Abstract
Bacterial vaginosis (BV) is the most commonly diagnosed vaginal infection in women of reproductive age, with most patients unaware that they have BV due to its asymptomatic nature. BV is a dysbiotic condition defined by a deviation from the healthy Lactobacillus dominance to a polymicrobial anaerobic bacterial community that increases the risk of sexually transmitted infections and adverse reproductive outcomes, including spontaneous preterm birth. The increasing number of infectious agents in BV, biofilm persistence and antibiotic resistance in the vaginal canal hinder effective treatments with antibiotics leading to consistent recurrence of BV in many women (30–70%). Like in the gut, these vaginal drug-microbiome interactions termed pharmacomicrobiomics could alter drug disposition, mechanism of action, and toxicity that reduce the efficacy of antibiotics and increase the risk of persistent and recurrent BV and its sequelae. For instance, both vaginal epithelial and bacterial cells co-exist and possess enzymes that metabolize antibiotics, and transporter proteins that expel drugs and toxins, rendering them ineffective. Despite significant progress on pharmacomicrobiomics in the gut, little is known about this phenomenon in the vaginal microenvironment, which harbors a consequential microbiota and a major source of infection and antibiotic resistance. Therefore, to improve therapeutic outcomes and reduce the rate of persistent/recurrent BV and infection-associated preterm birth, we present an overview of the evidence pertaining to the effect of vaginal microbiome-drug interactions and efficacy of antibiotics against recurrent BV. We also highlight plausible mechanistic underpinnings of these interactions and implications for treatment modalities to combat infection-associated preterm birth.
Similar content being viewed by others
Introduction
Bacterial vaginosis (BV) is the commonest vaginal infection in women of reproductive age1,2 with most patients unaware that they have BV due to its asymptomatic nature3. Bacterial vaginosis represents an infectious milieu that has posed severe health risks in women of reproductive age, particularly during pregnancy4,5. BV patients may not develop any symptoms but are at risk of serious reproductive health issues later, with limited therapeutic options3,4,5. BV is a dysregulated microbiota condition defined by a deviation from the optimal Lactobacillus dominance to a polymicrobial anaerobic bacterial community that increases the risk of sexually transmitted infection (STI) and adverse reproductive outcomes, including pelvic inflammatory disease, preeclampsia, and preterm birth1,6,7,8,9. BV rates vary from 30% in the US2,3,10,11,12 to more than 50% in sub-Saharan Africa5,8,10,13 and significantly impact the individual’s quality of life2,14,15,16,17. Furthermore, it is estimated that the cost of BV is US $4.8 billion annually2. There is no known single causative agent of BV18,19, hence, it is diagnosed microbiologically using the Nugent Score, which is rarely performed at the point of care because it requires trained personnel, more time, and other paraphernalia20,21. BV can also be diagnosed clinically by identification of at least 3 Amsel criteria: characteristic homogeneous milk-like vaginal discharge, “fishy” odor, >20% clue cells, and pH >4.522,23; and more recently, by nucleic acid amplification tests (NAATs)20,24,25. However, routine screening for BV in asymptomatic women is not recommended26,27,28, as treatment does not mitigate adverse obstetric/reproductive outcomes, especially infection-associated preterm birth29,30,31,32,33,34,35,36.
Recommended treatment for BV involves antibiotic therapy with metronidazole or clindamycin27,37,38,39, with a 1- to 4-week cure rate of 55%–90%40,41,42,43. However, 30%–70% of women experience BV recurrence within 6 months after antibiotic treatment41,44. Tinidazole, another recommended antibiotic27, has a recurrence rate of 20%–40% within 1–2 months after treatment for 7 days45. These high recurrence rates are due to the persistence of protective bacterial biofilm46,47,48,49,50,51,52,53, and the development of antibiotic resistance within the biofilm and vaginal canal46,54. Continuous exchange of pathogenic bacterial vaginosis-associated bacteria (BVAB) between sexual partners pre/post-treatment55,56, failure to re-establish an optimal lactobacilliary vaginal microbiome, vaginal colonization by extravaginal reservoirs of BVAB, and patient non-adherence to multidose therapy also contribute to recurrent BV27. Consequently, another antibiotic with a longer serum half-life, secnidazole, which has similar antibiotic activity as metronidazole and tinidazole but spares lactobacilli, has been used to treat BV57,58,59. While there is an intense search and application of alternative treatment regimens for recurrent BV7,25,27,41,60,61, the effect of vaginal microbiome variations or microbiome-drug interactions on the effectiveness of antibiotics against the condition has received very limited attention. This has stimulated our interest in reviewing and conceptualizing the concept of pharmacomicrobiomics in the context of persistent and recurrent BV, which significantly contributes to the inefficiency of antibiotics in the treatment and prevention of infection-associated preterm birth29,30,35,36.
Pharmacomicrobiomics was initially defined as the effect of microbiome variations on drug disposition, action, and toxicity62,63. It has progressed to an emerging discipline that explores the interaction between drugs and microbes64,65 to expand the scope of precision medicine63. That is the human microbiome from various anatomic sites such as skin, mouth, nose, and lungs, gut, and vagina66 may improve or hinder the efficacy of drugs and personalized therapeutics, especially drugs administered directly to these body sites65. In addition to interindividual variations in the microbiome, there are intraindividual variations, which could be spatial, temporal, seasonal, developmental, hormonal, dietary, or drug-dependent67,68. As expected, the concept of pharmacomicrobiomics is exemplified in the impact of gut microbiota on cardiovascular drugs, chemotherapeutic agents, and natural products, including plant xenobiotics and dietary supplements63. This includes facilitating drug efficacy, abrogating and compromising drug effects, and mediating toxicity by modulating host response to drugs64. Research has highlighted the importance of improving therapeutic outcomes through personalized therapy, especially in cancer treatment69. Although the mechanisms and genetic basis for the microbiome-drug interactions remain largely elusive, mechanisms associated with some drugs, including acetaminophen70, digoxin71, and cyclophosphamide72, have been unraveled. However, the data growth rate in the PharmacoMicrobiomics portal (http://www.pharmacomicrobiomics.org), a publicly available web resource initiated to collate available literature for microbiome interactions and classify them73, is hampered by the number of curators and published drug–microbiome interactions. There have been efforts to integrate the available data with biochemical pathways involved in the drug–microbiome interactions from the SEED74 and KEGG75 databases. Plans to directly link the interactions to existing pharmacogenomics databases such as PharmGKB76, CTD77,78, and PACdb79, as well as human microbiome sequence databases have also been initiated80,81.
Despite the significant progress made in studying drug–microbiome interactions in the gut70,82,83,84,85,86,87,88, little is known about this phenomenon in the vaginal microenvironment63, which harbors about the third most populous microbiome in the body (albeit derived from/crosstalk with the gut microbiota)89,90,91, a major source of infection and route of drug administration. Moreover, the observation that overexpression of a DNA repair protein (RecA) in Bacteroides fragilis, a gut and vaginal commensal bacteria89, increases resistance to metronidazole86,87 is thought-provoking. Oral metronidazole is an ineffective long-term treatment for recurrent BV as it only temporarily reinstates healthy vaginal microbiota in patients with recurrent BV6,40,60,92,93. High abundance of Prevotella prior to treatment and Gardnerella immediately after treatment is associated with an increased risk of BV recurrence94. Since the vagina and gut harbor similar bacterial species, though with different and sometimes opposite functional characteristics89, we hypothesize that the vaginal microbiome can also alter drug disposition, mechanism of action, and toxicity. This could contribute to antibiotic resistance and failure of antibiotic therapy in treating recurrent BV and reducing adverse obstetric/reproductive outcomes associated with genital tract infections. Therefore, to improve the therapeutic outcomes, reduce the rate of recurrent BV, and alleviate the resultant adverse obstetric/reproductive outcomes, available evidence on the effect vaginal microbiome-drug interactions or pharmacomicrobiomics on the efficacy of antibiotics against recurrent BV was reviewed. Possible mechanistic underpinnings of these interactions were also suggested.
Assessment of pharmacomicrobiomics in women’s vaginal health
Although pharmacomicrobiomics has been extensively explored in the gut microbiome, some important drug–microbiome interactions have been reported in the vaginal space63. An important question that has not been answered is the presence of confounding viral and bacterial infections in the female reproductive tract. One such discovery is the metabolism of the anti-HIV drug, tenofovir (TFV), by Gardnerella vaginalis. TFV reduced HIV incidence by only 18% in African women with G. vaginalis-dominated (BV-like) microbiota and 61% in women with Lactobacillus-dominant microbiota95. G. vaginalis and Prevotella spp. metabolized TFV, thereby decreasing its bioavailability in women with a Lactobacillus-deficient and G. vaginalis-dominated microbiota95. With the 3-fold reduction in the efficacy of TFV in the group with G. vaginalis-dominated microbiota, the authors concluded that having a Lactobacillus-dominant vaginal microbiota was associated with higher preexposure efficacy of vaginal 1% TFV gel95. These findings were sequel to the observations of varying concentrations of TFV in the vagina of a South African cohort96. Lower preexposure efficacy was observed in the women with low vaginal TFV concentrations96.
Another evidence of a link between vaginal microbiota and microbicide efficacy of systemically administered drugs was reported in a predominantly African American population of HIV-infected women97. The authors tested the hypothesis that the concentration of antiretroviral drugs would be increased in Lactobacillus-dominated vaginal microbiota97. The study reports that the female genital tract/plasma ratios of ritonavir-boosted atazanavir (ATV) and TFV were lower (<half) in women with low- (Lactobacillus-dominant) and high- (Lactobacillus-deficient and high BV) microbial community diversity compared to women with intermediate-diversity (lower Lactobacillus proportion). Another drug, emtricitabine (FTC, pKa 2.65), showed similar results for low- vs intermediate-diversity community types but not for high- vs intermediate-diversity97. Because the systemic distribution of drugs into different body compartments is influenced by both host-specific and drug-specific factors98,99, the authors opined that a dysbiotic female genital tract characterized by increased microbial diversity and BV is capable of increasing local pH and altering other factors that can determine the movement of drugs across the female genital tract compartment97.
The healthy Lactobacillus-dominant vagina is naturally acidic with a pH of 3.5–4.54,100, which increases during infection4,99. By “ion trapping”, acidic drugs such as ATV (pKa of 4.7)101,102 and tenofovir disoproxil fumarate (TDF, pKa 3.75)103 may be ionized and rendered ineffective by a more basic BV-like vaginal pH. On the other hand, these drugs are more likely to remain unionized, more lipid soluble, and better absorbed in a Lactobacillus-dominant vaginal environment with acidic pH99,104. For instance, TVF uptake by human cells is reduced with increased vaginal pH104. In addition to metabolizing TFV to adenine95, G. vaginalis inhibited TFV endocytosis by human cells through the release of adenine104. T-cell uptake of TFV in the vaginal space is reduced with increased extracellular pH to 6.5–8.2, as observed in BV104,105. As pH increases from 4.5 to 7.5 and 8.2, uptake of TFV by T cells decreased by >50%, whereas TDF uptake increased by the same margin104. T-cell uptake of TFV may be determined by changes in vaginal microbiota and pH, which could contribute to inconsistent drug efficacy in subpopulations, including adolescents or BV-positive individuals105. Another study demonstrated faster TDF permeation of vaginal tissues at pH 5.0 but decreased absorption at pH 3.8 due to partial ionization of the drug103. This ion trapping effect and the ability of G. vaginalis and other anaerobes to metabolize TFV may contribute to TFV’s decreased efficacy in reducing the incidence of HIV in the studies by Klatt et al.95 and Donahue, Carlson et al.97. The observation of similar concentrations of ATV and TFV in individuals with Lactobacillus-dominant and Lactobacillus-deficient microbiotas97 supports the assertion that specific microbes may also alter the movement of drugs across the genital tract by altering local drug transporters in a pH-dependent or independent manner97,106. For instance, unlike L. jensenii and L. iners, which have been linked to dysbiosis, BV, and preterm birth107,108,109,110,111, L. crispatus takes up TFV by an organic anion transporter (OAT)-dependent mechanism and reduces extracellular concentration of TFV by >75%104,105. However, vaginal drug pharmacokinetics is complicated by the ability of some strains of L. crispatus to transport and metabolize TFV actively104.
The effect of vaginal pH on drug efficacy has also been demonstrated in labor induction for term or preterm birth. Vaginal pH >4.5 (range, 4.0–6.0) was a positive factor for the effectiveness of dinoprostone (pKa = 4.9)112,113,114, but pH <5 did not have any effect when misoprostol (pKa = 14.68)115,116 was administered. Misoprostol (PGE1) and dinoprostone (PGE2) are used to induce labor - uterine contraction and cervical ripening, and dilation112,113,114,115,116. Moreover, based on observations from the gut microbiota, there are speculations that vaginal microbiota could indirectly influence the efficacy of drugs by altering the host drug metabolism and producing bacterial metabolites that compete with the drug receptors117 (Table 1). This is supported by the plausible microbiological metabolism of nicotine observed in the vagina of women with BV-like microbiota118. Nicotine (pKa 8.0)119 is concentrated in the acidic (pH 4.0–4.6)11 vaginal environment, whereas women with Lactobacillus-deficient vaginal microbiota (pH 5.3) show lower levels of nicotine118. Another metabolite (hippurate) derived from toluene, a by-product of cigarette smoke, is a known substrate for G. vaginalis120,121, a BV-associated bacteria4,23,122. Hippurate was reduced in women with Lactobacillus-deficient vaginal microbiota compared to those with Lactobacillus-dominant microbiota, and increased in non-smokers over smokers suggesting microbial (especially Gardnerella) utilization (hydrolysis) of hippurate118,120. Although larger confirmatory longitudinal studies are required, smoking may alter the vaginal environment123 to be conducive to the proliferation of G. vaginalis118,124,125, and smoking cessation could facilitate the reduction of recurrent BV54,124.
Taken together, vaginal microbiota may influence pharmacokinetics and contribute to inconsistent efficacy of locally administered drugs through mechanisms highlighted in Table 1104,106. In the context of BV and other STIs including HIV, suboptimal drug exposure associated with certain vaginal microbiota communities could be responsible for poor therapeutic responses to treatment in some women97. Hence, selection/utilization of drugs that are less prone to environmental factors, such as certain prodrugs, may lead to more consistent drug efficacy104.
Vaginal pharmacomicrobiomics and risk of recurrent bacterial vaginosis
Host-microbiome interplay shapes the vaginal microecosystem126. The host vaginal epithelium and microbiota coexist in a mutually beneficial relationship that supports a lactobacilliary community with low diversity4,126. Under the influence of estrogen, the host cells provide glycogen that is metabolized to lactic acid by Lactobacillus species to lower the vaginal pH and prevent growth of other potentially harmful bacteria4,126. Reproductive hormones regulate the community composition and population of the vaginal microbiome during the menstrual cycle and pregnancy127,128, and this directly impacts medicated vaginal inserts or pessaries65. Alterations in the vaginal microbiota towards communities with increased diversity and BV increase vaginal pH that can alter other local factors, which could impact the movement and metabolism of drugs in the female genital tract97. The gut microbiota has shown resilience by recovering to a stable but distinct community structure after disruption by antibiotic therapy. For instance, 2 years after clindamycin treatment, gut Bacteroides clonal diversity decreased significantly with the development of clindamycin-resistant clones129. B. fragilis also mounts resistance against metronidazole by overexpression of RecA86,87, nim genes (nitroreductases), multidrug efflux pumps (BmeRABC5) that are capable of pumping metronidazole out, and deficiency of ferrous iron transporter feoAB130. Lower levels of iron cause the deficiency of feoAB, which leads to decreased electron-mediated metronidazole activation130. B. fragilis is also associated with BV4,5 and could contribute to metronidazole resistance by mechanisms similar to those in the gut. Additionally, Gardnerella spp., which usually lays the foundation for biofilm formation5,25,50,52,53,131,132, also scavenges iron from the vaginal milieu, an action that deprives other bacterial species of iron133,134,135. Although, whether this also leads to decreased electron-mediated metronidazole activation in the vagina is unclear, an adherent Gardnerella spp. biofilm persist on the vaginal epithelium following metronidazole treatment53. Like B. fragilis in the gut, G. vaginalis may decrease metronidazole activation in the vagina by decreasing the level of iron necessary for its activation. Similar combination therapy with metronidazole, clarithromycin, and omeprazole significantly altered the microbial community for about 4 years, with the persistence of high levels of the macrolide resistance gene erm(B)136.
Another bacteria that shows synergism with Gardnerella spp. to form BV biofilms is Prevotella25. Gardnerella137,138 and Prevotella139,140,141 produce sialidase that degrades the cervical mucus to facilitate bacterial attachment to the vaginal epithelium and formation of biofilms5,137,142,143,144 characteristic of BV49, and probably contribute to treatment failure and recurrent BV47. This is by acting as a barrier to inhibit antibiotic penetration and expression of efflux pumps145. To support these assertions, a recent Melbourne study reported that Prevotella and Gardnerella contributed to treatment failure and high rates of recurrent BV following first-line antibiotic therapy – metronidazole and clindamycin94. A high abundance of Gardnerella post-treatment is associated with a high risk of recurrent BV94, while a sustained cure is associated with a low relative abundance of Gardnerella after treatment146,147,148. Metronidazole and clindamycin have limited ability to dislodge established multispecies Gardnerella biofilms149,150,151,152. During BV infection, Gardnerella and Prevotella are early colonizers of the vaginal environment and are able to evade host immune surveillance by sialidase production while establishing the biofilm that sets the stage for secondary colonizers, including Fannyhessea vaginae49,153,154,155, Sneathia spp. and other BVAB that are more potent triggers of host immune response to BV25. The poor performance of metronidazole and clindamycin against BV biofilms has triggered the clamor for antibiofilm agents to effectively treat BV47.
Factors that contribute to bacterial colonization and infection of host tissues
Efflux pumps and transporter proteins
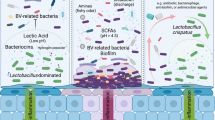
The expression of factors such as efflux pumps on vaginal epithelial and bacterial cells capable of expelling drugs and drug metabolites, thereby reducing their efficacy, requires further investigation156,157 (Fig. 1). Several ATP-binding cassette (ABC), solute carrier (SLC), and solute carrier organic anion (SLCO) transporters play crucial roles in maintaining a healthy lactobacilliary vaginal microbiota and in the pharmacokinetics of many drugs, including antiretroviral drugs156,157,158 (Table 2). However, studies on their roles in recurrent BV that can induce infectious spontaneous preterm birth are limited. As they regulate the entry (SLC and SLCO) and exit (ABC)156 of substances into the vaginal environment, disruption of vaginal transporter protein activity can lead to dysbiosis increasing the risk of infections, including BV156,159,160. Vaginal epithelial cells promote luminal acidification during acute bacterial infection via a TLR4-dependent pathway by up-regulating the expression of Na+/H+ exchanger-1 (NHE1) that pumps protons into the lumen161. ABCs such as P-gp, MRP4, and BCRP have been identified in the epithelial and vascular endothelial cells of human, mouse, and macaque cervicovaginal canal156,157,162 (Table 2). These are post-absorptive factors that could cause poor and variable absorption of drugs, such as the P-gp-mediated ATZ efflux from the gastrointestinal tract163. The microvilli on vaginal epithelial cells also contain concentrative nucleoside transporter 3 (CNT3), which could shuttle nucleoside analogs delivered to the vaginal tract from the epithelium to immune cells in lower layers of the tract164. Nucleoside analogs such as acyclovir are useful for treating and preventing viral STIs, and they need to accumulate within the cells; hence, the importance of CNT3 in their delivery, absorption, distribution, and efficacy160,164.
Both bacteria and human epithelial cells possess transporter proteins with which they take up and expel nutrients, toxins, and drugs. The cells also secrete extracellular vesicles that can also convey the transporter proteins, which can be exchanged between the host and bacterial cells. Together, these mechanisms can influence the state of the microbiota (healthy or dysbiotic) and drug efficacy, leading to antimicrobial resistance that is associated with recurrent bacterial vaginosis and a high incidence of infection-associated preterm birth. ABC ATP-binding cassette transporters, EVs extracellular vesicles, SLC solute carrier. Created in BioRender. https://BioRender.com/j51k900).
Bacterial transporter proteins
Bacteria can display resistance to multiple drugs and other cytotoxic agents that they have never encountered165. This is multidrug resistance (MDR) that has posed a major challenge to drug-based clinical treatment165, including recurrent BV. Transporter proteins are also found on bacterial cell membranes, where they are important virulent factors involved in nutrient uptake and secretion of toxins and antimicrobial agents159,160,165,166,167,168 (Fig. 1). Vaginal bacteria utilize transporter proteins to acquire nutrients such as sugars, glycogen, and lipids released by vaginal epithelial cells167,169,170. The jostle for nutrients may induce the expression of unique transporter proteins by certain bacteria to promote their dominance in the microbiota169. The bacterial membrane transport system is driven by ATP, proton motive force, and phosphoenolpyruvate (PEP); and includes ABC transporters, major facilitator superfamily (MFS) proton symporters, sodium solute symporters (SSS), enzyme II integral membrane subunits of the bacterial PEP-dependent phosphotransferase system (PTS)169. For example, vaginal microbiota dominated by lactobacilli (particularly L. crispatus) exhibit higher expression of glutamate, putrescine/spermidine, zinc, manganese, phosphate, and phosphonate transporters with which they promote their growth, protect themselves against oxygen toxigenicity, lower intracellular pH, and maintain acid tolerance to the detriment of their competitors171. G. vaginalis, a renowned BVAB,4 bears up to 7 members of the ABC superfamily on its membrane159. Efflux pumps and ABC transporters used for antimicrobial resistance172 were upregulated in G. vaginalis biofilms, e.g., genes encoding NLPA lipoprotein involved in ABC transporters173. G. vaginalis also has other efflux pumps, including MFS transporter, sulfonate ABC transporter, osmotic enzyme, and cobalt ABC transporter174. The high levels of lactic acid and hydrogen peroxide in Lactobacillus-dominant vaginal microbiota compels G. vaginalis to expend enormous energy to excrete these two antimicrobial molecules at the expense of its growth and proliferation. Hence, it is unable to form biofilms174. Metronidazole-resistant strains of Gardnerella (e.g., JNFY3, JNFY4, JNFY14, JNFY17, and JNFY28) possess an ABC-type multidrug transport system (YadH) and 5-methylcytosine-specific restriction endonuclease (McrA)175, that excrete and hydrolyze the methyl-nitryl group of metronidazole, respectively165,176. Similarly, Chlamydia trachomatis177 and group B Streptococcus178 also carry immunogenic ABC transporter proteins160.
Drug metabolizing enzymes
The ectocervix and vagina also express large amounts of drug metabolizing enzymes, including Phase I cytochrome P450 (CYP1A1, CYP1B1, CYP2C8, CYP3A4) and the Phase II UDP-glucuronosyltransferases (UGT1A1, -1A4, -1A7, -1A8, -1A10, UGT2B4, -2B15, -2B17) that metabolize antiretroviral and other drugs156,179,180,181. These enzymes metabolize the drugs and, in conjunction with the transporters, reduce microbicide exposure and efficacy in the cervicovaginal environment156. However, their roles in the inefficacy of the antibiotic treatments for recurrent BV and infection-associated preterm birth administered both vaginally and systemically is yet to be elucidated.
Taken together, we hypothesize that the transporters and enzymes in the lower genital tract and bacterial cell membranes may alter the tissue-to-lumen efflux and lumen-to-tissue drug distribution of both vaginally and systemically administered antimicrobial drugs. The mutualistic interactions between the bacterial species and host cells in the reproductive tract may include the temporal exchange of these drug efflux/transporter proteins, rendering the ecosystem resistant to both toxins and therapeutic agents. Such interactions may reduce drug efficacy, which could contribute to the inefficacy of current treatment for BV, high rates of recurrent BV, and poor performance of antibiotics in reducing the incidence of infectious preterm birth.
From the above evidence, efflux pumps, including ABC transporters, contribute to bacterial colonization and infection of host tissues, as well as multidrug resistance by actively expelling various antibiotics and metabolites from bacterial cells182 (Fig. 1, Table 1). These transporters can recognize and export multiple classes of antibiotics, including β-lactams, macrolides, and aminoglycosides182,183,184. Interestingly, this broad substrate range can lead to cross-resistance to multiple antibiotics and even some disinfectants185. Table 3 summarizes the role of efflux pumps in drug–microbiome interactions that require more exploration in the lower genital tract – vaginal epithelial cells, ectocervix and endocervix.
Discussion and future perspectives
As with other (including systemic) routes of drug administration, the efficacy of vaginally administered drugs depends on intrinsic factors of the vagina, including microbial composition, pH, fluid composition, viscosity, enzymatic metabolism, clearance, amongst others99. In order to prevent persistent and recurrent BV, there is a need to identify suitable microbicide agents186 as well as pharmaceutical vehicles/strategies that can improve the performance of drugs for better protection against such infections187,188,189,190. A few studies have demonstrated that the composition of the vaginal microbiota can alter the pH and metabolize anti-HIV drugs95,97,103,104,105, thereby reducing the efficacy of the drugs. However, these crucial drug-microbiota interactions have not been extensively explored in BV, which is not only the most common genital tract infection of reproductive-age women1,2 and a risk factor for adverse reproductive outcomes4,5,9,23,89,191,192, but has an alarmingly high recurrence rate after antibiotic therapy41,44,45.
We hypothesize that the efficacy of recommended antibiotics for treating BV may be reduced by vaginal microbiota-associated factors including pH and metabolism, leading to antibiotic resistance. Metronidazole resistance mechanisms exhibited by anaerobes, including Gardnerella spp., Bacteroides spp., Fusobacterium spp., Mobiluncus spp.4,5,23,193,194,195,196,197 include deletion, inactivation of genes including nitroreductase activity, drug inactivation, decreased drug uptake, increased efflux, altering drug targets, modulating DNA repair system, and increasing activity of oxygen-consuming enzymes198,199. Two bona fide BVAB (Bacteroides and Prevotella) that are highly resistant to metronidazole4,5,23,193,200 alter pyruvate fermentation, and so do not activate the prodrug86,198,199. Particularly, B. fragilis possesses nim genes (nimA-nimG) that encode 5-nitroimidazole reductase enzymes that convert metronidazole to a non-toxic compound193. B. fragilis also mounts resistance to clindamycin by modifying the 23S RNA using N6-methyltransferases encoded by the ermB, ermF, and ermG genes, as well as expelling the antibiotic using efflux pumps encoded by the msrSA and mefA genes193,201. Others, like G. vaginalis, possess endonucleases that hydrolyze the methyl-nitryl group from metronidazole165,175,176. Formation of biofilms and horizontal transfer of resistant genes202 among the constituent bacterial species produce a combined and more formidable antibiotic resistance that is responsible for the high recurrence of BV203. Insufficient drug exposure in the lower genital tract induced via the aforementioned mechanisms, amongst others, has led to suboptimal therapeutic outcomes. Consequently, some researchers administered a maintenance therapy to suppress recurrent BV and recorded low clinical recurrence of BV during the period patients used the treatment. This included a high dose of intravaginal metronidazole - 750 mg twice weekly for 3 months with additional follow-up for 3 months204. However, recurrence was high after cessation of suppression therapy204.
Because the pharmacokinetics of antibiotics for BV and bacterial STIs (which are often subclinical) is not often extensively assessed in clinical settings, the aforementioned drug-microbiota interactions have also contributed to the poor performance of antibiotic therapy in reducing the incidence of infection-associated spontaneous preterm birth, even when initiated earlier in gestation29,30,35,36. Therefore, to improve therapeutic outcomes and attenuate drug adverse effects, several strategies to selectively exploit microbiota were indicated. These include administration of probiotics (viable health-promoting microbial species), prebiotics (non-digestible compounds that selectively enhance the growth/activity of beneficial bacteria), synbiotics (a mixture of probiotics and prebiotics with improved effect compared to the sum of the two agents), postbiotics (nonviable but biological active microbial products or metabolites), and antibiotics in support of conventional treatments69. The gastrointestinal tract has benefited immensely from these therapeutic strategies69,205,206,207, a gesture that is practicable but underexplored in the genital tract.
Recently, a meta-analysis of 35 randomized controlled trials (RCTs), comprising 3751 patients, reported a significantly increased cure rate and reduced recurrence rate of BV when probiotics (L. crispatus, L. gasseri, L. rhamnosus, Bifidobacterium, etc.) are used as an adjuvant treatment with antibiotics (mostly metronidazole and clindamycin) compared to antibiotics alone208. Furthermore, postbiotics such as lactic acid, exopolysaccharides (EPS), and S-layer proteins produced by certain probiotic strains prevent colonization by harmful bacteria209 and reduce the rate of BV210. Newer alternative therapies such as bacteriophage-encoded endolysins (PM-477) have also shown high selectivity and effectiveness in eliminating Gardnerella, both in polymicrobial biofilms of BV patients and cultures of isolated strains, while sparing beneficial bacteria211. These therapeutic and preventative strategies hold great promise in reducing persistent and recurrent BV and require more comprehensive investigations.
There is also a need to study the feasibility of employing transporter and enzyme inhibitors to enhance drug exposure in the genital tract in relation to persistent and recurrent BV and prevention of infection-associated spontaneous preterm birth. The transporter proteins are differentially expressed across the lower reproductive tract. For instance, P-gp and MRP4 are predominantly localized in the cervicovaginal epithelial and vascular endothelial cells, while BCRP was predominantly found in the vascular endothelial cells157. This implies that different regions of the lower genital tract may require different levels of drug concentration for successful treatment and inhibition of transmission of infection157. The balance between uptake (SLC) and efflux (ABC) transporters in the cervicovaginal space may also be crucial to BV that is unresponsive to antimicrobial therapy. There could also be interindividual and intraindividual variability in cervicovaginal drug concentration and efficacy due to race, age, menstrual stage, and contraception choice-dependent changes in the expression and activity of transporters and enzymes156. As this may influence the pharmacokinetic profile and efficacy of administered drugs156, future experimental and clinical studies should account for such factors. Lastly, since bacterial and human cells can exchange extracellular vesicles and their cargo212, the exchange of uptake and efflux transporters between these cells is plausible. The implications of such interactions for the propagation of genital tract infection, multidrug resistance in the genital tract, and general maintenance of reproductive health deserve attention. Therefore, future studies should employ experimental strategies such as vaginal organ-on-chip models213,214,215 that can simulate host-microbiota-drug interactions as in vivo, combined with fluorescence in situ hybridization (FISH) susceptibility testing to determine optimal therapy choice as it permits unique efficacy assessment of individually adjusted topical therapy without microbial isolation216. The findings from such in vitro experiments can be validated by well-designed clinical studies such as longitudinal vaginal metagenomic-metabolomic profiling217 to investigate pharmacomicrobiomics mechanisms including pH and ion trapping, efflux/uptake, enzymatic inactivation, etc., in the context of persistent/recurrent BV.
In conclusion, various strategies that could improve antibiotic efficacy in the context of persistent/recurrent BV, which has overwhelmed recommended antibiotic therapy and increased the burden of spontaneous preterm birth, have been highlighted in this review. Through the concept of pharmacomicrobiomics or drug-microbe interactions, the gut has benefited from some of these interventions, giving credence to their translatability to maintaining genital tract eubiosis and reducing the incidence of persistent and recurrent bacterial vaginosis and infection-associated spontaneous preterm birth by designing new drugs, delivery systems, and dosing strategies.
Data availability
No datasets were generated or analyzed during the current study.
References
Muzny, C. A. & Sobel, J. D. Understanding and preventing recurring bacterial vaginosis: important considerations for clinicians. Int. J. Women’s. Health 15, 1317–1325 (2023).
Peebles, K., Velloza, J., Balkus, J. E., McClelland, R. S. & Barnabas, R. V. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex. Transm. Dis. 46, 304–311 (2019).
Koumans, E. H. et al. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex. Transm. Dis. 34, 864–869 (2007).
Amabebe, E. & Anumba, D. O. C. The vaginal microenvironment: the physiologic role of lactobacilli. Front. Med. https://doi.org/10.3389/fmed.2018.00181 (2018).
Amabebe, E. & Anumba, D. O. C. Mechanistic insights into immune suppression and evasion in bacterial vaginosis. Curr. Microbiol. 79, 84 (2022).
Gustin, A. T. et al. Recurrent bacterial vaginosis following metronidazole treatment is associated with microbiota richness at diagnosis. Am. J. Obstet. Gynecol. 226, 225 e221–225.e215 (2022).
Raba, G. et al. Efficacy of dequalinium chloride vs metronidazole for the treatment of bacterial vaginosis: a randomized clinical trial. JAMA Netw. Open 7, e248661 (2024).
McKinnon, L. R. et al. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res. Hum. Retroviruses 35, 219–228 (2019).
Jordan, M. M., Amabebe, E., Khanipov, K. & Taylor, B. D. Scoping review of microbiota dysbiosis and risk of preeclampsia. Am. J. Reprod. Immunol. 92, e70003 (2024).
Allsworth, J. E. & Peipert, J. F. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet. Gynecol. 109, 114–120 (2007).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 108, 4680–4687 (2011).
Schwebke, J. R., Nyirjesy, P., Dsouza, M. & Getman, D. Vaginitis and risk of sexually transmitted infections: results of a multi-center U.S. clinical study using STI nucleic acid amplification testing. J. Clin. Microbiol. 62, e0081624 (2024).
Kenyon, C., Colebunders, R. & Crucitti, T. The global epidemiology of bacterial vaginosis: a systematic review. Am. J. Obstet. Gynecol. 209, 505–523 (2013).
Chow, K. et al. Impact of (recurrent) bacterial vaginosis on quality of life and the need for accessible alternative treatments. BMC Womens Health 23, 112 (2023).
Watkins, E. et al. Bacterial vaginosis treatment patterns, associated complications, and health care economic burden of women with medicaid coverage in the United States. Ann. Pharmacother. 58, 480–493 (2024).
Watkins, E. et al. Treatment patterns and economic burden of bacterial vaginosis among commercially insured women in the USA. J. Comp. Eff. Res 13, e230079 (2024).
Bilardi, J. E. et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS ONE8, e74378 (2013).
Muzny, C. A. & Schwebke, J. R. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J. Infect. Dis. 214, S1–S5 (2016).
Reid, G. Is bacterial vaginosis a disease?. Appl. Microbiol. Biotechnol. 102, 553–558 (2018).
Muzny, C. A. et al. State of the art for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 61, e0083722 (2023).
Nugent, R. P., Krohn, M. A. & Hillier, S. L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29, 297–301 (1991).
Amsel, R. et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74, 14–22 (1983).
Chen, X., Lu, Y., Chen, T. & Li, R. The female vaginal microbiome in health and bacterial vaginosis. Front. Cell Infect. Microbiol. 11, 631972 (2021).
Muzny, C. A. et al. Diagnosis and management of bacterial vaginosis: summary of evidence reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines. Clin. Infect. Dis. 74, S144–S151 (2022).
Muzny, C. A., Laniewski, P., Schwebke, J. R. & Herbst-Kralovetz, M. M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 33, 59–65 (2020).
Muzny, C. A. & Schwebke, J. R. Asymptomatic bacterial vaginosis: to treat or not to treat? Curr. Infect. Dis. Rep. https://doi.org/10.1007/s11908-020-00740-z (2020).
Muzny, C. A. & Kardas, P. A narrative review of current challenges in the diagnosis and management of bacterial vaginosis. Sex. Transm. Dis. 47, 441–446 (2020).
Workowski, K. A. et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 70, 1–187 (2021).
Klebanoff, M. A. et al. Antibiotic treatment of bacterial vaginosis to prevent preterm delivery: systematic review and individual participant data meta-analysis. Paediatr. Perinat. Epidemiol. 37, 239–251 (2023).
McClure, E. M. & Goldenberg, R. L. Use of antibiotics to reduce preterm birth. Lancet Glob. Health 7, e18–e19 (2019).
Subtil, D. et al. Early clindamycin for bacterial vaginosis in pregnancy (PREMEVA): a multicentre, double-blind, randomised controlled trial. Lancet 392, 2171–2179 (2018).
Odendaal, H. J., Popov, I., Schoeman, J., Smith, M. & Grove, D. Preterm labour–is bacterial vaginosis involved?. S. Afr. Med. J. 92, 231–234 (2002).
Carey, J. C. et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 342, 534–540 (2000).
Vermeulen, G. M. & Bruinse, H. W. Prophylactic administration of clindamycin 2% vaginal cream to reduce the incidence of spontaneous preterm birth in women with an increased recurrence risk: a randomised placebo-controlled double-blind trial. Br. J. Obstet. Gynaecol. 106, 652–657 (1999).
Lamont, R. F. Advances in the prevention of infection-related preterm birth. Front. Immunol. https://doi.org/10.3389/fimmu.2015.00566 (2015).
Gravett, M. G. Successful treatment of intraamniotic infection/inflammation: a paradigm shift. Am. J. Obstet. Gynecol. 221, 83–85 (2019).
Workowski, K. A. Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin. Infect. Dis. 61, S759–S762 (2015).
Workowski, K. A., Bolan, G. A. & Centers for Disease, C. & Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 64, 1–137 (2015).
Sherrard, J., Wilson, J., Donders, G., Mendling, W. & Jensen, J. S. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int J. STD AIDS 29, 1258–1272 (2018).
Verwijs, M. C., Agaba, S. K., Darby, A. C. & van de Wijgert, J. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am. J. Obstet. Gynecol. 222, 157 e151–157.e113 (2020).
Armstrong-Buisseret, L. et al. Lactic acid gel versus metronidazole for recurrent bacterial vaginosis in women aged 16 years and over: the VITA RCT. Health Technol. Assess. 26, 1–170 (2022).
Mauck, C. et al. Single-dose, bioadhesive clindamycin 2% gel for bacterial vaginosis: a randomized controlled trial. Obstet. Gynecol. 139, 1092–1102 (2022).
Oduyebo, O. O., Anorlu, R. I. & Ogunsola, F. T. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst. Rev. 3, CD006055. https://doi.org/10.1002/14651858.CD006055.pub2 (2009).
Bradshaw, C. S. et al. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial. PLoS ONE7, e34540 (2012).
Schwebke, J. R. & Desmond, R. A. Tinidazole vs metronidazole for the treatment of bacterial vaginosis. Am. J. Obstet. Gynecol. 204, 211 e211–211.e216 (2011).
Muzny, C. A. & Sobel, J. D. The role of antimicrobial resistance in refractory and recurrent bacterial vaginosis and current recommendations for treatment. Antibiotics https://doi.org/10.3390/antibiotics11040500 (2022).
Muzny, C. A. & Schwebke, J. R. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin. Infect. Dis. 61, 601–606 (2015).
Sobel, J. D. et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am. J. Obstet. Gynecol. 194, 1283–1289 (2006).
Swidsinski, A. et al. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 106, 1013–1023 (2005).
Verstraelen, H. & Swidsinski, A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment. Curr. Opin. Infect. Dis. 26, 86–89 (2013).
Verstraelen, H. & Swidsinski, A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment: 2018 update. Curr. Opin. Infect. Dis. 32, 38–42 (2019).
Swidsinski, A. et al. Infection through structured polymicrobial Gardnerella biofilms (StPM-GB). Histol. Histopathol. 29, 567–587 (2014).
Swidsinski, A. et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 198, 97.e91–97.e96 (2008).
Abbe, C. & Mitchell, C. M. Bacterial vaginosis: a review of approaches to treatment and prevention. Front Reprod. Health 5, 1100029 (2023).
Bradshaw, C. S. et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin. Infect. Dis. 56, 777–786 (2013).
Vodstrcil, L. A. et al. Male-partner treatment to prevent recurrence of bacterial vaginosis. N. Engl. J. Med. 392, 947–957 (2025).
Petrina, M. A. B., Cosentino, L. A., Rabe, L. K. & Hillier, S. L. Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin. Anaerobe 47, 115–119 (2017).
Abd El Aziz, M. A., Sharifipour, F., Abedi, P., Jahanfar, S. & Judge, H. M. Secnidazole for treatment of bacterial vaginosis: a systematic review. BMC Womens Health 19, 121 (2019).
Elghazaly, S. M. et al. Efficacy and safety of single dose of oral secnidazole 2 g in treatment of bacterial vaginosis: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 238, 125–131 (2019).
Schwebke, J. R. & Desmond, R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am. J. Obstet. Gynecol. 196, 517 e511–516 (2007).
DeLong, K. et al. Conceptual design of a universal donor screening approach for vaginal microbiota transplant. Front. Cell Infect. Microbiol. 9, 306 (2019).
Rizkallah, M. R., Saad, R. & Aziz, R. K. The Human Microbiome Project, Personalized Medicine and the Birth of Pharmacomicrobiomics. Curr. Pharmacogenom. Pers. Med. 8, 182–193 (2010).
Aziz, R. K., Hegazy, S. M., Yasser, R., Rizkallah, M. R. & ElRakaiby, M. T. Drug pharmacomicrobiomics and toxicomicrobiomics: from scattered reports to systematic studies of drug-microbiome interactions. Expert Opin. Drug Metab. Toxicol. 14, 1043–1055 (2018).
Alexander, J. L. et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 14, 356–365 (2017).
ElRakaiby, M. et al. Pharmacomicrobiomics: the impact of human microbiome variations on systems pharmacology and personalized therapeutics. OMICS 18, 402–414 (2014).
Hou, K. et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 7, 135 (2022).
Aziz, R. K. Rethinking pharmacogenomics in an ecosystem: drug–microbiome interactions, pharmacomicrobiomics, and personalized medicine for the human supraorganism. Curr. Pharmacogenom. Pers. Med. 10, 258–261 (2012).
Ursell, L. K. et al. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J. Allergy Clin. Immunol. 129, 1204–1208 (2012).
Panebianco, C., Andriulli, A. & Pazienza, V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 6, 92 (2018).
Clayton, T. A., Baker, D., Lindon, J. C., Everett, J. R. & Nicholson, J. K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl. Acad. Sci. USA 106, 14728–14733 (2009).
Haiser, H. J. et al. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 341, 295–298 (2013).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Aziz, R. K. PharmacoMicrobiomics or how bugs modulate drugs: An educational initiative to explore the effects of human microbiome on drugs. BMC Bioinform. 12, A10 (2011).
Aziz, R. K. et al. SEED servers: high-performance access to the SEED genomes, annotations, and metabolic models. PLoS ONE 7, e48053 (2012).
Kanehisa, M., Goto, S., Sato, Y., Furumichi, M. & Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40, D109–D114 (2012).
Owen, R. P., Altman, R. B. & Klein, T. E. PharmGKB and the International Warfarin Pharmacogenetics Consortium: the changing role for pharmacogenomic databases and single-drug pharmacogenetics. Hum. Mutat. 29, 456–460 (2008).
Davis, A. P., Murphy, C. G., Rosenstein, M. C., Wiegers, T. C. & Mattingly, C. J. The Comparative Toxicogenomics Database facilitates identification and understanding of chemical-gene-disease associations: arsenic as a case study. BMC Med. Genom.1, 48 (2008).
Davis, A. P. et al. Comparative Toxicogenomics Database: a knowledgebase and discovery tool for chemical-gene-disease networks. Nucleic Acids Res. 37, D786–D792 (2009).
Gamazon, E. R. et al. PACdb: a database for cell-based pharmacogenomics. Pharmacogenet. Genom.20, 269–273 (2010).
Gevers, D. et al. The Human Microbiome Project: a community resource for the healthy human microbiome. PLoS Biol. 10, e1001377 (2012).
Huang, K. et al. MetaRef: a pan-genomic database for comparative and community microbial genomics. Nucleic Acids Res. 42, D617–D624 (2014).
Grundmann, O. & Yoon, S. L. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J. Gastroenterol. Hepatol. 25, 691–699 (2010).
Grundmann, O., Yoon, S. L. & Moshiree, B. Current developments for the diagnosis and treatment of irritable bowel syndrome. Curr. Pharm. Des. 16, 3638–3645 (2010).
Mathan, V. I., Wiederman, J., Dobkin, J. F. & Lindenbaum, J. Geographic differences in digoxin inactivation, a metabolic activity of the human anaerobic gut flora. Gut 30, 971–977 (1989).
Vermes, A., Kuijper, E. J., Guchelaar, H. J. & Dankert, J. An in vitro study on the active conversion of flucytosine to fluorouracil by microorganisms in the human intestinal microflora. Chemotherapy 49, 17–23 (2003).
Steffens, L. S. et al. Bacteroides fragilis RecA protein overexpression causes resistance to metronidazole. Res. Microbiol. 161, 346–354 (2010).
Steffens, L. S. et al. Corrigendum to: “Bacteroides fragilis RecA protein over expression causes resistance to metronidazole.” Res. Microbiol. 161, 346–354 (2010).
Strong, H. A., Renwick, A. G., George, C. F., Liu, Y. F. & Hill, M. J. The reduction of sulphinpyrazone and sulindac by intestinal bacteria. Xenobiotica 17, 685–696 (1987).
Amabebe, E. & Anumba, D. O. C. Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Front. Immunol. https://doi.org/10.3389/fimmu.2020.02184 (2020).
Amabebe, E. Analysis of Cervicovaginal Fluid Metabolome Microbiome in Relation to Preterm Birth. PhD thesis, Univ. Sheffield (2016).
Danielsson, D., Teigen, P. K. & Moi, H. The genital econiche: focus on microbiota and bacterial vaginosis. Ann. N. Y Acad. Sci. 1230, 48–58 (2011).
Goje, O., Shay, O. E., Markwei, M. & Padmanabhan, R. The effect of oral Metronidazole on the vaginal microbiome of patients with recurrent bacterial vaginosis: A pilot investigational study. Hum. Microbiome J. 20, 100081 (2021).
van de Wijgert, J. et al. Intermittent Lactobacilli-containing vaginal probiotic or metronidazole use to prevent bacterial vaginosis recurrence: a pilot study incorporating microscopy and sequencing. Sci. Rep. 10, 3884 (2020).
Plummer, E. L. et al. Prevotella and gardnerella are associated with treatment failure following first-line antibiotics for bacterial vaginosis. J. Infect. Dis. 228, 646–656 (2023).
Klatt, N. R. et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 356, 938–945 (2017).
Karim, S. S., Kashuba, A. D., Werner, L. & Karim, Q. A. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet 378, 279–281 (2011).
Donahue Carlson, R. et al. The female genital tract microbiome is associated with vaginal antiretroviral drug concentrations in human immunodeficiency virus-infected women on antiretroviral therapy. J. Infect. Dis. 216, 990–999 (2017).
Thompson, C. G., Cohen, M. S. & Kashuba, A. D. Antiretroviral pharmacology in mucosal tissues. J. Acquir Immune Defic. Syndr. 63, S240–S247 (2013).
Leyva-Gomez, G. et al. Modifications in vaginal microbiota and their influence on drug release: challenges and opportunities. Pharmaceutics https://doi.org/10.3390/pharmaceutics11050217 (2019).
Ventolini, G. Progresses in vaginal microflora physiology and implications for bacterial vaginosis and candidiasis. Womens Health12, 283–291 (2016).
Indulkar, A. S., Box, K. J., Taylor, R., Ruiz, R. & Taylor, L. S. pH-dependent liquid-liquid phase separation of highly supersaturated solutions of weakly basic drugs. Mol. Pharm. 12, 2365–2377 (2015).
Kis, O., Walmsley, S. L. & Bendayan, R. In vitro and in situ evaluation of pH-dependence of atazanavir intestinal permeability and interactions with acid-reducing agents. Pharm. Res. 31, 2404–2419 (2014).
Szymanska, E. et al. Chitosan-poly(ethylene oxide) nanofibrous mat as a vaginal platform for tenofovir disoproxyl fumarate - The effect of vaginal pH on drug carrier performance. Int. J. Biol. Macromol. 222, 856–867 (2022).
Taneva, E. et al. Vaginal microbiome modulates topical antiretroviral drug pharmacokinetics. JCI Insight https://doi.org/10.1172/jci.insight.99545 (2018).
Taneva, E. et al. Differential mechanisms of tenofovir and tenofovir disoproxil fumarate cellular transport and implications for topical preexposure prophylaxis. Antimicrob. Agents Chemother. 60, 1667–1675 (2015).
Thurman, A. R. et al. Vaginal microbiota and mucosal pharmacokinetics of tenofovir in healthy women using a 90-day tenofovir/levonorgestrel vaginal ring. Front. Cell Infect. Microbiol. 12, 799501 (2022).
Stafford, G. P. et al. Spontaneous preterm birth is associated with differential expression of vaginal metabolites by Lactobacilli-dominated microflora. Front. Physiol. https://doi.org/10.3389/fphys.2017.00615 (2017).
Zheng, N., Guo, R., Wang, J., Zhou, W. & Ling, Z. Contribution of Lactobacillus iners to vaginal health and diseases: a systematic review. Front. Cell Infect. Microbiol. 11, 792787 (2021).
Kindinger, L. M. et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 5, 6 (2017).
Petricevic, L. et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 4, 5136 (2014).
Chan, D. et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 13, 975 (2022).
Ramsey, P. S., Ogburn, P. L. Jr., Harris, D. Y., Heise, R. H. & Ramin, K. D. Effect of vaginal pH on efficacy of the dinoprostone gel for cervical ripening/labor induction. Am. J. Obstet. Gynecol. 187, 843–846 (2002).
Kurian, M., Rao, B. & Rao, A. A. S. Effect of vaginal pH on efficacy of dinoprostone gel for labour induction. Int. J. Reprod. Contracept. Obstet. Gynecol. 5, 1196–1201 (2016).
Kumari, A., Sharma, S. L., Pal, A. & Bhatia, V. Effect of vaginal pH on efficacy of the dinoprostone gel on labor induction and outcomes. J. Postgrad. Med. Educ. Res. 53, 72–74 (2019).
Chandra, S., Allen, V., Lee, W., Fanning, C. & Young, D. The effect of vaginal pH on labor induction with vaginal misoprostol. J. Matern. Fetal Neonatal Med. 17, 387–391 (2005).
Ramsey, P. S., Ogburn, P. L. Jr., Harris, D. Y., Heise, R. H. & Ramin, K. D. Effect of vaginal pH on efficacy of misoprostol for cervical ripening and labor induction. Am. J. Obstet. Gynecol. 182, 1616–1619 (2000).
Wilson, I. D. & Nicholson, J. K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 179, 204–222 (2017).
Nelson, T. M. et al. Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci. Rep. 8, 852 (2018).
Benowitz, N. L. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin. Pharmacol. Ther. 83, 531–541 (2008).
Piot, P., Van Dyck, E., Totten, P. A. & Holmes, K. K. Identification of Gardnerella (Haemophilus) vaginalis. J. Clin. Microbiol. 15, 19–24 (1982).
Piot, P. et al. Biotypes of Gardnerella vaginalis. J. Clin. Microbiol. 20, 677–679 (1984).
Qin, H. & Xiao, B. Research progress on the correlation between gardnerella typing and bacterial vaginosis. Front. Cell Infect. Microbiol. 12, 858155 (2022).
Tantengco, O. A. G., Vink, J., Medina, P. M. B. & Menon, R. Oxidative stress promotes cellular damages in the cervix: implications for normal and pathologic cervical function in human pregnancy†. Biol. Reprod. 105, 204–216 (2021).
Brotman, R. M. et al. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect. Dis. 14, 471 (2014).
Tuzil, J., Filkova, B., Malina, J., Kerestes, J. & Dolezal, T. Smoking in women with chronic vaginal discomfort is not associated with decreased abundance of Lactobacillus spp. but promotes Mobiluncus and Gardnerella spp. overgrowth - secondary analysis of trial data including microbio-me analysis. Ceska Gynekol. 86, 22–29 (2021).
Kwon, M. S. & Lee, H. K. Host and Microbiome Interplay Shapes the Vaginal Microenvironment. Front. Immunol. 13, 919728 (2022).
Aagaard, K. et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS ONE 7, e36466 (2012).
Consortium, T. H. M. P. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Jernberg, C., Lofmark, S., Edlund, C. & Jansson, J. K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 1, 56–66 (2007).
Ghotaslou, R. et al. Mechanisms of Bacteroides fragilis resistance to metronidazole. Infect. Genet. Evol. 64, 156–163 (2018).
Swidsinski, A. et al. Presence of a polymicrobial endometrial biofilm in patients with bacterial vaginosis. PLoS ONE8, e53997 (2013).
Marrs, C. N. et al. Evidence for Gardnerella vaginalis uptake and internalization by squamous vaginal epithelial cells: implications for the pathogenesis of bacterial vaginosis. Microbes Infect. 14, 500–508 (2012).
Jarosik, G. P., Land, C. B., Duhon, P., Chandler, R. Jr. & Mercer, T. Acquisition of iron by Gardnerella vaginalis. Infect. Immun. 66, 5041–5047 (1998).
Jarosik, G. P. & Land, C. B. Identification of a human lactoferrin-binding protein in Gardnerella vaginalis. Infect. Immun. 68, 3443–3447 (2000).
Khan, S., Voordouw, M. J. & Hill, J. E. Competition Among Gardnerella Subgroups From the Human Vaginal Microbiome. Front. Cell Infect. Microbiol. 9, 374 (2019).
Jakobsson, H. E. et al. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE5, e9836 (2010).
Hardy, L. et al. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS ONE12, e0172522 (2017).
Santiago, G. L. et al. Gardnerella vaginalis comprises three distinct genotypes of which only two produce sialidase. Am. J. Obstet. Gynecol. 204, 450 e451–450.e457 (2011).
Briselden, A. M., Moncla, B. J., Stevens, C. E. & Hillier, S. L. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J. Clin. Microbiol. 30, 663–666 (1992).
Ferreira, C. S. T., Marconi, C., Parada, C., Ravel, J. & da Silva, M. G. Sialidase activity in the cervicovaginal fluid is associated with changes in bacterial components of Lactobacillus-deprived microbiota. Front. Cell Infect. Microbiol. 11, 813520 (2021).
Gilbert, N. M. et al. Gardnerella vaginalis and Prevotella bivia trigger distinct and overlapping phenotypes in a mouse model of bacterial vaginosis. J. Infect. Dis. 220, 1099–1108 (2019).
Lewis, W. G., Robinson, L. S., Gilbert, N. M., Perry, J. C. & Lewis, A. L. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J. Biol. Chem. 288, 12067–12079 (2013).
Pleckaityte, M., Janulaitiene, M., Lasickiene, R. & Zvirbliene, A. Genetic and biochemical diversity of Gardnerella vaginalis strains isolated from women with bacterial vaginosis. FEMS Immunol. Med. Microbiol. 65, 69–77 (2012).
Novak, J. et al. Cervicovaginal Gardnerella sialidase-encoding gene in persistent human papillomavirus infection. Sci. Rep. 13, 14266 (2023).
Sharma, D., Misba, L. & Khan, A. U. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 8, 76 (2019).
Mollin, A., Katta, M., Sobel, J. D. & Akins, R. A. Association of key species of vaginal bacteria of recurrent bacterial vaginosis patients before and after oral metronidazole therapy with short- and long-term clinical outcomes. PLoS ONE17, e0272012 (2022).
Turner, E., Sobel, J. D. & Akins, R. A. Prognosis of recurrent bacterial vaginosis based on longitudinal changes in abundance of Lactobacillus and specific species of Gardnerella. PLoS ONE16, e0256445 (2021).
Armstrong, E. et al. Treatment success following standard antibiotic treatment for bacterial vaginosis is not associated with pretreatment genital immune or microbial parameters. Open Forum Infect. Dis. 10, ofad007 (2023).
Gottschick, C. et al. Screening of compounds against Gardnerella vaginalis biofilms. PLoS ONE11, e0154086 (2016).
Li, T. et al. Antimicrobial susceptibility testing of metronidazole and clindamycin against Gardnerella vaginalis in planktonic and biofilm formation. Can. J. Infect. Dis. Med. Microbiol. 2020, 1361825 (2020).
Johnston, W. et al. In vitro bacterial vaginosis biofilm community manipulation using endolysin therapy. Biofilm 5, 100101 (2023).
Rosca, A. S. et al. In vitro interactions within a biofilm containing three species found in bacterial vaginosis (BV) support the higher antimicrobial tolerance associated with BV recurrence. J. Antimicrob. Chemother. 77, 2183–2190 (2022).
Castro, J., Rosca, A. S., Muzny, C. A. & Cerca, N. Atopobium vaginae and Prevotella bivia Are Able to Incorporate and Influence Gene Expression in a Pre-Formed Gardnerella vaginalis Biofilm. Pathogens https://doi.org/10.3390/pathogens10020247 (2021).
Castro, J., Machado, D. & Cerca, N. Unveiling the role of Gardnerella vaginalis in polymicrobial Bacterial Vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J. 13, 1306–1317 (2019).
Hardy, L. et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLoS ONE10, e0136658 (2015).
Zhou, T., Hu, M., Cost, M., Poloyac, S. & Rohan, L. Short communication: expression of transporters and metabolizing enzymes in the female lower genital tract: implications for microbicide research. AIDS Res. Hum. Retroviruses 29, 1496–1503 (2013).
Zhou, T., Hu, M., Pearlman, A., Patton, D. & Rohan, L. Expression and localization of p-glycoprotein, multidrug resistance protein 4, and breast cancer resistance protein in the female lower genital tract of human and pigtailed macaque. AIDS Res. Hum. Retroviruses 30, 1106–1116 (2014).
Medina-Colorado, A. A. et al. Vaginal ecosystem modeling of growth patterns of anaerobic bacteria in microaerophilic conditions. Anaerobe 45, 10–18 (2017).
Marin, E. et al. Unraveling Gardnerella vaginalis surface proteins using cell shaving proteomics. Front. Microbiol. 9, 975 (2018).
Garmory, H. S. & Titball, R. W. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect. Immun. 72, 6757–6763 (2004).
Zhang, Y. L. et al. Lipopolysaccharide triggers luminal acidification to promote defense against bacterial infection in vaginal epithelium. Am. J. Pathol. 194, 2290–2301 (2024).
Zhou, T., Hu, M., Pearlman, A. & Rohan, L. C. Expression, regulation, and function of drug transporters in cervicovaginal tissues of a mouse model used for microbicide testing. Biochem. Pharma116, 162–175 (2016).
Berlin, M. et al. Advances and challenges in PBPK modeling–analysis of factors contributing to the oral absorption of atazanavir, a poorly soluble weak base. Eur. J. Pharm. Biopharm. 93, 267–280 (2015).
Webster, P., Saito, K., Cortez, J., Ramirez, C. & Baum, M. M. Concentrative nucleoside transporter 3 is located on microvilli of vaginal epithelial cells. ACS Omega 5, 20882–20889 (2020).
Lubelski, J., Konings, W. N. & Driessen, A. J. Distribution and physiology of ABC-type transporters contributing to multidrug resistance in bacteria. Microbiol. Mol. Biol. Rev. 71, 463–476 (2007).
Davidson, A. L. & Chen, J. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem 73, 241–268 (2004).
Rees, D. C., Johnson, E. & Lewinson, O. ABC transporters: the power to change. Nat. Rev. Mol. Cell Biol. 10, 218–227 (2009).
Wilkens, S. Structure and mechanism of ABC transporters. F1000Prime Rep. 7, 14 (2015).
Jeckelmann, J. M. & Erni, B. Transporters of glucose and other carbohydrates in bacteria. Pflug. Arch. 472, 1129–1153 (2020).
Abbott, D. W. et al. The molecular basis of glycogen breakdown and transport in Streptococcus pneumoniae. Mol. Microbiol. 77, 183–199 (2010).
France, M. T. et al. Insight into the ecology of vaginal bacteria through integrative analyses of metagenomic and metatranscriptomic data. Genome Biol. 23, 66 (2022).
Soto, S. M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 4, 223–229 (2013).
Castro, J. et al. Comparative transcriptomic analysis of Gardnerella vaginalis biofilms vs. planktonic cultures using RNA-seq. NPJ Biofilms Microbiomes 3, 3 (2017).
Zhang, K. et al. Transcriptomic and proteomic analysis of Gardnerella vaginalis responding to acidic pH and hydrogen peroxide stress. Microorganisms https://doi.org/10.3390/microorganisms11030695 (2023).
Zhang, K. et al. Antibiotic resistance and pathogenicity assessment of various Gardnerella sp. strains in local China. Front. Microbiol. 13, 1009798 (2022).
Lorca, G. L. et al. Transport capabilities of eleven gram-positive bacteria: comparative genomic analyses. Biochim. Biophys. Acta 1768, 1342–1366 (2007).
Bannantine, J. P. & Rockey, D. D. Use of primate model system to identify Chlamydia trachomatis protein antigens recognized uniquely in the context of infection. Microbiology 145, 2077–2085 (1999).
Hughes, M. J. et al. Novel protein vaccine candidates against group B streptococcal infection identified using alkaline phosphatase fusions. FEMS Microbiol. Lett. 222, 263–271 (2003).
Farin, F. M., Bigler, L. G., Oda, D., McDougall, J. K. & Omiecinski, C. J. Expression of cytochrome P450 and microsomal exposide hydrolase in cervical and oral epithelial cells immortalized by human papillomavirus type 16 E6/E7 genes. Carcinogenesis 16, 1670 (1995).
Yokose, T. et al. Immunohistochemical study of cytochrome P450 2C and 3A in human non-neoplastic and neoplastic tissues. Virchows Arch. 434, 401–411 (1999).
Patel, K. R. et al. Expression of cytochrome P450 enzymes in the cervix. An immunohistochemical study. Int. J. Gynecol. Cancer 3, 159–163 (1993).
Sharma, A., Gupta, V. K. & Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 149, 129–145 (2019).
Zwama, M. & Nishino, K. Ever-adapting RND efflux pumps in Gram-negative multidrug-resistant pathogens: a race against time. Antibiotics https://doi.org/10.3390/antibiotics10070774 (2021).
Nishino, K., Yamasaki, S., Nakashima, R., Zwama, M. & Hayashi-Nishino, M. Function and inhibitory mechanisms of multidrug efflux pumps. Front. Microbiol. 12, 737288 (2021).
Webber, M. A. & Piddock, L. J. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 51, 9–11 (2003).
Shattock, R. J. & Rosenberg, Z. Microbicides: topical prevention against HIV. Cold Spring Harb. Perspect. Med. 2, a007385 (2012).
Pandey, M. et al. Promising drug delivery approaches to treat microbial infections in the vagina: a recent update. Polymers https://doi.org/10.3390/polym13010026 (2020).
Wong, T. W., Dhanawat, M. & Rathbone, M. J. Vaginal drug delivery: strategies and concerns in polymeric nanoparticle development. Expert Opin. Drug Deliv. 11, 1419–1434 (2014).
Johal, H. S., Garg, T., Rath, G. & Goyal, A. K. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 23, 550–563 (2016).
Subi, M. T. M., Selvasudha, N. & Vasanthi, H. R. Vaginal drug delivery system: a promising route of drug administration for local and systemic diseases. Drug Discov. Today 29, 104012 (2024).
Amabebe, E. & Anumba, D. O. C. Psychosocial stress, cortisol levels, and maintenance of vaginal health. Front. Endocrinol.9, 568 (2018).
Haggerty, C. L. et al. Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin. Infect. Dis. 39, 990–995 (2004).
Shaskolskiy, B. et al. Drug resistance mechanisms in bacteria causing sexually transmitted diseases and associated with vaginosis. Front. Microbiol. 7, 747 (2016).
Schuyler, J. A., Mordechai, E., Adelson, M. E., Gygax, S. E. & Hilbert, D. W. Draft genome sequence of a metronidazole-resistant derivative of Gardnerella vaginalis strain ATCC 14019. Genome Announc https://doi.org/10.1128/genomeA.01345-15 (2015).
Schuyler, J. A. et al. Draft genome sequence of a metronidazole-resistant Gardnerella vaginalis isolate. Genome Announc https://doi.org/10.1128/genomeA.00992-15 (2015).
Schuyler, J. A. et al. Identification of intrinsically metronidazole-resistant clades of Gardnerella vaginalis. Diagn. Microbiol. Infect. Dis. 84, 1–3 (2016).
Nagaraja, P. Antibiotic resistance of Gardnerella vaginalis in recurrent bacterial vaginosis. Indian J. Med. Microbiol. 26, 155–157 (2008).
Lofmark, S., Edlund, C. & Nord, C. E. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin. Infect. Dis. 50, S16–S23 (2010).
Dingsdag, S. A. & Hunter, N. Metronidazole: an update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 73, 265–279 (2018).
Srinivasan, S. et al. Metabolic signatures of bacterial vaginosis. mBio 6, e00204–e00215 (2015).
Eitel, Z., Soki, J., Urban, E., Nagy, E. & Infection, E. S. G.oA. The prevalence of antibiotic resistance genes in Bacteroides fragilis group strains isolated in different European countries. Anaerobe 21, 43–49 (2013).
Van, T. T., Moutafis, G., Tran, L. T. & Coloe, P. J. Antibiotic resistance in food-borne bacterial contaminants in Vietnam. Appl. Environ. Microbiol. 73, 7906–7911 (2007).
Javed, A., Parvaiz, F. & Manzoor, S. Bacterial vaginosis: an insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microb. Pathog. 127, 21–30 (2019).
Aguin, T., Akins, R. A. & Sobel, J. D. High-dose vaginal maintenance metronidazole for recurrent bacterial vaginosis: a pilot study. Sex. Transm. Dis. 41, 290–291 (2014).
Pandey, K. R., Naik, S. R. & Vakil, B. V. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 52, 7577–7587 (2015).
Al-Habsi, N. et al. Health benefits of prebiotics, probiotics, synbiotics, and postbiotics. Nutrients https://doi.org/10.3390/nu16223955 (2024).
Martyniak, A., Medynska-Przeczek, A., Wedrychowicz, A., Skoczen, S. & Tomasik, P. J. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules https://doi.org/10.3390/biom11121903 (2021).
Abavisani, M., Sahebi, S., Dadgar, F., Peikfalak, F. & Keikha, M. The role of probiotics as adjunct treatment in the prevention and management of gynecological infections: An updated meta-analysis of 35 RCT studies. Taiwan J. Obstet. Gynecol. 63, 357–368 (2024).
Ali, M. S. et al. Probiotics and Postbiotics as an Alternative to Antibiotics: An Emphasis on Pigs. Pathogens 12, https://doi.org/10.3390/pathogens12070874 (2023).
Shen, X. et al. Postbiotic gel relieves clinical symptoms of bacterial vaginitis by regulating the vaginal microbiota. Front. Cell Infect. Microbiol. 13, 1114364 (2023).
Landlinger, C. et al. Engineered phage endolysin eliminates gardnerella biofilm without damaging beneficial bacteria in bacterial vaginosis ex vivo. Pathogens https://doi.org/10.3390/pathogens10010054 (2021).
Amabebe, E. et al. Cargo exchange between human and bacterial extracellular vesicles in gestational tissues: a new paradigm in communication and immune development. Extracell. Vesicles Circ. Nucleic Acids 5, 297–328 (2024).
Tantengco, O. A. G. et al. Modeling ascending Ureaplasma parvum infection through the female reproductive tract using vagina-cervix-decidua-organ-on-a-chip and feto-maternal interface-organ-on-a-chip. FASEB J. 36, e22551 (2022).
Tantengco, O. A. G. et al. Exosomes from Ureaplasma parvum-infected ectocervical epithelial cells promote feto-maternal interface inflammation but are insufficient to cause preterm delivery. Front. Cell Dev. Biol. 10, 931609 (2022).
Mahajan, G. et al. Vaginal microbiome-host interactions modeled in a human vagina-on-a-chip. Microbiome 10, 201 (2022).
Swidsinski, A. et al. Antimicrobial susceptibility of microbiota in bacterial vaginosis using fluorescence in situ hybridization. Pathogens https://doi.org/10.3390/pathogens11040456 (2022).
Cavanagh, M. et al. Vaginal host immune-microbiome-metabolite interactions associated with spontaneous preterm birth in a predominantly white cohort. NPJ Biofilms Microbiomes 11, 52 (2025).
Witkin, S. S. et al. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. mBio https://doi.org/10.1128/mBio.00460-13 (2013).
Huang, L. et al. Bacterial multidrug efflux pumps at the frontline of antimicrobial resistance: an overview. Antibiotics https://doi.org/10.3390/antibiotics11040520 (2022).
Laborda, P. et al. Mutations in the efflux pump regulator MexZ shift tissue colonization by Pseudomonas aeruginosa to a state of antibiotic tolerance. Nat. Commun. 15, 2584 (2024).
Alav, I., Sutton, J. M. & Rahman, K. M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 73, 2003–2020 (2018).
Fanelli, G., Pasqua, M., Colonna, B., Prosseda, G. & Grossi, M. Expression profile of multidrug resistance efflux pumps during intracellular life of adherent-invasive Escherichia coli strain LF82. Front. Microbiol. 11, 1935 (2020).
Acknowledgements
We thank the Department of Obstetrics and Gynecology, University of Texas Medical Branch at Galveston, for continued support. This study was supported by the Department of Obstetrics and Gynecology, University of Texas Medical Branch at Galveston, Seed Grant awarded to Dr. Emmanuel Amabebe. R.M. was supported by the NIH grant P42ES027704. S.S. is funded by R01 HD110408, R01AI141501.
Author information
Authors and Affiliations
Contributions
E.A. and the co-authors discussed the concept. E.A. designed the study and performed the initial literature search, and produced the first draft of the manuscript, which was reviewed and edited by M.T., A.K., L.R., B.T., S.S., and R.M. All authors have read and agreed to the submission of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Amabebe, E., Tatiparthy, M., Kammala, A.K. et al. Vaginal pharmacomicrobiomics modulates risk of persistent and recurrent bacterial vaginosis. npj Biofilms Microbiomes 11, 115 (2025). https://doi.org/10.1038/s41522-025-00748-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-025-00748-0