Abstract
Episodic memories are initially encoded in the hippocampus and subsequently undergo systems consolidation into the neocortex. The nature of memories stored in the hippocampus and neocortex differs, with the cortex encoding memories in more generalized forms. Although several brain regions encode social information, the specific cortical regions and circuits involved in the consolidation of social memories and the nature of the information encoded in the cortex remain unclear. Using in vivo Ca2+ imaging and optogenetic manipulations, we found that infralimbic (IL) neurons projecting to the nucleus accumbens shell (IL→NAcSh) store consolidated social memories in male mice. Inactivating IL→NAcSh neurons that responded to a familiar conspecific impaired the recognition of other familiar mice including littermates, demonstrating that these neuronal activities support social familiarity. Furthermore, inactivating hippocampal ventral CA1 neurons projecting to the IL region disrupted the consolidation of memory for newly familiarized mice while sparing the recognition of littermates. These findings demonstrate the critical role of hippocampal-cortical interactions in the consolidation of social memory.
Similar content being viewed by others
Introduction
Episodic memory is initially encoded and stored in the hippocampus and then consolidated into the cortex through intricate hippocampal-cortical interactions1,2. Prevalent models on the organization of memory, such as the complementary learning systems theory, propose that the interaction between the hippocampus and cortex during an “offline period” is crucial for memory storage, especially for recognition memory3. In addition, some memory models have proposed that different brain regions encode distinct aspects of memory4,5. For example, the cortex stores memory in more generalized or abstract forms compared to the hippocampus6.
Social memory, which refers to the ability to recognize and remember conspecifics, consists of the detection of familiarity and the recollection of social episodes7,8. The hippocampal dorsal CA2 (dCA2) region is a hub for social memory in rodents, with recent studies showing that the tuned geometries of dCA2 representations can support both familiarity and recollection8,9,10,11. The lateral entorhinal cortex sends projections conveying social information to the dCA2, which then sends projections to the ventral CA1 (vCA1)11,12. vCA1 projections to the nucleus accumbens shell (NAcSh) are critical for social memory13,14. While vCA1 projections to the mPFC have been implicated in social memory15,16, the precise role hippocampal-cortical interactions play in social memory consolidation remains largely unknown. It is also unclear whether the mPFC stores social memory in generalized forms.
Neural representations of social information have been reported in PFC neurons, encoding relevant social vs. nonsocial information, including information on sex or hierarchy17,18,19,20. Accordingly, social representations in the PFC are impaired in mouse models of autism spectrum disorder20,21,22. Several studies found that learning promoted task-relevant tuning and alterations in the dimensionality of neural representations in the PFC23,24,25. Still, our understanding of social representations in the PFC remains limited. Previously, we reported a subpopulation of mPFC infralimbic (IL) neurons projecting to the NAcSh that was activated by familiar mice. We found chemogenetic suppression of the activity of these neurons impaired social recognition, suggesting that these IL→NAcSh neurons are critical for processing social memory26. The stage of memory processing that requires these neurons remains unclear, as does the nature of the social information they encode.
In this study, we aimed to test our hypothesis that IL→NAcSh neurons store consolidated social memories in a generalized form via hippocampal-cortical interactions. To investigate this, we focused on the role of the vCA1-IL-NAcSh circuit in social memory consolidation in male mice using a combination of in vivo Ca2+ imaging, viral tracing and tagging, as well as both optogenetic and chemogenetic manipulations.
Results
Inactivation of IL→NAcSh neurons impairs social memory retrieval
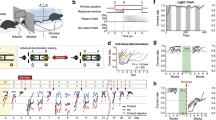
Previously, we showed that IL→NAcSh neurons play a critical role in social recognition26,27. The specific temporal window during which IL→NAcSh neurons are engaged, however, remains unclear. We used a social familiarization/recognition task to investigate social recognition memory in male mice (Fig. 1a). During social familiarization, we exposed each subject mouse to a novel conspecific mouse (FN) until it eventually became a familiar mouse (F). We then inactivated IL→NAcSh neurons in different phases of the social familiarization/recognition task via optogenetic or chemogenetic manipulation (Fig. 1b). To inactivate IL→NAcSh neurons during either the familiarization (encoding) or recognition (retrieval) phase, we expressed halorhodopsin (NpHR) or yellow fluorescent protein (YFP) in the IL and used optic fibers to bilaterally target the NAcSh (Fig. 1c). To demonstrate the functional inhibition of IL→NAcSh neurons via optogenetics, we expressed ChR2 with either YFP or NpHR in IL→NAcSh neurons and recorded optogenetically-induced excitatory postsynaptic currents (opto-EPSCs) in the NAcSh. We found that NpHR activation reduced opto-EPSCs in NAcSh neurons in ex vivo whole cell patch-clamp recordings, confirming the optogenetic inhibition functionally affected target region neurons (Supplementary Fig. 1a–c). Optogenetic inactivation of IL→NAcSh neurons during social familiarization on day 1 affected neither social familiarization nor recognition in NpHR or YFP-expressing mice (Fig. 1d and Supplementary Fig. 2a). On day 2, both groups showed significantly longer interaction times with novel conspecifics (N) than with either familiar conspecifics (F) or littermates (L). Chemogenetic inactivation of these neurons during the offline phase after social familiarization did not affect social preference for novel conspecifics during the social recognition session on day 2 (Fig. 1e, f, and Supplementary Fig. 2b). We also confirmed that CNO treatment reduced neuronal excitability in hM4Di-expressing IL→NAcSh neurons (Supplementary Fig. 1d–f). When we inactivated these neurons during the social recognition session on day 2, however, although the YFP-expressing mice showed normal preference for the novel conspecific, the NpHR-expressing mice exhibited impaired social recognition (Fig. 1g). The same mice showed normal social recognition in the absence of optogenetic manipulation (Supplementary Fig. 2c). These results indicate that IL→NAcSh neuronal activity is required for the retrieval of social memory. In addition, we found optogenetic manipulation had no effect on locomotive activity or real-time place preference (Supplementary Fig. 2d, e). Of note, we found most IL→NAcSh neurons in layer 5 of the IL cortex, but we also found a minor population in layers 2/3 (Fig. 1e and Supplementary Fig. 3a–c). Our virus injection strategy primarily labeled IL→NAcSh neurons, with minor labeling of other prefrontal subregions projecting to the NAc due to virus diffusion (Fig. 1e and Supplementary Fig. 3d–f).
a Social familiarization/recognition task. A subject mouse interacted with a novel conspecific three times (defined as FN, FN’, then FN”) for 5 min each with 5-min intervals. Next day, the subject mouse interacted with a familiarized (F), a novel (N), and a littermate (L) conspecific. b Timeline for the optogenetic and chemogenetic manipulation of IL→NAcSh neurons. c Left: Schematic for optogenetic manipulation. Right: mPFC (top) and NAcSh (bottom). Scale: 1000 μm and 500 μm. d Optogenetic inactivation during familiarization. Left: Both groups showed significantly reduced interaction times. Two-way ANOVA with repeated measures (RM): YFP/NpHR, n = 13/13 mice; F2,48 = 0.1333, p = 0.8755; Right: Both groups interacted longer with novel conspecifics. F2,48 = 3.509, *p = 0.0378. e Left: Schematic for chemogenetic manipulation. Right: IL (top) and NAcSh (bottom). Scale: 1000 μm and 500 μm. f Chemogenetic inactivation during offline. Left: Both groups showed reduced interactions during familiarization. Two-way RM ANOVA: mCherry/hM4Di, n = 14/12 mice; F2, 48 = 2.593, p = 0.0852. Right: Both groups interacted longer with novel conspecifics. F2, 48 = 0.6990, p = 0.5021. g Optogenetic inactivation during recognition. Left: Both groups showed reduced interactions during familiarization. Two-way RM ANOVA: YFP/NpHR, n = 13/13 mice; F2,48 = 0.3249, p = 0.7242. Right: NpHR mice showed similar interaction times with different conspecifics. F2,34 = 4648, p = 0.4648. Data presented as mean ± SEM; each dot represents one mouse. Statistics used two-way RM ANOVA test with two-sided Tukey-corrected post hoc comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a source data file. The mouse illustration in (a) and the schematic drawings of the brain in (c) and (e) are reprinted from Cell Reports, Volume 35, Issue 6, Park, et al., Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice, 109104, Copyright (2021), with permission from Elsevier.
IL→NAcSh neurons are activated by familiar conspecifics
Next, we investigated which aspects of social information are encoded by the IL→NAcSh neurons. Since the inactivation of IL→NAcSh neurons impaired social recognition, we wanted to examine whether these neurons encode the identity or social familiarity of social targets. To investigate social representations in IL→NAcSh neurons, we monitored Ca2+ activity in this prefrontal subpopulation using an open-source one-photon miniaturized endoscopic microscope during the social familiarization/recognition task (Supplementary Fig. 4a). In Ai148 mice expressing a Cre recombinase-dependent genetically-encoded Ca2+ indicator (GCaMP6f), we monitored calcium activity in IL→NAcSh neurons through a gradient-index (GRIN) lens attached to a right-angled optical prism implanted lateral to the prefrontal cortex, primarily targeting the infralimbic cortex (Fig. 2a, Supplementary Fig. 4b). After obtaining fluorescence signals (ΔF/F), we processed them and analyzed neuronal activity dynamics (Fig. 2b). During social familiarization, the subject mice showed a significantly reduced duration for each consecutive interaction with a novel conspecific (FN) (Fig. 2c). During the recognition session on day 2, the subject mice showed significantly longer interactions with a novel conspecific (N) compared to familiar conspecifics (F) or littermates (L) (Fig. 2c and Supplementary Fig. 4c). This suggests that the miniscope imaging did not affect performance in the social familiarization/recognition task. We imaged Ca2+ signals longitudinally during the task, achieving successful registration of over 80% of the GCaMP6f-expressing neurons across days (Supplementary Fig. 4d–f). Average Ca2+ transient frequency during the habituation sessions remained comparable with that during the social familiarization and recognition sessions (Supplementary Fig. 4g).
a Left: One-photon Ca2+ imaging of IL→NAcSh neurons expressing GCaMP6f in Ai148 mice. Right: GCaMP6f expression in IL with lens traces. Scale: 500 μm. b Imaging plane showing IL→NAcSh neurons (left) with pseudo-colored ROIs for Ca2+ analysis (left) and representative Ca2+ traces for those ROIs (right). c Mice with an endoscopic microscope showed normal social recognition. One-way RM ANOVA: n = 11; familiarization: F2,20 = 27.18, ****p < 0.0001, recognition: F2,20 = 22.82, ****p < 0.0001. d Numbers of social cells were comparable across sessions. One-way RM ANOVA: n = 11; familiarization, F2,20 = 2.418, p = 0.1147, recognition, F1.380,13.80 = 3.306, p = 0.0807. e Representative social cell activities responding to a familiar mouse. f The AUC for Interaction-dependent Ca2+ transient in social cells responding to familiar conspecifics increased 24 h after familiarization. Dot: average AUC of target-specific social cells per subject mouse. One-way RM ANOVA, n = 11; F1.596,15.96 = 68.94, ****p < 0.0001. g Ca2+ transients of social cells during interactions with F, N, and L. h The AUC for interaction-dependent Ca2+ transient in social cells responding to known social targets were higher than that of novel conspecific-social cells. One-way RM ANOVA, n = 11; F1.536,15.36 = 10.41, **p = 0.0024. i Social cell overlap between known targets (F∩L) was greater than other population overlaps. One-way RM ANOVA (left): n = 11; F1.982,19.82 = 0.1608, p = 0.8507. (right) F1.317,13.17 = 11.39, **p = 0.0030. j Schematic for the Ca2+ data processing. k An LSVM classifier successfully decode social targets. The performance for distinguishing between F and L was significantly lower than for other comparisons. One-way RM ANOVA: n = 10, F1.714,15.42 = 8.865, **p = 0.0037. Data presented as mean ± SEM; each dot represents one mouse. Box plots show median (center line), 25th–75th percentiles (box), and 5th–95th percentiles (whiskers). Statistics used one/two-way RM ANOVA with two-sided Tukey-corrected post hoc comparisons. p values presented in figures. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a source data file. The schematic drawing of the brain in (a) is reprinted from Cell Reports, Volume 35, Issue 6, Park, et al., Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice, 109104, Copyright (2021), with permission from Elsevier.
We used a receiver operating characteristic (ROC) analysis to define a population of social interaction-responsive cells among the IL→NAcSh neurons and classified them as ‘social cells’ (see the Methods section for details)17. We could classify 47.2% of all recorded cells (558 out of 1181) as social cells in at least one of the interaction sessions during the two days of the social familiarization/recognition task, with the number of social cells remaining comparable between sessions (Fig. 2d). Interestingly, when we analyzed the interaction-dependent Ca2+ transient area-under-the-curve (AUC) of familiar conspecific-social cells, we found a significant increase during the social recognition session and no change during the familiarization session (Fig. 2e, f). Repeated interactions with a conspecific within the familiarization session did not increase either the number of social cells nor the overlap between them (Fig. 2d, i). These data, along with our optogenetic inhibition results during the social memory encoding and retrieval phases, suggest a delayed emergence of neural representations in the IL for familiar mice 1 day after repeated social interactions.
Additionally, we found that the social cells responding to littermates and familiar conspecifics showed a significantly larger interaction-dependent Ca2+ transient AUC compared to the social cells responding to novel conspecifics, which is consistent with our previous finding that IL→NAcSh neurons show increased responses to familiar mice than novel mice (Fig. 2g, h)26. Thus, we next asked whether familiar mice and littermates activate the same or overlapping subpopulations of IL→NAcSh neurons during social recognition. We found that social cells responding to known conspecifics (F and L) showed a significantly larger overlap (F∩L) than other comparison groups, despite each group containing a similar number of social cells between sessions (Fig. 2d, i). These results led us to hypothesize that the activity of IL→NAcSh neurons encodes social familiarity, enabling them to more effectively distinguish between a novel mouse and a familiar mouse than between a familiar mouse and a littermate. To test this, we examined whether the population activity of IL→NAcSh neurons could decode the identity of social targets at different levels of familiarity. We trained a 3-dimensional linear support vector machine (LSVM) decoding algorithm with two single variables after linear dimensionality reduction with principal component analysis (PCA) (Fig. 2j). We found IL→NAcSh neuronal representations accurately discriminated between mice regardless of their familiarity levels, but the LSVM decoding performance for distinguishing two known social targets (F and L) was significantly lower than for other comparison groups (Fig. 2k). In conjunction with our ROC-based identification of social interaction-dependent cell populations and their properties, these results imply that IL→NAcSh neurons encode social memory of known social targets following social familiarization and subsequent memory consolidation.
IL→NAcSh neural activity represents social identity and familiarity
Recent studies have shown that neural ensembles in the PFC and hippocampus can simultaneously represent variables in an abstract format while still preserving their ability to decode other variables, including social familiarity and identity8,24. One recent study showed that CA2 activity encodes both social identity and familiarity by analyzing neural activity pattern arrangements, also known as neural representational geometries, in neural activity space8. Given the preferential activities of IL→NAcSh neurons towards known social targets and the decent decoding performance for distinguishing between two known social targets, we asked whether IL→NAcSh neurons can simultaneously represent both social familiarity and identity of familiar conspecifics. We monitored Ca2+ activity in IL→NAcSh neurons while subject mice were consecutively exposed to either two littermates or two novel mice, with the positions of the same target mice reversed between exposures (Fig. 3a, c). During the consecutive littermate recognition task, the subject mice interacted twice with each of two littermates, exhibiting a slight reduction in the interaction time for the second interaction with each littermate (Fig. 3a, b). We observed a similar proportion of social cells in each session (Supplementary Fig. 5a). Interestingly, we observed similar overlap for social cells responding to the same littermates in different sessions and for social cells responding to different littermates, suggesting that IL→NAcSh neurons are not consistently activated by a specific littermate (Supplementary Fig. 5b). Nevertheless, the LSVM classifier gave high decoding accuracy whether trained with social (identity) or spatial (position) information (Supplementary Fig. 5c–e). The LSVM classifier also showed high identity decoding performance whether trained with overlapping or non-overlapping social cells responding to two littermates (Supplementary Fig. 5f, g).
a Subject mice interacted with two littermates (L1 and L2) for 5 min each in two separate sessions with a 5-min interval. b Subject mice showed significantly reduced interaction times. One-way RM ANOVA: n = 13 mice; F3,36 = 5.113, **p = 0.0048. c Subject mice interacted with two novel conspecifics (N1 and N2) for 5 min in two sessions with a 5-min interval. d Subject mice interacting with novel conspecifics showed significantly reduced interaction times. One-way RM ANOVA: n = 13 mice; F2.006, 24.07 = 13.29, ***p = 0.0001. e Identity decoding using a CCGP analysis of the consecutive littermate recognition task data. f A representative image showing that IL→NAcSh neural activity from an exemplar subject supports a generalized decoding of the identity of a littermate. g Identity CCGP decoding with consecutive novel mouse interaction task data. h Representative image showing that IL→NAcSh neural activity does not support a generalized decoding of the identity of novel mice. i Social interaction-dependent neural activities during littermate interactions showed significantly higher CCGP decoding performance than those from a null model, while those during novel conspecific interactions showed similar CCGP performance to those from a null model. Two-way ANOVA with Sidak’s multiple comparison test: n = 11, F1,20 = 3.372, p = 0.0812, Littermate; Null-Actual: t20 = 2.801, *p = 0.0220, Novel mouse; Null-Actual: t20 = 0.2036, p = 0.9746. j Decoding familiarity using a CCGP analysis comparing social interaction-dependent IL→NAcSh neural activity during littermate and novel mouse interactions. k A representative image of social interaction-dependent IL→NAcSh neural activity from an exemplar subject in response to littermates and novel mice in the social familiarity CCGP analysis. l Social familiarity CCGP analysis comparing social interaction-dependent IL→NAcSh neural activity during interactions with littermates or novel mice exhibited significantly higher decoding performance than that of null data. Two-tailed paired t-test: n = 9 mice, CCGP; t8 = 6.500. ***p = 0.0002. Data presented as mean ± SEM; each dot represents one mouse. Statistics used one- or two-way RM ANOVA with two-sided Tukey-corrected post hoc comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a source data file. The mouse illustrations in (a) and (c) are reprinted from Cell Reports, Volume 35, Issue 6, Park, et al., Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice, 109104, Copyright (2021), with permission from Elsevier.
During the consecutive novel mouse interaction task, the subject mice alternately interacted twice with two novel mice (Fig. 3c). Interaction times with the novel mice were significantly higher than those with littermates during the first encounter but then decreased in the subsequent interactions to levels comparable to those with littermates (Fig. 3b, d). The number of social cells for novel conspecifics remained similar across sessions (Supplementary Fig. 5h). The degree of overlap between social cells responding to different novel mice and those responding to the same novel mouse was comparable (Supplementary Fig. 5i). Consistent with our social familiarization/recognition task results, we found that the Ca2+ responses of social cells responding to novel mice were substantially smaller than those of social cells responding to littermates (Supplementary Fig. 5j). Despite the overall lower Ca2+ activity of IL→NAcSh social cells responding to novel mice, both the social identity and position of the novel mice could be successfully decoded from the IL→NAcSh population activity (Supplementary Fig. 5k–m). In addition, the LSVM classifier showed high identity decoding performance when trained with overlapping social cells responding to different novel mice (Supplementary Fig. 5n, o).
After finding that we could decode social and non-social (i.e., spatial) features of social targets from the activity of the IL→NAcSh neurons via a linear classifier, we next explored whether social identity could be represented independently of position in the geometry supporting generalization. To answer this question, we used a modified linear SVM classifier called cross-condition generalization performance (CCGP) to train and determine a conceptual hyperplane that could separate an effectively generalized population of vectors illustrating different neural representations and use it for making predictions8,24. We measured identity CCGP by training the linear classifier to decode the identity of two littermates on the left side (L1left and L2left) and then tested it on data from littermates on the right side (L1right and L2right). This analysis was repeated with the training and testing datasets reversed, and the results were averaged (Fig. 3e). We also measured position CCGP in the similar manner. We found the identity CCGP showed much higher performance than the one trained on spatial information, suggesting that IL→NAcSh neural activity supports a generalized decoding of identity, but not the position of littermates (Fig. 3f, i and Supplementary Fig. 6a, b, and e). Interestingly, data from interactions with novel mice gave a CCGP performance that was as low as chance for both identity and position (Fig. 3g–i and Supplementary Fig. 6c–e). Despite high LSVM decoding performance, low CCGP decoding performance remains possible depending on the geometrical positioning of the clusters from novel mouse representations.
Next, we directly investigated the effect of familiarity of social targets on IL→NAcSh neuronal representations by comparing the population patterns from two behavioral paradigms, the littermate recognition task and the novel mouse interaction task. We registered cells from each behavioral paradigm (Supplementary Fig. 7a–d), trained the CCGP classifier using data from interactions with littermate 1 and novel mouse 1 on the left side (L1left and N1left), and then tested it with data from littermate 2 and novel mouse 2 on the right side (L2right and N2right). We did the same with the reversed positional configuration and averaged the results to account for any side-specific bias (Fig. 3j and Supplementary Movie 1). Considering the different social identities and positions of the target mice, the CCGP classifier specifically allowed us to determine any impact of familiarity beyond identity. We found that IL→NAcSh neural activity showed significant decoding performance depending on the familiarity of social targets with different identities but not depending on social target spatial location (Fig. 3j–l, and Supplementary Fig. 6f–h). These data suggest IL→NAcSh neural activity represents both a generalized social identity of littermates and a representation of familiarity. Of note, the LSVM decoder failed to distinguish the calcium activity patterns from the habituation sessions of the littermate recognition and novel mouse interaction tasks, which were performed over several days (Supplementary Fig. 7e–g). This indicates that decoding performance did not depend on the time difference between the two tasks. Decoding performance remained stable across multiple analytical conditions, including when dimensionality reduction or feature selection was omitted (Supplementary Fig. 8). Since our decoding analyses included the entire recorded neuronal population, including both overlapping and non-overlapping social cells, decoding performance could be influenced by both neuronal populations. To assess the specific contribution of each population, we attempted to decode the identity of social targets using either overlapping or non-overlapping social cells using CCGP decoder (Supplementary Fig. 9). When only overlapping cells from different littermates were used, decoding performance was at chance level (Supplementary Fig. 9a, b) Interestingly, non-overlapping social cells from different littermates were able to identify littermate identity with CCGP decoding (Supplementary Fig. 9c). In contrast, for novel social targets, decoding performance using either overlapping or non-overlapping social cells was close to chance (Supplementary Fig. 9d, e).
Inactivation of IL→NAcSh neurons activated by a familiar mouse impairs social recognition
We next asked whether selective inhibition of IL→NAcSh social cells activated by a familiar conspecific is sufficient to disrupt the neuronal representation of a single known social target and whether it extends to disrupting the recognition of other conspecifics. To investigate this question, we used a genetic technique known as targeted recombination in active population (TRAP)28. First, we injected wild-type mice with the fosCreERT2 virus into their IL cortex and with a retrograde Cre-dependent virus expressing hM4Di-mCherry (hereafter hM4Di) or mCherry into their NAcSh (Fig. 4a). This labeled (TRAPed) IL→NAcSh neurons activated by a familiar conspecific, such that their activities could subsequently be manipulated during the social recognition session (Fig. 4b). Subject mice were exposed to a novel conspecific for familiarization on day 1 and then re-exposed to the familiarized mouse for TRAP tagging on day 2 (Fig. 4c). One week later (day 9), when the TRAPed neurons expressed either hM4Di or mCherry, the subject mice were tested in the social familiarization/recognition task. In this task, the subject mice were familiarized with a novel mouse different from the one they interacted with on days 1 and 2 (Fig. 4c, d). Both hM4Di and mCherry-expressing groups showed similarly reduced interaction times during familiarization with a novel conspecific on day 9 regardless of CNO injection (Fig. 4d, Supplementary Fig. 10a, d). Injection of CNO prior to the social recognition session on day 10, however, inactivated the TRAPed IL→NAcSh neurons and induced a significant reduction in social recognition compared to controls. These controls included mCherry-expressing mice with or without CNO injection and hM4Di-expressing mice without CNO injection (Fig. 4d, Supplementary Fig. 10b, c, e, and f). Importantly, interaction times in CNO-injected hM4Di-expressing mice increased, not only with a familiarized mouse, but also with a littermate (Fig. 4d and Supplementary Fig. 10f). The inactivation of TRAPed IL→NAcSh neurons did not affect basal locomotor activity or total interaction times (Supplementary Fig. 10g, h). These data show that inactivation of IL→NAcSh neurons responding to a single familiar conspecific was sufficient to impair social recognition of other known social targets, demonstrating that IL→NAcSh neuronal activity supports social familiarity. In addition, we TRAPed IL→NAcSh neurons activated during interactions with a novel conspecific (Supplementary Fig. 11a–c). The inactivation of these novel mouse-dependent IL→NAcSh neurons did not impair social recognition, demonstrating the specificity of our other TRAP experiments (Supplementary Fig. 11d–g).
a TRAP tagging of IL→NAcSh neurons. b TRAP-mCherry expression in the mPFC after exposure to a familiar conspecific. ACC, anterior cingulate cortex; PL, prelimbic cortex; IL, infralimbic cortex. Scale: 500 μm, 100 μm. c TRAP labeling of familiar conspecific-dependent neurons. Day 1: A subject mouse was familiarized with a social target. Day 2: The subject mouse interacted with the familiarized target, followed by tamoxifen (4-OHT) injection. Days 9-10: The subject mouse underwent social familiarization/recognition task. d Both groups showed reduced interaction times during familiarization (left). Two-way RM ANOVA: mCherry CNO/hM4Di CNO, n = 18/22 mice; F2,76 = 0.1825, p = 0.8336. CNO-injected hM4Di group did not distinguish social targets, whereas mCherry-CNO mice interacted longer with novel conspecifics (right). CNO or saline was injected 40 min before recognition. Two-way RM ANOVA; F2,76 = 5.170, **p = 0.0079. e Induction of cFos in TRAP-labeled (TRAPed) mice in response to novel conspecifics or littermates. BL, baseline; N, novel mouse; L, littermate. f Mice exposed to novel conspecifics showed longer interaction times than those to littermates. Two-tailed unpaired t-test: TRAPed mice with littermates/novel conspecifics, n = 4/5; t7 = 3.571, **p = 0.0091. g IL sections showing TRAPed neurons (mCherry) and cFos (green) with DAPI (blue). Scale: 50 μm. h TRAPed neurons in littermate-exposed mice showed more overlap with cFos than those in baseline or novel conspecific-exposed mice. One-way ANOVA: Baseline/Novel/Littermate, n = 3/5/4 mice; F2,9 = 9.531, **p = 0.0060. i Comparable numbers of TRAPed neurons were observed across conditions. One-way ANOVA: F2,9 = 0.6061, p = 0.5663. Data presented as mean ± SEM; One dot represents one mouse. Statistics were made using either two-sided unpaired t-test, ordinary one- or two-way RM ANOVA with two-sided Tukey-corrected post hoc comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a source data file. The schematic drawing of the brain in (a) and the mouse illustrations in (c) and (e) are reprinted from Cell Reports, Volume 35, Issue 6, Park, et al., Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice, 109104, Copyright (2021), with permission from Elsevier.
To further investigate whether the TRAPed IL→NAcSh neuronal population represents social familiarity, we asked whether these neurons are reactivated during interactions with another familiar conspecific. After allowing the TRAPed mice to interact with either a novel conspecific or a littermate, we analyzed cFos expression (day 15-16, Fig. 4c, e). The TRAPed mice showed a strong preference toward novel conspecifics over littermates (Fig. 4f). We found that TRAPed mice showed a significantly higher percentage of TRAPed mCherry+ neurons co-labeled with cFos after interacting with a littermate than with a novel mouse or without any social interaction (Fig. 4g, h). The numbers of TRAP-labeled neurons (mCherry+) were comparable among groups (Fig. 4i). The increased overlap between TRAPed neurons and littermate interaction-induced cFos+ staining we observed was consistent with the high overlap of social cells that responded to known social targets in the Ca2+ imaging experiments (Fig. 2i). These results illustrate that IL→NAcSh neurons encode social memory with a certain level of overlap, which might support social familiarity.
vCA1 to IL projections are necessary for social memory consolidation
Given that the hippocampus plays crucial roles in multiple phases of social memory, including memory acquisition, and given that hippocampal-cortical interactions are critical for episodic memory, we hypothesized that the IL might interact with the hippocampus to process social memory. Among the subregions of the hippocampus engaged in social memory, the IL cortex receives direct inputs from vCA129. We used optogenetic and chemogenetic manipulation to investigate the temporal window during which the activities of IL-projecting vCA1 (vCA1→IL) neurons are required for social recognition. We expressed ChR2 with either NpHR or YFP in the vCA1 and inserted optic fibers bilaterally into the IL to selectively inactivate vCA1→IL neurons (Fig. 5a). This allowed us to validate the inhibitory effect of NpHR as we recorded opto-EPSCs in IL neurons in ex vivo whole cell patch-clamp recordings (Supplementary Fig. 12a–c). We found that illumination with a 594-nm laser during social familiarization did not affect social recognition in either NpHR- or YFP-expressing mice during the social recognition session (Fig. 5b and Supplementary Fig. 13a).
a Left: Optogenetic inactivation of vCA1→IL neurons. Right: mPFC (top) and vCA1 (bottom). Scale: 1 mm and 2 mm. b Left: Both groups showed reduced interaction times during familiarization. Two-way RM ANOVA: YFP/NpHR, n = 10/12 mice; F2, 40 = 1.396, p = 0.2593. Right: Both groups interacted more with novel conspecifics. Two-way RM ANOVA; F2, 40 = 0.2207, p = 0.8029. c Left: Chemogenetic inactivation of vCA1→IL neurons Right: mPFC (top) and vCA1 (bottom). Scale: 1 mm. d Left: Both groups showed reduced interaction times during familiarization. Two-way RM ANOVA: mCherry/hM4Di, n = 10/11 mice; F2, 38 = 1.384, p = 0.2630. Right: hM4Di mice showed similar interaction with familiar and novel conspecifics but shorter interaction with littermates, while mCherry mice interacted longer with novel conspecifics than familiar conspecifics or littermates. Two-way RM ANOVA: F2, 38 = 8.035, **p = 0.0012. e Left: Both groups showed reduced interaction times during familiarization. Two-way RM ANOVA: YFP/NpHR, n = 18/18 mice; F2, 68 = 1.165, p = 0.3179. Right: NpHR mice showed similar interaction with familiar and novel conspecifics but shorter interaction with littermates; YFP mice showed longer interaction with novel conspecifics than familiar conspecifics or littermates. F2, 68 = 2.402, p = 0.0982. f Left: Chemogenetic inhibition of vCA1→IL neuronal terminals. Right: IL (top) and vCA1 (bottom) sections. Scale: 1 mm. g Top: Social familiarization/recognition task with offline-phase chemogenetic inactivation. Bottom: Both groups showed reduced interaction times during familiarization (left). Two-way RM ANOVA: mCherry/hM4Di, n = 15/14 mice; F2,24 = 1.669, p = 0.1979. hM4Di mice showed comparable interaction times with familiar and novel conspecifics; mCherry mice showed significantly longer interaction times with novel conspecifics than familiar conspecifics or littermates (right). Two-way RM ANOVA: F2,54 = 9.197, ***p = 0.0004. Data presented as mean ± SEM; each dot represents one mouse. One or two-way RM ANOVA with two-sided Sidak or Tukey-corrected post hoc comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a source data file. The schematic drawings of the brain in (a), (c) and (f) are reprinted from Cell Reports, Volume 35, Issue 6, Park, et al., Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice, 109104, Copyright (2021), with permission from Elsevier.
To inhibit vCA1→IL neurons during the offline memory consolidation phase, we expressed hM4Di in vCA1→IL neurons (Fig. 5c). We confirmed that CNO treatment reduced the excitability of hM4Di-expressing vCA1→IL neurons (Supplementary Fig. 12d–f). By injecting CNO after social familiarization to inactivate these neurons, hM4Di-expressing mice showed deficits in social recognition. While they showed similar interaction times with familiar and novel conspecifics during the social recognition session (Fig. 5d), their interaction times with littermates remained as low as the controls (Fig. 5d and Supplementary Fig. 13b). We were also able to reproduce this behavioral phenotype by inactivating the hM4Di DREADD receptor during consolidation phase with deschloroclozapine (DCZ), an alternative DREADD agonist (Supplementary Fig. 14). In addition, optogenetic inhibition of vCA1→IL neurons during the retrieval phase also prevented NpHR-expressing mice from distinguishing between familiar and novel conspecifics during the social recognition session (Fig. 5e and Supplementary Fig. 13c). This is likely attributable to incomplete memory transfer from the hippocampus to the cortex rather than a block in memory recall, as the subject mice still showed lower interaction times with littermates (Fig. 5e). Both YFP and NpHR-expressing mice showed intact locomotor activity and place preference regardless of laser illumination (Supplementary Fig. 15a, b). Nor did chemogenetic inactivation of vCA1→IL neurons affect novel object recognition, suggesting a specific engagement of these projections in processing social context (Supplementary Fig. 15c).
As previously reported30, we observed that vCA1→IL neurons have collateral projections to the NAcSh (Fig. 5a, c, Supplementary Fig. 16). This raised the concern that the social memory deficits we observed in our previous experiment may have arisen from a blockade of the activity of vCA1 neurons that project to the NAcSh (Fig. 5d). To selectively inactivate vCA1→IL neurons, we expressed hM4Di in IL-projecting vCA1 neurons and infused CNO locally into the IL (Fig. 5f). While both mCherry- and hM4Di-expressing mice showed normal behaviors during the social familiarization session, hM4Di-expressing mice injected with CNO during the offline phase exhibited similar interaction times with familiar conspecifics and with novel mice, due mainly to increased investigation of familiar conspecifics (Fig. 5g, Supplementary Fig. 13d, e). Consistent with our systemic CNO injection result (Fig. 5d), hM4Di-expressing mice subjected to local CNO injection showed interaction times with littermates as low as controls. These data imply that the activity of IL-projecting vCA1 neurons during the offline phase is critical for the consolidation of memories of newly familiarized mice.
Interestingly, we found selective inactivation of vCA1 neuron terminals in the NAcSh during the offline phase also impaired the consolidation of newly formed social memories. hM4Di-expressing mice injected with CNO exhibited similar interaction times with familiar and novel conspecifics, while their interaction times with littermates remained low (Supplementary Fig. 17a, b). This result is consistent with our selective inactivation of vCA1-projecting neuron terminals in the IL (Fig. 5g). We found, however, that inactivation of vCA1→NAcSh neurons during social familiarization reduced social interaction times during the familiarization session, possibly causing the differences in the interaction times with familiar and novel conspecifics during the social recognition session to fall below the level of significance (Supplementary Fig. 17c). This result was consistent with a previous finding that chemogenetic inactivation of vCA1→NAcSh neurons disrupted social preference31. As vCA1→IL neuronal inactivation during social familiarization affected neither social memory encoding (familiarization) nor recall (recognition) (Fig. 5g), these results suggest overlapping but distinct roles for the vCA1→IL and vCA1→NAcSh neuronal populations.
To further determine whether projections from vCA1 are anatomically connected to IL→NAcSh neurons, we labeled both IL→NAcSh neurons and vCA1→IL neurons in the same mouse. We found that 20% of IL→NAcSh neurons are directly connected to vCA1 neurons (Supplementary Fig. 18).
In addition, to validate whether the inactivation of vCA1→IL neurons during the offline consolidation phase directly affects familiar conspecific-dependent cFos induction in IL→NAcSh neurons, we expressed the hM4Di DREADD protein in vCA1→IL neurons and labeled IL→NAcSh neurons with a retrograde AAV-GFP injected into the NAcSh (Supplementary Fig. 19a, b). After familiarizing hM4Di-expressing mice with a novel conspecific one day before being re-exposing them to the same conspecific, we sacrificed the animals to examine cFos expression in their IL→NAcSh neurons (Supplementary Fig. 19c). Consistent with our earlier result (Fig. 5d), the inactivation of vCA1→IL neurons during the consolidation phase increased interaction times with familiar conspecifics, indicating impaired social recognition (Supplementary Fig. 19d). Moreover, the CNO-injected group exhibited significantly less IL→NAcSh neuronal cFos expression than vehicle-treated controls, demonstrating that inactivation of vCA1→IL neurons during the consolidation phase directly affected the activity of the IL→NAcSh neurons (Supplementary Fig. 19e–g).
Inactivation of vCA1→IL neurons during the offline phase impairs social recognition and social representation in IL→NAcSh neurons
Since hippocampal inactivation during the offline period prevents consolidation of spatial or contextual memories32, we next examined the impact of vCA1→IL neuron inactivation during the offline phase on the neural representation of social information in the IL cortex. First, we used the Cre-Lox and fLp-FRT recombination systems to selectively express GCaMP protein in IL→NAcSh neurons and hM4Di or mCherry (control) in vCA1→IL neurons (Fig. 6a). Then, we recorded the Ca2+ activity of IL→NAcSh neurons during an extended version of the social familiarization/recognition task (Fig. 6b and Supplementary Fig. 20a–d). In this task, a subject mouse was familiarized with two social targets during the social familiarization session and then subjected to interaction with the same two familiarized mice, along with two novel conspecifics or two littermates on the next day to test its social recognition. In this extended social familiarization/recognition task, mCherry-expressing control mice injected with CNO showed significantly longer interaction times with the two novel mice than with either the two familiar mice or the two littermates (Fig. 6c). Consistent with our previous results (Fig. 5d, g), hM4Di-expressing mice injected with CNO during the offline phase did not distinguish familiar mice from novel mice (Fig. 6d). These mice still showed significantly shorter interaction times with their littermates than with familiar or novel mice, further demonstrating that vCA1 inactivation had prevented the subject mice from recognizing familiarized conspecifics as familiar (Fig. 6d).
a Left: Schematic for the expression of GCaMP6f in IL→NAcSh and hM4Di in vCA1→IL neurons. Right: GCaMP6f in IL (top, scale: 500, 100 µm); hM4Di in vCA1 (bottom, scale: 1000 µm). b Extended social familiarization/recognition task with offline vCA1→IL inactivation. Mice met two novel mice (FN1 and FN2) during familiarization; interacted with familiar (F1, F2), novel (N1, N2), and littermate (L1, L2) conspecifics during recognition. c Left: mCherry mice showed reduced interaction time during familiarization. One-way RM ANOVA: F2.800,19.60 = 9.807, ***p = 0.0004. Right: mCherry mice showed longer interaction with novel mice during recognition. F5,35 = 21.59, ****p < 0.0001. d Left: hM4Di mice showed reduced interaction times during familiarization. One-way RM ANOVA: F2.929,23.43 = 17.23, ****p < 0.0001. Right: hM4Di mice showed comparable interaction with familiar and novel conspecifics, but less interaction with littermates during recognition. F3.259,26.07 = 10.61, ****p < 0.0001. e mCherry mice exhibited increased overlapping social cells after familiarization, whereas hM4Di mice did not. Two-way RM ANOVA: F2, 30 = 3.384, *p = 0.0473. f Familiarity CCGP (familiar vs. novel) decoding. g Geometry from a mCherry mouse with high familiarity CCGP performance. h Geometry from a hM4Di mouse with chance-level performance. i High familiarity CCGP performance in mCherry, but not hM4Di mice. Two-way RM ANOVA: F1,15 = 9.343, **p = 0.0080. j Top: Familiarity decoding (familiar vs. FN). Bottom: High CCGP performance in mCherry only. Two-way RM ANOVA: F1, 15 = 6.444, *p = 0.0227. k Top: Familiarity decoding (littermate vs. FN). Bottom: Both groups showed high decoding performance. Two-way RM ANOVA: F1, 15 = 4.013, p = 0.0636. l Top: Familiarity decoding (littermate vs. novel). Bottom: Both groups showed high decoding performance. Two-way RM ANOVA: F1, 15 = 1.550, p = 0.2322. m Top: Cross-familiarity CCGP (familiar/littermate vs. novel). Bottom: High performance in mCherry only. Two-way RM ANOVA: F1, 15 = 4.858, *p = 0.0436. mCherry/hM4Di, n = 8/9 mice. Data presented as mean ± SEM; each dot represents one mouse. One- or two-way RM ANOVA with two-sided Sidak or Tukey-corrected post hoc comparisons. p values presented in figures. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a source data file. The schematic diagram of the brain in (a) and the mouse illustration in (b) are reprinted from Cell Reports, Volume 35, Issue 6, Park, et al., Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice, 109104, Copyright (2021), with permission from Elsevier.
After confirming the behavioral deficits of mice with vCA1→IL neuron inactivation in the extended social familiarization/recognition task, we analyzed social representations in IL→NAcSh neurons. mCherry- and hM4Di-expressing mice exhibited similar numbers of social cells (Supplementary Fig. 20f, g). Consistent with our results (Fig. 2i), we observed significantly more overlap of social cells for familiar conspecifics and littermates (F∩L) in vCA1 mCherry-expressing mice than in vCA1 hM4Di-expressing mice (Fig. 6e), suggesting vCA1 inactivation changes neuronal representations of social targets in IL→NAcSh neurons, subsequently impairing social recognition. We also found that an LSVM classifier was capable of predicting social identity with an accuracy above the level of chance even after vCA1→IL neuron inactivation (Supplementary Fig. 20h).
Next, we used a CCGP classifier to examine the impact of vCA1 inactivation on the representation of social familiarity in IL→NAcSh neural activity. We trained the CCGP classifier using data from interactions with familiar mouse 1 and novel mouse 2 on one side (e.g., F1left and N2left) during the social recognition session and tested it with data from familiar mouse 2 and novel mouse 1 on the opposite side (e.g., F2right and N1right) (Fig. 6f). The analysis was also repeated with training and testing datasets reversed, and the results were averaged. We found that IL→NAcSh neural activity showed high decoding performance for familiarity in mCherry-expressing mice even after CNO injection (Fig. 6g, i). Consistent with our behavioral data (Fig. 6d), however, vCA1 inactivation reduced the CCGP performance for decoding familiarity using IL→NAcSh neural activity in hM4Di-expressing mice (Fig. 6h, i). Moreover, after CA1 inactivation, the CCGP classifier failed to decode social targets before and after familiarization (F vs. FN, Fig. 6j). Both mCherry and hM4Di mice showed significantly higher CCGP performances for classifying littermates versus other social targets (L vs. FN, L vs. N; Fig. 6k, l). Last, to determine whether the CCGP classifier could decode familiarity even for cases in which the familiarity level differed between training and testing sets, we trained the classifier using data from interactions with a littermate and novel conspecific on one side (e.g., L1left and N2left) and then tested on data from interactions with a familiar conspecific and another novel conspecific on the opposite side (e.g., F2right and N1right). The mCherry-expressing mice exhibited a significantly higher decoding performance for familiarity, indicating that IL→NAcSh neural activity represents social familiarity regardless of the familiarity level (Fig. 6m). In hM4Di-expressing mice, however, the CCGP classifier failed to distinguish between familiar and novel mice when it was trained using data from interactions with littermates and novel mice (Fig. 6m). It is possible that the representations of IL→NAcSh neurons could produce a high performance CCGP if they encoded the passage of time across days. But when we trained and tested the CCGP with reference to time, it produced a decoding performance close to the level of chance for both mCherry- and hM4Di-expressing mice (Supplementary Fig. 20i). This demonstrates that the representation of IL→NAcSh neurons does not generalize due to the passage of time. In addition, we found CCGP decoding performance for the classification of interactions between littermates and novel mice was significantly higher than a null control when we trained and tested the decoder using data that were all acquired on the same day (Fig. 6l). Meanwhile, the performance of position-based CCGPs remained at the level of chance when comparing the neural representations of different social targets in both mCherry- and hM4Di-expressing mice (Supplementary Fig. 21). This suggests the neural representation of spatial information is not generalized in IL→NAcSh neurons. Together, these results demonstrate that inactivation of vCA1→IL neurons after familiarization prevents the formation of representations of newly familiarized conspecifics without disrupting social information previously stored in IL→NAcSh neurons.
Discussion
While the role of the hippocampus in social memory has been extensively investigated10,14,33, it has remained unclear where social memories are stored in the neocortex. In this study, we identified a subpopulation of mPFC IL neurons that stores consolidated memory of familiarized conspecifics, including littermates. The neural activity of vCA1→IL neurons during the offline phase is also critical for these cortical social memory representations in male mice. These representations support the discrimination of familiar and novel conspecifics.
Previously, we showed that mouse IL→NAcSh neurons are activated by familiar conspecifics and that their activity is required for social recognition but not object recognition26. It remains unclear, however, whether the activity of these neurons represents social memory, and if so, which aspects of social memory they encode and at which stage of social memory processing they act. We found that inactivating IL→NAcSh neurons during the recognition phase of the social familiarization/recognition task, but not the familiarization or consolidation offline phases, impairs social recognition 24 h after familiarization. This demonstrates that these neurons store long-term social memory and are required for its retrieval.
Our results are consistent with the complementary learning system model proposed by O’Reilly and colleagues, which suggests that hippocampal representations are gradually transformed into neocortical representations due to the temporal gap in learning between brain regions3,34. We found that inactivating vCA1 to IL neural projections during the offline consolidation phase impaired recognition memory of newly familiarized conspecifics without affecting memory of littermates, supporting the critical role of vCA1-IL interactions in consolidating newly established social memories. Previous studies showed that dCA2 to vCA1 projections are crucial for encoding, consolidation, and recall of social memory11. Our study extends the known dCA2-vCA1 social memory circuits by connecting vCA1 to the IL and uncovers differences in the temporal engagements and representational dynamics of social information in these brain regions. Our data further demonstrate the relatively slow learning of prefrontal subpopulations, which allows for the formation of neural representations of social targets 24 h after familiarization. We also found that optogenetic inhibition of vCA1→IL neurons during the social memory recognition session impaired recognition of newly familiarized conspecifics. This suggests that the transfer of social memories from the hippocampus to the cortex may not be completed within 24 h, as has been shown for other forms of memory2. Additionally, previous studies showed that hippocampal dCA2 activity is necessary for recognizing littermates10,11, indicating that the hippocampus may play a sustained role in social memory retrieval. It is still unclear, however, whether the hippocampus eventually disengages or remains permanently engaged in social memory retrieval.
We asked which aspects of social information are encoded by IL→NAcSh neurons, finding that their activity can simultaneously represent the identity of familiar conspecifics as well as familiarity in general by tuning the geometry of neural representations in neural activity space. Moreover, our TRAP experiment results showing that inactivation of IL→NAcSh neurons responding to a familiar mouse impaired recognition of other familiar mice (including littermates) strongly indicate that IL→NAcSh neurons encode social familiarity. This aligns with the previously established notion that learning induces a generalization within prefrontal representations with potential mixed selectivity6,35,36,37,38. Intriguingly, we also found that IL→NAcSh neuronal activity supports the decoding of littermate identity. Our decoding analyses demonstrate that non-overlapping social cells specific to different littermates are the main contributors to identity decoding, as indicated by CCGP results (Supplementary Fig. 9). These findings suggest that distinct neuronal populations selective for different familiar individuals encode social identity of familiar mice, while overlapping IL social cells represent generalized social familiarity.
Our study also highlights the distinct functional contributions of the IL→NAcSh and PL→NAc pathways in social behavior, suggesting that prefrontal cortical subregions engage in dissociable yet complementary roles. A previous study demonstrated that activation of NAcC-projecting PL neurons impaired social preference39. In addition, we previously showed that inactivation of PL→NAcSh neurons impaired both social preference and recognition26. The inactivation of IL→NAcSh neurons, however, selectively impaired social recognition, highlighting a functional distinction between NAc-projecting IL and PL neurons26. Consistent with these results, we found in this study that IL→NAcSh neuronal inactivation affected neither social preference nor total social exploration time in the social familiarization/recognition task (Fig. 1d, g).
Although we demonstrated that IL→NAcSh neurons play a critical role in social recognition memory, we also found that IL→NAcSh neurons constitute a small proportion of IL neurons (Supplementary Fig. 18). Considering previous studies showing that the IL is involved in social recognition memory40,41, it is possible that other IL subpopulations also play roles in social recognition memory. For example, Noelia and colleagues showed that corticotropin-releasing hormone released from IL neurons that project to the lateral septum (LS) modulates social interaction time with familiar social targets42. In addition, the IL shares reciprocal connections with the basolateral amygdala (BLA), which is implicated in reward-related behavior, and IL–BLA interactions were found to influence social preference43,44. Another recent study found a role for the cerebellar-IL pathway in social recognition, showing that increased cerebellar activity activated IL neurons, as evidenced by elevated c-Fos expression45. The specific roles these IL subpopulations play in social recognition memory require further investigation. While our findings underscore the functional specificity of IL→NAcSh neurons in social recognition, we cannot completely exclude the possibility that some PL neurons were included in our in vivo imaging study of the IL. To address this, we examined the locations of field of view for our miniscope imaging experiments and found that they were focused primarily within the IL (Supplementarys Figs. 4b, 20a).
The hippocampal dCA2 region is necessary for social memory across the encoding, consolidation, and retrieval phases10,11. In contrast, IL→NAcSh neurons are selectively required for social memory retrieval. We also found that vCA1→IL neurons are required for the consolidation of social memory. These findings suggest social memory processing involves multiple brain circuits, with each component playing distinct but also overlapping roles. For example, whereas dCA2 neurons respond primarily to novel social targets10,46, IL→NAcSh neurons are activated by familiar conspecifics26. Hassan et al. demonstrated that dCA2’s generalization capacity allows it to distinguish social versus non-social odor cues9, suggesting it plays a broader role in social memory encoding.
It is worth noting that dCA2 and IL neurons represent social targets in distinct ways. A recent study demonstrated that dCA2 encodes novel animals using low-dimensional representations that facilitate identity discrimination independent of spatial location, whereas it encodes littermate mice with high-dimensional representations8. Moreover, while Boyle and colleagues were able to use a CCGP classifier to successfully predict the identities and spatial positions of novel mice, its performance for littermates did not exceed the level of chance8. This finding suggests CA2 neural activity supports a generalized decoding of both identity and spatial position only in the exploration of novel mice, consistent with the hypothesis that dCA2 encodes episodic memories integrating both “what” and “where” information. In contrast, IL→NAcSh projections appear to support a generalized (abstract) representation of the identity of familiar conspecifics rather than novel mice. Notably, for these projections, we were unable to decode mouse position using a similar CCGP classifier approach, regardless of familiarity. This is again consistent with previous studies showing that the prefrontal cortex encodes memory in a more generalized manner36,47. Together, these results indicate that the IL and dCA2 process social information differently. While dCA2 specializes in novel identity–position associations, the IL preferentially represents familiar identities. Given that IL neurons receive hippocampal inputs—including from vCA1, which is itself innervated by dCA2—this connectivity may facilitate the transformation of detailed social memory traces into broader behavioral and decision-making contexts, particularly through the storage of information about familiar conspecifics in the IL11,15.
We remain curious about other brain regions and their connections that play a role in social memory processing. vCA1 projects not only to the prefrontal cortex, but also to other brain regions, including the NAc and amygdala, via its collaterals30,48. Indeed, vCA1 to NAcSh projections were found to store social memories in mice14. Interestingly, we found that vCA1 projections to the NAcSh also play a role in social memory consolidation (Fig. 5, Supplementarys Figs. 16, 17). Silencing vCA1→NAcSh neurons during consolidation disrupted memory of newly familiarized conspecifics (Supplementary Fig. 17b), like vCA1→IL inactivation (Fig. 5g). Notably, vCA1→NAcSh inactivation during the encoding phase reduced social discrimination the following day, potentially due to the reduction in social interaction times with novel conspecifics as recently reported (Supplementary Fig. 17c)31. Inactivation of vCA1→IL neurons during encoding did not, however, affect social recognition (Fig. 5g), highlighting the distinct functional contributions of these pathways. Despite these collateral projections, there may be functional distinctions between these two circuits. The vCA1→NAcSh pathway may be more critical for regulating approach decisions during motivational conflicts31, whereas vCA1→IL projections may be more specialized for modulating context-dependent affective memory48. Understanding these differential roles will be crucial for clarifying hippocampal-cortical mechanisms underlying social memory. This multi-system organization likely enhances efficiency in processing social interactions, allowing the brain to navigate complex social dynamics without overloading any single region. Future studies employing computational modeling and electrophysiological recordings will clarify how these regions interact to encode familiarity.
Changes in vCA1 neuronal ensembles supporting social memory have been reported in mouse models of autism spectrum disorder (ASD)49,50. Previously, we demonstrated that chronic social isolation during the juvenile period produces impaired social recognition in mice accompanied by a selective reduction in the excitability of IL→NAcSh neurons26. This, in combination with our current results, hints that an examination of the dynamics of neural representations of IL→NAcSh neurons in ASD mouse models may provide insight into the neural mechanisms underlying social recognition deficits in ASD.
Methods
Animals
Male C57BL/6NCrljOri mice were purchased from Orient Bio at PND (postnatal day) 35 and introduced to the animal facility at Seoul National University College of Medicine. Four male mice were allocated into each standard mouse cage. The mice were habituated in the animal facility, with some subjected to surgical procedures at PND 42, depending on experimental need.
B6.Cg-Igs7tm148.1(tetO-GCaMP6f,CAG-tTA2)Hze/J(Ai148D) mice were obtained from the Jackson Laboratory (USA, Strain #:030328) and were bred in the specific-pathogen-free (SPF) animal facility at Seoul National University College of Medicine. Homozygous mutant males were mated with wild-type C57BL/6NSNU females. Their heterozygous offspring were weaned at PND 21 before being used in experiments. Four or five mice from the same parents were allocated into a standard mouse cage and raised until PND 42. Wild-type Ai148 mice were used for miniscope recordings with chemogenetic manipulation. Mice were randomly assigned to receive viral vectors or chemical injections (DREADD agonist or vehicle).
All mice were kept under a fixed 12-h light and dark cycle (lights on: 8:00 am–8:00 pm; lights off: 8:00 pm–8:00 am the next day), while food and water were provided ad libitum. Experiments were conducted in accordance with the regulations of Seoul National University, College of Medicine, Department of Physiology and were approved by the Institutional Animal Care and Use Committee of Seoul National University (IACUC #: SNU-240103-10-1).
Surgical procedures
Subject mice were anesthetized with a mixture of Zoletil (30 mg/kg) and Rompun (10 mg/kg) via intraperitoneal (i.p.) injection of a volume that varied by subject mouse body weight. The skin above the mouse skull was gently rubbed with 70% (v/v) ethanol to sanitize it and the fur on the corresponding area was thoroughly shaved. Then, the anesthetized mouse was put into a rodent stereotaxic apparatus. The designated region of the mouse skull was drilled with a stereotaxically-fixed hand drill assembled with a 0.3-mm drill bit, and then virus was injected bilaterally.
The infralimbic cortex (AP +2.4 mm from bregma, ML ±1.0 mm from midline, DV −2.3 mm from skull; 10° angled injection to prevent virus expression in the prelimbic cortex), shell region of nucleus accumbens (AP +1.94 mm from bregma, ML ±0.55 mm from midline, DV −4.15 mm from skull), and CA1 region of ventral hippocampus (AP −3.3 mm from bregma, ML ±3.1 mm from midline, DV −3.9 mm from skull) were targeted for virus injection. For the virus injections, glass capillaries (#504949, WPI, USA) were pulled to the appropriate diameter with a Dual-Stage Glass Micropipette Puller (NARISHIGE, USA) and filled with mineral oil (M5904, Sigma-Aldrich, USA). Virus was then added to the front side of each glass capillary. All viruses were purchased from Addgene (USA) except AAV8-fosCreERT2-PEST (Stanford GVVC, USA).
For GCaMP6f expression in Ai148 mice, AAVrg-Ef1a-mCherry-IRES-Cre was injected at 5 × 1012 GC/mL (250 nL) into the NAcSh. For TRAP labeling, AAV8-fosCreERT2-PEST was injected at 1 × 1013 GC/mL (200 nL) into the IL and AAVrg-DIO-hM4Di-mCherry or AAVrg-DIO-mCherry (for control) was injected at 5 × 1012 GC/mL (200 nL) into the NAcSh. For optogenetics, AAV5-hsyn-NpHR3.0-YFP or AAV5-hsyn-eYFP (for control) was injected into the IL or vCA1 at 2.5 × 1012 GC/mL (200 nL). For chemogenetic manipulation, AAVPHP.eB-hsyn-DIO-hM4Di-mCherry or AAV8-hsyn-DIO-mCherry (for control) at 5 × 1012 GC/mL was injected into the IL (200 nL) or vCA1 (300 nL) and AAVrg-Ef1a-eGFP-IRES-Cre was injected at 5 × 1012 GC/mL (200 nL) into the NAcSh or the IL. For the collateral tracing experiments, AAV8-DIO-synaptophysin-mRuby at 5 × 1012 GC/mL (200 nL) was injected into vCA1 and the same titer and volume of AAVrg-hsyn-HI-eGFP-Cre was injected into either the IL or the NAcSh. For the vCA1-IL-NAcSh connectivity tracing experiments, AAV1-Cre at 5 × 1012 GC/mL (300 nL) was injected into vCA1, a mixture of AAV2-DIO-YFP at 1 × 1013 GC/mL (100 nL) and AAV8-fDIO-mCherry at 1 × 1013 GC/mL (100 nL) was injected into IL, and AAVrg-Flp at 5 × 1012 GC/mL (200 nL) was injected into the NAcSh. For the miniscope recordings during vCA1 chemogenetic inactivation, wild-type Ai148 mice were injected with 250 nL of a 1:1 mixture of AAVrg-Ef1a-Flpo and AAV2-flex-GCaMP6f at 5 × 1012 GC/mL into the IL. AAVrg-Ef1a-Cre was injected at 5 × 1012 GC/mL (200 nL) into the NAcSh and AAV8-hsyn-fDIO-hM4Di-mCherry or AAV8-hsyn-fDIO-mCherry (for control) was injected at 5 × 1012 GC/mL (300 nL) into vCA1. The virus was diluted with filtered ACSF to the desired titer before use. The virus was delivered to the target region of interest at a rate of 20–30 nL/min. After virus injection, the glass capillary was kept still within the brain for at least 5 min before being slowly withdrawn. The incised skin was then sutured with sterilized suture thread and treated with additional Betadine to prevent infection. The mice were then placed on a heating pad until the anesthesia wore off and returned to their home cages for further rest.
Integrated GRIN lens preparation
To observe neural activity from the pyramidal layer of the IL cortex while preventing damage to the prelimbic cortex, a GRIN lens with a 1 mm diameter and 4 mm length (Inscopix, USA) was attached to a 1 mm right-angle prism with an aluminum coat (#86-621, Edmund optics, USA) using a droplet of optical adhesive (NOA65, Edmund optics, USA). The assembly was UV-cured for at least 10 min and then immersed in 70% ethanol to sterilize it before use.
Ca2+ imaging surgery
Two weeks after virus injection, the injected mice were subjected to an additional stereotaxic surgery to prepare them for Ca2+ imaging. First, a sterilized steel screw with a 3 mm diameter was fastened to the skull prior to the craniotomy. To implant the GRIN lens, a 1.3 mm × 1.3 mm rectangular craniotomy was made above the IL viral injection site. The cortical tissue adjacent to the IL was carefully aspirated using a 28-gauge blunt needle. Cold saline was constantly applied during cortical aspiration to prevent tissue desiccation and further cell death. After aspiration of 1.5 mm of cortical tissue, a custom 1.2 mm laser-cut disposable blade was inserted into the cortex, adjacent to the IL, to create an additional 1 mm sheath for the integrated GRIN lens. After inserting the laser blade and gently moving it away from the midline, cold saline was applied to irrigate any excess bleeding. Once bleeding stopped, the integrated GRIN lens was quickly inserted to a depth of 2.5 mm. Following lens implantation, tissue adhesive (3M Vetbond) was applied to the junction between the GRIN lens and the skull, and dental cement (SUN MEDICAL, Japan) was applied in an appropriate amount to secure the lens and cover the exposed skull. After allowing at least 15 min for the dental cement to harden, a baseplate for miniscope recording was placed on top of the GRIN lens and cemented into position. A plastic dummy cap was then locked onto the baseplate to prevent photobleaching and lens damage. Dexamethasone (0.3 mg/kg) was administered during surgery and for a week post-surgery.
Electrophysiology
Electrophysiological recordings were performed as described previously26. Coronal slices of the mPFC (300 μm thick) were obtained by a vibratome (VT1200s, Leica) after isoflurane anesthesia and decapitation. Slices were cut in ice-cold cutting solution containing (in mM): 93 N-Methyl-D-glucamine (NMDG), 2.5 KCl, 10 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 30 NaHCO3, 25 glucose, 10 HEPES, 5 Na ascorbate, 2 thiourea, 3 Na pyruvate, 12 L- acetyl - cysteine, perfused with 95% O2 and 5% CO2. The slices immediately transferred to the same cutting solution at 32 °C for 10 min, and then transferred to artificial cerebrospinal fluid (ACSF) at room temperature containing (in mM): 125 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, perfused with 95% O2 and 5% CO2. For vCA1 slices (300 μm thick), ice0cold sucrose-CSF solution containing (in mM): Sucrose 230, NaCl 1.3, KCl 2.5, CaCl2 0.5, MgSO4∙7H2O 5, NaH2PO4 1.25, glucose 10, NaHCO3 25, perfused with 95% O2 and 5% CO2, was used as cutting solution before transferred to ACSF. Slices were recovered in ACSF for 1 h before the experiment. All recordings were done within 5 h from recovery. Brain slices were placed in a submerged chamber and perfused with ACSF for at least 10 min before recording. All recordings were made at 32 °C. We used recording pipettes (4–5 MΩ) filled with (in mM): 135 K-gluconate, 5 KCl, 2 NaCl, 10 HEPES, 0.1 EGTA, 5 Mg ATP, 0.4 Na3GTP and 10 Tris(di) phosphocreatine (pH 7.20 adjusted by KOH). Data were acquired using an EPC-9 patch-clamp amplifier (HEKA Elektronik) and PatchMaster software (HEKA Elektronik) with a 20 kHz sampling rate, and the signals were filtered at 2 kHz. Synaptic current data were analyzed using Mini Analysis (Synaptosoft). Excitability data were analyzed by customized LabView (National Instruments) analysis programs (https://github.com/parkgilbong/Minhee_Analysis_Pack).
For opto-EPSC recording, a terminal of optic fiber shining 10 mW 473nm-light was attached to a headstage connected to an optopatcher (A-M Systems, US), while a terminal of optic fiber shining 10 mW 594nm-light was held by 3-axis manipulator on the other side. Both lasers were connected to D/A outputs of EPC-9 patch-clamp amplifier for TTL. To validate the effect of DREADD agonists in hM4Di-expressing neurons, CNO or DCZ was diluted into the final concentration as 10 µM or 5 µM. While generating 10 action potentials with specific step current injection while whole cell recording of a fluorescent-expressing neuron (either hM4Di or control mCherry), CNO or DCZ was given to the slice to observe any change in its APs.
Cannula implantation
To induce local chemogenetic or optogenetic inactivation of neural terminals, the mice were subjected two weeks after virus injection to an additional stereotaxic surgery for target site implantation of either a drug cannula or an optic cannula. In brief, a 3 mm diameter sterilized steel screw was fastened to the skull to prevent movement artifacts. The designated region of the mouse skull was then drilled bilaterally using a stereotaxically fixed hand drill with a 0.5 mm drill bit. Either a guide cannula (#62003, RWD, USA) for micro-infusion or an optic cannula (#R-FOC-L200C-39NA, RWD, China) for local optogenetic silencing were slowly implanted at the target site using a 3D-printed cannula holder. For the micro-infusion in NAc, double guide cannula (#62025, RWD) was implanted at the designated site. After the implantation, tissue adhesive was applied to the gap between the bottom of the cannula and the skull, ensuring it did not spread to other skull holes. After waiting for 5 min to allow the adhesive to fully dry, the cannula was gently detached from the cannula holder. The other cannula was implanted in the remaining hole with a similar slow approach, and tissue adhesive was applied before the cannula holder was removed.
After cannula implantation, dental cement was applied in an appropriate amount to secure the cannula in the desired location and to cover the exposed skull. Once the dental cement had fully dried, a sterilized plastic dummy cap (#62102 or #62125, RWD) was inserted and tightly joined with each guide cannula to prevent any clogging of the guide cannula pathway during recovery for the micro-infusion. Dexamethasone (0.3 mg/kg) was administered during surgery and for three consecutive days post-surgery. Constant monitoring during recovery was necessary because mice tend to nibble on and remove the dummy cap. Dummy caps that were damaged or removed were replaced with new sterilized caps.
DREADD agonist administration
Clozapine N-oxide (CNO) dihydrochloride (#HB6149, Hellobio, UK) or deschloroclozapine (DCZ) dihydrochloride (#HB9126) were used to activate Designer Receptors Exclusively Activated by Designer Drugs (DREADDs). CNO or DCZ was dissolved in 0.9% normal saline to yield a stock concentration of 4 mg/mL and stored in a −20 °C freezer for up to 6 months. For i.p. injection, CNO was diluted with 0.9% normal saline to achieve a final concentration of 1 mg/mL and injected into subject mice at 3 mg/kg body concentration. DCZ was diluted into same final concentration with same solvent while injected into subject mice at 1 mg/kg body concentration. The same volume of saline was injected into controls. Behavioral assessments began 40 min after drug injection.
For local administration, after the subject mouse was isolated in a cage with clean bedding, 500 nL of 1 mM diluted CNO solution was infused per site over 5 min using a syringe pump (Pump11, Harvard Apparatus, USA) mounted on a 10 µL syringe (#701, Hamilton, USA). This allowed the mouse to move freely during the infusion. Then, the infusion cannula (#62203, RWD, USA) was gently removed, and the original dummy cap was tightly secured 3 min after the end of the micro-infusion to prevent drug reflux. The subject mouse was then returned to its home cage. To prevent any side effects from drug hydrolysis, at least a two-day interval was given between treatments with counterbalanced drugs.
Social target – specific engram labeling using TRAP
We employed viral-mediated Targeted Recombination in Active Population (TRAP) technology to specifically tag neurons activated during interactions with a particular social target. Experiments were carried out according to published protocols28. For TRAP tagging, subject mice were habituated to home cage isolation for 1 h each day for 5 days. On day 6, each subject mouse was isolated, and then a perforated acrylic box containing a novel social target was introduced to allow the subject to investigate the novel conspecific for 30 min. After 30 min, the target mice were returned to their home cages, while the subject mice remained isolated for an additional 30-min post learning session before being returned to their home cages.
On the day of the experiment, 4-hydroxytamoxifen (4-OHT; #H6278, Sigma-Aldrich, USA) was dissolved at 2 mg/ml with 2% tween-80 (#P1754, Sigma-Aldrich, USA) and 5% DMSO (#D2650, Sigma-Aldrich, USA) in 0.9% normal saline. The subject mouse was isolated for 30 min prior to social investigation. As on previous days, the same social target was introduced, this time without the perforated chamber. During the subsequent 10-min investigation period, subject mice that exhibited excessive aggression towards the social target were excluded from further experiments. After the 10-min investigation period, the target conspecific was removed from the cage, while the subject mouse remained for an additional hour. Following this, the subject mouse was injected with the prepared 4-OHT (body concentration: 10 mg/kg) via i.p. injection to tag neurons that had been activated during the social interaction. Two hours after the 4-OHT injection, the subject mice were returned to their home cages. Behavior assessments to induce the expression of the Fos-dependent hM4Di designer protein began one week after the TRAP tagging procedure.
Behavior tests
All behavior tests described below were performed in a soundproof chamber with dim light illumination during the light-on cycle. The order of subject mice for the behavior tests was randomized and the order of target mice was also randomized. All behavior tests were recorded via camcorder while the experimenters were blinded to the experimental conditions. Both the subject and target mice were habituated to handling prior to any behavior test. For the optogenetic inactivation of IL→NAcSh neurons or vCA1→IL neurons, the bilaterally-implanted optic fibers located above either the NAcSh or IL were connected to a laser (594 nm wavelength at 6 mW) with mating sleeves. The same experiment was repeated to assess the effect of inactivating the target circuit during the whole or only part of the behavior paradigm, as well as any effect of the laser treatment itself. The laser was administered to the subject mice in a randomly counterbalanced manner. For the social familiarization/recognition task, the same mice were used to observe the effect of neural inactivation during the social familiarization or recognition session, except for an additional batch subjected to vCA1→IL inactivation during the social recognition task (Fig. 5e and Supplementary Fig. 7c). For Ca2+ imaging, each subject mouse was fully habituated to handling and miniscope application for at least a week prior to the actual experiment. A custom LabVIEW program was used to synchronize mouse behavior (as monitored by a Basler camera) with Ca2+ activity, while using an active commutator to prevent any tension on the miniscope coaxial cable. Mouse behavior was manually analyzed by an experienced blind experimenter.
Social familiarization/recognition task
The social familiarization/recognition task was conducted over two days to examine the functional role of specific neural circuits during different stages of memory consolidation. During the social behavior test, mice were handled gently to minimize anxiety. Instead of being held by their tails, they were transferred by hand to reduce stress between test sessions.
At least two days before the task, littermates or conspecifics (6–8 weeks C57BL/6N mice with more than 10% less weight than subject mice) designated as target mice were habituated to the perforated acrylic target chamber (10 × 10 cm) for 10 min, twice per day, with a 3-h interval. This habituation period reduced unwilling anxiety of target mice.
On the test day, the subject mouse was isolated in a cage with fresh bedding for 5 min before testing. At the start of the task, the subject mouse was placed in a white opaque acrylic chamber (10 × 60 cm) for free exploration39. After a 5-min habituation period, a target mouse was introduced into the target chamber on a randomly determined side to prevent location bias. The chamber was covered with a transparent perforated lid to prevent any chance of distraction or escape. The subject and target mice interacted for 5 min before being returned to their isolation cages for a short rest. This interaction cycle was repeated twice more, with the target chamber position switched randomly each time. After three interaction sessions, the subject and target mice were returned to their original cages. After 24 h, the subject mouse underwent a social recognition test in the same linear chamber. In this test, the subject mouse was exposed to a familiar conspecific, a novel conspecific, or a littermate in a randomized order, with two 5-min intervals between sessions.
Depending on the study requirements, some subject mice were equipped with an integrated GRIN lens to monitor IL→NAcSh neuron activity using an open-source miniscope (UCLA Miniscope V4, Open Ephys, USA). If necessary, these subject mice were familiarized with two novel social targets during the social familiarization session and then allowed to interact with two familiar conspecifics, two novel conspecifics, or two littermates during the social recognition session. In addition, a DREADD agonist was administered to some mice via intraperitoneal (i.p.) injection or an infusion cannula implanted in the skull.
Consecutive littermate recognition task
The same behavior chamber from the social familiarization/recognition task was used to carry out the consecutive littermate recognition task. At least two days prior to the behavior tests, littermates to be used as target mice were placed inside a perforated chamber within the interaction chamber so they could be habituated to the interaction chamber while the subject mouse watched. On the test day, the subject mouse was isolated in a cage with fresh bedding until the behavior task. The subject was placed in the behavior chamber and allowed to freely explore. For the familiarization session, two of the subject mouse’s littermates were isolated as target mice in separate cages with fresh bedding prior to the behavior task. After 5 min of familiarization, one of the target mice was placed in the perforated acrylic chamber on either the left or the right side. After 5 min of interaction, the subject and target mice were returned to their respective isolation cages to rest. Then, the subject mouse was moved back to the linear chamber with a different target mouse in the opposite perforated chamber for a second interaction. This cycle of the subject mouse interacting with its littermates was repeated (two more littermate interactions) with a different position in the linear track compared to the initial session. After these social interactions, both the subject and target mice were returned to their original cages.
Consecutive novel mouse interaction task
The consecutive novel mouse interaction task was carried out like the consecutive littermate recognition task except for using two novel conspecifics as target mice.
Open field test
A subject mouse was placed in the center of a white opaque acrylic cylinder with a 20 cm diameter and allowed to move freely for 5 min. This was then followed by an additional 5 min of exposure to yellow light. The order of these yellow-light and no-light sessions were randomly switched to avoid artifacts.
Real-time place preference assay
A subject mouse was placed in the center of a white opaque acrylic box (33 × 33 cm) and allowed to move freely for 10 min. During this period of exploration, the location of the subject mouse was tracked live. When the subject mouse entered a randomly determined quadrant of the behavior chamber, the live-monitoring system sent an external TTL signal that activated a 594 nm yellow laser (ADR-800A, Shanghai Laser and Optics Century, China) attached to an optical stimulation cannula. This caused the targeted brain area to receive a 10 mW pulse of 594 nm yellow light when the subject mouse entered the intended quadrant area.
Novel object recognition test
The training and test sessions for the novel object recognition test were conducted on the same day. A subject mouse was placed in the center of a white opaque acrylic box (33 × 33 cm) to move freely for 10 min of familiarization. After the familiarization session, two identical objects were placed in the behavior chamber, and the subject mouse was allowed to investigate them for the duration of a 10-min training session. After the training session, the subject mouse was removed from the behavior chamber, and one of the objects was randomly replaced with a novel object. To avoid providing any scent cues, the remaining original object placed inside the test area was wiped with 70% ethanol. Immediately after object replacement, the subject mouse was reintroduced to the behavior chamber and allowed to explore for 10 min. To investigate the effect of chemogenetic inactivation, each subject mouse underwent the novel object recognition test twice with two different pairs of objects.
Immunohistochemistry
After the behavior experiments, the brain of each subject mouse was extracted following transcardial perfusion with cold PBS and then a 4% PFA solution (T&I, Korea). The perfused brains were immersed in 4% PFA and stored at 4 °C for 24 h. Then, the fixed brains were immersed in 30% (w/v) sucrose solution for 48 h at 4 °C. The brains were then frozen at −20 °C in frozen section compound (#FSC22, Leica, Germany), cut into 30-μm sections, and stored in cryopreservation solution until the next usage. To stain for cFos, brain slices were incubated for 40 min in a blocking solution containing 4% (v/v) goat serum, 0.1% (v/v) Triton X-100, and PBS. The brain slices were then incubated in primary antibody (#2250, Cell signaling Technology, USA) diluted in blocking solution at 4 °C and gently shaken at 30 RPM for the next 48 h. After that, the brain slices were incubated with Alexa Fluor 488 donkey anti-Rabbit IgG secondary antibody (#A-21206, Invitrogen, USA) diluted in blocking solution at room temperature for 4 h and then washed with PBS. A solution of 0.01% (v/v) DAPI (#62247, Thermo Scientific, USA) was used for counterstaining. Fluorescent images were obtained using an Axio Scan Z1 (Zeiss, Germany), and fluorescently labeled cells were counted manually by an experimenter blind to mouse identity. If necessary, fluorescence was measured with the software Zen (Zeiss, Germany).
Ca2+ data processing
After obtaining Ca2+ imaging video at 20–30 FPS from the open-source miniscope during rodent behavior, the videos were concatenated and converted to a TIFF format with the Image J software package. A custom-modified Constrained Nonnegative Matrix Factorization for micro-Endoscopic data (CNMF-e) was used to extract the fluorescence signals from individual regions of interests (ROIs). For each trial with a subject mouse, Ca2+ transients from the custom-modified CNMF-e were used to analyze neuronal activity with further processing. Cell activities from multiple days were registered, showing more than 80% ROI preservation without discernible GCaMP expression artifacts (Supplementarys Figs. 4c–f, 7a–d, 20a–d). Ca2+ transients from each subject mouse were synchronized with the behavior data obtained via manual scoring. The behavior data were classified with binary labels of either 0 or 1, with 0 indicating no interaction and 1 indicating social interaction. The 11 Ai148 mice in Fig. 2 exhibited 558 social cells out of a total of 1181 detected cells. In Fig. 6, the 8 mCherry-expressing mice exhibited 454 social cells out of a total of 1038 detected cells, and the 9 hM4Di-expressing mice exhibited 361 social cells out of a total of 862 detected cells.
Receiver operating characteristic (ROC) analysis
To identify whether an individual IL→NAcSh neuron is socially responsive, all Ca2+ traces were z-scored and a binary event vector was generated based on the presence (1) or absence (0) of social stimuli. The activity of each neuron based on the range of binary social stimulus thresholds was calculated for an ROC analysis51. By applying a binary threshold to the Ca2+ signal and comparing it to a binary event vector representing behavioral bouts, the detection of behavioral events based on neural activity can be summarized in a true positive rate (TRP) and a false positive rate (FPR) for individual neurons. ROC curves were then drawn based on the TRP against the FPR over the binary threshold range17. The area under each neuron’s ROC curve (auROC) was compared to 1000 auROCs obtained from randomly circular permuted Ca2+ signals of the same cell. Neurons were considered responsive “social cells” if their auROC values fell above the 95th percentile of the random distribution. The identities of these social cells were recorded and compared among different interaction sessions to define the overlapping social cell population of the behavioral paradigm.
To examine the effect of familiarization, the interaction-dependent Ca2+ AUCs of social cells responding to familiar mice were obtained during social familiarization (FN, FN’, and FN”) and recognition (F) tasks. To determine whether the reactivity of these social cells depended on familiarity, the interaction-dependent Ca2+ AUCs were calculated individually for social cells responding to familiar conspecifics (F), novel conspecifics (N), or littermates (L) during interactions with their corresponding social targets.
Population decoding analysis
The population activity decoding analysis was performed with reference to a published method8,24. A linear classifier based on a support vector machine (SVM) was used with custom MATLAB and Python scripts based on the scikit-learn SVC package for cluster visualization52.
First, the interaction times for different sessions were examined to balance the amount of Ca2+ transient data between trials. For example, if the interaction time with conspecific A lasted 30 s and the interaction time with conspecific B lasted 60 s, we used only the first 30 s worth of data from the interaction with conspecific B so that the duration would match that of the interaction with conspecific A.
We applied data expansion using pseudo-populations from the neural activity data8. By removing temporal dependencies and reducing potential cell-to-cell interaction correlations (e.g., those arising from gap junctions), this approach allowed us to focus on the relationship between social stimuli and their corresponding neural responses instead of on time-based dynamics. This is because it minimized potentially confounding session-to-session variations. Second, the neural activity recordings made during social interactions contained both stimulus-evoked responses and noise53. Assuming the noise had a mean of zero, we used random selection-based data expansion to reduce noise correlations within each session. This step ensured the noise did not artificially inflate correlations, improving the reliability of the decoding analysis54,55.
For pseudo-trials, Ca2+ transient data from the total population responding to social interaction bouts were used and divided into 200-ms time bins. Mean event activity was calculated by averaging all Ca2+ transients within each bin. 75% of the pseudo-trial data were used to train the classifier and the remaining 25% were used to test the trained classifier and assess decoding performance for each subject. Pseudo-population activity vectors were obtained from both the training and testing datasets. q randomly sampled population vectors (where q = 5 unless otherwise noted) from all neurons in the training dataset for each subject were concatenated into a single qn-long vector, where n is the total number of recorded neurons for each subject. The same procedure was repeated to construct a training dataset of pseudo-population activity vectors. For a meaningful comparison of decoding results across experiments, only subject mice that spent more than 3 s exploring in each session were included in the decoding analysis8.
To assess the statistical significance of the decoding performance, a null model was constructed in which the binary labels (0 or 1 as described above) for the pseudo-trials were randomly shuffled. The same procedures were repeated for each decoder to obtain the decoding performance with the null model.
Dimensional reduction and cluster metrics
Dimensionality was reduced to three dimensions to visualize the population vectors in 3D space as a means of improving reader comprehension of the functional representation of IL→NAcSh neurons. To visualize the pseudo-population activity vectors in a reduced-dimensional space, a principal component analysis (PCA) was performed on the training and testing datasets. Then, the resulting dimensional reduction was projected in a 3-dimensional space.
For each trial, the mean of each cluster sample (\(x\)) as the position of the corresponding centroid (\({C}_{t}\)) was defined via the following expression:
with t as the trial and S as the number of samples in the reduced dataset.
The mean distance from all samples to the centroid of each cluster was defined as the radius (\({R}_{t}\)) of the corresponding cluster and calculated via the following expression:
in which \({||}{{{\rm{x}}}}_{{{\rm{i}}}}-{{{\rm{C}}}}_{{{\rm{t}}}}{||}\) represents the Euclidean distance from the centroid to the samples.
Cross-condition generalization performance (CCGP)
CCGP was computed as described8,24. To make training and testing datasets, pseudo-population activity vectors for each trial were constructed to extract features as described above. To carry out CCGP, the features extracted from the experimental datasets were grouped into pairs for the training and testing datasets. Each training and testing dataset contained binary labels (0 or 1) corresponding to the decoding trial conditions (identity or position). Then, CCGP was calculated by averaging the decoding performances from e epochs (where e = 5 unless otherwise noted) with two randomly extracted pairs from the training and testing datasets. For the CCGP analyses (except the one assessing cross-position), the training and testing datasets were reversed, and the decoding performance was averaged. For example, identity CCGP for littermates was measured by training a linear classifier to decode the identity of two littermates on the left side (L1left and L2left) and tested on data from littermates on right side (L1right and L2right). The process was repeated by training the classifier with data from the littermates on the right side and testing it on data from the left side, with the decoding performances averaged together. The CCGP analysis for cross-familiarity was performed based on the target conspecific’s orientation. For example, CCGP was measured by training a linear classifier to decode the identity of two conspecifics on the left side (L1left and N2left) and then tested on data from different conspecifics on the right side (F2right and N1right). In addition, the decoding performance of the linear classifier was tested by training it on two conspecifics on the right side (L2right and N1right) and testing it on data from the left side (F1left and N2left). The two resulting CCGP performances were then averaged. For the cross-position analysis, CCGP was measured by training a linear classifier to decode the position of two known conspecifics (F1left and L2right) and then tested on data from two novel conspecifics (N1left and N2right).
Quantification and statistical analysis
To assess the effect of experimental manipulation, 2–3 independent batches of experiments using 8–10 mice per group were performed. Our chosen sample size as based on standard practice in similar behavioral/neuroscience studies14,21, which typically use sample sizes within this range to achieve reliable and reproducible results while minimizing animal use.
Mouse behavior and Ca2+ data were assessed via paired t-tests, one-sample t-tests, one-way and two-way repeated-measure analyses of variance (ANOVA) as appropriate and followed by the appropriate post-hoc adjustments for multiple comparisons. When analyzing the data via ANOVA, we applied the Greenhouse-Geisser correction for cases in which the Greenhouse-Geisser epsilon value fell below 0.75. Statistical analysis was carried with Prism 9 (Graphpad Software Inc, USA). Detailed descriptions and results of the statistical analyses are provided in Supplementary Table 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All raw data and reagents are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
All software or algorithms used in this study are available and described in the methods section. Custom Matlab and Python scripts for LSVM and CCGP classifiers used in this manuscript are available on Zenodo (https://doi.org/10.5281/zenodo.16592402).
References
Frankland, P. W. & Bontempi, B. The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 (2005).
Kitamura, T. et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017).
Norman, K. A. & O’Reilly, R. C. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychol. Rev. 110, 611–646 (2003).
Yonelinas, A. P. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 46, 441–517 (2002).
Nadel, L. & Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 7, 217–227 (1997).
Preston, A. R. & Eichenbaum, H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 23, R764–R773 (2013).
Mandler, G. Recognizing - the judgment of previous occurrence. Psychol. Rev. 87, 252–271 (1980).
Boyle, L. M., Posani, L., Irfan, S., Siegelbaum, S. A. & Fusi, S. Tuned geometries of hippocampal representations meet the computational demands of social memory. Neuron 112, 1358–1371.e9 (2024).
Hassan, S. I., Bigler, S. & Siegelbaum, S. A. Social odor discrimination and its enhancement by associative learning in the hippocampal CA2 region. Neuron 111, 2232–2246.e2235 (2023).
Hitti, F. L. & Siegelbaum, S. A. The hippocampal CA2 region is essential for social memory. Nature 508, 88–92 (2014).
Meira, T. et al. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun. 9, 4163 (2018).
Lopez-Rojas, J., de Solis, C. A., Leroy, F., Kandel, E. R. & Siegelbaum, S. A. A direct lateral entorhinal cortex to hippocampal CA2 circuit conveys social information required for social memory. Neuron 110, 1559–1572.e1554 (2022).
Okuyama, T. Social memory engram in the hippocampus. Neurosci. Res. 129, 17–23 (2018).
Okuyama, T., Kitamura, T., Roy, D. S., Itohara, S. & Tonegawa, S. Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016).
Phillips, M. L., Robinson, H. A. & Pozzo-Miller, L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. eLife 8, e44182 (2019).
Sun, Q. et al. Ventral hippocampal-prefrontal interaction affects social behavior via parvalbumin positive neurons in the medial prefrontal cortex. iScience 23, 100894 (2020).
Kingsbury, L. et al. Cortical representations of conspecific sex shape social behavior. Neuron 107, 941–953.e947 (2020).
Zhou, T. et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 357, 162–168 (2017).
Padilla-Coreano, N. et al. Cortical ensembles orchestrate social competition through hypothalamic outputs. Nature 603, 667–671 (2022).
Levy, D. R. et al. Dynamics of social representation in the mouse prefrontal cortex. Nat. Neurosci. 22, 2013–2022 (2019).
Xu, P. et al. Pattern decorrelation in the mouse medial prefrontal cortex enables social preference and requires MeCP2. Nat. Commun. 13, 3899 (2022).
Kim, J., Jung, M. W. & Lee, D. Reward learning improves social signal processing in autism model mice. Cell Rep. 42, 113228 (2023).
Bartolo, R., Saunders, R. C., Mitz, A. R. & Averbeck, B. B. Dimensionality, information and learning in prefrontal cortex. PLoS Comput. Biol. 16, e1007514 (2020).
Bernardi, S. et al. The geometry of abstraction in the hippocampus and prefrontal cortex. Cell 183, 954–967.e921 (2020).
Rigotti, M. et al. The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590 (2013).
Park, G. et al. Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice. Cell Rep. 35, 109104 (2021).
Park, G. & Lee, Y.-S. In Learning and Memory: A Comprehensive Reference (Third Edition) Ch. 2.31 (Academic Press, 2025).
Giannotti, G., Heinsbroek, J. A., Yue, A. J., Deisseroth, K. & Peters, J. Prefrontal cortex neuronal ensembles encoding fear drive fear expression during long-term memory retrieval. Sci. Rep. 9, 10709 (2019).
Liu, X. & Carter, A. G. Ventral hippocampal inputs preferentially drive corticocortical neurons in the infralimbic prefrontal cortex. J. Neurosci. 38, 7351 (2018).
Ciocchi, S., Passecker, J., Malagon-Vina, H., Mikus, N. & Klausberger, T. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348, 560–563 (2015).
Patterson, D. et al. Ventral hippocampus to nucleus accumbens shell circuit regulates approach decisions during motivational conflict. PLoS Biol. 23, e3002722 (2025).
Goto, A. et al. Stepwise synaptic plasticity events drive the early phase of memory consolidation. Science 374, 857–863 (2021).
Kong, E., Lee, K.-H., Do, J., Kim, P. & Lee, D. Dynamic and stable hippocampal representations of social identity and reward expectation support associative social memory in male mice. Nat. Commun. 14, 2597 (2023).
McClelland, J. L., McNaughton, B. L. & O’Reilly, R. C. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457 (1995).
Sun, W., Advani, M., Spruston, N., Saxe, A. & Fitzgerald, J. E. Organizing memories for generalization in complementary learning systems. Nat. Neurosci. 26, 1438–1448 (2023).
Tang, W., Shin, J. D. & Jadhav, S. P. Geometric transformation of cognitive maps for generalization across hippocampal-prefrontal circuits. Cell Rep. 42, 112246 (2023).
Tye, K. M. et al. Mixed selectivity: cellular computations for complexity. Neuron 112, 2289–2303 (2024).
Tse, D. et al. Schemas and memory consolidation. Science 316, 76–82 (2007).
Murugan, M. et al. Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171, 1663–1677.e1616 (2017).
Lüscher Dias, T., Fernandes Golino, H., Oliveira, V. E. M. D., Dutra Moraes, M. F. & Schenatto Pereira, G. c-Fos expression predicts long-term social memory retrieval in mice. Behav. Brain Res. 313, 260–271 (2016).
Tanimizu, T. et al. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J. Neurosci. 37, 4103–4116 (2017).
de León Reyes, N. S. et al. Corticotropin-releasing hormone signaling from prefrontal cortex to lateral septum suppresses interaction with familiar mice. Cell 186, 4152–4171.e31 (2023).
Huang, W.-C., Zucca, A., Levy, J. & Page, D. T. Social behavior is modulated by valence-encoding mPFC-amygdala sub-circuitry. Cell Rep. 32, 107899 (2020).
Yu, H. et al. Infralimbic medial prefrontal cortex signalling to calbindin 1 positive neurons in posterior basolateral amygdala suppresses anxiety- and depression-like behaviours. Nat. Commun. 13, 5462 (2022).
Chao, O. Y. et al. Social memory deficit caused by dysregulation of the cerebellar vermis. Nat. Commun. 14, 6007 (2023).
Donegan, M. L. et al. Coding of social novelty in the hippocampal CA2 region and its disruption and rescue in a 22q11.2 microdeletion mouse model. Nat. Neurosci. 23, 1365–1375 (2020).
Sekeres, M. J., Winocur, G. & Moscovitch, M. The hippocampus and related neocortical structures in memory transformation. Neurosci. Lett. 680, 39–53 (2018).
Kim, W. B. & Cho, J.-H. Synaptic targeting of double-projecting ventral CA1 hippocampal neurons to the medial prefrontal cortex and basal amygdala. J. Neurosci. 37, 4868 (2017).
Tao, K. et al. Disrupted social memory ensembles in the ventral hippocampus underlie social amnesia in autism-associated Shank3 mutant mice. Mol. Psychiatry 27, 2095–2105 (2022).
Piskorowski, R. A. et al. Age-dependent specific changes in area CA2 of the hippocampus and social memory deficit in a mouse model of the 22q11.2 deletion syndrome. Neuron 89, 163–176 (2016).
Kingsbury, L. et al. Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell 178, 429–446.e416 (2019).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Averbeck, B. B., Sohn, J.-W. & Lee, D. Activity in prefrontal cortex during dynamic selection of action sequences. Nat. Neurosci. 9, 276–282 (2006).
Courellis, H. S. et al. Abstract representations emerge in human hippocampal neurons during inference. Nature 632, 841–849 (2024).
Meyers, E. M., Freedman, D. J., Kreiman, G., Miller, E. K. & Poggio, T. Dynamic population coding of category information in inferior temporal and prefrontal cortex. J. Neurophysiol. 100, 1407–1419 (2008).
Acknowledgements
This work was supported by grants to Y.-S.L. (RS-2023-NR076980 and RS-2025-02303740), S.J.K. (NRF-2018R1A5A202596423), and G.P. (RS-2023-00271950) from the National Research Foundation of Korea, and to D.L. (IBS-R001-D2) from the Institute for Basic Science of Korea. This research was also supported by KBRI basic research program through Korea Brain Research Institute funded by Ministry of Science and ICT (25-BR-07-01). The authors thank Min-Gyun Kim, and Yeji Song for their assistance with rodent stereotaxic surgeries and behavioral analyses, and Dr. Sung-Phil Kim, Dr. Paul Frankland, Dr. Joseph A. Gogos, Dr. Steven Kushner and all lab members for their valuable comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Y.-S.L. and G.P. conceived the study and designed the experiments with contributions from S.J.K. and D.L. G.P. performed most of the experiments. G.P. performed most of the data analyses with advice from M.S.K., Y.-B.L., D.L., and Y.-S.L. S.S. wrote the code for miniscope data acquisition. G.P., D.L., and Y.-S.L. wrote the manuscript with input from M.S.K., T.K., Y.-B.L., and S.J.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Takashi Kitamura, Alfred Robison and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, G., Kim, M.S., Lee, YB. et al. Hippocampal-cortical interactions in the consolidation of social memory. Nat Commun 16, 8430 (2025). https://doi.org/10.1038/s41467-025-64264-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-64264-7