Abstract
Psychiatric disorders present a significant global health burden with limited effective medications. Observing the widespread comorbidities between diabetes and psychiatric disorders, we explored the potential of repurposing antidiabetic drug targets for psychiatric treatments. We identified 32 target genes of 60 antidiabetics and performed Mendelian randomization analyses using expression and protein quantitative trait loci data from brain tissues alongside summary data for seven psychiatric disorders. Additionally, we conducted colocalization analyses, replication analyses in blood and at the single-cell level, single-cell gene annotation, developmental trajectory analysis, and various functional assessments. We found that elevated GANC expression in the putamen basal ganglia, nucleus accumbens basal ganglia, cortex, and whole blood was associated with a reduced risk of bipolar disorder (OR, 0.532–0.877; P, 4.04 × 10−5 to 1.45 × 10−7), implying that antagonism of GANC by the antidiabetic drug miglitol could increase bipolar risk. Conversely, increased ABCC8 expression in the cortex, cerebellum, cerebellar hemisphere, and VIP- and LAMP5-expressing inhibitory neurons was linked to a higher risk of schizophrenia (OR, 1.054–1.119; P, 1.46 × 10−3 to 4.42 × 10−5), suggesting that ABCC8 inhibition by sulfonylureas or glinides may lower the risk of schizophrenia. Colocalization analysis further confirmed the above associations. GANC and ABCC8 displayed specific developmental trajectories, and functional analyses revealed that they affected psychiatric risk through pathways related to potassium ion channels, insulin secretion, and glucose metabolism. Our findings highlight GANC and ABCC8 as potential targets, suggesting caution in miglitol use for bipolar disorder and the potential repurposing of sulfonylureas and glinides for schizophrenia.
Similar content being viewed by others
Introduction
Psychiatric disorders such as attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), anorexia nervosa (AN), obsessive-compulsive disorder (OCD), bipolar disorder (BD), schizophrenia (SCZ), and major depressive disorder (MDD) impose an enormous burden on global public health and economy1. However, available medications for psychiatric disorders are limited and often ineffective, with most approved drugs targeting a narrow range of specific molecular targets, such as the type 2 dopaminergic receptor antagonized by almost all antipsychotics2,3. This highlights the urgent need to identify more viable targets and expand the range of drug options to effectively treat these disorders.
Previous studies indicate a widespread comorbidity between diabetes and psychiatric disorders. Patients with diabetes are more prone to psychiatric disorders such as SCZ, BD, MDD, ASD, and ADHD compared to healthy controls4,5. Conversely, individuals with psychiatric disorders such as SCZ, MDD, and ADHD also face a higher risk of developing diabetes6,7,8. Abnormalities in glucose metabolism and insulin secretion link diabetes and psychiatric disorders, playing a crucial role in their interaction. Patients with SCZ and BD exhibit altered brain glucose metabolism9,10. Impaired glucose metabolism and insulin resistance may contribute to disrupted neurodevelopment in SCZ through the activation of the mechanistic target of rapamycin mitochondrial pathway11. Evidence suggests that antidiabetic drugs may help treat certain neurocognitive disorders, such as Alzheimer's disease12,13. However, the potential causal relationship between the use of antidiabetic drugs and psychiatric disorders, as well as the underlying mechanisms, still remains unclear.
Developing new drugs involves huge time and economic costs, whereas drug repurposing, which tests approved drugs for new uses, provides a quick and cost-effective method to discover potential treatments for psychiatric disorders using drugs with established mechanisms and safety profiles14. Additionally, selecting therapeutic targets based on genetically supported target genes could significantly enhance the success rate of drug development15. Mendelian randomization (MR) is a promising tool for identifying new uses for approved drugs, enabling cost-effective testing of costly or time-consuming interventions. Drug target MR analysis utilizes quantitative trait loci (QTL) data of drug target genes as instrumental variables (IVs) for exposures and summary data from genome-wide association studies (GWAS) of psychiatric disorders for outcomes to investigate whether genetic variants causally affect diseases through changes in gene expression, thereby aiding drug repurposing efforts16,17.
A previous study investigated the association between seven antidiabetic target genes and psychiatric disorders but reported no significant findings. This could be attributed to the limited coverage of approved antidiabetic drugs and their corresponding target genes, as well as the fact that most IVs were derived from whole blood rather than brain tissues18. Therefore, this study integrated summary data of psychiatric disorders and molecular QTLs, including gene expression in various brain regions, cell types, and whole blood, as well as protein abundance in brain tissues, to perform MR and assess the causal effects of genetic variants in antidiabetic drug targets on psychiatric risk. Colocalization and replication MR analyses were performed to ensure stability. We then conducted single-cell gene expression annotation, developmental trajectory analysis, phenome-wide association studies (PheWAS), enrichment analysis, and drug-gene interaction (DGI) analysis to explore mechanisms linking drug targets to psychiatric risk. We hypothesize that antidiabetic drug targets could provide promising novel targets for psychiatric disorders.
Methods
Study design
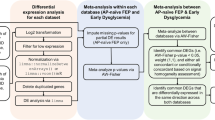
This study utilized Mendelian randomization analysis, colocalization analysis, and various functional analyses, leveraging publicly available summary data from expression quantitative trait loci (eQTL), protein quantitative trait loci (pQTL), and GWAS for exposures and outcomes (Additional file 2: Table S1). For accurate causal estimation in MR analysis, three critical assumptions must be met: (1) a strong association between the IVs and the drug target genes (relevance), (2) independence of IVs on confounders (exchangeability), and (3) no direct effects of IVs on psychiatric risk except via the drug target genes (exclusion restriction). The framework of our study design and diagram of the MR analysis are presented in Fig. 1. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) reporting guideline (Additional file 1: Supplementary Methods, Figs. S1–S7, and STROBE-MR checklist)19.
A Workflow for identifying antidiabetic drug target genes. B Diagram of the MR analysis. C Colocalization analyses confirming significant MR signals. D Replication MR analyses using multiple datasets. E Functional analyses, including single-cell gene annotation, developmental trajectory mapping, phenome-wide association studies, gene set enrichment, and drug–gene interaction analyses. eQTL expression quantitative trait loci, pQTL protein abundant quantitative trait loci, GTEx genotype-tissue expression, ROSMAP Religious Orders Study and Rush Memory and Aging Project, PGC Psychiatric Genomics Consortium, MR Mendelian randomization, PP.H4 posterior probability for hypothesis 4, SCZ schizophrenia, BD bipolar disorder, eQTLGen eQTLGen Consortium, UMAP uniform manifold approximation and projection, DGIdb drug-gene interaction database, GO gene ontology, KEGG Kyoto Encyclopedia of Genes and Genomes.
Ethics statement
Only publicly available data were used in this study. Ethical approval and consent to participation were available in the original studies.
Identification of antidiabetic drug targets
We utilized the Anatomical Therapeutic Chemical (ATC) classification system from the World Health Organization Collaborating Center (WHOCC) for drug statistics methodology, which provided comprehensive coverage of globally approved antidiabetic drug categories. We identified a total of 60 antidiabetic drugs (Additional file 2: Table S2) and determined their target genes using the DrugBank20 and ChEMBL21 databases. After removing target genes with unknown pharmacological actions in the DrugBank and duplications in two databases, 32 target genes were selected for subsequent analyses (Additional file 2: Table S3).
Selection of instruments for drug targets
We mainly focused on target gene expression in brain tissues when studying psychiatric disorders. Therefore, we extracted brain eQTL data from Genotype-Tissue Expression version 8 (GTEx v8), including 114–209 subjects with genotypes in brain tissues, with 24.7%–38.1% being females (Additional file 2: Table S1)22. The subjects from the GTEx v8 database mostly originated from European ancestry (85%)22. To minimize horizontal pleiotropy brought by trans-eQTLs23, we only utilized cis-eQTLs as IVs. We identified antidiabetic drug target genes with significant eQTLs (eQTL P < 1 × 10−4) in the brain, and the top eQTL of each target in each brain tissue was used as an IV (Additional file 2: Table S4).
We also extracted pQTL summary data from the Religious Orders Study and Rush Memory and Aging Project (ROSMAP) to select IVs for drug target proteins24. A total of 912,253 SNP-protein expression pairs from the dorsolateral prefrontal cortex of post-mortem brain samples donated by 376 people of European ancestry, of whom 69.7% were female, were included in the ROSMAP pQTL dataset25. We selected SNPs significantly associated with target proteins as IVs (pQTL P < 0.05).
To avoid weak IV bias, we calculated the F-statistic for each IV using the SNP-exposure association (beta) and the standard error (SE) of the SNP-exposure association for each SNP by the formula: F = (beta2/SE2)26.
GWAS summary data for psychiatric disorders
Genetic proxies for psychiatric disorders were obtained from publicly available GWAS datasets, including ADHD (20,183 cases and 35,191 controls)27, ASD (18,381 cases and 27,969 controls)28, AN (3495 cases and 10,982 controls)29, OCD (2688 cases and 7037 controls)30, BD (41,917 cases and 371,549 controls)31, SCZ (53,386 cases and 77,258 controls)32 from the Psychiatric Genomics Consortium (PGC), and MDD (170,756 cases and 329,443 controls) from PGC and UK Biobank (excluding 23andme)33. All individuals were of European descent.
Primary MR analysis
Prior to the primary analyses, power for each MR test was calculated using the mRnd web tool (https://sb452.shinyapps.io/power/), based on study-specific sample size, case/control ratio, and instrument R2 (Additional file 2: Tables S1 and S4)34,35. Assuming a two-sided α = 0.05 and the default causal effect (odds ratio (OR) = 1.20), 93.6% of the MR analyses achieved >80% power (minimum = 0.612), supporting the adequacy of the study design.
In the MR analysis for antidiabetic drug targets, the Wald ratio method was employed for targets with one top SNP as an IV, while the inverse-variance weighted (IVW) regression method was utilized for those with multiple top SNPs as IVs36. All MR analyses were conducted using the R package TwoSampleMR37. Results of the MR analyses were presented as ORs along with their 95% confidence intervals (CIs), indicating the risk of outcomes linked to unit changes in gene expression. In this study, we applied the Bonferroni correction to determine the statistical significance of the MR effect estimations. MR results that achieved a nominal P-value below 0.05 but did not pass the Bonferroni adjustment were regarded as suggestive of potential causality. For MR results that achieved Bonferroni-corrected significance, we re-conducted the analysis with an instrument variable threshold of 5 × 10−8 as a sensitivity analysis.
Colocalization analysis
To confirm the results of MR analysis, we performed Bayesian colocalization analyses using the R package coloc (Additional file 1: Supplementary Methods)38, which strengthened the causal inference by mitigating the influence of linkage disequilibrium (LD)39. Associations with a posterior probability for hypothesis 4 (PP.H4) exceeding 80% were considered significant evidence of colocalization40. The R package LocusCompare was used to visualize colocalization results.
Replication MR analysis
To ensure the stability of the primary MR analysis, we utilized brain cis-eQTL summary data exclusively from the European population in GTEx v8 and conducted MR analyses for Bonferroni-corrected significant results as outlined above. Additionally, we performed MR analysis using the IVW method, with significant cis-eQTLs (P < 5 × 10−8) from whole blood in the eQTLGen consortium (n = 31,684) as IVs41. To further pinpoint specific cell types whose gene expression levels were associated with psychiatric disorders, we leveraged single-cell eQTLs (sc-eQTLs) from the prefrontal cortex of 388 individuals, 35.1% of whom were female, in the PsychENCODE consortium Phase II42 and conducted MR analyses. For drug target genes with significant sc-eQTLs (P < 1 × 10−5) across various brain cell types, the top sc-eQTL for each target in each cell type was selected as the IV.
Single-cell gene expression annotation
We utilized single-nucleus RNA sequencing (snRNA-Seq) data from PsychENCODE Phase II (https://www.psychencode.org/home) to investigate the expression levels of target genes identified through MR analysis, mapped to the brain cell types corresponding to the sc-eQTLs42. Using snRNA-Seq data from post-mortem adult prefrontal cortex samples of patients with SCZ (n = 47) and controls (n = 53), this dataset provided annotations of target gene expression across 27 distinct brain cell types.
Spatiotemporal gene expression trajectory analysis
To explore how the expression of genes identified through MR and colocalization analyses varied across developmental stages and brain regions, we conducted a spatiotemporal brain expression trajectory analysis. We utilized processed mRNA-seq data from PsychENCODE Phase I, comprising 607 tissue samples from 41 post-mortem brains spanning 8 postconceptional weeks (PCW) to 40 postnatal years (PY), with 43.9% of the donors being female43. The data were divided into nine developmental windows following PsychENCODE methodology: 8–9 PCW, 12–13 PCW, 16–17 PCW, 19–22 PCW, 35 PCW-0.3 PY, 0.5–2.6 PY, 2.8–10.7 PY, 13–19 PY, and 21–40 PY, providing a comprehensive view of gene expression patterns across the lifespan. Brain regions were categorized into the hippocampus (HIP), amygdala (AMY), cerebellar cortex (CBC), striatum (STR), mediodorsal nucleus of the thalamus (MD), and cortex, following PsychENCODE Phase I guidelines. For each of these six regions, we aggregated gene expression levels from their respective subregions, measured in RPKM. Aggregated values were log2-transformed and normalized within each region. Finally, smoothed LOESS curves were plotted to visualize the expression trajectories44.
Phenome-wide association study
For drug targets significantly associated with psychiatric disorders, as identified through Bonferroni-corrected MR results and colocalization analysis, we performed a phenome-wide association study (PheWAS) using the GWAS ATLAS resource (https://atlas.ctglab.nl/)45. This approach allowed us to systematically examine the multifunctionality of target genes and explore their associations with a broad spectrum of traits.
GO/KEGG enrichment analysis
To further investigate the potential mechanisms by which antidiabetic drug targets affected psychiatric disorders, target genes that achieved a nominal P-value below 0.05 in the MR analysis were selected and annotated using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses. The analysis was completed using the R package clusterProfiler and biomaRt46,47.
Drug-gene interaction analysis
We further performed DGI analysis to investigate the associations between existing practical drugs and the identified target genes significantly associated with psychiatric disorders. Using the Drug-Gene Interaction Database 5.0 (DGIdb 5.0, https://dgidb.org)48, we identified potential drug candidates targeting these genes, thereby enhancing our understanding of possible therapeutic options for psychiatric disorders.
Results
Causal effects of antidiabetic drugs on psychiatric disorders
In total, 114 index SNPs were selected from GTEx v8 to genetically predict antidiabetic drug target genes. F-statistics for these genetic instruments were all larger than 10, indicating strong instruments49 (Additional file 2: Table S4). Given the 923 MR estimates, a Bonferroni-corrected P-value was set as 0.05/923 (5.42 × 10−5).
In Additional file 2: Tables S5–S11, results from MR analyses found suggestive evidence for the association between antidiabetic drug target genes and ADHD, ASD, AN, BD, MDD, OCD, and SCZ, respectively. Among these results, higher expression levels of Glucosidase Alpha Neutral C (GANC) across nine brain regions (amygdala, cerebellar hemisphere, cerebellum, cortex, frontal cortex, hippocampus, nucleus accumbens basal ganglia, putamen basal ganglia, and substantia nigra) were significantly associated with a lower risk of BD after Bonferroni correction (OR, 0.764–0.877; P, 4.04 × 10−5 to 1.45 × 10−7), while higher expression levels of ATP-Binding Cassette Subfamily C Member 8 (ABCC8) across three brain regions (cerebellar hemisphere, cerebellum, and cortex) were significantly associated with a higher risk of SCZ after Bonferroni correction (OR, 1.069–1.119; P = 4.42 × 10−5; Additional file 2: Table S12 and Fig. 2). There is no overlap between the exposure and outcome datasets.
Limiting instruments to genome-wide significant variants (P < 5 × 10−8) left suitable proxies only for GANC expression in the cerebellar hemisphere and for ABCC8 expression in the cerebellar hemisphere, cerebellum, and cortex. MR analyses based on these variants remained Bonferroni-significant for bipolar disorder and schizophrenia, respectively (Additional file 2: Table S12). No genome-wide significant instruments were available for the other tissue-trait combinations.
When examining the associations between antidiabetics target proteins, proxied by brain pQTLs from the ROSMAP dataset, and psychiatric disorders, valid IVs were available for only 12 of the 32 targets Among these, protein levels of PRKAB1, GPD2, GANAB, and HRH1 showed suggestive associations with certain psychiatric disorders (P, 0.002 to 0.041; Additional file 2: Table S13). However, none of these associations remained significant after Bonferroni correction (threshold = 0.05/71 = 7.04 × 10−4).
Colocalization analysis
To validate the MR results, we performed colocalization analyses between ABCC8 or GANC expression in the brain regions identified above and SCZ or BD risk. We observed evidence of highly likely colocalization, with a posterior probability exceeding 80% for shared genetic variants between GANC expression across cortex (PP.H4 = 81.9%), nucleus accumbens basal ganglia (PP.H4 = 87.4%), and putamen basal ganglia (PP.H4 = 92.8%) and BD risk, as well as between ABCC8 expression across cortex (PP.H4 = 87.3%), cerebellum (PP.H4 = 88.6%), and cerebellar hemisphere (PP.H4 = 88.4%) and SCZ risk (Additional file 2: Table S14 and Fig. 3). LocusCompare plots comparing eQTL results for GANC or ABCC8 and GWAS for BD or SCZ are presented in Additional file 1: Fig. S1. Sensitivity analyses of colocalization ensured the robustness of the results (Additional file 1: Figs. S2–S7).
A The plot displays the lead SNP, which has the highest posterior probability for H4 in the colocalization analysis between GANC expression in the putamen basal ganglia and BD risk, with other SNPs colored according to their LD r² with the lead SNP. B The plot displays the lead SNP, which has the highest posterior probability for H4 in the colocalization analysis between GANC expression in the nucleus accumbens basal ganglia and BD risk, with other SNPs colored according to their LD r² with the lead SNP. C The plot displays the lead SNP, which has the highest posterior probability for H4 in the colocalization analysis between GANC expression in the cortex and BD risk, with other SNPs colored according to their LD r² with the lead SNP. D The plot displays the lead SNP, which has the highest posterior probability for H4 in the colocalization analysis between ABCC8 expression in the cortex and SCZ risk, with other SNPs colored according to their LD r² with the lead SNP. E The plot displays the lead SNP, which has the highest posterior probability for H4 in the colocalization analysis between ABCC8 expression in the cerebellum and SCZ risk, with other SNPs colored according to their LD r² with the lead SNP. F The plot displays the lead SNP, which has the highest posterior probability for H4 in the colocalization analysis between ABCC8 expression in the cerebellar hemisphere and SCZ risk, with other SNPs colored according to their LD r² with the lead SNP. GWAS genome-wide association study, BD bipolar disorder, eQTL expression quantitative trait loci, GANC glucosidase alpha neutral C, SCZ schizophrenia, ABCC8 ATP-binding cassette subfamily C member 8, PP.H4 posterior probability for hypothesis 4.
Replication MR analysis
To ensure the stability of the primary analysis, we repeated the MR analyses using the GTEx dataset restricted to the European population, yielding results consistent with the primary findings (Additional file 2: Table S15). In the eQTLGen dataset, we demonstrated that higher GANC expression levels in blood were significantly associated with a reduced risk of BD (IVW OR = 0.532; 95% CI, 0.417–0.678; P = 3.36 × 10−7, Additional file 2: Table S16 and Fig. 4A). In the PsychENCODE dataset, we demonstrated that higher ABCC8 expression levels in vasoactive intestinal peptide (VIP)-expressing inhibitory neurons and lysosome-associated membrane protein family member 5 (LAMP5)-expressing inhibitory neurons were significantly associated with an increased risk of SCZ (OR, 1.054–1.066; P, 1.46 × 10−3 to 1.20 × 10−3; Additional file 2: Table S17 and Fig. 4A). Notably, gene expression of ABCC8 was not found in eQTLGen, while GANC expression was absent in PsychENCODE.
A Replication MR analyses between GANC or ABCC8 expression in blood or at the single-cell level and psychiatric disorders. B Brain single-cell expression of ABCC8. C Brain single-cell expression of ABCC8 by cell type. D Developmental trajectories of ABCC8. E Developmental trajectories of GANC. OR odds ratio, CI confidence interval, VIP vasoactive intestinal peptide, LAMP5 lysosome-associated membrane protein family member 5, BD bipolar disorder, SCZ schizophrenia, UMAP uniform manifold approximation and projection, HIP hippocampus, AMY amygdala, CBC cerebellar cortex, STR striatum, MD mediodorsal nucleus of the thalamus, PCW postconceptional week, PY postnatal year, MR Mendelian randomization.
Single-cell gene expression annotation
Using snRNA-Seq data from PsychENCODE, we mapped ABCC8 expression across 27 distinct brain cell types. The highest expression level was observed in layer 6 intratelencephalic projecting excitatory neurons expressing Car3, while the lowest was found in immune cells. Notably, in VIP-expressing inhibitory neurons and LAMP5-expressing inhibitory neurons—both of which showed ABCC8 expression levels associated with SCZ risk—ABCC8 exhibited similar expression patterns (Fig. 4B, C).
Developmental trajectories for identified target genes
Using spatiotemporal brain gene expression data from PsychENCODE, we examined the developmental trajectories of ABCC8 and GANC expression across six brain regions (Fig. 4D, E). Based on Bonferroni-corrected MR and colocalization results, our analysis focused on ABCC8 expression in the CBC (encompassing the cerebellum and cerebellar hemisphere) and cortex, as well as GANC expression in the STR (encompassing the nucleus accumbens and putamen basal ganglia) and cortex. ABCC8 expression in the cortex reached its peak during the mid-gestation period (window 4, 19–22 PCW), followed by a slight decline during windows 5–6 (35 PCW-2.6 PY), and subsequently increased after window 6. In contrast, ABCC8 expression in the CBC peaked at window 6 (0.5–2.6 PY) and then gradually declined. GANC expression in the cortex declined from the early prenatal period, reaching its lowest level during the mid-gestation period (window 4, 19–22 PCW), followed by a slight increase and stabilizing into a plateau phase after window 5 (35 PCW-0.3 PY). GANC expression in the STR showed a sharp decline from the early prenatal period, reaching its lowest level at window 3 (16–17 PCW), followed by a gradual increase and stabilizing into a plateau phase after window 6 (0.5–2.6 PY).
Phenome-wide association study
Using the GWAS ATLAS platform, we conducted a PheWAS analysis to aggregate traits associated with GANC and ABCC8 (Fig. 5A, B and Additional file 2: Table S20). For GANC, the top 20 associated traits were primarily related to activity (e.g., general risk tolerance, usual walking pace), psychiatry (e.g., BD, SCZ, ASD), and metabolism (e.g., fatty acid metabolism, fasting insulin). For ABCC8, the top associated traits were mainly in the domains of metabolism (e.g., type II diabetes mellitus (T2D), body mass index (BMI), whole-body impedance) and immunology (e.g., white blood cell count, neutrophil count, eosinophil count, granulocyte count). Notably, the PheWAS analysis also revealed a suggestive association between ABCC8 and SCZ.
A PheWAS of GANC. B PheWAS of ABCC8. C KEGG enrichment of suggestive causal genes for BD. D KEGG enrichment of suggestive causal genes for SCZ. E GO enrichment of suggestive causal genes for BD. F GO enrichment of suggestive causal genes for SCZ. GO gene ontology, KEGG Kyoto Encyclopedia of Genes and Genomes, PheWAS phenome-wide association study.
GO/KEGG enrichment
We identified eight suggestive causal genes for SCZ (ABCC8, PRKAB1, DPP4, GANC, SLC5A1, PPARG, KCNJ11, KCNJ1) and nine for BD (GANC, ABCC8, GANAB, SLC5A2, PPARG, AMY2A, RAMP1, KCNJ11, INSR), with nominal P-values < 0.05 (Additional file 2: Tables S8 and S11). Our GO and KEGG enrichment analyses showed that these antidiabetic targets affected SCZ risk through pathways including import across the plasma membrane, potassium channel complex, inward rectifier potassium channel activity, T2D, and insulin secretion. Similarly, BD risk was affected through pathways such as import across the plasma membrane, potassium channel complex, hydrolase activity hydrolyzing O-glycosyl compounds, T2D, and starch and sucrose metabolism (Fig. 5C–F).
Drug-gene interaction
Evidence from DrugBank and ChEMBL indicates that sulfonylureas and glinides act as inhibitors of ABCC8, while miglitol functions as an antagonist of GANC. Using the DGIdb 5.0 database, we identified existing drugs potentially interacting with GANC and ABCC8. For ABCC8, 20 candidate drugs were identified, including 12 approved sulfonylurea and glinide drugs. Additionally, one potentially effective drug (Prunetin, not approved) targeting GANC was identified (Additional file 2: Table S21).
Discussion
We investigated the effects of genetic variants in antidiabetic drug targets on psychiatric disorders using public QTL and GWAS summary data. Our findings indicate that elevated GANC expression (a target gene of miglitol) in the putamen basal ganglia, nucleus accumbens basal ganglia, cortex, and blood is associated with a reduced risk of BD. Additionally, increased ABCC8 expression (a target gene of sulfonylurea and glinide drugs) in the cortex, cerebellum, cerebellar hemisphere, and in VIP-expressing and LAMP5-expressing inhibitory neurons is linked to an elevated risk of SCZ. Colocalization analysis further validated the associations between GANC and ABCC8 expression with BD and SCZ risk, respectively.
GANC encodes alpha-glucosidase, a key enzyme in glucose metabolism, and inhibitors of this enzyme could reduce postprandial hyperglycemia50. Patients with BD show altered cerebral glucose metabolism, including increased activity in the right precentral gyrus and decreased activity in the left superior temporal gyrus, left middle temporal gyrus, and cerebellum, indicative of disease-related functional abnormalities in emotion and cognition10.
Gene expression trajectory analysis revealed that GANC expression in the CBC and cortex declined during the early prenatal period and remained relatively stable postnatally. Considering the negative association between GANC expression and BD risk, abnormally reduced GANC expression during the prenatal period may contribute to an increased risk of BD, aligning with the neurodevelopmental pathways of BD51. PheWAS associations between GANC and glucose-lipid metabolism as well as physical activity traits suggest that GANC may reduce BD risk by enhancing energy utilization and behavioral activation, both of which are often impaired in BD10,52. Its link to general risk tolerance reflects the impulsivity observed in manic episodes, offering a neurobehavioral clue to GANC’s potential protective role53. Through enrichment analyses, we identified pathways linking GANC to BD risk, involving galactose and sucrose metabolism. Galactose-1-phosphate is associated with BD risk, and exogenous administration of galactose might mimic the effects of Li+ in BD treatment54. Sucrose intake may also be linked to BD, potentially affecting the pathophysiology through microcapillary impairments and reduced brain glucose uptake55. According to the DrugBank database, miglitol is an antagonist of GANC. Given the causal link between higher GANC expression and lower BD risk, using miglitol might increase BD risk, but no clinical study has explored this relationship. After searching the DrugBank, ChEMBL, and DGIdb databases, we found no approved medication that acted as an agonist or activator of GANC. Future research should explore the potential protective effects of GANC in treating BD, and we recommend caution regarding the use of miglitol in diabetes patients with comorbid BD.
The protein encoded by ABCC8, a member of the multidrug resistance-associated proteins subfamily within the ATP-binding cassette transporters56, functions as a modulator of ATP-sensitive potassium (K-ATP) channels. ABCC8 has been shown to form the K-ATP channel together with potassium inwardly rectifying channel subfamily J member 11 (KCNJ11/Kir6.2), which is also a target of sulfonylurea and glinide drugs57. The structure of K-ATP channels in the brain is similar to that in the pancreas, consisting of the pore-forming subunit KCNJ11 and the regulatory subunit ABCC8. These channels are widely expressed in various brain regions, including the hypothalamus, hippocampus, and prefrontal cortex, as well as in neurons and astrocytes58. Research using Kir6.2 knockout mice demonstrates that K-ATP channels play a role in modulating the acute symptoms of SCZ triggered by MK-801, related to neural excitability59. Additionally, clinical studies indicated that brain K-ATP channels had effects on neuropsychological features. Patients with permanent neonatal diabetes mellitus caused by KCNJ11 mutations often exhibited neuropsychological abnormalities, including learning challenges, lower intelligence, and motor impairments, while patients with other mutations seldom displayed neurological issues60. Furthermore, potassium channel openers/activators like diazoxide may be effective adjuvant agents in managing SCZ by hyperpolarizing cells during metabolic stress, as evidenced by their superior efficacy in reducing positive and general psychopathology symptoms compared to treatment with antipsychotics alone61.
Increased ABCC8 expression in VIP- and LAMP5-expressing inhibitory neurons, cortex, and CBC is linked to higher SCZ risk. VIP- and LAMP5-expressing inhibitory neurons share similar ABCC8 expression patterns. VIP and its receptors have been implicated in SCZ susceptibility62, with SCZ-related cognitive impairments linked to alterations in VIP-expressing inhibitory neurons63. Additionally, dysregulation of LAMP5-expressing neurons has been observed in patients with SCZ64, further highlighting their potential role in SCZ. Gene expression trajectory analysis showed that ABCC8 expression in the CBC and cortex increased during the early prenatal period. Given the positive association between ABCC8 expression and SCZ risk, abnormally elevated ABCC8 expression during this critical developmental phase may contribute to a heightened risk of SCZ, indicating potential neurodevelopmental dysregulation in patients with SCZ65. Furthermore, ABCC8 expression in the cortex was observed to increase gradually after infancy, raising the possibility that postnatal adverse environmental factors might abnormally activate ABCC8 expression and potentially contribute to increased SCZ risk. This hypothesis aligns with the two-hit model of SCZ66, although further studies are needed to substantiate this conjecture.
ABCC8 is enriched in T2D and insulin secretion, playing an important role in glucose metabolism67,68. Patients with SCZ have a high incidence of T2DM69. Accumulating evidence suggests that SCZ and T2DM share genetic vulnerabilities that affect key biological processes such as abnormal inflammation, oxidative stress, and dysfunction of the hypothalamic-pituitary-adrenal axis6. The PheWAS analysis also links ABCC8 to BMI, T2D, and quantitative traits of various innate immune cell subtypes, highlighting its central role in the metabolic-immune interface. Given the consistent involvement of metabolic syndrome and immune dysregulation in SCZ70,71, ABCC8 may contribute to disease development through converging neuro-metabolic-immune pathways. Additionally, central nervous system insulin metabolism affects SCZ by regulating neurotransmitter activities like dopamine and glutamate through the AKT signaling pathway and enhancing insulin-dependent glucose utilization in key brain regions72. Evidence from MR analysis showed that increased fasting insulin levels contributed to a higher risk of SCZ, consistent with findings from observational studies73. Furthermore, a previous study found tentative polygenic associations of SCZ with glucose metabolism abnormalities using polygenic risk scores and LD score regression74. A meta-analysis of PET imaging studies found significantly reduced glucose metabolism in the frontal cortex of patients with SCZ, particularly in chronic and medicated cases, supporting the hypofrontality hypothesis in SCZ9.
Given the causal association between increased ABCC8 expression and elevated SCZ risk, sulfonylureas and glinides that inhibit the ABCC8 subunit of K-ATP channels, especially in brain tissues, may represent promising candidates for managing SCZ. These antidiabetic drugs reduce blood glucose levels by binding to and inhibiting the ABCC8 subunit of the K-ATP channel on pancreatic β-cell membranes. This inhibition leads to K-ATP channel closure, membrane depolarization, and subsequent insulin secretion75. Therefore, the use of sulfonylurea and glinide drugs might affect SCZ, partly due to their influence on insulin release and glucose metabolism. Besides, sulfonylureas, by regulating pathways associated with oxidative stress, might affect dopamine transmission in patients with SCZ76. This is suggested by the mechanism where glutamate activates AMPA receptors to generate hydrogen peroxide, which can diffuse from postsynaptic sites and open sulfonylurea-sensitive potassium channels, thereby inhibiting presynaptic dopamine release. Additionally, sulfonylurea analogs, by inhibiting the nucleotide-binding oligomerization domain-like receptor protein 3-linked inflammatory pathways, show promise for preventing inflammation-associated neurological disorders such as SCZ through reducing inflammatory responses and microglial activation77. After searching the DrugBank, ChEMBL, and DGIdb databases, we found no approved medications, other than sulfonylurea and glinide drugs, that act as antagonists or inhibitors of ABCC8. Future research should investigate whether sulfonylurea and glinide drugs could be effective adjunctive treatments for SCZ, necessitating further clinical studies to explore their association with SCZ risk.
The lack of Bonferroni-significant effects for ADHD, ASD, OCD, AN, and MDD may reflect the higher SNP-based heritability and larger GWAS sample sizes for SCZ and BD31,32, which confer greater power for MR. Additionally, greater diagnostic heterogeneity in traits like ADHD, ASD, OCD, and AN may dilute genetic signals27,28,29,30, reducing the ability to detect modest causal effects after multiple testing correction. Moreover, the lack of Bonferroni-significant pQTL signals likely reflects limited genomic and anatomical coverage (only 12 of 32 proteins quantified, primarily in the dorsolateral prefrontal cortex, excluding GANC and key regions like the basal ganglia), signal dilution due to the small fraction of disease-relevant VIP/LAMP5 interneurons in bulk cortical homogenate78, the age gap between ROSMAP donors and the typical onset of schizophrenia and bipolar disorder, as protein regulation changes with age79, and the decoupling of mRNA and protein levels due to post-transcriptional and post-translational regulation80.
Our study offers several key strengths. First, it ensures a comprehensive investigation of potential associations by encompassing globally approved antidiabetic drugs, their target genes, and a wide range of molecular QTL data, including gene expression across diverse brain regions, cell types, and whole blood, as well as protein abundance in brain tissues. Additionally, our findings were rigorously validated through Bonferroni correction, colocalization analysis, and replication MR analyses, demonstrating the robustness and reliability of the results. Lastly, this MR analysis employs lifelong genetic proxies for drug exposure, effectively minimizing confounding by distinguishing the effects of antidiabetic drugs from diabetes itself, achieved through the random allocation of genetic variants at conception.
Our study has several limitations. First, our study can only predict the on-target effects of antidiabetic drugs and cannot infer effects that are not mediated by these targets. Second, all datasets were drawn from participants of European ancestry, and the QTL cohorts are modest in size relative to typical GWAS, which may restrict the sensitivity of our analyses and the generalizability of the findings, particularly to non-European populations. Replication in larger cohorts with greater ethnic and age diversity is therefore warranted. Third, using only top SNPs as IVs may reduce the efficiency of MR. Therefore, we employed strong IVs, colocalization validation, and replication MR analyses. Lastly, given the limited blood-brain barrier permeability of commonly used oral sulfonylurea formulations and the absence of large-scale clinical trials providing direct evidence81,82, caution is warranted in repurposing existing sulfonylurea and glinide drugs for SCZ.
Overall, our study suggests that GANC may serve as a protective gene for bipolar disorder, while ABCC8 may be a risk gene for schizophrenia. These findings identify novel targets for the treatment of psychiatric disorders and propose the use of sulfonylurea and glinide drugs as potential adjunct therapies for managing schizophrenia. Further clinical studies are required to explore the impact of antidiabetic drugs on psychiatric disorders prospectively, focusing on their mechanisms, efficacy, and safety.
Data availability
The GWAS data, eQTL data, and pQTL data sources are listed in Additional file 2: Table S2. All other data and codes in this study are available upon reasonable request to the corresponding author.
Code availability
All analyses were performed using R version 4.3.1. All code used in this study is available from the corresponding author upon reasonable request.
References
GBD 2019 Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9, 137–150 (2022).
Cipriani, A. et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366 (2018).
Miyamoto, S., Miyake, N., Jarskog, L. F., Fleischhacker, W. W. & Lieberman, J. A. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry 17, 1206–1227 (2012).
Anderson, R. J., Freedland, K. E., Clouse, R. E. & Lustman, P. J. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diab. Care 24, 1069–1078 (2001).
Chen, M.-H. et al. Type 1 diabetes mellitus and risks of major psychiatric disorders: a nationwide population-based cohort study. Diab. Metab. 48, 101319 (2022).
Mizuki, Y. et al. Mechanisms underlying the comorbidity of schizophrenia and type 2 diabetes mellitus. Int. J. Neuropsychopharmacol. 24, 367–382 (2021).
Tao, H. et al. Psychiatric disorders and Type 2 diabetes mellitus: a bidirectional Mendelian randomization. Eur. J. Clin. Invest. 53, e13893 (2023).
Campayo, A., Gómez-Biel, C. H. & Lobo, A. Diabetes and depression. Curr. Psychiatry Rep. 13, 26–30 (2011).
Townsend, L. et al. Brain glucose metabolism in schizophrenia: a systematic review and meta-analysis of 18FDG-PET studies in schizophrenia. Psychol. Med. 53, 4880–4897 (2023).
Wu, C. et al. Cerebral glucose metabolism in bipolar disorder: a voxel-based meta-analysis of positron emission tomography studies. Brain Behav. 11, e02117 (2021).
Bryll, A. et al. Oxidative-antioxidant imbalance and impaired glucose metabolism in schizophrenia. Biomolecules 10, 384 (2020).
Tang, B. et al. Genetic variation in targets of antidiabetic drugs and Alzheimer disease risk: a Mendelian randomization study. Neurology 99, e650–e659 (2022).
Hsu, C.-C., Wahlqvist, M. L., Lee, M.-S. & Tsai, H.-N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimers Dis. 24, 485–493 (2011).
Corbett, A. et al. Drug repositioning for Alzheimer’s disease. Nat. Rev. Drug Discov. 11, 833–846 (2012).
Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015).
Zhao, G. et al. Association of intestinal anti-inflammatory drug target genes with psychiatric disorders: a Mendelian randomization study. J. Adv. Res. 70, 545–553 (2024).
Lu, Z. et al. The association of redox regulatory drug target genes with psychiatric disorders: a Mendelian randomization study. Antioxidants 13, 398 (2024).
Chen, Z. et al. A comprehensive assessment of the association between common drugs and psychiatric disorders using Mendelian randomization and real-world pharmacovigilance database. EBioMedicine 107, 105314 (2024).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 326, 1614–1621 (2021).
Wishart, D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2018).
Mendez, D. et al. ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. 47, D930–D940 (2019).
GTEx Consortium The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Pietzner, M. et al. Genetic architecture of host proteins involved in SARS-CoV-2 infection. Nat. Commun. 11, 6397 (2020).
Bennett, D. A. et al. Religious orders study and rush memory and aging project. J. Alzheimers Dis. 64, S161–S189 (2018).
Wingo, A. P. et al. Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat. Genet. 53, 143–146 (2021).
Bowden, J. et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol. 45, 1961–1974 (2016).
Demontis, D. et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 51, 63–75 (2019).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Duncan, L. et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am. J. Psychiatry 174, 850–858 (2017).
International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS) Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry 23, 1181–1188 (2018).
Mullins, N. et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 53, 817–829 (2021).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Howard, D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352 (2019).
Burgess, S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 43, 922–929 (2014).
Brion, M.-J. A., Shakhbazov, K. & Visscher, P. M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 42, 1497–1501 (2013).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, e34408 (2018).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Zuber, V. et al. Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches. Am. J. Hum. Genet. 109, 767–782 (2022).
Zhang, N., Li, Y., Sundquist, J., Sundquist, K. & Ji, J. Identifying actionable druggable targets for breast cancer: Mendelian randomization and population-based analyses. EBioMedicine 98, 104859 (2023).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53, 1300–1310 (2021).
Emani, P. S. et al. Single-cell genomics and regulatory networks for 388 human brains. Science 384, eadi5199 (2024).
Li, M. et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 362, eaat7615 (2018).
Liu, M. et al. Investigating the shared genetic architecture between depression and subcortical volumes. Nat. Commun. 15, 7647 (2024).
Watanabe, K. et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 51, 1339–1348 (2019).
Durinck, S. et al. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 21, 3439–3440 (2005).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Cannon, M. et al. DGIdb 5.0: rebuilding the drug-gene interaction database for precision medicine and drug discovery platforms. Nucleic Acids Res. 52, D1227–D1235 (2024).
Pierce, B. L., Ahsan, H. & Vanderweele, T. J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 40, 740–752 (2011).
Wee, A. S., Nhu, T. D., Khaw, K. Y., Tang, K. S. & Yeong, K. Y. Linking diabetes to Alzheimer’s disease: potential roles of glucose metabolism and alpha-glucosidase. Curr. Neuropharmacol. 21, 2036–2048 (2023).
Kloiber, S. et al. Neurodevelopmental pathways in bipolar disorder. Neurosci. Biobehav. Rev. 112, 213–226 (2020).
Fletcher, K., Parker, G. & Manicavasagar, V. Behavioral activation system (BAS) differences in bipolar I and II disorder. J. Affect Disord. 151, 121–128 (2013).
Karlsson Linnér, R. et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat. Genet. 51, 245–257 (2019).
Bhat, P. J. Galactose-1-phosphate is a regulator of inositol monophosphatase: a fact or a fiction?. Med Hypotheses 60, 123–128 (2003).
Hirai, S. et al. High-sucrose diets contribute to brain angiopathy with impaired glucose uptake and psychosis-related higher brain dysfunctions in mice. Sci. Adv. 7, eabl6077 (2021).
Sodani, K., Patel, A., Kathawala, R. J. & Chen, Z.-S. Multidrug resistance associated proteins in multidrug resistance. Chin. J. Cancer 31, 58 (2012).
De Franco, E. et al. Update of variants identified in the pancreatic β-cell KATP channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes. Hum. Mutat. 41, 884–905 (2020).
Avshalumov, M. V. & Rice, M. E. Activation of ATP-sensitive K+ (K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc. Natl. Acad. Sci. USA 100, 11729–11734 (2003).
Zhou, Y. et al. Enhanced MK-801-induced locomotion in Kir6.2 knockout mice. Neurosci. Res. 74, 195–199 (2012).
Bowman, P. et al. Cognitive, neurological, and behavioral features in adults with KCNJ11 neonatal diabetes. Diabetes Care 42, 215–224 (2019).
Akhondzadeh, S. et al. Diazoxide in the treatment of schizophrenia: novel application of potassium channel openers in the treatment of schizophrenia. J. Clin. Pharm. Ther. 27, 453–459 (2002).
Harmar, A. J. et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 166, 4–17 (2012).
Okuda, T. et al. Alterations in inhibitory neuron subtype-selective transcripts in the prefrontal cortex: comparisons across schizophrenia and mood disorders. Psychol. Med. 54, 1–10 (2024).
Cai, W. et al. An integrative analysis of identified schizophrenia-associated brain cell types and gene expression changes. Int. J. Mol. Sci. 23, 11581 (2022).
Lewis, D. A. & Levitt, P. Schizophrenia as a disorder of neurodevelopment. Annu. Rev. Neurosci. 25, 409–432 (2002).
Maynard, T. M., Sikich, L., Lieberman, J. A. & LaMantia, A. S. Neural development, cell-cell signaling, and the ‘two-hit’ hypothesis of schizophrenia. Schizophr. Bull. 27, 457–476 (2001).
Butnariu, L. I. et al. Congenital hyperinsulinism caused by mutations in ABCC8 gene associated with early-onset neonatal hypoglycemia: genetic heterogeneity correlated with phenotypic variability. Int. J. Mol. Sci. 25, 5533 (2024).
Goto, K., Mineta, K., Miyazaki, S. & Gojobori, T. Significant variants of type 2 diabetes in the Arabian Region through an Integration of exome databases. PLoS ONE16, e0249226 (2021).
Stubbs, B., Vancampfort, D., De Hert, M. & Mitchell, A. J. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr. Scand. 132, 144–157 (2015).
Jackson, A. J. & Miller, B. J. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr. Scand. 142, 18–26 (2020).
Mitchell, A. J. et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders-a systematic review and meta-analysis. Schizophr. Bull. 39, 306–318 (2013).
Agarwal, S. M. et al. Brain insulin action in schizophrenia: something borrowed and something new. Neuropharmacology 163, 107633 (2020).
Li, Z. et al. Glucose and insulin-related traits, type 2 diabetes and risk of schizophrenia: a Mendelian randomization study. EBioMedicine 34, 182–188 (2018).
So, H.-C., Chau, K.-L., Ao, F.-K., Mo, C.-H. & Sham, P.-C. Exploring shared genetic bases and causal relationships of schizophrenia and bipolar disorder with 28 cardiovascular and metabolic traits. Psychol. Med. 49, 1286–1298 (2019).
Lv, W., Wang, X., Xu, Q. & Lu, W. Mechanisms and characteristics of sulfonylureas and glinides. Curr. Top. Med. Chem. 20, 37–56 (2020).
Avshalumov, M. V., Chen, B. T., Marshall, S. P., Peña, D. M. & Rice, M. E. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J. Neurosci. 23, 2744–2750 (2003).
Zhang, C. et al. Targeting NLRP3 signaling by a novel-designed sulfonylurea compound for inhibition of microglial inflammation. Bioorg. Med. Chem. 58, 116645 (2022).
Tasic, B. et al. Shared and distinct transcriptomic cell types across neocortical areas. Nature 563, 72–78 (2018).
Klaips, C. L., Jayaraj, G. G. & Hartl, F. U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 217, 51–63 (2018).
Vogel, C. & Marcotte, E. M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13, 227–232 (2012).
Guo, L. & Xiao, X. Guideline for the management of diabetes mellitus in the elderly in China (2024 Edition). Aging Med. 7, 5–51 (2024).
Lahmann, C., Kramer, H. B. & Ashcroft, F. M. Systemic administration of glibenclamide fails to achieve therapeutic levels in the brain and cerebrospinal fluid of rodents. PLoS ONE 10, e0134476 (2015).
Acknowledgements
We thank GTEx, eQTLGen, and PsychENCODE for providing the eQTL transcriptome dataset; ROSMAP for providing the pQTL proteome dataset; PGC for providing the psychiatry-related phenotype datasets; and all cited studies for sharing data, software, and platform. This work was supported by the National Natural Science Foundation of China (82330042); National Key R&D Program of China (2023YFE0119400, 2021YFF1201100); Capital’s Funds for Health Improvement and Research (2024-1-4111); Fundamental Research Funds for the Central Universities (Peking University Medicine Fund for world’s leading discipline and discipline cluster development, BMU2022DJXK007); Beijing Municipal Health Commission Research Ward Programme (3rd batch); Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2023-PT320-08). This work was also supported by Grant BX20240029 from the National Postdoctoral Program for Innovative Talents (Zhe Lu). The funders did not have any role in the design of the study, and collection, analysis, and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Rui Yuan: conceptualization, methodology, data curation, formal analysis, visualization, writing - original draft; Guorui Zhao: conceptualization, methodology, data curation, formal analysis, writing - review & editing; Zhe Lu: conceptualization, methodology, formal analysis, writing - review & editing, supervision, funding acquisition; Yunqing Zhu: methodology, data curation, resources, writing - review & editing, visualization; Zhewei Kang: methodology, software, resources, writing - review & editing; Yuyanan Zhang: resources, visualization, writing - review & editing; Yaoyao Sun: resources, writing - review & editing; Yang Yang, Yundan Liao, Xiaoyang Feng, Junyuan Sun, Jing Guo: writing - review & editing; Weihua Yue: conceptualization, investigation, resources, writing - review & editing, supervision, project administration, funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical
Only publicly available data were used in this study. Ethical approval and consent to participation were available in the original studies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41537_2025_664_MOESM1_ESM.pdf
Additional file 1. Supplementary Methods, Figs. S1–S7, and STROBE-MR checklist
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, R., Zhao, G., Lu, Z. et al. Association between antidiabetic drug targets and psychiatric disorders. Schizophr 11, 116 (2025). https://doi.org/10.1038/s41537-025-00664-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-025-00664-4