Abstract
Addressing levodopa-unresponsive freezing of gait (FOG) in Parkinson’s disease (PD) presents a significant challenge. A randomized double-blinded trial evaluated the effects of repetitive transcranial magnetic stimulation (rTMS) in conjunction with transcutaneous magnetic spinal cord stimulation among 57 PD individuals experiencing levodopa-unresponsive FOG. Patients were randomized to receive dual-site stimulation involving bilateral primary motor cortex of the lower leg (M1-LL) and the lumbar spinal cord, single-site stimulation targeting bilateral M1-LL alone, or sham stimulation for 10 sessions. Low-frequency rTMS induced remarkable improvements in FOG, gait, and motor functions compared to sham at 1 day and 1 month postintervention. Notably, the dual-site protocol demonstrated superior efficacy in mitigating FOG and improving gait compared to the single-site approach, which correlated with a pronounced increase in short-interval intracortical inhibition of the abductor pollicis brevis. These findings underscore the potential of the cerebrospinal dual-site regimen as a promising approach for levodopa-unresponsive FOG and gait in PD.
Similar content being viewed by others
Introduction
Addressing freezing of gait (FOG), particularly the subtype unresponsive to levodopa, poses a significant challenge for clinicians1,2. While the neural mechanisms underpinning FOG in Parkinson’s disease (PD) are obscure, several models have been proposed3. To date, the most accepted model remains the “Interference model” proposed by Lewis and Baker4, and named by Nieuwboer and Giladi5, which indicates that FOG may arise from convergence dysfunction and overload of segregate cognitive, limbic and motor cortical-basal ganglia (BG) pathways. Targeting affected motor cortices could potentially rebalance the connectivity of motor cortex with the motor striatum and subthalamic nucleus (STN), thereby relieving FOG. Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive brain stimulation (NIBS) technique modulating brain connectivity in specific, distributed, cortico-subcortical networks6. Actually, the primary motor cortex of the lower leg (M1-LL) has been considered as one of the candidate stimulus targets for gait and FOG in PD7,8,9. However, its efficacy remained limited and controversial10. Therefore, improving the current rTMS therapeutic regimen is sorely warranted. Impaired corticomotor inhibition, particularly reduced short-interval intracortical inhibition (SICI) of the tibialis anterior (TA) muscle, was found to be associated with FOG pathogenesis in a recent study11. Low frequency rTMS over M1 has demonstrated the capacity to enhance corticomotor inhibition, as evidenced by a reduction in motor evoked potential (MEP) amplitude12 and an extension of the cortical silent period (CSP)13,14. Thus, our initial objective was to explore whether the cumulative application of 1 Hz rTMS over the bilateral M1-LL could ameliorate FOG in PD.
Recently, spinal cord stimulation (SCS) has emerged as a promising treatment for FOG in PD. A pilot study involving the implantation of electrodes in the epidural space of the upper thoracic spine (T2-T4) in 4 PD patients demonstrated that SCS improved gait and FOG15. This promising outcome was corroborated in another pilot study, where mid-thoracic SCS (T8-T10) was applied to 5 PD patients with levodopa-unresponsive FOG, confirming its beneficial effects16. Additionally, a recent Asian case highlighted the role of SCS (T9-T10) in reducing FOG again17. Although the precise mechanisms remain elusive, SCS may modulate neuronal firing in the supplementary motor area (SMA), a key motor hub for controlling gait initiation18. Despite the invasive nature of SCS, an intriguing recent trial has explored the feasibility of transcutaneous magnetic SCS at the level of fifth thoracic vertebra (T5). This noninvasive approach has shown promise in attenuating FOG in PD without severe adverse effects19. Encouraged by these preliminary findings, we posited a hypothesis: the synergistic combination of rTMS with transcutaneous magnetic SCS could prove more efficacious than rTMS alone in addressing levodopa-unresponsive FOG.
To substantiate our two hypotheses, we designed a randomized, double-blind, controlled trial to evaluate the safety and efficacy of cumulative low-frequency rTMS over bilateral M1-LL, as well as its synergistic combination with transcutaneous magnetic SCS targeting lower thoracic vertebra, in treating levodopa-unresponsive FOG in PD patients. Concurrently, we employed measures of corticomotor excitability of both upper and lower extremities to delve into the underlying neurophysiological mechanisms driving these interventions.
Results
Participant characteristics
The demographic, baseline clinical, and electrophysiological characteristics of all participants were summarized in Table 1. Participants assigned to the three distinct interventions groups, which included dual-site (DS: real bilateral M1-LL + real lumbar spinal cord), single-site (SS: real bilateral M1-LL + sham lumbar spinal cord), and no-site (NS: double sham) stimulation, exhibited similar demographic, baseline clinical, and gait characteristics. Furthermore, no statistically significant differences were detected in the electrophysiological measures across the three groups.
Adverse effects
No severe adverse events were reported. However, 5 subjects reported experiencing side effect following rTMS, all described mild, transient headaches during their initial rTMS session. Four of these individuals proceeded to complete the full interventions and follow-up without further headache episodes. Nevertheless, one participant, while not experiencing severe headaches, expressed apprehension that culminated in the decision to decline further treatment. The distribution of adverse events was similar in the DS (n = 3) and SS (n = 2) intervention groups.
Changes in FOG assessments
Repeated analysis of variance (ANOVA) clarified significant effects of TIME (FTIME = 26.148, p < 0.001, partial η2 = 0.506) and GROUP (FGROUP = 4.343, p = 0.018, partial η2 = 0.143), and a significant TIME × GROUP interaction (FTIME × GROUP = 30.727, p < 0.001, partial η2 = 0.542) in the FOG questionnaire (FOG-Q). DS and SS groups showed remarkable improvements at 1 day (Post), and 1 month (Post1m) postintervention relative to Baseline (all p < 0.01) (Table 2). Furthermore, SS group (Δ FOG-Q at Post: − 2.22 ± 1.59; Δ FOG-Q at Post1m: −1.39 ± 1.20) relieved more FOG symptoms compared with sham (Δ FOG-Q at Post: 0.44 ± 2.09; Δ FOG-Q at Post1m: 0.89 ± 1.60) (both p = 0.001), whereas DS group (Δ FOG-Q at Post: −4.05 ± 2.17; Δ FOG-Q at Post1m: −3.37 ± 2.09) induced a greater FOG-Q reduction than both SS and NS groups (all p < 0.05) (Fig. 1A). Hence, low-frequency rTMS, when administered independently, mitigated FOG both during intensive treatment phase and throughout the subsequent one-month follow-up period. Nonetheless, the dual-site protocol demonstrated a superior capacity to diminish FOG symptoms.
A FOG-Q, (B) UPDRS-III, (C) gait speed, (D) stride length, (E) stride time variability, and (F) double support time percentage. Abbreviations: DS dual-site, SS single-site, NS no-site, FOG-Q freezing of gait questionnaire, Post 1 day postintervention, Post1m 1 month postintervention, UPDRS-III Unified Parkinson’s Disease Rating Scale part III. *p < 0.05, **p < 0.01, ***p < 0.001.
Changes in motor function assessments
Repeated ANOVA revealed significant TIME effect and TIME × GROUP interaction in the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) (FTIME = 14.877, p < 0.001, partial η2 = 0.222; FTIME × GROUP = 5.586, p = 0.001, partial η2 = 0.177); however, no main effect of GROUP was detected. Similar to FOG-Q, both DS and SS groups led to significantly reduced UPDRS-III scores at Post and Post1m relative to Baseline (all p < 0.001) (Table 2). Compared with sham (Δ UPDRS-III at Post: 0.17 ± 5.80; Δ UPDRS-III at Post1m: 1.5 ± 6.97), both DS (Δ UPDRS-III at Post: −5.84 ± 7.16; Δ UPDRS-III at Post1m: −5.68 ± 6.72) and SS (Δ UPDRS-III at Post: −5.94 ± 5.13; Δ UPDRS-III at Post1m: −5.39 ± 3.78) groups showed significant motor improvements at intensive phase and 1 month follow-up (all p < 0.05) (Fig. 1B). Nevertheless, there was no significant difference between DS and SS interventions.
Changes in objective gait characteristics
Repeated ANOVAs disclosed significant effects of TIME and GROUP, and TIME × GROUP interactions in gait speed (FTIME = 10.565, p < 0.001, partial η2 = 0.297; FGROUP = 4.526, p = 0.016, partial η2 = 0.151; FTIME × GROUP = 18.683, p < 0.001, partial η2 = 0.423), stride length (FTIME = 5.501, p = 0.005, partial η2 = 0.097; FGROUP = 4.040, p = 0.024, partial η2 = 0.137; FTIME × GROUP = 10.924, p < 0.001, partial η2 = 0.300), and double support time percentage (FTIME = 11.031, p < 0.001, partial η2 = 0.306; FGROUP = 6.469, p = 0.003, partial η2 = 0.202; FTIME × GROUP = 8.469, p = 0.001, partial η2 = 0.249). Relative to Baseline, DS group showed remarkable improvements in gait speed and stride length at Post and Post1m (all p < 0.001); SS group increased gait speed only at Post (p = 0.006); however, NS group aggravated gait speed at Post1m (p = 0.011) and disimproved stride length at both intensive phase (p = 0.039) and 1 month follow-up (p = 0.002) (Table 2). For double support time percentage, compared to Baseline, DS and SS groups led to a decrease at Post (p = 0.002 and p = 0.016), whereas NS group resulted in unfavorable increases at Post1m (p = 0.008). Further, DS group was superior to NS group in ameliorating gait speed, stride length and double support time percentage, and to SS in improving gait speed and stride length (all p < 0.05) (Fig. 1C–F). In summary, the SS group experienced a degree of improvement in gait characteristics, yet the DS group exhibited superior gait performance.
Changes in cortical excitability
Given the role of dopaminergic therapy in corticomotor excitability20,21,22, levodopa equivalent daily dose (LEDD) was entered as a regressor in the present study. However, there were no significant differences in the cortical excitability of the TA before and after interventions. Interestingly, a significant TIME × GROUP interaction emerged for the SICI of the abductor pollicis brevis (APB), denoted as SICIAPB (FTIME × GROUP = 12.340, p < 0.001, partial η2 = 0.330) (Table S1 in supplementary material). Specifically, both the single-site and dual-site intervention groups showed increased SICIAPB relative to baseline (p < 0.01). Furthermore, SS protocol led to an improvement in SICIAPB when contrasted with sham (p = 0.005), whereas the DS regimen facilitated a more pronounced recovery of SICIAPB relative to both SS (p = 0.048) and NS (p < 0.001) programs (Fig. 2B). Nonetheless, no significant variances were discerned in the MEP amplitude at 120% resting motor threshold (RMT) intensity (AMP), CSP, intracortical facilitation (ICF), and short-latency afferent inhibition (SAI) of the APB muscle among the three trials before and after the interventions.
A SICIAPB was tested from 3 participants receiving DS (male, aged 69 years, SICIAPB at Baseline: 98.7% control MEP, SICIAPB at Post: 63.1% control MEP), SS (female, aged 63 years, SICIAPB at Baseline: 88.7% control MEP, SICIAPB at Post: 76.4% control MEP) and NS (male, aged 74 years, SICIAPB at Baseline: 93.1% control MEP, SICIAPB at Post: 107% control MEP) protocols. B After 10-session interventions, SS group induced an improvement in SICIAPB compared with sham, whereas DS group promoted greater SICIAPB recovery than both SS and NS groups. C Relationship between changes in SICIAPB and gait speed in DS group. D Relationship between changes in SICIAPB and stride length in DS group. Abbreviations: SICI short-interval intracortical inhibition, APB abductor pollicis brevis, MEP motor evoked potentials, DS dual-site, SS single-site, NS no-site, Post 1 day postintervention; FDR false discovery rate. *p < 0.05, **p < 0.01, ***p < 0.001.
Correlation analyses
For the DS group, greater rehabilitation in SICIAPB was correlated with greater increases in gait speed (r = −0.738, p = 0.004) and stride length (r = -0.740, p = 0.007) (Fig. 2C, D). This correlation indicated that improvements in gait performance were tightly coupled with the reestablishment of cortical inhibitory function.
Discussion
This investigation scrutinized the safety and efficacy of low-frequency rTMS applied over bilateral M1-LL, as well as its synergistic combination with transcutaneous magnetic SCS, in mitigating levodopa-unresponsive FOG in PD. Notably, no serious adverse effects were observed. The cumulative administration of 1 Hz rTMS over the bilateral M1-LL yielded improvements in FOG, gait and motor functions, accompanied by a marked enhancement in SICIAPB. Moreover, the integration of rTMS with transcutaneous magnetic SCS demonstrated superior efficacy over rTMS alone in alleviating freezing and gait symptoms. This cerebrospinal protocol elicited a more substantial increase in SICIAPB compared to the SS regimen, which was further associated with gait improvements. Collectively, these findings affirmed the effectiveness of 1 Hz rTMS over the bilateral M1-LL and highlighted consecutive rTMS and transcutaneous magnetic SCS as a promising therapeutic strategy for addressing levodopa-unresponsive FOG and gait dysfunction in PD.
Cumulative application of 1 Hz rTMS over the bilateral M1-LL using a “double-cone” coil effectively curtailed freezing episodes (-2.22 points in FOG-Q at Post; −1.39 points in FOG-Q at Post1m), improved motor function (-5.94 points in UPDRS-III at Post; −5.39 points in UPDRS-III at Post1m), and ameliorated gait abnormalities in individuals with PD, with the therapeutic effects persisting for at least one month. Moreover, real rTMS elicited a notable elevation of SICIAPB, indicating its potential role in modulating corticomotor inhibition. A reduction in SICI could signify cortical disinhibition and potentially facilitate heightened levels of excitatory cortical output. In a prior study23, we illustrated that cortical disinhibition drove FOG in PD, implying that cortical disinhibition in FOG could potentially serve as a compensatory reaction originating from abnormal BG output to the M1. Accordingly, rTMS applied over the bilateral M1-LL might alleviate freezing and bradykinesia by modulating the BG-cortical loop and reinstating the diminished cortical inhibition. Consistent with this notion, previous evidence has shown that consecutive days of low-frequency rTMS could induce alterations in corticomotor excitability and enhance inhibitory activity14. Interestingly, although our stimuli were specifically directed at the bilateral M1-LL, the alterations in corticomotor excitability primarily manifested in the hand region of the M1. This intriguing outcome could conceivably be elucidated by the indirect impact of bilateral M1-LL rTMS through network-level interactions. It is widely recognized that the impact of rTMS extends beyond the local immediate stimulation site, influencing interconnected brain areas and activity within the motor control neural networks.
Given the constrained efficacy of single rTMS protocols, there is a burgeoning interest in investigating the potential efficacy of dual-mode or multi-focal NIBS techniques for motor or non-motor symptoms in PD24,25,26. Chang et al. have determined the potential of dual-mode NIBS to simultaneously modulate two different cortices in PD patients with FOG, combining rTMS over the M1-LL with anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex24. In a similar vein, our research targeted both the cerebral motor cortex and spinal cord concurrently through a cerebrospinal DS intervention. We found that the DS intervention resulted in more substantial improvements in FOG (-4.05 points in FOG-Q at Post; −3.37 points in FOG-Q at Post1m) and gait during the intensive phase and 1 month follow-up, as compared to rTMS alone and sham conditions. These findings confirmed our hypothesis that the combination of low-frequency rTMS and transcutaneous magnetic SCS represents a promising therapeutic approach for levodopa-unresponsive FOG and gait impairments in patients with PD. It should be noted that the effects of dual cerebrospinal stimulation may still be transient, as the improvement was most pronounced on the first day postintervention and exhibited a declining trend at 1 month follow-up. Nonetheless, the precise duration of the sustained effects of cerebrospinal stimulation merits further investigation.
Furthermore, the cerebrospinal protocol exhibited a greater increase in SICIAPB compared to rTMS alone, which correlated with the observed gait improvements. This suggested that transcutaneous magnetic SCS may bolster the restoration of cortical inhibition through ascending pathways linking the spinal cord and the brain, thereby contributing to the alleviation of gait disturbances. Although the exact mechanisms underlying transcutaneous magnetic SCS are unclear, it is postulated that SCS affects the cortical motor circuit, especially the SMA, which played a role in FOG pathogenesis18. Previous animal studies have revealed that SCS could enhance locomotion in PD27 and increase neuronal firing in the M1 while reducing pathological cortico-striatal synchronous low-frequency waves28. These findings suggested that SCS modulated oscillatory activity in brain motor circuits. Meanwhile, inputs from ascending leminiscal and extralemniscal pathways to the brainstem and thalamus, which may modulate the SMA, are also highly connected to the pedunculopontine nucleus, a brainstem region involved in the control of movement initiation and body equilibrium, which is also implicated in FOG pathogenesis29. Consequently, it is plausible that ascending stimulation of the BG-cortical loop delivered by transcutaneous magnetic SCS can lead to the recovery of cortical inhibition. When combined with rTMS over bilateral M1-LL, it may disrupt the aberrant inhibition of globus pallidus interna onto thalamus and pedunculopontine nucleus, thereby facilitating the reticulospinal tract and subsequently activating central pattern generators in the spinal cord, ultimately meliorating FOG29,30.
Despite the valuable insights gained from our study, several potential limitations warrant consideration. Firstly, the neuro-navigation system was not incorporated in our rTMS interventions and the electromyography (EMG)-based evaluations. While effective EMG responses to cortical modulation were observed, it is crucial to recognize that this method is considerably less optimal than navigating the cortical areas using a frameless stereotaxic navigation system operated with the aid of the patient’s own 3D MRI brain imagery. Therefore, the future application of rTMS in navigation systems is anticipated to enhance therapeutic outcomes and elucidate the underlying mechanisms more effectively. Secondly, our sham design may be considered sub-optimal, as our sham protocol did not generate comparable peripheral sensory stimulation, and the efficacy of blinding was not formally evaluated. However, the fact that all participants were rTMS-naïve likely mitigated some of these concerns. Thirdly, the lack of a dedicated transcutaneous magnetic SCS group precluded a clear delineation of the standalone effects of this modality. However, notwithstanding the uncertain impact of transcutaneous magnetic SCS in isolation, the notable contrast in FOG-Q and gait characteristics between DS and SS implied that cerebrospinal dual stimulation offered a more robust therapeutic strategy for FOG compared to sole cortical rTMS, aligning with the primary aim of this trial. Fourthly, the use of a doble-cone coil for deeper stimulation, also introduced the possibility of co-stimulation of brain regions beyond the bilateral M1-LL, due to its reduced focality. Fifthly, while we employed ear-plugs and sham stimulation to mimic the sounds of real stimulation, the adaptable auditory control might be a better approach for minimizing auditory confounds, which warrants ample considerations in subsequent research endeavors31. Sixthly, in this study, stimulation was delivered via the TMS manufacturer’s dashboard. Transitioning to an automated approach in future research could potentially enhance the objectivity, reliability and repeatability of our experimental procedures32. Lastly, while our study has investigated the cortical excitability changes post-rTMS treatment, further investigations employing advanced imaging techniques such as functional MRI or magnetic resonance spectroscopy could be instrumental in validating alterations in network connectivity and shifts in neural activity related to relevant neurotransmitters.
In conclusion, this study underscored the efficacy of cumulative low-frequency rTMS applied over bilateral M1-LL in addressing levodopa-unresponsive FOG, gait and motor symptoms in PD. The study posited that the combination of low-frequency rTMS and transcutaneous magnetic SCS represents a promising rehabilitation approach for PD patients suffering from levodopa-unresponsive FOG.
Methods
Participants
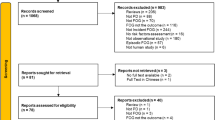
Sixty-eight PD patients with levodopa-unresponsive FOG were consecutively recruited from the First Affiliated Hospital of Nanjing Medical University. Participants were diagnosed with idiopathic PD according to the clinical criteria of the Movement Disorder Society33. Inclusion criteria were (1) aged 40 or older, (2) independent walking 30 m or longer, (3) stable and optimized antiparkinsonian treatment for > 4 weeks, and (4) confirmation of FOG based on (i) FOG-Q ≥ 134 and (ii) clinical freezing episodes confirmed by two researchers (KZ Zhang and LN Wang) during 10-m walking, turning or going through a narrow doorway. Among eligible FOG patients, we further ascertained levodopa-unresponsive FOG individuals by the following steps: (1) identification of drug “off” and “on” states based on the periodic recurrence of motor and non-motor symptoms of PD and their response to drug intake; (2) recognition of FOG subtypes based on the following question “When do you experience FOG?”. Patients with levodopa-unresponsive FOG experienced FOG in both medication “on” and “off” states, that is, the effect of dopaminergic stimulation is not enough to prevent FOG even if other parkinsonian motor signs are improved in the “on” state5. Exclusion criteria were (1) contraindications of TMS, (2) severe dyskinesia or tremors disturbing stimulation, (3) dementia (Mini-mental State Examination [MMSE] < 24)35, (4) a history of psychiatric disorders, (5) no recordable MEPs with TMS, (6) intake of benzodiazepines, neuroleptics or anti-depressant drugs, and (7) other disorders interfering with gait, such as primary progressive freezing gait. Prior to randomization, 5 were excluded from the study due to the presence of brain lesions and psychiatric disorders, 4 withdrew due to personal reasons, whereas 2 were lost to follow up. Therefore, a total of 57 PD patients with levodopa-unresponsive FOG participated in the present clinical trial (Fig. 3).
This study was approved by the Ethical Committee of the First Affiliated Hospital of Nanjing Medical University (2021-SR-209). All participants provided written informed consent in accordance with the Declaration of Helsinki. The trial was registered on ClinicalTrail.gov with the identifier NCT05174299.
Study design
This was a randomized, double-blind, sham-controlled and parallel-group study. Participants were randomly divided into 3 experimental groups, to receive DS, SS and NS stimuli for 10 sessions over 2 weeks (Fig. S1 in supplementary material). We used a random permuted block design, where envelopes were sealed by a research team member who was not the principal investigator or physiotherapist (M Ji). Patients were blinding to intervention conditions and all were naïve to TMS before this study. Participants were informed that the intensity of therapeutic stimuli was different from the intensity used for electrophysiological evaluation.
Participants were clinically assessed at the same time of day at Baseline and Post, and Post1m. Cortical excitability measures were evaluated at Baseline and Post. All assessments were conducted with patients in medication “on” state (1–2 h after antiparkinsonian medications). Two investigators (LN Wang and HM Sun) blinded to intervention conditions performed all neurologic and psychiatric evaluations. Throughout the present study, participants continued their antiparkinsonian mediations without dose adjustments.
The primary outcome measures in this study were changes in FOG-Q at Post and Post1m after the completion of 10 sessions compared to Baseline. The required sample size was calculated based on our pre-experiment (Table S2 in supplementary material). Sample size was calculated using G*Power 3.1.9.7 (effect size F = 0.61; α = 0.01; power = 0.95; number of groups = 3; number of measurements = 2) for a 2-way repeated ANOVA. To account for a predicted dropout rate of 20.0%, the required total sample size exceeded 57 participants.
Treatment regimen and course
Interventions were delivered by a trained physiotherapist (H Zhang). Each subject received 10 intervention sessions over 2 weeks, 1 session per day for 5 consecutive days per week. Participants received intervention in medication “on” state. DS and SS groups both received 1 Hz rTMS over bilateral M1-LL with a 90 mm “double-cone” coil connected to a magnetic stimulator (Neurosoft, Russia) while lying. The coil was placed at optimal position over M1-LL for eliciting MEPs in the targeted TA muscle. The more-affected side was treated first, followed by the less-affected side. Each participant received 800 rTMS pulses in 13 minutes 20 seconds on each hemisphere at 120% RMT. RMT is defined as the lowest intensity required to elicit MEPs of >50 μV in at least 5 of 10 consecutive trials while the target muscle is relaxed. Sham rTMS was applied with a disconnected coil and another active coil behind the subjects to mimic true stimulation sound effects without brain stimulation. Meanwhile, all patients wore ear-plugs during the stimulation to reduce the effect of noise. Immediately after rTMS, participants in DS groups proceeded with 1500 pulses 1 Hz transcutaneous magnetic SCS over the lower thoracic vertebra (T10-T12, corresponding to the pyramidal position of lumbar spinal cord) with “figure-of-eight” coil. The intensity used was just sufficient to elicit noticeable muscle twitching in the lower extremities. The treatment time is 25 minutes, and a sham condition implemented as previously described36,37, with the coil positioned 90°.
Adverse effects assessments
Following each intervention session, a comprehensive assessment of side effects was conducted, encompassing anxiety, fear, headache, tinnitus, dizziness, hearing loss, fainting, nausea, and vomiting.
Behavioral assessments
Primary outcome measures were changes in FOG-Q at Post and Post1m after the completion of 10 sessions compared to Baseline. Secondary outcome measures included the UPDRS-III scores.
Objective gait assessment
Objective gait features were collected at Baseline, Post and Post1m using a portable Inertial Measurement Unit system (GYENNO Science, China) while performing a 5 m timed Up-and-Go test. The test involved standing up from a chair, walking 5 m, turning around to walk back to the chair, and sitting back down38. Subjects were instructed to walk at a comfortable, self-selected pace. The test was performed once; nonetheless, each subject underwent two practice sessions before the formal test. The gait characteristics of interest included gait speed, stride length, stride time variability (expressed as the coefficient of variation) and double support time percentage.
Transcranial magnetic stimulation techniques and electromyographic recordings
In addition to the corticomotor excitability of the TA, the cortical excitability of the APB was also examined to elucidate the therapeutic mechanism. All these indicators were recorded from the more-affected side. To be specific, RMT, SICI and ICF were recorded from the TA. Meanwhile, RMT, AMP, CSP, SICI, ICF and SAI were recorded from the EMG of the APB. The EMG signals were recorded and stored after processing, amplified, and filtered (bandwidth 20 Hz to 2000 Hz), with a sampling rate of 5 kHz.
Single- and paired-pulse TMS was administered using a “butterfly-shaped” coil. In this investigation, SICI and ICF were quantified using a subthreshold conditioning stimulus at 80% RMT and a supra-threshold test stimulus at 130% RMT. The interstimulus interval (ISI) for SICI was set at 4 ms, whereas for ICF, it was 15 ms39. Each ISI condition was repeated for 10 trials. SICI and ICF were expressed as the percentage ratio of the test to the conditioning MEPs. Moreover, AMP refers to the mean peak-to-peak amplitude of MEPs obtained at 120% RMT for 5 single stimuli. The CSP was assessed in the 20% tonically activated APB muscle by stimulating the M1 at an intensity of 150% RMT. CSP measurements were determined by the duration from the stimulus pulse output to the reemergence of any voluntary EMG activity, with ten trials conducted to calculate the average CSP for each subject. These protocols facilitate the examination of the interplay between inhibitory and facilitatory mechanisms within the motor cortex, shedding light on corticomotor excitability and plasticity.
The methodology delineated by Tokimura et al. was utilized to investigate SAI40. Conditioning stimuli were single electrical pulses (width 200 μs) applied to the median nerve at the wrist using a constant current stimulator through adhesive electrodes (cathode proximal). The intensity of the conditioning peripheral stimulus was set at just over the motor threshold to evoke a visible twitch of the APB muscle. The N20 wave of cortical somatosensory response was recorded through adhesive electrodes applied to the scalp (contralateral to median nerve stimulation) after appropriate skin preparation, using the active electrode 2 cm posterior to C4/C3 (10–20 System) and reference electrode placed on the forehead. A total of 500–2000 responses were averaged twice and superimposed to identify the latency of the N20 peak. The intensity of the test cortical magnetic shock was adjusted to evoke an MEP in relaxed APB muscle with peak-to-peak amplitude of ~1 mV. SAI was tested at different ISIs determined on the basis of the N20 wave latency. ISIs ranged from 0 to 8 ms after N20 latency in 4 ms steps. For each ISI, we calculated the amplitude of the basal MEP (average of five consecutive responses obtained after cortical stimulation alone) and the amplitude of the conditioned MEP (average of five consecutive responses obtained after the conditioning peripheral electrical stimulus). The amplitude of conditioned MEP, expressed as a percentage of the basal MEP amplitude at each ISI, was used to evaluate the amount of SAI. All subjects utilized audiovisual feedback of EMG signal at high gain to maintain complete relaxation during experiments.
Statistical analyses
Gender differences were analyzed using Chi-square test. We used histograms and Shapiro-Wilk tests to check data normality. Based on the data normality, demographic, baseline clinical and electrophysiological characteristics were compared via one-way ANOVA or Kruskal-Wallis test. Two-way repeated ANOVAs were applied to compare outcome measures evaluated at different TIME (within factor: Baseline, Post and Post1m) and across different GROUP (between factor: DS, SS and NS groups). Repeated analyses of covariance (ANCOVAs) were performed to investigate neural electrophysiological alterations obtained at different TIME and across different GROUP during medication “on” state, with LEDD as the covariate. The level of significance was set at p < 0.05. When there was an interaction effect between GROUP and TIME, post hoc analyses were performed, applying Bonferroni correction for multiple comparisons. Further, the improvements of each value at Post (Δ value at Post = [value at Post - value at Baseline]) and Post1m (Δ value at Post1m = [value at Post1m - value at Baseline]) were calculated for each group and analyzed one-way ANOVAs or Kruskal-Wallis tests based on the data normality. False discovery rate (FDR) correction was performed due to multiple testing in post-hoc analyses. Lastly, partial correlation analyses were applied to establish the relationships between the electrophysiological and behavioral alterations, with LEDD as a regressor. SPSS Statistics version 27 (IBM Corp., Armonk, NY) was used for the statistical analyses.
Data availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
References
Cosentino, C. et al. Effectiveness of physiotherapy on freezing of gait in Parkinson’s disease: a systematic review and meta-analyses. Mov. Disord. 35, 523–536 (2020).
Fujikawa, J. et al. Therapeutic devices for motor symptoms in Parkinson’s disease: current progress and a systematic review of recent randomized controlled trials. Front Aging Neurosci. 14, 807909 (2022).
Taylor, N. L. et al. The contribution of noradrenergic activity to anxiety-induced freezing of gait. Mov. Disord. 37, 1432–1443 (2022).
Lewis, S. J. & Barker, R. A. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat. Disord. 15, 333–338, (2009).
Nieuwboer, A. & Giladi, N. Characterizing freezing of gait in Parkinson’s disease: models of an episodic phenomenon. Mov. Disord. 28, 1509–1519, (2013).
Siebner, H. R. et al. Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain 126, 2710–2725 (2003).
Lee, S. Y. et al. Effects of repetitive transcranial magnetic stimulation on freezing of gait in patients with Parkinsonism. Restor. Neurol. Neurosci. 32, 743–753 (2014).
Kim, M. S. et al. Efficacy of cumulative high-freq.uency rTMS on freezing of gait in Parkinson’s disease. Restor. Neurol. Neurosci. 33, 521–530 (2015).
Chung, C. L., Mak, M. K. & Hallett, M. Transcranial magnetic stimulation promotes gait training in Parkinson disease. Ann. Neurol. 88, 933–945 (2020).
Rektorova, I. et al. Repetitive transcranial stimulation for freezing of gait in Parkinson’s disease. Mov. Disord. 22, 1518–1519 (2007).
Lee, Y. Y., Li, M. H., Tai, C. H. & Luh, J. J. Corticomotor excitability changes associated with freezing of gait in people with Parkinson disease. Front Hum Neurosci. 14, 190 (2020).
Casula, E. P. et al. Low-frequency rTMS inhibitory effects in the primary motor cortex: insights from TMS-evoked potentials. Neuroimage 98, 225–232 (2014).
Lang, N. et al. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin. Neurophysiol. 117, 2292–2301 (2006).
Filipovic, S. R., Rothwell, J. C. & Bhatia, K. Slow (1 Hz) repetitive transcranial magnetic stimulation (rTMS) induces a sustained change in cortical excitability in patients with Parkinson’s disease. Clin. Neurophysiol. 121, 1129–1137, (2010).
Pinto de Souza, C. et al. Spinal cord stimulation improves gait in patients with Parkinson’s disease previously treated with deep brain stimulation. Mov. Disord. 32, 278–282 (2017).
Samotus, O., Parrent, A. & Jog, M. Spinal cord stimulation therapy for gait dysfunction in advanced Parkinson’s disease patients. Mov. Disord. 33, 783–792 (2018).
Zhou, P. B. & Bao, M. Spinal cord stimulation treatment for freezing of gait in Parkinson’s disease: a case report. Brain Stimul. 15, 76–77 (2022).
de Lima-Pardini, A. C. et al. Effects of spinal cord stimulation on postural control in Parkinson’s disease patients with freezing of gait. Elife 7, e37727 (2018).
Reis Menezes, J. et al. Transcutaneous magnetic spinal cord stimulation for freezing of gait in Parkinson’s disease. J. Clin. Neurosci. 81, 306–309 (2020).
Filipovic, S. R., Kacar, A., Milanovic, S. & Ljubisavljevic, M. R. Neurophysiological predictors of response to medication in Parkinson’s disease. Front Neurol. 12, 763911 (2021).
Kolmancic, K. et al. Continuous dopaminergic stimulation improves cortical maladaptive changes in advanced Parkinson’s disease. Mov. Disord. 37, 1465–1473 (2022).
Suppa, A. et al. The effect of L-dopa in Parkinson’s disease as revealed by neurophysiological studies of motor and sensory functions. Expert Rev. Neurother. 17, 181–192 (2017).
Sun, H. et al. Cortical disinhibition drives freezing of gait in Parkinson’s disease and an exploratory repetitive transcranial magnetic stimulation study. Mov. Disord. 38, 2072–2083 (2023).
Chang, W. H. et al. Effect of dual-mode and dual-site noninvasive brain stimulation on freezing of gait in patients with Parkinson disease. Arch. Phys. Med. Rehabil. 98, 1283–1290 (2017).
Manor, B. et al. Multitarget transcranial electrical stimulation for freezing of gait: a randomized controlled trial. Mov. Disord. 36, 2693–2698 (2021).
Brys, M. et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial. Neurology 87, 1907–1915 (2016).
Fuentes, R. et al. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science 323, 1578–1582 (2009).
Bentley, L. D. et al. Brain activity modifications following spinal cord stimulation for chronic neuropathic pain: a systematic review. Eur. J. Pain 20, 499–511 (2016).
Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 10, 1–17 (2017).
Brudzynski, S. M., Wu, M. & Mogenson, G. J. Decreases in rat locomotor activity as a result of changes in synaptic transmission to neurons within the mesencephalic locomotor region. Can. J. Physiol. Pharmacol. 71, 394–406 (1993).
Russo, S. et al. TAAC - TMS adaptable auditory control: a universal tool to mask TMS clicks. J. Neurosci. Methods 370, 109491 (2022).
Hassan, U., Pillen, S., Zrenner, C. & Bergmann, T. O. The brain electrophysiological recording & stimulation (BEST) toolbox. Brain Stimul. 15, 109–115 (2022).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Shine, J. M. et al. Assessing the utility of freezing of gait questionnaires in Parkinson’s disease. Parkinsonism Relat. Disord. 18, 25–29 (2012).
Scheffels, J. F., Frohlich, L., Kalbe, E. & Kessler, J. Concordance of mini-mental state examination, montreal cognitive assessment and Parkinson neuropsychometric dementia assessment in the classification of cognitive performance in Parkinson’s disease. J. Neurol. Sci. 412, 116735 (2020).
Mi, T. M. et al. High-frequency rTMS over the supplementary motor area improves freezing of gait in Parkinson’s disease: a randomized controlled trial. Parkinsonism Relat. Disord. 68, 85–90 (2019).
Ma, J. et al. Repetitive transcranial magnetic stimulation does not improve the sequence effect in freezing of gait. Parkinsons Dis. 2019, 2196195 (2019).
Criminger, C. & Swank, C. Impact of dual-tasking on mobility tasks in Parkinson’s disease as described through 2D kinematic analysis. Aging Clin. Exp. Res. 32, 835–840 (2020).
Peurala, S. H., Muller-Dahlhaus, J. F., Arai, N. & Ziemann, U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clin. Neurophysiol. 119, 2291–2297 (2008).
Tokimura, H. et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J. Physiol. 523, 503–513 (2000).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82271273) and the Jiangsu Social Development Project (No. BE2022808). We are grateful to all the participants for their patience and understanding, as well as all members of the neurology for their cooperation and support.
Author information
Authors and Affiliations
Contributions
Lina Wang: conception and design of the study, acquisition and analysis of the data, drafting and revising the work, final approval of the completed version. Huimin Sun: acquisition and analysis of the data, final approval of the completed version. Heng Zhang: acquisition of the data, revising the manuscript, final approval of the completed version. Min Ji: acquisition of the data, final approval of the completed version. Caiting Gan: analysis of the data, revising the manuscript, final approval of the completed version. Aidi Shan: analysis of the data, revising the manuscript, final approval of the completed version. Xingyue Cao: revising the manuscript, final approval of the completed version. Yongsheng Yuan: revising the manuscript, final approval of the completed version. Kezhong Zhang: conception and design of the study, revising the work, final approval of the completed version, accountability for all aspects of the work, obtaining funding. Lina Wang, Huimin Sun, and Heng Zhang contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, L., Sun, H., Zhang, H. et al. Effect of cerebrospinal dual-site magnetic stimulation on freezing of gait in Parkinson’s disease. npj Parkinsons Dis. 10, 183 (2024). https://doi.org/10.1038/s41531-024-00792-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-024-00792-1
This article is cited by
-
An L-shaped flexible neural implant for chronic ECoG signal acquisition in M2 region of control and Parkinsonian rat models
Scientific Reports (2025)
-
Advancements in invasive and non-invasive neuromodulation for Parkinson’s disease: current findings and future directions
npj Parkinson's Disease (2025)