Dot Light-Emitting Diodes. J. Am. Chem. Soc. 145, 24972–24980 (2023).
Wu, L., Huang, J., Pu, K. & James, T. D. Dual-locked spectroscopic probes for sensing and therapy. Nat. Rev. Chem. 5, 406–421 (2021).
Chen, Z., Su, L., Wu, Y. & Song, J. Design and synthesis of a small molecular NIR-II chemiluminescence probe for in vivo-activated H2S imaging. Proc. Natl. Acad. Sci. USA 120, e2205186120 (2023).
Nohara, Y., Masuda, S., Nakashima, K. K., Matsumoto, M. & Yoshiya, T. Dioxetane derivative containing carboxy group as a chemiluminophore-introducing reagent. Chem. Bio. Chem. 23, e202200556 (2022).
Watanabe, N., Ijuin, H. K., Takatsuka, H. & Matsumoto, M. Solvent effect on base-induced chemiluminescent decomposition of bicyclic dioxetanes bearing a 3-hydroxyphenyl group. Tetrahedron 88, 132147 (2021).
Li, S., Zhang, G., He, Y., Yang, L. & Wang, X. Emission wavelength-tunable bicyclic dioxetane chemiluminescent probes for precise in vitro and in vivo imaging. Anal. Chem. 95, 13191–13200 (2023).
Shi, M., Chen, J., Wu, Y., Jin, P. & Wang, X. A bicyclic dioxetane chemiluminescence nanoprobe for peroxynitrite imaging in vivo. Anal. Chem. 96, 19109–19116 (2024).
Franke, R. M., Kosloske, A. M., Lancaster, C. S. & Filipski, C. A. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-D-glucosaminidase. Clin. Cancer Res. 16, 4198–4206 (2010).
Nastos, C. et al. Global consequences of liver ischemia/ reperfusion injury. Oxid. Med. Cell. Longev. 2014, 906965 (2014).
Koci, J. & Renard, P. Y. A novel and unusually long-lived chemiluminophore based on the 7-hydroxycoumarin scaffold. Chem. Commun. 47, 6713–6715 (2021).
Liu, Y. & Huang, J. Molecular engineering of activatable NIR-II hemicyanine reporters for early diagnosis and prognostic assessment of inflammatory bowel disease. ACS Nano 18, 8437–8451 (2024).
Zhao, L. & Liu, S. L. A cyanine with 83.2% photothermal conversion efficiency and absorption wavelengths over 1200 nm for photothermal therapy. Adv. Healthc. Mater. 13, 2304421 (2024).
Strassel, K. & Bauer, M. Shortwave infrared-absorbing squaraine dyes for all-organic optical upconversion devices. Sci. Technol. Adv. Mater. 22, 194–204 (2021).
Acknowledgements
J.H. thanks the introduced innovative R&D team project of “The Pearl River Talent Recruitment Program” (2021ZT09Y544), National Natural Science Foundation of China (22207130, 22474160), the National Key R&D Program of China (2022YFA0912700), Guangzhou Municipal Science and Technology Project (2025A04J7148), the Key Research and Development Plan of Guangzhou City (202206080007) for the financial support. J.H. acknowledges the award of “The Recruitment Program of Global Youth Experts” and the support from the start-up funding of Sun Yat-Sen University. H.Z. thanks the National Natural Science Foundation (82470787) for the financial support.
Author information
Authors and Affiliations
Contributions
J.H. and B.R. conceived and designed the study. B.R. and J.C. performed the probe synthesis and in vitro experiments. B.R., W.D., and S.Y. performed in vivo experiments, histology experiments and cell imaging experiments. J.L. performed a computer calculation. B.R., W.X., Y.Z., Y.J., P.X., H.H.Z., H.W.Z., and J.H. analyzed the data. B.R. and J.H. drafted the manuscript. All authors contributed to the writing of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ruan, B., Deng, W., Che, J. et al. Unimolecular near-infrared chemiluminescent reporter for cascaded multiplex imaging of ischemia-reperfusion injury in the liver-kidney axis. Nat Commun 16, 7743 (2025). https://doi.org/10.1038/s41467-025-62348-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62348-y