Abstract
Alpha-synuclein (aSyn) post-translational modifications (PTM), especially phosphorylation at serine 129 and C-terminal truncations, are highly enriched in Lewy bodies (LB), Lewy neurites, and other pathological aggregates in Parkinson’s disease and synucleinopathies. However, the precise role of these PTM in pathology formation, neurodegeneration, and pathology spreading remains unclear. Here, we systematically investigated the role of post-fibrillization C-terminal aSyn truncations in regulating uptake, processing, seeding, and LB-like inclusion formation using a neuronal seeding model that recapitulates LB formation and neurodegeneration. We show that C-terminal cleavage of aSyn fibrils occurs rapidly post exogenous fibril internalization and during intracellular LB-like inclusion formation. Blocking cleavage of internalized fibrils does not affect seeding, but inhibiting enzymes such as calpains 1 and 2 alters LB-like inclusion formation. We show that C-terminal truncations, along with other PTMs, regulate fibril interactome remodeling, shortening, lateral association, and packing. These findings reveal distinct roles of C-terminal truncations at different aggregation stages on the pathway to LB formation, highlighting the need for consideration of stage‑specific strategies to target aSyn proteolytic cleavages.
Similar content being viewed by others
Introduction
The intracellular accumulation of aggregated forms of alpha-synuclein (aSyn) in neurons and glial cells represents one of the main pathological hallmarks of Parkinson’s disease (PD) and related synucleinopathies, including dementia with Lewy bodies (DLB) and multiple system atrophy (MSA)1. PD and DLB are characterized by the presence of Lewy bodies (LB) in neurons, while in MSA, aSyn inclusions are mainly detected in glial cells and are referred to as glial cytoplasmic inclusions (GCIs). Although it is widely believed that aSyn plays a central role in the pathogenesis of synucleinopathies, our understanding of the molecular and cellular processes that trigger and govern the misfolding, fibrillization, inclusion formation, and spread of aSyn in the brain remains incomplete.
Several post-translational modifications (PTMs), including phosphorylation2,3, C-terminal (C-ter) truncation2,4,5,6,7, ubiquitination8, and nitration9, have been consistently associated with aSyn aggregates and LB found in postmortem brains of patients with PD. This suggests that these modifications represent either markers of Lewy pathologies or key events that regulate the initiation of aSyn misfolding, aggregation, and/or inclusion formation. The identification of PTMs that enhance or interfere with any of these processes could open new avenues for targeting aSyn and developing strategies to prevent or delay pathology formation and disease progression.
Several studies by our group and others10,11,12,13,14,15,16,17,18 have investigated the role of PD-associated PTMs in regulating aSyn aggregation. Collectively, these studies have shown that most pathology-associated PTMs either inhibit or do not affect aSyn fibril formation in vitro and in different synucleinopathy models, with the only notable exception being C-terminal truncations2,4,19,20,21,22. Although several studies have shown that the C-terminal cleavage of aSyn occurs under both physiological23,24 and pathogenic4,6,22,25 conditions, studies in cell-free systems26,27,28,29,30,31,32 and cellular and animal models25,29,30,31,32,33,34,35,36,37,38,39 of synucleinopathies have consistently shown that C-terminally truncated aSyn monomeric variants aggregate much faster than the full-length (FL) protein and accelerate aSyn aggregation and pathology formation. Furthermore, C-terminally truncated aSyn aggregates retain the ability to seed the aggregation of FL aSyn30.
The development of antibodies that specifically recognized the C-terminally truncated fragments enabled further confirmation of their presence by IHC in the LB2,35,40,41,42,43 and GCIs2. Consistent with previous studies2,4,40,44,45,46, confocal imaging40 and super-resolution microscopy45 combined with the use of these C-terminal antibodies demonstrated that the C-terminally truncated species (1–119 and 1-122) were not always randomly distributed in LB, pale bodies, and Lewy neurites (LN), In specific types of LBs, these aSyn variants are found in the inner core of these pathological inclusions whereas FL and pS129-positive aSyn were found in the outer layers. These observations suggest that the FL and truncated aSyn distribution inside the inclusions is highly orchestrated and may reflect sequential events. Furthermore, studies47,48,49 using monoclonal antibodies targeting neo-epitopes of C-terminally truncated aSyn, combined with multiplex immunoassays47,48 or real-time quaking-induced conversion assay49, have uncovered significant variations in αSyn C-terminal truncations and pS129 phosphorylation patterns that are specific to the disease, brain region, and cell type within synucleinopathies such as PD, DLB, Alzheimer’s disease with amygdala Lewy bodies (AD/ALB), and MSA. These studies demonstrated that C-terminally truncated aSyn species are more prevalent than previously thought in the brain and revealed unique biochemical signatures for each synucleinopathy, suggesting that the heterogeneity in aSyn pathology may contribute to the distinct clinical manifestations and progression observed in these diseases. Moreover, we recently demonstrated that astrocytic aSyn inclusions in PD and other synucleinopathies are made of a nonfibrillar form of aSyn that is nitrated or phosphorylated at Y39 and cleaved at both N-terminal (N-ter) and C-terminal domains of the protein (first 30 a.a. and last 30 a.a. are absent)50,51. This is the first report of aSyn accumulation that is made purely of C-terminally truncated forms of aSyn, i.e., no FL aSyn species could be detected in aSyn astrocytic inclusions. Altogether, these data reinforce the potential role of N- and C-terminal truncation as a key driver in the formation of aSyn aggregates and biogenesis of LB, and that they may have cell-specific functions in PD and synucleinopathies22,52. However, the contributions of C-terminal truncation during the various stages of aSyn pathology formation and their role in the evolution and maturation of LB have not been systematically investigated.
The majority of previous studies on aSyn truncations have focused primarily on exploring how C-terminal truncations and other PTMs influence the kinetics and aggregation properties of monomeric aSyn. However, increasing evidence suggests that several PTMs, including phosphorylation (pS129)53,54,55,56,57,58 and C-terminal cleavage56,57,58, could also occur after aSyn fibrillization in mammalian cell lines53,56, neurons in culture54,57,58 and in vivo55,57. Therefore, post-fibrillization PTMs, such as C-terminal truncations, could also play important roles in regulating secondary aggregation processes, including secondary nucleation, lateral association of aSyn aggregates and their interactome, LB formation, and maturation.
To decipher the roles of aSyn C-terminal cleavage at different stages of LB formation, we performed systematic studies to elucidate the nature of post-aggregation PTMs that take place upon the (1) uptake of preformed fibril seeds (PFF) into neurons; (2) after the generation of newly formed fibrils by endogenous aSyn, and (3) during the maturation of LB-like inclusions. These studies were performed in the neuronal seeding model54,59, which we have shown previously to recapitulate many of the key events and processes that govern aSyn seeding, aggregation, and LB formation43,44. Our studies revealed differential C-terminal cleavage and stability of exogenously added PFF and endogenously seeded aSyn aggregates, suggesting a differential role of this PTM at various stages of LB formation. Furthermore, we show that C-terminal cleavages in combination with other PTMs play important roles in remodeling aSyn fibrils during the formation and evolution of LB. These findings, combined with the abundance of aSyn-truncated species in LB, have significant implications for ongoing efforts to investigate aSyn pathological diversity, quantitative mapping of aSyn PTMs, and developing therapeutic strategies based on targeting the C-terminus of aSyn or proteolytic processing of this region.
Results
Truncation of aSyn PFF seeds is an early event that occurs rapidly after neuronal internalization
To investigate the role of C-terminal truncation in regulating the seeding activity of aSyn, we took advantage of the neuronal seeding model54,59. In this model, aSyn intracellular aggregation is triggered by the addition of a nanomolar concentration (70 nM) of extracellular mouse aSyn PFF58 (Supplementary Fig. 1), which, upon internalization, induces the formation of intracellular LB-like inclusions in a time-dependent manner58, in wild-type (WT) hippocampal primary neurons (Fig. 1a). As previously shown54,58, immunocytochemistry (ICC, antibodies used are described in Supplementary Fig. 2) confirmed that these inclusions contained aSyn pS129 (Fig. 1a–c) and were also positive for two other well-established LB markers60, namely ubiquitin (ub) and p62 (Fig. 1d, e). We have also recently shown that the seeded aggregates are also partially phosphorylated at residues Y39, Y133, and Y13650. Moreover, in line with previous reports54,58, the increase in phosphorylated aSyn at S129 (at 7–10 days) and its colocalization with other LB markers, coincides with the shift over time of endogenous aSyn from the soluble (Supplementary Fig. 3a) to the insoluble fraction in the PFF-treated neurons (Fig. 1f and Supplementary Fig. 3b). Western blot (WB) analyses of the insoluble fraction showed a time-dependent increase in high-molecular-weight (HMW) species (~23, 37, 40, and 50 kDa and smear >50 kDa) which started to appear 4–7 days after treatment with PFF and were positively stained by pS129 specific antibodies (Fig. 1f, middle panel). The use of the pan-synuclein antibody (SYN-1, epitope 91–99) uncovered an additional prominent band at ~12 kDa (Fig. 1f, top panel, single red asterisk), which was detected by aSyn N-ter and non-amyloid component (NAC) domain antibodies (Fig. 1g–i) but not by antibodies specific for pS129 (Fig. 1f middle panel) or raised against the C-terminal residues 116 to 138 (Fig. 1g, i). This indicates that these species correspond to C-terminally truncated forms of aSyn (Supplementary Fig. 4), which persisted for up to 21 days (D21) (Supplementary Fig. 3c)58.
a Seeding model in primary hippocampal neurons. 70 nM of mouse PFF were added to neurons at DIV 5 (days in vitro). Control neurons were treated with Tris buffer (Tris) used to prepare PFF. After 4 days of treatment, positive pS129-aSyn aggregates were detected in the extension of the neurons. After 7 days of treatment, the aggregates appeared in the cytosol of the neurons. The number of LB-like inclusions increased over time, as shown at 10 days of treatment. Scale bars = 40 and 5 μm. ICC analysis of the LB-like inclusions formed 10 days after adding mouse PFF to aSyn KO neurons (b) or WT neurons (c–e). Aggregates were detected using pS129 (MJF-R13) in combination with total aSyn (c, SYN-1), ubiquitin (ub, d), or p62 (e) antibodies. Neurons were counterstained with microtubule-associated protein (MAP2) antibody, and the nucleus was counterstained with DAPI staining. Scale bars = 5 μm. f WB analyses of the insoluble fraction of PFF-treated WT neurons treated with 70 nM of PFF for D1, D2, D4, D7, and D10. Control neurons were treated with Tris. After sequential extractions of the soluble and insoluble fractions, cell lysates were analyzed by immunoblotting. Total aSyn, pS129, and actin were detected by SYN-1, pS129 (MJF-R13), and actin antibodies, respectively. WB band intensities of total aSyn (15 kDa, indicated by a double red asterisk; 12 kDa indicated by a single red asterisk or HMW) or pS129-aSyn were quantified by densitometry, normalized to actin levels, and expressed as fold change relative to D1. Purple arrows indicate the intermediate aSyn-truncated fragments. The graphs represent the mean ± SD of 3 independent experiments. *p < 0.01, ***p < 0.0001 (ANOVA followed by Tukey HSD post hoc test, Tris vs. PFF-treated neurons) and #p < 0.01, ##p < 0.001 (ANOVA followed by Tukey HSD post hoc test, PFF-treated neurons D1 vs. other time point). g Epitope mapping of antibodies raised against the NAC, N-terminal, or C-terminal domains of aSyn. h N-terminal antibodies raised against residues 1–5 or residues 1–20 could detect full-length (15 kDa, indicated by a double red asterisk) or truncated (~12 kDa, indicated by a single red asterisk) aSyn in the insoluble fraction of WT neurons treated with 70 nM of PFF up to D10. Only the LASH-EGT 1–20 was able to detect the HMW at 25, 35, and 40 kDa. i Mapping of the C-terminal cleaved product using antibodies raised against the NAC and the C-terminal domains of aSyn. Immunoblots of insoluble fractions of WT neurons treated with aSyn PFF up to D10 showed that the fragment 1–114 generated in these neurons was well recognized by the NAC antibodies [(FL-140; 61–95) and (SYN-1; 91–99)] and a C-terminal antibody raised against the residues 108–120. However, it was not recognized by antibodies raised against peptides bearing residues after 116 in the C-terminal domain [(ab6162; 116–131); (ab131508; 134–138) and (ab52168; 131–135)]. j WB analyses of the insoluble fractions of aSyn KO primary neurons treated with 70 nM of mouse PFF for up to 48 h using SYN-1 and pS129 (MJF-R13) antibodies. WB band intensities of total aSyn (15 kDa, indicated by a double red asterisk) and C-terminally cleaved aSyn species (12 kDa, indicated by a single red asterisk) were quantified by densitometry, normalized to actin levels, and expressed as fold change relative to 1 h (for the 15 kDa species, *p < 0.0001; ANOVA followed by Tukey HSD post hoc test) or 48 h (for the 12 kDa species, ###p < 0.0001; ANOVA followed by Tukey HSD post hoc test) after PFF addition. Purple arrows indicate intermediate aSyn-truncated fragments. Graphs represent the mean ± SD from three independent experiments. k, l Insoluble fractions of aSyn KO primary neurons treated with 70 nM of mouse PFF for 4 or 14 h were separated on a 16% Tricine gel. After Coomassie staining, two bands at ~15 (indicated by a black dashed box) and 12 kDa (indicated by a purple dashed box) were extracted from 16% Tricine gels (See Supplementary Fig. 4). Isolated bands were selected based on the size of the proteolytic fragments observed by WB (f) and subjected to proteolytic digestion followed by LC-MS/MS analysis. Proteomic analysis revealed that aSyn fragments generated in KO neurons transduced with PFF originate from C-terminal truncation, rather than N-terminal cleavage, of the PFF seeds. The diagram in (i) shows the different aSyn fragments generated upon C-terminal truncation and their relative position in a WB. Three fragments (1–135, 1–129, and 1–119) were detected in the upper band, and one main fragment (1–114) was found in the lower band. Original uncropped and unprocessed WB scans are provided in Supplementary Fig. 17. In (h–i), multiplexed detection (LiCor technology) allowed simultaneous antibody probing and multiple detections from the same membrane; thus, some immunoblots share the same actin control. See Supplementary Fig. 17 for a detailed explanation.
Importantly, the cleaved aSyn species were never detected in the corresponding soluble fractions (Supplementary Fig. 3a), suggesting that they represent C-terminally cleaved PFF or C-terminal truncations that occur post-fibrilization of endogenous aSyn.
We consistently observed that the C-terminal truncation fragments appeared rapidly after the internalization of aSyn PFF into the neurons and before the first newly formed aggregates were detected (Fig. 1f, Day 1). Therefore, we sought to determine if C-terminal truncation represents an early event required to initiate the seeding process in neurons. First, we monitored the extent of PFF cleavage and PTMs of PFF after internalization into aSyn knockout (KO) neurons by WB and confocal imaging approaches. The use of aSyn KO neurons allowed us to monitor the fate of aSyn PFF without interference from the formation of the new aSyn fibrils and LB-like inclusions since these processes do not occur in the absence of endogenous aSyn54 (Fig. 1b and Supplementary Fig. 3b). Interestingly, the internalized aSyn PFF did not undergo phosphorylation at residue S129 (Supplementary Fig. 3b, d) or residues Y39, Y133, or Y13650, ubiquitination (Supplementary Fig. 3e) or show any colocalization with p62 (Supplementary Fig. 3f) as commonly observed with the newly formed fibrils (Fig. 1c–e and Supplementary Fig. 3g). These findings suggest that the exogenous aSyn PFF are processed differently compared to the fibrils formed by endogenous aSyn. The absence of N-terminal modifications such as ubiquitination and p62 signal in the internalized aSyn PFF suggests that modifications at the C-terminus may play an important role in priming N-terminal PTMs and/or the interactome of aSyn aggregates with other proteins in neurons. WB analyses revealed that the internalized PFF were truncated into four fragments with apparent molecular weights (MWs) between 15 and 12 kDa during the first hours after internalization in the KO neurons (Fig. 1j) with complete loss of FL aSyn (15 kDa indicated by the double red asterisk) over time (Supplementary Fig. 3b) and the appearance of the 12 kDa band (single red asterisk) as the main species within 24 h (Fig. 1j). As in WT neurons (Fig. 1h, i), the 12 kDa band was recognized by the Nter and the NAC antibodies but not the C-terminal antibodies targeting residues beyond amino acid 116 (Supplementary Fig. 3h, i). Interestingly, the 12 kDa aSyn is detected in the insoluble fraction of the PFF-treated KO neurons as the dominant species for up to 21 days post-treatment (Supplementary Fig. 3c).
C-terminal truncation of aSyn PFF seeds occurs at multiple sites, leading to the accumulation of primary aSyn truncated at residue 114
To more precisely map the cleavage sites of mouse aSyn PFF seeds, aSyn bands from the insoluble fractions were digested by trypsin or Glu-C, and liquid chromatography-tandem mass spectrometry (LC-MS/MS) was performed as described in materials and methods61. In line with the WB data in Fig. 1, our proteomic analyses demonstrated that while PFF had an intact N-terminal domain, they were cleaved at several sites within the C-terminal domain: D135, S129, and D119 were detected in the upper band extracted at ~13–15 kDa, whereas a cleavage occurred at E114 in the band around ~12 kDa (Fig. 1k and Supplementary Fig. 4a, b). LC-MS/MS analyses also established that the main fragment that accumulates as the predominant species in neurons seeded with mouse PFF ends at residue 114 (1–114, MW = 11567.20 Da) (Supplementary Fig. 4c). Interestingly, similar cleavages, including the 1–114 fragment, were identified, using WB and proteomic approaches, for human PFF added to the KO neurons (Supplementary Fig. 4d–f) or in the human mammalian HeLa cell line (Supplementary Fig. 4g–k). Notably, the cleavage sites of the human PFF were almost identical to those previously identified in aSyn human neuroblastoma cell line seeding model56 or in LB from human brain tissue2,62 (Supplementary Fig. 4l, m). Our results suggest that the C-terminal truncation of the internalized aSyn PFF represents a generic cellular response to the uptake of PFF that could enhance their seeding activity or is necessary to signal their internalization.
aSyn PFF seeds are cleaved in the endo-lysosomal pathway or in the cytosol
To better understand where PFF cleavage occurs, we next performed confocal imaging analyses using mouse PFF fluorescently labeled with Atto 488 (PFF488) (Supplementary Fig. 1). Consistent with previous findings63,64,65, we observed that the PFF were predominantly internalized through the endo-lysosomal pathway and accumulated in late endosomes that were positively stained for the Lysosomal-associated membrane protein 1 (LAMP1) marker (Fig. 2a). Additional staining using N-ter (1–20 and 34–45) or C-ter (134–138 and 116–131) aSyn antibodies revealed that C-terminal cleavage occurs in the LAMP1-positive organelles (Fig. 2b and Supplementary Fig. 5a, b) with ~15% and ~45% (Supplementary Fig. 5c) of the PFF488 seeds not being recognized by the C-ter (116–131) antibody at D1 and D3, respectively (Supplementary Fig. 5b, c). This suggests that some of the PFF are processed in the endo-lysosomal compartments, which contain several proteases that are known to cleave aSyn66,67,68,69, such as cathepsin70 and asparagine endopeptidase (AEP)32,70,71. In line with this hypothesis, the activity of cathepsin B (Fig. 2c), but not cathepsin D and L (Supplementary Fig. 6a, b), was significantly increased during the first 6 h after the addition of the PFF to WT or aSyn KO neurons (Supplementary Fig. 6). Previous studies have also implicated the endo-lysosomal AEP in the cleavage32,70,71 of aSyn at residue 103 to generate a shorter C-terminal fragment 1-103. Therefore, we also assessed the activity of this enzyme and whether the PFF is cleaved at residue 103. The enzymatic activity of AEP was significantly increased in both aSyn KO and WT neurons during the first 24 h after the addition of the PFF (Supplementary Fig. 6c, d). Intriguingly, an additional band at ~10 kDa was detected in the insoluble fraction of the WT (Fig. 1i, blue arrow) and KO (Supplementary Fig. 3i, blue arrow) PFF-treated neurons by SYN-1 antibody but not by the N- or C-terminal antibodies targeting residues, 1–5 and 1–20 or between 108 and 138, respectively (Supplementary Fig. 7a, blue arrow).
a, b aSyn KO neurons were treated for up to 72 h with WT fluorescently labeled PFF488. The internalization and the truncation of the seeds were evaluated by confocal imaging. a One hour after addition to the KO neurons, we observed that most of the intracellular PFF488 were co-stained by an antibody raised against the extremity of aSyn C-terminal domain (epitope: 134–138, yellow arrows). C-terminal truncation of the seeds over time was confirmed by the loss of detection of the seeds by the C-terminal aSyn antibody (134–138, red; green arrows). b The internalization of the seeds via the endo-lysosomal pathway was confirmed by the detection of the fluorescently labeled PFF488 seeds in LAMP1-positive (late endosome, red) compartments over time. a, b Neurons were counterstained with MAP2 antibody, and the nucleus with DAPI stain. Scale bars = 10 μm. c Cathepsin B activity was measured in KO neurons treated with 70 nM of WT PFF seeds for up to 48 h. Control neurons were treated with Tris buffer. The graphs represent the mean ± SD of 3 independent experiments. The level of Cathepsin B activity is expressed as a fold change relative to Tris. **p < 0.001, ***p < 0.0001 (ANOVA followed by Tukey HSD post hoc test, Tris vs. PFF-treated neurons). d Truncation of aSyn PFF in the cytosol is confirmed by microinjection. The diagram on the left-hand side shows the experimental approach used to microinject WT PFF488 in KO neurons. Cells were fixed after 24 h and immunostained using the N-terminus antibody (aSyn 1–20) or C-terminus antibody (aSyn 134–138). Confocal imaging showed that WT PFF488 were detected by the N-terminus antibody (yellow arrows, merge), but not the C-terminus antibody (green arrows, merge). Neurons were counterstained with MAP2 antibody, and the nucleus with DAPI stain. Scale bars = 40 μm.
This ~10 kDa fragment exhibited similar migration in SDS-PAGE gels as recombinant aSyn 1-103, was recognized by the N103 antibody71 (Supplementary Fig. 7b, c), and was detected at a low, albeit similar, level from D1 to D21 (Supplementary Fig. 7a, SYN-1 antibody). These results demonstrate that the generation of aSyn 1-103 during the processing of PFF might result from the AEP cleavage, as previously described32,70,71 in the neurons (Supplementary Fig. 7), and suggest that the 1-103 fragment results from the truncation of a small subset of the PFF.
Next, we assessed whether the cleavage of the aSyn PFF could also occur once they escape from the endocytic pathway63,72 or upon their uptake by alternative routes, including receptor-mediated processes73,74,75. Toward this goal, we used a microinjection technique76 to deliver fluorescently labeled PFF488 directly into the cytoplasm of individual KO neurons (Fig. 2d). 24 h post-injection, the neurons were fixed and stained with N-terminal (epitope: 1–20) and C-terminal (epitope: 134–138) aSyn antibodies. The colocalization of the aSyn PFF488 signal with aSyn was observed using antibodies that target the N-terminal residues of aSyn (Fig. 2d, yellow arrow). An antibody against the extreme C-terminal residues 134–138 failed to detect the PFF488 (Fig. 2d, green arrows). After our findings, similar observations have been reported by other research groups, further supporting the validity and relevance of our results43,77. Altogether, our data confirm that aSyn PFFs are not only cleaved in the endo-lysosomal pathway but can also be processed once they reach the cytosol of the neurons.
PFF cleavage and generation of aSyn truncation fragments occur in different cell types and rodent models of aSyn pathology formation
Next, we sought to determine whether aSyn PFFs are subjected to differential cleavage in different cell types and various models of aSyn pathology formation (Figs. 3 and S8).
WB analyses of the truncation pattern of aSyn in primary neurons (a, b), in vivo after injection of human PFF in the striatum of WT and aSyn KO mice (c), in iPSC-derived neurons from a healthy control individual transduced with human PFF (d). Hippocampal (HIPP) and cortical (CTX) primary neurons from mice or (a) hippocampal (HIPP), cortical (CTX), or striatal (STR) primary neurons from rats (b) were treated for 10 days with 70 nM of mouse PFF. iPSC-derived neurons were treated with 70 nM of human PFF for 1, 3, 7, 10, and 14 days (d). Control neurons were treated with Tris buffer (−). The striatum of WT or aSyn KO mice was dissected after 1 h or 1, 3, or 7 days after injection with human PFFWT (c). Cell lysates were analyzed by immunoblotting after sequential extractions of the soluble and insoluble fractions. The levels of total aSyn (SYN-1, 4B12, or 134–138 antibodies) (15 kDa, indicated by a double red asterisk; 12 kDa indicated by a single red asterisk or HMW) were estimated by measuring the WB band intensity and normalized to the relative protein levels of actin (Supplementary Fig. 9). Purple arrows indicate the intermediate aSyn-truncated fragments. All the original uncropped and unprocessed WB scans are available in Supplementary Fig. 17.
Towards this goal, we assessed the extent of aSyn truncation in several neuronal seeding models (Fig. 3a–d and Supplementary Fig. 9a–c) as well as in a pure primary mouse cortical neuron cultures l b (Supplementary Fig. 9d). Truncation occurred in all the PFF-treated neurons, including hippocampal or cortical primary neurons from mice (Fig. 3a and Supplementary Fig. 9a) or hippocampal, cortical, or striatal primary neurons from rats (Fig. 3b and Supplementary Fig. 9b) but not in the astrocytes (Supplementary Fig. 9d). WB analyses clearly showed a similar truncation pattern and efficiency in all the types of neurons with aSyn cleaved into ~12 kDa fragments. Interestingly, we observed a similar HMW band pattern in the insoluble fraction (~23, 37, 40, and 50 kDa) in all types of neurons.
Strikingly, a similar truncation pattern was also observed in vivo after injecting human PFF in the striatum of C57BL/6J WT mice or aSyn KO mice (Figs. 3c and S9c). To follow the fate of the seeds specifically, we used an antibody raised against human aSyn (clone 4B12). As early as 1 day after injection, four cleaved fragments running at sizes similar to those observed in primary neurons were detected in the insoluble striatal fractions of the rodent brains using an aSyn antibody raised against the epitope 103–108 (clone 4B12), but not with an antibody specific for the C-terminal domain (epitope: 134–138). This is consistent with these fragments resulting from the C-terminal cleavage of aSyn species.
To explore the pathophysiological relevance of our findings further, we next investigated the processing of fibrillar aSyn in human induced pluripotent stem cells (iPSCs) that were differentiated into dopaminergic neurons78,79,80. We used a line derived from a healthy control individual, which showed dense processes and expressed markers consistent with human midbrain neurons (Fig. 3d and Supplementary Fig. 9e–g). Twenty-four hours after adding human PFF to the iPSC-derived human neuronal culture (50 days in vitro), aSyn was cleaved into a ~12 kDa fragment (Fig. 3d and Supplementary Fig. 9e), similar to our observations of mouse neurons. Altogether, our data demonstrate that the truncation of aSyn fibrils is a general and early event that occurs after the internalization of propagating fibrils and during the formation of intracellular LB-like aSyn inclusions in all neuronal seeding models used.
Preventing aSyn cleavage at residue 114 does not impact the seeding capacity of PFF in primary neurons
Given the rapid proteolytic processing of PFF upon internalization, we initially hypothesized that the C-terminal cleavage of the PFF might be a prerequisite for the seeding and initiation of endogenous aSyn aggregation in neurons. To test this hypothesis, we used PFF prepared from aSyn carrying a single amino acid substitution at residues 114 (E114A) or 115 (D115A) (Supplementary Fig. 1, mouse E114A, and D115A PFF), which were designed to specifically block C-terminal cleavage at residue 114. Figure 4a shows that aSynE114A PFF, but not aSynD115A PFF, underwent cleavage to generate the 1–114 fragment. Therefore, we used PFF generated from this mutant to investigate the role of C-terminal cleavage of PFF in regulating their seeding activity. In aSyn KO neurons, PFFD115A underwent cleavage only at residues 135, 129, and 119, and the resulting fragments were cleared over time (Fig. 4b).
aSyn KO neurons were treated for 14 h (a) or up to 21 days (b), or aSyn WT neurons were treated for 10 days (c) with PFFWT, PFFE114A, PFFD115A, or with PFF1–114. Neurons were lysed at the indicated time, and the insoluble fractions were analyzed by WB. The total aSyn (SYN-1 antibody) levels were estimated by measuring the WB band intensity normalized to the relative protein levels of actin. A double red asterisk indicates the 15 kDa species (full-length), and a single red asterisk indicates the C-terminal truncated species running at 12 kDa. The purple arrows indicate the intermediate aSyn-truncated fragments. d–e Newly formed inclusions were detected using pS129 antibody (81a) in WT neurons after 10 days of treatment. Neurons were counterstained with MAP2 antibody, and the nucleus was counterstained with DAPI staining. d Representative confocal images. Scale bar = 5 μm. e Quantification of images acquired by a high-throughput wide-field cell imaging system. For each independent experiment, triplicate wells were acquired per condition, and nine fields of view were imaged for each well. Each experiment was reproduced at least 3 times independently. Images were then analyzed using Cell profile software to identify and quantify the level of LB-like inclusions (stained with pS129 antibody, 81a clone) formed in neurons (MAP2-positive cells). The graphs (a, c, e) represent the mean ± SD of three independent experiments. a, c aSyn species were quantified by densitometry, normalized to actin levels, and expressed as fold change relative to PFFWT-treated neurons. e pS129 level was expressed as fold change relative to PFFWT-treated neurons. a, c, e *p < 0.01, **p < 0.001, ***p < 0.0001 (ANOVA followed by Tukey HSD post hoc test, Tris vs. PFF-treated neurons). ##p < 0.005 (ANOVA followed by Tukey HSD post hoc test, PFFWT vs. mutants PFF-treated neurons). All the original uncropped and unprocessed WB scans are available in Supplementary Fig. 17.
Next, we compared the seeding capacity of PFFE114A and PFFD115A to that of PFFWT after 10 days of treatment in WT neurons. PFFWT, PFFE114A, and PFFD115A all induced a similar level of seeding in WT neurons (Fig. 4c–e). These findings were confirmed by the WB analyses of the insoluble fractions extracted from WT neurons treated with PFFWT, PFFE114A, or PFFD115A, in which a similar level of pS129 and HMW signals was detected (Fig. 4c).
Consistent with the data, we observed similar reductions in the levels of soluble proteins in neurons treated with PFFE114A, PFFD115A, and PFFWT (Fig. 4c). These findings were further confirmed by quantitative ICC. Furthermore, we did not observe significant differences in the pS129 levels (Fig. 4e) or the morphology of the newly formed aggregates (Fig. 4d) in neurons treated with PFFWT or with PFF mutants for 10 days.
This is in line with previous studies reporting that the deletion of various aSyn regions other than the NAC domain does not inhibit the formation of seeded aggregates53,54. Our results demonstrate that blocking the C-terminal truncation of the seeds does not prevent the seeding and the recruitment of endogenous aSyn or the formation of aggregates in neurons.
The newly formed aSyn fibrils are processed differently and undergo more complex PTMs compared to exogenous PFF
Having established that C-terminal cleavage is not essential for the initial seeding events, we then sought to determine if the newly formed fibrils are also subjected to proteolysis and whether this modification is important for the transition from fibrils to LB-like inclusions58. Toward this goal, we used fluorescently labeled PFF488 to enable distinguishing exogenous PFF from newly formed fibrils. Using ICC and confocal imaging, we observed that the internalized seeds that appear to be incorporated in/or colocalized with the newly formed aggregates represent a minor species (Supplementary Fig. 10a). This suggests that the majority of the fibrils present in the seeded aggregates are derived from newly recruited endogenous aSyn. Next, to discriminate the PFF seeds from the newly formed fibrils by WB analyses, mouse WT neurons were treated with human PFF for 10 days, and the insoluble fraction was analyzed by WB using a combination of human- and mouse-specific antibodies, 4B12 and Glu105, respectively (Supplementary Fig. 10b). Our data clearly indicated that ~90% of the ~12 kDa species detected by WB were composed of the exogenous PFF seeds, while the newly formed aggregates were represented by the HMW bands (~23, 37, 40, and 50 kDa) but also by the species >170 kDa and trapped in the stacking gel (Supplementary Fig. 10b). These results and the absence of a truncated mouse aSyn band suggest that the newly formed and post-translationally modified fibrils are more stable and exhibit higher resistance to sodium dodecyl sulfate (SDS) compared to the aSyn PFF seed, which readily disassociates in SDS buffers.
We next used quantitative proteomic analyses (Fig. 5a, b and Supplementary Fig. 10d) combined with WB (Figs. 1h, i and 5c and Supplementary Fig. 10c) and ICC (Fig. 5d–g and Supplementary Fig. 10d, e) analyses to determine how the newly formed fibrils are cleaved during the aggregation process in comparison to the exogenous PFF. At day 10, both the proteomic (Fig. 5a, b and Supplementary Fig. 10d) and WB analyses (Figs. 1h, i and 5c and Supplementary Fig. 10c) showed that the processing of aSyn in WT neurons was, in all respects, similar to that observed for the PFF added to the KO neurons (Fig. 1j and Supplementary Fig. 3a), i.e., presence of aSyn species, that accumulate at ~12 kDa (Figs. 1h, i and 5c) were cleaved after residue 114 and bore an intact N-terminal domain (Figs. 1h, i and 5c).
Identification of C-terminal truncated fragments by proteomic analysis (a, b), WB (c and Fig. 1h, i), or confocal imaging (d–g). WT neurons were treated with 70 nM of mouse PFF for 10 days. a, b The insoluble fractions of PFF-treated neurons were separated on a 16% Tricine gel. After Coomassie staining, 8 bands were extracted from the Tricine gel (a). Isolated bands were subjected to proteolytic digestion using trypsin for C-terminal truncation identification137, followed by LC-MS/MS analysis. b Proteomic analyses showed the presence of the 1–114 and 1–119 C-terminal truncated fragments in the HMW species. c Table summarizing the capacity of NAC domain, N-terminal, and C-terminal antibodies to detect full-length aSyn (15 kDa), the C-terminally cleaved fragment of aSyn (~12 kDa), and the HMW formed in WT neurons after 10 days of treatment with PFF (see WB in Fig. 1h, i). Antibody mapping of the newly formed inclusions by confocal imaging using pS129 antibody (81a clone) in combination with N-terminal (d, epitope 1–20) or C-terminal (e–g, respective epitopes 108–120, 116–131, or 134–138) antibodies revealed the presence of aSyn-positive aggregates that were not pS129 positive or only partially phosphorylated at S129 residue (e, f). Neurons were counterstained with MAP2 antibody, and the nucleus with DAPI stain. The white arrows indicate the sub-populations of aggregates localized near the pS129-positive inclusions, and the white asterisk those inside the pS129-positive filamentous structures. Scale bars = 10 μm.
In addition, mass spectrometry analyses revealed that the HMW newly formed aggregates were composed of both FL aSyn and C-terminally cleaved aSyn species (1–114 and 1–119) (Fig. 5a, b and Supplementary Fig. 10d). All aSyn species present in the HMW were also ubiquitinated at multiple lysine residues from D7 to D21 post-treatment (Supplementary Fig. 10d).
Next, we compared the immunoreactivity of aSyn-seeded aggregates by ICC using a set of antibodies targeting the N- and C-terminal regions of aSyn (Fig. 5d–g and Supplementary Fig. 10d, e). After 10 days of treatment, the N-terminal antibody (1–20) and the C-terminal antibodies raised against the 108–120 or the 116–131 region uncovered the presence of aSyn-positive accumulations that were not pS129 positive (Fig. 5d, e) or only partially phosphorylated at the S129 residue (Fig. 5f). These sub-populations of aggregates were either localized near the pS129-positive inclusions (Fig. 5d, e, white arrows) or inside the pS129-positive filamentous structures (Fig. 5f, white asterisk). As expected, the C-terminal antibody (aa 134–138) that recognizes only FL aSyn by WB (Figs. 1h, i and 5c) detected the pS129 positive aggregates (Fig. 5g). These observations suggest that some of the newly formed aggregates are not yet phosphorylated at S129 or that the detection of pS129-modified fibrils is masked due to the presence of neighboring modifications.
We have recently developed and validated an expanded aSyn antibody toolset to profile aSyn pathology in postmortem human brain tissues and in the neuronal and in vivo seeding model50. Using these antibodies, we showed that while the PFF seeds are exclusively C-terminally truncated, the seeded aggregates contain a diversity of aSyn species phosphorylated mainly at S129 residue but also partially phosphorylated at N-ter and C-ter tyrosine residues (Y39, Y125, Y133, Y136)50,81,82,83, or nitrated at residue Y3950,84, or C-terminally truncated2,45,48,50,62,85,86. Altogether, our data demonstrate that the newly formed fibrils are processed differently in neurons and exhibit different biochemical properties compared to the exogenous recombinant PFF, which undergo only C-terminal cleavage but lack phosphorylation, ubiquitination, or other PTMs.
C-terminal truncations of aSyn occur post-fibrillization, induce fibrils’ lateral association, and cause changes in their interactome
Our previous correlative light electron microscopy (CLEM) studies showed that the process of inclusion formation in seeded neurons is accompanied by the transient lateral association of the newly formed fibrils, followed by interactions of fibrils with lipids, organelles, and endomembrane structures into LB-like structures58. The term “lateral association” refers to the process by which fibrils interact along their length, bundling together to form higher-order assemblies, as indicated by the red arrows in Fig. 6. Given that the highly negatively charged C-terminal domain is exposed and decorates the surfaces of the fibrils, we hypothesized that C-terminal cleavages of the newly formed fibrils could drive their lateral association.
PFF were added for 10 (a), 14 (b), and 21 (c–e) days to the extracellular media of hippocampal neurons plated on dishes with alpha-numerical searching grids imprinted on the bottom, which allowed the localization of the cells. At the indicated time, neurons were fixed and imaged by confocal microscopy (top images), and the selected neurons were embedded and cut by an ultramicrotome. Serial sections were examined by EM. Representative newly formed aSyn fibrils are indicated with a red arrow. Autophagolysosomal-like vesicles are indicated by a yellow asterisk, and the mitochondrial compartments by a green asterisk. The nucleus is highlighted in blue. a, b Scale bars = 500 nm; c, d Scale bars = 1 μm; e Scale bar = 2 μm.
We postulated that this post-fibrillization processing might be required for the packing and sequestration of fibrils during LB formation and could explain the accumulation of C-terminal cleavage aSyn in the core of LB43,45,87. Furthermore, the C-terminal domain of aSyn serves as a major interactome hub for aSyn monomers and fibrils. Therefore, removal of this domain is expected to disrupt the interactome of the fibrils, which is expected to regulate the interactions of the fibrils with other cellular proteins and components that co-accumulate with aSyn within LB.
To investigate the interplay between the C-terminal cleavage of fibrils and their interactome, lateral association, and LB formation, we first investigated changes in the morphology of the newly formed fibrils over time. We imaged neurons treated for 10, 14, or 21 days with mouse WT PFF by CLEM (Fig. 6).
Previously, we showed that at 7 days, newly formed fibrils exist as single-long filaments with lengths ranging between 600 nm and up to 2 μm58. As shown in Fig. 6, at D10 (Fig. 6a, red arrows) and D14 (Fig. 6b, red arrows), the newly formed aSyn fibrils were shorter in length as described previously58 and reorganized into tightly packed bundles of fibrils. These clusters of fibrils were not formed by randomly arranged fibrils but rather by fibrils that were closely associated and aligned in parallel. From D14, organelles such as mitochondria and endo-lysosomal-like vesicles were detected close to the laterally associated bundles of fibrils. At D21, long filamentous-like structures (Fig. 6c) and ribbon-like inclusions (Fig. 6d) of aSyn newly formed fibrils were still appearing as tightly packed bundles of fibrils that appeared to be closely associated with mitochondria, autophagosomes, and endoplasmic reticulum. Conversely, in neurons in which aSyn aggregates have completely transitioned into inclusions with a round LB-like morphology (Fig. 6e), the laterally associated fibrils were no longer observed, and only short aSyn filaments randomly organized were detected in the center of the inclusion. Our data suggest that newly formed fibrils undergo progressive fragmentation over time. Under the same experimental conditions, we previously quantified a reduction in fibril length from 1 to 2 µm at early stages (Day 7) to an average of ~300 nm at later stages (Day 21)88. This fragmentation represents a key temporal feature of aSyn pathology formation and maturation. Altogether, our data suggest that once fibril fragmentation has reached its minimum size, the lateral association and packing of the newly formed fibrils are no longer sustainable or require fibril disassembly88. Alternatively, other PTMs or cellular proteostasis mechanisms, such as the action of chaperones89,90,91, could induce disruption of fibril-to-fibril association or induce the disaggregation of the fibrils during LB formation and maturation. Our observation that newly formed fibrils contain both FL (pS129-immunoreactive) and C-terminally truncated aSyn suggests that truncation occurs after fibrillization. Alternatively, truncated species might form early aggregates that seed FL aSyn aggregation. We hypothesized that if truncated monomers primarily act as seeds, their levels within fibrils should remain constant over time, with no significant changes in the aSyn C-terminal interactome. However, if truncation occurs after fibrillization and contributes to fibril maturation into LB-like inclusions, a time-dependent decrease in the aSyn C-terminal interactome would be expected, indicating progressive cleavage of FL fibrils.
To test this hypothesis, we first identified the interacting partners of intact recombinant PFF by incubating cortical mouse brain lysates with PFF derived from aSyn that was site-specifically biotinylated at its N-terminal residue (Supplementary Fig. 11a–g). Biotinylated fibrils were then specifically pulled down using streptavidin beads, and putative interacting partners were determined by LC-MS/MS analysis (Supplementary Fig. 11i). As expected, ~80% of the previously reported putative aSyn C-terminal interacting partners were found to bind to FL PFF in the pull-down assay (Supplementary Fig. 11i).
Next, we conducted quantitative proteomic studies on the insoluble fraction of PFF-treated neurons (Supplementary Fig. 11i, j). We monitored the changes in the levels of the 76 previously reported C-terminal aSyn-interacting proteins over time (Supplementary Fig. 11i, left-handed column) and determined which C-terminal interacting proteins were lost under conditions where truncations were detected in the neuronal seeding model (Supplementary Fig. 11i, purple boxes). After 14 days of PFF treatment, ~25% of the proteins identified as putative C-terminal interactors were significantly enriched in the insoluble fraction of the PFF-treated neurons (Supplementary Fig. 11i, j, green boxes). After 21 days, only 9% of these C-terminal interactors were still present in the insoluble fraction of the PFF-treated neurons (Supplementary Fig. 11i, j, green boxes). These findings are in agreement with our hypothesis that C-terminal truncations of aSyn occur post-fibrillization and lead to the disruption of the aSyn interactome involving the C-terminal region of the protein. This mostly includes proteins involved in the cytoskeleton architecture (e.g., MAP1B, Tubb3, Tubb4, Tubb5, Tubb6, Myh10, and Rap1a), suggesting that the remodeling of the newly formed fibrils by C-terminal cleavage leads to a loss of physical interactions with the cytoskeleton and other partners.
C-terminal truncation promotes the lateral association of aSyn fibrils in vitro
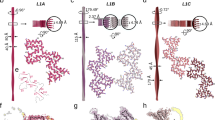
To test our hypothesis that C-terminal cleavage of fibrils promotes their lateral association, we investigated the impact of C-terminal truncations on the morphology of aSyn fibrils in vitro. First, we assessed the in vitro aggregation properties of the fibrils formed after the incubation of FL aSyn monomers (1–140) or C-terminal truncated variants corresponding to those detected in our neuronal model and the human brain (1–135, 1–133, 1–124, 1–120, 1–115, or 1–111) at 37 °C under agitation conditions using EM (Fig. 7a, b). After 6 days, EM imaging showed that the PFF1–140 and PFF1–135 formed predominantly well-dispersed single fibrils. In contrast, PFF formed by monomers derived from shorter C-terminally truncated fragments (PFF1–124, PFF1–120, PFF1–115, and PFF1–111) exhibited a high tendency to associate laterally and pack together (Fig. 7b), forming micrometer-sized dense aggregate clumps. The PFF1–133 exhibited an intermediate behavior with less dispersed fibrils compared with PFFWT (Fig. 7b). As expected, single (Δ111–115, Δ120–125 or Δ133–135) or double deletions (Δ111–115/Δ133–135) that do not substantially decrease the number of negative charges compared to WT aSyn did not favor the lateral association of these PFF (Fig. 7b). Interestingly, well-dispersed single fibrils were observed when the charge state of PFF1–114 was changed to −14 [comparable to that of the WT protein (−12)] by aggregating the protein at pH 10 (Fig. 7c). However, upon the adjustment of the pH to 7.5, the PFF1–114 underwent rapid lateral association and packed together in aggregate clusters.
a aSyn protein structure. The acidic C-terminal domain (purple, residues: 96–140) is negatively charged (z = −12), while the N-terminal domain (green, residues: 1–60) together with the central hydrophobic core named NAC (red, residues: 61–95) are positively charged. Interaction of unfolded aSyn monomers leads to the formation of (1) dimers that grow into (2) oligomers and protofibrils, which convert to (3) fibrils. During fibrilization, the C-terminal domain is exposed on the surface. b Full-length recombinant aSyn, C-terminal truncated aSyn (1–135, 1–133, 1–124, 1–120, 1–115 or 1–111), and aSyn with single (Δ111–115, Δ120–125 or Δ133–135) and double deletions (Δ111–115Δ133–135) were incubated at 37 °C for 6 days under shaking conditions and imaged by EM. We observed that the removal of charges in the C-terminal truncated proteins induces lateral association of the PFF, resulting in highly packed fibrils. The level of lateral association is stronger for the proteins with a lower number of negative charges in their C-termini. The number of C-terminal charges is indicated on the right side of each diagram. Scale bar = 200 nm. The lateral association is not observed for the PFF1–135, for which the number of negative charges remains comparable to that of the WT protein. Likewise, PFF with single (Δ111–115, Δ120–125 or Δ133–135) and double deletions (Δ111–115Δ133–135) do not laterally associate, as the number of negative charges remains comparable to that of the WT protein. Scale bars = 100 nm or 200 nm. c Fibrils formation was induced by incubating C-terminally truncated aSyn 1–114 at 37 °C and pH 10.5 in 50 mM Tris and 150 mM NaCl under constant agitation at 1000 rpm on an orbital shaker. After 6 days, PFF were sonicated, and the pH was re-adjusted to 7.5. After 2 h at room temperature, PFF laterally associated and formed large, dense aggregate clumps. Scale bars = 500 nm. Semisynthesis (d) and strategy (e) of photocleavable aSyn at position 120. f–i Photolysis of aSyn monomers (f) and PFF (g) results in the rapid generation of C-terminally truncated aSyn. h EM confirmed that photolysis of photocleavable aSyn PFF enabled the lateral association and clumping of the PFF, as also depicted in the diagram in (i). Scale bar = 200 nm.
These results demonstrate that the level of lateral association, the size, and the density of the resulting fibril clumps appeared to depend on the charge state of the C-terminal domain. Monomers with the shortest C-terminal domains (i.e., the lowest number of C-terminal negative charges) exhibit a higher propensity to undergo lateral association and form clusters of densely packed fibrils.
Given that we postulated that C-terminal cleavages also occur after aSyn fibrillization, we also assessed the effect of site-specific post-fibrillization cleavage on fibril morphology and lateral association with great precision. Towards this goal, we developed a novel semisynthetic form of aSyn with a (2-nitrophenyl)propanoic acid between residues 120 and 122 (aSyn-D121Anp), allowing the temporal regulation of site-specific aSyn cleavage. The incorporation of this unnatural amino acid allows the photocleavage of aSyn, specifically at position 120 (Fig. 7d, e). We first verified that photoactivation leads to site-specific cleavage of the monomeric aSyn-D121Anp using ultraviolet (UV) light. As shown in Fig. 7f, cleavage of aSyn monomers occurred within a few seconds (Fig. 7f). Next, we prepared fibrils derived from aSyn-D121Anp PFF (Fig. 7g) and showed that they could be successfully cleaved following exposure to UV light. As shown in Fig. 7h, the site-specific C-terminal photocleavage of FL aSyn-D121Anp PFF and removal of the last 20 amino acids led to their tight lateral association and the formation of fibrils that resemble those seen with the C-terminal truncated proteins (Fig. 7h, i). Taken together, our findings show that C-terminal cleavages of monomeric or fibrillar aSyn promote the lateral association of fibrils.
Calpains 1 and 2 play active roles in the remodeling of aSyn fibrils and the formation or maturation of LB
Having established that aSyn C-terminal cleavage plays a critical role in the lateral association and determined the morphology of the newly formed fibrils and their remodeling, we next sought to investigate the impact of blocking C-terminal cleavage of endogenous aSyn on PFF-mediated seeding and formation of LB-like inclusions. As a first step towards achieving this goal, we investigated which proteases were involved in regulating the C-terminal cleavage of aSyn fibrils.
Several enzymes have been reported as potential proteases that regulate aSyn C-terminal truncations20,52,92,93,94,95,96, including cathepsin D68,97, neurosin98, metalloproteinases99,100,101, caspase 129,94,96, calpain 152,92,93, and calpain 293,102. Among these, it has been shown that calpains 1 and 2 cleave aSyn fibrils predominantly at the amino acids 114 and 12293 in vitro. Consistent with this report, we confirmed that aSyn fibrils were cleaved by calpain in the 111–115 region (Supplementary Fig. 12a). To evaluate the potential role of calpains 1 and 2 in regulating aSyn seeding and inclusion formation, we first examined whether these enzymes were activated in WT neuronal primary cultures following treatment with aSyn PFF. Specifically, we investigated whether aSyn PFF cleavage could occur extracellularly before internalization. Western blot analysis of conditioned media collected at various time points post-PFF or PBS treatment (Supplementary Fig. 12b) revealed no detectable calpain presence. Moreover, no calpain activity was measured in the extracellular media of primary cultures (Fig. 8a and Supplementary Fig. 12b, c). Additionally, analysis of conditioned media for truncated aSyn revealed faint ~12 kDa bands at early time points (Days 1–3) following PFF addition, but no HMW, suggesting that these bands may represent C-terminally truncated aSyn species released from neurons that had already internalized the PFF. Notably, significant intracellular activation of calpains 1 and 2 was observed as early as 6 h after PFF treatment (Fig. 8b). While this increase suggests their involvement in aSyn processing, it does not conclusively prove they are the sole contributors, as basal calpain activity may be sufficient for cleavage, and other enzymes could also play a role. Nonetheless, our findings strongly indicate that calpain-mediated aSyn processing predominantly occurs intracellularly, with minimal contribution from extracellular mechanisms. Calpains are calcium-dependent proteases103. Therefore, we next measured calcium homeostasis in PFF-treated neurons. Calcium imaging using Fura-2 measurement showed that cytosolic calcium started to increase as early as 8 h after the addition of the PFF to the neurons (Fig. 8d, e), when calpain activity also started to rise significantly (Fig. 8b).
Calpains 1 and 2 are activated in the soluble (b) and insoluble fractions (c) but not in the extracellular media (a) of PFF-treated WT neurons or control neurons (Tris). Activity levels of calpains 1 and 2 were assessed at the indicated times. The graphs represent the mean ± SD of 3 independent experiments. p < 0.001 = **, p < 0.0001 = *** (ANOVA followed by Tukey HSD post hoc test, Tris vs. PFF-treated neurons). d–f Calcium homeostasis is affected in the PFF-treated neurons. Intracellular calcium levels were measured in Tris- or PFF-treated neurons at early time points (d, 1 h, 3 h, 6 h, 8 h) or at late time points (e, D1, D2, D7, D14, D21) after the addition of the seeds to the neurons. The Fura-2 340/380 ratio was measured in each condition. Each dot represents one neuron subjected to Fura-2 calcium imaging. The data are represented mean ± SD, *p < 0.05, **p < 0.005, ***p < 0.0005 (ANOVA followed by Tukey HSD post hoc test). f Temporal transcriptomic analysis of the gene expression level in PFF-treated neurons58 (accession no. GSE142416) available in the Gene Expression Omnibus (GEO) database. Differentially expressed genes were plotted against −log10 P value (t-test, PBS-treated neurons vs. PFF-treated neurons). Log2 fold changes of the calcium gene expression levels are represented over time. “+” and “−” indicate a significant upregulation or downregulation in the gene expression level. g Confocal imaging confirmed the recruitment of calpains 1 and 2 inside the LB-like inclusions, positively stained for pS129-aSyn. Neurons were counterstained with MAP2 antibody, and the nucleus with DAPI staining. Scale bar = 10 μm. h Temporal proteomic analyses showing the enrichment of calpain 2 protein in the insoluble fraction of PFF-treated WT neurons. Transcriptional regulation of calpain 1 (i) and calpain 2 (j) in WT neurons was treated with PFF for up to 21 days. Quantitative RT-qPCR was performed with primers specific for calpain 1 (i) and calpain 2 (j) at the indicated time points after adding PFF to WT neurons. Results are presented as fold increases in comparison to the respective level in control neurons treated with Tris buffer. mRNA levels were normalized to the relative transcriptional levels of GAPDH and actin housekeeping genes. The graphs represent the mean ± SD of three independent experiments. *p < 0.05 (ANOVA followed by Tukey HSD post hoc test, Tris vs. PFF-treated neurons).
Interestingly, the increase in calpain activity and calcium level correlated well with the extent of aSyn cleavage and the appearance of the ~12 kDa fragment, which starts to accumulate within the first 6 h after the addition of PFF seeds to the neurons (Fig. 1j). This suggests that the PFF entry into neurons results in calpain activation, with the cleavage of the PFF as a consequence. Consistent with this hypothesis, pharmacological inhibition of the enzymatic activity of calpain 1 (by ALLN or PD150606) or calpain 2 (by calpain inhibitor IV) in aSyn KO neurons significantly reduced the truncation level of aSyn PFF in a concentration-dependent manner (Supplementary Fig. 12d). Calpains 1 and 2 activity (Fig. 8b) and calcium increase (Fig. 8d, e) were concomitantly upregulated in the soluble fraction of PFF-treated neurons up to D7, suggesting that these enzymes might also be involved in the processing of aSyn monomers when recruited to the seeds during the early stages of the fibrilization.
We next determined whether the cleavage of aSyn mediated by calpains 1 and 2 was only restricted to the cleavage of soluble monomeric aSyn or whether it could also be involved in the truncation of aSyn seeds and/or the newly formed fibrils present in the insoluble fraction. Remarkably, according to WB, a significant increase in calpains 1 and 2 activity was first observed on day 7 in the insoluble fraction of the PFF-treated neurons, which coincided with the formation of LB-like inclusions in the neurites and cell bodies, as well as the first appearance of HMW aSyn species (Fig. 1f). Furthermore, the enzymatic activity of calpains 1 and 2 greatly increased at D14 and D21 post-treatment (Fig. 8c). However, no change in calcium level was observed at D14 and D21, suggesting that the activation of the calpains in the insoluble fraction at late stages might result from local calcium increase inside the LB-like inclusions that Fura-2 cannot measure. However, the expression of genes related to calcium signaling pathways was disturbed in PFF-treated neurons in a time-dependent manner, with most of the significant changes quantified at D14 and D21 (Fig. 8f). Thus, altogether our results suggest that adding PFF to the neurons induces a tight regulation of the calcium homeostasis pathways with the activation of calpains as a consequence and the cleavage of newly formed aSyn aggregates as an endpoint.
Consistent with this hypothesis, calpains 1 and 2 were detected inside the LB-like inclusions, and their signals showed extensive colocalization with pS129 immunoreactivity (Fig. 8g). The significant enrichment of calpain 2 level in the seeded-aggregates fraction was confirmed by LC-MS/MS analysis (proteomics dataset, accession no. PXD016850, ProteomeXchange)58 (Fig. 8h). Finally, gene expression profiling showed that calpain 1 but not calpain 2 mRNA level was markedly upregulated in the early (D3) and late (D21) stages of LB formation and maturation (Fig. 8i, j). Altogether, our data support the hypothesis that the calpains play an active role in the remodeling of aSyn fibrils and the formation or maturation of LB.
Inhibiting calpain 1 activity accelerates the packaging of the fibrils and their sequestration into small and rounded inclusions, highly toxic to the neurons
Having established that calpains play important roles in regulating C-terminal cleavage of aSyn fibrils, we then investigated the extent to which modulating the activity of calpains influences the conversion of aSyn fibrils into inclusions. Calpain inhibitor I or DMSO (negative control) was added to PFF-treated neurons at D7 (when the newly formed filaments are not yet cleaved58) until D10 (Fig. 9a and Supplementary Fig. 12d, e). In PFF-treated neurons incubated with DMSO, the newly formed fibrils were organized as long and/or compact filamentous structures54,58,73,104,105,106 (Fig. 9b, c). Conversely, in the presence of the calpain I inhibitor, the morphology of the seeded aggregates was significantly remodeled into small and numerous rounded inclusions at D10 (Fig. 9b, c). This contrasts with the PFF-treated neurons, where a single large LB-like inclusion is formed over time. Our data suggest that inhibition of calpain activation could accelerate the conversion of the newly formed fibrils into LB-like inclusions, which are usually observed only after 21 days in PFF-seeded neurons. Further characterization of these inclusions using a panel of C-terminal antibodies in combination with pS129 immunostaining confirmed that the C-terminal truncation of the newly formed fibrils was ok upon treatment with the calpain inhibitor I.
a Experimental design to study the role of calpain 1 in the maturation of the newly formed fibrils. DMSO or calpain inhibitor I was added to PFF-treated neurons at D7. b–d Level and morphology of the pS129-positive inclusions formed in PFF-treated WT neurons in the presence of DMSO or calpain inhibitor I were assessed after immunostaining using pS129 (81a) antibody in combination with total aSyn N-terminal (1–20) or C-terminal (134–138) antibodies. Neurons were counterstained with MAP2 antibody, and the nucleus with DAPI staining. Each experiment was reproduced at least 3 times independently. b Representative images by confocal imaging. Scale bars = 5 μm. c Newly formed aggregates were classified into two groups based on their shapes: long filamentous inclusions (b, top panel) or small and rounded inclusions (b, bottom panel). A minimum of 60 neurons was counted for each condition. d Quantification of images acquired by a high-throughput wide-field cell imaging system. For each independent experiment, duplicated wells were acquired per condition, and nine fields of view were imaged for each well. Each experiment was reproduced at least 3 times independently. Images were then analyzed using the CellProfiler software to identify and quantify the level of LB-like inclusions (stained with pS129 antibody, 81a clone) formed in neurons (MAP2-positive cells). e WB analyses of the insoluble fraction of Tris- or PFF-treated WT neurons treated with DMSO or calpain inhibitor I between day 7 and day 10. After sequential extractions of the soluble and insoluble fractions, cell lysates were analyzed by immunoblotting. Total aSyn, pS129, and actin were detected by SYN-1, pS129 (MJF-R13), and actin antibodies, respectively. WB band intensities of total aSyn (15 kDa, 12 kDa, and HMW species) and pS129-aSyn were quantified by densitometry, normalized to actin levels, and expressed as fold change relative to PFF + DMSO-treated primary neurons. f Cell death level was assessed over time based on lactate dehydrogenase (LDH) release. c–e, f The graphs represent the mean ± SD of 3 independent experiments. *p < 0.05, **p < 0.005, ***p < 0.0005 (ANOVA followed by Tukey HSD post hoc test, DMSO-Tris-treated neurons vs. the other conditions). #p < 0.05, ##p < 0.005, ###p < 0.0005 (ANOVA followed by Tukey HSD post hoc test, DMSO-PFF-treated neurons vs. Calpain inhibitor I-PFF-treated neurons). All the original uncropped and unprocessed WB scans are available in Supplementary Fig. 17.
This was evidenced by using the C-terminal antibody raised against the 108-120 region that allowed detection of the aSyn-positive aggregates composed of C-terminally cleaved aSyn fibrils that were not pS129 positive (Fig. 5e). Conversely, in calpain inhibitor I-treated neurons, all the small and rounded pS129-positive inclusions were positively stained by the aSyn antibody (epitope: 108–120), suggesting that these inclusions are mainly composed of FL aSyn (Fig. 9b). In line with these data, all the pS129-positive inclusions were also positively stained with the C-terminal antibody (epitope: 134–138) (Fig. 9b, bottom panel). High-content imaging-based quantification (Fig. 9d) and WB analyses (Fig. 9e) showed that the pS129 level was significantly lower in the PFF-seeded neurons treated with calpain Inhibitor I. Finally, in the presence of the Calpain inhibitor I, the precocious formation of the round inclusions at D10 induced higher cell death levels in these neurons at D14 and D21 than in those treated with DMSO (Fig. 9f). Altogether, our results demonstrate that calpains are key regulators of post-fibrillization C-terminal cleavage of aSyn, which has newly formed and contributes to their efficient packaging and sequestration into LB-like inclusions.
Implications of post-fibrilization C-terminal truncation for investigating aSyn pathology formation and pathological diversity in synucleinopathies
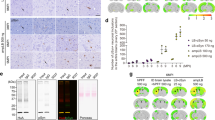
Although several studies have demonstrated the presence of truncated aSyn aggregates in the brains of patients with PD2,4,6,22,24,62,85,86 and DLB2,4,6,22,23,86,107, very few studies have explored the role of C-terminal cleavage in MSA2,6. To address this knowledge gap, we analyzed tissue homogenates from postmortem brains of MSA patients (Fig. 10a and Supplementary Fig. 13a) and confirmed the presence of C-terminally truncated aSyn species, which are detectable with an N-terminal antibody targeting residues 1–20 or a human-specific antibody (clone 4B12) targeting residues 103–108, but not an antibody against residues 134–138.
a WB analyses of the truncation pattern of aSyn in human brain tissue from MSA patients and healthy controls. Cell lysates were analyzed by immunoblotting after sequential extractions of the soluble and insoluble fractions. aSyn species were detected using 1–20, SYN-1, or 134–138 or pS129 antibodies. A double red asterisk indicates the 15 kDa band, the 12 kDa band by a single red asterisk, and the purple arrows indicate the intermediate aSyn-truncated fragments. WB band intensities of total aSyn (15 and 12 kDa) were quantified in the insoluble fraction by densitometry and normalized to actin levels. The level of the 15 kDa aSyn species in the insoluble fraction of the MSA patients is expressed as fold change relative to healthy controls. a.u (arbitrary units). b, c Serial sections from the midbrains of PDD (pars compacta) and SNCA duplication (tegmentum) cases were stained with aSyn antibodies raised specifically against the N-terminal (epitope: 1–20), the NAC (91–99), the C-terminal (epitopes: 110–115 and 134–138) or pS129 (EP1536Y) regions. Scale bars = 50 μm. All the original uncropped and unprocessed WB scans are available in Supplementary Fig. 17.
In addition, we stained serial midbrain sections of PD with dementia (PDD) (substantia nigra pars compacta, Fig. 10b and S13b for lower magnification), PD with white matter pathology (Supplementary Fig. 13c), and SNCA duplication (tegmentum, Fig. 10c and Supplementary Fig. 13c for lower magnification) with antibodies against aSyn phosphorylated at residue S129 or the NAC, the N- or the C-terminal domains of the protein.
Strikingly, this set of antibodies revealed different types of aSyn inclusions in the patients’ midbrain tissues. Specifically, while heavier and more diverse pathology was being revealed by SYN-1 (cytoplasmic punctae and extracellular aSyn as well as frequent LB and LN), the antibody raised against the last C-terminal amino acids (134–138) displayed rare cytoplasmic inclusions and neuritic pathology. Altogether, these observations suggest that C-terminal antibodies alone may not capture the diversity of aSyn pathology in human brain tissues, which could explain why C-terminally truncated species have always been viewed as a minor component in aSyn pathological aggregates despite their strong representation in proteomic data and WBs2,4,6,7,22 or by imaging43,45,50,51 when the appropriate antibodies are used.
Discussion
Several aSyn PTMs are consistently associated with LB and pathological inclusions, suggesting that these modifications are either markers of pathology or play important roles in initiating aSyn misfolding, aggregation, and formation of pathological inclusions inside the neurons. Cryo-EM studies of aSyn fibrils from recombinant proteins and brain-derived fibrils have consistently shown that the C-terminal domain of aSyn remains flexible and accessible to enzyme modifications or cleavage by proteases108,109. These observations suggest that post-fibrillization PTMs are likely to occur and influence aSyn fibril structure, interactome, and pathogenicity. Consistent with this hypothesis, we recently reported that post-fibrillization nitration induces fibril fragmentation, alters the biochemical surface properties of the fibrils, and inhibits aSyn seeding activity110. Furthermore, studies from our lab and others have also shown that C-terminal cleavage of aSyn fibrils also occurs post-aSyn fibrillization. However, the identity of the enzymes and processes that regulate the C-terminal cleavage of fibrils, as well as the role of these modifications in aSyn seeding, LB formation, and maturation, remains unknown. To address this knowledge gap, we employed a well-established neuronal seeding model that recapitulates all the stages leading to LB formation and neurodegeneration.
First, we showed that the post-fibrilization cleavage of aSyn PFF is a general phenomenon that occurs within 6–12 h after their internalization in different models of aSyn pathology formation and spreading, including mammalian cell lines, several types of rodent primary neurons, human iPSC-derived dopaminergic neurons, but also in vivo in WT and aSyn KO mice. Cleavage of aSyn PFF has been consistently observed after its internalization into the cells31,57,58,70. A recent report43 also confirmed our original findings57, showing that aSyn PFF seeds are mainly cleaved at residue 114. Next, we investigated the enzymes involved in the cleavage of aSyn PFF upon their internalization in neurons. Our results confirmed previous findings demonstrating that cathepsins67,70 and the AEP70 are involved in the processing of the PFF. Furthermore, we showed that calpains 1 and 2 are also involved in regulating the C-terminal cleavage of both exogenous aSyn PFF and newly formed fibrils, suggesting an active role of these enzymes in the processing and/or remodeling of aSyn fibrils and possibly their conversion into LB.
Interestingly, despite the systematic and rapid proteolytic processing of the PFF upon internalization into neurons, blocking the C-terminal truncation of the seeds (e.g., PFF carrying point mutations that prevent their cleavage) did not prevent or significantly alter the seeding and the recruitment of endogenous aSyn or the formation of fibrils and LB-like inclusions in neurons. Thus, C-terminal cleavage of the PFF is not a prerequisite for initiating aSyn seeding and aggregation in neurons. In line with our findings, the addition of C-terminally truncated PFF did not accelerate nor increase the seeding level in HEK cells (1–11031, 1–12031 or 1–13031), primary neurons (1–11457, 1–11031, 1–12031,54 or 1–13031) or PFF-injected mice (1–110111,112, 1–115113, 1–119113, 1–12031,114, 1–122113, 1–125113, 1–129113, 1–130111) suggesting that the deletion of various aSyn regions other than the NAC domain does not prevent the initiation of the seeding in cells and does not inhibit the formation of the LB-like structures31,53,54,57. Only one report suggested that human PFF lacking the last 30 amino acids has higher seeding activity in SH-SY5Y cells overexpressing mouse WT aSyn31. Interestingly, in this study, the aSyn 1–120 PFF, exhibited reduced seeding activity in cells. Our in vitro aggregation results suggest that the high propensity of the C-terminally truncated aSyn species to laterally associate may hinder their uptake and/or reduce the surface of fibrils that catalyze secondary nucleation events, thus resulting in reduced seeding activity. Altogether, these reports demonstrate that fibrils generated from truncated monomers or fibrils that undergo post-fibrillization C-terminal cleavage retain their seeding activity in neurons and can induce the spread of the pathology in vivo. Furthermore, our results suggest that the cleavage of the internalized aSyn PFF seeds may serve other functional or signaling purposes but is not essential for regulating aSyn seeding in neurons.
Interestingly, we observed that the exogenous aSyn PFF seeds and the newly formed aSyn fibrils are processed differently by the neurons and possess distinct biochemical properties, including PTMs (Supplementary Fig. 14). Internalized PFF do not undergo N-terminal cleavage or phosphorylation on pS129 residue, nor do they colocalize with p62 or ubiquitin, in contrast to the newly formed fibrils showing all these attributes. These observations suggest that C-terminal PTMs may be required to prime N-terminal PTMs or that aSyn PTMs occur during the process of fibril growth and LB formation and not simply in response to the presence of fibrils. Consistent with this hypothesis, adding aSyn PFF to the aSyn KO neurons does not induce toxicity58. These findings, combined with our studies on the internalization of aSyn PFF in aSyn KO neurons, suggest that the mere presence of fibrils in the absence of the formation of new fibrils by the endogenous protein is not toxic to neurons.
Post-fibrillization truncation of aSyn impacts the structural and physical properties of the fibrils, and thus it is reasonable to speculate that the PTMs on the surface of aSyn fibrils could significantly influence their interactome, toxicity, and maturation into LB-like inclusions15,20,110. In line with this hypothesis, our current work clearly demonstrates that post-fibrillization C-terminal truncation plays an important role in regulating the processing and structural reorganization of the newly formed fibrils from their lateral assembly into higher-order aggregates, as well as in the formation and maturation of LB58. More specifically, we show that the newly formed aSyn fibrils in the seeding model undergo three major changes over time: (1) increased lateral association; (2) fragmentation as evidenced by the significant decrease in fibril length over time58 resulting in short aSyn filaments randomly organized in the center of the LB-like inclusions at later stages; and (3) loss of C-terminal interacting proteins over time, in particular during the formation of LB-like inclusions. Based on these observations, we hypothesize that aSyn C-terminal truncations may also occur as post-fibrillization events that play important roles in regulating the interactome of the newly formed fibrils and trigger their lateral association, which favors their packaging into LB-like inclusions. Consistent with this hypothesis, site-specific C-terminal photocleavage of FL PFF and removal of the last 20 amino acids led to their rapid and tight lateral association. Several studies have also shown that treatment of PFF with proteases (e.g., calpain 1115, trypsin116, cathepsin B or D67,70, Supplementary Fig. 15), which target the C-terminus of aSyn, decreased fibril width67,115,116 or height67,115,116 and promoted their lateral association67,115,116. Interestingly, some of these proteases, such as calpain 152 and the proteasome117, are found sequestered in the bona fide LB in human brain tissues. In addition, the truncated fragments observed upon cleavage of fibrils in vitro or in cells are similar to those detected in vivo, in the isolated LB or the insoluble fractions extracted from PD2,4,6,22,24,25,33,40,41,42,52,71,86,118,119, DLB2,4,23,25,71,86,107,118,120, and MSA2,6,57,119,121 patients’ brain tissues. These observations suggest that specific enzymes can cleave the surface-exposed regions of aSyn fibrils, thereby significantly affecting the interactome and structural properties of the fibrils.
The abundance of C-terminally truncated aSyn species within pathological aggregates in the brain has significant implications for the detection, quantification, and targeting of aSyn pathologies in both human brain tissue and preclinical models of aSyn pathology formation and spreading122,123. First, our work underscores the limitations of relying on pS129 antibodies as the primary tools to assess and quantify aSyn aggregates and pathology formation, especially since recent studies have also shown that the level and types of C-terminally truncated aSyn vary in aSyn pathologies in different brain regions47,48. The use of multiple antibodies targeting pS129-aSyn species and the N- and C-terminal regions of the aSyn is essential to capture the full diversity and complexity of aSyn pathology in PD brain tissues and to more precisely map the distribution of aSyn species in aSyn pathologies51,124 (Fig. 10 and Supplementary Fig. 13). This limitation could also potentially extend to the study of physiological aSyn modified by pS129125, although the extent of cleavage of post-pS129 phosphorylation remains unknown. This conclusion is supported by recent studies from our group and others using different libraries of aSyn antibodies targeting different aSyn PTMs and epitopes to profile aSyn pathology in different brain regions and synucleinopathies45,47,48,51,124,126. Second, we show that C-terminal truncations play a critical role in the aSyn inclusion formation and maturation processes. Third, C-terminal truncations significantly alter the interactome of aSyn fibrils and may influence their clearance, interactions with organelles, and pathogenic properties (Supplementary Fig. 16). Finally, C-terminus-targeting therapeutic antibodies may not engage all aSyn pathological aggregates, particularly astrocytic aSyn aggregates, which lack the C-terminal domain of the protein. That being said, it should be emphasized that truncated aSyn species are always found associated with FL phosphorylated aSyn, which is consistent with post-fibrillization cleavage as the major driver for the generation of these species. The only aSyn inclusions that have been identified to be composed primarily of C-terminally truncated aSyn species are astrocytic aSyn inclusions, although these inclusions appear to be nonfibrillar and are not positive for any known LB markers51,122. Further studies using antibodies specific to the different truncated forms of aSyn51,111,124 are essential to determine the true abundance and distribution of aSyn-truncated aggregates in various brain regions and to investigate whether they are secreted and play important roles in the spreading of aSyn pathology. These studies are essential to determine whether inhibiting or promoting aSyn C-terminal cleavage at specific stages of LB formation represents a viable therapeutic strategy for treating PD and synucleinopathies (Supplementary Fig. 16).
In conclusion, although several studies have shown that the C-terminal cleavage of aSyn occurs under both physiological23,24 and pathological4,6,22,25 conditions, studies in cell-free systems26,27,28,29,30,31,32 and in cellular and animal models25,29,30,31,32,33,34,35,36,37,38,39 of synucleinopathies have consistently shown that C-terminal truncations of aSyn monomers accelerate aSyn aggregation and pathology formation. Previous studies have consistently shown that C-terminal truncations at the monomer level accelerate aSyn fibrillization and may contribute to initiating aSyn fibrillization in the brain. Although these findings suggest that inhibiting this process could protect against aSyn toxicity, it remains unclear whether C-terminal cleavage is the dominant mechanism for triggering aSyn aggregation, as several other biochemical and cellular processes have also been implicated in this process. The work presented here demonstrates that C-terminal truncations are not limited to monomeric aSyn but can also occur at the level of the fibrils and provides strong evidence that post-fibrillization C-terminal cleavages act as master regulators of fibril processing, PTMs, morphology (lateral association), and the formation and maturation of the LB. Further studies are required to dissect the role and impact of C-terminal cleavages coupled with other post-fibrillization PTMs on aSyn pathology formation, spreading, and neuronal loss in different brain regions. This work, along with recent studies from our group, highlights the crucial importance of focusing on the pattern of PTMs on aSyn fibrils and suggests that the specific pattern of PTMs, rather than merely the presence of the fibrils themselves, plays a key role in determining their pathogenicity. Finally, this is also crucial as many of the current diagnostic immunoassays127 and therapeutic strategies targeting aSyn pathological aggregates are based on targeting the C-terminal domain of the protein, which harbors the majority of disease-associated PTMs, which also influence aSyn fibril dynamics, structure, and interactome. Thus, underscoring the importance of accounting for PTMs in the development of aSyn diagnostic and therapeutics aimed at targeting the diversity of aSyn pathology.
Finally, one major limitation of this study is the use of unmodified fibrils derived from recombinant proteins, whereas aSyn fibrils formed in human brain neurons undergo extensive modifications at multiple N- and C-terminal residues. While PFFs are a well-established tool for inducing seeding in cellular and in vivo models to study aggregation and pathology propagation, they serve as an experimental approximation rather than an exact replication of patient brain pathology. The presence, size, and concentration of PFF-like entities in the human brain remain uncertain, and the relevance of the 70 nM concentration used in our experiments should be considered in this context. Although 70 nM may seem relatively low, this concentration was chosen based on prior studies using cellular models of PD and represents a sub-saturating dose that reliably induces seeding and aggregation. We acknowledge that the concentration of endogenous aSyn aggregates in patient brains is unknown, and extrapolating from in vitro conditions to in vivo scenarios should be approached with caution. Additionally, the number of PFF-like structures that a patient neuron might realistically encounter and their source within the brain remain unresolved questions. Despite these limitations, our study provides valuable insights into the fundamental mechanisms underlying aSyn aggregation and seeding. Furthermore, the use of PFF has enabled the development of the neuronal seeding model that robustly reproduces many key features of bona fide LB. Moreover, it is possible that the PTMs that occur in patients could alter the kinetics and extent of aSyn uptake, processing, clearance, and seeding activity. Recent studies by Marotta et al.128 showed that aSyn fibrils derived from PDD and AD brains, when added to neuronal cultures, result in predominantly cell body pathology, whereas unmodified recombinant aSyn fibrils give rise to predominantly neuritic pathology. These observations underscore the role of PTMs and other Lewy pathology-associated cofactors in aSyn seeding, pathology formation, and spreading. Therefore, future studies should systematically examine how PTMs found in aSyn aggregates isolated from different synucleinopathies’ brains influence aSyn uptake, seeding, and LB formation and maturation. Given the challenges in accessing brain tissues and the small amount of material that could be isolated from postmortem human brains, we propose the use of neuronal models of seeding as an alternative source of native, modified aSyn fibrils, since fibrils produced in these models recapitulate the PTM pattern found in LB.
Methods
Antibodies and compounds
Information and RRID of the primary antibodies used in this study are listed in Supplementary Fig. 2, which includes their clone names, catalog numbers, vendors, and respective epitopes.
Expression and purification of human and mouse aSyn
pT7-7 plasmids were used for the expression of recombinant human and mouse aSyn in E.coli. Human and mouse wild-type (WT) aSyn or mouse aSyn mutants (1–135, 1–133, 1–124, 1–120, 1–115, 1–114, 1–111 or 1–101, E114A, D115A, Δ111–115, Δ120–125, Δ133–135, Δ111–115Δ133–135) were expressed and purified using anion exchange chromatography (AEC) followed by reverse-phase High-Performance Liquid Chromatography. Recombinant aSyn proteins were fully characterized as described previously by Fauvet et al.11. For aSyn 1–114, the AEC purification was replaced by cation exchange chromatography due to the lack of the negatively charged C-terminus. The protein was then further purified and characterized in a manner similar to that of the other proteins.
Preparation of WT, deletion mutants, and C-terminally truncated PFF for in vitro studies
Lyophilized FL, C-terminally truncated (1–135, 1–133, 1–124, 1–120, 1–115, 1–114 or 1–111) single residue deletion (Δ111–115, Δ120–125 or Δ133–135) or double residue deletion (Δ111–115Δ133–135) aSyn proteins were dissolved in 50 mM Tris, 150 mM NaCl. The pH was adjusted to 7.5 using a 1 M sodium hydroxide solution, and the proteins were subsequently filtered through 100 kDa MW-cut-off filters. The concentration of aSyn in solution was determined using its UV absorption at 280 nm, while, due to the low number of remaining tyrosine residues, the concentration of the C-terminally truncated aSyn mutants and aSyn deleted mutants was determined using the PierceTM bicinchoninic acid (BCA) Protein Assay kit (Thermo Scientific). Fibril formation was induced by incubating the proteins at a final concentration of 20 µM at 37 °C and pH 7.5 (unless stated otherwise) in 50 mM Tris and 150 mM NaCl under constant agitation at 1000 rpm on an orbital shaker for 6 days.
Preparation of aSyn PFF for cell treatments
Monomeric human or mouse WT, or biotinylated aSyn monomers, were dissolved in 500 μL of Tris buffer (50 mM Tris, 150 mM NaCl, pH 7.5), filtered using a 100 kDa filter (Millipore, Switzerland) at a final concentration of 1–2 mg/mL. Proteins were incubated under constant orbital agitation at 1000 rpm (PEQLAB Biotechnologie GMBH) at 37 °C for 5 days129, leading to the formation of aSyn fibrils. These fibrils were sonicated with a fine probe 4 times for 5 s at an amplitude of 20% (Sonic Vibra Cell, Blanc Labo, Switzerland). Their biophysical and structural properties were then assessed using EM, ThT fluorescence, SDS-PAGE, and Coomassie blue staining130. For long-term storage, sonicated PFF were snap-frozen in liquid nitrogen and kept at −80 °C.
Preparation of fluorescently labeled mouse aSyn PFF
aSyn mouse WT PFF were diluted at a concentration of 250 μM in a final volume of 500 μl of PBS. The pH was adjusted to 7.5. One equivalent of Atto488 maleimide (Atto-Tec, Switzerland) was added and incubated at 4 °C overnight. The labeled PFF were then ultracentrifuged at 100,000 × g for 1 h at 4 °C. The supernatant was collected, and the pellet was resuspended in PBS. This wash step was repeated until the dye in excess was removed. PFFs were loaded onto an SDS-PAGE gel, and labeling was confirmed by scanning the gel using Typhoon FLA 7000 (GE Healthcare Life Sciences, Switzerland) with respective excitation and emission wavelengths of 400 and 505 nm. Labeled fibrils were then fragmented via sonication 4 times at 5 s at an amplitude of 20% (Sonic Vibra Cell, Blanc Labo, Switzerland). PFF were then snap-frozen in liquid nitrogen and stored at −80 °C for long-term storage. The biophysical and structural properties of the fibrils were then assessed by EM, SDS-PAGE/ Coomassie staining, and thioflavin T assays.
Preparation of biotinylated aSyn monomers
Recombinant FL M1C aSyn (10 mg) was placed in 1.0 mL of degassed buffer (200 mM Tris, 6.0 M Gdn at pH 6.5). To ensure that cysteines were in the reduced form, the protein was treated with 1.0 equivalent of Tris(2-carboxyethyl)phosphine (TCEP) and incubated at 37 °C for 20 min. The reaction was monitored by LC-MS to confirm that dimers were reduced to monomers. Then, 3.0 equivalents of biotin maleimide (10 µL of a 200 mM solution in dimethylformamide) were added and stirred at 37 °C for 60 min at pH 6.5. The progression of the labeling was monitored by electrospray ionization (ESI) LC/MS, and after complete consumption of the starting M1C aSyn protein, the reaction was quenched by lowering the pH from 6.5 to 5. The reaction mixture was cooled to 5 °C and purified by C8 semi-preparative column chromatography using a 20%–80% acetonitrile gradient over 60 min at a flow rate of 3.0 mL/min. The purity of the fractions was verified by ultra-performance liquid chromatography (UPLC), and the biotin-labeled aSyn was lyophilized. The purity and identity of the final product were confirmed by UPLC, SDS-PAGE, and ESI-LC/MS analyses.
Semisynthesis, aggregation, and photolysis of aSyn photocleavable at residue 120
The semisynthesis of aSyn photocleavable at residue 120 (aSyn-D121Anp) was carried out as previously described14. First, we dissolved 1-106-SR in 6 M guanidine and 200 mM Na2PO4 supplemented with 30 mM TCEP buffer. Then, 1.5 equivalents of A107C-140 D121Anp peptides were added to the reaction mixture. Next, the native chemical ligation was performed with shaking at 1000 rpm at 37 °C for 3 h. When the ligation was complete, as monitored by mass spectrometry and SDS-PAGE, the protein was purified as previously described14. To prepare monomeric aSyn-D121Anp, 100 µg of the protein was dissolved in phosphate-buffered saline (PBS). For the preparation of fibrils, 500 µg of the protein was dissolved in 100 µL of PBS, and the fibrils were formed by incubating the protein under constant orbital agitation at 1000 rpm (Peqlab, Thriller, Germany) at 37 °C for 5 days129. The photocleavage of both the fibrils and monomer was performed using a Panasonic UP50, 200 W mercury Xenon lamp with a bandpass filter of 300–410 nm at 100% power.
Electron microscopy (EM)
3.5 μl of sonicated or unsonicated fibrils (final concentration 10 μM) were applied onto glow-discharged formvar/carbon-coated 200-mesh copper grids (Electron Microscopy Sciences, United Kingdom) for 1 min. The grids were blotted off before the two washes with ultrapure water. The grids were then incubated in a staining solution of 0.7% (w/v) uranyl formate for 30 s. The grids were blotted and dried. Samples were imaged using a Tecnai Spirit BioTWIN operated at 80 kV and equipped with a LaB6 filament and a 4K × 4K FEI Eagle CCD camera.
Primary culture of hippocampal neurons and treatment with mouse aSyn fibrils
Pregnant C57BL/6Jrj females (Janvier Labs, France) were imported upon request, and C57BL/6JOlaHsd females (Envigo, France) were bred in the EPFL animal facility to generate experimental animals. Neonatal pups (P0–P1) were euthanized by decapitation immediately prior to brain collection. All experiments were conducted ex vivo. Whenever possible, dams were reassigned to the EPFL Organ/Tissue Sharing Program (Optimice) as part of a 3Rs initiative. All procedures were approved by the Swiss Federal Veterinary Office (authorization numbers VD 3392 and VD 4029). Primary hippocampal neurons were prepared from brains collected from P0 pups from WT mice (C57BL/6JRj) or aSyn KO mice (C57BL/6J OlaHsd) and cultured as previously described58. The neurons were seeded in 6-well plates or onto coverslips (CS) (VWR, Switzerland) that had been previously coated with 0.1% w/v poly-L-lysine in water (Brunschwig, Switzerland) at a density of 300,000 cells/mL. After 5 days in culture, the WT or aSyn KO neurons were treated with extracellular aSyn fibrils to a final concentration of 70 nM as previously described58.
Cell lysis and WB analyses of primary hippocampal neurons
After aSyn PFF treatment, primary hippocampal neurons were lysed as previously described54,58,59. Briefly, treated neurons were lysed in 1% Triton X-100/Tris-buffered saline (TBS) (50 mM Tris, 150 mM NaCl, pH 7.5) supplemented with protease inhibitor cocktail (Roche, Switzerland), 1 mM phenylmethane sulfonyl fluoride (PMSF), and phosphatase inhibitor cocktail 2 and 3 (Sigma-Aldrich, Switzerland). Cell lysates were sonicated 10 times with a fine probe for 0.5-s pulses at an amplitude of 20% (Sonic Vibra Cell, Blanc Labo, Switzerland) and then incubated for 30 min on ice. The cell lysates were then centrifuged at 100,000 × g for 30 min at 4 °C. The supernatant (soluble fraction) was collected while the pellet was resuspended in 1% Triton X-100/TBS. The pellet was then sonicated as described above and centrifuged for 30 min at 100,000 × g. The supernatant was discarded, whereas the pellet (insoluble fraction) was resuspended in 2% SDS/TBS supplemented with protease inhibitor cocktail (Roche, Switzerland), 1 mM PMSF, and phosphatase inhibitor cocktail 2 and 3 (Sigma-Aldrich, Switzerland). The insoluble fraction was then sonicated using a fine probe 15 times at a 0.5-s pulse with an amplitude of 20%. The protein concentration of the soluble and insoluble fractions was quantified using the BCA protein assay. Laemmli buffer (4% SDS, 40% glycerol, 0.05% bromophenol blue, 0.252 M Tris-HCl, pH 6.8, and 5% β-mercaptoethanol) was then added to the soluble and insoluble fractions. Each data point in the bar graph represents an independent experiment conducted using separately prepared primary neuronal cultures on different days, weeks, or even months. Each point corresponds to samples from distinct wells, and due to space constraints on the gels, independent experiments were not run on the same membrane. Instead, they were processed on separate gels on different days or weeks. Total aSyn (15 kDa, 12 kDa, or HMW) and pS129-aSyn levels were quantified by measuring band intensity in Western blots using ImageJ software (U.S. National Institutes of Health, Maryland, USA; RRID:SCR_001935). These values were normalized to actin protein levels. Densitometry graphs display the fold increase in aSyn species relative to the control, as specified in the figure legends.
AEP activity assay in primary neurons
AEP activity was measured in KO and WT neurons treated for up to 21 days with PBS (negative control) or mouse WT PFF at a final concentration of 70 nM, as previously reported71. Briefly, the primary cell cultures were lysed in the assay buffer (20 mM citric acid, 60 mM Na2HPO4, 1 mM EDTA, 0.1% CHAPS, and 1 mM DTT, pH 6.0), which contains 20 μM AEP substrate Z-Ala-Ala-Asn coupled to the AMC (7-Amino-4-methylcoumarin) fluorescent dye (Bachem, Switzerland) and incubated at 37 °C for 1 h. AMC release was measured using the Tecan Infinite M200 Pro plate reader (Tecan, Maennedorf, Switzerland) with respective excitation and emission wavelengths of 380 and 460 nm.
Cathepsin B, D, and L activity assay in primary neurons
Cathepsin B, D, and L activity was measured in KO and WT neurons treated for up to 48 h with PBS (negative control) or mouse WT PFF at 70 nM using fluorescence-based assays (Abcam, UK) as per the manufacturer’s instructions. Briefly, the primary neurons were lysed and incubated at 37 °C for 2 h, protected from light with the cathepsin-B substrate sequence RR labeled with AFC (amino-4-trifluoromethyl coumarin) or with the cathepsin-D substrate sequence GKPILFFRLK(Dnp)-D-R-NH2, labeled with MCA (7-methoxycoumarin-4-acetic acid) or with the preferred cathepsin-L substrate sequence FR labeled with AFC. The released AFC or MCA was quantified using a Tecan Infinite M200 Pro plate reader (Tecan, Maennedorf, Switzerland). The following wavelengths were used: for cathepsin B assay (Ex/Em = 400/505 nm), cathepsin D assay (Ex/Em = 328/460 nm), and cathepsin L (Ex/Em = 400/505 nm).
Calpain 1 and calpain 2 activity assay in primary neurons
The calpain activity assay kit (Abcam, UK) measured calpain activity in primary cultures treated with Tris buffer (negative control) or with aSyn WT PFF for 3 h to 21 days. At each indicated time point, 100 μL of the extracellular medium was collected before harvesting the neurons. Collected extracellular media and neurons were then lysed in the extraction buffer provided in the kit and centrifuged at 4 °C at 13,000 rpm for 5 min. Soluble fractions were collected and handled following the manufacturer’s instructions. Pellets from the lysed neurons were resuspended in the extraction buffer provided in the kit, and mild sonication was performed for 10 s at 20% amplitude (Sonic Vibra Cell, Blanc Labo, Switzerland) to ensure complete dispersion of the pellet. A calpain activity assay was then performed according to the supplier’s instructions. Fluorescein emission was quantified using a Tecan Infinite M200 Pro plate reader (Tecan, Männedorf, Switzerland) with respective excitation and emission wavelengths of 400 and 505 nm.
Calpain 1 cleavage of aSyn PFF in vitro
Cleavage of aSyn PFF was performed as described previously93. Briefly, 0.15 U of active calpain 1 recombinant protein (Abcam, UK) was added to aSyn PFF diluted to a final concentration of 50 μM in the reaction buffer containing 40 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) at pH 7.5 and 5 mM dithiothreitol (DTT) at 37 °C. The reaction was initiated by the addition of CaCl2 (1 mM final). Samples were collected before the addition of CaCl2 (time 0) or 2, 5, 15, or 30 min after calpain 1 activation. Cleavage of aSyn by calpain 1 was assessed by Coomassie staining after the samples were separated onto 16.5% SDS-PAGE gels.
Microinjection of PFF in primary hippocampal neurons
Primary aSyn KO hippocampal neurons were isolated and cultured according to the methods described above. Mouse Alexa Fluor488-labeled aSyn PFF were prepared as described in the methods above. aSyn PFF were diluted to 9.2 µM (0.13 µg/µl) in distilled H2O. Microinjection was performed using the INJECT + MATIC Sàrl injector (Geneva, Switzerland) under the Leica light transmission microscope. A pre-pulled glass microinjection needle was loaded with 2 µl of fibrils, and fibrils were injected at 2 times units/1 pressure unit/neuron (~6.5 ng in 50 nl/neuron). Cells were incubated for 24 h post-injection. Cells were fixed using 4% formaldehyde solution (Sigma-Aldrich, Switzerland) for 20 min, washed twice in 1X PBS, then permeabilized and blocked in 1% saponin and 5% bovine serum albumin in PBS with 0.01% NaN3 for 4 h at room temperature (RT), then stained with specific anti-aSyn antibodies (epitopes: 1–20 or 134–138) and anti-MAP2 overnight at 4 °C. Cells were washed twice in PBS and incubated in secondary antibody and DAPI stain to visualize nuclei for 2 h at RT. Secondary antibodies coupled with Alexa Fluor dyes (Thermo Fisher Scientific, USA) were applied for 1 h at RT. Cells were washed twice in PBS, and coverslips were mounted onto microscopy slides using a Mowiol mounting medium (Sigma-Aldrich, Switzerland). The cells were examined with a confocal laser-scanning microscope (LSM 700, Carl Zeiss Microscopy, Germany) with a 40× objective and analyzed using Zen software (RRID:SCR_013672).
Immunocytochemistry (ICC)
HeLa cells or primary hippocampal neurons treated with PFF were washed twice with PBS before being fixed in 4% PFA for 20 min at RT. Cells were then immunostained as previously described58. The antibodies used are indicated in the corresponding legend section of each figure. The source and dilution of each antibody can be found in Supplementary Fig. 2. The cells were examined using a confocal laser-scanning microscope (LSM 700, Carl Zeiss Microscopy, Germany) with a 40x objective and analyzed with Zen software (RRID:SCR_013672).
Quantitative high-throughput wide-field cell imaging screening (HTS)
After aSyn PFF treatment, primary hippocampal neurons plated in clear black bottom 96-well plates (BD, Switzerland) were washed twice with PBS, fixed in 4% PFA for 20 min at RT, and immunostained as described above. Images were acquired using the Nikon 10X/0.45 Plan Apo CFI/60 objective of the IN Cell Analyzer 2200 (GE Healthcare, Switzerland), a high-throughput imaging system equipped with a high-resolution 16-bit sCMOS camera (2048 × 2048 pixels), with a 2 × 2 binning. For each independent experiment, duplicated wells were acquired per condition, and nine fields of view were imaged for each well. Each experiment was reproduced at least 3 times independently. Images were then analyzed using Cell Profiler 3.0.0 software (RRID:SCR_007358) to identify and quantify the level of LB-like inclusions (stained with the pS129 antibody) formed in MAP2-positive neurons, as previously described58. Each data point in the HTS bar graphs represents an independent experiment using primary neuronal cultures prepared in different weeks or months. Within each experiment, at least three wells per condition were analyzed as internal replicates, and these were averaged to generate a single data point per experiment.
Correlative light electron microscopy (CLEM)
Mouse primary hippocampal neurons were grown on 35 mm dishes with alphanumeric searching grids etched to the bottom glass (MatTek Corporation, Ashland, MA, USA) and treated with WT PFF. At the indicated time, cells were fixed for 2 h with 1% glutaraldehyde and 2.0% PFA in 0.1 M phosphate buffer (PB) at pH 7.4. After washing with PBS, ICC was performed (for more details, see the corresponding section in the Materials and Methods). Neurons with LB-like inclusions (positively stained for pS129) were selected with a fluorescence confocal microscope (LSM700, Carl Zeiss Microscopy, Germany) for ultrastructural analysis. The precise position of the selected neuron was recorded using the alphanumeric grid etched on the dish bottom. The cells were then fixed further with 2.5% glutaraldehyde and 2.0% paraformaldehyde in 0.1 M PB at pH 7.4 for another 2 h. After washing five times for 5 min each with 0.1 M cacodylate buffer at pH 7.4, cells were post-fixed with 1% osmium tetroxide in the same buffer for 1 h and then washed with double-distilled water before being contrasted with 1% uranyl acetate stain for 1 h. The cells were then dehydrated in increasing concentrations of alcohol (2 × 50%, 1 × 70%, 1 × 90%, 1 × 95%, and 2 × 100%) for 3 min each. Dehydrated cells were infiltrated with Durcupan resin diluted with absolute ethanol at 1:2 for 30 min, at 1:1 for 30 min, and 2:1 for 30 min, and twice with pure Durcupan (Electron Microscopy Sciences, Hatfield, PA, USA) for 30 min each. After 2 h of incubation in fresh Durcupan resin, the dishes were transferred into a 65 °C oven for the resin to polymerize overnight. Once the resin had hardened, the glass CS on the bottom of the dish was removed by repeated immersion in hot (60 °C) water, followed by liquid nitrogen. The cell of interest was then located using the previously recorded alphanumeric coordinates, and a razor blade cut this region away from the rest of the resin. This piece was then glued to a resin block with acrylic glue, trimmed with a glass knife using an ultramicrotome (Leica Ultracut UCT, Leica Microsystems), and then ultrathin sections (50–60 nm) were cut serially from the face with a diamond knife (Diatome, Biel, Switzerland) and collected onto 2 mm single-slot copper grids coated with formvar plastic support film. Sections were contrasted with uranyl acetate and lead citrate and imaged with a transmission electron microscope (Tecnai Spirit EM, FEI, The Netherlands) operating at 80 kV acceleration voltage and equipped with a digital camera (FEI Eagle, FEI).
HeLa cell culture, plasmid transfection, and treatment with human aSyn PFF
HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), high glucose, pyruvate, and GlutaMAX™ supplemented with 10% FBS and 10 μg/mL penicillin and streptomycin in a humidified incubator, 5% CO2, 37 °C. According to the manufacturer’s protocol, HeLa cells were transfected with a pAAV vector encoding human aSyn using Lipofectamine 2000 transfection reagent (Life Technologies, Switzerland). Twenty-four hours post-transfection, human aSyn fibrils were added to the cells at a final concentration of 500 nM.
Cell lysis and WB analyses of HeLa cells
HeLa cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (150 mM sodium chloride, 50 mM Tris pH 8.0, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) supplemented with protease inhibitor cocktail (cOmpleteTM, Roche, Switzerland), 1 mM PMSF, and phosphatase inhibitor cocktail 2 and 3 (Sigma-Aldrich, Switzerland). Cell lysates were cleared by centrifugation at 4 °C for 15 min at 13,000 rpm. The pellet (insoluble fraction) was resuspended in SDS/TBS supplemented with a protease inhibitor cocktail (cOmpleteTM, Roche, Switzerland), 1 mM PMSF, and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich, Switzerland), and then sonicated using a fine probe 15 times at a 0.5-s pulse with an amplitude of 20%. A BCA protein assay was performed to quantify the protein concentration in the soluble and insoluble fractions before the addition of Laemmli buffer 4x. Proteins from the soluble and insoluble fractions were then separated on a 16% Tricine gel, transferred onto a nitrocellulose membrane (Thermo Fisher Scientific, Switzerland) using a semi-dry system (Bio-Rad, UK), and immunostained as previously described130.
iPSC-derived neurons, treatment with human aSyn PFF, WB, and ICC analyses
iPSCs were derived from dermal fibroblasts. The line used in this study originated from a healthy 78-year-old female control and was reprogrammed using the CytoTune-iPS Sendai Reprogramming kit (Invitrogen). The iPSC line has been characterized in previous studies as SFC856-03-04 (control 4)131,132. The iPSCs were kept at 37 °C, 5% CO2 in feeder-free culture conditions with daily changes of mTeSR™1 (StemCell Technologies, UK), on hESC-qualified Matrigel-coated plates (BD Biosciences, UK) and passaged as single cells using TrypLE Express incubation (Life Technologies, UK) and ROCK inhibitor (10 µM Y-27632) (Bio-Techne, Tocris, UK). IPSCs were differentiated into dopaminergic neurons using a floor-plate-based culture established by Kriks et al.79 and modified as previously described78,80 (see below Table 1 for media and reagents used during the differentiation).
The cells were seeded in 6-well plates coated with Geltrex (Life Technologies, UK) and grown to confluency. The medium was changed completely every 2 days, with half of the volume changing every other day, until day in vitro 20 (DIV20). The cells were then dissociated with StemPro Accutase (Life Technologies, UK) and replated onto Geltrex-coated 12-well plates (Corning, UK) in an even monolayer of 3 × 105 cells/cm2 in a medium containing a ROCK inhibitor (10 µM Y-27632). Cultures were treated with 1 µg/ml mitomycin C (Bio-Techne, Tocris, UK) in neurobasal (NB) medium for 1 h to remove proliferating cells and washed with NB medium before adding fresh NB medium. After a full medium change 3 days later to remove dead cells, the medium was half-changed every 2–3 days until DIV50, when 70 nM of PFF was added to neurons 14, 10, 7, 3, and 1 day before fractionation analyses at DIV64. For WB analyses, cultures were washed in phosphate-buffered saline (PBS), and detached by scraping into fresh 1% Triton X-100/TBS (50 mM Tris, 150 mM NaCl, pH 7.5) with protease (cOmpleteTM, Roche, Switzerland) and phosphatase inhibitors (PhosSTOPTM, Roche, Switzerland) and subjected to a previously described fractionation protocol59. After sonication using a fine probe, 0.5-s pulse at an amplitude of 20%, 10 times (UP50H, Hielscher, Germany), cell lysates were incubated on ice for 30 min and centrifuged (Optima TLX ultracentrifuge, TLA-110 rotor, Beckman Coulter, USA) at 100,000 × g for 30 min at 4 °C and the supernatant (soluble fraction) collected.
The pellet (insoluble fraction) was resuspended in 2% sodium dodecyl sulfate (SDS)/TBS supplemented with protease (cOmpleteTM, Roche, Switzerland) and phosphatase inhibitors (PhosSTOPTM, Roche, Switzerland) and sonicated using a fine probe (0.5-s pulse at an amplitude of 20%, 15 times). 10 ug of protein from each fraction was loaded on Criterion™ TGX™ precast gel 4–15% (Bio-Rad) and transferred onto Trans-Blot® Turbo™ polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Membranes were blocked in 5% milk in PBS containing 0.1% Tween for 30 min and incubated overnight in blocking solution with a total aSyn antibody (SYN-1, BD Biosciences; 1:500). The secondary anti-mouse HRP (Bio-Rad; 1:5000) and loading control β-actin-HRP (Abcam, 1:50,000) were diluted in blocking solution and applied for 1 h at RT. The membrane was exposed to Immobilon Western Chemiluminescent HRP Substrate and developed with the ChemiDocTM System (Bio-Rad). Protein levels were measured with the Image Lab Software (RRID:SCR_014210) (Bio-Rad) and analyzed with GraphPad Prism (RRID:SCR_002798).
Identification of N- and C-terminal truncation sites by quantitative proteomic analyses
After aSyn WT PFF treatment, primary hippocampal neurons (treated for 14 or 21 days) were lysed as previously described54,58,59 and separated by 16% Tricine gel, which was then stained with Coomassie Brilliant Blue (Life Technologies, USA). Each gel lane was entirely sliced, and proteins were in-gel digested as previously described133, skipping the reduction and alkylation procedures. Peptides were desalted on stageTips134 and dried under a vacuum concentrator. For LC-MS/MS analysis, the resuspended peptides were separated by reversed-phase chromatography using a Dionex Ultimate 3000 RSLC nano UPLC system, which was connected in line with an Orbitrap Lumos (Thermo Fisher Scientific, Waltham, USA). Raw data were processed using Mascot (Matrix Science, Boston, USA), MS-Amanda135, and SEQUEST in Proteome Discoverer v.1.4 (RRID:SCR_014477) against the UniProt Mouse protein database. Data were further processed and inspected in Scaffold4 (Scaffold Proteome Software, RRID:SCR_014345), and spectra of interest were manually validated.
Fura-2 calcium imaging
Primary hippocampal neurons treated with Tris and PFF at various time points were incubated in growth media with 1 μM Fura-2 AM (Abcam, UK) for 30 min, followed by incubation without Fura-2 AM for 30 min. After this, fluorescent images were collected by exciting at 340 and 380 nm, and emitting at 510 nm for ~4–5 min. At the end of each condition, ionomycin was added as a positive control to verify an increase in intracellular calcium levels. Fura-2 AM calcium imaging analysis was performed using an Olympus cellSens Software (RRID:SCR_014551) ratio analysis tool, as per the instruction manual, to collect Fura-2 340/380 ratio values.
Quantitative real-time RT-PCR
Primary hippocampal neurons were treated with WT PFF or PBS buffer for 7, 14, and 21 days. Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Switzerland) according to the manufacturer’s protocol. The concentration of each sample was measured using NanoDrop (NanoDrop Technologies, Wilmington, DE, USA), and the purity was confirmed using the ratios at 260/280 nm and 260/230 nm. Two micrograms of RNA were used to synthesize cDNA using the High-Capacity RNA-to-cDNA Kit (Life Technologies, USA) following the manufacturer’s instructions. To quantify aSyn, calpain 1, and calpain 2 mRNA levels (see primers in Table 2), we used the SYBR Green PCR master mix (Power SYBR Green PCR Master Mix, Life Technologies, USA). qRT/RT-PCR assay was performed using the following primers synthesized by Microsynth (Balgach, Switzerland).
40 cycles of amplification were then performed in an ABI Prism 7900 (Applied Biosystem, Foster City, USA) using a 384-wells plate, which simultaneously allowed the analysis of the genes of interest and the housekeeping genes (β-actin and GAPDH) that serve as references for the normalization step. For each independent experiment, triplicate wells were used per condition, and each experiment was replicated at least three times independently. The expression level of the genes of interest was quantified in the different conditions tested; the comparative 2−ΔΔCT method was used, where ΔΔCT = ΔCT(target gene)−ΔCT(reference gene) and ΔCT = CT(target gene)−CT(reference gene). Results were expressed as the fold change relative to control neurons (2−ΔΔCT). The geNorm method (RRID:SCR_006763, https://genorm.cmgg.be/) was performed to assess the most stable reference gene for normalizing gene expression136.
Temporal transcriptomic analyses
Our previously published transcriptomic dataset58 (accession no. GSE142416), available in the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo), was re-analyzed to examine gene expression related to calcium signaling.
Quantification of LDH release in primary neurons
Using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Switzerland), the release of lactic acid dehydrogenase (LDH) into culture supernatants was measured according to the manufacturer’s instructions. After a 30 min coupled enzymatic reaction, which results in the conversion of a tetrazolium salt (INT) into a red formazan product, the amount of color formed, which is proportional to the number of damaged cells, was measured using Tecan Infinite M200 Pro plate reader (Tecan, Maennedorf, Switzerland) at a wavelength of 490 nm.
Intra-striatal stereotaxic injection of mouse and human aSyn PFF into mice brain
Animal experiments were conducted in accordance with the European Directive 2010/63/EU guidelines and Belgian legislation. The ethical committee for animal experimentation from UCB Biopharma SPRL (LA1220040 and LA2220363) approved the experimental protocol. All surgical procedures were performed on wild-type male mice from the C57BL/6J strain and male aSyn KO mice (The Jackson Laboratory; #023837). Surgeries were performed under general anesthesia using a mixture of 50 mg/kg of ketamine (Nimatek, Eurovet Animal Health B.V.) and 0.5 mg/kg of medetomidine (Domitor, Orion Corporation) injected intraperitoneally. Additionally, 2.5 mg/kg atipamezole (Antisedan, Orion Corporation) was administered to support awakening. The recombinant purified FL human PFF were thawed and sonicated at RT by probe sonication (Q500 sonicator from Qsonica; 20 kHz; 65% power, for 30 pulses of 1 s ON, 1 s OFF). C57BL/6 wild-type and aSyn KO mice were anaesthetized and stereotactically injected into the right striatum (coordinates: anterior-posterior, 0.2 mm; mediolateral, −2 mm; dorsoventral, −3.2 mm relative to bregma and dural surface) using a glass capillary attached to a 25 µl Hamilton microsyringe. Five micrograms of human PFF (2.5 µg/µl in sterile PBS) were administered at a constant rate of 0.2 µl per minute, and the needle was left in place for an additional 2.5-min period before its slow retraction. Mice were euthanised at 0, 1, 3, and 7 days post-injection. After anesthesia using a mixture of 50 mg/kg of ketamine (Nimatek, Eurovet Animal Health B.V.) and 0.5 mg/kg of medetomidine (Domitor, Orion Corporation) injected intraperitoneally, the animals were perfused through the ascending aorta with a mixed solution (30 ml/mouse) made of PBS and heparin (10 U/ml, 4 °C). Brains were removed from the skull, and the right striatum was dissected out. Brain tissues were snap-frozen in nitrogen and stored at −80 °C.
Cell lysis and WB analyses of human PFF-injected mice brains and human brain tissues
For biochemistry analyses, the soluble and insoluble fractions of mouse and human brain tissues were prepared according to previously described protocols54,59. Briefly, tissues were homogenized in TBS-T lysis buffer containing 50 mM Tris-HCl, 1% Triton X-100, 150 mM NaCl, and a cocktail of protease and phosphatase inhibitors (Roche, Switzerland). The samples were then sonicated at 20% power for 20 s using a Q500 sonicator (Qsonica, USA). Homogenates were centrifuged at 100,000 × g for 30 min at 4 °C. The supernatant corresponded to the soluble fraction. The pellet was resuspended in TBS-T lysis buffer, sonicated at 20% power for 20 s, and then centrifuged at 100,000 × g for 30 min at 4 °C. The supernatant was discarded. The insoluble fraction was prepared by resuspending the pellet in TBS-T lysis buffer containing 2% SDS and then sonicated at 20% power for 40 s. Protein concentration was determined using the BCA assay. Twenty micrograms of total protein were loaded onto Tris-glycine 16% gels (Novex Thermo, USA) and migrated at 100 V. The gels were then transferred onto Immobilon polyvinylidene difluoride membranes (Merck-Millipore, Germany) at 25 V for 30 min using the Trans-Blot Turbo (Bio-Rad Laboratories, USA). Membranes were blocked for 1 h with Odyssey blocking buffer (LiCor, USA) and then incubated overnight at 4 °C with different primary antibodies diluted in the same blocking buffer. After rinsing with TBS-Tween 0.1%, membranes were incubated with IRDye® conjugated secondary antibodies (1:5,000; LiCor, USA) for 1 h at RT, and visualization was performed by fluorescence using Odyssey CLx from LiCor. Signal intensity was quantified using Image Studio 3.1 from LiCor (RRID:SCR_013715). Primary antibodies were directed to human aSyn epitope 103–108 (4B12, 1:1000, Thermo FisherScientific, USA), mouse aSyn (D37A6, 1:1000, Cell Signaling Technology, USA), aSyn epitope 1–20 (1:750, homemade), aSyn epitope 91–99 (clone 42, SYN-1, 1:1000, Becton Dickinson, USA), aSynuclein epitope 134–138 (1:1500, Abcam, UK), phospho-serine 129 aSyn (1:1500, Abcam, UK), actin (1:3000, Cell Signaling Technologies, USA).
Human brain samples
Case demographics are presented in Supplementary Fig. 13a. Frozen human brain samples from MSA patients were obtained from the Netherlands Brain Bank (NBB), a facility of the Netherlands Institute for Neuroscience (Amsterdam, the Netherlands; open access: www.brainbank.nl), after approval of the project by the Netherlands Brain Bank’s ethical committee. All material was collected from donors who provided written informed consent for brain autopsy, and the Netherlands Brain Bank obtained permission to use the material and associated clinical information for research purposes. The PDD and SNCA duplication cases selected from the Queen Square Brain Bank (QSBB) at the University College London (UCL, UK), Institute of Neurology, were collected following protocols approved by the London Multicentre Research Ethics Committee. Written informed consent was obtained from all donors before brain collection. The samples were stored under a license approved by the Human Tissue Authority (HTA; license number 12198). Ethical approval for the research was granted by the National Research Ethics Service (NRES) Committee London Central.
Immunohistochemistry on postmortem human tissue
Eight-micrometer-thick paraffin-embedded sections were cut sequentially. For selected primary antibodies (Supplementary Fig. 2a), immunohistochemical staining was performed as described previously51,124. Briefly, sections were deparaffinized, treated with 80% formic acid for 10 min and/or citrate buffer at pH 6.0 under pressure for 10 min at 121 °C, and then treated with 3% hydrogen peroxide. Blocking was carried out for 30 min in fetal bovine serum (10%). Sections were incubated in primary antibodies overnight at 4 °C (Supplementary Fig. 2) and in secondary antibody-horseradish peroxidase complex (REAL EnVision detection kit, Dako #K5007) for 1 h at RT. Visualization was carried out with 3,3’-diaminobenzidine (DAB) and counterstaining with hematoxylin. Sections were imaged using Olympus VS120 (RRID:SCR_018411) and analyzed on QuPath (RRID:SCR_018257).
Statistical analysis
Statistical analyses were performed using Student’s t-test or ANOVA, followed by the Tukey-Kramer post hoc test in KaleidaGraph (RRID:SCR_014980). Statistical significance was set at p < 0.05.
Figure preparation
Figures 1a, 2d and 9a, and Supplementary Fig. 11 include elements created with BioRender.com (license ID: zl1lvt5, https://BioRender.com/zl1lvt5), and were assembled and finalized in PowerPoint. Supplementary Fig. 15 was fully created using BioRender.com (license ID: hu7wmf6, https://BioRender.com/hu7wmf6). The generated images were used in accordance with BioRender’s academic license and publication guidelines.
Data availability
The data obtained in this research are available from the corresponding author upon reasonable request.
References
Goedert, M., Jakes, R. & Spillantini, M. G. The synucleinopathies: twenty years on. J. Parkinsons Dis. 7, S53–S71 (2017).
Anderson, J. P. et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281, 29739–29752 (2006).
Fujiwara, H. et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 (2002).
Baba, M. et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 152, 879–884 (1998).
Crowther, R. A., Jakes, R., Spillantini, M. G. & Goedert, M. Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett. 436, 309–312 (1998).
Campbell, B. C. et al. The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J. Neurochem. 76, 87–96 (2001).
Alafuzoff, I. et al. Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol. 117, 635–652 (2009).
Hasegawa, M. et al. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J. Biol. Chem. 277, 49071–49076 (2002).
Giasson, B. I. et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 (2000).
Burai, R., Ait-Bouziad, N., Chiki, A. & Lashuel, H. A. Elucidating the role of site-specific nitration of alpha-synuclein in the pathogenesis of Parkinson’s disease via protein semisynthesis and mutagenesis. J. Am. Chem. Soc. 137, 5041–5052 (2015).
Fauvet, B. et al. Characterization of semisynthetic and naturally Nα-acetylated α-synuclein in vitro and in intact cells: implications for aggregation and cellular properties of α-synuclein. J. Biol. Chem. 287, 28243–28262 (2012).
Fauvet, B. & Lashuel, H. A. Semisynthesis and enzymatic preparation of post-translationally modified alpha-synuclein. Methods Mol. Biol. 1345, 3–20 (2016).
Haj-Yahya, M. et al. Synthetic polyubiquitinated α-Synuclein reveals important insights into the roles of the ubiquitin chain in regulating its pathophysiology. Proc. Natl. Acad. Sci. USA 110, 17726–17731 (2013).
Hejjaoui, M. et al. Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: alpha-synuclein phosphorylation at tyrosine 125. J. Am. Chem. Soc. 134, 5196–5210 (2012).
Levine, P. M. et al. α-Synuclein O-GlcNAcylation alters aggregation and toxicity, revealing certain residues as potential inhibitors of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 116, 1511–1519 (2019).
Liu, Y., Qiang, M., Wei, Y. & He, R. A novel molecular mechanism for nitrated {alpha}-synuclein-induced cell death. J. Mol. Cell Biol. 3, 239–249 (2011).
Lu, Y., Prudent, M., Fauvet, B., Lashuel, H. A. & Girault, H. H. Phosphorylation of alpha-synuclein at Y125 and S129 alters its metal binding properties: implications for understanding the role of alpha-synuclein in the pathogenesis of Parkinson’s Disease and related disorders. ACS Chem. Neurosci. 2, 667–675 (2011).
Samuel, F. et al. Effects of serine 129 phosphorylation on alpha-synuclein aggregation, membrane association, and internalization. J. Biol. Chem. 291, 4374–4385 (2016).
Sanyal, A. et al. Alpha-synuclein is a target of fic-mediated adenylylation/AMPylation: possible implications for Parkinson’s disease. J. Mol. Biol. 431, 2266–2282 (2019).
Levine, P. M. et al. O-GlcNAc modification inhibits the calpain-mediated cleavage of alpha-synuclein. Bioorg. Med. Chem. 25, 4977–4982 (2017).
Cook, C. & Petrucelli, L. A critical evaluation of the ubiquitin-proteasome system in Parkinson’s disease. Biochim. Biophys. Acta 1792, 664–675 (2009).
Li, W. et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc. Natl. Acad. Sci. USA 102, 2162–2167 (2005).
Muntane, G., Ferrer, I. & Martinez-Vicente, M. alpha-synuclein phosphorylation and truncation are normal events in the adult human brain. Neuroscience 200, 106–119 (2012).
Killinger, B. A. et al. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci. Transl. Med. 10, https://doi.org/10.1126/scitranslmed.aar5280 (2018).
Liu, C. W. et al. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J. Biol. Chem. 280, 22670–22678 (2005).
Hoyer, W., Cherny, D., Subramaniam, V. & Jovin, T. M. Impact of the acidic C-terminal region comprising amino acids 109-140 on alpha-synuclein aggregation in vitro. Biochemistry 43, 16233–16242 (2004).
Levitan, K. et al. Conserved C-terminal charge exerts a profound influence on the aggregation rate of alpha-synuclein. J. Mol. Biol. 411, 329–333 (2011).
Murray, I. V. et al. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry 42, 8530–8540 (2003).
Ma, L. et al. C-terminal truncation exacerbates the aggregation and cytotoxicity of alpha-synuclein: a vicious cycle in Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 3714–3725 (2018).
Sorrentino, Z. A. et al. Physiological carboxy-truncation of alpha-synuclein potentiates the prion-like formation of pathological inclusions. J. Biol. Chem. https://doi.org/10.1074/jbc.RA118.005603 (2018).
Terada, M. et al. The effect of truncation on prion-like properties of alpha-synuclein. J. Biol. Chem. 293, 13910–13920 (2018).
van der Wateren, I. M., Knowles, T. P. J., Buell, A. K., Dobson, C. M. & Galvagnion, C. C-terminal truncation of alpha-synuclein promotes amyloid fibril amplification at physiological pH. Chem. Sci. 9, 5506–5516 (2018).
Tofaris, G. K. et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. J. Neurosci. 26, 3942–3950 (2006).
Daher, J. P. et al. Conditional transgenic mice expressing C-terminally truncated human alpha-synuclein (alphaSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Mol. Neurodegener. 4, 34 (2009).
Games, D. et al. Axonopathy in an alpha-synuclein transgenic model of Lewy body disease is associated with extensive accumulation of C-terminal-truncated alpha-synuclein. Am. J. Pathol. 182, 940–953 (2013).
Hall, K., Yang, S., Sauchanka, O., Spillantini, M. G. & Anichtchik, O. Behavioural deficits in transgenic mice expressing human truncated (1-120 amino acid) alpha-synuclein. Exp. Neurol. 264, 8–13 (2015).
Michell, A. W. et al. The effect of truncated human alpha-synuclein (1-120) on dopaminergic cells in a transgenic mouse model of Parkinson’s disease. Cell Transpl. 16, 461–474 (2007).
Ulusoy, A., Febbraro, F., Jensen, P. H., Kirik, D. & Romero-Ramos, M. Co-expression of C-terminal truncated alpha-synuclein enhances full-length alpha-synuclein-induced pathology. Eur. J. Neurosci. 32, 409–422 (2010).
Wakamatsu, M. et al. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol. Aging 29, 574–585 (2008).
Prasad, K., Beach, T. G., Hedreen, J. & Richfield, E. K. Critical role of truncated alpha-synuclein and aggregates in Parkinson’s disease and incidental Lewy body disease. Brain Pathol. 22, 811–825 (2012).
Moors, T. E. et al. The orchestration of subcellular alpha-synuclein pathology in the Parkinson’s disease brain revealed by STED microscopy. Preprint at bioRxiv https://doi.org/10.1101/470476 (2019).
Lewis, K. A. et al. Abnormal neurites containing C-terminally truncated alpha-synuclein are present in Alzheimer’s disease without conventional Lewy body pathology. Am. J. Pathol. 177, 3037–3050 (2010).
Quintin, S. et al. Cellular processing of α-synuclein fibrils results in distinct physiological C-terminal truncations with a major cleavage site at residue Glu 114. J. Biol. Chem. 299, 104912 (2023).
Lashuel, H. A. Do Lewy bodies contain alpha-synuclein fibrils? And does it matter? A brief history and critical analysis of recent reports. Neurobiol. Dis. 104876. https://doi.org/10.1016/j.nbd.2020.104876 (2020).
Moors, T. E. et al. The subcellular arrangement of alpha-synuclein proteoforms in the Parkinson’s disease brain as revealed by multicolor STED microscopy. Acta Neuropathol. 142, 423–448 (2021).
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M. & Goedert, M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 95, 6469–6473 (1998).
Hass, E. W. et al. Disease-, region- and cell type specific diversity of α-synuclein carboxy terminal truncations in synucleinopathies. Acta Neuropathol. Commun. 9, 146 (2021).
Moors, T. E. et al. Multi-platform quantitation of alpha-synuclein human brain proteoforms suggests disease-specific biochemical profiles of synucleinopathies. Acta Neuropathol. Commun. 10, 82 (2022).
Poggiolini, I. et al. RT-QuIC using C-terminally truncated α-synuclein forms detects differences in seeding propensity of different brain regions from synucleinopathies. Biomolecules 11, https://doi.org/10.3390/biom11060820 (2021).
Altay, M. F. et al. Development and validation of an expanded antibody toolset that captures alpha-synuclein pathological diversity in Lewy body diseases. Preprint at bioRxiv https://doi.org/10.1101/2022.05.26.493598 (2023).
Altay, M. F., Liu, A. K. L., Holton, J. L., Parkkinen, L. & Lashuel, H. A. Prominent astrocytic alpha-synuclein pathology with unique post-translational modification signatures unveiled across Lewy body disorders. Acta Neuropathol. Commun. 10, 163 (2022).
Dufty, B. M. et al. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am. J. Pathol. 170, 1725–1738 (2007).
Luk, K. C. et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA 106, 20051–20056 (2009).
Volpicelli-Daley, L. A. et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011).
Luk, K. C. et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Pieri, L. et al. Cellular response of human neuroblastoma cells to alpha-synuclein fibrils, the main constituent of Lewy bodies. Biochim. Biophys. Acta 1860, 8–19 (2016).
Mahul-Mellier, A.-L. et al. The making of a Lewy body: the role of α-synuclein post-fibrillization modifications in regulating the formation and the maturation of pathological inclusions. Preprint at bioRxiv https://doi.org/10.1101/500058 (2018).
Mahul-Mellier, A. L. et al. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 117, 4971–4982 (2020).
Volpicelli-Daley, L. A., Luk, K. C. & Lee, V. M. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 9, 2135–2146 (2014).
Kuusisto, E., Parkkinen, L. & Alafuzoff, I. Morphogenesis of Lewy bodies: dissimilar incorporation of alpha-synuclein, ubiquitin, and p62. J. Neuropathol. Exp. Neurol. 62, 1241–1253 (2003).
Mollenhauer, B. et al. Antibody-based methods for the measurement of α-synuclein concentration in human cerebrospinal fluid—method comparison and round robin study. J. Neurochem 149, 126–138 (2019).
Kellie, J. F. et al. Quantitative measurement of intact alpha-synuclein proteoforms from post-mortem control and Parkinson’s disease brain tissue by intact protein mass spectrometry. Sci. Rep. 4, 5797 (2014).
Karpowicz, R. J. Jr et al. Selective imaging of internalized proteopathic alpha-synuclein seeds in primary neurons reveals mechanistic insight into transmission of synucleinopathies. J. Biol. Chem. 292, 13482–13497 (2017).
Bayati, A. et al. Rapid macropinocytic transfer of α-synuclein to lysosomes. Cell Rep. 40, 111102 (2022).
Luk, K. C. et al. Molecular and Biological Compatibility with Host Alpha-Synuclein Influences Fibril Pathogenicity. Cell Rep. 16, 3373–3387 (2016).
Kiely, A. P. et al. Exploring the putative role of kallikrein-6, calpain-1 and cathepsin-D in the proteolytic degradation of alpha-synuclein in multiple system atrophy. Neuropathol. Appl. Neurobiol. https://doi.org/10.1111/nan.12512 (2018).
McGlinchey, R. P. & Lee, J. C. Cysteine cathepsins are essential in lysosomal degradation of alpha-synuclein. Proc. Natl. Acad. Sci. USA 112, 9322–9327 (2015).
Sevlever, D., Jiang, P. & Yen, S. H. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry 47, 9678–9687 (2008).
Tsujimura, A. et al. Lysosomal enzyme cathepsin B enhances the aggregate forming activity of exogenous alpha-synuclein fibrils. Neurobiol. Dis. 73, 244–253 (2015).
McGlinchey, R. P. et al. C-terminal alpha-synuclein truncations are linked to cysteine cathepsin activity in Parkinson’s disease. J. Biol. Chem. 294, 9973–9984 (2019).
Zhang, Z. et al. Asparagine endopeptidase cleaves alpha-synuclein and mediates pathologic activities in Parkinson’s disease. Nat. Struct. Mol. Biol. 24, 632–642 (2017).
Karpowicz, R. J. Jr., Trojanowski, J. Q. & Lee, V. M. Transmission of alpha-synuclein seeds in neurodegenerative disease: recent developments. Lab. Investig. https://doi.org/10.1038/s41374-019-0195-z (2019).
Mao, X. et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, https://doi.org/10.1126/science.aah3374 (2016).
Ihse, E. et al. Cellular internalization of alpha-synuclein aggregates by cell surface heparan sulfate depends on aggregate conformation and cell type. Sci. Rep. 7, 9008 (2017).
Chen, K. et al. LRP1 is a neuronal receptor for α-synuclein uptake and spread. Mol. Neurodegener. 17, 57 (2022).
Zhang, Y. & Yu, L. C. Single-cell microinjection technology in cell biology. Bioessays 30, 606–610 (2008).
Karim, M. R. et al. Internalized α-synuclein fibrils become truncated and resist degradation in neurons while glial cells rapidly degrade α-synuclein fibrils. Preprint at bioRxiv https://doi.org/10.1101/2024.06.05.597615 (2024).
Beevers, J. E. et al. MAPT genetic variation and neuronal maturity alter isoform expression affecting axonal transport in iPSC-derived dopamine neurons. Stem Cell Rep. 9, 587–599 (2017).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–551 (2011).
Sandor, C. et al. Transcriptomic profiling of purified patient-derived dopamine neurons identifies convergent perturbations and therapeutics for Parkinson’s disease. Hum. Mol. Genet. 26, 552–566 (2017).
Imam, S. Z. et al. Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson’s disease. J. Neurosci. 31, 157–163 (2011).
Kiely, A. P. et al. alpha-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy?. Acta Neuropathol. 125, 753–769 (2013).
Fayyad, M. et al. Investigating the presence of doubly phosphorylated α-synuclein at tyrosine 125 and serine 129 in idiopathic Lewy body diseases. Brain Pathol. 30, 831–843 (2020).
Danielson, S. R. et al. Preferentially increased nitration of alpha-synuclein at tyrosine-39 in a cellular oxidative model of Parkinson’s disease. Anal. Chem. 81, 7823–7828 (2009).
Bhattacharjee, P. et al. Mass spectrometric analysis of lewy body-enriched α-synuclein in Parkinson’s disease. J. Proteome Res. 18, 2109–2120 (2019).
Ohrfelt, A. et al. Identification of novel α-synuclein isoforms in human brain tissue by using an online nanoLC-ESI-FTICR-MS method. Neurochem. Res. 36, 2029–2042 (2011).
Suthar, S. K. & Lee, S. Y. Truncation or proteolysis of α-synuclein in Parkinsonism. Ageing Res. Rev. 90, 101978 (2023).
Smith, J. W. et al. Polymer-peptide conjugates convert amyloid into protein nanobundles through fragmentation and lateral association. ACS Appl. Nano Mater. 3, 937–945 (2020).
Gao, X. et al. Human Hsp70 disaggregase reverses Parkinson’s-linked alpha-synuclein amyloid fibrils. Mol. Cell 59, 781–793 (2015).
Tittelmeier, J. et al. The HSP110/HSP70 disaggregation system generates spreading-competent toxic α-synuclein species. EMBO J. e103954. https://doi.org/10.15252/embj.2019103954 (2020).
Mannini, B. & Chiti, F. Chaperones as suppressors of protein misfolded oligomer toxicity. Front. Mol. Neurosci. 10, 98 (2017).
Diepenbroek, M. et al. Overexpression of the calpain-specific inhibitor calpastatin reduces human alpha-synuclein processing, aggregation and synaptic impairment in [A30P]alphaSyn transgenic mice. Hum. Mol. Genet. 23, 3975–3989 (2014).
Mishizen-Eberz, A. J. et al. Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J. Neurochem. 86, 836–847 (2003).
Bassil, F. et al. Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy. Proc. Natl. Acad. Sci. USA 113, 9593–9598 (2016).
Nuber, S. & Selkoe, D. J. Caspase-1 clipping causes complications for alpha-synuclein. Proc. Natl. Acad. Sci. USA 113, 9958–9960 (2016).
Wang, W. et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein alpha-synuclein. Proc. Natl. Acad. Sci. USA 113, 9587–9592 (2016).
Takahashi, M. et al. Oxidative stress-induced phosphorylation, degradation and aggregation of alpha-synuclein are linked to upregulated CK2 and cathepsin D. Eur. J. Neurosci. 26, 863–874 (2007).
Kasai, T. et al. Cleavage of normal and pathological forms of alpha-synuclein by neurosin in vitro. Neurosci. Lett. 436, 52–56 (2008).
Sung, J. Y. et al. Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J. Biol. Chem. 280, 25216–25224 (2005).
Levin, J. et al. Increased alpha-synuclein aggregation following limited cleavage by certain matrix metalloproteinases. Exp. Neurol. 215, 201–208 (2009).
Choi, D. H. et al. Role of matrix metalloproteinase 3-mediated alpha-synuclein cleavage in dopaminergic cell death. J. Biol. Chem. 286, 14168–14177 (2011).
Mouatt-Prigent, A., Karlsson, J. O., Agid, Y. & Hirsch, E. C. Increased M-calpain expression in the mesencephalon of patients with Parkinson’s disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death?. Neuroscience 73, 979–987 (1996).
Croall, D. E. & DeMartino, G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol. Rev. 71, 813–847 (1991).
Karampetsou, M. et al. Phosphorylated exogenous alpha-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice. Sci. Rep. 7, 16533 (2017).
Tanik, S. A., Schultheiss, C. E., Volpicelli-Daley, L. A., Brunden, K. R. & Lee, V. M. Lewy body-like alpha-synuclein aggregates resist degradation and impair macroautophagy. J. Biol. Chem. 288, 15194–15210 (2013).
Henderson, M. X. et al. Unbiased proteomics of early Lewy body formation model implicates active microtubule affinity-regulating kinases (MARKs) in synucleinopathies. J. Neurosci. 37, 5870–5884 (2017).
Waxman, E. A., Duda, J. E. & Giasson, B. I. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta Neuropathol. 116, 37–46 (2008).
Guerrero-Ferreira, R. et al. Cryo-EM structure of alpha-synuclein fibrils. eLife 7, https://doi.org/10.7554/eLife.36402 (2018).
Li, Y. et al. Amyloid fibril structure of α-synuclein determined by cryo-electron microscopy. Cell Res. 28, 897–903 (2018).
Donzelli, S. et al. Post-fibrillization nitration of alpha-synuclein abolishes its seeding activity and pathology formation in primary neurons and in vivo. Preprint at bioRxiv https://doi.org/10.1101/2023.03.24.534149 (2023).
Sorrentino, Z. A., Xia, Y., Gorion, K. M., Hass, E. & Giasson, B. I. Carboxy-terminal truncations of mouse α-synuclein alter aggregation and prion-like seeding. FEBS Lett. 594, 1271–1283 (2020).
Rey, N. L. et al. α-Synuclein conformational strains spread, seed and target neuronal cells differentially after injection into the olfactory bulb. Acta Neuropathol. Commun. 7, 221 (2019).
Sorrentino, Z. A. et al. Carboxy-terminal truncation and phosphorylation of α-synuclein elongates survival in a prion-like seeding mouse model of synucleinopathy. Neurosci. Lett. 135017. https://doi.org/10.1016/j.neulet.2020.135017 (2020).
Luk, K. C. et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209, 975–986 (2012).
Mishizen-Eberz, A. J. et al. Cleavage of alpha-synuclein by calpain: potential role in degradation of fibrillized and nitrated species of alpha-synuclein. Biochemistry 44, 7818–7829 (2005).
Qin, Z., Hu, D., Han, S., Hong, D. P. & Fink, A. L. Role of different regions of alpha-synuclein in the assembly of fibrils. Biochemistry 46, 13322–13330 (2007).
Wakabayashi, K. et al. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 47, 495–508 (2013).
Culvenor, J. G. et al. Non-Abeta component of Alzheimer’s disease amyloid (NAC) revisited. NAC and alpha-synuclein are not associated with Abeta amyloid. Am. J. Pathol. 155, 1173–1181 (1999).
Tong, J. et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: a comparative investigation. Brain 133, 172–188 (2010).
Gai, W. P., Power, J. H., Blumbergs, P. C., Culvenor, J. G. & Jensen, P. H. Alpha-synuclein immunoisolation of glial inclusions from multiple system atrophy brain tissue reveals multiprotein components. J. Neurochem. 73, 2093–2100 (1999).
Dickson, D. W. et al. Widespread alterations of alpha-synuclein in multiple system atrophy. Am. J. Pathol. 155, 1241–1251 (1999).
Lloyd, G. M. et al. A multiverse of α-synuclein: investigation of prion strain properties with carboxyl-terminal truncation specific antibodies in animal models. Acta Neuropathol. Commun. 12, 91 (2024).
Pagano, G. et al. Trial of prasinezumab in early-stage Parkinson’s disease. N. Engl. J. Med. 387, 421–432 (2022).
Altay, M. F. et al. Development and validation of an expanded antibody toolset that captures alpha-synuclein pathological diversity in Lewy body diseases. npj Parkinsons Dis. 9, 161 (2023).
Ramalingam, N., Haass, C. & Dettmer, U. Physiological roles of α-synuclein serine-129 phosphorylation—not an oxymoron. Trends Neurosci. 47, 480–490 (2024).
Wiseman, J. A. et al. Aggregate-prone brain regions in Parkinson’s disease are rich in unique N-terminus α-synuclein conformers with high proteolysis susceptibility. npj Parkinsons Dis. 10, https://doi.org/10.1038/s41531-023-00614-w (2024).
Petricca, L. et al. Comparative analysis of total alpha-synuclein (αSYN) immunoassays reveals that they do not capture the diversity of modified αSYN proteoforms. J. Parkinsons Dis. 12, 1449–1462 (2022).
Marotta, N. P. et al. Alpha-synuclein from patient Lewy bodies exhibits distinct pathological activity that can be propagated in vitro. Acta Neuropathol. Commun. 9, 188 (2021).
Fauvet, B. et al. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 287, 15345–15364 (2012).
Mahul-Mellier, A. L. et al. Fibril growth and seeding capacity play key roles in alpha-synuclein-mediated apoptotic cell death. Cell Death Differ. 22, 2107–2122 (2015).
Haenseler, W. et al. A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Rep. 8, 1727–1742 (2017).
Haenseler, W. et al. Excess alpha-synuclein compromises phagocytosis in iPSC-derived macrophages. Sci. Rep. 7, 9003 (2017).
Dorin-Semblat, D. et al. Malaria parasite-infected erythrocytes secrete PfCK1, the plasmodium homologue of the pleiotropic protein kinase casein kinase 1. PLoS ONE 10, e0139591 (2015).
Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 13, 2513–2526 (2014).
Dorfer, V. et al. MS Amanda, a universal identification algorithm optimized for high accuracy tandem mass spectra. J. Proteome Res. 13, 3679–3684 (2014).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002).
Schmid, A. W., Fauvet, B., Moniatte, M. & Lashuel, H. A. Alpha-synuclein post-translational modifications as potential biomarkers for Parkinson disease and other synucleinopathies. Mol. Cell. Proteom. 12, 3543–3558 (2013).
Acknowledgements
This work was supported by funding from EPFL and UCB (H.A.L, A.L.M.M, F.A, N.M, N.A.B, A.C, G.L, S.J, Y.J and S.N) and the Fondation Bru (A.L.M.M and Y.J). R.W.-M and S.V were supported by the Joint Program for Neurodegenerative Diseases (JPND) from the UK Medical Research Council (R.W.-M.). J.H and C.S were supported by the Multiple System Atrophy Trust; the Multiple System Atrophy Coalition; Fund Sophia, managed by the King Baudouin Foundation; and Karin & Sten Mortstedt CBD Solutions. Queen Square Brain Bank for Neurological Disorders is supported by the Reta Lila Weston Institute for Neurological Studies and the Medical Research Council UK. This research was supported in part by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. J.Y.L and C.H were supported by the NNSF-81430025, Swedish Research Council, EU-JPND (aSynProtec, REfrAME) and EU-ITN (Syndegen). We acknowledge Elena Gasparotto, Jérémy Campos, and Lorène Aeschbach for their valuable technical assistance, respectively: Elena and Lorène for their continuous support with the preparation of the primary culture, Elena for the cloning of the mutated aSyn, and Jérémy for the expression and purification of WT or mutant aSyn proteins. We are grateful to Dr. Arne Seitz and his staff at the Bio-imaging Core Facility (EPFL) for their technical support. We are grateful to the individuals and their families for consenting to donate to QSBB and NBB.
Author information
Authors and Affiliations
Contributions
H.A.L. conceived and supervised the study and designed all the experiments. H.A.L. and A.L.M.M. designed all the cellular studies and wrote the paper with all the authors contributing. A.L.M.M. performed and analyzed the experiments shown in Figs. 1, 2a–c, 3a, b, 4–6, 8a–c, f–j,9 and Supplementary Figs. 2, 3, 4a–f, i–m, 6, 7b, c, 8, 9a, b, d, 10, 12d, e, 14, 15, 16 and 17. M.F.A. designed, performed, and analyzed the experiments shown in Fig. 10b, c and Supplementary Figs. 1, 4g–k, 12b, c and 13. N.M. designed, performed, and analyzed the experiments shown in Supplementary Fig. 11. N.A.B. and A.C. designed, performed, and analyzed the experiments shown in Fig. 7. A.C. produced and characterized aSyn-biotin PFF shown in Supplementary Fig. 1 and used in Supplementary Fig. 11. G.L., S.J., and S.N. designed, performed, and analyzed the experiments shown, respectively, in Figs. 2d and 8d, e and Supplementary Fig. 7a. S.V. and R.W.M. designed Fig. 3d and Supplementary Fig. 9e–g. S.V. performed and analyzed the experiment shown in Fig. 3d and Supplementary Fig. 9e–g. R.H. performed and analyzed the LC-MS/MS of the experiments shown in Figs. 1I, 5a, b and Supplementary Figs. 4 and 10c. J.H. and C.S. contributed to the analysis of Fig. 10b, c and Supplementary Fig. 13a–c. J.Y.L. and C.H. performed and analyzed the experiment shown in Supplementary Fig. 13c. M.C. prepared the samples for CLEM analysis and acquired EM images in Fig. 6. G.K. supervised the experiments shown in Fig. 6 and contributed to the interpretation of the data. G.M.C. designed and analyzed the experiments shown in Figs. 3c and 10a, and Supplementary Fig. 9c. L.W. performed and analyzed the experiments shown in Figs. 3c and 10a, and Supplementary Fig. 9c. A.M. designed and performed the experiments shown in Fig. 3c. P.D. and M.C. supervised the study performed in Figs. 3c and 10a, and Supplementary Fig. 9c. All authors reviewed and contributed to the writing.
Corresponding author
Ethics declarations
Competing interests
G.M.C., A.M., P.D., and M.C. are employees of UCB Pharma. H.A.L. is the co-founder and chief scientific officer of ND BioSciences, Epalinges, Switzerland, a company that develops diagnostics and treatments for neurodegenerative diseases (NDs) based on platforms that reproduce the complexity and diversity of proteins implicated in NDs and their pathologies. H.A.L. has received funding from industry to support research on neurodegenerative diseases, including Merck Serono, UCB, and AbbVie. These companies had no specific role in the conceptualization, preparation, and decision to publish this manuscript. H.A.L. is the associate editor of npj Parkinson’s Disease. H.A.L. was not involved in the journal’s review of, or decisions related to, this manuscript. All other authors declare no competing financial interests in association with this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahul-Mellier, AL., Altay, M.F., Maharjan, N. et al. Differential role of C-terminal truncations on alpha-synuclein pathology and Lewy body formation. npj Parkinsons Dis. 11, 261 (2025). https://doi.org/10.1038/s41531-025-01084-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-01084-y