Abstract
Neuromorphic engineering, originally focused on replicating the biophysics of neurons and synapses in hardware, has progressively expanded to explore novel computational principles, materials, and applications. With their unique ability to emulate brain functions, neuromorphic devices are emerging as prime candidates to advance the treatment of brain disorders, addressing the current limitations of electroceutical-based strategies, particularly their lack of flexibility and personalization. In this Perspective, we introduce and elaborate on the concept of the ‘Neuromorphic Twin’ and explain why this emerging technology is both timely and relevant. By integrating Digital Twin approaches for modelling the brain’s functions with neuromorphic engineering, Neuromorphic Twins offer the potential to address major challenges, such as dealing with brain complexity in real-time, enabling adaptive and personalized interventions, and tracking the progression of neurological diseases over time. Moreover, they can be embedded in low-power devices, thus marking a transformative shift in biomedical interventions and promising to open new frontiers for neuroengineering and brain repair.
Similar content being viewed by others
Introduction
Brain disorders and their debilitating effects on patients have emerged as one of the most significant public health challenges of the 21st century, and the situation will worsen in the years to come due to the ageing of the population1. In response to this pressing issue, the scientific and medical community has been actively explored neuroengineering-based alternatives, often centered on electroceutical approaches2 to treat neurological diseases or their symptoms, support rehabilitation, reduce healthcare costs, and, most importantly, improve patients’ quality of life. Significant progress has been made in the last decades, resulting in neurotechnologies such as Brain Machine/Computer Interfaces (i.e., BCI for simplicity), brain modulators and neuroprostheses able to replace and retrain either brain3,4 or somatosensory functions5,6,7, block seizures in epilepsy8 and relieve symptoms in neurodegenerative diseases, such as Parkinson’s9,10. Interestingly, implanted neuroprostheses, at both preclinical and clinical levels, have proven to be a promising tool for neurorehabilitation post brain11 or spinal cord injury12,13. A different type of neuroprostheses (i.e., cognitive neuroprostheses), aimed at replacing the function of damaged brain circuits in the hippocampus, have been tested in vivo14,15,16 and in humans17 for memory restoration. Recently, commercial initiatives such as Neuralink, founded by E. Musk (https://neuralink.com/), have also announced the implantation of BCI devices in humans, although detailed information on their intended clinical applications and scientific validation remains still limited.
Even if important results have been achieved so far, current neuroengineering solutions still present drawbacks, including lack of flexibility and personalization, and difficulty in tracking both disease progression and/or possible side effects of the therapeutic treatment. Indeed, state-of-the-art electroceutical strategies are associated with stimulation parameters that may change over time due to the physiological process of development or ageing and with the progression of the disease, potentially resulting in a decline in stimulation effectiveness and thus requiring several sessions of manual reprogramming18.
Digital Twin technology has recently emerged as a promising and powerful solution to tackle the above limitations19. By creating dynamic replicas, these in silico models of the brain (i.e. Virtual Brain Twins) can offer adaptable and personalized support for patients20,21, taking into account the multiscale nature of the brain22. Nevertheless, they require the collection of several data types, such as MRI and electrophysiology, and their use is currently limited to specific medical decision-making and actions, such as identifying the best location for surgery in drug-resistant epilepsy23,24 or the optimal stimulus location for Parkinson’s disease25. Importantly, Virtual Brain Twins developed so far, although dynamic and capable of simulating complex neural processes, differ from classical Digital Twin technology26,27,28, as they do not enable the bi-directional communication in real-time with their physical counterparts. Instead, they function as powerful predictive tools to simulate disease progression and treatment effects, possibly supporting in silico experimentation and virtual clinical trials29,30. Therefore, while valuable, this modeling approach does not provide the continuous, adaptive interaction required for treating chronic brain injuries or neurodegenerative diseases, highlighting the need for a new class of Digital Twins that can interactively co-evolve with the brain over time.
In this Perspective, we introduce a still nascent but potentially revolutionary technology named ‘Neuromorphic Twin’, which aims to overcome the above-mentioned limitations. The Neuromorphic Twin is a digital model designed to emulate, interact with, and adaptively co-evolve alongside a biological neural network. The biomimetic approach is central for the emulation component because the Neuromorphic Twin requires mechanistic fidelity that brain-inspired abstractions cannot provide (cf. Box 1 for more details). It integrates real-time neuromorphic hardware with adaptive software components to enable continuous bi-directional communication and long-term personalization. Its main features are: (i) real-time, bi-directional coupling with the biological system, enabling continuous information exchange; (ii) a hardware-level biomimetic emulation of neuronal and synaptic activity across neurons, synapses, and network scales; (iii) a software layer for non-real-time personalization, which periodically tunes the network topology and parameters to maintain alignment with the evolving biological counterpart.
Neuromorphic engineering was initially conceived as an interdisciplinary field, aiming at building hardware systems by mimicking the intricate biophysics of real neurons and synapses31. Recent advancements have expanded its horizons, exploring novel computational principles, materials, and applications32,33,34,35,36,37. With their unique ability to emulate even complex brain functions, neuromorphic devices emerge as the prime candidates for the realization of physical (i.e., hardware) neural digital twins. The term Neuromorphic Twin is not entirely new, as it has been recently used in contexts such as system and communication engineering38,39. Within the field of neuroengineering, the neuromorphic components and means to create the Neuromorphic Twins are partially in place40,41,42,43 and some ideas have been introduced, even mentioning the term itself44,45. However, the holistic integration and vision that would bring the Neuromorphic Twins to fruition within the neuroengineering context is currently lacking. Indeed, only a few concrete examples exist to date46,47,48,49. Inspired by the use of neuromorphic technology for biomimetic stimulation to restore sensations49, we foresee that a similar strategy could be expanded to create Neuromorphic Twins capable of restoring neural functions to a broader extent. This technology can also benefit from Artificial Intelligence (AI), which would form the software core of the twin, allowing it to adaptively track and co-evolve with the brain’s dynamics over extended timescales. This is the central aim of this Perspective, which is organized as follows. First, we describe the hardware and software architecture of the Neuromorphic Twin, emphasizing the critical real-time bi-directional communication between the Neuromorphic Twin and the brain network(s). The closed-loop interaction with the living brain, mirroring the way industrial digital twins continuously communicate with their physical counterparts, is a key aspect of the Neuromorphic Twin. Moreover, the tight integration of software and hardware components is fundamental to its functioning, enabling it to operate as a dynamic extension of the brain. Therefore, we present applications for this technology, focusing on its potential impact on healthcare and on the development of biohybrid intelligence, which could represent a crucial driver for the next generation of AI. Finally, we propose a roadmap for the adoption of the Neuromorphic Twin technology, outlining future challenges, a prospective timeline, and ethical considerations.
We are confident that this technology has the potential to advance biomedical interventions by driving a global therapeutic shift in the treatment of neural disorders, thereby representing a transformative leap forward for the field of Neuroengineering.
Architecture of the Neuromorphic Twin
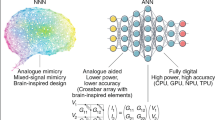
The Neuromorphic Twin relies on an architecture composed of three main elements (Fig. 1), two implemented in hardware and one in software. In this section, we first focus on the hardware for neural feature extraction (cf., “Hardware-based signal processing”), we then describe the hardware biomimetic network component (cf., “Hardware biomimetic network”), we present the software module for automatic tuning of the network parameters (cf., “Software automatic tuning”) and finally we describe the interaction among all these elements (cf., “Communication between interconnected layers”).
Neural recording. Electrodes from an implanted multichannel probe capture single neuron activity across one or more brain regions. The acquired analog signals constitute the input for the Neuromorphic Twin. Neuromorphic Twin. The Neuromorphic Twin consists of three main blocks. Starting from the neural measurements coming from the implanted brain, all feature extractions (e.g., sorted spikes) from the recording channels are performed by the signal-processing hardware (typically, Spiking Neural Network (SNN)-based) and then sent as input to both the biomimetic-network hardware and the software for automatic tuning blocks. The biomimetic network, implemented as an SNN and configured offline, emulates the brain network(s) and reproduces the neuronal activity at the single neuron level, operating in real time to maintain a closed-loop interaction with the biological brain. To personalize and accurately replicate the biological component, the SNN output is also fed into the automatic-tuning software block, which uses ANN (Artificial Neural Network)/SNN-based optimization to adjust the model parameters and plasticity rules online, enabling adaptive tuning in response to the current state of the brain. This update does not need to operate in real-time; a delay of minutes or even hours/days is sufficient for rewiring the biomimetic SNN’s network and the topology. Adaptive Stimulation. The digital output of the Neuromorphic Twin, directly coming from the biomimetic SNN, consists of a train of events through which adaptive, personalized stimulation is generated to treat the impaired brain network(s).
Hardware-based signal processing
A key feature of the Neuromorphic Twins lies in their ability to process and decode neural signals in real-time, enabling feedback that can modulate or replace neural functions within a closed-loop architecture36. This process poses several technological challenges, particularly the requirement for rapid and complex computation of extensive data, along with the extraction of meaningful features for stimulation control50. Indeed, current acquisition systems allow to record from a large number of electrodes (Fig. 1, Neural Recording block), i.e., hundreds up to thousands at the same time, both in vivo51,52,53 and in humans54,55. To tackle these challenges, the integration of neuromorphic-based systems for edge computing functions, such as detection and classification of neural events, can greatly enhance the overall performance of the Neuromorphic Twins (Fig. 1, Neuromorphic Twin–Hardware for signal processing).
To date, real-time neural processing remains limited by the lack of fully unsupervised algorithms capable of handling large volumes of neural data autonomously. While Deep Learning (DL) has shown impressive performance in pattern recognition tasks with large labeled datasets, its applicability in real-time, closed-loop neural interfaces is hindered by its dependence on labeled data and high computational demands, often requiring specialized hardware such as GPUs. These constraints make DL unsuitable for closed-loop neuro-engineering applications, where rapid calibration (within minutes) and millisecond-level adaptive stimulation are essential. In contrast, Spiking Neural Networks (SNNs) offer a compelling alternative. Their event-based processing makes them particularly well-suited for real-time analysis of neuronal signals. SNNs rely on biologically inspired plasticity rules like Spike-Timing-Dependent Plasticity (STDP) and often require fewer parameters and less data to train than conventional DL models. Several neuromorphic circuit architectures have already been developed to extract specific features of interest directly from neural signals. These circuits operate at the hardware level to perform low-latency, energy-efficient processing by mimicking the biophysics of neural computation. For instance, implementations of circuits such as event-driven edge detectors, adaptive threshold spike detectors, or temporal correlation filters have been used for real-time spike detection, burst classification in multi-electrode recordings56. Recent studies have demonstrated the efficacy of SNNs in real-time detection and classification of neural activity57,58,59,60,61, showing their potential to autonomously self-configure using local learning rules to recognize hidden patterns in data. Table 1 reports the various applications of SNNs for detecting and classifying different forms of biological events, such as spikes, spike trains, Local Field Potentials (LFPs), and even Electrocorticogram (ECoG) and Electroencephalography (EEG) signals.
Despite their promise, practical implementations of neuromorphic systems (Table 1), especially SNNs, face challenges in scaling to large numbers of parallel channels. Current implementations often remain limited to a small number of channels62,63. However, the coupling of analog-mixed signal (AMS) circuits with the high parallelism of Field Programmable Gate Arrays (FPGAs) offers a promising path forward. Particularly, FPGA-based System-on-Chip (SoC) architecture combining real-time hardware processing with Linux-based software environments provide an adaptable platform capable of real-time tuning for the Neuromorphic Twin. These advances not only enable detection but also allow sophisticated classification of spatiotemporal patterns, such as spike sorting64, vocal65 or neuropathological66 patterns (e.g., epileptic seizures), which are crucial for understanding neural dynamics and guiding the Neuromorphic Twin’s response.
For example, in spike sorting, a fundamental task in neural signal processing aimed at identifying the actual sources of the acquired neuronal signals, SNNs can detect and classify spikes from raw extracellular recordings. A simple SNN architecture67, comprising layers for signal encoding, pattern detection, and classification, uses STDP and low-threshold spiking (LTS) neurons to learn and recognize spike patterns efficiently (Fig. 2). This architecture processes raw input without requiring precise spike timing, ensuring no events are missed, and achieves real-time classification across multiple channels using only minimal FPGA resources (e.g., <6% LUT and <15% URAM on the Kria KR260 SoC). Unlike traditional spike sorting pipelines, which cannot scale efficiently to multi-channel real-time processing, this SNN-based approach provides a lightweight, parallelizable, and energy-efficient solution—ideal for embedded neuromorphic applications such as the Neuromorphic Twin. Still, challenges remain in scaling these models further while maintaining low power consumption and unsupervised learning capabilities, particularly for complex time-varying pattern extraction.
Hardware biomimetic network
Choice of neural network, synapse, and neuron models
To emulate neural dynamics in the brain, several neuroscience-inspired computational structures can be used. Winner-Take-All (WTA) circuits with lateral inhibition capture aspects of sensory cortex competition68; canonical microcircuits simplify the organization of various cortical regions69,70, Central Pattern Generators (CPGs)71, and Kuramoto oscillators72 model rhythmic or oscillatory behavior; and Liquid State Machines (LSMs) reproduce recurrent cortical dynamics73. These models are attractive because they allow fast implementation and tuning on microcontrollers.
For the initial biomimetic implementation of the Neuromorphic Twin, Artificial Neural Networks (ANNs) can serve as a practical starting point. ANNs provide an efficient computational framework capable of reproducing certain aspects of neural processing. They are widely used for tasks such as image recognition, classification, or facial identification through Feedforward Neural Networks (FNNs)74 or Convolutional Neural Networks (CNNs)75. For example, Kheradpisheh et al.76 demonstrated STDP-based CNNs for object recognition. Recurrent Neural Networks (RNNs)77, on the other hand, support applications involving sequential data, such as speech recognition or time-series prediction. However, despite these advances78, traditional ANNs remain fundamentally limited for biologically realistic modeling. They are optimized for specific computational tasks rather than for reproducing the fine-grained temporal and biophysical dynamics of real neurons. In particular, they do not naturally encode or exploit temporal information, which is essential for implementing biologically plausible unsupervised learning rules. These limitations have motivated the development of Spiking Neural Networks (SNNs), which emulate neuronal communication through discrete action potentials79. By capturing both spatial and temporal aspects of neural activity, SNNs provide a more faithful representation of biological neuronal networks than conventional ANNs. This is achieved through sophisticated neural connectivity patterns and plasticity mechanisms that reflect the brain’s natural processes. Unsupervised learning remains one of the most biologically plausible and computationally efficient strategies for training SNNs, making it particularly well-suited for neuromorphic systems. Among the most prominent mechanisms is STDP, a local rule that adjusts synaptic weights based solely on the relative timing of spikes, enabling networks to autonomously discover and encode meaningful spatiotemporal patterns in the input data. Such learning rules operate without the need for labeled datasets or global error signals, aligning naturally with the architecture and operational constraints of neuromorphic hardware. As highlighted in these review articles80,81, unsupervised plasticity mechanisms enable continual learning, adaptability, and low-power consumption in embedded systems.
A table comparing the advantages and disadvantages of both ANNs and SNNs, and the best choice for each criterion for the Neuromorphic Twin technology, is reported below (cf. Table 2). Table 2 highlights that, aside from learning and implementation criteria, SNNs emerge as the most suitable candidate for biomimetic emulation. While ANNs currently have an advantage in terms of learning algorithms and ease of implementation, this gap is expected to narrow over time as SNN frameworks continue to evolve.
In terms of emulation of biological networks, SNNs can operate in real-time, providing a sufficiently high level of biological coherence to accurately depict and mimic the dynamics of biological networks82. They are able to autonomously evolve through learning and plasticity rules83,84, similar to how the brain learns through experience. This is achieved through specific rules that allow the network to change its structure or function based on what it learns. Because of this, SNNs can improve their performance on their own, without needing to be reprogrammed. SNNs can also model different types of neurons and their interactions, which is important for accurately simulating the complex dynamics of the brain, where different neurons have specialized roles. Additionally, SNNs are good at capturing both where neurons are located and how they connect (spatial organization), as well as the timing of their signals (temporal organization). This makes them particularly useful for tasks that involve understanding patterns over time and space. Hardware SNNs employ generic biological parameters for updating the neural model and aim at emulating biological neural networks as accurately as possible in experiments. The use of complex models that closely mimic biology and have the same parameters allows for easier communication and interactions with neuroscientists. In particular, conductance-based models (see Table 3) such as the Hodgkin-Huxley type allow for precise identification of neuron parameters like the equilibrium voltage by biologists, thereby facilitating smooth interdisciplinary communication between designers and biologists. Furthermore, for emulation purposes and especially for reproducing neurological diseases, conductance-based models are essential, as certain disorders, such as ALS, involve dysfunctions at the level of ionic currents. Finally, since our goal is to replicate brain function, the backpropagation plasticity rule is not compatible with biological reality. We therefore turn to biologically plausible forms of plasticity, such as STDP or Hebbian learning.
The choice then remains between single or multi-compartment models to reproduce the topology and the various interactions between neurons. Single-compartment models, such as the Hodgkin-Huxley model85, allow for high prediction rates of action potentials and biophysical coherence86. Several cortical simulations exist, particularly using specialized software such as NEURON and NEST. However, real-time execution is generally not achieved, except in rare cases using huge GPU system87 or supercomputers88, which are incompatible with the Neuromorphic Twin approach in terms of cost and embedded system.
Multicompartmental models offer a more comprehensive and biologically realistic approach, thus providing deeper insights into neuronal function and information processing. It is particularly important as regions such as dendrites are the center of vital computations linked to their spatial morphology89 and are affected by some neurodegenerative diseases like Amyotrophic Lateral Sclerosis -ALS90. Multicompartmental modeling also allows the investigation of the role of dendrites in neurons. They are also known to display physiological and morphological abnormalities during postnatal development in motor neurons with ALS91. However, there are still few real-time multicompartmental neuron implementations in the state of the art92.
Hardware implementation
Several commercial neuromorphic systems have emerged in the recent years. Neuromorphic chips, such as Intel’s Loihi and IBM’s TrueNorth93, are designed to execute SNNs more efficiently than traditional processors by mimicking neuronal and synaptic structures. However, these chips were primarily developed for SNN-based processing rather than for reproducing and emulating biological systems. Notably, they lack support for complex neuronal models. The most well-known hardware systems are SpiNNaker, a digital hardware platform, and BrainScaleS, an analog hardware platform, both developed during the Human Brain Project. Some real-time implementations have successfully replicated cortical models94,95,96. Recent articles show some interesting results in real-time emulation of biological networks. The Neurogrid system, which is a hybrid analog-digital architecture that simulates various cortical cell types and dentritic effects97,98. BioemuS system99 emulates with high precision 1000 HH neurons and millions of synapses for brain organoid emulation. DYNAP-SE2100 implements 1024 AdEx I&F neurons for part of somatosensory cortex.
Several technologies can be used to implement these SNNs in hardware. They can be analog, digital, or a combination of both. However, emerging technologies could be the next target, particularly for synapses and plasticity. Indeed, spintronic101,102 and memristor103,104 technologies enable reduced power consumption and a smaller implementation footprint. Moreover, these systems inherently integrate both synapses and plasticity. However, these technologies are not yet mature enough for easy integration with analog or digital neurons. The challenge with these models remains their implementation, which requires significant hardware resources while maintaining real-time computation. The substantial number of parameters that require tuning also poses a problem. Leveraging AI to efficiently explore the parameter space can greatly accelerate the discovery of optimal network parameters that accurately replicate biological dynamics. Consequently, the Neuromorphic Twin and the biological network will establish a connection facilitating mutual adaptation, learning, and self-correction (Fig. 1 - Software automatic Tuning and section “Hardware biomimetic network”). Being intrinsically adaptive, the Neuromorphic Twin can thus naturally follow the evolution of the biological neural network and take the corrective action to counterbalance disease progression, by delivering the electrical therapy with the updated stimulation parameters.
Software automatic tuning
To design the Neuromorphic Twin, the biomimetic SNN must be able to adapt over time. The objective is therefore to continuously adjust the network’s dynamics based on biological recordings. To achieve this, several optimization algorithms can be used, including metaheuristic algorithms, Bayesian optimization, and gradient descent. Table 4 presents the advantages and disadvantages of the different possible methods.
The objective is to readjust the dynamics in case of a significant change in the biological network, while not knowing the future dynamics in advance. Table 4 shows that optimizing the parameters of an SNN using evolutionary algorithms is an effective approach to reproducing the spike frequencies of a biological network. Among these algorithms, Particle Swarm Optimization (PSO) is particularly well-suited for small-scale SNNs, as is the case for the Neuromorphic Twin, offering fast convergence and easy embedded implementation, although it may be sensitive to local optima. For more complex models, Evolution Strategies (ES) provide a robust alternative, capable of handling a large number of parameters and well-suited for execution on GPUs, TPUs, or FPGAs. On the other hand, Differential Evolution (DE) is useful for exploring complex parameter spaces, but it converges more slowly. However, the resources required for implementation and the simulation time are significant. Thus, it is necessary to carefully consider which software/hardware platform and programming language will be most effective.
For software implementation, frameworks such as PyTorch105 (used via Norse or Tonic) and SpikingJelly106 are well-suited for SNN optimization, because they support GPU-based parallel computation and biomimetic learning algorithms such as STDP. In contrast, PyNN107 is mainly designed for simulating biologically realistic SNNs on platforms such as NEST and NEURON, making it less relevant for our Neuromorphic Twin application.
As previously discussed, the Neuromorphic Twin already includes SNNs for signal processing and the replication of biological networks. The choice of hardware for implementing these algorithms can be based on: (i) FPGAs, which offer high performance but require a very long development time; (ii) GPUs or AI SoCs, which provide more flexible software execution but are less optimized for low latencies and get less I/Os; (iii) neuromorphic chips like Loihi108 and Akida109, which are well-suited for SNNs with bio-inspired learning rules, although native support for ES or PSO is limited. A hybrid approach would involve training the SNN with PSO or ES in PyTorch or SpikingJelly on a GPU, then exporting it to an FPGA or neuromorphic chip for embedded execution. This solution combines efficient optimization and embedded system, making it ideal for integration into a biological setup. Recent articles are using optimization algorithms based on DE110,111 or Bayesian optimization112 implemented on GPU for the hyperparameter optimization of SNN. Ethernet or SPI communication can be used from the GPU to the SoC FPGA device.
Communication between interconnected layers
In the previous sections, we introduced the three interconnected layers of the Neuromorphic Twin (Fig. 1): the signal processing module (cf. “Hardware biomimetic network”), the emulation module (cf. “Hardware biomimetic network”), and the optimization layer for parameter tuning (cf. “Software automatic tuning”).
The signal processing block acts as an edge-level preprocessor (edge computing), implemented on FPGA-SoC platforms or through mixed-signal analog–digital neuromorphic circuits (Fig. 1, green box). It runs algorithms for neural signal feature extraction, such as spike detection, burst classification, and spectral estimation, in real time, achieving latencies on the order of 1 ms for event detection and ~10 ms for classification. The extracted features are transmitted directly to the emulation module (Fig. 1, blue box), which hosts the biomimetic SNN hardware reproducing the dynamics of biological neural networks in real time (approximately 100 µs to emulate synaptic and ionic current dynamics). Using SNN-based architectures for both the signal processing and the emulation layers (although relying on different neuronal and synaptic models) offers the advantage of co-implementing them on the same hardware platform, thereby simplifying communication protocols, reducing latency, and enabling fully embedded operations. The two hardware blocks are functionally coupled through a software optimization layer (Fig. 1, black box), based on either ANN or SNN architecture, which adaptively tunes neuronal and synaptic parameters. The latency associated with this update does not need to operate in real time; update cycles on the order of minutes/hours are sufficient since neuronal dynamic changes occur over slow timescales. Usual communications such as ZeroMQ can be used as the usual latency is around 300 µs, large enough for the update timing.
This three-tier co-design ensures continuous adaptation of emulation fidelity while maintaining high energy efficiency. From a system-level perspective, this hardware–software co-optimization defines the operational metrics of the Neuromorphic Twin. Real-time performance and latency are primarily governed by the hardware interface between the signal processing unit and the SNN core, where analog front-ends enable fast feature encoding, while FPGA or neuromorphic ASICs handle large-scale parallel computation. High-bandwidth interconnects, such as AXI buses between microcontrollers and FPGAs or Ethernet links between recording and stimulation systems, can be leveraged to sustain low-latency real-time communication (range of 40 µs for an AXI DMA in loopback clocked at 200 MHz113).
Energy efficiency and scalability depend on the hardware technology choice: hybrid analog–digital architectures typically achieve one to two orders of magnitude higher energy efficiency than GPU-based simulations. For reference, the average power consumption of SNNs on FPGA is in the range of 3 W for the emulation part on a KR260 kit99. Therefore, the selection of the FPGA target defines the trade-off between energy efficiency, flexibility, and embedded capability. A future ASIC can be designed to optimized performances.
Overall, the Neuromorphic Twin demonstrates how hardware–software co-optimization can bridge low-level signal processing with high-level biomimetic computation. The interplay between analog neuromorphic circuits, FPGA-based SNN acceleration, and evolutionary software adaptation defines the main design trade-offs in terms of real-time capability, power efficiency, and scalability. These considerations are central to advancing neuromorphic hardware for next-generation brain–machine interfaces and digital twins of the nervous system. Finally, this hierarchical co-design enables the Neuromorphic Twin to maintain long-term biologically faithful personalization of neural emulation without compromising its real-time responsiveness, a prerequisite for future clinical and biohybrid applications.
Neuroengineering applications of Neuromorphic Twins
The applications of Neuromorphic Twins encompass a paradigm shift in neuroengineering, offering a diverse array of possibilities. The first example of a Neuromorphic Twin was envisaged in recent paper44 under the name “evolving neuromorphic Twin” (enTwin). The authors proposed that the hypothetical chip enTwin would be based on flexible neuromorphic arrays featuring learnable synaptic connectivity and a neuromorphic architecture, designed for implantation in the human body114 and the brain115. Even if they did not provide any timeline for such a development and the description was extremely general, they were confident about its feasibility just by looking at the state of contemporary neuromorphic research. Indeed, research in the last few years showed promising advancements in terms of components for the Neuromorphic Twin36,37,116, including biohybrid systems for brain repair as a novel tool for regenerative medicine36,117. We have already presented examples of neuromorphic-based solutions for the real-time processing. Another notable application lies in the real-time emulation of biological neural networks, which not only aids in understanding natural neural processes but can support investigating the influence of neurological and psychiatric disorders on the network dynamics, as recently underlined118.
Computational models capable of replicating natural neural responses can be adopted to guide neural stimulation strategies. By modulating stimulation across multiple parameters and channels in space and time, it becomes possible to evoke more complex and natural patterns of activity in the recruited neuronal population. Potential applications range from the sole peripheral interventions49,119,120 to central nervous system interfaces12. The evolution then extends to smart neuromodulators for treating a disease and its symptoms, where the Neuromorphic Twin can contribute to restoring the correct neural dynamics thanks to its ability to deliver a neural-like/biomimetic stimulation. Indeed, the importance of biomimicry in tailoring the neurostimulation protocols has been very recently underlined for both restoring naturalistic sensations49,120 and for improving personalization in vagal nerve stimulation121. Tangible progress in the biomimetic emulation of brain circuits lies at the forefront of current research. A recent study48 realized the neuromorphic implementation of a bio-realistic model of layer IV of the somatosensory cortex of the rodent brain (Fig. 3A top). That Neuromorphic Twin is an ASIC featuring 1024 neurons distributed across four cores, each implementing the AdEx I&F model (Fig. 3A bottom). According to the study, this physical emulation of cortical circuits provides a powerful tool for understanding and predicting the behavior of the somatosensory cortex under neurostimulation.
A Top. Model of the brain architecture within layer-IV of the somatosensory cortex. Bottom Left. Microphotograph of the ASIC (Application-Specific Integrated Circuit) called DYNAP-SE2 used to implement the Neuromorphic Twin of the somatosensory cortex, depicted on the top panel. Bottom Right. Diagram of the tuning scheme within each core of both neuron and synaptic parameters. B Top Left. Position of the nodes (neurons) in a simulated model of a neuronal network emulating the functional behavior of the premotor cortex of a rat. Top Right. Connections among the nodes of network reported on the left. Bottom Left. Block diagram of the BioemuS system architecture, detailing each component and indicating software and hardware elements with pink and red symbols, respectively. Bottom Right. Microphotograph of the board hosting the configured FPGA (Field-Programmable Gate Arrays). C Radar plot of selected network features computed by the analysis of spiking activity obtained through the simulation of 325 SNNs (Spiking Neural Networks) emulating the rat premotor cortex (i.e., the Biological Neural Network - BNN). They include the Root Mean Square Error of the Inter-Spike Interval, Mean Firing Rate, Mean Bursting Rate, Pearson’s Correlation Coefficient, and Burstiness Index. Each configured SNN is shown in grey. Three representative SNN are highlighted: SNN1, in red, exhibits the largest area; SNN2, in purple, shows a median area; and SNN3, in blue, covers one of the smallest areas. D Raster plots of the SNNs highlighted on the left, together with the BNN of origin. A Is reproduced with permission under CC BY 4.0 license from ref. 48. B–D Is reproduced with permission from ref. 122, copyright 2024, IEEE.
Along the same line, another study demonstrated that a hardware-based biomimetic SNN made up of 1,024 Hodgkin-Huxley neurons successfully reproduced key electrophysiological features of the rat premotor cortex, i.e., the Biological Neural Network - BNN122. By exploiting the Bioemus framework99, a small-world like topology123 was designed (Fig. 3B top). Using this set-up, 325 different SNN configurations, varying in neuron types, excitation/inhibition balance, were deployed on an FPGA board (Fig. 3B bottom). A comparative analysis was carried out between a set of BNNs, considered as the reference, and the 325 SNNs (Fig. 3 C). The SNN better approaching the key electrophysiological features was indeed matching well the global activity patterns, as depicted in the representative raster plots of Fig. 3D. Using a former version of the Bioemus set-up99, a hardware-based SNN was exploited to deliver neural-like stimulation patterns to deeply anesthetized healthy rats124 according to a purely open-loop modality. That study reported that the SNN-based stimulation increased spontaneous firing in both the primary somatosensory and in the premotor area, a result typically seen only with closed-loop stimulation125,126. All the previous studies highlight the feasibility of the Neuromorphic Twin technology, also for delivering brain-like stimulation patterns.
Given the above results, it is then clear that the Neuromorphic Twins can pave the way for a new generation of neuro/brain prostheses where the “twin” can replace the damaged brain network/region/area, providing adaptive and restorative treatment, typically in the form of stimulation. Neuromorphic Twins interacting bi-directionally with their biological counterpart are schematically depicted in Fig. 4A. Preliminary examples of their implementation show the closed-loop interaction between a neuromorphic-based model and neuronal cultures coupled to Micro-Electrode Arrays - MEAs in vitro46,127,128, to mimic the restoration after a traumatic lesion. Other applications involving brain slices of the hippocampus42 as well as in vivo animal models16,42, have been also presented in the literature. Even if those studies claim the use of such a technology for restoring neural activity after brain injury, most of the current implementations are still limited to healthy animal models.
A Neuromorphic Twin at the preclinical level. This diagram illustrates the key components required to integrate Neuromorphic Twin technology with in vivo experiments in rodent models. Neural recordings are obtained from a specific brain area using an implanted Micro Electrode Array (MEA). The collected data creates a library of electrophysiological parameters necessary for designing the biomimetic SNN, which is then deployed into the Neuromorphic Twin. The Twin also contains the hardware needed to process neural signals in real time and can be periodically updated based on network dynamics adjustments from monitoring the animal’s activity and behavior. The output of the Neuromorphic Twin is a signal, or a combination of signals, coming from a subset of neurons of the biomimetic SNN, which serves as a trigger for delivering stimulation to the animal via a second implanted MEA. Modified from refs. 99,124. B Top Left. Schematic of a biohybrid interaction between a brain organoid and its artificial counterpart (i.e., biomimetic SNN). B Two connected organoids (Bottom Left) are placed over a planar high-density MEA. The Neuromorphic Twin (Right) allows the artificial communication between the two via a biomimetic SNN emulating one organoid, implemented thanks to the Bioemus framework99. Neural events of interest (e.g., synchronized spikes, network burst, or even single spikes) are used to trigger stimulation from the left (i.e., purple shaded) organoid to the SNN and from the SNN to the right (i.e., green shaded) organoid. B takes inspiration from ref. 99.
For clinical applications in humans, we foresee that the architecture of Neuromorphic Twins, with their advanced properties, will enable complex analysis of neuronal signals, replication of biological behavior, and personalized treatments especially in the case of stroke, epilepsy and neurodegenerative disease such as Parkinson’s (cf. see also the Roadmap section). These treatments may include electroceuticals47 and other types of brain stimulation strategies, such as ultrasound129 or optogenetics130,131.
In parallel with clinical applications, the neuromorphic community has progressively advanced the development of increasingly bio-inspired and biomimetic models like SNN and efficient architectures, exploring various approaches at the intersection of electronics, chemistry, and biology. Three main research directions are currently emerging. The first involves using technologies other than electronics, such as chemistry, with molecular networks replicating neuromorphic architectures. For example, Okumura132 developed DNA-encoded enzymatic neurons with tunable weights and biases, assembled into multilayer architectures capable of classifying non-linearly separable regions. Similarly, Cherry and Qian133 implemented Winner-Take-All neural networks based on DNA strand displacement reactions for pattern recognition. The second is using organic technology for designing neuromorphic architecture. The use of organic materials offers many advantages, notably better integration with biological systems even at ionic level, thereby facilitating sensing, signal processing, and stimulation within a close-loop system134. Harikesh135 presented organic electrochemical neurons (OECNs) with ion-modulated spiking, integrated with organic electrochemical synapses exhibiting both short-term and long-term plasticity. The last axis will take advantage of biological intelligence and its tremendous energy efficiency (approximately 20 W for a brain with billions of neurons). An innovative approach involves the direct employment of biological neurons for computational tasks136,137,138. This is made possible by the synergistic emergence of bidirectional hybrid systems99 and biologically realistic AI algorithms139, further expanding the concept of Neuromorphic Twin and combining it with the idea of organoid intelligence140,141. Indeed, it is possible to create novel forms of intelligence where biological and artificial systems are able to dialogue in a truly bi-directional way (Fig. 4B, top left). A recent work by Beaubois and colleagues99 showcased this hypothesis and anticipated a preliminary concept of Neuromorphic Twin, by putting in communication a connectoid (i.e. two connected cortical organoids from human induced pluripotent stem cells) and a biomimetic organoid implemented as a biomimetic SNN (Fig. 4B, bottom left and right). These innovative approaches hold immense promise in advancing neuroprosthetic technologies, opening avenues for unprecedented levels of integration and adaptability in the realm of neural interfaces.
Roadmap to support the feasibility of Neuromorphic Twin technology
In the previous paragraphs, we have provided a picture of the current status of the research related to the new technology named Neuromorphic Twin, as witnessed by the reported results. Recent advances in this technology have shown significant potential for modeling brain dynamics, with early applications targeting small-scale, well-defined neural circuits and specific pathologies. These models have enabled researchers to explore the feasibility of personalized preliminary therapeutic interventions at the preclinical level, laying the foundation for more complex and adaptable systems. However, it is clear that the Neuromorphic Twin still is in the early stages of development, with most applications confined to the research settings. Although promising, the path toward the clinical adoption of the technology remains long and challenging. Looking ahead, the goal is to create fully personalized Neuromorphic Twins that can be used routinely in clinical environments for diagnostics, treatment planning, and real-time monitoring. Achieving this will require a well-defined roadmap that includes progressive refinement of models, extensive preclinical validation, and ultimately, regulatory approval for clinical use. In this section we present the technical limitations and challenges for the full exploitation of the technology together with a possible timeline for the use of the Neuromorphic Twin in clinics.
Technical barriers and challenges to technology adoption
Real-time signal processing
Recent advances in electrode array technologies, increasing from 64 to 102453 and even up to 10,000142 and 300,000 electrodes143, pose significant challenges for real-time signal processing. Edge computing (including detection and classification) will become essential to extract only the relevant biological signatures for analysis. Emerging techniques, such as attention-based SNNs144, show promise in classifying action potential shapes with greater precision. A key focus for ongoing research is the development of methods capable of extracting multivariate temporal patterns in a fully unsupervised manner, which would significantly enhance our understanding of complex neural dynamics. Despite the demonstrated potential of SNN hardware in applications like spike sorting, adaptive control, and brain signal recording, existing systems still fall short in terms of accuracy and online adaptability achieved by modern AI techniques. Bridging this gap will require the development of algorithms capable of real-time, parallel computation that are also suitable for low-power embedded systems, enabling the full potential of SNNs to be realized.
Real-time emulation
Regarding emulation, research efforts focus either at the single-cell level, which involves the electrical reproduction of neurons and networks145, particularly through the neuron model used, or at the functional level of the brain146, which focuses on topologies, connected neural circuits, and oscillations between networks. To achieve an efficient emulation that encompasses the different hierarchical levels, it is essential to combine these two approaches147. Real-time emulation is possible through hardware implementations of parallel computing such as GPUs, SoC FPGAs, ASICs—whether analog or digital—and neuromorphic chips. To maintain a real-time closed-loop interaction with biological systems, integrating the SNN-based signal processing solutions from section 6.1.1 allows both the interface and the emulation to be implemented within a single neuromorphic system.
Scalability
Fortunately, new technologies, particularly SoCs148 and the development of analog and mixed neuromorphic chips100,149,150, make it possible to address challenges related to scalability, power consumption, and parallelism, which are mandatory requirements for the Neuromorphic Twin. Networks with generic parameters are essential to allow the tuning of dynamics over time. The reproduction and personalization of networks are achieved through the internal parameters of the SNN and evolve with their plasticity; however, occasional re-adjustment of the network may be necessary to re-fit the real biological dynamics of the patient. This re-adjustment does not need to occur in real time, as it operates on a slower timescale (from few seconds to some hours for the fully rewired network and the updated topology).
Hardware miniaturization
While neuromorphic chips are powerful, they still face challenges related to both scalability and miniaturization, which are critical for integration into usable biomedical applications. To bridge this technological gap, the SNN hosting the Twin can be miniaturized using an analog or digital ASIC, enabling its deployment in medical implants151. The generic parameters can be uploaded and modified over time via wireless transmission to embedded hardware (such as SoC or microcontroller board). A hybrid system (ASIC – SoC – IoT network) appears to be an ideal solution, allowing for miniaturization (ASIC implant), parameter control and plasticity (SoC), and parameter and network tuning (IoT network). An IoT network152,153,154 applied to an ASIC brain implant enables continuous communication between the implant and external devices, such as a smartphone, a computer, or a cloud infrastructure. Thanks to this connectivity, brain activity can be monitored in real time, allowing for dynamic and remote adjustment of stimulation or tuning parameters. Learning or parameter optimization algorithms for the digital twin, hosted in the cloud, can adapt the implant’s behavior to the patient’s specific and evolving needs. Finally, medical doctors can track the progression of the electroceutical protocol and modify remotely, enhancing both the responsiveness and personalization of care.
Invasiveness
The problem related to electrodes’ invasiveness into brain tissue has been extensively faced in previous studies since the birth of brain-computer interface technologies, highlighting strategies to reduce tissue reaction and prolong the lifetime of chronic implants155,156. Invasiveness in the context of Neuromorphic Twin is important as both recording and stimulation need to as much selective as possible49. In a recent review, Shen and co-workers157 examined the key features of neural probes that may support the clinical translation of invasive neural interfaces, by focusing on abiotic and biotic factors that can lead to their failure, and highlighting emerging architectures in neural interface design. Notably, to support the use of invasive electrodes for the exploitation of Neuromorphic Twin, it is important to acknowledge that some studies already reported about patients having long-term implants without experiencing adverse reactions158,159. We feel that, in the years to come, this will become a less demanding issue, considering all the progress done so far.
Integration of multimodal data
The current approach for the Neuromorphic Twin implementation focuses on electrophysiological signals as the primary data input. However, additional data types, such as MRI scans, can be used to improve the model (i.e., the Twin) at its initial definition, providing important details related to the anatomical (i.e., structural) and functional connectivity. In the coming years, as both neuromorphic and imaging technologies continue to advance, the Neuromorphic Twin will be able to integrate a broader range of data to refine its models over time, an approach already explored in recent studies on data and sensor fusion with neuromorphic systems37,160. Moreover, behavioral and performance-related inputs, such as motor task execution, also represent a key dimension. Incorporating this data into the loop can improve patient monitoring and enable adaptive therapy adjustments based on real-time functional status, also thanks to AI-related markerless algorithms, which can accurately track behavior and performance without the need for wearable sensors161,162,163.
Long term-personalization
Brain networks’ emulation over long-term scales is necessary to guarantee a personalized therapy as the neural disease progresses. Thanks to the integration of multimodal data, even acquired through the patient’s body (cf. section “Integration of multimodal data”), and the scalability of the system (cf. section “Scalability”), it will be possible for the Neuromorphic Twin to co-evolve and continuously adjust the stimulation’s parameters for the electroceutical treatment. Occasional refinement of the Twin’s parameters can occur through the software for automatic tuning block (cf. Fig. 2), but it does not need to operate in real time.
Ethical issues
As new technologies emerge, especially before they become fully integrated into society, it is crucial to engage in early ethical and social reflection. The development of advanced neural interfaces based on a real-time, two-way connection between the brain and autonomous ANNs, such as the case of the Neuromorphic Twins, prompts critical questions about how those technologies might affect human subjectivity, i.e., the continuous process by which individuals develop identity, agency, and self-awareness, thus remaining the “subject” of their life158. This specific topic has been recently discussed by Yvert and Fourneret45. To protect the user’s autonomy, these technologies must be designed to clearly explain how they make decisions, to ensure that the human user stays in control. If this is not guaranteed, the device could act more like an independent agent than a simple tool, contributing to ongoing debates about the ethical and legal status of advanced non-human systems164. Similarly, a recent research paper first introduced the concept of the enTwin (basically our Neuromorphic Twin, cf. “Introduction”) and discussed the ethical related issues. The authors suggested that artificial brains designed thanks to neuromorphic engineering, as in the case of the enTwin, are becoming more and more similar to biological ones44. They also introduced a new model, called the Conductor Model of Consciousness, that explains how both humans and machines might develop an inner understanding of the world by organizing and interpreting information. The model also poses ethical questions, especially as artificial systems become more human-like in cognition and emotion, calling for a new ethical framework to guide human-AI relationships. In general, a multidisciplinary approach, incorporating neuroscience, philosophy, and technology policy, is essential to ensure that the integration of humans and machines benefits society as a whole165. Of course, the ethical framework for adopting the Neuromorphic Twin is tightly related to ongoing discussions about brain–body interface technologies166,167 and AI168. These fields have already raised important questions about human autonomy, agency, safety, and responsibility164,169,170. As the Neuromorphic Twin integrates elements of both advanced brain technology and AI, it inherits the ethical concerns of both domains and the importance of maintaining human control. Establishing a clear and anticipatory ethical framework will be essential to ensure that these technologies support human well-being, respect for individual rights, and are treated in a socially responsible way. Moreover, adaptive neuromorphic systems, such as the Neuromorphic Twins, present specific ethical challenges because the biomimetic model can evolve over time in response to neural activity, potentially altering its behavior after initial deployment171. This adaptability raises further concerns regarding patient autonomy, ongoing consent, and the ability to monitor or predict system adaptations172. To address these issues, strategies such as dynamic informed consent173,174, where patients are periodically updated on changes in the system’s behavior, and clinician-mediated oversight of the system’s adaptive behavior can be implemented. These approaches aim to ensure that patient autonomy is respected, ethical standards are maintained, and regulatory requirements are met throughout the system’s lifecycle175,176.
Clinical translation timeline and adoption pathway
Although several open issues remain, spanning technical, experimental, clinical, and ethical dimensions, the development of the Neuromorphic Twin is steadily progressing. As previously mentioned in this Perspective, the fundamental components required to enable biohybrid interaction between the Twin and the central nervous system are already in place4,36,117,177. However, these elements must be coherently integrated within a structured development framework. This calls for the definition of a clear and realistic timeline to guide the transition from current prototypes to functional systems that can be tested in relevant biological and, later on, clinical settings. To this end, we propose a phased roadmap for the adoption of the Neuromorphic Twin in clinical applications. The timeline spans approximately a decade and is structured into four main stages, with early-stage clinical testing potentially starting within the next 7 to 10 years (cf. Table 5).
As outlined in the reported Roadmap, Neuromorphic Twins are expected to represent a major innovation in neuroengineering by enabling real-time interaction with, and emulation of, complex brain dynamics. By integrating digital twin technology with neuromorphic engineering, this approach has the potential to overcome key limitations of current electroceutical strategies, enabling more adaptive and personalized interventions. Although still at an early stage of development, Neuromorphic Twins open promising clinical perspectives, ranging from the restoration of impaired neural functions to the management of neurological disorders. Their energy efficiency and suitability for implantable implementations further support their use in a new generation of compact, closed-loop neuroprosthetic systems. Overall, advances in this field may drive a substantial shift toward truly personalized neurotherapies and improved patient quality of life.
References
Béjot, Y. & Yaffe, K. Ageing population: a neurological challenge. Neuroepidemiology 52, 76–77 (2019).
Famm, K., Litt, B., Tracey, K. J., Boyden, E. S. & Slaoui, M. A jump-start for electroceuticals. Nature 496, 159–161 (2013).
Micera, S., Caleo, M., Chisari, C., Hummel, F. C. & Pedrocchi, A. Advanced neurotechnologies for the restoration of motor function. Neuron 105, 604–620 (2020).
Panuccio, G. et al. Progress in Neuroengineering for brain repair: new challenges and open issues. Brain Neurosci. Adv. 2, 2398212818776475 (2018).
Iberite, F. et al. Restoration of natural thermal sensation in upper-limb amputees. Science 380, 731–735 (2023).
Raspopovic, S., Valle, G. & Petrini, F. M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 20, 925–939 (2021).
Losanno, E., Mender, M., Chestek, C., Shokur, S. & Micera, S. Neurotechnologies to restore hand functions. Nat. Rev. Bioeng. 1, 390–407 (2023).
Geller, E. B. et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia 58, 994–1004 (2017).
Pycroft, L., Stein, J. & Aziz, T. Deep brain stimulation: an overview of history, methods, and future developments. Brain Neurosci. Adv. 2, 2398212818816017 (2018).
Milekovic, T. et al. A spinal cord neuroprosthesis for locomotor deficits due to Parkinson’s disease. Nat. Med. 29, 2854–2865 (2023).
Guggenmos, D. J. et al. Restoration of function after brain damage using a neural prosthesis. Proc. Natl. Acad. Sci. USA 110, 21177–21182 (2013).
Rowald, A. et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat. Med. 28, 260–271 (2022).
Courtine, G. & Sofroniew, M. V. Spinal cord repair: advances in biology and technology. Nat. Med. 25, 898–908 (2019).
Berger, T. W. et al. A cortical neural prosthesis for restoring and enhancing memory. J. Neural Eng. 8, 046017 (2011).
Hampson, R. E. et al. Facilitation and restoration of cognitive function in primate prefrontal cortex by a neuroprosthesis that utilizes minicolumn-specific neural firing. J. Neural Eng. 9, 056012 (2012).
Hogri, R. et al. A neuro-inspired model-based closed-loop neuroprosthesis for the substitution of a cerebellar learning function in anesthetized rats. Sci. Rep. 5, 8451 (2015).
Hampson, R. E. et al. Developing a hippocampal neural prosthetic to facilitate human memory encoding and recall. J. Neural Eng. 15, 036014 (2018).
Carè, M., Chiappalone, M. & Cota, V. R. Personalized strategies of neurostimulation: from static biomarkers to dynamic closed-loop assessment of neural function. Front. Neurosci. 18, 1363128 (2024).
Amunts, K. et al. Linking brain structure, activity, and cognitive function through computation. eNeuro 9, 0316-21 (2022).
Einevoll, G. T. et al. The scientific case for brain simulations. Neuron 102, 735–744 (2019).
Makin, S. Brain simulation. Nature 571, S9 (2019).
D’Angelo, E. & Jirsa, V. The quest for multiscale brain modeling. Trends Neurosci. 45, 777–790 (2022).
Wang, H. E. et al. Delineating epileptogenic networks using brain imaging data and personalized modeling in drug-resistant epilepsy. Sci. Transl. Med. 15, eabp8982 (2023).
Jirsa, V. et al. Personalised virtual brain models in epilepsy. Lancet Neurol. 22, 443–454 (2023).
Meier, J. M. et al. Virtual deep brain stimulation: Multiscale co-simulation of a spiking basal ganglia model and a whole-brain mean-field model with The Virtual Brain. Exp. Neurol. 354, 114111 (2022).
Niederer, S. A., Sacks, M. S., Girolami, M. & Willcox, K. Scaling digital twins from the artisanal to the industrial. Nat. Comput. Sci. 1, 313–320 (2021).
Tao, F., Zhang, H., Liu, A. & Nee, A. Y. Digital twin in industry: State-of-the-art. IEEE Trans. Ind. Inform. 15, 2405–2415 (2018).
Liu, M., Fang, S., Dong, H. & Xu, C. Review of digital twin about concepts, technologies, and industrial applications. J. Manuf. Syst. 58, 346–361 (2021).
Moingeon, P., Chenel, M., Rousseau, C., Voisin, E. & Guedj, M. Virtual patients, digital twins and causal disease models: Paving the ground for in silico clinical trials. Drug Discov. today 28, 103605 (2023).
Pathmanathan, P. et al. Credibility assessment of in silico clinical trials for medical devices. PLOS Comput. Biol. 20, e1012289 (2024).
Mead, C. Neuromorphic electronic systems. Proc. IEEE 78, 1629–1636 (1990).
Furber, S. Large-scale neuromorphic computing systems. J. neural Eng. 13, 051001 (2016).
Marković, D., Mizrahi, A., Querlioz, D. & Grollier, J. Physics for neuromorphic computing. Nat. Rev. Phys. 2, 499–510 (2020).
Van De Burgt, Y., Melianas, A., Keene, S. T., Malliaras, G. & Salleo, A. Organic electronics for neuromorphic computing. Nat. Electron. 1, 386–397 (2018).
Bartolozzi, C., Indiveri, G. & Donati, E. Embodied neuromorphic intelligence. Nat. Commun. 13, 1024 (2022).
Christensen, D. V. et al. 2022 roadmap on neuromorphic computing and engineering. Neuromorphic Comput. Eng. 2, 022501 (2022).
Kudithipudi, D. et al. Neuromorphic computing at scale. Nature 637, 801–812 (2025).
Ahmadvand, R., Sharif, S. S. & Banad, Y. M. Neuromorphic digital-twin-based controller for indoor multi-UAV systems deployment. In Proc. Conference on AI, Science, Engineering, and Technology (AIxSET) 204–207 (IEEE, 2025).
Boche, H., Schaefer, R. F., Poor, H. V. & Fitzek, F. H. On the need of neuromorphic twins to detect denial-of-service attacks on communication networks. In Proc. IEEE/ACM Transactions on Networking (IEEE, 2024).
Aboumerhi, K., Güemes, A., Liu, H., Tenore, F. & Etienne-Cummings, R. Neuromorphic applications in medicine. J. Neural Eng. 20, 041004 (2023).
Donati, E. & Indiveri, G. Neuromorphic bioelectronic medicine for nervous system interfaces: from neural computational primitives to medical applications. Prog. Biomed. Eng. 5, 013002 (2023).
Pisarchik, A. N. et al. Advanced neuromorphic engineering approaches for restoring neural activity after brain injury: innovations in regenerative medicine. Regenerat. Med. Rep. 1, 195–210 (2024).
Broccard, F. D., Joshi, S., Wang, J. & Cauwenberghs, G. Neuromorphic neural interfaces. in Handbook of Neuroengineering 1–33 (Springer, 2022).
Benitez, F., Pennartz, C. & Senn, W. The conductor model of consciousness, our neuromorphic twins, and the human-AI deal. AI Ethics, 5, 1–22 (2024).
Yvert, B. & Fourneret, E. Neuromorphic brain interfacing and the challenge of human subjectivation. Nat. Rev. Bioeng. 1, 380–381 (2023).
Buccelli, S. et al. A neuromorphic prosthesis to restore communication in neuronal networks. IScience 19, 402–414 (2019).
Chiappalone, M. et al. Neuromorphic-based neuroprostheses for brain rewiring: state-of-the-art and perspectives in neuroengineering. Brain Sci. 12, 1578 (2022).
Ramirez, H., Donati, E., von der Behrens, W., Indiveri, G. & Valle, G. A physical emulation of somatosensory cortex as a Neuromorphic Twin for neural prostheses. Research Square. https://doi.org/10.21203/rs.3.rs-4389036/v2 (2024).
Donati, E. & Valle, G. Neuromorphic hardware for somatosensory neuroprostheses. Nat. Commun. 15, 556 (2024).
Forro, C. et al. Electrophysiology read-out tools for brain-on-chip biotechnology. Micromachines 12, 124 (2021).
Steinmetz, N. A. et al. Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Ye, Z. et al. Ultra-high density electrodes improve detection, yield, and cell type identification in neuronal recordings. Neuron 113, 3966–3982 (2025).
Angotzi, G. N. et al. Multi-shank 1024 channels active SiNAPS probe for large multi-regional topographical electrophysiological mapping of neural dynamics. Adv. Sci. 12, 13 (2025).
Paulk, A. C. et al. Large-scale neural recordings with single neuron resolution using Neuropixels probes in human cortex. Nat. Neurosci. 25, 252–263 (2022).
Coughlin, B. et al. Modified Neuropixels probes for recording human neurophysiology in the operating room. Nat. Protoc. 18, 2927–2953 (2023).
Dominguez-Morales, J. P. et al. Real-time detection of bursts in neuronal cultures using a Neuromorphic Auditory Sensor and Spiking Neural Networks. Neurocomputing 449, 422–434 (2021).
Burelo, K. et al. A spiking neural network (SNN) for detecting high frequency oscillations (HFOs) in the intraoperative ECoG. Sci. Rep. 11, 6719 (2021).
Sharifshazileh, M., Burelo, K., Sarnthein, J. & Indiveri, G. An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG. Nat. Commun. 12, 3095 (2021).
Baek, E. et al. Neuromorphic dendritic network computation with silent synapses for visual motion perception. Nat. Electron. 7, 454–465 (2024).
Costa, F. et al. Robust compression and detection of epileptiform patterns in ECoG using a real-time spiking neural network hardware framework. Nat. Commun. 15, 3255 (2024).
Tian, F., Yang, J., Zhao, S. & Sawan, M. NeuroCARE: a generic neuromorphic edge computing framework for healthcare applications. Front. Neurosci. 17, 1093865 (2023).
Yoo, J. et al. An 8-channel scalable EEG acquisition SoC with patient-specific seizure classification and recording processor. IEEE J. Solid State Circ. 48, 214–228 (2012).
Van Helleputte, N. et al. 18.3 A multi-parameter signal-acquisition SoC for connected personal health applications. In Proc. IEEE International Solid-state Circuits Conference Digest Of Technical Papers (ISSCC) 314–315 (IEEE, San Francisco, CA, USA, 2014) https://doi.org/10.7264/9qdm-en61.
Cheslet, J. et al. Fpga implementation of a spiking neural network for real-time action potential and burst detection. In Proc. IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–5 (IEEE, 2023).
Pokala, S. D. et al. A frugal Spiking Neural Network for unsupervised multivariate temporal pattern classification and multichannel spike sorting. Nat. Commun. 16, 9218 (2025).
Ronchini, M., Rezaeiyan, Y., Zamani, M., Panuccio, G. & Moradi, F. NET-TEN: a silicon neuromorphic network for low-latency detection of seizures in local field potentials. J. Neural Eng. 20, 036002 (2023).
Cheslet, J., Bernert, M., Beaubois, R., Yvert, B. & Lévi, T. Real-Time spike sorting using an optimized STDP Spiking Neural Network on FPGA. In Proc. International Joint Conference on Neural Networks (IJCNN) 1–7 (IEEE, Yokohama, Japan, 2024) https://doi.org/10.1109/IJCNN60899.2024.10650675.
Linares-Barranco, A. et al. Towards hardware Implementation of WTA for CPG-based control of a Spiking Robotic Arm. 2022 IEEE International Symposium on Circuits and Systems (ISCAS). 1057–1061 (IEEE).
Luo, L. Architectures of neuronal circuits. Science 373, eabg7285 (2021).
Bastos, A. M. et al. Canonical microcircuits for predictive coding. Neuron 76, 695–711 (2012).
Ambroise, M., Levi, T., Joucla, S., Yvert, B. & Saïghi, S. Real-time biomimetic central pattern generators in an FPGA for hybrid experiments. Front. Neurosci. 7, 215 (2013).
Breakspear, M., Heitmann, S. & Daffertshofer, A. Generative models of cortical oscillations: neurobiological implications of the Kuramoto model. Front. Hum. Neurosci. 4, 190 (2010).
Pan, W., Zhao, F., Zeng, Y. & Han, B. Adaptive structure evolution and biologically plausible synaptic plasticity for recurrent spiking neural networks. Sci. Rep. 13, 16924 (2023).
Svozil, D., Kvasnicka, V. & Pospichal, J. Introduction to multi-layer feed-forward neural networks. Chemom. Intell. Lab. Syst. 39, 43–62 (1997).
O’shea, K. & Nash, R. An introduction to convolutional neural networks. arXiv e-prints 1511.08458 (2015).
Kheradpisheh, S. R., Ganjtabesh, M., Thorpe, S. J. & Masquelier, T. STDP-based spiking deep convolutional neural networks for object recognition. Neural Netw. 99, 56–67 (2018).
Medsker, L. R. & Jain, L. Recurrent neural networks. Des. Appl. 5, 2 (2001).
Pulvermüller, F., Tomasello, R., Henningsen-Schomers, M. R. & Wennekers, T. Biological constraints on neural network models of cognitive function. Nat. Rev. Neurosci. 22, 488–502 (2021).
Indiveri, G. et al. Neuromorphic silicon neuron circuits. Front. Neurosci. 5, 73 (2011).
Rahman, N. A. & Yusoff, N. Modulated Spike-Time Dependent Plasticity (STDP)-based Learning for Spiking Neural Network (SNN): A review. Neurocomputing, 129170 (2024).
Schuman, C. D. et al. Opportunities for neuromorphic computing algorithms and applications. Nat. Comput. Sci. 2, 10–19 (2022).
Khoyratee, F., Grassia, F., Saïghi, S. & Levi, T. Optimized real-time biomimetic neural network on FPGA for bio-hybridization. Front. Neurosci. 13, 377 (2019).
Rostami, A., Vogginger, B., Yan, Y. & Mayr, C. G. E-prop on SpiNNaker 2: Exploring online learning in spiking RNNs on neuromorphic hardware. Front. Neurosci. 16, 1018006 (2022).
Qiao, N. et al. A reconfigurable on-line learning spiking neuromorphic processor comprising 256 neurons and 128K synapses. Front. Neurosci. 9, 141 (2015).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500 (1952).
Brette, R. What is the most realistic single-compartment model of spike initiation?. PLoS Comput. Biol. 11, e1004114 (2015).
Kuriyama, R., Casellato, C., D’Angelo, E. & Yamazaki, T. Real-time simulation of a cerebellar scaffold model on graphics processing units. Front. Cell. Neurosci. 15, 623552 (2021).
Yamaura, H., Igarashi, J. & Yamazaki, T. Simulation of a human-scale cerebellar network model on the K computer. Front. Neuroinform. 14, 16 (2020).
Forrest, M. P., Parnell, E. & Penzes, P. Dendritic structural plasticity and neuropsychiatric disease. Nat. Rev. Neurosci. 19, 215–234 (2018).
Fogarty, M. J. et al. Cortical synaptic and dendritic spine abnormalities in a presymptomatic TDP-43 model of amyotrophic lateral sclerosis. Sci. Rep. 6, 37968 (2016).
Martin, E., Cazenave, W., Cattaert, D. & Branchereau, P. Embryonic alteration of motoneuronal morphology induces hyperexcitability in the mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 54, 116–126 (2013).
Beaubois, R., Cheslet, J., Ikeuchi, Y., Branchereau, P. & Levi, T. Real-time multicompartment Hodgkin-Huxley neuron emulation on SoC FPGA. Front. Neurosci. 18, 1457774 (2024).
Helias, M. et al. Supercomputers ready for use as discovery machines for neuroscience. Front. Neuroinform. 6, 26 (2012).
Van Albada, S. J. et al. Performance comparison of the digital neuromorphic hardware SpiNNaker and the neural network simulation software NEST for a full-scale cortical microcircuit model. Front. Neurosci. 12, 291 (2018).
Rhodes, O. et al. Real-time cortical simulation on neuromorphic hardware. Philos. Trans. R. Soc. A 378, 20190160 (2020).
Bogdan, P. A. et al. Towards a bio-inspired real-time neuromorphic cerebellum. Front. Cell. Neurosci. 15, 622870 (2021).
Benjamin, B. V., Steinmetz, N. A., Oza, N. N., Aguayo, J. J. & Boahen, K. Neurogrid simulates cortical cell-types, active dendrites, and top-down attention. Neuromorph. Comput. Eng. 1, 013001 (2021).
Benjamin, B. V. et al. Neurogrid: A mixed-analog-digital multichip system for large-scale neural simulations. Proc. IEEE 102, 699–716 (2014).
Beaubois, R. et al. BiœmuS: A new tool for neurological disorders studies through real-time emulation and hybridization using biomimetic Spiking Neural Network. Nat. Commun. 15, 5142 (2024).
Richter, O. et al. DYNAP-SE2: a scalable multi-core dynamic neuromorphic asynchronous spiking neural network processor. Neuromorph. Comput. Eng. 4, 014003 (2024).
Zhou, J. & Chen, J. Prospect of spintronics in neuromorphic computing. Adv. Electron. Mater. 7, 2100465 (2021).
Wang, D. et al. Spintronic leaky-integrate-fire spiking neurons with self-reset and winner-takes-all for neuromorphic computing. Nat. Commun. 14, 1068 (2023).
Duan, X. et al. Memristor-based neuromorphic chips. Adv. Mater. 36, 2310704 (2024).
Aguirre, F. et al. Hardware implementation of memristor-based artificial neural networks. Nat. Commun. 15, 1974 (2024).
Spilger, P. et al. hxtorch. snn: Machine-learning-inspired spiking neural network modeling on BrainScaleS-2. Proceedings of the 2023 Annual Neuro-Inspired Computational Elements Conference. 57-62.
Fang, W. et al. Spikingjelly: An open-source machine learning infrastructure platform for spike-based intelligence. Sci. Adv. 9, eadi1480 (2023).
Manna, D. L., Vicente-Sola, A., Kirkland, P., Bihl, T. J. & Di Caterina, G. Frameworks for SNNs: a review of data science-oriented software and an expansion of spyketorch. In Proc. International Conference on Engineering Applications of Neural Networks 227–238 (Springer).
Davies, M. et al. Loihi: A neuromorphic manycore processor with on-chip learning. IEEE Micro 38, 82–99 (2018).
Posey, B. M. What is the Akida event domain neural processor? (2022).
Ibad, T., Abdulkadir, S. J., Aziz, N., Ragab, M. G. & Al-Tashi, Q. Hyperparameter optimization of evolving spiking neural network for time-series classification. New. Gen. Comput. 40, 377–397 (2022).
Nanami, T., Grassia, F. & Kohno, T. A metaheuristic approach for parameter fitting in digital spiking silicon neuron model. J. Robot., Netw. Artif. Life 5, 32–36 (2018).
Firmin, T., Boulet, P. & Talbi, E.-G. Parallel hyperparameter optimization of spiking neural networks. Neurocomputing 609, 128483 (2024).
Townsend, T. J. Vivado design interface: enabling CAD-tool design for next generation Xilinx FPGA devices. (Brigham Young University, 2017).
Mariello, M., Kim, K., Wu, K., Lacour, S. P. & Leterrier, Y. Recent advances in encapsulation of flexible bioelectronic implants: Materials, technologies, and characterization methods. Adv. Mater. 34, 2201129 (2022).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604 (2020).
Forbes, I. & Indiveri, G. Neuromorphic computing and engineering’s coming of age. Neuromorph. Comput. Eng. 4, 030202 (2024).
Panuccio, G., Semprini, M. & Chiappalone, M. Intelligent biohybrid systems for functional brain repair. New. Horiz. Transl. Med. 3, 162–174 (2016).
Raikar, A. S. et al. Neuromorphic computing for modeling neurological and psychiatric disorders: Implications for drug development. Artif. Intell. Rev. 57, 318 (2024).
Romeni, S., Valle, G., Mazzoni, A. & Micera, S. Tutorial: a computational framework for the design and optimization of peripheral neural interfaces. Nat. Protoc. 15, 3129–3153 (2020).
Valle, G. et al. Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron 100, 37–45. e37 (2018).
Ciotti, F. et al. Towards enhanced functionality of vagus neuroprostheses through in silico optimized stimulation. Nat. Commun. 15, 6119 (2024).
De Venuto, G. et al. Recapitulating the electrophysiological features of in vivo biological networks by using a real-time hardware Spiking Neural Network. In Proc. 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 1–4 (IEEE, 2024).
Watts, D. J. & Strogatz, S. H. Collective dynamics of ‘small-world’networks. Nature 393, 440–442 (1998).
Di Florio, M. et al. Design of an experimental setup for delivering intracortical microstimulation in vivo via Spiking Neural Network. In Proc. 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) 1–4 (IEEE, 2023).
Averna, A. et al. Differential effects of open-and closed-loop intracortical microstimulation on firing patterns of neurons in distant cortical areas. Cereb. Cortex 30, 2879–2896 (2020).
Averna, A. et al. Entrainment of network activity by closed-loop microstimulation in healthy ambulatory rats. Cereb. Cortex 31, 5042–5055 (2021).
Mosbacher, Y. et al. Toward neuroprosthetic real-time communication from in silico to biological neuronal network via patterned optogenetic stimulation. Sci. Rep. 10, 7512 (2020).
Keren, H., Partzsch, J., Marom, S. & Mayr, C. G. A biohybrid setup for coupling biological and neuromorphic neural networks. Front. Neurosci. 13, 432 (2019).
Darmani, G. et al. Non-invasive transcranial ultrasound stimulation for neuromodulation. Clin. Neurophysiol. 135, 51–73 (2022).
Delbeke, J., Hoffman, L., Mols, K., Braeken, D. & Prodanov, D. And then there was light: perspectives of optogenetics for deep brain stimulation and neuromodulation. Front. Neurosci. 11, 663 (2017).
Sahel, J.-A. et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 27, 1223–1229 (2021).
Okumura, S. et al. Nonlinear decision-making with enzymatic neural networks. Nature 610, 496–501 (2022).
Cherry, K. M. & Qian, L. Scaling up molecular pattern recognition with DNA-based winner-take-all neural networks. Nature 559, 370–376 (2018).
Tuchman, Y. et al. Organic neuromorphic devices: Past, present, and future challenges. MRS Bull. 45, 619–630 (2020).
Harikesh, P. C. et al. Organic electrochemical neurons and synapses with ion mediated spiking. Nat. Commun. 13, 901 (2022).
Cai, H. et al. Brain organoid reservoir computing for artificial intelligence. Nat. Electron. 1–8 (2023).
Kagan, B. J. et al. In vitro neurons learn and exhibit sentience when embodied in a simulated game-world. Neuron 110, 3952–3969. e3958 (2022).
Yada, Y., Yasuda, S. & Takahashi, H. Physical reservoir computing with FORCE learning in a living neuronal culture. Appl. Phys. Lett. 119 (2021).
D’Agostino, S. et al. DenRAM: neuromorphic dendritic architecture with RRAM for efficient temporal processing with delays. Nat. Commun. 15, 3446 (2024).
Smirnova, L. et al. Organoid intelligence (OI): the new frontier in biocomputing and intelligence-in-a-dish. Front. Sci. 1, 1017235 (2023).
Shi, H. et al. Organoid intelligence: integration of organoid technology and artificial intelligence in the new era of in vitro models. Med. Nov. Technol. Devices 21, 100276 (2024).
Dutta, B. Eavesdropping on the brain: With 10,000 electrodes, this neural implant senses more than ever before. IEEE Spectr. 59, 30–35 (2022).
Suzuki, I. et al. Large-Area Field Potential Imaging Having Single Neuron Resolution Using 236 880 Electrodes CMOS-MEA Technology. Adv. Sci. 10, 2207732 (2023).
Bernert, M. & Yvert, B. An attention-based spiking neural network for unsupervised spike-sorting. Int. J. Neural Syst. 29, 1850059 (2019).
Vardalakis, N., Aussel, A., Rougier, N. P. & Wagner, F. B. A dynamical computational model of theta generation in hippocampal circuits to study theta-gamma oscillations during neurostimulation. Elife 12, RP87356 (2024).
Schirner, M. et al. Brain simulation as a cloud service: The Virtual Brain on EBRAINS. Neuroimage 251, 118973 (2022).
Yang, S. et al. BiCoSS: toward large-scale cognition brain with multigranular neuromorphic architecture. IEEE Trans. Neural Netw. Learn. Syst. 33, 2801–2815 (2021).
Canas-Moreno, S. et al. Towards neuromorphic FPGA-based infrastructures for a robotic arm. Auton.s Robot. 47, 947–961 (2023).
Davies, M. et al. Advancing neuromorphic computing with Loihi: A survey of results and outlook. Proc. IEEE 109, 911–934 (2021).
Gonzalez, H. A. et al. SpiNNaker2: a large-scale neuromorphic system for event-based and asynchronous machine learning. arXiv preprint https://doi.org/10.48550/arXiv.2401.04491 (2024).
Biyan, Z., Sun, P.-S. V. & Basu, A. Combining SNNs with filtering for efficient neural decoding in implantable brain-machine interfaces. Neuromorph. Comput.Eng. 5, 014013 (2025).
Maji, P., Patra, R., Dhibar, K. & Mondal, H. K. Snn-based neuromorphic computing towards healthcare applications. IFIP International Internet of Things Conference. 261–271 (Springer).
Bhuiyan, M. N., Rahman, M. M., Billah, M. M. & Saha, D. Internet of Things (IoT): A review of its enabling technologies in healthcare applications, standards protocols, security, and market opportunities. IEEE Internet Things J. 8, 10474–10498 (2021).
Chen, J. et al. Generative AI-driven human digital twin in IoT-healthcare: A comprehensive survey. IEEE Internet Things J. (2024).
Leach, J., Achyuta, A. K. H. & Murthy, S. K. Bridging the divide between neuroprosthetic design, tissue engineering and neurobiology. Front. Neuroeng. 2, 1107 (2010).
Gori, M., Vadalà, G., Giannitelli, S. M., Denaro, V. & Di Pino, G. Biomedical and tissue engineering strategies to control foreign body reaction to invasive neural electrodes. Front. Bioeng. Biotechnol. 9, 659033 (2021).
Shen, K., Chen, O., Edmunds, J. L., Piech, D. K. & Maharbiz, M. M. Translational opportunities and challenges of invasive electrodes for neural interfaces. Nat. Biomed.l Eng.g 7, 424–442 (2023).
Ienca, M., Valle, G. & Raspopovic, S. Clinical trials for implantable neural prostheses: understanding the ethical and technical requirements. Lancet Digit. Health (2025).
Valle, G. et al. Tactile edges and motion via patterned microstimulation of the human somatosensory cortex. Science 387, 315–322 (2025).
Isik, M., Tiwari, K., Eryilmaz, M. B. & Dikmen, I. C. Accelerating sensor fusion in neuromorphic computing: A case study on loihi-2. In Proc. IEEE High Performance Extreme Computing Conference (HPEC) 1–7 (IEEE, 2024).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Lauer, J. et al. Multi-animal pose estimation, identification and tracking with DeepLabCut. Nat. Methods 19, 496–504 (2022).
Moro, M., Marchesi, G., Hesse, F., Odone, F. & Casadio, M. Markerless vs. marker-based gait analysis: A proof of concept study. Sensors 22, 2022 (2011).
Carrillo, M. R. Artificial intelligence: From ethics to law. Telecommun. Policy 44, 101937 (2020).
Togooch, B.-E. & Norovsambuu, G. Transhumanism and the Future of Consciousness: The Integration of Humans and Machines. Eur. J. AI, Comput. Inform. 1, 12–17 (2025).
Sahasrabudhe, A., Cea, C. & Anikeeva, P. Multifunctional bioelectronics for brain–body circuits. Nat. Rev. Bioeng. 1–20 (2025).
Glannon, W. Ethical and social aspects of neural prosthetics. Prog. Biomed. Eng. 4, 012004 (2021).
Van Stuijvenberg, O. C., Samlal, D. P., Vansteensel, M. J., Broekman, M. & Jongsma, K. R. The ethical significance of user-control in AI-driven speech-BCIs: a narrative review. Front. Hum. Neurosci. 18, 1420334 (2024).
Shaw, J. Artificial intelligence and ethics. Harv. Mag. 30, 1–11 (2019).
Hendrycks, D. Introduction to AI safety, ethics, and society. (Taylor & Francis, 2025).
Haag, L., Starke, G., Ploner, M. & Ienca, M. Ethical gaps in closed-loop neurotechnology: a scoping review. NPJ Digit. Med. 8, 510 (2025).
Robertson, L. J., Higgins, N. L., Maier, M. J., Carter, A. & Gardner, J. G. Ethical Governance Strategies For The Responsible Innovation Of Neurotechnologies: A Scoping Review. J. Bioethic. Inquiry, 1–18 (2025).
Tauginienė, L., Hummer, P., Albert, A., Cigarini, A. & Vohland, K. Ethical challenges and dynamic informed consent. Sci. Citizen Sci. 397 (2021).
Lay, W., Gasparini, L., Siero, W. & Hughes, E. K. A rapid review of the benefits and challenges of dynamic consent. Res. Ethics 21, 180–202 (2025).
Lin, S. Y., Mahoney, M. R. & Sinsky, C. A. Ten ways artificial intelligence will transform primary care. J. Gen. Intern. Med. 34, 1626–1630 (2019).
Mennella, C., Maniscalco, U., De Pietro, G. & Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 10 (2024).
Bruno, U. et al. From neuromorphic to neurohybrid: transition from the emulation to the integration of neuronal networks. Neuromorph. Comput. Eng. 3, 023002 (2023).
Grassia, F., Levi, T., Doukkali, E. & Kohno, T. Spike pattern recognition using artificial neuron and spike-timing-dependent plasticity implemented on a multi-core embedded platform. Artif. Life Robot. 23, 200–204 (2018).
Neftci, E. O., Mostafa, H. & Zenke, F. Surrogate gradient learning in spiking neural networks: Bringing the power of gradient-based optimization to spiking neural networks. IEEE Signal. Process. Mag. 36, 51–63 (2019).
Grassia, F. et al. Tunable neuromimetic integrated system for emulating cortical neuron models. Front. Neurosci. 5, 134 (2011).
Carvalho, M. & Ludermir, T. B. Particle swarm optimization of neural network architectures andweights. In Proc. 7th International Conference on Hybrid Intelligent Systems (HIS 2007) 336–339 (IEEE, 2007).
Snoek, J., Larochelle, H. & Adams, R. P. Practical bayesian optimization of machine learning algorithms. Adv. Neural Inf. Process. Syst. 25 (2012).
Pospischil, M. et al. Minimal Hodgkin–Huxley type models for different classes of cortical and thalamic neurons. Biol. Cybern. 99, 427–441 (2008).
Palumbo, A., Vizza, P., Calabrese, B. & Ielpo, N. Biopotential signal monitoring systems in rehabilitation: A review. Sensors 21, 7172 (2021).
Guo, L. et al. 68-channel neural signal processing system-on-chip with integrated feature extraction, compression, and hardware accelerators for neuroprosthetics in 22 nm FDSOI. Front. Neurosci. 18, 1432750 (2024).
Zitzler, E. & Künzli, S. Indicator-based selection in multiobjective search. In Proc. International Conference on Parallel Problem Solving From Nature 832–842 (Springer).
Cranmer, K., Brehmer, J. & Louppe, G. The frontier of simulation-based inference. Proc. Natl. Acad. Sci. 117, 30055–30062 (2020).
Gonçalves, P. J. et al. Training deep neural density estimators to identify mechanistic models of neural dynamics. Elife 9, e56261 (2020).
Pehle, C. et al. The BrainScaleS-2 accelerated neuromorphic system with hybrid plasticity. Front. Neurosci. 16, 795876 (2022).
Ahmed, A. A. et al. Interfacing with the brain: How nanotechnology can contribute. ACS Nano 19, 10630–10717 (2025).
Keene, S. T. et al. A biohybrid synapse with neurotransmitter-mediated plasticity. Nat. Mater. 19, 969–973 (2020).
George, R. et al. Plasticity and adaptation in neuromorphic biohybrid systems. iScience 23 (2020).
Quintana, F. M., Perez-Pena, F. & Galindo, P. L. Bio-plausible digital implementation of a reward modulated STDP synapse. Neural Comput. Appl. 34, 15649–15660 (2022).
Shaikh, S. & Basu, A. Intelligent intracortical brain-machine interfaces: Next generation of scalable neural interfaces. In Handbook of Biochips: Integrated Circuits and Systems for Biology and Medicine 869–889 (Springer, 2022).
Shaik, T. et al. Remote patient monitoring using artificial intelligence: Current state, applications, and challenges. Wiley Interdiscip.Rev. Data Min. Knowl. Discov. 13, e1485 (2023).
Liu, S. et al. An ultrasmall organic synapse for neuromorphic computing. Nat. Commun. 14, 7655 (2023).
Matrone, G. M. et al. A modular organic neuromorphic spiking circuit for retina-inspired sensory coding and neurotransmitter-mediated neural pathways. Nat. Commun. 15, 2868 (2024).
Boufidis, D., Garg, R., Angelopoulos, E., Cullen, D. K. & Vitale, F. Bio-inspired electronics: Soft, biohybrid, and “living” neural interfaces. Nat. Commun. 16, 1861 (2025).
Boulingre, M., Portillo-Lara, R. & Green, R. A. Biohybrid neural interfaces: improving the biological integration of neural implants. Chem. Commun. 59, 14745–14758 (2023).
Szczecinski, N. S. Biomimetic and Biohybrid Systems. Vol. 14930 (Springer Nature, 2024).
Mazzolai, B., Walker, I. & Speck, T. Generation growbots: Materials, mechanisms, and biomimetic design for growing robots. Front. Robot. AI 8, 711942 (2021).
Yang, Y., He, Z., Jiao, P. & Ren, H. Bioinspired soft robotics: How do we learn from creatures?. IEEE Rev. Biomed. Eng. 17, 153–165 (2022).
Wang, S. & Yu, S.-H. Advances in biomimetic materials. Small Methods 8, e2301487 (2024).
Suárez, L. E., Richards, B. A., Lajoie, G. & Misic, B. Learning function from structure in neuromorphic networks. Nat. Mach. Intell. 3, 771–786 (2021).
Ham, D., Park, H., Hwang, S. & Kim, K. Neuromorphic electronics based on copying and pasting the brain. Nat. Electron. 4, 635–644 (2021).
Cheslet, J. et al. Biomimetic snake locomotion using central pattern generators network and bio-hybrid robot perspective. Artif. Life Robot. 29, 479–485 (2024).
Okorokova, E. V., He, Q. & Bensmaia, S. J. Biomimetic encoding model for restoring touch in bionic hands through a nerve interface. J. Neural Eng. 15, 066033 (2018).
Valle, G. et al. Biomimetic computer-to-brain communication enhancing naturalistic touch sensations via peripheral nerve stimulation. Nat. Commun. 15, 1151 (2024).
Acknowledgements
This work was supported by Università Italo-Francese/Université Franco-Italienne PHC Galileo as part of the ‘NEUROHYSTIM’ project (to MC and TL), by the NATO Science for Peace and Security Programme ‘BIONIC’ (#G8447, to MC and TL), by the French ANR-23-CE45-0001 IRVIN (to TL), and by the European Union - NextGenerationEU and by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.5, project “RAISE - Robotics and AI for Socio-economic Empowerment” (ECS00000035) to MC. MC is part of RAISE Innovation Ecosystem. The authors would like to thank Dr Romain Beaubois and Dr Jeremy Cheslet for useful discussion and careful proofreading the manuscript. The authors would like to thank professional product and visual designer Silvia Chiappalone for creating the original graphic elements used in Figs. 1 and 4, and in the figure included in Box 1.
Author information
Authors and Affiliations
Contributions
MC and TL co-wrote this perspective and made the figures. Both authors edited and proofread the manuscript and authorized the submission of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wilfred van der Wiel, Xumeng Zhang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chiappalone, M., Levi, T. Advancing neuroengineering with Neuromorphic Twins. Nat Commun 17, 1938 (2026). https://doi.org/10.1038/s41467-026-68923-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-026-68923-1