Abstract
Cold acclimation is critical for the survival of plants in temperate regions under low temperatures, and C-REPEAT BINDING FACTORs (CBFs) are well established as key transcriptional factors that regulate this adaptive process by controlling the expression of cold-responsive genes. Here we demonstrate that CBFs are involved in modulating alternative splicing during cold acclimation through their interaction with subunits of the spliceosome complex. Under cold stress, CBF proteins accumulate and directly interact with SKI-INTERACTING PROTEIN (SKIP), a key component of the spliceosome, which positively regulates acquired freezing tolerance. This interaction facilitates the formation of SKIP nuclear condensates, which enhances the association between SKIP and specific cold-responsive transcripts, thereby increasing their splicing efficiency. Our findings uncover a regulatory role of CBFs in alternative splicing and highlight their pivotal involvement in the full development of cold acclimation, bridging transcriptional and post-transcriptional regulatory mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the main text or the supplementary tables. The biological materials used in this study are available from the corresponding author upon reasonable request. The sequencing data (RNA-seq) for gene expression and AS analysis have been deposited in the publicly available repository Sequence Read Archive under accession code PRJNA990533. The structures of the CBF2 and SKIP proteins can be found in the AlphaFold Protein Structure Database (https://alphafold.com/). Major genes mentioned in this study can be found in the TAIR database (https://www.arabidopsis.org/) under the following accession numbers: AT1G77180 (SKIP), AT3G13200 (CWC15), AT1G13030 (COILIN), AT1G49590 (ZOP1), AT1G04510 (MAC3A), AT3G50670 (U1-70K), AT4G03430 (STA1), AT1G16610 (SR45), AT1G08890 (ESL3.05), AT1G51430, AT5G28770 (BZIP63), AT4G25490 (CBF1), AT4G25470 (CBF2), AT4G25480 (CBF3), AT5G53010, AT5G06520 (SWAP), AT4G01400 (MISF74), AT5G63370 (CDKG1), AT1G25682 (CWC16A), AT1G08115 (U1A snRNA), AT3G56705 (U2.6 snRNA), AT1G61275 (U12 snRNA), AT5G40395 (U6ACAT snRNA) and AT5G42300 (UBL5). Source data are provided with this paper.
References
Ding, Y. et al. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 13, 544–564 (2020).
Thomashow, M. F. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571–599 (1999).
Shi, Y. et al. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 23, 623–637 (2018).
Jia, Y. et al. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 212, 345–353 (2016).
Song, Y. et al. The direct targets of CBFs: in cold stress response and beyond. J. Integr. Plant Biol. 63, 1874–1887 (2021).
Laloum, T. et al. Alternative splicing control of abiotic stress responses. Trends Plant Sci. 23, 140–150 (2018).
Staiger, D. & Brown, J. W. S. Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell 25, 3640–3656 (2013).
Calixto, C. P. G. et al. Rapid and dynamic alternative splicing impacts the Arabidopsis cold response transcriptome. Plant Cell 30, 1424–1444 (2018).
Filichkin, S. A. et al. Genome-wide mapping of alternative splicing in Arabidopsis thaliana. Genome Res. 20, 45–58 (2010).
James, A. B. et al. Alternative splicing mediates responses of the Arabidopsis circadian clock to temperature changes. Plant Cell 24, 961–981 (2012).
Wahl, M. C. et al. The spliceosome: design principles of a dynamic RNP machine. Cell 136, 701–718 (2009).
Carrasco-Lopez, C. et al. Environment-dependent regulation of spliceosome activity by the LSM2-8 complex in Arabidopsis. Nucleic Acids Res. 45, 7416–7431 (2017).
Lee, B.-h. et al. STABILIZED1, a stress-upregulated nuclear protein, is required for pre-mRNA splicing, mRNA turnover, and stress tolerance in Arabidopsis. Plant Cell 18, 1736–1749 (2006).
Legen, J. et al. A prion-like domain is required for phase separation and chloroplast RNA processing during cold acclimation in Arabidopsis. Plant Cell 36, 2851–2872 (2024).
Guan, Q. et al. A DEAD box RNA helicase is critical for pre-mRNA splicing, cold-responsive gene regulation, and cold tolerance in Arabidopsis. Plant Cell 25, 342–356 (2013).
Zhong, Y. et al. Pan-transcriptomic analysis reveals alternative splicing control of cold tolerance in rice. Plant Cell 36, 2117–2139 (2024).
Banani, S. F. et al. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Fang, X. & Li, P. SnapShot: condensates in plant biology. Cell 187, 2894–2894 (2024).
Tong, J. J. et al. ALBA proteins confer thermotolerance through stabilizing messenger RNAs in cytoplasmic granules. Nat. Plants 8, 778–791 (2022).
Zhu, P. et al. Cold-induced Arabidopsis FRIGIDA nuclear condensates for FLC repression. Nature 599, 657–661 (2021).
Wang, B. et al. Condensation of SEUSS promotes hyperosmotic stress tolerance in Arabidopsis. Nat. Chem. Biol. 18, 1361–1369 (2022).
Wang, X. et al. SKIP is a component of the spliceosome linking alternative splicing and the circadian clock in Arabidopsis. Plant Cell 24, 3278–3295 (2012).
Zhao, C. et al. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 171, 2744–2759 (2016).
Tian, T. et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 45, W122–W129 (2017).
Merico, D. et al. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE 5, e13984 (2010).
Chen, W. & Moore, M. J. Spliceosomes. Curr. Biol. 25, R181–R183 (2015).
Emenecker, R. J. et al. Emerging roles for phase separation in plants. Dev. Cell 55, 69–83 (2020).
Bhat, P. et al. Genome organization around nuclear speckles drives mRNA splicing efficiency. Nature 629, 1165–1173 (2024).
Lamond, A. I. & Spector, D. L. Nuclear speckles: a model for nuclear organelles. Nat. Rev. Mol. Cell Biol. 4, 605–612 (2003).
Lv, J. et al. Reciprocal regulation between the negative regulator PP2CG1 phosphatase and the positive regulator OST1 kinase confers cold response in Arabidopsis. J. Integr. Plant Biol. 63, 1568–1587 (2021).
Lim, G. H. et al. A putative novel transcription factor, AtSKIP, is involved in abscisic acid signalling and confers salt and osmotic tolerance in Arabidopsis. New Phytol. 185, 103–113 (2010).
Feng, J. et al. SKIP confers osmotic tolerance during salt stress by controlling alternative gene splicing in Arabidopsis. Mol. Plant 8, 1038–1052 (2015).
Li, Y. et al. The SNW domain of SKIP is required for its integration into the spliceosome and its interaction with the Paf1 complex in Arabidopsis. Mol. Plant 9, 1040–1050 (2016).
Krainer, G. et al. Reentrant liquid condensate phase of proteins is stabilized by hydrophobic and non-ionic interactions. Nat. Commun. 12, 1085 (2021).
Zhang, H. et al. Liquid–liquid phase separation in biology: mechanisms, physiological functions and human diseases. Sci. China Life Sci. 63, 953–985 (2020).
Oono, Y. et al. Monitoring expression profiles of Arabidopsis genes during cold acclimation and deacclimation using DNA microarrays. Funct. Integr. Genomics 6, 212–234 (2006).
Provart, N. J. et al. Gene expression phenotypes of Arabidopsis associated with sensitivity to low temperatures. Plant Physiol. 132, 893–906 (2003).
Slawinski, L. et al. Responsiveness of early response to dehydration six-like transporter genes to water deficit in leaves. Front Plant Sci. 12, 708876 (2021).
Khan, A. et al. Vacuolar sugar transporter EARLY RESPONSE TO DEHYDRATION6-LIKE4 affects fructose signaling and plant growth. Plant Physiol. 193, 2141–2163 (2023).
Thomashow, M. F. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol. 154, 571–577 (2010).
Pajoro, A. et al. Histone H3 lysine 36 methylation affects temperature-induced alternative splicing and flowering in plants. Genome Biol. 18, 102 (2017).
Huertas, R. et al. Arabidopsis SME1 regulates plant development and response to abiotic stress by determining spliceosome activity specificity. Plant Cell 31, 537–554 (2019).
Bhat, P. et al. Nuclear compartmentalization as a mechanism of quantitative control of gene expression. Nat. Rev. Mol. Cell Biol. 22, 653–670 (2021).
Liu, L. et al. A conserved interaction between SKIP and SMP1/2 aids in recruiting the second-step splicing factors to the spliceosome in Arabidopsis. Mol. Plant 9, 1660–1663 (2016).
Xing, D. H. et al. Transcriptome-wide identification of RNA targets of Arabidopsis SERINE/ARGININE-RICH45 uncovers the unexpected roles of this RNA binding protein in RNA processing. Plant Cell 27, 3294–3308 (2015).
Jia, J. et al. Post-transcriptional splicing of nascent RNA contributes to widespread intron retention in plants. Nat. Plants 6, 780–788 (2020).
Zhang, G. et al. mTOR regulates phase separation of PGL granules to modulate their autophagic degradation. Cell 174, 1492–1506 (2018).
Jiang, B. et al. Light-induced LLPS of the CRY2/SPA1/FIO1 complex regulating mRNA methylation and chlorophyll homeostasis in Arabidopsis. Nat. Plants 9, 2042–2058 (2023).
Novillo, F. et al. CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc. Natl Acad. Sci. USA 104, 21002–21007 (2007).
Liu, Z. Y. et al. Plasma membrane CRPK1-mediated phosphorylation of 14-3-3 proteins induces their nuclear import to fine-tune CBF signaling during cold response. Mol. Cell 66, 117–128 (2017).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Shi, Y. et al. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 24, 2578–2595 (2012).
Wang, Z. et al. Protocol for analyzing protein liquid–liquid phase separation. Biophys. Rep. 5, 1–9 (2019).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Ding, Y. et al. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 32, 278–289 (2015).
Xue, B. et al. PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochim. Biophys. Acta 1804, 996–1010 (2010).
Varadi, M. et al. AlphaFold Protein Structure Database in 2024: providing structure coverage for over 214 million protein sequences. Nucleic Acids Res 5, 368–375 (2024).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Fang, X. et al. Arabidopsis FLL2 promotes liquid–liquid phase separation of polyadenylation complexes. Nature 569, 265–269 (2019).
Davis, M. P. et al. Kraken: a set of tools for quality control and analysis of high-throughput sequence data. Methods 63, 41–49 (2013).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y. et al. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I. et al. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Shen, S. et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-seq data. Proc. Natl Acad. Sci. USA 111, E5593–E5601 (2014).
Wierzbicki, A. T. et al. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135, 635–648 (2008).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013); https://www.r-project.org/
Acknowledgements
We thank L. Ma for providing skip-1, skip-1 proSKIP::SKIPNSNW–GFP, and skip-1 proSKIP::SKIP–GFP seeds; and X. Fang, Y. Miao, J. Li and J. Hua for helpful suggestions and discussions. This work was supported by the National Natural Science Foundation of China (grant nos 32230005, 32022008 and 31921001), and the Pinduoduo–China Agricultural University Research Fund (PC2023B01001).
Author information
Authors and Affiliations
Contributions
Y. Shi conceived and supervised the project. D.F., S.Y. and Y. Shi designed the experiments. D.F. and Y. Shi carried out most of the experiments with assistance from Y. Song, S.W., Y.P., Y.M., Z.L., X.Z., W.S., Z.S. and Z.G. D.F., Y. Shi and S.Y. analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
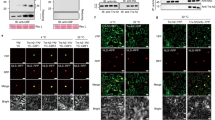
Extended Data Fig. 1 CBFs associate with spliceosome-associated proteins.
a, A list of spliceosome-associated proteins identified as CBF2-interacting proteins. b, BiFC assays showing SKIP/CBF2 co-expressed with SR45–mCherry in the nuclei of N. benthamiana leaf cells. nYFP-GUS and cYFP-GUS serve as controls. Scale bars, 10 μm. c, Co-IP assays showing the interactions between SKIP and CBFs in Arabidopsis protoplasts.
Extended Data Fig. 2 SKIP positively regulates acquired freezing tolerance and forms nuclear condensates in vivo.
a-c, Freezing tolerance of the skip-1, skip-1 proSKIP::SKIP-GFP and Col-0 under cold-acclimated (CA) conditions. Representative plant images of seedlings (a), survival rates (b), and ion leakage rates (c) are shown. In (b) and (c), data are means ± s.d. (n = 3 biologically independent experiments); means were compared by one-way ANOVA with Tukey’s multiple comparison tests. d, Subcellular localization of SKIP-GFP in the nuclei of N. benthamiana leaf cells under normal conditions. proSKIP::SKIP-GFP and pSuper::SKIP-GFP were transiently expressed in N. benthamiana leaf cells with different concentrations of Agrobacterium solution. e,f, Subcellular localization of SKIP-GFP under the indicated conditions in 7-d-old skip-1 proSKIP::SKIP-GFP seedlings. White arrows indicate representative condensates in the stele cells in the root elongation zone after cold treatment. Representative images (e), and quantification of cells with condensates (f) are shown. g,h, skip-1 proSKIP::SKIP-GFP seedlings grown at 22 °C for 7 days were treated at 4 °C for 6 h, and then shifted to 22 °C for recovery. Representative images of the stele cells in the root elongation zone (h), and quantification of cells with condensates (g) are shown. In (f) and (g), data are means ± s.d. (n = 3 root elongation zone analyzed in three independent experiments; ~30 nuclei in each root were examined). ND, not detected. Scale bars are indicated in figures.
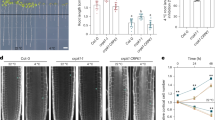
Extended Data Fig. 3 SKIP is highly disordered and possesses liquid-like properties in vivo.
a, Prediction of disorder regions of SKIP by PONDR (VLXT). The scores are assigned between 0 and 1, and a score above 0.5 indicates disorder. b, Structure of SKIP protein predicted by AlphaFold. c,d, Cold-induced SKIP condensates are sensitive to 1,6-hexanediol. Representative images in the stele cells in the root elongation zone of cold-treated Arabidopsis with or without 10% 1,6-hexanediol for 90 s (c), and quantification of cells with condensates (d) are shown. In (d), data are means ± s.d. from three biologically independent experiments; statistical significance was determined using a two-tailed t-test. e,f, Time course of FRAP of SKIP nuclear condensates in the nuclei of N. benthamiana leaf cells under normal conditions (e), and in the nuclei of skip-1 proSKIP::SKIP-GFP root cells under cold conditions (f). Time 0 indicates the time of the photobleaching pulse. Data are means ± s.d. (n = 7 independent experiments), and representative images are shown. (g) Quantification of cells with condensates in Fig. 3a. Data are means ± s.d. (n = 3 root elongation zones analyzed in three independent experiments; ~30 nuclei in each root were examined). ND, not detected.
Extended Data Fig. 4 In vitro phase separation and in vivo protein levels of SKIP with or without cold treatment.
a,b, In vitro phase separation of recombinant His-GFP-SKIP and His-SKIP incubated at 22 °C for 10 min. Representative images (a), and coomassie blue staining of indicated proteins (b) are shown. His-GFP serves as a control. c, In vitro phase separation of recombinant His-GFP-SKIP incubated at 22 °C for 10 min at different concentrations. d, In vitro phase separation of recombinant His-GFP-SKIP incubated at 22 °C and 4 °C for 1 h. His-GFP serves as a control. e, Detection of the SKIP protein level in two-week-old Col-0 and skip-1 using anti-SKIP antibody. f, Detection of the SKIP protein level in two-week-old Col-0 with or without 4 °C treatment. Nuclear proteins were extracted, and H3 was used as a loading control.
Extended Data Fig. 5 CBFs co-localize with SKIP in nuclear condensates.
a, Subcellular localization of CBF3-GFP under cold conditions in 7-d-old pSuper::CBF3-GFP transgenic Arabidopsis plants. Red arrows indicate the nucleolus, and yellow arrows indicate the nuclear condensates. b, Live imaging of pSuper::CBFs-mCherry co-expressed with proSKIP::SKIP-GFP in the nuclei of N. benthamiana leaf cells. c, Fluorescence profiles of SKIP-GFP and CBFs-mCherry over the white line shown in (b). d, Immunoblot analysis of SKIP-GFP abundance in 7-d-old proSKIP::SKIP-GFP and cbfs-1 proSKIP::SKIP-GFP seedlings incubated at 22 °C or 4 °C for 6 h. Nuclear proteins were extracted, and H3 was used as a loading control.
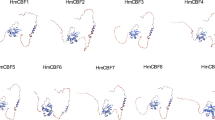
Extended Data Fig. 6 CBFs contain IDRs and promote SKIP condensation.
a, The proportion of cells with different numbers of SKIP nuclear condensates in Fig. 3h. b,c, In vitro phase separation of recombinant His-CBF-mCherry (10 μM) incubated at 22 °C for 10 min. Representative images (b), and coomassie blue staining of indicated proteins (c) are shown. His-GFP-SKIP serves as a positive control. d, Prediction of IDRs of CBFs by PONDR (VLXT). The scores are assigned between 0 and 1.0, and a score above 0.5 indicates disorder. e, The proportion of cells with different numbers of SKIP nuclear condensates in Fig. 3m.
Extended Data Fig. 7 Differentially expressed analysis of RNA-seq.
a, Principal component analysis of gene expression of two-week-old Col-0, cbfs-1, and skip-1 Arabidopsis seedlings under normal (22 °C) and cold conditions (4 °C for 6 h). b, Volcano plot of differentially expressed genes between cold-treated and non-treated WT (Col-0) plants. c,d, Volcano plot of differentially expressed genes between Col-0 and mutant (skip-1, cbfs-1) plants with or without cold treatment at 4 °C. In (b-d), the R package DESeq2 was used to analyze the differential expression of transcriptomes. And the differential expression analysis employs a two-tailed Wald test to assess the statistical significance of the log2 fold changes between different comparisons. e, Venn diagrams showing the number of up-regulated genes in Col-0 that were down-regulated in cbfs-1 and skip-1 compared to Col-0 under cold stress, and down-regulated genes in Col-0 that were up-regulated in cbfs-1 and skip-1 compared to Col-0 under cold stress. f, The ratio of CRDE genes regulated by CBFs and SKIP. g, Heat map representation and gene ontology analysis of CBFs- or SKIP-activated cold response genes under cold stress. Representatively enriched GO terms were determined by using AgriGO v.2.0. The P values are from Fisher’s exact test. h, Heat map representation of cold-activated genes that were regulated by CBFs but not by SKIP (71 genes in Extended Data Fig. 7e, up-regulated) under cold conditions. In (g) and (h), the z-score scale represents mean-subtracted regularized TPMs.
Extended Data Fig. 8 AS analysis of RNA-seq, and freezing tolerance of esl3.05 under cold-acclimated conditions.
a, Gene ontology analysis of 1,102 CBFs and SKIP co-regulated CRDS genes. The P values are from Fisher’s exact test. b, Heat map representation of AS events with decreased splicing efficiency for Col-0 after cold treatment and AS patterns in skip-1 and cbfs-1 compared to Col-0 in the same AS sites (222 AS events corresponding to 195 genes). Inclusion level differences were used to quantify the splicing efficiency. c, An RNA motif identified by MEME analysis using introns (entire introns plus 50 bp of 5′- and 3′-flanking exon sequences) with increased splicing efficiency in Supplementary Table 7. d, Integrative Genomics Viewer genome browser view showing the detected RNA-seq signals in the indicated conditions. Differentially spliced AS events were detected in the fifth intron (I5) of AT5G28770, the third intron (I3) of AT1G51430, the eleventh exon (E11), and the 3′SS in exon 24 (E24). Black arrows indicate the positions of designed primers. e, Characterization of esl3.05 T-DNA insertion mutants. f, RT-PCR analysis of ESL3.05 and EF1α expression in WT, esl3.05-1, and esl3.05-2 mutants. g-i, Freezing tolerance of the esl3.05 mutants under cold-acclimated conditions. Representative plant images (g), survival rates (h), and ion leakage rates (i) are shown. In (h) and (i), the values are the means ± s.d. (n = 3 independent experiments). Means were compared by two-tailed Student’s t-tests.
Supplementary information
Supplementary Tables
Supplementary Tables 1–9.
Source data
Source Data Fig. 2
Unprocessed western blots for Fig. 2.
Source Data Fig. 3
Unprocessed western blots for Fig. 3.
Source Data Fig. 4
Unprocessed gels for Fig. 4.
Source Data Extended Data Fig. 1
Unprocessed western blots for Extended Data Fig. 1.
Source Data Extended Data Fig. 4
Unprocessed western blots for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Unprocessed western blots for Extended Data Fig. 5.
Source Data Extended Data Fig. 8
Unprocessed gels for Extended Data Fig. 8.
Source Data Figs. 2–4 and Extended Data Figs. 2, 3, 5, 6 and 8
Statistical source data for Figs. 2–4 and Extended Data Figs. 2, 3, 5, 6 and 8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, D., Song, Y., Wu, S. et al. Regulation of alternative splicing by CBF-mediated protein condensation in plant response to cold stress. Nat. Plants 11, 505–517 (2025). https://doi.org/10.1038/s41477-025-01933-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-01933-x
This article is cited by
-
A receptor–kinase cascade confers cold-induced root growth inhibition in Arabidopsis
Nature Plants (2025)