Abstract
Plant pathogens secrete numerous effectors to promote host infection, but whether any of these toxic proteins undergoes phase separation to manipulate plant defence and how the host copes with this event remain elusive. Here we show that the effector FolSvp2, which is secreted from the fungal pathogen Fusarium oxysporum f. sp. lycopersici (Fol), translocates a tomato iron-sulfur protein (SlISP) from plastids into effector condensates in planta via phase separation. Relocation of SlISP attenuates plant reactive oxygen species production and thus facilitates Fol invasion. The action of FolSvp2 also requires K205 acetylation that prevents ubiquitination-dependent degradation of this protein in both Fol and plant cells. However, tomato has evolved a defence protein, SlPR1. Apoplastic SlPR1 physically interacts with and inhibits FolSvp2 entry into host cells and, consequently, abolishes its deleterious effect. These findings reveal a previously unknown function of PR1 in countering a new mode of effector action.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The LC–MS/MS raw data are publicly available via figshare (FolSvp2 peptides obtained from FolSvp2-GFP expressed in Fol, https://doi.org/10.6084/m9.figshare.24903507.v1 (ref. 60); target proteins obtained from the GFP or FolSvp2-GFP copurified, https://doi.org/10.6084/m9.figshare.24903546.v1 (ref. 61); FolSvp2 peptides obtained from FolSvp2-GFP expressed in tobacco leaves, https://doi.org/10.6084/m9.figshare.24903540.v1 (ref. 62); identification of FolSvp2 K205 acetylation, https://doi.org/10.6084/m9.figshare.25909660 (ref. 63)). Source data are provided with this paper.
References
Zhang, J., Coaker, G., Zhou, J.-M. & Dong, X. Plant immune mechanisms: from reductionistic to holistic points of view. Mol. Plant 13, 1358–1378 (2020).
Zipfel, C. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 20, 10–16 (2008).
Trösch, R. et al. Commonalities and differences of chloroplast translation in a green alga and land plants. Nat. Plants 4, 564–575 (2018).
Trösch, R. et al. Fast and global reorganization of the chloroplast protein biogenesis network during heat acclimation. Plant Cell 34, 1075–1099 (2022).
Shumskaya, M., Bradbury, L. M., Monaco, R. R. & Wurtzel, E. T. Plastid localization of the key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. Plant Cell 24, 3725–3741 (2012).
Trösch, R., Mühlhaus, T., Schroda, M. & Willmund, F. ATP-dependent molecular chaperones in plastids — More complex than expected. Biochim. Biophys. Acta 1847, 872–888 (2015).
Xu, Q. et al. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nat. Commun. 10, 1–13 (2019).
Foyer, C. H. & Hanke, G. ROS production and signalling in chloroplasts: cornerstones and evolving concepts. Plant J. 111, 642–661 (2022).
Mittler, R. ROS are good. Trends Plant Sci. 22, 11–19 (2017).
Lan, Y. et al. PetM is essential for the stabilization and function of the cytochrome b6f complex in Arabidopsis. Plant Cell Physiol. 62, 1603–1614 (2021).
Balk, J. & Pilon, M. Ancient and essential: the assembly of iron–sulfur clusters in plants. Trends Plant Sci. 16, 218–226 (2011).
Wang, Y., Pruitt, R. N., Nürnberger, T. & Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 20, 449–464 (2022).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Hyman, A. A., Weber, C. A. & Jülicher, F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014).
Nakamura, T. et al. Phase separation of FSP1 promotes ferroptosis. Nature 619, 371–377 (2023).
Alberti, S., Gladfelter, A. & Mittag, T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176, 419–434 (2019).
Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012).
Boeynaems, S. et al. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 28, 420–435 (2018).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Kong, X. et al. Antagonistic interaction between auxin and SA signaling pathways regulates bacterial infection through lateral root in Arabidopsis. Cell Rep. 32, 108060 (2020).
Li, J. et al. Enhancing tomato resistance by exploring early defense events against Fusarium wilt disease. Phytopathol. Res. 5, 24 (2023).
Breen, S., Williams, S. J., Outram, M., Kobe, B. & Solomon, P. S. Emerging insights into the functions of pathogenesis-related protein 1. Trends Plant Sci. 22, 871–879 (2017).
Chen, Y.-L. et al. Quantitative peptidomics study reveals that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell 26, 4135–4148 (2014).
Sung, Y. C. et al. PR1-mediated defence via C-terminal peptide release is targeted by a fungal pathogen effector. New Phytol. 229, 3467–3480 (2021).
Niderman, T. et al. Pathogenesis-related PR-1 proteins are antifungal. Isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiol. 108, 17–27 (1995).
Woloshuk, C. P., Meulenhoff, J. S., Sela-Buurlage, M., Elzen, P. & Cornelissen, B. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell 3, 619–628 (1991).
Choudhary, V. & Schneiter, R. Pathogen-Related Yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proc. Natl Acad. Sci. USA 109, 16882–16887 (2012).
Gamir, J. et al. The sterol-binding activity of pathogenesis-related protein 1 reveals the mode of action of an antimicrobial protein. Plant J. 89, 502–509 (2016).
Li, J. et al. Acetylation of a fungal effector that translocates host PR1 facilitates virulence. Elife 11, e82628 (2022).
Yang, G. et al. A cerato-platanin protein SsCP1 targets plant PR1 and contributes to virulence of Sclerotinia sclerotiorum. New Phytol. 217, 739–755 (2018).
Luti, S., Sella, L., Quarantin, A., Pazzagli, L. & Baccelli, I. Twenty years of research on cerato-platanin family proteins: clues, conclusions, and unsolved issues. Fungal Biol. Rev. 34, 13–24 (2020).
Michielse, C. B. & Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 10, 311–324 (2009).
Li, J., Gao, M., Gabriel, D. W., Liang, W. & Song, L. Secretome-wide analysis of lysine acetylation in Fusarium oxysporum f. sp. lycopersici provides novel insights into infection-related proteins. Front. Microbiol. 11, 559440 (2020).
Carabetta, V. J., Greco, T. M., Cristea, I. M., & Dubnau, D. YfmK is an Ne-lysine acetyltransferase that directly acetylates the histone-like protein HBsu in Bacillus subtilis. Proc. Natl Acad. Sci. USA 116, 3752–3757 (2019).
Lim, J.-H., Park, J.-W. & Chun, Y.-S. Human arrest defective 1 acetylates and activates beta-catenin, promoting lung cancer cell proliferation. Cancer Res. 66, 10677–10682 (2006).
Tan, X. et al. Coronavirus subverts ER-phagy by hijacking FAM134B and ATL3 into p62 condensates to facilitate viral replication. Cell Rep. 42, 112286 (2023).
Ngou, B. P. M., Ding, P. & Jones, J. D. Thirty years of resistance: zig-zag through the plant immune system. Plant Cell 34, 1447–1478 (2022).
Yang, Q. et al. Broad-spectrum chemicals block ROS detoxification to prevent plant fungal invasion. Curr. Biol. 32, 3886–3897. e3886 (2022).
Zhang, N. et al. Pathogenic fungi neutralize plant-derived ROS via Srpk1 deacetylation. EMBO J. 42, e112634 (2023).
He, Q., McLellan, H., Boevink, P. C. & Birch, P. R. J. All roads lead to susceptibility: the many modes of action of fungal and oomycete intracellular effectors. Plant Commun. 1, 100050 (2020).
Li, Q. et al. A Phytophthora capsici virulence effector associates with NPR1 and suppresses plant immune responses. Phytopathol. Res. 1, 6 (2019).
Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227 (2005).
Yin, W., Wang, Y., Chen, T., Lin, Y. & Luo, C. Functional evaluation of the signal peptides of secreted proteins. Bio Protoc. 8, e2839 (2018).
Chen, C. et al. Pyrophosphate-fructose 6-phosphate 1-phosphotransferase (PFP1) regulates starch biosynthesis and seed development via heterotetramer formation in rice (Oryza sativa L.). Plant Biotechnol. J. 18, 83–95 (2020).
Yu, G. et al. The Arabidopsis E3 ubiquitin ligase PUB4 regulates BIK1 and is targeted by a bacterial type-III effector. EMBO J. 41, e107257 (2022).
Deng, L. et al. Efficient generation of pink-fruited tomatoes using CRISPR/Cas9 system. J. Genet. Genomics 45, 51–54 (2018).
Martin, K. et al. Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J. 59, 150–162 (2009).
Du, F. et al. Leaflet initiation and blade expansion are separable in compound leaf development. Plant J. 104, 1073–1087 (2020).
Qiu, X. et al. The Phytophthora sojae nuclear effector PsAvh110 targets a host transcriptional complex to modulate plant immunity. Plant Cell 35, 574–597 (2023).
Yu, G. et al. A bacterial effector protein prevents MAPK-mediated phosphorylation of SGT1 to suppress plant immunity. PLoS Pathog. 16, e1008933 (2020).
Ji, H.-M. et al. Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. New Phytol. 232, 705–718 (2021).
Shabab, M. et al. Fungal effector protein AVR2 targets diversifying defense-related cys proteases of tomato. Plant Cell 20, 1169–1183 (2008).
Dong, S. et al. Effector specialization in a lineage of the Irish potato famine pathogen. Science 343, 552–555 (2014).
Egelhofer, T. A. et al. An assessment of histone-modification antibody quality. Nat. Struct. Mol. Biol. 18, 91–93 (2011).
He, K. et al. Gasdermin D licenses MHCII induction to maintain food tolerance in small intestine. Cell 186, 3033–3048.e3020 (2023).
Ji, L. et al. AKAP1 deficiency attenuates diet-induced obesity and insulin resistance by promoting fatty acid oxidation and thermogenesis in brown adipocytes. Adv. Sci. 8, 2002794 (2021).
Schopper, S. et al. Measuring protein structural changes on a proteome-wide scale using limited proteolysis-coupled mass spectrometry. Nat. Protoc. 12, 2391–2410 (2017).
Yu, D. et al. A novel, easy and rapid method for constructing yeast two-hybrid vectors using In-Fusion technology. Biotechniques 64, 219–224 (2018).
Oates, M. E. et al. D2P2: database of disordered protein predictions. Nucleic Acids Res. 41, D508–D516 (2012).
Li, J. FolSvp2-GFP fungal expressed and purified LC-MS/MS data. figshare https://doi.org/10.6084/m9.figshare.24903507.v1 (2023).
Li, J. The GFP or FolSvp2-GFP copurified LC-MS/MS data. figshare https://doi.org/10.6084/m9.figshare.24903546.v1 (2023).
Li, J. FolSvp2-GFP planta expressed and purified LC-MS/MS data. figshare https://doi.org/10.6084/m9.figshare.24903540.v1 (2023).
Li,J. The raw LC–MS/MS data for identifying secretory acetylated proteins of Fusarium oxysporum f. sp. lycopersici. figshare https://doi.org/10.6084/m9.figshare.25909660.v1 (2024).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (32272489 (J.L.), 32325043 (W.L.)), Key Research and Development Program of Shandong Province (2022CXGC020709) to W.L. and Taishan Scholar Construction Foundation of Shandong Province (tshw20130963) to W.L.
Author information
Authors and Affiliations
Contributions
W.L. and J.L. conceived the idea and supervised the project. W.L., J.L. and L.Y. designed the experiments. J.L. and L.Y. performed most of the experiments and analysed data. S.D. and M.G. helped in making some constructs and Fusarium transformation and performed Y2H assays. Y.Y., G.Y. and Y.Z. helped in making constructs. J.L. and W.L. wrote the paper, and all authors contributed to revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Yet-Ran Chen, Yushi Luan and Lay-Sun Ma for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Generation and analysis of FolSvp2 transformants.
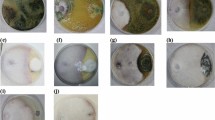
a, Expression profile of FolSvp2 in Fol with or without two-week-old tomato root incubation. The data are presented as the means ± SEs of three independent replicates. b, Genomic DNA was analyzed via PCR to determine which strains were harboring GFP, FolSvp2-GFP, or a K205 mutant (FolSvp2Q-GFP and FolSvp2R-GFP) under the RP27 constitutive promoter. The Histone H4 (FOXG_09402) was used as a positive PCR control. c, Secretion of FolSvp2 in the presence or absence of tomato roots. Conidia of strains harboring FolSvp2-GFP were inoculated into 5% liquid YEPD in the presence or absence of tomato roots. Forty hours after inoculation, proteins were extracted from mycelia or culture supernatants and probed with α-GFP and α-Actin. Coomassie brilliant blue (CBB) or silver staining was used to determine protein loading in each lane. d, Functional validation of the secreted signal peptide (SP) of FolSvp2. The yeast strains were cultured on CMD-W or YPRAA medium for 3 days. TTC was added to the culture supernatant to measure the enzymatic activity for reducing TTC to the red TPF. e, Secretion of FolSvp2 proteins in the presence of tomato roots. Protein extraction and identification were performed as described in (c). f, Homologous recombination-based deletion of FolSvp2. g, PCR analysis to identify the ∆FolSvp2 (KO1, KO9, KO26 and KO37) knockout and wild-type (WT) strains with the primer pairs FolSvp2-out-F/R, FolSvp2-in-F/R and H4-qRT-F/R. h, PCR analysis to identify the WT, ∆FolSvp2, complementation (∆FolSvp2-C) and K205 mutant (∆FolSvp2-CQ, ∆FolSvp2-CR) strains with the native promoter. The primer pairs FolSvp2-pQB-F/R, FolSvp2-pQB-F/pYF11-cGFP-R, FolSvp2-out-F/R and H4-qRT-F/R were used as described above. i, Mycelial growth of the WT, ∆FolSvp2 (∆FolSvp2-KO1), ∆FolSvp2-C, ∆FolSvp2-CQ and ∆FolSvp2-CR mutant strains on the PDA media, complete media (CM), and minimal media (MM) after 5 days of cultivation. Scale bars = 2 cm. j, Mycelial diameter and conidial production of the indicated strains in the indicated media or at the indicated times. No significant difference (ns) was indicated according to multiple comparison tests (mean ± SEM, n = 3). The experiments were performed three times with similar results obtained.
Extended Data Fig. 2 Identification of the ubiquitinated sites in FolSvp2 expressed in Fol and in planta.
a, LC-MS/MS identification of FolSvp2 ubiquitination in Fol. After MG132 treatment for 2 hours, the immunoprecipitated FolSvp2R-GFP was subjected to LC‒MS/MS analysis. The coverage (as highlighted in bold) of the FolSvp2 protein sequence was 75.9%. The ubiquitinated residues identified are highlighted in blue. b, Spectra showing FolSvp2 ubiquitination at K107 and K215. The representative y21 and y10 ions represent the assignment of K107 and K215 ubiquitination, respectively. c, LC-MS/MS identification of FolSvp2 ubiquitination in N. benthamiana. After MG132 treatment for 4 hours, the immunoprecipitated FolSvp2-GFP was subjected to LC‒MS/MS analysis. The coverage (as highlighted in bold) of the FolSvp2 protein sequence was 82.7%. The ubiquitinated residues identified are highlighted in blue. d, Spectrum showing FolSvp2 ubiquitination at K215. The representative y2 ion represents the assignment of K215 ubiquitination.
Extended Data Fig. 3 Localization of FolSvp2 and phenotypic analysis of FolSvp2 overexpression tomato seedlings.
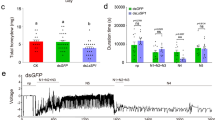
a, Subcellular localization of the WT and K205 mutant FolSvp2-GFP proteins with or without the native signal peptide (ΔSP) in tobacco. The indicated constructs were transiently expressed in nuclear (Nu) marker histone H2B-RFP-overexpressing N. benthamiana, and images were obtained at 3 DAI. The fluorescence intensity profiles of GFP and RFP were assessed along the transects shown as the green and magenta lines. Y-axis, fluorescence intensity (arbitrary units); X-axis, transect length (μm). Scale bars, 50 μm. b, Subcellular localization of GFP and FolSvp2-GFP in tomato. The FolSvp2-GFP protein were overexpressed in two tomato lines (FolSvp2-GFP-OE: #1, #3), and fluorescence was observed in the leaves and roots. The GFP were overexpressed (GFP-OE) in tomato as a control. Scale bars, 40 μm. c, Western blot analysis showing the protein expression described in (b). The extracted proteins were immunoblotted with α-GFP and α-actin. d, DAB staining showing H2O2 production in the indicated tomato root tissues infected with the Fol strain at 3 DAI (top). Microscopy observation of H2O2 accumulation after DAB staining in tomato roots (bottom). Scale bars = 100 µm. e, Quantification of H2O2 in the tomato roots in (d). The mean values (± SEM) of four replicates are shown. f, Resistance of WT, GFP-OE or FolSvp2-GFP-OE tomato seedlings to Fol strain infection at 14 DAI. g, Disease indices were scored at 14 DAI for the 14 plants in (f). (d) to (g) were generated as described in Fig. 5. All the experiments were performed three times with similar results obtained.
Extended Data Fig. 4 Subcellular localization of the WT and CP mutant FolSvp2-GFP proteins in tobacco leaves.
a, Schematic drawing of the WT and CP mutant FolSvp2 constructs without the native signal peptide (ΔSP). b, Subcellular localization of the WT and CP mutant FolSvp2-GFP proteins in N. benthamiana leaves. Images were taken at 3 DAI. Scale bars = 50 µm. c, Western blot analysis showing the expression of FolSvp2 protein in tobacco leaves in (b). Total proteins extracted were probed with α-GFP and α-actin. For (b) and (c), the experiments were repeated three times with similar observations.
Extended Data Fig. 5 Subcellular localization and functional analysis of SlISP in planta.
a, The SlISP-GFP or GFP construct was transiently expressed in N. benthamiana, and images were taken at 3 DAI. Scale bars, 20 μm. b, Western blot analysis showing the expression of the indicated proteins in (a). Total proteins extracted were probed with α-GFP. CBB staining was used to measure protein loading in each lane. c, Fluorescence observation of the roots of the GFP-ovexpressing (GFP-OE) or SlISP-GFP-overexpressing (SlISP-OE4) tomato line. Scale bars, 50 μm. d, Western blot analysis showing the expression of GFP or SlISP-GFP in (c). Total proteins and plastid proteins were extracted and probed with α-GFP and α-actin. Plastid proteins were immunoblotted with α-PetC to visualize the endogenous SlISP. e, Schematic of the WT SlISP target and the two selected guide RNA sequences (gRNA1 and gRNA2). The spacer sequence is shown in the dashed box. Three CRISPR-Cas9-induced SlISP mutants (Slisp-2, Slisp-5, and Slisp-10) in tomato plants were obtained through genetic transformation. The genotype of the mutation in Slisp-2 is based on base substitution and base deletion in the gRNA1 spacer sequence. The genotype of Slisp-5 is a 296-base deletion from positions 50 to 345 of the SlISP sequence. The genotype of Slisp-10 is a single-base deletion in the gRNA1 sequence, as shown by the dashed line. f, Western blot analysis showing the expression of SlISP proteins in the mutant lines. The extracted proteins were probed with anti-SlISP (α-PetC) and α-actin. g, Resistance of WT and CRISPR-Cas9-edited SlISP tomato seedlings to Fol infection. Disease indices of 10 plants were scored at 14 DAI. h, Western blot analysis showing GFP-overexpressing (GFP-OE) and three SlISP-GFP-overexpressing (SlISP-OE2, SlISP-OE4, and SlISP-OE21) tomato lines. Total proteins were extracted and probed with α-GFP and α-actin. i, Resistance of the indicated tomato seedlings to Fol infection. Disease indices (n=10) were scored as described in (g). All the experiments were repeated three times with similar observations.
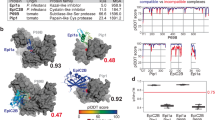
Extended Data Fig. 6 BiFC assays showing the interaction of SlISP with the WT, IDR2 and CP mutant FolSvp2 proteins.
The indicated constructs were transiently expressed in N. benthamiana leaves for 3 days, after which images were taken. cYFP, C-terminal region of YFP; nYFP, N-terminal region of YFP. Scale bars = 20 µm. The experiments were repeated three times with similar observations.
Extended Data Fig. 7 Interaction of putative tomato PR1 proteins with FolSvp2.
a, Y2H assays showing the interaction of thirteen predicted PR1 proteins with FolSvp2. b, Amino acid sequence alignment of SlPR1 homologs in tomato using DNAMAN. The black line indicates the C-terminal CAPE peptide. The dashed box in red shows the CAPE cleavage motif. The three red asterisks indicate the N-terminal differential sites between SlPR1 and SlP14b. c, Y2H assays showing the interaction of WT and mutant (S101D, K114M and R116G) SlPR1 proteins with FolSvp2. SlP14b was used as the control. For (a) and (c), the experiments were performed three times with similar results.
Extended Data Fig. 8 BiFC assays showing the interaction of the full-length or truncated SlPR1 proteins with FolSvp2 containing the SP of SlPR1.
A schematic of the recombinant FolSvp2 and truncated SlPR1 constructs is shown (left). The indicated constructs were transiently expressed in N. benthamiana leaves for 3 days. Plasmolysis was visualized after treatment with 800 mM mannitol for 6 hours. Scale bars = 50 µm. Experiments were performed three times with similar results.
Extended Data Fig. 9 Interaction of SlPR1 with the WT and mutant FolSvp2 proteins.
a, Y2H assays showing the interaction of SlPR1 with the WT and mutant FolSvp2 proteins. b, Split-LUC assays (left) showing the interaction of SlPR1 with the WT and mutant FolSvp2 proteins without the native signal peptide. Scale bars = 1 cm. Western blot analysis (right) was performed to show the expression of the indicated proteins. Proteins extracted were probed with α-LUC and α-actin. c, Co-IP assays showing the interaction of SlPR1 with the WT and mutant SlPR1SPFolSvp2 proteins. The indicated constructs were transiently expressed in N. benthamiana leaves for 3 days, and the proteins copurified with GFP-Trap beads were probed with α-GFP and α-RFP. The input proteins were subjected to Western blotting with α-GFP, α-RFP and α-actin. All the experiments were repeated three times with similar observations.
Extended Data Fig. 10 Subcellular localization of SlPR1, SlP14b and FolSvp2 in tobacco leaves.
a, Fluorescence images of SlPR1-RFP, CAPE1 mutant SlPR1-RFP and SlP14b-RFP proteins. b, Fluorescence observation of GFP and SlPR1SPFolSvp2-GFP. For (a) and (b), the indicated constructs were transiently expressed in WT or H2B-RFP-overexpressing N. benthamiana leaves for 3 days. Plasmolysis was visualized after treatment with 800 mM mannitol for 6 hours. The experiments were performed three times with similar results. Scale bars = 50 µm.
Supplementary information
Supplementary Table 1
FolSvp2 peptides obtained from FolSvp2-GFP expressed in Fol after LC–MS/MS analysis.
Supplementary Table 2
Target proteins obtained from the GFP or FolSvp2-GFP copurified LC–MS/MS data.
Supplementary Table 3
FolSvp2 peptides obtained from FolSvp2-GFP expressed in tobacco leaves after LC–MS/MS analysis.
Supplementary Table 4
Annotation of 22 candidate FolSvp2-interacting proteins identified via Y2H screening.
Supplementary Table 5
Primers used in this study.
Supplementary Table 6
Synthesized genes used in Y2H study.
Supplementary Data 1
The source data for the charts or graphs.
Source data
Source Data Fig. 1
Unprocessed western blots and gels.
Source Data Fig. 2
Unprocessed western blots and gels.
Source Data Fig. 3
Unprocessed western blots and gels.
Source Data Fig. 4
Unprocessed western blots and gels.
Source Data Fig. 5
Unprocessed western blots and gels.
Source Data Fig. 6
Unprocessed western blots and gels.
Source Data Extended Data Fig. 1
Unprocessed western blots and gels.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots and gels.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Yang, L., Ding, S. et al. Plant PR1 rescues condensation of the plastid iron-sulfur protein by a fungal effector. Nat. Plants 10, 1775–1789 (2024). https://doi.org/10.1038/s41477-024-01811-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-024-01811-y
This article is cited by
-
The pathogen effector BcSSP2 suppresses the NPC phase separation to facilitate Botrytis cinerea infection
Nature Communications (2025)
-
Ubiquitination of susceptibility factor drives broad-spectrum resistance
Science China Life Sciences (2025)
-
A fungal effector hijacks a plastid protein to dampen plant immunity; PR1 is here for rescue
Stress Biology (2025)