Abstract
Neurofilament light chain protein (NfL) is a valuable biomarker for the differential diagnosis between Parkinson’s disease (PD) and atypical parkinsonian disorders (APD). Here, we compared the performance of four cerebrospinal fluid (CSF) and three plasma NfL immunoassays in 253 PD and 265 APD. We measured NfL by ELISA in CSF and by SiMoA, CLEIA, and ELLA in both CSF and plasma. Additionally, we assessed Lewy body pathology by CSF α-synuclein real-time quaking-induced conversion assay (α-syn-RT-QuIC). In each biofluid, the tested assays showed comparable precision; however, CSF NfL showed higher diagnostic accuracy than plasma NfL for discriminating PD from APD (AUC range 0.966–0.974 vs 0.917–0.924). Combining CSF NfL and α-syn-RT-QuIC increased diagnostic accuracy. These results confirm the high diagnostic value of NfL in patients with parkinsonism, even when different assays are used. Combining CSF NfL and α-syn-RT-QuIC provides the highest accuracy, followed by CSF NfL and plasma NfL.
Similar content being viewed by others
Introduction
The neurofilament light chain protein (NfL), expressing neuroaxonal injury, is an established valuable biofluid marker supporting the differential diagnosis between Parkinson’s disease (PD) and atypical parkinsonian disorders (APD), the latter including multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). In contrast to PD, APD patients manifest a significant increase in NfL levels since the early disease stage, allowing their discrimination1,2,3,4,5.
Following the application of enzyme-linked immunosorbent assays (ELISA) to measure NfL in cerebrospinal fluid (CSF), the development of the ultrasensitive technology single molecule array (SiMoA) detecting protein concentrations up to the femtomolar/sub picogram range allowed for the first time the accurate measurement of NfL in blood and showed a significant correlation of the biomarker levels in the two biofluids6. More recently, however, other automated platforms exploiting chemiluminescent enzyme immunoassay (CLEIA) or ELISA (ELLA) have recently become available to measure NfL in blood, creating the foundation for the widespread use of the biomarker in clinical practice.
However, to date, no study has systematically compared the performance of the available assays in both matrices in a cohort with homogeneous preanalytical sample handling and standardized clinical measures. Moreover, the initial results on the high diagnostic value of NfL across neurodegenerative Parkinsonism, and especially the comparison of the biomarker performance in CSF and blood, still need to be extended to sizeable cohorts before translating the test to the clinical setting.
Here, we performed a head-to-head comparison of four CSF and three plasma NfL assays in a large, monocentric cohort representative of the full spectrum of neurodegenerative Parkinsonism. We assessed each assay’s ability to discriminate PD from APD. Additionally, we expanded initial findings on the added diagnostic value of combining CSF NfL dosage with the α-synuclein (α-syn) real-time quaking-induced conversion (RT-QuIC) assay, an established biomarker of Lewy body pathology.
Results
Participants
Of the 585 participants with Parkinsonism, 231 (39.3%) were women, and their mean age at biofluid sampling was 64.8 ± 9.7 years. Males were more frequent in the PD/PD cognitively impaired (PD-CI) cohort compared with the other groups (P < 0.0001), and patients with PD-CI, and PSP/CBS were significantly older than those with PD and MSA (P < 0.001 for all comparisons). The baseline demographic and clinical features of the study cohort and biomarker levels are summarized in Table 1. NfL was significantly associated with age in both CSF and plasma (β range, 0.120-0.385, P < 0.01 for all analyses), and with body mass index (BMI, β range, −0.153 to −0.184, P < 0.01 for all analyses), estimated Glomerular Filtration Rate (eGFR β range, −0.311 to −0.334, P < 0.001 for all analyses), and chronic kidney disease (CKD) (odd ratio range, 1.03–1.07, P < 0.001 for all analyses) in plasma. Additionally, CSF and plasma NfL levels were associated with disease duration (β range, 0.262-0.305, P < 0.001), Mini-Mental state examination score (MMSE: β range, −0.220 to −0.313, P < 0.01), Unified Parkinson’s Disease Rating Scale-section III score (β range, 0.329–0.457, P < 0.01) and Hoen & Yahr stage (β range, 0.242–0.355, P < 0.01) in the PD and PD-CI groups, independently from used assay. Finally, there were no significant associations with diabetes, history of cerebrovascular and/or cardiovascular disease and biomarker levels for both biofluids.
Correlations of NfL values between assays and biofluids
We first compared the NfL values measured by the different assays in each biofluid. Then, we explored the correlation between CSF and plasma NFL values obtained by each assay (i.e., NfLCLEIA, NfLSIMOA, and NfLELLA) (Fig. 1).
a Heatmap of Spearman correlation between assays in the whole cohort. b–d CSF/plasma correlations, stratified for disease, of NfLCLEIA, NfLSIMOA, and NfLELLA. CSF cerebrospinal fluid, MSA multiple system atrophy, PD Parkinson’s disease, PD-CI PD-cognitively impaired, PSP/CBS progressive supranuclear palsy/corticobasal syndrome.
There was a high concordance between NfL levels measured by different assays in CSF (ρ range, 0.965–0.982) and, to a lesser extent, in plasma (ρ range, 0.907–0.931). In contrast, the correlation between CSF and plasma NfL values (ρ range, 0.747–0.775) was moderate overall and weaker in MSA than in the other groups.
Diagnostic value of CSF and plasma NfL in the differential diagnosis of patients with parkinsonism
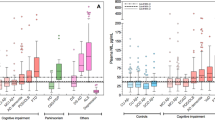
Independent of the assay used, patients with APD showed significantly higher CSF and plasma NfL levels than those with PD and PD-CI. Individuals with MSA showed higher mean CSF values than those with PSP/CBS, but the difference was no longer evident in plasma (Fig. 2).
a–d NfL analyses in CSF. e–g NfL analyses in plasma. Violin plots show value distributions; the horizontal bar indicates the median value. To improve readability, only statistically significant values reaching p ≤ 0.001 (***) are shown. CSF cerebrospinal fluid, HC healthy controls, MSA multiple system atrophy, PD Parkinson’s disease, PD-CI PD-cognitively impaired, PSP/CBS progressive supranuclear palsy/corticobasal syndrome.
ROC curve analysis demonstrated high accuracy for both CSF NfL and plasma NfL in distinguishing PD from APD with no significant differences between assays in CSF (AUC range, 0.966–0.974, P > 0.05 for all comparisons) and plasma (AUC range, 0.917–0.924, P > 0.05 for all comparisons) (Fig. 3, Tables 2 and 3).
a ROC curves showing the discrimination between PD and APD (i.e., APD = MSA + PSP/CBS), and (b) AUC value for each assay. ROC curve analysis for the differential diagnosis of (c) PD vs MSA, and (d) PD vs PSP/CBS. APD atypical parkinsonian disorder, AUC area under the curve, CSF cerebrospinal fluid, MSA multiple system atrophy, PD Parkinson’s disease, PSP/CBS progressive supranuclear palsy/corticobasal syndrome, ROC receiver operating characteristic.
Similarly, different CSF and plasma assays performed similarly in discriminating between PD and APD groups, and all reached the highest accuracy in distinguishing PD and MSA.
Compared to cognitively unimpaired PD, the diagnostic value of NfL, especially in plasma, was lower in discriminating PD-CI from the other groups (Tables 2 and 3). Consistently, merging the PD and PD-CI groups reduced the accuracy of all measures (PD/PD-CI vs APD: ΔAUC range, −0.010 to −0.014 in CSF; −0.026 to −0.031 in plasma).
Detailed diagnostic performances and cut-offs for each CSF and plasma NfL assay are reported in Table 4.
Diagnostic value of combining CSF NfL and α-syn RT-QuIC in parkinsonism
In line with previous studies5,7, the α-syn RT-QuIC assay showed high sensitivity in PD and PD-CI patients (249 positives out of 272, 91.5%) while showing a negative response in most MSA and PSP/CBS participants (5 positives out of 241, 2.1%) (raw data of aggregation kinetics in Supplementary Figure 1 and Supplementary Table 1). Following a previously described approach,53 we evaluated the diagnostic value of the combined CSF NfL and α-syn RT-QuIC analysis. We found significantly greater sensitivity and specificity of the combined test than CSF NfL alone in discriminating the merged PD/PD-CI from APD (Δaccuracy range, +7.2% to +10.7%). The added value of the combined test was most pronounced in the PD-CI group due to the higher median CSF NfL values and higher sensitivity of the α-syn RT-QuIC in PD-CI than in PD (Table 5).
Discussion
The NfL protein has emerged as the most valuable biomarker for detecting and measuring neuroaxonal injury in patients with neurological disorders. The availability of highly sensitive analytical platforms enabling reliable quantification of NfL in blood samples has laid the basis for using NfL as a diagnostic and prognostic biomarker in clinical practice. Using one of the largest cohorts studied to date, we sought to directly compare currently available assays for NfL determination in CSF and blood to establish their relative accuracy in the differential diagnosis between PD and APD.
All tested assays displayed relatively high and consistent diagnostic accuracy, with CSF NfL performing slightly better than plasma NfL. CSF and plasma biomarker values showed a good overall correlation, which, however, was weaker in MSA due to a lower relative increase in NfL levels in plasma than in CSF compared to the other APD groups. Although there is no definitive explanation for the latter divergent behaviour of MSA, one possibility could be the lower leakage of proteins into the blood compared to CSF from brain regions (e.g., the cerebellum) that show significant degeneration only in MSA.
In addition to confirming previous findings on the diagnostic values of both CSF and blood NfL in patients with parkinsonism3,4,5, our results support the use of all tested assays and provide potential reference cut-offs for each diagnostic platform and biofluid to be tested in the interlaboratory setting (Table 4). Of note, despite the comparable accuracy, we observed assay differences in absolute biomarker levels that are more pronounced in plasma and likely depend on the intrinsic analytic features of each platform. Accordingly, discriminative cut-offs slightly differ between the different immunoassays and are not interchangeable. Nonetheless, all NfL assays we tested in CSF and plasma showed a comparable ability to discriminate between neurodegenerative parkinsonism, supporting their use in clinical practice.
Besides the well-established association between NfL levels and increased age8, we also confirmed that plasma NfL is negatively associated with BMI and eGFR, meaning that the biomarker concentrations are expected to be decreased in obese patients while increasing in those with CKD9,10,11. Moreover, disease duration12, severity of motor symptoms13, and MMSE14 are all associated with NfL levels in PD, as we also confirmed in our cohort. Therefore, the diagnostic value of NfL decreases in older PD patients or those showing signs of advanced disease. Accordingly, we observed a drop in the diagnostic accuracy of NfL in discriminating PD-CI and APD (i.e., PD-CI participants were older, had longer disease duration and lower MMSE scores than cognitively unimpaired PD).
Our results also confirm in a larger cohort the added value of combining the assessment of CSF NfL with α-syn RT-QuIC5. The combined evaluation resulted in a noticeable increase in sensitivity and specificity and, above all, in a comparable accuracy in distinguishing between PD and PD-CI groups from APD.
The α-syn RT-QuIC assay we applied in this study is highly specific for the seeds of misfolded α-syn associated with Lewy body disease (LBD) but does not help in the distinction between MSA and PSP/CBS or other non-LBD parkinsonism since it does not recognize the misfolded α-syn targeting oligodendrocytes as glial cytoplasmic inclusions (GCIs) in MSA, which has a different structure15,16. Recently, a novel standardized fast α-syn seed amplification (SAA) for detecting and differentiating MSA from LBD synucleinopathies based on the amplification pattern has shown a good performance17. However, in this assay, the outcome must be determined according to two signal thresholds (negative vs MSA-like vs. LBD-like) rather than one (negative vs LBD positive), which might negatively affect specificity. Therefore, It would be interesting to explore whether the combined analysis with NfL we have applied here would have an added diagnostic value also using the α-syn SAA protocol, which discriminates between LBD and MSA.
The present study is not free of limitations. First, the diagnoses were based on clinical criteria. However, physicians specialized in movement disorders formulated the diagnoses at baseline and over time with reassessments at each follow-up visit. Moreover, the inclusion of patients only fulfilling the criteria for “probable” or “clinically established” disease to assure the highest likelihood of a correct diagnosis may limit the generalization of our findings to the clinical practice setting.
In conclusion, we show relatively high and consistent accuracy for several NfL immunoassays for both CSF and plasma. However, the plasma NfL assays’ meaningful lower performance than CSF NfL, especially in MSA, must be considered. We also confirm the superior performance of combining NfL determination with α-syn RT-QuIC. The findings support using NfL assays as a biomarker for neurodegenerative parkinsonism in drug trials and clinical practice.
Methods
Participants
The study included 585 individuals with parkinsonism enrolled at the Istituto delle Scienze Neurologiche di Bologna between 2007 and 2023 (retrospective cohort from 2007 to 2020 and prospective cohort from 2021 to 2023), and 40 healthy controls. We included patients with at least one biofluid (CSF or plasma) available and final clinical diagnosis of “probable” or “clinically established” according to current internationally established criteria (PD18, n = 253; PD-CI19, n = 67; MSA20, n = 141; PSP/CBS21,22, n = 124). The PD-CI group included patients with dementia or mild cognitive impairment at the CSF collection. Among them, 514 participants had CSF and 444 blood (both biomatrices were available in 373). All healthy controls had blood available. A movement disorder specialist (GCB, GG, LS, SC, PC) formulated the clinical diagnosis at baseline and re-evaluated it at each follow-up visit (mean follow-up 2.0 ± 2.5 years).
In all cases, sex (assigned, not self-reported), age at disease onset, time from disease onset to lumbar puncture, follow-up duration, CKD defined by an eGFR <60 mL/minute/1.73 m2), history of cerebrovascular disease (i.e., neuroradiologic assessment), cardiovascular disease (angina, heart attack) disease were collected. eGFR was calculated using the EPI-CKD equation23. Additionally, the scores of MMSE24, Unified Parkinson’s Disease Rating Scale-section III25, and Hoehn & Yahr stage26, were recorded, when available, in the PD and PD-CI groups.
Sample collection
CSF samples were obtained in the morning under fasting conditions by lumbar puncture at the L3/L4 or L4/L5 level, collected in 5 ml polypropylene tubes (Sarstedt, code number 63.504.027). Samples showing signs of blood contamination (even minimal) were centrifuged at room temperature at 2000 g for 10 min. Each sample (supernatant or non-centrifuged CSF) was divided into aliquots and stored in polypropylene tubes at − 80 °C until analysis.
EDTA plasma samples were collected by venous puncture, aliquoted, and stored at −80 °C according to standard procedures27.
Biomarker analyses
All biomarker analyses were performed by personnel blinded to the clinical diagnostic group. The samples were analyzed randomly to avoid bias due to the effect of interassay variability on specific patient groups.
In blood and CSF, NfL analyses were performed by single molecule array (NfLSIMOA) immunoassays, automated chemiluminescent enzyme immunoassay (NfLCLEIA), and ELLA automated microfluidic-based ELISA (NfLELLA). In CSF, NfL was additionally measured by standard manual ELISA (NfLELISA).
CSF NfLELISA was quantified by a validated commercial enzyme-linked immunosorbent assay (NfL ELISA kit, IBL, Hamburg, Germany)28. The mean intra- and inter-assay coefficients of variation (CVs) were 4% and 10%.
NfLSIMOA analyses were performed on a SiMOA SR-X analyzer platform (Quanterix, Billerica, MA, USA) using the SiMOA NF-light kit. The mean intra- and inter-assay CVs were 6% and 15% for CSF and 3% and 10% for plasma, respectively.
NfLCLEIA was measured on the Lumipulse G600II platform (Fujirebio, Gent, Belgium) using the Lumipulse® G NfL CSF and Lumipulse® G NfL blood kits. The mean interassay CVs were 7% for CSF and 11% for plasma, respectively.
NfLELLA was analyzed using a commercial kit for the ELLA microfluidic system (BioTechne, Minneapolis, USA). The mean interassay CVs were 5% for CSF and 7% for plasma, respectively.
Approximately half of the patients (n = 355) were included in a previous study reporting results of NfL analyzed by standard ELISA (NfL ELISA) in the CSF and by SiMoA (NfL SIMOA) in plasma5. CSF and plasma samples generating the NfLELISA and plasma NfLSIMOA data included in our previous study5 were not re-analyzed. To evaluate the effect of different batches and sample storage duration on the reproducibility of NfL levels, we included the same internal quality control samples (i.e., three samples loaded in duplicate) in all plates. We did not observe significant variations of the intra- and inter-assay CVs between the two study periods [CSF NfLELISA interassay CVs 2% (2021) vs. 4% (2024), interassay CVs 10% (2021) vs. 10% (2024); plasma NfLSIMOA interassay CVs 4% (2021) vs. 3% (2024), interassay CVs 11% (2021) vs. 10% (2024)].
All CSFs were tested by the α-syn RT-QuIC assay. The purification of recombinant wild-type α-syn was performed according to a previously published protocol29. For analyses, we followed a previously published protocol30. Briefly, six 0.8-mm silica beads (OPS Diagnostics) per well were pre-loaded into black 96-well plates with a clear bottom (Nalgene Nunc International). CSF samples were thawed and vortexed for 10 seconds before use. Then, 15 μl CSF was added to 85 μl of reaction mix composed of 40 mM PB, pH 8.0, 170 mM NaCl, 10 µM thioflavin-T (Sigma), 0.0015% SDS (Bio-Rad) and 0.1 g l − 1 of filtered recombinant α-syn (100-kDa Amicon centrifugal filters, Merck Millipore). Plates were closed with a plate sealer film (Nalgene Nunc International) and incubated into a FLUOstar Omega plate reader (BMG Labtech) at 42 °C with intermittent double orbital shaking at 400 r.p.m. for 1 min, followed by 1 min rest. Fluorescence was measured every 45 min with 450 nm excitation and 480 nm emission filters during the 30-hour test run. We ran each CSF sample in quadruplicate. Each plate was designed to include internal positive (min, n = 1, to max, n = 3) and negative (n = 1) controls. Controls were the same in each set of experiments. The test run duration was set at 30 h. The threshold to define a positive replicate among tested samples was arbitrarily set at 30% of the median of the maximum fluorescence intensity of each plate’s positive replicates of control samples and evaluated within the first 25 h. CSF samples were deemed “positive” when at least 2 out of 4 replicates reached the threshold, “negative” when none reached the threshold, and “unclear” when only 1 out of 4 replicates reached the threshold. “Unclear” cases were re-analysed up to three times (12 replicates); then, cases showing ≥ 4 positive replicates out of 12 were classified “positive”, otherwise they were deemed “negative”29.
Statistical analyses
SPSS version 22 (IBM) and GraphPad were used for statistical analyses. Outliers (n = 6) with NfL values above 5 standard deviations of the mean were excluded. Correlations between biomarkers were assessed with the Spearman test (p). Univariate linear regression analyses were used to test the association of clinical/demographic variables with NfL: the results are presented as Beta coefficients (β) and 95% CI. Binomial logistic regressions were used to test the association with comorbidities: results are presented as odd ratios and 95% CI. Differences between diagnostic groups were tested using the Kruskal-Wallis test (followed by Dunn’s post hoc test) or the chi-square test as appropriate. NfL concentrations in CSF and plasma were transformed by natural log (ln) to fulfil the normal distribution. All statistical analyses were performed on transformed data: NfL differences between diagnostic groups were investigated with univariate general linear models adjusting for age and sex. Unadjusted 2-sided P values < 0.05 were considered statistically significant. Discrimination accuracies of biomarkers were determined with receiver operating characteristic (ROC) curve analysis. The optimal cut-off value for NfL in both CSF and plasma was chosen using Youden’s Index. The two tests were also analysed in parallel to investigate the performance of the CSF NfL and α-syn RT-QuIC assays in combination. The rationale behind this approach relies on the different nature of the two markers. While NfL is a marker of neurodegeneration not specific to a given pathology, which can increase in conditions unrelated to parkinsonism or disease severity (e.g., increased NfL levels in “true” PD patients might prompt an incorrect APD diagnosis), the α-syn RT-QuIC is a highly sensitive and specific marker of LBD that is expected to be positive in most PD patients. Therefore, the combined approach aims to increase NfL specificity, using α-syn RT-QuIC to maintain high sensitivity. To this aim, we first chose a new cut-off for CSF NfL that maximizes its specificity (see also Table 5). Then, we formulated a diagnosis of PD when at least one of the two tests was positive (i.e., CSF NfL lower than the cut-off value and/or positive α-syn RT-QuIC seeding activity) and a diagnosis of APDs when both tests were negative (for PD). De Long test was used to compare the AUC between the tests performed individually and the combined test.
Ethics approval
This study involved human participants and was approved by the local ethics committee (approval numbers AVEC: 09070, 17093, 18025, and 18027). Written informed consent was given by study participants or by the next of kin for patients unable to communicate.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Abdo, W. F., Bloem, B. R., Van Geel, W. J., Esselink, R. A. J. & Verbeek, M. M. CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiol. Aging 28, 742–747 (2007).
Constantinescu, R., Rosengren, L., Johnels, B., Zetterberg, H. & Holmberg, B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson’s disease and atypical Parkinsonian disorders. Parkinsonism Relat. Disord. 16, 142–145 (2010).
Hansson, O. et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 88, 930–937 (2017).
Marques, T. M. et al. Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 92, e1479–e1486 (2019).
Quadalti, C. et al. Neurofilament light chain and α-synuclein RT-QuIC as differential diagnostic biomarkers in parkinsonisms and related syndromes. NPJ Parkinsons Dis. 7, 93 (2021).
Barro, C., Chitnis, T. & Weiner, H. L. Blood neurofilament light: a critical review of its application to neurologic disease. Ann. Clin. Transl. Neurol. 7, 2508–2523 (2020).
Rossi, M. et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 140, 49–62 (2020).
Bridel, C. et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 76, 1035–1048 (2019).
Dittrich, A. et al. Association of Chronic Kidney Disease With Plasma NfL and Other Biomarkers of Neurodegeneration: The H70 Birth Cohort Study in Gothenburg. Neurology 101, e277–e288 (2023).
Manouchehrinia, A. et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann. Clin. Transl. Neurol. 7, 139–143 (2020).
Pichet Binette, A. et al. Confounding factors of Alzheimer’s disease plasma biomarkers and their impact on clinical performance. Alzheimers Dement 19, 1403–1414 (2023).
Lerche, S. et al. CSF NFL in a Longitudinally Assessed PD Cohort: Age Effects and Cognitive Trajectories. Mov. Disord. 35, 1138–1144 (2020).
Aamodt, W. W. et al. Neurofilament Light Chain as a Biomarker for Cognitive Decline in Parkinson Disease. Mov. Disord. 36, 2945–2950 (2021).
Lin, C.-H. et al. Blood NfL: A biomarker for disease severity and progression in Parkinson disease. Neurology 93, e1104–e1111 (2019).
Shahnawaz, M. et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578, 273–277 (2020).
Schweighauser, M. et al. Structures of α-synuclein filaments from multiple system atrophy. Nature 585, 464–469 (2020).
Concha-Marambio, L., Pritzkow, S., Shahnawaz, M., Farris, C. M. & Soto, C. Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nat. Protoc. 18, 1179–1196 (2023).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Poewe, W. et al. Diagnosis and management of Parkinson’s disease dementia. Int J. Clin. Pr. 62, 1581–1587 (2008).
Wenning, G. K. et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 37, 1131–1148 (2022).
Höglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 32, 853–864 (2017).
Armstrong, M. J. et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503 (2013).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern Med 150, 604–612 (2009).
Measso, G. et al. The mini-mental state examination: Normative study of an Italian random sample. Develop. Neuropsychol. https://doi.org/10.1080/87565649109540545. (1993).
Fahn, S., Elton, R. & Members of the UPDRS Development Committee Unified Parkinson’s Disease Rating Scale. Recent Dev. Parkinson’s Dis. 2, 293–304 (1987).
Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression and mortality. Neurology 17, 427–442 (1967).
Baiardi, S. et al. Diagnostic value of plasma p-tau181, NfL, and GFAP in a clinical setting cohort of prevalent neurodegenerative dementias. Alzheimers Res Ther. 14, 153 (2022).
Abu-Rumeileh, S. et al. The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res Ther. 10, 3 (2018).
Mammana, A. et al. Improving protocols for α-synuclein seed amplification assays: analysis of preanalytical and analytical variables and identification of candidate parameters for seed quantification. Clin. Chem. Lab Med. https://doi.org/10.1515/cclm-2023-1472. (2024).
Quadalti, C. et al. Clinical effects of Lewy body pathology in cognitively impaired individuals. Nat. Med 29, 1964–1970 (2023).
Acknowledgements
The study was supported by the grant “Ricerca Corrente” funded by the Italian Ministry of Health, and the #NextGenerationEU (NGEU), funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006).
Author information
Authors and Affiliations
Contributions
S.B. and P.P. contributed to the conception and design of the study. S.B., M.R., G.G., A.M., B.P., L.S., F.M., A.M., P.C., S.C., G.C.B., and P.P. contributed to the acquisition and analysis of the data. S.B. and P.P. contributed to drafting the text and preparing the figures. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Baiardi, S., Rossi, M., Giannini, G. et al. Head-to-head comparison of four cerebrospinal fluid and three plasma neurofilament light chain assays in Parkinsonism. npj Parkinsons Dis. 11, 98 (2025). https://doi.org/10.1038/s41531-025-00951-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-00951-y