Abstract
Powdery mildew is a devastating disease that affects wheat yield and quality globally. Here, we identify a powdery mildew resistance locus MlIW39 from wild emmer wheat through map-based cloning, mutagenesis, and stable genetic transformation. Unlike many other cloned Pm genes, the MlIW39-mediated resistance is conferred by the combined effect of two complementary nucleotide-binding and leucine-rich repeat (NLR) genes, encoding a canonical coiled-coil (CC) type NLR protein (MlIW39-R1) and an atypical NLR protein (MlIW39-R2) with an unknown domain (CC-like), respectively. Overexpression of the NLR pair induces cell death in Nicotiana benthamiana, whereas MlIW39-R1 or MlIW39-R2 alone does not. The MlIW39-R1 and MlIW39-R2 proteins physically interact with each other. MlIW39-R1 and MlIW39-R2 likely originate independently and become neighborly located during evolution. Our findings shed light on the significance of NLR pairs in plant immunity and can facilitate wheat disease-resistance breeding using the developed MlIW39 introgression lines and functional marker.
Similar content being viewed by others
Introduction
Common wheat (Triticum aestivum L.) is an important food crop, providing > 20% of calories and proteins, as well as dietary fibers, vitamins, and phytochemicals in the human diet1. Wheat powdery mildew disease, caused by the biotrophic fungus Blumeria graminis f. sp. tritici (Bgt), poses a huge threat to grain yield and quality, with an estimated global yield loss of 7.6–19.9% annually2. Deploying broad-spectrum powdery mildew (Pm) resistance genes in wheat varieties is a sustainable and environmentally friendly strategy to manage this disease. Unfortunately, in practice, Bgt-resistant wheat cultivars often carry single resistance genes that are easily vulnerable to existing or newly evolved resistance-breaking pathogen strains3. Therefore, expanding resistant resources and utilizing novel disease-resistance genes (R genes) in wheat breeding programs is an ongoing task.
Until now, more than 110 Pm resistance genes have been documented in Triticeae species4, but only twenty-one Pm genes have been cloned. Thirteen Pm genes encode NLR proteins, including Pm1a5, Pm26, Pm3b7/Pm88/Pm179, RXL and Pm5e pair10, Pm1211/Pm2112, MlIW170/Pm26 (TdCNL1 and TdCNL5 pair)13, Pm4114, Pm5515, Pm6016/MlIW17217/MlWE1818, Pm6919, PmTR1/PmTR320, PmAeu121, and Pm6Sl22 with an extra zinc finger BED domain. However, Pm423 and Pm1324,25 encode kinase fusion proteins (KFP); Pm2426 and WTK4 27 encode tandem kinase proteins (TKP); and Pm3628 and Pm5729 encode TKPs fused with integrated domains. Notably, Pm38 (Yr18/Lr34/Sr57) and Pm46 (Yr46/Lr67/Sr55) encode an ATP-binding cassette (ABC) transporter and a hexose transporter, respectively. The latter two genes confer broad-spectrum, adult-plant resistance to multiple diseases30,31.
Generally, a singleton NLR can directly or indirectly sense a specific pathogen-derived avirulent (avr) effector to trigger plant immunity32,33. Intriguingly, growing evidence shows that neighboring or head-to-head paired complementary NLRs are required for plant disease resistance34,35. For example, Arabidopsis NLR pairs CHS3/CSA136 and RPS4/RRS137,38 confer resistance to Hyaloperonospora arabidopsidis and multiple pathogens, respectively. Rice (Oryza sativa L.) NLR pairs Pik-1/Pik-239, RGA4/RGA540, and Pii-2/Pii-141 can each mediate resistance to Magnaporthe oryzae. Soybean (Glycine max) NLR pair Rpp6907-7/Rpp6907-4 confers broad-spectrum resistance to Asian soybean rust42. The genetically linked RGA2 gene is required in wheat for Lr10 resistance to leaf rust43. A pair of atypical NLR genes, TdNLR1 and TdNLR2, are responsible for Yr84/YrTD121-mediated stripe rust resistance44,45. The RXL and Pm5e pair and TdCNL1 and TdCNL5 pair confer resistance against wheat powdery mildew10,13.
Wild emmer wheat (WEW, Triticum turgidum ssp. dicoccoides, genome AABB, 2n = 4x = 28), the progenitor of both cultivated tetraploid and hexaploid wheat46, is a valuable resource for wheat improvement, especially for disease resistance47. To date, around dozens of WEW-derived Pm genes have been reported, and five (Pm4114, MlIW17217/MlWE1818, Pm6919, Pm3628, MlIW170/Pm2613) have been cloned. Previously, we identified a dominant Pm gene MlIW39 from WEW, and mapped it to a 460.3 kb physical interval in chromosome (chr.) arm 2BS48. The genetic interval for Pm68 in tetraploid wheat overlaps with that of MlIW3949, but their allelic status remained unclear until the cloning of them.
In the current study, we clone MlIW39 and find that two tightly linked complementary NLR genes are required for resistance. MlIW39-R1 is a typical CC NLR protein, while MlIW39-R2 contains an uncharacterized N-terminal domain, whose predicted 3D structure is similar to the CC domain. Haplotype analysis reveals six distinct haplotypes at the MlIW39 locus, and MlIW39 and Pm68 represent two different functional alleles of the same gene locus. We introgress MlIW39 into elite common wheat cultivar Gaoyou2018, conferring powdery mildew resistance without obvious negative linkage drag. This study provides insights into the role of paired NLRs in plant immunity.
Results
High-resolution genetic map of powdery mildew resistance gene MlIW39
Seedlings of the common wheat introgression line 8D49 (Han87-1/IW39//2*Han87-1) containing the dominant powdery mildew resistance gene MlIW39 derived from WEW accession IW3948 conferred wide-spectrum resistance to 30 genetically distinct Bgt isolates (Supplementary Fig. 1 and Supplementary Data 1). MlIW39 was mapped to an approximate 460.3 kb interval in the terminal region of chr.2BS, flanked by the Indel markers 7seq610 and 7seq705 in a previous study48.
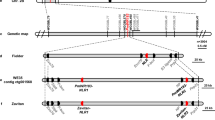
To narrow the MlIW39 interval, we used the markers 7seq610 and 7seq705 to screen 5,088 F3 individuals derived from crosses (Apogee × 8D49 and Liaochun10 (LC10) × 8D49) (Fig. 1a). Twenty-one recombinants were identified and genotyped by two additional Indel markers, 7seq622 and 7seq727, resulting in nine recombinant types (Supplementary Data 2). These progenies of recombinants were phenotyped using Bgt isolate E09, which is an avirulent isolate to MlIW39. Finally, MlIW39 was fine-mapped to an interval flanked by markers 7seq622 and 7seq705, corresponding to a 298 kb genomic region in the WEW reference genome Zavitan (WEW_v1.0)50, and showed co-segregation with 7seq727 (Fig. 1c, d).
a Infection types produced on 8D49 (highly resistant), Apogee (highly susceptible), and LC10 (highly susceptible) infected with Bgt isolate E09 at 15 days post inoculation (dpi). b Genetic map of MlIW39 on the short arm of chr.2B. c Physical positions of molecular markers used for fine mapping MlIW39 on the Zavitan WEW_v1.0 reference genome. d Genotypes and phenotypes of five recombinant types; R, resistant; or S, susceptible. Black, white, and gray blocks indicate homozygous segments from 8D49, homozygous segments from the susceptible parents, and heterozygous segments, respectively. e Seven annotated genes in the MlIW39 interval based on WEW_v1.0. A serine/threonine-protein kinase (STPK, TRIDC2BG003950), a typical NLR protein (NLR-1, TRIDC2BG003970), a NLR protein lacking coiled-coil (CC) domain (NLR-2, TRIDC2BG003990), a U4/U6 small nuclear ribonucleoprotein Prp31 (PRPF31, TRIDC2BG004000), a sulfate adenylyl transferase (SAT, TRIDC2BG004010), and two unknown proteins (UN-1 and UN-2, TRIDC2BG003960 and TRIDC2BG003980).
The MlIW39 interval in the Zavitan reference genome contained five predicted high-confidence protein-coding genes and two undescribed proteins (UN-1 and UN-2, TRIDC2BG003960 and TRIDC2BG003980), of which these five genes encode a serine/threonine protein kinase (STPK, TRIDC2BG003950), a typical CC-NLR protein (NLR-1, TRIDC2BG003970), an atypical NLR without a CC domain (NLR-2, TRIDC2BG003990), a U4/U6 small nuclear ribonucleoprotein Prp31 (PRPF31, TRIDC2BG004000), and a sulfate adenylyl transferase (SAT, TRIDC2BG004010), respectively (Fig. 1e). Collinearity analysis exhibited that the MlIW39 interval in different Triticeae genomes showed good collinearity, except for the two NLRs and the STPK gene, which were absent in several reference genomes (Supplementary Fig. 2). RT-PCR analysis showed that the NLR-1, NLR-2, PRPF31, and SAT genes were expressed in 8D49 seedlings at 24 h post inoculation (hpi) with Bgt isolate E09 (Supplementary Fig. 3).
We designed gene-specific markers based on the five candidate genes, all of which were positively amplified in 8D49. However, the markers for STPK, NLR-1, and NLR-2 failed to amplify PCR products in the susceptible parents Apogee and LC10 (Supplementary Fig. 3 and Supplementary Data 3), suggesting that the susceptible parents lacked or contained sequence variations in these three candidate genes. Sanger sequencing revealed that STPK from 8D49 and Zavitan shared identical protein sequences, and PRPF31 proteins from 8D49 and Apogee were identical, thus excluding these two genes as MlIW39 candidates due to the susceptibility of Zavitan and Apogee to Bgt isolate E09 (Fig. 2a). Compared to 8D49, a missense mutation (p.H775R) in NLR-1 and a nonsense mutation (p.E371*) in NLR-2 were identified in Zavitan (Fig. 2b), and one amino acid substitution (p.K362E) of the SAT protein was identified in Apogee and LC10 (Supplementary Fig. 4). Based on these results, NLR-1, NLR-2, and SAT were considered as MlIW39 candidates.
a Powdery mildew responses of 8D49, Zavitan and five susceptible mutants at 15 days post-inoculation (dpi) with Bgt isolate E09. b Predicted structures of the NLR-1 and NLR-2 genes and locations of the independent mutations. Gray rectangles indicate coding exons. Positions of the mutations are indicated by black lines. The coding sequence (c.) changes and their predicted effects on the translated protein (p.) are indicated below the mutants and Zavitan. c, d Three-dimensional (3D) models of NLR-1 and NLR-2 predicted by AlphaFold 3. The red spheres indicate amino acid substitutions from EMS mutagenesis and from Zavitan. Source data are provided as a Source Data file.
Analysis of MlIW39 mutants
To verify MlIW39 gene candidates, we treated approximately 10,000 seeds of 8D49 with 0.5% ethyl methane sulfonate (EMS) and harvested 4400 M1 plants. Tests of 30 M2 seedlings from each M1 plant identified five families segregating for reaction to Bgt isolate E09. Homozygous susceptible M3 lines were used for further analysis (Fig. 2a).
Full-length Sanger sequencing of STPK, NLR-1, NLR-2, PRPF31, and SAT in each mutant line showed that only NLR-1 and NLR-2 contained single-nucleotide polymorphisms (SNPs). The predicted coding sequences (CDS) structures of NLR-1 (2742 bp) and NLR-2 (3399 bp) were validated by Sanger sequencing using 8D49 cDNA, which showed no introns in their CDS regions (Fig. 2b). All five mutants showed G/C to A/T transitions, typical of EMS mutagenesis. Two mutants, M-2886 and M-2998, had a premature stop codon (p.W341*) and one amino acid change (p.R763K) in the NLR-1 protein, respectively. The other three mutants, M-1120, M-1784, and M-1867, harbored nonsynonymous mutations in the NLR-2 protein (Fig. 2b). These results suggested that both NLR-1 and NLR-2 might be required for MlIW39 resistance.
We used AlphaFold 351 to construct three-dimensional (3D) models to explore the structural characteristics of the two NLRs (Fig. 2c, d). The 3D structure of the N-terminal unknown domain from NLR-2 was composed principally of four α helices and exhibited similarity to the canonical CC domain of Arabidopsis ZAR1, thus was named the CC-like domain (Supplementary Fig. 5). A BLASTP search was performed in NCBI using three different domains (CC-like, NBS, and LRR) of NLR-2, only the CC-like domain showed significant sequence diversity, demonstrating the novelty of the NLR-2 CC-like domain (Supplementary Data 4). The missense and nonsense mutations in the susceptible mutants and Zavitan were present in the NBS and LRR domains of the NLR pair, and resulted in loss-of-function of Bgt resistance (Fig. 2b–d).
Paired NLRs conferred MlIW39 resistance
To further validate NLR-1 and NLR-2 function, transgenic expression constructs containing the CDS of NLR-1 (2742 bp) and NLR-2 (3399 bp), driven by the maize ubiquitin (Ubi) promoter, were separately transformed into susceptible wheat cultivar Fielder. Four and five independent T0 positive transgenic plants carrying NLR-1 and NLR-2, respectively, were identified (Fig. 3a, b). Transcription of NLR-1 and NLR-2 in respective T1 transgenic plants was confirmed by quantitative reverse transcription (qRT)-PCR analysis (Supplementary Fig. 6a). However, these T1 lines expressing either NLR-1 or NLR-2 alone were all susceptible to Bgt isolate E09 (Fig. 3a, b).
Given the results from transgenic and EMS mutagenesis studies, we predicted that both NLR-1 and NLR-2 were essential for Bgt resistance. To validate genetic interaction between NLR-1 and NLR-2, we crossed loss-of-function mutants of NLR-1 (M-2998) and NLR-2 (M-1784). The F1 hybrids exhibited high resistance to Bgt isolate E09, while the parental mutant lines were susceptible, suggesting functional complementation between these two NLRs (Supplementary Fig. 7). Similarly, F1 plants derived from a cross of NLR-1 with NLR-2 T1 transgenic plants were highly resistant to Bgt isolate E09 (Fig. 3c and Supplementary Fig. 8). The F2 population segregated for resistance and susceptibility, and only those plants pyramiding both NLR-1 and NLR-2 displayed resistance (Supplementary Fig. 9 and Table 1). Based on the qRT-PCR analysis, we found that both NLR-1 and NLR-2 were expressed in the resistant pyramiding F2 transgenic lines (Supplementary Fig. 6b).
Furthermore, we phenotyped the transgenic plants with five additional Bgt isolates. The transgenic plants carrying either NLR-1 or NLR-2 alone showed susceptibility, whereas the pyramided plants (NLR-1 × NLR-2) exhibited high resistance to all tested Bgt isolates, which is similar to that of 8D49 (Supplementary Fig. 10). Diamino benzidine (DAB) staining and trypan blue (TPN) staining revealed accumulation of reactive oxygen species (ROS) and cell death in the Bgt-challenged host cells of 8D49 and the transgenic plants carrying two NLRs. In contrast, neither ROS production nor cell death was detected in single-gene transgenic plants or the susceptible control Fielder (Supplementary Fig. 11).
Taken together, results from the EMS mutagenesis, transgenic experiments, and genetic hybridization collectively confirmed that MlIW39-mediated powdery mildew resistance depends on the cooperative function of the two head-to-head oriented (spaced 196 kb apart) NLRs, which were renamed as MlIW39-R1 and MlIW39-R2, respectively.
Expression analysis, subcellular localizations, protein interaction, and cell death-inducing activity of the NLR pair
The qRT-PCR analysis revealed similar up-regulated expression pattern of MlIW39-R1 and MlIW39-R2 following inoculation with Bgt isolate E09 compared to mock-inoculated controls, with peak expression observed at 3 dpi (Supplementary Fig. 12). To determine the subcellular locations of MlIW39-R1 and MlIW39-R2, we fused each full-length CDS (without the terminal codon) to the N-terminus of a green fluorescent protein (GFP) reporter gene and obtained recombinant constructs MlIW39-R1-GFP and MlIW39-R2-GFP. When transiently overexpressed in wheat leaf protoplasts, both NLR proteins exhibited fluorescence signals localized to the cytoplasm (Supplementary Fig. 13).
Previous studies indicated that overexpression of functional NLR genes in N. benthamiana, such as barley MLA1052 and wheat Pm6016 induces cell death. To check whether a single or NLR pair of MlIW39-R1/MlIW39-R2 can trigger cell death, we performed transient expression assays in N. benthamiana leaves. Only overexpression of the NLR pair resulted in hypersensitive responses (Fig. 4a and Supplementary Fig. 14), supporting that MlIW39-R1 and MlIW39-R2 must work together to activate immune signaling.
a Cell death response in N. benthamiana triggered by co-expression of MlIW39-R1 and MlIW39-R2, whereas MlIW39-R1 or MlIW39-R2 alone did not. The Autofluorescence indicative of cell death under ultraviolet light is shown. Protein expression was detected by immunoblotting, and Ponceau S staining of Rubisco served as a loading control. Experiments were performed twice with similar results. b–e Protein-protein interaction between the CC domains of MlIW39-R1 was detected by Y2H, Luc, BiFC and Co-IP assays. AD, activation domain; BD, binding domain; Positive control (53 + T); Negative control (empty vectors AD + BD). The cLUC-TaSUMO1+nLUC-TaHsfA1 were used as a positive control in the Luc assay. Experiments were performed twice with similar results. f, g Interaction between MlIW39-R1CC and MlIW39-R2CC-like was confirmed by Y2H and Co-IP assays. Experiments were performed twice with similar results. Source data are provided as a Source Data file.
To evaluate potential interaction between these two full-length NLR proteins, we conducted split-luciferase complementation (Luc) and co-immunoprecipitation (Co-IP) assays. The Luciferase signal was detected when MlIW39-R1-nLUC/cLUC-MlIW39-R2 were co-expressed, but not for the negative controls MlIW39-R1-nLUC/cLUC-GUS, or GUS-nLUC/cLUC-MlIW39-R2 (Supplementary Fig. 15a). Co-IP results demonstrated that MlIW39-R1 specifically co-immunoprecipitated MlIW39-R2, and further revealed interactions between distinct domains of MlIW39-R1 (CC/NBS/LRR) and full-length MlIW39-R2. These results indicated that MlIW39-R1 and MlIW39-R2 physically interact with each other (Supplementary Fig. 15b, c).
Upon recognition of effector proteins, NLRs oligomerize into pentameric or hexameric wheel-like complexes, commonly referred to as resistosomes, to initiate immune responses53. Therefore, we predicted pentameric and hexameric 3D models of these two NLR proteins using the oligomerization domains (CC-NBS/CC-like-NBS) via AlphaFold 3. The 3D models of MlIW39-R1CC-NBS achieved higher predicted template modeling (pTM) scores than those of MlIW39-R2CC-like-NBS (Supplementary Fig. 16). Notably, structural modeling showed that MlIW39-R1CC formed a funnel-shaped structure, as seen in Arabidopsis ZAR154, while MlIW39-R2CC-like did not (Supplementary Fig. 16). Building on this finding, we predicted the pentameric assembly of full-length MlIW39-R1, showing wheel-like architecture similar to ZAR1 resistosome (Supplementary Fig. 17). These structural parallels suggest that MlIW39-R1 likely functions similarly to ZAR1 in plant immune.
Moreover, we assessed the self-association ability of MlIW39-R1CC using Yeast two-hybrid (Y2H), bimolecular fluorescence complementation (BiFC), Luc and Co-IP assays, and detected an interaction for MlIW39-R1CC (Fig. 4b–e), supporting its capacity for oligomerization. To further investigate whether MlIW39-R1CC interacts with other domains, we employed the Y2H assay. This result suggested that MlIW39-R1CC associates with MlIW39-R2CC-like, which was subsequently confirmed by Co-IP assay (Fig. 4f, g and Supplementary Fig. 18). All together, we propose that full-length MlIW39-R1 and MlIW39-R2 proteins functionally interact, and MlIW39-R1CC specifically associates with MlIW39-R2CC-like.
Haplotype analysis of the NLR pair
A specific Indel marker 7IND2 was designed and used to screen for the presence of MlIW39 in 61 WEW and 276 other tetraploid and hexaploid wheat accessions. The target band was detected only in 27 WEW accessions, suggesting that MlIW39 originated from WEW. Two gene-specific primer pairs were used to amplify the CDS of the two NLRs, and the 27 WEW lines showed positive amplification for both MlIW39-R1 and MlIW39-R2. This result also supported that the two NLRs always appeared together in the MlIW39 interval (Supplementary Data 5). Based on sequences of the two NLRs, the 27 WEW accessions were classified into six different haplotype combinations (MlIW39_h1 to MlIW39_h6) (Supplementary Data 6, Supplementary Figs. 19 and 20).
In parallel with this study, He et al. cloned Pm68, which is located in the same chromosome interval as MlIW39 and has the same NLR pair (Pm68-1 and Pm68-2), but represents different haplotypes55. Compared to the MlIW39 NLR pair, there are two amino acid changes (p.H775R in MlIW39-R1 and p.M535T in MlIW39-R2) in Pm68 (Supplementary Fig. 21). Moreover, no MlIW39 haplotype from our WEW collection was the same as Pm68. We developed two diagnostic markers, MlIW39-R1-STARP and MlIW39-R2-CAPS, which differentiated the corresponding NLR gene pair within wheat accessions carrying Pm68 and MlIW39 (Supplementary Fig. 22).
Eight WEW accessions had the same haplotype as 8D49, designated as MlIW39_h1 (functional). Four WEW accessions shared an identical haplotype with Zavitan (MlIW39_h2, non-functional). Ten resistant accessions with MlIW39_h3 shared MlIW39-R1 with Pm68 and MlIW39-R2 with MlIW39. Four resistant WEW accessions carried three other haplotypes (MlIW39_h4 to MlIW39_h6); however, the functionality of these haplotypes in conferring resistance will require further study. Compared with MlIW39_h1, the MlIW39-R1 in the other six haplotypes (MlIW39_h2 to MlIW39_h6 and Pm68) had one common amino acid change (p.H775R). Hence, MlIW39-R1-STARP can be an ideal marker to identify MlIW39_h1 (MlIW39) from the other haplotypes (Supplementary Fig. 23). The haplotypic variants of MlIW39 (MlIW39_h1 to MlIW39_h6) were predominantly distributed among the WEW accessions collected from three regions in Israel (Tabigha, Yehudiyya, and Mount Hermon). In addition, one WEW accession carrying MlIW39_h1 originated from Diyarbakir of Southeastern Turkey (Supplementary Data 6 and Supplementary Fig. 24).
Phylogenetic analysis of MlIW39-R1 and MlIW39-R2
To study the evolutionary relationships of MlIW39-R1 and MlIW39-R2 with other known R genes, we compared their protein sequences with 115 cloned R proteins from wild relatives and various crop species (Supplementary Data 7). MlIW39-R1 was closest to Sr46 (66% identity) and MlIW39-R2 was closest to Yr27 (20% identity) in the phylogenetic tree, which has low protein sequence identity suggesting the novelty of MlIW39-R1 and MlIW39-R2 protein sequences (Supplementary Fig. 25). Phylogenetic analysis of MlIW39 NLR pair with other known paired NLRs showed that MlIW39-R1/MlIW39-R2 pair was closest to the Pii-1/Pii-2 (18%/18% identity) and Pi5-1/Pi5-2 (18%/15% identity) pairs in rice (Supplementary Fig. 26 and Supplementary Data 8). However, the low sequence identity suggests that they are unlikely to be homologs.
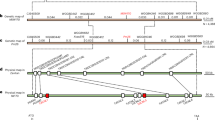
Blasting of the CDS of MlIW39 NLR pair in multiple published assemblies of Triticeae species showed MlIW39-R2 homologs presented in chromosomes group 2 (2A, 2B, 2D and 2E) with 80%-90% sequence identities, whereas homologs of MlIW39-R1 were identified mainly in chromosomes group 7 (7A, 7D, 7E, 7H, and 7S) with 81–87% sequence identities (Supplementary Data 9 and 10). Both MlIW39-R1 and MlIW39-R2 underwent multiple duplication events during wheat evolution. Phylogenetic analysis performed in representative Triticeae species showed that MlIW39-R1 was closest to copy-2 (87% identity) from H. vulgare in group I (Fig. 5a), and MlIW39-R2 was closest to copy-4 (88% identity) of Th. elongatum in group C (Fig. 5b). MlIW39-R1 group Ⅰ could be identified in T. dicoccoides, T. durum, and H. vulgare, and the MlIW39-R2 group C was identified in T. dicoccoides, T. durum, and Th. elongatum, indicating MlIW39-R1 and MlIW39-R2 evolved from different ancient NLRs (Fig. 5c). Considered overall, MlIW39-R1 and MlIW39-R2 likely originated from different chromosomes and became genetically linked in T. dicoccoides during evolution.
a, b Phylogenetic trees of MlIW39-R1 (a), MlIW39-R2 (b) and homologs in T. dicoccoides (Zavitan), T. durum (Svevo), Th. elongatum (D-3458) and H. vulgare (Morex). Homologs from the same group are displayed in the same color. c Microcollinearity plots of MlIW39-R1, MlIW39-R2, and their homologs among four Triticeae genomes. The dashed pentagons represent homologous copies of the two NLR genes. The colors of the pentagram and the Roman numerals under the pentagons correspond to the different groups in (a, b). The Arabic numerals in the pentagons represent the different homologs corresponding to each genome. The dashed and solid gray lines indicate possible routes resulting the neighborhood of MlIW39-R1 and MlIW39-R2 in the genomic context.
Development of MlIW39 introgression lines
To accelerate the breeding utilization of MlIW39, 8D49 was crossed and backcrossed with susceptible elite wheat cultivar Gaoyou2018 (GY2018), and Indel marker 7IND2 was used for marker-assisted selection (MAS) (Supplementary Fig. 27). The obtained BC3F1-derived line (GY2018 + MlIW39) homozygous for MlIW39 showed high resistance to Bgt isolate E09 (Fig. 6a). To evaluate the breeding potential of MlIW39, we compared the major agronomic traits between these introgression lines and the recurrent parent GY2018. The result revealed no negative effects on plant height, spike length, spikelet number per spike, thousand-kernel weight, grain length and grain width associated with the presence of MlIW39 (Fig. 6b–d), indicating that MlIW39 has great potential for application in wheat resistance breeding programs.
a Infection types of the BC3F1 plants (GY2018 + MlIW39) to Bgt isolate E09. The MlIW39 introgression lines showed high resistance to Bgt isolate E09. b, c Visual phenotypes of MlIW39 introgression lines (GY2018 + MlIW39, BC3F2) and the parent GY2018 under field conditions. d Statistical analysis of the agronomic traits in GY2018 + MlIW39 and its parental Line GY2018 under field conditions. The statistical significance of differences was determined using the two-sided student’s t test (ns = no significant, P > 0.05). Source data are provided as a Source data file.
Discussion
Cloning R genes from wild wheat relatives enriches the genetic resources available for breeding and deepens our understanding of how they work. Introgressing R genes into elite wheat varieties will effectively reduce yield losses caused by wheat diseases56. The considerable genetic diversity of R genes among WEW makes it an important resource for resistance breeding. So far, several ‘landmark resistance genes’ have been introgressed to bread wheat from WEW, including the stripe rust resistance genes Yr1557 (WTK1) encoding a tandem kinase-pseudokinase protein, and Yr3658 (WKS1) encoding a protein with kinase and START lipid-binding-like domains, and Yr84/YrTD121 (TdNLR1 and TdNLR2 pair)44,45 and powdery mildew resistance genes Pm4114, MlIW17217/MlWE1818, Pm6919, Pm36 (WTK7-TM)28 and Pm26 (TdCNL1 and TdCNL5 pair)13. Here, we report the map-based cloning of MlIW39 from WEW and confirm that an NLR pair (MlIW39-R1 and MlIW39-R2) is required for Bgt resistance. Our finding provides a new resistance gene resource from WEW for wheat resistance improvement and highlight the role of paired NLR genes in wheat immunity.
NLRs are critical intracellular immune receptors with hundreds of distinct members in the plant kingdom that can effectively respond to pathogen invasion and activate immune responses32. Typically, NLR proteins consist of two conserved domains: a central nucleotide-binding site (NBS) domain with AAA ATPase activity and a C-terminal leucine-rich repeat (LRR) domain. Based on the characteristics of the N-terminal, plant NLRs are further divided into three classes: toll and interleukin-1 receptor (TIR)-type NLR (TNL), coiled-coil (CC)-type NLR (CNL), or RPW8-like CC-type NLR (RNL)59. In the current study, MlIW39-R1 encodes a conventional CNL, while MlIW39-R2 lacks a CC domain predicted by NCBI. Interestingly, the 3D structure analysis showed that the N-terminal unknown domain of MlIW39-R2 forms a four-helix bundle structurally similar to the CC domain (Supplementary Fig. 5). The BLASTP analysis in NCBI showed lower sequence similarities when using the MlIW39-R2CC-like domain as a query, indicating that this domain differs from that in existing NLR proteins found in plants (Supplementary Data 4). The structural specificity of the N-terminal of MlIW39-R2 emphasizes its evolutionary diversity and potential functionality. Moreover, some truncated NLRs lacking a canonical domain also function in plant immunity. For example, YrSP, having lost most of the LRR region, still provides effective field-based resistance to wheat stripe rust60; in Arabidopsis, NRG1C, missing the RPW8 CC domain and most of the NBS domain, antagonized immunity mediated by its full-length neighbors NRG1A and NRG1B61.
Most reported NLRs act as singletons for Effector-Triggered Immunity (ETI), consistent with the gene-for-gene theory first proposed in the 1950s62. However, some NLRs cooperate as gene pairs in conferring resistance and are generally organized in head-to-head orientation33. In some cases, one member of the pairs is fused with an integrated domain (ID) that recognizes the pathogen effector and serves as a sensor NLR (sNLR), whereas the other gene encodes a typical NLR, which acts in immune signaling transduction as a helper NLR (hNLR)33,34. For example, in the Arabidopsis RPS4/RRS1 pair, RRS1 acts as a sensor NLR with a WRKY ID for recognizing effectors AvrRps4 from Pseudomonas syringae and PopP2 from Ralstonia solanacearum, whereas the RPS4 triggers immune signal activation34,37,63. In the RGA4/RGA5 and Pik-1/Pik-2 pairs of rice, RGA5 and Pik-1 are fused with a heavy metal-associated (HMA) ID for binding effectors from the blast pathogen Magnaporthe oryzae40,64. In the wheat RXL/Pm5e pair, putative sNLR Pm5e has an intrinsically disordered region (IDR)10. In the TdCNL1/TdCNL5 pair, TdCNL1 incorporates a new potassium-dependent sodium-calcium exchanger (NCKX) domain within the NB-ARC domain13. In our work, the MlIW39 NLR pair contains no ID, whereas MlIW39-R2 contains an uncharacterized functional domain (CC-like) at its N-terminal.
A recent study emphasized that AlphaFold 3 can serve as an effective predictive tool for distinguishing sNLRs from hNLRs in paired NLRs based on their oligomerization potential65. Protein structure prediction indicates that MlIW39-R1 is more likely than MlIW39-R2 to form a ZAR1-like pentameric resistosome. Crucially, only the MlIW39-R1 CC domain forms a funnel-shaped structure essential for Ca²⁺ channel formation, indicating MlIW39-R1 likely functions similarly to ZAR1 in plant immune responses (Supplementary Figs. 16 and 17). The oligomerization ability of MlIW39-R1CC was validated through multiple biochemical assays (Fig. 4b–e). Based on these findings, we inferred that MlIW39-R1 likely functions as a helper NLR to activate downstream immune signaling, while MlIW39-R2 specializes in recognizing powdery mildew effectors. Like RPS4/RRS1, RGA4/RGA5, and Pik-1/Pik-2 pairs that interact via their CC or TIR domains, MlIW39-R1CC associates with MlIW39-R2CC-like (Fig. 4f, g). However, whether they can form a heteromeric complex still needs further studies.
Overexpression of Arabidopsis RPS4 in tobacco or Arabidopsis resulted in suppressed defense activation in the presence of RRS137. Rice RGA4 mediated AVR-independent cell death in rice protoplasts and N. benthamiana was repressed when co-expressed with RGA540. In wheat, the Pm5e CC domain specifically suppresses HR induced by the RXL CC domain10. In contrast, the rice Pikp-1/Pikp-2 pair behaved differently; cell death occurred only when both genes were expressed together66. Remarkably, co-expression of the MlIW39-R1/MlIW39-R2 pair also triggered cell death in N. benthamiana (Fig. 4a). In the TdNLR1/TdNLR2 pair, the CC domain of TdNLR1 triggers cell death and exhibits self-association in planta44. Interestingly, although AlphaFold 3 predicts the N-terminal α1 helix of MlIW39-R1CC could form a Ca2+ channel, and we experimentally confirmed the autoactivation capacity of its CC domain, overexpression of this domain in N. benthamiana leaves failed to induce cell necrosis (Fig. 4b–e and Supplementary Fig. 14). Collectively, these observations suggest that the immune signaling activation mechanism of MlIW39 NLR pair may differ from previously reported paired NLRs (RPS4/RRS1, RGA4/RGA5, Pm5e/RXL and TdNLR1/TdNLR2).
In most examples, it is apparent that the two NLR members did not evolve from simple duplication events, but originated from different phylogenetic branches34. However, the rice NLR pair Pi5-1/Pi5-2 likely originated from a recent duplication event, owing to their high sequence similarity67. Phylogenetic analysis showed that MlIW39-R1 and MlIW39-R2 had distinct origins, whereby MlIW39-R1 had a homologous copy in chromosome 7 of H. vulgare, whereas the closest homolog of MlIW39-R2 was present in chromosome 2 of Th. elongatum (Fig. 5). This indicated that the NLR pair was not generated by a simple gene duplication event and might have originated from different Triticeae chromosomes and became linked in wild emmer wheat. An unknown evolutionary event enabled the two phylogenetically diverged genes to arrange together; it was also known as the ‘true love’ model34, which may be a reasonable explanation, and this model could be beneficial to natural selection, as both of them were required for Bgt resistance and tightly linked.
The isolation of the MlIW39 NLR pair provides a solid foundation for further understanding the molecular mechanism underlying paired NLRs function in plant immunity. Furthermore, we have successfully introduced MlIW39 into the susceptible bread wheat cultivar GY2018, and these introgression lines exhibit no significant adverse effects on the major agronomic traits compared the recurrent parent (Fig. 6). These introgression lines, along with the functional marker, represent valuable resources for wheat breeding to improve powdery mildew resistance.
Methods
Plant materials
Line 8D49 was developed from the cross Han87-1/IW39//2*Han87-1; WEW accession IW39 was the powdery mildew resistance gene MlIW39 donor, and Han87-1 was a susceptible common wheat line48. Mapping populations were developed by crossing 8D49 with susceptible lines Apogee and LC10 (Apogee × 8D49 and LC10 × 8D49)48, and 5,088 F3 individuals derived from heterozygous F2 plants were used to fine-map MlIW39. The common wheat line Xuezao was used as the susceptible control in the powdery mildew assessments. The susceptible wheat cultivar Fielder was used for genetic transformation. The susceptible cultivar GY2018 was used as the recipient parent for introducing MlIW39. A total of 337 wheat accessions were used for haplotype analysis, in which 276 wheat accessions were stocked at China Agricultural University, China, and 61 WEW accessions, as well as WEW IW39, that originated from the University of Haifa, Israel (Supplementary Data 5).
Bgt evaluation assays
Bgt isolate E09 and another 30 Bgt isolates collected from geographically different regions of China were used to evaluate the powdery mildew response of 8D49 (Supplementary Data 1). Bgt isolate E09, maintained on seedlings of the susceptible line Xuezao, was used for all other assays in this study. Five additional Bgt isolates were used to evaluate the powdery mildew response of transgenic plants. Wheat plants were inoculated with Bgt isolate E09 at the three-leaf stage under greenhouse conditions. Infection types (ITs) were evaluated at 15 days post-inoculation (dpi) on a scale of 0–4 (0, immune; 0, necrotic fecks; 1, high resistance; 2, moderate resistance; 3, moderate susceptibility and 4, high susceptibility)68. The phenotypes were classified into two categories, resistant (R, IT 0-2) and susceptible (S, IT 3-4).
High-resolution mapping of MlIW39
For fine mapping of MlIW39, a segregating pooled population of 5088 plants were genotyped with MlIW39-flanking Indel markers 7seq610 and 7seq705 for selecting recombinant plants. The recombinants were then screened with 7seq622 and 7seq727 (Supplementary Data 2). High confidence genes within the MlIW39 region were extracted based on the WEW Zavitan reference genome (WEW_v1.0)50. Total genomic DNA was extracted from leaves using the CTAB method69. Genotyping of the recombinants was performed in 10 µL volumes containing 5 μL 2 × Taq PCR StarMix (GenStar, Beijing), 1 µL DNA template (50–100 ng/µL), 1 µL primer (mixture of left and right primers, 2 μM) and 3 µL ddH2O. PCR amplification was performed as follows: 94 °C for 5 min; 34 cycles of 94 °C for 30 s, 56–58 °C primer annealing for 30 s (depending on different primers), 72 °C for 30–35 s, with a final extension at 72 °C for 5 min.
Gene expression analysis
At the three-leaf stage, the 8D49 plants were either mock or inoculated with Bgt isolate E09 in two independent plant growth chambers under identical environmental conditions (22 °C day/18 °C night and 16 h light/8 h dark). Total RNA samples were extracted from the uninfected or post-infected leaves at 0, 1, 2, 3, 4, and 5 days post-inoculation (dpi) using RNAiso Plus (TaKaRa, Beijing, China). The first-strand cDNA was synthesized using 1 μg of total RNA by Evo M-MLV reverse transcriptase Mix (Accurate Biology, Hunan, China) following the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) analysis was performed using SYBR Green Premix Pro Taq HS (Accurate Biology, Hunan, China). The TaActin gene was used as an internal control, and relative gene expression was calculated by the 2(-ΔΔCT) method70. The primers used to evaluate transcript levels are listed in Supplementary Data 3. All qRT-PCR samples were performed with three technical replicates, and each was conducted with three independent biological replicates. RT-PCR analysis was conducted to determine whether the five annotated genes were expressed, RNA samples were extracted from three-leaf stage seedling leaves of 8D49, Apogee and LC10 at 24 hpi, and the first-strand cDNA was synthesized as mentioned above. TaActin was used as the endogenous control.
5’- and 3’-RACE
Total RNA was isolated from inoculated three-leaf stage seedling leaves of 8D49 at 24 hpi, using RNAiso Plus (TaKaRa, Beijing, China). A rapid amplification of cDNA ends (RACE) was performed to obtain full-length coding regions of NLR-1 and NLR-2 genes. First-strand cDNA of 3’ and 5’ RACE was synthesized using HiScript-TS 5’/3’ RACE Kit (Vazyme, Nanjing, China). Specific amplification products from 5’ and 3’ RACE of the two NLR genes were cloned using a pEASY-Blunt Cloning Kit (TransGen Biotech, Beijing, China), and positive colonies were selected for Sanger sequencing.
EMS mutagenesis
Approximately 10,000 seeds of 8D49 were soaked in distilled water for 12 h and treated with 0.5% ethyl methane sulfonate (EMS) solution with shaking at 150 rpm for 12 h at room temperature, then rinsed with running water for 8 h. A total of 4400 M1 plants were harvested, and 30 M2 seedlings from each M1 line were tested for powdery mildew response at the three-leaf stage under greenhouse conditions. Susceptible M2 plants were selected, and M3 progenies were phenotyped to confirm their susceptibility. The full-length genomic sequences of five annotated genes in the MlIW39 interval were amplified from each M3 mutant and compared with wild-type 8D49 using DNAMAN 8 software (Lynnon Biosoft, San Ramon, CA, USA) to identify SNPs.
Genetic transformation of wheat
The full-length NLR-1 and NLR-2 coding sequences were amplified from cDNA of 8D49 and cloned into BamHI-digested pMWB110 vector to generate overexpression vectors: proUbi: NLR-1 and proUbi: NLR-2. These constructs were each transformed into the Agrobacterium strain EHA105 and transformed into the susceptible wheat cultivar Fielder by the Agrobacterium-mediated method71. T0 and T1 plants were tested for the presence of NLR-1 or NLR-2 by PCR amplification using specific primers (OE1JC-2 and OE2JC). The expression levels of NLR-1 and NLR-2 were evaluated by qRT-PCR. TaActin was used as an endogenous control.
Detection of ROS accumulation and cell death in Bgt-inoculated wheat leaves
To detect the accumulation of ROS, the leaves from 8D49, Fielder, transgenic plants at 3 dpi with Bgt isolate E09 were immediately incubated in a diamino benzidine (DAB) solution (1 mg/mL, pH 5.8) for 12 h at 25 °C in darkness, bleached in absolute ethanol and then stained with a 0.6% (w/v) Coomassie blue solution for 30 s and washed with distilled water. To detect cell death, the Bgt-inoculated leaves at 3 dpi were incubated in a 0.4% Trypan blue solution for 5 min in boiling water, bleached for 24 h in chloral hydrate solution (2.5 g/mL), stained in a 0.6% (w/v) Coomassie blue solution for 30 s, and then washed with distilled water72. The treated leaves were viewed under a light microscope.
Protein 3D modeling prediction and domain analysis
The domains of the NLR-1 and NLR-2 proteins were predicted based on the conserved domain database from the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). A BLASTP analysis with different domains of NLR-2 across plant species was performed in NCBI with the default setting, and the top 30 protein hits from each search were displayed (Supplementary Data 4). Three-dimensional (3D) structure models of NLR-1 and NLR-2 were performed using AlphaFold 351. The 3D models from the ranked sample with the best-predicted Template Modeling (pTM) score were selected. The structural graphics and the positions of amino acid substitutions were visualized using PyMOL (v.2.6.0a0).
Subcellular localization assays
To determine the subcellular locations of MlIW39-R1 and MlIW39-R2, the coding sequences of the two NLRs were separately cloned and inserted into XbaI-digested pCAMBIA1300-GFP vector driven by the CaMV35S promoter. An empty vector, pCAMBIA1300-GFP (Ev-GFP), was used as a negative control. A total of 10 μg of each plasmid construct (MlIW39-R1-GFP, MlIW39-R2-GFP and Ev-GFP) was delivered into wheat protoplasts via polyethylene glycol (PEG)-mediated method73, followed by overnight incubation at 25 °C. The fluorescence signals of transformed protoplasts were visualized using a confocal laser scanning microscope (LSM880, Carl Zeiss).
Cell death assays and Western blotting analysis
In the cell death induction assay, the full-length MlIW39-R1 and MlIW39-R2, or different domains, were inserted into the pCAMBIA-35S-MYC, pCAMBIA-35S-GFP and pCAMBIA-35S-Flag vectors for the expression of fusion proteins with MYC, GFP and FLAG epitope tags in N. benthamiana, respectively. The cells carrying target plasmids were inoculated in 5 mL LB liquid medium at 28 °C for 24-48 h, and were collected by centrifugation for 5 min at 4500 × g (room temperature). The Agrobacteria were suspended in infiltration buffer (10 mM MgCl2, 10 mM MES, 200 μM AS, pH = 5.6) to OD600 = 0.6–0.8, then incubated for 1–3 h at 28 °C. Equal volumes of mixed bacterial suspension were injected into N. benthamiana leaves. The leaves were photographed under ultraviolet light at 48–72 hpi to observe cell necrosis. Transiently expressed recombinant proteins were detected by western blotting using anti-GFP (TransGen Biotech, Beijing, China, HT801; 1:5000), anti-MYC (TransGen Biotech, Beijing, China, HT101; 1:5000) and anti-Flag (TransGen Biotech, Beijing, China, HT201; 1:5000) antibodies following the manufacturer’s instructions.
Yeast two-hybrid (Y2H) assays
The different domains of MlIW39-R1 and MlIW39-R2 were cloned into the yeast two-hybrid (Y2H) system vectors pGBKT7 and pGADT7, respectively. These constructs were co-transformed into yeast strain Y2Hgold, and then selected on SD medium lacking leucine and tryptophan (SD/-L/-T) at 30 °C for 3 days. Liquid cultures started from positively transformed colonies were resuspended in distilled water, then diluted to a 1, 10−1, 10−2, 10−3 gradient, and spotted on SD medium lacking leucine, tryptophan, histidine, and adenine (SD/-L/-T/-H/-A), then incubated at 30 °C for three to five days.
Split-luciferase complementation (Luc) assays
The Luc assays were performed to investigate the interactions between MlIW39-R1 and MlIW39-R2 using constructs pCAMBIA-35S-nLUC and pCAMBIA-35S-cLUC. The resulting constructs were transformed into the Agrobacterium strain GV3101 and injected into N. benthamiana leaves as described above. Approximately 48 h after infiltration, the LUC signal was analyzed using the NightShade LB 985 plant imaging system (Berthold Technologies) or the CCD imaging system (PlantView100). cLUC-TaSUMO1+TaHsfA1-nLUC74 were used as a positive control. At least three independent leaf samples were detected with consistent results.
BiFC assays
The CC domain of MlIW39-R1 was fused with pCAMBIA1300-35S-cYFP and pCAMBIA1300-35S-nYFP to generate fusion constructs. These constructs were transformed into the Agrobacterium strain GV3101 and then infiltrated into N. benthamiana leaves as described above. Yellow Fluorescent Protein (YFP) fluorescence was observed using a confocal laser scanning microscope (LSM880, Carl Zeiss) at 48 hpi.
Co-immunoprecipitation assay
The recombinant vectors used for cell death assays were subsequently employed in Co-IP assays to investigate the interaction between MlIW39-R1 and MlIW39-R2. For the Co-IP assays of these two full-length proteins, MlIW39-R2 was additionally cloned into the pE1776-HA vector. N. benthamiana leaves were collected at 20 hpi, and total proteins were extracted with lysis buffer (50 mM Tris-HCl [pH = 7.5], 150 mM NaCl, 5 mM EDTA [pH = 8.0], 0.1% Triton X-100, 0.2% NP-40, 0.6 mM PMSF, 20 μM MG132, 10 mM DTT and 1 × protease inhibitor cocktail). The immunoprecipitated proteins were separated in 10% SDS-PAGE gels and detected using the aforementioned antibodies (anti-GFP, anti-MYC and anti-Flag) along with an anti-HA antibody (TransGen Biotech, Beijing, China, HT301; 1:5000) following the manufacturer’s instructions.
Haplotype analysis and functional marker development
Specific Indel marker 7IND2 was used to test the distribution of the NLR gene pair in 337 wheat germplasms. The regions covering the coding sequences of MlIW39-R1 and MlIW39-R2 were amplified from WEW accessions DNA using gene-specific primers (Supplementary Data 3). PCR amplification was performed in a 30 µL reaction mixture containing 0.6 µL ApexHF HS DNA Polymerase CL, 15 µL 2×ApexHF CL Buffer (Accurate Biology, Hunan, China), 0.2 μM of each primer and 50-100 ng of genomic DNA. Then, thermocycling conditions were performed following the manufacturer’s instructions. All PCR products were sequenced using the Sanger chain termination method and analyzed using DNAMAN 8 software (Lynnon Biosoft, San Ramon, CA, USA).
One semi-thermal asymmetric reverse PCR (STARP) marker75 (MlIW39-R1-STARP) and a Cleaved Amplified Polymorphic Sequences (CAPS) marker76 (MlIW39-R2-CAPS) were developed based on specific SNPs in the MlIW39 NLR pair. MlIW39-R1-STARP amplification was performed using a touchdown PCR protocol77, and then separated on 10% non-denaturing polyacrylamide gels. PCR products of MlIW39-R2-CAPS were digested with HpyCH4Ⅳ restriction enzymes (New England Biolabs, Beijing, China) by following the manufacturer’s instructions, and were separated on 1% agarose gels.
Collinearity analysis and phylogenetic analysis
Collinearity analysis of the MlIW39 region among different species or sub-genomes was performed using the online tool Triticeae-GeneTribe78 with default parameters. Homologs of the NLRs in common wheat and related species were acquired by blasting the NLR CDS against the current Triticeae reference genomes using the WheatOmics1.0 platform79. Protein and CDS sequences were aligned using MAFFT (v7.471) with the options “--maxiterate 1000 --localpair”80. The phylogenetic trees were created using IQ-TREE (v1.6.12)81 with 1000 ultrafast bootstrap replicates by the maximum likelihood and were then visualized using itol82.
Agronomic trait evaluation of MlIW39 introgression lines
For agronomic trait evaluation, the MlIW39 introgression lines (GY2018 + MlIW39, BC3F2) and the recurrent parent GY2018 were cultivated in an experimental field at Shangzhuang, Beijing. Each wheat line was planted in six replicated rows (1.5 × 0.3 m per row), with 15 seeds sown per row. At least three plants were randomly selected from each row to measure plant height, spike length, spike numbers per plant, thousand-kernel weight, grain length, and grain width. Thousand-kernel weight, grain length, and grain width were quantified using a crop scanning test system (Wanshen SC-G seed detector). The statistical significance of differences among means of the agronomic traits was analyzed using the two-sided student’s t test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. The CDS sequences of two NLRs (MlIW39-R1 and MlIW39-R2) from wheat line 8D49 and their alleles from WEW accessions were deposited in the GenBank of NCBI under the accession numbers (PQ614233, PQ614234, PQ614235, PQ614236, PQ614237, and PQ614238, for MlIW39-R1; PQ614239, PQ614240, PQ614241, PQ614242, PQ614243, and PQ614244 for MlIW39-R2). Source data are provided in this paper.
References
Shewry, P. R. & Hey, S. J. The contribution of wheat to human diet and health. Food Energy Secur. 4, 178–202 (2015).
Sotiropoulos, A. G. et al. Global genomic analyses of wheat powdery mildew reveal association of pathogen spread with historical human migration and trade. Nat. Commun. 13, 4315 (2022).
Singh, R. P. et al. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 54, 303–322 (2016).
Su, F. Y. et al. Identification of a Pm4 allele conferring powdery mildew resistance in wheat breeding Line GR18-1. Plant Dis. 107, 2104–2111 (2023).
Kloppe, T. et al. Two pathogen loci determine Blumeria graminis f. sp. tritici virulence to wheat resistance gene Pm1a. New Phytol. 238, 1546–1561 (2023).
Sánchez-Martín, J. et al. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 17, 221 (2016).
Yahiaoui, N., Srichumpa, P., Dudler, R. & Keller, B. Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 37, 528–538 (2004).
Hurni, S. et al. Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J. 76, 957–969 (2013).
Singh, S. P. et al. Evolutionary divergence of the rye Pm17 and Pm8 resistance genes reveals ancient diversity. Plant Mol. Biol. 98, 249–260 (2018).
Guo, G. H. et al. The wheat NLR pair RXL/Pm5e confers resistance to powdery mildew. Plant Biotechnol. J. 23, 1260–1276 (2025).
Zhu, S. Y. et al. Orthologous genes Pm12 and Pm21 from two wild relatives of wheat show evolutionary conservation but divergent powdery mildew resistance. Plant Commun. 4, 100472 (2023).
He, H. G. et al. Pm21, encoding a typical CC-NBS-LRR Protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol. Plant 11, 879–882 (2018).
Zhu, K. Y. et al. An atypical NLR pair TdCNL1/TdCNL5 from wild emmer confers powdery mildew resistance in wheat. Nat. Genet. 57, 1553–1556 (2025).
Li, M. M. et al. A CNL protein in wild emmer wheat confers powdery mildew resistance. New Phytol. 228, 1027–1037 (2020).
Lu, C. T. et al. Wheat Pm55 alleles exhibit distinct interactions with an inhibitor to cause different powdery mildew resistance. Nat. Commun. 15, 503 (2024).
Zou, S. H., Wang, H., Li, Y. W., Kong, Z. S. & Tang, D. Z. The NB-LRR gene Pm60 confers powdery mildew resistance in wheat. New Phytol. 218, 298–309 (2018).
Wu, Q. H. et al. Functional characterization of powdery mildew resistance gene MlIW172, a new Pm60 allele and its allelic variation in wild emmer wheat. J. Genet. Genom. 49, 787–795 (2022).
Wu, Q. H. et al. Bulked segregant CGT-Seq-facilitated map-based cloning of a powdery mildew resistance gene originating from wild emmer wheat (Triticum dicoccoides). Plant Biotechnol. J. 19, 1288–1290 (2021).
Li, Y. H. et al. Dissection of a rapidly evolving wheat resistance gene cluster by long-read genome sequencing accelerated the cloning of Pm69. Plant Commun. 5, 100646 (2024).
Han, G. H. et al. Two functional CC-NBS-LRR proteins from rye chromosome 6RS confer differential age-related powdery mildew resistance to wheat. Plant Biotechnol. J. 22, 66–81 (2024).
He, H. G. et al. An integrated pipeline facilitates fast cloning of a new powdery mildew resistance gene from the wheat wild relative Aegilops umbellulata. Plant Commun. 5, https://doi.org/10.1016/j.xplc.2024.101070 (2024).
Ma, C. et al. An Aegilops longissima NLR protein with integrated CC-BED module mediates resistance to wheat powdery mildew. Nat. Commun. 15, 1–13 (2024).
Sánchez-Martín, J. et al. Wheat Pm4 resistance to powdery mildew is controlled by alternative splice variants encoding chimeric proteins. Nat. Plants 7, 327–341 (2021).
Li, H. H. et al. Wheat powdery mildew resistance gene Pm13 encodes a mixed lineage kinase domain-like protein. Nat. Commun. 15, 2449 (2024).
He, H. G. et al. A kinase fusion protein from Aegilops longissima confers resistance to wheat powdery mildew. Nat. Commun. 15, 6512 (2024).
Lu, P. et al. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 11, 680 (2020).
Gaurav, K. et al. Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement. Nat. Biotechnol. 40, 422–431 (2022).
Li, M. M. et al. A membrane associated tandem kinase from wild emmer wheat confers broad-spectrum resistance to powdery mildew. Nat. Commun. 15, 3124 (2024).
Liu, W. X. et al. Pm57 from Aegilops searsii encodes a novel tandem kinase protein conferring powdery mildew resistance in bread wheat. Nat. Commun. 15, 4796 (2023).
Krattinger, S. G. et al. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323, 1360–1363 (2009).
Moore, J. W. et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 47, 1494–1498 (2015).
Huang, S. J. et al. NLR signaling in plants: from resistosomes to second messengers. Trends Biochem. Sci. 48, 776–787 (2023).
Xi, Y. X., Cesari, S. & Kroj, T. Insight into the structure and molecular mode of action of plant paired NLR immune receptors. Essays Biochem. 66, 513–526 (2022).
van Wersch, S. & Li, X. Stronger when together: Clustering of plant NLR disease resistance Genes. Trends Plant Sci. 24, 688–699 (2019).
Li, Y. H., Govta, L., Sung, Y. C., Coaker, G. & Fahima, T. The spectrum of diverse disease-resistance genes cloned and characterized in the triticeae tribe. Annu. Rev. Phytopathol. 63, https://doi.org/10.1146/annurev-phyto-121323-031121 (2025).
Xu, F. et al. Autoimmunity conferred by chs3-2D relies on CSA1, its adjacent TNL-encoding neighbour. Sci. Rep. 5, 8792 (2015).
Huh, S. U. et al. Protein-protein interactions in the RPS4/RRS1 immune receptor complex. PLoS pathog. 13, e1006376 (2017).
Narusaka, M., Iuchi, S. & Narusaka, Y. Analyses of natural variation indicates that the absence of RPS4/RRS1 and amino acid change in RPS4 cause loss of their functions and resistance to pathogens. Plant Signal Behav. 12, e1293218 (2017).
Ashikawa, I. et al. Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm specific rice blast resistance. Genetics 180, 2267–2276 (2008).
Césari, S. et al. The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance. Embo J. 33, 1941–1959 (2014).
Fujisaki, K. et al. Binding of a pathogen effector to rice Exo70 proteins tethered to the NOI/RIN4 integrated domain of the NLR receptor Pii2 confers immunity against fungi. Preprint at https://doi.org/10.1101/239400 (2024).
Hao, Q. et al. An pair of an atypical NLR encoding genes confer Asian soybean rust resistance in soybean. Nat. Commun. 15, 3310 (2024).
Loutre, C. et al. Two different CC-NBS-LRR genes are required for Lr10-mediated leaf rust resistance in tetraploid and hexaploid wheat. Plant J. 60, 1043–1054 (2009).
Hu, Y. L. et al. A head-to-head NLR gene pair from wild emmer confers stripe rust resistance in wheat. Nat. Genet. 57, 1543–1552 (2025).
Klymiuk, V. et al. Coordinated function of paired NLRs confers Yr84-mediated stripe rust resistance in wheat. Nat. Genet. 57, 1535–1542 (2025).
Budak, H., Kantar, M. & Kurtoglu, K. Y. Drought tolerance in modern and wild wheat. Sci. World J. https://doi.org/10.1155/2013/548246 (2013).
Ji, X. L. et al. Identification and genetic mapping of a powdery mildew resistance gene in wild emmer (Triticum dicoccoides) accession IW72 from Israel. Euphytica 159, 385–390 (2008).
Qiu, L. N. et al. Fine mapping of a powdery mildew resistance gene MlIW39 derived from wild emmer wheat (Triticum turgidum ssp. dicoccoides). Theor. Appl. Genet. 134, 2469–2479 (2021).
He, H. G. et al. Characterization of Pm68, a new powdery mildew resistance gene on chromosome 2BS of Greek durum wheat TRI 1796. Theor. Appl. Genet. 134, 53–62 (2021).
Avni, R. et al. Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 357, 93–96 (2017).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Bai, S. et al. Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 8, e1002752 (2012).
Wang, J. Z. et al. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364, 6435 (2019).
Bi, G. Z. et al. The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell 184, 3528–3541 (2021).
He, H. G. et al. An NLR pair in the Pm68 locus confers powdery mildew resistance in durum and common wheat. (Companion manuscript).
Sánchez-Martín, J. & Keller, B. Contribution of recent technological advances to future resistance breeding. Theor. Appl. Genet. 132, 713–732 (2019).
Klymiuk, V. et al. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 9, 3735 (2018).
Fu, D. L. et al. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 323, 1357–1360 (2009).
Adachi, H., Derevnina, L. & Kamoun, S. NLR singletons, pairs, and networks: evolution, assembly, and regulation of the intracellular immunoreceptor circuitry of plants. Curr. Opin. Plant Biol. 50, 121–131 (2019).
Marchal, C. et al. BED-domain-containing immune receptors confer diverse resistance spectra to yellow rust. Nat. Plants 4, 662–668 (2018).
Wu, Z. S. et al. The N-terminally truncated helper NLR NRG1C antagonizes immunity mediated by its full-length neighbors NRG1A and NRG1B. Plant Cell 34, 1621–1640 (2022).
Flor, H. H. The complementary genic systems in flax and flax rust. Adv. Genet.8, 29–54 (1956).
Deslandes, L. et al. Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA 100, 8024–8029 (2003).
Zhai, C. et al. Function and interaction of the coupled genes responsible for Pik-h encoded rice blast resistance. Plos ONE 9, e98067 (2014).
Toghani, A. et al. Can AI modelling of protein structures distinguish between sensor and helper NLR immune receptors?. New Phytol. 248, 17–23 (2024).
Zdrzalek, R., Kamoun, S., Terauchi, R., Saitoh, H. & Banfield, M. J. The rice NLR pair Pikp-1/Pikp-2 initiates cell death through receptor cooperation rather than negative regulation. Plos ONE 15, e0238616 (2020).
Vo, K. T. X. et al. Pi5 and Pii paired NLRs are functionally exchangeable and confer similar disease resistance specificity. Mol. Cells 42, 637–645 (2019).
Wang, Z. L. et al. Seedling and adult plant resistance to powdery mildew in chinese bread wheat cultivars and lines. Plant Dis. 89, 457–463 (2005).
Saghaimaroof, M. A., Soliman, K. M., Jorgensen, R. A. & Allard, R. W. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 81, 8014–8018 (1984).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Ishida, Y., Tsunashima, M., Hiei, Y. & Komari, T. Wheat (Triticum aestivum L.) transformation using mature embryos. Methods Mol. Biol. 1223, 199–209 (2015).
Li, Y. H. et al. Intracellular reactive oxygen species-aided localized cell death contributing to immune responses against wheat powdery mildew pathogen. Phytopathology 113, 884–892 (2023).
Shan, Q. W., Wang, Y. P., Li, J. & Gao, C. X. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 9, 2395–2410 (2014).
Wang, H. R. et al. Thermosensitive SUMOylation of TaHsfA1 defines a dynamic ON/OFF molecular switch for the heat stress response in wheat. Plant Cell 35, 3889–3910 (2023).
Long, Y. M., Chao, W. S., Ma, G. J., Xu, S. S. & Qi, L. L. An innovative SNP genotyping method adapting to multiple platforms and throughputs. Theor. Appl. Genet.130, 597–607 (2017).
Shavrukov, Y. et al. Comparison of SNP and CAPS markers application in genetic research in wheat and barley. BMC Plant Biol. 16, https://doi.org/10.1186/s12870-015-0689-9 (2016).
Wei, W. X. et al. Mapping of powdery mildew resistance genes transferred to common wheat from wild emmer wheat revealed three functional Pm60 haplotypes. Crop J. 12, 540–548 (2024).
Chen, Y. M. et al. A collinearity-incorporating homology inference strategy for connecting emerging assemblies in the Triticeae Tribe as a pilot practice in the plant pangenomic era. Mol. Plant 13, 1694–1708 (2020).
Ma, S. W. et al. WheatOmics: A platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol. Plant 14, 1965–1968 (2021).
Katoh, K., Asimenos, G. & Toh, H. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 537, 39–64 (2009).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Letunic, I. & Bork, P. Interactive Tree of Life (iTOL) v6: recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 52, W71–W82 (2024).
Acknowledgements
The authors thank Prof. Rongqi Liang, China Agricultural University, China, for providing the 276 wheat accessions; Prof. Hongjie Li, Institute of Biotechnology, Xianghu Laboratory, Hangzhou, Zhejiang, China, for providing the resistance spectrum information of Bgt isolates; Prof. Robert McIntosh, the University of Sydney, Australia, for helpful suggestions; Dr. Can Yin, China Agricultural University, China, for professional support. This study was supported by the National Key Research and Development Program of China (2022YFF1001501 to C.X.; 2024YFD1201202 to Y.L.), the National Natural Science Foundation of China (32301817 to L.Q.), the Natural Science Foundation of Tianjin (22JCQNJC01470 to L.Q.), the Science and Technology Bureau of Chengdu City (2024-YF05-01624-SN to Y.L.) and the Sichuan Science and Technology Program (2024NSFSC1968 to Y.L.).
Author information
Authors and Affiliations
Contributions
C.X. conceived this research. L.Q. and Z.Y. developed mapping populations and screened the mutants; Z.Y. and L.Q. cloned MlIW39; Z.Y., N.L., W.X.W., J.S., W.P., J.Y., M.S., and W.D.W. performed phenotyping for Bgt response and haplotype analysis; Z.Y. and Y.B. performed biochemical assays to investigate protein-protein interactions. Z.Y., Y.L., and X.M.X. conducted the evolutionary analysis; T.F. provided WEW germplasm; Z.Y. and Y.L. wrote the manuscript. C.X., Y.L., H.K., T.F., Q.S., J.M., S.S., W.G., J.G., Z.H., X.D.X., and B.L. reviewed and edited the manuscript; C.X., Y.L., and L.Q. were responsible for the coordination and funding acquisition. All authors contributed to the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Adachi Hiroaki, Javier Sánchez-Martín and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Z., Liu, N., Xie, X. et al. Two complementary NLRs from wild emmer wheat confer powdery mildew resistance. Nat Commun 16, 9041 (2025). https://doi.org/10.1038/s41467-025-64052-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64052-3