Abstract
Heterozygous GBA1 variants increase Parkinson’s disease (PD) risk with variable penetrance. We investigated the interaction between genome-wide polygenic risk scores (PRS) and severity of pathogenic GBA1 variants (GBA1PVs) to assess their combined impact on PD risk. GBA1 variants were identified from whole exome sequencing in the UK Biobank and targeted PacBio sequencing in the Luxembourg Parkinson’s Study, with PRS calculated using genome-wide significant SNPs. GBA1PVs were present in 8.8% of PD patients in the UK Biobank and 9.9% in LuxPark, with carriers showing consistently higher PD risk across all PRS categories. In the highest PRS category, PD risk increased 2.3-fold in the UK Biobank and 1.6-fold in LuxPark. Severe and mild GBA1 variants conferred nearly double the risk of PD compared to risk variants. Our findings demonstrate the impact of PRS on GBA1PVs penetrance, highlighting implications for genetic counseling and clinical trial design in GBA1-associated PD.
Similar content being viewed by others
Introduction
Pathogenic variants in the GBA1 gene encoding the lysosomal enzyme glucocerebrosidase (GCase) are the most common genetic risk factors for Parkinson’s disease (PD)1,2. Initially associated with Gaucher’s disease (GD) in its biallelic form, GBA1 variants also increase PD risk in heterozygous carriers. GBA1 variants are prevalent in 5–20% of PD patients in different populations worldwide, with a recent study identifying a common intronic variant in 40% of African PD patients3. The risk of developing PD follows a gradient, with severe GD-causing variants (e.g., L483P) raising PD risk 9- to 10-fold, while mild variants (e.g., N409S) increase PD risk approximately 4-fold2,4. Additionally, non-GD-causing GBA1 variants, such as E365K and T408M, are more frequently observed in PD patients and are considered significant risk factors for PD2,4. GBA1-associated PD (GBA1-PD) is typically characterized by earlier onset, more rapid progression, and a higher frequency of non-motor symptoms5.
The penetrance of GBA1-PD is variable and age-dependent, with estimates ranging from approximately 7.6% at 50 years to 29.7% at 80 years under a dominant model6. This gradual increase in risk complicates accurate risk assessment for carriers7. Therefore to define individual risk, it is essential to understand the role of a ‘genetic background’ as defined by common variants associated with PD risk and identified by genome-wide association studies (GWAS)8 among GBA1 carriers, such as the polygenic background has been found to modulate the PD risk of GBA1 variants in carriers of p.E365K, p.T408M and p.N409S variants, and decreasing the age of onset (AAO) of PD9. Moreover, variants in the PD genetic risk score were more frequent in GD patients who developed PD, suggesting that common variants may play a role in shared biological pathways underlying both conditions10.
Our study extends previous research by analyzing the impact of polygenic risk scores (PRS) across a broader range of GBA1 pathogenic variant carriers (GBA1PVs) in the UK Biobank (UKB), with validation in the Luxembourg Parkinson’s Study (LuxPark), focusing on how PRS and GBA1PV severity influence PD risk.
Results
Demographic characteristics and distribution of GBA1 variants in UKB and LuxPark
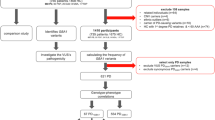
After QC, the final UKB dataset included 185,225 individuals with available WES and genotyping data at the analysis time. Descriptive statistics of the study population after filtering are shown in Table 1. The dataset consisted of 1636 PD cases, with a mean AAO of 64.6 years, and 183,589 healthy control participants, with a mean age at assessment (AAA) of 64.1 years. In the LuxPark replication cohort, a total of 1430 individuals were included, comprising 653 PD cases with a mean AAO of 62.4 years and 767 healthy controls with a mean AAA of 59.6 years. The LuxPark cohort exhibited a significantly higher proportion of individuals with a positive family history of PD (FH + ) compared to the UKB cohort (chi-squared test P < 0.01). In UKB, 57 GBA1PVs were identified in 9019 (4.8%) controls and 145 PD cases (8.8%). The mean of AAO for GBA1PVs carriers was 66.2 ± 7.2 years for PD patients and the mean of AAA was 56.6 ± 7.97 for healthy controls. Out of the 57 GBA1PVs, 40 were classified as severe, 14 as mild, and three as risk variants, with risk variants being the most common (n = 7990 carriers, Table 2). In the LuxPark cohort, targeted PacBio sequencing of the GBA1 gene previously revealed that 12.1% (77/637) of PD patients carried GBA1 variants, with 10.5% (67/637) harboring known pathogenic variants, including severe, mild, and risk variants11. For this study, we focused on a subset of individuals who were both genotyped and GBA1 PacBio-sequenced, identifying 99 carriers of GBA1PVs. This subset comprised 65 PD cases (9.9%) and 34 controls (4.4%). The mean AAO for PD cases was 61.5 ± 11.7 years, while the mean AAA for healthy controls was 59.4 ± 13.2 years. Of the 12 GBA1PVs detected, nine were classified as severe, one as mild, and two as risk variants, with risk variants being the most common (n = 68, Table 2).
Combined effect of PRS and GBA pvs status and severity on PD risk
We calculated the PRS using a panel of SNPs to investigate the influence of the genetic background on PD risk in PD GBA1PVs carriers. Our analysis revealed that the PRS was significantly higher in PD patients compared to healthy controls in both cohorts (Wilcoxon test P < 0.01).
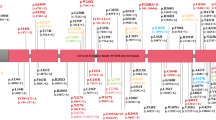
We assessed the influence of PRS and GBA1PVs carrier status on PD risk in both cohorts by calculating ORs for PD across PRS categories, using non-carriers with intermediate-PRS as the reference group. PD risk was consistently higher in GBA1PVs carriers compared to non-carriers across all PRS categories in both cohorts (Fig. 1). In UKB, non-carriers with low- or high-PRS had PD ORs of 0.75 (0.73–0.77) or 1.34 (1.32-1.36) respectively (Fig. 1). Among GBA1PVs carriers, those with high-PRS category exhibited OR of 2.34 (95% CI, 2.08–2.63) compared to carriers with low-PRS (OR: 1.13; 95% CI, 0.85–1.49) (Fig. 1). Similarly, in LuxPark, GBA1PVs carriers with high-PRS had PD ORs of 1.67 (95% CI, 1.55–1.79) compared to those with low PRS (OR: 1.25; 95% CI, 1.07–1.43), although the effect was less pronounced than in UKB. No significant interaction between GBA1PVs carrier status and PRS was observed in either UKB (P = 0.73) or LuxPark (P = 0.48). The Cox proportional hazards analysis confirmed the combined effect of carrier status and PRS on PD risk (Fig. 2, Supplementary Table 1). In UKB, among GBA1PVs carriers, individuals with a high PRS had the highest cumulative incidence of PD, reaching 12.8% by the age of 70. In contrast, carriers with a low PRS had a lower cumulative incidence of approximately 5.8% at the same age. At the same age, the cumulative incidence of the disease is consistently higher in GBA1PVs carriers compared to non-carriers. Similar PRS-stratified trends were observed in the LuxPark cohort; corresponding estimates are provided in the Supplementary Fig. 1. We performed a fisher test for each of 42 SNPS in cases compared to controls, to identify potential penetrance modifiers. After applying FDR correction for multiple testing, no SNPs showed significant differences in GBA1 carriers. However, in non-carriers, eight SNPs were significantly enriched in PD patients (FDR-adjusted p value < 0.05). Among these, only rs34311866 was replicated in the LuxPark cohort. The full results are provided in Supplementary Table 2.
Odds ratio for PD were estimated using logistic models, while conditioning on the sex, age at assessment and the first four ancestry principal components in both UK Biobank (A) and the Luxembourg Parkinson’s Study (B). Non-carriers with intermediate PRS served as the reference group. Carriers and non-carriers were categorized into categories based on their PRS.
Cumulative incidence was estimated using Cox proportional hazards models, adjusted for sex and the first four ancestry principal components. Participants were stratified by GBA1 carrier status as well as by PRS categories, in the UK Biobank. Red lines indicate GBA1 carriers and green lines indicate non-carriers. Line styles represent PRS categories, with solid lines for high PRS, dashed lines for intermediate PRS, and dotted lines for low PRS.
To gain a deeper understanding of how disease severity is influenced by PRS, we further examined the combined effect of PRS and GBA1PVs severity, categorized in two groups: “severe+mild” and “risk”. In both cohorts, we found an association between the severity of GBA1PVs and a higher OR for PD (Fig. 3). Carriers of severe+mild GBA1PVs tended to have higher risk of PD (almost twofold or higher) compared to carriers of risk GBA1PVs, regardless of their PRS category. In UKB, ORs for carriers of “severe+mild” GBA1PVs ranged from 2.05 (95% CI, 1.48–2.83) to 3.69 (95% CI, 2.68–5.04) across the PRS categories, while for carriers of risk GBA1PVs, ORs ranged from 1.07 (95% CI, 0.91–1.25) to 2.13 (95% CI, 1.87–2.42, Fig. 3). Similarly, in LuxPark, ORs for carriers of “severe+mild” GBA1PVs ranged from 1.73 (95% CI, 1.37–2.09) to 1.98 (95% CI, 1.77–2.18) across the PRS categories, while for carriers of risk GBA1PVs, ORs ranged from 1.03 (95% CI, 0.79–1.26) to 1.49 (95% CI, 1.29–1.68, Fig. 3). No significant interaction between GBA1PVs severity (respectively for severe+mild or risk) and PRS was observed in either UKB (P = 0.99 and P = 0.26) or LuxPark (P = 0.44 and P = 0.26). We performed an additional analysis grouping mild with risk variants (risk+mild) separately from severe variants. The results showed the same overall trend, with the odds of PD increasing when severe variants were analyzed independently, specifically in UK biobank (Supplementary Figs. 2a, 2b).
Discussion
In this study, we investigated the combined effect of polygenic background and PD-associated GBA1 variants on the risk of developing PD across two cohorts. Our findings show that both PRSs and GBA1PVs independently contribute to PD risk. Individuals carrying GBA1PVs consistently showed a higher baseline risk of developing PD compared to non-carriers, regardless of their PRS group. Notably, PRSs independently elevated the odds of developing PD in both carriers and non-carriers. These results suggest that the effects of GBA1PVs and PRSs are additive, where the presence of GBA1PVs contributes a fixed increase in baseline risk, and PRS independently further increases the overall risk of PD. At older ages, cumulative incidence of PD is consistently higher in GBA1PVs compared to non-carriers, therefore, highlighting the increased risk associated with carrying GBA1PVs.
Among GBA1PVs carriers, the cumulative incidence is higher in the high-PRS group compared to the low-PRs group, a trend also observed non-carriers. This suggests that PRS have an additive effect on the penetrance of GBA1PVs, amplifying the overall risk of PD. The earlier observed risk in the LuxPark cohort compared to the UKB likely results from differences in study design and recruitment strategies. The UKB represents a general population study, where early-onset PD is underrepresented, potentially leading to a later onset of PD. In contrast, LuxPark focuses on PD patients, including individuals with both early- and later-onset forms of the disease. Since such studies do not capture the full population at risk, clinically relevant cumulative incidence estimates are not suitable here; however, the data from LuxPark reflect a higher proportion of individuals with early-onset PD, leading to an earlier observed risk compared to the UKB.
There is notable variability in cumulative risk estimates for GBA1 carriers across studies, as shown in Supplementary Table 3. For example, Rana et al.11 reported a 1.0% risk at age 60, Anheim et al.6 found a 21.4% risk at age 70 in familial cohorts, while Alcalay et al.12 reported risks in an Ashkenazi Jewish cohort, with 5.2% risk in GBA1 carriers and 9.1% at age 70 in patients with Gaucher disease. In the UKB, we report a 1.7% risk in GBA1 carriers at age 60 and 10.1% at age 70. This variability likely arises from differences in study design, and cohort characteristics. Furthermore, the variance observed across PRS categories, particularly in GBA1 carriers, may contribute to these differences in risk estimates. PRS modulates this risk, as our results show a higher cumulative incidence at age 70 for GBA1 carriers with high PRS (12.8%) compared to those with low PRS (5.8%), contributing to the observed variance in risk estimates.
Using the same approach, we investigated the specific effect of GBA1PVs severity, classifying them into two groups: “severe+mild” and “risk” variants. Carriers of severe+mild GBA1PVs exhibited the highest baseline risk for developing PD, followed by those with risk variants, further emphasizing the dose-dependent effect of GBA1PVs on disease susceptibility. Importantly, PRS continued to elevate PD risk independently and additively, regardless of variants severity.
Our results suggest a clear gradient of risk driven by both GBA1PVs severity and PRS. Individuals carrying severe GBA1PVs in combination with a high PRS have the highest risk of developing PD, while those with risk variants and a low PRS have a lower, but still elevated, risk compared to non-carriers. Notably, non-carriers with a high PRS also have a substantial risk, but it remains lower than that of any variant carriers with similar PRS levels. These results highlight the complex interplay between polygenic risk and GBA1PVs, underscoring the importance of considering both factors in genetic assessments of PD risk.
Our findings are consistent with previous studies demonstrating that PRS not only modifies PD risk but also reduces the AAO in carriers of GBA1 variants, specifically for the two risk variants p.E365K and p.T408M, and the mild p.N409S variant9. Additionally, variants included in the PD PRS were found to be more frequent in patients with GD type 1 who developed PD, suggesting that common genetic risk variants may influence shared underlying biological pathways10.
A key finding of this study is that GBA1 variants and PRS independently and additively increase the risk of PD, with both factors separately contributing to the overall risk. This highlights the importance of considering PRS in clinical trial designs for GBA1-related PD, using pre-trial genetic analysis to stratify patients by both GBA1 variants and overall genetic risk9,13,14. Incorporating PRS alongside whole-exome or targeted gene sequencing in initial diagnoses could streamline healthcare costs, expand cohort sizes, and support the integration of genetic risk data into routine clinical practice15. Furthermore, combining genetic information with non-genetic factors, such as family history and presymptomatic phenotypes, could improve disease prediction and promote the development of multifactorial risk models16,17.
The results of PRS studies can be influenced by factors such as study design and recruitment strategies18. In this study, the effects were more pronounced in the discovery cohort (UKB), a population-based sample, compared to the replication cohort (LuxPark), a PD-specific case-control cohort. While both cohorts showed similar trends, the differences are likely due to specific cohort characteristics and recruitment methods. Notably, a significant discrepancy in family history between PD cases and controls was observed in the UKB but not in the Luxembourg cohort, further emphasizing the impact of study design on the outcomes.
This study has several limitations. The UKB cohort is not PD-specific and lacks age and sex matching between cases and controls; however, we mitigated this by including age and sex as covariates in the regression analysis. Additionally, the sample size is limited in certain PRS or GBA1PVs severity categories, which may lead to overestimation of effect sizes. These factors should be carefully considered when interpreting the findings. The LuxPark cohort is enriched for individuals with PD and includes relatively few GBA1 carriers without PD. Consequently, it is not appropriate for estimating population-level age-specific risk or cumulative incidence. Cumulative risk estimates presented for LuxPark in the supplementary material should be interpreted with caution, as they reflect cohort-specific trends rather than generalizable risk estimates.
Overall, this study shows that both PRS and GBA1PVs contribute independently and additively to the risk of PD. Carriers of GBA1PVs consistently show a higher baseline risk, particularly those with severe variants and high PRS, with PRS influencing GBA1PVs penetrance. These results highlight the need to integrate PRS and GBA1PVs into PD genetic assessment for better risk stratification. By deepening our understanding of this genetic landscape, especially variant severity, we can pave the way for personalized therapeutic strategies. Ultimately, this approach will bring us closer to tailoring treatments to individual genetic profiles, optimizing outcomes and improving patients’ quality of life.
Methods
The UK biobank cohort
UKB is a large, long-term prospective study comprising over 500,000 participants19. For this study, we included 185,225 individuals (1636 PD patients and 183,589 healthy controls) of European ancestry with both genotyping and whole-exome sequencing (WES) data available. Participants were genotyped using the UKB Axiom Array, and imputation was performed with the Haplotype Reference Consortium and UK10K + 1000 Genomes reference panels. Whole-exome sequencing was conducted using the IDT xGen Exome Research Panel v1.020. Ethics approval for the UK Biobank (UKB) study was obtained from the Northwest Multicentre for Research Ethics Committee (MREC). The UKB ethics statement is available at the following website (https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics). All UKB participants provided informed consent at recruitment. PD diagnosis was based on self-reports by participants or the International Classification of Diseases (ICD-10) diagnosis codes. This included individuals with self-reported code 1262 or ICD-10 code of G20 in hospitalization records. Quality control (QC) followed standard procedures. We excluded outliers with putative sex chromosome aneuploidy (field 22019), high heterozygosity or missing genotype rates (field 22027), and discordant reported versus genotypic sex (field 22001). The analysis was restricted to unrelated individuals to the second degree. The dataset is available for research purposes, and all participants provided documented consent. UKB analyses were conducted using a protocol approved by the Partners HealthCare Institutional Review Board. All study participants provided written informed consents. Participants carrying pathogenic variants in other PD-associated genes (SNCA, LRRK2, VPS35, PRKN, PINK1 and PARK7) were excluded.
The Luxembourg Parkinson’s study
For independent replication, we used 653 PD patients and 767 healthy controls from LuxPark21,22, a longitudinal monocentric study within the framework of the NCER-PD (National Center for Excellence in Research in PD). All NCER-PD participants provided written informed consent, and the study was approved by the National Research Ethics Committee (CNER Ref: 201407/13). Genotyping was carried out using the NeuroChip platform23, while GBA1 variants were identified using the GBA1-targeted PacBio sequencing method24. QC for the genotyping data has been previously described22. Participants carrying pathogenic single nucleotide variants (SNVs) or copy number variants (CNVs) in PD-associated genes (SNCA, LRRK2, VPS35, PRKN, PINK1 and PARK7) were excluded22. Additionally, we excluded individuals harboring variants of uncertain significance (VUS) or synonymous variants in the GBA1 gene. All subjects gave written informed consent. The study was approved by the National Research Ethics Committee (CNER Ref: 201407/13).
Classification of GBA1 variants
We classified GBA1 variants based on their pathogenicity in relation to PD and GD, categorizing them as risk, mild, or severe, following the classification of Höglinger and colleagues4, without further stratification by allele count. Variants identified as pathogenic for GD were classified as either mild or severe for PD, with severe variants exhibiting an odds ratio (OR) of 10–15 for developing PD, while mild variants had an OR of ≤525,26. Common variants not considered pathogenic for GD but known to increase PD risk (e.g., p.E365K, p.T408M) were categorized as risk variants. Frameshift and nonsense variants in GBA1 were classified as severe. Variants not classified by Höglinger et al. were further categorized (as severe, mild, or risk) according to the classification of Parlar and colleagues2 using the online GBA1 variant browser (https://pdgenetics.shinyapps.io/gba1browser/). Notably, carriers were identified by the presence of variants, regardless of zygosity. In the LuxPark cohort, two PD patients were found to be homozygous for the E365K risk variant.
Polygenic risk score
To generate the PRS, we used a list of 44 SNPs available from a previous meta-analysis of PD27. These SNPs were identified in independent datasets, minimizing bias from UK Biobank sample overlap with the Nalls et al. 2019 GWAS8. Variants within the GBA1 (rs35749011) and LRRK2 (rs76904798) loci were excluded from the PRS calculation, resulting in a final set of 42 SNPs used for PRS calculation. We computed PRS using PRSice228, where SNPs genotypes were treated as allele count (0, 1, 2) and weighted by their respective effect sizes, while accounting for allele-flipping. The analysis was performed using the ‘–no-regress’ and ‘–no-clumping’ options alongside default parameters.
Statistical analysis
To investigate the association between PRS, GBA1PVs carrier status, and PD risk, we performed logistic regression and Cox proportional hazards regression with disease occurrence as the outcome variable. Participants were stratified based on their PRS and GBA1PVs carrier status. Initially, individuals were categorized into equal tertiles based on their PRS distribution. Those in the lowest tertile were assigned to the low-PRS group, those in the highest tertile group to the high-PRS group, and those in the middle tertile to the intermediate-PRS group.
For both cohorts, ORs were estimated using a logistic regression model, conditioning on covariates such as sex, age at assessment, and the first four principal components. In the UK Biobank dataset, we used the principal components (PC1–4) provided by the UK Biobank, whereas for the Luxembourg Parkinson’s Study, we calculated PCs using PLINK. We applied Cox proportional hazard regression model to evaluate time-to-event data and estimate cumulative incidence risk of PD. Afterwards, we additionally incorporated interactions between GBA1PVs status and PRS by introducing an interaction term within the logistic regression model. Finally, we further categorized the GBA1PVs carriers’ participants into two groups: (1) mild and severe GBA1PVs carriers and (2) risk GBA1PVs carriers. This classification allowed to evaluate the association between different levels of GBA1PVs severity and the risk of developing PD. The reference category is given by non-carriers with intermediate-PRS. We used R v4.2.2 for all statistical analyses.
Data availability
Data used to prepare this article were obtained from UKB, and the National Center of Excellence in Research: Early diagnosis and stratification of Parkinson’s Disease (NCER-PD or the LuxPark https://www.parkinson.lu). Restrictions apply to the availability of these data for UKB, which were used under license for the current study (Project ID: 73507). Patient data used in the preparation of this manuscript were obtained from the National Center of Excellence in Research on Parkinson’s Disease (NCER-PD). NCER-PD datasets are not publicly available, as they are linked to the Luxembourg Parkinson’s Study and its internal regulations. The NCER-PD Consortium is willing to share its available data. Its access policy was devised based on the study ethics documents, including the informed consent form, as approved by the national ethics committee. Requests to access datasets should be directed to the Data and Sample Access Committee via email: request.ncer-pd@uni.lu.
References
Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361, 1651–1661 (2009).
Parlar, S. C., Grenn, F. P., Kim, J. J., Baluwendraat, C. & Gan-Or, Z. Classification of GBA1 Variants in Parkinson’s Disease: The GBA1 -PD Browser. Mov. Disord. 38, 489–495 (2023).
Rizig, M. et al. Identification of genetic risk loci and causal insights associated with Parkinson’s disease in African and African admixed populations: a genome-wide association study. Lancet Neurol. 22, 1015–1025 (2023).
Höglinger, G. et al. GBA-associated PD: chances and obstacles for targeted treatment strategies. J. Neural Transm. 129, 1219–1233 (2022).
Gan-Or, Z., Liong, C. & Alcalay, R. N. GBA-Associated Parkinson’s Disease and Other Synucleinopathies. Curr. Neurol. Neurosci. Rep. 18, 44 (2018).
Anheim, M. et al. Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology 78, 417–420 (2012).
Balestrino, R. et al. Penetrance of Glucocerebrosidase (GBA) Mutations in Parkinson’s Disease: A Kin Cohort Study. Mov. Disord. 35, 2111–2114 (2020).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Blauwendraat, C. et al. Genetic modifiers of risk and age at onset in GBA associated Parkinson’s disease and Lewy body dementia. Brain 143, 234–248 (2020).
Blauwendraat, C. et al. Polygenic Parkinson’s Disease Genetic Risk Score as Risk Modifier of Parkinsonism in Gaucher Disease. Mov. Disord. 38, 899–903 (2023).
Rana, H. Q., Balwani, M., Bier, L. & Alcalay, R. N. Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med. 15, 146–149 (2013).
Alcalay, R. N. et al. Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol. 71, 752–757 (2014).
Leonard, H. et al. Genetic variability and potential effects on clinical trial outcomes: perspectives in Parkinson’s disease. J. Med. Genet 57, 331–338 (2020).
Arena, G. et al. Polygenic Risk Scores Validated in Patient-Derived Cells Stratify for Mitochondrial Subtypes of Parkinson’s Disease. Ann. Neurol. 96, 133–149 (2024).
Lewis, A. C. F. et al. Patient and provider perspectives on polygenic risk scores: implications for clinical reporting and utilization. Genome Med. 14, 114 (2022).
Nalls, M. A. et al. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol. 14, 1002–1009 (2015).
Hassanin, E. et al. Clinically relevant combined effect of polygenic background, rare pathogenic germline variants, and family history on colorectal cancer incidence. BMC Med Genomics 16, 42 (2023).
Dueñas, N. et al. Ability of a polygenic risk score to refine colorectal cancer risk in Lynch syndrome. J. Med. Genet 60, 1044–1051 (2023).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Wang, Q. et al. Rare variant contribution to human disease in 281,104 UK Biobank exomes. Nature 597, 527–532 (2021).
Hipp, G. et al. The Luxembourg Parkinson’s Study: A Comprehensive Approach for Stratification and Early Diagnosis. Front. Aging Neurosci. 10, 326 (2018).
Landoulsi, Z. et al. Genetic landscape of Parkinson’s disease and related diseases in Luxembourg. Front. Aging Neurosci. 15, 1282174 (2023).
Blauwendraat, C. et al. NeuroChip, an updated version of the NeuroX genotyping platform to rapidly screen for variants associated with neurological diseases. Neurobiol. Aging 57, 247.e9–247.e13 (2017).
Pachchek, S. et al. Accurate long-read sequencing identified GBA1 as major risk factor in the Luxembourgish Parkinson’s study. npj Parkinsons Dis. 9, 156 (2023).
Iwaki, H. et al. Genetic risk of Parkinson disease and progression: An analysis of 13 longitudinal cohorts. Neurol. Genet 5, e348 (2019).
Straniero, L. et al. The SPID-GBA study: Sex distribution, penetrance, incidence, and dementia in GBA-PD. Neurol. Genet 6, e523 (2020).
Chang, D. et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet 49, 1511–1516 (2017).
Choi, S. W. & O’reilly, P. F. PRSice-2: Polygenic risk score software for biobank-scale data. GigaScience 8, giz082 (2019).
Acknowledgements
Data used to prepare this article were obtained from the UKB, and the National Center of Excellence in Research: Early diagnosis and stratification of Parkinson’s Disease (NCER-PD) (https://www.parkinson.lu/). This research has been conducted using the UK Biobank Resource under application number 73507. The NCER-PD is funded by the Luxembourg National Research Fund (FNR/NCER13/BM/11264123). We would like to thank all participants of the Luxembourg Parkinson’s Study for their important support to our research. Furthermore, we acknowledge the joint effort of the NCER-PD Consortium members from the partner institutions Luxembourg Center for Systems Biomedicine, Luxembourg Institute of Health, Center Hospitalier de Luxembourg, and Laboratoire National de Santé generally contributing to the Luxembourg Parkinson’s Study as listed below in the Author Information section. Parts of the computational analysis were done on the High-Performance Computing cluster of the University of Luxembourg (https://hpc.uni.lu/). The FNR supported P.M. as part of the National Center of Excellence in Research on Parkinson’s disease (NCER-PD, FNR11264123) and the DFG Research Units FOR2715 (INTER/DFG/17/11583046) and FOR2488 (INTER/DFG/19/14429377). The Fonds National de Recherche (FNR) supported D.R.B through the Industrial fellowship program of Luxembourg (FNR14323864).
Author information
Authors and Affiliations
Consortia
Contributions
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution; (3) Data: A. Acquisition B. Curation; (4) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique. E.H.: 1A, 1B, 1C, 2A, 2B, 4A, 4B. Z.L.: 3A, 3B, 4A, 4B. S.P.: 3A, 3B, 4A, 4B. R.A.: 1A, 4B. R.K.: 3A, 4B. P.K.: 4B. C.M.: 1A, 1B,1C, 4B. P.M.: 1A, 1B, 1C, 3A, 3B, 4A, 4B. D.R.B.: 1A, 1B, 1C, 4A, 4B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassanin, E., Landoulsi, Z., Pachchek, S. et al. Penetrance of Parkinson’s disease in GBA1 carriers depends on variant severity and polygenic background. npj Parkinsons Dis. 11, 162 (2025). https://doi.org/10.1038/s41531-025-00997-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-00997-y