Abstract
Bacterial adherence to the intestinal epithelium plays a role in niche establishment in the gut lumen. Through sampling natural populations of Caenorhabditis, we discovered several bacterial species that adhere to the intestinal epithelium via polar, intimate association, best described as attachment. These bacteria had varying effects on host fitness and physiology, with one species having negative effects, and the others exhibiting neutral effects. These bacteria can actively divide in the gut lumen, either replicating throughout the gut simultaneously or anteroposteriorly. In competition assays, animals pre-colonized with an attaching commensal bacteria reduced colonization by the pathogenic bacteria, but this effect was not seen when animals were colonized by both species simultaneously. Regardless of the colonization paradigm, populations exposed to both bacteria showed a near-identical mitigation of the pathogenic effects. Altogether, these strains illustrate the capacity of microbiome bacteria to attach, replicate, and establish a niche across the entire intestinal lumen.
Similar content being viewed by others
Introduction
The community of microbes in the gut lumen is affected by their ability to persist and survive the gastrointestinal environment1,2,3. These bacteria must evade the host immune system, digestion, and harsh conditions like low pH. Bacteria better adapted to resist displacement from the host are more likely to successfully establish a niche in the intestine. For example, some gut microbes can modify their outer membranes with lipids4 or proteins5 to endure harsh conditions and host inflammation. One common method to survive the GI tract is through adherence to the intestinal epithelia.
The ability to adhere to the intestinal epithelial surface strongly affects bacterial survival, niche formation, and persistence in the gut lumen3,6. In fact, bacterial adherence to the mammalian intestinal epithelium is a common mechanism utilized by pathogens, like Salmonella enterica serovar Typhimurium7 and enterohemorrhagic E. coli (EHEC)8, although their access to the epithelia usually requires dysbiosis9. Beneficial bacteria can also colonize the intestinal epithelium, such as species of Bacteroides10 and Lactobacillus that colonize mucus layers and potentially attach to the glycocalyx11,12. In vitro work suggests that more adhesive microbes can outcompete less adhesive ones13; for example, bacteria with the capacity to adhere to microfluidics devices can displace non-adherent bacteria. Consequently, in these experiments planktonic, or non-adhering, bacteria are unable to colonize this occupied space. According to mathematical modelling14, even slow-growing adhering bacteria may have a competitive advantage over planktonic species due to better establishment of their niche within the intestine. However, we do not fully understand the role that adherence plays on governing bacterial colonization and niche establishment in the intestine.
Caenorhabditis elegans has emerged as an excellent model to study host-microbe interactions in the gut15,16,17, being genetically tractable18 and easy to maintain. The C. elegans intestine shares numerous functional and morphological similarities to mammalian intestines. These include the presence of baso-apical polarization of intestinal cells, microvilli, and apical junctions that resemble mammalian cell-cell junctions19,20. Yet, C. elegans intestines are simpler to study as they are comprised of a population of twenty non-renewable cells that are largely considered to be a single cell type20. Due to C. elegans body transparency, intestinal colonization can be easily visualized in vivo using differential interference contrast (DIC) microscopy or RNA fluorescent in situ hybridization (FISH)21. Gut microbiome bacteria22 and intracellular pathogens, such as microsporidia23 and Orsay virus24, naturally colonize and infect the intestines of wild-caught C. elegans17,25. Recently, a simplified natural core microbiome of twelve bacteria was established in C. elegans26,27, called CeMbio. However, while the bacterial species comprising CeMbio bacteria have been shown to persist in the intestinal lumen, none have been shown to attach to the intestinal cells22,26,28,29,30.
Through ecological sampling of wild Caenorhabditis isolates, we discovered bacterial species that bind to the glycocalyx of the intestine, forming a direct, polar interaction with the epithelial cells, which we refer to as attachment. These represent the first members of the Caenorhabditis gut microbiome capable of extensive attachment to intestinal cells. Each of these bacterial species is horizontally transmitted amongst the host population and has the capacity to replicate and persist in the intestinal lumen without the need for constant bacterial uptake. We identified three distinct bacterial species in this collection, two members of Enterobacterales order and one member of Rickettsiales. We discovered that they have differential effects on host fitness, from neutral to negative, when colonizing independently. Co-colonization studies showed that an attaching commensal bacterium prevented colonization of an attaching pathogenic bacteria, though this had a detrimental effect on host reproductive fitness. Altogether, this unique set of bacteria presents an opportunity to utilize the C. elegans in vivo system to dissect the molecular mechanisms governing bacterial attachment to the gut, visualize the microbial biogeography in the intestines, and study the physiological impacts of this interaction on the host.
Results
Discovery of attaching bacteria in the gut lumen of Caenorhabditis nematodes
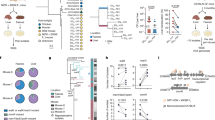
By collecting wild Caenorhabditis nematodes, chiefly in decomposing plant substrates, we and others found them associated with an array of microorganisms22,26,27,31. In addition to using bacterial rDNA sequencing or bacterial isolation, we established clonal lines from single nematodes picked onto standard NGM plates containing E. coli OP50-1 as food. Their self-progeny was observed using differential interference contrast (DIC) microscopy. Over ten years of sampling around the world, we observed natural Caenorhabditis isolates that were colonized with microbes that attach to the intestinal epithelium in the gut lumen and here, we characterize seven of them (Table 1, Fig. 1a–c). We first categorized these attaching bacteria into three distinct phenotypes in the host: (1) bacilli that cause severe anterior distension of the host lumen, (2) thin bacilli that are densely packed and hard to distinguish from their neighbors by DIC, and (3) bacilli that colonize with a comb-like appearance, i.e. where individual bacteria can be distinguished from their neighbors by DIC. The presence of three distinct morphologies in the intestine suggests the presence of three different bacterial species. Fluorescent in situ hybridization (FISH) of these wild strains using a universal probe (EUB338)21 against the bacterial small ribosomal subunit (16S) rRNA revealed that all these microbes are bacteria (Fig. 1d–f, left). After preliminary identification of these bacteria through 16S sequencing, we designed three specific FISH probes to unique regions of each 16S and found that each of the above categories corresponded to three distinct bacterial species or genera (Fig. 1d–f, right).
a–c DIC microscopy images of the intestinal lumen (lu) of Caenorhabditis isolates demonstrating bacteria (indicated by brackets) attaching to the intestinal epithelium, denoted by arrows. Images to the right are insets of the dashed boxes seen on the left. a Attaching bacteria in C. briggsae (JU3205) that causes severe anterior distension of the lumen, later identified as Ca. Lumenectis limosiae (LUAb1). Scale bars are 10 μm. b Thin bacteria in C. tropicalis (JU1848), later identified as Ca. Enterosymbion pterelaium (LUAb2). Scale bars are 5 μm. c Wild C. elegans (LUA21) colonized with attaching bacteria forming a comb-like appearance, later identified as Lelliottia jeotgali (LUAb3). Scale bars are 5 μm. d–f FISH using a universal 16S rRNA probe, EUB338 (green) and a species-specific FISH probe designed to the 16S rRNA (red) of either LUAb1, LUAb2, or LUAb3, respectively. Scale bars are 100 μm. Nuclei are stained via DAPI (blue).
We selected one representative wild strain of each of the three morphological categories and selectively cleaned them to enrich for the attaching bacteria32. These representative strains are LUAb1, LUAb2, and LUAb3, named for being the first, second, and third bacterial isolates found in the Luallen Lab (using standard C. elegans strain naming). By forcing the animals into dauers, bacteria in the intestine are protected while external contaminants are removed through a harsh overnight wash of detergent and antibiotics. After enrichment, we assigned the designation LUAb1 to the bacteria that causes anterior distention, LUAb2 to the thin bacteria, and LUAb3 to the comb-like bacteria (Supplementary Fig. 1a–c). LUAb3 was determined to be culturable in vitro after seeing persistent colonies on the NGM plates following selective cleaning. By contrast, the nematode strains containing LUAb1 (JU3205) and LUAb2 (JU1808) showed no obvious contamination on the plates after cleaning, suggesting that these bacteria were unculturable in vitro. Indeed, testing multiple culture media and techniques failed to result in bacterial growth for these two species.
To characterize these bacterial species, we conducted whole genome sequencing. LUAb3 was cultured in vitro while LUAb1 and LUAb2 were grown in the host gut lumen (C. elegans N2). We assembled their genomes de novo and analyzed their average nucleotide identity (ANI) with known bacteria, which indicated that LUAb1 and LUAb2 are yet unidentified taxa (Supplementary Fig. 2a, b). Phylogenomics using 92 up-to-date bacterial core genes (UBCG) describe LUAb1 as a novel bacterium in the Enterobacterales order, that we named Candidatus Lumenectis limosiae, due to the characteristic distension in colonized animals (Fig. 2a). We newly describe LUAb2 as a novel bacterium belonging to the Rickettsiales order that we named Candidatus Enterosymbion pterelaium after the King Pterelaus in Greek mythology (Fig. 2b). Finally, we identified LUAb3 as an isolate of the existing species Lelliottia jeotgali33 in the Enterobacteriaceae family (Fig. 2a, Supplementary Fig. 2c).
a Phylogenomic tree of sequenced Enterobacterales spp. with outgroup Pseudomonas aeruginosa. Branch lengths are the number of substitutions per site and branch points indicate percentage of trees with clustering of associated taxa. b Phylogenomic tree of sequenced Alphaproteobacteria spp. Branch lengths are the number of substitutions per site and branch points indicate percentage of trees with clustering of associated taxa. c Sequenced whole genomic DNA from clean C. elegans ERT413 colonized with LUAb1 or LUAb2 were analyzed via CZ-ID (formerly IDseq). Pie charts show the percent of non-C. elegans reads that were identified to belong to a particular bacterial species or genus that represented >0.01% of the total reads. d 16S PCR and amplicon sequencing of C. elegans ERT413 colonized with LUAb1 or LUAb2, with uncolonized N2 as a control.
Members of Rickettsiales are typically obligate intracellular parasites34. Ca. E. pterelaium does not show any evidence of intracellular invasion (0% invasion incidents out of 101 animals across 3 independent replicates, scored in fully colonized animals stained by FISH). However, we have found that this bacterium cannot be cultured in vitro and does not survive well outside of the host, as our standard protocol to make bacterial preparations from host lysate fails to result in viable bacteria (see below). Its membership in Rickettsiales may explain the obligate-like nature of this bacterium.
Despite not being able to culture Ca. L. limosiae LUAb1 or Ca. E. pterelaium LUAb2 in vitro, several results indicate that we were able to remove a majority of contaminating bacteria and establish a population of C. elegans N2 that remains persistently colonized. First, after cleaning as described above, the cultures of C. elegans N2 reference strain containing either LUAb1 or LUAb2 showed no obvious external contamination growing on the NGM plates. Second, when comparing species-specific FISH to generic bacterial FISH (EUB338), we saw no detectable colonization in the gut by any other species (see Fig. 1d, e). Third, we analyzed the read distribution after whole genome sequencing in C. elegans. For LUAb1, we found that the vast majority of non-C. elegans reads were either from E. coli (OP50-1) or LUAb1, with ~1% of reads distributed to other microbes. Similar results were seen for LUAb2, with ~6% of reads coming from other microbes (Fig. 2c). Fourth, similar levels of microbial contamination were seen when we conducted 16S amplicon sequencing in clean Ca. L. limosiae- and LUAb2-colonized strains. E. coli reads were predominant in the LUAb2-C. elegans culture likely due to the 16S PCR primers used that target Gammaproteobacteria and do not amplify Alphaproteobacteria efficiently (Fig. 2d). These data show that despite not being able to culture LUAb1 or LUAb2 in vitro, we were able to remove a majority of contaminating bacteria and establish a population of C. elegans N2 that remains persistently colonized with these microbiome bacteria.
Finally, from our collection of seven wild Caenorhabditis strains from around the world with attaching bacteria, we utilized our bacterial-specific FISH probes to determine their relative relationship to these bacteria. We found a total of four C. elegans or C. briggsae isolates from France, New Zealand, and the USA were colonized with Lelliottia spp. similar to LUAb3, two C. briggsae isolates from India and Mayotte were colonized with a LUAb1-like species, and only one C. tropicalis isolate from French Guiana was colonized with LUAb2 bacteria (Table 1). Interestingly, each of these bacterial isolates was capable of stably colonizing wild-type C. elegans (N2) as measured by FISH, suggesting that there is no strict host specificity for any of these three bacterial species (Table 1). Overall, we have isolated and identified three divergent attaching bacterial species found in the intestinal lumen of wild-isolated Caenorhabditis nematodes from around the globe. In total, we isolated four Lelliottia spp. (LUAb3, LUAb14, LUAb15, LUAb16), two Ca. L. limosiae isolates (LUAb1, LUAb47), and one Ca. E. pterelaium (LUAb2). We have further validated all the Lelliottia spp. by isolation in pure culture and whole genomes sequencing, while LUAb47 was only validated with LUAb1-specific FISH probes.
Contrasting effects of three attaching bacteria on host reproductive fitness and life history traits
To determine whether these attaching bacteria affect C. elegans fitness, we assayed the effects of the bacteria on host reproductive fitness, specifically on reproductive lifespan and brood size. To better visualize the colonization within the intestine, we used C. elegans strain ERT41335,36 expressing GFP specifically in intestinal cells. We found that ERT413 animals colonized with Ca. L. limosiae LUAb1 exhibited a substantial decrease in reproductive lifespan compared to uncolonized ERT413, with only ~20% of animals surviving to day 8 of adulthood compared to ~80% of non-colonized controls (Fig. 3a). However, colonization with Ca. E. pterelaium LUAb2 or L. jeotgali LUAb3 does not appear to affect host lifespan, compared to the uncolonized control (Fig. 3b, c). A similar result was observed with brood size assays, as LUAb1 colonized animals showed a ~ 45% decrease in total brood size (Fig. 3d), while LUAb2 and LUAb3 did not show a significant decrease (Fig. 3e-f). There was a small, significant decrease in brood size for LUAb3 colonized animals when specifically looking at daily brood sizes, but this was at a time point after most of the brood had been laid (day 3 and day 4, Fig. 3f). Overall, these data suggest that under these conditions, LUAb1 is a pathogen to C. elegans, while LUAb2 and LUAb3 behave as commensal-like microorganisms.
a–c Reproductive lifespan of ERT413 colonized with LUAb1, LUAb2, or LUAb3, respectively. Sample size, n, = 60 in 3 independent experiments (N). Error bars show standard deviation (SD) from the mean. Statistical analysis performed by Mantel-Cox test, where ****p < 0.0001 and ns = not significant. d–f Brood size of ERT413 colonized with LUAb1, LUAb2, or LUAb3, respectively, n = 60 in 3 independent experiments. Statistical analyses performed by unpaired two-tailed t-test where ns = not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. g–i Representative images for “Smurf” assay testing of intestinal barrier function assay. g Uncolonized C. elegans N2 fed E. coli OP50-1. h C. elegans N2 colonized with LUAb1. i C. elegans colonized with LUAb3. j Quantification of G-I, showing percent of animals that experienced leakage of blue dye from the intestine into the germline and/or body cavity, where N = 3 and n = 87-108. Statistical analyses performed by ordinary one-way ANOVA where ns = not significant and ****p < 0.0001. k Mortal germline (Mrt) phenotype describing the number of generations until sterility at 25°C of wild C. elegans strain JU775 either fed only E. coli OP50-1 (grey), colonized first with LUAb3 and transferred to E. coli OP50-1 (orange), or fed only LUAb3 (brown). Wilcoxon rank tests ***p < 0.001 and ****p < 0.0001 across at least 12 replicates for each of the three conditions.
Next, we measured the intestinal barrier function of C. elegans when colonized with LUAb1 and LUAb3 through a “Smurf” assay37,38 in which animals are fed a blue dye that can pass into the body cavity if the intestinal epithelium is compromised (Fig. 3g–j). Due to difficulties culturing LUAb1 in vitro, we adopted an existing protocol used with microsporidia spores23 for LUAb1 to better control the amount of exposure to the bacteria. Unfortunately, we were unable to prepare LUAb2 in a similar manner. Overall, we found that at 2 days post colonization C. elegans N2 adults colonized with LUAb1 exhibited an increase in intestinal leakiness resulting in the presence of blue dye in the body cavity compared to uncolonized controls fed only OP50-1 (Fig. 3g, h, j). On the other hand, there was no significant difference in intestinal leakage between LUAb3 colonized animals and the uncolonized control animals (Fig. 3i, j).
Finally, we tested whether LUAb3 could suppress the mortal germline (Mrt) phenotype. This Mrt phenotype is seen in some Caenorhabditis elegans wild isolates where the population becomes sterile after several generations at 25°C, which would be detrimental for the lineage in the wild. Several bacterial species, including another L. jeotgali isolate (JUb276), were demonstrated to suppress the Mrt phenotype39,40. We found that colonization with L. jeotgali LUAb3 could also suppress the Mrt phenotype of wild C. elegans strain JU775 when grown at high temperatures, demonstrating a beneficial effect (Fig. 3k). Altogether, these data support the pathogenic nature of LUAb1 and the commensal nature of LUAb3 on C. elegans, with LUAb3 having a benefit to the host in certain contexts.
Attaching bacteria cause structural changes to the host intestine
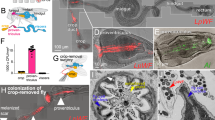
Because of the close proximity between the host intestinal epithelial cells and the attaching bacteria, we aimed to better visualize this intimate interaction and its physiological impacts on the host through transmission electron microscopy (TEM), focusing on Ca. L. limosiae LUAb1 and L. jeotgali LUAb3 colonized C. elegans N2. In N2 uncolonized animals fed OP50-1 bacteria, we did not see live bacteria in the gut lumen, but rather circular, electron-dense structures of various sizes that may represent digested bacteria (Fig. 4a). The intestinal cells contain characteristically long microvilli surrounded by a moderately electron-dense mucus layer, which corresponds to the glycocalyx. By contrast, animals colonized with LUAb1 contained intact bacteria, with a clustering of bacterial cells in contact with the glycocalyx that surrounds dramatically shortened intestinal microvilli (Fig. 4b). Intact bacterial cells were also observed in the lumen of animals colonized with LUAb3, some of which were also directly adjacent to the intestinal glycocalyx (Fig. 4c). We did not observe the organized comb-like adherence pattern seen via DIC microscopy (Fig. 1c), although this may be attributed to the orientation of the focal plane as dark bacterial structures can be seen along the perimeter of the intestinal epithelia near the glycocalyx. The TEM images demonstrate that both the pathogenic and commensal-like attaching bacteria likely interact with the glycocalyx layer covering the intestinal epithelium.
a-c Representative TEM images of C. elegans intestine uncolonized or colonized with attaching bacteria. Bacteria undergoing septation without contact with the glycocalyx are indicated by a red dashed oval and bacteria dividing with contact to the glycocalyx are indicated by a black dashed oval. lu indicates the intestinal lumen, arrowheads indicate the apical side of the glycocalyx, and arrows denote the microvilli. Scale bars indicated are 1 µm. a Intestine of uncolonized C. elegans N2. b C. elegans ERT413 (jySi21[spp-5p::GFP; Cbr-unc-119( + )] II) colonized with LUAb1 seen as the dark rod and spherical structures in the lumen. c Image of the intestine of L. jeotgali LUAb3 colonized C. elegans N2. Individual LUAb3 cells are seen as dark round or ovoid structures in the lumen. d–f TEM images showing the intestinal distension in C. elegans N2 colonized LUAb1 and LUAb3 in the intestine. The intestinal lumen is outlined in black. Scale bars are 5 µm. d Cross section of intestine of uncolonized C. elegans N2. e C. elegans ERT413 colonized with LUAb1. f Image of the intestine of LUAb3-colonized C. elegans N2. g Quantification of the intestinal brush border depth from TEM images of uncolonized (independent sections observed, n = 56 across 4 total animals, N), LUAb1 colonized animals (N = 3, n = 35), and LUAb3-colonized (N = 3, n = 36). h. Quantification of the area of the intestinal lumen for uncolonized (N = 4, n = 20), LUAb1 colonized (N = 3, n = 17) and LUAb3-colonized (N = 3, n = 15), animals. Statistical analyses performed by ordinary one-way ANOVA where ns = not significant and ****p < 0.0001.
LUAb1 colonization caused a significant reduction of the intestinal brush border depth, with a depth of 0.43 µm compared to the 1.026 µm depth seen in uncolonized and LUAb3 colonized animals (Fig. 4d–f, quantified in Fig. 4g). Effacement of the intestinal microvilli and loss of the glycocalyx are expected to impact the intestinal integrity and increase intestinal leakiness as seen in the “Smurf” assay (see Fig. 3g–j). By contrast, LUAb3 colonized animals did not display a significant change in intestinal brush border depth, with a depth of 0.98 µm (Fig. 4f, g). Separately, we found that both attaching bacteria caused varying levels of intestinal dilation, where the natural elliptical shape of the lumen became more circular (Fig. 4d–f, h). This effect was quantified by measuring the area of the intestinal lumen. We found that the intestinal dilation in animals colonized with LUAb1 is nearly 4-fold higher than in uncolonized animals (Fig. 4e), with a concomitant decrease in the volume of the intestinal cell. LUAb3 colonized animals also had a more circular lumen, with a small but significant increase in the luminal area (Fig. 4f). This partial distension may be attributed to the presence of intact bacteria colonizing the lumen.
Attaching microbiome bacteria replicate in the gut to colonize the entire lumen
A hallmark of gut microbiome bacteria is the ability to survive and proliferate in the host intestine. We developed a pulse-chase colonization assay to visually assess whether these attaching bacteria can replicate in C. elegans. In this assay, axenic animals were pulse exposed to bacteria prior to propagation on E. coli OP50-1 lawns and chased for colonization over the course of 48 h. Although we propagate these animals on lawns of live OP50-1, L. jeotgali LUAb3 is still able to colonize C. elegans fed only heat-killed OP50-1 (Supplementary Fig. 3). All other experiments were performed on live lawns of OP50-1 as the animals did not develop normally when fed dead bacteria. For the pulse-chase colonization assay, we quantified colonization by the intensity of fluorescence using 16S-specific FISH probes for each bacterial species21,41. Using this technique with L. jeotgali LUAb3, we found an increase in fluorescence from 0 to 48 h post exposure (hpe) (Fig. 5a–d). LUAb3 appeared to colonize simultaneously throughout the lumen and colonized ~99% of the population by 48 hpe (Fig. 5e). To determine the number of viable bacteria cells in the lumen per animal at 0 hpe, 24 hpe, and 48 hpe, we performed a colony forming unit (CFU) assay after surface sterilization using the same pulse-chase assay. LUAb3-pulsed animals showed a strong increase in bacterial load over time, with an average of ~57,000 CFUs per animal at 48 hpe (Fig. 5f). In parallel, we tested colonization with a CeMBio strain in the same genus, Lelliottia amnigena JUb66a26. This strain showed no significant increase in CFUs after 48 hpe (Fig. 5f), and we observed no bacteria in the gut lumen after 48 hpe by DIC (Supplementary Fig. 4). To observe JUb66a colonization in the intestine, we had to use the grinder mutant eat-15 to allow more bacteria to survive and persist in the intestine, and we found that JUb66a does not show attachment to the C. elegans intestine compared to LUAb3 colonized animals (Supplementary Movie 1 and Supplementary Movie 2, respectively).
a-c Representative RNA FISH-stained images of C. elegans N2 briefly exposed to LUAb3 and fixed 0 h post exposure (hpe), 24 hpe, and 48 hpe respectively. Red fluorescence correlates with LUAb3 specific RNA FISH probes with a Cal-610 fluorophore. Scale bars are 100 µm. d Quantification of fluorescence intensity of LUAb3 specific fluorescent probes for each fixed time point. n = 30 in 3 independent experiments. Statistical analysis performed by Tukey’s multiple comparisons test in a one-way ANOVA where **p < 0.05 and ****p < 0.001. e Percentage of C. elegans N2 colonized with LUAb3 in the intestine at 24 hpe and 48 hpe (N = 3, n = 30). f Quantification of the colony forming units (CFUs) per L. jeotgali LUAb3 or L. amnigena JUb66a colonized animal up to 48 hpe. Data is normalized to animals exposed to only E. coli OP50-1 and consist of two independent experiments performed in biological triplicate. Statistical analysis performed by Tukey’s multiple comparisons test in a One-Way ANOVA where ****p < 0.0001 and ns = not significant. g Categories of directional LUAb1 (red fluorescence) colonization in C. elegans, where 0 represents no colonization, 1 represents LUAb1 present only in the anterior end, 2 is LUAb1 detected between the anterior and midline of the animal (vulva), 3 is LUAb1 found from the anterior end past the midline, and 4 is LUAb1 colonizing the entire anterior-posterior length of the intestine. Scale bars are 100 µm. h–j Representative RNA FISH-stained images of C. elegans N2 exposed to LUAb1 briefly and fixed at 24 hpe, 48 hpe, and 72 hpe. Scale bars are 100 µm. k Percent of LUAb1 colonized animals in each biogeographical category for each time point. l Quantification of the percentage of animals colonized past the midline (categories 3 and 4) in each time point. n is at least 90 animals in two independent experiments. Statistical analysis performed by Tukey’s multiple comparisons test in a One-Way ANOVA where *p < 0.05, **p < 0.005, and ns = not significant.
In our TEM data for L. jeotgali (LUAb3) we observed binary fission events, where bacterial cells display a clear cleavage furrow indicative of septa formation (Fig. 4c). This demonstrates that the bacteria are actively replicating in the intestines of C. elegans even in the absence of continuous feeding with LUAb3. To understand where in the gut lumen bacterial division was taking place, we counted binary fission events occurring with bacteria in contact with host glycocalyx (black dashed oval) versus those occurring more in the center of the lumen with no contact to the glycocalyx (red dashed oval) (Fig. 4b, c, e). A total of 19 septation events were seen in LUAb3 cells attached to the glycocalyx compared to 9 events seen in the lumen (N = 3, n = 36). Given that LUAb3 shows polar colonization to epithelial cells, it is likely that bacterial division by attaching L. jeotgali LUAb3 cells produces a daughter cell that disperses into the central lumen.
We conducted a similar pulse-chase assay with LUAb1. Surprisingly, LUAb1 was observed to have different biogeography and dynamics of colonization as they colonized in a directional manner, starting at the anterior end of the intestine and moving posteriorly. We quantified this progression into different categories (from 0 to 4) depending on the extent of anterior-to-posterior colonization, with 0 representing no bacterial colonization, 1 at the anterior end only, 2 between the anterior end and the vulva, 3 from the anterior end past the vulva, and 4 representing colonization of the entire gut lumen (Fig. 5g). LUAb1 colonization became more severe over time. Between 24 to 72 hpe, we saw a progression of LUAb1 colonization from the anterior end to past the midline of the intestine (Fig. 5h–j). And at 72 hpe, 100% of the animals were colonized past the midline (Fig. 5k, l). Unlike LUAb3, we observed fewer binary fission events of attached LUAb1 cells dividing by TEM (Fig. 4b, e). Instead, division occurred more frequently in the lumen with 29 septation events occurring in non-attached cells versus 10 events in attached cells (N = 3, n = 35). It is likely that LUAb1 replication happens primarily in the intestinal lumen and then new daughter cells attach to the epithelia.
Altogether, the contrast between the dynamics of colonization of L. jeotgali LUAb3 and Ca. L. limosiae LUAb1 supports the idea that these natural members of the nematode microbiome have distinct mechanisms for replication and persistence in the intestine. Additionally, these data demonstrate two distinct attachment patterns between different bacteria as they establish a niche in the intestine.
Effects of microbiome bacterial competition on the biogeography of gut lumen colonization
Colonization by beneficial or commensal bacteria in the intestine can aid in the defense of C. elegans against invading pathogens30,42. In the first experimental paradigm, we tested whether commensal L. jeotgali LUAb3 can provide colonization resistance against an invading pathogen, Ca. L. limosiae LUAb1. We exposed axenic C. elegans L2 larvae to LUAb3 for 24 h prior to challenging with LUAb1 at the L4 stage for 2 h. Following the 2 h exposure period, the samples were washed and grown on OP50-1 seeded NGM plates for 24 h. Animals were then fixed, and RNA FISH-stained using LUAb3 and LUAb1 specific fluorescent probes (Fig. 6a, top). We observed that pre-colonization with LUAb3 severely reduced LUAb1 colonization past the midline at 24 and 48 hpe (Fig. 6b, c, f). By contrast, L. jeotgali LUAb3 colonization showed no obvious change in colonization at 48 hpe when LUAb1 was added (Supplementary Fig. 5).
a Graphic representation of the pre-colonized and competition assay, with Ca. L. limosiae LUAb1 used from frozen bacterial preps and L. jeotgali LUAb3 from in vitro culture. Created with BioRender. b, c Representative RNA FISH-stained images of C. elegans N2 pre-colonized with LUAb3 and pulse exposed to LUAb1. Animals shown are fixed 24- and 48 hpe, respectively. Only the red fluorescence indicating LUAb1 is shown in all RNA FISH images. DAPI is shown in blue and scale bars are 100 µm. d, e Representative RNA FISH-stained images of N2 exposed to LUAb3 and LUAb1 simultaneously for 2 h. The animals shown are fixed at 24 hpe and 48 hpe. f Quantification and comparison of the percent of LUAb1 colonized past the midline in either pre-colonized or competed animals at two time points. Statistical analyses performed via Tukey’s multiple comparisons test in a one-way ANOVA where **p < 0.005 and ****p < 0.0001 for three independent experiments where n is at least 98 animals. g Total brood size of LUAb3 and competed animals in comparison to uncolonized animals across the span of 4 days. n = 60 for 3 independent experiments. h Lifespan during the most reproductively active period of uncolonized, LUAb3 pre-colonized, and competed animals where n = 60 for N = 3 independent experiments. Lifespan curves were analyzed by Mantel–Cox test, ****p < 0.0001.
In the second experimental paradigm, we tested whether simultaneously competing an attaching commensal and pathogenic bacteria can affect pathogenic colonization. Axenic C. elegans L4 larvae were exposed for 2 h to Ca. L. limosiae LUAb1 and L. jeotgali LUAb3 (Fig. 6a, bottom). Under this paradigm, we found that neither bacterium was dramatically affected in its colonization capacity by the other. At 48 hpe, ~80% of animals were colonized by LUAb1 past the midline (Fig. 6d-f). At both 24- and 48 h post exposure, we see that LUAb1 continues to initiate colonization at the anterior end of the intestine (Fig. 6b–e). Comparing the two experiments, LUAb3 pre-colonization led to a nearly 7-fold reduction in the prevalence LUAb1 colonizing past the midline at 48 hpe when compared to the simultaneous competition paradigm (Fig. 6f). These data suggest that pre-colonization with an attaching bacteria can prevent the colonization by a subsequent species.
Using these two paradigms, we next tested the effect on host reproductive lifespan and brood size. Due to the reduction of LUAb1 colonization past the midline seen in the pre-colonization paradigm, we predicted that the animals would fare better in reproductive lifespan and brood size assays than the animals in the bacterial competition paradigm. Surprisingly, reproductive fitness decreased similarly in comparison to uncolonized animals in both experimental situations (Fig. 6g, h). Overall, even though LUAb3 pre-colonization led to a severe decrease in pathogenic LUAb1 colonization compared to simultaneous competition, there was no concomitant benefit to host fitness. These data suggest that either the pathogenic effects of Ca. L. limosiae LUAb1 are mitigated by the presence of commensal L. jeotgali LUAb3, or that LUAb3 has cryptic virulence that emerges when a pathogenic bacterium is present.
Discussion
We have characterized three bacterial species that colonize the C. elegans gut by specifically attaching to the apical side of intestinal cells. Ca. E. pterelaium LUAb2 and L. jeotgali LUAb3 have commensal features as they show no significant effect on host fitness, while Ca. L. limosiae LUAb1 displays clear pathogenicity. Though other animal models are used to study the gut microbiome, C. elegans offers a distinct advantage in its transparent body, which allows for easy visualization of the dynamics of bacterial colonization in the intestine of a whole animal. We were able to observe the organization and spatial distribution, or biogeography, of attaching bacteria and observe differences among them.
Attachment to the apical side of the intestinal epithelia is very efficient for colonization of the lumen. Indeed, an initial 2-h exposure window is sufficient for L. jeotgali LUAb3 and Ca. L. limosiae LUAb1 persistence and proliferation throughout the host reproductive life span. While planktonic gut microbes need to withstand the defecation cycle that may empty most of the intestinal lumen every 45 s43,44, attachment is a mechanism enabling persistence in the intestine. Most bacteria adhere by utilizing mucus-binding proteins and/or cell surface appendices, like fimbriae or pili45, that bind to the mucosal layer protecting the intestinal epithelium. Currently, it is unknown if C. elegans has a loose mucus layer for trapping and dispelling microbes, similar to secreted mucus produced by goblet cells in the many vertebrate intestines. However, the presence of an electron dense layer covering the microvilli of the intestine can be seen via electron micrographs20,46. This layer presumably corresponds to a glycocalyx composed of glycoproteins that protect the microvilli.
Adherence to the mucus layer covering the intestine may thus provide a further resource advantage over less adherent species. In fact, pathogens like Pseudomonas aeruginosa47 have been shown to use mucin-derived monosaccharides as a nutrition source when the bacteria colonize the intestine in C. elegans. The robust persistence of our attaching bacteria in the intestine throughout the lifespan of C. elegans may be attributed to their ability to better persist in the mucus layer and then potentially utilize mucins or intestinal cells for nutrition48. For example, lysed E. coli OP50-1 in the lumen and/or extraction of nutrients from the host intestinal cells could feed them. Specific to adhering bacteria, they may feed off the mucus layer itself, comprised of primarily highly glycosylated mucins45. In this study, we found that L. jeotgali LUAb3 does not have a preferred niche and will initiate colonization throughout the intestine, whereas Ca. L. limosiae LUAb1 initiates colonization only at the anterior end and progresses to the posterior. It is likely that distinct differences between the anterior and posterior niche play a role for this preference by LUAb1, as there are differences in the physical structure of the first intestinal segment (int1)49, and the pH is less acidic in the anterior intestine compared to the posterior50. Bacteria that can attach and utilize nutrition from the host to establish a niche in the intestine are more likely to resist displacement from the host.
Several other bacterial species are studied as gut microbiome bacteria in C. elegans, including members of CeMbio and BIGbiome, providing an opportunity to study how the complexity of host-bacteria and bacteria-bacteria interactions influence microbiome composition and host physiology and fitness26,27,51. Many of these bacteria were shown to persist in the C. elegans gut, with a host-adapted Pseudomonas lurida strain showing improved persistence associated with increased biofilm formation28. The attaching bacteria in this study appear to be distinct from many other microbiome bacteria in C. elegans, as LUAb1, LUAb2, and LUAb3 form an intimate, polar interaction directly with epithelial cells that can be visualized by DIC light microscopy. In fact, when we observe LUAb3 in a live animal by DIC, a single bacterium remains in a fixed location for at least 15 minutes. Consequently, these bacteria appear to be very successful in colonizing, as evidenced by their capacity to colonize the entire anteroposterior length of the gut, actively replicate in the lumen, and exponentially increase in numbers over time (as seen for L. jeotgali LUAb3). Interestingly, a related CeMbio bacterium, Lelliottia amnigena (JUb66a), did not appear to persist over 48 h when tested in our hands (Fig. 5f). This may be due to different experimental paradigms, as we pulse colonize animals for 2 hours before removing them to their normal food source (OP50-1), while other studies28,29,30,51,52 continuously feed the animals with the bacteria on plates before subsequent analyses, like CFUs or microscopy.
Evolution of such strong attachment by the commensal bacteria may be due to the host providing protection, as well as possibly facilitating bacterial dissemination as wild nematodes travel to subsequent rotting substrates for food. In fact, the procedure we used to clean the wild isolates involved forcing animals into dauer and harsh detergent cleaning32, showing that these bacteria not only can colonize dauers but also survive within them. This suggests a possible mechanism for co-evolving a relationship with a host for dissemination, as overlapping ecologies can lead to the enrichment of certain microbes in dispersing animals53, allowing for the evolution of more specific associations with a host.
We found three distinct bacterial lineages that evolved the ability to attach to C. elegans intestinal cells. The most prevalent is Lelliottia (Table 1), which we found in several Caenorhabditis strains around the world. Lelliottia falls within the Enterobacterales order, which contains both commensals and pathogens, with the most notable species being Escherichia coli54. Amongst the four attaching Lelliottia isolates, LUAb3, LUAb14, and LUAb15 are L. jeotgali and LUAb16 is L. nimipressuralis (Table 1). Lelliottia species are often associated with water sources55, crops56,57, and in some cases human clinical samples58. Both L. nimipressuralis and L. amnigena are known plant pathogens causing soft rot in dangshen roots57 and onion bulb decay56, respectively. This potentially explains how wild Caenorhabditis nematodes may encounter these Lelliottia species. Interestingly, in the same genus, Lelliottia amnigena JUb66a is a member of CeMbio26 and was also found colonizing the C. elegans cuticle59 with no evidence that it attaches to the epithelial cells in the gut. We provide evidence that L. jeotgali LUAb3 displays commensal-like behavior in wild C. elegans and is beneficial under some conditions. First, it provides colonization resistance against Ca. L. limosiae LUAb1. The extent of this protective effect still remains unclear. However, pathogens like P. aeruginosa that utilize intestinal mucins in C. elegans to colonize may be affected. Second, it can delay the mortal germline phenotype of some C. elegans wild isolates. Another isolate of L. jeotgali (JUb276), isolated from C. elegans strain JU3224, was also shown to suppress the mortal germline of its wild cognate strain39. These findings suggest that under certain environmentally-relevant contexts L. jeotgali LUAb3 can provide benefits for its host.
The second bacterial lineage we found that displayed commensal-like behavior in the tested conditions is Ca. Enterosymbion pterelaium LUAb2. Amazingly, genome sequencing of the LUAb2 strain placed it within the clade of Rickettsiales bacteria, which were once thought to be exclusively intracellular symbionts, comprising for instance Wolbachia, Rickettsia and Ca. Midichloria60; bacteria of the Wolbachia genus are known to infect some parasitic nematodes61. Recently a novel Rickettsiales bacterium, Ca. Deianiraea vastatrix62, was found to externally colonize a ciliate protist and replicate outside the host cell. This species was found to possess a higher capability to synthesize amino acids compared to other Rickettsiales. The discovery of LUAb2 as another extracellular symbiont opens the debate on whether these species evolved from an intracellular to an extracellular obligate symbiotic association, or whether the Rickettsiales ancestor was an extracellular bacterium with independent lineages evolving intracellularity.
The last bacterial lineage represented by Ca. L. limosiae LUAb1 was found to strongly reduce host brood size and is found within Enterobacteriaceae, like Lelliottia. Our genomic analysis places it as an outgroup to a clade that includes among others Escherichia, Citrobacter, Lelliottia, Serratia, Erwinia or Pantoea, bacteria that are particularly prevalent in the intestinal lumen of various animals but are also found in decomposing vegetal matter where C. elegans proliferates22,27,30.
We found that L. jeotgali LUAb3 pre-colonization led to a severe decrease in LUAb1 colonization compared to simultaneous competition, showing that LUAb3 can provide colonization resistance against a pathogen. This data suggests that there is competition between these attaching bacteria for ‘real estate’ along the intestinal epithelial cells. Alternatively, other potential mechanisms may play a role in this colonization resistance, as other commensal microbiome bacteria in C. elegans can protect the host from pathogens. For example, microbiota isolates of Pseudomonas lurida can protect C. elegans from Bacillus thuringiensis infection by producing a cyclic lipopeptide that inhibits pathogen growth, while an isolate of P. fluorescens seems to mediate protection in an indirect, potentially host-mediated manner42. A commensal Enterobacter cloacae strain can also protect from gram-positive Enterococcus faecalis infection63. It would be interesting to distinguish whether L. jeotgali LUAb3 could provide resistance to these and other C. elegans pathogens, independently of competition for space along the epithelial cells.
Even though LUAb3 pre-colonization limited pathogenic LUAb1 colonization, there was no concomitant benefit to host fitness, suggesting that the pathogenic effects of LUAb1 are mitigated by LUAb3, or that bacterial competition induces cryptic virulence in LUAb3. This result is not due to numerical dominance of any one bacterial species over the other, as the “pre-colonized” animals have higher LUAb3 bacterial levels but lower LUAb1 bacterial levels than the “competed” animals (Fig. 6h and Supplementary Fig. 5), yet both host populations have the same fitness. This capacity of commensal bacteria to display cryptic virulence has been demonstrated for many C. elegans microbiome bacteria, including CeMbio isolates, P. lurida, and E. cloacae, often in genomic contexts where the animals were immune compromised in different pathways64,65,66. Overall, these studies along with our results demonstrate the complexity of the gut microbiome, even when reduced to one or two microbial members, with host and bacterial context playing a role in fitness and virulence.
Commensal and pathogenic gut microbes capable of attachment are more persistent residents of the intestine. Uncovering how bacteria use attachment to colonize the gut will provide insight into how different microbes can become a part of the gut microbiome. C. elegans provides a simplified model system in which we can study these host-microbe and microbe-microbe interactions. We present a collection of bacteria to further our understanding of bacterial attachment and colonization dynamics in vivo. This has the potential to translate to future work regarding identification of host and bacterial factors facilitating attachment, limitations of commensal bacterial attachment, and further characterization of the C. elegans glycocalyx.
Methods
Nematode and Bacterial Strains
Ca. Lumenectis limosiae (LUAb1) was discovered in C. briggsae strain JU3205 found on a rotting banana stem from the University of Agricultural Sciences in Bangalore, India on December 23, 2016. To transfer LUAb1 to C. elegans N2, we co-cultured the cleaned C. briggsae colonized with LUAb1 with C. elegans ERT413 (jySi21[spp-5p::GFP; Cbr-unc-119( + )] II. Caenorhabditis strains containing LUAb1 were maintained on nematode growth media (NGM) plates seeded with E. coli OP50-1 for the food source at 20°C under standard conditions. Isolation of LUAb1 was performed via an adapted protocol for microsporidia spore preparation23. We collected and washed gravid adult C. elegans N2 colonized with Ca. L. limosiae LUAb1 in M9 and lysed 100 µl of C. elegans pellets through bead beating with silicon beads. The lysate was filtered using 5 µM filters to remove lysed nematode debris, and aliquoted into 125 µl. We added 25 µl of 10% glycerol to each aliquot and stored the preps at -80°C for further use.
Ca. Enterosymbion pterelaium LUAb2 was found in C. tropicalis JU1848 isolated from rotten palm tree fruits sampled in the Nouragues Forest in the French Guiana on November 22, 2009. It was transferred to C. elegans ERT413 using the same co-culturing method described for Ca. L. limosiae LUAb1. Strains containing LUAb2 were maintained on NGM plates with OP50-1 at 20°C under standard conditions. LUAb2 did not withstand the bead-beating and filtration protocol described above for Ca. Lumenectis limosiae LUAb1.
L. jeotgali LUAb3 was isolated from wild C. elegans LUA21 which was found on a rotting leopard plant stem (Ligularia tussilaginea) collected on March 18, 2019 from the San Diego State University campus. After selective cleaning of LUA21 (see below), non-E. coli OP50-1 colonies grew on the nematode growth media (NGM) plates in the absence of C. elegans. These colonies were isolated via streak plating. LUAb3 cultures were grown at 32°C in LB media prior to use for experimentation. Animals colonized with LUAb3 were maintained on NGM plates at 20°C. We isolated additional Lelliottia spp. using a similar technique, with L. jeotgali LUAb14 isolated from wild C. elegans LUA11, L. jeotgali LUAb15 isolated from wild C. elegans JU3224, and L. nimipressuralis LUAb16 isolated from wild C. briggsae JU2222 (Table 1).
Selective cleaning of wild Caenorhabditis strains
To enrich for the attaching bacteria within the intestine, while removing external contamination, we selectively cleaned32 the wild Caenorhabditis strains as described previously. In summary, animals colonized with attaching bacteria were forced into dauer to seal off the intestine. Dauers were then washed overnight (ON) in an M9 buffer solution containing 0.25% SDS, 50 µg/mL of carbenicillin, 25 µg/mL of kanamycin, 12.5 µg/mL of tetracycline, 100 µg/mL of gentamycin, 50 µg/mL of streptomycin, 37.5 µg/mL of chloramphenicol, and 200 µg/mL of cefotaxime. Once the antibiotic and SDS solution was removed, single clean dauers were propagated to individual NGM plates. Colonization with attaching bacteria was verified through DIC imaging or RNA FISH.
Genome sequencing, assembly, and analysis
Cleaned N2 with Ca. L. limosiae LUAb1 or Ca. E. pterelaium LUAb2 were grown on standard 10 cm NGM + E. coli OP50-1 plates. We harvested 10 plates in 15 ml tubes, washed 3 times with H2O and isolated total genomic DNA using Qiagen DNeasy Blood and Tissue kit. Whole genome sequencing and assembly was conducted largely as described previously67. Briefly, DNA libraries were made using Illumina Nextera DNA Library and sequenced on Illumina MiSeq at 250 bp paired end reads. Reads were process with cutadapt v3.4 using the following parameters, cutadapt -u 4 -u -6 -U 4 -U -6 --no-indels -q 15,1068. Then, bowtie2 v2.5.4 was used to map away C. elegans reads69 (genome WBcel123) followed by E. coli OP50-1 reads (ADBT01.1) with the parameters --fast --no-mixed -X 2000 --un-conc-gz. Genomes were assembled with Spades70 v3.15.5 using ‘careful’ parameter and testing 21, 33, 55, 77, 99, and 127 kmers. Annotation was conducted with prokk71 v1.14.0 with the parameters ‘addgenes’ and an e-value of 1e-06. To analyze the percent reads belonging to different microbial taxon in these samples, we used CZ-ID (formerly IDseq)72. Briefly, C. elegans reads were subtracted, and reads were aligned to reference databases to acquire reads per million (rPM) that align to a taxon. rPMs were used to determine the percent of the total population each microbial taxon represented in the sample, removing all taxa that represented less than 0.01%. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the BioProject accession PRJNA116858, BioSample accession SAMN44052871 (LUAb1), SAMN44052872 (LUAb2), and Genome Accession JBIEOG000000000 (LUAb1) and JBIEOF000000000 (LUAb2).
Lelliottia spp. strains were cultured from a single colony in LB and DNA was purified using the Qiagen DNeasy Blood and Tissue Kit. Whole genome sequencing was conducted on Oxford Nanopore R10.4.1 flow cells after v14 Library Preparation by Plasmidsaurus. Reads were filtered and downsampled to 250 Mb using Filtlong v0.2.1 (default parameters) and a rough sketch of the assembly was made with Miniasm v0.3. Using information acquired from the Miniasm assembly73, reads were downsampled to ~100x coverage with heavy weight applied to remove low quality reads. Flye74 v2.9.1 was used for genome assembly with parameters selected for high quality ONT reads. The assembly was polished with Flye via Medaka v1.8.0 using the reads generated from the sketch assembly. Annotation was conducted using Bakta75 v1.6.1. Genome completeness and contamination was analyzed by CheckM76 v1.2.2. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the BioProject accession PRJNA1168587; BioSample accession SAMN44052892 (LUAb3), SAMN44052893 (LUAb14), SAMN44052894 (LUAb15), SAMN44052895 (LUAb16); and Genome Accession CP171285-CP171286 (LUAb3), CP171283-CP171284 (LUAb15), CP171281-CP171282 (LUAb16).
Average nucleotide identity (ANI) was measured using JSpeciesWS77. For this procedure, draft genomes were searched for related genomes with the Tetra Correlation Search function, then the top 12 closest-related genomes were selected for pairwise ANI based on BLAST (ANIb)78. Bacterial strains LUAb3, LUAb14, LUAb15, and LUAb16 were classified as specific Lelliottia spp. based on their highest, greater than 96% ANI.
Phylogenomic analysis
Phylogenomic analysis was conducted largely as described previously67, with the UBCG79 pipeline, using 92 up-to-date bacterial core genes isolated from the genomes analyzed. Genomes were chosen based on preliminary 16S phylogenetic tree analysis to see the predicted, closest related species. Alignments were made using UBCG (version 3.0) with parameter -a aa (using RAxML alignment based on amino acid sequences). Trees were made in MEGA X v10.0.5 using maximum likelihood with 500 bootstraps using AA substitution via JTT model and tree inference using Nearest-Neighbor-Interchange. Ca. L. limosiae strain LUAb1 and L. jeotgali strain LUAb3 were compared to the genomes of several divergent species in Enterobacterales. Ca. E. pterelaium LUAb2 was fairly divergent from any known species and was compared to the genomes of representative species in every Order in the Class Alphaproteobacteria.
RNA Fluorescent in situ hybridization (FISH)
RNA FISH was performed as described previously21. Animals colonized with their respective attaching bacteria were collected and washed in M9 + 0.05% TritonX. Following an additional wash in 1x PBS + 0.1% Tween-20 (PBS-T), animals were fixed for 30–45 min in 4% paraformaldehyde (PFA). Samples were washed four times with PBS-T to remove PFA and then put in a thermal shaker at 46°C at 1200 rpm overnight in hybridization buffer (900 mM NaCl, 20 mM Tris pH 7.5, 0.01% SDS) containing species-specific and universal bacterial FISH probes. Following overnight hybridization, animals were pelleted down and washed with wash buffer (hybridization buffer + 5 mM EDTA) once. Samples were then pelleted down and incubated in wash buffer for two 30-minute periods in a thermal shaker at 48°C, 1200 rpm. After the second 30-minute incubation, the animals are pelleted and washed in PBS-T. Animals are mounted in Vectashield, antifade mounting media with DAPI. Samples were imaged using the Eclipse Ni microscope (Nikon).
The FISH probes target the 16S rRNA of bacteria and are conjugated to either 5-Carboxyfluorescein (FAM) or CAL Fluor Red 610 (CF610). Probes used are universal bacterial EUB338 (GCTGCCTCCCGTAGGAGT), Ca. L. limosiae specific 16S b001 (GAAAATAAGTATATTACCCTTATCTCC), Lelliottia-specific 16S b003 (CTCTCTGTGCTACCGCTCG), and Ca. E. pterelaium-specific 16S b002 (TGTACCGACCCTTAACGTTC). Each species-specific FISH probe was designed by aligning the 16S DNA sequence of LUAb1, LUAb2, LUAb3, and E. coli OP50-1 using MUSCLE. Unique regions for each species were selected based on containing 9+ mismatches to each other species across 20-27 mer probes. Probes were synthesized and conjugated to fluorophores as described above. Probes were validated for species specificity by conducting FISH of animals colonized with LUAb1, LUAb2 or LUAb3. For example probe b001 was validated for positive signal in LUAb1 colonized animals and negative signal in uncolonized, and LUAb2- and LUAb3-colonized animals.
Fitness assays
Prior to the lifespan assay, bleached80 C. elegans ERT413 were grown to the L2 stage on 10 cm NGM plates seeded with E. coli OP50-1. To bleach animals, gravid C. elegans N2 were grown on NGM plates and collected into a 15 mL conical with M9. Once collected, animals were resuspended in 1 mL of M9. 800 µL of 5.65% sodium hypochlorite solution and 200 µL of 5 M sodium hydroxide were added and the animals were incubated at room temperature for about 2 minutes. Once 50% of the animals have burst and released eggs, 13 mL of M9 are added to begin the washing process. The sample was then spun down and then subsequently washed 3 times in 14 mL M9. After the final wash, the eggs are resuspended in 5 mL of M9 and left on the rotator at room temperature to hatch overnight. After 18–24 h, the newly hatched L1s are plated onto NGM plates seeded with E. coli OP50-1.
For Ca. L. limosiae LUAb1 and L. jeotgali LUAb3 lifespan assays, L2 larvae were exposed to either one aliquot of Ca. L. limosiae lysate preps or 500 µL of an overnight of LUAb3 overlaid onto the NGM + OP50-1 plate containing the L2 larvae. The animals were grown overnight at 20°C until they reached the L4 stage. LUAb2 colonized animals were propagated on NGM + E. coli OP50-1 plates until they reached the L4 stage. Uncolonized animals were bleached and fed only OP50-1. Assays were performed in technical triplicate over three separate trials with n = 20 animals per 6 cm NGM plate. Animals were scored every 24 h and were considered dead if they did not move following touch with a pick. The animals were picked onto new NGM plates every 24 h and were maintained at 20°C. Death due to user handling and missing animals were censored from the final count. Life span assays were conducted over the course of the reproductive period, 10 days. Statistical analysis was performed using Mantel-Cox test by Oasis 281. GraphPad Prism (version 10.1.0 (316)) was used to make the life span curves.
Brood size assays were performed similarly, except n = 20 animals were divided across four 3.5 cm NGM plates. The adults were moved to new NGM plates seeded with OP50-1 every 24 h. Only the hatched, and thus viable, progeny were counted every day over the course of 4 days. Data was analyzed by unpaired two-tailed t-test using GraphPad Prism (version 10.1.0 (316)).
Colonization on heat-killed E. coli OP50-1
An overnight culture of E. coli OP50-1 was grown at 32°C in LB. The culture was concentrated via centrifugation followed by resuspension of the bacterial pellet in a tenth of the initial LB volume. Bacteria were then heat-killed by submerging the concentrated culture in boiling water (80-90°C) for 30 minutes. 200 µl of live and heat-killed OP50-1 were seeded onto 6 cm NGM plates and incubated at room temperature for 2 days. Bleached80 C. elegans N2 L1s were pulse exposed to an overnight culture of L. jeotgali LUAb3 for 3 h and then maintained on the live and heat-killed OP50-1 NGM plates. Day 1 adults were FISH-stained using Lelliottia-specific 16S b003 with a red fluorophore. Imaging was performed using the fluorescent Eclipse Ni microscope (Nikon).
Mortal germline phenotype assay
Wild C. elegans strain JU775 was grown on NGM plates seeded with E. coli OP50-1 at 15°C. Three L4 larvae were transferred to NGM plates seeded with either OP50-1 or L. jeotgali LUAb3 and then grown for six days at 15°C. Three adults from the new generation were transferred to a new NGM plate and then grown at 25°C to begin the assay, either on OP50-1 or on L. jeotgali LUAb3. Three adults from each of the subsequent generations were transferred to new plates seeded with either OP50-1 or L. jeotgali LUAb3 until no larva was produced. The number of days to sterility was compared using unpaired two-tailed Wilcoxon rank tests.
Transmission electron microscopy
High pressure freezing and transmission electron microscopy was conducted as described previously46. Briefly, N2 animals and clean ERT413 animals colonized with Ca. L. limosiae LUAb1 were grown under standard NGM growth on E. coli OP50-1. Animals were colonized with L. jeotgali LUAb3 by overlaying an overnight culture of bacteria on standard NGM + E. coli OP50-1 plates. Samples were subjected to high-pressure freezing (HPM live µ, CryoCapCell) followed by freeze substitution, flat embedding, targeting and sectioning. Each adult animal was sectioned in several different places, every 10 μm and ultrathin sections (70 nm) were collected on Formvar-coated slot grids (FCF2010-CU, EMS). Grids were observed using a JEM-1400 transmission electron microscope (JEOL) operated at 120 kV, equipped with a Gatan Orius SC 200 camera (Gatan) and piloted by the Digital Micrograph program.
Proliferation assay
An overnight culture of L. jeotgali LUAb3 was grown in LB broth at 32°C prior to the start of the proliferation assay. Bleached and synchronized C. elegans N2 L4s on NGM plates seeded with E. coli OP50-1 were overlaid with 200 µl of LUAb3 culture with an OD = 2. After 2 h of exposure to LUAb3, the L4s were harvested with M9 + 0.05% Triton-X and washed twice in M9. Once washed, the animals were plated on new 10 cm NGM plates seeded with OP50-1. At 24 hpe, the animals were harvested and washed again. Half of the washed animals were plated on another 10 cm NGM + OP50-1 plate, and the remaining half were fixed, and FISH-stained using the b003 FISH probe. At 48 hpe, the animals were collected, washed, fixed, and FISH-stained again. To quantify the percentage of animals colonized with LUAb3, bleached C. elegans N2 were prepared as described for proliferation assay. The number of animals where LUAb3 was detected in the intestine via RNA FISH probe within the first 30 animals seen were counted at 24 hpe and 48 hpe.
Ca. L. limosiae LUAb1 proliferation was conducted similarly, except bleached N2 L2s were overlaid with one LUAb1 lysate prep. The animals were exposed for 24 h, then washed and transferred to new 10 cm NGM plates seeded with OP50-1. At 48 hpe, the animals were washed and transferred to new NGM plates seeded with OP50-1 and maintained at 20°C for another 24 h. Animals were fixed and FISH-stained with the b001 FISH probes at 24 hpe, 48 hpe, and 72 hpe.
Imaging was performed using the fluorescent Eclipse Ni microscope (Nikon) at 20x magnification. The exposure times were 200 ms for DAPI and 3 s for RFP. Fluorescence intensity was quantified as previously described (ref) using FIJI (Version: 2.14.0/1.54 f). GraphPad Prism (version 10.1.0 (316)) was used to perform statistical analyses.
Colony forming unit (CFU) assay
The CFU assay was performed using a protocol adapted from Dirksen et al., 2020, with animals treated as described previously in the proliferation assay with a pulse chase on the tested bacteria. In brief, bleached80 C. elegans N2 were grown under standard conditions on NGM plates seeded with OP50-1 until they reached the L4 stage. 200 µl of L. jeotgali LUAb3 and L. amnigena JUb66a (with OD = 2) overnight culture were overlaid on the NGM plates with the bleached L4s. Animals grown solely on E. coli OP50-1 were included as a control. After 2 h, the animals were harvested in 1.5 mL microtubes with M9 + 0.05% Triton-X and washed twice in M9. The animals were incubated at room temperature on a rotator for an hour to allow for digestion and defecation. Following the incubation period, approximately two thirds of the animals were plated onto new OP50-1 seeded NGM plates and grown for 24 and 48 h. The remaining one third of the animals were harvested for the 0 hpe time point. Note that in this proposal we are using strain JUb66a (L. amnigena), which represents the original CemBio strain discovered and sequenced (NCBI Accession: NZ_RJVC02000001.1)26. There is another circulating JUb66 strain, now called JUb66n for L. nimipressuralis, that was distributed to a number of labs. We validated our copy of JUb66a as L. amnigena through whole genome sequencing and ANI (99.97% ANI to NZ_RJVC02000001.1).
For lysis of the animals and plating of the bacteria for the CFU assay, the animals were washed twice and paralyzed using 100 µl 10 mM tetramisole. Once the animals stopped moving (about 2 minutes), 200 µl of 2% bleach were added. After 2 minutes, animals were washed four times in M9. The number of animals in 20 µl of the solution was counted to infer the total number of animals in the sample. The rest of the sample was brought to a total volume of 400 µl with M9 and lysed via bead beating on the Disruptor Genie for 3 minutes. Samples were serially diluted (from 1:10 to 1:10000) and 50 µl of each dilution was plated on 3 LB agar plates and left to incubate at 32°C for 24 h.
LB agar plates with dilutions showing distinct CFUs with 50-500 colonies were used to estimate the number of bacterial cells in the original sample. Data were normalized to the OP50-1 control by subtracting the number of CFUs from animals grown only on OP50-1 from both the LUAb3 and JUb66a exposed animals. This number was then divided by the number of animals in the sample to obtain the number of CFUs per animal. Data analysis was performed using GraphPad Prism version 10.4.1 (627).
Intestinal barrier function assay
C. elegans N2 were grown on NGM plates seeded with OP50-1 at 20°C. Gravid adults were bleached with an alkaline hypochlorite solution80. After the embryos hatched in M9, synchronized L1s were transferred to 10 cm NGM plates seeded with E. coli OP50-1 at 20°C and grown for 48 h until they reached the L4 stage. L4 larvae were collected using M9 and placed onto 10 cm NGM plates seeded with either OP50-1, L. jeotgali LUAb3, or a OP50-1 + Ca. L. limosiae LUAb1 bacterial prep. They were exposed to the subsequent bacteria for a 2 h period at 20°C. For each experimental condition, 60 animals were picked and transferred to four 6 cm NGM plates seeded with OP50-1 following the 2 h exposure. After 24 h, the animals were transferred to new plates and grown for an additional 24 h until they reached day 2 of adulthood.
The intestinal barrier function was assessed at day 2 via the “Smurf” assay37,38. In a 96-well plate, 250 µL of a 5.0% wt/vol mixture of OP50-1 in LB + blue food dye (Spectrum FD&C Blue #1 PD110) was pipetted into each well. For every treatment, 30-40 animals were placed in the wells using a pick and maintained at 20°C for 3 h. The animals were then resuspended in the dye mixture and pipetted evenly onto 6 cm unseeded NGM plates. After drying in a flow hood with the lids ajar for 20 minutes, the adults were picked and transferred to 6 cm NGM plates seeded with OP50-1 where each animal was dropped into ~10 µL of 15 mM sodium azide. We assessed the “Smurf” phenotype using a Nikon SMZ18 stereomicroscope under 20x and 40x magnification. The animals were scored + or - for “Smurf” phenotype. Smurf phenotype was determined by the presence of blue dye within the body cavity or both the body cavity and germline. Data analysis was performed using GraphPad Prism (version 10.2.1 (395)).
Competition assay
Bleached and synchronized C. elegans N2 were grown on 10 cm NGM plates seeded with OP50-1 at 20°C. At the L2 stage, 200 µL (OD = 2) of L. jeotgali LUAb3 grown in LB broth overnight at 32°C was overlaid on top of the NGM plate to allow for LUAb3 colonization. Once the animals reached L4 stage, LUAb3 pre-colonized animals were overlaid with 150 µL prep of Ca. L. limosiae LUAb1. For competition, L4 animals were overlaid with 200 µL of LUAb3 culture (OD = 2) and one 150 µL prep of LUAb1. After a 2h exposure, the animals were collected from their respective plates and washed twice in M9. The animals were plated onto new E. coli OP50-1 seeded NGM plates. At 24 h post-exposure, the animals were collected and washed twice in M9. Half of the population was then transferred to new NGM OP50-1 seeded plates. The remaining population was FISH-stained using red LUAb1-specific 16S b001 probe and green LUAb3-specific 16S b003 probe. After 48 hpe, the remaining animals were collected and FISH-stained using the probes described above. Colonization of LUAb1 was binned into 5 categories determined by the location of bacterial presence. 0 is no bacteria detected, 1 is bacteria present only in the anterior end, 2 is bacteria present between the anterior and midline of the intestine, 3 is bacteria found between the midline and the posterior end, and 4 is bacteria colonizing the entire anterior-posterior length of the intestine.
Lifespan and reproductive brood size assays were conducted as described in the fitness assays (see above) using LUAb3 pre-colonized or LUAb1- LUAb3 competed C. elegans N2 at L4 stage. The fitness assays were performed in technical triplicate over three independent trials. Animals declared missing or killed due to user handling were censored. Data analysis was performed using GraphPad Prism (version 10.2.1 (395)).
Taxonomic summary for Candidatus Lumenectis limosiae
Lumenectis defines a new genus
The Lumenectis genus comprises bacteria that colonize the lumen of the genus Caenorhabditis through attachment. This genus belongs in the Enterobacteriaceae family. The mode of infection is likely horizontal, as standard bleaching of Caenorhabditis strains to obtain surface sterilized embryos fails to result in colonized progeny. The site of infection is the host intestinal lumen. The etymology of the generic name,” lumen expanding” denotes the phenotype seen by DIC and electron microscopy whereby colonization by these bacteria leads to severe luminal expansion.
Candidatus Lumenectis limosiae defines a new species
Candidatus Lumenectis limosiae was initially found in wild Caenorhabditis briggsae strain JU3205, isolated from a rotting banana sampled from the University of Agricultural Sciences in Bangalore, India on December 23, 2016 (coordinates 13.0836, 77.57745). This bacterium is horizontally transmitted to other uninfected animals via the fecal-oral route and shows no evidence of vertical transmission. Ca. L. limosiae was transmitted to C. elegans by co-culturing. At the time of publication, this bacterium is not culturable in vitro. The bacterial genome was assembled in silico and phylogenomic analysis newly describe this as a novel bacterium within the new Lumenectis genus, named after the distended lumen characteristic this bacterium exhibits during colonization. Species etymology comes from the Greek goddess of starvation, Limos, as the bacterium causes a pathogenic effect on the animal.
We discovered this bacterium via DIC light microscopy as bacilli attaching to the apical side of intestines. Animals colonized with this bacterium experienced symptoms of infection such as severely distended anterior lumens, sluggish movement, and clear intestinal cells, which are indicative of a loss of stored intestinal granules that diffract light in DIC microscopy. RNA fluorescence in situ hybridization (FISH) images using specific 16S rRNA probes revealed that over time this bacterium colonizes the entire anterior to posterior length of the intestine.
Although this bacterium cannot grow outside of the host, we could enrich its presence within the intestinal lumen through selective cleaning overnight. Bacterial preparations can be made by allowing the bacterium to colonize a large population of C. elegans, lysing the colonized animals, and removing lysed nematode debris via 5 μm filter. These Ca. L. limosiae preparations could then be stored in the -80°C for subsequent infections.
Taxonomic summary for Candidatus Enterosymbion pterelaium
Enterosymbionaceae defines a new family
The Enterosymbionaceae family comprises a bacterium that colonize the lumen of the genus Caenorhabditis through attachment. This new family belongs in the Rickettsiales order. The mode of infection is likely horizontal, as standard bleaching of Caenorhabditis strains to obtain surface sterilized embryos fails to result in colonized progeny. The site of infection is the host intestinal lumen. The etymology of the family name, “intestinal symbiont” denotes that this bacterium is an organism living in close association with the host in the intestine.
Enterosymbion defines a new genus
The Enterosymbion genus comprises bacteria that colonize the lumen of the genus Caenorhabditis through attachment. This genus belongs to the Enterosymbionaceae family. The mode of infection is horizontal and the site of infection is the host intestinal lumen. The etymology of the generic name, “intestinal symbiont” denotes that this bacterium lives in close association with the host in the intestine.
Enterosymbion pterelaium defines a new species
The bacterium Candidatus Enterosymbion pterelaium was first observed in the wild Caenorhabditis tropicalis JU1848 strain isolated from rotten palm tree fruits collected on November 22, 2009 next to a small river in the Nouragues Forest in French Guiana. Ca. E. pterelaium is horizontally transmissible with no evidence of vertical transmission. Ca. E. pterelaium was transferred to C. elegans using the same co-culturing methods as for Ca. L. limosiae. Currently, Ca. E. pterelaium is not culturable in vitro. Instead, the bacterium was enriched in C. tropicalis and selectively cleaned before genomic DNA isolation. Ca. E. pterelaium could then be transferred to C. elegans via co-culturing with C. tropicalis colonized animals. Unlike Ca. L. limosiae, lysate from Ca. E. pterelaium colonized animals does not result in re-colonization of uncolonized nematodes, emphasizing an obligate nature characteristic of other members of Rickettsiales. The bacterial genome was assembled in silico, and phylogenomic analysis newly described his bacterium as a novel species belonging to the Rickettsiales order. Based on the analysis of 92 core bacterial genes we have placed this bacterium into a novel genus named Enterosymbion for its obligate symbiotic nature within the animal intestinal lumen. Due to the hair-like appearance of this bacterium, the etymology of the species comes from the Greek King Pterelaus, who possessed a golden lock of hair that rendered him immortal.
Under DIC light microscopy, this bacterium was originally discovered as thin, hair-like bacilli that directionally attach to the intestinal epithelium of C. tropicalis. RNA FISH staining using specific FISH probes designed to the 16S rRNA of Ca. E. pterelaium shows that this bacterium colonizes the entire length of the animal intestine. Animals colonized with this bacterium appear to be healthy, albeit with slightly distended intestinal lumens.
Data availability
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the BioProject accession PRJNA116858 (LUAb1-2) and PRJNA1168587 (LUAb3, LUAb15-16); BioSample accession SAMN44052871 (LUAb1), SAMN44052872 (LUAb2), SAMN44052892 (LUAb3), SAMN44052893 (LUAb14), SAMN44052894 (LUAb15), SAMN44052895 (LUAb16); and Genome Accession JBIEOG000000000 (LUAb1), JBIEOF000000000 (LUAb2), CP171285-CP171286 (LUAb3), CP171283-CP171284 (LUAb15), CP171281-CP171282 (LUAb16). Other data generated and analyzed during this study are available from the corresponding authors upon reasonable request.
References
Obeng, N., Bansept, F., Sieber, M., Traulsen, A. & Schulenburg, H. Evolution of microbiota-host associations: the microbe’s perspective. Trends Microbiol. 29, 779–787 (2021).
Naissinger da Silva, M., Tagliapietra, B. L., Flores, V. D. A. & Pereira Dos Santos Richards, N. S. In vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 4, 320–325 (2021).
Fontana, L., Bermudez-Brito, M., Plaza-Diaz, J., Muñoz-Quezada, S. & Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 109, S35–S50 (2013).
Pienaar, J. A., Singh, A. & Barnard, T. G. Membrane modification as a survival mechanism through gastric fluid in non-acid adapted enteropathogenic Escherichia coli (EPEC). Microb. Pathogenesis 144, 104180 (2020).
Hingorani, K. S. & Gierasch, L. M. How bacteria survive an acid trip. Proc. Natl Acad. Sci. USA 110, 5279–5280 (2013).
de Wouters, T., Jans, C., Niederberger, T., Fischer, P. & Rühs, P. A. Adhesion potential of intestinal microbes predicted by physico-chemical characterization methods. PloS ONE10, e0136437 (2015).
Viswanathan, V. K., Hodges, K. & Hecht, G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat. Rev. Microbiol. 7, 110–119 (2009).
McWilliams, B. D., & Torres, A. G. (2014). Enterohemorrhagic Escherichia coli Adhesins. Microbiol. Spectrum 2, https://doi.org/10.1128/microbiolspec.EHEC-0003-2013 (2013).
Bäumler, A. J. & Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93 (2016).
Huang, J. Y., Lee, S. M. & Mazmanian, S. K. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe 17, 137–141 (2011).
Vélez, M. P., De Keersmaecker, S. C. & Vanderleyden, J. Adherence factors of Lactobacillus in the human gastrointestinal tract. FEMS Microbiol. Lett. 276, 140–148 (2007).
Nishiyama, K., Sugiyama, M. & Mukai, T. Adhesion properties of lactic acid bacteria on intestinal mucin. Microorganisms 4, 34 (2016).
Schluter, J., Nadell, C. D., Bassler, B. L. & Foster, K. R. Adhesion as a weapon in microbial competition. ISME J. 9, 139–149 (2015).
McLoughlin, K., Schluter, J., Rakoff-Nahoum, S., Smith, A. L. & Foster, K. R. Host selection of microbiota via differential adhesion. Cell host microbe 19, 550–559 (2016).
Pukkila-Worley, R. & Ausubel, F. M. Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium. Curr. Opin. Immunol. 24, 3–9 (2012).
Balla, K. M. & Troemel, E. R. Caenorhabditis elegans as a model for intracellular pathogen infection. Cell. Microbiol. 15, 1313–1322 (2013).
Zhang, R. & Hou, A. Host-microbe interactions in Caenorhabditis elegans. ISRN Microbiol. 2013, 356451 (2013).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Leung, B., Hermann, G. J. & Priess, J. R. Organogenesis of the Caenorhabditis elegans intestine. Developmental Biol. 216, 114–134 (1999).
McGhee, J. D. The C. elegans intestine (2007), WormBook, ed. The C. elegans Research Community, WormBook, https://doi.org/10.1895/wormbook.1.133.1
Rivera, D. E., Lažetić, V., Troemel, E. R., & Luallen, R. J. (2022). RNA Fluorescence in situ Hybridization (FISH) to Visualize Microbial Colonization and Infection in Caenorhabditis elegans Intestines. J. Vis. Exp. https://doi.org/10.3791/63980.
Samuel, B. S., Rowedder, H., Braendle, C., Félix, M. A. & Ruvkun, G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proc. Natl Acad. Sci. USA 113, E3941–E3949 (2016).
Troemel, E. R., Félix, M. A., Whiteman, N. K., Barrière, A. & Ausubel, F. M. Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol. 6, 2736–2752 (2008).
Félix, M. A. & Wang, D. Natural viruses of Caenorhabditis nematodes. Annu. Rev. Genet. 53, 313–326 (2019).
Clark, L. C. & Hodgkin, J. Commensals, probiotics and pathogens in the Caenorhabditis elegans model. Cell. Microbiol. 16, 27–38 (2014).
Dirksen, P. et al. CeMbio - The Caenorhabditis elegans microbiome resource. G3 (Bethesda, MD)10, 3025–3039 (2020).
Berg, M. et al. Assembly of the Caenorhabditis elegans gut microbiota from diverse soil microbial environments. ISME J. 10, 1998–2009 (2016).
Obeng, N. et al. Bacterial c-di-GMP has a key role in establishing host-microbe symbiosis. Nat. Microbiol. 8, 1809–1819 (2023).
Kissoyan, K. A. B. et al. Exploring Effects of C. elegans Protective Natural Microbiota on Host Physiology. Front. Cell. Infect. Microbiol. 12, 775728 (2022).
Dirksen, P. et al. The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC Biol. 14, 38 (2016).
Schulenburg, H. & Félix, M. A. The natural biotic environment of Caenorhabditis elegans. Genetics 206, 55–86 (2017).
Morgan, E., Longares, J. F., Félix, M. A., Luallen, R. J. (2021). Selective cleaning of wild caenorhabditis nematodes to enrich for intestinal microbiome bacteria. J. Vis. Exp. 174, e62937. https://doi.org/10.3791/62937.
Yuk, K. J., Kim, Y. T., Huh, C. S. & Lee, J. H. Lelliottia jeotgali sp. nov., isolated from a traditional Korean fermented clam. Int. J. Syst. Evolut. Microbiol. 68, 1725–1731 (2018).
Salje, J. Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle. Nat. Rev. Microbiol. 19, 375–390 (2021).
Luallen, R. J. et al. Discovery of a natural microsporidian pathogen with a broad tissue tropism in Caenorhabditis elegans. PLoS Pathog. 12, e1005724 (2016).
Frøkjaer-Jensen, C. et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40, 1375–1383 (2008).
Martins, R. R., McCracken, A. W., Simons, M. J. P., Henriques, C. M. & Rera, M. How to catch a smurf? - ageing and beyond… in vivo assessment of intestinal permeability in multiple model organisms. Bio-Protoc. 8, e2722 (2018).
Gelino, S. et al. Intestinal Autophagy Improves Healthspan and Longevity in C. elegans during Dietary Restriction. PLoS Genet. 12, e1006135 (2016).
Frézal, L. et al. Genome-wide association and environmental suppression of the mortal germline phenotype of wild C. elegans. EMBO Rep. 24, e58116 (2023).
Frézal, L., Demoinet, E., Braendle, C., Miska, E. & Félix, M. A. Natural genetic variation in a multigenerational phenotype in C. elegans. Curr. Biol. : CB 28, 2588–2596.e8 (2018).
Poirier, K. M., Luallen, R. J., & Rivera, D. E. RNA fluorescence in situ hybridization (FISH) as a method to visualize bacterial colonization in the C. elegans gut. microPublication Biol. 2024, https://doi.org/10.17912/micropub.biology.001044.
Kissoyan, K. A. B. et al. Natural C. elegans Microbiota Protects against Infection via Production of a Cyclic Lipopeptide of the Viscosin Group. Curr. Biol.29, 1030–1037.e5 (2019).
Ghafouri, S. & Mcghee, J. D. Bacterial residence time in the intestine of Caenorhabditis elegans. Nematology 9, 87–91 (2007).
Liu, D. W. & Thomas, J. H. Regulation of a periodic motor program in C. elegans. J. Neurosci.14, 1953–1962 (1994).
Sicard, J. F., Le Bihan, G., Vogeleer, P., Jacques, M. & Harel, J. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 7, 387 (2017).
Bidaud-Meynard, A. et al. High-resolution dynamic mapping of the C. elegans intestinal brush border. Dev. (Camb., Engl.) 148, dev200029 (2021).
Hoffman, C. L., Lalsiamthara, J. & Aballay, A. Host Mucin Is Exploited by Pseudomonas aeruginosa to provide monosaccharides required for a successful infection. mBio 11, e00060–20 (2020).
Singh, A., & Luallen, R. J., (2024). Understanding the factors regulating host–microbiome interactions using Caenorhabditis elegans. Phil. Trans. R. Soc. B37920230059 https://doi.org/10.1098/rstb.2023.0059.
McGhee, J. D. The C. elegans intestine (March 27, 2007), WormBook, ed. The C. elegans Research Community, WormBook, https://doi.org/10.1895/wormbook.1.133.1, http://www.wormbook.org.
Chauhan, V. M., Orsi, G., Brown, A., Pritchard, D. I. & Aylott, J. W. Mapping the pharyngeal and intestinal pH of Caenorhabditis elegans and real-time luminal pH oscillations using extended dynamic range pH-sensitive nanosensors. ACS nano 7, 5577–5587 (2013).
Zhang, F. et al. Natural genetic variation drives microbiome selection in the Caenorhabditis elegans gut. Curr. Biol.: CB 31, 2603–2618.e9 (2021).
Ortiz, A., Vega, N. M., Ratzke, C. & Gore, J. Interspecies bacterial competition regulates community assembly in the C. elegans intestine. ISME J. 15, 2131–2145 (2021).
Sieber, M., Traulsen, A., Schulenburg, H. & Douglas, A. E. On the evolutionary origins of host-microbe associations. Proc. Natl Acad. Sci. USA 118, e2016487118 (2021).
Brenner, D. J., & Farmer III, J. J. Family I. in Bergey’s Manual of Systematic Bacteriology 2nd edn, 2, 587–607. (eds Brenner, D. J. et al.) (Springer, 2005).
Kämpfer, P. et al. Lelliottia aquatilis sp. nov., isolated from drinking water. Int. J. Syst. Evolut. Microbiol. 68, 2454–2461 (2018).
Liu, S., Tang, Y., Wang, D., Lin, N., & Zhou, J. (2016). Identification and characterization of a new Enterobacter onion bulb decay caused by Lelliottia amnigena in China. Applied Microbiology: Open Access, 2. https://doi.org/10.4172/2471-9315.1000114.
Zhao, X. et al. Identification and characterization of pathogenicity of Lelliottia nimipressuralis causing soft rot of Codonopsis pilosula (dangshen) roots in China. Plant Pathol. 71, 1801–1811 (2022).
Li, A. et al. Characterization and identification of a novel chromosomal class C β-lactamase, LAQ-1, and comparative genomic analysis of a multidrug resistance plasmid in Lelliottia amnigena P13. Front. Microbiol. 13, 990736 (2022).
Haghani, N. B., Lampe, R. H., Samuel, B. S., Chalasani, S. H. & Matty, M. A. Identification and characterization of a skin microbiome on Caenorhabditis elegans suggests environmental microbes confer cuticle protection. Microbiol Spectr. 12, e00169-24 (2024).
Weyandt, N., Aghdam, S. A. & Brown, A. M. V. Discovery of early-branching wolbachia reveals functional enrichment on horizontally transferred genes. Front. Microbiol. 13, 867392 (2022).
Taylor, M. J., Bandi, C. & Hoerauf, A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv. Parasitol. 60, 245–284 (2005).
Castelli, M. et al. Deianiraea, an extracellular bacterium associated with the ciliate Paramecium, suggests an alternative scenario for the evolution of Rickettsiales. ISME J. 13, 2280–2294 (2019).
Berg, M., Zhou, X. Y. & Shapira, M. Host-specific functional significance of Caenorhabditis gut commensals. Front. Microbiol. 7, 1622 (2016).
Berg, M. et al. TGFβ/BMP immune signaling affects abundance and function of C. elegans gut commensals. Nat. Commun. 10, 604 (2019).
Gonzalez, X. & Irazoqui, J. E. Distinct members of the Caenorhabditis elegans CeMbio reference microbiota exert cryptic virulence that is masked by host defense. Mol. Microbiol. 122, 387–402 (2024).
Griem-Krey, H., Petersen, C., Hamerich, I. K. & Schulenburg, H. The intricate triangular interaction between protective microbe, pathogen and host determines fitness of the metaorganism. Proc. Biol. Sci. 290, 20232193 (2023).
Tran, T. D. et al. Bacterial filamentation as a mechanism for cell-to-cell spread within an animal host. Nat. Commun. 13, 693 (2022).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet j. 17, 10 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. methods 9, 357–359 (2012).
Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A. & Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinforma. 70, e102 (2020).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics30, 2068–2069 (2014).
Kalantar, K. L. et al. IDseq-An open source cloud-based pipeline and analysis service for metagenomic pathogen detection and monitoring. GigaScience 9, giaa111 (2020).
Li, H. Minimap and miniasm: fast mapping and de novo assembly for noisy long sequences. Bioinformatics32, 2103–2110 (2016).
Kolmogorov, M., Yuan, J., Lin, Y. & Pevzner, P. A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 37, 540–546 (2019).
Schwengers, O. et al. Bakta: rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. genomics 7, 000685 (2021).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Richter, M., Rosselló-Móra, R., Oliver Glöckner, F. & Peplies, J. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformtics32, 929–931 (2016).
Richter, M. & Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl Acad. Sci. USA 106, 19126–19131 (2009).
Na, S. I. et al. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. (Seoul., Korea) 56, 280–285 (2018).
Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A. & Cerón, J. Basic Caenorhabditis elegans methods: synchronization and observation. J. Vis.Exp.10, e4019 (2012).
Han, S. K. et al. OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 7, 56147–56152 (2016).
Acknowledgements
The authors thank Christian Braendle, Jonathan Ewbank, Nathalie Pujol, Vincent Debat, Allowen Evin and Lise Frézal for helping sample strains with adhering bacteria. This work was supported by NIH grant R35 GM146836 and NSF IOS CAREER grant 2143718 to RJL., and Rees-Stealy Research Fellowship to DER. MAF is supported by the Centre National de la Recherche Scientifique. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The authors thank WormBase. TEM Imaging was performed at the Microscopy Rennes Imaging Center (MRiC, Biosit, Rennes, France), a member of the National Infrastructure France-BioImaging supported by the French National Research Agency (ANR-10-INBS-04).
Author information
Authors and Affiliations
Contributions
RJL and MAF conceived of the project. DER, RJL and MAF designed and analyzed the experiments and cowrote the paper, with consultation of all authors. DER, KP, SM, AS, EM, JFL, MAF, and RJL conducted experiments. GM and ON conceived the methods and conducted TEM. MAF provided novel reagents. RJL provided funding and mentorship.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rivera, D.E., Poirier, K., Moore, S. et al. Dynamics of gut colonization by commensal and pathogenic bacteria that attach to the intestinal epithelium. npj Biofilms Microbiomes 11, 70 (2025). https://doi.org/10.1038/s41522-025-00696-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-025-00696-9