Abstract
Acidic CO2 electroreduction to multi-carbon (C2+) products using Cu-based catalyst has attracted considerable attention for CO2 recycling due to high single-pass CO2 utilization. However, its development is drastically limited by the poor stability, especially at high current density, caused by Cu dissolution/reconstruction during the reaction. Herein, we find the trace dissolved oxygen in the electrolyte accounts for the Cu dissolution/reconstruction and report an in-situ passivation strategy to prevent oxygen adsorption for inhibiting Cu dissolution/reconstruction for high stability CO2-to-C2+ conversion. Theoretical and in situ spectroscopy demonstrate that aluminum citrate (AC) passivation layer decreases the adsorption of oxygen on Cu surface to effectively prevent the Cu oxidation, which is beneficial for the formation and adsorption of linearly bonded *CO toward C-C coupling. As the result, the Cu catalysts with AC layer achieve over 60% Faradaic efficiency C2H4 and 38.7% energy efficiency to C2+ for over 150 h stability at 500 mA cm−2 in strong acidic electrolyte.
Similar content being viewed by others
Introduction
The excessive consumption of fossil fuels has caused an upsurge in CO2 emissions, which leads to increasingly serious climate and environmental problems1,2,3,4,5,6,7. Renewable electricity-driven CO2 electroreduction reaction (CO2RR) to high-value-added fuels or chemicals is the most promising strategy for the effective reduction of CO2 emissions and the recycling of carbon resources8,9,10, in which multi-carbon (C2+) products have attracted worldwide attention due to their high energy density and economic value11,12,13,14,15,16. Among various catalysts, Cu is recognized as a preeminent catalyst for CO2-to-C2+ conversion, owing to its abilities for moderate *CO adsorption and effective carbon–carbon (C−C) coupling17,18,19,20. On the other hand, the CO2RR performance is also affected by electrolyte, i.e., carbonate formation in alkaline or neutral electrolytes leads to insufficient CO2 gas supply and thus seriously affects the CO2RR activity and stability21,22. Although acidic electrolytes can eliminate the carbonate formation due to the presence of abundant protons, resulting in high single-pass CO2 utilization23,24, Cu-based catalysts generally suffer from unavoidable surface dissolution/reconstruction during the CO2RR process leading to serious stability issue, especially at high current density25,26,27,28,29,30,31.
Many strategies have been adopted to address the dissolution/reconstruction of Cu-based catalysts to improve their stability, such as surface modification, alloying, and dopant modification26,32,33,34,35,36. Okatenko et al. designed GaCu alloy catalysts by introducing Ga metals with high oxophilicity and low electronegativity37. The more stable Ga–Cu bonds effectively prevented the dissolution/reconstruction of Cu, thereby increasing the stability from 2 to 20 h. Albertini et al. encapsulated Cu catalysts with an organic/inorganic alumina hybrid shell (Cu/AlOx) to lock a fraction of the Cu surface into a reduction-resistant state38, thus inhibiting the structural reconstruction of Cu and extending the stability of CO2RR from 5 to 24 h. In addition, Fan et al. designed a cationic group-modified Cu catalyst to extend its stability from 1 h to over 100 h at 150 mA cm−2 in a strong acidic electrolyte by reducing the concentration of alkali cations39. However, the stability of Cu-based catalysts at the industrial level current density is still unappealing, because the cause for its dissolution/reconstruction is still unclear and thus, lacks an effective strategy for surface protection5,40,41,42.
In this work, we found the trace dissolved oxygen in the electrolyte accounts for the dissolution/reconstruction of Cu to affect its stability and thus, developed an in-situ passivation strategy to inhibit dissolution/reconstruction of Cu caused by trace dissolved oxygen with aluminum citrate (AC), thereby achieving long-term stable CO2-to-C2+ conversion at industrial level current density in strong acidic electrolyte. Density functional theory (DFT) calculations proved that citrate decreased the adsorption of oxygen on the Cu surface effectively. Electron microscopy and OH− adsorption analysis proved that the AC on Cu surface effectively inhibited dissolution/reconstruction of Cu by weakening the adsorption of oxygen to maintain the shape of catalysts. In situ Raman spectra analyses demonstrated that oxide-derived Cu with an AC passivation layer (AC-OD-Cu) keeped its metallic state even at open circuit potential (OCP), while OD-Cu (oxide-derived Cu without an AC passivation layer) was rapidly reoxidized to Cu2O within 60 s. In addition, in situ attenuated total reflection infrared spectroscopy (ATR-IR) revealed the formation and adsorption of the *CO intermediate were favored on the AC-OD-Cu electrode, which was one of the key steps to accelerate C–C coupling for C2+ products. As a result, the AC-OD-Cu exhibited a remarkable CO2-to-C2H4 conversion with Faradaic efficiency (FE) of 60% and 38.7% energy efficiency (EE) to C2+ for above 150 h at 500 mA cm−2 in a strong acidic electrolyte. This study reveals the reason for Cu dissolution/reconstruction and provides new guidance for improving the stability of Cu-based catalysts in strongly acidic CO2RR.
Results and discussion
Dissolved oxygen
To monitor the effect of dissolved oxygen on Cu dissolution/reconstruction, both Winkler’s method and gas chromatography (GC) were used to measure the O2 level in the electrolyte during CO2RR (Fig. 1a and Supplementary Table 1). It can be found that the dissolved O2 in the electrolyte was kept at 2.0 mg L−1 during the CO2RR process with continuous CO2 gas purging, indicating the dissolved O2 cannot be avoided under typical CO2RR conditions.
a The spectra of Gas chromatograph (GC) for O2 by AC-OD-Cu during the long-term CO2RR test. b In situ Raman spectra of OD-Cu reoxidation in 3 M KCl + 1 M KCl with pH = 1.0. c Cu content OD-Cu in electrolyte after the same reaction time at different O2 flow rates. TEM image of OD-Cu in 3 M KCl +1 M HCl electrolyte (pH = 1.0) with different O2 concentration: d 3.0 sccm; e 5.0 sccm; f 10.0 sccm; g 20.0 sccm.
To investigate the effect of dissolved O2 on Cu catalysts, in situ Raman spectroscopy and O2 concentration tests were conducted. In situ Raman results presented that the vibrational bands of Cu2O at ~220, ~525, and ~630 cm−1 appeared within 60 s over Cu catalysts even switching the potential from −0.5 V vs. RHE to OCP, and their intensities increased with time, indicating rapid oxidation of Cu to Cu2O (Fig. 1b)41,43. Furthermore, the Cu content in the electrolyte increased from 0.12 mg L−1 (0.9 ± 0.6 ppm at 0 sccm O2 flow rate) to 0.28 mg L−1 (6.9 ± 0.5 ppm at 20 sccm O2 flow rate), confirming that Cu dissolution was accelerated with the increase of O2 concentration (Fig. 1c and Supplementary Table 2). Transmission electron microscopy (TEM) images showed that Cu cubes gradually changed to irregular Cu nanoparticles as O2 concentration increased in the electrolyte (Fig. 1d–g), proving that dissolved O2 promoted the dissolution/reconstruction of Cu by accelerating the Cu oxidation.
To mitigate the detrimental effects of dissolved oxygen on Cu catalysts, an AC passivation layer was introduced to prevent dissolved oxygen adsorption on the Cu surface and inhibit its dissolution/reconstruction. AC, with its carboxylic acid groups, forms stable dinuclear Cu(II) carboxylate coordination compounds with Cu, preventing oxygen from adsorbing on the Cu surface (Fig. 2a)44,45. The AC passivation layer serves to stabilize the Cu catalyst and enhance its long-term performance during CO2RR under industrial current density.
a Schematic diagram of Cu dissolution/reconstruction by dissolved oxygen and the construction aluminum citrate passivation layer for anti-oxidation. b Adsorption energy of AC on Cu(111), Cu(110), and Cu(100) surface. Insets are the corresponding adsorption models. c Adsorption energy of O2 on OD-Cu and AC-OD-Cu surfaces. Insets are the corresponding adsorption models. Orange red: Cu; purple: Al; dark gray: C; red: O; white: H.
Theoretical calculations
DFT calculations were employed to study the impact of the AC passivation layer on Cu surface properties and its effect on O2 adsorption. To investigate the effect of AC passivation layer on inhibiting the Cu dissolution/reconstruction by trace dissolved oxygen, DFT calculations were carried out using the explicit solvent model (Supplementary Fig. 1). As shown in Fig. 2b and Supplementary Fig. 2, the adsorption energy of citrate molecules on Cu(111), (110), and (100) were found to be −3.04, −3.86, and −3.43 eV, respectively. The lower adsorption energy allows the citrate molecule to readily adsorb on the Cu surface to form passivation layers, thereby protecting the catalyst structure to maintain stable CO2RR performance28,46. After adsorption of AC, the adsorption energy of O2 on Cu(111), (110), and (100) surface increased from −1.65, −2.39, and −2.24 eV to −1.11, −1.93 and −1.88 eV, respectively, suggesting that AC passivation layer is effective at reducing O2 interaction with Cu and mitigates the effects of dissolved oxygen on Cu dissolution/reconstruction (Fig. 2c; Supplementary Figs 3, and 4 and Supplementary Table 3)34.
Evolution of catalyst structure
Inspired by the theoretical results, oxide-derived Cu (OD-Cu) catalysts with well-defined cubic shapes were synthesized for CO2 reduction to C2+ products. The AC passivation layer was then applied to OD-Cu via an in-situ electrostatic adsorption process (see details in Methods). Scanning electron microscopy (SEM), TEM and high-resolution TEM (HRTEM) showed that both pristine OD-Cu and AC-OD-Cu exhibited cubic shapes with (100) crystal planes (Fig. 3a–d and Supplementary Fig. 5). Notably, HRTEM and energy-dispersive X-ray spectroscopy (EDS) linear scans revealed that the AC passivation layer was uniformly covered on the surface of AC-OD-Cu with a thickness of ~2 nm (Supplementary Fig. 6). X-ray diffraction (XRD) patterns showed that OD-Cu and AC-OD-Cu were composed of Cu and Cu2O phases (Supplementary Fig. 7).
a TEM image of initial OD-Cu. b TEM image of AC-OD-Cu after 0.5 h reaction. c, d TEM image of OD-Cu and AC-OD-Cu after 2.0 h reaction at 500 mA cm−2, respectively. e C1s XPS spectra of OD-Cu and AC-OD-Cu after 2.0 h reaction. f The FTIR spectra of OD-Cu and AC-OD-Cu after 2.0 h reaction. Inset is the structural formula of citrate. g Enlarged CV curves showing OHad-(100) adsorption peaks on the OD-Cu and AC-OD-Cu.
To prove the presence of AC layer on the AC-OD-Cu surface, X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FT-IR) were conducted after 2.0 h reaction. The Cu2p, O1s and Cu LMM spectra of OD-Cu and AC-OD-Cu had no significant changes, indicating that the AC passivation layer did not affect the chemical states of Cu and O (Supplementary Fig. 8). However, C1s spectra of AC-OD-Cu exhibited distinct peaks at 286.3 eV and 288.3 eV, corresponding to C−O and O−C=O bonds, respectively (Fig. 3e)27, which were not observed in the C 1s spectra of OD-Cu, indicating these peaks were attributed to the hydroxyl and carbonyl groups in AC and thus verifying the cover of AC over AC-OD-Cu. FT-IR further supported this observation, showing strong peaks at 1585 and 1395 cm−1, which correspond to the O−C=O symmetric stretching vibration of AC (Fig. 3f and Supplementary Figs. 9 and 10), confirming that AC was well covered on the AC-OD-Cu surface4,45.
To evaluate the oxophilicity of the catalysts, cyclic voltammetry (CV) measurements were performed in 0.1 M KOH aqueous solution. Three peaks of adsorbed hydroxide ions were observed and attributed to OHad-(100), OHad-(110), OHad-(111) (Supplementary Fig. 11)28,46. Notably, the peak of OHad-(100) for AC-OD-Cu was located at 344 mV, higher than the peak location 335 mV of OD-Cu (Fig. 3g), indicating a reduced oxophilicity and thus a reduced dissolved oxygen interaction over AC-OD-Cu catalyst19.
Up unraveling the existence of the AC passivation layer and weak oxophilicity, the dissolution and morphology evolutions of OD-Cu and AC-OD-Cu catalysts were further evaluated at 500 mA cm−2 in 3 M KCl +1 M HCl electrolyte with pH = 1.0 for different reaction times. ICP data showed that the Cu content in the electrolyte was lower for AC-OD-Cu compared to OD-Cu at the same reaction time, confirming that the AC passivation layer effectively inhibited Cu dissolution (Supplementary Fig. 12 and Table 3). After 15 h of CO2RR, the OD-Cu underwent rapid dissolution, resulting in the formation of hollow and irregular Cu nanoparticles (Fig. 3c and Supplementary Figs. 13 and 14), indicating that the dissolution/reconstruction of Cu was extremely serious during the CO2RR process. Inversely, the AC-OD-Cu maintained its cube shape even after ~100 h of long-term reaction (Fig. 3d and Supplementary Figs. 15–17), proving that the AC passivation layer effectively prevents Cu dissolution/reconstruction during the CO2RR process.
In situ spectroscopy
To determine AC passivation layer inhibits Cu dissolution/reconstruction by preventing Cu oxidation, in situ Raman spectroscopies were carried out over OD-Cu and AC-OD-Cu. Prior to in-situ Raman testing, the electrode was subjected to a strict electrochemical pre-reduction treatment (−1.34 V vs. RHE for 10 min) to eliminate the influence of residual Cu2O. No characteristic peak of Cu2O was found on the electrode after electrochemical pre-reduction treatment. (Supplementary Fig. 18). For the OD-Cu, the characteristic peaks of Cu2O phase at ~220, ~525, and ~630 cm−1 disappeared at negative −0.5 V vs. RHE and re-generated at OCP over OD-Cu during five cycle measurements, indicating that OD-Cu was rapidly reoxidized (Fig. 3a)41,43. For the AC-OD-Cu, the characteristic peaks of Cu2O phase never appeared at −0.5 V vs. RHE and OCP during the five-cycle measurements, indicating that the reoxidation of Cu was effectively inhibited by preventing the O2 adsorption (Fig. 4b and Supplementary Fig. 19)41,43. Additional Raman measurements in a fully deoxygenated electrolyte were conducted to confirm that Cu2O forms were induced by O2 (Supplementary Fig. 20). Moreover, compared to OD-Cu, the Cu dissolution/reconstruction of the AC-OD-Cu catalyst was inhibited, and the catalyst morphology still maintained the cube shape in the electrolyte with high O2 concentration (Fig. 1d–f and Supplementary Figs. 21 and 22). These results indicated that O2 was the main factor leading to the Cu dissolution/reconstruction, and in situ constructed AC passivation layer on the Cu surface effectively inhibited the reoxidation of Cu, thereby preventing its dissolution/reconstruction.
a, b In situ Raman spectra of OD-Cu and AC-OD-Cu under loop tests with a reduction potential at −0.5 V vs. RHE for 60 s and reoxidation potential at OCP for 60 s in CO2-saturated 3 M KCl +1 M HCl electrolyte with pH = 1.0. c In situ ATR-IR spectra of AC-OD-Cu with a voltage window ranges from −0.74 to −1.54 V vs. RHE in CO2-saturated 3 M KCl +1 M HCl electrolyte with pH = 1.0. d In situ ATR-IR spectra of AC-OD-Cu with different times at −1.34 V vs. RHE in CO2-saturated 3 M KCl +1 M HCl electrolyte with pH = 1.0.
To prove that AC passivation layer can promote CO2RR to C2+ products while inhibiting Cu dissolution/reconstruction, in situ ATR-IR over OD-Cu and AC-OD-Cu was measured in CO2-saturated 3 M KCl +1 M HCl electrolyte with pH = 1.0 (Supplementary Fig. 23). Compared to OD-Cu (Supplementary Fig. 24), the distinct IR peak belonging to the linearly bonded *CO (*COL) intermediate at 2080 cm−1 appeared on AC-OD-Cu at −0.84 vs. RHE (Fig. 4c). With the applied potentials stepping down from −0.84 to −1.54 V vs. RHE, the *COL absorption peak areas showed a volcanic trend, indicating that AC passivation layer increased the coverage of *COL on the catalyst surface and further accelerated the *COL consumption for a fast *COL dimerization process46,47. Furthermore, the peaks of C=O at 1585 cm−1 and C−O at 1395 cm−1 for AC were significantly enhanced at −1.34 V vs. RHE with increasing reaction time, indicating that AC was stably adsorbed on the OD-Cu surface (Fig. 4d)48. These results demonstrated that the AC-OD-Cu surface was beneficial for the formation and adsorption of *COL intermediates and accelerated *COL dimerization to C2+ products.
Electrochemical CO2 reduction performance
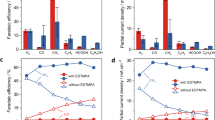
To assess the CO2RR performance of the OD-Cu and AC-OD-Cu catalyst, electrochemical tests were conducted in 3 M KCl +1 M HCl electrolyte with pH = 1.0 using flow cell (Supplementary Fig. 25). As shown in Fig. 5a–c, the AC-OD-Cu exhibited larger current density and better CO2RR to C2+ products than those of OD-Cu. At 500 mA cm−2, the AC-OD-Cu achieved C2+ selectivity up to 82.9 ± 2.1% (61.2 ± 1.4% towards C2H4, 15.3 ± 1.2% towards Et-OH and 6.4 ± 0.6% towards acetate), which was better than that of OD-Cu (FEC2+ of 51.3 ± 0.8%). Moreover, the AC-OD-Cu obtained an EE of 38.7% for acidic CO2RR towards C2+ products. The electrochemical surface areas (ECSAs) of all catalysts were measured via the CV method. ECSA slightly increases with the presence of the AC layer, indicating that AC-OD-Cu can provide more surface active sites (Supplementary Fig. 26). In addition, to confirm the source of the carbon products, an isotopic experiment with 13CO2 as the substrate and AC-OD-Cu as the catalyst has been carried out. As depicted in Supplementary Fig. 27, the mass spectrum signals at m/z = 29, m/z = 30, and m/z = 45 can be assigned to 13CO, 13C2H4, and 13CO2, definitely confirming that the C in carbon products indeed derives from the CO2 reduction.
a LSV curves recorded in Ar-saturated and CO2-saturated 3 M KCl +1 M HCl electrolyte with pH = 1.0. b Distribution of CO2RR products of OD-Cu in 3 M KCl +1 M HCl electrolyte with pH = 1.0. Error bars represent the standard deviation based on three independent measurements. c Distribution of CO2RR products of AC-OD-Cu in 3 M KCl +1 M HCl electrolyte with pH = 1.0. Error bars represent the standard deviation based on three independent measurements. d Long-term stability test of AC-OD-Cu and OD-Cu at 500 mA cm−2 (85% iR-correction, Resistance = 0.95 Ω and 1.05 of AC-OD-Cu and OD-Cu, respectively). e Long-term stability test with additional oxygen of AC-OD-Cu and OD-Cu at 500 mA cm−2 (85% iR-correction, Resistance = 0.96 Ω and 1.01 Ω of AC-OD-Cu and OD-Cu, respectively).
We further evaluated the stability of the catalyst in acidic CO2RR (CO2-saturated 3 M KCl +1 M HCl electrolyte with pH = 1.0). As shown in Fig. 5d and Supplementary Figs. 28–31, the AC-OD-Cu maintained a FE of ~60% toward C2H4 at 500 mA cm−2 for over 150 h, which was 10 times of OD-Cu (15 h) and 15 times of only adding citrate or Al3+ (about 10 h), revealing the importance of AC layer for preventing dissolution/reconstruction of the Cu catalysts. At 250 mA cm−2, AC-OD-Cu maintained a FE for ethylene above 50% for 150 h, indicating that the presence of the AC passivation layer can prolong the stability of Cu-based catalysts at both high and low current density (Supplementary Fig. 32). After stability test, AC-OD-Cu was mainly composed of irregular granular metal Cu, and AC still coexisted with Cu substrate (Supplementary Figs. 33–36). Moreover, in different bulk pH system, although the stability of AC-OD-Cu decreased from 150 h at bulk pH = 1.0–30 h at bulk pH = 5.0, it was still much higher than OD-Cu (Supplementary Figs. 37 and 38). At the same current density, this stability represented a 10 times improvement over the reported acidic CO2RR system (Supplementary Table 5)22,23,24,32,49.
Moreover, with increasing the O2 level in the electrolyte, the CO2RR activity and stability of AC-OD-Cu can still be maintained at the identical levels (Fig. 5e), but a drastic decrease could be observed over OD-Cu (5 h), proving the effective passivation of the AC layer for preventing Cu dissolution/reconstruction under high current density. These results demonstrated that the AC passivation layer on the Cu surface effectively inhibited the effect of oxygen on Cu, thereby preventing its dissolution/reconstruction for high activity and long-term stability.
Conclusion
In summary, we developed an in-situ passivation strategy to inhibit dissolution/reconstruction of Cu caused by trace dissolved oxygen with AC, thereby achieving long-term stable CO2-to-C2+ conversion at industrial level current density in strong acidic electrolyte. DFT calculation, electron microscope and OH− adsorption proved that the AC passivation layer improved the anti-oxidation ability of Cu catalyst, effectively inhibiting Cu dissolution/reconstruction to maintain the shape of the catalysts. In situ Raman and in situ ATR-IR spectroscopy showed that the AC passivation layer prevented the Cu dissolution/reconstruction by inhibiting the reoxidation of Cu, which was facilitated the formation and adsorption of *COL intermediates to achieve rapid C−C coupling. The AC-OD-Cu achieved significant CO2-to-C2 conversion with a Faraday efficiency of over 80% and EE of 38.7% for over 150 h at 500 mA cm−2. This study provided a new approach for extending the stability of acidic CO2RR under industrial current density. Furthermore, the influence of some potential factors (such as Cl−, pH, etc.) on the Cu dissolution/reconstruction still requires further study to comprehensively and accurately understand the mechanism of Cu dissolution/reconstruction.
Methods
Chemicals
CuSO4·5H2O (99.99% metals basis), Al(NO3)3·9H2O (A. R. grade), sodium citrate tribasic dehydrate (A. R. grade), Na2S2O3 (A. R. grade), MnCl2 (A. R. grade) and KI (A. R. grade) were purchased from Aladdin. KCl (A. R. grade), NaOH (A. R. grade), KOH (A. R. grade) and acetone (A. R. grade) were provided by Sinopharm Chemical Reagent Co., Ltd, China. L-(+)-Ascorbic acid (99 %) and deuterium oxide (D2O) (99.9 atom %) were obtained from Alfa Aesar China Co., Ltd.
Synthesis of Cu2O
Cu2O cube was synthesized by a modified wet chemical reduction method50. Firstly, 2.0 mL of 0.9 M sodium citrate tribasic dehydrate was added into 150 mL distilled water and stirring for 5 min. Then 1.0 mL of 1.2 M CuSO4·5H2O were added to the above solution successively and stirring for 10 min. Next, 1.0 mL of 4.8 M NaOH was injected rapidly into the mixed solution and stirring for 10 min. Finally, 1.0 mL of 1.2 M L-(+)-ascorbic acid solution was injected rapidly into the above prepared colloidal suspension and stirring for another 30 min to obtain the precipitates. The obtained precipitates were collected by centrifugation and subsequently washed three times with deionized water and one time with acetone. Afterwards, the sample was immediately dried under vacuum. The OD-Cu and AC-OD-Cu were synthesized through the reduction of Cu2O catalysts in 3 M KCl (pH = 1.0) electrolyte without or with AC for 10 min at −1.1 V vs. RHE, respectively.
Characterizations
The morphology and corresponding EDS elemental mapping images were studied by SEM (FEI Helios Nanolab 600i). TEM images were obtained from FEI Tecnai G2 F20 field emission transmission electron microscope operated at 200 kV. Phases structure of the prepared catalysts was characterized by XRD (Rigaku Miniflex 600, Cu-Kα radiation with λ = 1.51484 Å) with a 2θ range from 10° to 80° and a scan rate of 5° min−1. XPS measurements were performed on Thermo Fisher Scientific Escalab 250 XI, and all the binding energies were calibrated by the C 1s peak at 284.8 eV. Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Agilent 7700 s) was used to measure the content of metal atoms in the samples. The gas phase products after bulk electrolysis were quantified by online Gas chromatograph (GC, Shimadzu, Model 2014).
Measurements of O2 level of electrolytes
The O2 level was measured with Winkler’s method51. 47.5 mL of CO2-saturated KCl electrolytes was added to a 50 mL centrifuge tube, followed by adding 0.3 ml 3 M MnCl2 solution and 0.6 ml mixed solution (4 M KOH + 2 M KI). The tube was sealed and shaken vigorously to allow completely fixation of dissolved O2 as precipitate. Then 0.3 mL 5 M H2SO4 was added to acidify the solution to generate I3− accompanied by the reduction of fixed O2, which was further titrated by a Na2S2O3 solution with a starch indicator to obtain the O2 level of electrolytes. To improve the accuracy and reproducibility of the O2 level measurements, reagents from the same batch should be used, and all the samples should be measured at the same time. The calculated standard deviations of three measurements were in the reasonable range of ~0.5 mg L−1, validating the measured O2 levels in the samples. Use the following formula to calculate the O2 level:
Where C is the concentration of Na2S2O3 solution. V is the volume of Na2S2O3 solution consumed by titration.
In situ Raman spectroscopy
Raman spectra were tested by in DXR3 (Thermo Scientific, US) with a 532 nm laser as the excitation source52. The measurements were performed in a custom-designed three-electrode electrochemical cell equipped with a quartz window. The working, reference, and counter electrodes consisted of the as-prepared Cu electrode, an Ag/AgCl electrode (3.0 M KCl), and a graphite electrode, respectively. Prior to each test, the electrolyte was continuously pumped into the cell at a flow rate of 20 mL min−1. A built-in optical microscope was employed to capture real-time microscopic images of the Cu electrodes during Raman characterization.
In situ attenuated total reflection-infrared spectroscopy (ATR-IR)
ATR-IR was carried out on a Nicolet iS50 FT-IR spectrometer equipped with an MCT detector cooled with liquid nitrogen15,53. First, the Si prism was prepared by polishing with a 0.3 μm Al2O3 slurry, followed by sequential sonication in acetone and deionized water. To clean the prism of organic contaminants, the Si prism was soaked in a piranha solution (3: 1 volumetric ratio of 98% H2SO4 and H2O2) for 60 min. The reflective surface was immersed in the gold plating solution at 60 °C for 7 min to prepare the Au electrode. Five miligrams catalyst was mixed with 1 mL mixture solution (Vethanol:VH2O = 1:1) and 50 μL Nafion solutions (5 wt%, Sigma-Aldrich) followed by sonication of 30 min to form a homogeneous solution. Then 80 μL of catalyst ink was cast onto the Au film modified Si prism reflecting surface. In situ ATR-IR spectra were recorded during stepping the working electrode potential.
Electrochemical measurements
All the electrochemical measurements were operated with an electrochemical station and at 25 °C. The flow cell measurements were performed on a home-made cell. The flow cell comprises a gas diffusion electrode (GDE, YSL-30T), a sandwich of flow frames, gaskets, counter electrode, reference electrode and exchange membrane (117 µm-thickness Nafion 117). Before using, Nafion 117 was treated with 5% hydrogen peroxide at 80 °C for 1 h, then boiled in 5% dilute sulfuric acid at 80 °C for 1 h. The preparation of the working electrode was home-made. Briefly, 10 mg catalyst was mixed with 1 mL mixture solution (Vethanol:VH2O = 1:1) and 50 μL Nafion solutions (5 wt%, Sigma-Aldrich) followed by sonication of 30 min to form a homogeneous solution. Then 150 μL of catalyst ink was sprayed on a hydrophobic GDE (3 cm2) and then dried at 50 °C for 2 h. The loading of the catalyst is 0.5 mg cm−2 and the area contacting electrolyte is 1.0 cm2. The IrO2-coating titanium foil is employed as counter electrode and an Ag/AgCl (with saturated 3.5 M KCl) electrode as reference electrode. The cathode electrolyte is divided into two types: (1) 75 mL 3 M KCl + 1 M HCl electrolyte with pH = 1.0; (2) 75 mL 3 M KCl + 1 M HCl electrolyte with pH = 1.0 contains 1 mL 0.9 M sodium citrate and 0.5 mL 0.12 M Al3+. 60 mL 0.05 M H2SO4 solution (pH = 1.0) was used as anolyte. Before the test, all the electrolyte is stored in the refrigerator. The electrolyte in the cathode and anode was circulated at a rate of 30 mL min−1 by a peristaltic pump. During the measurements, high-purity CO2 (99.999%) gas was continuously supplied to the gas chamber at a rate of 20 sccm by a mass flowmeter. On the basis of the original test, the oxygen corrosion experiment continued to inject O2 (99.999%) into the cathode electrolyte with a flow rate of 3 sccm. All potentials were measured against an Ag/AgCl reference electrode, and converted to the reversible hydrogen electrode (RHE) reference scale via Nernst function with iR compensation as below:
All the electrochemical tests were carried out using an electrochemical workstation (DongHua Analytical, DH7002A). The linear sweep voltammetry (LSV) experiments were scanned in Ar-saturated and CO2-saturated 3 M KCl + 1 M HCl electrolyte (pH = 1.0) without/with AC. The scan rate is 10 mV s−1 and the scan range is −0.74 to −1.74 V vs. RHE. The electrochemical impedance spectroscopy study was investigated by applying an open circuit voltage in a frequency range from 10−1 to 105 Hz with an amplitude of 10 mV. Electrochemical OH− adsorption was performed in Ar-saturated 1 M KOH electrolyte using CV curves at a sweep rate of 100 mV s−1 for the Cu2O electrode. The potential ranged from −0.2 to 0.6 V vs. RHE. The electrochemical active surface area (ECSA) of the electrode was measured by the double-layer capacitor method, which was obtained by the CV method. The potential window of the CV curves was 0.25–0.35 V vs. RHE for OD-Cu and AC-OD-Cu with different scan rates from 10 to 80 mV s−1. The geometric area of electrodes exposed to electrolytes was controlled at 1.0 cm2.
CO2 reduction products analysis
The gaseous products released from the electrolytic cell were analyzed by GC, with sampling performed at 15 min intervals. The quantification of gas-phase products was based on the corresponding peak areas in the GC spectra. Faraday efficiency characterizes the selectivity of the reduction product, and the formula for calculating FE (%) for quantitative analysis of gases follows:
Where n is the number of transferred electrons of the target product, V is the volume of the target product of the 1 mL sample detected by the GC, S is the gas flow rate, 20 mL min−1, P is pressure, F is Faradaic constant, 96485 C mol−1, R is the ideal gas constant, 8.314 Pa m3 mol−1 K−1, T is room temperature, 298 K, 25 °C, Itotal is the current recorded by the electrochemical workstation.
The liquid products were analyzed using 1H NMR spectroscopy with water suppression. According to the approximate concentration of the liquid product, the standard curve is developed by measuring the nuclear magnetic peak area of the known concentration. We used D2O as the lock solvent. The FE was calculated using the equation below:
where Ni is the number of electrons transferred, F is the Faradaic constant, ni is the total moles of products, and Q = I × t is the total charged passed during the experiment.
The EE for the formation of ethylene is calculated as follows:
where FEC2H4, FEEt-OH, and FEacetate denotes the FE of C2H4, Et-OH and n-PrOH, \({E}_{{{{\rm{a}}}}}^{\theta }\), \({{{\mbox{E}}}}_{{\mbox{b}}}^{\theta }\), \({E}_{{{{\rm{c}}}}}^{\theta }\) and \({{{\mbox{E}}}}_{{\mbox{d}}}^{\theta }\) are the standard reduction potentials for the anode and cathode (CO2-to-C2H4, Et-OH and acetate) reactions, respectively. Eb, Ec, and Ed are the potential for CO2RR to C2H4, Et-OH and acetate.
DFT computational details
All calculations were carried out by spin-polarized DFT with the Vienna Ab initio Simulation Package (VASP)54,55. Electron exchange-correlation was expressed by the Perdew–Burke–Ernzerhof (PBE) functional within the generalized gradient approximation56. To describe the ionic cores, the projector augmented wave pseudopotential was applied57,58. All calculations were performed at the Gamma point using a single k-point sampling scheme. The plane wave energy cutoff, and convergence criterion for electronic energy and forces were set as 450 eV, 10−5 eV, and 0.02 eV/Å. Van der Waals interactions were corrected using the D3 method of Grimme59. The atomic structure was visualized using the VESTA package58.
The (111), (100), and (110) surfaces were constructed based on the cubic unit cell. All slab models consisted of three atomic layers, with the bottom layer fixed and the upper two layers fully relaxed during geometry optimizations. The (111) surface was modeled using a 6 × 4 supercell containing 144 Cu atoms, the (100) surface used a 6 × 8 supercell containing 144 Cu atoms, and the (110) surface employed a 4 × 8 supercell containing 96 Cu atoms, unless otherwise specified. A vacuum layer of 20 Å was applied along the z direction to avoid spurious interactions between periodic images.
Implicit solvation effects were considered by employing the VASPsol implicit solvent model59,60. The Debye length was set to 3 Å to simulate the experimental acid conditions (1 M). The dielectric constant for water was set to a relative permittivity value of 80. In addition, a monolayer of water molecules was pre-adsorbed on the Cu surfaces to account for the explicit influence of interfacial hydrogen bonding. The initial Cu surface model with pre-adsorbed water molecules is shown in Fig. S1.
For the adsorption of citrate and oxygen, the calculations were performed by replacing three water molecules with citrate and one water molecule with oxygen, respectively60. The specific formulas are as follows:
where Ecu+adsorbate is the total energy of the Cu surface with the adsorbate, Esurface vacancies is the energy of the Cu surface after removing the corresponding number of water molecules (vacancies), and Eadsorbate is the energy of the isolated adsorbate.
Data availability
Full data supporting the findings of this study are available within the article and its Supplementary Information, as well as from the corresponding author upon reasonable request. Source data are provided with this paper Source data are provided with this paper.
References
Nguyen, T. N. et al. Gas diffusion electrode design for electrochemical carbon dioxide reduction. Chem. Soc. Rev. 49, 7488–7504 (2020).
Liu, K. et al. Single-atom transition metals supported on black phosphorene for electrochemical nitrogen reduction. Nanoscale 12, 4903–4908 (2020).
Chen, G. et al. CoS2 needle arrays induced a local pseudo-acidic environment for alkaline hydrogen evolution. Nanoscale 13, 13604–13609 (2021).
Zhu, Y. et al. Nano-manganese oxides-modified biochar for efficient chelated copper citrate removal from water by oxidation-assisted adsorption process. Sci. Total Environ. 709, 136154 (2020).
Zhang, X. et al. Selective and high current CO2 electro-reduction to multicarbon products in near-neutral KCl electrolytes. J. Am. Chem. Soc. 143, 3245–3255 (2021).
Wang, X. et al. Promoted CF4 decomposition via enhanced tricoordinated al active sites. ACS EST Eng.4, 1142–1148 (2024).
Chen, Q. et al. Significantly enhanced photocatalytic CO2 reduction by surface amorphization of cocatalysts. Small 17, e2102105 (2021).
Fu, J. et al. Graphitic carbon nitride based single-atom photocatalysts. Front. Phys. 15, 33201 (2020).
Zhang, X.-D. et al. Pseudo-copper Ni-Zn alloy catalysts for carbon dioxide reduction to C2 products. Front. Phys. 16, 63500 (2021).
Chen, Q. et al. Electrocatalytic CO2 reduction to C2+ products in flow cells. Adv. Mater. 36, 2303902 (2024).
Chen, K. et al. Ligand engineering in nickel phthalocyanine to boost the electrocatalytic reduction of CO2. Adv. Funct. Mater. 32, 2111322 (2021).
Zi, X. et al. Breaking K+ concentration limiton Cu nanoneedles for acidic electrocatalytic CO2 reduction to multi-carbon products. Angew. Chem. Int. Ed. 62, e202309351 (2023).
Ge, W. et al. Dynamically formed surfactant assembly at the electrified electrode–electrolyte interface boosting CO2 electroreduction. J. Am. Chem. Soc. 144, 6613–6622 (2022).
Pham, T. H. M. et al. Enhanced electrocatalytic CO2 reduction to C2+ products by adjusting the local reaction environment with polymer binders. Adv. Energy Mater. 12, 2103663 (2022).
Wang, Q. et al. Asymmetric coordination induces electron localization at Ca sites for robust CO2 electroreduction to CO. Adv. Mater. 35, 2300695 (2023).
Chen, Q. et al. Ordered ag nanoneedle arrays with enhanced electrocatalytic CO2 reduction via structure-induced inhibition of hydrogen evolution. Nano Lett. 22, 6276–6284 (2022).
Zhou, Y. et al. Vertical Cu nanoneedle arrays enhance the local electric field promoting C2 hydrocarbons in the CO2 electroreduction. Nano Lett. 22, 1963–1970 (2022).
Wang, X. et al. Gas diffusion in catalyst layer of flow cell for CO2 electroreduction toward C2+ products. Nano Res. 7, 1101–1106 (2024).
Liu, Z. et al. Switching CO2 electroreduction toward ethanol by delocalization state-tuned bond cleavage. J. Am. Chem. Soc. 146, 14260–14266 (2024).
Fang, M. et al. Hydrophobic, ultrastable Cuδ+ for robust CO2 electroreduction to C2 products at ampere-current levels. J. Am. Chem. Soc. 145, 11323–11332 (2023).
Zhang, T. et al. Acidic CO2 electrolysis addressing the “alkalinity issue” and achieving high CO2 utilization. Chem. Eur. J. 29, e202301455 (2023).
Zhao, Y. et al. Conversion of CO2 to multicarbon products in strong acid by controlling the catalyst microenvironment. Nat. Synth. 2, 403–412 (2023).
Xie, Y. et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Nat. Catal. 5, 564–570 (2022).
Ma, Z. et al. CO2 electroreduction to multicarbon products in strongly acidic electrolyte via synergistically modulating the local microenvironment. Nat. Commun. 13, 7596 (2022).
Wu, H. et al. Toward durable CO2 electroreduction with Cu-based catalysts via understanding their deactivation modes. Adv. Mater. 36, 2403217 (2024).
Popovic, S. et al. Stability and degradation mechanisms of copper-based catalysts for electrochemical CO2 reduction. Angew. Chem. Int. Ed. 59, 14736–14746 (2020).
Kim, J. et al. Vitamin C-induced CO2 capture enables high-rate ethylene production in CO2 electroreduction. Nat. Commun. 15, 192 (2024).
Wua, M. et al. Sequential *CO management via controlling in situ reconstruction for efficient industrial-current-density CO2-to-C2+ electroreduction. Proc. Natl. Acad. Sci. USA 120, e2302851120 (2023).
DuanMu, J.-W. et al. A critical review of operating stability issues in electrochemical CO2 reduction. Sci. China Mater. 67, 1721–1739 (2024).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Chen, P. C. et al. Chemical and structural evolution of agcu catalysts in electrochemical CO2 reduction. J. Am. Chem. Soc. 145, 10116–10125 (2023).
Zhang, J. et al. Accelerating electrochemical CO2 reduction to multi-carbon products via asymmetric intermediate binding at confined nanointerfaces. Nat. Commun. 14, 1298 (2023).
Weng, Z. et al. Self-cleaning catalyst electrodes for stabilized CO2 reduction to hydrocarbons. Angew. Chem. Int. Ed. 56, 13135–13139 (2017).
Zhu, J. et al. Surface passivation for highly active, selective, stable, and scalable CO2 electroreduction. Nat. Commun. 14, 4670 (2023).
Chen, X. et al. Highly stable layered coordination polymer electrocatalyst towards efficient CO2-to-CH4. Adv. Mater. 36, 2310273 (2023).
Ma, X. et al. Carbonate shell regulates CuO surface reconstruction for enhanced CO2 electroreduction. Nat. Synth. 4, 53–66 (2025).
Okatenko, V. et al. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical CO2 reduction reaction. J. Am. Chem. Soc. 145, 5370–5383 (2023).
Albertini, P. P. et al. Hybrid oxide coatings generate stable Cu catalysts for CO2 electroreduction. Nat. Mater. 23, 680–687 (2024).
Fan, M. et al. Cationic-group-functionalized electrocatalysts enable stable acidic CO2 electrolysis. Nat. Catal. 6, 763–772 (2023).
Kreizer, V. et al. The effect of oxygen on copper dissolution during cathodic polarization. Prot. Met. 39, 30–33 (2003).
Mu, S. et al. Hydroxyl radicals dominate reoxidation of oxide-derived Cu in electrochemical CO2 reduction. Nat. Commun. 13, 3694 (2022).
Pan, B. et al. Close to 90% single-pass conversion efficiency for CO2 electroreduction in an acid-fed membrane electrode assembly. ACS Energy Lett. 7, 4224–4231 (2022).
Zhao, Y. et al. Speciation of Cu surfaces during the electrochemical CO reduction reaction. J. Am. Chem. Soc. 142, 9735–9743 (2020).
Peng, J. et al. Surface coordination layer passivates oxidation of copper. Nature 586, 390–394 (2020).
Li, Q. et al. Efficient removal of Cu(II) and citrate complexes by combined permanent magnetic resin and its mechanistic insights. Chem. Eng. J. 366, 1–10 (2019).
Han, Z. et al. Steering surface reconstruction of copper with electrolyte additives for CO2 electroreduction. Nat. Commun. 13, 3158 (2023).
Yang, B. et al. Accelerating CO2 electroreduction to multicarbon products via synergistic electric-thermal field on copper nanoneedles. J. Am. Chem. Soc. 144, 3039–3049 (2022).
Acharya, A. et al. Ultrathin covalent organic overlayers on metal nanocrystals for highly selective plasmonic photocatalysis. Nat. Commun. 14, 7667 (2023).
Cao, Y. et al. Surface hydroxide promotes CO2 electrolysis to ethylene in acidic conditions. Nat. Commun. 14, 2387 (2023).
Li, P. et al. P-d orbital hybridization induced by p-block metal-doped Cu promotes the formation of C2+ products in ampere-level CO2 electroreduction. J. Am. Chem. Soc. 145, 4675–4682 (2023).
Sastry, G. S. et al. Spectrophotometric determination of dissolved oxygen in water. Anal. Chem. 41, 857–858 (1969).
Wang, Q. et al. Lanthanide single-atom catalysts for efficient CO2-to-CO electroreduction. Nat. Commun. 16, 2985 (2025).
Wang, Q. et al. Cooperative alkaline hydrogen evolution via inducing local electric field and electron localization. Chin. J. Catal. 54, 229–237 (2023).
Kresse, G. et al. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. et al. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P. et al. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. et al. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Grimme, S. et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phy. 132, 154104 (2010).
Liao, W. et al. Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm-2. Nat. Commun. 15, 1264 (2024).
Acknowledgements
We gratefully thank the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 52121004), Natural Science Foundation of China (Grant Nos. 22376222, 52372253, and 22403108), Science and Technology Innovation Program of Hunan Province (Grant No. 2023RC1012), the Natural Science Foundation of Hunan Province for Excellent Youth Scholars (Grant No. 2024JJ4051), the Natural Science Foundation of Hunan Province (Grant No. 2024JJ6484), Central South University Research Programme of Advanced Interdisciplinary Studies (Grant No. 2023QYJC012), Central South University Innovation-Driven Research Program (Grant No. 2023CXQD042). The Fundamental Research Funds for the Central Universities of Central South University (Grant No. 2024ZZTS0097). We are grateful for resources from the High Performance Computing Center of Central South University.
Author information
Authors and Affiliations
Contributions
M.L., J.F., and Q.C. conceived the idea of this work and designed the experiments. Q.C., X.W., and Y.T. performed the material synthesis and characterizations. Q.C. and X.W. performed catalytic CO2 reduction performance measurements. Y.T. and K.L. performed DFT theoretical calculations. Q.C., X.W., Q.W., K.L., H.L, Y.T, L.C., M.L., and Z.L. analyzed the data and discussed the results. Q.C. wrote and revised the manuscript. M.L. and Z.L. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Antonia Herzog, Xinchen Kang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Q., Tan, Y., Wang, X. et al. Long-term stable acidic electroreduction of CO2 to C2 products at industrial current density using passivated copper. Nat Commun 16, 8468 (2025). https://doi.org/10.1038/s41467-025-63318-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63318-0