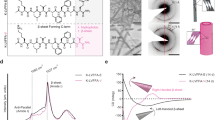
Abstract
Chiral pesticides have captivated considerable interest in agriculture, yet the integration of chirality into multifunctional supramolecular materials within this sector remains uncharted. Here, we show the fabrication of chiral AIM-12S/R@β-CD by encapsulating AIM-12S/R within β-cyclodextrin (β-CD), designed to enhance foliar adhesion and biofilm disruption for effective management of rice bacterial blight. Upon assembly in aqueous media, the chiral disparities in foliar affinity and biofilm disruption, initially present in AIM-12S/R, are amplified, as evidenced by a 67° lower contact angle for AIM-12R@β-CD relative to its S-enantiomer, and an 8.5-fold increase in biofilm eradication at 12.5 μg·mL⁻¹. Despite comparable in vitro potency, which often obscures chiral influences on other traits, enantioselective interactions between the enantiomers with leaf surfaces and biofilms dictate the divergence in in planta efficacy. This work provides a demonstration of chiral discrimination in supramolecular agrochemicals, presenting valuable insights into the further deployment of chiral supermolecules in agriculture.
Similar content being viewed by others
Introduction
Bacterial leaf blight (BLB) of rice, incited by Xanthomonas oryzae pv. oryzae (Xoo), ranks among the most destructive rice diseases globally1,2, inflicting yield losses of 20–50% annually and, under severe outbreaks, leading to total crop failure3. Chemical control remains the most effective and widely adopted strategy4,5. However, conventional agrochemicals suffer from poor deposition due to the hydrophobicity of rice foliage, with merely ~10% of the active ingredient reaching the target and as little as 0.1% being absorbed6,7. Simultaneously, surface runoff contributes to environmental contamination and human health risks8,9. Recent efforts to overcome these limitations have focused on incorporating adjuvants or engineering nanocarriers10,11,12,13. While adjuvants may enhance adhesion and retention, their excessive use raises ecological and toxicological concerns14; nanocarriers, though promising, often require intricate manufacturing processes and incur high production costs15,16. Furthermore, even when agrochemicals manage to reach the afflicted regions through considerable effort, the notorious biofilms evolved by pathogens shield symbiotic bacteria from adversarial agents17,18,19, further undermining efficacy20,21,22. Consequently, neither of these strategies provides a holistic solution to the multifaceted issues surrounding the pesticide-applying process, making it necessary to develop multifunctional pesticide formulations that combine high bioavailability, cost-efficiency, environmental sustainability, and biofilm-disrupting capabilities.
Supramolecular chemistry offers a promising approach for achieving the aforesaid goals23. Propelled by non-covalent forces, tailored guest molecules spontaneously assemble with host structures in aqueous media, yielding functional supramolecular architectures24,25. These assemblies simplify conventional formulation processes and reduce reliance on excessive additives, thereby advancing environmental and human health safety26,27. Beyond convenience for preparation, supramolecular integration confers emergent properties inaccessible to individual building blocks, enabling synergistic functionalities between host and guest28. Yet, despite its potential, the exploration of chirality within supramolecular systems remains largely confined to structural characterization, with practical applications, particularly in agriculture, still in their infancy.
In recent decades, the widespread emergence of chiral-center-bearing pesticides has brought chiral agrochemicals to the forefront of research29. Due to their stereospecificity, enantiomers frequently differ in bioactivities and environmental fates, with intended biological effects typically confined to a single enantiomer, as seen in deltamethrin, metalaxyl, and metoclopramide30,31,32. Nevertheless, the vast majority of chiral pesticides are commercialized as racemic mixtures33, with merely ~7% available in enantiomerically pure forms34,35,36. This practice results in the unintended environmental release of inactive isomers, which may threaten non-target organisms and disrupt ecological balance37. To address this, conventional agents are increasingly being reengineered into chiral-specific variants to enhance efficacy and reduce ecological burden37,38. The development of stereochemically defined agrochemicals is now regarded as a pivotal step toward sustainable plant protection39. However, current efforts have predominantly focused on the influence of chirality on biological activity, while its impact on foliar affinity (bioavailability) remains comparatively underexplored, despite its crucial relevance to environmental and human health40,41,42. Concurrently, to our knowledge, the application of chiral supramolecular materials in agriculture remains largely uncharted. It is therefore imperative to determine whether the chirality-dependent properties of small molecules are preserved or even amplified upon integration into supramolecular assemblies.
To engineer chiral supramolecular assemblies, we employed β-cyclodextrin (β-CD), a fundamental building block in supramolecular chemistry, as the host scaffold. This cost-effective and environmentally benign cyclic oligosaccharide features a hydrophobic inner cavity and a hydrophilic outer surface, enabling the selective entrapment of bioactive guests in aqueous media. Departing from the conventional reliance on pre-existing scaffolds, we designed and synthesized long-chain cationic guests de novo, each bearing a chiral center. These tailor-made molecules were endowed with predefined functionalities, including stereospecificity, antibacterial potency, and anti-biofilm activity. Among these compounds, AIM-12S/R exhibited superior potency over other series analogues, identifying them as prime candidates for self-assembly with β-CD. Upon forming multifunctional supramolecular materials (AIM-12S/R@β-CD) in aqueous media, the chiral disparities in bioavailability and biofilm disruption, initially evident in AIM-12S/R, were strikingly amplified, with AIM-12R@β-CD outperforming its counterpart. The enantioselective interactions between the drug and both the target and biofilm further accentuated the in planta divergence in efficacy, with AIM-12R@β-CD conferring greater protection against BLB than its S-enantiomer (Fig. 1). Collectively, this work pioneers the elucidation of chiral dependence in agricultural supramolecular systems, with respect to bioavailability, anti-biofilm activity, and in vivo efficacy, thus presenting a precedent for the fabrication and deployment of other chiral supramolecules in agriculture.
AIM-12S and AIM-12R engage in host–guest recognition with β-CD to yield multifunctional chiral supramolecular assemblies (AIM-12S/R@β-CD) of distinct morphologies: rod-like needles (AIM-12S@β-CD) and thin films (AIM-12R@β-CD). These architectural divergences translate into strikingly different deposition behaviors on rice leaves, as indicated by their contact angles (116° for AIM-12S@β-CD versus 49° for AIM-12R@β-CD). Owing to its stronger deposition, AIM-12R@β-CD penetrates and disrupts bacterial biofilms more efficiently, thereby eradicating resident bacteria and achieving superior suppression of bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae (Xoo). Furthermore, earthworm safety assays confirm that AIM-12R@β-CD exhibits favorable environmental compatibility. Live and dead bacterial cells are visualized as green and grey signals, respectively.
Results and discussion
The design, preparation, and in vitro activity evaluation of chiral molecules
To develop an innovative chiral antibacterial structure that maximizes efficacy while minimizing environmental hazards, we integrated azobenzene as the recognition module for β-cyclodextrin (β-CD) and introduced isopropanolamine, a common antibacterial component, at the terminal to conveniently incorporate a chiral carbon center. Moreover, a cost-effective, positively charged imidazole cation, known for its ability to bind to the negatively charged biofilm extracellular matrix43,44,45, disrupt the biofilm structure, and inactivate the bacteria within, was conjugated with isopropanolamine to achieve targeted bactericidal activity. Furthermore, a long-chain moiety, resembling a surfactant, was appended to the terminal end of the structure to enhance the compound’s bioavailability. In brief, through the connection and stacking of cost-effective fragments with diverse functional attributes, we were able to engineer a class of molecules that not only embody desirable properties but also adhere to the agricultural preference for streamlined, efficient, and economical agrochemicals.
Following the design strategy, we efficiently synthesized a series of azobenzene derivatives featuring chiral centers, AIM-10S/R to AIM-18S/R, via a streamlined and highly effective three-step process (Supplementary Fig. 1). These intermediates and compounds were characterized and confirmed by 1H NMR, 13C NMR, and HRMS (Supplementary Figs. 35–68), and the absolute configuration of the compound was characterized by HPLC and crystal structure (Supplementary Figs. 2, 3 and Supplementary Table 1), while their antibacterial efficacy in vitro against the recalcitrant pathogen Xoo was evaluated via the classic turbidimetric approach. Commercially obtainable agents thiodiazole-copper (TC) and kasugamycin (KSM) served as positive controls. It was found that chiral compounds possessing carbon chain lengths of 10, 12, and 14 exhibited markedly superior antibacterial efficacy compared to TC and KSM, with EC50 values ranging from 1.24 to 2.20 μg·mL⁻¹ (Table 1 and Supplementary Table S2). Moreover, compounds with the R-configuration showed enhanced potency relative to their S-configured counterparts. Although this pattern less prominent in compounds with shorter carbon chains, the activity difference between configurations became increasingly evident with increasing chain length, indicating a certain degree of chirality dependence in the activity. Among the chiral compounds, AIM-12R and AIM-12S exhibited the most pronounced activity within their respective configurations, positioning them as prime candidates for the fabrication of host-guest supramolecular materials.
Fabrication and characterization of chiral supramolecular materials AIM-12S@β-CD and AIM-12R@β-CD
The preparation of the supramolecular materials AIM-12S@β-CD and AIM-R@β-CD was accomplished with both simplicity and efficiency. AIM-12S/R (4 μL, 87.47 mM) was dissolved in acetonitrile, and afterward, was dripped into 1.0 mL of deionized water wherein β-CD (0.35 mM) was present, followed by vigorous stirring. Upon natural evaporation, the resulting supramolecular assemblies exhibited modestly enhanced antibacterial activity against Xoo relative to the guest molecules alone, whereas the host remained inactive even at the highest tested concentration (Table 1).
Scanning electron microscopy (SEM) was employed to observe the morphology of the guest molecules and supramolecular complexes, while dynamic light scattering (DLS) was used to quantify their particle size distributions. As depicted in Fig. 2A, B and Supplementary Fig. 4, the guest molecules AIM-12S and AIM-12R displayed sheet-like structures of varying thicknesses, with relatively large particle sizes, measuring 1641 nm and 1275 nm, respectively. Upon complexation with β-CD, the supramolecular material AIM-12S@β-CD transformed into a rod-like needle shape, whereas AIM-12R@β-CD assumed a thin film structure, with peak particle sizes of 361 nm and 326 nm, respectively. Above information presents the most direct evidence of supramolecular formation. Furthermore, variation in particle size suggests that encapsulation by β-CD enhances the dispersion of guest molecules in aqueous media, with the R-configuration supramolecular material demonstrating superior dispersibility relative to its S-configuration counterpart.
A SEM images of AIM-12S, AIM-12S@β-CD, AIM-12R and AIM-12R@β-CD on conducting glass. B Particle size distributions of AIM-12S@β-CD and AIM-12R@β-CD in aqueous solution (200 µg·mL−1). C UV-vis titration curves of AIM-12S (50 μM) with increasing molar equivalents of β-CD (0.0-2.0 eq) in aqueous solution. D HRMS spectrum of AIM-12S@β-CD. E Job’s plots for ΔA at 344 nm, with a total concentration of 50 μM for AIM-12S and β-CD in aqueous solution. F UV-vis titration curves of AIM-12R (50 μM) with increasing molar equivalents of β-CD (0.0-2.0 eq) in aqueous solution. G HRMS spectrum of AIM-12R@β-CD. H Job’s plots for ΔA at 344 nm, with a total concentration of 50 μM for AIM-12R and β-CD in aqueous solution. I Benesi–Hildebrand plot of [ΔA]−1 versus [β-CD]−1 for AIM-12S. J Benesi–Hildebrand plot of [ΔA]−1 versus [β-CD]−1 for AIM-12R. K Zeta-potentials of AIM-12S, AIM-12S@β-CD, AIM-12R and AIM-12R@β-CD. The concentration of AIM-12S/R in each system was maintained at 200 μg·mL−1, with molar ratios of AIM-12S/R to β-CD set at 1:1. For panels (K), data are presented as mean ± SD. Statistical significance was analyzed by one-way ANOVA followed by Duncan’s multiple range test, with distinct lowercase letters indicating significant differences (n = 3, p < 0.05). All experiments were performed with a minimum of three biological replicates, with sample sizes indicated in the corresponding figures. Source data are available in the Source Data file.
After verifying the formation of the host-guest material, the assembly behavior of AIM-12S/R with β-CD was investigated via UV-vis titration across a range of β-CD concentrations, allowing for the preliminary determination of the stoichiometric ratio. For AIM-12S, it exhibited a characteristic absorption peak at 344 nm, matching the π-π* electronic transition of azobenzene. As β-CD was incrementally introduced, the absorption at 344 nm steadily diminished, reaching a plateau at equimolar concentrations, beyond which no detectable changes were observed. This behavior signifies a 1:1 stoichiometric relationship between the host and guest (Fig. 2C). This conclusion was corroborated by HRMS (Fig. 2D), indicating a molecular weight of 1625.7010, closely aligning with the theoretical value corresponding to [AIM-12S@β-CD-Br]+, featuring a minimal deviation of -4.21 ppm, and further validated by Job’s plot (Fig. 2E). In this plot, the ΔA reached its peak when the β-CD molar fraction was 0.5. Comparable ideal outcomes were achieved for AIM-12R through both HRMS and Job’s plot analysis, unequivocally confirming the 1:1 complexation ratio of AIM-12R with β-CD (Fig. 2F–H).
From titration experiments, the Benesi-Hildebrand equation yielded a binding affinity of 4.3967 × 10⁴ M⁻¹ for AIM-12S@β-CD and 1.4245 × 10⁵ M⁻¹ for AIM-12R@β-CD (Fig. 2I, J), highlighting the robust interactions between AIM-12S/R and β-CD, with the R-configuration exhibiting superior binding stability. Moreover, Zeta potential analysis revealed an elevation for the supramolecular materials compared to the individual guest molecules (Fig. 2K), indicating that encapsulation by β-cyclodextrin (β-CD), enriched with hydrophilic hydroxyl groups, enhances the stability of the guest molecules in aqueous environments. Additionally, the Zeta potential for the R-enantiomeric supramolecule was marginally higher than that of the S-enantiomer, suggesting a potentially superior stability profile for the R-configuration. Moreover, under aqueous conditions at 15, 25 and 35 °C, both AIM-12R@β-CD and AIM-12S@β-CD exhibited less than 15% degradation, indicating their aqueous-phase stability across this temperature range (Supplementary Figs. 5–7).
To clarify the encapsulation position of chiral molecules inside the β-CD cavity and preliminarily identify the driving force behind self-assembly, 1H NMR titration was conducted at diverse molar ratios (Supplementary Fig. 8). The results revealed that the two proton signals corresponding to the benzene moiety (d and e) shifted upfield, with chemical shift variations (Δδ) of +0.01, +0.03 ppm and +0.02, +0.04 ppm when molar ratios were 1:0.5 and 1:1, respectively, suggesting that these proton groups were successfully encapsulated. In contrast, the other three benzene ring proton signals (a-c) shifted downfield, with Δδ of -0.03, -0.01,-0.03 ppm and -0.03, -0.01, -0.03 ppm at molar ratios of 1:0.5 and 1:1, respectively, a displacement attributable to the deshielding effect arising from their exclusion from β-CD encapsulation at the portal, thereby leaving these protons exposed to the external environment. These results suggest that β-CD encapsulated the benzene ring directly linked to the isopropylamine fragment after traversing the terminal phenyl group of the molecule, thereby creating a larger hydrophilic surface that facilitates the self-assembly of the molecules. Collectively, the stoichiometry, spatial binding orientation, and interaction mechanism converge to support the formation of a 1:1 host–guest inclusion complex, predominantly governed by hydrophobic interactions.
Chirality-dependent foliar affinity of supramolecular materials on superhydrophobic leaves
Effective foliar deposition and retention are pivotal in enhancing pesticide efficacy and minimizing ecological repercussions. However, rice leaves are superhydrophobic, covered by a waxy hydrophobic layer consisting of macromolecules and lipids, along with micro-papillae and nano-spike structures arranged in an orderly manner46. Conventional pesticides frequently suffer from poor wettability on superhydrophobic rice leaves, resulting in issue such as splashing, bouncing, fragmentation, and sliding, all of which compromise deposition and potency. In this context, the development of a class of antibacterial agents with superior foliar affinity to superhydrophobic surfaces is of critical importance. Herein, we investigated the foliar adhesion properties of the structured AIM-12S/R and their supramolecular materials, AIM-12S/R@β-CD.
Typically, the contact angle serves as the primary metric for assessing the wettability of droplets47,48. Accordingly, we monitored the temporal variation in contact angles of droplets infused with target molecules at 200 μg·mL⁻¹ on rice leaf surfaces (Fig. 3A and B). Throughout the 180-second observation period, the dynamic contact angles of AIM-12S droplet remained largely unchanged, whereas those of AIM-12R droplets demonstrated considerable fluctuations, declining from 117° to 94° and ultimately stabilizing at an equilibrium contact angle of 94 ± 0.87°, indicating the high influence of chirality on the contact angles of the guest molecules in isolation. Further elucidation is provided by measuring the static contact angle of different proportions of enantiomers (Supplementary Fig. 9). For AIM-12S@β-CD, despite integration of β-CD, the contact angle of the droplets displayed no severe fluctuation over time, decreasing from 126° to 120° within 180 seconds, with a mean contact angle reaching 116 ± 0.5°. In stark contrast, upon the integration of β-CD, the contact angle of the R-configured supramolecular material, AIM-12R@β-CD, dropped sharply over time, with an average contact angle of merely 49 ± 1.3°. This reduction underscores an enhanced wetting ability, further indicating a chirality-dependent influence on the contact angle within the supramolecular context. Additionally, a preliminary comparison between β-CD and calix[4]arene (Cas: 74568-07-3), a host with a larger cavity, was conducted to evaluate their influence on wettability (Supplementary Fig. 10). Calix[4]arene-based formulations offered negligible improvement in spreading for the S-enantiomer and even diminished wetting performance for the R-enantiomer. These results suggest that, relative to calixarenes, β-CD confers superior foliar affinity to guest molecules and was therefore selected for subsequent investigations.
A Time-lapse images of dynamic contact angle evolution over 180 s. B Contact angle values over time within 180 s. C Dynamic surface tension (DST) over 38 s. D Macroscopic comparison between untreated and treated leaves. E, F High-speed imaging of droplet splashing from 30 cm (E) and rebound from 15 cm (F). G Time-resolved normalized contact diameter (Dt/D0), derived from Supplementary Movie 2). H Time-resolved normalized rebound height (Ht/D0), derived from Supplementary Movie 2. I Liquid holding capacities of rice leaves after spraying at a 30° angle with various formulations. J SEM images showing surface deposition. The concentration of AIM-12S/R in each system was maintained at 200 μg·mL−1, with molar ratios of AIM-12S/R to β-CD set at 1:1. In panels (I), data are presented as mean ± SD. Statistical significance was determined by one-way ANOVA followed by Duncan’s multiple range test. Distinct lowercase letters indicate significant differences for the immersion method, and uppercase letters for the 30° spraying method (n = 10, p < 0.05). All experiments were performed with a minimum of three biological replicates, with sample sizes indicated in the corresponding figures. Source data are available in the Source Data file.
Surface tension exerts a pivotal influence during droplet impact, where its reduction facilitates the rapid migration and adsorption of compounds onto the newly established solid-liquid interface within seconds upon impact, potentially triggering a wetting transition from the Cassie state to the Wenzel state49. Consequently, we employed the Wilhelmy technique to quantify dynamic surface tension (DST) for all four formulations (Fig. 3C), revealing that, over a 38-second timespan, the surface tension of the supramolecular complexes consistently remained lower than that of their respective guest molecules, thereby indicating that the supramolecular assembly improves surface tension. Furthermore, the surface tension of R-configured molecules, whether as guest molecules or within supramolecular assemblies, persistently registered lower values than their S-configured counterparts, revealing a distinct chirality-dependent influence on surface tension. More importantly, the assembly of supramolecular complexes further amplified this chirality-driven effect. To further substantiate the influence of chirality on surface tension and contact angle, we measured equilibrium contact angles and surface tensions for other chiral compounds, with the results, as illustrated in Supplementary Figs. 11A and B, closely aligning with the aforementioned conclusions.
To visually assess the wetting properties of each group and examine the macroscopic effects of variations in contact angle and surface tension, a post-spray evaluation was performed12. The results, shown in Fig. 3D, indicate that droplets of water, β-CD, AIM-12S, and AIM-12S@β-CD remained spherical on the leaf surface, leaving most of the leaf surface unwetted. In contrast, the leaves sprayed with AIM-12R solution displayed smaller, uniformly distributed droplets with higher liquid density. Notably, leaves treated with AIM-12R@β-CD were predominantly coated with transparent droplets across most regions, attributable to its low contact angle and DST, thereby indicating impressive leaf wetting properties.
In the course of the spraying process, droplets impinging upon the superhydrophobic leaf surface frequently undergo splitting, splashing, or rebounding, which contribute to reduced pesticide efficacy and off-target contamination50. Consequently, we conducted a thorough investigation into droplet splashing and rebound behavior on superhydrophobic rice foliage. Initially, the splashing behavior of droplets released from 30 cm was recorded using a high-speed camera (Fig. 3E and Supplementary Movie 1). Upon contact with the rice leaf surface, droplets of water, β-CD, and AIM-12S initially spread to their maximal extent before retracting, fragmenting, and rebounding from the surface, leaving behind only a sparse deposition of minute spherical droplets. Conversely, AIM-12R droplets displayed slight fragmentation, culminating in a greater deposition of droplets, revealing a chirality-dependent influence on the splashing dynamics of the guest molecules. Furthermore, the S/R-type supramolecular materials exhibited distinct capacities in mitigating droplet splashing. AIM-12S@β-CD droplets, while exhibiting reduced fragmentation compared to the guest molecules alone and leading to greater residual deposition on the leaf surface, still lost a considerable proportion of the droplets and formed larger contact angles. In contrast, AIM-12R@β-CD droplets exhibited negligible splitting, with virtually no discernible bouncing, ultimately depositing notable liquid on the rice leaves, characterized by reduced contact angles and enhanced wetting behavior.
Subsequently, droplets were released from a height of 15 cm, with their bouncing dynamics captured (Fig. 3F and Supplementary Movie 2), allowing for the quantification of the normalized spreading diameter (Dt/D0) and the normalized rebound height (Ht/D0) throughout this process (Fig. 3G and H). Regarding Dt/D0 over time, droplets of water, β-CD, AIM-12S, and AIM-12S@β-CD showed comparable maximum spreading diameters (Dmax/D0) of 2.66, 2.68, 2.55, and 2.66, respectively. AIM-12R droplets exhibited a relatively enhanced Dmax/D0 of 2.90, and encapsulation of the R-configured molecule by β-CD further amplified this effect, reaching a Dmax/D0 of 3.17. Regarding Ht/D0, droplets of water, β-CD, and AIM-12S exhibited full rebound, with maximum rebound heights (Hmax/D0) of 1.97, 1.90, and 1.90, respectively. In stark contrast, AIM-12R droplets demonstrated a lower Hmax/D0 of 1.11, outperforming the S-configured guest molecules. Upon introduction of β-CD to the S-configured guest molecule, rebound in AIM-12S@β-CD droplets was suppressed, reducing Hmax/D0 to 1.45, though still exceeding that of AIM-12R droplets. Notably, encapsulation of the R-configured guest molecules by β-CD resulted in the strongest suppression of rebound, with AIM-12R@β-CD droplets achieving a Hmax/D0 (0.53) that was merely half of the unencapsulated guest molecule. The combination of maximal spreading diameter and minimal rebound height indicates that AIM-12R@β-CD droplets maintained prolonged contact with the leaf surface, thereby promoting improved interaction and adhesion.
To better approximate the natural configuration of rice leaves during pesticide application, we inclined the leaves at an angle of 30 degrees and dispensed droplets from a height of 15 cm, documenting their dynamic behavior. As illustrated in Supplementary Fig. 12 and Supplementary Movie 3, water, β-CD, and AIM-12S droplets bounced forward upon impact with the leaf surface, ultimately detaching from it. Upon encapsulation by β-CD, the droplets ceased to exhibit such bouncing behavior. Although much of the AIM-12S@β-CD liquid ultimately departed from the leaf surface, a small fraction persisted at the point of contact. Moreover, it was observed that AIM-12R droplets rolled forward upon contact, forming an elliptical deposition on the leaf surface. The introduction of β-CD further accentuated this deposition phenomenon, as AIM-12R@β-CD droplets exhibited reduced sliding distances, resulting in notable retention of liquid on the leaf surface. These findings reveal that the deposition and adhesion of guest molecules on the leaf surface are chirality-dependent, with this effect amplified by the supramolecular strategy.
Moreover, the liquid holding capacity (LHC) serves as an essential parameter for assessing the efficacy of active ingredient deposition28. Accordingly, formulations were applied via direct spraying onto rice leaves positioned horizontally and inclined at 30°, with the variation in leaf mass pre- and post-application quantified to ascertain the LHC. As illustrated in Fig. 3I, relative to droplets containing only the guest molecules, the deposition and adhesion capabilities of both S- and R-configured supramolecular droplets exhibited varying degrees of enhancement on the superhydrophobic surface. Furthermore, for both the guest molecules and their supramolecular complexes, the R-configured droplets consistently demonstrated superior performance compared to their S-configured counterparts, in concordance with the findings from prior experiments.
SEM imaging was subsequently used to observe the behavior of guest molecules and supramolecular materials on rice leaves (Fig. 3J). Rice leaves without treatment showed distinct features, including the waxy cuticle, trichomes, and stomata. Application of AIM-12S, extensive, irregular block-like deposits formed, unevenly scattered across the leaf surface, leaving considerable regions exposed. Conversely, AIM-12R, while also producing block-like deposits, resulted in thinner layers and reduced uncovered areas. Following the introduction of β-CD, AIM-12S@β-CD continued to generate block-like deposits on the leaf surface, with a more extensive area of deposition, although numerous gaps remained discernible. In contrast, AIM-12R@β-CD exhibited minimal block formation and fewer prominent gaps. Under higher magnifications, AIM-12R@β-CD adhered tightly to the rice leaf surface, conforming to the sinusoidal grooves and aligning with the organized pattern of micropillars and nanospikes, thereby promoting a more uniform distribution and coverage. The divergent behaviors of the S- and R-configured molecules on rice leaves highlight the chirality-dependent interactions between these enantiomeric molecules and the rice leaf surface. To investigate the impact of environmental factors on the supramolecular stability, AIM-12R@β-CD and AIM-12S@β-CD were applied to uniformly cultivated rice plants and exposed to ambient field conditions. Residual supramolecular content was quantified at defined intervals via high-performance liquid chromatography (HPLC). As depicted in Supplementary Figs. 13 and 14, after 10 days, AIM-12R@β-CD and AIM-12S@β-CD exhibited degradation rates of 12.24% and 35.62%, respectively. These results indicate the intrinsic stability of both constructs under natural conditions, with the R-enantiomer demonstrating superior persistence, thereby underpinning its sustained antimicrobial efficacy in planta.
Chirality-dependent inhibition of biofilm formation by supramolecular materials
Bacterial biofilms are pivotal in plant bacterial infections, offering bacteria a protective shield against external threats, thus diminishing the efficacy of bactericidal agents51,52. Therefore, preempting the formation of Xoo biofilms and disassembling established biofilms represent critical strategies for bacterial eradication and the alleviation of plant bacterial diseases. To this end, the inhibitory potential of various molecules on Xoo biofilm formation was assessed via the conventional crystal violet staining assay. Following a 48-h incubation of Xoo cells with graded concentrations of the molecules, biofilm quantification was conducted at OD570 nm using a microplate reader.
As illustrated in Fig. 4A–D and Supplementary Figs. 15 and 16, the treatment groups comprising AIM-12S, AIM-12R, AIM-12S@β-CD, and AIM-12R@β-CD, administered at 0.5–8 × EC50 concentrations, displayed Xoo biofilm inhibition that strengthened progressively with increasing concentrations. Strikingly, across all concentrations, the R-enantiomers consistently demonstrated superior biofilm inhibition compared to their S-enantiomeric counterparts, regardless of whether the compounds were in their free or supramolecular forms. Interestingly, at concentrations below 1.0 × EC50, AIM-12R, AIM-12R@β-CD, and AIM-12S@β-CD exhibited negligible effects on bacterial proliferation, yet maintained potent efficacy in curtailing biofilm formation. In contrast, at elevated concentrations, an enhanced inhibition was observed for both biofilm formation and bacterial growth (Supplementary Fig. 16). These findings reveal the potential of supramolecular materials to function as biofilm inhibitors without compromising bacterial proliferation at lower concentrations, while at elevated concentrations, they perform a dual role by concurrently impeding both biofilm development and bacterial proliferation.
A–D Quantification of Xoo biofilm formation quantified by crystal violet staining (OD570 nm) after 48 h incubation with varying concentrations of AIM-12S (A), AIM-12S@β-CD (B), AIM-12R (C), AIM-12R@β-CD (D). E CLSM 3D images of Xoo stained with acridine orange and Xoo colonies grown on NA solid medium following treatment with the same agents for 48 h. F Quantification of green fluorescence intensity from CLSM images, indicating Xoo growth and biofilm biomass, analyzed using ImageJ. G Statistical analysis of Xoo colony numbers on the upper layer of NA medium. In A–D, F, G data are presented as mean ± SD. One-way ANOVA with LSD post-hoc analysis was used to determine statistical differences, and significance levels were indicated by asterisks (n ≥ 3, *p < 0.05, **p < 0.01, ***p < 0.001; ns no significance). All experiments were performed with a minimum of three biological replicates, with sample sizes indicated in the corresponding figures. Source data are available in the Source Data file.
To further validate the inhibitory potential of various molecules on biofilm formation and their bactericidal activity against encapsulated bacteria, acridine orange (AO) staining was employed53,54, followed by three-dimensional imaging of live cells via confocal laser scanning microscopy (CLSM). As illustrated in Fig. 4E and F, at equivalent concentrations (1-4 × EC50), the green fluorescence in the supramolecular-treated groups was reduced compared to both the control and groups treated solely with the guest molecules, indicating an enhancement in biofilm inhibition and bactericidal efficacy conferred by the supramolecular materials. Of particular note, the R-enantiomer supramolecular complex AIM-12R@β-CD exhibited the highest activity, with green fluorescence nearly undetectable at 4 × EC50 concentration. The bactericidal effect, as indicated by fluorescence intensity, was further validated through colony density analysis (Fig. 4E, G). Both visual inspection and statistical evaluation revealed that, at identical concentrations (1-4 × EC50), in the AIM-12R@β-CD treatment group, colony density was less than in all other groups, with the colony count dropping to as little as 3.33 × 105 CFU·mL⁻¹ at 4 × EC50 concentration.
Chirality-dependent eradication of mature biofilms by supramolecular materials
Despite extensive research efforts aimed at preventing biofilm formation, the effective elimination of established mature biofilms continues to present a formidable challenge. Herein, we advanced our investigation into the capacity of various molecules to eradicate mature biofilms by culturing Xoo for 48 h to allow biofilm maturation, followed by a 48-h treatment with varying concentrations of the molecules to assess their capacity to dismantle these resilient structures (Fig. 5A–F). The results indicated that the dense architecture of mature biofilms exhibited high resistance to disruption by AIM-12S at lower concentrations, with eradication only commencing at a threshold concentration of 50 μg·mL⁻¹. In striking contrast, AIM-12S@β-CD initiated biofilm eradication at considerably lower concentrations, beginning at 12.5 μg·mL⁻¹, while AIM-12R demonstrated efficacy starting at 6.1 μg·mL⁻¹. Notably, AIM-12R@β-CD exhibited its efficacy at 3.1 μg·mL⁻¹, with the eradication rate surging to 80.23% at 12.5 μg·mL⁻¹, demonstrating superior eradication capability compared to all other groups. To further evaluate the efficacy of various molecules in biofilm eradication and the subsequent elimination of the bacteria encapsulated within, a quantitative assessment was conducted utilizing the spread plate method (SPM) (Fig. 5G, H). Data indicated that AIM-12R@β-CD showed greater bactericidal efficacy than the other groups at the same concentrations, effectively eliminating nearly all bacterial cells at 50 μg·mL⁻¹, with a colony count reduced to as low as 3.33 × 105 CFU·mL⁻¹. These results provide preliminary evidence of the distinct capacities of R- and S-enantiomers in biofilm disruption and bactericidal activity, with their differential effects intensified by the incorporation of β-CD.
A Crystal violet staining images showing biofilm eradication following treatment with AIM-12S, AIM-12S@β-CD, AIM−12R, and AIM−12R@β-CD. B–E Quantification of residual biofilm by optical density at 570 nm after 48 h incubation with varying concentrations of AIM-12S (B), AIM−12S@β-CD (C), AIM−12R (D), and AIM-12R@β-CD (E). F Biofilm eradication rates for each formulation against pre-formed biofilms. G, H Statistical colonies and colony growth of biofilm-embedded Xoo on agar plates after 48 h biofilm establishment in 96-well plates and subsequent 48 h exposure to varying concentrations of each agent. In B–G data are presented as mean ± SD. One-way ANOVA with LSD post-hoc analysis was used to determine statistical differences, and significance levels were indicated by asterisks (n ≥ 3, *p < 0.05, **p < 0.01, ***p < 0.001; ns no significance). All experiments were performed with a minimum of three biological replicates, with sample sizes indicated in the corresponding figures. Source data are available in the Source Data file.
The 3D imaging technique, using AO to label live cells and PI to label dead cells, further assessed the biofilm eradication potential and bactericidal efficacy of the compounds and supramolecular materials. As illustrated in Supplementary Figs. 17A, C and D, a progressive increase in the concentration of the molecules (from 12.5 to 50 μg·mL⁻¹) resulted in a gradual attenuation of green fluorescence alongside a concomitant intensification of red fluorescence across all groups, signifying a heightened biofilm eradication, accompanied by a decrease in live bacterial cells and a corresponding rise in bacterial mortality. Notably, at an equivalent concentration, AIM-12R@β-CD consistently demonstrated diminished green fluorescence and intensified red fluorescence relative to the other groups, underscoring its superior efficacy in biofilm eradication and bactericidal potency. Moreover, the disruption of biofilms may allow the escape and survival of planktonic bacteria, potentially leading to recolonization55. Therefore, preventing the survival of these escaped planktonic cells is essential for achieving comprehensive biofilm eradication. As depicted in Supplementary Fig. 17B, Supplementary Figs. 18 and 19, quantitative analysis of viable planktonic bacteria using the spread plate method (SPM) revealed that AIM-12R@β-CD exhibited superior efficacy in suppressing planktonic bacteria compared to AIM-12S@β-CD, effectively eliminating all viable cells at 25 μg·mL⁻¹. This trend, where the R-configuration consistently outperforms the S-configuration, is likewise replicated in the comparison of guest molecules.
To evaluate the long-term efficacy of the supramolecular systems against biofilms and the embedded bacteria, the eradication assay was extended by withdrawing the agents following initial exposure and continuing incubation for an additional 48 h. Both confocal imaging and colony counting revealed that AIM-12R and AIM-12R@β-CD treatments sustained effective suppression of bacterial regrowth within biofilm matrix, indicating their potential to mitigate disease recurrence in practical agricultural settings (Supplementary Fig. 20).
Mechanisms underlying biofilm interventions: attenuation of biofilm-associated virulence factors
The formation and persistence of Xoo biofilms are intricately entwined with extracellular polysaccharides (EPS), which provide structural stability and protection56,57; and extracellular enzymes such as amylase, which aids in nutrient acquisition and tissue degradation, thereby facilitating bacterial invasion and colonization58. We initially investigated the effects of various molecules on EPS by Xoo, and as illustrated in Figs. 6A, B and Supplementary Fig. 21, AIM-12R@β-CD, at a concentration of 1 × EC50 that does not affect bacterial growth, demonstrated a inhibitory effect on EPS synthesis, with inhibition rates of 58.47%, respectively, which were notably higher than those observed for AIM-12R (46.19%), AIM-12S (36.44%), and the supramolecular material AIM-12S@β-CD (41.94%). Upon evaluating the impact of various molecules on extracellular amylase activity (Fig. 6C), we observed a contraction in the hydrolysis zone diameter following AIM-12R@β-CD treatment, signifying a profound attenuation in extracellular amylase levels that far surpassed the reductions seen in other treatment groups. These findings suggest that this rationally engineered supramolecular material holds considerable potential to attenuate virulence and pathogenicity, with AIM-12R@β-CD displaying the most potent attenuation effects within a chirality-dependent context.
A Growth curves of Xoo treated with different agents at concentrations ranging from 0 to 8.0 × EC50. B Inhibition of extracellular polysaccharide (EPS) production by AIM-12S, AIM-12S@β-CD, AIM-12R and AIM-12R@β-CD across concentrations from 0.5 to 4.0 × EC50. C Representative images and quantitative analysis of extracellular amylase activity, measured as hydrolytic zone diameter, following treatment with β-CD, AIM-12S, AIM-12S@β-CD, AIM-12R and AIM-12R@β-CD at 1.0 × EC50. D Representative images and quantification of swimming motility, assessed by motility zone diameter under the same treatment conditions. E Photographs of Xoo-infected rice leaves after treatment with the tested agents at 1.0 × EC50. F Lesion length from the pathogenicity assay in (E) was quantified. In panels (A–D, F), data are presented as mean ± SD. For B–D, F, one-way ANOVA with LSD post-hoc analysis was used to determine statistical differences, and significance levels were indicated by asterisks (n ≥ 3, *p < 0.05, **p < 0.01, ***p < 0.001; ns not significant). All experiments were performed with a minimum of three biological replicates, with sample sizes indicated in the corresponding figures. Source data are available in the Source Data file.
The motility of plant pathogenic bacteria enables their infiltration through water pores or wound sites, facilitating subsequent colonization and occlusion of the xylem, which culminates in bacterial disease manifestation. Hence, evaluating the influence of the synthesized molecules on bacterial motility is of critical importance. As depicted in Fig. 6D, administration of AIM-12R@β-CD at a concentration of 1 × EC50 yielded a motility diameter of 9.15 mm, smaller than those recorded for AIM-12R (14.60 mm), AIM-12S (21.15 mm), and AIM-12S@β-CD (16.53 mm) treatments. This reduction in motility highlights the superior inhibitory potency of AIM-12R@β-CD against bacterial movement.
To visually assess the degree of pathogenicity attenuation induced by various molecules, we conducted pathogenicity assays. As illustrated in Fig. 6E, F, the mean lesion lengths observed on rice leaves treated with DMSO, β-CD, AIM-12S, AIM-12S@β-CD, AIM-12R, and AIM-12R@β-CD were 14.6 cm, 15.4 cm, 13.4 cm, 10.8 cm, 10.5 cm, and 6.61 cm, respectively. The shortest lesion length, indicative of the lowest pathogenicity, suggests that AIM-12R@β-CD achieves the most potent attenuation of virulence and pathogenicity. This aligns with the results previously observed in the biofilm formation inhibition and mature biofilm eradication experiments.
Mechanisms Underlying Antibacterial Activity: Disruption of Redox Homeostasis and Membrane Permeability
Disruption of intracellular redox balance is a key mechanism in bacterial inactivation, primarily by triggering overproduction of reactive oxygen species (ROS)59. To further elucidate the extent of redox disruption, intracellular ROS levels were quantified as a direct indicator of oxidative stress and consequent cellular damage. Initially, the ROS levels in bacterial cells exposed to AIM-12S, AIM-12S@β-CD, AIM-12R, and AIM-12R@β-CD across varying concentrations were quantified using a ROS assay kit. As illustrated in Fig. 7A–D, the progressive elevation in fluorescence intensity signifies an increase in intracellular ROS levels following treatment with the aforementioned molecules, suggesting their capacity to disrupt the redox equilibrium within pathogens, thereby causing redundant ROS accumulation. Furthermore, analysis of the fluorescence data at 522 nanometers revealed a dose-dependent relationship in ROS production by the activated cells (Fig. 7E). Subsequently, to further investigate the potential mechanism by which these molecules disrupt redox homeostasis, the activities of three major enzymes, catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD), responsible for maintaining redox equilibrium in bacterial cells, were measured following treatment with varying concentrations of the molecules, using their respective assay kits60,61. As illustrated in Fig. 7F–H, the enzymatic activities of CAT, POD, and SOD in Xoo were profoundly diminished following exposure to the four molecules, with CAT activity declining by 54.89%, 62.54%, 61.09%, and 74.76%, POD activity by 54.73%, 60.69%, 73.17%, and 79.64%, and SOD activity by 69.39%, 69.44%, 71.72%, and 71.39% at a concentration of 25 μg·mL⁻¹, respectively.
A–D Intracellular ROS accumulation in Xoo after treatment with increasing concentrations of AIM-12S (A), AIM-12S@β-CD (B), AIM-12R (C) and AIM-12R@β-CD (D), monitored using a ROS detection kit (Ex = 490 nm). E Quantification of fluorescence intensity at 522 nm corresponding to (A–D). In the “Relative intensity (a.u.)” shown in panels A-E, a.u. = arbitrary units. F–H Enzymatic activity of catalase (CAT, F), peroxidase (POD, G), and superoxide dismutase (SOD, H) in Xoo following treatment with increasing doses of each formulation. I, J Electrical conductivity of Xoo suspensions treated with 0.00-50.0 μg·mL-1 of AIM-12S and AIM-12S@β-CD (I), or AIM-12R and AIM-12R@β-CD (J). K, L Quantitative analysis of conductivity data shown in (I, J). In E–H, I–L data are presented as mean ± SD. For F–H one-way ANOVA with LSD post-hoc analysis was used to determine statistical differences, and significance levels were indicated by asterisks (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001; ns,no significance). For E, K, L significance was determined by one-way ANOVA followed by Duncan’s multiple range test, with distinct lowercase letters indicating significantly different groups (n = 3, p < 0.05). All experiments were performed with a minimum of three biological replicates, with sample sizes indicated in the corresponding figures. Source data are available in the Source Data file.
Additionally, recognizing that these molecules may employ alternative mechanisms to exert bactericidal effects, the permeability of the bacterial membrane and the resultant electrolyte leakage were further assessed by analyzing electrical conductivity62. Following an 8-h exposure to the aforementioned molecules at 25 μg·mL⁻¹, the relative conductivity of the Xoo solution increased from 0.13 (0 μg·mL⁻¹) to 0.55, 0.64, 0.68, and 0.68, respectively, with a further elevation observed at 50 μg·mL⁻¹ (Fig. 7I, J). The conductivity values plateaued at the 2-h mark, indicating that the four molecules irreversibly compromised bacterial membrane permeability, leading to electrolyte leakage (Figs. 7K, L). These findings suggest that the potential antibacterial mechanisms of the four molecules entail disruption of redox homeostasis, resulting in the excessive accumulation of ROS, and alterations in membrane permeability, causing electrolyte leakage, both of which may culminate in bacterial cell death.
Supramolecular Materials Exhibit High In Vivo Potency against Xoo Infection
Given the robust in vitro potency, elevated foliar affinity, and high anti-biofilm activity of the engineered supramolecular materials, all of which exhibited a chirality-dependent pattern, we proceeded to evaluate their potential in protecting and treating bacterial blight caused by Xoo in vivo. Employing the standard leaf-clipping inoculation method63,64, we sought to ascertain whether the chirality-dependent bioactivity exhibited in vitro would be consistently maintained in vivo. As depicted in Fig. 8A–C, while the control and β-CD groups exhibited clear disease symptoms, treatment with low concentrations (200 μg·mL⁻¹) of AIM-12S, AIM-12S@β-CD, AIM-12R, and AIM-12R@β-CD resulted in varying degrees of symptom alleviation, achieving curative and protective efficiencies of 30.83% and 31.44%, 34.16% and 36.38%, 39.17% and 40.50%, and 52.59% and 51.60%, respectively. The results outperformed commonly employed commercial bactericides, such as TC suspension, which demonstrated a protective efficacy at 34.72% and a curative efficacy at 33.75%, as well as KSM formulation, with a protective efficacy of 33.89% and a curative efficacy of 31.66%. Notably, AIM-12R demonstrated superior protective and curative efficacy relative to AIM-12S, in alignment with their respective in vitro activities and foliar affinity profiles, indicating that the chirality-dependent behavior of the designed molecules extends to in vivo activity. Furthermore, the incorporation of β-CD augmented the efficacy of the guest molecules in mitigating bacterial blight, with AIM-12R@β-CD standing out as the most potent treatment across all cohorts, suggesting that the chirality dependence is not only preserved but also amplified within the supramolecular constructs in vivo.
A Representative images of disease symptoms on rice leaves after treatment with AIM-12S, AIM-12S@β-CD, AIM-12R, AIM-12R@β-CD, or commercial bactericides TC and KSM (200 μg·mL⁻¹), following 14 days of continuous cultivation post- application. B Quantification of protective efficacy for each treatment at 200 μg·mL−1, based on lesion severity observed in (A). C Quantification of curative efficacy for the same agents at 200 μg·mL−1, also derived from data in (A). In B, C data are presented as mean ± SD. Statistical difference was determined by one-way ANOVA followed by Duncan’s multiple range test, with distinct lowercase letters denoting significantly different groups (n = 3, p < 0.05). All experiments were performed with a minimum of three biological replicates, with sample sizes indicated in the corresponding figures. Source data are available in the Source Data file.
Supramolecular Materials Exhibit Broad Applicability and Photoisomerization Behavior
To demonstrate the broader applicability of our supramolecular system, we extended the scope of evaluation to include Pseudomonas syringae pv. actinidiae (Psa), the pathogen responsible for kiwifruit bacterial canker, a disease characterized by its intractability. Initial in vitro assays confirmed that both the free guest molecules and their assembled complexes elicited high anti-Psa potency, with EC₅₀ values ranging from 11.03 to 14.54 μg·mL⁻¹, outperforming the commercial agent TC (82.56 μg·mL⁻¹) (Supplementary Table 3). Moreover, contact angle measurements on the hydrophobic surface of kiwifruit leaves revealed favorable spreading and wetting behaviors of the supramolecular complexes. Notably, a distinct chiral dependence emerged, with AIM-12S@β-CD and AIM-12R@β-CD exhibiting contact angles of 55.33° and 39.33°, respectively, corresponding to a 16° difference (Supplementary Fig. 22). Further in planta evaluations showed that AIM-12S, AIM-12S@β-CD, AIM-12R, and AIM-12R@β-CD conferred protective efficacies of 34.49%, 47.55%, 53.66%, and 79.08%, respectively, and therapeutic efficacies of 37.21%, 59.30%, 47.01%, and 69.25% against kiwifruit bacterial canker, all surpassing those of TC (30.24% and 29.04%). Notably, despite comparable in vitro activities, the R-configured complexes consistently outperformed its S-counterpart in planta, with 31.53% and 9.95% higher protective and therapeutic effects, respectively (Supplementary Fig. 23). These results not only underscore the efficacy of our supramolecular systems against Psa but also reveal the pivotal role of chirality in modulating antimicrobial performance. Overall, the demonstrated effectiveness across different crops and pathogens supports the generalizability of this chiral supramolecular strategy and its potential for broad application in the management of bacterial plant diseases.
Azobenzene derivatives are renowned for their capacity to undergo photoinduced isomerization, enabling reversible modulation of their physicochemical and biological properties. Firstly, to determine whether the chiral supramolecular assemblies undergo photoisomerization upon UV irradiation, we monitored the UV-vis spectra of AIM-12S/R@β-CD before and after exposure to ultraviolet light for varying durations (Supplementary Fig. 24). UV irradiation induced a hypochromic and blue shift in the π–π* transition band at 345 nm, along with an increased absorbance of the n–π* transition at 440 nm. These spectral changes plateaued within 20 seconds and mirrored those of the UV-irradiated free AIM-12S/R, indicating complete release of the cis-isomeric guests under the applied conditions. Subsequently, the wetting behavior of the UV-irradiated systems (AIM-12S + UV, AIM-12S@β-CD + UV, AIM-12R + UV, and AIM-12R@β-CD + UV) on rice leaf surfaces was examined. As shown in Supplementary Figs. 25, 26 and Supplementary Movie 4, the increased contact angles and enhanced droplet rebound observed after UV exposure indicate a reduced foliar affinity for these cis-isomers. Furthermore, across all tested concentrations, both the R- and S-configured agents displayed weaker biofilm eradication and bactericidal activities compared to their non-irradiated counterparts (Supplementary Figs. 27–29). Notably, despite the detrimental effects of UV irradiation on in vitro performance, the cis-R-isomer consistently outperformed its S-counterpart. Additionally, due to the light-induced dissociation of the guest molecules from the β-CD cavity, the in vitro profiles of the AIM-12S/R@β-CD + UV and AIM-12S/R + UV systems were largely indistinguishable. To evaluate the in planta efficacy of the cis-isomers against rice bacterial blight, both curative and protective assays were performed. Despite retaining in vitro potency comparable to their trans-counterparts (EC₅₀ = 0.88-1.34 μg·mL⁻¹; Supplementary Table 4), the cis-isomers exhibited therapeutic and protective effects in planta that were merely equivalent to those of the commercial bactericides TC and KSM (Supplementary Fig. 30). This attenuated efficacy likely stems from compromised foliar affinity and weakened biofilm-disrupting capacity. Collectively, pre- and post-irradiation comparisons consistently demonstrate the superior performance of trans-R-isomers across multiple dimensions, including foliar adhesion, biofilm eradication, and in planta activity. Moreover, UV-triggered release of the guest molecules from the host cavity further impaired both in vitro and in planta performance. Consequently, further characterization of the supramolecular system post-irradiation was not pursued.
Supramolecular materials ensure safety for both targeted and non-targeted species
To assess their safety for both target plants and non-target species, toxicity tests were performed. Initially, the influence of various molecular treatments on rice seed germination and growth was evaluated65. As illustrated in Supplementary Figs. 31 and 32A, exposure to AIM-12S, AIM-12S@β-CD, AIM-12R, and AIM-12R@β-CD at 200 μg·mL⁻¹ did not yield any detectable impact on seed germination, with germination rates consistently exceeding 95% across all treatment groups, and no discernible differences observed among them. A more detailed assessment of the molecular effects on root and shoot growth indicated a slight inhibitory effect of AIM-12S and AIM-12R at 200 μg·mL⁻¹, where root and shoot lengths averaged 22.82 mm and 30.65 mm, and 28.93 mm and 29.61 mm, respectively, compared to the control group. In contrast, AIM-12S@β-CD and AIM-12R@β-CD exhibited no appreciable impact on root and shoot development at the same concentration, with mean lengths reaching 38.08 mm and 38.15 mm, and 31.23 mm and 31.32 mm, respectively (Supplementary Figs. 32B and 32C). These findings imply that the encapsulation of guest molecules within β-CD effectively attenuated their inhibitory effects on rice seed growth and development. Furthermore, the direct application of these molecules at 200 μg·mL⁻¹ to rice plants did not impair normal growth (Supplementary Fig. 32D). Even after a week of exposure, no obvious variations were detected across the treatment groups, nor were there any manifestations of phytotoxicity, such as foliar scorching, morphological abnormalities, or chlorosis. In addition, at 200 μg·mL⁻¹, none of the tested molecules exerted deleterious effects on the viability or physiological activity of earthworms following a 48-h exposure66 (Supplementary Figs. 32E and 32F), with no detectable deviations observed relative to the control group, underscoring their commendable biocompatibility and safety for soil-dwelling organisms. In parallel, we characterized the degradation profile of AIM-12R@β-CD in soil. As illustrated in Supplementary Figs. 33 and 34, the complex underwent 31.32% degradation after 10 days, attesting to its biodegradability and affirming its environmental benignity.
In summary, this work explores the enantioselective behavior of chiral supramolecules in agricultural settings, with particular emphasis on their efficacy against Xoo-induced bacterial blight and the underlying mechanisms driving these differences. Prior to supramolecular assembly, the de novo–designed chiral guests AIM-12S/R exhibited stereoselective differences in foliar adhesion and biofilm disruption. Upon complexation with β-CD in aqueous media to form multifunctional supramolecular materials AIM-12S/R@β-CD, these differences were further amplified. Specifically, the R-configured AIM-12R@β-CD outperformed its S-configured counterpart not only in foliar affinity, evidenced by reduced contact angle, improved wettability, enhanced deposition, and suppressed splashing and rebound, but also in biofilm eradication efficiency at equivalent concentrations. Accordingly, despite comparable in vitro anti-Xoo activity, the superior in planta efficacy of the R-configuration was anticipated, consistent with its enhanced physicochemical and biological properties. This represents an account of chiral supramolecules demonstrating enantioselective in vivo efficacy in agricultural context, driven by differences in foliar affinity and biofilm disruption, despite their comparable in vitro antibacterial potency, an equivalence that often conceals broader enantioselective effects on other biofunctional properties. Consequently, this study furnishes a critical reference for the rational design of chiral supramolecules and underscores the need to elucidate the enantioselective influences of their ostensibly inert properties in agricultural systems. Collectively, this study furnishes a critical reference for the rational design of chiral supramolecules and underscores the need to elucidate the enantioselective influences of their ostensibly inert properties in agricultural systems.
Methods
Preparation of supramolecular complex AIM-12S/R@β-CD
AIM-12S/R (4 μL, 87.47 mM) in acetonitrile was gradually introduced into 1.0 mL deionized water with β-CD (0.35 mM), then stirred thoroughly and allowed to evaporate naturally, yielding the supramolecular complex AIM-12S/R@β-CD.
In vitro antibacterial experiment
Target compounds and commercial control agents were prepared in five gradient concentrations (e.g., 100, 50, 25, 12.5, 6.25 μg·mL-1). One milliliter of each solution was added to test tubes containing 4 mL of NB liquid medium, thereby preparing sterile NB liquid media with the drugs. The absorbance of the sterile NB liquid media containing the drugs was measured using a microplate reader, and the OD values (OD595) were recorded. Subsequently, 40 μL of NB liquid medium containing the pathogen (Xanthomonas oryzae pv. oryzae (Xoo) and Pseudomonas syringae pv. actinidiae (Psa)) causing rice bacterial leaf blight was added (cultured to the logarithmic phase) to each test tube. The mixtures were incubated at 28 °C and 180 rpm in a constant-temperature shaker for 24 to 48 h. After incubation, the OD values (OD595) of the bacterial cultures at each concentration were measured using a microplate reader. Drug concentration was converted to log (x), and inhibition rate data was transformed to odds (y). The toxicity regression equation (\({{\rm{y}}}={{\rm{ax}}}+{{\rm{b}}}\)) and correlation coefficient (R2) were calculated using Excel to determine the inhibitory semi-inhibitory concentration value (EC50).
Fabrication and characterization of chiral supramolecular materials AIM-12S@β-CD and AIM-12R@β-CD
SEM images of compounds morphology
A compound solution under 200 μg·mL⁻¹ conditions was prepared. Then, 30 μL of the compound solution was placed on conductive glass and allowed to dry naturally. Then the morphological structure was detected after the gold sputtering treated.
DLS and zeta potential determination
The samples were prepared at 200 μg·mL⁻¹, and their particle size and Zeta potential were determined using a dynamic light scattering device. AIM-12S/R and AIM-12S/R@β-CD solutions (200 μg·mL⁻¹) were similarly analyzed for these parameters.
1H NMR titration experiment
AIM-12S (5.0 mM), β-CD, and their inclusion complexes were prepared at molar ratios of 1:0, 1:1, and 1:1.5, and analyzed by 1H NMR to examine molecular interactions.
UV-vis titration experiment
The UV-visible spectrum was acquired using a 3.0 mL colorimetric dish containing a 50 μM aqueous solution of AIM-12S/R. A 10 mM aqueous solution of β-CD was prepared and added incrementally to the colorimetric dish for the UV-visible spectroscopic analysis. The Benesi-Hildebrand (B-H) equation was employed to determine the binding constant (Ka).
This equation involves measuring the change in absorbance values before and after inclusion (ΔA), the total concentration of β-CD (c), and a constant (α). To obtain the binding constant (Ka), plot 1/ΔA against 1/c (β-CD) and calculate the slope and intercept of the resulting straight line.
Job’s plot experiment
Several solutions of AIM-12S/R and β-CD, totaling 0.05 mM in a volume of 3.0 mL, were prepared in various ratios. In this process, AIM-12S/R was present in different molar fractions (xa). The difference in UV absorbance was measured for each concentration, both with and without AIM-12S/R, yielding the variation ΔAxa. Job’s curve was generated by plotting ΔAxa against the molar ratio of Nβ-CD to NAIM-12S/R+β-CD.
Foliar affinity of supramolecular materials on superhydrophobic leaves
Dynamic contact angle (DCA)
Measurements were performed with an OCA 20 system (DataPhysics) equipped with analysis software to determine the dynamic contact angle. For each test, 2 μL droplets of control solution (0.4% DMSO or β-CD) or treatment solution (200 μg·mL⁻¹) were placed on rice leaf surfaces, and readings were taken at 25 ± 1 °C.
Dynamic surface tension (DST)
The DST of the drug solution (200 μg·mL⁻¹) was measured using the Wilhelmy method with a surface tension meter (DCAT 21, DataPhysics) at 28 ± 1 °C.
Equilibrium contact angle (ECA)
A contact angle measuring instrument (JC 2000D1) with analysis software was used to measure the ECA. A 100 μL microliter syringe was employed to dispense droplets of the control group (0.4% DMSO or β-CD) or treatment group (200 μg·mL⁻¹) to their maximum size. The lifting platform was rotated to bring a glass slide attached to the rice leaf into contact with the droplet, which was then allowed to rest. The droplet angle was measured using the angle measurement method. Each experiment was repeated three times, and the average value and standard deviation were recorded.
Equilibrium surface tension (EST)
Measurements were performed using a contact angle instrument (JC 2000D1). A 100 μL syringe dispensed droplets of control solution (0.4% DMSO or β-CD) or treatment solution (200 μg·mL⁻¹) until the maximum droplet size was reached. Images were recorded, and droplet surface tension was determined via the hanging drop method. Each test was repeated over ten times, and mean values with standard deviations were calculated.
Spray macroscopic observation experiment
AIM-12S/R and AIM-12S/R@β-CD (200 μg·mL⁻¹) were uniformly applied to rice leaves, and photographs were captured to evaluate droplets distribution.
Bouncing behavior assay
The droplet impact on rice leaves was recorded at 2000 fps using a high-speed camera (Cyclone-2-2000-C, Germany). Water, β-CD, AIM-12S/R, and AIM-12S/R@β-CD (200 μg·mL⁻¹) were dispensed via a microliter syringe. The normalized diameter (Dt/D0) and rebound height (Ht/D0) were determined from video analysis using i-SPEED Suite software. For splash and bounce tests, droplet release heights were 30 cm and 15 cm, respectively, at 25 °C. A rice leaf affixed to a slide on a 30° stand received droplets released from a fixed needle (outer diameter 0.51 mm, inner diameter 0.25 mm) at 15 cm. The entire impact sequence was captured at 2000 fps and analyzed with i-SPEED Suite software.
Liquid holding capacity on rice leaf
Rice leaves (with known area and mass) were immersed in solutions of water, β-cyclodextrin (β-CD), AIM-12S/R, and AIM-12S/R@β-CD under 200 μg·mL-1 conditions. After 20 seconds, the leaves were lifted vertically with tweezers until no droplets remained, and their mass was measured using an analytical balance. A glass slide with attached rice leaves (with known area and mass) was fixed on a 30° bracket. The leaves were sprayed five times with a spray bottle from a distance of 15 cm, and the mass retention of the leaves was calculated using the following equation, after performing all tests ten times:
where M0 and M1, represent the weights of the leaves before and after soaking or sparying, respectively, and S is the surface area of the leaves. The area of rice leaves was measured using coordinate paper.
Dispersion and deposition on rice leaf
The surface morphology of water (containing 0.4% DMSO), β-CD, AIM-12S/R, and AIM-12S/R@β-CD under 200 μg·mL-1 conditions on rice leaves was characterized using SEM. Briefly, 20 μL of droplets were sprayed onto the rice leaves fixed on the loading platform. After drying at room temperature, all samples were characterized under the SEM.
Exploration of antibiofilm mechanisms
Biofilm inhibition experiment
Xoo cultures in NB medium were diluted with sterilized nutrient broth (NB) to an OD595 of 0.1. DMSO (0.17%) or test compounds were mixed with the bacterial suspension to final concentrations of 0.5, 1, 2, 4, and 8 × EC50. Subsequently, 200 μL of each mixture was transferred into 96-well plates and incubated at 28 °C for 48 h. After incubation, non-adherent cells were removed, and wells were rinsed three times with sterile water, followed by drying at 45 °C for 2 h. Each well was treated with 200 μL of 0.1% crystal violet (w/v) for 30 min, followed by washing with sterile water until the stain was completely removed. Plates were dried again at 45 °C for 2 h, and bound crystal violet was solubilized with 95% ethanol. Biofilm content was quantified by measuring OD570 using an ELISA reader.
Biofilm eradication experiment
Xoo cultures in NB medium were diluted with sterilized NB to an OD595 of 0.1. Then, 200 μL of bacterial suspension was dispensed into 96-well plates and incubated at 28 °C for 48 h. DMSO (0.4%) or test compounds were added to sterilized NB to final concentrations of 200, 100, 50, 25, 12.5, 6.25, and 3.125 μg·mL⁻¹. Subsequently, 200 μL of each solution was transferred into 96-well plates and incubated again at 28 °C for 48 h. After incubation, non-adherent cells were removed, and wells were rinsed three times with sterile water, followed by drying at 45 °C for 2 h. Each well then received 200 μL of 0.1% crystal violet (w/v) for 30 min. Excess stain was removed with sterile water until no floating color remained. Plates were dried again at 45 °C for 2 h, and bound crystal violet was solubilized in 95% ethanol. Biofilm biomass was quantified by measuring OD570 using an ELISA reader.
Xoo colonies on agar plates from biofilm inhibition experiment
Xoo cultures in NB medium were diluted with sterilized NB to an OD595 of 0.1. DMSO (0.17%) or test compounds were mixed with the bacterial suspension to final concentrations of 0.5, 1, 2, 4, and 8 × EC50. Then, 200 μL of each mixture was transferred into 96-well plates and incubated at 28 °C for 48 h. After incubation, the supernatant was removed, fresh medium was added, and wells were agitated to release bacteria from the biofilm. The resulting suspension was diluted 1 × 105-fold in PBS, and 10 μL was spread onto agar plates. Plates were incubated at 28 °C for 36–48 h, photographed, and colonies were counted.
CLSM 3D imaging
Biofilm inhibition: Xoo was cultured at 28 °C with shaking (180 rpm) until OD595 reached 0.6, then adjusted to 0.1 in liquid medium. Sterilized conductive glass was placed in 12-well plates, and 5 mL of bacterial suspension was added per well. DMSO (0.11%) or test compounds were introduced to final concentrations of 1×, 2×, and 4× EC50. Plates were statically incubated at 28 °C for 48 h. Supernatant was removed, and samples were rinsed with sterile water before sequential staining with acridine orange (0.1%, 15 min) and pyridinium iodide (0.01%, 10 min). The glass was mounted on slides for CLSM analysis using 488 nm or 543 nm lasers.
Biofilm eradication: Xoo was cultured and adjusted as above. Conductive glass was placed in 12-well plates with 5 mL of bacterial suspension and incubated statically at 28 °C for 48 h to form biofilms. Medium was replaced with fresh sterilized NB containing DMSO (0.1%) or test compounds at 50, 25, or 12.5 μg·mL⁻¹, followed by another 48 h static incubation. Staining and CLSM analysis were performed as in the inhibition assay. Fluorescence intensity was quantified with ImageJ software.
The biofilm recurrence study after treatment cessation
Sterilized conductive glass was placed in 6-well plates, and Xoo suspensions (OD595 = 0.1) were added, followed by static incubation at 28 °C for 48 h to establish initial biofilms. The medium was then replaced with fresh NB containing AIM-12S, AIM-12S@β-CD, AIM-12R, or AIM-12R@β-CD at 12.5, 25, or 50.0 μg·mL⁻¹, and incubation continued for another 48 h. After treatment, wells were rinsed 2–3 times with PBS to remove non-adherent cells, replenished with bactericide-free medium, and incubated at 28 °C for 48 h to evaluate regrowth. Biofilms were stained with acridine orange (0.1%, 15 min) and imaged by CLSM (488 nm and 543 nm lasers). Fluorescence intensity was quantified using ImageJ software.
For CFU enumeration, 200 μL of Xoo suspension (OD595 = 0.1) was inoculated into 96-well plates and incubated at 28 °C for 48 h. Following medium removal and PBS washing, fresh NB containing the same bactericides and concentrations was added, and plates were incubated for another 48 h. The treatment medium was replaced with bactericide-free NB, and cultures were incubated at 28 °C for a further 48 h. Biofilm-associated cells were then resuspended, serially diluted (105-fold), and 10 μL aliquots were plated onto agar. Plates were incubated at 28 °C for 3 days before colony counting.
Exploration of bactericidal mechanisms
Determination of extracellular polysaccharide
The initial OD595 of Xoo cells in NB liquid medium was adjusted from 1 to 0.1 using sterilized NB medium. A 30 mL bacterial suspension with an OD595 value of 0.1 was transferred to a 50 mL Erlenmeyer flask, and DMSO (0.11%) or compounds were added to the sterilized NB medium to achieve concentrations of 1 × EC50, 2 × EC50, and 4 × EC50, respectively. The flask was placed on a shaker to incubate for 4 days at 28 °C and 180 rpm. After incubation, the precipitate of all samples was removed by centrifuging at 7080 g for 10 min. Subsequently, 30 mL of 95% ethanol was mixed with 10 mL of supernatant and refrigerated at 4 °C for 12 h. The supernatant was discarded to collect the precipitate, which was then centrifuged at 7080 g for 10 min. Finally, all the precipitates were dried at 60 °C and weighed.
Determination of extracellular amylase formation
First, the bacterial suspension with an OD595 value of 0.1 was transferred to a sterilized 2 mL centrifuge tube, and compounds were added to achieve concentrations of 1 × EC50, with a bacterial solution containing 0.028% DMSO as a blank control. Next, a layer of water agar was added to a 90 mm culture dish, and an Oxford cup was placed on its surface after it had solidified. Subsequently, 10 mL of melted NA solid culture medium containing 0.1% starch was added to it. The Oxford cup was removed after the NA medium had solidified, and 2 μL of bacterial culture containing compounds was added to the hole. The culture dish was incubated at 28 °C for 3 days. Finally, the culture dish was stained for 10 min using a 1% I₂/KI solution, and photos were taken to measure the hydrolysis circle diameter after washing away the excess I₂/KI solution.
Swimming motility assay
A 30 mL of melted NA solid culture medium was added to a culture dish, along with compounds to achieve concentrations of 1 × EC50. Then, 2 μL of Xoo cells, incubated to an OD595 value of 0.5, was added to the NA medium. The culture dish was incubated at 28 °C for 3 days. Finally, the diameter of the bacterial colony was measured with a ruler.
Pathogenicity experiment
Firstly, 20 mL bacterial suspension with OD595 value of 0.1 was added to 50 mL erlenmeyer flask and AIM-12S/R, AIM-12S/R@β-CD were added to achieve concentrations of 1 × EC50, with 0.028% DMSO as a blank control. The Erlenmeyer flask was placed on a shaker for 24 h to achieve Xoo cells with an OD595 value of 0.8 (28 °C, 180 rpm). Subsequently, rice plants were infected using the leaf cutting method. After 14 days of infection, the length of the infection was measured and photographed.
Antibacterial mechanism experiment
ROS detection: The accumulation of reactive oxygen species (ROS) was assessed using a ROS assay kit (Genmed Scientific Inc., USA). Xoo bacterial solutions in the logarithmic phase were collected via centrifugation (2549 g, 5 min, 4 °C), washed three times with phosphate-buffered saline (PBS, 10 mM, pH 7.2), and resuspended in PBS. Subsequently, Xoo was mixed with compounds at varying concentrations (6.25, 12.5, 25.0, and 50.0 μg·mL⁻¹), with DMSO as the control group. After shaking for 14 h, 200 μL of the bacterial suspension was incubated with 5 μL of the Genmed staining solution in the dark at room temperature for 20 min. Samples were then analyzed using a Fluoromax 4cp fluorescence spectrophotometer (excitation wavelength: 490 nm). Enzyme activities detection: Xoo cells were cultivated to the early logarithmic phase (OD595 = 0.2) and supplemented with a DMSO solution containing compounds to achieve final concentrations of 0, 6.25, 12.5, 25, and 50 μg·mL⁻¹. Following incubation at 28 °C for 14 hours, relevant pathogens were collected and washed with precooled PBS (0.1 M, pH 7.2). Pathogenic bacteria were obtained by sonication (3 seconds on, 10 seconds off, repeated 60 times at 0 °C) and resuspended in the same PBS buffer. Bacterial fragments were removed by centrifugation (7080 g, 10 min, 4 °C), and protein concentration was determined using the Bradford method. The enzyme activities of catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) were monitored and calculated using respective assay kits (Solarbio Life Sciences or Suzhou Comin Biotech, China) according to the manufacturer’s instructions, with OD values detected using an enzyme labeling instrument. Determination of conductivity: Twenty milliliters of Xoo bacterial suspension (OD595 = 0.6–0.8) were centrifuged overnight at 4 °C and 637 g for 10 min. The supernatant was discarded, and the bacterial cells were washed three times with 5% glucose solution to remove the culture medium. Fresh 5% glucose solution was added until the conductivity approximated that of a 5% glucose solution, referred to as the isotonic fluid. Different doses of compounds were then added to a 5% glucose solution to achieve final concentrations of 6.25, 12.5, 25, and 50 μg·mL⁻¹, with an equal volume of DMSO as a negative control. After mixing, the conductivity was measured and recorded as L1. Next, the corresponding doses of compounds and DMSO were added to the isotonic solution, mixed thoroughly, and placed in a constant temperature shaker at 28 °C and 180 rpm. Conductivity measurements were taken every hour for a total of 8 h, recorded as L2. Finally, the isotonic solution was boiled for 5 min, cooled, and its conductivity measured, recorded as L0. Relative conductivity was calculated as follows:
In vivo against rice bacterial leaf blight (BLB) and Psa infection
BLB: The in vivo efficacy of agents against rice bacterial leaf blight was assessed via the leaf-cutting method. A 20 mL bacterial suspension (OD595 = 0.8) was prepared. For protective assays, rice leaves were sprayed with AIM-12S/R, AIM-12S/R@β-CD, KSM, or 20% TC SC at 200 μg·mL⁻¹. After 24 h, leaf tips (1–2 cm) were excised with sterilized scissors and immersed in the Xoo suspension. For curative assays, leaf tips were first cut and immersed in the bacterial suspension, followed 24 h later by spraying with the same treatments. All rice plants were incubated for 14 days in an artificial climate (28 °C, 90% RH). After 14 days of infection, the length of infection was measured, and photographs were taken. Control efficacy was assessed using the classification standardized counting method, and the disease index (C or T) was calculated as follows:
Control efficiency (I) for anti-Xoo activity was calculated using:
Where C is the disease index of the blank control and T is the treatment group.
Psa Infection: Uniform-length leaves were collected, rinsed three times with sterile water, and blotted dry on filter paper. Using a piercing mill, 1.2 cm diameter leaf discs were prepared, avoiding major veins. All discs were immersed in 10 mL of Psa cell suspension (OD = 0.1) for 1 h to facilitate bacterial inoculation. After air-drying, the discs were individually submerged in 10 mL solutions of 200 μg·mL-1 AIM-12S, AIM-12S@β-CD, AIM-12R or AIM-12R@β-CD, and 20% TC SC for 4 h. An equal volume of DMSO served as the blank control. Treated samples were incubated in an artificial climate chamber (28 °C, 90% relative humidity). After 5 days, photographs were taken to document lesion development on the leaf discs, and lesion areas were quantified.
Biosafety assessment
Rice seed germination and rice growth assay: AIM-12S/R and AIM-12S/R@β-CD were dissolved in pure water to prepare treatment solutions with a compound concentration of 200 μg·mL⁻¹. Each culture dish received 10 mL of the treatment solution and was seeded with 40 healthy rice seeds, with three replicates for each treatment. A 0.4% DMSO aqueous solution served as the blank control. The dishes were incubated in a light incubator (26 °C, 12 h light/dark cycle, 95% humidity), and treatment solutions were changed daily. The number of germinated seeds was counted after 2 days to determine the germination rate, while root and shoot lengths were measured after 5 days, with 20 seeds per culture dish measured using a ruler.
Mature rice safety assay: AIM-12S/R and AIM-12S/R@β-CD were dissolved in pure water to prepare treatment solutions with a concentration of 200 μg·mL⁻¹, with a 0.4% DMSO aqueous solution as the blank control. The treatment solution was sprayed on 40-day-old rice plants. One week later, the plants were observed for any speckling.
Earthworm safety assay: Filter paper was placed at the bottom of a 4.8 cm diameter glass bottle. Two milliliters of the 200 μg·mL⁻¹ compound solution were added to the bottom, with β-CD at a 1:1 molar ratio to AIM-12S/R as the negative control, and a 0.4% DMSO aqueous solution as the blank control. The filter paper was dried to evenly distribute the compound or other components. After wetting the filter paper with 2 mL of pure water, two earthworms were placed in each glass bottle, with five parallel groups totaling ten earthworms. The condition of the earthworms was observed and recorded after 48 h, defining earthworms unresponsive to mechanical stimuli as dead, those exuding yellow body fluid as poisoned, and those showing no symptoms as normal.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics 27 and Origin 2021 (Windows). Unless stated otherwise, experiments were independently repeated at least three times, yielding similar results. Values are expressed as mean ± standard deviation (SD), with sample sizes detailed in the corresponding figure legends. Differences among means were evaluated by one-way ANOVA, followed by Duncan’s multiple range test or least significant difference (LSD) post-hoc test. For all analyses, n ≥ 3; *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. All statistical tests were two-sided.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All relevant data supporting this study are contained in the main manuscript and supplementary materials. Detailed information on the compounds, including numbering, physical state, color, and spectroscopic profiles (1H, 13C), is provided in the Supplementary Information. Additional details can be obtained from the corresponding authors upon request. Source data for Figs. 1–8, Table 1, Supplementary Figs. 1–34, and Table 1–4 are included in the accompanying source data file and are available within this paper. Source data are provided with this paper.
References
Jiang, N. et al. Resistance genes and their interactions with bacterial blight/leaf streak pathogens (Xanthomonas oryzae) in rice (Oryza sativa l.)—an updated review. Rice 13, 3 (2020).
Shamsunnaher et al. Rice immune sensor XA21 differentially enhances plant growth and survival under distinct levels of drought. Sci. Rep. 10, 16938–16938 (2020).
Peng, J. et al. Design, synthesis, antibacterial activity, and mechanism of novel resveratrol derivatives containing an 1,3,4-oxadiazole moiety. Pest. Biochem. Physiol. 193, 105457 (2023).
Vishakha, K., Das, S. & Ganguli, A. Photodynamic antibacterial and antibiofilm activity of riboflavin against Xanthomonas oryzae pv oryzae: an ecofriendly strategy to combat bacterial leaf blight (BLB) rice disease. Arch. Microbiol. 204, 566 (2022).
Wu, Z., Shi, J., Chen, J., Hu, D. & Song, B. Design, synthesis, antibacterial activity, and mechanisms of novel 1,3,4-thiadiazole derivatives containing an amide moiety. J. Agric. Food Chem. 69, 8660–8670 (2021).
Das, A. & Ghosh, S. Stimuli-responsive self-assembly of a naphthalene diimide by orthogonal hydrogen bonding and its coassembly with a pyrene derivative by a pseudo-intramolecular charge-transfer interaction. Angew. Chem. Int. Ed. 53, 1092–1097 (2013).
Liu, B. et al. Construction of a controlled-release delivery system for pesticides using biodegradable PLA-based microcapsules. Colloids Surf. B Biointerfaces 144, 38–45 (2016).
Bao, Z. et al. The simple strategy to improve pesticide bioavailability and minimize environmental risk by effective and ecofriendly surfactants. Sci. Total Environ. 851, 158169 (2022).
Ghormade, V., Deshpande, M. V. & Paknikar, K. M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 29, 792–803 (2011).
Anjaneyulu, B., Chauhan, V., Mittal, C. & Afshari, M. Innovative nanocarrier systems: a comprehensive exploration of recent developments in nano-biopesticide formulations. J. Environ. Chem. Eng. 12, 113693 (2024).
Li, Z. et al. Regulating droplet impact and wetting behaviors on hydrophobic weed leaves by a double-chain cationic surfactant. ACS Sustain. Chem. Eng. 9, 2891–2901 (2021).
Bao, Z. et al. Molecular selection and environmental evaluation of eco-friendly surfactants to efficiently reduce pesticide pollution. J. Clean. Prod. 416, 137954 (2023).
Luo, S. et al. Uniform spread of high-speed drops on superhydrophobic surface by live-oligomeric surfactant jamming. Adv. Mater. 31, 15214095 (2019).
Song, R. et al. Preparation and characterization of an oil-in-water microemulsion of thiamethoxam and acetamiprid without organic solvent for unmanned aerial vehicle spraying. Colloid Surf. Physicochem. Eng. Asp. 607, 125485 (2020).
Shamay, Y. et al. Quantitative self-assembly prediction yields targeted nanomedicines. Nat. Mater. 17, 361–368 (2018).
Shangguan, W. et al. Making the complicated simple: a minimizing carrier strategy on innovative nanopesticides. Nano-Micro Lett. 16, 193 (2024).
Bodelón, G. et al. Detection and imaging of quorum sensing in Pseudomonas aeruginosa biofilm communities by surface-enhanced resonance Raman scattering. Nat. Mater. 15, 1203–1211 (2016).
Conlon, B. P. et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 503, 365–370 (2013).
Liu, J. et al. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 523, 550–554 (2015).
Vishwakarma, A., Dang, F., Ferrell, A., Barton, H. A. & Joy, A. Peptidomimetic polyurethanes inhibit bacterial biofilm formation and disrupt surface established biofilms. J. Am. Chem. Soc. 143, 9440–9449 (2021).
Lv, X. et al. Recent nanotechnologies to overcome the bacterial biofilm matrix barriers. Small 19, e2206220 (2022).
Hu, D., Deng, Y., Jia, F., Jin, Q. & Ji, J. Surface charge switchable supramolecular nanocarriers for nitric oxide synergistic photodynamic eradication of biofilms. ACS Nano 14, 347–359 (2019).
Mattia, E. & Otto, S. Supramolecular systems chemistry. Nat. Nanotechnol. 10, 111–119 (2015).
Webber, M. J., Appel, E. A., Meijer, E. W. & Langer, R. Supramolecular biomaterials. Nat. Mater. 15, 13–26 (2015).
Hendry, E. et al. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 5, 783–787 (2010).
Wei, K. et al. Natural glycyrrhizic acid-tailored nanoparticles toward the enhancement of pesticide bioavailability. Adv. Funct. Mater. 34, 2315493 (2024).
Liu, P. et al. Promoting the spreading of droplets on a superhydrophobic surface by supramolecular amphiphilic complex-based host–guest chemistry. J. Agric. Food Chem. 69, 9545–9550 (2021).
Dai, H., Yang, J., Fan, L., Luo, M. & Wang, P. Excellent foliar deposition of cyclodextrin-encapsulated furyl/thienyl-engineered ingredients: novel effective supramolecular agrochemicals for combating plant bacterial and fungal infections. Adv. Funct. Mater. 34, 2403823 (2024).
Morrow, S. M., Bissette, A. J. & Fletcher, S. P. Transmission of chirality through space and across length scales. Nat. Nanotechnol. 12, 410–419 (2017).
Müller, M. D., T. P. & Buser, H.-R. Isolation and identification of the metolachlor stereoisomers using high-performance liquid chromato. J. Agric. Food Chem. 49, 42–49 (2000).
Aiello, F., Simons, M. G., van Velde, J. W. & Dani, P. New insights into the degradation path of deltamethrin. Molecules 26, 3811 (2021).
Jing, X. et al. Enantioselective degradation and chiral stability of metalaxyl-M in tomato fruits. Chirality 28, 382–386 (2016).
Lucci, E. et al. A liquid chromatography-mass spectrometry method for the enantioselective multiresidue determination of nine chiral agrochemicals in urine using an enrichment procedure based on graphitized carbon black. Anal. Bioanal. Chem. 416, 1127–1137 (2023).
Aboul-Enein, H. Y. & Ali, I. Comparison of the chiral resolution of econazole, miconazole, and sulconazole by HPLC using normal-phase amylose CSPs. Fres. J. Anal. Chem. 370, 951–955 (2001).
Ali, I., Al-Othman, Z. A., Nagae, N., Gaitonde, V. D. & Dutta, K. K. Recent trends in ultra-fast HPLC: new generation superficially porous silica columns. J. Sepn. Sci. 35, 3235–3249 (2012).
Aboul-Enein, H. Y. & Ali, I. HPLC enantiomeric resolution of nebivolol on normal and reversed amylose based chiral phases. De. Pharmazie 56, 214–216 (2001).
Vashistha, V. K. et al. Stereoselective analysis of chiral pesticides: a review. Environ. Monit. Assess. 196, 153 (2024).
Yang, X., Jiang, S., Jin, Z. & Li, T. Application of asymmetric catalysis in chiral pesticide active molecule synthesis. J. Agric. Food Chem. 72, 17153–17165 (2024).
Chen, Y., Li, T., Jin, Z. & Chi, Y. R. New Axially chiral molecular scaffolds with antibacterial activities against Xanthomonas oryzae pv. oryzae for Protection of Rice. J. Agric. Food Chem. 70, 6050–6058 (2022).
Ali, I. & Aboul-Enein, H. Y. Enantioseparation of some clinically used drugs by HPLCusing cellulose Tris (3,5-dichlorophenylcarbamate) chiralstationary phase. Biomed. Chromatogr. 17, 113–117 (2003).
Ali, I., Gupta, V. K. & Aboul-Enein, H. Y. Chirality: a challenge for the environmental scientists. Curr. Sci. 84, 152–156 (2003).
Ali, I., Sanagi, M. M. & Aboul-Enein, H. Y. Advances in chiral separations by nonaqueous capillary electrophoresis in pharmaceutical and biomedical analysis. Electrophoresis 35, 926–936 (2013).
Pang, C. et al. Self-assembled borneol-guanidine-based amphiphilic polymers as an efficient antibiofilm agent. ACS Appl. Mater. Interfaces 16, 38429–38441 (2024).
Zhou, Z. et al. pH-activated nanoparticles with targeting for the treatment of oral plaque biofilm. J. Mat. Chem. B 6, 586–592 (2018).
Zheng, L. et al. Efficient pesticide formulation and regulation mechanism for improving the deposition of droplets on the leaves of rice (Oryza sativa L.). Pest Manag. Sci. 77, 3198–3207 (2021).
Zhang, C., Zhao, X., Lei, J., Ma, Y. & Du, F. The wetting behavior of aqueous surfactant solutions on wheat (Triticum aestivum) leaf surfaces. Soft Matter 13, 503–513 (2017).
Gauthier, A., Symon, S., Clanet, C. & Quéré, D. Water impacting on superhydrophobic macrotextures. Nat. Commun. 6, 8001 (2015).
Liu, H., Liu, D., Li, P., Zeng, Y. & Jin, H. Direct observation of the wetting state of Cassie and Wenzel. Mater. Lett. 340, 134182 (2023).
Song, Y. et al. The use of folate/zinc supramolecular hydrogels to increase droplet deposition on chenopodium album l. leaves. ACS Sustain. Chem. Eng. 8, 12911–12919 (2020).
Zhao, M. et al. pH/redox dual responsive from natural polymer-based nanoparticles for on-demand delivery of pesticides. Chem. Eng. J. 435, 134861 (2022).
Gupta, A. et al. Engineered Polymer Nanoparticles with Unprecedented Antimicrobial Efficacy and Therapeutic Indices against Multidrug-Resistant Bacteria and Biofilms. J. Am. Chem. Soc. 140, 12137–12143 (2018).
Qiu, D. et al. Enzymolysis and photothermal-mediated synergistic antimicrobial nanoplatform with programmed EPS degradation and biofilm penetration capabilities for eradication of biofilm wound infections. Chem. Eng. J. 477, 147217 (2023).
Wang, H. et al. Mechanically robust dissolving microneedles made of supramolecular photosensitizers for effective photodynamic bacterial biofilm elimination. ACS Appl. Mater. Interfaces 15, 25417–25426 (2023).
Qin, M. et al. Engineered probiotic bio-heterojunction with robust antibiofilm modality via “eating” extracellular polymeric substances for wound regeneration. Adv. Mater. 36, 2402530 (2024).
Mei, J. et al. Biofilm Microenvironment-Responsive Self-Assembly Nanoreactors for All-Stage Biofilm Associated Infection through Bacterial Cuproptosis-like Death and Macrophage Re-Rousing. Adv. Mater. 35, 2303432–2302432 (2023).
Singh, A., Gupta, R., Tandon, S. & Pandey, R. Thyme oil reduces biofilm formation and impairs virulence of Xanthomonas oryzae. Front. Microbiol. 8, 1074 (2017).
Tayi, L. et al. A mutation in an exoglucanase of Xanthomonas oryzae pv. oryzae, which confers an endo mode of activity, affects bacterial virulence, but not the induction of immune responses, in rice. Mol. Plant Pathol. 19, 1364–1376 (2017).
Wang, H. et al. A transcriptional regulator gar regulates the growth and virulence of Xanthomonas oryzae pv. oryzae. Curr. Microbiol. 80, 279–279 (2023).
Huang, X. et al. Rational Optimization of 1,2,3-triazole-tailored carbazoles as prospective antibacterial alternatives with significant in vivo control efficiency and unique mode of action. J. Agric. Food Chem. 69, 4615–4627 (2021).
Chen, F., Huang, G. & Huang, H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydr. Polym. 252, 117179 (2021).
Chen, F., Huang, S. & Huang, G. Preparation, activity, and antioxidant mechanism of rice bran polysaccharide. Food Funct. 12, 834–839 (2021).
Kong, M. et al. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids Surf. B Biointerfaces 65, 197–202 (2008).
Ke, Y., Hui, S. & Yuan, M. Xanthomonas oryzae pv. oryzae Inoculation and Growth Rate on Rice by Leaf Clipping Method. Bio-Protoc. 7, e2568 (2017).
Li, P. et al. Design, Synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J. Agric. Food Chem. 66, 3093–3100 (2018).
Yang, L. et al. Pectin-coated iron-based metal–organic framework nanoparticles for enhanced foliar adhesion and targeted delivery of fungicides. ACS Nano 18, 6533–6549 (2024).
Zhang, X. et al. A pH- and enzymatic-responsive nanopesticide to control pea aphids and reduce toxicity for earthworms. Sci. Total Environ. 861, 160610 (2023).
Acknowledgements
We acknowledge funds from the National Key Research and Development Program (2022YFD1700300) to P.W., Innovation Program for High-level Talents of Guizhou Province (No. GCC[2023]008) to P.W., Guizhou Provincial S&T Project (ZK[2022]017) to P.W., Research and Innovation Team of Guizhou University (Guidakechuangtuan[2023]03) to P.W., Natural Science Special Project of Guizhou University (Guidazhuanjihe[2024]02) to P.W., and the National Natural Science Foundation of China (82404431 and 22467025) to K.L.
Author information
Authors and Affiliations
Contributions
P.W. conceived the work, reviewed and edited the manuscript. M.L. and J.L. performed the experiments. M.L., J.Y., K.L. and P.W. analyzed the data. M.L. and K.L. completed the initial draft. All authors read and approved to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors, P.W., M.L., K.L., J.Y., and J.L., declare that they have no financial or non-financial competing interests related to the design, experiments, data analysis, manuscript preparation, or publication of this study.
Peer review
Peer review information
Nature Communications thanks Xixi Mao and the other anonymous reviewer(s) for their contribution to the peer review of this work. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, M., Liu, K., Yang, J. et al. Chiral dependence of multifunctional supramolecular materials in crop protection. Nat Commun 16, 8668 (2025). https://doi.org/10.1038/s41467-025-63677-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63677-8