Abstract
Transparent electro-optical neural interfacing technologies offer simultaneous high-spatial-resolution microscopic imaging, and high-temporal-resolution electrical recording and stimulation. However, fabricating transparent, flexible, and mechanically robust neural electrodes with high electrochemical performance remains challenging. In this study, we fabricated transparent (72.7% at 570 nm), mechanically robust (0.05% resistance change after 50k bending cycles) ultrathin Au microelectrodes for micro-electrocorticography (µECoG) using a hexadentate metal-polymer ligand bonding with an EDTA/PSS seed layer. These transparent µECoG arrays, fabricated with biocompatible gold, exhibit excellent electrochemical properties (0.73 Ω·cm2) for neural recording and stimulation with long-term stability. We recorded brain surface waves in vivo, maintaining a low baseline noise and a high signal-to-noise ratio during acute and two-week recordings. In addition, we successfully performed optogenetic modulation without light-induced artifacts at 7.32 mW/mm2 laser power density. This approach shows great potential for scalable, implantable neural electrodes and wearable optoelectronic devices in digital healthcare systems.
Similar content being viewed by others
Introduction
With increasing interest in developing multi-modal neurotechnology for a deeper understanding of our brain and treatment of neurological disorders, the need for transparent, flexible neural interfacing electrodes is greater than ever1,2,3,4,5,6,7,8,9,10. For example, high-resolution functional deep brain imaging such as three-photon microscopy with simultaneous electrophysiological signal recording could provide high spatiotemporal resolution investigation of the nervous systems11. Optical neural stimulation methods such as optogenetics and photothermal stimulation provide versatile control of neural cell activities with high specificity and spatial resolution10,12,13,14. These approaches must be accompanied by high-density, multichannel neural recording electrodes that are transparent to the illuminated light for reliable data analysis without false positive signals caused by photoelectric artifacts10,14,15,16,17,18,19. The transparent electrodes must be highly flexible for mechanically reliable long-term recordings in chronic implantation5,20.
However, fabricating highly transparent, high-density, and flexible electrodes is difficult because of the limitation of the materials in terms of good electrochemical characteristics and mechanical flexibility, as well as the incompatibility with mass-producible conventional microfabrication processes. To overcome the mechanical brittleness of the most well-known transparent electrode, indium tin oxide (ITO)21,22, conductive polymer-based flexible materials such as PEDOT:PSS14,16,23, polypyrrole24, and polyaniline25, 2-dimentional (2D) materials such as graphene5,26,27, and porous structures such as metallic nanowires6,28, carbon nanotube (CNT)15,29 and nanomesh17,18,30 are suggested. However, conductive polymers have low electrical conductivity compared to the other metallic electrodes, limiting the high-density array design. 2D materials and 1D network showed excellent transparency and flexibility. However, the wafer-scale deposition and high-resolution micropatterning of the transparent 2D and 1D materials are still not generally available in conventional microfabrication. Further, the nanomesh structures require low throughput nanoscale hole patterning using e-beam lithography or unconventional nanospheres, which is unsuitable for general mass production.
Recently, an ultrathin gold microelectrode, a new way of fabricating highly transparent, flexible, and conductive electrodes using a conventional gold thin film vacuum deposition process, has been proposed to overcome the aforementioned limitations31,32,33. This method involves pre-coating and patterning of biocompatible polyelectrolyte multilayers (PEM) as the vacuum-deposited gold nucleation-inducing polymer seed layer followed by ultrathin (<10 nm) Au film deposition. Owing to the electrostatic attraction and adsorption between the amine side chains of the polymer seed layer and the gold, the ultrathin gold formed highly continuous electrode characteristics despite its less than 10 nm thickness33. Because of the significant light penetration in less than 10 nm thick continuous metal film, these ultrathin gold electrodes reported good optical transparency as well. Despite the advantages, the previous works in ultrathin gold electrodes using PEM still showed limitations for high-resolution transparent µECoG arrays33. Since photolithography patterning of photoresist on the PEM layer was challenging due to the pattern smearing after photoresist development or swelling of the PEM layer during the photoresist development, the ultrathin gold microelectrodes in the previous work were fabricated by shadow mask lithography, limiting the minimum line width at 50 µm and tight line spacing for high-density microelectrode arrays. Therefore, there is a need to develop a thinner but more effective nucleation-inducing seed layer to form the ultrathin Au microelectrodes.
In transparent optoelectronic devices, ethylene diamine tetraacetic acid (EDTA) has been recently suggested as an inter-layer to improve the efficiency of solar cells by promoting electron transport in tin oxides34,35. In these works, the specialized hexagonal structure of the EDTA was demonstrated for efficiently capturing metal atoms in tin oxides with lots of chemical bonding parts36. Therefore, the stronger chemical interaction of the EDTA with metal ions can be a good candidate for a more efficient nucleation-induced seed layer than the PEM in ultrathin gold electrode fabrication, requiring fewer layers for a more stable photolithography process on top of the gold electrodes.
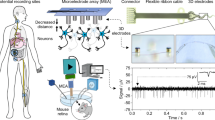
In this work, we fabricated an 8 nm-thick ultrathin gold microelectrode by forming an EDTA-metal complex on a 25-µm-thick flexible substrate (Fig. 1a). Despite single-layer coating, the EDTA/PSS seed layer complex effectively prevents the random migration of thermally evaporated Au particles because of its hexadentate ligand with a long-chain structure (Fig. 1b). This ultrathin Au electrode showed excellent conductivity, transparency, low electrochemical impedance, long-term stability in aqueous solutions, and stable mechanical reliability under tensile stress. By integrating this process with inkjet printing, we patterned PEDOT:PSS to less than 100 µm in diameter for fabricating a µECoG array with excellent electrochemical performance, while maintaining transparency. Further, we optically imaged the brain surface through the transparent µECoG while simultaneously recording mouse brain wave signals in vivo, which exhibited 3.3 µV of low root-mean-square noise (RMS) and 43.28 dB of high signal-to-noise ratio (SNR). We also successfully performed 2 weeks of recording without significant recording quality degradation. Finally, we quantified the optogenetic stimulation effect through our transparent electrode on ChR2 transgenic mice by comparing it with a commercial opaque electrode. We successfully confirmed more efficient optogenetic modulation through our transparent µECoG array without light-induced artifacts. The results confirm that our transparent electrode array can be sufficiently used for electro-optical neural interfaces (Fig. 1c).
a Overall layer structures of ultrathin metal-based flexible, transparent 16-channel neural electrodes. b Schematic for EDTA/PSS seed layer-based ultrathin gold film formation mechanism. EDTA (Orange color) forms a strong hexagonal bond with gold molecules, and PSS (Green color) forms a long chain structure to strengthen the bonding between EDTA and gold molecules. Each sphere represents a chemical part (Hydrogen-Sky blue, Oxygen-Red, Nitrogen-Purple, Gold-yellow, Sulfur-light green) c Multi-modal capability of transparent electro-optical µECoG arrays optical recording, simultaneous electrical two-week recording with light-induced artifact-free optogenetic modulation.
Results
EDTA/PSS seed layer-based transparent ultrathin Au film
We fabricated an ultrathin Au electrode based on the strong hexagonal bonding between Au and ethylenediaminetetraacetic acid/polystyrene sulfonate (EDTA/PSS) seed layer (Fig. 2a). Initially, the thermally evaporated 8-nm-thick Au on the bare PEN substrate exhibited isolated island structures of Au with non-conductivity. According to the Volmer–Weber film growth mechanism, this random migration of thermally evaporated Au atoms is attributed to the attraction between the deposited Au atoms themselves37,38. To overcome the random migration issue, we developed a simple yet efficient spin-coated EDTA/PSS seed layer on a bare PEN substrate surface to fabricate a uniform, ultrathin Au film. Figure 2b shows the chemical reaction mechanism of EDTA-inactive (deprotonated [EDTA]0-, pH-adjusted active (protonated [EDTA]4-) chemical structures and the formation of the Au/(EDTA/PSS) tricomponent system, respectively. EDTA at a low pH value does not dissolve in water (Supplementary Fig. S1a). The white precipitate EDTA solution had an inactive deprotonated chemical structure that cannot chemically interact with metal atoms. After adjusting the pH to 9–10 using NH3, the EDTA solution appeared as a clear liquid (Supplementary Fig. S1b). The active-protonated [EDTA]4- can strongly chemically interact with metal atoms and molecules39,40,41. The formation of chelating complexes played a significant role in the chemical interaction between the EDTA seed layer and thermally evaporated Au, wherein EDTA acted as a multidentate ligand and coordinated with the metal. Au-EDTA forms a hexadentate chelate in which six of its oxygen and nitrogen atoms surround the Au atoms, thereby creating a stable ring-like structure, which is helpful in forming ultrathin Au film by preventing random migration (Fig. 2b). Moreover, the EDTA/PSS plays a pivotal role in the remarkable synergy of the Au/(EDTA/PSS) tricomponent system: this triad encompasses EDTA as a robust chelating agent, PSS as a polymeric electrolyte with enhanced adhesion between substrate surface and tricomponent system, surface uniformity properties, and the PSS acts as a bridge to enhance the bonding affinity between EDTA and Au because of its charged sulfonate groups, and the Au as a noble metal forming the biocompatible neural electrodes.
a Schematic of EDTA/PSS seed layer-based ultrathin Au film formation. b Activation of EDTA for forming EDTA-metal complex. Refer to Fig. 1b for which colored circle represents which material. c SEM image of 8 nm Au/PEN film (Scale bar: 500 nm). d SEM image of 8 nm Au/(EDTA/PSS)/PEN (Scale bar: 500 nm). e FT-IR spectra for (i) EDTA/PEN, (ii) EDTA/PSS/PEN, and (iii) 8 nm Au/EDTA/PSS/PEN. f XPS spectra in the Au-4f region for Au/(EDTA/PSS)/PEN, Au/EDTA/PEN, Au/PEN. g Transmittance spectrum of Au/(EDTA/PSS)/PEN film with various Au thicknesses. h Electro-optical performance of Au/(EDTA/PSS)/PEN with different Au thicknesses.
Without this polymer seed coating layer, Au forms separate island structures that are non-conductive when we deposit 8 nm of Au on the bare PEN flexible substrate (Fig. 2c). However, the same 8-nm-thick Au forms a continuous film with fewer grain boundaries on the pre-polymer (EDTA/PSS)-coated PEN, thereby providing remarkable electrical conductivity (8 ± 0.5 Ω/sq, n = 20) (Fig. 2d). To deeply understand the chemical bonding between Au and the polymer seed layer, we took a Fourier transform infrared spectra (FT-IR) on all the samples (Fig. 2e). EDTA/PEN shows (i) a broad peak at 2966−1 (O-H stretch) and an intense peak at 1350−1 (O-H stretch), attributed to the carboxylic components of EDTA. The strong peak at 1609−1 (N-H bending) and medium intense peak at 1202−1 (C-N stretching) were attributed to the amine components of EDTA. Further, (EDTA/PSS)/PEN (ii) also showed intense peaks at 1609−1 (N-H bending), which was attributed to the amine components of the EDTA seed layer. The O-H stretching peak shift from 2966−1 to 3416−1 is attributed to the active carboxylic group of EDTA/PSS. The 8 nm Au/(EDTA/PSS)/PEN (iii) showed intense peaks with a shift in O-H stretching from 2966−1 to 3416−1, and C = O stretching from 1704−1 to 1712 cm−1. These shifts can be attributed to the reduction of Au and the capping of the evaporated Au nanolayers on the polymer seed layer. The chemical binding energy between EDTA, PSS, and Au is a manifestation of the intricate electrostatic and chelation interactions. This manifests as a cohesive force that strongly anchors EDTA/PSS to the Au surface. The EDTA/PSS complex exhibits a greater negative shift in bonding with Au than with only EDTA (Fig. 2f). This phenomenon contributed not only to the functionalized chemical bonding part of EDTA but also to the (-) sulfur group of PSS with long chains, which help form a dense polymer-metal complex. Compared to the multilayer-by-layer polyelectrolyte (PEI/PSS)5 coating, which we used in our previous work, we induce a greater negative shift in bonding with the EDTA/PSS composite33. Therefore, considering that EDTA/PSS composited coating only requires one bi-layer, which is significantly less than a minimum of 10 layers of the previous polyelectrolyte multilayer coating (PEI/PSS)5, EDTA/PSS polymer seed layer can significantly simplify the overall fabrication processes while being able to provide more efficient formation of the ultrathin metal film.
Transmittance was leveled depending on the Au thicknesses of 6–15 nm (Fig. 2g). The electro-optical performance for various ultrathin Au thicknesses shows a trade-off relationship between sheet resistance and transmittance (Fig. 2h). Finally, we selected 8 nm of Au as the optimized thickness, which shows 8 Ω/sq sheet resistance and 73% transmittance at 530 nm wavelength. We decreased the sheet resistance of 8 nm Au/(EDTA/PSS)/PEN film from 8 Ω/sq to 6.2 Ω/sq after thermal treatment (150 °C, 20 min) by improving the bonding between Au structures. The sheet resistance did not significantly decrease over 20 min of thermal treatment (Supplementary Fig. S2).
Mechanical robustness of the transparent, ultrathin Au Film
To apply our 8 nm Au/(EDTA/PSS)/PEN film for in vivo neural recording, we verified the mechanical robustness of our ultrathin Au film by performing stress analysis and tensile strain flexibility tests. The stress-strain (SS) curve is obtained from the tensile test of the (EDTA/PSS)/PEN film to define the elastic/plastic boundary point (Fig. 3a, Supplementary Fig. 3a). The 120-µm-thick (EDTA/PSS)/PEN substrate is operated in the elastic region up to ~18 MPa (~2.5% strain) with Young’s modulus of 662 MPa and shows a linear increase in the elastic region. Further, to verify the electrical breakdown point of ultrathin 8 nm Au/(EDTA/PSS)/PEN film, we measured electrical resistance change depending on the various strains with comparison samples Au/Ti (50 nm/5 nm) PEN and 50 nm Au/(EDTA/PSS)/PEN (Fig. 3b, Supplementary Fig. 3b). The 8 nm Au/(EDTA/PSS)/PEN film exhibited linear and small resistance increase (~15%) until the mechanical fracture point of the PEN substrate (~150% strain). Also, 8 nm Au/(EDTA/PSS)/PEN shows electrical stability within the elastic region (~2.5%), which is less than 0.2% of the resistance increase. This phenomenon can be attributed to stress relaxation caused by the low elastic modulus of the EDTA/PSS polymer layer between the ultrathin 8 nm Au film and the PEN substrate. As a counterexample, the resistance change of Au/Ti (50 nm/5 nm)/PEN was significantly higher compared to the 8 nm Au/(EDTA/PSS)/PEN film, which shows a 56% resistance increase on 50% of applied stress. Although 50 nm Au/(EDTA/PSS)/PEN film showed a smaller resistance increase than the Au/Ti (50 nm/5 nm)/PEN from 0 to 120% strain, it still showed a much higher resistance increase compared to the 8 nm Au/(EDTA/PSS)/PEN film. We further analyzed the SEM images of three different metal films after 50% of tensile strain. As a result, 8 nm Au/(EDTA/PSS)/PEN film shows small propagation of micro-cracks which originated from the PEN substrate. On the other hand, the 50 nm Au/(EDTA/PSS)/PEN film shows a big propagation crack, and Au/Ti (50/5 nm)/PEN film showed serious delamination (Supplementary Fig. 4a–d). Further, we implemented stress analysis using a finite-element method based on the extracted mechanical properties to verify the flexible bending safety margin (Fig. 3c). The tensile stress applied to the PEN substrate was calculated based on the radius of the curvature. The substrate can elastically deform up to a bending radius of ~4.2 mm; the minimum operational bending radius was set to 5 mm, considering safety margins. The two-dimensional stress distribution of the (EDTA/PSS)/PEN substrate was simulated at a radius of curvature of 5 mm (Fig. 3d). We found that 8 N/mm2 of maximum tensile stress is applied to the top of the substrate, which is within the allowable elastic deformation range as shown in the inset of Fig. 3a. Also, there was no significant change in resistance when the radius of curvature was reduced from 50 to 5 mm in the simulation (Fig. 3e). Therefore, we verified that the device could operate without a significant change in resistance within the chosen bending condition (~5 mm).
a stress-strain curve from the tensile test with (EDTA/PSS)/PEN film. b Electrical resistance changes during the strain test on the various films. c Calculated stress and strain applied to the substrate according to the various radius of curvature of 8 nm Au/(EDTA/PSS)/PEN film. d Maximum tensile stress of 8 nm Au/(EDTA/PSS)/PEN film. e Resistance change during tensile stress on the film with various bending radii for 8 nm Au/(EDTA/PSS)/PEN film. f Resistance changes during various bending cycles. g–i SEM images of each metal film before/after 50,000 times of bending.
Finally, we performed a cyclic bending test at a previously determined bending radius of 5 mm to test the performance degradation caused by the cyclic fatigue failure (Fig. 3f). The 8 nm Au/(EDTA/PSS)/PEN film shows less than 0.05% resistance increase even after 50k bending cycles due to the strong hexagonal bonding with the polymer seed layer and small applied stress on 8 nm ultrathin film. In contrast, both 50 nm Au/(EDTA/PSS)/PEN and Au/Ti (50 nm/5 nm)/PEN exhibited critical and linear resistance increases of 26.8% and 36.4%, respectively, after 50k bending cycles. We believe it is because of the larger thickness of the gold films, which leads to higher tensile stress and, thus, more accumulated fatigue on the metal film. The most well-known transparent 50 nm ITO/PEN film shows immediate failure (~100% resistance increment) in less than 50 bending. After 50k bending, the 8 nm Au/(EDTA/PSS)/PEN film does not show significant cracks on the SEM image (Fig. 3g), whereas the 50-nm-thick Au/(EDTA/PSS)/PEN film and Au/Ti (50 nm/5 nm)/PEN film are severely damaged (Fig. 3h, i). These are inferred from the stress concentrations occurring in many microcracks and gaps in the thick electrodes.
Electrochemical properties of µECoG array
For more efficient local field potential (LFP) recording, lower impedance at low frequency, 100 Hz or below, is required4,10,42. Because single metal electrodes often cannot satisfy the requirement, functionalized electrode materials have been used to improve the impedance for successful LFP neural recording. Pt8, Ti2C3 MXene43, IrO217 and nanoporous structures44 are good examples of lowering impedance and also improving charge transfer characteristics such as cyclic voltammetry (CV) for neural stimulation, thereby enabling simultaneous electrical stimulation and neural recording. However, there is a trade-off between the electrode performance and optical transparency for these functional electrode materials.
We fabricated two versions of transparent 16-channel µECoG arrays for the LFP recording of the brain surface based on the pre-characterized 8 nm Au/(EDTA/PSS)/PEN ultrathin metal film. Firstly, we fabricated an ultrathin Au-only µECoG array based on the 8 nm Au/(EDTA/PSS) structure with a 100 µm diameter. Secondly, to further improve electrochemical characteristics while maintaining optical transparency, we additionally inkjet-printed a transparent PEDOT:PSS conductive polymer on the 16 microelectrodes of the Au 8 nm/(EDTA/PSS) µECoG array without any photomask (Fig. 4a, b). The detailed fabrication steps are provided in Supplementary Fig. 5. The PEDOT:PSS ink jetted stably without any satellite drops (Supplementary Fig. 6a). Owing to the 78.32° of high contact angle of gold film, we successfully printed a 3 × 3 dot pattern with a 25 µm drop spacing of PEDOT:PSS ink without any spreading to SU-8 passivated region while maintaining high throughput in the vertical direction (Supplementary Fig. 6b). The 5 and 10 printing layers of the PEDOT:PSS dot patterns show 80 µm and 140 µm diameter and concave structure with 900 nm, 1500 nm peak thickness, and 500 nm and 900 nm averaged thickness, respectively (Supplementary Fig. 6c). Due to the designed diameter of our µECoG as 100 µm, we chose 80 µm as the diameter of printed PEDOT:PSS. The drop-on-demand inkjet printing-based patterning strategy can easily micro-pattern the conductive polymer at room temperature without any photomask. Also, complicated conventional microfabrication processes such as etching45, electroplating17, and parylene-C induced peel-off technique16,46 are not required. Our 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG array shows 55% and 43% transmittance at the 473 nm (blue) and 580 nm (amber) wavelengths, which are frequently used wavelengths for optogenetic modulation and optical imaging (Fig. 4c)10,17,47,48. Owing to the transparency of both ultrathin 8 nm Au and the PEDOT:PSS, µECoG shows adjustable transparency, which is high enough to read the letters located behind the µECoG array (Fig. 4c, Supplementary Fig. 7).
a Schematic of inkjet printed PEDOT:PSS on the ultrathin Au based transparent µECoG array. b Optical image of the PEDOT:PSS inkjet patterned ultrathin Au µECoG array (Scale bar: 100 µm). c Transmittance spectrum of PEDOT:PSS printed transparent µECoG array (500 nm: average thickness, 900 nm: peak thickness). Inset images show representative samples of the two different regions. And the 473 nm (blue) and 580 nm (amber) wavelength ranges used for optogenetics modulation are displayed. d Magnitude of Bode plots of representative EIS data. The thickness of PEDOT:PSS reported as the averaged value. e AFM scanning results of Au/Ti (50 nm/5 nm) (left) and 8 nm Au/(EDTA/PSS) (right) film (Scale bar : 200 nm). f Phase angle in the Bode plots of the representative EIS. g Cyclic voltammetry results of 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG array at various voltage scan rates. h Charge storage capacity of µECoG arrays at various voltage scan rates. i Long-term stability of µECoG array in PBS immersed environment (Totally 4 weeks, 37 °C) j Comparison of area-normalized electrochemical impedance and RMS noise among recently developed transparent/opaque microelectrodes for neural recording. (Minimum noise detection level of Intan recording: 2.4 µV).
The electrochemical properties of both 8 nm Au/(EDTA/PSS) µECoG and PEDOT:PSS interfaced 8 nm Au/(EDTA/PSS) µECoG were also characterized. Even without the printed PEDOT:PSS, the 8 nm Au/(EDTA/PSS) µECoG shows 112 kΩ of impedance at 1 kHz, which is 3.8 times lower compared to the opaque Au/Ti (50 nm/5 nm) µECoG at 1 kHz (Fig. 4d). To more deeply understand the effect of impedance improvement, we scanned the 8 nm Au/(EDTA/PSS) film and Au/Ti (50 nm/5 nm) film using atomic force microscopy (AFM) (Fig. 4e, Supplementary Fig. 8a, b). The surface roughness of the 8 nm Au/(EDTA/PSS) film is 7.26 nm (Rq), which is 6 times higher than that of the Au/Ti film. A larger surface roughness induced a larger double-layer capacitance due to the larger effective surface area, thereby contributing to lowering the impedance33. 800 nm-thick inkjet-printed PEDOT: PSS-based transparent µECoG showed 9.13 kΩ of impedance at 1 kHz, which is 53 times and 14 times lower than the 1 kHz impedance of Au/Ti and 8 nm Au/(EDTA/PSS) µECoG, respectively (Fig. 4d). Both 500 nm and 800 nm thick PEDOT:PSS added µECoG showed nearly no differences in the impedance. The baseline impedance of the flexible transparent electrodes saturated in the frequency range above 200 Hz, and is directly related to the electrical resistance of the ultrathin Au film. Therefore, optimizing the sheet resistance shown in Fig. 2h while maintaining the electrode’s transparency was crucial for reducing the electrode’s electrical and electrochemical impedance. The PEDOT:PSS ultrathin Au µECoGs showed less capacitive characteristics, while the Au/Ti showed the most capacitive characteristics over the entire frequency range, and 8 nm Au/(EDTA/PSS) µECoG showed intermediate characteristics (Fig. 4f). There are several reasons why PEDOT:PSS printed on 8 nm Au exhibits low impedance characteristics. The PEDOT:PSS interface coating induces a larger electroactive surface area (ESA), which increases the area-proportional double layer capacitance (Cdl) compared to non-porous metal electrodes, thereby lowering the overall impedance of the µECoG. Additionally, the charge transfer resistance (Rct), which is connected in parallel with Cdl, is also reduced due to the increase in charge on the surface induced by the larger ESA. This is further enhanced by the material properties of PEDOT:PSS, which facilitate more efficient oxidation-reduction reactions compared to bulk gold. Moreover, PEDOT:PSS promotes rapid and efficient ion diffusion at the interface, as related to the Warburg diffusion element (Zw), which helps lower the Faradic charge transfer resistance, even at low frequency regions17.
To verify the feasibility of electrical stimulation, we performed cyclic voltammetry (CV) testing with a 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG at voltage scan rates of 20–1000 mV/s (Fig. 4g). There was no significant degradation of the CV area after 1000 times of voltage cycling (Supplementary Fig. 9). In addition, due to the wider window and higher peak current density than those of the opaque Au/Ti and 8 nm Au/(EDTA/PSS) µECoGs, 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG showed 117.0 times higher charge storage capacity (CSC) than the Au/Ti µECoG, and 28.1 times higher than 8 nm Au/(EDTA/PSS) µECoGs at 20 mV/s scan rate (Fig. 4h). The 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG shows 945.5 µC/mm2 of CSC at a voltage scan rate of 1000 mV/s, 10.9 times higher than previously reported graphene µECoG work (87.8 µC/mm2) that performed electrical stimulation with spatiotemporal neural response optical imaging on cortex of mouse in −0.8 V to 0.6 V voltage scan range26. Moreover, 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG shows less than 10% change of impedance after immersing in a phosphate-buffered saline (PBS) solution at 37.5 °C for 4 weeks (Fig. 4i). PEDOT:PSS interfaced surface showed no serious delamination after the 4 weeks in the PBS solution (Supplementary Fig. 10). Stabilization of PEDOT:PSS interface was achieved by adding 0.2% of (3-Glycidy loxypropyl) trimethoxysilane (GOPS) crosslinker in the PEDOT:PSS ink49. We expected that the 10% impedance variation of µECoG was from the hydrate swelling property of PSS as previously reported50.
Finally, we compared the root mean square (RMS) noise and area-normalized impedance of our transparent µECoG with other in vivo MEAs (Fig. 4j)4,7,14,17,21,27,30,33,51,52. 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG shows an average impedance of only 0.73 Ω·cm2 and a 3.3 µV of RMS noise, which is close to the baseline noise floor of the electronic recording setup (~2.4 µV), and the best among the transparent in vivo MEA group (blue colored data in Fig. 4j). By comparing the opaque in vivo MEA group (red colored data in Fig. 4j), 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG shows only 7 times higher impedance compared to the inkjet printed nanoporous Au interface which is reported as the lowest area-normalized impedance (0.1 Ω·cm2 at 1 kHz) in all the MEAs. In sum, our 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG still belongs to the best performance group while providing optical transparency.
In vivo epicortical recording with µECoG array
Performing waveform analysis with in vivo brain recording signals is important for predicting and analyzing animal behavior in response to external stimuli and neurological disorders such as Alzheimer’s and Parkinson’s diseases4,8,10,14,15,53. Additionally, the flexibility of the electrodes is also important depending on the targeted recording parts, such as the brain surface, muscles, and various organs5,7,10,14. To test the feasibility of using the optimized transparent, flexible, ultrathin Au µECoG, we performed an in vivo brain surface recording. We placed the transparent 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS), µECoG array onto the parietal cortex of the mouse brain and then recorded the surface of the cortex signal while optically imaging the vessels of the brain below the µECoG array (Fig. 5a). In contrast, optical imaging was impossible with an opaque 200 nm platinum µECoG array (Fig. 5b). After integrating a µECoG array with a commercial electrophysiological recording system (Supplementary Fig. 11), the area-normalized impedance of each 8 nm Au/(EDTA/PSS) and 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG array were 3.4 and 75.3 times lower than that of a 200 nm platinum µECoG array at 100 Hz and 1 kHz despite their optical transparency (Fig. 5c).
a Optical images of transparent 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG array (right zoom, scale bar: 100 µm) on mouse brain with a reference electrode: 8 nm Au/EDTA-PSS electrode (left zoom, scale bar: 500 µm). b Optical images of 200 nm thickness of platinum (opaque) µECoG array on mouse brain (scale bar: 100 µm). c Impedance measurement results of various µECoG arrays at 100 Hz and 1000 Hz frequencies after integration with a recording system. d Local field potentials (LFPs) in four representative channels of 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG array after 200 Hz of low-pass filtering. e Time-domain analysis of the short-time Fourier transform and f Power spectral density (PSD) analysis in the frequency domain for each µECoG array recording. g Image of the mouse with the transparent µECoG array implanted 2 weeks after surgery, along with in vivo recordings from the transparent and opaque µECoG arrays. h Root mean square noise of the transparent and opaque µECoG arrays after surgery. i, j PSD analysis of the transparent and opaque µECoG arrays over a period of 2 weeks.
We recorded 16 channels of local field potential (LFP) for 2 min with 200 Hz of lowpass filtering using the two different µECoG arrays. Note that we recorded the brain wave of the same mouse, but not simultaneously. The approximate interval between the two different µECoG array recordings is 10 min. The exemplary 2-second window of the recorded signals for 4 channels using our 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG array is displayed in Fig. 5d. The peak-to-peak voltage scale and waveform of the recorded LFP signals showed similarity with other groups’ works of in vivo brain signal recording4,10. All channels show similar LFP waveforms on the same time scale (Supplementary Fig. 12a). The recorded signal with 200 nm platinum µECoG also shows similar LFP waveforms in the same time window (Supplementary Fig. 12b)
Next, we analyzed the recorded signals by color-mapped power spectra density (PSD) of both 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG array and 200 nm platinum µECoG array (Fig. 5e). During in vivo recording for 60 s, spontaneous peak-to-peak signals of 400 to 800 µV are observed with excellent SNR (~43.28 dB) in both the 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG array and the 200 nm platinum µECoG array by calculating RMS of the peak-to-peak signals with respect to the baseline RMS noise (Supplementary Fig. 12c)54. The spontaneous spikes detected during the in vivo brain surface recording might have been caused by the injected ketamine drug to anesthetize the mouse before in vivo neural recording to minimize the noise artifact from the movement of the mouse55,56. To additionally verify whether the recorded spikes are electrical noise or not, we performed the recording in PBS to immerse both 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG and 200 nm platinum µECoG for 22 s, and there was no spontaneous spikes and baseline fluctuation on both µECoG (Supplementary Fig. 13). The power spectral density (PSD) of neural signals shows sharp peaks simultaneously with spontaneous spikes and low-frequency oscillations (<6 Hz) on both recorded signals from each µECoG (Fig. 5e). However, the waveforms recorded from each µECoGs were slightly different, possibly because the two µECoG recordings were not performed simultaneously, and the recording electrode sizes are different, 100 µm diameter for 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG and 200 µm diameter for the commercial platinum µECoG. We additionally analyzed the PSD of the signal in the frequency domain (Fig. 5f). 500 nm PEDOT:PSS/8 nm Au/(EDTA/PSS) µECoG and 200 nm platinum µECoG show very similar PSD distribution, and both recorded signals showed high delta frequency oscillation (0.5–3 Hz) without any significant power differences in all frequency ranges.
To verify the long-term stability and recordability of our transparent µECoG array in vivo, we performed recordings over a 2-week period using both the transparent and opaque µECoG arrays (Fig. 5g). Both µECoG arrays showed stable brain wave recording performance with minimal degradation of RMS noise levels even after 2 weeks. Both arrays exhibited an approximately 3-fold increase in RMS noise after 7 days, which is expected as surgical effects resulting from the recovery process of surrounding tissues (Fig. 5h). At day 14, both µECoG arrays showed less than 10% of RMS noise increase compared to day 7, while still exhibiting suitable values for in vivo recording (transparent: 11.2 µV, opaque: 8.5 µV), despite the transparent µECoG array being four times smaller in area than the opaque µECoG array. Frequency band analysis of the recorded signals revealed that the transparent µECoG array exhibited an overall increase in PSD across all frequency bands after day 7, but the increase was minimal, less than one order of magnitude, and there was no significant difference of PSD between day 7 and day 14 (Fig. 5i). The opaque µECoG array showed a slight increase in PSD in the frequency range above 10 Hz after 7 days compared to day 0, and this trend was maintained until day 14 (Fig. 5j). Both µECoG arrays exhibited similar PSD values within the same frequency range, with no significant differences, confirming that both arrays are suitable for two-week in vivo brain signal recording (Supplementary Fig. 14).
Neural recording and 2D mapping using optogenetics
To further verify the adjustability of our transparent µECoG array as an electro-optical neural interface, we performed optogenetic modulation using channelrhodopsin-2 (ChR2), photo-activated cation channel,-expressing mice using adeno-associated viral (AAV) vector. We placed our transparent µECoG array on the AAV-ChR2-injected brain region and irradiated it with a 473-nm wavelength blue laser using a pulse train of 50 on/off cycles (15 ms/500 ms) at a 7.32 mW/mm² of laser power density (Fig. 6a). We also conducted the same procedure with an opaque µECoG array on the AAV-ChR2-injected brain region at the same laser power density (Fig. 6b). After repeatedly irradiating the blue laser on the transparent µECoG array region, we observed up to 800 µV of evoked brain surface potential changes at the laser-aligned stimulation spot (Fig. 6c). However, the maximal response with opaque µECoG was limited to 350 µV, indicating that the opaque µECoG array shows lower optogenetic modulation performance compared to the transparent µECoG array. This highlights the advantage of the transparent µECoG array in terms of optogenetic modulation efficiency at the same laser power density (Fig. 6d).
a, b Images of the optogenetic modulation setup with the transparent µECoG array and opaque µECoG array on the ChR2 transgenic mouse brain. The distance between the laser fiber and the electrode array is 1 mm, and optogenetic modulated was performed at 7.32 mW/mm² of laser power density. c, d 2D electrophysiological mapping of optogenetic stimulation from the 16-channel transparent µECoG array and 16-channel opaque µECoG array, showing the exact arrangement of the arrays and scale information. The color maps display voltage changes (ΔVoltage) calculated the differences between before and after 15 ms of 473 nm laser irradiation, with a potential range of (−100–800 μV). The 473 nm pulsed laser was modulated with 50 cycles of on/off (15 ms/400 ms). e Optically evoked neural signals from the ChR2 transgenic mouse at various laser power densities (2.35, 4.07, 7.32 mW/mm²) for both the transparent µECoG array and the opaque µECoG array. f Neural signals from the EGFP injection region (control) under the same 473 nm laser irradiation.
Additionally, to verify the efficiency of optogenetic modulation and quantify light-induced artifacts, we performed optogenetic modulation at various laser power densities (Fig. 6e). At a laser irradiation power density of 2.35 mW/mm², the transparent µECoG array showed about 150 µV evoked voltage changes on the transgenic region, while no evoked voltage responses were observed in the opaque µECoG array. At 4.07 mW/mm² and 7.32 mW/mm² laser power densities, the transparent µECoG array showed higher levels of evoked voltage changes (600 µV and 750 µV, respectively), while the opaque µECoG array exhibited lower changes of 150 µV and 350 µV, respectively. These results indicate that the transparent µECoG array is much more efficient for optogenetic modulation at all examined laser power density range, with a better sensitivity. Moreover, at 4.07 mW/mm² and 7.32 mW/mm² laser power densities, the opaque µECoG array showed repeated positive light-induced artifacts after laser irradiation, which interferes the precise quantification of optogenetic modulation results. To verify whether the induced voltage changes were due to optogenetic modulation or light-induced artifacts, we performed repeated laser irradiation on both the transparent and opaque µECoG arrays at a laser power density of 7.32 mW/mm² in the AAV-Enhanced Green Fluorescent Protein (EGFP)-injected brain region, which does not react to the 473 nm wavelength laser (Fig. 6f). Brain surface signals recorded with the transparent µECoG array showed no light-induced artifacts with the repeated laser irradiation, whereas the brain signals recorded with the opaque µECoG array exhibited repeated light-induced artifacts. The reason why the photoelectric artifact is larger in the EGFP control region (600–1200 µV) compared to the ChR2 injection region (300–400 µV) under opaque ECoG conditions is that the EGFP control region does not respond to the blue laser (473 nm wavelength). Therefore, the negative voltage change from optogenetic modulation does not cancel out the existing photoelectric artifact in the EGFP control region, resulting in a larger voltage change compared to the ChR2 injection region.
To further investigate light-induced artifacts in both transparent and opaque µECoG arrays, we performed baseline-free recordings in a PBS buffer solution with the laser placed 6 mm distance from each µECoG array with 3 mm diameter laser irradiation spot (Supplementary Fig. 15a). The transparent µECoG array showed 2–4 times lower light-induced artifacts compared to the opaque µECoG array from 0.75 to 68.9 mW/mm2 blue laser power density during repeated turn on/off (20/80 ms) cycles (Supplementary Fig. 15b, c). Generally, optogenetic modulation is performed in a power density range of 1–30 mW/mm9,15,57,58. At 7.02 mW/mm2, the transparent µECoG array shows small (~5 µV) light-induced artifacts owing to its transparency, whereas the opaque µECoG array shows a 20 µV of artifacts at the same laser power density (Supplementary Fig. 15d). At 13.8 and 20.9 mW/mm2, the transparent µECoG array shows 10 µV and 23 µV of light-induced artifacts, which are 2–3 times lower compared to the opaque µECoG array (30 µV at 13.8 mW/mm2, 50 µV at 20.9 mW/mm2). The same trend was observed during 580 nm amber laser irradiation on both µECoGs (Supplementary Fig. 15e).
In previous studies of light-induced artifact characterization on PEDOT:PSS/Au bilayer nanomesh electrodes, there were no significant differences in the light-induced artifacts on both PEDOT:PSS/Au bilayer nanomesh and Au nanomesh at the same laser power density (5–20 µV), which means that PEDOT:PSS does not mainly contribute to the light-induced artifact17. The same results are reported on all PEDOT:PSS fabricated µECoG, which reports that there was no light-induced artifact even at high laser power density (80.88 mW/mm2)14. Since our transparent µECoG consists of 500 nm PEDOT:PSS/8 nm Au, the light-induced artifacts mainly originate from the 8 nm Au, not the 500 nm PEDOT:PSS. Moreover, owing to the transparency of our 8 nm ultrathin Au film, the light-induced artifact is much smaller than the thick Au film. Our transparent µECoG did not show the light artifact at 0.75 mW/mm2, while opaque µECoG still showed 15 µV of artifact at the same laser power density. For the most representative optical recordings, such as diffuse optical tomography59 and calcium imaging11,16, typical excitation laser power density is around 0.13–0.23 mW/mm2, which means our transparent µECoG is transparent enough to perform these optical recordings with simultaneous high SNR electrical recording without any light-induced artifacts.
Inflammatory response to µECoG array
In vivo electrophysiological recordings were conducted for two weeks post-surgery, followed by immunohistochemical analysis using Ionized calcium-binding adapter molecule 1(Iba1) and Glial fibrillary acidic protein(GFAP), markers specific for microglia and astrocytes, respectively, to assess inflammatory responses (Fig. 7a, b Supplementary Fig. 16a, b). Sections of the implanted cortex were compared with those of the contralateral, non-implanted cortex. Iba1 staining revealed no significant differences between the implanted and non-implanted cortices for both the transparent µECoG array and the opaque µECoG array, indicating the absence of microglial activation and suggesting minimal or negligible inflammation. Conversely, GFAP staining demonstrated a more pronounced astrocyte response in the implanted cortex for both electrode array types, implying that the physical insertion of the µECoG array induced mild tissue damage. Altogether, while GFAP expression exhibited marked differences, the absence of significant variation in Iba1 staining between the implanted and non-implanted cortices suggests that µECoG array implantation results in minor tissue damage and supports the biocompatibility of the transparent µECoG array.
a DAPI, Iba1, and GFAP staining in the implanted cortex with the transparent µECoG array, showing markers for cell nuclei, microglia, and astrocytes, respectively. Scale bar = 100 µm. b DAPI, Iba1, and GFAP staining in the contralateral, non-implanted cortex for the transparent µECoG array, illustrating baseline levels of microglia and astrocytes (scale bar = 100 µm).
Discussion
In this work, we fabricated flexible, transparent, conductive, and mechanically robust ultrathin Au µECoG arrays by introducing hexagonal chemical bonding between (EDTA/PSS) and ultrathin 8 nm Au film. With the additional inkjet printing of PEDOT:PSS on µECoG interface directly, we successfully improved the impedance of the µECoG and its long-term stability in an aqueous solution while maintaining transparency. Our µECoG successfully recorded neural signals from the brain surface of the cortical area, similar to the waveforms of commercial 200 nm platinum µECoG, and showed a high SNR value and long-term stability during 2 weeks of recording. Finally, we performed optogenetic modulation with the transgenic mouse. The optogenetic stimulation effect through our transparent µECoG array was more efficient than the opaque electrodes at the same laser power density without light-induced artifacts. With the simple wafer-scale fabrication processes, our ultrathin 8 nm Au electrode arrays will be a core technology as flexible, transparent bioelectronics such as implantable neural probes along with high expandability to flexible transparent optoelectronics such as display panels, solar cells, and smart windows.
Methods
EDTA and PSS hybrid composite ink mixing
EDTA ink: 1 M concentrated EDTA solution was prepared in 100 ml of volume. The optimization chemical composition is 18.60 g of 4 N EDTA (Sigma-Aldrich) mixed into 80 ml Milly-Q water and the solution was vigorously stirred by a magnetic stirrer. Then we slowly added the Ammonium solution (NH3(aq), 25%) up to clear the solution apprentice and adjust the pH around 9 to 10. The final transparent solution was filtered by a 1 µm PALL filter to remove any dust and contaminated particles. PSS ink: 10 grams of 4 N PSS and 0.5 grams of 4 N NaCl microparticles (Sigma-Aldrich, South Korea) were dissolved in 50 ml volume of water solvent. Once dissolved, add more water to bring the total volume up to 100 ml. EDTA and PSS hybrid composite ink: The EDTA and PSS separately prepared each solution was mixed with a 3:1 (EDTA: PSS) ratio in a 100 ml bottle at room temperature. The mixed hybrid composite solution was vigorously stirred at 400 RPM for 5 h by a magnetic stirrer, and the final solution was filtered by a 1 µm PALL filter to remove any dust and contaminated particles. The fresh hybrid composite solution always shows better performance than the long-time storage mixed solution.
PEDOT:PSS ink optimization
We mixed PEDOT:PSS (Clevios PH 1000) solution and DI at a 1:1 ratio and added 5 wt% of Ethylene glycol (EG) (Sigma-Aldrich) to induce conductivity by separating PSS from PEDOT. We also added 0.2 wt% of GOPS as a crosslinker to enhance the durability of the transparent µECoG array in an aqueous solution. The prepared ink was stirred at 25 °C, 200 rpm, for 6 h with a magnetic bar for the uniform mixture. The viscosity of our ink was 9.55 cPs (DV3T, Brookfield), which is the reasonable value for inkjet printing.
Fabrication of transparent µECoG array
We cleaned the 25-µm thick PEN substrates by sonication in acetone and IPA for 10 min. Then, we spin-coated EDTA/PSS to form a uniform polymer seed layer, following the patterning of the negative photoresist (NLoF 2035, 70 mJ/cm2). Both EDTA/PSS and NLoF were spin-coated at 3500 rpm for 40 s. 8 nm of Au was thermal evaporated 0.1 Å/s (Korea Vacuum Tech, South Korea) and patterned by lift-off. We passivated the electrode with 700 nm thick biocompatible insulator SU-8 (Microchem/2000.5) at 3000 rpm of spin coating speed and soft-baked at 95 °C for 1 min. Afterward, UV exposure (365 nm) was performed with an exposure energy of 70 mJ/cm2, and post-exposure bake was done at 95 °C for 2 min. After that, SU-8 was developed using SU-8 developer for 1 min and rinsed with DI water. We printed PEDOT:PSS ink on the 100 µm diameter of the transparent µECoG array, 3 by 3 dots with 25 µm drop spacing, and 5 printing layers. We used a drop-on-demand inkjet printer (DMP 2850, Dimatix Fujifilm) and a 10-pL nozzle head cartridge (Dimatix DMC-11601). The jetting voltage was 20 V, and the single drop velocity was 7−9 m/s, suitable for stable printing. The PEDOT:PSS patterns were thermally crosslinked by GOPS after the printing (100 °C, 1 h on a hot plate).
Material properties
To analyze the morphology of the surface of electrodes, we used FE-SEM (Hitachi/SU8230) and atomic force microscopy (AFM) (Park Systems/XE-150). The SEM image was taken at an acceleration voltage of 1.6 kV and a 5.4–6 mm working distance. The bonding energy and peaks between the metal and polymer seed layer were characterized by XPS (Thermo-Scientific/ESCALAB 250Xi) and FT-IR (Thermo Scientific/ Nicolet Continuum). The contact angle of the transparent µECoG array for PEDOT:PSS ink drop was characterized by a contact angle measurement system (SEO/Phoenix-MT).
Mechanical properties
The alterations in the stain and stress (SS) curve and resistance throughout the tensile procedure were quantified employing an (SFM-100kN) state-of-the-art universal testing apparatus (United Calibration). Real-time monitoring of resistance fluctuations was achieved by interfacing a specialized jig and a digital multimeter (Keithley 2001) through a copper conductor. An automatic bending machine was used to measure the mechanical stability of ultrathin gold microelectrodes (25 μm-thick PET substrates). Bending was performed up to 50,000 times under a bending radius of 5 mm. To assess fatigue failure through cyclic bending, we employed a cutting-edge 1-axis motion controller (MS Tech, South Korea).
Electrochemical properties
EIS measurements were done using an LCR meter (NF Model ZM2410/NF corporation) on a probe station (Model 8000, MS Tech, South Korea). One terminal was connected to the contact pad of a microelectrode, and the other terminal was connected to a large ref. 8 nm Au/EDTA-PSS electrode. All the electrodes were covered with Dulbecco’s Phosphate Buffered Saline (DPBS) solution (Sigma-Aldrich, 806552) to form electrode/electrolyte interfaces. Electrochemical impedance and phase angle were measured using a 100-mV sine wave signal with a frequency range from 20 Hz to 100 kHz. Multichannel potentiostat (Ivium-n-Stat, IVIUM Technology) was used to evaluate the cyclic voltammetry (CV) window and charge storage capacity.
Animal preparation
All procedures were approved by the Institutional Animal Care and Use Committee of Daegu Gyeongbuk Institute of Science and Technology (DGIST) in compliance with DGIST’s laboratory safety guidelines (DGIST-IACUC-24121906-0004). C57BL/6 J mice were housed in standard mouse cages (16 × 36 × 12.5 cm³) with food and water provided ad libitum. Mice were weaned at 3–4 weeks of age and housed with sex-matched siblings (up to four per cage) in a 12:12-h light/dark cycle at 22 ± 1 °C. A total of five male adult mice were used (two for acute recording, two for two-week recording, and one for optogenetics). No animals or data points were excluded from the analysis.
Surgical procedures
All surgeries were performed with mice anesthetized by intraperitoneal injection of a ketamine-xylazine mixture (100 mg/kg and 10 mg/kg, respectively) and secured in a stereotaxic apparatus. As the transparency of the uECoG used in the surgery was visually evident, the researcher was aware of whether the transparent uECoG or the opaque uECoG was being utilized in the experiment. For electrophysiological recordings, a craniotomy was performed on the right skull to expose the parietal cortex for electrode placement (E16-500-5-200; NeuroNexus, MI, USA, PEDOT:PSS 500 nm/Au 8 nm/EDTA/PSS). Two bone screws were inserted into the skull over the cerebellum as reference and ground electrodes. For two-week recordings, Hemospon (Technew, São Paulo, Brazil) moistened with artificial cerebrospinal fluid (ACSF; Tocris Bioscience, Bristol, UK) was placed on top of the electrode. Kwik-Sil and Kwik-Cast (World Precision Instruments, Sarasota, FL, USA) were applied to seal the area. Finally, Super-Bond (Sun Medical Co., Ltd., Shiga, Japan) and dental cement were used to secure and insulate the assembly. For optogenetic experiments, AAV-hSyn-hChR2(H134R)-EYFP was injected into the left cortex and AAV-hSyn-EGFP into the right cortex (bilateral injections of 250 nL each) using a Nanoliter injector system (WPI, Sarasota, FL, USA) at a rate of <0.1 μL/min. After a three-week recovery, electrophysiological recordings with optical stimulation were conducted.
In vivo neural recording
We integrate our transparent neural electrode with flexible PCB (FPCB) by anisotropic conduction film (ACF) bonding following 220 °C of temperature during 30 s in the bonding region (Hitachi chemical/AC-7246LU-18). We integrated the FPCB with a customized PCB, which attached the omnetics 2114 connector to integrate with the Intan recording system (RHS 2116 Stimulation/Recording System). Neural recordings were conducted using the Intan RHS stimulation/recording system (M4016, M4200; Intan Technologies, Los Angeles, CA, USA). Signals were recorded at 25 kHz and filtered with a 0.98 Hz–7.60 kHz band-pass filter and a 60 Hz notch filter. The signal was obtained after at least 30 min of acclimatisation in the recording room. The recorded signal’s power spectra density (PSD) was calculated and displayed by MATLAB.
Optogenetics
For optical stimulation, a 473 nm wavelength laser was used. Light was delivered via an optical fiber (core diameter: 100 µm, NA: 0.22; Doric Lenses, Quebec, Canada) positioned 1 mm above the electrode. The laser was operated using the Intan system, delivering 15 ms light pulses at 2.5 Hz with various power densities.
Immunohistochemistry and confocal microscopy
After all experimental sessions, mice were perfused intracardially with 4% paraformaldehyde and post-fixed overnight at 4 °C. Brains were then dehydrated in 30% sucrose in phosphate-buffered saline (PBS) for 2 days at 4 °C, embedded in optimal cutting temperature compound (Scigen, Paramount, CA, USA), and frozen at −80 °C. Coronal sections (20 µm thick) were obtained with a Leica CM3050 S cryostat (Leica Microsystems, Wetzlar, Germany). Primary antibodies used included mouse anti-GFAP (3670 S, 1:200, Cell Signaling Technology, Danvers, MA, USA) and rabbit anti-IbaI (019-19741, 1:1000, Wako Chemicals USA, Inc., Richmond, VA, USA). Secondary antibodies were Alexa 488 donkey anti-rabbit (711-545-152, 1:1000) and Cy3 donkey anti-mouse (715-165-150, 1:1000) from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). DAPI (Sigma-Aldrich, St. Louis, MO, USA) was used for nuclear counterstaining, and images were captured on a Nikon C2+ confocal microscope (Nikon, Tokyo, Japan).
Photoelectric artifact characterization
We pre-performed power calibration of various laser knobs with an optical power meter (Cohernet/pm10) for the 473 nm and 580 nm wavelength (Shanghai Laser/ORS-1) lasers, respectively. For the characterization of photoelectric artifact in PBS solution, we placed a fiber-type laser with a wavelength of 473 nm at a distance of 6 mm from the transparent/opaque electrode and then irradiated the laser. The diameter of the laser spot was 3 mm. The turn on/off (20 ms/80 ms) of the laser was controlled by TTL pulses generated from a multichannel recording system. For the characterization of photoelectric artifacts during in vivo brain signal recording, the laser was operated using the Intan system, delivering 15 ms light pulses at 2.5 Hz repeated modulation.
Data availability
All data needed to evaluate the discussions in the paper are present in the paper and/or the Supplementary Materials. Other raw data are available from the corresponding authors upon request.
References
Hong, J. W., Yoon, C., Jo, K., Won, J. H. & Park, S. Recent advances in recording and modulation technologies for next-generation neural interfaces. iScience 24, 103550 (2021).
Zeng, Q. & Huang, Z. Challenges and opportunities of implantable neural interfaces: from material, electrochemical and biological perspectives. Adv. Funct. Mater. 33, 2301223 (2023).
Cho, Y. U., Lim, S. L., Hong, J. H. & Yu, K. J. Transparent neural implantable devices: a comprehensive review of challenges and progress. npj Flex. Electron. 6, 53 (2022).
Lee, J. M. et al. The ultra-thin, minimally invasive surface electrode array NeuroWeb for probing neural activity. Nat. Commun. 14, 7088 (2023).
Viana, D. et al. Nanoporous graphene-based thin-film microelectrodes for in vivo high-resolution neural recording and stimulation. Nat. Nanotechnol. https://doi.org/10.1038/s41565-023-01570-5 (2024).
Tybrandt, K. et al. High-density stretchable electrode grids for chronic neural recording. Adv. Mater. 30, e1706520 (2018).
Ramezani, M. et al. High-density transparent graphene arrays for predicting cellular calcium activity at depth from surface potential recordings. Nat. Nanotechnol. https://doi.org/10.1038/s41565-023-01576-z (2024).
Ganji, M. et al. Selective formation of porous Pt nanorods for highly electrochemically efficient neural electrode interfaces. Nano Lett. 19, 6244–6254 (2019).
Jhang, J. et al. Anterior cingulate cortex and its input to the basolateral amygdala control innate fear response. Nat. Commun. 9, 1–16 (2018).
Seo, J. W. et al. Artifact-Free 2D mapping of neural activity in vivo through transparent gold nanonetwork array. Adv. Funct. Mater. 30, 1–11 (2020).
Streich, L. et al. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nat. Methods 18, 1253–1258 (2021).
Wu, X. et al. Tether-free photothermal deep-brain stimulation in freely behaving mice via wide-field illumination in the near-infrared-II window. Nat. Biomed. Eng. 6, 754–770 (2022).
Wang, Y., Garg, R., Cohen-Karni, D. & Cohen-Karni, T. Neural modulation with photothermally active nanomaterials. Nat. Rev. Bioeng. 1, 193–207 (2023).
Cho, Y. U. et al. Ultra-low cost, facile fabrication of transparent neural electrode array for electrocorticography with photoelectric artifact-free optogenetics. Adv. Funct. Mater. 32, 2105568 (2022).
Zhang, J. et al. Stretchable transparent electrode arrays for simultaneous electrical and optical interrogation of neural circuits in vivo. Nano Lett. 18, 2903–2911 (2018).
Middya, S. et al. Microelectrode arrays for simultaneous electrophysiology and advanced optical microscopy. Adv. Sci. 8, 1–9 (2021).
Qiang, Y. et al. Bilayer nanomesh structures for transparent recording and stimulating microelectrodes. Adv. Funct. Mater. 27, 1704117 (2017).
Qiang, Y. et al. Transparent arrays of bilayer-nanomesh microelectrodes for simultaneous electrophysiology and two-photon imaging in the brain. Sci. Adv. 4, eaat0626 (2018).
Lee, W. et al. Transparent, conformable, active multielectrode array using organic electrochemical transistors. Proc. Natl. Acad. Sci. USA 114, 10554–10559 (2017).
Tringides, C. M. et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 16, 1019–1029 (2021).
Chen, Z. et al. Flexible and Transparent Metal Oxide/Metal Grid Hybrid Interfaces for Electrophysiology and Optogenetics. Adv. Mater. Technol. 5, 1–8 (2020).
Gilshtein, E. et al. Inkjet-Printed Conductive ITO Patterns for Transparent Security Systems. Adv. Mater. Technol. 5, 2000369 (2020).
Dijk, G., Kaszas, A., Pas, J. & O’Connor, R. P. Fabrication and in vivo 2-photon microscopy validation of transparent PEDOT:PSS microelectrode arrays. Microsyst. Nanoeng. 8, 90 (2022).
Lan, L. et al. Highly flexible polypyrrole electrode with acanthosphere-like structures for energy storage and actuator applications. Chem. Eng. J. 455, 140675 (2023).
Zheng, W. et al. Nanocellulose-mediated hybrid polyaniline electrodes for high performance flexible supercapacitors. J. Mater. Chem. A 5, 12969–12976 (2017).
Park, D. W. et al. Electrical neural stimulation and simultaneous in vivo monitoring with transparent graphene electrode arrays implanted in GCaMP6f Mice. ACS Nano 12, 148–157 (2018).
Kuzum, D. et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 5, 1–10 (2014).
Kim, C. L., Jung, C. W., Oh, Y. J. & Kim, D. E. A highly flexible transparent conductive electrode based on nanomaterials. NPG Asia Mater. 9, e438–e438 (2017).
Wang, L. et al. Green manufacturing of flexible sensors with a giant gauge factor: bridging effect of CNT and electric field enhancement at the percolation threshold. ACS Appl. Mater. Interfaces 14, 26024–26033 (2022).
Seo, K. J. et al. Transparent electrophysiology microelectrodes and interconnects from metal nanomesh. ACS Nano 11, 4365–4372 (2017).
Kang, H., Jung, S., Jeong, S., Kim, G. & Lee, K. Polymer-metal hybrid transparent electrodes for flexible electronics. Nat. Commun. 6, 1–7 (2015).
Kim, D. et al. Inkjet-Printed polyelectrolyte seed layer-based, customizable, transparent, ultrathin gold electrodes and facile implementation of photothermal effect. ACS Appl. Mater. Interfaces 15, 20508–20519 (2023).
Hong, W. et al. Ultrathin gold microelectrode array using polyelectrolyte multilayers for flexible and transparent electro-optical neural interfaces. Adv. Funct. Mater. 32, 1–11 (2022).
Tao, J. et al. Functionalized SnO2 films by using EDTA-2 M for high efficiency perovskite solar cells with efficiency over 23. Chem. Eng. J. 430, 132683 (2022).
Yang, D. et al. High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2. Nat. Commun. 9, 3239 (2018).
Park, G., Kim, D., Kim, G. & Jeong, U. High-Performance Indium-Tin Oxide (ITO) electrode enabled by a counteranion-free metal-polymer complex. ACS Nanosci. Au 2, 527–538 (2022).
Bi, Y. G. et al. Ultrathin metal films as the transparent electrode in ITO-Free organic optoelectronic devices. Adv. Opt. Mater. 7, 1–23 (2019).
Kaiser, N. Review of the fundamentals of thin-film growth. Appl. Opt. 41, 3053 (2002).
Kafshboran, H. R. & Ghasemi, S. Designing magnetic catalysts based on gold nanoparticles supported by ethylenediamine tetraacetic acid functionalized amino-modified poly(N-isopropyl acrylamide) for reduction of nitro compounds in water. Polym. Int. https://doi.org/10.1002/pi.6625 (2024).
Kovács, A., Nemcsok, D. S. & Kocsis, T. Bonding interactions in EDTA complexes. J. Mol. Struct. THEOCHEM 950, 93–97 (2010).
Dozol, H. et al. On the synthesis of Au nanoparticles using EDTA as a reducing agent. J. Phys. Chem. C. 117, 20958–20966 (2013).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Driscoll, N. et al. MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci. Transl. Med. 13, 1–14 (2021).
Kim, R., Hong, N. & Nam, Y. Gold nanograin microelectrodes for neuroelectronic interfaces. Biotechnol. J. 8, 206–214 (2013).
Kim, S. M. et al. High-performance, polymer-based direct cellular interfaces for electrical stimulation and recording. NPG Asia Mater. 10, 255–265 (2018).
Susloparova, A. et al. Low impedance and highly transparent microelectrode arrays (MEA) for in vitro neuron electrical activity probing. Sensors Actuators, B Chem. 327, 128895 (2021).
Neto, J. P. et al. Transparent and flexible electrocorticography electrode arrays based on silver nanowire networks for neural recordings. ACS Appl. Nano Mater. 4, 5737–5747 (2021).
Zhang, F. et al. Multimodal electrocorticogram active electrode array based on zinc oxide-thin film transistors. Adv. Sci. 10, 1–10 (2023).
Lee, Y. et al. Tunable organic active neural probe enabling near-sensor signal processing. Adv. Mater. 35, 1–11 (2023).
Bießmann, L. et al. Monitoring the swelling behavior of PEDOT:PSS electrodes under high humidity conditions. ACS Appl. Mater. Interfaces 10, 9865–9872 (2018).
Kim, Y. H. et al. In vitro extracellular recording and stimulation performance of nanoporous gold-modified multi-electrode arrays. J. Neural Eng. 12, 066029 (2015).
Chik, G. K. K. et al. Flexible multichannel neural probe developed by electropolymerization for localized stimulation and sensing. Adv. Mater. Technol. 7, 1–12 (2022).
Driscoll, N. et al. Two-dimensional Ti3C2 MXene for high-resolution neural interfaces. ACS Nano 12, 10419–10429 (2018).
Cho, Y. et al. Hybrid bionic nerve interface for application in bionic limbs. Adv. Sci. 10, 2303728 (2023).
Michelson, N. J. & Kozai, T. D. Y. Isoflurane and ketamine differentially influence spontaneous and evoked laminar electrophysiology in mouse V1. J. Neurophysiol. 120, 2232–2245 (2018).
Średniawa, W. et al. Network and synaptic mechanisms underlying high frequency oscillations in the rat and cat olfactory bulb under ketamine-xylazine anesthesia. Sci. Rep. 11, 6390 (2021).
Park, D. W. et al. Fabrication and utility of a transparent graphene neural electrode array for electrophysiology, in vivo imaging, and optogenetics. Nat. Protoc. 11, 2201–2222 (2016).
Cardin, J. A. et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 5, 247–254 (2010).
Zeff, B. W., White, B. R., Dehghani, H., Schlaggar, B. L. & Culver, J. P. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proc. Natl Acad. Sci. USA 104, 12169–12174 (2007).
Acknowledgements
This work was supported in part by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (No. RS-2022-NR069917, RS-2024-00416319), in part by the ‘DGIST intramural grant’ (25-IRJoint-03) and in part by an Ideas Grant from the National Health and Medical Research Council (NHMRC) of Australia (APP1188414), and in part by the Interdisciplinary Research Initiatives Program from College of Engineering and College of Medicine, Seoul National University (grant no. 800-20240490). The authors would like to thank the Institute of Next-generation Semiconductor convergence Technology (INST), Nano Fab Support Team and Advanced Convergence Analysis Team in DGIST. Part of Fig. 1c is drawn with BioRender.com.
Author information
Authors and Affiliations
Contributions
D. Kim, M. B., and B. Kim contributed equally to this work. D. Kim, M. B., B. Kim, and H. Kang conceived and designed the project. D. Kim and M. B. fabricated and characterized the transparent ultrathin gold µECoG. D. Kim, B. Kim, H. Kang and H. K. Choe planned animal experiments, and D. Kim and B. Kim performed all in vivo experiments, including neural acute/two-week recording, optogenetic modulation and immunohistochemistry. N. Hong contributed examination of brain recorded signal, J. Park contributed CV, CSC characterization, H. Lim contributed mechanical property characterization, Junhee Lee contributed impedance measurement, Jungha Lee contributed electrode noise analysis, Y. K. Kim contributed optogenetic modulation, and Y. Cho contributed inkjet printing. K. Lee, Junghyup Lee, J.-H. Yoon, H. Kwon, D. Tsai, S. Lee, J. Jang, H. K. Choe, and H. Kang provided analysis of the experimental data and resources for the experiments. D. Kim, M. B., B. Kim, and H. Kang wrote the initial draft. The final manuscript was prepared with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, D., Bissannagari, M., Kim, B. et al. Hexagonal metal complex based mechanically robust transparent ultrathin gold µECoG for electro-optical neural interfaces. npj Flex Electron 9, 31 (2025). https://doi.org/10.1038/s41528-025-00403-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41528-025-00403-w
This article is cited by
-
Optical wireless data transfer through skin for implantable biomedical electronics
npj Flexible Electronics (2025)