Abstract
Chloroplasts regulate plant development and immunity. Here we report that potato chloroplast elongation factors StTuA and StTuB, targeted by Phytophthora infestans RXLR effector Pi22926, positively regulate immunity and growth. Plants expressing Pi22926, or silenced for TuA/B, show increased P. infestans susceptibility and decreased photosynthesis, plant growth and tuber yield. By contrast, StTuA/B overexpression reduces susceptibility, elevates chloroplast-derived reactive oxygen species production and increases photosynthesis and potato tuber yield by enhancing chloroplast protein translation. Another plant target of Pi22926, StMAP3Kβ2, interacts with StTuB, phosphorylating it to promote its translocation into chloroplasts. However, Pi22926 attenuates StTuB association with StMAP3Kβ2 and phosphorylation. This reduces StTuB translocation into chloroplasts, leading to its proteasome-mediated turnover in the cytoplasm. We uncover new mechanisms by which a pathogen effector inhibits immunity by disrupting key chloroplast functions. This work shows that StTuA/B break the growth–immunity trade-off, promoting both disease resistance and yield, revealing the enormous potential of chloroplast biology in crop breeding.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in this published article (and its Supplementary Information files). The biological materials used in this study are available from Z.T. on reasonable request. Source data are provided with this paper.
References
He, Z., Webster, S. & He, S. Y. Growth–defense trade-offs in plants. Curr. Biol. 32, R634–R639 (2022).
Fan, M. et al. The bHLH transcription factor HBI1 mediates the trade-off between growth and pathogen-associated molecular pattern-triggered immunity in Arabidopsis. Plant Cell 26, 828–841 (2014).
Wang, J. et al. A single transcription factor promotes both yield and immunity in rice. Science 361, 1026–1028 (2018).
Liu, M. et al. Inducible overexpression of Ideal Plant Architecture1 improves both yield and disease resistance in rice. Nat. Plants 5, 389–400 (2019).
Lu, Y. & Yao, J. Chloroplasts at the crossroad of photosynthesis, pathogen infection and plant defense. Int. J. Mol. Sci. 19, 3900 (2018).
Wu, A., Hammer, G. L., Doherty, A., von Caemmerer, S. & Farquhar, G. D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 5, 380–388 (2019).
Breen, S. et al. Chloroplasts play a central role in facilitating MAMP-triggered immunity, pathogen suppression of immunity and crosstalk with abiotic stress. Plant Cell Environ. 45, 3001–3017 (2022).
Grant, M. R. & Jones, J. D. G. Hormone (dis) harmony moulds plant health and disease. Science 324, 750–724 (2009).
Nomura, H. et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 3, 926 (2012).
Mullineaux, P. M., Exposito-Rodriguez, M., Laissue, P. P., Smirnoff, N. & Park, E. Spatial chloroplast-to-nucleus signalling involving plastid–nuclear complexes and stromules. Phil. Trans. R. Soc. Lond. B 375, 20190405 (2020).
Caplan, J. L. et al. Chloroplast stromules function during innate immunity. Dev. Cell 34, 45–57 (2015).
Liu, J. et al. Chloroplast immunity: a cornerstone of plant defense. Mol. Plant 17, 686–688 (2024).
Gao, L. L., Hong, Z. H., Wang, Y. & Wu, G. Z. Chloroplast proteostasis: a story of birth, life and death. Plant Commun. 4, 100424 (2022).
Krab, I. M. & Parmeggiani, A. Mechanisms of EF-Tu, a Pioneer GTPase. Prog. Nucleic Acid Res. Mol. Biol. 71, 513–551 (2002).
Cai, L. et al. Nuclear encoded elongation factor EF-Tu is required for chloroplast development in rice grown under low-temperature conditions. J. Genet. Genomics 49, 502–505 (2022).
Li, X. et al. Plastid translation elongation factor Tu is prone to heat-induced aggregation despite its critical role in plant heat tolerance. Plant Physiol. 176, 3027–3045 (2018).
Kunze, G. et al. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16, 3496–3507 (2004).
Zipfel, C. et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125, 749–760 (2006).
Breen, S., McLellan, H., Birch, P. R. J. & Gilroy, E. M. Tuning the wavelength: manipulation of light signaling to control plant defense. Int. J. Mol. Sci. 24, 3803 (2023).
de Torres Zabala, M. et al. Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 1, 15074 (2015).
Xu, Q. et al. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nat. Commun. 10, 5571 (2019).
Liu, R. et al. A Plasmopara viticola RXLR effector targets a chloroplast protein PsbP to inhibit ROS production in grapevine. Plant J. 106, 1557–1570 (2021).
Gao, C. et al. Pathogen manipulation of chloroplast function triggers a light-dependent immune recognition. Proc. Natl Acad. Sci. USA 117, 9613–9620 (2020).
Ren, Y. et al. Phytophthora infestans RXLR effectors target parallel steps in an immune signal transduction pathway. Plant Physiol. 180, 2227–2239 (2019).
McLellan, H. et al. Exploiting breakdown in nonhost effector-target interactions to boost host disease resistance. Proc. Natl Acad. Sci. USA 119, e2114064119 (2022).
Wang, S. et al. Phytophthora infestans RXLR effectors act in concert at diverse subcellular locations to enhance host colonization. J. Exp. Bot. 70, 343–356 (2019).
Zavaliev, R., Mohan, R., Chen, T. & Dong, X. Formation of NPR1 condensates promotes cell survival during the plant immune response. Cell 182, 1093–1108 (2020).
Van Hoewyk, D. Use of the non-radioactive SUnSET method to detect decreased protein synthesis in proteasome inhibited Arabidopsis roots. Plant Methods 12, 20 (2016).
Amanda, P. et al. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 377, 851–854 (2022).
Thomson, S. M., Pulido, P. & Jarvis, R. P. Protein import into chloroplasts and its regulation by the ubiquitin-proteasome system. Biochem. Soc. Trans. 48, 71–82 (2020).
Lamberti, G., Drurey, C., Soll, J. & Schwenkert, S. The phosphorylation state of chloroplast transit peptides regulates preprotein import. Plant Signal. Behav. 6, 1918–1920 (2011).
Boevink, P. C., Birch, P. R. J., Turnbull, D. & Whisson, S. C. Devastating intimacy: the cell biology of plant–Phytophthora interactions. New Phytol. 228, 445–458 (2020).
Liu, S. et al. The transcription factor StMYB113 regulates light-induced greening by modulating steroidal glycoalkaloid biosynthesis in potatoes (Solanum tuberosum L.). Hortic. Adv. 2, 7 (2024).
Qi, Y. et al. Heterologous overexpression of StERF3 triggers cell death in Nicotiana benthamiana. Plant Sci. 315, 111149 (2022).
Yan, P. et al. Nimble cloning: a simple, versatile, and efficient system for standardized molecular cloning. Front. Bioeng. Biotechnol. 7, 460 (2019).
Saunders, D. G. et al. Host protein BSL1 associates with Phytophthora infestans RXLR effector AVR2 and the Solanum demissum immune receptor R2 to mediate disease resistance. Plant Cell 24, 3420–3434 (2012).
Gilroy, E. M. et al. Involvement of cathepsin B in the plant disease resistance hypersensitive response. Plant J. 52, 1–13 (2007).
Li, H. et al. Pathogen protein modularity enables elaborate mimicry of a host phosphatase. Cell 186, 3196–3207 (2023).
Zhao, Y. & Zhou, J. M. Luciferase complementation assay for detecting protein interactions. Chin. Bull. Bot. 55, 69–75 (2020).
Wellburn, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144, 307–313 (1994).
Klinkenberg, J. Extraction of chloroplast proteins from transiently transformed Nicotiana benthamiana leaves. Bio Protoc. 4, e1238 (2014).
Li, X. et al. Deciphering the regulatory network of the NAC transcription factor FvRIF, a key regulator of strawberry (Fragaria vesca) fruit ripening. Plant Cell 35, 4020–4045 (2023).
Zurbriggen, M. D. et al. Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J. 60, 962–973 (2009).
Li, L. et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15, 329–338 (2014).
Cikos, S., Bukovska, A. & Koppel, J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol. Biol. 8, 113 (2007).
Acknowledgements
We acknowledge the financial support from the National Key R&D Program of China (grant no. 2023YFF1000404), the National Natural Science Foundation of China (grant nos. 32372172, 32072121, 31761143007 to Z.T.) and the Science and Technology Planning Projects of Zhejiang Province (grant no. 2024SSYS0100 to Y.Q.). P.R.J.B was supported by European Research Council (ERC) Advanced Grant PathEVome 787764; Biotechnology and Biological Sciences Research Council Grants BB/S003096/1, BB/P020569 and BB/L026880/1.
Author information
Authors and Affiliations
Contributions
Z.T., Y.Q. and P.R.J.B. planned and designed the research. Y.Q., J.W., Z.Y., H.L., L.L., H.W., X.S., Xinya Wu, J.N., J.Z., M.X., Xintong Wu, R.Y., D.C., Q.S., H.Q. and Y.Z. performed the experiments. Y.Q., S.B., P.R.J.B. and Z.T. wrote the paper with input from all authors. Y.Q., S.B., P.C.B., P.R.J.B. and Z.T. analysed data and revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. On the basis of the results in this study, the authors have applied for patents (application no. 202210963447.3).
Peer review
Peer review information
Nature Plants thanks Dong-Lei Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Phenotype of OE-Pi22926 transgenic N. benthamiana lines.
a, b, Western blots show the protein levels of GFP-Pi22926 in transgenic overexpression potato and N. benthamiana lines using anti-GFP antibody. Protein loading is indicated by Ponceau stain. The experiments were repeated at least twice with similar results. c, Representative OE-Pi22926 transgenic N. benthamiana and wild type N. benthamiana plants. Images taken at 4 w after planting. Scale bar, 2 cm. d, Graph showing that the heights of OE-Pi22926 lines were lower than that of N. b (n = 6 individual transgenic plants). e, Graph showing chlorophyll content of OE-Pi22926 transgenic N. benthamiana lines (n = 5 biological replicates). f, Graph showing biomass of OE-Pi22926 transgenic N. benthamiana lines (n = 6 individual transgenic plants). g, Representative images showing the P. infestans infection areas on OE-Pi22926 transgenic N. benthamiana leaves. Scale bar, 2 cm. h, Graph shows a significant increase in P. infestans lesion sizes in transgenic lines compared to N. benthamiana control which was measured at 4 dpi and 5 dpi (Three biological replicates, n = 40). Centre lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend to 1.5 × the interquartile range from the 25th and 75th percentiles, each point represents the lesion area of an individual leaf with precise n numbers in figure. i Graph showing the flg22-induced ROS production in OE-Pi22926 N. benthamiana leaves (n = 8 biological replicates, error bars are ± SE). Leaves were treated with 10 μM flg22 before ROS production was measured. RLU, relative luminescence units. In d, e, f and g, data are presented as mean values +/− SEM with different asterisks refer to significant difference based on one-way ANOVA, comparing the mean of each column with the mean of the control N. b (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001). Exact P values are shown in source data with 95% confidence intervals.
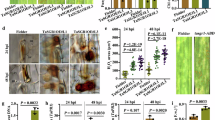
Extended Data Fig. 2 TuA and TuB positively regulate plant immunity.
a, b, VIGS of both NbTuA and NbTuB in N. benthamiana significantly increased P. infestans lesion sizes compared to TRV2:GFP control (n represents the total number of measured lesions with 3 biological replicates, **** P < 0.0001, unpaired t test). Left leaves in (b) show the phenotype of VIGS plants. Right leaves are P. infestans infected leaf images under UV light. c–f, Graph shows a significant decrease in P. infestans lesion and sporangia after inoculation with HB0914-2 on StTuA-GFP and StTuB-GFP transgenic potato lines compared to the Desiree control (for sporangia counting, n = 9). g–j, Transgenic StTuA-GFP and StTuB-GFP N. benthamiana lines showed significantly increased late blight resistance. k, l, Transgenic N. benthamiana lines overexpressing StTuA-GFP and StTuB-GFP showed a significant decrease in P. capsici lesion size compared to the N. benthamiana (WT) control. In c-f, g, i, k, data are presented as mean values +/− SEM with different asterisks refer to significant difference based on one-way ANOVA, comparing the mean of each column with the mean of the control N. b (n represents the total number of measured lesions with 3 biological replicates, ** P < 0.01, *** P < 0.001, **** P < 0.0001). The white circle represents the P. capsici lesion area. h, j, l, representative leaf images taken under UV light. In a, c, e, g, I and k, centre lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend to 1.5 × the interquartile range from the 25th and 75th percentiles, the top line represents maxima and bottom line represents minima, each point represents the lesion area of an individual leaf with precise n numbers in figures. Exact P values are shown in source data with 95% confidence intervals.
Extended Data Fig. 3 Formation of chloroplast stromules in StTuA/B overexpressing N. benthamiana leaves.
a, Chloroplast stromules (indicated by arrows) formed in leaves of OE-StTuB transgenic N. benthamiana lines. Transgenic N. benthamiana leaves were treated with 10 μM flg22, DMSO, H2O or P. infestans. Fluorescence was detected by confocal microscopy 30 min after flg22 treatment or 48 h after P. infestans infection. Bar = 5 μm. This experiment was repeated independently at least twice with similar results. b, Chloroplast stromules formed after flg22 treatment in N. benthamiana leaves transiently expressing StTuB-GFP or cTP-GFP. GFP fluorescence shows the expression of StTuB-GFP or cTP-GFP, and the merged images contain the fluorescence of GFP, RFP-H2B and chlorophyll. c, Graph shows the percentage of chloroplasts showing stromule formation in StTuB-GFP or cTP-GFP-expressing plants treated with flg22. Bar = 10 μm. Data are presented as mean values +/− SEM with different asterisks refer to significant difference based on unpaired t-test (*** P < 0.001, n = 5 biological replicates). Exact P values are shown in source data with 95% confidence intervals.
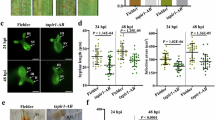
Extended Data Fig. 4 StTuA and StTuB overexpression increases potato plant height, chlorophyll content and N. benthamiana plant biomass.
a, Representative images of OE-StTuA, OE-StTuB and StTuA/B-RNAi transgenic and Desiree (WT) plants taken under white light, bar = 5 cm. b–d, Chlorophyll a/b content of OE-StTuA, OE-StTuB and StTuA/B-RNAi transgenic lines and control Desiree potato (n = 3 biological replicates). Data are presented as mean values +/− SEM with different asterisks refer to significant difference based on one-way ANOVA, comparing the mean of each column with the mean of the control Desiree (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001). Bar = 1 μm. e, Representative plants and leaves of OE-StTuB N. benthamiana transgenic lines and WT (left; N. b), bar = 5 cm. f, Bar graph shows a significant biomass increase in OE-StTuB transgenic lines compared to the WT control. Data are presented as mean values +/− SEM with different asterisks refer to significant difference based on one-way ANOVA, comparing the mean of each column with the mean of the control N. b (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, n = 6 individual transgenic plants). Exact P values are shown in source data with 95% confidence intervals.
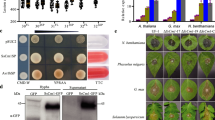
Extended Data Fig. 5 Overexpression of StTuA and StTuB enhances chloroplast protein translation.
a, Chloroplast protein synthesis efficiency in leaves of wild type (Desiree) and OE-StTuA/B transgenic potato plants. Total chloroplast protein was determined by antipuromycin (α-Pur) western blot following a 1- h time course after puromycin treatment. Western blot probed with anti-GFP shows the amount of StTuA/B-GFP in each sample. The protein loading in each lane was 90 μg. The experiment was repeated twice with similar results. b, Heatmap showing relative fold-changes of chloroplast protein from Desiree (WT) vs OE-StTuB (line 12, 18) plant leaves under light and flg22 treatment (from chloroplast proteomics data). The proteins in the red frame are related to photosynthesis, in the blue frame are enzymes linked to ROS production, and in the green frame are disease resistance-related proteins. c, The presence of PsbD, GLYK, FSD1, tAPX which appeared in the proteomics data (b) was confirmed by western blotting using chloroplast protein extraction from transgenic line OE-StTuB12 and 18). The amount of the protein loaded in each lane was 50 μg. Mean fold change of protein level of PsbD, GLYK, FSD1, tAPX from proteome mass spectrometry analysis was presented at the bottom of the band. All of protein level of WT under light and flg22 was normalised to 1.00. The experiments were repeated independently at least twice with similar results.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17 with figure legends and unprocessed blots in Supplementary Figs. 1, 2, 9, 11, 13–16.
Supplementary Tables
Supplementary Table 1. Targets of Pi22926 by Y2H screening. Supplementary Table 2. PSMs of TuA with and without cTP. Supplementary Table 3. The sequence of StTuA/B-RNAi, StTuA-RNAi and StTuB-RNAi fragment. Supplementary Table 4. Proteome sequencing data of StTuB12/18 under light and flg22 treatment. Supplementary Table 5. Primers used to construct vectors and RT–qPCR. Supplementary Table 6. Information on genes used in the paper.
Supplementary Data
Statistical source data for Supplementary Figs. 4–6, 8, 10, 12 and 15.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, Y., Wu, J., Yang, Z. et al. Chloroplast elongation factors break the growth–immunity trade-off by simultaneously promoting yield and defence. Nat. Plants 10, 1576–1591 (2024). https://doi.org/10.1038/s41477-024-01793-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41477-024-01793-x
This article is cited by
-
Genetic and transcriptomic identification of WPG1 controlling nitrogen allocation-related leaf chlorisis and premature senescence in wheat
Theoretical and Applied Genetics (2026)
-
Histone ZmH2B regulates resistance to the Southern corn leaf blight pathogen Bipolaris maydis in maize
BMC Plant Biology (2025)
-
Low-intensity laser exposure enhances rice (Oryza sativa L.) growth through physio-biochemical regulation, transcriptional modulation, and microbiome alteration
BMC Plant Biology (2025)