Abstract
Mepolizumab (anti-IL5 therapy) reduces asthma exacerbations in urban children with exacerbation-prone eosinophilic asthma. We previously utilized nasal transcriptomics to identify inflammatory pathways (gene co-expression modules) associated with asthma exacerbations despite this therapy. In this study, we applied differential gene correlation analysis on these targeted gene co-expression modules to gain better insight into the treatment effects on correlation structure within gene networks. Mepolizumab treatment resulted in loss of correlation amongst eosinophil-specific genes but conservation and even strengthening of correlation amongst mast cell-specific genes, T2 cytokines, and airway epithelial inflammatory genes. Notably, mepolizumab induced significant gain in correlation of genes associated with multiple aspects of airway epithelial inflammation including those related to extracellular matrix production and nitric oxide synthesis, and this change was associated with a poor clinical response to mepolizumab. These findings highlight that using differential gene correlation analysis offers insight into the molecular regulatory effects of treatment on gene interactions and may lead to better understanding of disease mechanisms and therapeutic responses. ClinicalTrials.gov ID: NCT03292588.
Similar content being viewed by others
Introduction
Asthma exacerbations are a major aspect of asthma morbidity in children. A significant proportion of children with asthma, particularly those living in socioeconomically disadvantaged urban neighborhoods1, have uncontrolled symptoms despite use of conventional therapies such as inhaled corticosteroids with or without long-acting beta-agonists. Mepolizumab, a humanized monoclonal antibody directed at interleukin-5 (IL-5), was developed as a targeted therapy for patients with elevated numbers of eosinophils in the blood and/or airway. The anti-IL5 mechanism of mepolizumab blocks a component of the T2 inflammatory pathway by reducing down-stream effects of eosinophil activation. The MUPPITS-2 randomized clinical trial (Mechanisms Underlying Asthma Exacerbations Prevented and Persistent with Immune Based Therapy: a Systems Approach Phase 2) assessed the efficacy, safety and mechanisms of therapy with mepolizumab added to guideline-based care for children with exacerbation-prone eosinophilic asthma living in low-income, urban areas in the USA2. The MUPPITS-2 study demonstrated a 27% relative reduction in rate of severe exacerbations which was lower than the approximately 50% reduction seen in the clinical trials of mepolizumab in adult participants2,3,4.
The MUPPITS-1 study was a prospective, longitudinal case-control study assessing gene expression changes during colds that progressed to exacerbations versus those that resolved without intervention5. In this study, we identified upper airway transcriptome modules, which are networks of co-expressed and functionally related genes, associated with asthma exacerbations in urban children5. In the MUPPITS-2 study, using upper airway transcriptome profiling and modular analysis, we identified that expression of eosinophil inflammatory modules decreased while on mepolizumab therapy but surprisingly, expression of multiple non-T2 airway inflammatory modules associated with the airway epithelium increased in expression while on mepolizumab therapy, hypothesized as a possible reason for the relatively diminished clinical efficacy observed2. These findings indicated that the mechanism(s) of action of a highly targeted drug like mepolizumab can, in fact, have broader molecular impacts beyond the anticipated effects on eosinophilic inflammation. Following this result, we have sought to investigate whether the incomplete prevention of asthma exacerbations with mepolizumab therapy was a result of other residual components of T2 inflammation (e.g., mast cell activation) and/or unchecked non-T2 airway epithelium inflammation.
Differential expression analysis gives insight into a single point in time but does not reveal gene interactions. In contrast, differential gene correlation analysis (DGCA) identifies changes in measures of association of gene networks across biological conditions (such as treatment effect)6. To better understand how treatment alters the interactions amongst genes, we can leverage DGCA, which assesses alterations in the correlation structure among genes, defining the impacts of therapy on the coordinated regulatory processes at the level of transcription.
In the current study, we utilize DGCA7 to identify significant alterations in gene correlation structure after IL-5 blockade by assessing how genes may have fallen out of coregulation (loss of correlation/coherence) or maintain or increase coregulation with one another (maintenance/gain of correlation/coherence) on therapy. We hypothesize that by revealing differentially coherent subnetworks of genes, we may identify inflammatory pathways in the airway with increased coregulation despite anti-IL5 therapy. Additionally, if we were to consider every gene pair interaction of the transcriptome, there would be millions of gene pair combinations to consider. In order to make sense of these gene pair interactions in the context of a treatment effect, we narrow our focus to a subset of genes that are within the modules of clinical interest identified as being associated with exacerbation risk in the MUPPITS-2 study (modules associated with eosinophilic inflammation and modules associated with non-T2 epithelial inflammation). Critically, the inclusion of a matched placebo arm for comparison allows us to identify gene regulatory network changes attributable to mepolizumab therapy. Our analysis characterizes complex dynamic changes in the behavior of regulatory networks in the airway initiated by mepolizumab that we have shown are otherwise well conserved across cohorts and over time5.
Results
Study design and participant characteristics
The MUPPITS-2 study was a randomized, double-blind, placebo-controlled, parallel-group trial of urban children (6–17 years) (n = 290) with exacerbation prone-eosinophilic asthma and participants were randomized in a 1:1 ratio to receive either mepolizumab or matching placebo added to guideline-based care for 52 weeks2. In this current analysis, we compared RNA-sequencing data from nasal lavage samples at baseline (before treatment) to a composite timepoint (on treatment) in order to maximize the number of paired samples for participants receiving mepolizumab (n = 134) or placebo (n = 135) (Supplementary Table 1). Samples were collected at two scheduled on treatment timepoints (week-8 and week-52). To maximize the number of individuals with paired samples but to avoid statistical biases that might arise from repeated on treatment samples per individual, we used one on treatment sample per participant favoring the week-8 sample if available and otherwise the week-52 sample (see “methods” for details) (see Supplementary Fig. 1A for sample numbers).
Mepolizumab treatment decreased gene correlations for a T2 inflammation module and increased correlations for epithelial associated modules
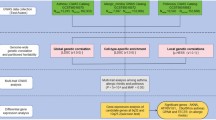
To assess whether there were global changes in the correlation structures of modules after mepolizumab therapy in biological pathways of interest, we assessed the within-module pairwise gene-gene Pearson correlation coefficients from before treatment versus on treatment for 6 modules of interest: T2 inflammation module (eos1), Eosinophil activation & mucus hypersecretion (eos3), Eicosanoid metabolism (eos5), EGFR signaling & cell-cell adhesion (epi1), Extracellular matrix production & cell membrane (m13), and TGFβ, Smad3 cell differentiation (m27). These 6 modules were selected for this analysis because we had previously found them to be associated with asthma exacerbation risks in urban children and with positive or negative responses to mepolizumab therapy2,5. We first confirmed that these modules had evidence of overall preservation from the MUPPITS-1 to the MUPPITS-2 study using the established composite preservation statistic (Zsummary > 10)8 allowing interpretation of the differential expression effects of mepolizumab on the overall module expression values (Supplementary Fig. 1). Comparing the within-module pairwise gene-gene Pearson correlation coefficients showed that the T2 inflammation (eos1) and Eicosanoid metabolism (eos5) modules had decreased within-module correlations on mepolizumab treatment, compared to before treatment. In contrast, the Eosinophil activation & mucus hypersecretion (eos3), EGFR signaling & cell-cell adhesion (epi1), Extracellular matrix production & cell membrane (m13) and TGFβ, Smad3 cell differentiation (m27) modules showed an increase in the median Pearson correlation on mepolizumab treatment. The Pearson correlations remained stable across timepoints on placebo therapy for each of these 6 modules (Fig. 1a), confirming this observation was a specific effect of mepolizumab therapy. Sensitivity analyses subset to the 8-week and 52-week treatment time points yielded congruent results (Supplementary Fig. 1b).
a Module boxplots (median ± interquartile range with whiskers 1.5 times the interquartile range). N participants; Before treatment placebo: 124; On treatment placebo: 120; Before treatment mepolizumab: 125; On treatment mepolizumab: 110. * empirical p < 0.05 (calculated with differential modular connectivity; two-sided test); all other contrasts not significant. p-values (left to right) = 0.32; 0.02; 0.63; 0.01; 0.32; 0.08; 0.93; 0.03; 0.86; 0.02; 0.12; 0.11. b Modules are listed by their cell association and annotation; further details about each module are in Supplementary Table 2. c Total participant numbers shown for each treatment group (before treatment and on treatment).
Eosinophil-associated modules had negative differential gene correlation and epithelial-associated modules had positive differential correlation after mepolizumab treatment
Given the observed module-level changes in median pairwise correlation on mepolizumab treatment, we sought to further interrogate the subnetwork changes in module intergene pair correlations using DGCA, a method that identifies differential correlations among genes across conditions and thereby coherent coregulated subnetworks and hub genes under different biological conditions7. Using the pairwise gene-gene Pearson correlations, individual gene pairs within-module were first classified as having significant positive across-sample correlation, no significant correlation, or significant negative correlation at each time point (before treatment or on treatment) for each treatment group (mepolizumab or placebo) based on a Pearson correlation p < 0.05. Next, the gene-pair differential correlations between time-points, which are the changes in correlation from before treatment to on treatment within each group, were classified as either a gain in correlation (GOC), no change in correlation, or loss in correlation (LOC) by calculating the difference in Z-transformed correlation coefficients for each gene pair (differential correlation z-score) and using a threshold of differential correlation significance of a false discovery rate corrected p value (FDR) < 0.05 adjusted within-module (Supplementary Fig. 3). Finally, the median differential correlation z-score for each individual gene was calculated. A median differential correlation z-score below 0 indicated an overall loss of correlation between the given gene and the remainder of the genes in the module before treatment compared to on treatment, and similarly above 0 indicated an overall gain of correlation.
We assessed differential gene correlation at the module-level by contrasting the median differential correlation across all gene pairs within a module (modular differential connectivity). Four modules had significant modular differential connectivity in the mepolizumab group with empirical p < 0.05 (indicated by * in Fig. 1a). The T2 inflammation (eos1) module had a significant negative median differential correlation z-score difference indicating an overall loss of correlation of gene pairs within the module on treatment raising the possibility that gene interactions within this network were being disrupted. The eosinophil activation & mucus hypersecretion (eos3), EGFR signaling & cell-cell adhesion (epi1) and Extracellular matrix production & cell membrane (m13) modules had significant positive median differential correlation z-scores indicating an overall gain of correlation of gene pairs within the respective modules raising the possibility that gene interactions within these networks were being strengthened (Fig. 1a, Supplementary Table 3). These effects were not explained by changes in cell composition as the same analysis done after regressing out variance related to cell proportions provided similar results (Supplementary Fig. 4). None of the 6 modules had significant differences when assessing the cross-sectional before treatment comparisons between the placebo and mepolizumab groups, indicating there were no baseline differences in module correlation between the two groups (Supplementary Table 4).
Differential gene correlation is a distinct measurement from differential gene expression
For participants receiving mepolizumab, the changes in module-level gene expression from before treatment to on treatment were consistent with our previously published analysis of the full MUPPITS-2 cohort and they were directionally congruent in the module-level differential gene correlations (Supplementary Table 3). The module-level gene expression changes were consistent using either the log average of expression of genes in the module or the WGCNA eigengene9 (which is the first principal component derived from the expression of the module genes), which we show are functionally equivalent as a summary metric of module expression (Supplementary Fig. 5).
Given a directional congruence between changes in average module correlation and module expression due to mepolizumab therapy, we assessed whether gene level differential correlation was congruent with gene level differential expression or whether these metrics provided unique information about the relative effect of mepolizumab therapy on the gene components of modules. Overall, for participants receiving mepolizumab treatment, there was little association between change in differential gene expression and median differential correlation z-score; the adjusted R2 for the 6 modules ranged from <0.01 to 0.26 demonstrating these two metrics provide unique information about the effects of mepolizumab on gene subnetworks in the modules (Supplementary Fig. 7). The genes with the greatest median differential correlation z-score were not among the genes with the greatest differential gene expression indicating that differential correlation identifies distinct leading edge genes. As an example, in the T2 inflammation (eos1) module, PAPSS1, a gene expressed in eosinophils and responsive to TGFß−1 and IL-3310,11, had the largest median differential correlation z-score (−3.23) in mepolizumab treated participants, despite a fairly small decrease in gene expression (−0.33 log2 fold change). In contrast, traditional effector molecules of eosinophils such as CCL23, RNASE2, CLC, had the largest differential gene expression (−1.36 to −1.94) but relatively small decreases in in median differential correlation z-score in mepolizumab treated participants (−1.25 to −1.91) (Fig. 2a). It was also apparent that within this module, the eosinophil expressed genes had more negative median differential correlation z-scores compared to mast cell and T2 cytokine genes suggesting that the gene interactions amongst eosinophil genes were disrupted whereas preserved amongst mast cell and T2 cytokine genes. For the Extracellular matrix production/cell membrane (m13) module, P2RY1, a purinergic receptor highly expressed in airway epithelial cells, macrophages12, and granulocytes and NOS2, nitric oxide synthase 2, an inflammation induced enzyme that produces nitric oxide and a marker of airway inflammation13, had high differential correlation z-scores of 2.29 and 2.23, respectively, though, had relatively low increases in expression when comparing before to on treatment (0.34 and 0.44 log2 fold change, respectively) (Fig. 2b). Past works have shown that differential correlations identify differentially coherent subnetworks of genes and unique regulatory hub genes based on the differential correlations7,14,15, in particular how genes may have fallen out of coregulation with one another (loss of coherence) or maintained or increased coregulation with one another (maintenance or gain of coherence) and our results are consistent with this metric providing a unique assessment that is generally independent of differential expression.
Subnetwork of genes gaining correlation identified within the T2 inflammation (eos1) module after mepolizumab treatment
To understand which subnetworks of each module experienced changes in correlation structure due to mepolizumab therapy, we assessed the proportion of genes within each module that had significant change in their gene correlations with any other gene before treatment compared to on treatment and we contrasted this to the placebo group. We noted there was large variation among modules in the proportion of genes within the modules that had significant changes in correlation with mepolizumab treatment even amongst the 4 modules that had significant differential modular connectivity (ranging from 20% of the 209 genes in Extracellular matrix production & cell membrane (m13) to 92% of the 242 genes in the T2 inflammation (eos1) module) (Fig. 3). Comparing these results to the parallel analyses in participants receiving placebo, we observed these modules had zero genes with significant changes in differential gene correlation between the two time points.
Gain of correlation (GOC) with other genes represented in red. Loss of correlation (LOC) with other genes represented in blue. Degree of color gradient represents the proportion of the genes within the module with any significant differential correlations (FDR < 0.05) (more opaque reflects a greater percentage of genes in the module having significant differential correlation). DGC = differentially correlated genes.
We additionally conducted cross-sectional contrasts of the mepolizumab and placebo participants at each timepoint. We found the T2 inflammation (eos1) module had the highest percentage of significant differential gene correlations on treatment between the participants receiving mepolizumab and placebo (76% of all genes in the module had loss of correlation with another gene) (Supplementary Fig. 8). These cross-sectional contrasts further indicated that the module most impacted by mepolizumab treatment for differential correlation was the T2 inflammation module.
We identified that while the vast majority of the gene pairs had differential correlation changes that matched the direction of modular differential connectivity (i.e., negative modular differential connectivity corresponded to mainly loss of correlation amongst gene pairs), there were also instances of genes that had correlation changes in the opposite direction. Most notably, of the 222/242 genes in the T2 inflammation (eos1) module that had significant differential modular connectivity, 204 genes had only loss of correlation with another gene indicating probable loss of coregulation, whereas 18 genes had GOC with at least one other gene in the module after treatment with mepolizumab indicating probable greater coregulation, alongside the 20 genes in this module that maintained correlations. Given the unique subnetwork correlation findings within these modules and specific to mepolizumab therapy, we next performed an in-depth investigation on the correlation changes at the gene level. To highlight the biological impact of conducting this analysis, we focus on two modules, T2 inflammation (eos1) and Extracellular matrix production & cell membrane (m13), so as to focus on high value probable gene regulatory changes resulting from mepolizumab’s effects.
Eosinophil-specific genes lose correlation and mast cell-specific genes/T2 cytokines gain correlation within T2 inflammation module after mepolizumab treatment
We used tower plots to visualize differential correlations among gene pairs in a network graph diagram to better understand the underlying structure and molecular pathways within each module (Figs. 4, 5). We first evaluated the T2 inflammation (eos1) module for participants receiving mepolizumab treatment which is composed of eosinophil-specific genes, mast-cell specific genes, T2 cytokine genes, and epithelial inflammatory genes (Fig. 4). In participants receiving mepolizumab, the vast majority of the genes in the module lost correlation with other genes (these genes are located in the left half of Fig. 4a). Eosinophil-specific genes, including for example CCR3, CCL23, RNASE3, HIC1, PAPSS1, had high absolute median differential correlation z-scores indicating that these genes had a greater tendency towards loss of correlation with other gene pairs in the module. In clear contrast, mast cell-specific genes, including HDC, FCER1A, CPA3, MS4A2, GATA2, and TPSAB1, T2 cytokine genes including IL13, IL4 and IL5, and epithelial inflammatory genes including POSTN, MMP10, CCL13, and CCL17 showed both gains of correlation and maintenance of correlation (no significant change in correlation) with the other genes and were characterized by lower absolute median differential correlation z-scores, which reflects that these genes generally maintained coregulation with one another on treatment with mepolizumab.
a Tower plot for participants receiving mepolizumab comparing before treatment to on treatment. Each gene is represented by a plot point. Each line corresponds to significant differential correlation between the gene pairs (FDR < 0.05). Blue lines-loss of correlation (LOC). Red lines-gain of correlation (GOC). Isolated plot points-genes with no significant differential correlations. X-axis represents composition of differential correlations (complete LOC on left, complete GOC on right). Y-axis reflects the absolute median differential correlation z-score for each gene. Genes highlighted in yellow are eosinophil-specific genes. Genes highlighted in pink are mast cell-specific genes or T2 cytokine genes. b Highlighted genes of interest and brief description. See supplementary Table 5 for gene description references. See supplementary data 1 for DGCA results for all significant gene pairs associated with genes listed in tables and coordinate information for each gene.
a Tower plot for participants receiving mepolizumab comparing before treatment to on treatment. Each gene is represented by a plot point. Each line corresponds to significant differential correlation between the gene pairs (FDR < 0.05). Red lines represent a gain of correlation (GOC). Isolated plot points represent genes with no significant differential correlations. X-axis represents composition of differential correlations (complete LOC on left, complete GOC on right). Y-axis reflects the absolute median differential correlation z-score for each gene. Genes highlighted in green are associated with airway inflammation. b Highlighted genes of interest and brief description. See supplementary Table 5 for gene description references. See supplementary data 2 for DGCA results for all significant gene pairs shown in figure and coordinate information for each gene.
Genes associated with airway inflammation gain correlation within Extracellular matrix production/cell membrane module after mepolizumab treatment
The tower plot network visualization of the Extracellular matrix production/cell membrane (m13) module for participants receiving mepolizumab reflects differential correlations largely composed of gains of correlation (Fig. 5). In participants receiving mepolizumab therapy, the genes with significant gain of correlation with other genes (located on the right half of Fig. 5a) were mainly associated with airway epithelial inflammation. NOS2, nitric oxide synthase 2 (a marker of airway inflammation), displayed high absolute median differential correlation z-score, and other notable genes with significant gain of correlation included COL1A1 an asthma associated collagen/pro-fibrotic marker16, EPHA1 a tyrosine kinase linked to pulmonary inflammation17,18, and SMAD1, TRPM4, VIPR1 associated with airway smooth muscle growth and contraction19,20,21.
Down-regulation of stress response pathways, conservation of immune effector pathways, and up-regulation of cellular morphogenesis pathways after mepolizumab treatment
We next performed a tailored hypergeometric enrichment analysis within-module to better understand which annotated gene pathways were implicated in the observed changes to within-module correlation structure following treatment with mepolizumab (Fig. 6). Within-module, genes were quartile divided using their median differential correlation z-score, in order to compare enrichment of the lower quartile (partition 1; the quartile of genes with the most negative median differential correlation z-scores) to the upper quartile of genes (partition 2; the quartile of genes with the most positive median differential correlation z-scores) (Fig. 6/Supplementary Fig. 9). We utilized the Gene Ontology Biological Pathways gene sets with our full genome-wide bulk RNASeq dataset as background for pathway investigation22,23. For the T2 Inflammation (eos1) module, in the genes with the largest positive (or least negative) median differential correlation z-score (partition 2), the top pathways differentially enriched between partitions (empirical p < 0.05) included positive regulation of immune effector process/cell activation and regulation of immune effector process/leukocyte mediated immunity (Fig. 6A). In the genes with the largest negative median differential correlation z-score (partition 1), the top pathways differentially enriched between partitions (empirical p < 0.05) included response to oxidative stress/nitrogen compound/organic cyclic compound, cellular response to chemical stress.
Enrichment of top Gene Ontology Biological Processes (GOBP) pathways (ranked by enrichment difference p value) for participants receiving mepolizumab therapy comparing before treatment to on treatment: Partitioned by top/bottom quartile of median differential z-score (1 = most negative, 2 = most positive/least negative). Size of point reflects partition enrichment score. Dashed line = significant in full module (p < 0.05). P values were obtained from two-sided tests and adjustments were not made for multiple comparisons. GOBP pathway genes present in each partition labeled by partition color (blue = 1, red = 2). a Type 2 inflammation module (eos1). b Extracellular Matrix Production & Cell Membrane Module (m13). See supplementary data 3 for full enrichment results for eos1, m13 modules (tab 1,2 respectively).
For the Extracellular matrix production/cell membrane (m13) module, in the genes with the largest positive median differential correlation z-score (partition 2), the top pathways differentially enriched between partitions (empirical p < 0.05) included cellular component morphogenesis, developmental growth, positive regulation of multicellular organismal process, and cellular response to organic cyclic compound (Fig. 6B). In the genes with the least positive median differential correlation z-score (partition 1), the top pathways differentially enriched between partitions (empirical p < 0.05) included leukocyte differentiation, mononuclear differentiation, regulation of cellular response to growth factor stimulus, immune system development and cellular ketone metabolic process.
Participants with repeated exacerbations after mepolizumab therapy have the greatest increase in correlation of the Extracellular matrix production/cell membrane module
We next assessed whether the change in module correlation resultant from mepolizumab was associated with the treatment response by stratifying the population into exacerbation frequency subgroups, comparing individuals with 0 exacerbations (n = 41), 1 exacerbation (n = 45), and ≥2 exacerbations (n = 24) during mepolizumab treatment. Through a bootstrapping sensitivity analysis we assessed the effect each subgroup had on the within-module correlation change due to therapy - this was accomplished by iterative removal of participants from each subgroup or equally from all three (see “methods”). Participants who had poor clinical response to mepolizumab therapy (≥2 exacerbations) significantly contributed the most to the increase in correlation of the Extracellular matrix production/cell membrane (m13) module, whereas individuals with a good clinical response (0 exacerbations) significantly contributed the least (Fig. 7). Congruent with this observation, the differential gene correlation at the mepolizumab on treatment time point was significantly highest in the subgroup with ≥2 exacerbations and significantly lowest in the subgroup with 0 exacerbations (empirical p < 0.05), a finding that was not observed in the placebo group (Supplementary Fig. 10). We did not see a relationship of the correlation structure of the T2 inflammation (eos1) module with exacerbation frequency.
Boxplots depict the distribution of median gene-gene Pearson correlation values from the Extracellular Matrix Production & Cell Membrane module (m13) when calculated by iterative downsampling in the mepolizumab treatment arm at each time point. For each iteration, 15 individuals were randomly removed from one of 3 within treatment arm exacerbation subgroups (those with either 0, 1, or ≥2 exacerbations during the study), and the median correlation among the remaining samples was recorded. This was repeated 1000 times for each exacerbation subgroup. The resultant distribution of these median values indicates the relative contribution of each exacerbation subgroup on the overall observed module correlation for the group, thereby highlighting the relative effect of each subgroup on the module median correlation. Notably, in the mepolizumab on treatment group, when individuals were removed from the ≥2 exacerbation subgroup the correlations were lower than the group-level median indicating individuals with ≥2 exacerbations disproportionately drive the observed higher module correlation on mepolizumab treatment. The opposite is true for individuals in the 0 exacerbation subgroup, they contribute the least to the observed higher module correlation on mepolizumab treatment. Boxes show the interquartile range (IQR), whiskers extend to 1.5 × IQR. Solid black lines correspond to the overall group level within-module pairwise Pearson correlation for each time point corresponding to Fig. 1 (not stratified by exacerbation frequency). The left-hand boxplot (gray) indicates the effect of balanced random downsampling of 15 individuals where 5 individuals come from each of the 3 exacerbation subgroups on each iteration as comparison. Statistical annotations indicate mean p < 0.05* and mean p < 0.01** in two-sided t-test contrasting the full DGC Z-Score distributions for each exacerbation group downsampling relative to the balanced downsampling, and are averaged across 1000 random iterations. p-values (left to right) = 0.0118; 0.0176; 0.0107; 0.0094; 0.0125; 0.0062.
Discussion
In this study, we demonstrated that mepolizumab therapy alters the correlation structure of airway gene co-expression modules that are associated with asthma exacerbation risk and thereby gained insights into airway gene network regulatory alterations caused by blocking IL-52. These results shed insight into specific persistent or augmented airway inflammatory pathways that remain despite targeted IL-5 blockade. We further show how this finding relates to the incomplete clinical effect of this therapy in this urban pediatric population. Broadly, we found that mepolizumab treatment resulted in loss of correlation amongst eosinophil-specific genes but conservation and even strengthening of correlation amongst mast cell-specific genes, T2 cytokines, and airway epithelial inflammatory genes, all of which appeared co-expressed as part of a T2 inflammation gene network in the absence of mepolizumab treatment. Interestingly we also found that mepolizumab induced significant GOC of genes specifically associated with multiple aspects of airway epithelial inflammation and the dysregulation of this module directly associated with exacerbation risk in the mepolizumab group. Specifically, at the module-level, we found that mepolizumab treatment caused an unbalanced loss of correlation structure within an eosinophil-associated module enriched for T2 inflammation genes (eos1) and an increase in correlation structure within epithelial-associated modules enriched for Extracellular matrix production/cell membrane (m13) and EGFR signaling/cell-cell adhesion (epi1) genes. In contrast, the correlation structure of these modules was unchanged on placebo therapy. These changes due to mepolizumab therapy were not attributable to changes in cell composition, such as reduction of eosinophils, thus indicating fundamental alterations in gene expression network coherence due to therapy.
Interpreting high gene correlations to indicate coregulation, the disruption of eosinophil gene coregulation appears to result in potentially a reciprocal induction and greater coregulation of T2 cytokines with airway mast cells and epithelial inflammation, which indicates the persistence or even augmentation of other non-eosinophil components of T2 inflammation mediated by mast cell activation and likely their interactions with the airway epithelium despite this T2 directed therapy. Several previous studies have shown that mast cell signatures have been associated with an eosinophilic asthma phenotype including one study showing quantitative gene expression of sputum mast cell genes (including GATA2, CPA3, HDC, FCER1A) was associated with eosinophilic airway inflammation and blood eosinophils in severe asthma and another study demonstrating that patients with high enrichment scores for FcɛRI-activated mast cell signatures have distinct characteristics associated with severe eosinophilic inflammation24,25. Additional studies have further substantiated this concept of eosinophils and mast cells being closely coupled in the airway in T2 asthma26,27. Our findings of uncoupling the coregulation of these responses by blocking the IL-5 pathway, suggests this treatment can accentuate mast cell inflammation in the airway. This concept of eosinophil and mast cell coregulation is well supported in other atopic diseases including eosinophilic esophagitis and aspirin exacerbated respiratory disease/nasal polyposis. Notably in eosinophilic esophagitis studies, mast cell activation and persistence of disease has been noted despite eosinophil depletion congruent with our current result28,29,30. In aspirin exacerbated respiratory disease studies, mast cells accumulate in nasal/bronchial mucosa at higher concentrations than controls driven by both innate and adaptive pathways and polyp mast cell proliferation has been shown to have direct correlation with peripheral blood eosinophilia31,32. The T2 cytokine genes (IL4, IL13) gain of correlation with other genes suggests ongoing potential activation of IL-4/13 which can act to induce B-cell class switching to IgE production, induce further Th2 cell development, and upregulate inducible nitric oxide synthase33. As such, biologics like omalizumab (anti-IgE monoclonal antibody) or dupilumab (monoclonal antibody directed against the IL-4R chain, thereby blocking the downstream effects of both IL-4 and IL-13) are therapeutic agents that could potentially have broader influence on these pathways left unaffected or augmented by mepolizumab treatment and therefore may be expected to have superior clinical benefit in this population.
We additionally found that the pathways involving response to stress (oxidative, nitrogen, chemical) lost correlation with mepolizumab treatment and all pathways included CAT (catalase). Catalase, found in alveolar type II pneumocytes/macrophages and eosinophils as a granule-stored enzyme, functions as a protective antioxidant enzyme which may be diminished after anti-IL5 therapy34,35,36.
Notably, for the Extracellular matrix production/cell membrane (m13) module we found that mepolizumab treatment was associated with significant gains in correlation amongst the genes linked to inflammatory pathways indicating increased coregulation of those pathways. NOS2 and P2RY1 had the highest positive correlation with other genes in the module (including one another). Nitric oxide is known to be a critical factor in inflammatory lung disease and the majority of nitric oxide production in the inflammatory state derives from inducible nitric oxide synthase (NOS2) in the airway epithelium37,38. FeNO is a clinical biomarker of T2 inflammation primarily reflecting IL-13 activity. This increased coregulation of NOS2 with epithelial barrier genes while on mepolizumab therapy may give mechanistic insight into the clinical finding that mepolizumab therapy does not result in reduction of FeNO despite a reduction of T2 inflammation, which was also observed in the MUPPITS-2 cohort2,39. P2RY1 is a purinergic receptor involved in adenosine 5’-triphosphate (ATP) energy transfer and highly expressed in airway epithelial cells, macrophages, and granulocytes. In the airway, purinergic signaling is known to be important for mucociliary clearance, mucin secretion, ciliary beat frequency, modulating airway diameter through airway smooth muscle, recruitment/activation of immune cells (alveolar macrophages, lung dendritic cells, lung mast cells) and previous studies have shown respiratory diseases, like asthma, to have aberrant purinergic signaling12,40. Other notable genes gaining correlation included COL1A1 an asthma-associated collagen/pro-fibrotic marker16 and EPHA1 a tyrosine kinase linked to pulmonary inflammation via increased vascular permeability17,18. Genes associated with airway smooth muscle growth and contraction (SMAD1, TRPM4, VIPR1) were also found to be gaining correlation with the caveat that these results are from upper airway samples (not direct sampling from lower airway)19,20,21. Critically, we observed that this augmented correlation of the Extracellular matrix production/cell membrane (m13) module was disproportionately driven by those mepolizumab treated individuals who had multiple exacerbations during the study, linking a direct adverse clinical outcome to this molecular finding. We hypothesize that enhanced coordinated regulation of this pathway, an unanticipated effect of mepolizumab, exacerbates airway epithelial inflammation responsible for continued poor clinical asthma control.
While we found a directional congruence between changes in average module correlation and module expression on mepolizumab therapy, at the individual gene level, there was little association between change in differential gene expression and differential gene correlation. Our findings revealed these metrics identified different leading-edge genes consistent with past works that have shown differential correlation analyses identify unique regulatory hub genes while additionally revealing gene regulatory interactions7,14,15. More broadly, DGCA may be a useful addition to gene expression analyses in pharmacogenomics studies to better understand treatment impacts from a mechanistic perspective. Importantly, by implementing this analysis in the framework of a placebo-controlled trial, we found a stark difference in the differential gene correlations in the mepolizumab and placebo groups, indicating that mepolizumab treatment fundamentally changes coregulation of gene pathways.
This descriptive analysis was performed to define changes in gene interactions among conserved pathways after mepolizumab treatment. Differential correlation analyses are relatively uncommon compared to differential expression analyses, and lack of historical studies providing insight into the clinical relevance/effect thresholds for differential correlations between gene pairs limits the interpretability of our results. However, we would argue this work strongly motivates the need to better explore these approaches in clinical-translational studies. The statistical approach used does not identify the molecular or cellular etiologies of changes in gene coregulation. These could be mediated by transcription factors, epigenetic modifications, changes in cell composition or cell cross-talk, among other possibilities. Unfortunately, the method is not currently suited to assess how these covariates, such as cell counts measured in these same samples, might impact the observed changes. To investigate the molecular and cellular mechanisms underlying these changes in gene connectivity, future studies could integrate single-cell or spatial transcriptomics to localize pathway activity to specific cell types or niches. In addition, perturbation-based functional studies (e.g., CRISPR screens and in vitro airway epithelial co-culture models) may help identify causal drivers of altered gene network regulation that could provide insights into targetable molecules. Another limitation of our study was analysis of nasal airway samples as a surrogate for lower airway pathophysiology, though, prior studies have shown strong associations of upper airway sampling to lower airway pathophysiology41,42. Lastly, since the study population selected for T2-high asthma participants from low-income urban centers, the results may not represent the response to mepolizumab in other asthma demographics or phenotypes.
In conclusion, targeted anti-IL5 treatment with mepolizumab perturbed conserved gene co-expression modules, disrupting the correlation structure of co-expressed genes, thereby identifying differentially coherent subnetworks among gene pathways. We revealed the anticipated loss of coregulation amongst eosinophil specific genes after IL-5 blockade, but also discovered conserved and increased coregulation of mast cell genes, T2 cytokine genes and epithelial T2 response genes indicating persistence of alternate aspects of T2 inflammation. Moreover and adding key clinical context, we observed mepolizumab therapy to augment coregulation of epithelial specific matrix, remodeling, and inflammation genes, which directly associated with a poor clinical response to therapy in some individuals. These indicate an unanticipated, and potentially adverse secondary effect of the therapy in this population.
Overall, this study provides application of DGCA to a pediatric randomized clinical trial with mepolizumab, revealing functional alteration of multiple airway inflammatory pathways after anti-IL5 therapy, which could provide insights towards future therapeutic strategies for children with exacerbation-prone asthma.
Methods
This research complies with all relevant ethical regulations. The MUPPITS-2 study protocol was approved by the Western Institutional Review Board2.
Study design
The MUPPITS-2 (Mechanisms Underlying Asthma Exacerbations Prevented and Persistent with Immune Based Therapy: a Systems Approach Phase 2) study (conducted November 2017 to March 2020) was a randomized, double-blind, placebo-controlled, parallel-group trial to assess the efficacy, safety and mechanisms of therapy with mepolizumab added to guideline-based therapy for children with exacerbation-prone eosinophilic asthma living in low-income urban areas performed at nine urban medical centers in the USA. The study was approved by the Institutional Review Board2.
The MUPPITS-1 study was a prospective, longitudinal case-control study assessing gene expression changes during colds that progressed to exacerbations versus those that resolved without intervention. In this study, we identified upper airway transcriptome modules, which are networks of co-expressed and functionally related genes, associated with asthma exacerbations in urban children. The MUPPITS-1 study (conducted October 2015 to January 2017) was performed across nine urban medical centers in the USA. The study was approved by the Institutional Review Board5.
Participant characteristics
For the MUPPITS-2 study, inclusion criteria included children and adolescents (6–17 years) with exacerbation-prone asthma and blood eosinophil count of 150 or more cells per µL who lived in prespecified low-income US census tracts. Participants had been diagnosed by a medical doctor as having asthma for at least 1 year, had two or more exacerbations treated with systemic corticosteroids in the previous year, required a minimum inhaled corticosteroid regimen of twice-daily treatment with fluticasone propionate 250 µg or equivalent (6–11 years) or fluticasone propionate/salmeterol 250/50 µg or equivalent (12–17 years). Parents approved written informed consent and children aged 12–17 years provided written assent and children aged 7–11 years provided verbal assent2.
For the MUPPITS-1 study, inclusion criteria included children and adolescents (6–17 years) with exacerbation-prone asthma and blood eosinophil count of ≥150 cells/µL who received treatment at one of nine identified urban medical centers. Participants had been diagnosed by a medical director as having asthma for at least 1 year, had two or more exacerbations treated with systemic corticosteroids in the previous year, required a minimum of inhaled corticosteroid regimen of twice-daily treatment with fluticasone propionate 250 µg or equivalent (6–11 years) or fluticasone propionate/salmeterol 250/50 µg or equivalent for those aged 12–17 years. Parents approved written informed consent and children provided assent if applicable5.
MUPPITS-2 study randomization
After screening, eligible participants were enrolled in a 4-week run-in period and the study teams assumed management of the participants’ asthma care according to a guideline-line based treatment algorithm. Participants were then randomized in a 1:1 ratio to receive either mepolizumab (6–11 years: 40 mg; 12–17 years: 100 mg) or matching placebo, by subcutaneous injections once every 4 weeks for 52 weeks added to their guideline-based care2.
Sample collection
Nasal lavage samples were collected during pre-scheduled visits before treatment (baseline/week-0) and on treatment (week-8, week-52). To maximize the number of individuals with paired samples but to avoid statistical biases that might arise from repeated on treatment samples per individual, we used one on treatment sample per participant favoring the week-8 sample if available and otherwise the week-52 sample (Supplementary Fig. 1A). The visit03/week-8 sample was favored because it had the larger number, which was a result of the study concluding during the start of the COVID-19 pandemic, which prevented collection of nasal lavage samples during visit14/week-52 on a non-trivial number of participants. Both week-8 and week-52 samples were obtained at least 7 weeks after starting mepolizumab treatment and 2 doses of therapy. None of the samples were obtained while the participants were actively receiving oral corticosteroids. Nasal lavage was collected immediately after a nasal blow procedure detailed as follows and previously described5. Briefly, the participants squeezed sterile sodium bicarbonate-buffered normal saline from a 240 mL sinus rinse bottle (NeilMed Sinus Rinse) into each nostril while 15-20 mL of fluid was collected from the other side. Samples were kept on ice during/after collection. The collected lavage was centrifuged at 1300 × g for 10 min at 4 °C, then resuspended, strained (100-micron strainer—MACS SmartStrainer) and homogenized via pipette mixing. 1.3 mL was removed and set aside for histology slide generation, and the remainder was centrifuged at 1300 × g for 10 min at 4 °C, the supernatant was removed, and 1 mL of RNAprotect Saliva Reagent (Qiagen) was added and the pellet resuspended. Samples were kept on ice and/or at 4 °C during processing5.
Nasal cell differentials
Each nasal lavage sample was used to create 6 slides using a Cytospin 4 Cytocentrifuge (ThermoFisher Scientific) using a previously established protocol5. Briefly, 2 slides were made at each of 3 serial dilutions of the sample. Slides were air dried for 10 min, then fixed and stained using a Hema3 Stain Set (Thermo Fisher Scientific) following the manufacturer’s protocol. Nasal cell differentials were performed by manual review of the 6 cytospin slides per sample, followed by manual read of 2 slides with optimal cell density to distinguish among neutrophils, lymphocytes, macrophages, eosinophils, respiratory epithelium, and squamous cells using previously established morphologic criteria5. Two sequential counts were performed on each sample. All slides were read by a single experienced individual who was blinded to clinical and outcome variables2.
RNA-seq library preparation and sequencing
RNA-sequencing was performed on RNA extracted from nasal lavage cell pellets and is publicly available at the US National Center for Biotechnology Information Gene Expression Omnibus (GEO) accession number GSE1928612. The RNA sequencing methods detailed as follows were previously described5. Total RNA was isolated from nasal cell pellets in RNAprotect Saliva Reagent (Qiagen). Samples were centrifuged for 10 min at 10,000 × g, supernatant was removed, and the cell pellet was resuspended in 350 μL of RLT buffer + 1% β-mercaptoethanol. Samples were vortexed for 1–2 s, sonicated for 30 s, spun through a QIAshredder column (Qiagen), and then extracted using the RNeasy MinElute spin column (Qiagen) following the manufacturer’s protocol.
RNA quality was assessed by RNA electrophoresis (Agilent) and NanoDrop 1000 (NanoDrop Products, Thermo Fisher Scientific). Sequencing libraries were constructed from total RNA with TruSeq RNA Sample Preparation Kit v2 (Illumina) and clustered onto a flow cell by using the cBOT amplification system with the HiSeq SR v4 Cluster Kit (Illumina). Single-read sequencing was carried out on a HiSeq2500 sequencer (Illumina), by using the HiSeq SBS v4 kit to generate 58-base reads, with a target of approximately 10 million reads per sample. Samples were sequenced in three batches using repeated samples across batches to allow assessment for batch effects. The resulting bcl files were deconvoluted and converted to fastq format by using Casava from Illumina. fastq files were aligned to the Ensembl version of the human genome (GRCh38; GenBank assembly GCA_000001405.15) using TopHat (version 1.4.1)43. The single-paired flag was set to “single”, while all other TopHat parameters were set to default. HTSeq-count44 was used to generate gene counts with the mode set to “Intersection (nonempty)” and the minimum alignment quality set to 0 and otherwise set to default. For quality control, samples were kept that had human aligned counts >250,000 and median CV coverage of <1.1. Genes were filtered to include those with trimmed mean of M value normalization count of at least 1 in at least 10% of libraries and were classified as protein coding using BioMart45. Normalized counts were transformed to log2 counts per million along with observations level weights by using voomWithQualityWeights from the “limma” R package version 3.4646,47. Processing batch did not contribute meaningfully to gene expression variability, explaining 1% of gene expression by Principal Variance Component Analysis, and Pearson correlations between technical replicates (N = 58 pairs) ranged from 0.97 to >0.99, so for replicate samples, the replicate with the highest aligned counts was included for analysis and other replicates removed. Age, sex, batch, and technical quality variables (log base 2 of aligned sequences, log base 2 of total reads, median CV coverage), did not confound treatment arm assignment at either timepoint and would not be expected to bias downstream analyses. RNA sequencing was performed by the same methodology in MUPPITS-1 and MUPPITS-22,5.
Module definition
In the MUPPITS-1 study, RNA-sequencing data were summarized into cell-type associated gene co-expression modules with functional annotation using the following previously published and validated module analysis framework6. Briefly, cell type deconvolution was performed by assigning genes to cell types on the basis of statistically significant positive Pearson correlation between gene expression and cell differentials according to CellCODE48. For the nasal samples, 13672 unique protein-coding genes were identified and 6 cell types (eosinophil, epithelial, lymphocyte, neutrophil, macrophage, squamous, unmatched/unassigned). Following cell assignment, weighted gene co-expression network analysis, WGCNA9, was run on each separate cell type, resulting in a total of 52 nasal modules ranging in size from 36 to 1041 genes. The WGCNA parameters used were minCorKME = 0.7 (module required block of genes with Pearson correlation of at least 0.7) and minKMEtoStay = 0.5 (all genes in a module required Pearson correlation of ≥0.5 with the module’s eigengene). Module-level expression values were calculated by using the geometric mean for the genes assigned to the module. The biological function of the modules was investigated using Database for Annotation Visualization and Integrated Discovery version 6.8 and STRING version 10.549,50,51,52. A summary annotation of each module was derived from manual inspection, functional enrichment, and interaction network. Details about the size, gene composition, and annotations of each module can be found in Supplementary Table 2.
Module preservation analysis (MUPPITS-1/2)
Module preservation analyses were performed to assess whether these upper airway transcriptome modules were generally conserved between the MUPPITS-1 and MUPPITS-2 studies, and could be sensibly interpreted when applied to the MUPPITS-2 transcriptome data8.
Module preservation statistics (median rank and Zsummary) were calculated using the WGCNA package to assess whether the modules of co-expressed genes created using the MUPPITS-1 dataset were generally preserved within the MUPPITS-2 dataset8.
Median rank (medianRank), a composite module preservation statistic useful for comparing relative preservation among multiple modules, was calculated for each module. A module with lower median rank tended to exhibit stronger observed preservation statistics than a module with higher median rank8.
A permutation test procedure randomly permuted the module assignment in the test data. The mean and variance of the preservation statistic under the null hypothesis of no relationship between the module assignments in reference data and test data were calculated based on the permutation test. A Z statistic for the preservation statistic was then defined by standardizing each observed preservation with regard to the mean and variance. Each Z statistic approximately followed the standard normal distribution if the module was not preserved. The higher the value of a Z statistic, the stronger the evidence that the observed value of the preservation statistic was significantly higher than expected by chance8.
For correlation networks based on quantitative variables, density preservation statistics were summarized by Zdensity, and connectivity based statistics were summarized by Zconnectivity, and all individual Z statistics were summarized by Zsummary defined as follows:
The WGCNA package guidelines suggested the following thresholds for Zsummary: if Zsummary > 10 there was strong evidence of module preservation; if 2 < Zsummary < 10 there was weak to moderate evidence of preservation; if Zsummary < 2, there was no evidence of module preservation8.
46 out of 52 modules described using the MUPPITS–1 dataset exhibited strong evidence of preservation in the MUPPITS-2 dataset (Zsummary > 10) and 6 out of 52 modules had weak to moderate evidence of preservation in the MUPPITS-2 dataset (2 < Zsummary < 10)8. Module size (number of genes assigned to each module) was compared to preservation median rank score and preservation Zsummary (Supplementary Fig. 2). There was not an association between module size and median rank score (Supplementary Fig. 2a). Modules with lower median rank tend to exhibit stronger observed preservation statistics (modules with lowest rank = neut5, neut6, neu4, neu2, neut3, squa1).
To assess module preservation across treatment groups (mepolizumab versus placebo), module Zsummary preservation statistics were then calculated for the endpoints of each MUPPITS-2 treatment arm (mepolizumab or placebo) individually (Supplementary Fig. 2c). 38 out of 52 modules displayed better preservation in the MUPPITS-2 placebo group, while 14 out of 52 modules were better preserved in the MUPPITS-2 mepolizumab treatment group. The differential in Zsummary was calculated for each module. The modules with a positive change (neut3, m18) indicated the module were better preserved in the mepolizumab group and the modules with a negative change (eos1, eos4) were better preserved in the placebo group (Supplementary Fig. 2d).
We noted a general trend toward weaker preservation of modules in the MUPPITS-2 mepolizumab group relative to placebo, which served as the basis for further interrogation of changes in gene co-expression patterns in this study. The MUPPITS-2 cohort dataset was used for the remainder of the analyses.
Within-module pairwise correlation
Median gene pair Pearson correlation was calculated for each module before treatment and on treatment for both treatment groups (mepolizumab versus placebo).
Classifying differentially correlated gene pairs
Individual gene pairs were first classified as having significant positive correlation, no significant correlation or significant negative correlation in each condition (before treatment and on treatment) (using a Pearson correlation significance threshold of p < 0.05) (Supplementary Fig. 3). Next when comparing the gene-gene correlations across time-points (before treatment and on treatment), the differential correlation between the gene pairs could be categorized as gain of correlation, loss of correlation, no significant change in correlation or a switch (eg; change from positive correlation before treatment to loss of correlation on treatment) using differential gene correlation analysis (DGCA, R package)7. For the purposes of our evaluation, the switches in correlation were considered to be loss of correlation, though these were uncommon in our analysis. The threshold of significance was set to FDR < 0.05 (adjusted p value using the Benjamini-Hochberg false discovery rate (FDR) within modules).
Average differential correlation between gene pairs
The median difference in z-score for each gene was calculated from pairwise differential gene correlation analyses by quantifying the median difference in z-transformed correlation coefficients of a gene-pair within a given module between before treatment and on treatment. A median difference in z-score above 0 indicated a tendency towards a gain of correlation between the given gene and the rest of the genes in the module before treatment compared to on treatment. A median difference in z-score below 0 indicated a tendency towards a loss of correlation between the given gene and the rest of the genes in a module before treatment compared to on treatment. To measure the significance of the median change in correlation, we used 10,000 permutations of sample assignments within contrast (eg. Mepolizumab before treatment vs Mepolizumab on treatment) to calculate empirical p values7.
Modular differential connectivity
DGCA was also used to calculate the modular differential connectivity as defined by the median difference in z-score across all gene pairs within a module before treatment compared to on treatment for each treatment group (mepolizumab versus placebo). A median difference in z-score above 0 indicated a tendency toward a gain of correlation between the gene-pairs within the module as a whole when comparing before treatment to on treatment. A median difference in z-score below 0 indicated a tendency towards a loss of correlation between gene pairs within the module as a whole when comparing before treatment to on treatment. Significance of the median differential z-scores within-module was then evaluated by randomly permuting the sample assignments across contrasts and recalculating differential correlation z-scores to generate a null distribution from which to draw empirical p values (10,000 permutations). At the module level, in order to calculate a two-sided p value, the median was taken over all of the gene pairs7.
To assess for the relevance of cell proportions on these results, this analysis was also repeated after regressing out variance due to cell composition. Principal component analysis was performed on the matrix of cell proportions (centered, scaled). PC1 (33.7% explanatory), PC2 (23.4%), or both were used to adjust gene expression counts using limma removeBatchEffect47. Residual counts were then used in the DGCA analysis (Supplementary Fig. 4A).
Comparing module gene expression metrics
We compared different metrics for relative gene expression of a module by assessing the correlations between module eigengene, geometric mean expression of each module and mean scaled expression of each module. The geometric mean of the module was defined as the overall gene expression of a module determined from the geometric mean expression of all constituent genes. Expression levels represent the log base 2 of the geometric mean for normalized expression of all genes within the module. We compared geometric mean expression to the eigengene and mean scaled expression metrics as calculated by the WGCNA package using the moduleEigengenes function8. In both the MUPPITS-1 and MUPPITS-2 cohorts, we found that Pearson correlations for each module in all expression contrasts were >0.98 suggesting that all of these metrics are comparable for relative module expression (Supplementary Fig. 5).
Additionally, we assessed module membership between the MUPPITS-1 and MUPPITS-2 cohorts by comparing eigengene-based connectivity kME for each module between the two cohorts. Module membership (eigengene-based connectivity kME) was defined as: kMEi module membership represented by correlation of genei expression to module summary value. 47 out of 52 modules had correlation (r) > 0.7 suggesting similar module connectivity metrics between the two cohorts (Supplementary Fig. 6).
Gene expression versus median differential Z-score
Differential gene expression was calculated for individual genes within a module (expressed as log2 fold change). Differential expression was then compared to median differential correlation z-score for each individual gene comparing before treatment versus on treatment for participants receiving mepolizumab (Fig. 2, Supplementary Fig. 7). At the gene level, a simple linear model used for comparison of median differential correlation z-score and differential expression.
Proportion of module with differential correlation
For each module, we calculated the percent of genes within the module that had any significant differential correlation with another gene pair. Differential correlation of gene pairs was considered significant when FDR < 0.05. Correlation outcomes were classified as loss of correlation (negative differential correlation between gene pairs), gain of correlation (positive differential correlation between gene pairs), switch (e.g., positive correlation before treatment became negative correlation on treatment) or no significant differential correlation (Fig. 3, Supplementary Fig. 8).
Visualizing differential correlations
Tower plots were created to better visualize differential correlations between gene pairs from before treatment to on treatment for modules of interest. Individual genes were represented by plot points. The size of each plot point represented the percent of significant gene pair correlation changes. Edges in the network graphs connect genes (vertices) that have a significant pairwise differential correlation comparing before treatment to on treatment (FDR < 0.05); A loss of correlation between the gene pairs is represented by a blue edgeline whereas a gain of correlation is represented by a red edgeline. Placement of each vertex reflects each gene’s median differential correlation z-score (y-axis, absolute value), and the composition of its significant differential correlations with the rest of the module (x-axis; ranging from 100% losses of correlation to 100% gains of correlation). Genes located in the middle of the plot without any other genes connected by lines did not have any significant differential correlations with other genes (Figs. 4, 5).
Gene enrichment analysis
We first calculated enrichment scores in the Gene Ontology Biological Processes (GO:BP) for all genes within the module with a background of all 13672 unique protein-coding genes from the MUPPITS-1 dataset with fenr R package53,54. Pathways were considered significant at permutation p < 0.01. We then split the module into quadrants based on median differential correlation z-score for individual genes. We compared the quadrant with the most negative differential correlation z-scores (partition 1) to the quadrant with the positive/least negative differential correlation z-scores (partition 2). Hypergeometric enrichment analysis was performed individually on each partition of the module, and 10,000 permutations of the gene assignments were used to randomly repartition the module and assign empirical p value to the observed difference in enrichments between partition 1 and partition 2. This approach allowed us to identify biological pathways enriched in the observed correlation changes, while controlling for existing background enrichments already present at the module level. Enrichment score and enrichment significance of the GO:BP pathways were calculated for each partition using the MUPPITS-1 unique protein-coding genes as the background (Fig. 6, Supplementary Fig. 9).
Exacerbation frequency analysis
We assessed the association of module coherence to patient exacerbation outcomes using a statistical resampling approach. We used random bootstrapping to better characterize how participants within three different exacerbation subgroups contribute to the observed changes in group level coherence. A series of randomized downsamplings was performed in which 15 individuals were randomly removed from one of each of the three within treatment arm exacerbation subgroups (those with either 0, 1, or ≥2 exacerbations throughout the study duration), and the median within-module correlation across the remaining individuals was recorded. The distribution of recorded median values across 1000 bootstrapped permutations for each subgroup indicated the relative contribution of each exacerbation subgroup to the overall group level observed module correlation, thereby highlighting the relative effect of each exacerbation subgroup on the module median correlation. As part of this sensitivity analysis, we additionally performed the bootstrap downsampling with removal of 5 participants from each exacerbation subgroup (15 individuals total) to capture any observable bias of downsampling generally (Fig. 7). Mean p across all iterations was calculated with correlation values relative to the balanced downsample. We further assessed differential gene correlation (differential modular connectivity/median differential z-score using the DGCA package) with the exacerbation subgroups to assess whether the observed differences among subgroups was statistically significant, with the caveat that the subgroups had different sample sizes in this analysis (Supplementary Fig. 10). There were no differences in demographics between the exacerbation groups for the participants receiving mepolizumab (Supplementary Table 6).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All study design information and data used in the analyses can be accessed through ImmPort (https://www.immport.org/) at accession number SDY1903. The raw RNA-sequencing FASTQ format data and minimum information about a high-throughput nucleotide sequencing experiment have been deposited with the US National Center for Biotechnology Information Gene Expression Omnibus at accession number GSE192861. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request. Clinical registry number and link: A Trial of Mepolizumab Adjunctive Therapy for the Prevention of Asthma Exacerbations in Urban Children (MUPPITS-2) ClinicalTrials.gov ID: NCT03292588.
References
Zahran, H. S., Bailey, C. M., Damon, S. A., Garbe, P. L. & Breysse, P. N. Vital signs: asthma in children—United States, 2001-2016. MMWR Morb. Mortal. Wkly. Rep. 67, 149–155 (2018).
Jackson, D. J. et al. Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet 400, 502–511 (2022).
Chupp, G. L. et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir. Med. 5, 390–400 (2017).
Ortega, H. G. et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 371, 1198–1207 (2014).
Altman, M. C. et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat. Immunol. 20, 637–651 (2019).
Ahn, S., Grimes, T. & Datta, S. A pseudo-value regression approach for differential network analysis of co-expression data. BMC Bioinform. 24, 8 (2023).
McKenzie, A. T., Katsyv, I., Song, W. M., Wang, M. & Zhang, B. DGCA: a comprehensive R package for differential gene correlation analysis. BMC Syst. Biol. 10, 106 (2016).
Langfelder, P., Luo, R., Oldham, M. C. & Horvath, S. Is my network module preserved and reproducible?. PLoS Comput. Biol. 7, e1001057 (2011).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 9, 559 (2008).
Sridhar, S., Liu, H., Pham, T. H., Damera, G. & Newbold, P. Modulation of blood inflammatory markers by benralizumab in patients with eosinophilic airway diseases. Respir. Res. 20, 14 (2019).
Shen, Z. J., Hu, J., Esnault, S., Dozmorov, I. & Malter, J. S. RNA Seq profiling reveals a novel expression pattern of TGF-β target genes in human blood eosinophils. Immunol. Lett. 167, 1–10 (2015).
Thompson, R. J., Sayers, I., Kuokkanen, K. & Hall, I. P. Purinergic receptors in the airways: potential therapeutic targets for asthma?. Front Allergy 2, 677677 (2021).
Corcoran, T. E. et al. Mucociliary clearance differs in mild asthma by levels of type 2 inflammation. Chest 160, 1604–1613 (2021).
Singh, A. J., Ramsey, S. A., Filtz, T. M. & Kioussi, C. Differential gene regulatory networks in development and disease. Cell Mol. Life Sci. 75, 1013–1025 (2018).
Lea, A. et al. Genetic and environmental perturbations lead to regulatory decoherence. Elife 8, https://doi.org/10.7554/eLife.40538 (2019).
Savin, I. A., Markov, A. V., Zenkova, M. A. & Sen’kova, A. V. Asthma and post-asthmatic fibrosis: a search for new promising molecular markers of transition from acute inflammation to pulmonary fibrosis. Biomedicines 10, https://doi.org/10.3390/biomedicines10051017 (2022).
Coulthard, M. G. et al. Eph/Ephrin signaling in injury and inflammation. Am. J. Pathol. 181, 1493–1503 (2012).
Wohlfahrt, J. G. et al. Ephrin-A1 suppresses Th2 cell activation and provides a regulatory link to lung epithelial cells. J. Immunol. 172, 843–850 (2004).
Banerjee, P. et al. Network and co-expression analysis of airway smooth muscle cell transcriptome delineates potential gene signatures in asthma. Sci. Rep. 11, 14386 (2021).
Dietrich, A., Chubanov, V., Kalwa, H., Rost, B. R. & Gudermann, T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharm. Ther. 112, 744–760 (2006).
Sotty, J. et al. Toxicological effects of ambient fine (PM(2.5-0.18)) and ultrafine (PM(0.18)) particles in healthy and diseased 3D organo-typic mucocilary-phenotype models. Environ. Res 176, 108538 (2019).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet 25, 25–29 (2000).
Aleksander, S. A. et al. The Gene Ontology knowledgebase in 2023. Genetics 224, https://doi.org/10.1093/genetics/iyad031 (2023).
Winter, N. A. et al. Sputum mast cell/basophil gene expression relates to inflammatory and clinical features of severe asthma. J. Allergy Clin. Immunol. 148, 428–438 (2021).
Tiotiu, A. et al. Association of Differential mast cell activation with granulocytic inflammation in severe asthma. Am. J. Respir. Crit. Care Med. 205, 397–411 (2022).
Al-Shaikhly, T. et al. Location of eosinophils in the airway wall is critical for specific features of airway hyperresponsiveness and T2 inflammation in asthma. Eur. Respir. J. 60, https://doi.org/10.1183/13993003.01865-2021 (2022).
Altman, M. C. et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J. Clin. Invest 129, 4979–4991 (2019).
Ben-Baruch Morgenstern, N. et al. Single-cell RNA sequencing of mast cells in eosinophilic esophagitis reveals heterogeneity, local proliferation, and activation that persists in remission. J. Allergy Clin. Immunol. 149, 2062–2077 (2022).
Alvarado, D. et al. Strong association of mast cells with eosinophilic esophagitis-specific signatures. Allergy 78, 583–586 (2023).
Zhang, S., Caldwell, J. M., Rochman, M., Collins, M. H. & Rothenberg, M. E. Machine learning-based identification and characterization of mast cells in eosinophilic esophagitis. J. Allergy Clin. Immunol. 153, 1381–1391.e1386 (2024).
Laidlaw, T. M. & Boyce, J. A. Updates on immune mechanisms in aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 151, 301–309 (2023).
Dwyer, D. F. et al. Human airway mast cells proliferate and acquire distinct inflammation-driven phenotypes during type 2 inflammation. Sci. Immunol. 6, https://doi.org/10.1126/sciimmunol.abb7221 (2021).
Castro, M. et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med. 378, 2486–2496 (2018).
Ansar, M., Ivanciuc, T., Garofalo, R. P. & Casola, A. Increased lung catalase activity confers protection against experimental RSV infection. Sci. Rep. 10, 3653 (2020).
Kinnula, V. L. & Crapo, J. D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 167, 1600–1619 (2003).
Hogan, S. P. et al. Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy 38, 709–750 (2008).
Mehta, S. The effects of nitric oxide in acute lung injury. Vasc. Pharm. 43, 390–403 (2005).
Kobzik, L. et al. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am. J. Respir. Cell Mol. Biol. 9, 371–377 (1993).
Hearn, A. P. et al. The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma. J. Allergy Clin. Immunol. Pr. 9, 2093–2096.e2091 (2021).
Burnstock, G., Brouns, I., Adriaensen, D. & Timmermans, J. P. Purinergic signaling in the airways. Pharm. Rev. 64, 834–868 (2012).
Kicic, A. et al. Assessing the unified airway hypothesis in children via transcriptional profiling of the airway epithelium. J. Allergy Clin. Immunol. 145, 1562–1573 (2020).
Poole, A. et al. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J. Allergy Clin. Immunol. 133, 670–678.e612 (2014).
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Smedley, D. et al. The BioMart community portal: an innovative alternative to large, centralized data repositories. Nucleic Acids Res. 43, W589–W598 (2015).
Liu, R. et al. Why weight? Modelling sample and observational level variability improves power in RNA-seq analyses. Nucleic Acids Res. 43, e97 (2015).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Chikina, M., Zaslavsky, E. & Sealfon, S. C. CellCODE: a robust latent variable approach to differential expression analysis for heterogeneous cell populations. Bioinformatics 31, 1584–1591 (2015).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Snel, B., Lehmann, G., Bork, P. & Huynen, M. A. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 28, 3442–3444 (2000).
Szklarczyk, D. et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43, D447–D452 (2015).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Gierlinski, M. Fenr: Fast functional enrichment for interactive applications. https://doi.org/10.18129/B9.bioc.fenr, R package version 1.6.1, https://bioconductor.org/packages/fenr (2024).
Acknowledgements
The research reported in this publication was supported by NIH-NIAID award numbers 5UM1AI114271, UM1AI160040, and UM2AI117870, with additional support provided through award numbers UL1TRG01422, UL1RR025741, UL1TR000150, UL1TR001422, UL1 TR002535, UL1TR001876, 5UL1TR001425–03 and T32AI007635. Additional support was also provided through an unrestricted grant from GlaxoSmithKline. The manuscript content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH. We are grateful to all participants and their families who took part in this study.
Author information
Authors and Affiliations
Contributions
M.C.A., R.M.S., D.J.J. and C.L.G. participated in study design; D.J.J. and P.M.B. did the project administration; L.B.B., M.A.G., J.S., A.H.L., R.T.C., G.K.K.H., M.G.S., A.L., R.K., E.M.Z., S.J.T. and M.K. did the data collection; R.M.S., M.C.A., C.L.G., K.D.M. and D.J.J. oversaw or did the data analysis; R.M.S., C.L.G., K.D.M., A.C. and M.C.A. directly accessed and verified the underlying data; C.L.G. wrote the first draft of the manuscript; D.J.J., M.C.A., R.M.S., P.M.B., A.T., R.K., E.M.Z., K.D.M., S.J.T. and W.W.B. provided initial manuscript review and revisions; All authors critically reviewed the manuscript and approved submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
All authors, with the exceptions of P.M.B. and A.T. report grants from the US National Institutes of Health (NIH)–NIAID during the study. P.M.B. and A.T. have nothing to disclose outside of the submitted work. L.B.B. reports personal fees from GlaxoSmithKline, Genentech–Novartis, Teva, Boehringer Ingelheim, AstraZeneca, WebMD–Medscape, Merck, DBV Technologies, Circassia, Sanofi–Regeneron, and Vectura, all outside the submitted work. M.C.A. reports personal fees from Sanofi–Regeneron, outside the submitted work. W.W.B. reports consulting fees and payment from GlaxoSmithKline, Sanofi and Regeneron and royalties from Elsevier outside the submitted work. M.A.G. reports monetary compensation from the American Academy of Pediatrics for her work teaching the biannual Pediatrics board review course: PREP The Course. G.K.K.H. reports grants from Adare during the study. D.J.J. reports personal fees from Genentech, Pfizer, Sanofi, Avillion, AstraZeneca and Areteia as well as grants and personal fees from GlaxoSmithKline, Regeneron and OM Pharma, outside the submitted work. M.K. reports personal fees from Regeneron, outside the submitted work. A.H.L. reports personal fees from Phadia ThermoFisher as consulting honoraria; grants and non-financial support from ResMed–Propeller Health; non-financial support from Revenio; grants and personal fees from Avillion; and personal fees from Labcorp, all outside the submitted work. S.J.T. reports grants from NIH–Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH–NHLBI, and EJF Philanthropies and personal fees from Uptodate, all outside the submitted work. All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Rodney Britt, who co-reviewed with Maria Ford; Javier Perez-Garcia and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gaberino, C.L., Segnitz, R.M., Dill-McFarland, K.A. et al. Mepolizumab alters gene regulatory networks of nasal airway type-2 and epithelial inflammation in urban children with asthma. Nat Commun 16, 8191 (2025). https://doi.org/10.1038/s41467-025-63629-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63629-2