Abstract
We conducted a retrospective analysis of 213 patients with HER2+ T1micN0 breast cancer. Patients with ≥5 foci of microinvasion received more aggressive treatment with higher rates of sentinel lymph node biopsy and adjuvant chemotherapy/HER2-targeted therapy (CT/H2TT) than patients with 1–4 foci. We did not detect significant differences in invasive disease-free survival between patients with 1–4 and ≥5 foci, or between patients with ≥5 foci who did and did not receive adjuvant CT/H2TT.
Similar content being viewed by others
In breast cancer (BC), microinvasion (MI), defined as one or more foci of invasion ≤1 mm in size, is most commonly encountered in the setting of high-grade ductal carcinoma in-situ (DCIS)1. Due to the low incidence of DCIS with MI (DCIS-MI, comprising ~1% of all BC cases)2, data regarding disease characteristics and clinical outcomes are limited. Although the prognosis of DCIS-MI is reported to be similar or slightly worse than that of patients with pure DCIS1,3,4, microinvasive BC is more likely to be human epidermal growth factor receptor 2-positive (HER2+) and less likely to be estrogen/progesterone receptor-positive (ER/PR+) than DCIS5. In an analysis of 1530 patients with microinvasive BC stratified by hormone receptor (HR) and HER2 subtypes, the HR−/HER2+ subgroup had the worst outcomes6.
The standard of care (SOC) for stage IA HER2+ BC consists of breast-conserving surgery (BCS) and adjuvant radiotherapy (RT) or mastectomy, and antiestrogen therapy if the invasive carcinoma is ER/PR+7. In addition, adjuvant paclitaxel and trastuzumab (TH) is now SOC for T1b+ (>5 mm) and strongly considered for T1a after the single-arm APT trial demonstrated a 3-year invasive disease-free survival (iDFS) rate of 98.7%8. However, only 9 of the 406 patients in the trial had T1mic disease. No randomized trials have specifically investigated optimal treatment for the T1micN0 HER2+ subgroup. Thus, management remains controversial. National Comprehensive Cancer Network Guidelines recommend to “consider adjuvant chemotherapy with trastuzumab (category 2B)” for patients with stage IA HER2+ tumors ≤5 mm in size, and a footnote states that “the absolute benefit of HER2-based systemic chemotherapy is likely negligible in patients with HR+ cancers and tumor size bordering on T1mic (<1 mm)”7. Guidelines do not indicate whether management should vary between cases with single or multiple foci of MI.
In this single-center study of patients with HER2+ T1micN0 BC, our objectives were to define patient and disease characteristics, treatment patterns, and clinical outcomes. We hypothesized that patients with many foci of MI would have worse survival outcomes, receive more aggressive treatment, and derive more clinical benefit from adjuvant chemotherapy and HER2-targeted therapy (CT/H2TT) than patients with single or rare/few foci of MI.
We searched the Memorial Sloan Kettering Cancer Center pathology database for patients with a histologically confirmed, final diagnosis of T1micN0 HER2+ (immunohistochemistry [IHC] 3+, or amplified by fluorescence in-situ hybridization [FISH]) BC on core needle biopsy (CNB) and/or excision between 1/1/2009–9/30/2023. We excluded patients who, at time of index diagnosis, had known metastatic disease including macro- or micrometastatic lymph node involvement (isolated tumor cells only were permitted), or macroinvasive BC (spanning >1 mm) either concurrently or within the preceding 20 years (Supplementary Fig. 1). The outcome of interest was iDFS, defined as time from date of surgery to event (distant or locoregional recurrence, new microinvasive or macroinvasive BC, death due to any cause). Patients who were alive and event-free were censored at the last known date of no evidence of disease (NED).
We identified 213 patients who met the above criteria. Clinicopathologic variables are included in Table 1. The median age at diagnosis was 55 years (range, 25–85 years). Two hundred twelve patients (99.5%) had DCIS-MI, and one (0.5%) had lobular carcinoma in-situ (LCIS) with lobular microinvasive carcinoma. The DCIS component was of high nuclear grade in 169 (79.3%) cases, with moderate, central, or marked necrosis in 196 (92.0%) cases. Two hundred eleven patients (99.1%) had 3 + HER2 staining of the microinvasive component, and two (0.9%) had 1–2+ staining with amplification by FISH. One hundred fifty-five patients (72.8%) had multiple foci of MI. Eighty-five patients (39.9%) underwent BCS, of whom 82 (96.5%) received adjuvant RT. One hundred twenty-eight patients (60.1%) underwent total mastectomy, of whom 48 (37.5%) underwent contralateral mastectomy. One hundred eighty-four patients (86.4%) underwent sentinel lymph node biopsy (SLNB). Thirty-five patients (16.4%) received adjuvant CT and/or H2TT, the predominant regimen being TH (n = 33, 94.3%). Fifty-two patients (24.4%) had ER/PR+ microinvasive carcinoma, of whom 40 (76.9%) received adjuvant antiestrogen therapy. Fifteen (7.0%) patients had ER/PR-low positive (1–10% of tumor cells) microinvasive carcinoma, of whom 4 (26.7%) received adjuvant antiestrogen therapy.
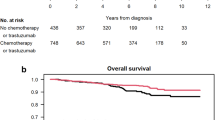
The median follow-up for the overall cohort was 5.5 years (range, 0.1–15.7 years). There were 13 iDFS events: two distant recurrences, one contralateral HER2-negative new breast primary, seven local recurrences, two non-BC related deaths, and one death due to unknown cause. Details regarding events are included in Table 2. Prognosis was excellent, with an estimated 5-year iDFS rate of 95% (95% confidence interval [CI]: 91, 98). We did not detect significant differences in iDFS between patients with single vs. multiple (≥2) foci of MI (p = 0.40), or between patients with ≥2 foci of MI who received vs. did not receive adjuvant CT/H2TT (p = 0.69) (Supplementary Fig. 2a, b).
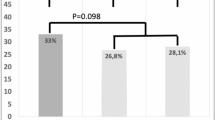
Upon review of the original pathology reports, we found that qualitative/semi-quantitative descriptors of DCIS-MI (e.g. “rare”, “few”, “multiple”, “at least X”) were used to communicate extent of microinvasive disease in 84 cases (39.4%). Prior studies have compared outcomes between patients with single and multiple (≥2) foci of MI6,9,10,11. However, we hypothesized that prognosis and treatment patterns would vary by number of foci (e.g. 2 vs. 12) such that comparisons of single vs. ≥2 foci would be too broad to capture these differences. To obtain more precise data, we retrieved available glass and/or digital slides to quantify exact number of microinvasive foci for 55 (25.8%) cases where the pathology report used a qualitative/semi-quantitative descriptor that did not allow us to definitively determine whether there were <5 or ≥5 foci (e.g. “multiple”, “at least 3”). Patients were subsequently classified as having 1–4 vs. ≥5 foci (Table 1). We were unable to categorize number of foci as <5 vs. ≥5 for seven cases (3.3%) due to unavailable material; these patients were removed from analyses comparing these subgroups. The distribution of cases by number of microinvasive foci is shown in Supplementary Fig. 3.
Of the 155 patients with multiple foci of MI, 65 (41.9%) had ≥5 foci. We did not detect a significant difference in iDFS between patients with ≥5 vs. 1–4 foci of MI (HR 0.73, 95% CI: 0.20, 2.72; p = 0.64; Fig. 1a). Compared to patients with 1–4 foci of MI, those with ≥5 foci were more likely to present with DCIS that was high grade (p = 0.01) and larger in size (p < 0.01), to be diagnosed with microinvasive disease on CNB (p < 0.01), to undergo SLNB (p < 0.01), and to receive adjuvant CT/H2TT (p < 0.01). More patients with ≥5 foci underwent total mastectomy; this difference was marginally significant (p = 0.06). There were no significant differences in rates of ER/PR positivity of the microinvasive component, antiestrogen therapy, adjuvant RT, and contralateral mastectomy by focality (Table 1). While these data suggest that patients with many foci receive more aggressive treatment than those with single/few foci, there are no prospective data to support escalation of therapy for this subgroup. Thus, we investigated whether adjuvant CT/H2TT is associated with an iDFS benefit.
The median follow-up of patients who received adjuvant CT/H2TT and those who did not were 4.0 (range, 1.3–11.1 years) and 6.1 years (range, 0.1–15.7 years), respectively, due to the adoption of adjuvant TH as SOC for stage I HER2+ BC starting in 2015. Few patients with 1–4 foci of MI (n = 9) received adjuvant CT/H2TT, precluding evaluation of potential clinical benefit from CT/H2TT in this subgroup. Among patients with ≥5 foci of MI, we did not detect a significant difference in iDFS (p = 0.35; Fig. 1b) between patients who received adjuvant CT/H2TT (n = 25) and those who did not (n = 39). However, we acknowledge the separation of iDFS curves (Fig. 1b) and in light of the small sample size, we cannot rule out that the lack of iDFS benefit is due to insufficient statistical power.
We characterized the frequency and nature of distant recurrences given the potential for curative salvage therapy in the case of locoregional recurrence (Table 2). Patient #1 had 9 foci of MI at time of diagnosis of HER2+/HR− DCIS-MI. She did not receive any adjuvant systemic therapy. She developed a HER2+/HR− recurrence in the liver 18.9 months after surgery. Patient #2 had 2 foci of MI at time of diagnosis of triple-positive DCIS-MI. She underwent BCS with re-excision for close margins. Five weeks after surgery, she began treatment with adjuvant TH, but paclitaxel was discontinued at approximately 9 weeks due to progressive neuropathy. She was found to have leptomeningeal disease 2 weeks after the last dose of TH (3.8 months after surgery). As she did not have neurologic symptoms at time of index diagnosis, imaging and evaluation of the central nervous system were not done as part of initial staging and thus, we are unable to rule out that she had occult metastatic disease. Overall, one patient in each subgroup (1–4 and ≥5 foci of MI) experienced a distant recurrence.
Prior studies have investigated the association between multifocality and clinical outcomes in DCIS-MI with mixed conclusions1,6,9,10,11,12. However, they are limited by categorization of foci as single vs. multiple6,9,10,11,12, lack of data regarding systemic therapy administered1,6,9,10,11, or the exclusion of patients who received chemotherapy from survival analyses12. Large database studies have investigated the benefit of adjuvant chemotherapy in stage I HER2+ BC. One study included 626 patients with T1mic disease and found that patients who received adjuvant chemotherapy had worse 5-year overall survival (OS) those who did not, but drug-specific data, including whether or not H2TT was administered, were unavailable13. Another study, which included 1184 patients with T1mic through T1c N0 disease, found that adjuvant H2TT ± CT was associated with an iDFS and OS benefit over observation, but only 14 patients had T1mic disease14. Finally, a retrospective analysis of 833 patients with T1mic HER2+ BC (96% pN0) and a median follow-up of 9.9 years did not detect an iDFS benefit associated with the receipt of adjuvant chemotherapy, but only six patients received H2TT15. In all three studies13,14,15, focality of MI was not reported. To our knowledge, our study is the first to assess treatment patterns of patients with HER2+ T1micN0 BC according to number of microinvasive foci beyond the overly limiting categorization of single vs. multiple, and to assess the frequency and clinical benefit of administering adjuvant TH to this important subgroup.
This study has several limitations. First, our sample size of 213 for a disease with excellent prognosis and a low event rate limits our statistical power, particularly for the analysis of CT/H2TT benefit in the even smaller subgroup of 65 patients with ≥5 microinvasive foci. Efforts are underway to confirm our findings in a large multi-center cohort. Second, its retrospective nature precludes definitive guidance regarding which patients should and should not receive adjuvant CT/H2TT. A prospective study would be necessary, but we believe this is unlikely to occur due to sample size considerations. Third, the median follow-up of patients who received CT/H2TT was short. We plan to update this analysis when we report results of the multi-center study.
At our center, patients with HER2+ T1micN0 BC and ≥5 foci of MI receive more aggressive treatment than those with single/few foci do. However, the risk of distant recurrence is low even in cases with many foci of MI. Our data do not currently support the practice of administering adjuvant CT/H2TT to this subgroup.
Methods
Study design, data sources, and variables
This study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB #24-010). The requirement to obtain written informed consent for participation in this retrospective study was waived given minimal risk to patients and removal of all identifying information. This study complied with all relevant ethical regulations regarding patient data, in line with ethical norms and standards in the Declaration of Helsinki.
The pathology laboratory information system (Cerner CoPathPlus) was queried to identify patients with DCIS-MI. Diagnostic and predictive/prognostic biomarker data were retrieved from patient respective data in the laboratory information system. Pathology report review was completed to extract relevant information. Clinical variables including age, sex, type of surgery, lymph node evaluation, time elapsed from surgery to event (locoregional or distant recurrence, death due to any cause) or last known NED status in the absence of an event, cause of death, site of recurrence, chemotherapy, HER2-targeted therapy, radiotherapy, and antiestrogen therapy were obtained by review of electronic medical records. Follow up of patient outcomes in our dataset are updated as of 2/1/2025.
Statistical analyses and methods
Patient and disease characteristics were tabulated by number of microinvasive foci (1–4 vs. ≥5). Continuous variables were summarized using median and interquartile range, and compared using Wilcoxon rank sum test. Categorical variables were summarized using frequency and percentage, and compared using Fisher’s exact test and Pearson’s chi-squared test. The outcome of interest was iDFS, defined as time from date of surgery to date of distant or locoregional recurrence, new microinvasive or macroinvasive BC, or death due to any cause. Living patients without disease recurrence were censored at time of last known NED status. The Kaplan–Meier method was used to estimate iDFS. The log-rank test was used to compare iDFS between groups. Cox regression was used to examine the association between focality and iDFS.
Data availability
Deidentified data will be made available upon reasonable request at the discretion of the corresponding author.
References
Shatat, L. et al. Microinvasive breast carcinoma carries an excellent prognosis regardless of the tumor characteristics. Hum. Pathol. 44, 2684–2689 (2013).
Hoda, S. A., Chiu, A., Prasad, M. L., Giri, D. & Hoda, R. S. Are microinvasion and micrometastasis in breast cancer mountains or molehills?. Am. J. Surg. 180, 305–308 (2000).
Parikh, R. R., Haffty, B. G., Lannin, D. & Moran, M. S. Ductal carcinoma in situ with microinvasion: prognostic implications, long-term outcomes, and role of axillary evaluation. Int J. Radiat. Oncol. Biol. Phys. 82, 7–13 (2012).
Shaaban, A. M. et al. The presentation, management and outcome of patients with ductal carcinoma in situ (DCIS) with microinvasion (invasion ≤1 mm in size)—results from the UK Sloane Project. Br. J. Cancer 127, 2125–2132 (2022).
Kim, M. et al. Microinvasive Carcinoma versus Ductal Carcinoma In Situ: A Comparison of Clinicopathological Features and Clinical Outcomes. J. Breast Cancer 21, 197–205 (2018).
Lee, S. Y. et al. Clinical significance of microinvasive breast cancer across the different subtypes and human epidermal growth factor receptor 2 expression levels. Breast Cancer Res. Treat. 200, 47–61 (2023).
National Comprehensive Cancer Network Guidelines. Breast Cancer (Version 3.2024), https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2024).
Tolaney, S. M. et al. Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer. N. Engl. J. Med. 372, 134–141 (2015).
Lee, S. Y. et al. Characteristics and risk factors of axillary lymph node metastasis of microinvasive breast cancer. Breast Cancer Res. Treat. 206, 495–507 (2024).
Matsen, C. B. et al. Extent of microinvasion in ductal carcinoma in situ is not associated with sentinel lymph node metastases. Ann. Surg. Oncol. 21, 3330–3335 (2014).
Si, J. et al. Multiple Microinvasion Foci in Ductal Carcinoma In Situ Is Associated With an Increased Risk of Recurrence and Worse Survival Outcome. Front. Oncol. 10, 607502 (2020).
Fang, Y. et al. Biologic behavior and long-term outcomes of breast ductal carcinoma in situ with microinvasion. Oncotarget 7, 64182–64190 (2016).
Parsons, B. M., Uprety, D., Smith, A. L., Borgert, A. J. & Dietrich, L. L. A US Registry–Based Assessment of Use and Impact of Chemotherapy in Stage I HER2-Positive Breast Cancer. J. Natl Compr. Cancer Netw. J. Natl Compr. Canc Netw. 16, 1311–1320 (2018).
Johnson, K. C. C. et al. The survival benefit of adjuvant trastuzumab with or without chemotherapy in the management of small (T1mic, T1a, T1b, T1c), node negative HER2+ breast cancer. npj Breast Cancer 10, 49 (2024).
Shin, Y. et al. Clinical outcomes of patients with HER2-positive microinvasive breast cancer. J. Clin. Oncol. 42, 544–544 (2024).
Acknowledgements
This study received funding support from the Judah Gubbay Memorial Fund. We would also like to thank our patients, their families, and all staff members who cared for them.
Author information
Authors and Affiliations
Contributions
A.D.S. developed the concept for this study. R.H. and M.G.H. performed an institutional pathology database search to generate the initial dataset. C.Y., R.H., A.K., E.B., and A.D.S. filtered this dataset according to pre-defined inclusion/exclusion criteria. C.Y., A.K., and A.D.S. performed data collection of clinical variables. R.H. and E.B. performed data collection of pathologic variables, including retrieval of glass and/or digital slides and precise quantification of microinvasive foci for select cases. C.Y., C.W., and Y.C. designed and performed the statistical analyses. All authors analyzed and interpreted the data. C.Y. wrote the manuscript. All authors read, made modifications to, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yeh, C., Han, R., Kulkarni, A. et al. Treatment patterns and clinical outcomes of patients with HER2-positive T1micN0 breast cancer: a single-center analysis. npj Breast Cancer 11, 47 (2025). https://doi.org/10.1038/s41523-025-00759-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41523-025-00759-2