Abstract
The vaginal microbiota is critical for reproductive health, and its disruption, particularly the loss of Lactobacillus spp. and dominance of anaerobes such as Mobiluncus mulieris (community state type IV, CST IV), is associated with bacterial vaginosis, sexually transmitted infections, and adverse reproductive outcomes, including preterm birth (PTB). While Gardnerella spp. have been widely studied, the role of M. mulieris remains poorly understood. This study used an unbiased discovery approach to examine host-microbe interactions driven by M. mulieris across distinct epithelial barriers of the lower reproductive tract. RNA sequencing revealed that live bacteria, cell-free supernatant, and bacterial extracellular vesicles (bEVs) each induced unique transcriptional responses in epithelial cells. All three components activated immune and inflammatory pathways, with bEVs eliciting the strongest response, particularly via toll-like receptor (TLR) 2 and TLR5 signaling. M. mulieris also altered extracellular matrix (ECM) remodeling pathways, including upregulation of matrix metalloproteinase 9 (MMP9), a key mediator linked to PTB. These findings were supported by clinical data showing elevated MMP9 in pregnant women with M. mulieris-containing vaginal microbiota. Collectively, these results highlight the broad impact of M. mulieris on epithelial responses and identify mechanisms by which specific anaerobes contribute to inflammation and ECM disruption in adverse reproductive outcomes.
Similar content being viewed by others
Introduction
The vaginal microbiota plays a critical role in reproductive health through its interactions with epithelial barriers and modulation of local immune responses1,2. Vaginal microbiota characterized by depletion of Lactobacillus spp. and a diverse array of anaerobic bacteria (e.g., Gardnerella spp., Mobiluncus, Fannyhessea vaginalis, Prevotella spp.) are classified as Community State type IV (CST IV)1,3. CST IV is commonly associated with bacterial vaginosis (BV) and has been linked to adverse health and reproductive outcomes, including preterm birth (PTB), infertility, sexually transmitted infections, and endometriosis1,2. While many studies have focused on Gardnerella spp. as a key driver of these outcomes1,4, our work and others suggest an important role for M. mulieris1,5,6,7.
Although M. mulieris is less abundant than G. vaginalis, it is rarely found in the vaginal microbiota of healthy women. Like G. vaginalis and many other vaginal anaerobes, M. mulieris is a gram-positive bacterium and induces molecular effects through Toll-like receptor (TLR) 2 activation via select components of its bacterial cell wall. However, M. mulieris is unique in its ability to express flagellin, which can activate TLR58. While this structural feature suggests potential immune-modulating effects, little is known about how M. mulieris influences host epithelial transcriptional responses in the lower reproductive tract. Initial studies, including our own, have shown that M. mulieris, like G. vaginalis, disrupts the epithelial barrier and induces immune responses9,10,11,12. However, these studies have not examined (1) the distinct epithelial surfaces of the lower reproductive tract or (2) the potential for M. mulieris to exert pathogenic effects not only through direct bacterial interactions but also via its bacterial-free supernatant (BFS) and bacterial extracellular vesicles (bEVs).
Host-microbe interactions are critical to health and disease, and understanding how vaginal microbiota interact with the lower reproductive tract epithelia is essential. This environment consists of distinct specialized epithelial barriers: the vagina is lined with non-keratinized squamous cells lacking tight junctions, whereas the cervical epithelium forms a columnar layer that produces mucus13,14,15,16,17. These differences suggest that vaginal microbes, including M. mulieris, may engage in unique interactions with each epithelial barrier, potentially mechanistically contributing to reproductive health and disease outcomes. Determining how M. mulieris affects these different epithelial surfaces is critical for understanding its role in the development of adverse reproductive outcomes.
In vivo, bacteria interact with the host through contact and their secreted products (BFS). Consistent with evidence from other biological niches18,19, BFS can have both harmful and protective effects on epithelial cells9,11. Our previous work has shown that vaginal microbes produced bEVs that can be isolated from the BFS12. BEVs are nanoscale membrane vesicles containing bioactive molecules that interact locally and systematically with target cells. BEVs from other gram-positive and gram-negative organisms have been implicated in biological processes driving health and disease20,21. Our studies have indicated that vaginal bEVs carry specialized proteomic cargo capable of influencing the functions of epithelial and immune cells12. Importantly, we found that M. mulieris bEVs contain flagellin12, suggesting they may trigger immune pathways distinct from those activated by whole bacteria. However, whether M. mulieris, its BFS, and/or its bEVs induce similar or distinct transcriptional responses across the lower reproductive tract remains unknown.
Given the complexity of host-microbe interactions in the cervicovaginal environment, this study aimed to comprehensively assess the transcriptional impact of a clinically relevant bacterium, M. mulieris. Using an unbiased RNA sequencing (RNA-seq) approach, we analyzed the transcriptional response to live M. mulieris, its BFS, and its bEVs in vaginal, ectocervical, and endocervical epithelial cells. We further investigated the mechanisms by which M. mulieris and its products are recognized by the host, with a particular focus on TLR pathways. Additionally, to translate the findings, we validated key gene pathways of interest in vitro experiments and human clinical samples.
Results
Viability of M. mulieris in culture
We tracked the viability of M. mulieris cells from the sample preparation steps up to 24 h after incubation in a CO2 incubator, as this is critical to interpret the impact of M. mulieris cell exposure. The viability of M. mulieris was maintained during the sample preparation (1.5 × 108 CFU/mL at T2, 1.1 × 108 CFU/mL at T3) compared to the original bacterial culture (1.9 × 108 CFU/mL at T1; Supplementary Fig. 1). However, 24 h of incubation in a CO2 incubator led to a 100-fold reduction in the viable CFU count (9.7 × 105 CFU/mL, T4).
M. mulieris live bacteria, BFS, and bEVs significantly altered gene expression in cervicovaginal epithelial cells
Exposure to live M. mulieris, their BFS, or bEV resulted in significant changes in gene expression in Ect, End, and VK2 epithelial cells (Supplementary Data 1–9). Among these, bEV exposure induced the highest number of differentially expressed genes (DEGs) (Live: 13, 44, 13; BFS: 33, 116, 69; bEV: 210, 381, 313 in Ect, End, VK2, respectively; Table 1). Most DEGs were upregulated, with pathway over-representation analysis revealing broader pathway alterations in bEV-exposed cells compared to exposure to live M. mulieris cells or their BFS (Fig. 1a, Supplementary Fig. 2). Common DEGs across all exposures (13, 41, and 13 common DEGs from Ect, End, and VK2; Fig. 1b–d, Supplementary Data 10) were SAA1, BIRC3, CXCL10, MMP9, and TNFAIP3. bEV exposure uniquely altered 179, 274, and 252 DEGs in Ect, End, and VK2 cells, respectively, with a significant enrichment in immune system-related pathways: Cytokine Signaling in Immune System, Signaling by Interleukins, and Interferon alpha/beta Signaling (Supplementary Data 11).
a Heatmap showing the number of entities associated with each main pathway category in the network view of the pathway over-representation analysis. Color intensity is proportional to the number of entities in each pathway, with darker colors representing higher numbers of entities. Venn diagrams representing the number of DEGs identified in response to three M. mulieris exposure in b Ect, c End, and d VK2 cell lines.
M. mulieris live bacteria, BFS, and bEVs altered more genes in vaginal compared to cervical epithelial cells
Gene set enrichment analysis (GSEA) was first used to understand the impact of M. mulieris on pathways at the highest hierarchical level (Fig. 2a). GSEA revealed that VK2 cells tended to exhibit the highest pathway FCs in response to M. mulieris exposures, with bEV exposure tending to result in the largest FCs compared to exposure to live bacteria and BFS (Fig. 2a). The three pathways with the highest FC with significant p values after M. mulieris bEV exposure (Supplementary Data 12) were cell-cell communication (overall FC = 1.14), ECM organization (overall FC = 1.13), and immune system (overall FC = 1.12) in VK2 cells.
a A summary of the results from GSEA, visualizing the impact of M. mulieris on pathways at the highest hierarchical level. Fold changes (FCs) were calculated by comparing to the control group. Only cell line and exposure sets showing significant alterations are included in the plot. Different shapes represent cell lines (circle for Ect, square for End, and triangle for VK2), and colors represent the three M. mulieris exposures (orange for live, blue for BFS, and green for bEV exposure). b Heatmap showing the sub-pathways under the main pathway “Cell-cell communication” and c the DEGs involved in these sub-pathways. d Heatmap for sub-pathways under “Extracellular matrix organization” and e the DEGs involved in these sub-pathways. f Heatmap for sub-pathways under the “Immune system” and g the DEGs involved in these sub-pathways. For all heatmaps, darker colors indicate higher FC compared to the control group. Annotations representing significant alterations are visualized while non-significant changes are left blank (white) in the plots, and p values from VK2 treated with M. mulieris bEV group are summarized in Supplementary Data 13 (pathways) and Supplementary Data 14 (DEGs).
Under the cell-cell communication pathway, the sub-pathways cell-junction organization (FC = 1.1) and signal regulatory protein family interactions (FC = 1.3) were significantly overexpressed in VK2 cells exposed to M. mulieris bEVs (Fig. 2b; p values are summarized in Supplementary Data 13). Key DEGs involved included CLDN1 (FC = 1.2), SIRPB1 (FC = 1.1), and ZC3H12A (FC = 1.6) (Fig. 2c; p values are summarized in Supplementary Data 14). Under the ECM organization pathway, exposure to live M. mulieris, BFS, and bEV significantly impacted collagen formation, non-integrin membrane ECM interactions, ECM degradation, and integrin cell surface interactions in Ect and VK2 cells (Fig. 2d). In contrast, End cells were predominantly affected by bEV exposure, except for collagen formation, which was altered by all three exposures. Specifically, bEV exposure significantly increased the expression of matrix metalloproteinase (MMP) gene family, including MMP9 (FC = 2.9), MMP1 (FC = 1.7), MMP10 (FC = 2.0), MMP13 (FC = 1.1), and MMP19 (FC = 1.2) in VK2 cells (Fig. 2e). Additionally, gene involved in collagen formation and degradation, such as COL12A1 (FC = 1.5) and CTSS (FC = 1.4), and other ECM-related genes (ICAM1, FC = 1.8; FGF2, FC = 1.4; PDGFB, FC = 1.5) were significantly upregulated in VK2 cells following bEV exposure.
Under the immune system pathway, all three M. mulieris exposures primarily affected innate immune system and cytokine signaling pathways, rather than adaptive immune system pathways, particularly in VK2 cells (Fig. 2f). Among the innate immune system sub-pathways, all exposures significantly elevated TLR cascades, NLR signaling pathways, and DDX58/IFIH1-mediated induction of interferon alpha/beta in all three epithelial cell lines. The antimicrobial peptides pathway was elevated in Ect and End cells.
Among the cytokine signaling sub-pathways, signaling by interleukins and growth hormone receptor signaling were significantly altered by all exposures in the three epithelial cell lines. In contrast, Interferon signaling and CSF3 signaling pathways’ activation were specific to End cells. In VK2 cells, bEV exposure significantly upregulated key DEGs associated with these pathways, including: Innate immune pathways: TLR signaling (TLR2, FC = 1.5), NLR signaling (BIRC3, FC = 2.9; IRAK2, FC = 1.8; MEFV, FC = 1.1; NFKB2, FC = 1.5; NLRP3, FC = 1.3; TNFAIP3, FC = 2.6), DDX58/IFIH1-mediated interferon induction (NFKBIA, FC = 1.6; S100A12, FC = 1.3), and antimicrobial peptides pathway (LCN2, FC = 1.7; S100A7, FC = 1.1); Cytokine signaling and signaling by interleukins pathways: Signaling by interleukins (CCL20, FC = 1.5; CXCL8/IL-8, FC = 2.2; IL-24, FC = 1.5; SAA1, FC = 2.4; SERPINEB2, FC = 1.8; TNF, FC = 1.2); and Growth hormone receptor signaling: SOCS1 (FC = 1.3) (Fig. 2g).
TLR2 signaling mediated an inflammatory response to M. mulieris exposures
Given the diverse immune response elicited by live M. mulieris, its BFS, and bEVs, we sought to assess whether this response was dependent on the activation of TLR212, and/or TLR5 through its flagellin, as would be suggested by the microbial structure8. We used TLR blockades to determine if the induction of select immune mediators (IL-6, IL-8, CCL20), which were found to be upregulated in the RNA-seq analysis (Fig. 2g), was dependent on TLR2 and/or TLR5 activation. Consistent with the RNA-seq analysis findings, live M. mulieris, its BFS, and bEVs all increased the protein levels of the three immune mediators. However, there were differences in the responses by cell type and between the different bacterial exposures.
Exposure to live M. mulieris increased all three immune mediators in End and VK2 cells, with no significant changes observed in Ect cells (Fig. 3a–c). In End cells, TLR2 blockade significantly reduced the M. mulieris-induced immune mediators. Blockade of TLR5 also limited the ability of M. mulieris to induce an immune response, resulting in a significant reduction in IL-6 and IL-8 levels. While live M. mulieris increased IL-6, IL-8, and CCL20 levels in VK2 cells, the immune response was not as robust as observed in Ect or End cells. Only TLR2 blockade reduced IL-8 levels, and it did not affect IL-6 or CCL20 levels induced by live M. mulieris exposure in VK2 cells. Notably, TLR5 blockade did not significantly reduce any immune mediator levels in VK2 cells exposed to live M. mulieris.
a IL-6, b IL-8, and c CCL20 levels in response to live M. mulieris exposure; d IL-6, e IL-8, and f CCL20 levels in response to BFS M. mulieris exposure; g IL-6, h IL-8, and i CCL20 levels in response to bEV M. mulieris exposure. The inhibitors for hTLR2 and hTLR5 are written as TLR2i and TLR5i. Bar plots represent the mean, the error bars for the standard deviation, and the dots represent the technical replicate (n = 3). One-way ANOVA was used, followed by Tukey’s multiple comparison test, with statistical significance denoted by “*” (p < 0.05), “**” (p < 0.01), “***” (p < 0.001), “****” (p < 0.0001).
M. mulieris BFS significantly increased IL-8 in Ect cells (Fig. 3d), all three mediators in Endo cells (Fig. 3e), and IL-8 and CCL20 in VK2 cells (Fig. 3f). In Ect cells, both TLR2 and TLR5 blockades significantly reduced IL-8 levels. In End cells, both TLR2 and TLR5 blockade reduced all three mediator levels, where statistical significance was observed for the reduction in all three mediators by TLR2 blockade and IL-6 and CCL20 levels by TLR5 blockade. In VK2 cells, the TLR2 blockade did not significantly reduce the levels of the immune mediators, but the TLR5 blockade significantly reduced the IL-8 and CCL20 levels. The combination of TLR2 and TLR5 blockades demonstrated a synergistic effect, reaching statistical significance for IL-8 and CCL20 levels in all cell lines and IL-6 levels in Ect and End cells.
M. mulieris bEVs significantly elevated the three immune mediators across the three cell lines (Fig. 3g–i). TLR2 blockade was sufficient to significantly reduce IL-6 and CCL20 levels in Ect cells, as well as IL-8 and CCL20 levels in End cells, with no significant impact from TLR5 blockade. In VK2 cells, TLR2 blockade significantly reduced IL-8 levels, and both TLR2 and TLR5 blockade significantly reduced CCL20 levels. An additive effect of TLR2 and TLR5 blockades was observed for IL-8 in all three epithelial cell lines.
As a quality control measure, neither TLR2 nor TLR5 blockades affected baseline immune mediators’ expression in any cell line (Supplementary Fig. 3a–c), except for a significant, but minor increase in CCL20 levels in Ect cells following TLR2 blockade compared to the control group, with a mean increase of only 10.5 pg/mL.
MMP9 production is induced by M. mulieris via TLR signaling
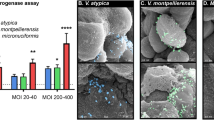
A key finding from the RNA-seq analysis was that M. mulieris increased expression of MMP9, a matrix metalloproteinase involved in ECM degradation and previously associated with PTB22. Since RNA-seq data showed that live M. mulieris, its BFS, and bEVs significantly upregulated MMP9 gene expression in all cervicovaginal epithelial cells tested in this study (Fig. 2e), we sought to determine whether any M. mulieris exposures also altered MMP9 protein levels by measuring the total MMP9 (including both active and inactive forms). We found that exposure to live M. mulieris, BFS, and bEV significantly increased MMP9 protein levels in VK2 cell culture supernatant (Fig. 4a–c), while MMP9 levels in Ect and End cells remained below the detection limit. No change in MMP9 production was noted with exposure to L. crispatus, an optimal vaginal bacterium (Supplementary Fig. 4).
MMP9 protein levels were measured in the culture media of VK2 cells exposed to a live, b BFS, and c bEV M. mulieris exposures, with and without the addition of hTLR2 and/or hTLR5 inhibitors (denoted as TLR2i and TLR5i). One-way ANOVA was used to compare MMP9 concentrations between groups. For statistically significant results (p < 0.05), post-hoc tests were applied. d MMP9 protein levels measured in the culture media of THP1 wild-type cells in response to d live, e bEV, and f BFS M. mulieris exposures. Unpaired t-tests were used to compare MMP9 concentrations between control and M. mulieris exposures. g MMP9 protein levels measured in the culture media of THP1 wild type and THP-TLR2KO exposed to g live and h bEV. mulieris exposures. White bars represent the results from THP1 cells, and black bars represent the results from THP1-TLR2KO cells. Bar plots represent the mean, the error bars for the standard deviation, and the dots represent the technical replicate (n = 3). Two-way ANOVA was applied to assess the impact of TLR2 knockout. The statistical significance was denoted by “*” (p < 0.05), “**” (p < 0.01), and “***” (p < 0.001).
The increase in MMP9 by live M. mulieris was significantly reduced by TLR2 blockade but not by TLR5 blockade (Fig. 4a). In contrast, the increase in MMP9 by M. mulieris BFS was significantly reduced by TLR5 blockade and, to a lesser extent, by TLR2 blockade (Fig. 4b). The combination of TLR2 and TLR5 blockades further decreased the MMP9 levels induced by M. mulieris BFS. The increase in MMP9 by M. mulieris bEVs was only significantly reduced when both TLR2 and TLR5 blockades were combined (Fig. 4c).
As a quality control measure, we confirmed that the addition of TLR2 and/or TLR5 inhibitors did not alter baseline MMP9 protein expression (Supplementary Fig. 3d).
To confirm the role of TLR2 in mediating M. mulieris-induced MMP9 production, we assessed MMP9 levels following exposure to live M. mulieris cells, BFS, and bEV in THP1 wild-type and THP1 TLR2-deficient cells (THP1-TLR2KO). Exposures to both live M. mulieris (Fig. 4d) and bEV M. mulieris (Fig. 4e), but not BFS (Fig. 4f), significantly increased MMP9 production. The absence of TLR2 significantly reduced MMP9 production in response to exposures to live M. mulieris (Fig. 4g) and M. mulieris bEVs (Fig. 4h).
M. mulieris abundance correlates with MMP9 levels in vaginal swabs from pregnant women
We compared MMP9 protein levels in vaginal samples from two groups: 20 pregnant women with a high abundance of L. crispatus and no detectable M. mulieris (high LC group) and 10 pregnant women with low L. crispatus and high M. mulieris abundance (high MM group). As shown in Table 2, there were no significant differences between the groups in race, age, gestational age at sample collection, infant birth weights, or gestational age at delivery between the groups (p > 0.05). The high MM group had significantly higher MMP9 levels than the high LC group (Fig. 5a). However, vaginal MMP9 concentration did not show a direct correlation with M. mulieris abundance (p = 0.38, r = 0.16). Within the high MM group, CST IV-A and CST V subtypes exhibited a trend toward higher MMP9 levels (Fig. 5b).
a A bar plot showing the MMP9 concentrations measured in vaginal swab samples from subjects in the high LC and high MM groups. Bar plots represent the mean and the error bars for the standard deviation. The Mann–Whitney test was applied to compare MMP9 levels between the two groups, with statistical significance indicated by “***” (p < 0.001). b Within the high MM group, MMP9 concentrations were visualized by finer CST groupings.
Discussions
The vaginal microbiota plays a critical role in both reproductive health and disease1,2. M. mulieris has been associated with various adverse reproductive outcomes1,5,6,7; however, the molecular mechanisms underlying its pathogenic effects remain unclear. This study presents the first unbiased approach to assess the transcriptional impact of M. mulieris on different epithelial barriers of the lower reproductive tract. Our analyses revealed that exposure to live M. mulieris and its BFS or bEVs significantly altered the transcriptional profile of cervicovaginal epithelial cells. Specifically, M. mulieris induced significant immune responses across epithelial cell types. However, in a pattern suggesting specificity in host-microbial interactions, M. mulieris bEVs had the most profound transcriptional impact, inducing the greatest number of DEGs. Additionally, exposure to M. mulieris had a more pronounced effect on vaginal epithelial cells compared to cervical epithelial cells, highlighting the tissue-specific nature of host-microbe interactions. As a mechanistic explanation for these molecular effects, we demonstrated that TLR2 and TLR5 activation are essential to M. mulieris-induced inflammation. Lastly, we provide in vivo evidence correlating M. mulieris abundance with elevated MMP9 levels, suggesting that the molecular effect observed in vitro also occurs in vivo.
Live M. mulieris, as well as its BFS and bEVs, significantly upregulated genes involved in immune responses, especially those related to inflammation via innate immune signaling and cytokine/chemokine pathways in cervicovaginal epithelial cells. Our findings further confirm M. mulieris’ role in inflammation, as exposure to M. mulieris activated pro-inflammatory cytokine and chemokine production, consistent with previous studies12,23. Novel to this study, we also found that M. mulieris exposures significantly elevated pathways related to cell-cell communication, potentially contributing to increased membrane permeability, as previously observed in response to M. mulieris BFS10. These findings suggest that M. mulieris exposures may weaken epithelial barriers, thereby increasing susceptibility to sexually transmitted infections (STIs) (Fig. 6). Indeed, previous studies reported that cervicovaginal fluid from women with BV can disrupt the endocervical epithelial membrane integrity, facilitating the transmigration of human immunodeficiency virus-124. Additionally, M. mulieris exposures significantly increased MMP9 gene and protein expression in cervicovaginal epithelial cells. Vaginal microbiota dysbiosis, along with increase in pro-inflammatory cytokines and chemokines such as IL-1, IL-8, and TNF, has been implicated in MMP9 level elevation25, all of which were upregulated in our unbiased RNA-seq analysis. Importantly, elevated MMP9 activity has been observed in pregnant women at increased risk of PTB22,25,26 (Fig. 6).
This figure provides a visual overview of the main results, illustrating the relationships between M. mulieris exposure and gene expression changes in immune system pathways, cell-cell communication, and extracellular matrix organization within the cervicovaginal space. These changes are compared to the condition with healthy vaginal microbiota, and the potential clinical relevance of these findings is also highlighted. All annotations are based on DEGs and significantly enriched pathways. Annotations in gray represent genes or pathways with a fold change of less than 1.5 compared to the control groups. This figure was generated with BioRender.com.
Consistent with this, we found that TLR2 blockade alone was sufficient to significantly reduce cytokines, chemokines, and MMP9 protein levels following exposure to live M. mulieris. In contrast, both TLR2 and TLR5 blockades were required to prevent inflammation triggered by M. mulieris BFS and bEV. These findings have significant therapeutic implications, as bacterial products, such as BFS and bEVs, undoubtedly have biological effects in vivo. Our results support a primary role for TLR2 in M. mulieris-induced inflammation, consistent with our previous study showing that M. mulieris bEVs trigger IL-8 production via TLR2-mediated NF-κB signaling12. Additionally, given that M. mulieris bEVs contain flagellin-family proteins12 recognized by TLR58, our findings suggest that M. mulieris activates both TLR5 and TLR2, contributing to inflammation in the lower reproductive tract. These results, along with our previous data, suggest that M. mulieris BFS, as well as bEVs isolated from BSF, contain a broader range of bacterial bioactive molecules, as evidenced by the wide range of bEV sizes (90–420 nm in diameter) isolated from M. mulieris BFS12,27. These diverse ligands and metabolites engage various innate immune receptors, providing redundant pathways through which M. mulieris propagates inflammation.
Our clinical findings support the in vitro results, demonstrating that vaginal swabs from women with low L. crispatus abundance and high M. mulieris abundance exhibit significantly elevated MMP9 levels compared to women dominated by L. crispatus. Notably, individuals whose vaginal microbiome was classified as CST IV-A and CST V exhibited the highest MMP9 concentrations, suggesting that M. mulieris may be more transcriptionally active within these microbiomes. Our results align with a prior report showing that cervicovaginal fluid from non-pregnant women with BV showed higher MMP levels24. Moreover, elevated MMP9 levels and resulting ECM degradation in the vagina have been associated with pelvic organ prolapse, a condition linked to significant health complications, reduced quality of life, increased health costs28,29 (Fig. 6). Although further research is needed, our study provides new insights into the potential role of vaginal M. mulieris in the development of different reproductive disorders associated with degradation of ECM such as premature cervical remodeling in PTB and pelvic floor dysfunction.
This study has some limitations, including the use of cell lines rather than primary tissues and the lack of investigation into M. mulieris’ impact on mucus formation in the cervix. Additionally, clinical findings may be confounded by other bacterial species present in the vaginal microbiota of the participants evaluated, as our primary focus was on M. mulieris and the sample size was relatively small. Future studies examining the full microbial repertoire will provide a more comprehensive understanding of the relationship between M. mulieris and MMP9, as well as its impact on the downstream TLR2 and TLR5 signaling pathways. Also, it is important to note that M. mulieris is an anaerobic bacterium, and therefore, live M. mulieris did not continue to grow or remain metabolically active when exposed to epithelial cells. Instead, viability significantly decreased over the 24-h exposure period. During the bacterial death phase, bEVs can be formed through lytic mechanisms, potentially carrying non-selective cargo whose composition may differ from the bEVs used in this study27. However, the concentration of bEVs produced by dying M. mulieris was much lower than that used in this study for bEV exposure (bEVs were isolated from 300 mL of bacterial culture, with 109 EVs loaded per well). Therefore, the effect observed with live M. mulieris exposure was likely due to a contact-dependent effect rather than the production of secreted products.
In conclusion, we report that M. mulieris is a potent vaginal anaerobe that activates various biological processes, particularly those related to inflammation and ECM remodeling. We also provide insights into the molecular mechanisms by which M. mulieris triggers immune responses through multiple pathways, suggesting its potential role in adverse reproductive outcomes, particularly in individuals with CST IV, where M. mulieris is both prevalent and transcriptionally active. Furthermore, our findings highlight the role of M. mulieris-derived bEVs in modulating local immune pathways and suggest their potential to be trafficked to distant cells and tissues, impacting broader reproductive health. Our findings emphasize the importance of maintaining an optimal vaginal microbiota dominated by Lactobacillus spp. and suggest potential therapeutic targets for mitigating M. mulieris-mediated reproductive risks. Further research into adjunctive therapies, including live biotherapeutics or targeted anti-inflammatory interventions, may help improve reproductive health outcomes in individuals colonized by M. mulieris.
Methods
Cell culture
Human ectocervical (Ect; American Type Culture Collection (ATCC) CRL-2614, Ect1/E6E7, Manassas, VA, USA), endocervical (End; ATCC CRL-2615, End1/E6E7), and vaginal (VK2; ATCC CRL-2616, VK2/E6E7) epithelial cell lines were cultured in keratinocyte-serum-free (KSF) media supplemented with 0.1 ng/mL epidermal growth factor, 50 μg/mL bovine pituitary extract (Gibco/Thermo Fisher Scientific, Waltham MA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). THP1-Null cells (THP1) and THP1-Dual KO-TLR2 cells (THP1-TLR2KO; InvivoGen, San Diego, CA, USA) were cultured in RPMI-1640 (Life Technologies, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin.
Bacterial culture and bacterial sample preparation
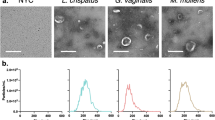
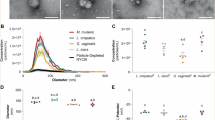
A clinical strain of M. mulieris (ATCC 35243), as well as L. crispatus (ATCC 33197) as the healthy bacteria control for matrix metalloproteinase 9 (MMP9) analysis, were used. To obtain live and BFS samples, M. mulieris and L. crispatus were cultured in NYCIII media with 5% horse serum (Gibco) for 5 and 2 days, respectively, at 37 °C in an anaerobic glove box. Bacterial culture media was then centrifuged at 3500 × g for 30 min to separate the live bacterial pellets from the BFS. The BFS was subsequently filtered through a 0.02 µm Nalgene vacuum filtration system (Fisher Scientific, Fair Lawn, NJ, USA). Separately, for bEV isolation, NYCIII media was supplemented with 1% serum, and ultracentrifugation was performed at 100,000 × g overnight to remove EVs from the media. Both bacteria were cultured in 300 mL of EV-depleted NYCIII media for 10 days. The bacterial culture media was centrifuged at 3500 × g for 30 min, after which the supernatant was filtered through a 0.02 µm filtration system, followed by bEV isolation via sequential ultracentrifugation, as previously described12. Briefly, the filtrate was centrifuged at 30,000 × g for 33 min to remove any remaining particles. The supernatant was then centrifuged at 100,000 × g for 70 min to collect the EV pellets, which were washed once with phosphate-buffered saline (PBS) and centrifuged again at 100,000 × g for 70 min. Finally, the bEV pellets were resuspended in bEV suspension media composed of 100 µL of 10 mM HEPES (Gibco) and 25 mM NaCl and stored at −80 °C until further analysis. BEVs were characterized by Nanoparticle Tracking Analysis using ZetaView (Particle Metrix, Germany) as previously reported12.
The viability of the live M. mulieris culture was tracked from the sample preparation steps to 24 h after incubation in a CO2 incubator (the same conditions used during bacterial exposure). Colony-forming unit (CFU) counts of the same bacterial culture were monitored at four different time points: before (T1) and after (T2) centrifuging the M. mulieris culture at 3500 × g for 30 min to prepare the live bacterial pellet; after resuspending the bacterial pellet in KSF media (the same conditions used to prepare the live bacterial sample for exposure to cervicovaginal epithelial cells, T3); and 24 h after incubation in a CO2 incubator (T4).
Cell exposure to live bacteria, BFS, and bEVs
The doses and duration of bacterial exposure to cells were determined based on cell death analysis using a lactate dehydrogenase assay, and significant elevation of inflammation mediators11,12 was ensured. Given that in our previous work, based on our earlier work, the qPCR PanBacterial data showed that a typical vaginal swab contains ~108 bacterial cells. In that same study, we identified a M. mulieris relative abundance threshold of 0.00358 that distinguishes spontaneous PTB from term birth. This corresponds to roughly 106 M. mulieris cells per swab. Considering that the surface area sampled by a vaginal swab is approximately twice larger than the surface area of a 24-well plate, 105 M. mulieris cells per well represents a physiologically relevant bacterial load, comparable to what is found in vivo on an equivalent surface area. Culture cells were plated at 1.5 × 105 cells per well in 24-well plates (n = 3 per group) and cultured at 37 °C in a 5% CO2 humidified incubator for 24 h. Cells were then treated with Live bacteria (105 CFU/well), BFS (1:100 dilution in cell culture media), or bEVs (109 bEVs/well) for 24 h. As the control group, cells were incubated with cell culture media for the Live group, cells received a 1:100 dilution of NYCIII media in cell culture media for the BFS group, and cells were incubated with cell culture media containing the same volume of bEV suspension media as the treated samples in the bEV groups. For protein analysis, cells were pre-treated with anti-hTLR2-IgA2 or anti-hTLR5-IgA2 (InvivoGen) at a final concentration of 1 µg/mL for 1 h, followed by live, BFS, or bEV exposures at the same dose described earlier for 24 h. These antibodies were validated for neutralizing activity using cellular assays, and their binding specificity was confirmed by flow cytometry (InvivoGen). After exposure to bacterial samples, cells were gently rinsed with 1 mL PBS per well and mixed with 400 µL TRIzol per well for RNA-seq analysis (described further below). For protein analysis, the culture supernatant was collected and stored at −80 °C until further analysis.
RNA-seq analysis
RNA extraction, library preparation, sequencing, and initial data processing and analyses were performed by Azenta Life Sciences (South Plainfield, NJ, USA). Total RNA was extracted using Qiagen RNeasy Plus Mini kit following the manufacturer’s instructions (Qiagen, Hilden, Germany). Post extraction, total RNA samples were quantified using Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA), and RNA integrity was checked using Agilent TapeStation 4200 (Agilent Technologies, Palo Alto, CA, USA) to confirm the RNA integrity values were confirmed to be above 9.
Strand-specific RNA-seq libraries were prepared by using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina following the manufacturer’s instructions (NEB, Ipswich, MA, USA). Briefly, the enriched RNAs were fragmented for 8 min at 94 °C. First-strand and second-strand cDNA were subsequently synthesized. The second strand of cDNA was marked by incorporating dUTP during the synthesis. cDNA fragments were adenylated at the 3′ends, and an indexed adapter was ligated to the cDNA fragments. Limited-cycle PCR was used for library enrichment. The incorporated dUTP in the second-strand cDNA quenched the amplification of the second strand, which helped to preserve the strand specificity. The sequencing library was validated on the Agilent TapeStation (Agilent Technologies) and quantified by using Qubit 2.0 Fluorometer (Thermo Fisher Scientific), as well as by quantitative PCR (KAPA Biosystems, Wilmington, MA, USA).
The sequencing libraries were multiplexed and clustered onto a flowcell on the Illumina NovaSeq instrument according to the manufacturer’s instructions. The samples were sequenced using a 2 × 150 bp Paired End configuration. Image analysis and base calling were conducted by the NovaSeq Control Software. Raw sequence data (.bcl files) generated from Illumina NovaSeq were converted into fastq files and de-multiplexed using Illumina bcl2fastq 2.20 software. One mismatch was allowed for index sequence identification.
After investigating the quality of the raw data, sequence reads were trimmed to remove possible adapter sequences and nucleotides with poor quality using Trimmomatic v.0.36. The trimmed reads were mapped to the reference genome available on ENSEMBL using the STAR aligner v.2.5.2b. BAM files were generated as a result of this step. Unique gene hit counts were calculated by using feature Counts from the Subread package v.1.5.2. Only unique reads that fell within exon regions were counted. After the extraction of gene hit counts, the gene hit counts table was used for downstream differential expression analysis. Using DESeq2, a comparison of gene expression between the groups of samples was performed. The Wald test generated P values and Log2 fold changes (FCs). Genes with false discovery rate-adjusted p values < 0.05 and absolute log2 FCs >1 were called DEGs for each comparison. We used the software GeneSCF to perform gene ontology (GO) analysis on the set of statistically significant genes (both up- and downregulated genes). The functional enrichment analysis identifies which biological processes are most associated with the genes. A human-specific GO annotation list was used to group the genes by biological process and assess which groups were significantly enriched. Over-representation analysis and GSEA were performed in Reactome (v90). For GSEA, normalization was conducted using the correlation-adjusted mean rank gene set test mode, which accounts for correlations among genes. The number of genes (entities) enriched in each pathway was quantified, and the results were visualized using GraphPad Prism (version 10.5.0).
Protein extraction from Dacron-tipped swabs
To investigate MMP9 protein levels in human vagina of characterized vaginal microbial taxonomy, Dacron swabs collected as part of the M&M study were used5. Written informed consent was obtained from all participants, with approval from the Institutional Review Boards (IRB) at the University of Pennsylvania (IRB #818914) and the University of Maryland School of Medicine (HP-00045398). Vaginal samples that had been previously shown to have a high abundance of L. crispatus (LC) and no detectable M. mulieris (MM) (“High LC” group: 97.3 ± 1.1% L. crispatus, 0 ± 0% M. mulieris) (N = 20) or high abundance of M. mulieris (n = 10) (“High MM” group: 0.9 ± 1.3% L. crispatus, 1.8 ± 2.0% M. mulieris) were used; bacterial abundance was previously reported5. Each Dacron swab was submerged in PBS containing a Pierce Protease Inhibitor tablet (Thermo Fisher Scientific) and incubated for 5 min to isolate the protein. The swab was then vigorously agitated in the solution. The isolation media was centrifuged at 10,000 × g for 5 min, and the supernatant was collected and stored at −80 °C until further analysis.
For the metadata of subjects who donated vaginal swabs, differences in race were assessed using Fisher’s exact test, age and gestational age at visit were compared using a t-test, and infant birth weight and gestational age at delivery were evaluated using the Wilcoxon test.
Measurement of inflammation mediators and MMP9 proteins
Analysis of inflammation mediators (Interleukin (IL)-6, IL-8, Chemokine (C-C motif) ligand 20 (CCL20)) and MMP9 levels were conducted by ELISA (R&D Systems, Minneapolis, MN, USA), following the manufacturer’s protocol. The MMP9 ELISA kit measured total human MMP9 levels, including the 92 kDa proenzyme (inactive form) and the 82 kDa active form, but did not detect the 65 kDa C-terminal truncated form.
A one-way ANOVA was used to compare the levels of proteins in Ect, End, and VK2 culture supernatant samples, followed by Tukey’s multiple comparison test. An unpaired t-test compared MMP9 levels in THP1 cell culture media and MMP9 levels following L. crispatus exposures. The impact of TLR2 knockout in THP1 cells was assessed using two-way ANOVA followed by Sidak’s multiple comparison test. MMP9 level in vaginal swab samples was analyzed using the Mann–Whitney test. Pearson correlation test was used to assess the correlation between MMP9 and M. mulieris abundance in vaginal swab samples. A p value < 0.05 was considered statistically significant for all tests.
Data availability
Data are provided within the manuscript or supplementary information files.
References
Al-Nasiry, S. et al. The interplay between reproductive tract microbiota and immunological system in human reproduction. Front. Immunol. 11, https://doi.org/10.3389/fimmu.2020.00378 (2020).
van de Wijgert, J. H. H. M. & Jespers, V. The global health impact of vaginal dysbiosis. Res. Microbiol. 168, 859–864 (2017).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 108, 4680–4687 (2011).
Gerson, K. D. et al. Gardnerella vaginalis induces matrix metalloproteinases in the cervicovaginal epithelium through TLR-2 activation. J. Reprod. Immunol. 152, 103648 (2022).
Elovitz, M. A. et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun. 10, 1305 (2019).
Holst, E., Goffeng, A. R. & Andersch, B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J. Clin. Microbiol. 32, 176–186 (1994).
Nelson, D. B. et al. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr. Perinat. Epidemiol. 28, 88–96 (2014).
Sun, H. et al. Targeting toll-like receptor 7/8 for immunotherapy: recent advances and prospectives. Biomark. Res. 10, 89 (2022).
Anton, L. et al. Common cervicovaginal microbial supernatants alter cervical epithelial function: mechanisms by which Lactobacillus crispatus contributes to cervical health. Front. Microbiol. 9, https://doi.org/10.3389/fmicb.2018.02181 (2018).
Dude, C. M., Saylany, A., Brown, A., Elovitz, M. & Anton, L. Microbial supernatants from Mobiluncus mulieris, a bacteria strongly associated with spontaneous preterm birth, disrupts the cervical epithelial barrier through inflammatory and miRNA mediated mechanisms. Anaerobe 61, 102127 (2020).
Anton, L. et al. Gardnerella vaginalis alters cervicovaginal epithelial cell function through microbe-specific immune responses. Microbiome 10, 119 (2022).
Joseph, A. et al. Extracellular vesicles from vaginal Gardnerella vaginalis and Mobiluncus mulieris contain distinct proteomic cargo and induce inflammatory pathways. npj Biofilms Microbiomes 10, 1–12 (2024).
Prendiville, W. & Sankaranarayanan, R. Anatomy of the uterine cervix and the transformation zone. in Colposcopy and Treatment of Cervical Precancer (International Agency for Research on Cancer, 2017).
Forsberg, J. G. Cervicovaginal epithelium: its origin and development. Am. J. Obstet. Gynecol. 115, 1025–1043 (1973).
Fritsch, H., Hoermann, R., Bitsche, M., Pechriggl, E. & Reich, O. Development of epithelial and mesenchymal regionalization of the human fetal utero-vaginal anlagen. J. Anat. 222, 462–472 (2013).
Kurita, T. Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation 82, 117–126 (2011).
Taherali, F., Varum, F. & Basit, A. W. A slippery slope: on the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 124, 16–33 (2018).
Abrehame, S. et al. Selection of fermentation supernatant from probiotic strains exhibiting intestinal epithelial barrier protective ability and evaluation of their effects on colitis mouse and weaned piglet models. Nutrients 16, 1138 (2024).
Zhang, Y.-J. et al. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 16, 7493–7519 (2015).
Kim, J. H., Lee, J., Park, J. & Gho, Y. S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 40, 97–104 (2015).
Toyofuku, M., Schild, S., Kaparakis-Liaskos, M. & Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol 21, 415–430 (2023).
Amabebe, E., Ogidi, H. & Anumba, D. O. Matrix metalloproteinase-induced cervical extracellular matrix remodelling in pregnancy and cervical cancer. Reprod. Fertil. 3, R177–R191 (2022).
McKenzie, R., Maarsingh, J. D., Łaniewski, P. & Herbst-Kralovetz, M. M. Immunometabolic analysis of Mobiluncus mulieris and Eggerthella sp. reveals novel insights into their pathogenic contributions to the hallmarks of bacterial vaginosis. Front. Cell. Infect. Microbiol. 11, 759697 (2021).
Cherne, M. D. et al. Matrix metalloproteinases expressed in response to bacterial vaginosis disrupt the endocervical epithelium, increasing transmigration of HIV. Infect. Immun. 88, e00041-20 (2020).
Romero, R., Dey, S. K. & Fisher, S. J. Preterm labor: one syndrome, many causes. Science 345, 760–765 (2014).
Chung, I.-H. et al. Overexpression of lipocalin 2 in human cervical cancer enhances tumor invasion. Oncotarget 7, 11113–11126 (2016).
Guo, J. et al. Opportunities and challenges of bacterial extracellular vesicles in regenerative medicine. J. Nanobiotechnol. 23, 4 (2025).
Wu, J. M. et al. Matrix metalloproteinase-9 genetic polymorphisms and the risk for advanced pelvic organ prolapse. Obstet. Gynecol. 120, 587–593 (2012).
Chen, S., Zheng, Q., Zhang, L., Chen, L. & Wang, J. Effect of vaginal microecological alterations on female pelvic organ prolapse. Int. Urogynecol. J. 35, 881–891 (2024).
Acknowledgements
This study was funded by the National Institutes of Health (NIH) National Institute of Child Health and Human Development (NICHD) (R01HD102318 and R01HD098867), as well as the National Institute for Nursing Research of the National Institutes of Health under award number R01NR014784. The funder played no role in study design, data collection, analysis, and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design of the work: Y.H., M.E.; data acquisition and analysis: Y.H., O.S., U.R., M.F., L.N., I.M.; writing: Y.H.; methodology: H.Y., A.H.; revisions: Y.H., I.M., A.H., J.R., M.E.; supervision: A.H., J.R., M.E. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
J.R. is co-founder of LUCA Biologics, a biotechnology company focusing on translating microbiome research into live biotherapeutic drugs for women’s health. All other authors declare that they have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hasegawa, Y., Swain, O., Rajpal, U. et al. Mobiluncus mulieris alters the transcriptomic profile of cervicovaginal epithelial cells, shedding light on molecular drivers of adverse reproductive outcomes. npj Biofilms Microbiomes 11, 154 (2025). https://doi.org/10.1038/s41522-025-00784-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41522-025-00784-w
This article is cited by
-
Vaginal bacteria-derived extracellular vesicles diffuse through human cervicovaginal mucus to enable microbe-host signaling
npj Biofilms and Microbiomes (2026)