Abstract
Psoriasis is a chronic, complex immune-mediated inflammatory disorder with cutaneous and systemic manifestations in which keratinocytes, dendritic cells and T cells have central roles. UBE2L3 may be a protective biomarker that regulates the pathogenesis of psoriasis. Here, we identify the IL-17A signaling similarity between human psoriatic skin and Ube2l3 conditional knockout mouse skin in the epidermis rather than dermis. IL-17A is regulated by CXCR6+ Vγ2+ γδT cells in mouse while CXCR6+ CD8+ T cells in human. CXCL16 is the only chemokine that binds to and stimulates CXCR6. Ube2l3 reduction in keratinocytes activates IL-1β and then promotes CXCL16 expression through STAT3 signaling. Up-regulated CXCL16 in keratinocytes and cDC2/mDC then attracts Vγ2+ γδT17 or CD8+ T cells to secrete IL-17A and form a positive feedback loop in keratinocytes supporting psoriatic lesions. Thus, UBE2L3 is a keratinocyte-intrinsic suppressor of epidermal IL-17 production in Vγ2+ γδT cells in mouse and CD8+ T cells in human through the CXCL16/CXCR6 signaling pathway in psoriasis.
Similar content being viewed by others
Introduction
Psoriasis is a chronic, complex immune-mediated inflammatory disorder with cutaneous and systemic manifestations1. Its pathogenesis is characterized by the involvement of keratinocytes, dendritic cells and T cells, which play a central role in the disease process2. To facilitate research on the mechanisms of action that drive inflammatory and autoimmune processes associated with psoriasis, it would be highly beneficial to have a suitable animal model3,4. Moreover, keratinocytes may be a crucial factor in both the onset and perpetuation of psoriasis5. Our and other previous research have indicated that diminished Ubiquitin Conjugating Enzyme E2 L3 (UBE2L3) expression is linked to elevated Interleukin 1β (IL-1β) production in the epidermis of psoriasis.6,7. The epidermal immune environment is of pivotal importance in the pathogenesis of psoriasis8. Furthermore, UBE2L3 interacts with the ubiquitin enzyme 3, including TRIP12 and AREL1, to facilitate the turnover of the precursor IL-1β (pro-IL-1β)9. This process is of critical importance to the functioning of the immune system. Consequently, we established a mouse model of epidermal Ube2l3 deficiency in order to gain further insight into the pathogenesis of psoriasis. This mouse model offers a valuable opportunity to explore the pathogenesis of psoriasis10,11. It is of great importance to compare the mutant mice to psoriatic patient samples at the level of bulk RNA, single cell RNA, and protein.
In contrast to adaptive IL-17-producing T cells, dermal IL-17-producing γδT (γδT17) cells are programmed for the earliest IL-17 response to various pathogens12. In these settings, γδT17 cells have been demonstrated to respond to cytokines, predominantly IL-23 and IL-1β13. Vγ2 T (IL-17A producers, Heilig & Tonegawa nomenclature) cells have been demonstrated to contribute to the pathogenesis of autoimmune diseases by through the production of IL-17A14. Integration of single cell sequencing (scRNA-seq) data and spatial-seq transcriptomic CD8+IL17+T (Tc17) enabled the definition of Tc17, which was identified as a principal source of IL-17A in psoriatic skin15,16. However, the function of γδT17 and Tc17 in epidermal psoriatic lesions remains to be elucidated.
Chemokine (C-X-C motif) ligand 16 (CXCL16) functions as a ligand for C-X-C chemokine receptor type 6 (CXCR6), and both are all upregulated in psoriasis. They mediate cutaneous recruitment of human CD8+T cells17,18. The expression of CXCR6 has been observed in Th17 cells and Tc17 with increased levels of CXCL1619. Furthermore, CXCL16 has been demonstrated to regulate the migration of CXCR6-expressing CD8+ T cells and promote Tc17 differentiation20,21. Additionally, CXCL16 is secreted by macrophages and dendritic cells (DCs) to attract memory T cells22. Molecular studies demonstrated that fibrosus cells exposed to IL-1ß, exhibited a notable increase in CXCL16 expression compared to control cells23. The precise function of CXCL16 in dendritic cells (DCs) and CXCR6 in γδT and CD8+ T cells, particularly within the context of the epidermal psoriatic milieu, remains to be elucidated.
In this study, we demonstrated an epidermal Ube2l3 conditional knockout mouse model closely recapitulates the human psoriatic phenotype. This was achieved through the analysis of using bulk RNA levels, protein levels and single-cell RNA sequencing (scRNA-seq). In conclusion, UBE2L3 functions as a keratinocyte-intrinsic suppressor of epidermal IL-17 production in mouse Vγ2+ γδT cells and human CD8+ T cells via the CXCL16/CXCR6 signaling pathway in psoriasis.
Results
Cutaneous phenotypes of epidermal deficiency of Ube2l3 mouse models exhibit a high degree of similarity to that observed in human psoriasis
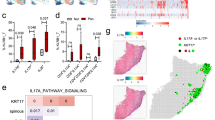
Previously, our research demonstrated that in patients with psoriasis, UBE2L3 is predominantly downregulated in the epidermis, with the exception of the dermis7. To gain further insight into the underlying mechanisms, we established a Ube2l3 epidermal deficiency mouse model (Ube2l3△Epi, Fig. 1a). The cutaneous phenotype of Ube2l3△Epi murine models exhibited notable erythema, thickness, and scales as the induction time by tamoxifen (TAM) was prolonged (Fig. 1b–d). The substantial downregulation of Ube2l3 in the epidermis of Ube2l3△Epi group was confirmed through the implementation of western blot, bulk RNA sequencing, immunofluorescence and immunohistochemistry staining (Figs. 1e, f and S1a, S1b). A typical psoriasis-like skin phenotype was established in back, ear and tail (Fig. 1g), measured by significantly increased epidermal thickness in Ube2l3△Epi mice (Fig. 1h–j), comparable to human psoriatic skin lesions (Fig. 1k). The increased angiogenesis (CD31) (Fig. 1l), proliferation (K14, Ki-67) (Fig. S1c, d), lymphocytes infiltration (CD3, CD8) (Fig. S1g, h) and dendritic cells infiltration (Fig. S1i), which corresponded to the psoriasis-like phenotype and inflammatory stage, thus provide a insight into the ability to mimic psoriasis disease in the mouse model.
a Procedure of generating Ube2l3△Epi mice from Ube2l3fl/fl-K14creERT mice by oral tamoxifen (TAM; 200 mg/kg) for 6 weeks. b Representative phenotype progression of Ube2l3△Epi mice at weeks 2, 4, and 6 post-TAM induction. c, d Incidence rate of psoriasis-like phenotype in the back and ear of Ube2l3△Epi mice during 6-week TAM induction. The average change of Psoriasis Area and Severity Index (PASI) on the back of Ube2l3△Epi mice. e Western blot results from epidermis between Ube2l3△Epi mice group and Ctrl group in epidermis. Image J analysis of western blot of Ctrl group (n = 6) and Ube2l3△Epi group (n = 4). f FPKM of Ube2l3 between Ube2l3△Epi mice group (n = 8) and Ctrl group (n = 6) in epidermis by using RNA-seq analysis. g Phenotypic changes and and skin thickness measurements (using vernier calipers) of ear, back skin, and tail in Ctrl and Ube2l3△Epi mice post-TAM. h Changes of back skin and ear thickness in mouse skin after tamoxifen application (Ctrl, n = 7; Ube2l3△Ep, n = 5). i H&E staining to compare histopathological differences between Ctrl and Ube2l3△Epi mice after applying tamoxifen (scale bar is 50 μm in back skin, ear skin, scale bar is 250 μm in tail skin). j Quantification of epidermal thickness from H&E staning (μm) in back skin (Ctrl, n = 6; Ube2l3△Epi, n = 10), ear skin (Ctrl, n = 3; Ube2l3△Epi, n = 3), and tail skin (Ctrl, n = 4; Ube2l3△Epi, n = 5). k H&E staining of human skin between healthy donors (HD) and psoriatic lesions (PSO). l Statistics of immunofluoresence of vasculariation (CD31+; Ctrl, n = 3; Ube2l3△Epi, n = 3) and T cell infiltration (CD3+; Ctrl, n = 3; Ube2l3△Epi, n = 4), and immunohistology for keratinocyte proliferation (Ki-67; Ctrl, n = 3; Ube2l3△Epi, n = 4) in the back skin of Ctrl and Ube2l3△Epi mice (scale bar = 100 μm). TAM tamoxifen, H&E hematoxylin and eosin, PASI Psoriasis Area and Severity Index, FPKM Fragments Per Kilobase of transcript per Million mapped reads. Data are presented as mean ± SEM, and p-values were calculated using unpaired, two-tail student’s t-test. Figure a was created in BioRender. Chen, X. (2025) https://BioRender.com/o3b4rwp.
A comparative analysis of RNA and protein levels between Ube2l3 △Epi mice and human psoriasis skin
To investigate the molecular mechanisms involved in sustaining skin inflammation in the epidermis or dermis, we conducted ex vivo bulk-RNA sequencing and Illumina array analysis of epidermis and dermis respectively from Ube2l3△Epi mice and control (Ctrl) mice. The principal component analysis (PCA) demonstrated a distinct separation of the four groups (Fig. 2a). The number of genes in the dermis is significantly lower than those in the epidermis when using the same p-value threshold of less than 0.05 and an absolute log2 fold change greater than 1.5 are employed (Fig. 2b–d). The data were obtained from GSE166388, GSE68937 and GSE68923, and a comparison was made between human psoriatic lesional skin and normal skin from controls in the epidermis (Fig. S2a, b). The genes that were found to be upregulated were collated and analysed by using the Metascape website24 in order to identify all molecules and enrichment pathways. The Ube2l3△Epi mouse was found to be closely associated with human psoriasis in the epidermis, specifically in relation to the immune response (Fig. 2e, f). While enrichment analysis of epidermal differentially expressed genes (DEGs) showed clustered psoriasis-associated cytokines, DEGs in dermis also related with IL-17 signaling (Fig. S2c), and parallel analysis in the dermis demonstrated dispersed cytokine distribution (Fig. S2d). The genes found to be related to psoriasis belong to the majority of members of the cytokine and chemokine families, including interleukin-1 (IL-1), IL-17, IL-6/IL-2325, chemokines and their receptors, antimicrobial peptides (AMPs), tumour necrosis factor (TNF), toll-like receptors (TLRs), as well as representative Type I interferon receptors (IFNRs). The heatmap indicated that these families were all significantly upregulated in the epidermis of Ube2l3△Epi mice, which is analogous to human psoriasis (Fig. 2g, h). Luminex array analysis revealed increase in protein levels of AMPs (S100A8, S100A9), vascular endothelial growth factor (VEGF), chemokines (CCL2, CCL22, CXCL2), and cytokine (IL-17A) in the epidermis of the Ube2l3△Epi mouse (Fig. 2i). Furthermore, the iTRAQ analysis revealed an increase in antimicrobial peptides (AMPs), while the Luminex array analysis indicated a tendency towards an increase in tumour necrosis factor (TNF), interleukin 12/interleukin 23p40 (IL-12/IL23 P40), and IL-17A in the human dermis (Fig. 2j). However, no significant differences were observed in psoriasis-related families in the dermis as that in the epidermis, as indicated by both the enrichment analysis and the heatmap (Fig. S2c, d). Consequently, a comparison of RNA and protein levels between the Ube2l3△Epi mice and human psoriasis skin revealed a similarity.
a Principal component (PCA) analysis of epidermal (E) and dermal (D) samples from Ube2l3△Epi mice (U_E, n = 6; U_D, n = 6) and Ctrl mice (C_E, n = 8; C_D, n = 8). b Volcano plot of differentially expressed genes (DEGs) between the epidermis of Ube2l3△Epi mice and Ctrl mice ( | log₂FC | > 1.5; p < 0.05). Red: 1,631 upregulated genes; Green: 592 downregulated genes. c DEGs counts between the dermis of Ube2l3△Epi mice and Ctrl mice was analyzed (Up: 288; Down: 245). d The number of DEGs in all samples of epidermis and dermis of the two groups were compared. e Metascape enrichment of upregulated genes in Ube2l3△Epiepidermis. Color intensity reflects enrichment significance (−log₁₀(P-value)). f Metascape analysis of human up-regulated gene in GSE166388, GSE68937 and GSE68923. Heatmaps of FPKM expression for selected cytokine/chemokine families (IL-1, IL-17, IL-6/IL-23, AMPs, TNF, TLRs, IFNRs) in mouse (g) and human (h) epidermal samples. i Significant protein level of S100A8, Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 5, S100A9, Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 4, VEGF, Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 5, CCL3, Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 5, CCL22, Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 5, CXCL2, Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 4 in Luminx array analysis in epidermal Ctrl mice (Epi-Ctrl) and Ube2l3△Epi mice (Epi-Ube2l3△Epi). Elisa results of IL-17A in Epi-Ctrl and Epi-Ube2l3△Epi. Epi-Ctrl, n = 3, Epi- Ube2l3△Epi, n = 4. j iTRAQ results of S100A8 and S100A9 in epidermal healthy donor skin (Epi-HD) and psoriasis lesion (Epi-PSO). Epi-HD, n = 15, Epi-PSO, n = 1649. IL-17A, TNFα, Epi-HD, n = 4, Epi-PSO, n = 4, and IL-12/IL-23p40, Epi-HD, n = 3, Epi-PSO, n = 3. Luminx array results in epidermal and dermal of healthy donors (Der-HD) and psoriasis patients (Der-pso) respectively. Der-HD, n = 4, Der-PSO, n = 4. Data are presented as mean ± SEM, and p-values were calculated using unpaired, two-tail student’s t-test.
Mouse versus human epidermal lymphocytes subpopulations with respect to γδT and CD8+T cells secreting IL-17A
We next sought to compare subpopulations within the T lymphocytes compartments from human psoriasis skin lesion and Ube2l3△Epi mouse model. IL-17A, the principal downstream cytokine of IL-23, is most strongly implicated in the pathogenesis of psoriasis, with T lymphocytes secreting it in the greatest quantity26. scRNA-seq data showed a prevalence of infiltrating IL-17A secreting γδT (gdT17) cells in the epidermis of Ube2l3△Epi mice (UE), whereas dendritic epidermal T cells (DETC) were dominant in the epidermis of control mice (CE) (Fig. 3a, b). The gdT17 demonstrated a high level of expression of Il17a, Il17f and Tcrg-V4, whereas the DETC displayed a high relative expression of Cd3e, Trdv4, Tcrg-v5 and Fcer1g27. Both cell types displayed a lack of Cd4/Cd8a expression (Fig. 3c). Furthermore, Immune CellAI-mouse28 was employed to elucidate the infiltration score in various cell types in both the epidermis and dermis. The findings revealed that the infiltration of γδT was markedly more pronounced in the epidermis of Ube2l3△Epi mice in comparison to the dermis (Fig. S3a).
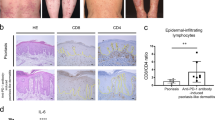
a UMAP visualization of 8545 lymphoid cells in mouse control epidermal skin (Mouse-CE, n = 3) and Ube2l3△Epi epidermal skin (Mouse-UE, n = 3). Cell clusters: CD8T (CD8+ T cells), DETC (dendritic epidermal T cells), NK (natural killer cells), ILC (innate lymphoid cells), Unknown (unknown cells), Treg (regulatory T cells), gdT17 (IL-17A+ γδT cells), Cyc-T (cycling T cells), CD4 (CD4+ T cell). b Comparative percentages of cell clusters in CE vs. UE. n = 3 per group. c Dot plot of cluster-defining marker genes. d Integrated UMAPs of 35502 lymphoid cells of human cells. Subpopulations: CCL20_Tc17 (CCL20-expressing cytotoxic CD8⁺ T cells), CXCL13_Th17 (CXCL13-expressing Th17 cells), FOXP3_Treg (FOXP3⁺ regulatory T cells), GNLY_Tc (GNLY-expressing cytotoxic CD8⁺ T cells). e Composition of 15 human T-cell subsets (grouped into 8 categories) in normal epidermis (NE) vs. psoriatic epidermis (PE) by scRNA-seq. f Dot plot of representative genes expressed in T-cell subsets. KEGG enrichment of DEGs in (g) mouse gdT17 (UE vs. CE) and (h) human Tc17 (PE vs. NE) subsets. Red arrows indicate conserved pathways: T-cell receptor signaling, Th17 differentiation, Th1/Th2 differentiation. i PySCENIC transcription factor regulon activity in mouse γδT17. Rorc: one of top upregulated TF. j Top 10 concordant pathway signatures (GSVA) between PE_Tc17 and UE_gdT cluster after cross-species gene ortholog mapping. Correlation: R = 0.65, p = 0.049. k Top 10 discordant pathway signatures between PE_Tc17 and UE_gdT clusters. Correlation: R = 0.99, p < 2.2e−16. l Flow cytometry of TNFα and IL-17A in Epi-Ctrl and Epi-Ube2l3△Epi. Bar graphs show proportions of: IL-17A⁺CD45⁺/CD45⁺ cells, IL-17A⁺CD3⁺/CD3⁺ T cells, IL-17A⁺αβT/αβT cells, IL-17A⁺γδT/γδT cells. n = 5/group. m, n Flow cytometry of TNFα and IL-17A in the epidermal lymphoid cells of healthy donors (Epi-HD) and psoriatic patients (Epi-PSO). Bar charts: IL-17A+ CD3+ /CD3+ T cells, TNFα+ CD3+ /CD3+ T, IL-17A+CD4+/IL-17A+CD3+ T cells, IL-17A+CD8+/ CD3+ T cells (HD, n = 5, PSO, n = 12). o Schematic of anti-IL-17A mAb or IgG on tamoxifen induced Ube2l3△Epi mice (IgG: isotype control). p Phenotype and H&E staining (Week 4). Bar: epidermal thickness (IgG, n = 3, IL-17A mAb, n = 3). q Immunofluorence staining of CD3 and Loricrin in two group (scar bar:100 μm). Data are presented as mean ± SEM, and p-values were calculated by unpaired, two-tail student’s t-test (l, m, p), and paired, two-tail student’s t-test (m). Figure o was created in BioRender. Chen, X. (2025) https://BioRender.com/nc6wucr.
In the historical context, IL-17-producing T cells have been predominantly regarded as CD4+ T cells. However, recent observations have provided growing evidence for the presence of IL-17 producing CD8+ T cells in psoriasis29. A similar isolation of human lymphocytes yielded 15 clusters, including IL-17 signalling-related CD8+ T cells (CCL20_Tc17, IL-17A_Tc17, IL-17F_Tc17 and IL26_Tc17), based on expression of CD4, CD8A, or CD8B (Fig. 3d–f), as observed in other recent studies30. Moreover, Th17/Tc17 cytokines, including IL17A, IL17F, IL2631, CCL20 and CXCL1332 were also employed (Fig. 3d–f, and Fig. S3b). Another group was defined as cytotoxic T cells (Tc), which were identified by the presence of GNLY, GZMH, HSPA1B and IFNG (Fig. 3d–f). The simultaneous up-regulation of the T cell receptor signalling pathway, Th17 cell differentiation and Th1 and Th2 cell differentiation was observed in both the mouse gdT17 and human Tc17 clusters (Fig. 3g, h). The nuclear receptor retinoid-related orphan receptor gamma t (RORγt, RORC) has been identified as a pivotal regulator of the differentiation and expansion of Th17 cells15. One of the most significant findings was that Rorc, a transcription factor (TF) with a pivotal role in the differentiation and expansion of Th17 cells, was identified as one of the top TFs in the mouse gdT17 cluster (Fig. 3i). This observation aligns with the characteristics of human psoriasis type 17 cell differentiation33. To further compare the similarities between the mouse gdT17 and human Tc17 clusters, we performed GSVA after mapping mouse genes to their human orthologs. Figure 3j presents the top ten biologically meaningful pathways that exhibit similar trends and minimal differences between humans and mice, with a strong correlation in signaling activity (R = 0.65). In contrast, Fig. 3k displays the top ten pathways with opposite trends and the greatest differences between the two specie. We proceeded to perform flow cytometry on IL-17A source cells derived from Ube2l3△Epi mice skin lesions and psoriasis patients skin biopsies, in comparison with those from site-matched control mice (Ctrl)/ Ube2l3△Epi mice. An increase in IL-17A-producing γδT cells was observed in the epidermal samples of Ube2l3△Epi mice (Epi-Ube2l3△Epi mice samples) in comparison with those of control mice (Epi-Ctrl). This was in contrast to the observed increase in TNFα secretion (Fig. 3l, Fig. S3c). In addition, an increase in IL-17A-producing CD8+ T cells (Tc17) was observed in epidermal psoriatic lesions (PSO-Epi) (Fig. 3m, n). The X-Y axis between TCR-γδ, CD4, CD8 and IL-17A, in epidermis and dermis respectively, showed IL-17A was mainly increased in epidermal TCR-γδ+ CD3+ cells (Fig. S3d). Following dermal conversion, no notable discrepancy in IL-17A secretion was discerned between the Ube2l3△Epi mice samples (Der-Ube2l3△Epi) and the control mice (Der-Ctrl) (Fig. S3e).
The administration of an antibody that neutralizes IL-17A to Ube2l3△Epi mice during the effector phase resulted in a reduction in epidermal thickness, a decrease in the infiltration of lymphocytes (CD3), and an enhanced tendency for epidermal keratinocytes to differentiate (Loricrin) (Fig. 3o–q). Therefore, the Ube2l3 conditional deficiency model of psoriasis in epidermal γδT cells is largely analogous to the IL-17A-secreting CD8+T cells observed in human psoriasis.
Crosstalk with CXCL16-CXCR6 signaling informed ligand receptor analysis
The maintenance of homeostasis requires intercellular communication between diverse cell types. An approach to assess such communication is provided by single-cell integration. The initial step involved the tabulation of inferred ligand-receptor interactions across keratinocytes, myeloid cells (myeloids) and NK/T cells (Fig. 4a–c). Subsequently, the most highly expressed ligand-receptor pairs from known major pathways operating in mouse epidermal skin were curated. Within the epidermis, one of the most prominent pairs of NK/T cells demonstrated crosstalk with myeloid cells or keratinocytes through Cxcl16-Cxcr6 signalling (Fig. 4d, e). Cxcr6 was previously demonstrated to be expressed in T cells, which were predominantly enriched in gdT17 clusters with high expression of Vγ2 and Vγ4 specified markers simultaneously (Tcrg-V4, 5830411N06Rik and Cd163l)34,35 (Fig. 4f). A greater proportion of IL-17A and CXCR6 was observed to be co-expressed in Epi-Ube2l3△Epi, as evidenced by flow cytometry analysis (Fig. 4g). The percentage of Vγ2+γδT cells within the γδT cell population was found to be significantly increased, while the frequency of Vγ3+ cells was observed to be abnormally decreased (Fig. 4h). The observed up-regulation of IL-17A+ Vγ2+γδT cells indicates that the increase in IL-17A proportion in T cells is driven by Vγ2+γδT upregulation (Fig. 4i). The intraperitoneal neutralisation of Vγ2 in Ube2l3△Epi psoriasis-like mice has led to the conclusion that Vγ2+γδT may play an important role in this psoriasis-like mouse model. Furthermore, a decreased trend towards psoriasis-like lesions, epidermal thickness (Fig. 4j, k) and IL-17A proportion of γδT in the epidermis of the anti-Vγ2 group (Figs. 4l and S4a, b) was observed. No significant difference was observed between the dermal IL-17A and TNFα proportions in the isotype and anti-Vγ2 groups (Fig. S4c, d). The decreased number of Vγ2+δT was observed in the epidermis, dermis lymphoid nodes (LN). In addition, the knock-out of Rag1 in Ube2l3△Epi mice (Ube2l3△Epi-Rag1 -/-) also exhibited a decreased epidermal thickness compared Ube2l3△Epi mice (Fig. S4f). These all indicated that Vγ2+γδT-secreting IL-17A played a pivotal role in the inflammatory environment of the mouse epidermis.
a UMAP visualization of 398188 cells epidermal cells across control (CE) and Ube2l3△Epi (UE) mice, classified into 6 immune/non-immune clusters via Louvain algorithm. Cell cluster: cyc-T, cycling T cell, Krt, keratinocytes, Mel, melanocytes, NK/T, natural kill T cells and T cells, SG, sebaceous gland. b Marker expression dot plot for the 6 cell populations. Dot size = expressing cell fraction; color intensity = mean scaled expression. c Compositional changes of epidermal cell states in CE vs. UE. d Predicted ligand-receptor interactions between clusters. Red arrow: upregulated Cxcl16 (myeloid)→Cxcr6 (NK/T) axis in UE. e Signaling activity of CXCR6-CXCL16 and top ligand-receptor pairs across cell types. f Dot plot showing the differentially expressed Vγ2 and Vγ4 specified marker (Tcrg-V4, 5830411N06Rik and Cd163l) in T-cell subsets. g Flow cytometry: Increased IL-17A⁺CXCR6⁺CD3⁺ T cells in Ube2l3△Epi mice epidermis. n = 6/group. h Flow cytometry plots and quantification of the percentage of Vγ2 and Vγ3 cells in γδT cells in Epi-Ctrl and Epi-Ube2l3△Epi groups. Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 6. i Flow cytometric analysis of the percentage of TNFa+ and IL-17A+ Vγ2+cells in Vγ2+ γδT cells between Epi-Ctrl and Epi-Ube2l3△Epi groups. Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 6. j Schematic experimental design showing Ube2l3△Epi mice treated with or without anti-Vγ2mAb (10ug/g/week) intraperitoneally in four weeks. k Representative phenotype of Ube2l3△Epi mice treated with or without anti-Vγ2mAb before (Week 0) and after 4-week treatment (Week 4). Histological analysis of H&E staining was calculated and showed a decrease in Vγ2 antibody group. (n = 5 per group). Immunofluorence analysis of Vγ2 (red) and CD3 (green) was showed. white triangle, double stained Vγ2 and CD3. l Proportion of L-17A+ γδT cells in γδT in Epi-Ctrl, Epi-Ube2l3△Epi and the epidermis of mouse Ube2l3△Epi group treated with anti-Vγ2mAb (Epi-Ube2l3△Epi- Vγ2Ab). Epi-Ctrl, n = 5, Epi-Ube2l3△Epi, n = 6, Epi-Ube2l3△Epi- Vγ2Ab, n = 3. m FPKM of CXCR6 in GSE53552 (left, NL, n = 23, LS, n = 25, LS-treated, n = 20), GSE117239 (middle, NL, n = 34, LS, n = 34, LS-treated, n = 30), and GSE117239 (right, NL, n = 50, LS, n = 49, LS-treated, n = 45). n UMAP plots of CXCR6 expression in human lymphoid cells in NE and PE group. o Heatmap results showed the average expression of CXCR6 and other markers in 8 groups in human lymphoid cells. p Immunofluorescence staining of psoriatic skin for CD3 (green), CXCR6 (red), CD8 (purple), and DAPI (blue). White triangle indicated CXCR6+ and CD8+ T cells (Scale bar: 50 um). q Representative flow cytometric plots and percentage of IL-17A+CXCR6+ T cells in CD3+ T cells in Epi-HD (n = 6) and Epi-PSO (n = 3). The proportion of CD4+ and CD8+ in IL-17A+CXCR6+T cells. Data are presented as mean ± SEM, and p-values were calculated by unpaired, two-tail student’s t-test (g, h, i, k and q), one-way ANOVA with Tukey’s multiple comparisons (l, m). The Figure j was created in BioRender. Chen, X. (2025) https://BioRender.com/854rwn9.
We next ascertain the relevance of human psoriasis in epidermal CXCR6+T cells. The expression of CXCR6 found to be elevated in psoriatic lesional skin at the baseline stage (LS-baseline) in comparison to non-lesional skin (NL) and following the administration of biologic treatment (LS-treated) (Fig. 4m). A comparison was made between lymphocytes isolated from both adult epidermal healthy skin and epidermal psoriasis samples from the scRNA-seq dataset. The spatial transcriptomics, dotplot analysis and heatmap in the scRNA-seq analysis revealed an abundance of CXCR6 enrichment in the Tc17 population in the epidermis (Fig. 4n, o, Fig. S4g, h), which is consistent with the immunofluorescence staining and flow cytometry analysis in the epidermis in human psoriasis (Fig. 4p, q). In conclusion, epidermal IL-17A signalling was demonstrated to be regulated by CXCR6+ Vγ2+ γδ T cells in the mouse model and by CXCR6+ CD8+ T cells in the human donor.
IL-17A and IL-1β stimulated secretion of CXCL16 in keratinocytes through STAT3 signaling
The scRNA-seq analyses of the Ube2l3ΔEpi psoriasis-like mouse model provided evidence to support the hypothesis that keratinocyte subpopulation dysfunction may play a role in the pathogenesis of psoriasis. The proportion of basal and spinous cells between healthy and psoriatic skin was analysed, revealing an increase in both proliferating basal cells (prolif.Basal), specified by Top2a, Mki6736, and cycling spinous cells (Spinous II) in the Ube2l3△Epi mouse epidermis (Mouse-UE) compared to the control epidermis (Mouse-CE). This suggests that the basal layer exhibited hyperproliferation (Fig. 5a–c). We therefore conducted a detailed investigation into the up-regulated expression of CXCL16 in Mouse-UE, which was predominantly expressed in the prolif.Basal cluster (Fig. 5d, e). This finding was consistent with the CXCL16 expression observed in CD45- cells, as analysed by flow cytometry (Fig. 5f). We therefore proceeded to investigate the relationship between Ube2l3 deficiency and CXCL16 secretion.
a The single cell profile of 28,725 Keratinocytes (Krt) about nine classes conserved epidermis across UE and CE, delineated by Louvain clustering. Basal, basal keratinocytes (Krt14 and Krt5), spinous cell (Krt1 and Krt10), granular cell (Spink5 and Calm5, Krt17, Krt79), hair follicle stem cells (HFSC) (Cd34, Postn, Ptn and Fst). b Proportional abundance of KC states in CE vs. UE. c Scatter plots defining KC clusters. d Top upregulated genes in krt subset in scRNA-seq of UE vs CEs. e Dot plot of cytokines/ chemokines (Cxcl16, Ccl20, Il23a, Tnf and Il1b) compared in each group in Krt subset. red arrow is Prolif.cell group. f Flow cytometric plots and percentage of CXCL16 expression in CD45- cells in Epi-Ctrl (n = 6), Epi-IMQ (n = 6) and Epi-Ube2l3△Epi group (n = 5). g The immunoblotting of UBE2L3, CXCL16 and β-actin in lysates, soluble CXCL16 (sCXCL16) in the supernatants (super.) of cultured primary mouse keratinocytes in control group (KC-Ctrl) and in Ube2l3 knockout group (KC-Ube2l3△Epi). h Quantification of (g). n = 3 biological replicates. i The immunoblotting results of UBE2L3, Pro-IL1β and β-actin in lysates, IL-1β p35 and IL-1β p17 in supernatants of KC-Ctrl and KC-Ube2l3△Epi. j Quantification of (i). n = 3 biological replicates. k Mouse control keratinocytes were stimulated with IL-1β (100 ng/ml), IL-17A (100 ng/ml) for 24 hours. Immunoblotting of STAT3, pSTAT3, CXCL16 and β-actin was carried out. l Quantification of (k). (n = 3 biological replicates). m The R&D Luminex assay showed that the protein level of CXCL16 was increased in Epi-Ube2l3△Epi group. n = 5. n, o Flow cytometric plots and percentage of TNFα and CXCL16 in the epidermis of healthy donors (Epi-HD, n = 3) and psoriatic patients (Epi-PSO, n = 3). p, q Normal human epidermal keratinocytes (NHKEs) were stimulated with rhIL1β (100 ng/ml) and rhIL-17A(100 ng/ml) for 24 hours and the STAT3, pSTAT3 and CXCL16 in lysates were measured by western blot. (n = 3 biological replicates). Data are presented as mean ± SEM, and p-values were calculated by unpaired, two-tail student’s t-test (h, j, m, and o), one-way ANOVA with Tukey’s multiple comparisons (m, q).
Previous studies have demonstrated that the overexpression of UBE2L3 reduces its binding to TRIM21, consequently leading to a decrease in STAT3 pathway activity and a reduction in the level of the IL-1β precursor (pro-IL-1β)7. Furthermore, molecular studies demonstrated that annulus fibrosus cells exposed to IL-1β, but not TNF-α, exhibited a notable elevation in CXCL16 expression relative to control cells23. The results demonstrated that both CXCL16 and IL-1β were elevated in Ube2l3-deficient keratinocytes (KC-Ube2l3△Epi) relative to control cells (KC-Ctrl) (Fig. 5g–j). When IL-1β was blocked by subcutaneous injection of its neutralization antibody, the epidermal thickness of Ube2l3△Epi mice decreased significantly (Fig. S5a, b). Compared to isotype Ube2l3△Epi mice, Vγ2+ γδT proportion of IL-1β antibody was down-regulated in the epidermis (Fig. S8a). Besides, the percentage of IL-17A+ CXCR6 + T cells, Vγ2+ IL-17A+ T cells and IL-17A+ T cells were also decreased in the epidermis rather than dermis (Fig. S8b-d). Upon stimulation of keratinocytes with or without recombinant mouse IL-17A (rmIL-17A) and recombinant IL-1β (rmIL-1β), the protein level of CXCL16 was markedly increased, exhibiting a similar trend to that of STAT3 signalling (Fig. 5k, l). Furthermore, CXCL16 was found to be upregulated in epidermal Ube2l3△Epi mice (Ube2l3△Epi-Epi) by Luminex assay (Fig. 5m). Similarly, the same up-regulated tendency of CXCL16 in psoriasis in epidermis (PSO-Epi) was observed in CD45- cells by flow cytometry (Fig. 5n, o). Upon stimulation of normal human epidermal keratinocytes (NHEKs) with IL-1β and IL-17A, a significant up-regulation of CXCL16 was observed in lysates, accompanied by activation of the STAT3 pathway (Fig. 5p, q). In conclusion, the data collectively indicate that CXCL16 is secreted by Ube2l3-deficient keratinocytes and stimulated by IL-1β and IL-17A. This suggests that CXCL16 may act as a chemoattractant for T cells, which in turn secrete IL-17A, forming a positive feedback loop in psoriatic keratinocytes.
Up-regulated CXCL16 secreted by cDC2/mDC controlling CXCR6+ γδT/CD8+T IL-17A secretion in psoriatic epidermis
scRNA-seq and bulk RNA sequencing analysis indicated the potential involvement of CXCL16-CXCR6 signalling between myeloid cells and natural killer (NK) and T cells (NK/T) in the epidermis of Ube2l3△Epi psoriasis-like mice, with no evident impact on the dermis (Figs. 4d, e and S6a–c). Therefore, we proceeded to evaluate the proportion of CXCL16 in myeloid cells, identifying a notable presence in the migrating dendritic cell (mDC) and cDC2 (type 2 DC) population, specifically marked by Itgam, Irf4, Fscn1, Cacnb3 and Ccr737 (Figs. 6a–c, and S6d). The results of the pseudo-time analysis indicated that Cxcl16 was predominantly expressed in mDC_cDC2 and Mac (macrophage) (Fig. 6d). The same trend was corroborated by flow cytometry, which demonstrated that cDC2 was the predominant cells secreting CXCL16 in the epidermis (Fig. 6e). Following the knockout of cDCs in Ube2l3△Epi mice by an intraperitoneal (i.p.) injection of diphtheriaetoxin (DT), Ube2l3△Epi-cDC-/- mice were generated after TAM induction (Fig. 6g). The decreased number of DCs in epidermis, dermis, lymphoid nodes and spleen showed a successful depletion of DCs by DT induction (Fig. S6f–i). The epidermal thickness of the psoriasis-like lesions was found to be decreased in conjunction with the down-regulation of CD11c and keratin 14 (K14) (Fig. 6g). Furthermore, we observed a notable reduction in the proportion of γδT cells secreting IL-17A in Ube2l3△Epi-cDC-/-(Fig. 6h). In a human myeloid single-cell analysis, a comparable population of mDC and cDC2 was identified based on the expression of CCR7, LAMP3, CLEC10A, CD1C38,39 (Figs. 6i and S6j, k). The results demonstrated that CXCL16 was markedly elevated in the epidermis of PSO (Epi-PSO) specimens, as evidenced by scRNA-seq heatmap analysis and flow cytometry (Fig. 6j, k). Similarly, a comparable trend is observed in myeloid cells, with the involvement of analogous pathways, such as the regulation of cell activation (Fig. S6l, m). Therefore, CXCL16 was released by the cDC2/mDC population simultaneously and may be involved in the regulation of γδT17 secretion in the psoriatic epidermis.
a UMAP of 1647 myeloid cells from control (Mouse-CE) and Ube2l3△Epi (Mouse-UE) epidermis, clustered into 9 subsets: mast cells/basophils (MC_Bas), neutrophils (Neu), Langerhans cells (LC), macrophages (Mac), type 1 conventional DCs (cDC1), type 2 conventional DCs (cDC2). b Proportional abundance of myeloid subsets in CE vs. UE. c Expression of cytokines (Il1b) and chemokine (Cxcl16, Cxcl10 and Adam10) in nine defined classes. Red arrow: Cxcl16 in mDC_cDC2 cluster. d Pseoudotime analysis of Cxcl16 gene in nine cluster above in myeloid cells. Blue circles indicate the enrichment of Cxcl16 in mDC_cDC2 and Mac. e Flow cytometry and percentage of CXCL16 in mouse DC group in Epi-ctrl and Epi- Ube2l3△Epi. Among CXCL16+ DC, the proportion of CD11b+ DC (cDC2) and CD103+DC (cDC1) in all CXCL16+DC. n = 6. f Schematic strategy for generating Ube2l3 Epi△-cDC -/- mice. g The phenotype of Ube2l3 Epi△mice and Ube2l3 Epi△-cDC -/- mice at week 0, 4, and 8. H&E staining (week 8). Epidermal thickness qualification in each group was calculated in Ube2l3 Epi△mice (n = 3) and Ube2l3 Epi△-cDC -/- mice (n = 4). Immunofluorescence of CD11C (red), K14 (green) and DAPI (blue), was done in two groups. scale bar= 50 um. h Flow cytometric plots and the percentage of IL17A+ in CD3+T, γδT and αβT in the epidermis of each group were showed. n = 3 per group. i UMAP visualization of 3237 human myeloid cells states found in human normal epidermis (NE) and human psoriatic epidermis (PE). pDC, Representative markers used in classifying mouse myeloid cells. pDC, plasmacytoid dendritic Cells. LC Langerhans cell cluster. Prolif.T, proliferated associated T cell. cDC2_mDC, type 2 DC and mature DC. Mac, macrophage. Pre-DC_cDC1, pre-DC and type 1 DC, B cell, B lymphocyte. j Heatmap of CXCL16 expression across human myeloid clusters. k Flow cytometry of IL-23p19 and CXCL16 expression in the epidermis of healthy donors (HD) and psoriatic patients (PSO). n = 3 per group. Data are presented as mean ± SEM, and p-values were calculated by unpaired, two-tail student’s t-test (h, e, g, h, and k). Figure f was created in BioRender. Chen, X. (2025) https://BioRender.com/riyjlc8.
CXCL16 facilitates Vγ2+ γδT/ CD8+T migration and IL-17A release in psoriatic epidermis
In wild-type mice, Vγ2+ γδT was observed to be absent in the epidermis and present in the dermis40. In psoriasis-like Ube2l3△Epi mice, the percentage of Vγ2+ γδT cells was significantly increased in the dermis (Fig. 7a). Epithelial cells lining the mucosal surfaces play a crucial role in this process by producing specific chemokines, such as CXCL16, which serve as attractants for γδT cells expressing the corresponding chemokine receptors41. Furthermore, IL-23R signalling is responsible for the generation of CXCR6+ cells from IL-17+ SLAMF6+ cells42. It was therefore postulated that there is a relationship between CXCL16 and CXCR6+Tc17/γδT17. The migration assay demonstrated that CXCL16 exerted a migratory effect on dermal Vγ2+ γδT (Fig. 7b, c). Next, epidermal Vγ2+ γδT cells were sorted and stimulated with recombinant mouse Cxcl16 (rmCxcl16) (Fig. 7d). The secretion of IL-17A was increased in Vγ2+ γδT cells in 6 week-TAM induced Ube2l3△Epi mice (Fig. 7e). To further investigate the potential origin of epidermal IL-17A⁺ Vγ2⁺ γδ T cells, we performed time-kinetic flow cytometry analysis on epidermal and dermal samples from control (Ctrl) and Ube2l3ΔEpi mice at 2 and 6-week post-tamoxifen administration. The results revealed that, in the epidermis, Vγ2⁺ cells were absent in Ctrl mice. In contrast, Ube2l3ΔEpi mice exhibited Vγ2⁺ cells at 2 weeks post-tamoxifen (Ube2l3ΔEpi-2W), albeit at low levels (Fig. S7a–g). By 6 weeks (Ube2l3ΔEpi-6W), Vγ2⁺ cell frequency peaked, and these cells were predominantly IL-17A⁺ (Fig. S7b, g). This tendency was consistent with CXCR6+ IL-17A+ cell, IL-17A+ cell, in contrary with CD4+ IL-17A+ and CD8+ IL-17A+ cell (Fig. S7c-g). In the dermis, Vγ2⁺ cells were detectable in all groups (Ctrl-2W, Ctrl-6W, Ube2l3ΔEpi-2W, Ube2l3ΔEpi-6W), with the highest frequency observed in the Ube2l3ΔEpi-6W group (Fig. S7h, n). All groups presented high expression of Vγ2⁺ IL-17A+ cell, CXCR6+IL-17A+ cell, IL-17A+ cell (Fig. S7i–n). In addition to it, Ube2l3ΔEpi-2W CD3+T cells showed a high expression of Ki-67 when stimulated with rmCXCL16 (Fig. S9h). These findings suggest that epidermal IL-17A⁺ Vγ2⁺ γδ T cells may originate from the dermis and subsequently expand within the epidermis following Ube2l3 deletion.
a The percentage of Vγ2+ γδT in γδT in Der-Ctrl and Der-Ube2l3△Epi group. b Schematic strategy for studying the migration of Vγ2+ γδT cells with or without Cxcl16 for 24 hours. c The migrated cell was viewed by immunofluorence staining with DAPI (blue), five independent views of lower chambers of insects were calculated between Der-Ctrl (n = 5) and Der-Ube2l3△Epi group (n = 5). d Next, epidermal Vγ2+ γδT cells were sorted and stimulated with rmCXCL16 for 48 hours, and flow cytometry to be carried out to analyze the IL-17A secretion percentage. e Percentage of Vγ2+ IL-17A+T in CD3+T in isotype and rmCXCL16 group. n = 3 per group. f, g Ube2l3△Epi mice were treated with isotype antibodies or anti-CXCL16 mAb. H&E staining of back skin and ear skin was performed. h Epidermal single cells were stimulated with or without recombinant human CXCL16 and flow cytometric analysis of IL-17A was showed. i The percentage of IL-17A+ CD3+T /CD3+Tcells, CD8+IL-17A+/IL-17A+ T cells, CD4+IL-17A+/IL-17A+T cells in isotype cytokine and rhCXCL16 cytokine. j In wild-type (WT) mice, intradermal injection of recombinant mouse CXCL16 (rmCXCL16) and rmIL-23 every other day for 14 days. k H&E staining of and ear skin was performed. l Skin thickness measurements (using vernier calipers) of ear skin in control ear, rmCXCL16 ear, n = 5 per group, and control ear, rmIL-23 group, n = 3 per group. m–o Flow cytometry analysis of Vγ2+ γδT cell and Vγ3+ γδT cells in γδT, IL-17A in CD3+T cells, and CXCR6+IL-17A+ in CD3+T cells. n = 4 per group. Data are presented as mean ± SEM, and p-values were calculated by unpaired, two-tail student’s t-test (a, c, e, g, l), paired, two-tail student’s t-test (i, k, m, n, o). The Figure d, f, h, j was created in BioRender. Chen, X. (2025) https://BioRender.com/a73juv2.
CXCL16 neutralization in Ube2l3△Epi mice resulted in a reduction in the epidermal thickness of psoriasis-like lesions (Fig. 7f, g). We subsequently assessed the proportion of Vγ2⁺ cells within the γδ T cell population. In the epidermis, CXCL16 neutralization led to a decreasing trend in the proportion of Vγ2⁺ γδ T cells (Fig. S8a). Similarly, reductions were observed in the percentages of IL-17A⁺CXCR6⁺ T cells, Vγ2⁺IL-17A⁺ T cells, and total IL-17A⁺ T cells in the epidermis of treated mice (Fig. S8b–d). However, neither CD4⁺ nor CD8⁺ T cells secreted detectable IL-17A in this compartment (Figs. S8e, f). In the dermis, CXCL16 neutralization significantly decreased the percentage of IL-17A⁺ CXCR6⁺ T cells (Fig. S8h). In contrast, no significant differences were detected between the treatment and control groups for the percentages of Vγ2⁺IL-17A⁺ T cells, total IL-17A⁺ T cells, IL-17A⁺CD4⁺ T cells, or IL-17A⁺CD8⁺ T cells (Fig. S8g-l).
Besides performing CXCL16 neutralization in Ube2l3△Epi mice, we also applied CXCL16 antibody into imiquimoid-induced mice, and CXCL16 antibody treatment partially decreased the epidermal thickness of imiquimoid induced psoriasis-like lesion (Fig. S9a, b) and the level of IL-17A secretion in epidermal Vγ2+ cells (Fig. S9c, f). CXCR6+ IL-17A+ cells and IL-17A+ cells level had a slight decrease (Fig. S9d, e).
Stimulation of CD3⁺ T cells isolated from human psoriatic epidermis (human epidermal CD3⁺ T cells) with recombinant human CXCL16 (rhCXCL16) increased the percentage of IL-17A-secreting cells, particularly among CD8⁺ T cells (Fig. 7h, i). In wild-type (WT) mice, intradermal injection of rmCXCL16 induced greater ear epidermal thickening compared to rmIL-23 injection in the contralateral ear (Fig. 7j–l). As Vγ2⁺ cells are absent in the epidermis of WT mice, we analyzed IL-17A secretion in the dermis. In the rmCXCL16-injected ears, we observed increased percentages of both IL-17A⁺CXCR6⁺ T cells and total IL-17A⁺ T cells (Fig. 7m-o), as well as an increase in Vγ2⁺IL-17A⁺ cells (Fig. S9g).
Therefore, CXCL16 can facilitate the migration, proliferation and IL-17A secretion of Vγ2+ γδT cells in mouse epidermis from dermis and CD8+T cells in human psoriatic epidermis.
Discussion
In this study, scRNA-seq from human psoriasis skin is integrated to map subpopulation composition and intercellular communication within the major compartments of the skin (keratinocytes, myeloid cells and NK/T cells), thereby generating a comprehensive resource for hypotheses regarding the epidermal immune environment in psoriasis. A psoriasis-like lesion mouse model is generated, which may facilitate further insights into the pathogenesis of psoriasis10. The data are compared with mouse skin scRNA-seq data to facilitate cross-species comparisons of differentiation dynamics and ligand-receptor pathways. In particular, the CXCL16-CXCR6 signaling pathway is identified as a highly active cluster in keratinocytes/myeloids and NK/T cells (Fig. 8).
A psoriasis-like lesion mouse model was generated by conditional knock out Ube2l3 in epidermis (Ube2l3△Epi), which was compared with human psoriasis scRNA-seq data and facilitate cross-species comparisons of differentiation dynamics and ligand-receptor pathways in epidermis. In particular, IL-17A was regulated by CXCR6+ Vγ2+ γδT in mouse while CXCR6+ CD8+ T in human. Ube2l3 reduction in keratinocytes activated IL-1β and then promote CXCL16 expression through STAT3 signaling. CXCR6+γδT17/ Tc17cells are prevalent within the epidermal immune microenvironment of psoriasis. Neutralization of CXCL16 inhibits the progression of psoriasis-like lesions in Ube2l3△Epi mice. (Created in BioRender. Chen, X. (2025) https://BioRender.com/c9dlkqv).
Some research has indicated that pathogenic CXCR6+ Th17 populations are induced in autoimmunity42. In the context of inflammatory diseases, the activation of CXCR6+ T cells has been identified as a key factor in the differentiation of T cells into Th17 and the subsequent secretion of pro-inflammatory cytokines43. In human, CXCR6-expressing cells were absent in the epidermis of healthy skin44. In contrast, CXCR6+ CD8+ T cells were significantly increased in both peripheral blood and skin in psoriasis patients43. In mouse model, previous sltudies have demonstrated that dermal γδT cells are the primary IL-17-producing cells in the skin that respond to IL-23 stimulation in an IMQ (imiquimod) induced psoriasis-like mouse model45. However, there is a paucity of research examining the epidermal CXCL16-CXCR6/IL-17 signalling pathway in psoriasis patients and psoriasis-like mouse models.
In addition to it, IL-17-induced effector cells in skin inflammation are keratinocytes46. In our studies, we found that CXCL16 was secreted by Ube2l3 -deficient keratinocytes, stimulated by IL-1β through the STAT3 pathway, together with CXCL16 from cDC2 and mDC, promoted the migration and proliferation of γδT17 cells in the epidermis of Ube2l3△Epi mice. Crucially, this pathway demonstrates cross-species conservation: human psoriatic epidermis exhibits analogous STAT3/CXCL16 activation, where CD8⁺Tc17 cells functionally replace γδT17 as key responders (Fig. 3). We first compared the psoriasis-like mouse model and human psoriasis patients in the epidermis and found both CXCL16-CXCR6 signaling played a crucial role in psoriatic cell communication. The increased epidermal Vγ2+ γδT17 was derived from the dermis and CXCL16 from keratinocytes/dendritic cells may further facilitate Vγ2+ γδT17 differentiation (Fig. 7d, e). We also found that cutaneous injection of CXCL16 neutralising antibody contributed to the amelioration of psoriasis-like lesion in Ube2l3△Epi mice (Fig. 7g). The CXCR6 ligand CXCL16 is expressed in Pso keratinocytes, epidermal anddermal antigen-presenting cells4, although it may be more prominent in our Ube2l3△Epi psoriasis-like mice epidermis. In mouse and human keratinocytes, IL-1β drives CXCL16 via STAT3 signaling (Fig. 6g–l). Outstanding questions include functional validation of UBE2L3 reconstitution on IL-1β/STAT3/CXCL16 signaling in knockout keratinocytes and Vγ2⁻ subset transcriptomic profiling to define its inflammatory role. Therefore, CXCL16 may be another therapeutic target and be suitable for use in a precision medicine approach to psoriasis treatment, especially in patients with elevated CXCL16.
Overall, our findings indicate that CXCR6+γδT17/ Tc17cells are prevalent within the epidermal immune microenvironment of psoriasis8,47. The elevated level of CXCL16 produced by Ube2l3-deficient serves to augment the differentiation and recruitment of γδT17/ Tc17cells, thereby establishing a positive feedback loop that facilitates keratinocyte advancement in psoriasis. Furthermore, we demonstrated that the deletion of CXCL16 inhibits the progression of psoriasis-like lesions in Ube2l3△Epi mice and exhibits a therapeutic effect in vivo. The findings of our study offer valuable insights that may inform the development of a therapeutic approach utilising anti-CXCL16 neutralising antibodies, with the potential to provide alleviation of psoriasis.
Methods
Mice and ethics
All participating patients with plaque psoriasis patients and healthy donors gave written informed consent before inclusion. A skin biopsy with a diameter of 1-2 cm was taken from healthy donors’ or psoriasis patients’ extremities and/or back. The study was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine (2020-No.135). All participated patients’ clinical characteristics were showed in Supplementary Data 1.
In the animal study, C57BL/6 mice was supplied by SLAC Laboratory Animal Co. (Shanghai, China), Zbtb46-DTR mice (Jackson Laboratories), Ube2l3fl/fl (Cyagen)and were housed under specific pathogen-free (SPF) conditions. Ube2l3fl/fl (mice were crossed to K14-CreERT mice (Jackson Laboratories) to generate Ube2l3 epidermis-specific knockout mice (Ube2l3fl/fl-K14-CreERT). When Ube2l3fl/fl-K14-CreER was induced by tamoxifen for 6 weeks, we called them Ube2l3△Epi mice. Ube2l3fl/fl-K14-CreERT were crossed to Zbtb46-DTR mice to generate DC knockout in Ube2l3△Epi mice (Ube2l3△Epi-cDC-/-). Ube2l3fl/fl-K14-CreERT were crossed to Rag1-/- mice (GemPharmatech) to generate Rag1 knockout in Ube2l3△Epi mice (Ube2l3△Epi-Rag1-/-). Sex- and age-matched animals between 12 and 15 weeks of age were used for experiments. Preliminary experiments were performed to determine proper sample size. All animal experiments were performed in accordance with protocols approved by the Second Affiliated Hospital, Zhejiang University School of Medicine Animal Care Committee (2019-No.072).
Flow cytometric analysis of skin tissue-derived cells
Mouse/human skin biopsy tissue was immersed in 0.5% dispase (Gibco, USA) at 4 °C overnight in order to separate the epidermis and dermis. The epidermis was digested with 0.25% trypsin (Thermo Fisher Scientific, USA), while the dermis was digested with 1 mg/ml collagenase (Sigma-Aldrich, USA). After neutralization with serum, the mixture was centrifuged at 1000 rpm for 5 min, and the cells were resuspended in PBS to obtain a single-cell suspension.
Epidermal and dermal single-cell suspensions were prepared as described above. The cells were first stimulated with Cell Activation Cocktail (with Brefeldin A, BioLegend) together with 2 μg/ml resiquimod (Sigma) for 6 hours. After stimulation, the cells were first stained with a Zombie UV Fixable Viability Kit (BioLegend) for 15 minutes and then incubated with the Fc receptor blocker TruStain FcX (BioLegend) for 10 minutes. Then, the cells were incubated with mixed cell surface antibodies for 30 minutes at 4 °C in the dark. For intracellular staining, cells were fixed and permeabilized by using the BD Cytofix/Cytoperm Kit (BD Biosciences) or Foxp3/Transcription Factor Staining Buffer Set (eBioscience) and then incubated with anti-cytokine antibodies for 30 minutes at 4 °C in the dark. The antibodies used were purchased from BioLegend, BD Biosciences, or eBioscience, as listed in supplementary methods. The data were acquired by CytoFLEX LX (Beckman Coulter) and analyzed by CytExpert Software (Beckman Coulter). All related antibodies were provided in Supplementary Data 2.
Single-cell RNA sequencing data analysis
Processed human single-cell RNA-sequencing (scRNA-seq) datasets from our datasets, sequenced by performed by PLTTech Inc. (Hangzhou, China). For human skin integration of the psoriatic epidermis (PE, n = 3 donors) and normal epidermis (NE, n = 2 donors) was analyzed with the Seurat R package (versions 3.0.0 and 4.0.3)48. Cells with >20% mitochondrial gene percentage, minimum of 350 genes and maximum of 4500 detected were filtered from downstream analyses. Samples were split by donor identity (referred to as sample in metadata in analysis scripts and Seurat objects) into individual objects by donor, which were each normalized and processed with the NormalizeData and FindVariableFeatures functions with default parameters. Integration anchors were determined with these donor objects using the first 30 dimensions and integrated using the IntegrateData function on the first 30 dimensions. Using this integrated object, dimensionality reduction was carried out after ScaleData with principal component analysis and Uniform manifold approximation and projection (UMAP) on the first 30 principal components to generate UMAP. T lymphocytes (Human NE, Human PE) clustering was performed at resolution 0.2 after FindNeighbors was run on the first 30 dimensions. Myeloid cells clustering was performed at resolution 0.5 after FnidNeighbors was run on the first 30 dimensions.
Similarly, for mouse skin integrated data (CE, n = 3 mice) and (UE, n = 3 mice), a Seurat object was created with 10x cellranger outputs. SingleCell Data Analysis was performed using the OmicStudio tools created by LC-BIO Co., Ltd (HangZhou, China) at https://www.omicstudio.cn/cell, and was analyzed with the Seurat R package (4.1.0, R version 4.1.3). Differentially Expressed Genes (DEGs) in each cluster in mouse and human showed in Supplementary Data 3.
Anti-IL-17A/ Vγ2/ CXCL16 / IL-1β treatment
Anti-IL-17A: Mice received subcutaneous injections (s.c.) of 50 µg/g/week hamster anti-mouse IL-17A mAb (clone 17F3, Bio X Cell) or hamster IgG isotype control (clone BE0091, Bio X Cell). The experimental design is shown in Fig. 3o.
Anti-Vγ2: Mice received intraperitoneal injections (i.p.) of 10 µg/g/week hamster anti-mouse Vγ2 mAb (clone UC3-10A6, Bio X Cell) or hamster IgG isotype control (clone BE0091, Bio X Cell). The experimental design is shown in Fig. 4j.
Anti-CXCL16: Mice received subcutaneous injections (s.c.) of 4 µg/g/week goat anti-mouse CXCL16 polyclonal antibody (AF503, R&D Systems) or goat IgG isotype control. Results are shown in Fig. 7g.
Anti-IL-1β: Mice received subcutaneous injections (s.c.) of anti-mouse IL-1β antibody (Bio X Cell) or its respective isotype control. Results are shown in Fig. S5.
Primary keratinocyte culture
The separated epidermis was digested with 0.25% trypsin (Thermo Fisher Scientific, USA). The digested epidermal keratinocytes were neutralized with FBS (Gibco, USA) and centrifuged at 400 g. Cells were cultured to 70% confluence in EpiGRO Human Epidermal Keratinocyte Complete Culture Media Kit (Millipore, USA, SCMK001) in a humidified incubator with 5% CO2 at 37 °C.
Recombinant mouse IL-23/CXCL16 treatment
Mice were injected intradermally (i.d.) with 20 μL PBS/0.1% bovine serum albumin (BSA) containing 0.5 μg recombinant mouse (rm)IL-23 or recombinant mouse (rm)CXCL16 in the right ear using a 29-gauge needle, every other day for 14 days.
Quantification and statistical analysis
Statistical analyses were performed with GraphPad Prism, versions 9-10 (GraphPad Software, San Diego, CA), or R, versions 3.5.1, 3.61, and 4.0.2. Parameters such as number of replicates; the number of independent experiments; measures of center, dispersion, and precision (mean SD or SEM); statistical test; and significance are reported in figures and figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing data in this paper are deposited in Genome Sequence Archive (GSA) and Gene Expression Omnibus (GEO). The raw human single-cell sequencing data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2024), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: HRA011011) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human/browse/HRA011011.The raw mouse bulk-RNA-seq data and scRNA-seq data are deposited to the NCBI Sequence Read Archive (SRA) as PRJNA1278800 and PRJNA1278886. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1278800, and https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1278886. All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. All other data are available in the article and its Supplementary files or from the corresponding author upon request. Source data are provided with this paper.
References
Griffiths, C. E. M., Armstrong, A. W., Gudjonsson, J. E. & Barker, J. Psoriasis. Lancet 397, 1301–1315 (2021).
Boehncke, W. H. & Schon, M. P. Psoriasis. Lancet 386, 983–994 (2015).
Zenz, R. et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437, 369–375 (2005).
Gangwar, R. S., Gudjonsson, J. E. & Ward, N. L. Mouse Models of Psoriasis: A Comprehensive Review. J. Investig. Dermatol 142, 884–897 (2022).
Zhou, X., Chen, Y., Cui, L., Shi, Y. & Guo, C. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 13, 81 (2022).
Eldridge, M. J. G., Sanchez-Garrido, J., Hoben, G. F., Goddard, P. J. & Shenoy, A. R. The Atypical Ubiquitin E2 Conjugase UBE2L3 Is an Indirect Caspase-1 Target and Controls IL-1beta Secretion by Inflammasomes. Cell Rep. 18, 1285–1297 (2017).
Chen, X. Y. et al. UBE2L3 Reduces TRIM21 Expression and IL-1beta Secretion in Epidermal Keratinocytes and Improves Psoriasis-Like Skin. J. Invest Dermatol 143, 822–831.e824 (2023).
Zhou, Y. et al. The epidermal immune microenvironment plays a dominant role in psoriasis development, as revealed by mass cytometry. Cell Mol. Immunol. 19, 1400–1413 (2022).
Mishra, V. et al. IL-1beta turnover by the UBE2L3 ubiquitin conjugating enzyme and HECT E3 ligases limits inflammation. Nat. Commun. 14, 4385 (2023).
Uluçkan, Ö & Wagner, E. F. Role of IL-17A signalling in psoriasis and associated bone loss. Clin. Exp. Rheumatol. 34, 17–20 (2016).
Schafer, P. S. L., Dimitrov, D., Villablanca, E. J. & Saez-Rodriguez, J. Integrating single-cell multi-omics and prior biological knowledge for a functional characterization of the immune system. Nat. Immunol. 25, 405–417 (2024).
Spidale, N. A. et al. Interleukin-17-Producing gammadelta T Cells Originate from SOX13(+) Progenitors that Are Independent of gammadeltaTCR Signaling. Immunity 49, 857–872.e855 (2018).
Papotto, P. H., Ribot, J. C. & Silva-Santos, B. IL-17(+) gammadelta T cells as kick-starters of inflammation. Nat. Immunol. 18, 604–611 (2017).
Li, Y., Wu, J., Luo, G. & He, W. Functions of Vgamma4 T Cells and Dendritic Epidermal T Cells on Skin Wound Healing. Front Immunol. 9, 1099 (2018).
Ma, F. et al. Single cell and spatial sequencing define processes by which keratinocytes and fibroblasts amplify inflammatory responses in psoriasis. Nat. Commun. 14, 3455 (2023).
Liu, J. et al. Single-cell RNA sequencing of psoriatic skin identifies pathogenic Tc17 cell subsets and reveals distinctions between CD8(+) T cells in autoimmunity and cancer. J. Allergy Clin. Immunol. 147, 2370–2380 (2021).
Ma, K. L. et al. Activation of the CXCL16/CXCR6 pathway promotes lipid deposition in fatty livers of apolipoprotein E knockout mice and HepG2 cells. Am. J. Transl. Res. 10, 1802–1816 (2018).
Gunther, C., Carballido-Perrig, N., Kaesler, S., Carballido, J. M. & Biedermann, T. CXCL16 and CXCR6 are upregulated in psoriasis and mediate cutaneous recruitment of human CD8+ T cells. J. Investig. Dermatol 132, 626–634 (2012).
Steel, K. J. A. et al. Polyfunctional, Proinflammatory, Tissue-Resident Memory Phenotype and Function of Synovial Interleukin-17A+CD8+ T Cells in Psoriatic Arthritis. Arthritis Rheumatol. 72, 435–447 (2020).
Bao, N. et al. Role of the CXCR6/CXCL16 axis in autoimmune diseases. Int Immunopharmacol. 121, 110530 (2023).
Wang, F. et al. Targeting IL-17A enhances imatinib efficacy in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia. Nat. Commun. 15, 203 (2024).
Steffen, S. et al. Toll-Like Receptor-Mediated Upregulation of CXCL16 in Psoriasis Orchestrates Neutrophil Activation. J. Investig. Dermatol. 138, 344–354 (2018).
Gruber, H. E., Marrero, E., Ingram, J. A., Hoelscher, G. L. & Hanley, E. N. Jr. The chemokine, CXCL16, and its receptor, CXCR6, are constitutively expressed in human annulus fibrosus and expression of CXCL16 is up-regulated by exposure to IL-1ß in vitro. Biotech. Histochem 92, 7–14 (2017).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
de Alcantara, C. C., Reiche, E. M. V. & Simao, A. N. C. Cytokines in psoriasis. Adv. Clin. Chem. 100, 171–204 (2021).
Furue, M., Furue, K., Tsuji, G. & Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21041275 (2020).
O’Brien, R. L. & Born, W. K. Dermal γδ T cells-What have we learned?. Cell Immunol. 296, 62–69 (2015).
Miao, Y. R. et al. ImmuCellAI-mouse: a tool for comprehensive prediction of mouse immune cell abundance and immune microenvironment depiction. Bioinformatics https://doi.org/10.1093/bioinformatics/btab711 (2021).
Mills, K. H. G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 23, 38–54 (2023).
Reynolds, G. et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 371, https://doi.org/10.1126/science.aba6500 (2021).
Itoh, T. et al. Biological Effects of IL-26 on T Cell-Mediated Skin Inflammation, Including Psoriasis. J. Invest Dermatol 139, 878–889 (2019).
Cook, C. P. et al. A single-cell transcriptional gradient in human cutaneous memory T cells restricts Th17/Tc17 identity. Cell Rep. Med. 3, 100715 (2022).
Chi, X. et al. RORgammat expression in mature T(H)17 cells safeguards their lineage specification by inhibiting conversion to T(H)2 cells. Sci. Adv. 8, eabn7774 (2022).
Tan, L. et al. Single-Cell Transcriptomics Identifies the Adaptation of Scart1(+) Vgamma6(+) T Cells to Skin Residency as Activated Effector Cells. Cell Rep. 27, 3657–3671.e3654 (2019).
Cai, Y. et al. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat. Commun. 5, 3986 (2014).
Frumm, S. M. et al. A Hierarchy of Proliferative and Migratory Keratinocytes Maintains the Tympanic Membrane. Cell Stem Cell 28, 315–330.e315 (2021).
Liu, Y. et al. Single-Cell Profiling Reveals Divergent, Globally Patterned Immune Responses in Murine Skin Inflammation. iScience 23, 101582 (2020).
Gao, Y. et al. Single-Cell Analysis Reveals the Heterogeneity of Monocyte-Derived and Peripheral Type-2 Conventional Dendritic Cells. J. Immunol. 207, 837–848 (2021).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Qu, G. et al. Comparing Mouse and Human Tissue-Resident gammadelta T Cells. Front Immunol. 13, 891687 (2022).
Chien, Y. H., Meyer, C. & Bonneville, M. gammadelta T cells: first line of defense and beyond. Annu Rev. Immunol. 32, 121–155 (2014).
Schnell, A. et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 184, 6281–6298.e6223 (2021).
Li, T., Pan, J., Chen, H., Fang, Y. & Sun, Y. CXCR6-based immunotherapy in autoimmune, cancer and inflammatory infliction. Acta Pharm. Sin. B 12, 3255–3262 (2022).
Scholz, F. et al. Constitutive expression and regulated release of the transmembrane chemokine CXCL16 in human and murine skin. J. Investig Dermatol 127, 1444–1455 (2007).
Cai, Y. et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 35, 596–610 (2011).
Ha, H. L. et al. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl Acad. Sci. USA 111, E3422–E3431 (2014).
Chen, X. Y., Wang, Z. Y., Zhou, Y., Ye, L. R. & Man, X. Y. Keratinoctye-neuro-immune-units (KNICUs): collaborative impact on the initiation and maintenance of psoriasis. Front Med (Lausanne) 10, 1191057 (2023).
Stuart, T. et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e1821 (2019).
Zhou, Y. et al. Quantitative Proteomic Profile of Psoriatic Epidermis Identifies OAS2 as a Novel Biomarker for Disease Activity. Front Immunol. 11, 1432 (2020).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 82230104, 81930089, 81773318, 82404117, 82103709 and 82303999).
Author information
Authors and Affiliations
Contributions
These authors contributed equally: Xue-Yan Chen, Li-Ran Ye, Ni-Chang Fu.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Amos Gilhar, Felix Lauffer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, XY., Ye, LR., Fu, NC. et al. Single cell transcriptomics of human psoriasis and epidermal specific Ube2l3 deficient mice highlight CXCL16/CXCR6 involvement in psoriasis development. Nat Commun 16, 9084 (2025). https://doi.org/10.1038/s41467-025-64106-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-64106-6