Abstract
We developed a highly sensitive and specific single-molecule array (Simoa) Homebrew assay for quantification of phosphorylated α-synuclein at serine 129 (pS129 α-syn) and evaluated its performance in human cerebrospinal fluid (CSF) and plasma. Using a cohort of patients with Parkinson’s disease (PD), Alzheimer’s disease (AD), and neurological controls with available CSF α-synuclein seed amplification assay (synSAA) outcome, we examined pS129 α-syn alongside N-terminal and C-terminal α-syn proteoforms. Our results showed that pS129 α-syn concentration was about 1% and 0.001% of the other α-syn species in CSF and plasma, respectively. We found no correlation between pS129 α-syn and synSAA outcome, indicating that soluble pS129 α-syn in CSF and plasma does not reflect presence of synucleinopathy. Interestingly, pS129 α-syn and other α-syn forms were significantly increased in AD compared to PD and controls, supporting the role of α-syn as biomarker of synaptic degeneration in AD.
Similar content being viewed by others
Introduction
Lewy body disease (LBD) is a distinctive neuropathological feature found in various neurodegenerative disorders, such as Parkinson’s disease (PD), PD with dementia (PDD), and dementia with Lewy bodies (DLB)1. Additionally, LBD is the most frequent co-pathology in Alzheimer’s disease (AD)2,3. Therefore, reliable biomarkers specifically reflecting LBD are needed for the molecular characterization of several neurodegenerative diseases4.
Intraneuronal Lewy bodies are enriched in α-synuclein (α-syn) often carrying post-translational modifications (PTMs) such as phosphorylation (at serine(S)87and 129, or tyrosine(Y)125), ubiquitination, and truncation at its C-terminus5. Therefore, α-syn PTMs represents a candidate target for biomarkers discovery in LBD. However, quantifying modified α-syn forms in cerebrospinal fluid (CSF) and blood is challenging due to their lower concentrations compared to unmodified α-syn, the reversible nature of certain modifications, and antibody specificity issues in immunoassays. For instance, it has been shown that the concentration of phosphorylated α-syn at S129 (pS129 α-syn) in CSF is between 4 and 10 times lower than that of total α-syn6 and that different antibodies for pS129 α-syn tested in immunoassays have different reactivity depending of several factors7,8. An important technical issue is that C-terminal modifications, and in particular the phosphorylation surrounding position Y125, can mask the detection of pS129 α-syn7. Due to these challenges, studies on pS129 α-syn in CSF and blood as a biomarker have led to conflicting results9. Magalhães and Lashuel reviewed studies where pS129 α-syn was measured across different cohorts, reporting high variability of analyte concentration in CSF and plasma, contrasting conclusions on the association between pS129 α-syn levels and synucleinopathy and, globally, a limited diagnostic value of this biomarker10. To overcome limitations due to low concentration, ultrasensitive assays based on electrochemiluminescence and Simoa technologies have been used to measure pS129 α-syn in CSF and plasma11,12.
In this work we describe a novel ultrasensitive immunoassay to measure pS129 α-syn in plasma and CSF and the thorough validation of its performance, including epitope mapping of the antibodies used, impact of additional phosphorylations close to the site of interest, and assessment, in a well-characterized cohort of PD, PDD and AD patients, of its diagnostic value as LBD biomarker. Finally, we tested the association between the levels of pS129 α-syn in CSF and the presence of synucleinopathy, defined by means of CSF α-syn seed amplification assay (CSF synSAA), the current reference technique for in-vivo detection of LBDs13,14.
Results
A summary of all developed assay formats is shown in Supplementary Table S1.
Peptide based epitope mapping of used antibody pair for the Homebrew Simoa pS129 α-synuclein assay
The pan α-synuclein mAb RD-080 (clone 25F8) was further characterized for epitope mapping using biotinylated synthetic peptides in indirect ELISA and revealed an epitope in the α-synuclein region 99–110 (Fig. S1, supplementary material). The detection antibody D1R1R and 4 additional commercially available pS129 α-synuclein mAbs were mapped by synthetic peptides that were phosphorylated at position S129 (Figs. 1A and S2). We performed studies on an array of peptides across the α-synuclein region between aa 113 and aa 140. The MJF-R13 antibody demonstrated signal-to-noise (S/N) ratios ranging from 155 to 201, compared to a blank value of 1, indicating a binding response to a region located N-terminal to S129. In contrast, the D1R1 antibody and other tested antibodies exhibited binding to a peptide sequence located further downstream, in a region C-terminal to S129. Based on the immunoreactivity to these synthetic peptides, the epitopes recognized by all antibodies are linear in nature but consist of distinct sequences. Collectively, the results suggest that the epitope for MJF-R13 is localized between amino acids 125 and 129 (including Y125 and excluding Y133), whereas the epitope for D1R1R spans amino acids 128–133 (excluding Y125 and including Y133). Additionally, the mAbs EP1536Y, pSyn#64, and 81 A exhibit epitopes that are more C-terminal, excluding Y125 and including Y133.
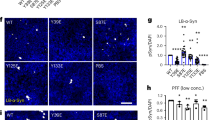
A Epitope mapping of pS129 α-Syn D1R1R, MJF-R13, EP1536Y, pSyn#64 and 81A monoclonal antibodies (mAbs). B For each of the five pS129α-syn mAbs, the picture shows a “-” for non-reactivity and a “+” for positive reactivity with the synthetic peptides with different phosphorylation combinations at Y125, S129, and Y133.
Specificity testing by indirect testing with synthetic peptides
In a next step, the specificity of the pS129 α-synuclein assay was assessed. Published data7 showed that phosphorylation of amino acids (pY125, pY133) adjacent to pS129 may affect the binding of anti-pS129 antibodies. Moreover, it has been reported that α-synuclein derived from pathological brain tissues from patients with synucleinopathies, can be heavily modified by hyperphosphorylation, truncation and other PTMs or conformational changes that can lead to protein aggregation into Lewy bodies or Lewy neurites5,15. Therefore, we assessed the influence of additional phosphorylations close to pS129 on the reactivity of the pS129 α-synuclein antibody used in our assay. We used non-phosphorylated and phosphorylated α-synuclein peptides at respectively position S129, pY125, and pY133 in indirect testing, and compared the specificity of 4 commercial antibodies commonly used in the field (see above). With reference to Figs. 1B and S3, all antibodies were non-reactive towards the single phosphorylation peptides pY125 and pY133 while showing a clear dose response towards the peptide with single phosphorylation at pS129, although the dose response of antibody pSyn#64 was weaker. The two controls (non-phosphorylated α-synuclein and Aβ x-40 as irrelevant protein) were negative as expected. However, D1R1R was non-reactive in case of pS129 and pY133 phosphorylation while reactive when both pY125 and pS129 were phosphorylated. MJF-R13 was non-reactive for phosphorylation of both pY125 and pS129 and reactive when using phosphorylated peptides at both pS129 and pY133. Among the other pS129 α-synuclein antibodies, EP1536Y, pSyn#64, and 81A showed an identical pattern as D1R1R (Fig. 1B).
Assay characteristics
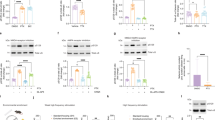
The following analytical test parameters were assessed for CSF analytes: intra- and inter-run variability, measuring range, parallelism and LLOQ (Figs. S4 and S5 and Table S2 Supplementary Material). The pS129 α-syn Homebrew assay showed an overall mean intra-run CV of 14.4%, based upon duplicate testing of QC CSF samples as described in the methods. The intra-run CVs varied between 11.8% (QC pool 3) and 18.5% (QC pool 1). It must be noted that the concentration of QC1, which showed higher variability, was close to the lowest calibrator point (1.1 pg/mL). Inter-assay variation was determined by testing the 3-QCs in 6 consecutive test runs in duplicate. Mean inter-assay CV was 14.1% and ranged between 11.8% (QC pool 3) and 17.7% (QC pool 1) over the 6 test runs. A measuring range between 0.125 and 100 pg/mL was used, including 8 non-zero calibrator points. For this exploratory study, all CSF and plasma samples could be measured within the measuring range of the assay. CSF and plasma samples were prediluted 3- and 4-fold, respectively. Parallelism was demonstrated by serial CSF dilution into assay diluent with a maximum of 20% relative deviation from the reference condition (3-fold dilution) up to a 12-fold sample dilution. Finally, a precision plot (Fig. S5 supplementary material) showed a CV below 20% across 6 test runs on back-calculated concentrations for all the calibrator points pointing to a preliminary LLOQ value of 0.125 pg/mL (the lowest calibrator point).
Specificity testing in sandwich immunoassay (Simoa)
Non-phosphorylated and phosphorylated full-length α-synuclein (Proteos) were tested in the Homebrew Simoa immunoassay. As expected, the D1R1R antibody showed no reactivity towards E. coli produced full length non-phosphorylated α-synuclein up to a concentration of 1000 pg/mL (Fig. S6A). Next the effect of alkaline phosphatase (AP) on phospho-reactivity was determined by incubation of the calibrator point of 25 pg/mL pS129 α-syn with increasing AP concentrations. A reduction in signal with over 50% was obtained at 500 Units AP/mL and higher, compared with the reference where no AP was added (Fig. S6B).
Blood contamination and analyte stability
We investigated the effect of CSF blood contamination on the levels of pS129 α-synuclein when measured with the Homebrew Simoa assay (Fig. S7) by spiking in whole blood into neat CSF from remnant samples at different dilution factors. When compared to non-spiked CSF the concentration of pS129 α-synuclein in spiked samples was at least 100 times higher and a dilution effect was observed over the dilution of the spiked samples. The data strongly suggest that whole blood contains high pS129 α-synuclein concentration. Analysis of analyte stability in the CSF matrix (freeze/thawing and hold time) (Fig. S8, Table S3) showed that the recovery of pS129 α-synuclein after 24 h at room temperature respectively +2–8 °C was between 102–136% and 72–113% suggesting that the analyte concentration is not or minimally affected by the hold time. There was no outspoken trend over the measured time points (2 h, 4 h, 24 h). The effect of freeze/thawing was investigated up to 5 cycles of freezing with respect to the reference condition. The pS129 α-synuclein concentrations in 3 QC pools were between 93% and 119% of the reference value after 5 freeze/thaw cycles and no trend was observed within this range of conditions.
Association of N-α-syn, C-α-syn and pS129 α-syn levels in CSF and plasma with clinical groups and CSF synSAA outcomes
The main demographic and clinical features of each diagnostic group of the study cohort are summarized in Table 1. Due to the well-known effect of blood contamination in CSF soluble α-syn quantification, CSF N-α-syn, CSF C-α-syn, and CSF pS129 α-syn corresponding to CSF hemoglobin (Hb) values >50 ng/mL were excluded from analyses16.
In the whole cohort, the median pS129 α-syn concentration was 1% and 0.5% of that of N-α-syn and C-α-syn, respectively. A much lower fraction was measured in plasma, i.e., 0.002% and 0.01% of N-α-syn and C-α-syn, respectively. CSF and plasma N-α-syn, C-α-syn, and pS129 α-syn were not significantly different between PD/PDD and CTRL and between patients with positive (S+) and negative (S−) CSF synSAA (Fig. 2, Table 2). Computing ratios between pS129 α-syn and N/C-α-syn did not produce any significant difference in the above-mentioned comparisons.
In box-plots, the horizontal line is representative of median, boxes of interquartile range (IQR, 25-75%) and whiskers of 1.5*IQR. No significant differences were observed between the S+ and S− groups. In contrast, higher levels of CSF α-synuclein species were found in AD patients compared to CTRL and PD/PDD patients, as determined by Dunn’s test. A For CSF N-α-syn: p-values were AD vs CTRL = 2.7 × 10⁻⁷, AD vs PD/PDD = 3.6 × 10⁻⁸, CTRL vs PD/PDD = 0.86. B For CSF C-α-syn: p-values were AD vs CTRL = 0.00034, AD vs PD/PDD = 0.00051, PD/PDD vs CTRL = 0.70. C For CSF pS129-α-syn: p-values were AD vs CTRL = 0.0032, AD vs PD/PDD = 2.7 × 10⁻⁵, CTRL vs PD/PDD = 0.5. D-F No statistically significant associations were observed for any of the α-syn species measured in plasma. Boxes represent the interquartile range, with horizontal lines indicating median values. Whiskers represent the 5th to 95th percentile.Abbreviations: AD Alzheimer’s disease, C-α-syn: C-terminal α-synuclein, CSF cerebrospinal fluid, CTRL neurological controls, N-α-syn N-terminal α-synuclein, PD Parkinson’s disease, PDD Parkinson’s disease with dementia, pS129-α-syn α-synuclein phosphorylated at serine 129, S+ positive CSF synSAA, S− negative CSF synSAA.
In S + AD and PD/PDD patients, no statistically significant associations were found between synSAA kinetic parameters and N-α-syn, C-α-syn, and pS129 α-syn.
Conversely, for all the α-syn species considered, higher CSF concentrations were found in AD with respect to PD/PDD and CTRL, as previously described17,18. Additionally, the concentrations of the three CSF α-syn species considered were found to be significantly correlated with CSF biomarkers of AD pathology and neurodegeneration (Aβ42/40, pTau181 and t-tau, SNAP-25) and, to a lesser extent, to plasma ones (Aβ42/40, pTau18, NfL). Among these the strongest associations were those with SNAP-25 (r = 0.62–0.79). Plasma N-α-syn, C-α-syn, and pS129 α-syn were found to be associated with each other but no other relevant associations were found (Fig. 3).
The correlation heatmap was created by considering Pearson’s correlation coefficients, columns and rows were clustered by means of hierarchical clustering using Ward’s linkage criterion. Correlation’s coefficients that had an FDR-adjusted p value < 0.05 are marked with an asterisk (*). Abbreviations. Aβ40: amyloid-β 1-40, Aβ42: amyloid-β 1-42, Aβ42/40: ratio between amyloid-β 1-42 and amyloid-β 1-40, C-α-syn: C-terminal α-synuclein, CSF: cerebrospinal fluid, N-α-syn: N-terminal α-synuclein, NfL: neurofilament light chain, pS129 α-syn: α-synuclein phosphorylated at serine 129, p-tau181: tau protein phosphorylated at threonine 181, SNAP-25: synaptosomal associated protein 25, t-tau: total tau protein.
Association of N-α-syn, C-α-syn and pS129 α-syn levels with clinical features
With respect to clinical features, when accounting for multiple testing, the three measured α-syn forms were not found to be associated with any baseline clinical parameter within PD/PDD (H&Y, UPDRS-III, MMSE, and MoCA) or AD (neuropsychological battery including MMSE), neither in CSF nor in plasma (Table S4 and Table S5). Follow-up data (all measures for PD/PDD, MMSE for AD) were available for a subgroup of patients (n = 20 AD and n = 23 PD). No relevant associations were found in PD with changes in H&Y, UPDRS-III, MMSE, and MoCA at 2-year follow-up (Table S4). Although the limited sample size and relatively short and variable follow-up time (2.4 ± 0.9 y), CSF C-α-syn was found to be moderately to strongly correlated with the rate of MMSE change (dMMSE/dt, r = 0.68, p = 0.0007, p adj. = 0.03, see Fig. 4, Table S5).
Discussion
In this study, we developed an analytically sensitive and specific Simoa assay for the measurement of pS129 α-syn in human CSF and plasma. The assay was extensively characterized: we assessed the specificity of pS129 α-syn antibodies, performed epitope mapping and evaluated several pre-analytical variables, including the impact of blood contamination on CSF measurements. Finally, the analytical performance was also assessed. In addition to pS129 α-syn, we quantified N-terminal and C-terminal α-syn proteoforms in CSF and plasma from a retrospectively selected cohort of PD and AD patients, who were comprehensively characterized with biomarkers of amyloidosis, tauopathy, and, crucially, α-syn aggregation via CSF synSAA.
Phosphorylation of α-syn is one of the most common PTM for this protein and phosphorylation at serine 129 has been shown to be the predominant α-syn PTM present in Lewy bodies19,20. Several studies have proposed different roles of this phosphorylation in the pathology of LBDs, including enhanced α-syn aggregation21,22, and association with disease progression and cognitive decline23,24. This view is contrasted by several other studies which showed opposite roles of pS129 α-syn in LBD pathology, like a neuroprotective function in model systems25,26, physiologic attenuation of neurotransmitter release27, or inhibition of α-syn aggregation28. Therefore, despite the clear-cut association with the structure of Lewy bodies, there is no agreement on the functional role of this α-syn PTM and its role within LBD pathology.
Given the proposed role of pS129 α-syn in LBDs, previous research has focused on the detection of this modification in biofluids as biomarker of LBDs. Whereas the measurement of pS129 α-syn in skin nerve fibers is gaining attention as noninvasive biomarker of synucleinopathies29, the measurement of pS129 α-syn in CSF and blood has been described over the last years with variable conclusions regarding absolute levels in various biofluids and pS129 α-syn in relation to disease status11,12,30,31,32. In most studies the changes were small, and the direction of change (increase/decrease/neutral) was not consistent11,12,30,31.
Our study provides additional evidence that pS129 α-syn is detectable and quantifiable in biofluids such as CSF and plasma; however, it does not serve as a reliable marker of α-syn aggregation or Lewy body disease (LBD) and likely reflects a physiological, minor fraction of the total soluble α-syn. We found no correlation between CSF pS129 α-syn levels and α-syn seeding activity as assessed by synSAA, either in the whole cohort or within PD/PDD patients. This finding strongly suggests that pS129 α-syn concentration in biofluids cannot be considered an indirect measure of α-syn aggregation. Moreover, while CSF pS129 α-syn levels were unchanged in PD/PDD compared to CTRL, they were significantly increased in AD patients, compared to both PD/PDD and CTRL, confirming previous studies15,33. This increase was not specific to phosphorylated α-syn, as N-terminal and C-terminal α-syn species were also elevated in AD. Moreover, the three α-syn species measured in CSF were significantly associated (moderately to strongly) with CSF levels of SNAP-25, a recognized biomarker of synaptic damage34,35. These findings, together with the inverse correlation we found between C-α-syn and MMSE, support the hypothesis that these soluble α-syn species in CSF may reflect synaptic degeneration rather than α-syn aggregation31. Indeed, α-syn is highly expressed at synapses, and its elevated concentrations in biofluids could be a bystander effect of synaptic damage and loss.
Our results reveal other important issues on the level of phosphorylated α-syn in biofluids. In CSF, the range of pS129 α-syn is between 1 and 3 pg/mL, showing that only a small fraction of the total synuclein present in CSF is phosphorylated at serine 129. While earlier studies showed relatively higher levels of pS129 α-syn in CSF (up to 200 pg/mL)36, recent work using a chemiluminescent method and the antibody MJF-R13 as detection antibody, obtained values for pS129 α-syn much more similar to those obtained in this work30. In plasma, the range in our results was between 5 and 40 pg/mL, possibly showing that in the periphery there might be other sources of pS129 α-syn contributing to the total plasma level of this form. The presence of other sources of phosphorylated α-syn in plasma is also indirectly confirmed by the different pattern of α-syn species across our diagnostic groups, with no discrimination of AD patients as observed in CSF. A recent study measured pS129 α-syn in plasma-derived extracellular vesicles (EVs) using a single molecule assay with the D1R1R as capture antibody37. The authors found that pS129 α-syn is present in plasma as free protein and enclosed in vesicles, and that phosphorylated α-syn was enriched in EVs. The measurement of pS129 α-syn loaded in brain-derived EVs might represent an interesting approach to limit the effect of peripheral α-syn.
Our detailed characterization of pS129 α-syn antibodies may also give important information on commercially available reagents for the detection of this phosphorylation site. It has been reported that hyperphosphorylation can result in underestimation of pS129 α-syn, especially in the case of presence of pY125 and pS133 α-syn12. We therefore characterized five pS129 α-syn monoclonal antibodies previously used in immunoassays to verify the effect of phosphorylations adjacent to S129. Consistent with previous findings12, MJF-R13 showed reduced reactivity in the presence of Y125 phosphorylation, while D1R1R and three other antibodies were affected by Y133 phosphorylation. Epitope mapping revealed that MJF-R13 targets the 125–129 region, whereas D1R1R and others recognize a more C-terminal epitope. This suggests that additional phosphorylation sites may reduce assay signals, as triple phosphorylation (pY125, pS129, pY133) is not detected by current assays12. To mitigate this, we selected D1R1R as the capture antibody due to its highest reactivity. Lastly, our data indicate that pS129 α-syn remains stable through up to five freeze/thaw cycles and 24 h at non-freezing temperatures, though further validation is needed to fully assess pre-analytical variability. In summary, our findings suggest that soluble α-syn species in CSF are not associated with LBD pathology, as assessed by CSF synSAA, but instead more broadly reflect neurodegeneration as it occurs in AD. Plasma α-syn species showed no correlation with any clinical variable examined. These results reinforce previous studies indicating that soluble α-syn proteoforms in CSF, including pS129 α-syn, are more indicative of AD-related synaptic degeneration than of brain synucleinopathy17,18.
Methods
Patients
The cohort investigated in this study was retrospectively selected from patients attending the Section of Neurology, University Hospital of Perugia, Italy. All patients underwent medical history, blood chemistry, physical and neurological examination, a thorough neuropsychological evaluation, brain imaging (computed tomography or magnetic resonance imaging), and lumbar puncture for the measurement of CSF core AD biomarkers, namely the β-amyloid (Aβ)1-42/Aβ1-40 ratio (Aβ42/40), phosphorylated tau at threonine 181 (p-tau181), and total tau (t-tau). CSF Aβ42/40 lower than 0.072 was considered indicative of an A+ profile, and CSF p-tau181 higher than 50 pg/mL was considered indicative of a T+ profile38. Additionally, Aβ42/40 and p-tau181 were measured also in plasma. Plasma neurofilament light chain (NfL) was also available for n = 126 subjects. AD biomarkers were all measured with Lumipulse G600-II assays as previously described38,39. CSF synaptosomal-associated protein 25 (SNAP 25) was also previously measured with Simoa in the same samples34. All biological samples were collected, handled, and stored according to international guidelines40. An aliquot of CSF from all patients and control participants (CTRL) included here were previously analyzed by synSAA developed by Concha-Marambio and colleagues at Amprion Inc14,41. Briefly, CSF samples were evaluated in triplicate (40 μL/well) in a clear bottom 96-well plate containing a reaction mix consisting of 0.3 mg/mL recombinant α-Syn (Amprion, cat# S2020), 100 mM PIPES pH 6.50 (Sigma, cat# 80,635), 500 mM NaCl (Lonza, cat# 51,202), 10 μM ThT (Sigma, cat# T3516), and two 1/8-inch Si3N4 beads (Tsubaki Nakashima). The assay was performed in a BMG FLUOstar Omega shaker/reader at 42 °C, with 15-min shaking/incubation cycles. Maximum fluorescence (Fmax) from three replicates was used for result determination; if all three replicates present Fmax higher than 3000 RFU, the sample is deemed positive (S+). If only two cross the 3,000 RFU threshold, the sample is considered inconclusive (S?). If one or no replicate presents Fmax higher than 3000 RFU, the sample is considered negative (S−). For synSAA-positive AD patients, the following SAA-derived parameters (averaged on three replicates) were included in kinetic analysis: Fmax, time-to-threshold (TTT, time to reach 3000 RFU), F24h (fluorescence at 24 h), AUFC (area under the fluorescence curve), Smax (maximum slope of the fluorescence curve), TSmax (time to reach the maximum slope), and AUFCdydx (area under the derivative of the fluorescence curve). Parameters were estimated using the Omega data analysis tool Mars (BMG). The data relative to these measurements has been previously published41.
PD/PDD patients (n = 47) were diagnosed according to the current diagnostic criteria42,43. Based on neuropsychological assessment, PD patients were categorized as cognitively normal PD (PD-CN) or PD with mild cognitive impairment (PD-MCI)44. A complete clinical characterization was performed, including a 2-year clinical follow-up (n = 23 patients). Both at baseline and follow-up, Mini-Mental State Examination score (MMSE) and Montreal Cognitive Assessment (MoCA) were administered. Motor assessment included MDS-Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) and Hoen & Yahr staging (H&Y).
AD patients (n = 80) were diagnosed according to the 2018 National Institute of Aging–Alzheimer’s Association criteria45, using the CSF biomarker profile A+/T+ classification. Combining the CSF profile (A+/T+) with neuropsychological evaluation and functional assessment, AD patients were grouped as preclinical AD (pre-AD) (n = 19), MCI-AD (n = 30) and dementia due to AD (AD-dem) (n = 31). All patients within the AD continuum underwent a neuropsychological evaluation including MMSE46, and Clinical Dementia Rating scale (CDR)47. Neuropsychological evaluation was repeated for n = 20 AD patients at approximately 2-year follow-up time (average: 2.4 y, standard deviation: 0.9 y). CTRL participants (n = 42) underwent lumbar puncture for CSF analysis as part of their routine diagnostic work-up for various clinical conditions, including headache, mononeuropathy, psychiatric disturbances, and subjective cognitive complaints. Their CSF profile was negative for AD (A−/T−). According to the neuropsychological evaluation, these subjects were either cognitively unimpaired (CTRL-CN) or had MCI (CTRL-MCI). Only CTRL-MCI subjects with stable cognitive performance at 2-year follow-up and a negative brain 18F-fluorodeoxyglucose-positron emission tomography were included.
The main demographic and clinical features of each diagnostic group are summarized in Table 1. For the technical validation all samples were leftovers from clinical samples with unknown preanalytical background like freeze/thaw cycles and time from collection to freezing. All samples were used neat without pretreatment. The three QC samples (QC1, QC2, and QC3) used for precision testing and analyte stability were non spiked pools from individual CSF samples. For the technical validation sample dilutions and transfers were performed in 0.5 mL polypropylene test tubes (Sarstedt Cat#72.730.105). The study was approved by the local Ethics Committee (registry N°: 3944/21, date: February 25, 2021).
pS129 α-syn Homebrew Simoa assay
The method used in this work is a Homebrew Simoa immunoassay developed by ADx NeuroSciences. The 3-step immunoassay consists of a capture step (60 min) in which α-syn present in the prediluted CSF or plasma sample is captured by a pan α-syn mouse monoclonal IgG2c antibody (Cat# RD-080; ADx NeuroSciences). The second step (10 min) is an incubation with a biotinylated pS129 α-syn specific monoclonal rabbit IgG antibody D1R1R (Cat#23706; Cell Signaling Technology). The third step is an incubation with streptavidin–β-galactosidase (SβG) conjugated enzyme for substrate signal generation (5 min). pS129 α-syn levels in CSF and plasma matrix were calculated from a ready-to-use calibrator curve (0.25–500 pg/mL) (5-PL curve with 1/Y² weighing) that is based upon full length pS129 α-syn (Cat#RP004; Proteos). The reported pS129 α-syn concentrations are based upon the concentration of the stock material provided by the supplier.
Epitope mapping of capture α-syn antibody RD-080 (ADx NeuroSciences)
RD-080 is an IgG2c monoclonal antibody and was generated by immunization of BALB/c mice with a KLH conjugated synthetic peptide spanning the C-terminal sequence of the α-syn protein. Spleen cells from the mouse giving highest titers towards the immunization antigen were fused with SP2/0 myeloma cells and a stable cell line was obtained after selecting the best reactive positive clone. A set of 7 overlapping synthetic peptides in region aa 89–112 of the canonical α-syn isoform (Uniprot α-synuclein sequence P37840-1) was produced for epitope mapping through indirect ELISA (Fig. S1). In short, the biotinylated synthetic peptides were bound to streptavidin coated 96-well microplates at 1 ng/mL. Next, antibody RD-080 was added (100 ng/mL in assay diluent) for 1 h at room temperature. After a wash step, the tracer antibody was added (Peroxidase coupled goat anti mouse H + L; Jackson Cat#115-035-166) at 5000-fold dilution into assay buffer and incubated for 30 min at room temperature. After a final wash step and colorimetric substrate (TMB) incubation for another 30 min at room temperature, the reaction was stopped with 100 µL of 1 M sulphuric acid. Optical density (OD) was measured at 450 nm and 630 nm (plate background) into a microplate reader (BMG Labtech Clariostar). The signal to noise value (S/N) is reported in Fig. S1 as the OD value of the specific peptide over the OD value of the blank value (no peptide added).
Epitope mapping of pS129 α-syn antibodies
A set of 14 overlapping synthetic peptides in the region 113-140 of the canonical α-syn isoform (Uniprot α-synuclein sequence P-37840-1) was produced for epitope mapping using indirect ELISA (Figs. 1A and S2 Supplementary Material). Control synthetic peptides covering the aa from 113 to 140 with and without S129 phosphorylation were also included in the analysis. In short, the biotinylated synthetic peptides were bound to streptavidin coated 96-well microplates at 1 ng/mL. Next, pS129 α-syn antibodies MJF-R13 (Abcam Cat#ab168381), D1R1R (CST Cat#23706), EP1536Y (Abcam Cat#ab51253), pSyn#64 (Fujifilm Wako Cat#015-25191) and 81 A (BioLegend Cat# 825701) were added (100 ng/mL in assay diluent) for 1 h at room temperature. After a wash step tracer antibody was added (Peroxidase coupled donkey anti rabbit H + L; Jackson Cat# 711-035-152 for rabbit species and Peroxidase coupled goat anti mouse H + L; Jackson Cat#115-035-166 for mouse species) at 5000-fold dilution into assay buffer and incubated for 30 min at room temperature. After a final wash step and colorimetric substrate (TMB) incubation for another 30 min at room temperature, the reaction was stopped with 100 µL 1 M sulphuric acid. ODs at 450–630 nm were measured with a microplate reader (BMG Labtech Clariostar). The signal to noise value (S/N) is reported in Fig. S2 as the OD value of the specific peptide over the OD value of the blank value (no peptide added).
Effect of the phosphorylation of adjacent amino acids (pY125, pY133) on pS129 α-syn antibody reactivity
Eight synthetic peptides biotinylated at their N-terminal region, representing the C-terminal part of the α-syn sequence (between aa116 and aa140), were tested in the same indirect ELISA approach for antibody epitope mapping (Figs. 1B and S3). The peptides were respectively not phosphorylated, single phosphorylated at position Y125, S129, Y133, phosphorylated at position Y125, S129, Y133 (triple), both Y125 and S129 (double), both Y125, and Y133 (double), and both S129 and Y133 (double). Four commercial antibodies were tested together with D1R1R at a concentration of 100 ng/mL: MJF-R13, EP1536Y, pSyn#64, and 81 A. A biotinylated peptide representing a β-amyloid sequence was included as negative control. The OD at 450–630 nm was measured with a microplate reader (BMG Labtech Clariostar) at varying peptide concentration.
Assay characteristics
The intra- and inter-run precision of the assay was assessed by running 6 independent test runs (Run 1 to Run 6) using 8 ready to use calibrator points (25–0.125 pg/mL), a blank and QC1, QC2 and QC3 (Fig. S4A Supplementary Material). Parallelism was determined by serial dilution into assay diluent of 4 EDTA plasma samples from healthy volunteers up to 20-fold dilution (Fig. S4B Supplementary Material). The measuring range was determined on the whole cohort (167 study CSF and 164 paired EDTA plasma samples (Fig. S4C and D Supplementary material). The measuring range was defined between the lowest and highest calibrator for which precision was below 20% (0.125–100 pg/mL). The lowest calibrator point (0.125 pg/mL) was considered as LLOQ based upon precision data of this point (Figure S5 supplementary material).
Since α-syn from red blood cells is a major confounder of α-syn measurement in CSF, we assessed this effect on the Homebrew pS129 α-syn assay by spiking whole blood into CSF at multiple dilutions and then analyzing the pS129 α-syn concentration (Fig. S6 Supplementary material). Whole blood from two healthy volunteers was frozen/thawed multiple times to lyse blood cells. A 1% v/v solution of whole blood was spiked into different CSF samples and serially diluted with assay diluent up to 96-fold dilution and then measured with the pS129 α-synuclein Homebrew assay. The reference condition without spike was included (labeled as “0”). The final concentrations tested were 0.01%, 0.02%, 0.04%, 0.08%, 0.017% and 0.033%. Two methods were applied to determine the specificity of the assay: i) Method using non-phosphorylated full-length recombinant α-syn protein. EndoClear™ human recombinant α-syn protein was used (Anaspec Cat# AS-55555-100; Uniprot nr. P37840; purified up to 95% purity from bacterial lysate) to prepare a serial dilution between 0.5 and 1000 pg/mL reflecting relevant α-syn concentrations in CSF. The recombinant α-syn material was reactive in ELISA (data not shown). In parallel, the calibrator points of the pS129 α-syn homebrew assay were tested (full-length α-syn phosphorylated at position S129; Proteos Cat# RP004). No cross reactivity was observed with α-syn indicating pS129 α-syn specificity of the assay for the used α-syn concentrations (Fig. S7 A Supplementary Material). ii) The second method consisted of alkaline phosphatase (AP) treatment of calibrator material to verify the effect on the readout in the Simoa Homebrew assay. Briefly, alkaline phosphatase (Merck Cat#P5521) was incubated at a concentration of respectively 0, 476, 952, 1429, and 1905 U/mL with α-syn phosphorylated at position S129 at a concentration of 25 pg/mL during an overnight incubation at +37 °C. After incubation, the treated calibrators were measured with the pS129 α-syn Homebrew assay Fig. S7B. Finally, we investigated analyte stability to estimate the robustness towards preanalytical parameters (Figure S8 supplementary material). A combination of two parameters, hold time and temperature was used in an isochrone approach to control the hold time stability. The reference samples were kept at −80 °C until the testing point. The experimental conditions were a combination of each temperature (RT = +18–25 °C) or cold room (+2–8 °C) with 3 time points (2, 4, and 24 h). Samples were kept frozen until the testing point where all conditions were analyzed in a single test run for pS129 α-syn with the Homebrew Simoa assay (Fig. S8A Supplementary Material).
To test freeze/thaw cycles the CSF samples were frozen respectively 1, 2 and 5 times and measured in a single test run with the pS129 α-syn Homebrew Simoa assay including a reference condition (freshly thawed) (Fig. S8B Supplementary Material).
N- and C-terminal α-synuclein ELISA
A research prototype N-terminal α-synuclein (N-α-syn) colorimetric ELISA was developed using mouse monoclonal antibodies by ADx NeuroSciences. The capture antibody RD-007 (ADx NeuroSciences; clone 24G6) maps in the amino acid region aa95 to aa104 and the detection antibody RD-009 (ADx NeuroSciences; clone 16A6) recognizes an epitope in the N-terminal part of the α-synuclein protein (aa1 to aa20). The calibrator points in the range of 25 and 400 pg/mL were based upon highly purified recombinant alpha-synuclein protein expressed in E. coli (Anaspec Cat# AS-55555-100). The immunoassay test procedure consists of a simultaneous incubation of 80 µL of prediluted sample (CSF 4-fold; plasma 1000-fold) and 20 µL of biotinylated detection antibody followed by a 3-h incubation step at room temperature. After a wash step, 100 µL of tracer is added (peroxidase-coupled streptavidin) and incubated for 30 min. After the next wash step, and TMB substrate incubation, the reaction was stopped with 100 µL of 1 M sulphuric acid. OD450-630nm values were obtained using a microplate reader (BMG Labtech, Clariostar). The immunoassay method for C-terminal α-syn (C-α-syn) detection used was previously described15. A summary of all assay formats is shown in Supplementary Table S1.
Statistical analysis
Statistical analyses were performed by using R v. 4.3.1. Two-way ANOVA was used to assess differences in α-syn species among synSAA-defined groups by controlling for clinical groups. Dunn’s test was used to assess pairwise differences in α-syn species among clinical groups by applying Benjamini-Hochberg corrections on p values. Correlations among biomarkers were assessed by means of Pearson’s correlation coefficients. The coefficients were then displayed on a heatmap where columns and rows were clustered by means of hierarchical clustering using Ward’s linkage criterion. Coefficients with a statistically significant adjusted p value (Benjamini-Hochberg correction) were marked with an asterisk (*). An adjusted p value < 0.05 was considered to be statistically significant across all the analyses.
Data availability
The immunoassay data generated during the current study are available from the corresponding author on reasonable request.
References
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M. & Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl Acad. Sci. USA 95, 6469–6473 (1998).
DeTure, M. A. & Dickson, D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 14, 32 (2019).
Bellomo, G. et al. Investigating alpha-synuclein co-pathology in Alzheimer’s disease by means of cerebrospinal fluid alpha-synuclein seed amplification assay. Alzheimer’s & Dementia 4, 2444–2452 (2024).
Gaetani, L. et al. CSF and Blood Biomarkers in Neuroinflammatory and Neurodegenerative Diseases: Implications for Treatment. Trends Pharmacol. Sci. 41, 1023–1037 (2020).
Schmid, A. W., Fauvet, B., Moniatte, M. & Lashuel, H. A. Alpha-Synuclein post-Translational modifications as potential biomarkers for parkinson disease and other synucleinopathies. Mol. Cell. Proteom. 12, 3543–3558 (2013).
Wang, Y. et al. Phosphorylated α-synuclein in Parkinson’s disease. Sci. Transl. Med. 4, 121ra20 (2012).
Lashuel, H. A. et al. Revisiting the specificity and ability of phospho-S129 antibodies to capture alpha-synuclein biochemical and pathological diversity. npj Parkinson’s Dis. 8, 136 (2022).
Arlinghaus, R., Iba, M., Masliah, E., Cookson, M. R. & Landeck, N. Specific detection of physiological S129 phosphorylated α-Synuclein in tissue using proximity ligation assay. J. Parkinson’s Dis. 13, 255–270 (2023).
Eusebi, P. et al. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord.: Off. J. Mov. Disord. Soc. 32, 1389–1400 (2017).
Magalhães, P. & Lashuel, H. A. Opportunities and challenges of alpha-synuclein as a potential biomarker for Parkinson’s disease and other synucleinopathies. NPJ Parkinsons Dis. 8, 93 (2022).
Dutta, S. et al. Development of a Novel Electrochemiluminescence ELISA for Quantification of α-Synuclein Phosphorylated at Ser 129 in Biological Samples. ACS Chem. Neurosci. 14, 1238–1248 (2023).
Norman, M., Gilboa, T. & Walt, D. R. High-Sensitivity Single Molecule Array Assays for Pathological Isoforms in Parkinson’s Disease. Clin. Chem. 68, 431–440 (2022).
Bellomo, G. et al. α-Synuclein Seed Amplification Assays for Diagnosing Synucleinopathies: The Way Forward. Neurology 99, 195–205 (2022).
Ma, Y. et al. Sensitivity and specificity of a seed amplification assay for diagnosis of multiple system atrophy: a multicentre cohort study. Lancet Neurol. 23, 1225–1237 (2024).
Chiasserini, D. et al. Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimers Res Ther. 9, 52 (2017).
Paciotti, S. et al. Cerebrospinal fluid hemoglobin levels as markers of blood contamination: Relevance for α-synuclein measurement. Clin. Chem. Lab. Med. 59, 1653–1661 (2021).
Barba, L. et al. CSF synaptic biomarkers in AT(N)-based subgroups of lewy body disease. Neurology 1, e50–e62 (2023).
Paolini Paoletti, F. et al. CSF neurochemical profile and cognitive changes in Parkinson’s disease with mild cognitive impairment. npj Parkinson’s Disease 9, 68 (2023).
Fujiwara, H. et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 (2002).
Anderson, J. P. et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281, 29739–29752 (2006).
Smith, W. W. et al. Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J. Neurosci. 25, 5544–5552 (2005).
Karampetsou, M. et al. Phosphorylated exogenous alpha-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice. Sci. Rep. 7, 16533 (2017).
Walker, D. G. et al. Changes in properties of serine 129 phosphorylated α-synuclein with progression of Lewy-type histopathology in human brains. Exp. Neurol. 240, 190–204 (2013).
Swirski, M. et al. Evaluating the relationship between amyloid-β and α-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson’s disease. Alzheimers Res Ther. 6, 77 (2014).
Gorbatyuk, O. S. et al. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc. Natl Acad. Sci. USA 105, 763–768 (2008).
Tenreiro, S. et al. Phosphorylation modulates clearance of alpha-synuclein inclusions in a yeast model of Parkinson’s disease. PLoS Genet 10, e1004302 (2014).
Parra-Rivas, L. A. et al. Serine-129 phosphorylation of α-synuclein is an activity-dependent trigger for physiologic protein-protein interactions and synaptic function. Neuron 111, 4006–4023.e10 (2023).
Ghanem, S. S. et al. α-Synuclein phosphorylation at serine 129 occurs after initial protein deposition and inhibits seeded fibril formation and toxicity. Proc. Natl Acad. Sci. USA 119, e2109617119 (2022).
Gibbons, C. H. et al. Skin Biopsy Detection of Phosphorylated α-Synuclein in Patients With Synucleinopathies. JAMA 331, 1298–1306 (2024).
Silva, A. M., Hickford, E. S. & Cutler, P. An immunoassay for the quantification of phosphorylated α-synuclein at serine 129 in human cerebrospinal fluid. Bioanalysis 16, 1125–1139 (2024).
Majbour, N. K. et al. Increased levels of CSF total but not oligomeric or phosphorylated forms of alpha-synuclein in patients diagnosed with probable Alzheimer’s disease. Sci. Rep. 7, 40263 (2017).
Cariulo, C. et al. Phospho-S129 Alpha-Synuclein Is Present in Human Plasma but Not in Cerebrospinal Fluid as Determined by an Ultrasensitive Immunoassay. Front. Neurosci. 13, 889 (2019).
Barba, L. et al. Cerebrospinal fluid β-synuclein as a synaptic biomarker for preclinical Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 94, 83–86 (2023).
Gaetani, L. et al. Influence of co-pathology on CSF and plasma synaptic markers SNAP25 and VAMP2 in Alzheimer’s disease and Parkinson’s disease. Alzheimer’s Res. Ther. 17, 115 (2025).
Zhang, C., Xie, S. & Malek, M. SNAP-25: A biomarker of synaptic loss in neurodegeneration. Clin. Chim. Acta 571, 120236 (2025).
Majbour, N. K. et al. Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov. Disord. 31, 1535–1542 (2016).
Gilboa, T. et al. Measurement of α-synuclein as protein cargo in plasma extracellular vesicles. Proc. Natl Acad. Sci. USA 121, e2408949121 (2024).
Bellomo, G. et al. Machine Learning Driven Profiling of Cerebrospinal Fluid Core Biomarkers in Alzheimer’s Disease and Other Neurological Disorders. Front. Neurosci. 15, 337 (2021).
Bellomo, G. et al. Fully automated measurement of plasma Aβ42/40 and p-tau181: Analytical robustness and concordance with cerebrospinal fluid profile along the Alzheimer’s disease continuum in two independent cohorts. Alzheimer’s Dement. 20, 2453–2468 (2024).
Teunissen, C. E. et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73, 1914–1922 (2009).
Bellomo, G. et al. Investigating alpha-synuclein co-pathology in Alzheimer’s disease by means of cerebrospinal fluid alpha-synuclein seed amplification assay. Alzheimer’s & dementia: the journal of the Alzheimer’s Association https://doi.org/10.1002/alz.13658 (2024).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov.Disord. 12,1591–601 (2015).
Emre, M. et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord.: Off. J. Mov. Disord. Soc. 22, 1689–1707 (2007).
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. https://doi.org/10.1002/mds.24893 (2012).
Jack, C. R. et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement.: J. Alzheimer’s Assoc. 14, 535–562 (2018).
Measso, G. et al. The mini-mental state examination: Normative study of an Italian random sample. Dev.Neuropsychol. 9, 77–85 (1993).
Morris, J. C. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 9, 173–176 (1997).
Acknowledgements
G.B. was supported by the Postdoctoral Fellowship for Basic Scientists grant of the Parkinson's Foundation (Award ID: PF-PRF-934916). L.P. and L.G. are funded by the European Union—Next Generation EU—PNRR M6C2––Investimento 2.1 Valorizzazione e potenziamento della ricerca biomedica del SSN (PNRR-MAD-2022-12376035).
Author information
Authors and Affiliations
Contributions
G.B. and E.S. designed the study. G.B., E.S., and D.C. wrote the main manuscript text. G.B. perfromed all the statistical analyses. G.B. prepared the main figures and tables. G.B., L.G., and F.P.P. selected the study cohort. F.P.P. and L.P. collected the clinical information of the study cohort. L.C.M., Y. M., and C. F. prformed CSF synSAA. E.S., J.V., L.D., C.F., and M.V. develped and characterized the apha-synuclein immunoassays and prepared the supplementary figures and tables. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing financial interests: G. Bellomo and L. Gaetani completed paid consultancies for Fujirebio Europe. E. Stoops, J. Vanbrabant, L. Demeyer, C. Francois, M. Vanhooren are employees of ADx NeuroSciences. L. Concha-Marambio, C. Farris, and Y. Ma are inventors of several patents related to SAA technology (PMCA) and are associated with Amprion Inc., a biotech company focused on the commercial utilization of SAA for diagnosis. L. Parnetti served as an Advisory Board Member for Fujirebio, IBL, Roche, and Merck. The other authors declare no financial and non-financial competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bellomo, G., Stoops, E., Vanbrabant, J. et al. Phosphorylated α-synuclein in CSF and plasma does not reflect synucleinopathy. npj Parkinsons Dis. 11, 232 (2025). https://doi.org/10.1038/s41531-025-01086-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01086-w