Abstract
The “gut-brain axis” is involved in many physiological processes. However, its role in regulating mammary gland (MG) development remains unknown. In this study, we established the mice model of bilateral subdiaphragmatic vagotomy (Vago) to clarify the effects of “gut-brain axis” on MG development in pubertal mice. The results showed that Vago reduced the ratio of Lactobacillus and Bifidobacterium, neuronal excitability in the nucleus of solitary tract (NTS), and synthesis and secretion of BDNF, thereby slowing MG development. Transplanting the gut microbiota of Vago mice to recipient mice replicated these effects, and transplanting the gut microbiota of Control mice to Vago mice did not alleviate these effects. Galacto-Oligosaccharide (GOS), which up-regulates the ratio of Lactobacillus and Bifidobacterium, supplementation elevated NTS neuron excitability, synthesis and secretion of BDNF, and MG development, but Vago reversed these benefits. In conclusion, GOS enhances BDNF-mediated mammary gland development in pubertal mice via the “gut-brain axis”.
Similar content being viewed by others
Introduction
The mammary gland (MG) development is a complex and delicate process in mammalian physiology that is not only a central component of reproductive biology, but also critical to the health and survival of the individual. The primary function of the MG is to produce milk to support the growth and development of the offspring. The normal development of the MG is therefore the basis for guaranteeing that the offspring will have access to primary nutrition and immunological protection. The MG development is divided into five periods, including the embryonic, puberty, pregnancy, lactation and degeneration period1. After birth, the MG undergoes its first stage of rapid development, puberty, which is the main period of ductal extension and branching, while alveolar proliferation and differentiation occur mainly during pregnancy and lactation2. It’s worth noting that the development of the MG during puberty has a decisive influence on the development of the MG and the function of lactation during the subsequent lactation period3. Therefore, the study of the physiological mechanism of MG development during puberty has important theoretical and practical value.
The development of the MG during puberty is affected by a variety of factors, including genetics, hormone regulation, environmental factors and nutritional status4,5,6,7. Over the past few decades, researchers have revealed a series of complex molecular and cellular mechanisms through their studies of this biological phenomenon. Recently, it has been shown that pruning of sensory nerve axons in the MG of male mice results in the underdevelopment of the MG in male mice8,9. Thus, the survival of sensory nerve fibers within the mammary fat pad (MFD) ensures the development of the ductal tree. Despite significant progress, however, certain aspects of MG development are still unknown. In particular, the specific mechanisms by which the external environment and internal physiological state interact to jointly influence MG development. The in-depth study of the interaction mechanisms between the external environment and the internal physiological state not only forms an important theoretical value in the field of basic science research, but also plays a key role in clinical medicine and public health management.
As one of the important ecosystems of the organism, the gut microbiota is the main channel of communication between the external and internal environment. With deeper studies of the microbiome and its host interactions, the roles of gut microbes in food digestion and energy production have all been revealed. However, with the introduction and development of the “gut-brain axis” concept10,11, attention has been focused on the connection between the gut and the distal organs. At the same time, there is growing evidence that the gut microbiota may have a regulatory effect on organ function in the body through the gut-brain axis. For example, changes in gut microbiota can affect the levels of hormones in the body, which in turn are directly involved in tissue growth and differentiation12,13. Meanwhile, the metabolites produced by gut microbiota may be transmitted to various parts of the body through the blood circulation, directly or indirectly affecting the development of the organism12,14. Moreover, a large number of vagus nerve (VN) axon endings are distributed in the mucosal and smooth muscle layers of the gut, and gut microbiota activate NTS neurons through VN. Then it projects to other brain regions such as hypothalamus and hippocampus, thus regulating changes in relevant physiological processes15. The “gut-brain axis (vagal pathway)” plays an important role in a variety of CNS-related behavioral abnormalities such as depression and anxiety. For example, when the gut microbiota of mice maintained in chronic mild stress for 8 W was transplanted into recipient mice, the recipient mice developed depressive-like behaviors, but these responses were absent in Vago mice16. Furthermore, Salmonella-induced anxiety-like behavior in mice and hypomotility in mice caused by depletion of the gut microbiota by ABX can be alleviated by Vago13,17.
Despite the fact that the link between the gut-brain axis regarding the development and function of distant organs is now becoming more widely recognised. However, the role of the gut-brain axis in the regulation of MG function remains unreported. Therefore, in the present study, we are going to conduct a series of studies on the gut-brain axis (vagus nerve pathway) on MG development in pubertal mice to verify the existence of the “gut-brain-mammary gland axis”.
Results
Inhibition of mammary gland development in pubertal mice by severing the gut-brain neural communication pathway
The vagus nerve (VN) as a major bidirectional neural communication pathway in the gut-brain axis. The signals originating from the gut microbiota are directly or indirectly transmitted to the nucleus of solitary tract (NTS) via the VN. Therefore, the differences in the development levels of MG in ICR mice were exerted with Vago (hereinafter referred to as Vago mice), ICR mice were exerted with sham surgery (hereinafter referred to as SS mice), and Control mice were evaluated. The results showed that the body weight and food intake were significantly higher in Vago mice, and the difference between SS mice and Control mice was not significant (Fig. 1b–e). Subsequently, MG development was examined in mice were exerted with SS/Vago at 4 weeks of age. The results showed that SS did not cause significant changes in the development levels of MG in mice compared to Control mice, but the development levels of MG in Vago mice was significantly lower than that in SS mice, and the same results were seen in mice were exerted with SS/Vago at 6 weeks of age (Fig. 1f–i). The enclosing radius, mammary epithelial area (MEA) and the number of Sholl intersections (Sum inters) were significantly reduced, the Sholl decay coefficients (Sholl decay) were significantly increased in Vago mice, but there was no significant change in branching density (Fig. 1j). Meanwhile, the Linear Sholl plots (Fig. 1k) and Sholl analysis bubble map (Fig. 1l) both indicate that Vago resulted in a decrease in various developmental indicators of the MG in mice.
a Schematic diagram of Vago/SS mouse model protocol. The grouping information is as follows: the blank control group (4-week-old pubertal mice) was not subjected to any treatment, the negative control group (4/6-week-old pubertal mice) was exerted with SS, and the experimental group (4/6-week-old pubertal mice) was exerted with Vago (n = 6); (b, d) Changes in body weight of mice during 4 W of rearing, weighed every 2 d; (c, e) Changes in food intake of mice during 4 W of rearing; (f) Representative image of whole mount staining, scale bar: 5 mm; (g) Quantitative schematic representation of MG development in mice; (h, i) Quantification of MG development; (j) Enclosing Radius, Mammary Epithelial Area (MEA), the number of Sholl intersections (Sum inters), Sholl decay coefficients (Sholl decay), and Branching Density; (k) Linear Sholl plots; (l) Sholl analysis bubble map. The mean ± SEM was used for all data presentations. Compared to the SS group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To further investigate the potential mechanism by which severing the VN inhibits MG development in mice, the development levels of MG in 8-week-old (adult) mice exerted with SS/Vago was evaluated. However, no significant difference in MG development was observed (Supplementary Fig. 1a–d). This suggests that the treatment of Vago in adult mice does not affect MG development, which may be due to the fact that the MG of mice are temporarily in a state of developmental arrest in adulthood. Moreover, in the present study, Vago was exerted on mice at 4 weeks of age, and the development levels of MG in Vago mice surviving to 9 W was similar to that of SS mice surviving to 8 W, and the development levels of MG in Vago mice surviving to 8 W was similar to that of SS mice surviving to 7 W (Supplementary Fig. 1e, f). The above results suggest that Vago leads to inhibition of MG developmental processes in pubertal mice.
The gut-brain axis mediates the development of mammary sensory nerve
At birth, all five pairs of MGs are innervated by sensory nerve fibers in female mice, but they are virtually undetectable in the MGs of male mice8. This suggests that one of the reasons for the initiation of MG development is the development of sensory nerve fibers. Whereas the results of pubertal mice in which the VN was severed showed suppressed the MG development. Therefore, we speculated that this may be related to the fact that the development of sensory nerve fiber is affected. In the next study, sensory nerve fibers extending from the dorsal root ganglia (DRG) of the spine to the MG were severed in 4-week-old (puberty) female mice. Whole mount staining of the MG showed that Sensory Neurotomy (SNeuro) inhibited MG development (Supplementary Fig. 2a, b). Western blotting results showed that SNeuro resulted in a significant reduction in the protein expression levels of the neuromarker β-III Tubulin (Tuj1) (Supplementary Fig. 2c, d). The result suggested a correlation between mammary sensory nerve fiber density and the development levels of MG.
Neurotrophic factors control neural innervation by regulating axon terminal growth and pruning18. Recent studies have revealed that BDNF plays a role in regulating the survival of sensory nerve axon terminals in the MG8,9. Therefore, we speculated that BDNF is an important neurotrophic factor that regulates the development of mammary sensory nerve. Next, the protein expression levels of Tuj1 and BDNF were examined in MG of mice were exerted with SS/Vago at 4/6 weeks, and the results showed that Vago significantly reduced their expression (Fig. 2a-c). ELISA results showed that BDNF levels were significantly reduced in the serum of Vago mice (Fig. 2d). To visualize mammary sensory nerve fibers and quantify their density more visually, Tuj1 immunofluorescence staining of MG sections was performed, which showed that the density of sensory nerve fibers in Vago mice was significantly lower than that in SS mice (Fig. 2e, f). These results suggest that BDNF reduction leads to the pruning of mammary sensory nerve axon. This ultimately inhibited the process of MG development in mice.
The blank control group (4-week-old pubertal mice) was not subjected to any treatment, the negative control group (4/6-week-old pubertal mice) was exerted with SS, and the experimental group (4/6-week-old pubertal mice) was exerted with Vago (n = 6); (a) The protein expression of Tuj1 and BDNF in MG were detected by Western blotting (n = 3); (b, c) Relative protein abundance of Tuj1 and BDNF (compared to β-actin) in Fig. 2a; (d) ELISA kit for measuring BDNF in serum; (e) MG frozen sections were stained with Tuj1 (red) and DAPI (blue) to visualize sensory nerve fibers (n = 5), scale bar: 100 μm; (f) Quantification of mammary sensory nerve fiber density. The mean ± SEM was used for all data presentations. Compared to the SS group, *p < 0.05, **p < 0.01, ***p < 0.001.
The gut-brain axis mediates NTS to affect BDNF synthesis
Major processes of neural development of the brain are accompanied by changes in the maternal and neonatal gut microbiota10. The VN senses gut signals and transmits them to the NTS, a key link in the regulation of neural development15. Therefore, immunofluorescence staining of NTS neurons by using c-Fos (a transcription factor associated with neuronal activity19) showed that Vago significantly inhibited the excitability of NTS neurons (Fig. 3a, b). Due to the significantly reduced levels of BDNF in MG and serum of Vago mice (Fig. 2a, c, d), which is synthesized mainly in the hippocampus, hypothalamus, and cortex20. Concurrently, VN plays a key role in sensing signals in the gut and regulating BDNF expression in the brain21,22,23. In this study, immunofluorescence staining of paraventricular nucleus of hypothalamic (PVH) regions and hippocampal regions (CA1, CA2, CA3, and DG) using BDNF showed that Vago significantly inhibited the synthesis and secretion of BDNF in the PVH regions (Fig. 3c, d), and the four regions of the hippocampus (CA1, CA2, CA3, and DG) showed the same as PVH region results (Fig. 3e–i). The results obtained were verified to be consistent whether Vago was exerted on mice at 4 or 6 weeks of age.
The blank control group (4-week-old pubertal mice) was not subjected to any treatment, the negative control group (4/6-week-old pubertal mice) was exerted with SS, and the experimental group (4/6-week-old pubertal mice) was exerted with Vago (n = 6), (a) NTS were stained with c-Fos (green) and DAPI (blue) to visualize the effect on NTS excitability (n = 3), scale bar: 200 μm; (b) Quantification of the activated neuron within the NTS based on c-Fos using ImageJ; (c) PVH were stained with BDNF (green) and DAPI (blue) to visualize the effect on BDNF synthesis and secretion in the PVH (n = 3), scale bar: 200 μm; (d) Quantification of the mean fluorescence intensity of BDNF within the PVH using ImageJ; (e) Hippocampus were stained with BDNF (green) and DAPI (blue) to visualize the effect on BDNF synthesis and secretion within four regions of the hippocampus (CA1, CA2, CA3, DG), scale bar: 200 μm; (f, g, h, i) Quantification of the mean fluorescence intensity of BDNF within four regions of the hippocampus (CA1, CA2, CA3, DG) using ImageJ. The mean ± SEM was used for all data presentations. Compared to the SS group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Changing the ratio of gut microbiota composition by severing the gut-brain neural communication pathway
Communication mechanisms exist between gut microbiota and multiple organs, while the gut-brain axis plays a huge role in them, namely, the gut-brain-hepatic axis24, the brain-gut-renal axis25 and so on. Revealing the intrinsic potential of bidirectional gut-brain communication in regulating the development and function of other organs. It was also shown that VN plays an important role in bidirectional gut-brain communication, so colonic faeces were sequenced for 16S rRNA after 4-week-old mice were exerted with SS/Vago. As the number of reads/samples rose, the sparse curve gradually reached the saturation plateau, which indicates sufficient sequencing depth (Fig. 4a). The α-diversity results showed that Chao1 index (Fig. 4b), Observed species (Fig. 4c) and Shannon index (Fig. 4d) were significantly higher in the Vago group compared to the SS group, suggesting that Vago resulted in the enhancement of the abundance and diversity of the mouse gut microbiota. The β-diversity results indicated that mice were exerted with Vago showed significant differences in gut microbiota compared to the SS group (Fig. 4e–g). The rank abundance curves showed that Vago caused the homogeneity of the mouse gut microbiota to be changed (Supplementary Fig. 3c). At the family level, the abundance of Lactobacillaceae was significantly reduced in the gut microbiota of Vago mice (Supplementary Fig. 3a, b); at the genus level, the abundance of Lactobacillus was significantly reduced in the gut microbiota of Vago mice (Fig. 4h, i), with decreasing trends in the abundance of Bifidobacterium and Bacteroides (Fig. 4h). In the heat map of species composition, mice exerted with Vago were significantly negatively correlated with the abundance of Lactobacillus and Bifidobacterium in the gut microbiota (Supplementary Fig. 3d). The Venn diagram results showed that 1683, 2898 different OTUs were identified in the SS and Vago groups, respectively, and the other OTUs were identical (Fig. 4j). After analyzing the diversity of the gut microbiota, LEfSe was then used in order to evaluate the specific effects of Vago on the structure of the gut microbiota in mice. The results showed that the dominant flora in the SS group was Lactobacillus and Bifidobacterium, while the dominant flora in the Vago group was Biloplila (Fig. 4k, l). The above findings suggest that the brain may play a crucial role in regulating the proportion of gut microbiota composition. However, this result could also be the result of differences in nutritional levels.
The blank control group (4-week-old pubertal mice) was not subjected to any treatment, the negative control group (4-week-old pubertal mice) was exerted with SS, and the experimental group (4-week-old pubertal mice) was exerted with Vago (n = 6), (a) The sparse curves based on OTU levels of gut microbiota; (b) Chao1 index; (c) Observed species; (d) Shannon index; (e) PCoA of gut microbiota based on the bray curtis distance algorithm; (f) PCoA of gut microbiota based on the weighted unifrac distance algorithm; (g) The nonmetric multidimensional scale (NMDS) analysis of gut microbiota based on bray curtis distance algorithm; (h) The relative abundance of species in each group of gut microbiota at the genus level (within-group means); (i) Differences in relative abundance of Lactobacillus between groups; (j) Venn diagram shows common and unique OTUs between groups; (k) Linear discriminant analysis (LDA) effect size (LEfSe) screening for differential gut microbiota; (l) LDA of LEfSe analysis (LDA > 3, P < 0.05). Compared to the SS group, *p < 0.05, **p < 0.01.
The development of mammary gland was inhibited by FMT
The brain senses gut microbiota as well as other gut signals through the VN. Therefore, in this study, the gut microbiota of Vago mice was transplanted to 4-week-old recipient mice using FMT to detect the effects of altered gut microbiota composition ratios on MG development in pubertal mice. Firstly, the body weight and food intake of mice in the Control (8 W) and Vago-FMT (8 W) groups were monitored, which showed no significant differences in body weight and food intake (Fig. 5b, c). The present results demonstrate that differences in MG development in mice were not due to differences in nutritional intake. Compared with mice in the Control (8 W) group, MG development was significantly inhibited in the Vago-FMT (8 W) group. However, when the mice in the Vago-FMT group grew to 10 weeks of age, their MG development was examined to be similar to that of mice in the Control (8 W) group. Moreover, there was no significant difference in MG development between mice in the Vago-FMT (8 W) group and mice in the Control (6 W) group (Fig. 5d–f). At the same time, the Linear Sholl plots (Fig. 5g) and Sholl analysis bubble map (Fig. 5h) both indicate that Vago-FMT reduced various developmental indicators of the MG. Finally, the Control (8 W) and Vago-FMT (8 W) groups were cross-fed between mothers and pups (Fig. 5i). Results demonstrated that pups born to Vago-FMT (8 W) female mice but fostered by Control (8 W) mice were normal in body weights, whereas pups born to Control (8 W) females but fostered by Vago-FMT (8 W) mice exhibited reduced growth (Fig. 5j). These results suggest that changing the proportion of gut microbiota composition in the presence of intact VN can still affect MG development in pubertal mice. Due to the suppression of the developmental process of the MG during puberty, this effect may also be carried over into lactation, with some degree of impact on the lactation function of the MG.
a Schematic diagram of the Vago-FMT mouse model protocol. The grouping information is as follows: the blank control group (4-week-old pubertal mice) was not subjected to any treatment, and the experimental group (4-week-old pubertal mice) was subjected to Vago-FMT (n = 9); (b, c) Changes in body weight and food intake of mice during 4 W of rearing; (d, e) Euthanised after 14/28/42 d of rearing, representative images of whole mount staining and quantification of MG development (n = 6), scale bar: 5 mm; (f) Enclosing Radius, MEA, Sum inters, Sholl decay, and Branching Density; (g) Linear Sholl plots; (h) Sholl analysis bubble map; (i) Schematic of cross-feeding after a female mice has given birth to her pups; (j) Weight change of pups during 4 W of rearing. The mean ± SEM was used for all data presentations. Vago-FMT (8 W) group compared to Control (8 W) group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To further determine whether altering the ratio of gut microbiota composition was effective in reversing the suppression of MG development caused by Vago, SS/Vago was exerted on 4-week-old pubertal mice and Control-FMT was carried out on some of them. However, with or without Control-FMT, the results showed that Vago mice had significantly higher body weight and food intake than SS mice (Fig. 6b, c). It is well demonstrated that VN has an irreplaceable and important role in transmitting satiety and hunger signals. Whole mount staining of the MG showed that inhibition of MG development caused by Vago was not relieved by Control-FMT intervention (Fig. 6d–h). The SS (8 W) and SS (8 W)+Control-FMT groups were cross-fed between mothers and pups. The Vago (8 W) and Vago (8 W)+Control-FMT groups were then cross-fed between mothers and pups (Fig. 6i). The results showed that the average body weight of pups fed by the Vago (8 W)/Vago (8 W)+Control-FMT female mice was lower than that of pups fed by the SS (8 W)/SS (8 W)+Control-FMT female mice (Fig. 6j). These results indicate that altering the proportion of gut microbiota composition after severing the gut-brain neural communication pathway fails to reverse the suppression of MG development caused by Vago. This result re-emphasizes that the VN is essential for the brain to perceive gut microbiota as well as other gut signals.
a Schematic diagram of the Control-FMT mouse model protocol. The grouping information is as follows: the control group (4-week-old pubertal mice) was subjected to SS/Vago followed by SS-FMT/Vago-FMT, and the experimental group (4-week-old pubertal mice) was subjected to SS/Vago followed by Control-FMT (n = 9); (b, c) Changes in body weight and food intake of mice during 4 W of rearing; (d, e) Euthanised after 14/28/42 d of rearing, representative images of whole mount staining and quantification of MG development (n = 6), scale bar: 5 mm; (f) Enclosing Radius, MEA, Sum inters, Sholl decay, and Branching Density; (g) Linear Sholl plots; (h) Sholl analysis bubble map; (i) Schematic of cross-feeding after a female mice has given birth to her pups; (j) Weight change of pups during 4 W of rearing. The mean ± SEM was used for all data presentations. Vago (8 W) group compared to SS (8 W) group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Vago (8 W)+Control-FMT group compared to SS (8 W)+Control-FMT group, #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001.
The gut-brain-mammary gland axis was inhibited by FMT
The study aims to investigate the effects of FMT on the NTS-PVH neural circuit in a state where the VN remains intact or is severed. Firstly, immunofluorescence staining of NTS using c-Fos showed that Vago-FMT significantly inhibited NTS neuron excitability. After Control-FMT intervention, the results showed that Control-FMT did not reverse the inhibition of NTS neuron excitability caused by Vago (Fig. 7a, b). Next, the synthesis and secretion of BDNF in the PVH region were detected by immunofluorescence, and the results showed that Vago-FMT significantly inhibited the synthesis and secretion of BDNF in the PVH region. After Control-FMT intervention, the results showed that Control-FMT still did not reverse the reduced levels of BDNF synthesis and secretion in the PVH region caused by Vago (Fig. 7c, d). Finally, ELISA results showed that BDNF levels were significantly lower in serum of mice in the Vago-FMT (8 W) group compared to the Control (8 W) group. Serum levels of BDNF were significantly lower in mice in the Vago (8 W)+Control-FMT group compared to the SS (8 W)+Control-FMT group (Fig. 7e).
4-week-old mice were raised by Vago-FMT until 8 weeks of age or 4-week-old mice were exerted with SS/Vago and then raised by Control-FMT until 8 weeks of age, (a) NTS were stained with c-Fos (green) and DAPI (blue) to visualize the effect on NTS excitability (n = 3), scale bar: 200 μm; (b) Quantification of the activated neuron within the NTS based on c-Fos using ImageJ; (c) PVH were stained with BDNF (green) and DAPI (blue) to visualize the effect on BDNF synthesis and secretion in the PVH (n = 3), scale bar: 200 μm; (d) Quantification of the mean fluorescence intensity of BDNF within the PVH using ImageJ; (e) ELISA kit for measuring BDNF in serum; (f) The protein expression of Tuj1 and BDNF in MG were detected by Western blotting (n = 3); (g, h) Relative protein abundance of Tuj1 and BDNF (compared to β-actin) in Fig. 7f; (i) MG frozen sections were stained with Tuj1 (red) and DAPI (blue) to visualize sensory nerve fibers (n = 5), scale bar: 100 μm; (j) Quantification of mammary sensory nerve fiber density. The mean ± SEM was used for all data presentations. Vago-FMT (8 W) group compared to Control (8 W) group and Vago (8 W) group compared to SS (8 W) group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Vago (8 W)+Control-FMT group compared to SS (8 W)+Control-FMT group, #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001.
In the present study, we found that FMT affects the NTS-PVH neural circuit and that the inhibitory effect of Vago on the NTS-PVH neural circuit could not be reversed by altering the proportion of gut microbiota composition. At the same time, it was determined that the gut-brain axis can have a positive or negative effect on MG development due to the inhibition of MG development caused by Vago-FMT (Fig. 5d, e) and the effect on the NTS-PVH neural circuit (Fig. 7a–d). Next, changes in the protein expression levels of Tuj1 and BDNF in the MG of mice were detected by western blotting. The results showed that the protein expression levels of Tuj1 and BDNF in the MG in the Vago-FMT (8 W) group were significantly reduced compared with those in the Control (8 W) group. The protein expression levels of Tuj1 and BDNF were significantly reduced in the MG in the Vago (8 W)+Control-FMT group compared with the SS (8 W)+Control-FMT group (Fig. 7f, g, h). Finally, immunofluorescence was carried out on MG sections, and Tuj1 staining showed that sensory nerve fiber density was significantly lower in Vago-FMT (8 W) mice than in Control (8 W) mice, whereas the reduction in sensory nerve fiber density in mice caused by Vago was not reversed after the Control-FMT intervention (Fig. 7i, j). The above results suggest that the GUT-VN-NTS-PVH pathway affects the expression levels of BDNF.
GOS promotes mammary gland development in pubertal mice via the gut-brain axis (vagal pathway)
GOS as prebiotic and also as food additive that selectively stimulate the proliferation of Bifidobacterium and Lactobacillus26,27,28. Therefore, in this study, GOS was chosen as prebiotic to change the proportional composition of the gut microbiota. Firstly, three concentrations of 681.5 mg/kg GOS (GOS-L), 1365 mg/kg GOS (GOS-M), and 2730 mg/kg GOS (GOS-H) were gavaged to the mice, and the effects on MG development were analysed by whole mount staining of the MG. The results showed that the promotion effect of GOS on MG development was more significant as the concentration increased, and GOS-H was selected as the optimal concentration in this study and used for subsequent experiments (Supplementary Fig. 4a, b). Next, ABX was employed in order to remove gut microbiota and to verify whether GOS could still be effective in the presence of significantly reduced gut microbiota. The results showed that the promotional effect of GOS on MG development disappeared (Supplementary Fig. 4c, d). Also, by 16S rRNA sequencing of colonic faeces, PCoA analysis showed that GOS significantly altered the structure of gut microbiota (Supplementary Fig. 4e). The α-diversity results showed that the Chao1 index (Supplementary Fig. 4f) of the mice in the GOS-H group, although not significantly changed, tended to decrease. Whereas the Shannon index (Supplementary Fig. 4g) was significantly altered when compared to the Control group, suggesting that the GOS-H intervention improved the gut microbiota abundance and diversity. Furthermore, the abundance of Bifidobacterium, Allobaculum and Bacteroides was significantly increased in the GOS-H group of mice (Supplementary Fig. 4h-j), and the abundance of Lactobacillus was not significantly altered (Supplementary Fig. 4h, l), but the above effects disappeared after ABX intervention. Finally, this study verified whether GOS has the ability to promote cell proliferation in mMECs and BMECs, respectively. CCK8 results showed that 5, 10, and 20 mg/mL GOS did not significantly promote cell proliferation compared to the Control group, while higher concentrations of GOS (40 mg/mL) significantly inhibited cell proliferation (Supplementary Fig. 5a, e). Western blotting results showed that the protein expression levels of Cyclin D1 and PCNA were not significantly increased after 5, 10, and 20 mg/mL GOS stimulation compared with the Control group, whereas 40 mg/mL GOS stimulation significantly inhibited the protein expression of Cyclin D1 and PCNA (Supplementary Fig. 5b–d, f–h).
To further investigate the mechanism by which GOS promotes MG development in pubertal mice, 4-week-old mice were exerted with SS/Vago and gavaged with GOS-H. Whole mount staining of the MG showed that GOS-H promoted MG development in pubertal mice, however, the effect of GOS in promoting MG development in pubertal mice disappeared after the treatment with Vago (Fig. 8b–f). Immunofluorescence staining of NTS neurons using c-Fos showed that GOS-H significantly increased NTS neuron excitability, and Vago reversed this effect (Fig. 8g, j). The synthesis and secretion of BDNF in the PVH region were detected by immunofluorescence, and the results showed that GOS-H significantly increased the synthesis and secretion of BDNF in the PVH region, whereas the positive effect of GOS-H disappeared after the treatment with Vago (Fig. 8h, k). The BDNF content in the serum of mice showed the same trend (Fig. 8l). Finally, immunofluorescence staining was carried out on MG sections, and Tuj1 staining showed that the mammary sensory nerve fiber density was significantly higher in the Control+GOS-H group of mice than in the Control group of mice. However, after the treatment with Vago, this result was reversed compared with mice in the SS + GOS-H group (Fig. 8i, m). These results suggest that GOS promotes MG development in pubertal mice via the gut-brain axis (vagal pathway).
a Schematic diagram of the mouse supplemented GOS model protocol. The grouping information is as follows: the blank control group (4-week-old pubertal mice) was not subjected to any treatment, the negative control group (4-week-old pubertal mice) was subjected to SS, and the experimental group (4-week-old pubertal mice) was subjected to Vago, gavage GOS-H (n = 6); (b, c) Representative images of whole mount staining and quantification of MG development, scale bar: 5 mm; (d) Enclosing Radius, MEA, Sum inters, Sholl decay, and Branching Density; (e) Linear Sholl plots; (f) Sholl analysis bubble map; (g) NTS were stained with c-Fos (green) and DAPI (blue) to visualize the effect on NTS excitability (n = 3), scale bar: 200 μm; (h) PVH were stained with BDNF (green) and DAPI (blue) to visualize the effect on BDNF synthesis and secretion in the PVH (n = 3), scale bar: 200 μm; (i) MG frozen sections were stained with Tuj1 (red) and DAPI (blue) to visualize sensory nerve fibers (n = 5), scale bar: 100 μm; (j) Quantification of the activated neuron within the NTS based on c-Fos using ImageJ; (k) Quantification of the mean fluorescence intensity of BDNF within the PVH using ImageJ; (l) ELISA kit for measuring BDNF in serum; (m) Quantification of mammary sensory nerve fiber density. The mean ± SEM was used for all data presentations. Control+GOS-H group compared to the Control group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Vago+GOS-H group compared to SS + GOS-H group, #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001.
Discussion
The most rapid and direct way for the gut microbiota to have an impact on the brain is through the vagus nerve (VN)29. In this study, the important role of the VN in the “gut-brain axis” was evaluated. The gut microbiota influences the synthesis and secretion of BDNF in the brain via the VN, thereby altering mammary gland developmental processes. When mice were exerted Vago, the proportion of Lactobacillus and Bifidobacterium in the gut microbiota decreased. Furthermore, the results showed that GOS significantly increased the excitability of NTS neurons and the synthesis and secretion of BDNF in the brain via the “gut-brain axis (vagal pathway)”. Thus, it effectively promoted the development of MG in pubertal mice (Fig. 9).
Gut microbiota are essential to the developmental process of the host CNS10,11. A significant reduction in the number of nerve cells and a slowing down of the nerve formation process in the hippocampal region of mice was observed after antibiotic cocktail (ABX) was fed to the mice30. BDNF promotes the maturation and differentiation of host neuronal cells, so it has an important regulatory role in the development of the host CNS, and it is also indispensable for the normal development of the peripheral nervous system (PNS)31,32,33. Recently, there has been increasing evidence that VN play a key role in regulating BDNF expression in the brain. Gut hormones such as cholecystokinin and glucagon-like peptide-1 (GLP-1) bind to specific receptors on the surface of the VN and regulate the gene expression levels of BDNF in the hippocampus, contributing to appetite control21,22. Moreover, the gene expression levels of BDNF in the hippocampus were significantly decreased in mice in which the VN was severed23. 4/6-week-old mice were exerted to Vago, which was shown to significantly inhibit NTS neuronal excitability and decrease synthesis and secretion of BDNF in the paraventricular nucleus of hypothalamic (PVH) and hippocampal regions. Meanwhile, a significant increase in body weight and food intake was observed in the mice, while a significant increase in body weight and food intake was also observed in the Vago mice transplanted with normal microbiota. This result suggests that VN has an important role in sensing gut signals and thus regulating appetite.
Compared with normal mice, germ-free mice showed significantly lower expression levels of BDNF in the hippocampus and amygdala regions, and the neural maturation process of the brain showed retardation34. Akkermansia muciniphila has been shown to improve hippocampal BDNF gene expression in mice model of depression by modulating the gut microbiota and metabolites35. In the present study, the excitability of NTS neurons was altered by Vago-FMT. Meanwhile, altering the proportion of gut microbiota composition by FMT significantly reduced BDNF synthesis and secretion, whereas even transplantation of normal flora could not reverse the reduction in BDNF synthesis and secretion caused by Vago. This likewise confirms that gut microbiota can influence BDNF synthesis and secretion in the brain of pubertal mice via the VN. However, the method of gut microbial colonisation was not effective in improving neurodevelopmental delays in adult germ-free mice36. The above studies suggest that early in life, gut microbiota may mediate the synthesis and secretion of BDNF via the VN. This process induces neuronal maturation and differentiation in different brain regions and plays a key role in regulating the development of the host CNS. It also alters the normal development of the host PNS, which in turn affects the development of other organs.
BDNF is continuously highly expressed in the MG during puberty and its level decreases with the end of puberty37. A large number of differences have been found in the CNS and PNS of male and female vertebrates, and this difference is thought to be the result of a combination of sex hormones and neurotrophic factors38. It has been shown that BDNF can bind to the Tropomyosin-related kinase B (TrkB) receptor on axons of mammary sensory nerves. Early in life, this process is necessary for the establishment and maintenance of mammary sensory nerve fibers in both female and male embryos. However, androgens produced by males block BDNF-TrkB signalling, which leads to pruning of mammary sensory nerve axon terminals and cessation of MG development8. Recent studies have shown that mammary sensory nerve fibers are regulated by a balance between BDNF and Semaphorin, and that Semaphorin-PlexinA4 signalling promotes axonal pruning after inhibition of BDNF-TrkB signalling9. In 4/6-week-old mice exerted with Vago, the protein expression levels of Tuj1 and BDNF in the MG were significantly reduced, whereas Tuj1 immunofluorescence staining of MG sections showed a significant reduction in mammary sensory nerve fiber density. The MG development was inhibited due to the reduced levels of BDNF allowing axon terminal pruning.
The VN plays a regulatory role in the levels of Lactobacillus and Bifidobacterium through parasympathetic activity, with sensory fibers potentially contributing to the modulation of the gut microbiota. For example, CCK acts on vagal sensory ganglia, resulting in the release of mucin from Brunner’s glands (BGs). This mucin has been demonstrated to serve as a proliferative substrate for Lactobacillus39. Moreover, the research has revealed that a subcortical region within the temporal lobes (CeA) regulates BGs via the VN, and its mucosal secretions facilitate the proliferation of Lactobacillus39. The results of this study showed that after mice were exerted with Vago, the 16S rRNA sequencing showed that the abundance of Lactobacillus in the gut microbiota of mice in the Vago group was significantly lower, and the abundance of Bifidobacterium and Bacteroides showed a decreasing trend compared to mice in the SS group. LEfSe analyses showed that the differential flora in the Vago group were Lactobacillus and Bifidobacterium compared to the SS group. Meanwhile, given that vagus sensory fibers are accountable for detecting alterations in the microbial constitution of the gut, this study proceeded with FMT experiments. Vago-FMT inhibits NTS neuronal excitability and reduces synthesis and secretion of BDNF in the brain. As a result, mammary sensory nerve axons are pruned, ultimately leading to inhibition of MG developmental processes, but the VN is integral to this pathway. BDNF is the only neurotrophic factor known to be associated with the gut microbiota and is strongly and positively correlated with levels of Lactobacillus and Bifidobacterium, etc40. This is consistent with our results.
In mice with lesions in the BGs, a specific dietary preference for fiber-rich pelleted feeds and solutions containing probiotics was observed. This phenomenon may be attributed to the reduced mucin secretion triggered by BGs lesions, which in turn inhibits the proliferation of probiotic bacteria such as Lactobacillus. The Vagal sensory fibers are capable of detecting alterations in the composition of the gut microbiota and transmit this information to specific neural circuits within the brain. It induces alterations in the physiological processes of the organism, thereby influencing dietary habits. At present, Lactobacillus and Bifidobacterium are the most widely studied probiotics39,40,41,42. The vast majority of prebiotic research focuses on the two beneficial bacteria. For instance, GOS26,27,28, polyphenols in pomegranate43, inulin44 and pectin45. The International Scientific Association for Probiotics and Prebiotics (ISAPP) defines prebiotics as fermented substrates that are selectively utilized by host microorganisms to achieve health benefits46. It has been shown that GOS can increase the average daily weight gain of Holstein dairy calves and improve the feed conversion ratio. At the same time, serum concentrations of two appetite-related hormones, cholecystokinin and GLP-1, were increased, so Holstein dairy calves growth performance was enhanced by GOS46.
Referring to the “Technical Standards for Testing & Assessment of Health Food” (the Ministry of Health of the People’s Republic of China) and previous experiments27, three dose groups were set. In the present study, it was found that as the concentration of GOS supplementation increased, the more significant the effect of promoting MG development. Moreover, when most of the gut microbiota was removed by ABX, the effect of GOS in promoting MG development disappeared. The levels of Bifidobacterium, Allobaculum and Bacteroides in the gut microbiota of mice were significantly increased after GOS treatment. Bifidobacterium, one of the most common probiotics, helps to improve intestinal functions26,27; Allobaculum is associated with the regulation of lipid metabolism47; Bacteroides maintains the balance of gut microbiota and plays an important role in preventing obesity48,49. Thus, GOS acts as a microbial fermentation substrate, and the intestinal environment is significantly altered, ensuring intestinal health and producing a significant beneficial effect on the host. Based on these findings, we determined that GOS effectively promotes the developmental process of the MG in pubertal mice by altering the proportion of gut microbiota composition. Subsequently, 4-week-old mice were exerted with Vago, and further experiments showed that Vago reversed the effect of GOS in promoting MG development in pubertal mice. The activation of the NTS-PVH neural circuit by GOS and the increase in mammary sensory nerve fiber density were similarly reversed. Therefore, the “gut-brain-mammary gland axis” can be activated by GOS. Furthermore, in the process of consuming GOS, the gut microbiota produce short-chain fatty acids, of which formic, acetic, propionic and butyric acids are the main short-chain fatty acids50. VN can be directly activated by short-chain fatty acids51, which may be one of the effective means of activating the “gut-brain axis (vagal pathway)” by GOS.
Our findings indicate that Vago exerts an inhibitory effect on mammary development in pubertal mice. Furthermore, Vago has been observed to alter the digestive capacity of mice, thereby influencing the nutritional status of the organism. Therefore, although the VN is a principal bidirectional neural communication pathway in the gut-brain axis, however, the findings of this study do not provide compelling evidence to support the conclusion that the brain regulates gut microbial composition through the VN. The current study indicates that alterations in the composition of the gut microbiota may influence the synthesis and secretion of BDNF in the brain. This suggests that further investigation into the potential use of this mechanism in the treatment of postpartum depression due to lactation in pregnant women may be of interest. In conclusion, GOS activates the VN by up-regulating Lactobacillus and Bifidobacterium. It promotes MG development in pubertal mice via the GUT-VN-NTS-PVH pathway. It remains unclear how Lactobacillus and Bifidobacterium activate the VN. We hypothesize that this may occur via two potential pathways: the presence of altered enteric nervous system/intestinal glands and/or altered metabolite levels.
Methods
Animals
The experimental animals used in this study were SPF grade ICR mice (3-4 weeks old) purchased from Liaoning Changsheng Biotechnology Co., Ltd (China). Protection of animal welfare during husbandry and experimental operations was carried out in accordance with the Jilin University Institutional Animal Care and Use Committee (Number of permit: SY202401004). Mice were given three days to acclimatize before the start of the experiment. All mice were housed in an artificially adjustable barrier environment (22-23 °C, 55 ± 10% humidity) with a 12 h light-dark cycle and provided with adequate food and water.
Experimental design
-
1.
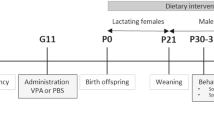
A batch of 4-week-old mice were randomly divided into 5 groups: Control (4 W) group (n = 6), SS (4 W) group (n = 6), Vago (4 W) group (n = 6), SS (6 W) group (n = 6), and Vago (6 W) group (n = 6). Firstly, the SS (4/6 W) and Vago (4/6 W) groups underwent sham surgery (SS) and bilateral subdiaphragmatic vagotomy (Vago) at week 4/6, respectively. Secondly, the mice were weighed at two-day intervals during the experiment. Finally, they were euthanized at 8 weeks of age. Moreover, serum and fecal samples (colon contents) were collected, as well as bilateral MGs and intact brain tissue were also gathered.
Another batch of 4-week-old mice were randomly divided into 3 groups: the Control (8 W) group (n = 6), the SS (8 W) group (n = 6), and the Vago (8 W) group (n = 6). The SS (8 W) and Vago (8 W) groups underwent SS and Vago at week 8, respectively. Finally, they were euthanized at 10 weeks of age. Moreover, serum and fecal samples (colon contents) were collected, as well as bilateral MGs and intact brain tissue were also gathered.
-
2.
A batch of 4-week-old mice was randomly divided into 6 groups: Control (8 W) group (n = 21), Vago-FMT (8 W) group (n = 21), SS (8 W) group (n = 21), SS (8 W)+Control-FMT group (n = 21), Vago (8 W) group (n = 21) and Vago (8 W)+Control-FMT group (n = 21). All groups were subjected to FMT (Vago-FMT: Vago derived Fecal Microbiota Transplant; Control-FMT: Control derived Fecal Microbiota Transplant) and reared until 8 weeks of age for subsequent experiments, during which the mice were weighed at two-day intervals.
Mice were randomly divided into two parts: (1) The first part was euthanized at 8 weeks of age. Moreover, serum was collected, as well as bilateral MGs and intact brain tissue were also gathered. (2) In the other part, two female mice were randomly placed in a cage with a male mouse, and the female mice were individually transferred to another cage after they became pregnant. After female mice gave birth to pups, they were cross-nursed as shown in Figs. 5i and 6i. Fixed feeding of 9 pups per female mouse for 4 weeks and pups were weighed at two-day intervals.
-
3.
Referring to Qiu et al. (2022)27, different concentrations of GOS (purity ≥95%, New Froncisco Biotechnology Co., Ltd, China) were supplemented by gavage according to the body weight of mice. GOS was dissolved in PBS solution, low-dose GOS (GOS-L): 681.5 mg/kg GOS, medium-dose GOS (GOS-M): 1365 mg/kg GOS, and high-dose GOS (GOS-H): 2730 mg/kg GOS. A batch of 4-week-old mice was randomly divided into 4 groups: Control group (n = 6), GOS-L group (n = 6), GOS-M group (n = 6), and GOS-H group (n = 6). They were euthanized at 8 weeks of age. Moreover, fecal samples (colon contents) were collected, as well as bilateral MGs was also gathered.
Another batch of 4-week-old mice were randomly divided into two parts and randomly grouped: (1) Antibiotic cocktail (ABX) group (n = 6), ABX + GOS-H group (n = 6). Metronidazole (1 g/L), neomycin (1 g/L), ampicillin (1 g/L), and vancomycin (0.5 g/L) were added to the drinking water to remove most of the gut microbiota. (2) Control group (n = 6), Control+GOS-H group (n = 6), SS + GOS-H group (n = 6), Vago+GOS-H group (n = 6). They were euthanized at 8 weeks of age. Moreover, serum was collected, as well as bilateral MGs and intact brain tissue were also gathered.
Bilateral subdiaphragmatic vagotomy (Vago)
Mice were fasted overnight before surgery and anesthetized using 10% urethane, ketoprofen (5 mg/kg, MyBioSource) was used for analgesia. Firstly, the hair near the abdominal midline was shaved and the skin was disinfected with an iodophor disinfectant. The skin and muscular layer were incised along the abdominal midline to expose the liver tissue, which was carefully and slowly pushed superiorly with a sterile cotton ball moistened with 0.9% saline to expose the esophagus and the required surgical field. Secondly, the subsequent operations were performed under an animal operating microscope: A ligature was performed at the junction of the stomach and esophagus for retraction to allow the operator to see the dorsal and ventral vagus nerve trunks extending along the esophagus, which were meticulously stripped and then severed. If there was no damage to the liver, esophagus, or other organs and no bleeding, restored the stomach and liver to their normal position. Finally, the muscular layer and skin were sutured layer by layer using 4-0 absorbable surgical sutures (PGA).
The success of Vago was verified using cholecystokinin-octapeptide (CCK-8, CLP0164, Beijing Solarbio Science & Technology Co., Ltd, China)52. Mice were fasted for 20 h before intraperitoneal injection of CCK-8 (8 μg/kg), and then the food intake was measured over a 2 h period. The Vago mice whose food intake was reduced by more than 30% were removed during the course of the experiment and were not involved in subsequent experiments. A 3-day surgical recovery period was given before proceeding to the next step of the experiment.
For sham surgery, incisions of uniform size were created at the same site in the mouse using the same treatment. The dorsal and ventral vagal trunks were exposed, but they were not severed. If there was no damage to the liver, esophagus, or other organs and no bleeding, restored the stomach and liver to their normal position. Finally, the abdominal incision was closed layer by layer using the same method.
Fecal microbiota transplant (FMT)
Refer to the method of Sun et al. (2018) for FMT53. Briefly, fresh fecal pellets were collected from mice at 8 weeks of age and then soaked (1 fecal pellet/mL for 15 min) using sterile PBS solution, with constant vibration shaking during the soaking period. The suspension was obtained by centrifugation at 1000 rpm, 4 °C for 5 min at the end of soaking. Firstly, the suspension was centrifuged at 8000 rpm, 4 °C for 5 min and filtered twice to obtain the final bacterial suspension. Secondly, for the final bacterial suspension, mixed with an equal volume of 40% glycerol, and stored at -80 °C for subsequent experiments. Finally, solid media was used under anaerobic conditions and colonies were counted by the dilution coating method to determine if the bacterial concentration of 108 CFU/mL was reached in each tube of storage solution.
Before FMT, metronidazole (200 mg/kg), neomycin (200 mg/kg), ampicillin (200 mg/kg), and vancomycin (100 mg/kg) were dissolved in sterile PBS solution according to the body weights of the mice and gavaged (200 μL at a time) to the mice for 5 days. Next, gavage (200 μL at a time) was carried out on day 6 using sterile PBS solution, and finally, FMT (200 μL at a time) was carried out on day 7 for 1 week, after which gavage was performed at 2-day intervals.
Whole mount staining of the MG and quantification of various developmental indicators of the MG
After the mice were euthanized, the limbs were immobilized and the skin was opened to expose the MGs. The fourth pair of MGs of mice were removed, carefully spread and placed on slides treated with poly-L-lysine solution (Sigma) and subsequently immersed in pre-prepared Carnoy’s solution (60% ethanol, 30% chloroform, and 10% glacial acetic acid) for 12 h of fixation. The MGs were immersed in 70%, 35%, 15% ethanol and distilled water for 10 min, and stained with carmine dye solution (2 g/L, Sigma) at room temperature for 12 h. After staining, the MGs were decolorized with 70%, 95%, 100%, and 100% ethanol for 10 min. The slides were then immersed in xylene, and the results were visualized by an optical microscope.
Quantitative method 1: Referring to the method of Goel et al. (2011)54. Briefly, the microscopic photograph was separated by six equally spaced imaginary lines (Fig. 1g), and the central lymph node was located approximately one-quarter of the way to the left of the whole MG. Then, the number of mammary ducts crossing each hypothetical line was determined to quantify MG development in mice.
Quantitative method 2: Referring to the method of Stanko et al. (2017)55. Briefly, Sholl analysis was carried out on the microscopic photograph of the whole MG using the Sholl analysis plugin in ImageJ, and the radius step size was set to 0.2 mm while performing the analysis. For which, for enclosing radius: the length of the MG was measured from the starting point as the primary mammary duct to the end point as the farthest terminal duct; for the mammary epithelial area (MEA): measurement of the area surrounded by the mammary ducts; for the number of Sholl intersections (Sum inters) and Sholl decay coefficients (Sholl decay): statistics by the Sholl analysis plugin; for branching density: Sum inters / (MEA - LNA).
Mammary gland sensory neurotomy (SNeuro)
Mice were fasted overnight before surgery and anesthetized using 10% urethane, ketoprofen (5 mg/kg, MyBioSource) was used for analgesia. Firstly, the hair near the abdominal midline was shaved and the skin was disinfected with an iodophor disinfectant. The skin is incised above the 1st-2nd lumbar vertebrae along the dorsal midline, and the skin is carefully and slowly separated from the abdominal wall using forceps, making sure not to damage the surrounding tissues. During this process, part of the sensory nerve extending from the dorsal root ganglia can be observed and severed. After no significant bleeding was observed, the skin was finally sutured using 4-0 absorbable surgical sutures (PGA). A 3-day surgical recovery period was given before proceeding to the next step of the experiment.
For sham surgery, incisions of uniform size were created at the same site in the mouse using the same treatment. The skin and abdominal wall are carefully and slowly separated using forceps, making sure not to damage the surrounding tissue and not to sever the sensory nerves. Finally, the back incision was closed in the same way.
Immunofluorescence and quantification
For brain tissue samples: firstly, the collected intact brain tissues were immersed in 4% paraformaldehyde (w/v) for 3 days, then embedded in paraffin and cut the tissue blocks into 5 μm sections. The paraffin sections underwent deparaffinization with xylene and ethanol.
For MG tissue samples: firstly, the collected MG tissues were embedded in OCT (Sakura Finetek USA, 4583), frozen at −80 °C, and the tissue blocks were cut into 8 μm sections. Secondly, the frozen sections were air-dried to dry out the water and fixed in cold acetone for 10 min and then washed with PBS three times after the acetone was completely dry.
Next, antigen repair was performed in 20 mM EDTA (PH = 8.0) for 9 min at medium heat, 8 min at cease-fire, and 7 min at medium-low heat in microwave. The sections were blocked with 3% BSA (w/v) for 30 min, primary antibody was incubated overnight at 4 °C, secondary antibody was incubated at room temperature for 1 h protected from light, DAPI was incubated at room temperature for 10 min protected from light, and the sections were sealed with fluoromount-G (Wuhan Servicebio Technology Co., Ltd, China). Finally, images were acquired using an upright fluorescent microscope (Nikon Eclipse C1). Primary antibody: anti-BDNF (1:500, Proteintech, 28205-1-AP), anti-c-Fos (1:500, Cell Signaling Technology, 2250S), anti-β-III Tubulin (Tuj1, 1:100, Selleck, A5107). Secondary antibody: Alexa Fluor 488-labeled goat anti-rabbit IgG (1:400, Wuhan Servicebio Technology Co., Ltd, GB25303), CY3-labeled goat anti-rabbit IgG (1:300, Wuhan Servicebio Technology Co., Ltd, GB21303).
Quantification:
C-Fos+ cells were calculated using the ImageJ.
Mean fluorescence intensity=Integrated Density (IntDen) / Area.
Referring to the method of Liu et al. (2012)8. MG sensory nerve fiber density was quantified using ImageJ. Briefly, Fiber density (%) = Area of Tuj1 staining within the band / Total area of 15 µm band surrounding the mammary duct × 100%.
Cell culture and treatment
The mouse mammary epithelial cells (mMECs, EPH4EV Cell) were purchased from American Type Culture Collection (ATCC CRL-3063TM), and the bovine mammary epithelial cells (BMECs, MAC-T) were purchased from Inner Mongolia Wanrui Biotechnology Co., Ltd. The cells were cultured in a humidified incubator at 37 °C with 5% CO2, and the medium was DMEM (Gibco) containing 10% FBS (Inner Mongolia Opcel Biotechnology Co., Ltd, China, JYK-FBS-301), penicillin and streptomycin (100 μg/mL, North China Pharmaceutical). Cell culture consumables (CellSafeTM) were purchased from Guangzhou Jet BioFiltration Co., Ltd. Once the cells reached 70% confluence, they were treated with varying concentrations of GOS (0, 5, 10, 20 and 40 mg/mL) for 24 h. Subsequently used for protein extraction or the effect of varying concentrations of GOS on the cell proliferative viability was assayed using the Cell Counting Kit-8 (Invigentech Inc, IV08-500) according to the manufacturer’s instructions.
Western blotting
Firstly, NP40 lysis buffer (Beyotime, P0013F) containing Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, 78443) was added to the sample tubes containing mouse mammary gland tissues, and fully grinded using a fully automated sample rapid grinder (Shanghai Jingxin Industrial Co., Ltd, JXFSTPRP-24L). The protein concentration was determined and partitioned using the BCA Protein Concentration Assay Kit (Proteintech, PK10026). Next, extracted proteins (30 μg/lane) were electrophoresed by 4% and 12% SDS-PAGE, and then the proteins were transferred to polyvinylidene fluoride (PVDF) membranes, which were blocked for 2 h with 5% skimmed milk (w/v). The PVDF membranes were incubated with primary antibody at 4 °C overnight and then washed five times with Tris-buffered saline with Tween 20 (TBST) for 10 min each time. The membrane was incubated for 1 h at room temperature with secondary antibody (1:5000, BOSTER, BA1056/BA1050) corresponding to the primary antibody, and then washed five times with TBST for 10 min each time. Finally, the secondary antibody was made to fluoresce with a hypersensitive luminescent solution (Applicygen, P1050), and then the protein content was visualized and photographed using a Tanon 4600 image analysis system. Primary antibody: anti-BDNF (1:500, Proteintech, 28205-1-AP), anti-β-III Tubulin (Tuj1, 1:1000, Selleck, A5107), anti-Cyclin D1 (1:1000, Proteintech, 60186-1-Ig), anti-PCNA (1:1000, Bioss, bs-2006R), anti-β-actin (1:5000, Proteintech, 66009-1-Ig).
Enzyme linked immunosorbent assay (ELISA)
The Mouse BDNF ELISA Kit (Thermo Fisher Scientific Inc, EEL088) was used to detect the changes in BDNF levels in mouse serum according to the manufacturer’s instructions.
16S rRNA sequencing
The 16S rRNA sequencing of fecal bacteria obtained from the experiment was done on the Illumina NovaSeq platform (Nanjing Personal Biotechnology Co., Ltd, China). Nonrepetitive sequences were clustered into different optional taxon units (OTUs) according to a 97% similarity.
A Venn diagram was drawn based on the OTUs shared and unique to each group. Both α-diversity index (Chao1 index, Observed species and Shannon index) and β-diversity were calculated based on OTU data. For β-diversity, the bray curtis and weighted unifrac distance algorithms, as well as the nonmetric multidimensional scale (NMDS) based on the bray curtis distance algorithm, were utilized for principal coordinate analysis (PCoA) to evaluate the differences in gut microbiota composition. Using the serial numbers of the OTUs arranged in order of abundance as the horizontal coordinate and the Log2 log-transformed value of the abundance value/mean value of each OTU in the group as the vertical coordinate, rank abundance curves were plotted to evaluate the homogeneity of the gut microbiota composition. Differential flora were screened by Linear discriminant analysis (LDA) effect size (LEfSe) (LDA > 2, P < 0.05). Online analytics using cloud platforms (https://www.genescloud.cn).
Statistical analysis
A minimum of 6 mice were present in each group of animal experiments, and all data are expressed as mean ± standard error of the mean (SEM). Bar graphs, box plots, line graphs, and bubble maps were performed using GraphPad Prism 9.0 software, and differences between groups were determined by t-test or one/two-way ANOVA, with p < 0.05 considered significant.
Data availability
All relevant data about this research can be requested from the corresponding author. The data for 16S rRNA gene sequencing have been deposited with links to BioProject accession number PRJNA1145029 in the NCBI BioProject database and will be accessed upon published.
References
Kaimala, S., Bisana, S. & Kumar, S. Mammary gland stem cells: more puzzles than explanations. J. Biosci. 37, 349–358 (2012).
Watson, C. J. & Khaled, W. T. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development 135, 995–1003 (2008).
Roche, J. R. et al. Fertility and the transition dairy cow. Reprod. Fertil. Dev. 30, 85–100 (2017).
Jaswal, S. et al. Critical review on physiological and molecular features during bovine mammary gland development: recent advances. Cells 11, 3325 (2022).
Luo, L. et al. Overexpression of igf-1 during early development expands the number of mammary stem cells and primes them for transformation. Stem Cells 40, 273–289 (2022).
Cao, Y. et al. Niacin stimulates eph4ev mammary epithelial cell proliferation and mammary gland development in pubertal mice through activation of akt/mtor and erk1/2 signaling pathways. Cell Tissue Res 384, 313–324 (2021).
Sundaram, S., Johnson, L. K. & Yan, L. High-fat diet alters circadian rhythms in mammary glands of pubertal mice. Front. Endocrinol. 11, 349 (2020).
Liu, Y. et al. Sexually dimorphic bdnf signaling directs sensory innervation of the mammary gland. Science 338, 1357–1360 (2012).
Sar, S. H., Goldner, R., Golan-Vaishenker, Y. & Yaron, A. Balance between bdnf and semaphorins gates the innervation of the mammary gland. Elife 8, e41162 (2019).
Sharon, G., Sampson, T. R., Geschwind, D. H. & Mazmanian, S. K. The central nervous system and the gut microbiome. Cell 167, 915–932 (2016).
Rutsch, A., Kantsjo, J. B. & Ronchi, F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 11, 604179 (2020).
Xia, T., He, W., Luo, Z., Wang, K. & Tan, X. Achyranthes bidentata polysaccharide ameliorates type 2 diabetes mellitus by gut microbiota-derived short-chain fatty acids-induced activation of the glp-1/glp-1r/camp/pka/creb/ins pathway. Int. J. Biol. Macromol. 270, 132256 (2024).
Lai, T. T. et al. The gut microbiota modulate locomotion via vagus-dependent glucagon-like peptide-1 signaling. Npj. Biofilms Microbomes 10, 2 (2024).
Margolis, K. G., Cryan, J. F. & Mayer, E. A. The microbiota-gut-brain axis: from motility to mood. Gastroenterology 160, 1486–1501 (2021).
Agirman, G. & Hsiao, E. Y. Snapshot: the microbiota-gut-brain axis. Cell 184, 2524 (2021).
Siopi, E. et al. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol. Psychiatr. 28, 3002–3012 (2023).
Zou, Q. et al. Alleviating effect of vagus nerve cutting in salmonella-induced gut infections and anxiety-like behavior via enhancing microbiota-derived gaba. Brain Behav. Immun. 119, 607–620 (2024).
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001).
Barros, V. N. et al. The pattern of c-fos expression and its refractory period in the brain of rats and monkeys. Front. Cell. Neurosci. 9, 72 (2015).
Chao, M. V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 (2003).
Berthoud, H. R., Albaugh, V. L. & Neuhuber, W. L. Gut-brain communication and obesity: understanding functions of the vagus nerve. J. Clin. Invest. 131, e143770 (2021).
Cork, S. C. The role of the vagus nerve in appetite control: implications for the pathogenesis of obesity. J. Neuroendocrinol. 30, e12643 (2018).
O’Leary, O. F. et al. The vagus nerve modulates bdnf expression and neurogenesis in the hippocampus. Eur. Neuropsychopharmacol. 28, 307–316 (2018).
Xu, F. et al. Silibinin decreases hepatic glucose production through the activation of gut-brain-liver axis in diabetic rats. Food Funct. 9, 4926–4935 (2018).
Yang, T., Richards, E. M., Pepine, C. J. & Raizada, M. K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14, 442–456 (2018).
Wu, Y. et al. Strain specificity of lactobacilli with promoted colonization by galactooligosaccharides administration in protecting intestinal barriers during salmonella infection. J. Adv. Res. 56, 1–14 (2024).
Qiu, S. et al. Gos ameliorates nonalcoholic fatty liver disease induced by high fat and high sugar diet through lipid metabolism and intestinal microbes. Nutrients 14, 2749 (2022).
Wang, K., Duan, F., Sun, T., Zhang, Y. & Lu, L. Galactooligosaccharides: synthesis, metabolism, bioactivities and food applications. Crit. Rev. Food Sci. Nutr. 64, 6160–6176 (2023).
Fulling, C., Dinan, T. G. & Cryan, J. F. Gut microbe to brain signaling: what happens in vagus…. Neuron 101, 998–1002 (2019).
Mohle, L. et al. Ly6c(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 15, 1945–1956 (2016).
Camuso, S., La Rosa, P., Fiorenza, M. T. & Canterini, S. Pleiotropic effects of bdnf on the cerebellum and hippocampus: implications for neurodevelopmental disorders. Neurobiol. Dis. 163, 105606 (2022).
Tessarollo, L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 9, 125–137 (1998).
Park, H. & Poo, M. M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 (2013).
Diaz, H. R. et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. Usa. 108, 3047–3052 (2011).
Ding, Y. et al. A next-generation probiotic: akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 105, 8411–8426 (2021).
Ogbonnaya, E. S. et al. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry 78, e7–e9 (2015).
Colitti, M. Expression of ngf, bdnf and their high-affinity receptors in ovine mammary glands during development and lactation. Histochem. Cell Biol. 144, 559–570 (2015).
Forger, N. G. Cell death and sexual differentiation of the nervous system. Neuroscience 138, 929–938 (2006).
Chang, H. et al. Stress-sensitive neural circuits change the gut microbiome via duodenal glands. Cell 187, 5393–5412 (2024).
Agnihotri, N. & Mohajeri, M. H. Involvement of intestinal microbiota in adult neurogenesis and the expression of brain-derived neurotrophic factor. Int. J. Mol. Sci. 23, 15934 (2022).
Wang, M. et al. Depression-associated gut microbes, metabolites and clinical trials. Front. Microbiol. 15, 1292004 (2024).
Xiong, R. G. et al. The role of gut microbiota in anxiety, depression, and other mental disorders as well as the protective effects of dietary components. Nutrients 15, 3258 (2023).
Yin, Y., Martinez, R., Zhang, W. & Estevez, M. Crosstalk between dietary pomegranate and gut microbiota: evidence of health benefits. Crit. Rev. Food Sci. Nutr. 64, 10009–10035 (2023).
Radziszewska, M., Smarkusz-Zarzecka, J., Ostrowska, L. & Pogodzinski, D. Nutrition and supplementation in ulcerative colitis. Nutrients 14, 2469 (2022).
Li, W., Zhang, K. & Yang, H. Pectin alleviates high fat (lard) diet-induced nonalcoholic fatty liver disease in mice: possible role of short-chain fatty acids and gut microbiota regulated by pectin. J. Agric. Food Chem. 66, 8015–8025 (2018).
Yu, X. et al. Effects of different galacto-oligosaccharide supplementation on growth performance, immune function, serum nutrients, and appetite-related hormones in holstein calves. Animals 13, 3366 (2023).
Zheng, Z. et al. Allobaculum involves in the modulation of intestinal angptlt4 expression in mice treated by high-fat diet. Front. Nutr. 8, 690138 (2021).
Yang, J. Y. et al. Gut commensal bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 10, 104–116 (2017).
Li, H. et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 16, 2304159 (2024).
Wenzel, T. J., Gates, E. J., Ranger, A. L. & Klegeris, A. Short-chain fatty acids (scfas) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 105, 103493 (2020).
Kasarello, K., Cudnoch-Jedrzejewska, A. & Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 14, 1118529 (2023).
O’Mahony, C., van der Kleij, H., Bienenstock, J., Shanahan, F. & O’Mahony, L. Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 297, R1118–R1126 (2009).
Sun, M. F. et al. Neuroprotective effects of fecal microbiota transplantation on mptp-induced parkinson’s disease mice: gut microbiota, glial reaction and tlr4/tnf-alpha signaling pathway. Brain Behav. Immun. 70, 48–60 (2018).
Goel, H. L. et al. Neuropilin-2 promotes branching morphogenesis in the mouse mammary gland. Development 138, 2969–2976 (2011).
Stanko, J. P. & Fenton, S. E. Quantifying branching density in rat mammary gland whole-mounts using the sholl analysis method. J. Vis. Exp. 19, 55789 (2017).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Project No. 32272955, 32172807, 32202766), Jilin Scientific and Technological Development Program (Project No. 20220101302JC).
Author information
Authors and Affiliations
Contributions
Y.G., J.L. and S.F. designed this experiment. Y.G. wrote the manuscript. Y.G., Y.C., F.L. and S.F. revised the manuscript. Y.G., J.Z. and J.W. participated in the experimental operation. Y.G., M.S., Y.L., X.L. and W.G. participated in the compilation of experimental data. All authors were informed and agreed to publish the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ge, Y., Cao, Y., Zhang, J. et al. GOS enhances BDNF-mediated mammary gland development in pubertal mice via the gut-brain axis. npj Biofilms Microbiomes 10, 130 (2024). https://doi.org/10.1038/s41522-024-00607-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-024-00607-4